Abstract
Only a small number of studies have assessed structural differences between the two hemispheres during childhood and adolescence. However, the existing findings lack consistency or are restricted to a particular brain region, a specific brain feature, or a relatively narrow age range. Here, we investigated associations between brain asymmetry and age as well as sex in one of the largest pediatric samples to date (n = 4265), aged 1–18 years, scanned at 69 sites participating in the ENIGMA (Enhancing NeuroImaging Genetics through Meta‐Analysis) consortium. Our study revealed that significant brain asymmetries already exist in childhood, but their magnitude and direction depend on the brain region examined and the morphometric measurement used (cortical volume or thickness, regional surface area, or subcortical volume). With respect to effects of age, some asymmetries became weaker over time while others became stronger; sometimes they even reversed direction. With respect to sex differences, the total number of regions exhibiting significant asymmetries was larger in females than in males, while the total number of measurements indicating significant asymmetries was larger in males (as we obtained more than one measurement per cortical region). The magnitude of the significant asymmetries was also greater in males. However, effect sizes for both age effects and sex differences were small. Taken together, these findings suggest that cerebral asymmetries are an inherent organizational pattern of the brain that manifests early in life. Overall, brain asymmetry appears to be relatively stable throughout childhood and adolescence, with some differential effects in males and females.
Keywords: adolescence, age, asymmetry, brain, childhood, cortical thickness, development, ENIGMA, gender, gray matter, sex
Effects of sex: Girls had more asymmetric regions than boys; boys had more asymmetric measurements than girls (we obtained more than one measurement per cortical region). Effects of age: Some asymmetries became weaker with increasing age, while others got stronger; sometimes asymmetries also reversed direction.
1. INTRODUCTION
Despite its striking overall symmetric appearance at first glance, a closer look at the human brain reveals a multitude of asymmetries (Ocklenburg & Gunturkun, 2018). These asymmetries not only exist functionally—that is, the preference of one hand over the other (Papadatou‐Pastou et al., 2020) or the lateralization of language to one hemisphere (Malik‐Moraleda et al., 2022)—but also structurally. The brain's most prominent structural asymmetry is the Yakovlevian torque—a forward warp of the right hemisphere and a backward warp of the left hemisphere (Kong et al., 2021; LeMay, 1976). However, several other left–right differences exist, as summarized elsewhere (Jancke, 2003; Toga et al., 2009). For example, the left Sylvian fissure is often longer and runs more horizontally than the right, whereas the left hemisphere often contains only one Heschl's gyrus, but the right hemisphere contains two. While some structural asymmetries are consistent across brains and stable over time, others have been reported to differ across individuals and to change with increasing age (Guadalupe et al., 2017; Kong et al., 2018; Kong et al., 2022).
The majority of asymmetry studies have been conducted in adult populations (Chiarello et al., 2016; Good et al., 2001; Guadalupe et al., 2017; Jancke et al., 1994; Koelkebeck et al., 2014; Kong et al., 2018; Kong et al., 2022; Luders et al., 2006; Maingault et al., 2016; Plessen et al., 2014; Sha, Pepe, et al., 2021; Toga et al., 2009; Toga & Thompson, 2003; Zhou et al., 2013), and some of those studies cover the entire lifespan, including childhood and adolescence, but do not explicitly focus on these earlier periods in life (Kong et al., 2018; Plessen et al., 2014; Zhou et al., 2013). So, it remains unclear when cerebral asymmetries arise and how they develop during childhood and adolescence. There is some evidence that cerebral asymmetries already exist in newborns (de Vareilles et al., 2022; Ge et al., 2022; Gilmore et al., 2007; Lehtola et al., 2019; Li et al., 2014; Li et al., 2015; Namburete et al., 2023; Steger et al., 2023) and even in the fetal brain (Abu‐Rustum et al., 2013; Corballis, 2013; de Kovel et al., 2017; Namburete et al., 2023; Steger et al., 2023; Vasung et al., 2020). However, to date there is limited research investigating brain asymmetries and their age‐related associations during childhood and adolescence. The few existing studies with an explicit focus on childhood and adolescence have resulted in inconsistent findings or in findings restricted to a particular brain region, a specific brain feature, or a relatively narrow age range (Levman et al., 2017; Raja et al., 2021; Shaw et al., 2009; Wang et al., 2015).
Our current study was designed to pool international data to provide crucial and high‐powered insights into brain asymmetry with a focus on the age range of 1–18 years, capturing a multitude of brain features (cortical thickness, cortical surface area, as well as cortical and subcortical volumes), reflecting both regional and global measures. Moreover, we set out to analyze whether there are significant differences in brain asymmetry between males and females, given the often conflicting outcomes in prior (mostly adult) studies (Fan et al., 2010; Good et al., 2001; Guadalupe et al., 2015; Guadalupe et al., 2017; Jancke et al., 1994; Kong et al., 2018; Kong et al., 2022; Kurth et al., 2017; Kurth et al., 2018; Levman et al., 2017; Luders et al., 2004; Luders et al., 2006; Plessen et al., 2014; Savic, 2014; Shaw et al., 2009; Takao et al., 2011; Toga et al., 2009; Toga & Thompson, 2003; Watkins et al., 2001; Zhou et al., 2013). If sex differences in brain asymmetry exist, such differences may already be present at their full extent early on in life or manifest only later (e.g., during puberty). Moreover, any existing sex differences may become stronger (or weaker) over time. Any significant change in sex differences in brain asymmetry over time would manifest as a sex‐by‐age interaction.
Altogether, this study was designed to answer the following four main questions: (1) are there any structural brain asymmetries in childhood and adolescence; (2) do brain asymmetries change with age during this period; (3) are there any sex differences in brain asymmetries in childhood and adolescence; and (4) do sex differences in brain asymmetries change during this period (sex‐by‐age interaction)?
2. METHODS
2.1. Dataset and sample
Our study sample consisted of typically developing children and adolescents aged 1–18 years (i.e., <19 years), scanned at 69 sites participating in the Enhancing NeuroImaging Genetics through Meta‐Analysis (ENIGMA) consortium (Boedhoe et al., 2018; Hoogman et al., 2019; Postema et al., 2019; Satterthwaite et al., 2014). At each site, data collection was performed after obtaining institutional ethics approval as well as informed consent/assent. The present study was conducted with additional approval from the University of Auckland Human Participants Ethics Committee (UAHPEC23851).
All brain scans were processed at the respective sites using FreeSurfer (Fischl, 2012), which resulted in a standard set of cortical thickness and surface area measures for 34 cortical regions‐of‐interest (ROIs) (Desikan et al., 2006) and of volume measures for 7 subcortical ROIs1 (Fischl et al., 2002) in each hemisphere. In addition, the mean thickness and total surface area for each hemisphere as well as the total intracranial volume (TIV) were calculated. These individual measures, together with other relevant information (sex, age, scanner/site, and handedness), were shared with a central analysis team in the form of spreadsheets. In total, the central analysis team received information on 4331 participants. Of those, 66 participants lacked measures of cortical thickness, cortical surface area, and/or subcortical volume, and thus were excluded. The final sample of 4265 participants had a mean (SD) age of 12.2 (3.2) years (range: 1–18 years) and consisted of 41.2% females and 58.8% males. Information on handedness was available for 2391 participants, with 2126 (88.9%) right‐handers reflecting the expected distribution of handedness in the population (Annett, 1973). Demographics of the final sample for each site are given in Supplementary Table 1.
2.2. Existing measures and new calculations
As mentioned above, the different sites provided the left and right regional measures (regional cortical thickness, regional surface area, and regional subcortical volume) as well as the left and right global measures (total cortical thickness and total surface area).2 The central analysis team calculated the following additional measures: (I) the left and right regional cortical volumes of the 34 ROIs in each hemisphere (thickness × surface area), (II) the left and right total cortical volume, and (III) the left and right total subcortical volume. Altogether, this resulted in 109 regional measures (34 × 3 cortical + 7 × 1 subcortical) as well as in four global measures in each hemisphere. Subsequently, for each of these hemispheric measures, the asymmetry index was calculated as AI = (Left − Right) / (0.5 × [Left + Right]), with resulting positive values indicating leftward asymmetry and negative values indicating rightward asymmetry (Guadalupe et al., 2017; Kong et al., 2018; Kurth et al., 2015; Kurth et al., 2018).
2.3. Statistical analysis
All analyses were run in Matlab 2018a (http://www.mathworks.com/products/matlab) using mixed models, where site was treated as a random effect3 and TIV as a variable of no interest. Overall, four analyses were run to assess (1) hemispheric asymmetry; (2) age‐related changes in asymmetry; (3) sex differences in asymmetry; and (4) sex‐by‐age interactions in asymmetry. For all four analyses, the dependent variables were the asymmetry indices for each measurement, while the independent variables varied according to the analysis performed (see next sections). For all four analyses, the results were corrected for multiple comparisons by controlling the false discovery rate (FDR) at p fdr ≤.05 (Benjamini & Yekutieli, 2001; Hochberg & Benjamini, 1990) within each set of measurements (i.e., [sub]cortical volumes, cortical thickness, and cortical surface areas).
