Abstract
Leishmaniasis, a disease of global relevance, results from infection with the protozoan parasite, Leishmania, which is transmitted to susceptible hosts through the bite of sand flies. Multiple forms of leishmaniasis may occur, including cutaneous, mucocutaneous, and visceral. Research with animal models remains an important approach to help define basic pathophysiologic processes associated with infection and disease. In this regard, mice and hamsters represent the most commonly used models. The severity of leishmaniasis in animal models depends on several factors, including genotype of the host and parasite and the dose and route of administration of the parasite to the host, and severity of outcome may range from subclinical to severe illness. This review provides basic background on leishmaniasis, relevant animal models, the pathophysiology and clinical signs in animals used as models of leishmaniasis, and general approaches to mitigate risk to personnel.
Abbreviations and Acronyms: CL, cutaneous leishmaniasis; MCL, mucocutaneous leishmaniasis; VL, visceral leishmaniasis
Introduction
Human leishmaniasis is caused by one of more than 20 obligate intracellular protozoa of the genus Leishmania spp., transmitted by infected female phlebotomine flies, known as the “sand fly.”43 It is worth noting that sand flies are not the same thing as sand fleas. There are approximately 1,000 sand fly species and subspecies, with 35 proven, and an additional 63 incriminated, as vectors of Leishmania to humans.17,44 The earliest known evidence for the presence of Leishmania organisms appears in Burmese amber dating to around 100 million years ago.53 The presence of leishmanial life stages within the alimentary tract of a blood-filled sand fly preserved in amber accompanied by the nucleated red blood cells of reptiles suggests that the complicated host-vector-parasite relationship is ancient. This might account for the broad dispersal and high variability of both sand flies and Leishmania organisms having had millions of years to evolve in various environments and hosts. Leishmaniasis exists contemporaneously in tropical regions of the Americas, East Africa, North Africa, and Western and Southeast Asia.
The term, leishmaniasis, is reserved for illness associated with Leishmania infection. Although the World Health Organization (WHO) indicates that more than 1 billion people live in areas endemic for leishmaniasis, it is believed that a very small proportion of individuals infected with the parasite will become clinically ill.73 Globally, an estimated 2 million patients are diagnosed with leishmaniasis each year, with an overall prevalence of 12 million.24 Although progress has been made with respect to efforts directed at medical surveillance for leishmaniasis, the reporting rates may not be optimal, thus complicating efforts to identify actual rates of infection, and without the benefit of routine medical surveillance, the true incidence is difficult to discern.56
Leishmaniasis, while often lumped into one entity, represents a constellation of disease manifestations, ranging from self-limiting cutaneous lesions to fatal visceral disease. There are 3 commonly recognized clinical presentations of the disease in humans: cutaneous leishmaniasis (CL), mucocutaneous leishmaniasis (MCL), and visceral leishmaniasis (VL). In Assamese, a language native to some areas of northeastern India, VL may be referred to as kala-azar, which translates into English as “black disease,” a term coined in the 1880s to describe the discoloration of the skin that occurs during infection.67 VL has also been referred to as Dumdum Fever, named by Dr. William Boog Leishman for the location where the parasite was first observed in smears from the spleen of a patient who succumbed to the disease, Dumdum, Calcutta, India.70 Post-kala-azar dermal leishmaniasis is a condition that develops in some patients, sometimes years, after recovery from VL, and manifests as dermal lesions.75 Individuals affected by CL typically have self-limiting cutaneous ulcerations that gradually heal and are replaced by scar tissue. In contrast, lesions associated with MCL are generally not self-limiting and may involve the destruction of mucous membranes of the throat, mouth, and nose. The CL and MCL versions of the disease, while lacking the colorful nomenclature applied to VL, are of great clinical significance due to the potential for disfiguring scarring and destruction of normal anatomy that can occur secondary to the infection. Further, individuals so affected may experience social isolation, as the lesions are sometimes confused with leprosy and fungal dermatitis.12,62
Overview of Leishmania
It is difficult not to admire the elegant evolutionary choreography that exists between parasites, hosts, and vectors. Leishmania spp. is no exception. A stylized depiction of the Leishmania life cycle is presented in Figure 1. The female sand fly must take a blood meal to obtain the energy for egg development.74 If the sand fly is infected with Leishmania, promastigotes can be transferred to the host.41 Of note, sand fly saliva facilitates blood meal acquisition through anticoagulant and immunomodulatory factors and can influence establishment of Leishmania parasites within the host.39 Sand fly salivary components vary among phlebotomine flies (sand flies) and contribute to the highly variable manifestation and presentation of leishmaniasis.39 Given the numerous Leishmania and sand fly species, the highly variable presentation of the disease entity is not surprising.
Figure 1.
Life cycle of Leishmania. Image courtesy of DPDx, Centers for Disease Control and Prevention (https://www.cdc.gov/dpdx.). Infected sand flies transmit promastigotes to a host when taking a blood meal. Following internalization by host phagocytic cells, the amastigote form of the parasite multiplies and can infect other cells. A subsequent blood meal taken by another sand fly results in transmission of the parasite to the vector where the amastigotes transform in the gut to promastigotes, which then migrate to the proboscis and prepare to infect other hosts during ingestion of a blood meal, thus completing the cycle.
