Abstract
Following intracerebral inoculation, the DA strain of Theiler’s virus sequentially infects neurons in the gray matter and glial cells in the white matter of the spinal cord. It persists in the latter throughout the life of the animal. Several observations suggest that the virus spreads from the gray to the white matter by axonal transport. In contrast, the neurovirulent GDVII strain causes a fatal encephalitis with lytic infection of neurons. It does not infect the white matter of the spinal cord efficiently and does not persist in survivors. The inability of this virus to infect the white matter could be due to a defect in axonal transport. Using footpad inoculations, we showed that the GDVII strain is, in fact, transported in axons. Transport was prevented by sectioning the sciatic nerve. The kinetics of transport and experiments using colchicine suggested that the virus uses microtubule-associated fast axonal transport. Our results show that a cardiovirus can spread by fast axonal transport and suggest that the inability of the GDVII strain to infect the white matter is not due to a defect in axonal transport.
Theiler’s murine encephalomyelitis virus (TMEV) belongs to the Cardiovirus genus within the Picornaviridae family (22, 24, 27). Most strains of TMEV, including the DA and BeAn strains, cause a biphasic disease of the central nervous system (CNS) after intracranial inoculation (17). The first phase, which occurs during the first 7 to 10 days, is an acute encephalomyelitis. At this time, the virus is found in the gray matter of the CNS, predominantly in neurons and in a small number of glial cells (2). Soon after, the virus disappears from the gray matter and infects the white matter of the spinal cord, where it persists, mainly in macrophage-microglial cells and, to a lesser extent, in oligodendrocytes (19, 26). Persistence in the white matter causes chronic inflammation and primary demyelination.
Although the routes of TMEV dissemination within the CNS have not been fully explored, there is a good indication that the virus may use axonal transport. Viral antigens have been found in axons by using ultrastructural immunohistochemistry (8), and the pattern of spread of the virus within the limbic system in mice is consistent with axonal transport (35). Furthermore, TMEV antigens are found rather early in the corticospinal tract, the main pathway from the brain to the anterior horn cells (35), and infected white matter macrophages have been found close to axons containing viral antigens (19).
In contrast to the DA and BeAn strains, the GDVII strain is highly neurovirulent and kills its host in a matter of days (33). It does not persist in the CNSs of the rare survivors (16). Attenuated variants of this strain have been used recently to confirm its incapacity to persist (12, 18). Importantly, the GDVII strain, which infects the gray matter, where it replicates almost exclusively in neurons (2, 30), does not infect the white matter (12). Viral recombinants between the persisting DA or BeAn strain and the virulent GDVII strain have been constructed by several groups in order to map viral genes responsible for persistence. The results obtained by different laboratories are, on the whole, consistent and show that the capsids of the DA and BeAn strains bear the main determinants of persistence (1, 20). Studies from our group showed that the ability to persist correlates with the ability to infect the white matter of the spinal cord (10). Thus, the capsid of the DA strain may determine persistence by allowing the infection of white matter glial cells. Among other possibilities, the DA capsid could allow the virus to be transported in axons. According to this hypothesis, the inability of the GDVII virus to infect the white matter and to persist could be due to defective axonal transport. There is a precedent for closely related strains of the same virus differing in their abilities to be transported in axons. Tyler et al. showed that reovirus type 3 reaches the CNS via fast axonal transport, whereas type 1 reaches it via the bloodstream, and that this difference maps to the gene coding for the viral hemagglutinin (34). In the present work, we tested the ability of the GDVII strain to be transported in axons, using the paradigm of transport to the spinal cord through the sciatic nerve after footpad inoculation.
Clinical signs after footpad inoculation.
The GDVII virus infects and kills CNS neurons efficiently. If it is able to spread from the periphery to the spinal cord through the sciatic nerve, it will cause paralysis, appearing first in the inoculated limb. Eleven 4- to 5-week-old SJL/J mice were inoculated in the left hind footpad with 50 μl of phosphate-buffered saline containing 5 × 106 PFU of the GDVII strain and were examined daily for clinical signs. All mice showed symptoms. The typical course of the disease was paralysis of the inoculated limb 5 to 6 days postinoculation (p.i.), followed by paralysis of the contralateral hind limb 1 to 2 days later. Death of the animals occurred 9 to 10 days p.i. These results were consistent with the spread of the GDVII strain from the periphery to the CNS via the sciatic nerve.