In a supplementary stream, the aforementioned analyses were repeated (a) in a subsample of 2126 right‐handers (45.9% female); (b) in a subsample of 2116 participants younger than 12 years of age (41.1% female); and (c) in a subsample of 2149 participants aged 12 years or older (41.2% female). The outcomes of these analyses are presented in Supplementary Tables 7–21 and Supplementary Figures 1–9.
2.3.1. Analysis 1: Hemispheric asymmetry
The first analysis used the intercept of the mixed model as the independent variable to determine the presence of asymmetries. The effect size was calculated as d = t/sqrt(df) (Rosnow & Rosenthal, 2003). To capture the direction of the asymmetry (leftward/rightward) we used the sign of the estimated intercept in the statistical model: a positive estimate signifies a leftward asymmetry, while a negative sign signifies a rightward asymmetry.
2.3.2. Analysis 2: Change of asymmetries with age
The second analysis used age and age‐squared as the independent variables to establish the link between asymmetry and age (age‐squared, respectively), where age‐squared was orthogonalized to age to avoid collinearity between the two regressors. The effect size was calculated as the partial correlation coefficient r = t /(sqrt[t 2+df]) (Rosnow & Rosenthal, 2003). To capture the direction of the asymmetry (leftward/rightward) as well as the trajectory of the asymmetry (increasing/decreasing), we used the fixed‐effects estimate from the statistical model at the minimum and the maximum age (as calculated from the betas): the sign of the estimate indicates the direction, with positive values for leftward asymmetries and negative values for rightward asymmetries. A higher absolute estimate at the maximum age compared to the minimum age signifies a more pronounced asymmetry with increasing age, while a lower absolute estimate signifies a less pronounced asymmetry with increasing age. A switch in the sign indicates a change in the direction of asymmetry with age.
2.3.3. Analysis 3: Sex differences in asymmetry
The third analysis used sex as the independent variable to determine if asymmetry was different between males and females. The effect size was calculated as d = t(n1+n2) / (sqrt[n1×n2] × sqrt[df]) (Nakagawa & Cuthill, 2007). To capture the direction of the asymmetry (leftward/rightward) as well as the direction of the sex difference (more asymmetric in males/more asymmetric in females), we used the fixed‐effects estimates from the statistical model for males and females (as calculated from the betas): a higher absolute value of the estimates in one sex signifies the direction of the sex effect (i.e., a more pronounced asymmetry than in the other sex). The sign of the estimate indicates the direction of the asymmetry, with positive values for leftward asymmetries and negative values for rightward asymmetries. Significant sex differences were followed up by assessing asymmetries within males and females, separately.
2.3.4. Analysis 4: Sex‐by‐age interactions in asymmetry
The fourth analysis used sex, age as well as the sex‐by‐age interaction as independent variables to determine whether any age‐related changes in asymmetry differ between males and females. Significant interactions were followed up by post hoc analyses assessing the Pearson correlation between asymmetry and age within males and females, separately.
3. RESULTS
3.1. Hemispheric asymmetry
When comparing both hemispheres, a multitude of significant left‐ and rightward asymmetries emerged both on a global and regional scale. More specifically, with respect to global measures, total cortical thickness and total subcortical volume showed a significant leftward asymmetry, total cortical surface area a significant rightward asymmetry, and total cortical volume no significant asymmetry. With respect to regional measures, as detailed in Table 1, all subcortical ROIs as well as the vast majority of cortical ROIs displayed significant asymmetries (for visualization of cortical effects, see Figure 1). Supplemental Table 2 provides statistics for all ROIs and sets of measurements, regardless of significance.
TABLE 1.
Significant asymmetries at the population level.
| ROI | Cortical volume | Cortical thickness | Cortical surface area | Subcortical volume | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Direction | d | p fdr | Direction | d | p fdr | Direction | d | p fdr | Direction | d | p fdr | |
| Banks superior temporal sulcus | Leftward | 0.32 | <.001 | Rightward | −0.50 | <.001 | Leftward | 0.58 | <.001 | – | – | n/a |
| Caudal anterior cingulate | Rightward | −0.41 | <.001 | Leftward | 0.40 | <.001 | Rightward | −0.62 | <.001 | – | – | n/a |
| Caudal middle frontal | Leftward | 0.52 | <.001 | – | – | n.s. | Leftward | 0.48 | <.001 | – | – | n/a |
| Cuneus | Rightward | −0.42 | <.001 | Rightward | −0.12 | <.001 | Rightward | −0.41 | <.001 | – | – | n/a |
| Entorhinal | Leftward | 0.40 | <.001 | Rightward | −0.27 | <.001 | Leftward | 0.56 | <.001 | – | – | n/a |
| Fusiform | Leftward | 0.24 | <.001 | Rightward | −0.06 | <.001 | Leftward | 0.31 | <.001 | – | – | n/a |
| Inferior parietal | Rightward | −1.58 | <.001 | – | – | n.s. | Rightward | −1.56 | <.001 | – | – | n/a |
| Inferior temporal | Leftward | 0.29 | <.001 | Rightward | −0.05 | .001 | Leftward | 0.36 | <.001 | – | – | n/a |
| Isthmus cingulate | Leftward | 0.62 | <.001 | Leftward | 0.11 | <.001 | Leftward | 0.61 | <.001 | – | – | n/a |
| Lateral occipital | Rightward | −0.09 | <.001 | Rightward | −0.16 | <.001 | Leftward | 0.17 | <.001 | – | – | n/a |
| Lateral orbitofrontal | Leftward | 0.12 | <.001 | Leftward | 0.12 | <.001 | Leftward | 0.09 | <.001 | – | – | n/a |
| Lingual | Rightward | −0.37 | <.001 | Rightward | −0.34 | <.001 | Rightward | −0.06 | <.001 | – | – | n/a |
| Medial orbitofrontal | Rightward | −0.05 | .002 | – | – | n.s. | Rightward | −0.08 | <.001 | – | – | n/a |
| Middle temporal | Rightward | −0.45 | <.001 | – | – | n.s. | Rightward | −0.56 | <.001 | – | – | n/a |
| Parahippocampal | Leftward | 0.35 | <.001 | Leftward | 0.16 | <.001 | Leftward | 0.29 | <.001 | – | – | n/a |
| Paracentral | Rightward | −0.88 | <.001 | Rightward | −0.07 | <.001 | Rightward | −0.93 | <.001 | – | – | n/a |
| Pars opercularis | Leftward | 1.09 | <.001 | – | – | n.s. | Leftward | 1.11 | <.001 | – | – | n/a |
| Pars orbitalis | Rightward | −0.47 | <.001 | Leftward | 0.05 | .001 | Rightward | −1.67 | <.001 | – | – | n/a |
| Pars triangularis | Rightward | −0.89 | <.001 | – | – | n.s. | Rightward | −0.95 | <.001 | – | – | n/a |
| Pericalcarine | Rightward | −0.70 | <.001 | Leftward | 0.12 | <.001 | Rightward | −0.85 | <.001 | – | – | n/a |
| Postcentral | Leftward | 0.47 | <.001 | Leftward | 0.17 | <.001 | Leftward | 0.43 | <.001 | – | – | n/a |
| Posterior cingulate | – | – | n.s. | Leftward | 0.12 | <.001 | Rightward | −0.12 | <.001 | – | – | n/a |
| Precentral | Leftward | 0.05 | .004 | Leftward | 0.13 | <.001 | – | – | n.s. | – | – | n/a |
| Precuneus | Rightward | −0.51 | <.001 | – | – | n.s. | Rightward | −0.51 | <.001 | – | – | n/a |
| Rostral anterior cingulate | Leftward | 1.26 | <.001 | Leftward | 0.11 | <.001 | Leftward | 0.45 | <.001 | – | – | n/a |
| Rostral middle frontal | – | – | n.s. | Leftward | 0.08 | <.001 | Rightward | −0.31 | <.001 | – | – | n/a |
| Superior frontal | Leftward | 0.15 | <.001 | Leftward | 0.07 | <.001 | Leftward | 0.43 | <.001 | – | – | n/a |
| Superior parietal | Leftward | 0.15 | <.001 | Leftward | 0.06 | <.001 | Leftward | 0.12 | <.001 | – | – | n/a |
| Superior temporal | Leftward | 0.22 | <.001 | Rightward | −0.06 | <.001 | Leftward | 0.74 | <.001 | – | – | n/a |
| Supramarginal | Leftward | 0.22 | <.001 | Leftward | 0.06 | <.001 | Leftward | 0.22 | <.001 | – | – | n/a |
| Frontal pole | Rightward | −0.45 | <.001 | Leftward | 0.20 | <.001 | Rightward | −0.60 | <.001 | – | – | n/a |
| Temporal pole | Leftward | 0.20 | <.001 | Rightward | −0.23 | <.001 | Leftward | 0.27 | <.001 | – | – | n/a |
| Transverse temporal | Leftward | 1.79 | <.001 | Rightward | −0.04 | .029 | Leftward | 1.96 | <.001 | – | – | n/a |
| Insula | Leftward | 0.04 | .009 | – | – | n.s. | – | – | n.s. | – | – | n/a |
| Thalamus | – | – | n/a | – | – | n/a | – | – | n/a | Leftward | 0.11 | <.001 |
| Caudate | – | – | n/a | – | – | n/a | – | – | n/a | Rightward | −0.17 | <.001 |
| Putamen | – | – | n/a | – | – | n/a | – | – | n/a | Leftward | 0.05 | .002 |
| Pallidum | – | – | n/a | – | – | n/a | – | – | n/a | Leftward | 0.11 | <.001 |
| Hippocampus | – | – | n/a | – | – | n/a | – | – | n/a | Rightward | −0.08 | <.001 |
| Amygdala | – | – | n/a | – | – | n/a | – | – | n/a | Rightward | −0.15 | <.001 |
| Nucleus Accumbens | – | – | n/a | – | – | n/a | – | – | n/a | Rightward | −0.04 | .012 |
| Total cortical | – | – | n.s. | Leftward | 0.09 | <.001 | Rightward | −0.13 | <.001 | – | – | n/a |
| Total subcortical | – | – | n/a | – | – | n/a | – | – | n/a | Leftward | 0.05 | .003 |
Note: Table restricted to ROIs, where at least one measure survived corrections for multiple comparisons.
