Abstract
OBJECTIVES:
The impact of age on hospital survival for patients treated with extracorporeal cardiopulmonary resuscitation (ECPR) for cardiac arrest (CA) is unknown. We sought to characterize the association between older age and hospital survival after ECPR, using a large international database.
DESIGN:
Retrospective analysis of the Extracorporeal Life Support Organization registry.
PATIENTS:
Patients 18 years old or older who underwent ECPR for CA between December 1, 2016, and October 31, 2020.
MEASUREMENTS AND MAIN RESULTS:
The primary outcome was adjusted odds ratio (aOR) of death after ECPR, analyzed by age group (18–49, 50–64, 65–74, and > 75 yr). A total of 5,120 patients met inclusion criteria. The median age was 57 years (interquartile range, 46–66 yr). There was a significantly lower aOR of survival for those 65–74 (0.68l 95% CI, 0.57–0.81) or those greater than 75 (0.54; 95% CI, 0.41–0.69), compared with 18–49. Patients 50–64 had a significantly higher aOR of survival compared with those 65–74 and greater than 75; however, there was no difference in survival between the two youngest groups (aOR, 0.91; 95% CI, 0.79–1.05). A sensitivity analysis using alternative age categories (18–64, 65–69, 70–74, and ≥ 75) demonstrated decreased odds of survival for age greater than or equal to 65 compared with patients younger than 65 (for age 65–69: odds ratio [OR], 0.71; 95% CI, 0.59–0.86; for age 70–74: OR, 0.84; 95% CI, 0.67–1.04; and for age ≥ 75: OR, 0.64; 95% CI, 0.50–0.81).
CONCLUSIONS:
This investigation represents the largest analysis of the relationship of older age on ECPR outcomes. We found that the odds of hospital survival for patients with CA treated with ECPR diminishes with increasing age, with significantly decreased odds of survival after age 65, despite controlling for illness severity and comorbidities. However, findings from this observational data have significant limitations and further studies are needed to evaluate these findings prospectively.
Keywords: cardiac arrest, extracorporeal cardiopulmonary resuscitation, extracorporeal membrane oxygenation, geriatrics, survival
Cardiac arrest (CA) is a leading cause of mortality worldwide (1). In the United States alone, approximately 370,000 out-of-hospital cardiac arrests (OHCAs) and 200,000 in-hospital cardiac arrests (IHCAs) occur yearly (2). Nearly half of CA patients are greater than or equal to 65 years old, and many are greater than or equal to 75 (3, 4). As the population ages, the occurrence rate of OHCA and IHCA is expected to rise, as are the rates of attempted cardiopulmonary resuscitation (CPR) (5, 6). Despite significant advances in CA care, mortality remains high: less than or equal to 10% of OHCA patients and 25% of IHCA patients will survive with a good neurologic outcome (2, 7). Numerous studies have demonstrated that older adults have a decreased likelihood of survival after CPR (8–17).
When extracorporeal membrane oxygenation (ECMO) is used for CA refractory to CPR, it is referred to as extracorporeal CPR (ECPR). Use of ECPR has grown substantially over the last decade (18, 19). Yet evidence supporting ECPR is mixed. Several observational studies have demonstrated a positive association between EPCR and survival (20–23), however, more recently, three randomized controlled trials have shown conflicting results for ECPR and survival (compared with conventional CPR) (24–26). Notably, the age of exclusion in these trials ranged from less than or equal to 65 to less than or equal to 75 (24–26).
The Extracorporeal Life Support Organization (ELSO) guidelines recommend using an ECPR age cutoff of 70 years, however, little evidence supports this recommendation (27). Literature addressing age and outcomes after ECMO for cardiac support in general is sparse, and primarily focuses on heart failure, not CA (28, 29). Only two previous studies look specifically at age and ECPR outcomes (27, 30). The first examined ECPR patients from 1998 to 2008, and found no association between age and mortality (30). However, this study made no attempt to adjust for covariates, and cannot account for the impact of modern CPR practices, and therefore interpretation of results from this study are extremely limited. The second study, and the basis for the ELSO recommendations, is a single study from Japan, and thus are quite limited in terms of generalizability (27).
Given that ECPR is logistically challenging, resource intensive, and not widely available, improved identification of who is likely to benefit from ECPR is paramount (31). Specifically, as the population ages and the occurrence rate of CA rises, it is of the utmost importance to understand the benefit of ECPR for older adults. Therefore, we sought to characterize the association between older age and outcomes from ECPR for CA, using the largest existing ECMO database, the ELSO registry (32).
MATERIALS AND METHODS
Data Source and Population
We queried the international ELSO Registry, the largest, international ECMO database, containing over 125,000 patients (32). A detailed description of the registry has been previously published (33). The study was reviewed and deemed exempt by the University of New Mexico Institutional Review Board (Study No. 21–105, 9/27/2021; ECMO for Cardiac Arrest), and followed the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) guidelines for reporting observational studies (34).
We included all patients from December, 1, 2016, to October 31, 2020, who were greater than or equal to 18 years, with ECPR, and whose initial cannulation configuration was peripheral venoarterial ECMO. Since our objective was to describe the association between age and hospital mortality among ECPR patients suffering from OHCA or IHCA, we excluded patients whose initial cannulation configuration was central (e.g., aortic), because central cannulations overwhelmingly occur in a select group of patients (e.g., cardiac surgery patients), who are unlikely to have undergone ECPR as a direct result of a primary diagnosis of CA. We also excluded patients whose initial configuration was for venovenous ECMO. Finally, we reported results for secular trends in ECPR from December 1, 2010, to October 31, 2020.
Outcome Variables
The primary outcome was the adjusted odds of in-hospital death. Secondary outcomes included the adjusted odds for the duration of ECMO support and the hospital length of stay (LOS).
Exposure Variables
The primary exposure was age. We performed bivariate analysis with prespecified, variables, based on the literature and availability: sex; pre-ECMO mechanical cardiac support; pre-ECMO renal replacement therapy (RRT); body mass index (BMI) (< 25, 25–29.9, and ≥ 30); and initial post-cannulation pH (< 7.0, 7.01–7.2, and > 7.2). We also included the 17 diagnoses in the Charlson Comorbidity Index (CCI) (35, 36), as well as the CCI category (0, 1–2, or ≥ 3 comorbidities). We chose to include comorbidities and overall CCI category because certain comorbidities pose a specific risk to ECPR patients (e.g., cardiac disease), while overall CCI score better approximates medical complexity. Finally, we evaluated “center volume” (total cases per year), and hospital mortality rates.