Detection of the virus in the sciatic nerve and the spinal cord.
If the GDVII virus spreads from the footpad to the spinal cord through the sciatic nerve, viral RNA should appear first in the sciatic nerve of the inoculated leg, then in the inferior spinal cord—the region containing the neurons innervating the skin and muscles of the footpad—and lastly in the superior spinal cord. In contrast, if the virus reaches the CNS via the bloodstream, it should appear in the inferior and superior regions of the spinal cord at the same time. The pattern of the spread of TMEV to the CNS was examined by inoculating SJL/J mice with 5 × 106 PFU of the GDVII strain in the left hind footpad and sacrificing them on days 1 through 4 p.i. The spinal cords and sciatic nerves were removed. The spinal cord was divided into a superior segment, which consisted of the cervical cord, and an inferior segment, which consisted of the thoracic and lumbosacral cords. Total RNA was extracted from tissues as previously described (6), and viral RNA was detected by reverse transcription (RT)-PCR. Briefly, RT was performed with 10 μg of spinal cord RNA or with the totality of RNA extracted from the sciatic nerve. Then, 30 cycles of PCR were performed with primers specific for part of the capsid-coding region of the viral genome. The primers corresponded to GDVII virus nucleotides 2889 to 2909 (5′-TGACCCCCCTGACCTACCCTT-3′) and 3969 to 3989 (5′-CGCCCATGCACACGAGCATTC-3′). After separation by electrophoresis, the PCR products were transferred to nylon membranes and hybridized with a 32P-labeled probe consisting of nucleotides 3297 to 3319. For each tissue sample examined, except for sciatic nerve because of the limited amount of RNA available, the housekeeping β-actin mRNA was amplified in parallel by RT-PCR to confirm the integrity of the RNA (data not shown).
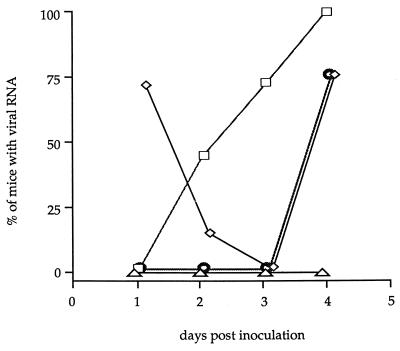
Viral RNA was first detected 1 day p.i. in the left sciatic nerves of five of seven inoculated mice (Fig. 1 and Table 1). At that time, viral RNA was not detected in the right sciatic nerve (Fig. 1). By 2 days p.i., viral RNA was also found in the inferior spinal cord segment. One day later, viral RNA had disappeared from the sciatic nerve and was detected only in the inferior spinal cord segment. By 4 days p.i., the whole spinal cord as well as the left sciatic nerve contained viral RNA (Fig. 1). As determined by using the Kaplan-Meier model and the log-rank test with a standard statistical threshold of a significance of 5%, the kinetics of appearance of viral RNA in the inferior spinal cord was statistically different from that observed in the superior spinal cord (P ≤ 0.002). The reappearance of viral RNA in the left sciatic nerve 4 days p.i. might have been due to axonal transport from the spinal cord back to the periphery.
FIG. 1.
Spread of the GDVII strain from the footpad to the sciatic nerve and the spinal cord. Shown are the percentages of mice inoculated in the footpad in which viral RNA was detected in the ipsilateral (◊) or contralateral (▵) sciatic nerve and in the inferior (□) or superior (○) spinal cord. The percentages were calculated from the data shown in Table 1.
TABLE 1.