The vector-parasite-host interaction is largely the same across more than 189 vertebrate host species, including the orders Carnivora, Chiroptera, Cingulata, Didelphimorphia, Diprotodontia, Lagomorpha, Eulipotyphla, Pilosa, Primates, and Rodentia.9 Once transferred from the vector to the host, the Leishmania promastigote is internalized by antigen presenting cells of the host, generally macrophages. The promastigote (Figure 2A) transforms into the amastigote form (Figure 2B) that is able to perpetuate within the host.36 By simple binary fission, Leishmania amastigotes multiply and are subsequently released either through cell rupture or exocytosis. Liberated amastigotes can then be phagocytized by host cells, thus perpetuating the infection. When the female sand fly takes a blood meal from an infected host, amastigotes within macrophages may also pass to the vector. Once the amastigotes reach the gut of the sand fly, the parasite transforms to its promastigote phase and continues to divide, eventually differentiating and migrating to, and blocking, the stomodeal valve, resulting in regurgitation of Leishmania infectious stage promastigotes into the host, thus repeating the life cycle.58,71
Figure 2.
Amastigotes of Leishmania sp. (A) In a biopsy specimen from a skin lesion, stained with hematoxylin and eosin. Leishmania sp. (B) Promastigotes from culture. Images courtesy of DPDx, Centers for Disease Control and Prevention (https://www.cdc.gov/dpdx.).
Promastigotes are elongated with a large central nucleus and a long, motile, anterior flagellum. The promastigote is found within the midgut of the sand fly and would not be expected to be present in the infected host animal. The amastigote, defined as a protozoan that lacks visible functional external flagella or cilia, is round to oval and can be recovered from macrophages throughout the body of the host. Leishmania amastigotes exhibit a rudimentary, nonmotile flagella compared with the promastigote phase.
Commonly Used Animal Models
A great need remains for greater insight toward the full understanding of Leishmania pathophysiology and therapeutic approaches to leishmaniasis. Animal models, while often lacking a direct correlation with disease in humans, offer the opportunity to study various steps in the pathogenesis of leishmaniasis.42 Further, current therapeutic approaches include treatment with compounds such as meglumine antimoniate or amphotericin B, both of which may result in serious adverse clinical effects; thus, investigations with animal models are needed to develop new clinical options that offer improved patient outcomes.16 Animals are primarily used to model CL and VL and there are no reported standardized models specific to MCL.
Nonhuman primates, particularly Macaca spp., acquire infection and develop clinical disease approximating that found in humans, making them a logical model for the study of leishmaniasis.29 Further, New World Monkeys have been used specifically for the study of VL.5 However, the significant challenges associated with using nonhuman primates as models of leishmaniasis remain their relative expense and availability for biomedical research. Questions remain regarding the existence of immunity and cross-immunity to previous exposure to Leishmania spp. further complicating the use of nonhuman primates sourced from the wild, given that many species are sourced from areas of the world where Leishmania spp. are endemic.54 These factors make the nonhuman primate a challenging model for leishmaniasis research except for the most critical of studies; thus, they are not commonly used.
Dogs may serve as a reservoir of leishmaniasis in endemic areas and exhibit some disease progression similar to other animals, including humans. Various factors including climate change, resulting in the expansion of vector habitats, and international travel have the potential to increase the importance of leishmaniasis in companion animals, including horses, representing a “One Health” opportunity to further the understanding of leishmaniasis epidemiology.46 In addition, companion animals, such as dogs, may serve as useful sentinels for leishmaniasis in the human population.
Dogs have been used for modeling of leishmaniasis because they are easy to handle, are well-characterized immunologically, and have reasonable pathophysiologic homology with human leishmaniasis. With the demonstration and subsequent confirmation of vertical transmission in dogs, and the more recent evidence of vertical transmission in mice, the use of the dog model may be useful in gaining a better understanding of the adaptability of Leishmania organisms.13,20,26,45,65
Rodents are the most widely used models of leishmaniasis, as they are well-characterized, and they provide the opportunity to explore very specific and nuanced processes in parasite transmission, disease pathogenesis, and immune response.42 Of interest, the Syrian golden hamster (Mesocricetus auratus) is often regarded as the best experimental model of VL.41,57 Early research using hamster cells in vitro helped explain the activity of Leishmania spp. amastigotes within macrophages, and ongoing work serves to highlight the immunomodulation that occurs in Leishmania spp. infections that allow the disease to develop.18,47 The hamster remains an important model for immunopathogenesis, drug discovery, and vaccine development for VL but has application in the study of CL as well.27,57
As with other types of biomedical research, the power of murine models for leishmaniasis benefits from the availability of genetically standardized and genetically modified animals. Importantly, this ready access allows researchers to perform controlled experiments without the confounding variable of conducting research on subjects that are genetically heterogeneous. Further, the relative ease of maintaining the animals in standardized conditions that are controlled for environmental variables adds value to the use rodent models. As well, distinct differences between mouse strains with respect to resistance and susceptibility to infection with Leishmania have allowed scientists to identify fundamental characteristics that confer resistance.2,40,50 Consequently, mouse models of leishmaniasis remain extremely important to sorting out the remaining questions surrounding disease development and potential interventions, but selection of the appropriate mouse model is of paramount importance.
The Pathophysiology and Clinical Signs in Animals Used as Models of Leishmaniasis
A basic precept for an animal model of infectious disease is that it should reasonably parallel the pathophysiology and clinical manifestations of the disease in humans; therefore, illness and adverse outcomes are often part of the experimental paradigm. In the case of Leishmania, impacts on animals depend to a great extent on whether the infection is visceral compared with cutaneous and on the species/isolate and dose of Leishmania used to initiate infection, the route of administration, as well as on host factors such as the genetic background of the host being used Here, descriptions are focused on mice, hamsters, and dogs as they are the most commonly used species for the study of leishmaniasis.
