Abstract
COVID-19 brought back to the attention of the scientific community that males are more susceptible to infectious diseases. What is clear for other infections—that sex and gender differences influence both risk of infection and mortality—is not yet fully elucidated for plague, particularly bubonic plague, although this knowledge can help find specific defences against a disease for which a vaccine is not yet available. To address this question, we analysed data on plague from hospitals in different parts of the world since the early eighteenth century, which provide demographic information on individual patients, diagnosis and course of the disease in the pre-antibiotic era. Assuming that the two sexes were equally represented, we observe a worldwide prevalence of male cases hospitalized at any age, a result which seems better explained by gender-biased (thus cultural) behaviours than biological sex-related factors. Conversely, case fatality rates differ among countries and geographic macro-areas, while globally, lethality appears slightly prevalent in young females and older adults (regardless of sex). Logistic regression models confirm that the main risk factor for bubonic plague death was the geographical location of the cases and being older than 50 years, whereas sex only showcased a slight trend.
Keywords: Yersinia pestis, epidemiology, sex-disaggregating selection
1. Introduction
Molecular evidence has shown that Yersinia pestis has affected people in Eurasia since the Neolithic and through the Bronze Age [1–5]. Conventionally, we distinguish three major plague events, called pandemics since they spread over two or more continents, although not continuously: the First Plague Pandemic (sixth and seventh centuries) hit Africa and Eurasia, the Second Plague Pandemic (1346−nineteenth century) spread from Central Asia [6,7] throughout Europe and to sub-Saharan Africa [8], whereas the Third Plague Pandemic, which has its origin in 1772 in the Yunnan region of China, spread all over the world from Hong Kong in 1894 [6,9]. Several ancient DNA studies have demonstrated that Y. pestis was the causative agent of all historic pandemics [10–25]. The ability of Y. pestis to generate three distinct forms of plague (bubonic, septicaemic and pneumonic) was also genetically acquired during its evolution time (reviewed in [26]). The bacillus adapted to distinct mechanisms of transmission mediated by parasites’ bites (bubonic when intradermic inoculation occurs, and septicaemic if Y. pestis enters the bloodstream), or by respiratory droplets (pneumonic plague) (e.g. [6,27]). In the bubonic form of plague, once the bacterium is inoculated intradermally, it should be cleared in the skin by the cells of the innate immune system recruited at the site of the wound [28,29]. From murine models, we know that neutrophils are the first arriving at site within few minutes or hours [30] and are important in this early phase and for the first 1–2 days after inoculation to control the development of bubonic plague possibly by apoptosis [29], whereas the transport of Y. pestis seems to depend on macrophages also recruited at site [30,31]. Using isolated human polymorphonuclear leukocytes incubated at 21°C, Spinner et al. [32] demonstrated that apoptotic neutrophils containing survived Y. pestis can be internalized by macrophages, in which they remain viable and replicate. In fact, Y. pestis is able to neutralize the phagocytosis process in the host’s macrophages (e.g. [27,33–36]) and the transit in the flea’s gut seems to enhance Y. pestis resistance to phagocytosis by macrophages [37]. Within the first 6–12 h, dying macrophages convey and release the bacteria to the next draining lymph node [30], where the pathogens start multiplying extracellularly, after having neutralized the phagocytotic activity of the neutrophils [38,39]. Bacteria multiplying in the lymph node cause the formation of a swollen, painful bubo. The necrosis of the lymph node causes bacteraemia, septicaemia and the production of endotoxins quickly producing shock, disseminated intravascular coagulation, coma and death [40]. Without treatment with antibiotics (introduced no earlier than the late 1940s [41]), the three forms differ in their mortality rate as well, being close to 100% for septicaemic and pneumonic plague and about 40–70% for bubonic plague [6].
Previous studies have tried to address in different populations questions about biological (versus cultural) parameters that might have driven patterns of plague lethality. In particular, sex, age, condition of frailty, as well as environmental factors (reviewed in [42], but also see [43]) have been considered potential biological causes of selective lethality. Sex-related mortality owing to plague has been investigated extensively using bio-archaeological markers on skeletons of plague victims, as well as mortality records (reviewed in [42], but also see [44–49]). All these works, which have reached controversial and non-univocal conclusions, were carried out on singular or multiple archaeo-anthropological and historical datasets. Some of these works have evidenced in early modern records a sex-disaggregating selection against females [50–55], whereas no trend was evidenced from the biological profile of plague victims of different epochs considered together [42]. The only trend discovered in this last complex dataset concerned a prevalence of deaths among individuals 5–10, but mostly 20–35 years of age [42]. The absence of any other detectable biological trend among different plague cemeteries of different epochs let the authors conclude that the differences in sex ratio observed in single graveyards may have been owing to varying compositions of the populations, a hypothesis which is also supported by recent historical work [56,57]. Another possibility is that distinct cultural behaviours of the two genders, with particular reference to their occupational activity (e.g. [57,58]) or gender-based discrimination or inequities (e.g. [55]) may have enhanced the risk of exposition and/or the risk of death for one gender or the other in the population under investigation. Nonetheless, some limitations are intrinsic to studies carried out on skeletal remains of plague victims and might have constrained the informativeness of the dataset itself [42]. In particular, the assumption of causality linking the number of infected and the risk of death, owing to the impossibility of calculating the case fatality rate (CFR, expressed by the percentage of deaths among cases). Moreover, the actual form of plague (bubonic, septicaemic or pneumonic) that caused the death of victims can only be conjectured from archaeological skeletons, as well as from many historical data of the pre-industrial era.
To overcome this constraint, we collected historical clinical data on plague patients from the period 1720 to 1945 from different parts of the world. Compared with earlier data, these modern records contain accurate information about sex and age of individual plague cases, diagnosis of the plague form the patients were suffering from, and their outcome in terms of death or recovery after infection. This dataset enabled us to calculate the CFR of bubonic plague for the two sexes, at different ages and in different countries. Further, having data from different geographical areas and different periods, we could discuss whether biological factors (resulting in homogeneous trends) or cultural factors (resulting in non-homogeneous trends) may have influenced our observations on pathogenicity and lethality of plague. From the analysis of the dataset, we observed that among the hospitalizations, males infected by plague, in particular bubonic plague, were prevalent, a situation that may possibly be more influenced by gender-related cultural reasons—whereas the slightly higher CFR observed in females might tentatively be better explained by biological factors. Local cultural differences may have influenced the great dissimilarities observed in mortality among geographic macro-areas—albeit genetic reasons could not be completely excluded by this study.
2. Results
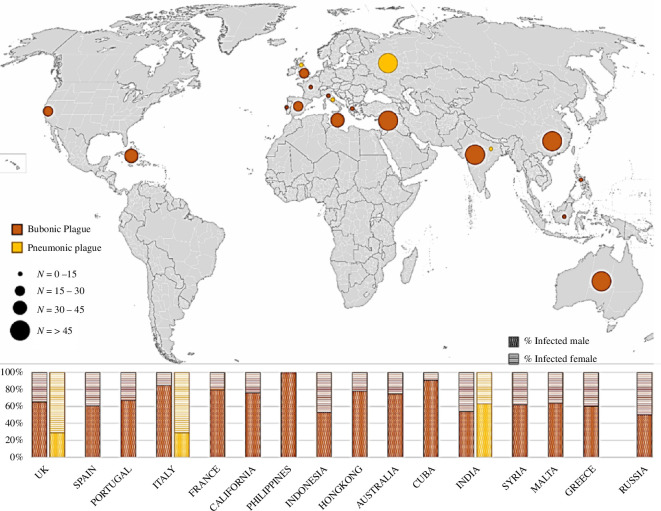
The complete dataset (electronic supplementary material, table S1) contains 1616 cases recorded during the eighteenth−twentieth centuries in hospitals of 17 countries on 5 continents. Of the 1086 male and 530 female cases, the only 100 who were affected and died of septicaemic plague were scattered in different geographical areas, thus not relevant for our purposes. Moreover, as the lethality is very close to 100% [59,60], septicaemic plague cases do not help to distinguish CFRs by sex and age (electronic supplementary material, S1). Septicaemic cases were therefore not considered for further analyses. The spatial distribution of the remaining bubonic and pneumonic cases is given in figure 1, while the relative data are summarized in electronic supplementary material, table S2.
Figure 1.
Spatial distribution and incidence of pneumonic and bubonic clinical plague cases collected from the literature for this study (Second and Third Pandemics). At the bottom of the panel are resumed percentages of cases disaggregated by sex for each country.