Abbreviations: d, Cohen's d; n.s., not significant; n/a, not applicable; p fdr, fdr corrected p‐value; ROI, region of interest.
FIGURE 1.
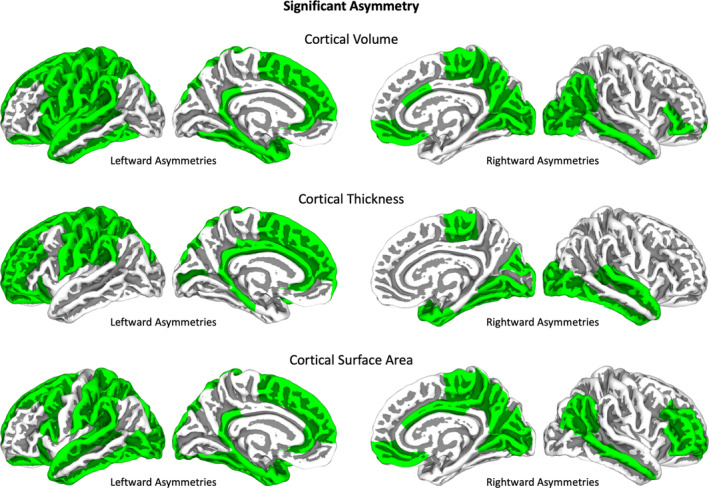
Hemispheric asymmetry. Cortical regions with significant asymmetries are indicated in green. The regions defined by the Desikan–Killiany atlas (Desikan et al., 2006) were projected onto the central surface of the FSAverage template using the CAT12 toolbox (Gaser et al., 2022). Rightward asymmetries are indicated on the right hemispheres, leftward asymmetries on the left hemispheres. All significant asymmetries are FDR‐corrected using a threshold of 0.05 (Benjamini & Yekutieli, 2001; Hochberg & Benjamini, 1990).
3.2. Change of asymmetries with age
There was no significant association between asymmetry and age for any global cortical measurement, but a significant change in asymmetry direction was observed for total subcortical volume. In contrast, several significant age‐related changes in asymmetry were detected for regional cortical and subcortical measures. As detailed in Table 2, 15 regions overall displayed a significant association between asymmetry and age. More specifically, of those 15 regions, five showed an increase and six a decrease in asymmetry (two of those had additional associations with age‐squared). One region showed a decrease in one measurement but a change in direction in another measurement. One region had a change in direction, and two others had an association with age‐squared. For visualization of cortical effects, see Figure 2. For statistics for all areas and sets of measurement regardless of significance, see Supplemental Table 3.
TABLE 2.
Significant effects of increasing age on asymmetry.
| Cortical volume | Age | Age2 | |||
|---|---|---|---|---|---|
| ROI | Direction | r | p fdr | r | p fdr |
| Fusiform | – | 0.01 | n.s. | −0.06 | .003 |
| Precuneus | Decrease rightward | 0.06 | .004 | −0.05 | .046 |
| Supramarginal | Decrease leftward | −0.05 | .009 | 0.02 | n.s. |
| Cortical thickness | Age | Age2 | |||
|---|---|---|---|---|---|
| ROI | Direction | r | p fdr | r | p fdr |
| Banks superior temporal sulcus | Decrease rightward | −0.04 | .043 | −0.05 | n.s. |
| Inferior parietal | Left to right | −0.05 | .013 | 0.01 | n.s. |
| Inferior temporal | Left to right | −0.04 | .043 | 0.03 | n.s. |
| Lateral orbitofrontal | Decrease leftward | −0.06 | .004 | 0.00 | n.s. |
| Parahippocampal | Increase leftward | 0.05 | .012 | 0.02 | n.s. |
| Paracentral | Increase rightward | −0.05 | .004 | −0.02 | n.s. |
| Rostral anterior cingulate | Decrease leftward | −0.05 | .004 | 0.00 | n.s. |
| Rostral middle frontal | Increase leftward | 0.05 | .004 | 0.02 | n.s. |
| Transverse temporal | Decrease rightward | 0.06 | .003 | −0.05 | .034 |
| Cortical surface area | Age | Age2 | |||
|---|---|---|---|---|---|
| ROI | Direction | r | p fdr | r | p fdr |
| Fusiform | – | 0.01 | n.s. | −0.05 | .021 |
| Inferior parietal | Decrease rightward | 0.05 | .042 | −0.03 | n.s. |
| Precuneus | Decrease rightward | 0.06 | .001 | −0.03 | n.s. |
| Subcortical volume | Age | Age2 | |||
|---|---|---|---|---|---|
| ROI | Direction | r | p fdr | r | p fdr |
| Thalamus | – | 0.03 | n.s. | −0.05 | .008 |
| Putamen | Increase leftward | 0.06 | .002 | 0.01 | n.s. |
| Nucleus accumbens | Increase rightward | −0.05 | .003 | −0.03 | n.s. |
| Total subcortical | Right to left | 0.05 | .003 | −0.03 | n.s. |
Note: Table restricted to ROIs, where at least one measure survived corrections for multiple comparisons.
Abbreviations: d, Cohen's d; n.s., not significant; p fdr, fdr corrected p‐value (main analyses); ROI, region of interest.
FIGURE 2.
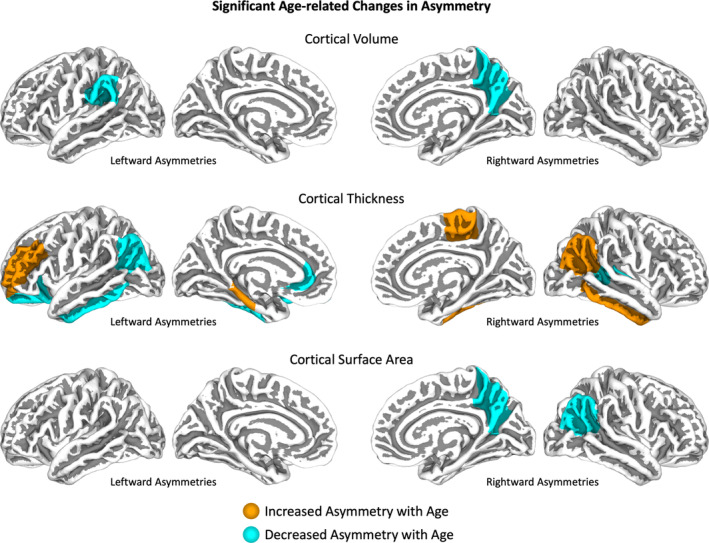
Age‐related changes in asymmetry. Cortical regions with significant age‐related changes in asymmetry are indicated in orange (increases) or cyan (decreases). Rightward asymmetries are indicated on the right hemispheres, leftward asymmetries on the left hemispheres. If asymmetry for a specific region changes in its direction with increasing age, the region is indicated in both left and right panels.
3.3. Sex differences in asymmetry
There was no significant association between asymmetry and sex for any global measurements. In contrast, significant sex differences in asymmetry were evident for regional cortical and subcortical measures. More specifically, as detailed in Table 3, males had significantly stronger asymmetries than females in two cortical ROIs (superior temporal gyrus and inferior parietal cortex), while females had significantly stronger asymmetries in two cortical (rostral anterior cingulate and insula) as well as two subcortical (thalamus and nucleus accumbens) ROIs. For the transverse temporal gyrus males and females showed asymmetries in opposite directions (for visualization of cortical effects, see Figure 3). Follow‐up analyses revealed that, except for the insula, asymmetries were significant when males and females were investigated separately. In other words, even if one sex displayed a more pronounced asymmetry than the other, the asymmetry was significant in both sexes. Supplemental Table 4 provides statistics for all areas and sets of measurements regardless of significance. Supplemental Table 5 provides additional statistics for the follow‐up analyses.
TABLE 3.