Models of CL.
Models of CL typically involve inoculation of live parasite organisms, usually promastigotes, subcutaneously or intradermally. Mice are the primary model for CL, although hamsters have been used in some cases. In mice, infections are usually initiated by subcutaneous administration of parasites in either the ear pinna or the rear footpad. Infections of the pinna may result in induration of the site and development of an ulcer that typically heals with time (Figure 3). Foot pad infections result in swelling of the foot (Figure 4), with assessments of treatment or other variables made by comparison of the degree of swelling, as determined by measurement of foot pack thickness with calipers or by plethysmometry, to the contralateral noninoculated foot pad.6,8,16,33 In resistant mouse strains, swelling is typically self-limiting and rarely results in lameness; in susceptible mouse strains, lesions progress with subsequent necrosis of pedal tissues and spontaneous amputation of the foot.3 The impact of mouse and parasite genotype on the outcome of cutaneous infections can be substantial. While BALB/c mice exhibit greater susceptibility to Leishmania major infection with development of large cutaneous ulcers leading to the spread of parasites to visceral sites, C3H/He, CBA, C57BL/6, and 129Sv/Ev mice are resistant to infection and develop small lesions, which typically heal in 10 to 12 wk.4,7,10,23 In contrast, although BALB/C mice are susceptible to infection with L. braziliensis, the resulting lesions are neither severe nor persistent, thereby demonstrating the impact of Leishmania species on outcome.22
Figure 3.

Induration and ulceration (arrow) of the pinna in a BALB/c mouse experimentally infected with Leishmania. Photo courtesy of Dr. Mary Ann McDowell.
Figure 4.
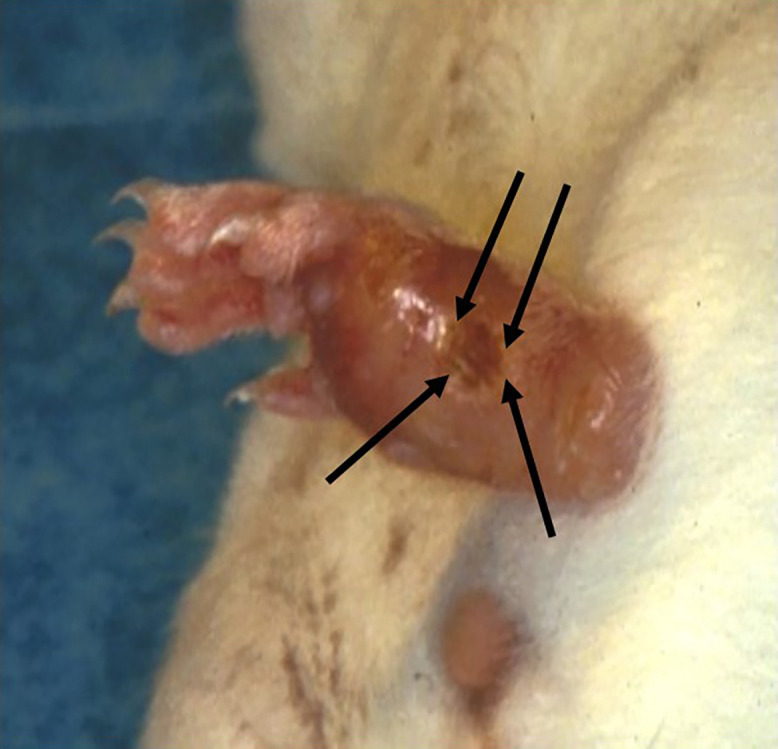
Induration and ulceration of the foot pad of a mouse experimentally infected with Leishmania. The arrows outline the area of cutaneous ulceration. Photo courtesy of Dr. Mary Ann McDowell.
CL has been modeled in golden hamsters (Mesocricetus auratus), with infections established following intradermal inoculation of Leishmania promastigotes at the lumbosacral area.28 In hamsters, pathologic changes and clinical illness depend, to at least some extent, on the route of inoculation and the genotype of the Leishmania organism being evaluated.48
Models of VL.