We further excluded 362 cases of pneumonic plague from the subsequent analyses on plague lethality, because the probability of dying from pulmonary plague was close to 100% in all countries as well—irrespective of sex and age. The only exception we found (electronic supplementary material, tables S2 and S3) was India during the Third Pandemic, but the number of cases recorded was too small (eight cases and five deaths) for the CFR to be considered representative. In our dataset, pneumonic plague cases were in general underrepresented during the Third Pandemic in comparison with Europe during the Second Pandemic (mainly represented by the outbreak of Vetlianka 1878–1879 [61]).
Consequently, we limited subsequent analyses to only those bubonic plague cases whose diagnosis had been explicitly stated in hospitals’ reports. Based on where the bubonic plague cases were reported, we also divided the data into four geographical macro-areas: Europe and the Mediterranean (which included data from the United Kingdom, Spain, Portugal, Italy, France, Malta and Greece, as well as Syria [61–78]); India [79]; East Asia (which included data from Philippines, Indonesia and Hong Kong [73,80–82]); and Australia [83–85] (table 1). Data from California and Cuba [86–88] (54 cases) were excluded from the subsequent dataset because they were insufficient to represent that of the United States.
Table 1.
Percentual CFR of bubonic plague cases of the Second and Third Pandemics in different geographic macro-areas, subdivided by sex. The prevalent values are indicated in bold.
| cases | deaths | CFR% | p-valuea | |||||
|---|---|---|---|---|---|---|---|---|
| males | females | males | females | males | females | M&F | ||
| Europe II P | 132 | 69 | 73 | 42 | 55.3 | 60.9 | 57.2 | 0.694 |
| East Asia III P | 171 | 50 | 131 | 37 | 76.6 | 74.0 | 76.0 | 0.885 |
| Europe III P | 91 | 26 | 36 | 15 | 39.6 | 57.7 | 43.6 | 0.319 |
| India III P | 117 | 99 | 66 | 68 | 56.4 | 68.7 | 62.0 | 0.371 |
| Australia III P | 259 | 86 | 88 | 22 | 34.0 | 25.6 | 31.9 | 0.290 |
| total | 770 | 330 | 352 | 166 | 45.7 | 50.3 | 47.1 | 0.439 |
| % | 70% | 30% | 51.2% | 55.6% | ||||
Comparison between males and females (chi-squared test).
In table 1, we summarize data and CFRs of all clinical cases of bubonic plague. The global CFR in the period considered is 47.1%, demonstrating that almost half of the hospitalized people affected by bubonic plague died worldwide. However, for the Third Pandemic (i.e. after 1894), the CFRs in the four macro-areas differ considerably, ranging from 31.9% in Australia, 43.6% in Europe, 62.0% in India, up to 76.0% in East Asia. This result showcases that mortality in Australia was extremely low compared with East Asia during the Third Pandemic. In Europe, we observed a reduction in mortality in the Third Pandemic in comparison to the Second Pandemic (43.6% versus 57.2%, respectively), yet the difference is not statistically significant (p = 0.183).
With regard to the data disaggregated by sex, we also observed that the absolute frequencies in all macro-areas showed a prevalence of male cases (770 infected males against 330 infected females were hospitalized, corresponding to 70% males against 30% females). The difference is relevant, especially when assuming a balanced sex ratio in the populations. This confirms the data observed in the histograms in the lower part of figure 1, where we see that the frequency of male cases is always higher than that of females in almost all countries, with the exception of Russia, for which the number of males and females is equivalent. In table 1, we noted that the CFRs differed substantially across the four macro-areas during the Third Pandemic, with a prevalence of female deaths in Europe (57.7% versus 39.6%) and India (68.7% versus 56.4%), and a prevalence of male deaths in Australia (34.0% versus 25.6%) and East Asia (76.6% versus 74.0%). However, the differences between the sexes are not statistically significant in any macro-area as well as in the global sample (table 1). Comparing the CFRs of the two sexes separately in Europe for the two pandemics, we also found no statistical difference (males: p = 0.211; females: p = 0.962). Therefore, we decided to merge the data from the two pandemics in Europe for the subsequent analyses.
Globally, we observed that females tended to die more from bubonic plague than males, albeit the difference is not statistically significant (table 1).
From this reduced dataset of 1100 cases of bubonic plague, with known sex, we further selected only those patients whose individual age was also known (967 individuals). Doing so, the total CFR ratio does not change (50.4% in males and 54.7% in females; table 2) and we were able to disaggregate the dataset into 10-year age groups, to observe whether there was a trend in their respective CFRs (table 2). Data not disaggregated by sex showcase slight differences in lethality among age classes, but for the older adults (50+), who have the highest CFR (61.4%), and for the 10–19 age class, who showed the lowest value of CFR (45.2%). Interestingly, while the CFR of the older adults slightly differs between the sexes (61.7% in males versus 60.9% in females), those of the subadults diverge by sex, albeit not significantly, with prevalent CFRs in females in both classes 0–9 years and 10–19 years.
Table 2.
Subdivision per sex and 10-year age classes of all cases of bubonic plague (Second and Third Pandemics merged). The prevalent CFRs are indicated in bold.
| males | females | total sample | p-valuea | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| cases | deaths | CFR% | cases | deaths | CFR% | cases | deaths | CFR% | ||
| total | 667 | 336 | 50.4 | 300 | 164 | 54.7 | 967 | 500 | 51.7 | 0.560 |
| 50+ | 47 | 29 | 61.7 | 23 | 14 | 60.9 | 70 | 43 | 61.4 | 0.862 |
| 40–49 | 70 | 36 | 51.4 | 45 | 23 | 51.1 | 115 | 59 | 51.3 | 0.885 |
| 30–39 | 118 | 59 | 50.0 | 42 | 22 | 52.4 | 160 | 81 | 50.6 | 0.997 |
| 20–29 | 205 | 115 | 56.1 | 81 | 43 | 53.1 | 286 | 158 | 55.2 | 0.890 |
| 10–19 | 185 | 76 | 41.1 | 78 | 43 | 55.1 | 263 | 119 | 45.2 | 0.254 |
| 0–9 | 42 | 21 | 50.0 | 31 | 19 | 61.3 | 73 | 40 | 54.8 | 0.751 |
Comparison between males and females (chi-squared test).
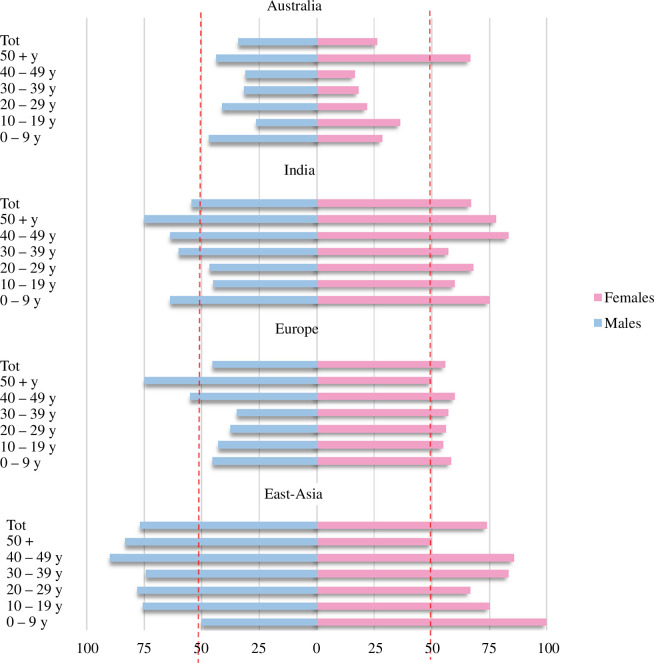
Furthermore, we drew histograms of the CFRs clustered in the 10-year groups for each macro-area (figure 2). The CFR value in Australia and East Asia showed a bias towards males (with the only exception of the 50+ group in Australia and children 0–9 years in East Asia), whereas in India and Europe, the bias is towards females (only exception, the class 50+ in Europe).
Figure 2.
CFRs subdivided for different geographic macro-areas, and by age (10-year groups) and sex (in blue, males; in pink, females) for bubonic plague. Dashed red lines mark the 50% of CFR for females and for males. Data for Europe from the Second and Third Pandemics were merged.