Statistical Analysis
We calculated descriptive statistics using medians and interquartile range (IQR) for continuous variables, and counts and percentages for categorical variables. For categorical variables, we performed bivariate analysis using chi-square or Fisher exact test. Age evaluated categorically (18–49, 50–64, 65–74, and ≥ 75), to allow for comparison with previous research and existing protocols (28, 30, 37). We performed a supplementary analysis using staggered age categories. Given that the registry assigns all patients greater than or equal to 80 an age of 80 (for de-identification), we did not analyze age as a continuous variable.
For numerical variables with missing values, (i.e., pH, BMI), we imputed the cohort median. For comorbidities, and pre-ECLS support, we assumed a value of zero indicated its absence. We excluded variables with greater than 50% missingness, a priori. Thus, witnessed arrest, initial heart rhythm, and arrest location were excluded.
Model 1 estimated unadjusted odds ratio (OR) of survival for each age category using bivariate logistic regression. Model 2 was constructed using variables selected a priori based on the strength of their association in the literature (CCI, cardiac disease, cardiac support, renal disease) (36, 37), as well variables reaching significance in bivariate analysis (p < 0.05), after testing for collinearity. For model 3, we adjusted only for those variables that reached significance in model 2 (p < 0.1), and included interaction terms of clinically relevant covariates to estimate marginal effects of survival, conditional on these covariates and age.
For the secondary outcomes (hours on ECMO and hospital LOS), we performed an unadjusted survival analysis, using product-limit Kaplan-Meier curves. For LOS analysis, we truncated analysis at 150 days, given that after this timepoint survival is no longer driven by initial CA or ECPR (38, 39) (i.e., these patients likely remain hospitalized due to nonmedical reasons like insurance status, bed availability, and geographic distance) (40). For the adjusted analyses of the secondary outcomes, we used the same age categories and variables from model 2 to fit Cox proportional hazards model. The proportional hazards assumption for the main exposure variable—age—was checked against the log-rank test. We used SAS Version 9.4 (SAS Institute, Cary, NC) and set significance levels to 0.05.
RESULTS
Overall Results
There were 5,120 patients in the analysis after exclusion (Fig. 1). The number of ECPR cases increased from 2010 to 2019 across all age groups (note that 2020 data are only through October) (Fig. 2). The majority (75.3%) of ECPR encounters occurred in the later portion of the study period (2016–2020). There was no significant proportional change in annual cases of ECPR for those greater than or equal to 65 as compared with those less than 65.
Figure 1.
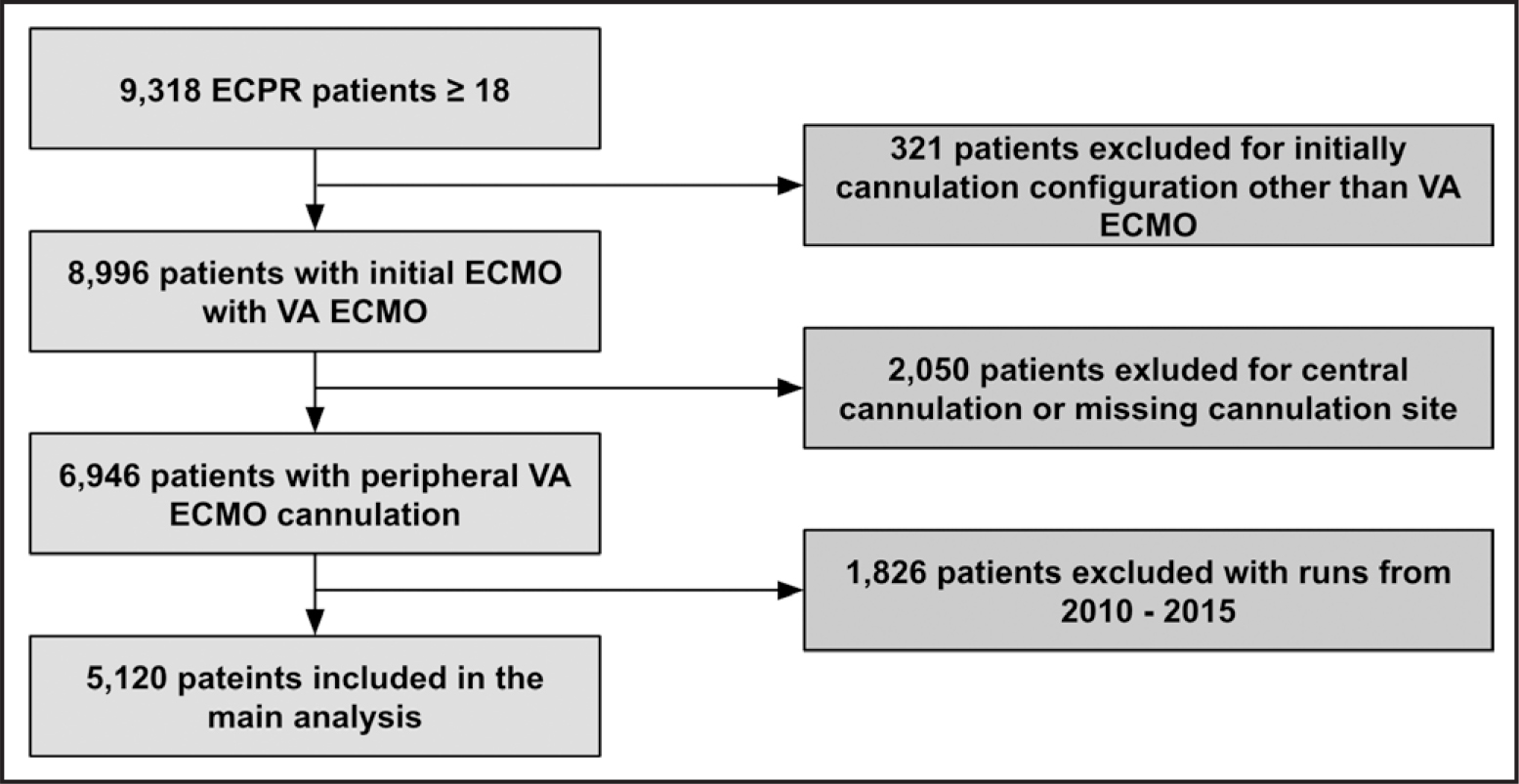
Flow diagram. ECMO = extracorporeal membrane oxygenation, ECPR = extracorporeal cardiopulmonary resuscitation, VA = venoarterial.
Figure 2.
