Abstract
Bronchiectasis presents a significant challenge due to its rising prevalence, associated economic burden and clinical heterogeneity. This review synthesises contemporary understanding and literature of bronchiectasis exacerbations, addressing the transition from stable state to exacerbations, underlining the importance of early and precise recognition, rigorous severity assessment, prompt treatment, and prevention measures, as well as emphasising the need for strategies to assess and improve early and long-term patient outcomes. The review highlights the interplay between stable state phases and exacerbations in bronchiectasis, introducing the concept of “exogenous and endogenous changes in airways homeostasis” and the “adapted island model” with a particular focus on “frequent exacerbators”, a group of patients associated with specific clinical characteristics and worse outcomes. The pathophysiology of exacerbations is explored through the lens of microbial and nonmicrobial triggers and the presence and the activity of comorbidities, elaborating on the impact of both exogenous insults, such as infections and pollution, and endogenous factors such as inflammatory endotypes. Finally, the review proposes a multidisciplinary approach to care, integrating advancements in precision medicine and biomarker research, paving the way for tailored treatments that challenge the traditional antibiotic paradigm.
Shareable abstract
Bronchiectasis exacerbations involve dynamic interactions between microbial communities and host factors. Effective management should rely on understanding these complex interplays to tailor individualised treatments. https://bit.ly/3V8E0od
Introduction
Bronchiectasis is a chronic respiratory disease characterised by irreversible airways dilation, chronic inflammation and impaired mucus clearance [1]. Clinical features of bronchiectasis include daily productive cough, dyspnoea, fatigue and increased susceptibility to respiratory tract infections [1, 2]. Bronchiectasis is a heterogeneous disease and, in recent years, international registries have informed the scientific community about patient demographics, clinical characteristics, comorbidities and microbiology across different geographical regions [3].
The occurrence and prevalence of bronchiectasis are increasing, especially among the elderly population [4]. Prevalence estimates range up to 566 cases per 100 000 people in the UK [4]. In a Belgian prospective cohort study, overall mortality in bronchiectasis patients was 20.4% in a 5-year follow-up [5]. The economic burden of bronchiectasis is far from insignificant: total annual healthcare costs per adult patient reach up to 82 000 USD, driven predominantly by exacerbations and hospitalisations [6].
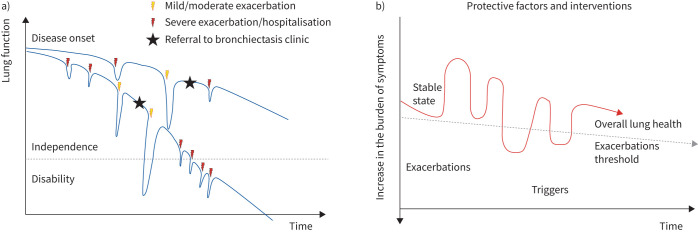
Bronchiectasis encompasses stable state phases interspersed with phases of acute worsening of signs and symptoms, in line with other chronic airway diseases (CADs) such as asthma and COPD [1, 2]. Landmark studies on CADs have shown that repeated exacerbations lead to faster lung function decline and higher mortality [7–10]. Additionally, the frequency and the severity of exacerbations are indicative of the effectiveness of disease control measures, highlighting the need for tailored treatment strategies to mitigate exacerbation risk and optimise long-term outcomes. In bronchiectasis, exacerbations have also been associated with increased mortality, impaired lung function and worsening quality of life [11–14] (figure 1). From a clinical perspective, the rate of exacerbations as well as the interval between them are evaluated as markers of bronchiectasis severity, activity and control. From a research perspective, exacerbations are recognised as the primary end-points in clinical trials [2].
FIGURE 1.
Disease decline and exacerbations in the natural history of bronchiectasis. a) Trajectory of lung function over time in bronchiectasis. The decline in lung function is associated with the occurrence of both mild/moderate and severe exacerbations. b) Oscillation between stable states and exacerbations over time, the influence of triggers and the impact of protective factors and interventions on maintaining overall lung health and raising the threshold for exacerbations.
Data from the European Bronchiectasis Registry (EMBARC) showed that over a third of patients across all regions can be identified as frequent exacerbators (defined as three or more exacerbations per year) [3]. Exacerbations are significant events in the clinical course of the disease and represent integral components of severity scoring systems such as the bronchiectasis severity index (BSI) and the E-FACED score (exacerbation–frequency, age, chronic infection and dyspnoea) [13, 14]. These scoring systems serve as valuable tools for clinicians in evaluating prognosis and guiding management of bronchiectasis patients.
This review will primarily focus on exacerbations in adults with bronchiectasis. We conducted a thorough and expansive search of the literature to ensure that all significant studies related to this topic were covered. We explore the complex interplay between stable state phases and exacerbations, introducing the concepts of “exogenous and endogenous homeostasis” and the “adapted island model” during exacerbation in the universe of bronchiectasis, as well as clinical manifestations and diagnostic challenges, providing useful tips for management and therapeutic interventions.
Methods
To conduct this review, we searched electronic databases, including PubMed, Embase and Scopus, from January 1980 up to April 2024. We used the following combination of keywords: “bronchiectasis”, “exacerbations” and “management”. The search was limited to articles published in English. With this approach, we tried to cover the literature related to the exacerbations of bronchiectasis as comprehensively as possible.
From suspicion to diagnosis of bronchiectasis exacerbation
Bronchiectasis presents a spectrum of clinical manifestations that vary not only between individuals but also within the same patient, even during the stable state. Some individuals exhibit typical symptoms such as chronic cough and daily sputum production, while others may present with less common manifestations such as wheezing, fatigue or other symptoms [1, 2]. Factors contributing to this heterogeneity of signs and symptoms include the underlying bronchiectasis aetiology, sudden changes in lung microbiome and/or homeostasis, and the presence/activity of comorbidities [15].
The heterogeneous nature of bronchiectasis is also evident when looking at the different ways exacerbations can occur across different patients. Various definitions of bronchiectasis exacerbations have been proposed over the years for both clinical and research purposes, as shown in table 1. Typically, bronchiectasis exacerbations are marked by the sudden appearance or worsening of symptoms, prompting an adjustment in respiratory therapy or the start of a specific treatment (e.g. antibiotics).
TABLE 1.
Current definitions of exacerbation for bronchiectasis, asthma and COPD
| Bronchiectasis | Asthma | COPD |
|---|---|---|
|
Hill [2] Deterioration in three or more key symptoms for at least 48 h# SEPAR Sustained clinical deterioration characterised by an increase in the usual cough and changes in the sputum characteristics (purulence, volume or viscosity), which may be accompanied by a change in other symptoms, changes in the respiratory examination or in patient's usual treatment or a significant decline in lung function [92] BTS Deterioration with worsening local symptoms such as cough, increased sputum volume or change in viscosity, with or without worsening of other symptoms [37] |
Progressive increase in dyspnoea, cough, wheezing or chest tightness, accompanied by a decline in lung function [18] | Dyspnoea and/or cough and sputum that worsen over <14 days [19, 20] |
#: This definition has been developed for clinical trials. BTS: British Thoracic Society; SEPAR: Sociedad Española de Neumología y Cirugía Torácica.
One reason explaining heterogeneity during bronchiectasis exacerbations might be coexistence with other CAD [3]. The two most recent analyses from the EMBARC registry revealed that 31% of bronchiectasis patients have a diagnosis of asthma, while 25% have COPD, highlighting significant overlap between these respiratory conditions [16, 17]. This co-existence not only significantly complicates the overall clinical picture in stable phases but also influences the frequency, severity and clinical characteristics of exacerbations, with an impact on overall patient health and treatment approaches [16, 17]. In patients with multiple CADs, distinguishing the role of each disease in characterising exacerbations poses a considerable challenge due to substantial overlap in clinical manifestations (increased cough, wheezing, dyspnoea and sputum modifications); however, it is uncertain whether it might change clinical management. As evidence of this, current definitions of exacerbations in bronchiectasis, asthma and COPD largely coincide, as shown in table 1 [18–20].
Among less frequent manifestations of bronchiectasis exacerbations, haemoptysis is a concerning complication. Severity can range from mild to massive and management ranges from domiciliary oral tranexamic acid to bronchial artery embolisation in urgent settings [21]. A recent single-centre observational prospective study from Seo et al. [22] found that bronchiectasis patients hospitalised with haemoptysis only and no signs of pulmonary or systemic sepsis had a lower bronchiectasis severity (measured by BSI and FACED), a lower rate of Pseudomonas (P.) aeruginosa infection and lower short-term mortality compared to those hospitalised due to an infective complication.
Moving from clinical phenotypes to endotypes, bronchiectasis patients have been clustered during stable states based not only on clinical features (phenotypes) [23] but also on molecular pathways of inflammation (endotypes) [24]. By extending this approach to exacerbations, it is possible that distinct clinical and inflammatory profiles could emerge among exacerbating patients, potentially unrelated to their stable state characteristics.
Bronchiectasis exacerbations are currently identified as primary end-points in the majority of phase II and III clinical trials. A specific definition of bronchiectasis exacerbation for clinical trials was developed in 2017 through a large expert consensus and includes deterioration in three or more pivotal symptoms for at least 48 h (table 1) [2]. Previous studies employed slightly different criteria for defining bronchiectasis exacerbations [25]; therefore, such differences might have influenced previous data analyses. This global definition of bronchiectasis exacerbation was introduced to standardise criteria for clinical research and improve the comparability of study outcomes by providing a clear and actionable framework for identifying exacerbations.
While Hill's [2] definition provides a solid framework for identifying exacerbations, it lacks validation studies and may not reflect patients’ personal experiences in real life. To overcome these issues, innovative tools that accurately reflect the patient's disease experience have been designed. The bronchiectasis exacerbation diary (BED) and the bronchiectasis exacerbation and symptom tool (BEST) are both designed to monitor exacerbations and daily symptoms as reported by patients themselves (patient-reported outcome). However, they were developed with different focuses and methodologies [26, 27]. The BED is an eight-item tracking system that aims to capture the variability of symptoms and the impact of exacerbations more qualitatively, whereas the BEST provides a structured approach for symptom monitoring with a scoring system, offering a more quantitative measure of exacerbation severity.