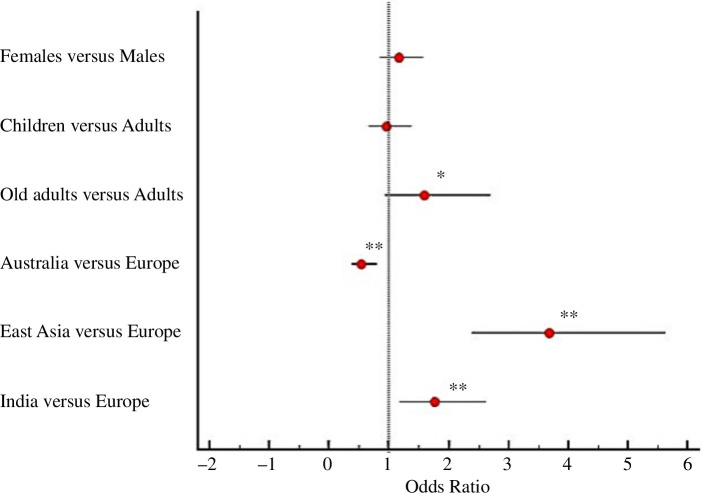
To enhance our understanding of bubonic plague lethality, we estimated the odds ratio (OR) of dying by bubonic plague using a logit model and adding as independent variables sex, age classes and geographic macro-areas. We decided to use as an independent variable the biological age, intended as childhood (0–14 years), adulthood (15–49 years) and ageing (50+), to test the influence of biological and physical changes owing to hormonal fluctuations (electronic supplementary material, table S3). As reported by the logit model (table 3) and the forest plot (figure 3), even if females appear to have slightly higher odds of dying of plague than males, the odds are not significantly different between the two sexes; on the other hand, older adults had significantly higher odds of dying in respect to adults (more than 1.5 times the odds). Moreover, significant ORs are reported for the four geographic macro-areas (table 3). Getting sick in East Asia and India increases the risk of dying compared with Europe (used as a reference), while being affected in Australia decreases the risk of dying. The overall variance explained by the model is 16% (based on Nagelkerke R2). As reported by the Hosmer and Lemeshow test (p > 0.05), the fitted model is adequate. Further attempts considering data only from the Third Pandemic or from the Second and Third Pandemics separately in Europe did not show relevant changes (electronic supplementary material, tables S4 and S5).
Table 3.
Logistic regression model (logit) representing the relative OR of dying by plague in relation to individuals’ biological characteristics and geographical area. The 95% confidence intervals (95% CI) are reported in parentheses.
| variables | OR (95% CI) | p‐value |
|---|---|---|
| sex | ||
| males | 1 (reference) | — |
| females | 1.156 (0.861; 1.553) | 0.335 |
| biological age classes | ||
| children (0−14 years) | 0.950 (0.670, 1.349) | 0.775 |
| adults (15−49 years) | 1 (reference) | — |
| old adults (50+ years) | 1.583 (0.934; 2.683)* | 0.088 |
| geographic area | ||
| Europe (II+III pandemic) | 1 (reference) | — |
| Australia | 0.541 (0.376; 0.779)*** | 0.001 |
| East Asia | 3.675 (2.401; 5.625)*** | <0.001 |
| India | 1.756 (1.183; 2.609)*** | 0.005 |
| R2 Cox and Snell | 0.12 | |
| R2 Nagelkerke | 0.16 | |
| Hosmer and Lemeshow test | ||
| chi-squared | 8.688 | |
| d.f. | 7 | |
| p‐value | 0.276 |
*p < 0.1; **p < 0.05; ***p < 0.01.
Figure 3.
Forest plot displaying the OR of being dead of plague versus being recovered from plague. An OR greater than 1 indicates a higher probability of dying of plague, while an OR less than 1 indicates a lower probability of dying of plague and a higher probability of recovering. Red dots indicate OR values. Horizontal bars represent 95% CI. *p < 0.1; **p < 0.01 (table 3).
3. Discussion
On the occasion of the COVID-19 pandemic, a strong bias towards older people and males was observed worldwide [89], recalling the general rule that males are more susceptible to be infected by pathogens and to have more negative outcomes than females (e.g. [90–95]). Although epidemic-prone infectious diseases like TBC, malaria and leishmaniosis have been proven to follow this general rule (summarized in [96]), no conclusive information has been provided for plague. Medical, anthropological and historical studies have inspected sex or gender differences during plague outbreaks of the past (reviewed in [42]) with contrasting results. Rarely was it possible to reconstruct mortality rates for pre-industrial plague epidemics: one exception is the valuable work of Alfani et al. [57] on the plague of Carmagnola (Italy, 1630), which was accomplished by cross-referencing several historical data to obtain a dataset with indication of sex, age and outcome, albeit without any indication of plague form.
To address the question as to whether to a high number of plague cases corresponds to a high number of deaths in one of the two biological sexes or at a certain age and to discuss the possible causes of the evidence, we collected modern medical plague datasets, which, in comparison to data from skeletons of plague victims and historical registers, provided us with additional information (besides sex and age) about plague form and outcome of the disease for people before the antibiotic era (1720–1945 CE). The data collected from different sources (electronic supplementary material, S1) concerned more than 1600 cases recorded worldwide. The goal of our work was to better understand whether biological or cultural reasons accounted for plague cases and deaths in the different macro-areas.
From an initial dataset, we excluded septicaemic and pneumonic plague and retained only those affected by bubonic plague whose sex, age and outcome were known (967 total cases).
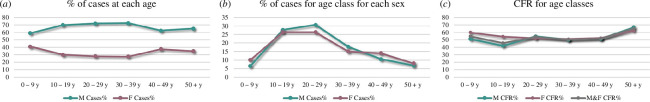
A first interesting finding concerns the prevalence of male individuals infected by plague in general (figure 1) and bubonic plague in particular (figure 4a), worldwide: in our database, 70% of hospitalized cases with evident buboes were male (while 30% were female), with a male prevalence within each macro-area (table 1) and for each age (table 2, figure 4a), with no exception. A similar trend was observed in the second half of the twentieth century in Madagascar, with plague affecting more men (57.1%) than women (42.9%) in a dataset of 20 900 notified cases [97]. Thus, at least for pathogenicity, bubonic plague apparently mirrors the tendency of other epidemic diseases.
Figure 4.
Trends of cases and CFR in both sexes at different ages. (a) Relative frequencies of male and female cases on the total of cases for each age class; (b) for each sex separately, frequencies of cases at each age class; and (c) CFR% of males and females at each age class.
Cultural aspects like gender-specific occupational and social activities are considered as factors that increase the exposure to infections in the review of WHO about sex-specific epidemic-prone diseases [58]. In general (although with exceptions), men are more exposed to infection because they tend to be outside home longer than females. Another reason for a larger number of males in our medical reports may be owing to a form of social discrimination or inequity towards females, who might have had less access to resources (see also [55]) like hospitals and treatments [58]. Females are also more often carer, at both familial and social levels, a condition which could also prevent hospitalization [55,57]. Regarding plague, if cases are not rapidly isolated, human-to-human contagion owing to direct contact with sick bodies or with vectors like fleas or lice may occur, a condition that has been recently re-proposed (e.g. [9,56,98–100]), and women could be infected at the same rate as men, but they remain home.
The observed trend (more male cases worldwide, including Madagascar [96]) might rather suggest that biological factors make the male sex more susceptible to bubonic plague. In fact, gender-specific behaviours and/or gender inequalities are not the only mechanism proposed by the WHO to explain the increased risk of infection for males; biological factors play an equally important role, in general. There are at least two biological mechanisms, which are known to work for infectious diseases. (i) At the genetic level, on the X-chromosome, regions containing immune-related genes are present, which may clear infections at early stages. Two X-chromosomes may provide females with greater plasticity in their immune response against pathogens than males (reviewed in [101]; see also [96]). (ii) The second mechanism is mediated by sexual hormones: testosterone inactivates the immune system, while oestrogen acts as an activator [102,103]. In fact, multiple immune-related cells (including neutrophils [92]) are partially controlled by oestrogen, for which they express ER-α and ER-β receptors (reviewed in [101]). Female neutrophils appear more activated and mature than male ones, owing to hormonal regulation [104], and might therefore provide more defence in the early phases of infection, thus resulting in negative or not identified plague cases. In the case of bubonic plague, a third defence mechanism for females might be owing to reduced compartmental iron content, a circumstance that may make females from adolescence less likely to be infected by bacteria like Y. pestis, which require extracellular iron for their growth and multiplication [105,106]. Notably, the absence of the Yersiniabactin (Ybt) siderophore-dependent system or the Yfe iron transporter attenuate the virulence of Y. pestis in mouse models [107,108]. Hypoferremia in females has long been associated with the menses, yet recent studies [109,110] have demonstrated that oestrogen actively reduces the uptake of iron from the intestine by promoting the production of hepcidin, whereas testosterone inhibits its production. Thus, oestrogen reduces in females the quantity of circulating iron from adolescence through maturity until menopause, perhaps contributing to diminishing female bubonic plague cases.