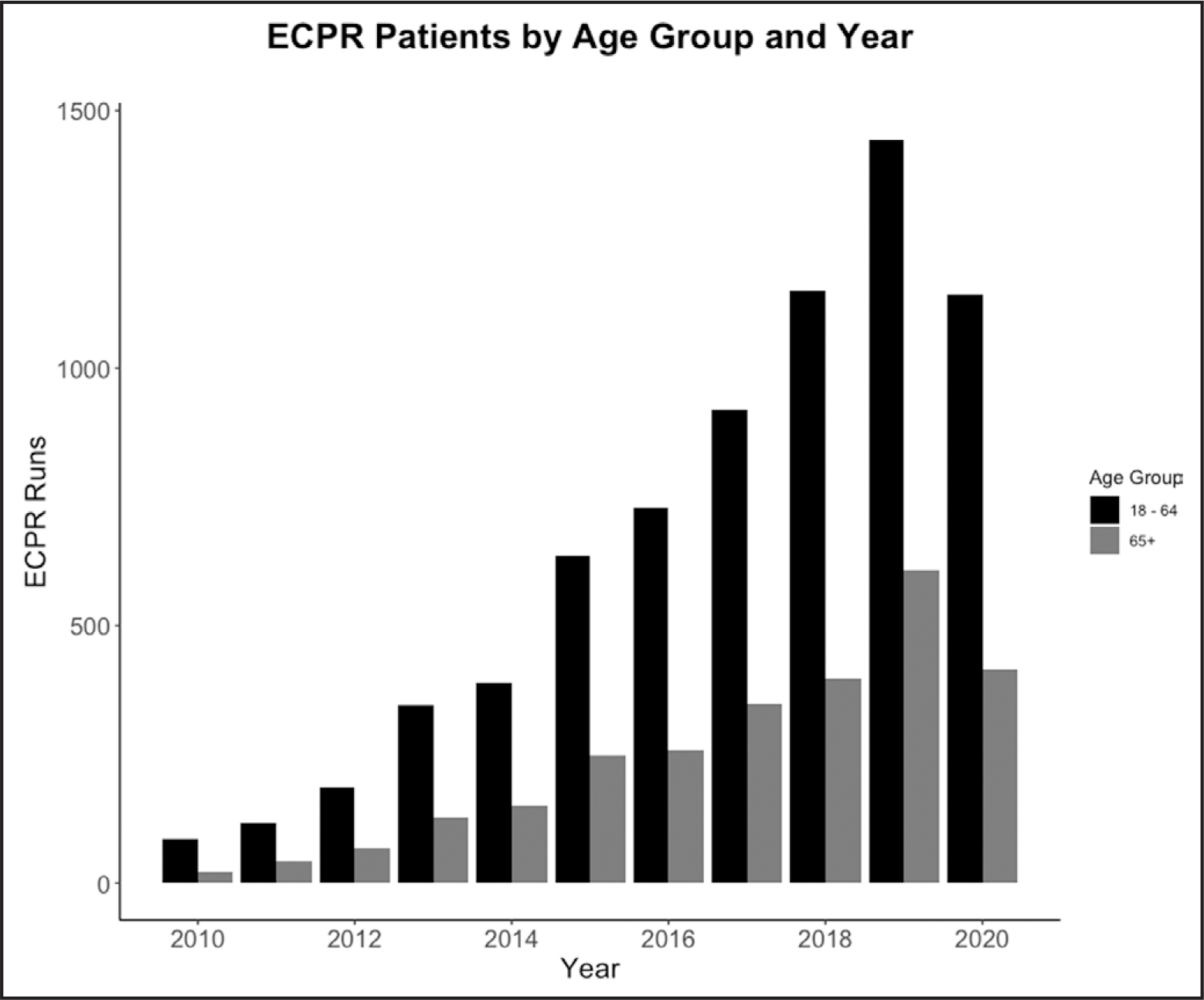
Extracorporeal cardiopulmonary resuscitation (ECPR) patients by age group and year.
The majority of patients were male (70%) and White (57.9%) (Table 1). The median age was 57 years (IQR, 46–66 yr). The overall mortality was 69.3%. Survivors were younger than decedents (55 yr [IQR, 45–64 yr] vs 58 yr [IQR, 46–66 yr], respectively [p < 0.0001]). The rate of discharge alive was lowest in those greater than or equal to 75 (23.5%; p < 0.001) compared with those 18–49, 50–64, and 65–74. Comorbidities were infrequent (Supplementary Table 1, http://links.lww.com/CCM/H418).
TABLE 1.
Patient Characteristics and Univariable Analysis of Hospital Survival
| Characteristic(s) | Overall, n (%) | Discharged Alive, n (%) | p |
|---|---|---|---|
|
| |||
| Total | 5,120 (100) | 1,572 (30.7) | |
|
| |||
| Sex | |||
| Male | 3,585 (70.0) | 1,085 (30.3) | 0.29 |
| Female | 1,512 (29.5) | 481 (31.8) | |
| Missing/unknown | 23 (0.004) | 6 (26.2) | |
|
| |||
| Age (yr) | |||
| Group 1 (18–49) | 1,638 (32.0) | 546 (33.3) | < 0.001 |
| Group 2 (50–64) | 2,018 (39.4) | 646 (32.0) | |
| Group 2 (65–74) | 1,069 (20.9) | 287 (26.9) | |
| Group 4 (≥ 75) | 395 (7.71) | 93 (23.5) | |
|
| |||
| Race | |||
| White | 2,965 (57.9) | 944 (31.8) | 0.823a |
| Asian | 830 (16.2) | 259 (31.15) | |
| African American | 477 (9.3) | 150 (31.5) | |
| Hispanic | 206 (4.0) | 58 (28.2) | |
| Other | 390 (7.6) | 118 (30.3) | |
| Missing/unknown | 252 (4.9) | 43 (17.1) | |
|
| |||
| Body mass index | |||
| < 25 | 1,398 (27.3) | 497 (35.6) | < 0.001 |
| 25–29.9 | 2,198 (42.9) | 622 (28.3) | |
| ≥ 30 | 1,524 (29.8) | 453 (29.7) | |
| Missing | 730 (14.3) | Not available | |
|
| |||
| pH (n = 3,229) | |||
| < 7.0 | 976 (19.1) | 240 (24.6) | < 0.001 |
| 7.01 –7.2 | 2,939 (57.4) | 899 (30.6) | |
| ≥ 7.2 | 1,205 (23.5) | 433 (35.9) | |
| Missing | 1,910 (37.3) | Not available | |
|
| |||
| Charlson Comorbidity Index | |||
| 0 | 2,978 (58.2) | 901 (30.3) | 0.656 |
| 1 –2 | 1,999 (39.0) | 624 (31.2) | |
| ≥ 30 | 143 (2.8) | 47 (32.9) | |
|
| |||
| Pre-extracorporeal membrane oxygenation interventions | |||
| Cardiac supportb | 920 (18.0) | 274 (29.8) | 0.504 |
| Renal replacement therapy | 217 (4.2) | 44 (20.3) | < 0.001 |
χ2 testing, does not include “missing/unknown race.”