Significant sex differences in asymmetry.
| ROI | Main analyses | Follow‐up analyses (within each sex separately) | |||||
|---|---|---|---|---|---|---|---|
| Sex effect | Asymmetry by sex | ||||||
| Direction | d | p fdr | Direction | Estimate | d | p | |
| Cortical volume | |||||||
| Inferior parietal | M > F: both rightward | 0.09 | .036 | M: rightward | −0.19 | −1.21 | <.001 |
| F: rightward | −0.18 | −0.94 | <.001 | ||||
| Rostral anterior cingulate | F > M: both leftward | 0.10 | .035 | M: leftward | 0.25 | 0.91 | <.001 |
| F: leftward | 0.28 | 0.81 | <.001 | ||||
| Superior temporal | M > F: both leftward | −0.13 | .002 | M: leftward | 0.05 | 0.23 | <.001 |
| F: leftward | 0.04 | 0.15 | <.001 | ||||
| Cortical thickness | |||||||
| Superior temporal | M > F: both rightward | 0.10 | .018 | M: rightward | −0.01 | −0.07 | <.001 |
| F: rightward | −0.01 | −0.04 | .023 | ||||
| Transverse temporal | M/F: opposite direction | 0.15 | <.001 | M: rightward | −0.01 | −0.08 | <.001 |
| F: leftward | 0.01 | 0.04 | .019 | ||||
| Insula | F > M: both leftward | 0.11 | .009 | M: leftward | 0.00 | 0.00 | n.s. |
| F: leftward | 0.01 | 0.04 | .006 | ||||
| Cortical surface area | |||||||
| Inferior parietal | M > F: both rightward | 0.12 | .002 | M: rightward | −0.18 | −1.20 | <.001 |
| F: rightward | −0.17 | −0.91 | <.001 | ||||
| Superior temporal | M > F: both leftward | −0.17 | <.001 | M: leftward | 0.07 | 0.61 | <.001 |
| F: leftward | 0.05 | 0.39 | <.001 | ||||
| Subcortical volume | |||||||
| Thalamus | F > M: both leftward | 0.08 | .049 | M: leftward | 0.04 | 0.11 | <.001 |
| F: leftward | 0.04 | 0.12 | <.001 | ||||
| Nucleus accumbens | F > M: both rightward | −0.08 | .049 | M: rightward | −0.02 | −0.03 | .045 |
| F: rightward | −0.03 | −0.05 | .001 | ||||
Note: Table restricted to ROIs, where at least one measure survived corrections for multiple comparisons in the main analysis.
Abbreviations: d, Cohen's d; F > M, females more asymmetric than males; F, females; M > F, males more asymmetric than females; M, males; n.s., not significant; p, uncorrected p‐value (follow‐up analyses); p fdr, fdr corrected p‐value (main analyses); ROI, region of interest.
FIGURE 3.
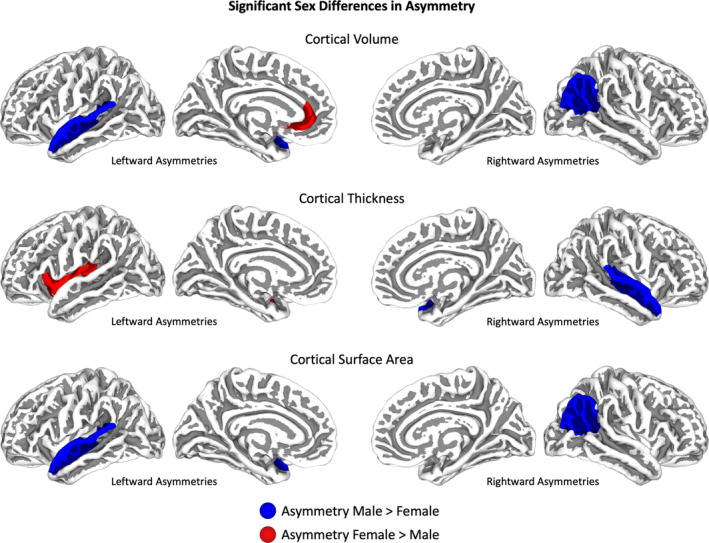
Sex differences in asymmetry. Cortical regions with significantly larger asymmetries in males are depicted in blue, and with significantly larger asymmetries in females in red. Rightward asymmetries are indicated on the right hemispheres, leftward asymmetries on the left hemispheres.
3.4. Sex‐by‐age interactions in asymmetry
There were no significant sex‐by‐age interactions for any global or regional measurement. Supplemental Table 6 provides statistics for all areas and sets of measurement regardless of significance.
4. DISCUSSION
In the present study we characterized cerebral gray matter asymmetries during childhood and adolescence. For this purpose, we analyzed a large sample of >4000 children and adolescents between 1 and 18 years of age, scanned at 69 sites around the world. Hemispheric differences were determined with respect to various morphological features—cortical thickness, cortical surface area, as well as cortical and subcortical volumes—capturing both regional and global measures.
4.1. Asymmetries
Asymmetries varied markedly between brain regions, ranging from minute differences between the hemispheres to extremely large differences, the latter observed, for example, for the cortical surface areas of the transverse temporal gyrus (34% larger on the left than on the right) and the inferior parietal cortex (20% larger on the right than on the left). Overall, effects were most pronounced for cortical surface area (some large and very large effects) and least pronounced for cortical thickness and subcortical volumes (only small and medium effects).
While, historically, asymmetry studies relied on smaller sample sizes, some recent studies were able to draw from larger pools of participants comprising thousands or even tens of thousands of brains. The overall pattern of asymmetries in cortical thickness and cortical surface area as presently observed is very similar to that reported in the large‐scale studies that were primarily based on adults (Guadalupe et al., 2017; Kong et al., 2018). More specifically with respect to cortical thickness, in accordance with Kong et al. (2018), there is a leftward asymmetry around the central sulcus which extends into frontal and prefrontal regions dorsolaterally and medially; and there is a rightward asymmetry in lateral and medial occipital cortical regions. With respect to cortical surface area, and also in accordance with Kong et al. (2018), there is a leftward asymmetry in superior frontal and inferior frontal (pars opercularis) regions, in perisylvian regions including postcentral and supramarginal gyrus, in superior temporal regions including Heschl's gyrus, as well as in inferior temporal, lateral occipital, retrosplenial, dorsomedial frontal, and pregenual regions; there is a rightward asymmetry in prefrontal, middle temporal and inferior parietal as well as in medial occipital and parietal regions. In addition, both Kong et al.'s study (2018) and the present study detected a leftward asymmetry for total cortical thickness and a rightward asymmetry for total cortical surface area. Similarly, when comparing the present results to the other lifespan study focusing on subcortical volumes (Guadalupe et al., 2017), the direction of asymmetry is identical in both studies for all six subcortical regions examined.
Relating our findings to the outcomes of other smaller‐scale asymmetry studies, there is a moderate degree of consistency. For example, the pattern of asymmetries in total cortical volume, thickness, and surface area matches the effects reported in children by Raja et al. (2021). Similarly, our finding of a leftward asymmetry in the cortical volume and surface area of the transverse temporal gyrus, the pars opercularis of the inferior frontal gyrus, and the region around the central sulcus matches the effects reported in children and adolescents by Levman et al. (2017). Finally, our finding of a rightward asymmetry in the volume of the caudal anterior cingulate and a leftward asymmetry of the superior frontal gyrus matches effects detected in a study in children that focused specifically on the dorsal anterior cingulate cortex (Wang et al., 2015). Nevertheless, there are also some differences between the current findings and other previously reported effects, such as a leftward asymmetry in inferior parietal and a rightward asymmetry in inferior frontal regions with respect to cortical thickness (Zhou et al., 2013), a leftward asymmetry in inferior frontal regions with respect to cortical thickness (Plessen et al., 2014), and a leftward asymmetry in the lateral occipital cortex with respect to cortical volume (Levman et al., 2017). Such discrepancies, however, might be attributable to differences in sample characteristics, applied methods and/or scanning equipment and parameters, as discussed elsewhere (Kong et al., 2022). Importantly, asymmetry effects for regions where the current study detected very large effect sizes (transverse temporal gyrus, etc.) have been largely consistent across studies (Kong et al., 2018; Levman et al., 2017; Plessen et al., 2014; Raja et al., 2021; Zhou et al., 2013).
4.2. Effects of age
Recent large‐scale studies addressing age‐related changes in brain asymmetry across the entire life span reported an increasing leftward asymmetry of the putamen (Guadalupe et al., 2017), superior temporal gyrus, and entorhinal cortex (Kong et al., 2018). However, some of these effects only reached significance when covering an age range of more than 20 years. Thus, these life‐time studies are not immediately comparable with the current study covering merely 18 years toward the beginning of the age spectrum (1–18 years), albeit there is some resemblance with respect to the effects, such as an increasing leftward asymmetry of the putamen with age (Guadalupe et al., 2017). In contrast to those adult studies (Guadalupe et al., 2017; Kong et al., 2018)—which reported only 3 out of 41 regions to be significantly associated with age—the current study revealed more widespread associations between age and asymmetries during childhood and adolescence, with significant effects in 15 out of 41 regions. However, the effects of age were small in general. The localization of the present results somewhat agrees with the study by Shaw et al. (2009) in children and adolescents, even though there is also some disagreement on the direction of the age‐related change. More specifically, with respect to cortical thickness, both the current study and Shaw's study (2009) detected a decreasing leftward asymmetry in the lateral orbitofrontal cortex. However, while Shaw et al. (2009) reported a decrease in rightward asymmetry in the middle occipital and angular gyri, our study revealed a change from leftward to rightward asymmetry in the inferior parietal cortex (i.e., a region that best matches the angular cluster described by Shaw et al., 2009).