The “frequent exacerbator” bronchiectasis phenotype
Chalmers et al. [28] defined the “frequent exacerbator phenotype” (three or more exacerbations per year) in bronchiectasis which is associated with worse disease outcomes, including poorer quality of life and increased mortality. The authors also identified that factors such as Haemophilus (H.) influenzae and P. aeruginosa infection, low forced expiratory volume in 1 s (FEV1), radiological severity, and coexisting COPD independently predict future exacerbation frequency. This phenotype shares similarities with those identified in COPD and asthma. The work from Hurst et al. [29] is well-known for its insights into the frequent exacerbator phenotype in COPD (two or more exacerbations per year), illustrating that severity of disease and history of exacerbations were the major predictors for new exacerbations. Furthermore, factors independently associated with exacerbations in this study were gastroesophageal reflux disease (GERD) and worse lung function. In asthma, frequent exacerbations are often associated with daily poor symptom control, medication noncompliance and environmental triggers [10]. Having had one or more exacerbations puts the patient at risk for future exacerbations, suggesting this phenotype exists also in asthmatic people. Factors underlying the frequent exacerbator phenotype in asthma are cigarette smoking, GERD, chronic rhinosinusitis and obesity [30]. Interestingly, although the “frequent exacerbator” phenotype is acknowledged across the three different CADs (bronchiectasis, asthma and COPD), the specific number of exacerbations needed vary by disease. Finally, according to Araújo et al. [31], the presence of P. aeruginosa infection further increases mortality in patients with bronchiectasis experiencing two or more exacerbations per year. However, it is unknown whether patients with P. aeruginosa have more exacerbations or whether frequently exacerbating patients have a poor prognosis, with frequent antibiotic courses leading to P. aeruginosa infection and poor outcomes.
The pathophysiology of bronchiectasis exacerbations
The “vicious cycle” model from Cole [32] describes how, regardless of underlying aetiology, four mechanisms contribute to bronchiectasis disease progression, namely recurrent and chronic bacterial infection, chronic airways inflammation, impaired mucociliary clearance, and structural lung damage. This model was subsequently updated to the “vicious vortex” as it was recognised that, rather than occurring sequentially, there is interaction and interdependence between these mechanisms, so targeting one component alone cannot simply halt the cycle [33]. Frequent exacerbations can accelerate the vicious vortex, leading to progressive structural lung damage and clinical decline [34]. Identifying exogenous and endogenous triggers that disrupt the patient's homeostasis and predispose to exacerbations may be important to guide treatment.
Exogenous triggers of exacerbations
Acute and chronic bacterial infection are known triggers for bronchiectasis exacerbations. The most common bacteria cultured in sputum from bronchiectasis patients include P. aeruginosa, H. influenzae, Moraxella catarrhalis, Enterobacteriales, Staphylococcus aureus and Streptococcus pneumoniae [3, 35].
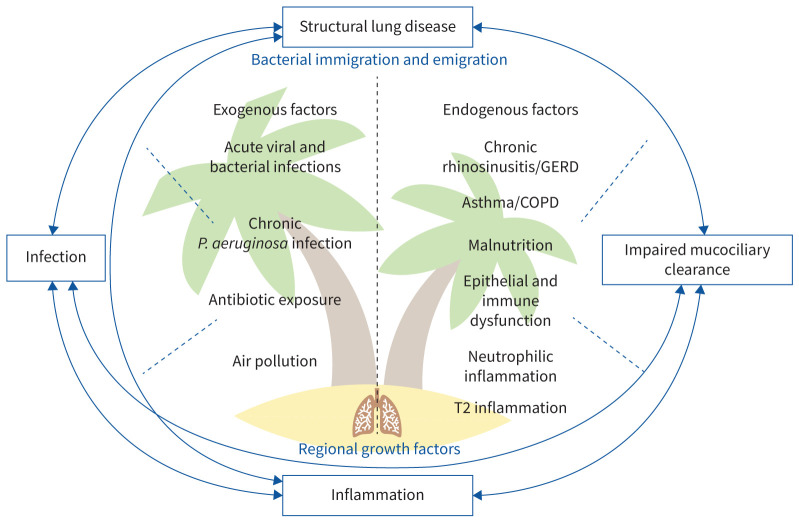
However, many of these bacteria are also cultured during stable state; thus, positive sputum culture alone is not a diagnostic criterion for exacerbations [36, 37]. Furthermore, novel culture-independent microbiological techniques, including DNA and rRNA sequencing, have demonstrated the complexity of the respiratory microbiome beyond individual cultured pathogens, even during exacerbations [38, 39]. The “adapted island model” provides a more nuanced understanding of these dynamics and describes how lung microbiological homeostasis is determined by a balance between microbial immigration and elimination processes, with strong interconnections between upper and lower airways and the gut [39, 40]. In bronchiectasis, this delicate balance may be easily disrupted by features of the vicious vortex, including architectural airway distortion, impaired mucociliary clearance and altered local immunity [38, 39, 41], as well as potentially many other endogenous and exogenous triggers (see figure 2).
FIGURE 2.
“Adapted island model” and the “vicious vortex/cycle” in bronchiectasis pathophysiology. This figure illustrates the dynamic homeostasis of lung inflammation, likened to an “island”, affected by both exogenous factors such as infections and pollution, and endogenous factors including underlying diseases and immune responses. One or more triggers may destabilise these interactions leading to exacerbations. These factors are also grouped according to the aspect of the vortex/island to which they predominantly relate. GERD: gastroesophageal reflux disease; P.: Pseudomonas.
Several studies have shown that the composition of the microbiome does not change significantly during or after treatment for exacerbations. Instead, dysregulation or “dysbiosis” of the microbiome is associated with exacerbation frequency [38, 42–44].
A case in point is P. aeruginosa, whose occurrence in sputum is associated with a higher risk of exacerbations and severe exacerbations requiring hospitalisation [31, 45]. Studies using 16S rRNA sequencing have further demonstrated that the predominance of proteobacteria, particularly P. aeruginosa, is associated with reduced microbiome diversity and a consequent increased exacerbation risk [43, 46, 47]. P. aeruginosa also affects other aspects of the bronchiectasis vicious vortex by activating inflammatory mediators, such as interleukin-1β, and stimulating mucin secretion, leading to mucus hyperviscosity and reduced mucociliary clearance [48, 49].
Antibiotics used to treat or prevent exacerbations may also alter the lung microbiome [38]. Post hoc analysis of the BLESS randomised controlled trial (RCT) showed that erythromycin treatment altered the microbiome with displacement of H. influenzae by macrolide-tolerant pathogens such as P. aeruginosa [50], which is one of many bacteria capable of virulence adaptations conferring resistance to antibiotic treatment [51]. Multidrug-resistant organisms are common in bronchiectasis and are associated with more severe exacerbations and higher mortality, both in prospective and retrospective analyses [52, 53].
There is a need for more studies investigating viruses in bronchiectasis exacerbations, but current evidence suggests they play a significant role [54]. A Spanish prospective observational multicentre study by Polverino et al. [55] comparing bronchiectasis exacerbations with and without pneumonic changes on chest X-ray found that respiratory viruses accounted for 19.4% of pneumonic exacerbations and 29% of nonpneumonic exacerbations. Other studies demonstrated viral infection in 14.5–49% of exacerbations. Rhinovirus, influenza A and B, and respiratory syncytial virus (RSV) were the most common viruses isolated [55–58].
During the coronavirus disease 2019 (COVID-19) pandemic, studies in the UK and USA demonstrated an over 40% reduction in bronchiectasis exacerbations, likely due to public health measures leading to reduced circulation of viruses, confirming the hypothesis that some bronchiectasis exacerbations are related to external exposure [59, 60]. On the other hand, bronchiectasis patients who did contract COVID-19 were at risk of more severe COVID-19 and of increased exacerbation frequency during a 12 month follow-up [61–63].
Fungi are also implicated in the pathophysiology of exacerbations. The Cohort of Matched Asian and European Bronchiectasis study characterised the respiratory mycobiome in 238 bronchiectasis patients and found Aspergillus sensitisation and colonisation, and serological allergic bronchopulmonary aspergillosis (ABPA) were associated with increased exacerbations [64]. In addition to their individual contributions, interactions between the respiratory virome, mycobiome and microbiome in bronchiectasis exacerbations are a growing area of study with potential to reveal new treatment approaches [47].
There is evidence that air pollution triggers bronchiectasis exacerbations. In a study of 432 patients, Goeminne et al. [65] found an 11.2% increased exacerbation risk associated with a 10 µg·m−3 increase in particulate matter particles <10 µm in diameter and a 4.7% increase per 10 µg·m−3 nitrogen oxide. Subsequent studies in Spain, China and Seoul have also shown a link between air pollutants, exacerbations and increased healthcare utilisation in bronchiectasis [66–68].
Lastly, recent research suggests that nutrition may play a significant role in bronchiectasis through its impact on the immune system and the gut–lung axis [69, 70]. In particular, malnutrition should be considered a critical concern/comorbidity, as it can lead to impaired airways epithelium barrier function, decreased cellular immunity and antibody production, thereby increasing the vulnerability to bacterial and viral infections that can trigger exacerbations in bronchiectasis patients [71, 72].
Endogenous triggers of exacerbations
Bronchiectasis is an inflammatory disease and distinct inflammatory endotypes have been linked to exacerbation risk. Neutrophilic inflammation is the most well-studied inflammatory process underlying exacerbations. Neutrophil elastase (NE), a protease released from the azurophil granules of activated neutrophils, increases during exacerbations [73]. High baseline NE predicts an increased exacerbation risk, reduced microbiome diversity and P. aeruginosa infection [74, 75]. NE is also reported to increase mucin secretion, with implications for mucociliary clearance [76]. Neutrophil extracellular traps, webs of DNA and granule contents released as part of a dysregulated neutrophilic response, also predict severe exacerbations and are reduced by intravenous (i.v.) antibiotics [46].
A T2-high bronchiectasis endotype, distinct from comorbid asthma, has also been demonstrated. This inflammatory endotype in bronchiectasis is associated with distinct microbiome profiles and blood eosinophils >300 cells·µL−1 are associated with shortened time to first exacerbation following antibiotic treatment for P. aeruginosa [77, 78].
Inflammatory endotypes may also explain the links between comorbid asthma and COPD and higher exacerbation risk [16, 17]. Both asthma and COPD have neutrophilic and eosinophilic endotypes, which may interact with the bronchiectasis vicious vortex [79, 80].
Further supporting the concept of the island model, the association of comorbid GERD and chronic rhinosinusitis with increased exacerbations underscores the dynamic movement of microbial communities into and out of the airways. This phenomenon may be attributed to dysphagia and associated micro-aspiration, as evidenced in many studies demonstrating interactions between gastro–intestinal and upper and lower airway microbiomes [38, 41, 81–83].