Summary of the patterns of the spread of the GDVII strain from the periphery to the CNS in SJL/J mice after different treatments of the sciatic nervea
| Day p.i. | No. of mice positive for viral RNA/no. examined
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Untreated
|
Treated through sciatic nerve sectioning
|
Treated with colchicine
|
||||||||
| 1 day before viral inoculation
|
10 days before viral inoculation
|
|||||||||
| RSN | LSN | ISC | SSC | ISC | SSC | ISC | SSC | ISC | SSC | |
| 1 | 0/7 | 5/7 | 0/7 | 0/7 | ND | ND | 0/8 | 0/8 | 0/8 | 0/8 |
| 2 | 0/6 | 1/7 | 3/7 | 0/7 | 0/4 | 0/4 | 0/8 | 0/8 | 8/8 | 1/8 |
| 3 | 0/2 | 0/5 | 5/7 | 0/7 | 0/6 | 0/5 | 2/11 | 0/11 | 6/6 | 0/6 |
| 4 | 0/4 | 3/4 | 4/4 | 3/4 | 0/7 | 0/7 | 5/8 | 2/8 | 6/6 | 5/6 |
RSN, right sciatic nerve; LSN, left sciatic nerve; ISC, inferior spinal cord; SSC, superior spinal cord; ND, not done.
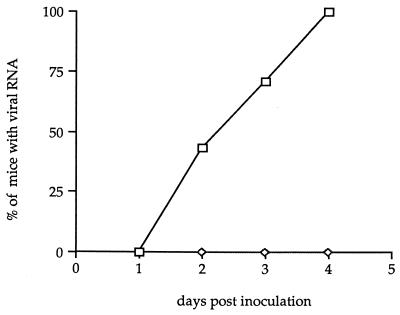
We examined if sectioning the sciatic nerve, which is the main neural pathway from the hind limb to the spinal cord, prevented the virus from reaching the CNS. Sciatic nerve transection was done as previously described (29, 34). Briefly, a small incision was made above the superficial gluteus muscle of the left hind leg, and the muscle layers were moved to expose the nerve. Approximately 1 mm of nerve was removed. Mice were checked for paresis after surgery. Two days after nerve transection, SJL/J mice were inoculated in the left hind footpad with 5 × 106 PFU of the GDVII strain, and they were sacrificed on days 2 through 4 p.i. for RT-PCR analysis. The sciatic nerves of approximately half of the mice were examined at necropsy. Transection was confirmed in all cases. As shown in Fig. 2 and Table 1, sectioning the nerve completely inhibited the spread of the virus to the spinal cord. Indeed, we were unable to detect viral RNA in the inferior spinal cord up to 4 days p.i.
FIG. 2.
Effect of sectioning the sciatic nerve on the spread of the GDVII strain to the inferior spinal cord segment. Shown are the percentages of mice inoculated in the footpad in which viral RNA was detected in the inferior spinal cord (◊). The control (□) corresponds to the spread of the GDVII virus to the inferior spinal cord in the absence of sectioning the sciatic nerve.
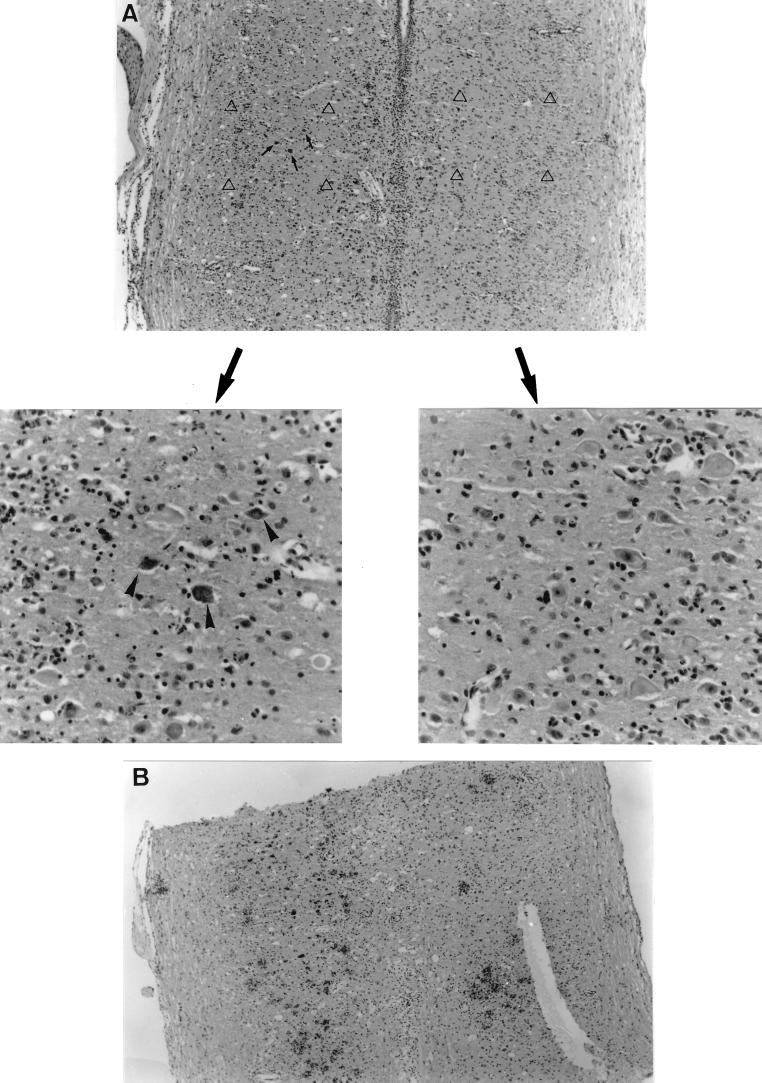