Observing figure 4a, we can see that, while male frequencies of cases are prevalent on female incidences at each age, female relative frequency of cases decreases in the reproductive period (age classes from 10–19 to 30–39 years) when the effect of hormones and iron combined might account for less susceptibility in women. Yet, comparing the relative frequencies of cases at each age class in both sexes (figure 4b), we do not see any differences: the tendency of females exactly mirrors that of males and shows the same risk of susceptibility for both sexes at any age class, suggesting that in the case of bubonic plague infection, biological differences cannot be responsible for the immune response to the pathogen. More likely, exposure owing to occupational and social activities or gendered behaviours, thus cultural aspects globally makes both sexes more prone to be affected and hospitalized during the age of 10–39 years. Indeed, in Kenya, Mozambique and Tanzania, 1986–2002 [111,112], women and children were reported to be mostly affected by plague. It should be noted that these studies did not differentiate for the form of plague, although septicaemic and pneumonic have different mechanisms and different lethality (in our dataset, for instance, females were more affected than males by pneumonic plague; figure 1). Regardless, the authors attribute this tendency to the habit of women and children sleeping on the ground, thus more in contact with parasites, whereas men sleep in beds [111]. Other examples of contrasting evidence for sex bias in bubonic plague incidence have also been collected by Pollitzer [113], whose authoritative review, which is the most recent work globally considering gender/sex-specific differences for plague, concluded that all sex differences observed for plague cases in different geographical contexts may be attributable to dissimilarities in exposure, thus to occupational, social and other cultural causes. Another cultural reason, unbalanced access to hospitalization by gender, could explain the large difference observed between males and females in our dataset.
Further, the majority of infectious diseases, which show higher susceptibility in males, show a higher mortality risk for males as well (e.g. [114]). Conversely, from our complete dataset of bubonic plague cases, we observe that infected females seem to be more prone to die in comparison to males (CFR 55.6% against 51.2%, data obtained from the sum of all macro-areas; table 1). Although the difference between sexes is not statistically significant, this evidence confirms that females are not more protected than men by their immune system in the course of the disease. This phenomenon is particularly evident in Europe (both Second and Third Pandemics; table 1) and India, but the opposite can be observed in Australia and East Asia. In fact, we observe globally (figure 4c), that females show a maximal CFR during childhood and adolescence when the hormones have not yet reached their peaks (sexual hormones peak in the mid- to late-20s), as expected. Yet, adults do not show sex bias, although the hormonal levels, and thus the protecting effect in females, should be higher. In fact, in (female) individuals with less iron in the extracellular compartment, hepcidin causes retention of iron within the macrophages owing to clearance of red blood cells. Thus, if the infection is not early cleared by neutrophils, it could be that Y. pestis finds in iron-overloaded macrophages a much more suitable environment for its proliferation, thus might develop in a more aggressive form of bubonic plague in females, owing to a higher number of bacteria in the lymph nodes, from adolescence (thus justifying the notable, although not significant, difference in CFR between adolescent males and females). While remaining a hypothesis, the presence of iron overload in the macrophages, which is hepcidin-dependent, should be enhanced under the influence of oestrogen and reduced under the effect of testosterone (thus from the mid-20s years of age). These contrasting effects of the hormones might explain the similar trend observed in male and female CFRs in adulthood.
Another hypothesis is that women of fertile age were admitted to hospitals only if they were pregnant, a condition that might enhance the risk of complications. In our dataset, pregnancy was reported in 12 cases worldwide, albeit only five were considered in our final dataset, of whose both age and outcome were recorded. In fact, there is no reason to hide a pregnancy in a medical report; conversely, the case of a plague-infected pregnant woman is interesting. In a systematic review [115] conducted worldwide on pregnant women between the ages of 18 and 40 infected with plague, the authors found at least 59 eligible papers published from 1897 to 2002, with 160 cases reported in detail, with information on the course and outcome of disease for both mother and fetus/child, also in the pre-antibiotic era.
In figure 4c, we can also see that older people (50+) are mostly prone to die from bubonic plague, regardless of sex, possibly owing to the reduced presence of hormones and to iron-loss. Thus, it could be that mortality owing to bubonic plague is affected by sex- and age-related factors owing to contrasting biological effects, although experimental work is necessary to verify this hypothesis.
Further, we tried to apply statistical models to detect eventual predicting factors of death for bubonic plague. To the best of our knowledge, only two studies [57,97] used logistic regression analysis to infer risk factors of dying for plague. The first one [57] found a prevalence of female cases in historical data from Carmagnola (seventeenth century), whereas the other [97] confirmed the prevalence of male cases at almost each age class previously observed in Madagascar [116,117]. The first work [57] observed a slight disadvantage of males in surviving plague and in individuals at age 41–60, regardless of sex. In the other work [97], neither sex (M versus F 0.98 : 1.00 OR, with 95% CI), nor the adolescent age (children 0–9 versus adolescents 10–19, 1.00 : 0.88 OR and ORa, with 95% CI) were associated with an enhanced risk of dying for plague in Madagascar. Although bubonic plague was prevalent (92.6 %) in the dataset of Migliani et al. [97], no disaggregation for plague form was performed in their logistic regression analysis, whereas in the Alfani et al. [57] dataset, the form of plague was unknown. In addition, in the case of Madagascar, the 4309 cases under investigation, which showed similar mortality rates in males (24.2 %) and females (24.5 %), were collected from 1957 to 2001, and the patients were in part treated with different antibiotic therapies (for plague therapies, effectively started in 1947; see [41]).
In the present work, logistic regression analysis confirmed the evidence that being old enhances the risk to die of bubonic plague (p‐value = 0.088). Apparently, sex does not significantly affect the risk of dying, yet females have a slightly higher probability, which may confirm the previously proposed biological mechanism. Regardless, the only significant risk factors evinced was the geographic macro-areas of infection and treatment, with a significantly higher risk to die from bubonic plague in India and East Asia and a significantly lower one in Australia, in comparison to Europe (Second and Third Pandemics). (We also tested the European Second and Third Pandemics separately, without observing any significant change—electronic supplementary material, table S5.) We cannot exclude that regional dissimilarities are owing to population genetic differences (this point would also deserve further investigation in light of new evidence [118]), but we should consider that the Australian population has a very recent European ancestry (end of the eighteenth century), which cannot explain the discordant results between the two macro-areas. Possibly, there are different cultural behaviours in distinct parts of the world that may influence the risk to die from plague. For instance, women might have been admitted to hospital only at a late stage of the disease, a condition which may have enhanced their probability of dying of bubonic plague and affected our observation of a higher risk for females. However, the benefit of being hospitalized at an earlier stage of bubonic plague in the pre-antibiotic era may only relate to better access to food and water (as proposed by [57]), since we found no evidence of antimicrobial therapies in the reports. Only in the late 1940s was carbolic acid experimentally employed in Hong Kong, although with mixed results [73] (electronic supplementary material, S1). Both in Australia and Europe (Third Pandemic) serum was used, albeit with discordant results: of the 341 total cases in Australia, 248 were treated with serum (Yersin-Roux serum produced by Pasteur Institute in Paris from 1901 to 1904; from 1904 to 1907, the same serum employed was produced by the Lister Institute of Preventive Medicine in London [84]). Table 4 showcases that the use of serum halved the general risk to die for bubonic plague in Australia (total CFR 25% against 51.6%, table 4), working more efficiently in females than in males (CFR 17.2% against 26.8%)—albeit the natural CFR is as well lower in females than in males in Australia (CFR 45.8% against 53.6%). In Europe, the Pasteur serum was employed at least during the outbreak in Taranto 1945 (Italy) [72] along with an antimicrobic therapy on 17 individuals, of whom only 7 survived. To our knowledge, the serum was not employed in the other European countries represented in our dataset.
Table 4.