Mice, hamsters, and dogs have been used as models of VL, with parasites usually being introduced via either intradermal or intravenous administration.23 Intravenous infection of susceptible mouse strains (BALB/c and C57BL/10) with L. donovani and L. infantum generally resulted in granulomatous inflammation in the spleen and liver with an initial increased parasite burden, followed by a spontaneous decline and, ultimately, clearance as the animals mounted a cellular response against the parasite.61
Like humans, golden hamsters are exceptionally susceptible to infection with L. donovani and may develop clinical illness characterized by cachexia and weight loss, similar to human infection. Following intracardiac inoculation of parasites, hamsters will become anemic and may develop hepatosplenomegaly and proliferative glomerulonephritis.1,48 The hamster has also been used to maintain isolates of Leishmania by intracardiac injection of parasites and subsequent harvesting of tissues to provide a renewable source of parasites.15 Hamsters infected with L. infantum exhibit elevated blood cortisol levels that correlate with increasing severity of infection as determined by splenic and hepatic parasite burden; consequently, one can speculate that the animals experienced increased distress as the severity of infection increased.11
The clinical presentation of natural Leishmania infections varies greatly in dogs and can range from the absence of clinical symptoms to weight loss, cutaneous lesions, and protein-losing nephropathy.21,60 Experimentally, dogs have primarily been used to study the visceral disease. Similar to other species, severity of disease in dogs can vary with several factors, including the species of Leishmania. Dogs infected intravenously with L. mexicanum developed disseminated disease that included cutaneous ulcerations and, in some dogs, nephritis and hepatic necrosis.19 Intravenous inoculation of dogs with L. donovani produced persistent infection that was characterized by weight loss, splenomegaly, lymphadenomegaly, and normocytic, normochromic anemia.34 In contrast, intravenous infection of dogs with L. infantum can produce asymptomatic infections, as PCR evaluation of blood and liver samples demonstrated persistence of Leishmania through at least 7 mo following infection.37
Sand flies are sometimes maintained to either specifically study interactions of the Leishmania parasite with the vector or, in some cases, to study transmission of the parasite from infected sand flies to animal hosts, as this represents a more natural route of infection.51,52 For both, sand flies are often maintained by providing a blood meal through access to heparinized mouse blood via an artificial membrane; however, feeding of sand flies on live animals, including mice, hamsters, rats, and rabbits, has also been used for purposes of colony maintenance and for studies involving transmission of Leishmania to animals.14,55 Because sand fly bites may cause discomfort, mice or hamsters are first anesthetized and then placed into a container with sand flies, which can then take a blood meal over 30 to 60 min (Figure 5).72 Feeding of sand flies on restrained rabbits within a specially constructed feeding cage over the course of 1 to 3 h has been described.64
Figure 5.

An adult sand fly containment system designed to facilitate blood meals on live, anesthetized animals. Sand flies are housed within the polycarbonate chamber, and anesthetized animals are placed in the cloth sleeve that is then inverted into the box through which sand flies can take a blood meal from the animal. Photo courtesy of Dr. Mary Ann McDowell.
Humane Considerations
A systematic review of literature describing research using animal models of leishmaniasis published between 2000 and 2020 demonstrated a general lack of provisions to enhance animal welfare.69 For example, approximately 10% of studies using mice or hamsters described the use of individual, rather than group, housing; approximately 5% of studies described any attempt to introduce principles related to the principles of replacement, reduction, and refinement (3Rs); and humane endpoints were not reported in any of the reviewed studies.
As described earlier, infection with Leishmania may have an impact on animal well-being depending on factors such as host and parasite genotype, parasite dose, and route of administration. Infection may result in outcomes that range from absence of clinical disease to severe disease, even death. In this regard, it is essential that potential adverse impacts on animals be clearly defined and articulated to the IACUC and research staff and that endpoints are defined at which animals will be treated, removed from the study, or euthanized.32 Typically, treatment for foot pad induration or open wounds is contraindicated due to the experimental paradigm, as data related to measurement of cutaneous lesion size, increased thickness of the foot pad, and blood inflammatory markers could be impacted. Although seldom described in published literature, endpoints that result in euthanasia of the animal should be included as part of the experimental paradigm for models of leishmaniasis and might include loss of body weight, presence of cutaneous ulceration, body condition, nonresponsiveness to external stimuli, and presence of ascites, as these are all possible clinical outcomes with CL or VL and have, in some instances, been applied as endpoint criteria.23,31,48 Importantly, if humane endpoint criteria are to be used, it is essential that personnel be trained to evaluate animals vis-à-vis such criteria and that they be empowered to actuate euthanasia when endpoints are met.
Although nonanimal models of leishmaniasis have not been described, one interesting approach has been applied to reduce the number of animals used in longitudinal studies. Specifically, the use of Leishmania organisms engineered to express luciferase for infection of mice and subsequent imaging of bioluminescence were shown to allow for repeated assessment of the progression of infection using fewer animals, compared with the need to euthanize animals at multiple time points.63
Management Approaches to Working with Animal Models of Leishmaniasis
Leishmania spp., as an agent that is associated with human disease and poses moderate risk to personnel, requires management practices consistent with BSL2 and animal management practices consistent with animal BSL2 (ABSL2).68 Laboratory-acquired Leishmania spp. infections have been associated with parenteral, mucous membranes, nonintact skin, animal bites, and “no known accident” exposures.30 In the research setting personnel can be exposed to Leishmania spp. promastigotes by exposure to infected sand flies or other infectious inocula. For example, L. infantum has been reported in cases of venereal transmission in dogs suggesting the possibility of mucous membrane transmission to personnel who work with infected dogs.49,59 Compliance with BSL2 standards requires that laboratory personnel receive specific training and are supervised by personnel competent in handling Leishmania spp.; that access to the laboratory or animal room is restricted when work is being conducted; and that procedures that may result in aerosol generation or splashes are conducted in a biosafety cabinet.68 Housing of rodents can be safely accomplished by using individually ventilated cages of the appropriate size for the species, but work with larger species requires increased reliance on room or facility engineering controls. Risk assessment of personnel should consider the specific tasks that will be performed and the likelihood of personnel exposure to infectious Leishmania organisms. Common personal protective equipment to be worn when handling animals or materials potentially containing Leishmania include eye and respiratory protection.