The pattern of the spread of the GDVII strain from the periphery to the spinal cord was confirmed by histological analysis performed as previously described (3). Viral antigens were first detected in the inferior spinal cord segment 3 days p.i. and in the superior spinal cord 1 day later. There was a good correlation between the pattern of infection and clinical signs. Five days p.i., most viral antigens were located on one side of the inferior spinal cord (Fig. 3). At this time, the mouse shown in Fig. 3A demonstrated paralysis of the inoculated limb. At 8 days p.i., a large amount of viral antigens was found on one side of the inferior spinal cord and some were found on the contralateral side (Fig. 3B). The mouse used for Fig. 3B demonstrated paralysis of both hind limbs. Taken together, the molecular and histological data indicate that the GDVII strain is able to spread from the periphery to the spinal cord via the sciatic nerve.
FIG. 3.
Histological findings in longitudinal sections of the inferior spinal cords of SJL/J mice after inoculation with 5 × 106 PFU of the GDVII strain in the left hind footpad. Viral antigens were detected in paraffin sections by immunocytochemistry. (A) Histological findings 5 days p.i. The top panel shows viral antigens (arrows) present on one side of the inferior spinal cord. Magnification, ×73. The bottom panels show higher-magnification views of symmetric fields designated by open triangles in the top panel. Arrowheads point to infected cells. Magnification, ×293. (B) Histological findings 8 days p.i. Viral antigens are present on both sides of the inferior spinal cord. Magnification, ×73.
Effect of colchicine on the transport of the virus through the sciatic nerve.
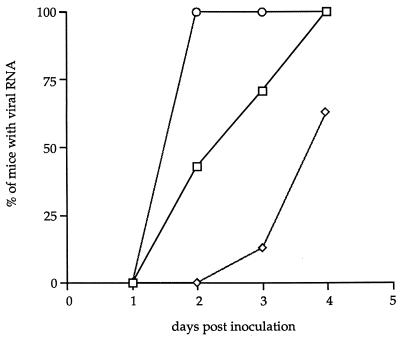
Our experiments did not identify the mechanism of transport of the GDVII virus in the sciatic nerve, although the presence of viral RNA in the inferior spinal cord as early as 2 days p.i. strongly suggested fast axonal transport. To examine this point in more detail, we studied the spread of the GDVII virus to the spinal cord after treatment of the sciatic nerve with colchicine, an agent which causes a reversible dissociation of microtubules and inhibits fast axonal transport (25). Briefly, 20 μg of colchicine in 40 μl of phosphate-buffered saline was injected into the left hind footpad. The animals were inoculated with 5 × 106 PFU of the GDVII strain 20 h later. The treatment caused a delay in the appearance of viral RNA in the spinal cord (Table 1 and Fig. 4). Viral RNA could not be detected in the spinal cord before 3 days p.i., and it was detected in only 2 of 11 mice. The difference between the kinetics of appearance of viral RNA in the inferior spinal cord with colchicine treatment and that without it was statistically significant (P ≤ 0.001).
FIG. 4.