Bi, right, or left ventricular assistance device, pacemaker, cardiopulmonary bypass, intra-aortic balloon pump, or percutaneous ventricular assist device.
For LOS, 56 patients were dropped from the cohort (54 had missing LOS, and two had negative values), leaving 5,064 patients for analysis. Median LOS in all comers and survivors was 8 days (IQR, 2–22 d) and 25 days (IQR, 15–42 d), respectively (Supplementary Table 2, http://links.lww.com/CCM/H418). Among descendants the median time-to-death was 4 days (IQR, 1–11 d). Decedents had both fewer hours on ECMO and shorter LOS than survivors (Supplementary Fig. 1, http://links.lww.com/CCM/H418).
Unadjusted Results by Age
In the unadjusted analysis of hospital survival by age (model 1), the two oldest groups (65–74 and ≥ 75) had the lowest odds of survival compared with the youngest group (18–49) (OR, 0.73; 95% CI, 0.62–0.87; p < 0.0004 and OR, 0.62; 95% CI, 0.48–0.79; p < 0.0002, respectively) (Table 2). There was no significant difference in odds of survival in those 50–64 compared with 18–49 (OR, 0.94; 95% CI, 0.82–1.08; p = 0.40) (Table 2). Between-category unadjusted odds of survival reflect this trend (Supplementary Table 3, http://links.lww.com/CCM/H418).
TABLE 2.
Survival Models of Extracorporeal Cardiopulmonary Resuscitation by Age Category
| Age Category | Estimatea | OR (Lower CI-Upper CI) | P |
|---|---|---|---|
|
| |||
| Model 1–Survival by age category, unadjusted | |||
| Group 2 (50–64) | −0.06 | 0.94 (0.82–1.08) | 0.40 |
| Group 3 (65–74) | −0.31 | 0.73 (0.62–0.87) | < 0.001 |
| Group 4 (≥ 75) | −0.48 | 0.62 (0.48–0.79) | < 0.001 |
|
| |||
| Model 2–Survival of ECPR by age, adjusted for prespecified variables | |||
| Group 2 (50–64) | −0.10 | 0.91 (0.79–1.05) | 0.19 |
| Group 3 (65–74) | −0.39 | 0.68 (0.57–0.81) | < 0.001 |
| Group 4 (≥ 75) | −0.62 | 0.54 (0.41–0.69) | < 0.001 |
|
| |||
| Model 3–Survival of ECPR by age, parsimonious model with interaction terms | |||
| Group 2 (50–64) | −0.57 | 0.58 (0.38–0.85) | < 0.001 |
| Group 2 (65–74) | −0.59 | 0.55 (0.33–0.94) | 0.03 |
| Group 4 (≥ 75) | −0.79 | 0.45 (0.17–1.20) | 0.11 |
ECPR = extracorporeal cardiopulmonary resuscitation, OR = odds ratio.
Group 1 (18–49 yr) referent category.
ECPR recipients across all age categories had similar unadjusted LOS and duration of their ECMO run (Fig. 3 and Table 3). This finding also was demonstrated after stratifying the analysis by survival and age (Supplementary Fig. 2, http://links.lww.com/CCM/H418).
Figure 3.
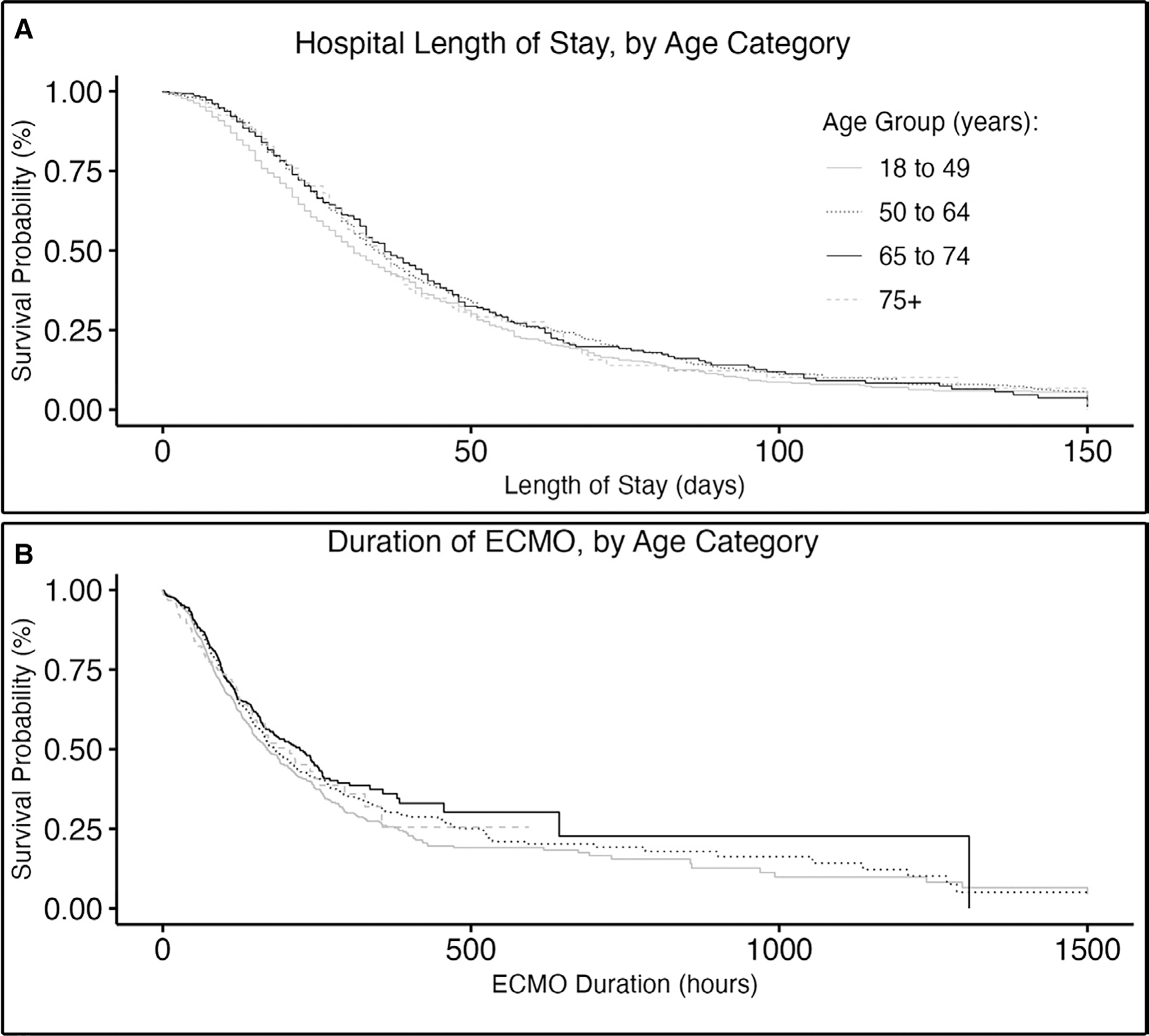
Duration of treatment by age category. ECMO = extracorporeal membrane oxygenation.
