Abstract
Sex differences are present in psychiatric disorders associated with disrupted dopamine function, and thus, sex differences in dopamine neurobiology may underlie these clinical disparities. In this chapter, we review sex differences in the dopaminergic system with a focus on substance use disorders, especially tobacco smoking, as our exemplar disorder. This chapter is organized into five sections describing sex differences in the dopaminergic system: (1) neurobiology, (2) role of sex hormones, (3) genetic underpinnings, (4) cognitive function, and (5) influence on addiction. In each section, we provide an overview of the topic area, summarize sex differences identified to date, highlight addiction research, especially clinical neuroimaging studies, and suggest avenues for future research.
INTRODUCTION
Dopamine is a monoamine, like serotonin and noradrenaline, that was first synthesized in 1910 (Fahn, 2008). However, dopamine wasn’t discovered in the human brain until 1957 (Montagu, 1957; Weil-Malherbe and Bone, 1957) and wasn’t recognized as a neurotransmitter until 1958 (Carlsson et al., 1958). In the brain, dopamine is implicated in processes that range from motor movement to reward. Disrupted dopaminergic signaling is central to neurologic conditions (i.e., Parkinson’s disease) and psychiatric disorders (i.e., addiction, depression, and schizophrenia). In this chapter, we describe sex differences in dopamine neurobiology, the influence of sex hormones on dopamine neurobiology, genetic underpinnings of dopamine sex differences, and the influence of dopamine sex differences on cognitive function and substance use disorders (SUDs).
Why study sex differences in dopamine?
The National Institutes of Health (NIH) Revitalization Act of 1993 mandated and established guidelines for including women and minorities in clinical research. In 1994, NIH mandated that women be included in NIH-funded clinical research. Prior to this mandate, women were often excluded from clinical trials, and sex was ignored in many research studies, even among studies specifically focused on women’s health (Zakiniaeiz et al., 2016). Since 1994, the number of manuscripts describing sex differences has increased more than six-fold. Now, as of January 2016, every NIH grant submitted must consider sex in “research designs, analyses, and reporting in vertebrate animal and human studies.” (NOT-OD-15–102: https://grants.nih.gov/grants/guide/notice-files/NOT-OD-15-102.html). Before summarizing the literature, we must first define sex and gender. Sex is a person’s biologic status, typically categorized as male, female, or intersex as determined by a number of indicators such as sex chromosomes, gonads, internal reproductive organs, and external genitalia. Gender pertains to the attitudes, feelings, and behaviors associated with a person’s biologic sex as reflected in social, cultural, and psychologic traits (American Psychological Association, 2012). We recognize clinical research is often limited to the research subject’s self-reported gender, which may not always reflect one’s biologic sex, but the focus of this chapter is the role of sex as a biologic variable.
Sex differences are present in psychiatric disorders associated with disrupted dopamine function, and thus, sex differences in dopamine neurobiology may underlie these clinical disparities. Throughout this chapter, we will refer to SUDs, especially tobacco smoking, as our exemplar disorder. Dopamine is known to play a central role in SUDs via reward and incentive motivation. Sex differences exist throughout the lifecycle of addiction—from age of initial drug experimentation to treatment response—for nearly all drugs of abuse. Men are more likely to report experimenting with drugs than women, across all drugs of abuse (Mental and Health Services Administration, 2017; Zakiniaeiz and Potenza, 2018). Yet, women progress from experimentation to dependence more rapidly than men (Hernandez-Avila et al., 2004), the so-called “telescoping effect,” and consequently, women often present to treatment with more severe clinical profiles (Greenfield et al., 2010). The clinical importance of these biologic differences is highlighted by treatment research. Women experience more barriers to treatment entry, more social stigma in treatment, and are more likely to relapse than men (reviewed elsewhere: Becker and Hu, 2008; Tuchman, 2010; Becker et al., 2017; Zakiniaeiz and Potenza, 2018). These disparities highlight the tremendous need to develop and implement sex-specific treatments among SUDs.
This chapter is organized into five sections describing sex differences in the dopaminergic system: (1) neurobiology, (2) role of sex hormones, (3) genetic underpinnings, (4) cognitive function, and (5) influence on addiction. In each section, we provide an overview of the topic area, summarize sex differences identified to date, highlight addiction research, especially clinical neuroimaging studies, and suggest avenues for future research.