Effect of colchicine treatment on the axonal transport of the GDVII strain. Colchicine was injected 1 (◊) or 10 (○) days before viral inoculation. Shown are the percentages of mice inoculated in the footpad in which viral RNA was detected in the inferior spinal cord. The control (□) corresponds to the spread of the GDVII virus to the inferior spinal cord without colchicine treatment.
As a control for these experiments, we examined, by electron microscopy, the effect of colchicine on the axonal microtubules of the sciatic nerve. Compared with the control, the axonal cytoskeleton showed disruption and microtubules disappeared 1.5 days after treatment with colchicine (Fig. 5). Another important control consisted of testing the effect of 500 μg of colchicine per ml (the concentration used for in vivo experiments) on the infectivity of viral particles and on viral replication in permissive BHK-21 cells. The virus was incubated with or without colchicine for 1 h at 37°C, and virus titers were measured before and after treatment. On the other hand, BHK-21 cells were treated with 500 μg of colchicine per ml or were left untreated, and they were infected with the GDVII strain of TMEV. Viral yields were measured after 24 h. No effect of colchicine on viral particles or virus yield was detected (data not shown).
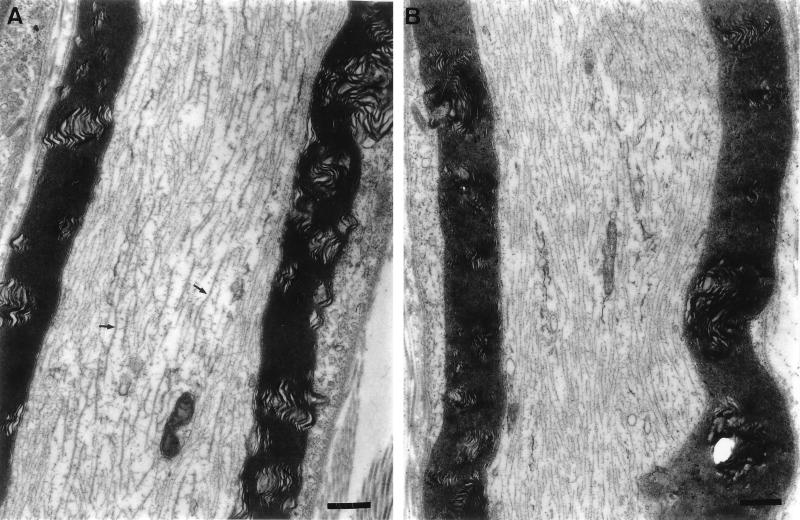
FIG. 5.
Effect of colchicine treatment on the microtubules of the sciatic nerve as observed by electron microscopy. (A) Longitudinal section of the sciatic nerve of an untreated mouse. The arrows point to some of the microtubules. Magnification, ×15,470. (B) Longitudinal section of the left sciatic nerve, 1.5 days after the injection of colchicine in the left hind footpad. Note the absence of microtubules. Magnification, ×15,470. Bars, 500 nm.
High doses of colchicine can cause axonal degeneration. The dose used in our experiments was in the range required for reversible inhibition of fast axonal transport and was below the threshold for nerve degeneration (4). Moreover, the detection of viral RNA in the inferior spinal cord 3 days p.i. after colchicine treatment suggested that colchicine did not cause nerve degeneration. However, to confirm this mice were inoculated with the virus 10 days after treatment with the drug. Under these conditions the spread of the GDVII virus to the spinal cord was not delayed (Fig. 4 and Table 1). The difference in kinetics of the spread to the CNS 1 and 10 days after colchicine treatment was statistically significant (P < 0.001). As expected, the difference between the kinetics observed 10 days after colchicine treatment and that without colchicine treatment was not statistically different (P > 0.1). Therefore, our results indicate that the spread of the GDVII virus through the sciatic nerve is microtubule dependent.
The axonal transport of the persisting DA and BeAn strains takes place in the CNS. The present work describes the axonal transport of the nonpersisting GDVII strain in the sciatic nerve, which belongs to the peripheral nervous system. Although there are well-known differences in the myelin sheaths of the CNS and peripheral nervous system, there is no evidence for a difference in the mechanism of axonal transport. Furthermore, viruses such as herpes simplex virus or rabies virus, for which peripheral axonal transport is well established, also spread within the CNS through axons and transneurally (4, 5, 15, 31). Thus, our results suggest that the inability of the GDVII strain to infect the white matter is not due to a defect of axonal transport in the CNS.