TABLE 3.
Duration of Treatment, by Age Category
| Age Category | Estimatea | Hazard Ratio (Lower CI-Upper CI) | P |
|---|---|---|---|
|
| |||
| Duration of ECMO (unadjusted model) | |||
| Group 2 (50–64) | −0.18 | 0.83 (0.71–0.98) | 0.025 |
| Group 3 (65–74) | −0.13 | 0.88 (0.73–1.06) | 0.179 |
| Group 4 (≥ 75) | 0.01 | 1.01 (0.82–1.30) | 0.914 |
|
| |||
| Duration of ECMO (adjusted model) | |||
| Group 2 (50–64) | −0.17 | 0.84 (0.72–0.99) | 0.036 |
| Group 3 (65–74) | −0.12 | 0.89 (0.73–1.07) | 0.207 |
| Group 4 (≥ 75) | −0.01 | 0.99 (0.80–1.23) | 0.951 |
|
| |||
| Hospital length of stay (unadjusted) | |||
| Group 2 (50–64) | −0.18 | 0.83 (0.71–0.98) | 0.028 |
| Group 2 (65–74) | −0.03 | 0.97 (0.80–1.17) | 0.745 |
| Group 4 (≥ 75) | −0.07 | 0.93 (0.75–1.15) | 0.503 |
|
| |||
| Hospital length of stay (adjusted) | |||
| Group 2 (50–64) | −0.15 | 0.86 (0.73–1.01) | 0.071 |
| Group 2 (65–74) | −0.04 | 0.97 (0.80–1.17) | 0.711 |
| Group 4 (≥ 75) | −0.07 | 0.93 (0.75–1.16) | 0.536 |
ECMO = extracorporeal membrane oxygenation.
Group 1 (18–49 yr) referent category.
Given that we found a threshold for decreased odds of survival at age 65 using our original categories, we performed a supplementary analysis using alternative age categories at 5-year increments to evaluate whether 65 years would remain the threshold (i.e., 50–64, 65–69, 70–74, and ≥ 75) (Supplementary Table 4, http://links.lww.com/CCM/H418). We found significant decrease in the odds of survival among those age greater than 65 as compared those less than 65. The largest drop off in odds of survival was between age 50–64 versus 65–69.
Adjusted Results by Age
Six variables (age, pH, BMI, RRT, heart failure, and peripheral vascular disease) were significant (p < 0.05) in the univariable analysis (Table 1; and Supplementary Table 1, http://links.lww.com/CCM/H418). There was no relationship between survival and center volume (Supplementary Fig. 3, http://links.lww.com/CCM/H418). In model 2, there was a significant decrease in the odds of survival by age category (adjusted OR [aOR] for 65–74, and ≥ 75 vs 18–49 was 0.68 [95% CI, 0.57–0.81] and 0.54 [95% CI, 0.41–0.69], respectively) (Table 2). As with the previous models, there were no significant differences in the odds of survival between those 18–49 versus age 50–64.
Finally, we made a third, parsimonious model, limited to those variables from model 2 with a p value of less than 0.1 (age, pH, BMI, and pre-ECMO RRT), also including interaction terms for each of the significant variables (i.e., age × pH, age × BMI, and age × RRT), to examine the extent to which comorbidities and illness severity had a differential effect on outcome depending on age. The relationship between increased age and decreased survival remained present. There was a decrease in the aOR of survival between age groups 50–64, 65–74, and greater than or equal to 75 versus ages 18–49 (aOR, 0.58 [95% CI, 0.38–0.85]; aOR, 55 [95% CI, 0.33–0.95]; and aOR, 0.45 [95% CI, 0.17–1.20], respectively). With only the comparison between group 1 and 4 not meeting significance (Table 2). aOR comparing each age group to each other for models 2 and 3 are included in Supplementary Tables 5 and 6 (http://links.lww.com/CCM/H418).
In the adjusted analysis for secondary outcomes (hospital LOS and duration of ECMO), there was a trend toward younger patients having a shorter ECMO run duration and shorter hospital LOS compared with older patients. However, few comparisons were significant (Table 3).
DISCUSSION
This is the largest analysis of the relationship between age and hospital survival for patients treated with ECPR for CA. In an adjusted model of age on mortality, increasing age was associated with decreased survival for patients greater than or equal to 65 years old. These findings are consistent with the multiple studies demonstrating that age is a predictor of mortality in CA (8–14), and in patients requiring ECMO for other indications (28, 29), and substantially improve the quality of evidence around age and survival with ECPR. Importantly, while the ELSO Registry is the single largest existing ECMO database, significant limitations in the data necessitate that future prospective studies is conducted to confirm these findings.
The need for evidence-based guidance regarding ECPR in the elderly is pressing (27). While multiple groups are working to improve the efficiency, quality, effectiveness, and safety of the modality with encouraging results (41, 42), ECPR is a finite resource is associated with high complication rates and significant burden on the healthcare system (43). Therefore, many questions remain surrounding its use.
Despite a lack of evidence, many ECMO centers have been using age as an eligibility criteria for ECPR. A recent systematic review of institutional ECPR protocols identified age as criteria for exclusion in 60% of protocols, with the upper limit for inclusion ranging from age 55–80 years, with 70 being the most common (37). This is reflected in ELSO’s latest guidelines recommending age greater than 70 as an exclusion (27). Indeed, we found no significant proportional change in terms of the age of those receiving ECPR over the last decade.
Our study supports the consideration of age to guide selection, with the threshold effect occurring at age 65 rather than age 70. However, it is important to note that neither our findings, nor previously published evidence, support a rigid interpretation of age as a cutoff for ECPR. Our study, while significantly more robust than the evidence upon which the ELSO guidelines are based, should be interpreted cautiously. Attention must be given to the individual patient and specific clinical circumstance.