GERD can exacerbate bronchiectasis symptoms and increase exacerbation frequency [81]. Notably, McDonnell et al. [81] showed how azithromycin can modulate this effect, potentially by reducing the inflammatory response induced by acid reflux in the airways. Furthermore, we can speculate that the beneficial effects of azithromycin in this context might be partially due to its ability to enhance gastric emptying, mitigating the reflux of gastric contents into the oesophagus and, subsequently, the airways.
These interactions underscore the importance of considering respiratory and nonrespiratory comorbid conditions such as GERD and malnutrition in the comprehensive management of bronchiectasis, as well as during an exacerbation.
Additionally, some evidence suggests dental health affects exacerbation risk in COPD, but this has not been studied in bronchiectasis [84].
Poor adherence to treatment, for both bronchiectasis and comorbid conditions, likely contributes to exacerbations. McDonnell et al. [85] showed that just 16% of patients were adherent to all three of inhaled antibiotics, other respiratory medicines and airway clearance and that treatment nonadherence led to increased exacerbations. Furthermore, McCullough et al. [86] explored predictors of adherence to treatment in bronchiectasis and found that concerns about medications and perceived necessity of treatment, significantly influence adherence. Older age and fewer prescribed medications also predicted better adherence, suggesting that interventions to improve adherence should consider both psychosocial and demographic factors.
Endotyping bronchiectasis exacerbations
There is significant overlap between the exogenous and endogenous causes of exacerbations. Identifying distinct endotypes that may respond to specific treatments has been a recent research focus. Integrating inflammatory pathways and microbiome data, Choi et al. [24] identified four inflammatory clusters with distinct microbiome and clinical profiles. Notably, clusters defined by severe neutrophilic inflammation and mixed neutrophilic and T2 inflammation displayed a predominance of proteobacteria and were associated with higher exacerbation risk. Similarly, the T2 predominant endotype or endotypes defined by coexistent CAD may respond to targeted therapies. Ongoing research into endotypes and the biomarkers defining them may aid future personalised therapies for bronchiectasis exacerbations [87].
Defining severity and site-of-care of bronchiectasis exacerbations
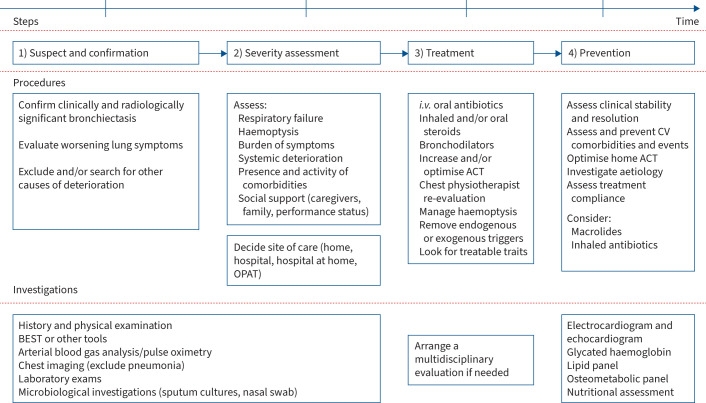
An important gap in bronchiectasis management is the lack of an instrument to assess exacerbation severity. We know from other lower respiratory tract acute conditions, such as COPD exacerbations and community acquired pneumonia (CAP), that having a risk tool to predict mortality is helpful to guide site of care and treatment. A robust prediction tool in COPD exacerbations is the DECAF score [88, 89]. DECAF stands for dyspnoea, eosinopenia, consolidation, acidaemia and atrial fibrillation, which are the variables that better predict the risk of adverse outcomes [88]. On the other hand, in CAP, the CURB-65 test only evaluates clinical parameters (confusion, urea level, respiratory rate, blood pressure and age 65 or older), whereas the pneumonia severity index incorporates a wider range of clinical variables (including comorbidities) to categorise patients into risk classes [90, 91]. These scores aid clinicians in making informed decisions about patient care, including the need for hospital admission or intensive care, by providing an objective assessment of the clinical picture. The absence of a severity score for bronchiectasis exacerbations underscores the need for research and development in this area to improve patient outcomes and care strategies. Currently, clinicians must rely on clinical judgment through evaluation of factors such as respiratory failure, severity of haemoptysis, sepsis or systemic deterioration, and coexistence of new or decompensated comorbidities (see figure 3) [92, 93].
FIGURE 3.
Stepwise approach in the management of bronchiectasis exacerbation. Comprehensive management pathway for bronchiectasis. This figure delineates a structured approach to the clinical management of bronchiectasis, highlighting key steps from initial suspicion and confirmation to severity assessment, followed by targeted treatment strategies and culminating in preventative measures to mitigate future exacerbations. ACT: airway clearance technique; BEST: bronchiectasis exacerbation and symptom tool; CV: cardiovascular; i.v.: intravenous; OPAT: outpatient parenteral antibiotic therapy.
The scientific and clinical communities agree that a bronchiectasis exacerbation leading to hospitalisation should be considered severe. Hospitalisation due to bronchiectasis exacerbation has been recognised as a major end-point in previous clinical trials [94]. Although this might be correct for most cases, some bronchiectasis patients could be admitted with a mild-to-moderate exacerbation only because of the need for i.v. antibiotics. Furthermore, the decision to admit a patient with acute exacerbation of bronchiectasis, as in other conditions, is not always solely determined by clinical features. Factors such as healthcare resources, social circumstances and caregiver support also influence the decision-making process.
Management strategies for bronchiectasis exacerbations are evolving and new approaches have been explored to enhance patients’ quality of life and reduce healthcare costs. One notable option is the use of outpatient parenteral antibiotic therapy (OPAT), which allows patients to receive i.v. antibiotics in an outpatient setting. In 2019, López-Cortés et al. [95] conducted a prospective observational cohort study demonstrating that 94% of patients with bronchiectasis exacerbation who were treated under an OPAT programme achieved resolution of exacerbation symptoms, with a significant reduction in hospital days and, therefore, health costs.
Another important issue is to decide whether a patient with an exacerbation needs chest imaging to search for consolidation, as bronchiectasis exacerbations and CAP share similar clinical features (see figure 3) [96]. Polverino et al. [55] compared 144 bronchiectasis patients with acute deterioration of symptoms with and without a new radiological infiltrate to verify the clinical significance of differentiating between CAP and a severe exacerbation. They found that CAP in bronchiectasis leads to more complications, such as atrial fibrillation and pleural effusion, and had higher hospitalisation rates, despite similar in-hospital, 30- and 90-day and 1-year mortality rates. Patients with pneumonia also had higher rates of fever, leucocytosis and high C-reactive protein (CRP); microbiologically, Streptococcus pneumoniae is the main cause of CAP, while P. aeruginosa was the main isolate of nonpneumonic exacerbations.
Investigations
Microbiological assessment is crucial during bronchiectasis exacerbations. Latest bronchiectasis guidelines and quality standards suggest, where possible, using sputum or bronchioalveolar lavage and nasopharyngeal swabs to detect bacterial and/or viral infection during acute exacerbations and before commencing antibiotic therapy [37, 97].
Specific blood tests are not recommended by the latest guidelines during bronchiectasis exacerbations. In a single-centre prospective study, Kwok et al. [98] enrolled 123 patients and found that an elevated serum CRP level (>0.35 mg·dL−1) at stable state was predictive of increased risks of bronchiectasis exacerbation and hospitalisations. Unfortunately, CRP is nonspecific and increases in multiple inflammatory conditions and therefore is not useful in guiding targeted treatment of exacerbations. On the other hand, given increasing data supporting a T2-high bronchiectasis endotype, we can speculate that, as in asthma and COPD where patients with eosinophilia or other markers of T2-high inflammation may benefit from corticosteroid therapy, a similar approach could be explored in bronchiectasis exacerbations [8, 99, 100].
Identifying reliable biomarkers is crucial for an effective management of bronchiectasis exacerbation. Studies by Good et al. [101, 102] demonstrated that procalcitonin levels in sputum are significantly higher than in serum during bronchiectasis exacerbations and elevated in stable bronchiectasis compared to healthy controls.
According to current guidelines, every patient should also be educated on recognising early signals of exacerbations, when and how to use medications (such as antibiotics and bronchodilators), lifestyle modifications (such as increasing chest physiotherapy), and managing haemoptysis (see figure 3) [37]. This “self-management plan” is aimed at empowering patients to take an active role in managing their condition.
Treatment of bronchiectasis exacerbations
Exacerbations require immediate management and a comprehensive approach combining pharmacological and nonpharmacological strategies (see figure 3).
Airway clearance techniques (ACTs)
ACTs are cornerstone interventions for managing bronchiectasis, as emphasised by British Thoracic Society, Sociedad Española de Neumología y Cirugía Torácica and European Respiratory Society guidelines [36, 37, 92]. During exacerbations, frequency and/or duration of ACT sessions may need to be increased. These techniques, including postural drainage, chest physiotherapy and positive expiratory pressure, aim to clear mucus from the airways, thereby reducing bacterial load and inflammation. All chest physiotherapy interventions should be tailored to the patient's symptoms, physical capability and disease characteristics. When a patient is experiencing an exacerbation, manual techniques can be employed to improve the clearance of sputum.
Antibiotic therapy
Oral, inhaled or i.v. antibiotics are used short-term for management of pulmonary exacerbation and eradication purposes, or long-term as suppressive therapy for maintenance of lung health [103]. Eradication refers to treatment with the intent to clear the airways of a pathogen and is only recommended for P. aeruginosa infection based on expert consensus, with higher eradication rates achieved by combining systemic and inhaled antibiotics [104].
Systemic antibiotics are a mainstay in the treatment of bronchiectasis exacerbations [36, 37, 92]. Nonetheless, studies assessing the effectiveness of different antibiotics in treating exacerbations among adults are lacking. Selection of an appropriate antibiotic (pathogen-directed therapy) should be guided by previous sputum bacteriology findings. In the absence of specific sputum microbiology, clinicians can be guided by local data on prevalent pathogens in bronchiectasis patients. To enhance diagnostic accuracy, it is recommended that sputum for microbiological analysis be collected before beginning treatment during exacerbations. Following identification of a pathogen, antibiotic therapy, referred to as targeted antibiotic therapy, should be adjusted in the absence of clinical improvement, guided by the results of sensitivity testing.