DOPAMINE NEUROBIOLOGY
In the context of addiction, dopamine neurobiology can be divided into three systems: nigrostriatal, mesolimbic, and mesocortical (Fig. 9.1). In the nigrostriatal system, cell bodies of dopaminergic neurons emanate from the substantia nigra (SN) and project primarily to the striatal targets. The mesolimbic and mesocortical systems both emanate from the ventral tegmental area (VTA) in the ventral midbrain. As their names suggest, mesolimbic dopamine neurons project from VTA primarily to subcortical limbic targets and mesocortical neurons project primarily to cortical targets. Dopaminergic projections for all three systems are predominantly ipsilateral; fewer than 5% of projections are contralateral; and none are bilateral (Loughlin and Fallon, 1982; Fallon, 1988).
Fig. 9.1.
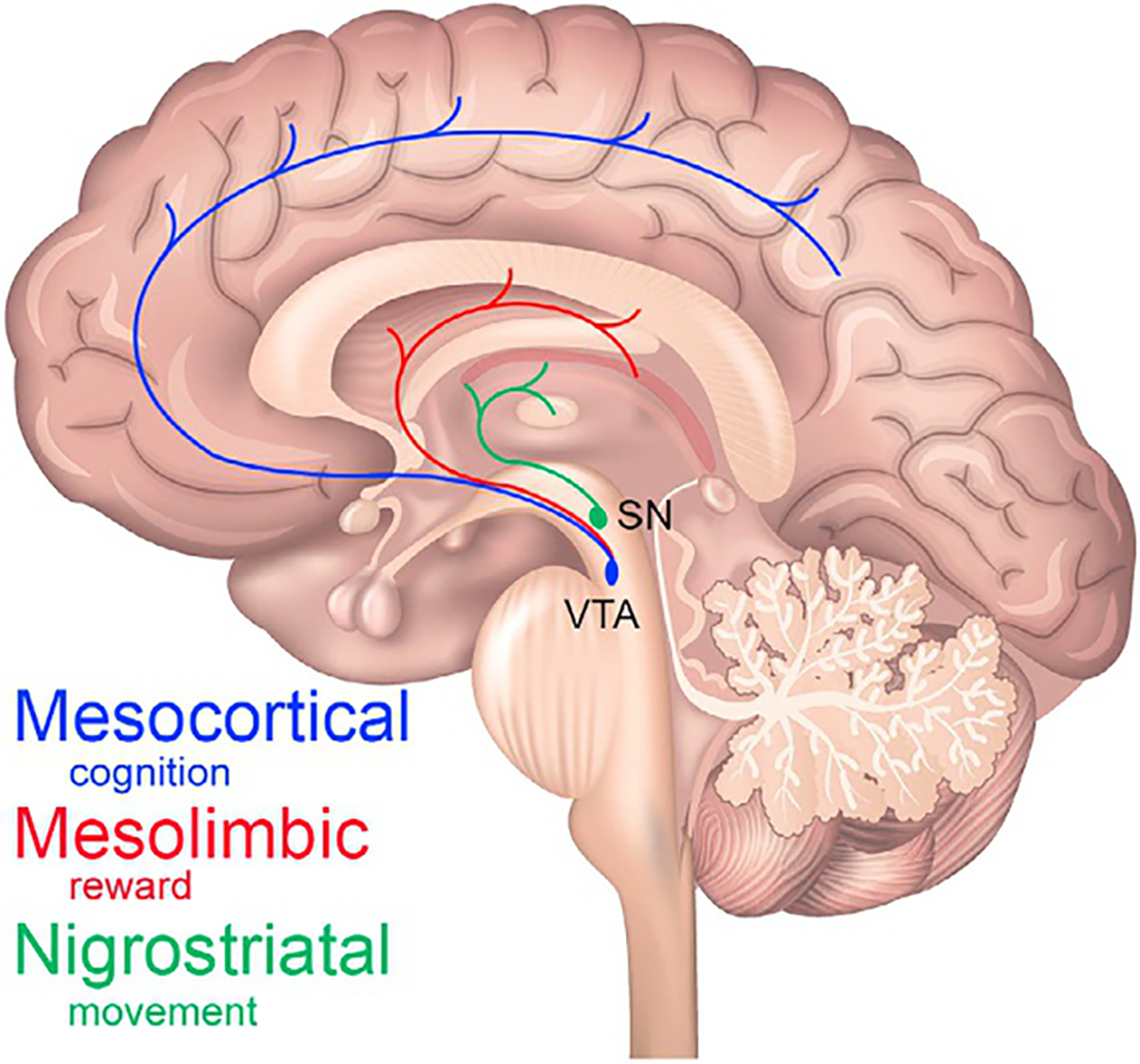
Dopamine systems. A cartoon depiction of the three dopamine systems on a midline sagittal slice. The nigrostriatal system (green) emanates from the substantia nigra (SN) and projects throughout the striatum. The mesolimbic (red) and mesocortical (blue) systems emanate from the ventral tegmental area (VTA) and project throughout the striatum and cortex, respectively. The prefrontal cortex receives most of the mesocortical dopamine projections.