Importantly, laboratories conducting BSL2 or ABSL2 work must have a means of decontamination of all waste. Autoclaving is often used except in the case where chemical components of research may prove hazardous under heat and pressure. Alternatively, incineration may be a consideration based on hazard assessment and risk management. In addition, personnel should have access to occupational medical services based on workplace hazards, risk assessment, and awareness of risks based on personal health status.68
Consideration should be given to both vector and animal hosts with respect to management of work involving Leishmania. Methods for initiating and maintaining sand fly colonies and record-keeping strategies to optimize the health of the sand fly have been published elsewhere.38 Sand fly colonies require appropriate housing and nutrition for all stages of their life cycle and are susceptible to a range of pathogens, including parasites, microsporidians, mites, fungus, bacteria, and nematodes. Currently, viruses that adversely affect sand flies are not described.
Sand flies may be provided a sugar solution or moist fruit as a food source except that the female sand flies must take a blood meal to lay eggs. Blood can be provided through access to heparinized blood via an artificial membrane; however, feeding success is generally lower than when live vertebrates are used as a blood source.25,38 Commonly, live mice are used for purposes of colony maintenance, although other species have been sometimes used for studies involving transmission of Leishmania to animals.14,55 Because sand fly bites may cause discomfort, mice are first anesthetized and then placed into a container with sand flies, which can then take a blood meal over 30 to 60 min (Figure 5).72 Feeding of sand flies on restrained rabbits within a specially constructed feeding cage over the course of 1 to 3 h has been described.64 Sand flies are typically sugar deprived for 24 h to encourage feeding. Blood donor animals are often euthanized after feeding to prevent any pain or distress secondary to blood feeding.
Sand flies have been characterized as weak fliers, traveling through short hopping flights.38 While sand flies appear to have the ability to fly further than previously appreciated, they are not reported to fly fast, with top speeds of 2.52 km/h or 1.566 mph.35 A recent study evaluating the flight behavior of sand flies demonstrated flights of ∼65 m/213 ft.66 Sand fly colonies should be maintained in facilities that can contain escaped flies with appropriately sized screens or seals over any possible egress. Double-door entry as well as air curtains may effectively contain escaped flies and prevent the research staff from accidentally carrying escaped flies beyond the containment barrier.
With respect to the management of vertebrate animals used in research on leishmaniasis, some animals may be maintained solely as a source of a blood meal. These animals should be free of infection with Leishmania spp. and housed under conditions appropriate for their species.48 Animals that are infected with Leishmania spp., including those that serve as the source of amastigotes to infect sand flies, should be held under containment conditions. Appropriate management should consider not only risks associated with Leishmania but also those inherent to the host species (for example, nonhuman primates).
Summary
Critical to the advancement of treatment and prevention of leishmaniasis are strategies that can only be developed through the use of animal models. While all models are but an approximation for humans, they remain an essential feature of both basic and translational work. The impact of Leishmania infection on animals varies with genotype of both parasite and animal, dose of parasite, and route of administration. Key to properly conducted studies are steps taken to enhance animal welfare, including IACUC oversight and review of procedures, clearly stated and understood humane endpoints, and training of personnel with respect to proper animal handling and recognition of endpoints.
Conflict of Interest
The authors have no conflicts of interest to declare.
Funding
This work was internally funded.
References
- 1.Agu WE, Farrell JP, Soulsby EJL. 1981. Proliferative glomerulonephritis in experimental Leishmania donovani infection of the golden hamster. Comp Immunol Microbiol Infect Dis 4:353–368. [DOI] [PubMed] [Google Scholar]
- 2.Aguilar-Torrentera F, Carlier Y. 2001. Immunological factors governing resistance and susceptibility of mice to Leishmania major infection. Rev Latinoam Microbiol 43:135–142. [PubMed] [Google Scholar]
- 3.Ahmed SB, Bahloul C, Robbana C, Askri S, Dellagi KA. 2004. Comparative evaluation of different DNA vaccine candidates against experimental murine leishmaniasis due to L. major. Vaccine 22:1631–1639. [DOI] [PubMed] [Google Scholar]
- 4.Alimohammadian MH, Darabi H, Ajdary S, Khaze V, Torkabadi E. 2010. Genotypically distinct strains of Leishmania major display diverse clinical and immunological patterns in BALB/c mice. Infect Genet Evol 10:969–975. [DOI] [PubMed] [Google Scholar]
- 5.André S, Rodrigues V, Picard M, Silvestre R, Estaquier J. 2020. Non-human primates and Leishmania immunity. Cytokine X 2:100038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aoki JI, Hong A, Zampieri RA, Floeter-Winter LM, Laranjeira-Silva MF. 2020. In vivo infection with Leishmania amazonensis to evaluate parasite influence in mice. J Vis Exp 156:e60617. [DOI] [PubMed] [Google Scholar]