Typically, a 14-day course of antibiotics is recommended, particularly for patients infected with P. aeruginosa [36, 37, 92]. However, shorter durations may be adequate for nonsevere patients. Due to a lack of strong evidence supporting specific antibiotic durations, a recent proof-of-concept RCT aimed to determine the feasibility of reducing the length of i.v. antibiotic treatment during exacerbations depending on bacterial load compared to a standard 14-day regimen [105]. It involved 47 patients receiving 14 days of antibiotics and 43 patients where treatment duration was determined by bacterial load measurements; in the latter group, 88% were able to discontinue antibiotics by the eighth day. The study noted a trend towards greater clinical improvement by the twenty-first day with the 14-day regimen, although the difference was not statistically significant. Intriguingly, the group with the bacterial load-guided shorter antibiotic course experienced a longer interval before the next exacerbation [105].
In patients experiencing frequent exacerbations or those with persistent airway infection caused by P. aeruginosa, there exists moderate-quality evidence supporting the recommendation for inhaled antibiotics, for a duration of 3 months or longer [103]. While inhaled antibiotics are a staple in managing stable bronchiectasis, they have no role in treating acute exacerbations.
Bronchodilators
For patients experiencing symptomatic airflow obstruction, use of long-acting beta-agonists and/or anticholinergics is suggested [36, 37, 92]. Due to the lack of data regarding the use of bronchodilators in the setting of exacerbations, their application is currently recommended for patients with coexisting CAD, where bronchial hyperresponsiveness is a concern.
Corticosteroids
The use of corticosteroids, either systemic or inhaled, in bronchiectasis exacerbations is a topic of ongoing debate. The CORTICO-COP and STARR2 trials recently showed that blood eosinophil-guided corticosteroid therapy in COPD exacerbation is a valuable option [99, 100]; however, no RCTs or observational studies regarding the use of systemic corticosteroids, either in stable conditions or during exacerbations, exist in bronchiectasis. However, studies indicate a potential role for inhaled corticosteroids (ICS) as a targeted treatment approach for eosinophilic exacerbations of bronchiectasis [106, 107].
Early and long-term outcomes in bronchiectasis exacerbations
Once treatment of bronchiectasis exacerbation is started, clinicians should be aware what clinical consequences to expect, both in short- and long-term metrics.
Regarding early outcomes, time to clinical stability refers to the duration it takes for a patient to improve and stabilise after initiation of treatment. However, a clear definition of clinical stability in bronchiectasis exacerbation is not provided in either current international guidelines or literature. This early end-point is critical for determining when the overall condition is coming under control, when it might be safe to simplify therapy and, in the case of hospitalised patients, to consider discharge. In CAP, this concept is well established by Halm et al.'s [108] criteria. On the other hand, clinical cure refers to resolution of clinical symptoms and signs, indicating that the patient has recovered completely. In bronchiectasis exacerbations, Brill et al. [109] provided 32 bronchiectasis outpatients with symptom diary cards and advised them to measure peak expiratory flow (PEF) during exacerbations. The median duration of exacerbation symptoms was 16 days, during which mean PEF dropped significantly by 10.6%. Interestingly, 16% had not fully recovered by 35 days. Something similar was observed in COPD patients where median duration of exacerbation symptoms was 10 days and 7.3% of patients did not recover PEF within 99 days [110].
Symptom resolution is a primary goal of treatment, but managing symptoms is only one aspect of care. Successful treatment also involves preventing complications such as to hospital readmissions and mortality.
Interesting data regarding characteristics and outcomes of patients requiring hospitalisation for exacerbations of bronchiectasis come from a prospective observational study from Suarez-Cuartin et al. [111]. The authors found that patients admitted had very severe disease with median BSI=15 and E-FACED=4 and high prevalence of respiratory failure (62%). These patients showed, respectively, 17 and 34% 30- and 90-day readmission rates with low mortality rates (3/60 patients enrolled, 5%). Similar mortality and re-admission rates were found by Polverino et al. [55] in their study evaluating pneumonic versus nonpneumonic exacerbations.
A larger sample of 1235 patients with severe bronchiectasis exacerbations (including pneumonia) were retrospectively analysed in a Taiwan cohort. The definition of severe exacerbation in this study was need for emergency room visits or hospital admission, and all patients had chest computed tomography. Overall, in-hospital mortality was 3.0%. Post-discharge, age, bronchiectasis aetiology and comorbidity indexscore ≥6, male sex and systemic corticosteroid usage were associated with significantly higher cumulative incidence of respiratory failure and mortality in a 1-year follow-up, while ACT was associated with a lower mortality risk [112].
Data in the literature show that 1-year mortality after a bronchiectasis exacerbation ranges up to 30% according to patient's exacerbating status, the number of hospitalisations in the previous year, FEV1 and co-existence of COPD or cigarette smoking [113, 114].
In recent years, an association between bronchiectasis, exacerbations and risk of cardiovascular events (CVEs) has been established in population-based studies [115, 116]. Navaratnam et al. [115] tried to clarify the weight of exacerbations on this outcome and found that a respiratory tract infection is associated with a significant increase in incident CVEs (myocardial infarction and stroke) during a 91-day follow-up. In a post hoc retrospective analysis of a prospective observational study enrolling 250 patients with bronchiectasis at two tertiary care clinics, Méndez et al. [117] found that almost 30% of bronchiectasis patients developed a CVE during 35-month median follow-up after an exacerbation. CVE was defined as any acute coronary syndrome, new or worsening heart failure, new or recurrent arrhythmia or cerebrovascular accident. Risk factors associated with CVE were age, hypertension and COPD, whereas severe exacerbations showed a trend (hazard ratio 1.85, 95% CI 0.98–3.52). Eventually, the patients with a CVE during follow-up had a greater risk of death than those who did not have one. In another study from Korea, two separate retrospective cohorts of patients with bronchiectasis experiencing first-time atherosclerotic cardiovascular disease or cerebrovascular disease were analysed. Within 1 year before the index events, more than 13.5% of patients in both cohorts had experienced an exacerbation necessitating emergency room access or hospitalisation. In the multivariate analysis, the exacerbators group showed increased 90-day and 1-year cardiovascular and cerebrovascular mortality compared to the nonexacerbators [118].
This evidence highlights the importance of comprehensive post-exacerbation care, addressing modifiable risk factors, such as minimising unnecessary corticosteroid use, and the need to routinely perform cardiovascular evaluations possibly in a standardised protocol that involves a multidisciplinary team.
Prevention of bronchiectasis exacerbations
As with acute management, preventing bronchiectasis exacerbations requires a multidisciplinary approach focussed on preventing infections, optimising mucociliary clearance and targeted treatment of comorbidities and inflammatory endotypes (figure 3).
The 2017 European guidelines recommend prophylactic macrolide therapy first line for patients with frequent exacerbations without P. aeruginosa infection and inhaled antibiotics for those with P. aeruginosa [36]. A 2019 meta-analysis subsequently showed macrolide therapy reduced exacerbation frequency and increased time to first exacerbation in patients with and without P. aeruginosa [119]. The effect on P. aeruginosa despite known macrolide resistance is attributed to immunomodulatory properties and broader impact on microbial communities [46, 47]. A recent meta-analysis investigating efficacy of inhaled antibiotics in bronchiectasis showed a comparable reduction in exacerbations in patients with and without P. aeruginosa [120]. These findings suggest that both macrolide therapy and inhaled antibiotics effectively prevent exacerbations irrespective of P. aeruginosa infection status.
The effect of vaccines on bronchiectasis exacerbations has not been extensively studied but the limited available evidence suggests benefit [121, 122]. Given the robust evidence for vaccination in other respiratory diseases and the high risk of exacerbations in bronchiectasis, influenza and pneumococcal vaccines are recommended [36, 65, 92]. Vaccinations against other viruses such as RSV are either on the market or in development and may benefit bronchiectasis patients in future [123, 124].
The drastic reduction in bronchiectasis exacerbations during COVID-19 lockdowns was attributed to reduced circulating viruses and reduced air pollution [59, 60]. Social, psychological, physical and economic effects mean these measures cannot be maintained long-term, but it is unknown whether less stringent preventative measures, such as mask-wearing in high-risk settings, could be an effective approach to reduce exacerbations.
As well as its role in acute management of excessive mucus during exacerbations, two RCTs have shown that practising regular airway clearance also prevents exacerbations [125, 126]. Mucolytics can also be suggested if beneficial. N-acetylcysteine reduced exacerbations in one study, with a further RCT underway [127, 128]. Exercise is also associated with a reduction in exacerbations and pulmonary rehabilitation reduced exacerbations over 12-month follow-up in one study [129].
Regular ICS therapy could be beneficial to prevent exacerbations in patients with a T2-high inflammatory endotype. In the EMBARC study, regular ICS reduced risk of hospitalisation in patients with comorbid bronchiectasis and asthma [16]. Furthermore, in a study of bronchiectasis patients without asthma, ICS reduced exacerbation frequency in those with high eosinophils but not in noneosinophilic patients [130].
However, current guidelines collectively advise against routine use of ICS in bronchiectasis, except for in coexisting COPD, asthma, ABPA, inflammatory bowel disease or significant bronchorrhea unresponsive to other treatments, based on low-quality evidence [36, 37, 92]. Their extended use must be carefully weighed against the risk of side-effects. For example, among nonselected bronchiectasis patients, regular ICS use is associated with increased exacerbation frequency and severity in one study [131]. This is concerning as ICS are commonly prescribed in COPD but 44.4% of patients in the EMBARC cohort diagnosed with COPD did not meet diagnostic criteria, raising concerns about inappropriate prescribing [17].
The role of bronchodilators in bronchiectasis without COPD or asthma remains unclear, and a trial of inhaled tiotropium showed improvement in lung function but not in exacerbations [132]. Bronchodilators are therefore not recommended routinely, but further studies are ongoing. On the other hand, it is advised to administer short-acting beta-agonists (such as salbutamol or terbutaline) prior to respiratory physiotherapy to enhance ACT and support the effectiveness of inhaled antibiotics.
New treatments, such as DPP-1 (dipeptidyl peptidase-1) inhibitors, highlighted by the phase II Willow trial using brensocatib, offer promising avenues for bronchiectasis management by targeting inflammation. This trial showed that brensocatib significantly reduced time to first exacerbation and reduced exacerbation risk by about 40% compared to placebo [133]. Such treatments could serve as alternatives to conventional antibiotic therapy, lessening reliance on antibiotics and potentially curbing the rise of antimicrobial resistance. Comparative studies between long-term macrolides, inhaled antibiotics and DPP-1 inhibitors are needed to elucidate the most effective maintenance strategies for preventing exacerbations in bronchiectasis.