Bubonic plague patients treated or not with Yersin–Roux serum during the Third Plague Pandemic in Australia. The prevalent CFRs are reported in bold.
| M | F | M&F | |||||||
|---|---|---|---|---|---|---|---|---|---|
| cases | deaths | CFR% | cases | deaths | CFR% | cases | deaths | CFR% | |
| serum | 190 | 51 | 26.8 | 64 | 11 | 17.2 | 248 | 62 | 25.0 |
| no serum | 69 | 37 | 53.6 | 24 | 11 | 45.8 | 93 | 48 | 51.6 |
| total | 259 | 88 | 34.0 | 86 | 22 | 25.6 | 341 | 110 | 32.2 |
Besides treating plague patients with serum, Australia also applied safety measures from the beginning of the plague in Hong Kong in 1894 to prevent the entry of plague through its ports, thereby significantly reducing the number of cases [85]. Government and public health authorities were able to stop the various outbreaks that struck Australia from 1900 to 1925. Similarly, previous work on data from the Third Plague Pandemic in Europe [9,119] had proposed that prevention and control measures, thus cultural behaviours, led to a reduced number of cases and to the end of plague in Europe.
In conclusion, and apparently, cultural differences as well as biological reasons may account for dissimilarities in infection and mortality risk owing to bubonic plague. Although further studies are needed to verify our hypotheses, it is clear that a gender/sex perspective might be of great help in better understanding cultural and biological phenomena behind infectious diseases. With WHO [58], we strongly recommend that data should always be accomplished separately by sex and along with information on age, disease outcome, occupational activity and other elements, which can help to better understand differences in disease course and outbreak evolution.
4. Material and methods
4.1. Material
The database used in this analysis includes individual data of 1616 cases of plague victims and their course. The data include cases of epidemics in several countries of the world from 1720 to 1945 (figure 1). Information was drawn from 33 publications (electronic supplementary material, table S1).
4.2. Data collection
We systematically searched for online publications (using online databases and collections such as Google Scholar, Internet Archive, Wellcome Collection, Google Books) that provided detailed information on the infection and course of plague patients. For our research, we used the following keywords: ‘plague’, ‘third plague pandemic in Europe’, ‘plague in’, ‘medical reports of plague’, ‘clinical data of plague victims’, ‘cases of plague’ in combination with ‘recovery’, ‘healed’, ‘sex’, ‘age’, ‘death’. We extended the research also to publications in different languages, like Italian, French and Spanish. We included all publications in which individual data of plague patients, such as age and sex, were reported and excluded all cases with an uncertain diagnosis of plague and whose outcome of the infection was not known. The 33 publications selected produced data on 1616 individuals from 17 different countries. For this study, we further selected our data to include only individuals infected by bubonic plague (i.e. all cases in which the presence of the bubo was specified), of known sex and age and whose disease course (death or recovery from plague) was indicated, for a total of 1154 cases.
4.3. Statistical analysis
Descriptive statistical analyses (means, standard deviations and frequencies) were performed for all the study variables. The CFR (i.e. percentage of individuals who died divided by diagnosed cases of plague) was calculated for each sex and age classes. Comparisons between categorical data were performed using chi-square test. Multivariate logistic regression model (logit model) analysis was performed to test the association between biological features (sex and age) and geographic macro-areas as categorical predictor variables and death by plague/recovering from plague being the binary outcome. Sex and age were included as categorical variables and age was divided into three age classes: juveniles (0–14 years), adults (15–49 years), and older adults (more than 50 years). Geographic macro-areas considered are Europe (Second and Third Pandemics), East Asia, Australia and India. Male category, middle age class (adults) and Europe were used as reference values. The results were reported as ORs with 95% CI. All the assumptions of the logistic regression were met (a binary dependent variable; no multicollinearity among the independent variables; independence of the observations; the sample size respects the ‘rule of thumb’). The significance of each regressor is assessed by the Wald test and its p‐value. Cox and Snell R2 and Nagelkerke R2 were reported. The validation of the model was performed using the Hosmer–Lemeshow goodness-of-fit test. Participants with missing data were excluded from the analysis.
Values of p < 0.10 were considered statistically significant [48,57]. All statistical analyses were conducted using STATISTICA (version 11, StatSoft, Tulsa, OK).
Acknowledgements
We thank Lars Walløe and Samuel Cohn for their suggestions. We thank the Department of Environmental and Prevention Sciences, University of Ferrara, for the departmental grant to N.Z. and the University Strategic Center for Studies on Gender Medicine, University of Ferrara, for its contribution to the funding of the publication. We are indebted to Giovangualberto Carducci for the historical information on the last plague in Taranto. We also thank two anonymous reviewers for their valuable suggestions.
Contributor Information
N. Rinaldo, Email: natascia.rinaldo@unife.it.
M. C. Manfrinato, Email: mariacristina.manfrinato@unife.it.
B. Bramanti, Email: barbara.bramanti@unife.it.
Ethics
This work did not require ethical approval from a human subject or animal welfare committee.
Data accessibility
Data are reported in the electronic supplementary material [120].
Declaration of AI use
We have not used AI-assisted technologies in creating this article.
Authors’ contributions
J.M.: data curation, formal analysis, writing—original draft; N.Z.: formal analysis, investigation, methodology, writing—original draft; N.R.: formal analysis, investigation, methodology, writing—review and editing; T.B.: conceptualization, funding acquisition, writing—review and editing; M.C.M.: writing—review and editing; Z.D.: methodology, writing—review and editing; R.Y.: methodology, writing—review and editing; N.C.S.: methodology, writing—review and editing; B.B.: conceptualization, data curation, funding acquisition, methodology, project administration, supervision, writing—original draft.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
We declare we have no competing interests.
Funding
No funding has been received for this article.
References
- 1. Rascovan N, Sjögren KG, Kristiansen K, Nielsen R, Willerslev E, Desnues C, Rasmussen S. 2019. Emergence and spread of basal lineages of Yersinia pestis during the Neolithic decline. Cell 176, 295–305. ( 10.1016/j.cell.2018.11.005) [DOI] [PubMed] [Google Scholar]
- 2. Rasmussen S, et al. 2015. Early divergent strains of Yersinia pestis in Eurasia 5,000 years ago. Cell 163, 571–582. ( 10.1016/j.cell.2015.10.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Spyrou MA, et al. 2018. Analysis of 3800-year-old Yersinia pestis genomes suggests Bronze Age origin for bubonic plague. Nat. Commun. 9, 2234. ( 10.1038/s41467-018-04550-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Susat J, et al. 2021. A 5,000-year-old hunter-gatherer already plagued by Yersinia pestis. Cell Rep. 35, 109278. ( 10.1016/j.celrep.2021.109278) [DOI] [PubMed] [Google Scholar]
- 5. Andrades Valtueña A, et al. 2017. The stone age plague and its persistence in Eurasia. Curr. Biol. 27, 3683–3691. ( 10.1016/j.cub.2017.10.025) [DOI] [PubMed] [Google Scholar]
- 6. Bramanti B, Stenseth NC, Walløe L, Lei X. 2016. Plague: a disease which changed the path of human civilization. In Yersinia pestis: retrospective and perspective(eds Yang R, Anisimov A), pp. 1–26. Dordrecht, The Netherlands: Springer. ( 10.1007/978-94-024-0890-4) [DOI] [PubMed] [Google Scholar]
- 7. Spyrou MA, et al. 2022. The source of the black death in fourteenth-century central Eurasia. Nature 606, 718–724. ( 10.1038/s41586-022-04800-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Green HM. 2018. Putting Africa on the black death map: narratives from genetics and history. Afriques 9, 1–46. ( 10.4000/AFRIQUES.2125) [DOI] [Google Scholar]