What could be the reason(s) why the GDVII virus does not infect glial cells in the white matter of the spinal cord, although it can be transported in axons? The virus infects neurons and is highly lytic as shown, e.g., by the presence in gray matter of numerous fragmented neuronal cell bodies with typical images of neuronophagia. It is possible that the rapid death of the neuron does not give the virus time to be transported down the axon. In contrast, the persisting DA strain exhibits a much more attenuated phenotype in neurons (11), which may allow the virus to reach the white matter by axonal transport. Alternatively, persisting strains may use different receptors on neurons and glial cells, and the GDVII strain might not recognize the latter. This is consistent with the presence of determinants of persistence at the surfaces of the capsids of the DA and BeAn strains (1, 20) and with the fact that the BeAn and GDVII virions bind differently to the surfaces of permissive cells (36). Obviously, a block of replication in glial cells, in particular in macrophage-microglial cells, could also occur at later steps, after attachment of the virus to the receptor. There is evidence for this from work done with macrophage cell lines. Jelachich et al. reported that the replication of the GDVII strain is highly restricted in two different murine macrophage cell lines (13). Moreover, recent studies showed that the GDVII virus does not replicate actively in another macrophage cell line, whereas the DA strain productively infected these cells (21, 32).
Since viral RNA was first detected in the lower spinal cord 2 days after inoculation into the footpad, the rate of transport in the sciatic nerve was approximately 20 mm/day, which is consistent with fast axonal transport (for a review, see reference 9). We confirmed that the virus uses fast axonal transport by using colchicine, a drug which depolymerizes microtubules. Colchicine has already been used to demonstrate fast axonal transport for reovirus type 3, herpes simplex virus, and rabies virus (4, 5, 15, 34). It had been conjectured for a long time that picornaviruses might use axonal transport (7, 14). Taking advantage of mice transgenic for the poliovirus receptor, two studies demonstrated axonal transport of poliovirus (28, 29). Recently, Ohka et al., although they did not use colchicine, also concluded that poliovirus is transported in the sciatic nerves of mice transgenic for the poliovirus receptor by the fast transport mechanism (23). However, several points concerning axonal transport of picornaviruses, such as the mechanism of entry at nerve terminals and of subsequent transport, remain unclear. There is evidence that the poliovirus receptor is required for entry at nerve terminals and that intact infectious virions are transported in the nerve.
In conclusion, we report that the GDVII strain of TMEV is transported in the sciatic nerve by a fast axonal transport mechanism. Therefore, the inability of this virus to infect the white matter of the spinal cord and to persist is most probably not due to a defect in axonal transport.
Acknowledgments
We thank Laurence Fiette for helpful discussions and Mireille Gau for secretarial assistance.
This work was supported by grants from the Institut Pasteur Fondation, the Centre National de la Recherche Scientifique, the Association pour la Recherche sur la Sclérose en Plaques, the National Multiple Sclerosis Society, and the EC Human Capital and Mobility program (contract no. CHRX-CT94-0670). C.M. is a recipient of a scholarship from the Ministère de la Recherche et de l’Enseignement Supérieur.