Finally, interventions to ensure treatment adherence are important. Self-management and electronic and digital technologies have shown promising results in other lung diseases but their impact on bronchiectasis exacerbations has not been studied [134].
Conclusions
The interplay between stable and exacerbation phases of bronchiectasis reveals a dynamic disease course, influenced by both exogenous and endogenous factors, including microbial immigration and emigration, as conceptualised in the adapted island model. Identification of the frequent exacerbator phenotype presents a critical opportunity for targeted interventions aimed at mitigating the adverse effects of recurrent exacerbations. Thus, exacerbations serve as important markers of disease activity reflecting the dynamic nature of chronic lung conditions and their impact on respiratory health. Moreover, exploration of molecular endotypes and their link to exacerbation patterns (phenotypes) offers a promising avenue for personalised treatment strategies, aligning with the field of precision medicine. Despite advancements in understanding and managing bronchiectasis, significant gaps remain, particularly in the definition of exacerbations and the standardisation of a comprehensive management approach (see table 2).
TABLE 2.
Questions for future research in the field of bronchiectasis exacerbation
| Summary of research needs and priorities |
|---|
| 1) Generate epidemiological data, including healthcare costs, across different countries and continents of bronchiectasis exacerbations, not only for patients treated in the hospital but also for those treated in a domiciliary setting |
| 2) Validation of Hill's [2] criteria of bronchiectasis exacerbation in a real-life setting |
| 3) Identification of clinical phenotypes among bronchiectasis patients who are exacerbating |
| 4) Identification of endotypes among bronchiectasis patients who are exacerbating |
| 5) Validation of DECAF or other existing tools in bronchiectasis exacerbation to define severity |
| 6) Derivation and validation of new tools to assess exacerbation severity |
| 7) Validation of tools for exacerbation severity in deciding the site-of-care |
| 8) Identification of exogenous and endogenous triggers for bronchiectasis exacerbations |
| 9) Studies comparing different antibiotic regimens during exacerbations |
| 10) Definition and validation of clinical stability as well as clinical cure and clinical failure in bronchiectasis exacerbations |
| 11) Definition of the proper duration of antibiotic therapy during an exacerbation based on each single patient's response to treatment |
| 12) Define safety and efficacy of systemic corticosteroids treatment during a bronchiectasis exacerbation in patients with or without eosinophilia |
| 13) Define safety and efficacy of bronchodilators during a bronchiectasis exacerbation |
| 14) Identification of a systematic bundle of interventions (e.g. ACT, antibiotic, steroids, bronchodilators, etc.) to improve outcomes during a bronchiectasis exacerbation |
| 15) Comparative studies between long-term macrolides, inhaled antibiotics and other immunomodulatory/anti-inflammatory agents to elucidate the most effective maintenance strategies for preventing exacerbations in bronchiectasis |
ACT: airway clearance technique; DECAF: dyspnoea, eosinopenia, consolidation, acidaemia and atrial fibrillation.
Footnotes
This article has an editorial commentary: https://doi.org/10.1183/16000617.0124-2024
Provenance: Commissioned article, peer reviewed.
Number 4 in the Series “World Bronchiectasis Conference 2024” Edited by James D. Chalmers, Felix C. Ringshausen and Pieter C. Goeminne
Previous articles in this series: No. 1: Perea L, Faner R, Chalmers JD, et al. Pathophysiology and genomics of bronchiectasis. Eur Respir Rev 2024; 33: 240055. No. 2: Mac Aogáin M, Dicker AJ, Mertsch P, et al. Infection and the microbiome in bronchiectasis. Eur Respir Rev 2024; 33: 240038. No. 3: Van Braeckel E, Bosteels C. Growing from common ground: nontuberculous mycobacteria and bronchiectasis. Eur Respir Rev 2024; 33: 240058.
Conflict of interest: A. De Angelis and E.D. Johnson have nothing to disclose. S. Sutharsan reports payment or honoraria for lectures, presentations, manuscript writing or educational events from Vertex Pharamceuticals, Insmed and Boehringer Ingelheim, and participation on a data safety monitoring board or advisory board with Vertex Pharamceuticals. S. Alberti reports grants from INSMED Incorporated, Chiesi, Fisher & Paykel and GSK, royalties or licences from McGraw Hill, consultancy fees from INSMED Incorporated, INSMED Italy, INSMED Ireland Ltd, INSMED Netherlands BV, ZAMBON Spa, AstraZeneca UK Limited, AstraZeneca Pharmaceutical LP, CSL Behring GmbH, Grifols, Fondazione Internazionale Menarini, Moderna Italy, Moderna TX, Boehringer Ingelheim, Chiesi farmaceutica Spa, MSD Italia S.r.l., Vertex Pharmaceuticals, BRAHMS GMBH, Physioassist SAS, AN2 Therapeutics and GlaxoSmithKline Spa, payment or honoraria for lectures, presentations, manuscript writing or educational events from GlaxoSmithKline Spa, Thermofisher Scientific, INSMED Italy, INSMED Ireland Ltd, Boehringer Ingelheim, Zambon, Vertex Pharmaceuticals and Fondazione Internazionale Menarini, and participation on a data safety monitoring board or advisory board with INSMED Incorporated, INSMED Italy, AstraZeneca UK Limited and MSD Italia S.r.l.
References
- 1.Aliberti S, Goeminne PC, O'Donnell AE, et al. Criteria and definitions for the radiological and clinical diagnosis of bronchiectasis in adults for use in clinical trials: international consensus recommendations. Lancet Respir Med 2022; 10: 298–306. doi: 10.1016/S2213-2600(21)00277-0 [DOI] [PubMed] [Google Scholar]
- 2.Hill AT, Haworth CS, Aliberti S, et al. Pulmonary exacerbation in adults with bronchiectasis: a consensus definition for clinical research. Eur Respir J 2017; 49: 1700051. doi: 10.1183/13993003.00051-2017 [DOI] [PubMed] [Google Scholar]
- 3.Chalmers JD, Polverino E, Crichton ML, et al. Bronchiectasis in Europe: data on disease characteristics from the European Bronchiectasis Registry (EMBARC). Lancet Respir Med 2023; 11: 637–649. doi: 10.1016/S2213-2600(23)00093-0 [DOI] [PubMed] [Google Scholar]
- 4.Quint JK, Millett ERC, Joshi M, et al. Changes in the incidence, prevalence and mortality of bronchiectasis in the UK from 2004 to 2013: a population-based cohort study. Eur Respir J 2016; 47: 186–193. doi: 10.1183/13993003.01033-2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goeminne PC, Nawrot TS, Ruttens D, et al. Mortality in non-cystic fibrosis bronchiectasis: a prospective cohort analysis. Respir Med 2014; 108: 287–296. doi: 10.1016/j.rmed.2013.12.015 [DOI] [PubMed] [Google Scholar]
- 6.Roberts JM, Goyal V, Kularatna S, et al. The economic burden of bronchiectasis. Chest 2023; 164: 1396–1421. doi: 10.1016/j.chest.2023.06.040 [DOI] [PubMed] [Google Scholar]
- 7.Wedzicha JA, Miravitlles M, Hurst JR, et al. Management of COPD exacerbations: a European Respiratory Society/American Thoracic Society guideline. Eur Respir J 2017; 49: 1600791. doi: 10.1183/13993003.00791-2016 [DOI] [PubMed] [Google Scholar]
- 8.Bourdin A, Bjermer L, Brightling C, et al. ERS/EAACI statement on severe exacerbations in asthma in adults: facts, priorities and key research questions. Eur Respir J 2019; 54: 1900900. doi: 10.1183/13993003.00900-2019 [DOI] [PubMed] [Google Scholar]
- 9.Miravitlles M, Ferrer M, Pont À, et al. Effect of exacerbations on quality of life in patients with chronic obstructive pulmonary disease: a 2 year follow up study. Thorax 2004; 59: 387–395. doi: 10.1136/thx.2003.008730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dougherty RH, Fahy JV. Acute exacerbations of asthma: epidemiology, biology and the exacerbation-prone phenotype. Clin Exp Allergy 2009; 39: 193–202. doi: 10.1111/j.1365-2222.2008.03157.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martínez-García MA, Soler-Cataluña JJ, Perpiñá-Tordera M, et al. Factors associated with lung function decline in adult patients with stable non-cystic fibrosis bronchiectasis. Chest 2007; 132: 1565–1572. doi: 10.1378/chest.07-0490 [DOI] [PubMed] [Google Scholar]
- 12.Guan WJ, Gao YH, Xu G, et al. Inflammatory responses, spirometry, and quality of life in subjects with bronchiectasis exacerbations. Respir Care 2015; 60: 1180–1189. doi: 10.4187/respcare.04004 [DOI] [PubMed] [Google Scholar]
- 13.Chalmers JD, Goeminne P, Aliberti S, et al. The bronchiectasis severity index. An international derivation and validation study. Am J Respir Crit Care Med 2014; 189: 576–585. doi: 10.1164/rccm.201309-1575OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martinez-Garcia MA, Athanazio RA, Girón R, et al. Predicting high risk of exacerbations in bronchiectasis: the E-FACED score. Int J COPD 2017; 12: 275–284. doi: 10.2147/COPD.S121943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Donnell AE. Bronchiectasis — a clinical review. N Engl J Med 2022; 387: 533–545. doi: 10.1056/NEJMra2202819 [DOI] [PubMed] [Google Scholar]
- 16.Polverino E, Dimakou K, Traversi L, et al. Bronchiectasis and asthma: data from the European Bronchiectasis Registry (EMBARC). J Allergy Clin Immunol 2024; 153: 1553–1562. doi: 10.1016/j.jaci.2024.01.027 [DOI] [PubMed] [Google Scholar]
- 17.Polverino E, De Soyza A, Dimakou K, et al. The association between bronchiectasis and chronic obstructive pulmonary disease: data from the European Bronchiectasis Registry (EMBARC). Am J Respir Crit Care Med 2024; 210: 119–127. [DOI] [PubMed] [Google Scholar]