- 9. Bramanti B, Dean KR, Walløe L, Stenseth NC. 2019. The third plague pandemic in Europe. Proc. R. Soc. B 286, 20182429. ( 10.1098/rspb.2018.2429) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Haensch S, et al. 2010. Distinct clones of Yersinia pestis caused the black death. PLoS Pathog. 6, e1001134. ( 10.1371/journal.ppat.1001134) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bos KI, et al. 2011. A draft genome of Yersinia pestis from victims of the black death. Nature 478, 506–510. ( 10.1038/nature10549) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bos KI, et al. 2016. Eighteenth century Yersinia pestis genomes reveal the long-term persistence of an historical plague focus. Elife 5, e12994. ( 10.7554/eLife.12994) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bramanti B, Wu Y, Yang R, Cui Y, Stenseth NC. 2021. Assessing the origins of the European plagues following the black death: a synthesis of genomic, historical, and ecological information. Proc. Natl Acad. Sci. USA 118, e2101940118. ( 10.1073/pnas.2101940118) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Feldman M, et al. 2016. A high-coverage Yersinia pestis genome from a sixth-century Justinianic plague victim. Mol. Biol. Evol. 33, 2911–2923. ( 10.1093/molbev/msw170) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Guellil M, et al. 2020. A genomic and historical synthesis of plague in 18th century Eurasia. Proc. Natl Acad. Sci. USA 117, 28328–28335. ( 10.1073/pnas.2009677117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Guellil M, Rinaldo N, Zedda N, Kersten O, Gonzalez Muro X, Stenseth NC, Gualdi-Russo E, Bramanti B. 2021. Bioarchaeological insights into the last plague of Imola (1630–1632). Sci. Rep. 11, 22253. ( 10.1038/s41598-021-98214-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Harbeck M, et al. 2013. Yersinia pestis DNA from skeletal remains from the 6th century AD reveals insights into justinianic plague. PLoS. Pathog. 9, e1003349. ( 10.1371/journal.ppat.1003349) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Morozova I, Kasianov A, Bruskin S, Neukamm J, Molak M, Batieva E, Pudło A, Rühli FJ, Schuenemann VJ. 2020. New ancient eastern European Yersinia pestis genomes illuminate the dispersal of plague in Europe. Phil. Trans. R. Soc. B 375, 20190569. ( 10.1098/rstb.2019.0569) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Namouchi A, et al. 2018. Integrative approach using Yersinia pestis genomes to revisit the historical landscape of plague during the medieval period. Proc. Natl Acad. Sci. USA 115, E11790–E11797. ( 10.1073/pnas.1812865115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Spyrou MA, et al. 2019. Phylogeography of the second plague pandemic revealed through analysis of historical Yersinia pestis genomes. Nat. Commun. 10, 4470. ( 10.1038/s41467-019-12154-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Spyrou MA, et al. 2016. Historical Y. pestis genomes reveal the European black death as the source of ancient and modern plague pandemics. Cell Host Microbe 19, 874–881. ( 10.1016/j.chom.2016.05.012) [DOI] [PubMed] [Google Scholar]
- 22. Susat J, Bonczarowska JH, Pētersone-Gordina E, Immel A, Nebel A, Gerhards G, Krause-Kyora B. 2020. Yersinia pestis strains from latvia show depletion of the pla virulence gene at the end of the second plague pandemic. Sci. Rep. 10, 14628. ( 10.1038/s41598-020-71530-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wagner DM, et al. 2014. Yersinia pestis and the plague of Justinian 541-543 AD: a genomic analysis. Lancet Infect. Dis. 14, 319–326. ( 10.1016/S1473-3099(13)70323-2) [DOI] [PubMed] [Google Scholar]
- 24. Bramanti B, Namouchi A, Schmid BV, Dean KR, Stenseth NC. 2019. Reply to Barbieri et al.: out of the land of darkness: plague on the fur trade routes. Proc. Natl Acad. Sci. USA 116, 7622–7623. ( 10.1073/pnas.1902274116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Keller M, et al. 2019. Ancient Yersinia pestis genomes from across Western Europe reveal early diversification during the first pandemic (541–750). Proc. Natl Acad. Sci. USA 116, 12363–12372. ( 10.1073/pnas.1820447116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Demeure CE, Dussurget O, Mas Fiol G, Le Guern AS, Savin C, Pizarro-Cerdá J. 2019. Yersinia pestis and plague: an updated view on evolution, virulence determinants, immune subversion, vaccination, and diagnostics. Genes Immun. 20, 357–370. ( 10.1038/s41435-019-0065-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pradel E, Lemaître N, Merchez M, Ricard I, Reboul A, Dewitte A, Sebbane F. 2014. New insights into how Yersinia pestis adapts to its mammalian host during bubonic plague. PLoS Pathog. 10, e1004029. ( 10.1371/journal.ppat.1004029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Marketon MM, DePaolo RW, DeBord KL, Jabri B, Schneewind O. 2005. Plague bacteria target immune cells during infection. Science 309, 1739–1741. ( 10.1126/science.1114580) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lukaszewski RA, Kenny DJ, Taylor R, Rees DGC, Hartley MG, Oyston PCF. 2005. Pathogenesis of Yersinia pestis infection in BALB/C mice: effects on host macrophages and neutrophils. Infect. Immun. 73, 7142–7150. ( 10.1128/IAI.73.11.7142-7150.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shannon JG, Hasenkrug AM, Dorward DW, Nair V, Carmody AB, Hinnebusch BJ. 2013. Yersinia pestis subverts the dermal neutrophil response in a mouse model of bubonic plague. MBio 4, e00170-13. ( 10.1128/mBio.00170-13) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mecsas J. 2019. Unraveling neutrophil–Yersinia interactions during tissue infection. F1000Res. 8, F1000 Faculty Rev-1046. ( 10.12688/f1000research.18940.1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Spinner JL, Winfree S, Starr T, Shannon JG, Nair V, Steele-Mortimer O, Hinnebusch BJ. 2014. Yersinia pestis survival and replication within human neutrophil phagosomes and uptake of infected neutrophils by macrophages. J. Leukoc. Biol. 95, 389–398. ( 10.1189/jlb.1112551) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Burrows TW, Bacon GA. 1956. The basis of virulence in Pasteurella pestis: the development of resistance to phagocytosis in vitro. Br. J. Exp. Pathol. 37, 286–299. [PMC free article] [PubMed] [Google Scholar]
- 34. Cavanaugh DC, Wheeler CM, Suyemoto W, Shimada T, Yamakawa Y. 1956. Studies on Pasteurella pestis in various flea species I. Infection of flea species with avirulent strains of Past. pestis. J. Infect. Dis. 98, 103–106. ( 10.1093/infdis/98.1.103) [DOI] [PubMed] [Google Scholar]
- 35. Grabenstein JP, Marceau M, Pujol C, Simonet M, Bliska JB. 2004. The response regulator PhoP of Yersinia pseudotuberculosis is important for replication in macrophages and for virulence. Infect. Immun. 72, 4973–4984. ( 10.1128/IAI.72.9.4973-4984.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pujol C, Grabenstein JP, Perry RD, Bliska JB. 2005. Replication of Yersinia pestis in interferon gamma-activated macrophages requires ripA, a gene encoded in the pigmentation locus. Proc. Natl Acad. Sci. USA 102, 12909–12914. ( 10.1073/pnas.0502849102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vadyvaloo V, Jarrett C, Sturdevant DE, Sebbane F, Hinnebusch BJ. 2010. Transit through the flea vector induces a pretransmission innate immunity resistance phenotype in Yersinia pestis. PLoS Pathog. 6, e1000783. ( 10.1371/journal.ppat.1000783) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Derbise A, Hanada Y, Khalifé M, Carniel E, Demeure CE. 2015. Complete protection against pneumonic and bubonic plague after a single oral vaccination. PLoS Negl. Trop. Dis. 9, e0004162. ( 10.1371/journal.pntd.0004162) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sebbane F, Lemaître N, Sturdevant DE, Rebeil R, Virtaneva K, Porcella SF, Hinnebusch BJ. 2006. Adaptive response of Yersinia pestis to extracellular effectors of innate immunity during bubonic plague. Proc. Natl Acad. Sci. USA 103, 11766–11771. ( 10.1073/pnas.0601182103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Titball RW, Leary SEC. 1998. Plague. Br. Med. Bull. 54, 625–633. ( 10.1093/oxfordjournals.bmb.a011715) [DOI] [PubMed] [Google Scholar]