REFERENCES
- 1.Adami C, Pritchard A E, Knauf T, Luo M, Lipton H L. A determinant for central nervous system persistence localized in the capsid of Theiler’s murine encephalomyelitis virus by using recombinant viruses. J Virol. 1998;72:1662–1665. doi: 10.1128/jvi.72.2.1662-1665.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aubert C, Brahic M. Early infection of the central nervous system by the GDVII and DA strains of Theiler’s virus. J Virol. 1995;69:3197–3200. doi: 10.1128/jvi.69.5.3197-3200.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aubert C, Chamorro M, Brahic M. Identification of Theiler’s virus infected cells in the central nervous system of the mouse during demyelinating disease. Microb Pathog. 1987;3:319–326. doi: 10.1016/0882-4010(87)90002-7. [DOI] [PubMed] [Google Scholar]
- 4.Bulenga G, Heaney T. Post-exposure local treatment of mice infected with rabies with two axonal flow inhibitors, colchicine and vinblastine. J Gen Virol. 1978;39:381–385. doi: 10.1099/0022-1317-39-2-381. [DOI] [PubMed] [Google Scholar]
- 5.Ceccaldi P E, Gillet J P, Tsiang H. Inhibition of the transport of rabies virus in the central nervous system. J Neuropathol Exp Neurol. 1989;48:620–630. doi: 10.1097/00005072-198911000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 7.Dal Canto M C, Barbano R L, Jubelt B. Ultrastructural immunohistochemical localization of poliovirus during virulent infection of mice. J Neuropathol Exp Neurol. 1986;45:613–618. doi: 10.1097/00005072-198611000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Dal Canto M C, Lipton H L. Ultrastructural immunohistochemical localization of virus in acute and chronic demyelinating Theiler’s virus infection. Am J Pathol. 1982;106:20–29. [PMC free article] [PubMed] [Google Scholar]
- 9.Hirokawa N. Mechanism of axonal transport. Neurosci Res. 1993;18:1–9. doi: 10.1016/0168-0102(93)90099-c. [DOI] [PubMed] [Google Scholar]
- 10.Jarousse N, Fiette L, Grant R A, Hogle J M, McAllister A, Michiels T, Aubert C, Tangy F, Brahic M, Peña Rossi C. Chimeric Theiler’s virus with altered tropism for the central nervous system. J Virol. 1994;68:2781–2786. doi: 10.1128/jvi.68.5.2781-2786.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jarousse N, Syan S, Martinat C, Brahic M. The neurovirulence of the DA and GDVII strains of Theiler’s virus correlates with their ability to infect cultured neurons. J Virol. 1998;72:7213–7220. doi: 10.1128/jvi.72.9.7213-7220.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jarousse N, Viktorova E G, Pilipenko E V, Agol V I, Brahic M. An attenuated variant of the GDVII strain of Theiler’s virus does not persist and does not infect the white matter of the central nervous system. J Virol. 1999;73:801–804. doi: 10.1128/jvi.73.1.801-804.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jelachich M L, Bandyopadhyay P, Blum K, Lipton H L. Theiler’s virus in murine macrophage cell lines depends on the state of differentiation. Virology. 1995;209:437–444. doi: 10.1006/viro.1995.1276. [DOI] [PubMed] [Google Scholar]
- 14.Jubelt B, Narayan O, Johnson R T. Pathogenesis of human poliovirus infection in mice. II. Age-dependency of paralysis. J Neuropathol Exp Neurol. 1980;39:149–159. doi: 10.1097/00005072-198003000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Kristensson K, Lycke E, Sjostrand J. Spread of herpes simplex virus in peripheral nerves. Acta Neuropathol. 1971;17:44–53. doi: 10.1007/BF00684740. [DOI] [PubMed] [Google Scholar]
- 16.Lipton H L. Persistent Theiler’s murine encephalomyelitis virus infection in mice depends on plaque size. J Gen Virol. 1980;46:169–177. doi: 10.1099/0022-1317-46-1-169. [DOI] [PubMed] [Google Scholar]
- 17.Lipton H L. Theiler’s virus infection in mice: an unusual biphasic disease process leading to demyelination. Infect Immun. 1975;11:1147–1155. doi: 10.1128/iai.11.5.1147-1155.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lipton H L, Pritchard A E, Calenoff M A. Attenuation of neurovirulence of Theiler’s murine encephalomyelitis virus strain GDVII is not sufficient to establish persistence in the central nervous system. J Gen Virol. 1998;79:1001–1004. doi: 10.1099/0022-1317-79-5-1001. [DOI] [PubMed] [Google Scholar]