- 18.Global Initiative for Asthma . Global strategy for asthma management and prevention 2023. Date last accessed: 1 February 2024. Date last updated: 10 July 2023. https://ginasthma.org/2023-gina-main-report/ [Google Scholar]
- 19.Global Initiative for Chronic Obstructive Lung Disease . Global strategy for prevention, diagnosis and management of COPD: 2024 report. Date last accessed: 1 February 2024. Date last updated: 2024. https://goldcopd.org/2024-gold-report/
- 20.Celli BR, Fabbri LM, Aaron SD, et al. An updated definition and severity classification of chronic obstructive pulmonary disease exacerbations: the Rome proposal. Am J Respir Crit Care Med 2021; 204: 1251–1258. doi: 10.1164/rccm.202108-1819PP [DOI] [PubMed] [Google Scholar]
- 21.Radchenko C, Alraiyes AH, Shojaee S. A systematic approach to the management of massive hemoptysis. J Thorac Dis 2017; 9: S1069–S1086. doi: 10.21037/jtd.2017.06.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seo H, Cha SI, Park J, et al. Hemoptysis as the presenting manifestation of bronchiectasis-associated hospitalization in Korea. J Thorac Dis 2023; 15: 3636–3645. doi: 10.21037/jtd-22-1541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aliberti S, Lonni S, Dore S, et al. Clinical phenotypes in adult patients with bronchiectasis. Eur Respir J 2016; 47: 1113–1122. doi: 10.1183/13993003.01899-2015 [DOI] [PubMed] [Google Scholar]
- 24.Choi H, Ryu S, Keir HR, et al. Inflammatory molecular endotypes in bronchiectasis: a European multicenter cohort study. Am J Respir Crit Care Med 2023; 208: 1166–1176. doi: 10.1164/rccm.202303-0499OC [DOI] [PubMed] [Google Scholar]
- 25.Sibila O, Laserna E, Shoemark A, et al. Heterogeneity of treatment response in bronchiectasis clinical trials. Eur Respir J 2022; 59: 2100777. doi: 10.1183/13993003.00777-2021 [DOI] [PubMed] [Google Scholar]
- 26.Artaraz A, Crichton ML, Finch S, et al. Development and initial validation of the bronchiectasis exacerbation and symptom tool (BEST). Respir Res 2020; 21: 18. doi: 10.1186/s12931-019-1272-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shih VH, Jison M, Bark E, et al. The Bronchiectasis exacerbation diary: a novel patient-reported outcome for non-cystic fibrosis bronchiectasis. ERJ Open Res 2023; 9: 00712-2022. doi: 10.1183/23120541.00712-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chalmers JD, Aliberti S, Filonenko A, et al. Characterization of the “frequent exacerbator phenotype” in bronchiectasis. Am J Respir Crit Care Med 2018; 197: 1410–1420. doi: 10.1164/rccm.201711-2202OC [DOI] [PubMed] [Google Scholar]
- 29.Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med 2010; 363: 1128–1138. doi: 10.1056/NEJMoa0909883 [DOI] [PubMed] [Google Scholar]
- 30.Denlinger LC, Heymann P, Lutter R, et al. Exacerbation-prone asthma. J Allergy Clin Immunol Pract 2020; 8: 474–482. doi: 10.1016/j.jaip.2019.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Araújo D, Shteinberg M, Aliberti S, et al. The independent contribution of Pseudomonas aeruginosa infection to long-term clinical outcomes in bronchiectasis. Eur Respir J 2018; 51: 1701953. doi: 10.1183/13993003.01953-2017 [DOI] [PubMed] [Google Scholar]
- 32.Cole PJ. Inflammation: a two-edged sword–the model of bronchiectasis. Eur J Respir Dis Suppl 1986; 147: 6–15. [PubMed] [Google Scholar]
- 33.Flume PA, Chalmers JD, Olivier KN. Advances in bronchiectasis: endotyping, genetics, microbiome, and disease heterogeneity. Lancet 2018; 392: 880–890. doi: 10.1016/S0140-6736(18)31767-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amati F, Simonetta E, Gramegna A, et al. The biology of pulmonary exacerbations in bronchiectasis. Eur Respir Rev 2019; 28: 190055. doi: 10.1183/16000617.0055-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang HY, Chung FT, Lo CY, et al. Etiology and characteristics of patients with bronchiectasis in Taiwan: a cohort study from 2002 to 2016. BMC Pulm Med 2020; 20: 45. doi: 10.1186/s12890-020-1080-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Polverino E, Goeminne PC, McDonnell MJ, et al. European Respiratory Society guidelines for the management of adult bronchiectasis. Eur Respir J 2017; 50: 1700629. doi: 10.1183/13993003.00629-2017 [DOI] [PubMed] [Google Scholar]
- 37.Hill AT, Sullivan AL, Chalmers JD, et al. British Thoracic Society guideline for bronchiectasis in adults. BMJ Open Respir Res 2018; 5: e000348. doi: 10.1136/bmjresp-2018-000348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dickson RP, Martinez FJ, Huffnagle GB. The role of the microbiome in exacerbations of chronic lung diseases. Lancet 2014; 384: 691–702. doi: 10.1016/S0140-6736(14)61136-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dickson RP, Erb-Downward JR, Freeman CM, et al. Spatial variation in the healthy human lung microbiome and the adapted island model of lung biogeography. Ann Am Thorac Soc 2015; 12: 821–830. doi: 10.1513/AnnalsATS.201501-029OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Natalini JG, Singh S, Segal LN. The dynamic lung microbiome in health and disease. Nat Rev Microbiol 2023; 21: 222–235. doi: 10.1038/s41579-022-00821-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Keir HR, Chalmers JD. Counterpoint: is chronic bacterial infection clinically relevant in COPD? No. Chest 2022; 162: 972–976. doi: 10.1016/j.chest.2022.07.009 [DOI] [PubMed] [Google Scholar]
- 42.Tunney MM, Einarsson GG, Wei L, et al. Lung microbiota and bacterial abundance in patients with bronchiectasis when clinically stable and during exacerbation. Am J Respir Crit Care Med 2013; 187: 1118–1126. doi: 10.1164/rccm.201210-1937OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rogers GB, Zain NMM, Bruce KD, et al. A novel microbiota stratification system predicts future exacerbations in bronchiectasis. Ann Am Thorac Soc 2014; 11: 496–503. doi: 10.1513/AnnalsATS.201310-335OC [DOI] [PubMed] [Google Scholar]
- 44.Dicker AJ, Lonergan M, Keir HR, et al. The sputum microbiome and clinical outcomes in patients with bronchiectasis: a prospective observational study. Lancet Respir Med 2021; 9: 885–896. doi: 10.1016/S2213-2600(20)30557-9 [DOI] [PubMed] [Google Scholar]
- 45.Finch S, McDonnell MJ, Abo-Leyah H, et al. A comprehensive analysis of the impact of Pseudomonas aeruginosa colonization on prognosis in adult bronchiectasis. Ann Am Thorac Soc 2015; 12: 1602–1611. [DOI] [PubMed] [Google Scholar]
- 46.Keir HR, Shoemark A, Dicker AJ, et al. Neutrophil extracellular traps, disease severity, and antibiotic response in bronchiectasis: an international, observational, multicohort study. Lancet Respir Med 2021; 9: 873–884. doi: 10.1016/S2213-2600(20)30504-X [DOI] [PubMed] [Google Scholar]
- 47.Aogáin M M, Narayana JK, Tiew PY, et al. Integrative microbiomics in bronchiectasis exacerbations. Nat Med 2021; 27: 688–699. doi: 10.1038/s41591-021-01289-7 [DOI] [PubMed] [Google Scholar]
- 48.Sun J, LaRock DL, Skowronski EA, et al. The Pseudomonas aeruginosa protease LasB directly activates IL-1β. EBioMedicine 2020; 60: 102984. doi: 10.1016/j.ebiom.2020.102984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alcaraz-Serrano V, Fernández-Barat L, Scioscia G, et al. Mucoid Pseudomonas aeruginosa alters sputum viscoelasticity in patients with non-cystic fibrosis bronchiectasis. Respir Med 2019; 154: 40–46. doi: 10.1016/j.rmed.2019.06.012 [DOI] [PubMed] [Google Scholar]
- 50.Rogers GB, Bruce KD, Martin ML, et al. The effect of long-term macrolide treatment on respiratory microbiota composition in non-cystic fibrosis bronchiectasis: an analysis from the randomised, double-blind, placebo-controlled BLESS trial. Lancet Respir Med 2014; 2: 988–996. doi: 10.1016/S2213-2600(14)70213-9 [DOI] [PubMed] [Google Scholar]
- 51.Qin S, Xiao W, Zhou C, et al. Pseudomonas aeruginosa: pathogenesis, virulence factors, antibiotic resistance, interaction with host, technology advances and emerging therapeutics. Signal Transduct Target Ther 2022; 7: 199. doi: 10.1038/s41392-021-00710-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Menéndez R, Méndez R, Polverino E, et al. Risk factors for multidrug-resistant pathogens in bronchiectasis exacerbations. BMC Infect Dis 2017; 17: 569. doi: 10.1186/s12879-017-2754-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chang CH, Chang CH, Huang SH, et al. Epidemiology and outcomes of multidrug-resistant bacterial infection in non-cystic fibrosis bronchiectasis. Ann Clin Microbiol Antimicrob 2024; 23: 15. doi: 10.1186/s12941-024-00675-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kartsiouni E, Chatzipanagiotou S, Tamvakeras P, et al. The role of viral infections in pulmonary exacerbations of patients with non-cystic fibrosis bronchiectasis: a systematic review. Respir Investig 2022; 60: 625–632. doi: 10.1016/j.resinv.2022.06.002 [DOI] [PubMed] [Google Scholar]
- 55.Polverino E, Rosales-Mayor E, Benegas M, et al. Pneumonic and non-pneumonic exacerbations in bronchiectasis: clinical and microbiological differences. J Infect 2018; 77: 99–106. doi: 10.1016/j.jinf.2018.04.006 [DOI] [PubMed] [Google Scholar]
- 56.Park YE, Sung H, Oh YM. Respiratory viruses in acute exacerbations of bronchiectasis. J Korean Med Sci 2021; 36: e217. doi: 10.3346/jkms.2021.36.e217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen C, Huang Y, Yuan J, et al. The roles of bacteria and viruses in bronchiectasis exacerbation: a prospective study. Arch Bronconeumol 2020; 56: 621–629. doi: 10.1016/j.arbres.2019.12.010 [DOI] [PubMed] [Google Scholar]