- 41. Butler T. 2014. Plague history: Yersin’s discovery of the causative bacterium in 1894 enabled, in the subsequent century, scientific progress in understanding the disease and the development of treatments and vaccines. Clin. Microbiol. Infect. 20, 202–209. ( 10.1111/1469-0691.12540) [DOI] [PubMed] [Google Scholar]
- 42. Bramanti B, Zedda N, Rinaldo N, Gualdi-Russo E. 2018. A critical review of anthropological studies on skeletons from European plague pits of different epochs. Sci. Rep. 8, 17655. ( 10.1038/s41598-018-36201-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zedda N, Rinaldo N, Gualdi-Russo E, Bramanti B. 2022. Overall frailty gauged in victims of the Italian plague (Imola, 1630-1632): was plague an indiscriminate killer? Archaeol. Anthropol. Sci. 14, 199. ( 10.1007/s12520-022-01670-8) [DOI] [Google Scholar]
- 44. Brennan EJ, DeWitte SN. 2022. Sexual stature difference fluctuations in pre- and post-black death London as an indicator of living standards. Am. J. Hum. Biol. 34, e23783. ( 10.1002/ajhb.23783) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Van KD, Dewitte SN. 2020. Covid-19 and the black death: nutrition, frailty, inequity, and mortality theoretical research in anthropology and the history of medicine. Ital. J. Interdiscip. Health. Soc. Dev. 5, 471–484. ( 10.19204/2020/cvdn3) [DOI] [Google Scholar]
- 46. DeWitte SN. 2014. Differential survival among individuals with active and healed periosteal new bone formation. Int. J. Paleopathol. 7, 38–44. ( 10.1016/j.ijpp.2014.06.001) [DOI] [PubMed] [Google Scholar]
- 47. DeWitte SN. 2009. The effect of sex on risk of mortality during the black death in London, A.D. 1349-1350. Am. J. Phys. Anthropol. 139, 222–234. ( 10.1002/ajpa.20974) [DOI] [PubMed] [Google Scholar]
- 48. Dewitte SN. 2012. Sex differences in periodontal disease in catastrophic and attritional assemblages from medieval London. Am. J. Phys. Anthropol. 149, 405–416. ( 10.1002/ajpa.22138) [DOI] [PubMed] [Google Scholar]
- 49. Dewitte SN, Hughes-Morey G. 2012. Stature and frailty during the black death: the effect of stature on risks of epidemic mortality in London, AD 1348–1350. J. Archaeol. Sci. 39, 1412–1419. ( 10.1016/j.jas.2012.01.019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Curtis DR, Roosen J. 2017. The sex-selective impact of the black death and recurring plagues in the southern Netherlands, 1349-1450. Am. J. Phys. Anthropol. 164, 246–259. ( 10.1002/ajpa.23266) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Frandsen KE. 2010. The last plague in the Baltic region, 1709-1713. Hamburg, Germany: Kovač. [Google Scholar]
- 52. Perez Moreda V. 1987. The plague of 1647-1657 in the western Mediterranean. Bol. Assoc. Demogr. Hist. 5, 14–25. [PubMed] [Google Scholar]
- 53. Signoli M, Séguy I, Biraben JN, Dutour O, Belle P. 2002. Paleodemography and historical demography in the context of an epidemic. Population 57, 829. ( 10.3917/pope.206.0829) [DOI] [Google Scholar]
- 54. Zapnik J. 2007. Pest und Krieg im Ostseeraum, der ‘Schwarze Tod’ in Stralsund während des Großen Nordischen Krieges (1700-1721). Hamburg, Germany: Kovac. [Google Scholar]
- 55. Curtis DR, Han Q. 2021. The female mortality advantage in the seventeenth-century rural low countries. Gend. Hist. 33, 50–74. ( 10.1111/1468-0424.12495) [DOI] [Google Scholar]
- 56. Alfani G, Bonetti M. 2019. A survival analysis of the last great European plagues: the case of Nonantola (northern Italy) in 1630. Popul. Stud. 73, 101–118. ( 10.1080/00324728.2018.1457794) [DOI] [PubMed] [Google Scholar]
- 57. Alfani G, Bonetti M, Fochesato M. 2024. Pandemics and socio-economic status. Evidence from the plague of 1630 in northern Italy. Popul. Stud. 78, 21–42. ( 10.1080/00324728.2023.2197412) [DOI] [PubMed] [Google Scholar]
- 58. World Health Organization . 2007. Addressing sex and gender in epidemic-prone infectious diseases. Geneva, Switzerland: WHO. [Google Scholar]
- 59. Butler T. 2013. Plague gives surprises in the first decade of the 21st century in the United States and worldwide. Am. J. Trop. Med. Hyg. 89, 788–793. ( 10.4269/ajtmh.13-0191) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Roussos D. 2002. Plague. Prim. Care Update Ob. Gyns. 9, 125–128. ( 10.1016/S1068-607X(02)00102-6) [DOI] [Google Scholar]
- 61. Radcliffe JN. 1881. Ninth annual report of the local government board 1879-80. Supplement containing report and papers submitted by the medical officer on the recent progress of the Levantine plague, and on quarantine in the Red Sea. London, UK: HMSO. [Google Scholar]
- 62. Bertrand JB. 1805. A historical relation of the plague at Marseilles in the year 1720. London, UK: Gregg. [Google Scholar]
- 63. Stafford W. 1816. Observations on the plague at Malta. Edinb. Med. Surg. J. 12, 13–21. [PMC free article] [PubMed] [Google Scholar]
- 64. Burrell WH. 1854. Appendix V to the second report on quarantine. In Report of Dr W. H. Burrell on the plague of Malta in 1813. London, UK: HMSO. [Google Scholar]
- 65. Chalmers MD. 1901. Report on certain cases of plague occurring in Glasgow, in 1900 by the medical officer of health. Glasgow, UK: Corporation of Glasgow. [Google Scholar]
- 66. Boyle J, Hope EW. 1901. Plague in Liverpool. Pub. Health Rep. 16, 2684–2686. [Google Scholar]
- 67. Walford E. 1900. Notes on the introduction of a case of plague into the neighbourhood of Cardiff. Br. Med. J. 2, 1232. ( 10.1136/bmj.2.2078.1232) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Coelho C.A. da Cunha. 1900. A Peste do Porto de 1899. Escola medico-cirurgica do porto. [Google Scholar]
- 69. Pellissier J. 1902. La Peste au Frioul, Lazaret de Marseille, en 1900 et 1901. [Google Scholar]
- 70. Cartana Castella P, Collado JG. 1934. Estudio de las ratas y de sus ectoparasitos en ocasion del brote epidemico de peste en Bactelona en 1931 57–63.
- 71. Van Zwanenberg D. 1970. The last epidemic of plague in England? Suffolk 1906–1918. Med. Hist. 14, 63–74. ( 10.1017/s0025727300015143) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Leone A. 2000. Taranto tra guerra e dopoguerra: il minamento della rada di Mar Grande (1943) e l’episodio epidemico di peste bubbonica (1945). Cenacolo XII (XXIV), 149–188. [Google Scholar]
- 73. Thomson JC. 1903. Plague cases treated in the Kennedy town hospital. Government report, Hong Kong. [Google Scholar]
- 74. De Piro GM. 1879. Ragguaglio storico della pestilenza che afflisse le Isole di Malta e Gozo negli anni 1813 e 1814. Malta: Tipografia del Risorgimento. [Google Scholar]
- 75. Privitera S. 1917. La Peste in Catania. Catania. [Google Scholar]
- 76. Faulkner AB. 1820. A treatise on the plague, designed to prove it contagious, from facts, collected during the author’s residence in Malta, when visited by that malady in 1813: with observations on its prevention, character and treatment: to which is annexed an appendix. London, UK: Longman, Hurst, Rees, Orme, and Brown. [Google Scholar]
- 77. Tully JD. 1821. The history of plague: as it has lately appeared in the islands of Malta, Gozo, Corfu, Cephalonia, etc. detailing important facts, illustrative of the specific contagion of that disease, with particulars of the means adopted for its eradication. London, UK: Longman, Hurst, Rees, Orme, and Brown. [Google Scholar]
- 78. Russell P. 1791. A treatise of the plague: containing an historical journal, and medical account, of the plague, at Aleppo, in the years 1760, 1761, and 1762. ( 10.24157/ARC_16891) [DOI] [Google Scholar]
- 79. Gatacre WF. 1898. Report on the bubonic plague in Bombay. The Lancet 151, 250–251. ( 10.1016/S0140-6736(01)97990-5) [DOI] [Google Scholar]
- 80. van Loghem JJ. 1912. Eenige Epidemiologische Gegevens Omtrent de pest op Java Mei-October 1911 [Some epidemiological facts concerning the plague in Java May-October 1911]. Batavia: Javasche Boekhandel and Drukkerij. [Google Scholar]
- 81. Ayres PBC, Lawson JA. 1894. Report on the outbreak of bubonic plague in Hongkong, 1894, to the International Congress of Hygiene and Demography held at Budapest. Hong Kong: China Mail Office. [Google Scholar]
- 82. Goff AP. 1913. Bubonic plague in Manila. J. Am. Med. Assoc. 60, 2042. ( 10.1001/jama.1913.04340260016009) [DOI] [Google Scholar]