- 7.Asadpour A, Riazi-Rad F, Khaze V, Ajdary S, Alimohammadian MH. 2013. Distinct strains of Leishmania major induce different cytokine mRNA expression in draining lymph node of BALB/c mice. Parasite Immunol 35:42–50. [DOI] [PubMed] [Google Scholar]
- 8.Attia H, Bali A, Sghaier RM, Martinez PAL, Mkannez G, Atri C, Chourabi K, Guerfali FZ, Laouini D. 2015. Comparison between plethysmometer and caliper methods to monitor lesion-size induced by Leishmania major infection in BALB/c mouse experimental model. Anim Vet Sci 3:179–185. [Google Scholar]
- 9.Azami-Conesa I, Gómez-Muñoz MT, Martínez-Díaz RA. 2021. A systematic review (1990-2021) of wild animals infected with zoonotic Leishmania. Microorganisms 9:1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Babay BEC, Louzir H, Kebaïer C, Boubaker S, Dellagi K, Cazenave PA. 2004. Inbred strains derived from feral mice reveal new pathogenic mechanisms of leishmaniasis due to Leishmania major. Infect Immun 72:4603–4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barros-Goncalves TD, Saavedra AF, Silva-Couto LD, Ribeiro-Romão RP, Bezerra-Paiva M, Gomes-Silva A, Carvalho VF, Da-Cruz AM, Pinto EF. 2021. Increased levels of cortisol are associated with the severity of experimental visceral leishmaniasis in a Leishmania (L.) infantum-hamster model. PLoS Negl Trop Dis 15:e0009987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bilgic-Temel A, Murrell DF, Uzun S. 2019. Cutaneous leishmaniasis: A neglected disfiguring disease for women. Int J Womens Dermatol 5:158–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boggiatto PM, Gibson-Corley KN, Metz K, Gallup JM, Hostetter JM, Mullin K, Petersen CA. 2011. Transplacental transmission of Leishmania infantum as a means for continued disease incidence in North America. PLoS Negl Trop Dis 5:e1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bongiorno G, Di Muccio T, Bianchi R, Gramiccia M, Gradoni L. 2019. Laboratory transmission of an Asian strain of Leishmania tropica by the bite of the southern European sand fly Phlebotomus perniciosus. Int J Parasitol 49:417–421. [DOI] [PubMed] [Google Scholar]
- 15.Bruhn KW, Birnbaum R, Haskell J, Vanchinathan V, Greger S, Narayan R, Chang P-L, et al. 2012. Killed but metabolically active Leishmania infantum as a novel whole-cell vaccine for visceral leishmaniasis. Clin Vaccine Immunol 19:490–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bustos MFG, Barrio A, Prieto GC, de Raspi DM, Cimino RO, Cardozo RM, Parada LA, et al. 2014. In vivo antileishmanial efficacy of miltefosine against Leishmania amazonensis. J Parasitol 100:840–847. [DOI] [PubMed] [Google Scholar]
- 17.Cecílio P, Cordeiro-da-Silva A, Oliveira F. 2022. Sand flies: Basic information on the vectors of leishmaniasis and their interactions with Leishmania parasites. Commun Biol 5:305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang KP, Dwyer DM. 1978. Leishmania donovani. Hamster macrophage interactions in vitro: Cell entry, intracellular survival, and multiplication of amastigotes. J Exp Med 147:515–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cruz-Chan JV, Aguilar-Cetina AC, Villanueva-Lizama LE, Martinez-Vega PP, Ramírez-Sierra MJ, Rosado-Vallado ME, Guillermo-Cordero JL, Dumonteil E. 2014. A canine model of experimental infection with Leishmania (L.) mexicana. Parasit Vectors 7:361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.da Silva SM, Ribeiro VM, Ribeiro RR, Tafuri WL, Melo MN, Michalick MS. 2009. First report of vertical transmission of Leishmania (Leishmania) infantum in a naturally infected bitch from Brazil. Vet Parasitol 166:159–162. [DOI] [PubMed] [Google Scholar]
- 21.de Jong MK, Rappoldt A, Broere F, Piek CJ. 2023. Survival time and prognostic factors in canine leishmaniosis in a non-endemic country treated with a two-phase protocol including initial allopurinol monotherapy. Parasit Vectors 16:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DeKrey GK, Lima HC, Titus RG. 1998. Analysis of the immune responses of mice to infection with Leishmania brazilensis. Infect Immun 66:827–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Oliveira CI, Teixeira MJ, Gomes R, Barral A, Brodskyn C. 2004. Animal models for infectious diseases caused by parasites: Leishmaniasis. Drug Discovery Today 1:81–86. [Google Scholar]
- 24.De Vries HJC, Schallig HD. 2022. Cutaneous leishmaniasis: A 2022 updated narrative review into diagnosis and management developments. Am J Clin Dermatol 23:823–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fatemi M, Saeidi A, Noruzian P, Akhavan AA. 2018. Designing and introducing a new artificial feeding apparatus for sand fly rearing. J Arthropod Borne Dis 12:426–431. [PMC free article] [PubMed] [Google Scholar]
- 26.Franssen SU, Sanders MJ, Berriman M, Petersen CA, Cotton JA. 2022. Geographic origin and vertical transmission of Leishmania infantum parasites in hunting hounds, United States. Emerg Infect Dis 28:1211–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gomes-Silva A, Valverde JG, Ribeiro-Romão RP, Plácido-Pereira RM, Da-Cruz AM. 2013. Golden hamster (Mesocricetus auratus) as an experimental model for Leishmania (Viannia) braziliensis infection. Parasitology 140:771–779. [DOI] [PubMed] [Google Scholar]