4.3. Effects of sex
Previous studies on sex differences in asymmetry revealed conflicting results (Good et al., 2001; Guadalupe et al., 2015; Guadalupe et al., 2017; Kong et al., 2018; Levman et al., 2017; Plessen et al., 2014; Shaw et al., 2009; Zhou et al., 2013), with findings indicating stronger asymmetries in men, stronger asymmetries in women, or no differences. Moreover, effects seem to depend on the brain region examined and the specific measures used. Recent large‐scale studies reported significant sex differences, particularly around the Sylvian fissure, but also inferior parietal, lateral occipital and medial frontal as well as in subcortical structures, such as the putamen and globus pallidus (Guadalupe et al., 2015; Guadalupe et al., 2017; Kong et al., 2018). Our current study is consistent with these findings in part, revealing an increased leftward asymmetry in males compared to females in the superior temporal gyrus (cortical volume and surface area) and an increased rightward asymmetry in males compared to females in the inferior parietal cortex (cortical surface area). However, we also observed more pronounced asymmetries in the rostral anterior cingulate, the insula, as well as the thalamus and nucleus accumbens in females compared to males. Interestingly, while the total number of regions exhibiting significant asymmetries was larger in females than in males, the magnitude of the significant asymmetries was larger in males. Moreover, the total number of measures indicating significant asymmetries was also larger in males (as we obtained more than one measurement per cortical region; that is, cortical thickness, cortical volume, and cortical surface area). Given that there were no significant sex‐by‐age interactions, these sex differences do not appear to change with age and remain stable throughout development.
5. CONCLUSION
The multitude of asymmetries detected in our large sample of children and adolescents suggests that cerebral asymmetries manifest early in life. In terms of age effects, asymmetries became both smaller and larger with increasing age and sometimes even reversed in direction, depending on region or measure. However, in general, age effects were small. In other words, the pattern of asymmetry seems relatively stable throughout childhood and adolescence, which may seem surprising given that cerebral development is rather dynamic during that time. Cerebral asymmetries may therefore reflect a fundamental organizational pattern of the brain rather than a result of brain development and regional specialization. This view might be supported by reports of cerebral asymmetries as early as the first and second trimester of pregnancy (Abu‐Rustum et al., 2013; Corballis, 2013; de Kovel et al., 2017; Namburete et al., 2023; Steger et al., 2023; Vasung et al., 2020) and by findings of asymmetries in gene activation (de Kovel et al., 2017; Francks, 2015; Karlebach & Francks, 2015; Ocklenburg et al., 2017). In addition, genes associated with variation in adult brain asymmetry, as identified in large‐scale genome‐wide association analyses, tend to be most active in the embryonic and fetal brain (Sha, Schijven, et al., 2021). In terms of sex differences, there were greater asymmetries in males than in females when investigating cortical volume and cortical surface area, while sex differences in the asymmetry of cortical thickness were more variable. However, these sex differences were small overall. Thus, brain asymmetry and its change over time appear to be relatively similar in males and females.
FUNDING INFORMATION
The study and authors were supported by the following entities: The Royal Society of New Zealand (Marsden 20‐UOA‐045), the NIH (R01AG058854, R01MH116147, P41EB015922, R01MH104648, R21MH101441, K23MH115206, MH119219, R01MH113550, R01MH120482, R01MH112847, RF1MH116920, MHR0159105, R01MH117601, R01MH13004, R01MH114879), the INPD (Fapesp 2014/50917‐0, 2021/05332‐8, CNPq 465550/2014‐2), the NIHR (NIHR130077), the ERC, the Max Planck Society, the National Natural Science Foundation of China (32171031), the Fundamental Research Funds for the Central Universities (2021XZZX006), the Information Technology Center of Zhejiang University, the Australian NHMRC (APP1172917, APP1158127, 1008522, 1065895), the Ontario Mental Health Foundation, the Canadian Institutes of Health Research, the Department of Psychiatry at the University of Toronto, the CAMH Foundation, the Innovative Medicines Initiative Joint Undertaking (115300 and 777394), the Tommy Fuss Center, the Marató TV3 Foundation (091710, 091710, 091810), the Medical Research Council, the Research Council of Norway (190544/H110), the Swedish Research Council, the Swedish Brain foundation (Hjärnfonden), the Stockholm Brain Institute, the Autism and Asperger Association Stockholm, the Queen Silvia Jubilee Fund, the Solstickan Foundation, the Pediatric Research Foundation at Astrid Lindgren Children's Hospital, the Swedish Foundation for Strategic Research, the Jerring Foundation, the Swedish Order of Freemasons, the Kempe‐Carlgrenska Foundation, the Jeansson Foundation, the Italian Ministry of Health (RC Linea 4 at IRCCS Stella Maris Foundation), the Penn‐CHOP Lifespan Brain Institute, the European Union's Seventh Framework Programme, the European Federation of Pharmaceutical Industries and Associations, the Meath Foundation, the National Children's Research Foundation, the UK Department of Health via the National Institute for Health Research (NIHR) Biomedical Research Centre (BRC) for Mental Health at South London and the Maudsley NHS Foundation Trust and Institute of Psychiatry, Psychology and Neuroscience, King's College London, the Riksbankens Jubileumsfond, Fundació La Marató de TV3‐2009 (project 091810), the Carlos III Health Institute (PI11/01419), the Ontario Brain Institute, the Natural Sciences and Engineering Research Council, the New Frontiers in Research Fund, Sunnderdahls Handikappsfond, PRIMA Child and Adult Psychiatry, Vinnova, Formas, FORTE, Alberta Innovates Translational Health Chair in Child and Youth Mental Health, STI 2030 (2021ZD0200409), Autism Speaks, Michael Smith Health Research BC, EU‐AIMS, AIMS‐2‐TRIALS, Horizon2020, EFPIA, Autistica, SFARI, CANDY (847818), EME, CNPq, FAPESP, CAPES, IOCDF, NOW (91619115), ZIHP, Tallaght Hospital (Dublin, Ireland), Our Ladies Hospital for Sick Children (Dublin, Ireland), the Government of India Department of Science and Technology (DST INSPIRE Faculty Grant No. IFA12‐LSBM26 and Grant No. SR/S0/HS/0016/2011), the Government of India Department of Biotechnology (Grant No. BT/06/IYBA/2012), ERDF Funds from the European Commission/European Union—NextGenerationEU (PMP21/00051 and PI19/01024). CIBERSAM, Madrid Regional Government (B2017/BMD‐3740 AGES‐CM‐2), European Union Structural Funds, European Union Seventh Framework Program, European Union H2020 Program under the Innovative Medicines Initiative 2 Joint Undertaking, Project PRISM‐2 (No. 101034377), Project AIMS‐2‐TRIALS (No. 777394), Horizon Europe, the National Institute of Mental Health of the National Institutes of Health under Award Number 1U01MH124639‐01 (Project ProNET) and Award Number 5P50MH115846‐03 (project FEP‐CAUSAL), Fundación Familia Alonso, and Fundación Alicia Koplowitz. The following provides additional information on support for the data used in the study (if not already mentioned above). ADHD/ASD Data: Support was received from the Medical Research Council (MRC GO300155); Medical Research Council UK Autism Imaging Multicentre Study (G0400061); UK Department of Health via the National Institute for Health Research (NIHR) Biomedical Research Centre (BRC) for Mental Health at South London and Maudsley NHS Foundation Trust and Institute of Psychiatry, King's College London. Additional support is received from the European Community's Seventh Framework Programme (FP7/2007‐2013) under grant agreements n° 602805 (Aggressotype), n° 603016 (MATRICS), n° 602450 (IMAGEMEND), and n° 278948 (TACTICS), and from the European Community's Horizon 2020 Programme (H2020/2014–2020) under grant agreements n° 643051 (MiND) and n° 667302 (CoCA). IMpACT Data: We acknowledge funding from the Netherlands Organization for Scientific Research (NWO), that is, the Veni Innovation Program (grant 016‐196‐115 to MH) and the Vici Innovation Program (grant 016–130‐669 to BF). The work was also supported by grant U54 EB020403 to the ENIGMA Consortium from the BD2K Initiative, a cross‐NIH partnership, and by the European College of Neuropsychopharmacology (ECNP) Network “ADHD Across the Lifespan.” ADHD WG: This initiative received support from the Medical Research Council (MRC GO300155); the National Institute for Health Research (NIHR) Biomedical, the Research Centre (BRC) for Mental Health at the South London and Maudsley NHS Foundation Trust (SLaM) and the Institute of Psychiatry at King's College, London; Lilly Pharmaceuticals. OCD WG: This initiative received support from the Medical Research Council (MRC GO300155). The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results. Any views expressed are those of the authors and not necessarily those of the funders.