Historically, each dopamine system has been associated with one or two primary functions: the nigrostriatal system with motor movement; the mesolimbic system with reward and incentive motivation; and the mesocortical with cognitive function. This is an oversimplification. Recent research suggests these systems overlap both anatomically and functionally. For example, there is evidence that the nigrostriatal system can mediate reward learning in the absence of mesocorticolimbic inputs (Wise, 2009). Thus, the dopamine system nomenclature should be thought of as shorthand and not an indication of three distinct systems.
In the brain, there are two types of chemical transmission: fast and slow synaptic transmission (Greengard, 2001). Fast synaptic transmission is mediated by ion channels and is roughly half excitatory (glutamatergic) and half inhibitory (GABAergic). Conversely, slow synaptic transmission is far more complex with 150+ neurotransmitter species (Greengard, 2001); of which, dopamine is one. Dopamine neurons are relatively sparse, composing 0.001% of all neurons (or fewer); yet they have considerable influence on brain function and psychiatric health (Greengard, 2001). Upon release from a presynaptic vesicle, dopamine molecules will bind either presynaptic auto-receptors, the dopamine transporter (DAT; located on either presynaptic neurons or glial cells), or postsynaptic receptors (Fig. 9.2). There are five dopamine receptor subtypes known—dopamine 1 receptor (D1R) through D5R—as well as trace amine-associated receptor 1 (TAAR 1) that modulates dopaminergic activity, all of which are seven-transmembrane G-protein coupled metabotropic receptors. Dopamine is a neuromodulator; thus, dopamine neurotransmission can be either excitatory or inhibitory depending on the nature of the postsynaptic neuron. According to the conventional model, tonic dopaminergic signaling is defined as slow and stable firing (<8Hz) that changes gradually (Volkow et al., 2017). Tonic signaling is associated primarily with D2R stimulation and determines arousal and sensitivity to external stimuli (Bromberg-Martin et al., 2010; Dreyer et al., 2010; Danjo et al., 2014). Conversely, phasic dopaminergic signaling is rapid (>15Hz) and transient (<500ms) (Volkow et al., 2017). Phasic dopamine signaling results in high concentrations of extrasynaptic dopamine, which can stimulate lower-affinity receptors (e.g., D1R and D3R) in addition to the higher-affinity D2R. Phasic dopamine signaling is associated with reinforcement, memory consolidation, and coordinated motor movements (Wise, 2004; Volkow et al., 2017). Again, this is an oversimplified view of the dopaminergic system that cannot explain subtleties and exceptions. Nevertheless, this conceptualization is helpful for understanding normative dopamine neurotransmission.