- 83. Watson A. 1903. Report on the outbreak of plague at Fremantle. Perth, Australia: Wm. Alfred Watson. [Google Scholar]
- 84. Burnett HB. 1907. Report on plague in Queensland, 1900-1907 (26th February, 1900, to 30th June, 1907). Queensland, Australia: Department of Public Health. [Google Scholar]
- 85. Thompson JA. 1906. On the epidemiology of plague. J. Hyg. 6, 537–569. ( 10.1017/s0022172400003156) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Kellogg WH. 1900. The bubonic plague in San Francisco. J. Am. Med. Assoc. 1280, 1235–1237. ( 10.1001/jama.1900.24610200021001g) [DOI] [Google Scholar]
- 87. Kellogg WH. 1900. Bubonic plague in san francisco: further case reports. J. Am. Med. Assoc. 35, 1280. ( 10.1001/jama.1900.24620460030001n) [DOI] [Google Scholar]
- 88. Guiteras J. 1915. Bubonic plague in Cuba. Havana, Cuba: Department of Sanitation & Charities. [Google Scholar]
- 89. Valenzuela Mayorga A, Silva Jiménez D, Silva-Sá N, Arjona Ramírez LA. 2023. Salud y masculinidades: el efecto de la pandemia y sus inequidades. Est. Avanz. 37, 60–71. ( 10.35588/estudav.v0i37.4935) [DOI] [Google Scholar]
- 90. Fish EN. 2008. The X-files in immunity: sex-based differences predispose immune responses. Nat. Rev. Immunol. 8, 737–744. ( 10.1038/nri2394) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Klein SL, Flanagan KL. 2016. Sex differences in immune responses. Nat. Rev. Immunol. 16, 626–638. ( 10.1038/nri.2016.90) [DOI] [PubMed] [Google Scholar]
- 92. Klein SL, Marriott I, Fish EN. 2015. Sex-based differences in immune function and responses to vaccination. Trans. R. Soc. Trop. Med. Hyg. 109, 9–15. ( 10.1093/trstmh/tru167) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Libert C, Dejager L, Pinheiro I. 2010. The X chromosome in immune functions: when a chromosome makes the difference. Nat. Rev. Immunol. 10, 594–604. ( 10.1038/nri2815) [DOI] [PubMed] [Google Scholar]
- 94. Neyrolles O, Quintana-Murci L. 2009. Sexual inequality in tuberculosis. PLoS Med. 6, e1000199. ( 10.1371/journal.pmed.1000199) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. vom Steeg LG, Klein SL. 2016. SeXX matters in infectious disease pathogenesis. PLoS Pathog. 12, e1005374. ( 10.1371/journal.ppat.1005374) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Gemmati D, Bramanti B, Serino ML, Secchiero P, Zauli G, Tisato V. 2020. COVID-19 and individual genetic susceptibility/receptivity: role of Ace1/Ace2 genes, immunity, inflammation and coagulation. Might the double X-chromosome in females be protective against SARS-Cov-2 compared to the single X-chromosome in males Int. J. Mol. Sci. 21, 3474. ( 10.3390/ijms21103474) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Migliani R, Chanteau S, Rahalison L, Ratsitorahina M, Boutin JP, Ratsifasoamanana L, Roux J. 2006. Epidemiological trends for human plague in Madagascar during the second half of the 20th century: a survey of 20 900 notified cases. Trop. Med. Int. Health. 11, 1228–1237. ( 10.1111/j.1365-3156.2006.01677.x) [DOI] [PubMed] [Google Scholar]
- 98. Dean KR, Krauer F, Schmid BV. 2019. Epidemiology of a bubonic plague outbreak in Glasgow, Scotland in 1900. R. Soc. Open Sci. 6, 181695. ( 10.1098/rsos.181695) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Dean KR, Krauer F, Walløe L, Lingjærde OC, Bramanti B, Stenseth NC, Schmid BV. 2018. Human ectoparasites and the spread of plague in Europe during the second pandemic. Proc. Natl Acad. Sci. USA 115, 1304–1309. ( 10.1073/pnas.1715640115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Bland DM, Long D, Rosenke R, Joseph Hinnebusch B. 2024. Yersinia pestis can infect the Pawlowsky glands of human body lice and be transmitted by louse bite. PLoS Biol. 22, e3002625. ( 10.1371/journal.pbio.3002625) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Schurz H, Salie M, Tromp G, Hoal EG, Kinnear CJ, Möller M. 2019. The X chromosome and sex-specific effects in infectious disease susceptibility. Hum. Genom. 13, 2. ( 10.1186/s40246-018-0185-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Cutolo M, Capellino S, Sulli A, Serioli B, Secchi ME, Villaggio B, Straub RH. 2006. Estrogens and autoimmune diseases. Ann. N. Y. Acad. Sci. 1089, 538–547. ( 10.1196/annals.1386.043) [DOI] [PubMed] [Google Scholar]
- 103. Klein SL. 2012. Sex influences immune responses to viruses, and efficacy of prophylaxis and treatments for viral diseases. Bioessays 34, 1050–1059. ( 10.1002/bies.201200099) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Gupta S, et al. 2020. Sex differences in neutrophil biology modulate response to type I interferons and immunometabolism. Proc. Natl Acad. Sci. USA 117, 16481–16491. ( 10.1073/pnas.2003603117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Chen Y, et al. 2022. Attenuation of Yersinia pestis fyuA mutants caused by iron uptake inhibition and decreased survivability in macrophages. Front. Cell Infect. Microbiol. 12, 874773. ( 10.3389/fcimb.2022.874773) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Nairz M, Haschka D, Demetz E, Weiss G. 2014. Iron at the interface of immunity and infection. Front. Pharmacol. 5, 152. ( 10.3389/fphar.2014.00152) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Iteman I, Guiyoule A, de Almeida AM, Guilvout I, Baranton G, Carniel E. 1993. Relationship between loss of pigmentation and deletion of the chromosomal iron-regulated Irp2 gene in Yersinia pestis: evidence for separate but related events. Infect. Immun. 61, 2717–2722. ( 10.1128/iai.61.6.2717-2722.1993) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Bearden SW, Perry RD. 1999. The Yfe system of Yersinia pestis transports iron and manganese and is required for full virulence of plague. Mol. Microbiol. 32, 403–414. ( 10.1046/j.1365-2958.1999.01360.x) [DOI] [PubMed] [Google Scholar]
- 109. Hou Y, Zhang S, Wang L, Li J, Qu G, He J, Rong H, Ji H, Liu S. 2012. Estrogen regulates iron homeostasis through governing hepatic hepcidin expression via an estrogen response element. Gene 511, 398–403. ( 10.1016/j.gene.2012.09.060) [DOI] [PubMed] [Google Scholar]
- 110. Yang Q, Jian J, Katz S, Abramson SB, Huang X. 2012. 17Β-estradiol inhibits iron hormone hepcidin through an estrogen responsive element half-site. Endocrinology 153, 3170–3178. ( 10.1210/en.2011-2045) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Davis S, Makundi RH, Machang’u RS, Leirs H. 2006. Demographic and spatio-temporal variation in human plague at a persistent focus in Tanzania. Acta Trop. 100, 133–141. ( 10.1016/j.actatropica.2006.10.006) [DOI] [PubMed] [Google Scholar]
- 112. Dennis DT, Gage KL, Gratz NG, Poland JD, Tikhomirov E. 1999. Plague manual: epidemiology, distribution, surveillance and control. Geneva, Switzerland: WHO. [Google Scholar]
- 113. Pollitzer R. 1954. Plague. WHO Monograph Series 22. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 114. Úbeda F, Jansen VAA. 2016. The evolution of sex-specific virulence in infectious diseases. Nat. Commun. 7, 13849. ( 10.1038/ncomms13849) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Fleck-Derderian S, et al. 2020. Plague during pregnancy: a systematic review. Clin. Infect. Dis. 70, S30–S36. ( 10.1093/cid/ciz1228) [DOI] [PubMed] [Google Scholar]
- 116. Brygoo ER. 1966. Epidémiologie de la peste à Madagascar. Arch. Inst. Pasteur Madag. 35, 9–147. [Google Scholar]
- 117. Chanteau S, Ratsitorahina M, Rahalison L, Rasoamanana B, Chan F, Boisier P, Rabeson D, Roux J. 2000. Current epidemiology of human plague in Madagascar. Microbes Infect. 2, 25–31. ( 10.1016/s1286-4579(00)00289-6) [DOI] [PubMed] [Google Scholar]
- 118. Klunk J, et al. 2022. Evolution of immune genes is associated with the black death. Nature 611, 312–319. ( 10.1038/s41586-022-05349-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Stenseth NC, et al. 2022. No evidence for persistent natural plague reservoirs in historical and modern Europe. Proc. Natl Acad. Sci. USA 119, e2209816119. ( 10.1073/pnas.2209816119) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Mongillo J, Zedda N, Rinaldo N, Bellini T, Manfrinato MC, Du Zet al. 2024. Supplementary material from: Differential pathogenicity and lethality of bubonic plague (1720-1945) by sex, age and place. Figshare ( 10.6084/m9.figshare.c.7358190) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are reported in the electronic supplementary material [120].