CONFLICT OF INTEREST STATEMENT
Paul Arnold receives research support from Biohaven Pharmaceuticals. Sven Bölte acted as an author, consultant or lecturer for Medice and Roche over the past 3 years. In addition, he receives royalties for textbooks and diagnostic tools from Hogrefe and Liber, and he is partner at NeuroSupportSolutions International AB. Rodrigo Bressan received personal fees from Sanofi, Ache, as well as institutional grants, personal fees and non‐financial support from Janssen unrelated to this manuscript. Barbara Franke and Martine Hoogman have received educational speaking fees from Medice. Azadeh Kushki has a patent for Anxiety Meter with royalties paid from Awake Labs. Pedro Pan received payment or honoraria for lectures and presentations in educational events for Sandoz, Daiichi Sankyo, Eurofarma, Abbot, Libbs, Instituto Israelita de Pesquisa e Ensino Albert Einstein, Instituto D'Or de Pesquisa e Ensino. Katya Rubia has received a grant from Takeda pharmaceuticals for an unrelated project and consulting fees from Supernus and Lundbeck. Lin Sorensen received a fee for speaking and conference support in 2023 from Medice Nordic Norway. Paul Thompson and Neda Jahanshad were funded in part by a research grant from Biogen, Inc., for research unrelated to this manuscript. Blair Simpson has received royalties from UpToDate, Inc., and a stipend from the American Medical Association for her role as Associate Editor at JAMA Psychiatry. Celso Arango has been a consultant to or has received honoraria or grants from Acadia, Angelini, Biogen, Boehringer, Gedeon Richter, Janssen Cilag, Lundbeck, Medscape, Menarini, Minerva, Otsuka, Pfizer, Roche, Sage, Servier, Shire, Schering Plough, Sumitomo Dainippon Pharma, Sunovion and Takeda.
Supporting information
Data S1. Supplementary figures.
Supplementary Table 1. Demographics of included studies. If studies used more than one scanner or more than one study site, the respective subgroups are listed as different sites.
Supplementary Table 2. Hemispheric asymmetry.
Supplementary Table 3. Change of asymmetries with age.
Supplementary Table 4. Sex differences in asymmetry.
Supplementary Table 5. Sex differences in asymmetry: Follow‐up.
Supplementary Table 6. Sex‐by‐age interactions in asymmetry.
Supplementary Table 7. Hemispheric asymmetry—(right handers).
Supplementary Table 8. Change of asymmetries with age—(right handers).
Supplementary Table 9. Sex differences in asymmetry—(right handers).
Supplementary Table 10. Sex differences in asymmetry: follow‐up—(right handers).
Supplementary Table 11. Sex‐by‐age interactions in asymmetry—(right handers).
Supplementary Table 12. Hemispheric asymmetry—Younger than 12 years of age.
Supplementary Table 13. Change of asymmetries with age—Younger than 12 years of age.
Supplementary Table 14. Sex differences in asymmetry—Younger than 12 years of age.
Supplementary Table 15. Sex differences in asymmetry: Follow‐up—Younger than 12 years of age.
Supplementary Table 16. Sex‐by‐age interactions in asymmetry—Younger than 12 years of age.
Supplementary Table 17. Hemispheric asymmetry—12 years and older.
Supplementary Table 18. Change of asymmetries with age—12 years and older.
Supplementary Table 19. Sex differences in asymmetry—12 years and older.
Supplementary Table 20. Sex differences in asymmetry: Follow‐up—12 years and older.
Supplementary Table 21. Sex‐by‐age interactions in asymmetry—12 years and older.
ACKNOWLEDGEMENTS
The authors would like to thank Daniel Brandeis, Lars Michels, Peter Klaver, Simon Schlomo‐Poil, and Ernst Martin for their contributions to the collection of data. The research was further supported by the Swedish Collegium for Advanced Study and the Erling‐Persson Family Foundation. Support for the creation of the author list and affiliations was provided by AuthorArranger, a tool developed at the National Cancer Institute. Open access publishing facilitated by The University of Auckland, as part of the Wiley ‐ The University of Auckland agreement via the Council of Australian University Librarians.
Kurth F., Schijven, D. , van den Heuvel, O. A. , Hoogman, M. , van Rooij, D. , Stein, D. J. , Buitelaar, J. K. , Bölte, S. , Auzias, G. , Kushki, A. , Venkatasubramanian, G. , Rubia, K. , Bollmann, S. , Isaksson, J. , Jaspers‐Fayer, F. , Marsh, R. , Batistuzzo, M. C. , Arnold, P. D. , Bressan, R. A. , … Luders, E. (2024). Large‐scale analysis of structural brain asymmetries during neurodevelopment: Associations with age and sex in 4265 children and adolescents. Human Brain Mapping, 45(11), e26754. 10.1002/hbm.26754
Footnotes
Some sites included the lateral ventricles as an 8th ROI, while others did not. Thus, in our analyses, the lateral ventricles were omitted, resulting in a total of 7 subcortical ROIs.
Any empty cells—a result of classifying individual measures as outliers at the original sites and excluding them—were coded as missing values.
If a site provided data from multiple scanners, the dataset from each scanner was treated as coming from a different site.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- Abu‐Rustum, R. S. , Ziade, M. F. , & Abu‐Rustum, S. E. (2013). Reference values for the right and left fetal choroid plexus at 11 to 13 weeks: An early sign of “developmental” laterality? Journal of Ultrasound in Medicine, 32, 1623–1629. [DOI] [PubMed] [Google Scholar]
- Annett, M. (1973). Handedness in families. Annals of Human Genetics, 37, 93–105. [DOI] [PubMed] [Google Scholar]
- Benjamini, Y. , & Yekutieli, D. (2001). The control of the false discovery rate in multiple testing under dependency. Annals of Statistics, 29, 1165–1188. [Google Scholar]
- Boedhoe, P. S. W. , Schmaal, L. , Abe, Y. , Alonso, P. , Ameis, S. H. , Anticevic, A. , Arnold, P. D. , Batistuzzo, M. C. , Benedetti, F. , Beucke, J. C. , Bollettini, I. , Bose, A. , Brem, S. , Calvo, A. , Calvo, R. , Cheng, Y. , Cho, K. I. K. , Ciullo, V. , Dallaspezia, S. , … Group, E.O.W . (2018). Cortical abnormalities associated with pediatric and adult obsessive‐compulsive disorder: Findings from the ENIGMA obsessive‐compulsive disorder working group. The American Journal of Psychiatry, 175, 453–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiarello, C. , Vazquez, D. , Felton, A. , & McDowell, A. (2016). Structural asymmetry of the human cerebral cortex: Regional and between‐subject variability of surface area, cortical thickness, and local gyrification. Neuropsychologia, 93, 365–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corballis, M. C. (2013). Early signs of brain asymmetry. Trends in Cognitive Sciences, 17, 554–555. [DOI] [PubMed] [Google Scholar]
- de Kovel, C. G. F. , Lisgo, S. , Karlebach, G. , Ju, J. , Cheng, G. , Fisher, S. E. , & Francks, C. (2017). Left‐right asymmetry of maturation rates in human embryonic neural development. Biological Psychiatry, 82, 204–212. [DOI] [PubMed] [Google Scholar]
- de Vareilles, H. , Riviere, D. , Sun, Z. Y. , Fischer, C. , Leroy, F. , Neumane, S. , Stopar, N. , Eijsermans, R. , Ballu, M. , Tataranno, M. L. , Benders, M. , Mangin, J. F. , & Dubois, J. (2022). Shape variability of the central sulcus in the developing brain: A longitudinal descriptive and predictive study in preterm infants. NeuroImage, 251, 118837. [DOI] [PubMed] [Google Scholar]
- Desikan, R. S. , Segonne, F. , Fischl, B. , Quinn, B. T. , Dickerson, B. C. , Blacker, D. , Buckner, R. L. , Dale, A. M. , Maguire, R. P. , Hyman, B. T. , Albert, M. S. , & Killiany, R. J. (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage, 31, 968–980. [DOI] [PubMed] [Google Scholar]
- Fan, L. , Tang, Y. , Sun, B. , Gong, G. , Chen, Z. J. , Lin, X. , Yu, T. , Li, Z. , Evans, A. C. , & Liu, S. (2010). Sexual dimorphism and asymmetry in human cerebellum: An MRI‐based morphometric study. Brain Research, 1353, 60–73. [DOI] [PubMed] [Google Scholar]
- Fischl, B. (2012). FreeSurfer. NeuroImage, 62, 774–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl, B. , Salat, D. H. , Busa, E. , Albert, M. , Dieterich, M. , Haselgrove, C. , van der Kouwe, A. , Killiany, R. , Kennedy, D. , Klaveness, S. , Montillo, A. , Makris, N. , Rosen, B. , & Dale, A. M. (2002). Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron, 33, 341–355. [DOI] [PubMed] [Google Scholar]