- 58.Gao YH, Guan WJ, Xu G, et al. The role of viral infection in pulmonary exacerbations of bronchiectasis in adults: a prospective study. Chest 2015; 147: 1635–1643. doi: 10.1378/chest.14-1961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Crichton ML, Shoemark A, Chalmers JD. The impact of the COVID-19 pandemic on exacerbations and symptoms in bronchiectasis: a prospective study. Am J Respir Crit Care Med 2021; 204: 857–859. doi: 10.1164/rccm.202105-1137LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Åstrand A, Kiddle SJ, Siva Ganesh Mudedla R, et al. Effect of COVID-19 on bronchiectasis exacerbation rates: a retrospective US insurance claims study. Ann Am Thorac Soc 2024; 21: 261–270. doi: 10.1513/AnnalsATS.202211-944OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Choi H, Lee H, Lee SK, et al. Impact of bronchiectasis on susceptibility to and severity of COVID-19: a nationwide cohort study. Ther Adv Respir Dis 2021; 15: 4–7. doi: 10.1177/1753466621995043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shteinberg M, Sibila O, Stein N, et al. Risk of SARS-CoV-2 infection and disease severity among people with bronchiectasis: analysis of three population registries. Chest 2024; 165: 79–83. doi: 10.1016/j.chest.2023.08.007 [DOI] [PubMed] [Google Scholar]
- 63.Kwok WC, Ho JCM, Tam TCC, et al. Increased exacerbations of bronchiectasis following recovery from mild COVID-19 in patients with non-cystic fibrosis bronchiectasis. Respirology 2024; 29: 209–216. doi: 10.1111/resp.14664 [DOI] [PubMed] [Google Scholar]
- 64.Aogáin MM, Chandrasekaran R, Hou Lim AY, et al. Immunological corollary of the pulmonary mycobiome in bronchiectasis: the CAMEB study. Eur Respir J 2018; 52: 1800766. doi: 10.1183/13993003.00766-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Goeminne PC, Cox B, Finch S, et al. The impact of acute air pollution fluctuations on bronchiectasis pulmonary exacerbation: a case-crossover analysis. Eur Respir J 2018; 52: 1702557. doi: 10.1183/13993003.02557-2017 [DOI] [PubMed] [Google Scholar]
- 66.Garcia-Olivé I, Stojanovic Z, Radua J, et al. Effect of air pollution on exacerbations of bronchiectasis in Badalona, Spain, 2008–2016. Respiration 2018; 96: 111–116. doi: 10.1159/000488646 [DOI] [PubMed] [Google Scholar]
- 67.Wang Z, Zhou Y, Zhang Y, et al. Association of hospital admission for bronchiectasis with air pollution: A province-wide time-series study in southern China. Int J Hyg Environ Health 2021; 231: 113654. doi: 10.1016/j.ijheh.2020.113654 [DOI] [PubMed] [Google Scholar]
- 68.Lee H, Kim SH, Lee SK, et al. Impact of air pollution on healthcare utilization in patients with bronchiectasis. Front Med 2023; 10: 1233516. doi: 10.3389/fmed.2023.1233516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gozzi-Silva SC, Teixeira FME, Duarte AdS, et al. Immunomodulatory role of nutrients: how can pulmonary dysfunctions improve? Front Nutr 2021; 8: 674258. doi: 10.3389/fnut.2021.674258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Narayana JK, Aliberti S, Aogáin MM, et al. Microbial dysregulation of the gut–lung axis in bronchiectasis. Am J Respir Crit Care Med 2023; 207: 908–920. doi: 10.1164/rccm.202205-0893OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chandra RK. Nutrition and the immune system: an introduction. Am J Clin Nutr 1997; 66: 460S–463S. doi: 10.1093/ajcn/66.2.460S [DOI] [PubMed] [Google Scholar]
- 72.Schaible UE, Kaufmann SHE. Malnutrition and infection: complex mechanisms and global impacts. PLoS Med 2007; 4: e115. doi: 10.1371/journal.pmed.0040115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chalmers JD, Moffitt KL, Suarez-Cuartin G, et al. Neutrophil elastase activity is associated with exacerbations and lung function decline in bronchiectasis. Am J Respir Crit Care Med 2017; 195: 1384–1393. doi: 10.1164/rccm.201605-1027OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shoemark A, Cant E, Carreto L, et al. A point-of-care neutrophil elastase activity assay identifies bronchiectasis severity, airway infection and risk of exacerbation. Eur Respir J 2019; 53: 1900303. doi: 10.1183/13993003.00303-2019 [DOI] [PubMed] [Google Scholar]
- 75.Oriano M, Gramegna A, Terranova L, et al. Sputum neutrophil elastase associates with microbiota and Pseudomonas aeruginosa in bronchiectasis. Eur Respir J 2020; 56: 2000769. doi: 10.1183/13993003.00769-2020 [DOI] [PubMed] [Google Scholar]
- 76.Zhou J, Perelman JM, Kolosov VP, et al. Neutrophil elastase induces MUC5AC secretion via protease-activated receptor 2. Mol Cell Biochem 2013; 377: 75–85. doi: 10.1007/s11010-013-1572-3 [DOI] [PubMed] [Google Scholar]
- 77.Shteinberg M, Chalmers JD, Narayana JK, et al. Bronchiectasis with chronic rhinosinusitis is associated with eosinophilic airway inflammation and is distinct from asthma. Ann Am Thorac Soc 2024; 21: 748–758. doi: 10.1513/AnnalsATS.202306-551OC [DOI] [PubMed] [Google Scholar]
- 78.Shoemark A, Shteinberg M, De Soyza A, et al. Characterization of eosinophilic bronchiectasis a European multicohort study. Am J Respir Crit Care Med 2022; 205: 894–902. doi: 10.1164/rccm.202108-1889OC [DOI] [PubMed] [Google Scholar]
- 79.David B, Bafadhel M, Koenderman L, et al. Eosinophilic inflammation in COPD: from an inflammatory marker to a treatable trait. Thorax 2021; 76: 188–195. doi: 10.1136/thoraxjnl-2020-215167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Crisford H, Sapey E, Rogers GB, et al. Neutrophils in asthma: the good, the bad and the bacteria. Thorax 2021; 76: 835–844. doi: 10.1136/thoraxjnl-2020-215986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.McDonnell MJ, O'Toole D, Ward C, et al. A qualitative synthesis of gastro–oesophageal reflux in bronchiectasis: current understanding and future risk. Respir Med 2018; 141: 132–143. doi: 10.1016/j.rmed.2018.06.031 [DOI] [PubMed] [Google Scholar]
- 82.Handley E, Nicolson CH, Hew M, et al. Prevalence and clinical implications of chronic rhinosinusitis in people with bronchiectasis: a systematic review. J Allergy Clin Immunol Pract 2019; 7: 2004–2012.. doi: 10.1016/j.jaip.2019.02.026 [DOI] [PubMed] [Google Scholar]
- 83.Perluk T, Abu Bandora E, Freund O, et al. Asymptomatic dysphagia and aspiration in patients with idiopathic bronchiectasis. Lung 2024; 202: 189–195. doi: 10.1007/s00408-024-00683-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kelly N, Winning L, Irwin C, et al. Periodontal status and chronic obstructive pulmonary disease (COPD) exacerbations: a systematic review. BMC Oral Health 2021; 21: 425. doi: 10.1186/s12903-021-01757-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.McDonnell MJ, Aliberti S, Goeminne PC, et al. Comorbidities and the risk of mortality in patients with bronchiectasis: an international multicentre cohort study. Lancet Respir Med 2016; 4: 969–979. doi: 10.1016/S2213-2600(16)30320-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.McCullough AR, Tunney MM, Stuart Elborn J, et al. Predictors of adherence to treatment in bronchiectasis. Respir Med 2015; 109: 838–845. doi: 10.1016/j.rmed.2015.04.011 [DOI] [PubMed] [Google Scholar]
- 87.Pembridge T, Chalmers JD. Precision medicine in bronchiectasis. Breathe 2021; 17: 210119. doi: 10.1183/20734735.0119-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Steer J, Gibson J, Bourke SC. The DECAF score: predicting hospital mortality in exacerbations of chronic obstructive pulmonary disease. Thorax 2012; 67: 970–976. doi: 10.1136/thoraxjnl-2012-202103 [DOI] [PubMed] [Google Scholar]
- 89.Echevarria C, Steer J, Heslop-Marshall K, et al. Validation of the DECAF score to predict hospital mortality in acute exacerbations of COPD. Thorax 2016; 71: 133–140. doi: 10.1136/thoraxjnl-2015-207775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.M VDE WL, W B RL, G T NK, et al. Defining community acquired pneumonia severity on presentation to hospital: An international derivation and validation study. Thorax 2003; 58: 377–382. doi: 10.1136/thorax.58.5.377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fine MJ, Auble TE, Yealy DM, et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med 1997; 336: 243–250. doi: 10.1056/NEJM199701233360402 [DOI] [PubMed] [Google Scholar]
- 92.Martínez-García MÁ, Máiz L, Olveira C, et al. Spanish guidelines on the evaluation and diagnosis of bronchiectasis in adults. Arch Bronconeumol 2018; 54: 79–87. doi: 10.1016/j.arbres.2017.07.015 [DOI] [PubMed] [Google Scholar]
- 93.Polverino E, Rosales-Mayor E, Torres A. Exacerbation of bronchiectasis. In: Chalmers J, Polverino E, Aliberti S, eds. Bronchiectasis. Cham, Springer International Publishing, 2018; pp. 205–222. doi: 10.1007/978-3-319-61452-6_15 [DOI] [Google Scholar]
- 94.Crichton ML, Aliberti S, Chalmers JD. A systematic review of pharmacotherapeutic clinical trial end-points for bronchiectasis in adults. Eur Respir Rev 2019; 28: 180108. doi: 10.1183/16000617.0108-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.López-Cortés LE, Ayerbe-Garciá R, Carrasco-Hernández L, et al. Outpatient parenteral antimicrobial treatment for non-cystic fibrosis bronchiectasis exacerbations: a prospective multicentre observational cohort study. Respiration 2019; 98: 294–300. doi: 10.1159/000501085 [DOI] [PubMed] [Google Scholar]
- 96.Alcaraz V, Polverino E, Rosales E, et al. Exacerbations and pneumonia in bronchiectasis: clinical and microbiological characterization. Eur Respir J 2015; 46: Suppl. 59, PA367. doi: 10.1183/13993003.congress-2015.PA367 [DOI] [Google Scholar]