Interestingly, our findings do not support two of the most commonly expressed hypotheses for why older adults face higher mortality rates in ECMO: 1) older adults heal more slowly, taking longer to recover, leading to longer ECMO runs and increased complication rates, that in turn lead to higher mortality rates and 2) a perception of poor prognosis in older adults would lead to quicker withdrawal of life-sustaining treatment, thus leading to shorter ECMO runs and higher mortality. However, this was not supported by our analysis, which showed no significant difference in duration of ECMO or LOS by age.
As noted above, the ELSO registry is the largest and most comprehensive existing ECMO database and thus plays an important role in providing preliminary and hypothesis-driving analysis of this and other ECMO-related questions. ECMO stakeholders will benefit from building more robust databases to address important questions such as age in ECPR. Finally, given that, ECPR is a cutting-edge technology, secular trends in ECPR practices and ECPR survival are of critical importance. However, there was no change in proportion of older adults undergoing ECPR over a 10-year period, suggesting that clinicians have not shifted their approach to age as a selection criterion. Similarly, we did not notice any shift in mortality rates by year. Other secular trends in management and patient selection may be unaccounted for in this study.
Despite decades of concerted efforts, standard CPR care models have led to marginal improvements in survival after CA (44). Meanwhile, ECPR, a novel therapy with relatively limited availability, has become increasingly popular, despite uncertain benefit (31). Survival estimates for ECPR ranging widely from less than 15% to greater than 50% (23, 45–48), with serious concerns for selection bias (31), compared with 10–20% survival rate for standard CPR (7, 49). The implications of expanding access to ECPR on healthcare costs and resource utilization are substantial. In addition to cost and resource considerations (50), recipients of ECPR often must endure numerous invasive procedures and encounter multiple complications—this type of “heroic” treatment may not be concordant with the values or preferences for care of many older adults (51, 52). Taken together, our findings diminish hope that ECPR may prove to be an effective therapy for the hundreds of thousands of older adults who die of CA each year (1).
Historically, age greater than 70 has been used as an exclusion criterion in clinical ECPR guidelines (27), but little evidence exists to supporting such a guideline (30). Our study findings should prompt reexamination of the current recommended age cutoff, 70 years, despite broad uptake of this recommendation, there is scant evidence to support it (27, 30). Findings from this study suggest that there might be an important threshold affect at age greater than or equal to 65 (not ≥ 70). While based on a significantly larger and more rigorous analysis than the previous studies, our study’s findings should still be interpreted with caution. In order to guide delivery of high-value care for older adults, it is critical that the impact of older age on ECPR outcomes for OHCA with prospective data, and further evaluate the impact of unmeasured confounders.
There are several important limitations to our study. First, retrospective analysis has inherent risks for bias, such as selection bias and confounding bias. Given that each center contributing data to ELSO may use different criteria and/or norms in their ECPR patient selection, it is not possible to know if patients were selected for ECPR. For instance, centers may be more likely to use ECPR for “healthy” older patients than “unhealthy” younger patients. We attempted to mitigate this bias by controlling for comorbidities in our adjusted analysis. However, clinical teams may not be aware of the patient’s comorbidities or may underreport them. Indeed, we observed low rates of comorbidities in this cohort (Supplementary Table 1, http://links.lww.com/CCM/H418). Selection bias for ECPR may be inherently limited by the urgency of the condition: the decision to initiate ECMO support in the setting of CA is extremely time sensitive, often with only limited information available to the cannulation team. Conversely, while urgency may “limit” comorbidity-based selection of ECPR candidates, it’s possible comorbidities influence the decision to withdraw treatment. However, we found no significant difference between age groups and duration of ECMO or hospital LOS.
Due to missingness in the data, we were not able to control for certain variables known to impact CA outcomes, such as the location of the arrest, the duration of CPR, or initial rhythm. At the time of our investigation, these data was not available in the ELSO database or any comparable database of ECPR; however, future studies should strive to include these and other key variables. Our goal was to significantly strengthen the data used to support existing ELSO recommendations (which comes from a single-center study from Japan) (27). By excluding variables with greater than 50% missingness, we reduced the potential bias. The impact of missing data was further mitigated where possible by using imputation techniques.
CONCLUSIONS
Current international guidelines recommend limiting ECPR to patients less than 70 years old; however, this recommendation is based on limited evidence. Our investigation represents the largest analysis of the relationship between older age and ECPR outcomes. We found that odds of hospital survival for patients undergoing ECPR for CA diminishes for those over age 65, despite controlling for illness severity and comorbidities. However, given the limitations of this retrospective dataset, future prospective studies are needed to confirm these findings.
Supplementary Material
KEY POINTS.
Question:
What is the association between older age and hospital survival after extracorporeal cardiopulmonary resuscitation (ECPR) for refractory cardiac arrest (CA)?
Findings:
In this retrospective analysis, there was a significant decrease in the adjusted odds of survival for those age 65–74 and greater than 75 compared with patients 18–49, despite controlling for illness severity and comorbidities. However, observational data has significant limitations and findings must be evaluated prospectively.
Meaning:
In this study, the largest analysis of the relationship between age and ECPR outcomes, the odds of hospital survival for patients with CA treated with ECPR diminishes with increasing age, particularly for patients over the age of 65.
ACKNOWLEDGMENTS
We would like to acknowledge the Extracorporeal Life Support Organization (ELSO) registry and the ELSO Scientific Oversight Committee.
Dr. George is funded in part by grant number CTSC008-12 from the Clinical & Translational Science Center at the University of New Mexico, under the National Center for Advancing Translational Sciences, National Institutes of Health grant number UL1TR001449. Dr. Ouchi is supported by National Institute on Aging (K76AG064434) and Cambia Health Foundation; he received funding from Jolly Good; they received support for article research from the National Institutes of Health. Dr. Myaskovsky’s work on this project is funded in part by grant number C-3924 from Dialysis Clinic, a national nonprofit dialysis provider. Dr. Kamdar received funding from the University of New Mexico, Stanford University, Stanford University, and Lucent Surgical. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccmjournal).