- 19.Lipton H L, Twaddle G, Jelachich M L. The predominant virus antigen burden is present in macrophages in Theiler’s murine encephalomyelitis virus-induced demyelinating disease. J Virol. 1995;69:2525–2533. doi: 10.1128/jvi.69.4.2525-2533.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McAllister A, Tangy F, Aubert C, Brahic M. Genetic mapping of the ability of Theiler’s virus to persist and demyelinate. J Virol. 1990;64:4252–4257. doi: 10.1128/jvi.64.9.4252-4257.1990. . (Author’s correction, 67:2427, 1993.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Obuchi M, Ohara Y, Takegami T, Murayama T, Takada H, Iizuka H. Theiler’s murine encephalomyelitis virus subgroup strain-specific infection in a murine macrophage-like cell line. J Virol. 1997;71:729–733. doi: 10.1128/jvi.71.1.729-733.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ohara Y, Stein S, Fu J, Stillman L, Klaman L, Roos R P. Molecular cloning and sequence determination of DA strain of Theiler’s murine encephalomyelitis viruses. Virology. 1988;164:245–255. doi: 10.1016/0042-6822(88)90642-3. [DOI] [PubMed] [Google Scholar]
- 23.Ohka S, Yang W-X, Terada E, Iwasaki K, Nomoto A. Retrograde transport of intact poliovirus through the axon via the fast transport system. Virology. 1998;250:67–75. doi: 10.1006/viro.1998.9360. [DOI] [PubMed] [Google Scholar]
- 24.Ozden S, Tangy F, Chamorro M, Brahic M. Theiler’s virus genome is closely related to that of encephalomyocarditis virus, the prototype cardiovirus. J Virol. 1986;60:1163–1165. doi: 10.1128/jvi.60.3.1163-1165.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paulson J C, McClure W O. Inhibition of axoplasmic transport by colchicine, podophyllotoxin, and vinblastine: an effect on microtubules. Ann N Y Acad Sci. 1975;253:517–527. doi: 10.1111/j.1749-6632.1975.tb19225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pena Rossi C, Delcroix M, Huitinga I, McAllister A, van Rooijen N, Claassen E, Brahic M. Role of macrophages during Theiler’s virus infection. J Virol. 1997;71:3336–3340. doi: 10.1128/jvi.71.4.3336-3340.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pevear D C, Calenoff M, Rozhon E, Lipton H L. Analysis of the complete nucleotide sequence of the picornavirus Theiler’s murine encephalomyelitis virus indicates that it is closely related to cardioviruses. J Virol. 1987;61:1507–1516. doi: 10.1128/jvi.61.5.1507-1516.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Racaniello V R, Ren R. Transgenic mice and the pathogenesis of poliomyelitis. Arch Virol Suppl. 1994;9:79–86. doi: 10.1007/978-3-7091-9326-6_9. [DOI] [PubMed] [Google Scholar]
- 29.Ren R, Racaniello V R. Poliovirus spreads from muscle to the central nervous system by neural pathways. J Infect Dis. 1992;166:747–752. doi: 10.1093/infdis/166.4.747. [DOI] [PubMed] [Google Scholar]
- 30.Simas J P, Dyson H, Fazakerley J K. The neurovirulent GDVII strain of Theiler’s virus can replicate in glial cells. J Virol. 1995;69:5599–5606. doi: 10.1128/jvi.69.9.5599-5606.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun N, Cassell M D, Perlman S. Anterograde, transneuronal transport of herpes simplex virus type 1 strain H129 in the murine visual system. J Virol. 1996;70:5405–5413. doi: 10.1128/jvi.70.8.5405-5413.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takata H, Obuchi M, Yamamoto J, Odagiri T, Roos R P, Iizuka H, Ohara Y. L* protein of the DA strain of Theiler’s murine encephalomyelitis virus is important for virus growth in a murine macrophage-like cell line. J Virol. 1998;72:4950–4955. doi: 10.1128/jvi.72.6.4950-4955.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Theiler M. Spontaneous encephalomyelitis of mice, a new virus disease. J Exp Med. 1937;55:705–719. doi: 10.1084/jem.65.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tyler K L, McPhee D A, Fields B N. Distinct pathways of viral spread in the host determined by reovirus S1 gene segment. Science. 1986;233:770–774. doi: 10.1126/science.3016895. [DOI] [PubMed] [Google Scholar]
- 35.Wada Y, Fujinami R S. Viral infection and dissemination through the olfactory pathway and the limbic system by Theiler’s virus. Am J Pathol. 1993;143:221–229. [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou L, Lin X, Green T J, Lipton H L, Luo M. Role of sialyloligosaccharide binding in Theiler’s virus persistence. J Virol. 1997;71:9701–9712. doi: 10.1128/jvi.71.12.9701-9712.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]