- Francks, C. (2015). Exploring human brain lateralization with molecular genetics and genomics. Annals of the New York Academy of Sciences, 1359, 1–13. [DOI] [PubMed] [Google Scholar]
- Gaser, C. , Dahnke, R. , Thompson, P. M. , Kurth, F. , & Luders, E. (2022). CAT—A computational anatomy toolbox for the analysis of structural MRI data. bioRxiv. 10.1101/2022.06.11.495736 [DOI] [Google Scholar]
- Ge, X. , Zheng, Y. , Qiao, Y. , Pan, N. , Simon, J. P. , Lee, M. , Jiang, W. , Kim, H. , Shi, Y. , & Liu, M. (2022). Hippocampal asymmetry of regional development and structural covariance in preterm neonates. Cerebral Cortex, 32, 4271–4283. [DOI] [PubMed] [Google Scholar]
- Gilmore, J. H. , Lin, W. , Prastawa, M. W. , Looney, C. B. , Vetsa, Y. S. , Knickmeyer, R. C. , Evans, D. D. , Smith, J. K. , Hamer, R. M. , Lieberman, J. A. , & Gerig, G. (2007). Regional gray matter growth, sexual dimorphism, and cerebral asymmetry in the neonatal brain. The Journal of Neuroscience, 27, 1255–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good, C. D. , Johnsrude, I. , Ashburner, J. , Henson, R. N. , Friston, K. J. , & Frackowiak, R. S. (2001). Cerebral asymmetry and the effects of sex and handedness on brain structure: A voxel‐based morphometric analysis of 465 normal adult human brains. NeuroImage, 14, 685–700. [DOI] [PubMed] [Google Scholar]
- Guadalupe, T. , Mathias, S. R. , vanErp, T. G. , Whelan, C. D. , Zwiers, M. P. , Abe, Y. , Abramovic, L. , Agartz, I. , Andreassen, O. A. , Arias‐Vasquez, A. , Aribisala, B. S. , Armstrong, N. J. , Arolt, V. , Artiges, E. , Ayesa‐Arriola, R. , Baboyan, V. G. , Banaschewski, T. , Barker, G. , Bastin, M. E. , … Francks, C. (2017). Human subcortical brain asymmetries in 15,847 people worldwide reveal effects of age and sex. Brain Imaging and Behavior, 11, 1497–1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guadalupe, T. , Zwiers, M. P. , Wittfeld, K. , Teumer, A. , Vasquez, A. A. , Hoogman, M. , Hagoort, P. , Fernandez, G. , Buitelaar, J. , van Bokhoven, H. , Hegenscheid, K. , Volzke, H. , Franke, B. , Fisher, S. E. , Grabe, H. J. , & Francks, C. (2015). Asymmetry within and around the human planum temporale is sexually dimorphic and influenced by genes involved in steroid hormone receptor activity. Cortex, 62, 41–55. [DOI] [PubMed] [Google Scholar]
- Hochberg, Y. , & Benjamini, Y. (1990). More powerful procedures for multiple significance testing. Statistics in Medicine, 9, 811–818. [DOI] [PubMed] [Google Scholar]
- Hoogman, M. , Muetzel, R. , Guimaraes, J. P. , Shumskaya, E. , Mennes, M. , Zwiers, M. P. , Jahanshad, N. , Sudre, G. , Wolfers, T. , Earl, E. A. , Soliva Vila, J. C. , Vives‐Gilabert, Y. , Khadka, S. , Novotny, S. E. , Hartman, C. A. , Heslenfeld, D. J. , Schweren, L. J. S. , Ambrosino, S. , Oranje, B. , … Franke, B. (2019). Brain imaging of the cortex in ADHD: A coordinated analysis of large‐scale clinical and population‐based samples. The American Journal of Psychiatry, 176, 531–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jancke, L. (2003). Current methods for cognitive neuroanatomy. In Hugdahl K. (Ed.), Experimental methods in neuropsychology (pp. 197–222). Springer. [Google Scholar]
- Jancke, L. , Schlaug, G. , Huang, Y. , & Steinmetz, H. (1994). Asymmetry of the planum parietale. Neuroreport, 5, 1161–1163. [DOI] [PubMed] [Google Scholar]
- Karlebach, G. , & Francks, C. (2015). Lateralization of gene expression in human language cortex. Cortex, 67, 30–36. [DOI] [PubMed] [Google Scholar]
- Koelkebeck, K. , Miyata, J. , Kubota, M. , Kohl, W. , Son, S. , Fukuyama, H. , Sawamoto, N. , Takahashi, H. , & Murai, T. (2014). The contribution of cortical thickness and surface area to gray matter asymmetries in the healthy human brain. Human Brain Mapping, 35, 6011–6022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong, X. Z. , Mathias, S. R. , Guadalupe, T. , Group, E.L.W , Glahn, D. C. , Franke, B. , Crivello, F. , Tzourio‐Mazoyer, N. , Fisher, S. E. , Thompson, P. M. , & Francks, C. (2018). Mapping cortical brain asymmetry in 17,141 healthy individuals worldwide via the ENIGMA consortium. Proceedings of the National Academy of Sciences of the United States of America, 115, E5154–E5163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong, X. Z. , Postema, M. , Schijven, D. , Castillo, A. C. , Pepe, A. , Crivello, F. , Joliot, M. , Mazoyer, B. , Fisher, S. E. , & Francks, C. (2021). Large‐scale phenomic and genomic analysis of brain asymmetrical skew. Cerebral Cortex, 31, 4151–4168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong, X. Z. , Postema, M. C. , Guadalupe, T. , de Kovel, C. , Boedhoe, P. S. W. , Hoogman, M. , Mathias, S. R. , van Rooij, D. , Schijven, D. , Glahn, D. C. , Medland, S. E. , Jahanshad, N. , Thomopoulos, S. I. , Turner, J. A. , Buitelaar, J. , van Erp, T. G. M. , Franke, B. , Fisher, S. E. , van den Heuvel, O. A. , … Francks, C. (2022). Mapping brain asymmetry in health and disease through the ENIGMA consortium. Human Brain Mapping, 43, 167–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurth, F. , Gaser, C. , & Luders, E. (2015). A 12‐step user guide for analyzing voxel‐wise gray matter asymmetries in statistical parametric mapping (SPM). Nature Protocols, 10, 293–304. [DOI] [PubMed] [Google Scholar]
- Kurth, F. , Jancke, L. , & Luders, E. (2017). Sexual dimorphism of Broca's region: More gray matter in female brains in Brodmann areas 44 and 45. Journal of Neuroscience Research, 95, 626–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurth, F. , Thompson, P. M. , & Luders, E. (2018). Investigating the differential contributions of sex and brain size to gray matter asymmetry. Cortex, 99, 235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehtola, S. J. , Tuulari, J. J. , Karlsson, L. , Parkkola, R. , Merisaari, H. , Saunavaara, J. , Lahdesmaki, T. , Scheinin, N. M. , & Karlsson, H. (2019). Associations of age and sex with brain volumes and asymmetry in 2–5‐week‐old infants. Brain Structure & Function, 224, 501–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeMay, M. (1976). Morphological cerebral asymmetries of modern man, fossil man, and nonhuman primate. Annals of the New York Academy of Sciences, 280, 349–366. [DOI] [PubMed] [Google Scholar]
- Levman, J. , MacDonald, P. , Lim, A. R. , Forgeron, C. , & Takahashi, E. (2017). A pediatric structural MRI analysis of healthy brain development from newborns to young adults. Human Brain Mapping, 38, 5931–5942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, G. , Lin, W. , Gilmore, J. H. , & Shen, D. (2015). Spatial patterns, longitudinal development, and hemispheric asymmetries of cortical thickness in infants from birth to 2 years of age. The Journal of Neuroscience, 35, 9150–9162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, G. , Nie, J. , Wang, L. , Shi, F. , Lyall, A. E. , Lin, W. , Gilmore, J. H. , & Shen, D. (2014). Mapping longitudinal hemispheric structural asymmetries of the human cerebral cortex from birth to 2 years of age. Cerebral Cortex, 24, 1289–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luders, E. , Gaser, C. , Jancke, L. , & Schlaug, G. (2004). A voxel‐based approach to gray matter asymmetries. NeuroImage, 22, 656–664. [DOI] [PubMed] [Google Scholar]
- Luders, E. , Narr, K. L. , Thompson, P. M. , Rex, D. E. , Jancke, L. , & Toga, A. W. (2006). Hemispheric asymmetries in cortical thickness. Cerebral Cortex, 16, 1232–1238. [DOI] [PubMed] [Google Scholar]
- Maingault, S. , Tzourio‐Mazoyer, N. , Mazoyer, B. , & Crivello, F. (2016). Regional correlations between cortical thickness and surface area asymmetries: A surface‐based morphometry study of 250 adults. Neuropsychologia, 93, 350–364. [DOI] [PubMed] [Google Scholar]
- Malik‐Moraleda, S. , Ayyash, D. , Gallee, J. , Affourtit, J. , Hoffmann, M. , Mineroff, Z. , Jouravlev, O. , & Fedorenko, E. (2022). An investigation across 45 languages and 12 language families reveals a universal language network. Nature Neuroscience, 25, 1014–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa, S. , & Cuthill, I. C. (2007). Effect size, confidence interval and statistical significance: A practical guide for biologists. Biological Reviews of the Cambridge Philosophical Society, 82, 591–605. [DOI] [PubMed] [Google Scholar]