REFERENCES
- 1.Graham R, McCoy MA, Schultz AM; Directions C on the T of CACS and F, Policy B on HS, Medicine I of: Understanding the Public Health Burden of Cardiac Arrest: The Need for National Surveillance. Washington, DC, National Academies Press (US), 2015 [Google Scholar]
- 2.Benjamin EJ, Muntner P, Alonso A, et al. ; on behalf of the American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee: Heart disease and stroke statistics—2019 update: A report from the American Heart Association. Circulation 2019; 139:e56–e528 [DOI] [PubMed] [Google Scholar]
- 3.McNally B, Robb R, Mehta M, et al. ; Centers for Disease Control and Prevention: Out-of-hospital cardiac arrest surveillance --- Cardiac Arrest Registry to Enhance Survival (CARES), United States, October 1, 2005--December 31, 2010. Morb Mortal Wkly Rep Surveill Summ Wash DC 2002 2011; 60:1–19 [PubMed] [Google Scholar]
- 4.Peberdy MA, Kaye W, Ornato JP, et al. : Cardiopulmonary resuscitation of adults in the hospital: A report of 14720 cardiac arrests from the national registry of cardiopulmonary resuscitation. Resuscitation 2003; 58:297–308 [DOI] [PubMed] [Google Scholar]
- 5.Nations United, Department of Economic and Social Affairs, Population Division: World Population Ageing, 2019 Highlights. 2020. Available at: https://www.un.org/en/development/desa/population/publications/pdf/ageing/WorldPopulationAgeing2019-Report.pdf. Accessed October 26, 2021
- 6.Merchant RM, Yang L, Becker LB, et al. ; American Heart Association Get With The Guidelines-Resuscitation Investigators: Incidence of treated cardiac arrest in hospitalized patients in the United States. Crit Care Med 2011; 39:2401–2406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Girotra S, Nallamothu BK, Spertus JA, et al. ; American Heart Association Get with the Guidelines–Resuscitation Investigators: Trends in survival after in-hospital cardiac arrest. N Engl J Med 2012; 367:1912–1920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herlitz J, Svensson L, Engdahl J, et al. : Characteristics of cardiac arrest and resuscitation by age group: An analysis from the Swedish Cardiac Arrest Registry. Am J Emerg Med 2007; 25:1025–1031 [DOI] [PubMed] [Google Scholar]
- 9.Andersen LW, Bivens MJ, Giberson T, et al. : The relationship between age and outcome in out-of-hospital cardiac arrest patients. Resuscitation 2015; 94:49–54 [DOI] [PubMed] [Google Scholar]
- 10.Pleskot M, Hazukova R, Stritecka H, et al. : Five-year survival of patients after out-of-hospital cardiac arrest depending on age. Arch Gerontol Geriatr 2011; 53:e88–e92 [DOI] [PubMed] [Google Scholar]
- 11.Winther-Jensen M, Pellis T, Kuiper M, et al. : Mortality and neurological outcome in the elderly after target temperature management for out-of-hospital cardiac arrest. Resuscitation 2015; 91:92–98 [DOI] [PubMed] [Google Scholar]
- 12.Joslyn SA, Pomrehn PR, Brown DD: Survival from out-of-hospital cardiac arrest: Effects of patient age and presence of 911 emergency medical services phone access. Am J Emerg Med 1993; 11:200–206 [DOI] [PubMed] [Google Scholar]
- 13.Bonnin MJ, Pepe PE, Clark PS: Survival in the elderly after out-of-hospital cardiac arrest. Crit Care Med 1993; 21:1645–1651 [DOI] [PubMed] [Google Scholar]
- 14.George N, Thai TN, Chan PS, et al. : Predicting the probability of survival with mild or moderate neurological dysfunction after in-hospital cardiopulmonary arrest: The GO-FAR 2 score. Resuscitation 2020; 146:162–169 [DOI] [PubMed] [Google Scholar]
- 15.Seder DB, Patel N, McPherson J, et al. ; International Cardiac Arrest Registry (INTCAR)-Cardiology Research Group: Geriatric experience following cardiac arrest at six interventional cardiology centers in the United States 2006–2011: Interplay of age, do-not-resuscitate order, and outcomes*. Crit Care Med 2014; 42:289–295 [DOI] [PubMed] [Google Scholar]
- 16.Grimaldi D, Dumas F, Perier MC, et al. : Short- and long-term outcome in elderly patients after out-of-hospital cardiac arrest: A cohort study*. Crit Care Med 2014; 42:2350–2357 [DOI] [PubMed] [Google Scholar]
- 17.Rogove HJ, Safar P, Sutton-Tyrrell K, et al. : Old age does not negate good cerebral outcome after cardiopulmonary resuscitation: Analyses from the brain resuscitation clinical trials. Crit Care Med 1995; 23:18–25 [DOI] [PubMed] [Google Scholar]
- 18.Kennedy JH: The role of assisted circulation in cardiac resuscitation. JAMA 1966; 197:615–618 [PubMed] [Google Scholar]
- 19.Gerke AK, Tang F, Cavanaugh JE, et al. : Increased trend in extracorporeal membrane oxygenation use by adults in the United States since 2007. BMC Res Notes 2015; 8:686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang CH, Chou NK, Becker LB, et al. : Improved outcome of extracorporeal cardiopulmonary resuscitation for out-of-hospital cardiac arrest – a comparison with that for extracorporeal rescue for in-hospital cardiac arrest. Resuscitation 2014; 85:1219–1224 [DOI] [PubMed] [Google Scholar]
- 21.Morimura N, Sakamoto T, Nagao K, et al. : Extracorporeal cardiopulmonary resuscitation for out-of-hospital cardiac arrest: A review of the Japanese literature. Resuscitation 2011; 82:10–14 [DOI] [PubMed] [Google Scholar]
- 22.Leick J, Liebetrau C, Szardien S, et al. : Door-to-implantation time of extracorporeal life support systems predicts mortality in patients with out-of-hospital cardiac arrest. Clin Res Cardiol 2013; 102:661–669 [DOI] [PubMed] [Google Scholar]
- 23.Stub D, Bernard S, Pellegrino V, et al. : Refractory cardiac arrest treated with mechanical CPR, hypothermia, ECMO and early reperfusion (the CHEER trial). Resuscitation 2015; 86:88–94 [DOI] [PubMed] [Google Scholar]
- 24.Yannopoulos D, Bartos J, Raveendran G, et al. : Advanced reperfusion strategies for patients with out-of-hospital cardiac arrest and refractory ventricular fibrillation (ARREST): A phase 2, single centre, open-label, randomised controlled trial. Lancet Lond Engl 2020; 396:1807–1816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suverein MM, Delnoij TSR, Lorusso R, et al. : Early extracorporeal CPR for refractory out-of-hospital cardiac arrest. N Engl J Med 2023; 388:299–309 [DOI] [PubMed] [Google Scholar]