- 97.Aliberti S, Hill AT, Mantero M, et al. Quality standards for the management of bronchiectasis in Italy: a national audit. Eur Respir J 2016; 48: 244–248. doi: 10.1183/13993003.00232-2016 [DOI] [PubMed] [Google Scholar]
- 98.Kwok WC, Teo KC, Lau KK, et al. High-sensitivity C-reactive protein level in stable-state bronchiectasis predicts exacerbation risk. BMC Pulm Med 2024; 24: 80. doi: 10.1186/s12890-024-02888-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sivapalan P, Lapperre TS, Janner J, et al. Eosinophil-guided corticosteroid therapy in patients admitted to hospital with COPD exacerbation (CORTICO-COP): a multicentre, randomised, controlled, open-label, non-inferiority trial. Lancet Respir Med 2019; 7: 699–709. doi: 10.1016/S2213-2600(19)30176-6 [DOI] [PubMed] [Google Scholar]
- 100.Ramakrishnan S, Jeffers H, Langford-Wiley B, et al. Blood eosinophil-guided oral prednisolone for COPD exacerbations in primary care in the UK (STARR2): a non-inferiority, multicentre, double-blind, placebo-controlled, randomised controlled trial. Lancet Respir Med 2024; 12: 67–77. doi: 10.1016/S2213-2600(23)00298-9 [DOI] [PubMed] [Google Scholar]
- 101.Good W, Mooney S, Zeng I, et al. Sputum procalcitonin levels in patients admitted to hospital with acute exacerbations of bronchiectasis. Heal Sci Reports 2020; 3: e203. doi: 10.1002/hsr2.203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Good W, Jeon G, Zeng I, et al. Sputum procalcitonin: a potential biomarker in stable bronchiectasis. ERJ Open Res 2021; 7: 00285-2021. doi: 10.1183/23120541.00285-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Somayaji R, Goss CH. Duration of antibiotic therapy in non-cystic fibrosis bronchiectasis. Curr Pulmonol Reports 2019; 8: 160–165. doi: 10.1007/s13665-019-00235-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Conceição M, Shteinberg M, Goeminne P, et al. Eradication treatment for Pseudomonas aeruginosa infection in adults with bronchiectasis: a systematic review and meta-analysis. Eur Respir Rev 2024; 33: 230178. doi: 10.1183/16000617.0178-2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bedi P, Cartlidge MK, Zhang Y, et al. Feasibility of shortening intravenous antibiotic therapy for bronchiectasis based on bacterial load: a proof-of-concept randomised controlled trial. Eur Respir J 2021; 58: 2004388. doi: 10.1183/13993003.04388-2020 [DOI] [PubMed] [Google Scholar]
- 106.Martínez-García MÁ, Oscullo G, García-Ortega A, et al. Inhaled corticosteroids in adults with non-cystic fibrosis bronchiectasis: from bench to bedside. a narrative review. Drugs 2022; 82: 1453–1468. doi: 10.1007/s40265-022-01785-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Martinez-Garcia MA. Inhaled corticosteroids and bronchiectasis: friend or foe? J Clin Med 2023; 12: 10–13. doi: 10.3390/jcm12093322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Halm EA, Fine MJ, Marrie TJ, et al. Time to clinical stability in patients hospitalized with community-acquired pneumonia: Implications for practice guidelines. JAMA 1998; 279: 1452–1457. doi: 10.1001/jama.279.18.1452 [DOI] [PubMed] [Google Scholar]
- 109.Brill SE, Patel ARC, Singh R, et al. Lung function, symptoms and inflammation during exacerbations of non-cystic fibrosis bronchiectasis: a prospective observational cohort study. Respir Res 2015; 16: 16. doi: 10.1186/s12931-015-0167-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Donaldson GC, Law M, Kowlessar B, et al. Impact of prolonged exacerbation recovery in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2015; 192: 943–950. doi: 10.1164/rccm.201412-2269OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Suarez-Cuartin G, Feliu A, Rodrigo-Troyano A, et al. Exacerbations of bronchiectasis requiring hospitalization; clinical characteristics and outcomes. Eur Respir J 2016; 48: Suppl. 60, PA1550. doi: 10.1183/13993003.congress-2016.PA1550 [DOI] [Google Scholar]
- 112.Huang HY, Chung FT, Lin CY, et al. Influence of comorbidities and airway clearance on mortality and outcomes of patients with severe bronchiectasis exacerbations in Taiwan. Front Med 2022; 8: 812775. doi: 10.3389/fmed.2021.812775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Scioscia G, Alcaraz-Serrano V, Méndez R, et al. Factors associated with one-year mortality in hospitalised patients with exacerbated bronchiectasis. Arch Bronconeumol 2022; 58: 773–775. doi: 10.1016/j.arbres.2022.04.008 [DOI] [PubMed] [Google Scholar]
- 114.Finklea JD, Khan G, Thomas S, et al. Predictors of mortality in hospitalized patients with acute exacerbation of bronchiectasis. Respir Med 2010; 104: 816–821. doi: 10.1016/j.rmed.2009.11.021 [DOI] [PubMed] [Google Scholar]
- 115.Navaratnam V, Millett ERC, Hurst JR, et al. Bronchiectasis and the risk of cardiovascular disease: a population-based study. Thorax 2017; 72: 161–166. doi: 10.1136/thoraxjnl-2015-208188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ledda RE, Balbi M, Milone F, et al. Cardiovascular outcomes after a respiratory tract infection among adults with non-cystic fibrosis bronchiectasis: a general population-based study. Ann Am Thorac Soc 2021; 3: 315–321. doi: 10.1513/AnnalsATS.201706-488OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Méndez R, Feced L, Alcaraz-Serrano V, et al. Cardiovascular events during and after bronchiectasis exacerbations and long-term mortality. Chest 2022; 161: 629–636. doi: 10.1016/j.chest.2021.10.013 [DOI] [PubMed] [Google Scholar]
- 118.Lee SC, Son KJ, Hoon Han C, et al. Cardiovascular and cerebrovascular-associated mortality in patients with preceding bronchiectasis exacerbation. Ther Adv Respir Dis 2022; 16: 17534666221144206. doi: 10.1177/17534666221144206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chalmers JD, Boersma W, Lonergan M, et al. Long-term macrolide antibiotics for the treatment of bronchiectasis in adults: an individual participant data meta-analysis. Lancet Respir Med 2019; 7: 845–854. doi: 10.1016/S2213-2600(19)30191-2 [DOI] [PubMed] [Google Scholar]
- 120.Cordeiro R, Choi H, Haworth CS, et al. The efficacy and safety of inhaled antibiotics for the treatment of bronchiectasis in adults. Chest 2024; 166: 61–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.O'Grady KAF, Cripps AW, Grimwood K. Paediatric and adult bronchiectasis: vaccination in prevention and management. Respirology 2019; 24: 107–114. doi: 10.1111/resp.13446 [DOI] [PubMed] [Google Scholar]
- 122.Chang CC, Singleton RJ, Morris PS, et al. Pneumococcal vaccines for children and adults with bronchiectasis. Cochrane Database Syst Rev 2009; 2009: CD006316. doi: 10.1002/14651858.CD006316.pub3 [DOI] [PubMed] [Google Scholar]
- 123.Schmoele-Thoma B, Zareba AM, Jiang Q, et al. Vaccine efficacy in adults in a respiratory syncytial virus challenge study. N Engl J Med 2022; 386: 2377–2386. doi: 10.1056/NEJMoa2116154 [DOI] [PubMed] [Google Scholar]
- 124.Walsh EE, Pérez Marc G, Zareba AM, et al. Efficacy and safety of a bivalent RSV prefusion F vaccine in older adults. N Engl J Med 2023; 388: 1465–1477. doi: 10.1056/NEJMoa2213836 [DOI] [PubMed] [Google Scholar]
- 125.Muñoz G, De Gracia J, Buxó M, et al. Long-term benefits of airway clearance in bronchiectasis: a randomised placebo-controlled trial. Eur Respir J 2018; 51: 1701926. doi: 10.1183/13993003.01926-2017 [DOI] [PubMed] [Google Scholar]
- 126.Murray MP, Pentland JL, Hill AT. A randomised crossover trial of chest physiotherapy in non-cystic fibrosis bronchiectasis. Eur Respir J 2009; 34: 1086–1092. doi: 10.1183/09031936.00055509 [DOI] [PubMed] [Google Scholar]
- 127.Qi Q, Ailiyaer Y, Liu R, et al. Effect of N-acetylcysteine on exacerbations of bronchiectasis (BENE): a randomized controlled trial. Respir Res 2019; 20: 73. doi: 10.1186/s12931-019-1042-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Liao Y, Wu Y, Zi K, et al. The effect of N-acetylcysteine in patients with non-cystic fibrosis bronchiectasis (NINCFB): study protocol for a multicentre, double-blind, randomised, placebo-controlled trial. BMC Pulm Med 2022; 22: 401. doi: 10.1186/s12890-022-02202-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lee AL, Hill CJ, Cecins N, et al. The short and long term effects of exercise training in non-cystic fibrosis bronchiectasis - a randomised controlled trial. Respir Res 2014; 15: 44. doi: 10.1186/1465-9921-15-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Martínez-García MÁ, Méndez R, Olveira C, et al. The U-shaped relationship between eosinophil count and bronchiectasis severity: the effect of inhaled corticosteroids. Chest 2023; 164: 606–613. doi: 10.1016/j.chest.2023.04.029 [DOI] [PubMed] [Google Scholar]
- 131.Håkansson KEJ, Fjaellegaard K, Browatzki A, et al. Inhaled corticosteroid therapy in bronchiectasis is associated with all-cause mortality: a prospective cohort study. Int J COPD 2021; 16: 2119–2127. doi: 10.2147/COPD.S311236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jayaram L, Vandal AC, Chang CL, et al. Tiotropium treatment for bronchiectasis: a randomised, placebo-controlled, crossover trial. Eur Respir J 2022; 59: 2102184. doi: 10.1183/13993003.02184-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chalmers JD, Haworth CS, Metersky ML, et al. Phase 2 trial of the DPP-1 inhibitor brensocatib in bronchiectasis. N Engl J Med 2020; 383: 2127–2137. doi: 10.1056/NEJMoa2021713 [DOI] [PubMed] [Google Scholar]
- 134.Thornton CS, Somayaji R, Lim RK. Assessment of factors and interventions towards therapeutic adherence among persons with non-cystic fibrosis bronchiectasis. ERJ Open Res 2022; 8: 00340-2022. doi: 10.1183/23120541.00340-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]