- 28.Gómez-Galindo AM, Delgado-Murcia LG. 2013. Body weight as a determinant of clinical evolution in hamsters (Mesocricetus auratus) infected with Leishmania (Viannia) panamensis. Rev Inst Med Trop Sao Paulo 55:357–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grimaldi G, Jr.. 2008. The utility of rhesus monkey (Macaca mulatta) and other non-human primate models for preclinical testing of Leishmania candidate vaccines – A review. Mem Inst Oswaldo Cruz 103:629–644. [DOI] [PubMed] [Google Scholar]
- 30.Herwaldt BL. 2001. Laboratory-acquired parasitic infections from accidental exposures. Clin Microbiol Rev 14:659–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iborra S, Carríon J, Anderson C, Alonso C, Sacks D, Soto M. 2005. Vaccination with the Leishmania infantum acidic ribosomal P0 protein plus CpG oligodeoxynucleotides induces protection against cutaneous leishmaniasis in C57BL/6 mice but does not prevent progressive disease in BALB/c mice. Infect Immun 73:5842–5852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals 8th ed. Washington (DC): The National Academies Press. [Google Scholar]
- 33.Kebaier C, Louzir H, Chenik M, Salah AB, Dellagi K. 2001. Heterogeneity of wild Leishmania major isolates in experimental murine pathogenicity and specific immune response. Infect Immun 69:4906–4915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keenan CM, Hendricks LD, Lightner L, Webster HK, Johnson AJ. 1984. Visceral leishmaniasis in the German shepherd dog. I. Infection, clinical disease, and clinical pathology. Vet Pathol 21:74–79. [DOI] [PubMed] [Google Scholar]
- 35.Killick-Kendrick R, Wilkes TJ, Bailly M, Bailly I, Righton LA. 1986. Preliminary field observations on the flight speed of a phlebotomine sandfly. Trans R Soc Trop Med Hyg 80:138–142. [DOI] [PubMed] [Google Scholar]
- 36.Kima PE. 2007. The amastigote forms of Leishmania are experts at exploiting host cell processes to establish infection and persist. Int J Parasitol 37:1087–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Konno H, Yokoyama N, Tamura Y, Aoshima K, Nakao R, Takiguchi M, Katakura K. 2022. An experimental challenge model for Leishmania donovani in beagle dogs, showing a similar pattern of parasite burden in the peripheral blood and liver. Parasitol Res 121:3569–3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lawyer P, Killick-Kendrick M, Rowland T, Rowton E, Volf P. 2017. Laboratory colonization and mass rearing of phlebotomine sand flies (Diptera, Psychodidae). Parasite 24:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lestinova T, Rohousova I, Sima M, de Oliveira CI, Volf P. 2017. Insights into the sand fly saliva: Blood-feeding and immune interactions between sand flies, hosts, and Leishmania. PLoS Negl Trop Dis 11:e0005600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lipoldová M, Demant P. 2006. Genetic susceptibility to infectious disease: Lessons from mouse models of leishmaniasis. Nat Rev Genet 7:294–305. [DOI] [PubMed] [Google Scholar]
- 41.Liu D, Uzonna JE. 2012. The early interaction of Leishmania with macrophages and dendritic cells and its influence on the host immune response. Front Cell Infect Microbiol 2:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Loría-Cervera EN, Andrade-Narváez FJ. 2014. Animal models for the study of leishmaniasis immunology. Rev Inst Med Trop Sao Paulo 56:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mann S, Frasca K, Scherrer S, Henao-Martinez A, Newman S, Ramanan P, Suarez JA. 2021. A review of leishmaniasis: Current knowledge and future directions. Curr Trop Med Rep 8:121–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maroli M, Feliciangeli MD, Bichaud L, Charrel RN, Gradoni L. 2013. Phlebotomine sandflies and the spreading of leishmaniases and other diseases of public health. Med Vet Entomol 27:123–147. [DOI] [PubMed] [Google Scholar]
- 45.Martín-Sánchez J, Torres-Medina N, Corpas-López V, Morillas-Márquez F, Díaz-Sáez V. 2020. Vertical transmission may play a greater role in the spread of Leishmania infantum in synanthropic Mus musculus rodents than previously believed. Transbound Emerg Dis 67:1113–1118. [DOI] [PubMed] [Google Scholar]
- 46.Martínez-Sáez L, Dulac Q, Montaner-Angoiti E, Marín-García PJ, Llobat L. 2023. Prevalence and factors related to Leishmania infantum infection in healthy horses (Equus caballus) from eastern Spain. Animals (Basel) 13:2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Melby PC, Tryon VV, Chandrasekar B, Freeman GL. 1998. Cloning of Syrian hamster (Mesocricetus auratus) cytokine cDNAs and analysis of cytokine mRNA expression in experimental visceral leishmaniasis. Infect Immun 66:2135–2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moreira ND, Vitoriano-Souz J, Roatt BM, Vieira PM, Coura-Vital W, Cardoso JM, Rezende MT, et al. 2016. Clinical, hematological, and biochemical alterations in hamster (Mesocricetus auratus) experimentally infected with Leishmania infantum through different routes of inoculation. Parasit Vectors 9:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Naucke TJ, Lorentz S. 2012. First report of venereal and vertical transmission of canine leishmaniosis from naturally infected dogs in Germany. Parasit Vectors 5:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pérez-Cabezas B, Cecílio P, Gaspar TB, Gärtner F, Vasconcellos R, Cordeiro-da-Silva A. 2019. Understanding resistance vs. susceptibility in visceral leishmaniasis using mouse models of Leishmania infantum infection. Front Cell Infect Microbiol 9:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Peters NC, Egen JG, Secundino N, Debrabant A, Kimblin N, Kamhawi S, Lawyer P, Fay MP, Germain RN, Sacks D. 2008. In vivo imaging reveals an essential role for neutrophils in leishmaniasis transmitted by sand flies. Science 321:970–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Peters NC, Kimblin N, Secundino N, Kamhawi S, Lawyer P, Sacks DL. 2009. Vector transmission of leishmania abrogates vaccine-induced protective immunity. PLoS Pathog 5:e1000484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Poinar G, Jr, Poinar R. 2004. Evidence of vector-borne disease of Early Cretaceous reptiles. Vector Borne Zoonotic Dis 4:281–284. [DOI] [PubMed] [Google Scholar]