- Namburete, A. I. L. , Papiez, B. W. , Fernandes, M. , Wyburd, M. K. , Hesse, L. S. , Moser, F. A. , Ismail, L. C. , Gunier, R. B. , Squier, W. , Ohuma, E. O. , Carvalho, M. , Jaffer, Y. , Gravett, M. , Wu, Q. , Lambert, A. , Winsey, A. , Restrepo‐Mendez, M. C. , Bertino, E. , Purwar, M. , … Kennedy, S. H. (2023). Normative spatiotemporal fetal brain maturation with satisfactory development at 2 years. Nature, 623, 106–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocklenburg, S. , & Gunturkun, O. (2018). The lateralized brain: The neuroscience and evolution of hemispheric asymmetries. Academic Press. [Google Scholar]
- Ocklenburg, S. , Schmitz, J. , Moinfar, Z. , Moser, D. , Klose, R. , Lor, S. , Kunz, G. , Tegenthoff, M. , Faustmann, P. , Francks, C. , Epplen, J. T. , Kumsta, R. , & Gunturkun, O. (2017). Epigenetic regulation of lateralized fetal spinal gene expression underlies hemispheric asymmetries. eLife, 6, e22784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadatou‐Pastou, M. , Ntolka, E. , Schmitz, J. , Martin, M. , Munafo, M. R. , Ocklenburg, S. , & Paracchini, S. (2020). Human handedness: A meta‐analysis. Psychological Bulletin, 146, 481–524. [DOI] [PubMed] [Google Scholar]
- Plessen, K. J. , Hugdahl, K. , Bansal, R. , Hao, X. , & Peterson, B. S. (2014). Sex, age, and cognitive correlates of asymmetries in thickness of the cortical mantle across the life span. The Journal of Neuroscience, 34, 6294–6302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postema, M. C. , van Rooij, D. , Anagnostou, E. , Arango, C. , Auzias, G. , Behrmann, M. , Filho, G. B. , Calderoni, S. , Calvo, R. , Daly, E. , Deruelle, C. , Di Martino, A. , Dinstein, I. , Duran, F. L. S. , Durston, S. , Ecker, C. , Ehrlich, S. , Fair, D. , Fedor, J. , … Francks, C. (2019). Altered structural brain asymmetry in autism spectrum disorder in a study of 54 datasets. Nature Communications, 10, 4958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raja, R. , Na, X. , Glasier, C. M. , Badger, T. M. , Bellando, J. , & Ou, X. (2021). Associations between cortical asymmetry and domain specific cognitive functions in healthy children. Annual International Conference of the IEEE Engineering in Medicine and Biology Society, 2021, 3127–3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosnow, R. L. , & Rosenthal, R. (2003). Effect sizes for experimenting psychologists. Canadian Journal of Experimental Psychology, 57, 221–237. [DOI] [PubMed] [Google Scholar]
- Satterthwaite, T. D. , Elliott, M. A. , Ruparel, K. , Loughead, J. , Prabhakaran, K. , Calkins, M. E. , Hopson, R. , Jackson, C. , Keefe, J. , Riley, M. , Mentch, F. D. , Sleiman, P. , Verma, R. , Davatzikos, C. , Hakonarson, H. , Gur, R. C. , & Gur, R. E. (2014). Neuroimaging of the Philadelphia neurodevelopmental cohort. NeuroImage, 86, 544–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savic, I. (2014). Asymmetry of cerebral gray and white matter and structural volumes in relation to sex hormones and chromosomes. Frontiers in Neuroscience, 8, 329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha, Z. , Pepe, A. , Schijven, D. , Carrión‐Castillo, A. , Roe, J. M. , Westerhausen, R. , Joliot, M. , Fisher, S. E. , Crivello, F. , & Francks, C. (2021). Handedness and its genetic influences are associated with structural asymmetries of the cerebral cortex in 31,864 individuals. Proceedings of the National Academy of Sciences of the United States of America, 118, e2113095118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha, Z. , Schijven, D. , Carrion‐Castillo, A. , Joliot, M. , Mazoyer, B. , Fisher, S. E. , Crivello, F. , & Francks, C. (2021). The genetic architecture of structural left‐right asymmetry of the human brain. Nature Human Behaviour, 5, 1226–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw, P. , Lalonde, F. , Lepage, C. , Rabin, C. , Eckstrand, K. , Sharp, W. , Greenstein, D. , Evans, A. , Giedd, J. N. , & Rapoport, J. (2009). Development of cortical asymmetry in typically developing children and its disruption in attention‐deficit/hyperactivity disorder. Archives of General Psychiatry, 66, 888–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steger, C. , Moatti, C. , Payette, K. , De Silvestro, A. , Nguyen, T. D. , Coraj, S. , Yakoub, N. , Natalucci, G. , Kottke, R. , Tuura, R. , Knirsch, W. , & Jakab, A. (2023). Characterization of dynamic patterns of human fetal to neonatal brain asymmetry with deformation‐based morphometry. Frontiers in Neuroscience, 17, 1252850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takao, H. , Abe, O. , Yamasue, H. , Aoki, S. , Sasaki, H. , Kasai, K. , Yoshioka, N. , & Ohtomo, K. (2011). Gray and white matter asymmetries in healthy individuals aged 21–29 years: A voxel‐based morphometry and diffusion tensor imaging study. Human Brain Mapping, 32, 1762–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toga, A. W. , Narr, K. L. , Thompson, P. M. , & Luders, E. (2009). Brain asymmetry: Evolution. In Squire L. R. (Ed.), Encyclopedia of neuroscience (pp. 303–311). Academic Press. [Google Scholar]
- Toga, A. W. , & Thompson, P. M. (2003). Mapping brain asymmetry. Nature Reviews. Neuroscience, 4, 37–48. [DOI] [PubMed] [Google Scholar]
- Vasung, L. , Rollins, C. K. , Yun, H. J. , Velasco‐Annis, C. , Zhang, J. , Wagstyl, K. , Evans, A. , Warfield, S. K. , Feldman, H. A. , Grant, P. E. , & Gholipour, A. (2020). Quantitative in vivo MRI assessment of structural asymmetries and sexual dimorphism of transient fetal compartments in the human brain. Cerebral Cortex, 30, 1752–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J. , Yang, N. , Liao, W. , Zhang, H. , Yan, C. G. , Zang, Y. F. , & Zuo, X. N. (2015). Dorsal anterior cingulate cortex in typically developing children: Laterality analysis. Developmental Cognitive Neuroscience, 15, 117–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins, K. E. , Paus, T. , Lerch, J. P. , Zijdenbos, A. , Collins, D. L. , Neelin, P. , Taylor, J. , Worsley, K. J. , & Evans, A. C. (2001). Structural asymmetries in the human brain: A voxel‐based statistical analysis of 142 MRI scans. Cerebral Cortex, 11, 868–877. [DOI] [PubMed] [Google Scholar]
- Zhou, D. , Lebel, C. , Evans, A. , & Beaulieu, C. (2013). Cortical thickness asymmetry from childhood to older adulthood. NeuroImage, 83, 66–74. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supplementary figures.
Supplementary Table 1. Demographics of included studies. If studies used more than one scanner or more than one study site, the respective subgroups are listed as different sites.
Supplementary Table 2. Hemispheric asymmetry.
Supplementary Table 3. Change of asymmetries with age.
Supplementary Table 4. Sex differences in asymmetry.
Supplementary Table 5. Sex differences in asymmetry: Follow‐up.
Supplementary Table 6. Sex‐by‐age interactions in asymmetry.
Supplementary Table 7. Hemispheric asymmetry—(right handers).
Supplementary Table 8. Change of asymmetries with age—(right handers).
Supplementary Table 9. Sex differences in asymmetry—(right handers).
Supplementary Table 10. Sex differences in asymmetry: follow‐up—(right handers).
Supplementary Table 11. Sex‐by‐age interactions in asymmetry—(right handers).
Supplementary Table 12. Hemispheric asymmetry—Younger than 12 years of age.
Supplementary Table 13. Change of asymmetries with age—Younger than 12 years of age.
Supplementary Table 14. Sex differences in asymmetry—Younger than 12 years of age.
Supplementary Table 15. Sex differences in asymmetry: Follow‐up—Younger than 12 years of age.
Supplementary Table 16. Sex‐by‐age interactions in asymmetry—Younger than 12 years of age.
Supplementary Table 17. Hemispheric asymmetry—12 years and older.
Supplementary Table 18. Change of asymmetries with age—12 years and older.
Supplementary Table 19. Sex differences in asymmetry—12 years and older.
Supplementary Table 20. Sex differences in asymmetry: Follow‐up—12 years and older.
Supplementary Table 21. Sex‐by‐age interactions in asymmetry—12 years and older.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