- 26.Belohlavek J, Smalcova J, Rob D, et al. ; Prague OHCA Study Group: Effect of intra-arrest transport, extracorporeal cardiopulmonary resuscitation, and immediate invasive assessment and treatment on functional neurologic outcome in refractory out-of-hospital cardiac arrest: A randomized clinical trial. JAMA 2022; 327:737–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Richardson ASC, Tonna JE, Nanjayya V, et al. : Extracorporeal cardiopulmonary resuscitation in adults. Interim guideline consensus statement from the extracorporeal life support organization. ASAIO J 2021; 67:221–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chung M, Zhao Y, Strom JB, et al. : Extracorporeal membrane oxygenation use in cardiogenic shock: Impact of age on in-hospital mortality, length of stay, and costs. Crit Care Med 2019; 47:e214–e221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lorusso R, Gelsomino S, Parise O, et al. : Venoarterial extracorporeal membrane oxygenation for refractory cardiogenic shock in elderly patients: Trends in application and outcome from the Extracorporeal Life Support Organization (ELSO) registry. Ann Thorac Surg 2017; 104:62–69 [DOI] [PubMed] [Google Scholar]
- 30.Mendiratta P, Wei JY, Gomez A, et al. : Cardiopulmonary resuscitation requiring extracorporeal membrane oxygenation in the elderly: A review of the extracorporeal life support organization registry. ASAIO J 2013; 59:211–215 [DOI] [PubMed] [Google Scholar]
- 31.Tonna JE, Selzman CH, Girotra S, et al. : Patient and institutional characteristics influence the decision to use extracorporeal cardiopulmonary resuscitation for in-hospital cardiac arrest. J Am Heart Assoc Cardiovasc Cerebrovasc Dis 2020; 9:e015522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Extracorporeal Life Support Organization: ECMO Registry of the Extracorporeal Life Support Organization (ELSO). 2022. Available at: https://www.elso.org/
- 33.Lorusso R, Alexander P, Rycus P, et al. : The extracorporeal life support organization registry: Update and perspectives. Ann Cardiothorac Surg 2019; 8:93–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Field N, Cohen T, Struelens MJ, et al. : Strengthening the Reporting of Molecular Epidemiology for Infectious Diseases (STROME-ID): An extension of the STROBE statement. Lancet Infect Dis 2014; 14:341–352 [DOI] [PubMed] [Google Scholar]
- 35.Charlson ME, Pompei P, Ales KL, et al. : A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis 1987; 40:373–383 [DOI] [PubMed] [Google Scholar]
- 36.Tseng LJ, Yu HY, Wang CH, et al. : Impact of age-adjusted Charlson comorbidity on hospital survival and short-term outcome of patients with extracorporeal cardiopulmonary resuscitation. J Clin Med 2018; 7:313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koen ‘ TJ, Nathanaël T, Philippe D: A systematic review of current ECPR protocols. A step towards standardisation. Resusc Plus 2020; 3:100018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iwashyna TJ, Hodgson CL, Pilcher D, et al. : Timing of onset and burden of persistent critical illness in Australia and New Zealand: A retrospective, population-based, observational study. Lancet Respir Med 2016; 4:566–573 [DOI] [PubMed] [Google Scholar]
- 39.Iwashyna TJ, Viglianti EM: Patient and population-level approaches to persistent critical illness and prolonged ICU stays. Crit Care Clin 2018; 34:493–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Makam AN, Nguyen OK, Xuan L, et al. : Factors associated with variation in long-term acute care hospital vs skilled nursing facility use among hospitalized older adults. JAMA Intern Med 2018; 178:399–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hsu CH, Meurer WJ, Domeier R, et al. : Extracorporeal Cardiopulmonary Resuscitation for Refractory Out-of-Hospital Cardiac Arrest (EROCA): Results of a randomized feasibility trial of expedited out-of-hospital transport. Ann Emerg Med 2021; 78:92–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lamhaut L, Hutin A, Puymirat E, et al. : A pre-hospital extracorporeal cardio pulmonary resuscitation (ECPR) strategy for treatment of refractory out hospital cardiac arrest: An observational study and propensity analysis. Resuscitation 2017; 117:109–117 [DOI] [PubMed] [Google Scholar]
- 43.Dennis M, Zmudzki F, Burns B, et al. ; Sydney ECMO Research Interest Group: Cost effectiveness and quality of life analysis of extracorporeal cardiopulmonary resuscitation (ECPR) for refractory cardiac arrest. Resuscitation 2019; 139:49–56 [DOI] [PubMed] [Google Scholar]
- 44.Wu L, Narasimhan B, Bhatia K, et al. : Temporal trends in characteristics and outcomes associated with in-hospital cardiac arrest: A 20-year analysis (1999–2018). J Am Heart Assoc 2021; 10:e021572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yannopoulos D, Bartos JA, Martin C, et al. : Minnesota resuscitation consortium’s advanced perfusion and reperfusion cardiac life support strategy for out-of-hospital refractory ventricular fibrillation. J Am Heart Assoc Cardiovasc Cerebrovasc Dis 2016; 5:e003732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sakamoto T, Morimura N, Nagao K, et al. ; SAVE-J Study Group: Extracorporeal cardiopulmonary resuscitation versus conventional cardiopulmonary resuscitation in adults with outof-hospital cardiac arrest: A prospective observational study. Resuscitation 2014; 85:762–768 [DOI] [PubMed] [Google Scholar]
- 47.Chen YS, Lin JW, Yu HY, et al. : Cardiopulmonary resuscitation with assisted extracorporeal life-support versus conventional cardiopulmonary resuscitation in adults with in-hospital cardiac arrest: An observational study and propensity analysis. Lancet 2008; 372:554–561 [DOI] [PubMed] [Google Scholar]
- 48.Maekawa K, Tanno K, Hase M, et al. : Extracorporeal cardiopulmonary resuscitation for patients with out-of-hospital cardiac arrest of cardiac origin: A propensity-matched study and predictor analysis. Crit Care Med 2013; 41:1186–1196 [DOI] [PubMed] [Google Scholar]
- 49.Goto T, Morita S, Kitamura T, et al. : Impact of extracorporeal cardiopulmonary resuscitation on outcomes of elderly patients who had out-of-hospital cardiac arrests: A single-centre retrospective analysis. BMJ Open 2018; 8:e019811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gravesteijn BY, Schluep M, Voormolen DC, et al. : Cost-effectiveness of extracorporeal cardiopulmonary resuscitation after in-hospital cardiac arrest: A Markov decision model. Resuscitation 2019; 143:150–157 [DOI] [PubMed] [Google Scholar]
- 51.Ramanathan K, Cove ME, Caleb MG, et al. : Ethical dilemmas of adult ECMO: Emerging conceptual challenges. J Cardiothorac Vasc Anesth 2015; 29:229–233 [DOI] [PubMed] [Google Scholar]
- 52.Steinhauser KE, Christakis NA, Clipp EC, et al. : Factors considered important at the end of life by patients, family, physicians, and other care providers. JAMA 2000; 284:2476–2482 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