- 54.Porrozzi R, Teva A, Amaral VF, Santos da Costa MV, Grimaldi G, Jr.. 2004. Cross immunity experiments between different species or strains of Leishmania in rhesus macaques (Macaca mulatta). Am J Trop Med Hyg 71:297–305. [PubMed] [Google Scholar]
- 55.Romano A, Inbar E, Debrabant A, Charmoy M, Lawyer P, Ribeiro-Gomes F, Barhoumi M, et al. 2014. Cross-species genetic exchange between visceral and cutaneous strains of Leishmania in the sand fly vector. Proc Natl Acad Sci USA 111:16808–16813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ruiz-Postigo JA, Jain S, Madjou S, Maia-Elkhoury AN, Valadas S, Warusavithana S, Osman M, et al. 2022. Global leishmaniasis surveillance: 2021, assessing the impact of the COVID-19 pandemic. Weekly Epidemiol Rec 45:575–590. [Google Scholar]
- 57.Saini S, Rai AK. 2020. Hamster, a close model for visceral leishmaniasis: Opportunities and challenges. Parasite Immunol 42:e12768. [DOI] [PubMed] [Google Scholar]
- 58.Schlein Y, Jacobson RL, Messer G. 1992. Leishmania infections damage the feeding mechanism of the sandfly vector and implement parasite transmission by bite. Proc Natl Acad Sci USA 89:9944–9948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Silva FL, Oliveira RG, Silva TM, Xavier MN, Nascimento EF, Santos RL. 2009. Venereal transmission of canine visceral leishmaniasis. Vet Parasitol 160:55–59. [DOI] [PubMed] [Google Scholar]
- 60.Slappendel RJ. 1988. Canine leishmaniasis. A review based on 95 cases in the Netherlands. Vet Q 10:1–16. [DOI] [PubMed] [Google Scholar]
- 61.Stern JJ, Oca MJ, Rubin BY, Anderson SL, Murray HW. 1988. Role of L3T4+ and LyT-2+ cells in experimental visceral leishmaniasis. J Immunol 140:3971–3977. [PubMed] [Google Scholar]
- 62.Swain SK, Behera IC, Sahu MC, Panda M. 2016. Isolated cutaneous leishmaniasis over face – A diagnostic dilemma. Alexandria J Med 52:343–346. [Google Scholar]
- 63.Thalhofer CJ, Graff JW, Love-Homan L, Hickerson SM, Craft N, Beverley SM, Wilson ME. 2010. In vivo imaging of transgenic Leishmania parasites in a live host. J Vis Exp 41:1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tiwary P, Singh SK, Kushwaha AK, Rowton E, Sacks D, Singh OP, Sunda S, Lawyer P. 2017. Establishing, expanding, and certifying a closed colony of Phlebotomus argentipes (Diptera: Psychodidae) for xenodiagnostic studies at the Kala Azar Medical Research Center, Muzaffarpur, Bihar, India. J Med Entomol 54:1129–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Toepp AJ, Bennett C, Scott B, Senesac R, Oleson JJ, Petersen CA. 2019. Maternal Leishmania infantum infection status has significant impact on leishmaniasis in offspring. PLoS Negl Trop Dis 13:e0007058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tonelli GB, Binder C, Margonari C, Andrade Filho JD. 2021. Sand fly behavior: Much more than weak-flying. Mem Inst Oswaldo Cruz 116:e210230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Torres-Guerrero E, Quintanilla-Cedillo MC, Ruiz-Esmenjaud J, Arenas R. 2017. Leishmaniasis: A review. F1000Res 6:750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.U.S. Department of Health and Human Services, Centers for Disease Control, National Institutes of Health. 2020. Biosafety in microbiological and biomedical laboratories 6th ed. Washington (DC): U.S. Government Printing Office. [Google Scholar]
- 69.Van der Ende J, Schallig HDFH. 2023. Leishmania animal models used in drug discovery: A systematic review. Animals (Basel) 13:1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vincent HM. 2017. William Boog Leishman: Parasitologist and politician. Parasitology 144:1582–1589. [DOI] [PubMed] [Google Scholar]
- 71.Volf P, Hajmova M, Sadlova J, Votypka J. 2004. Blocked stomodeal valve of the insect vector; similar mechanism of transmission in two trypanosomatid models. Int J Parasitol 34:1221–1227. [DOI] [PubMed] [Google Scholar]
- 72.Volf P, Volfova V. 2011. Establishment and maintenance of sand fly colonies. J Vector Ecol 36 Suppl 1:S1–S9. [DOI] [PubMed] [Google Scholar]
- 73.World Health Organization. [Internet]. 2024. Health topics: Neglected tropical diseases. [Cited 21 March 2024]. Available at: https://www.who.int/health-topics/neglected-tropical-diseases#tab=tab_.
- 74.Yared S, Gebresilassie A, Abbasi I, Aklilu E, Kirstein OD, Balkew M, Brown AS, et al. 2019. A molecular analysis of sand fly blood meals in a visceral leishmaniasis endemic region of northwestern Ethiopia reveals a complex host-vector system. Heliyon 5:e02132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zijlstra EE, Musa AM, Khalil EA, el-Hassan IM, el-Hassan AM. 2003. Post-kala-azar dermal leishmaniasis. Lancet Infect Dis 3:87–98. [DOI] [PubMed] [Google Scholar]