Fig. 9.2.
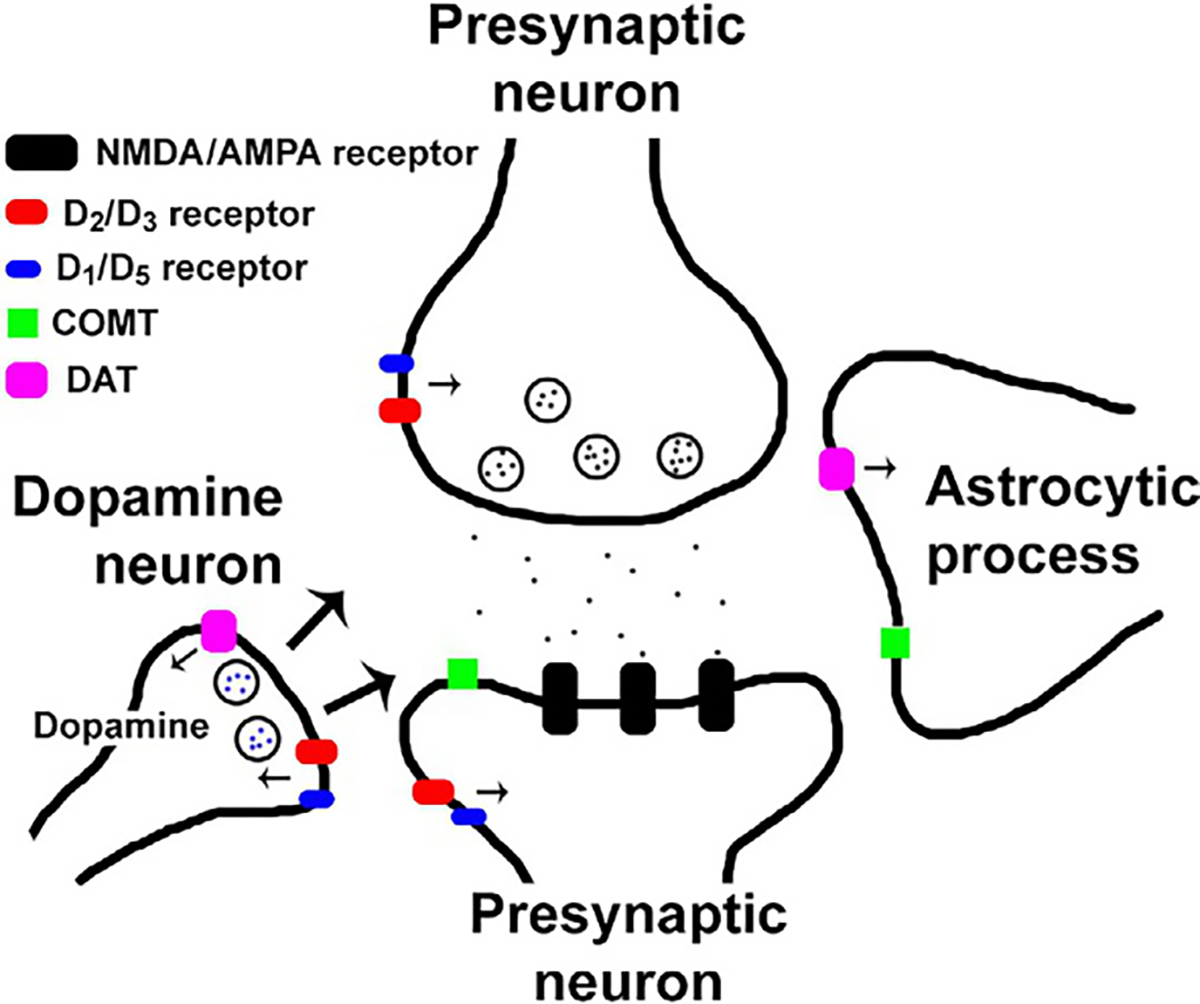
Dopamine synapse. A cartoon depiction of a modulatory dopaminergic synapse. Once dopamine is released in the synapse (arrows depict possible dopamine pathways), molecules can bind D1 or D5 receptors (blue), D2 or D3 receptors (red), the dopamine transporter (DAT; pink), or COMT (catabolized; green).
Investigating dopamine neurobiology in humans
In humans, there are two approaches for studying the dopamine system in vivo: positron emission tomography (PET) and single-photon emission computed tomography (SPECT) imaging. Briefly, PET imaging involves labeling a molecule with a radioactive isotope, typically either carbon-11 [11C] or fluorine-18 [18F] and injecting that radiotracer into research subjects. As the radiotracer decays, positrons collide with nearby electrons and emit two gamma rays in opposite directions. The gamma rays are detected by the PET scanner (coincidence detection) and reconstruction algorithms determine the location of the annihilation event so that we can infer the location of the molecule of interest (Morris et al., 2013). Thus, PET imaging provides a quantitative map of the available sites for radiotracer binding. Similarly, SPECT imaging involves the injection of a radioactive pharmaceutical and measurement of gamma radiation. However, SPECT directly measures gamma rays emitted from the decaying radiotracer using detectors that rotate around the sample, e.g., a subject’s head. Relative to PET imaging, SPECT has poorer spatial and temporal resolution. Clinical SPECT imaging spatial resolution is 8–12mm while clinical PET imaging yields 4–6 mm resolution. Head-only PET scanners can achieve even higher spatial resolution: 2–3 mm. The temporal resolution of both SPECT and PET imaging is on the order of minutes.
In studying the dopamine system, there are numerous radiotracers (or ligands) that broadly fall into one of two categories; striatal-only and extrastriatal (Table 9.1). Due to radiotracer properties, which dictates signal to background contrast levels, some radiotracers exhibit measurable specific binding only in certain brain regions, e.g., striatum. Conversely, extrastriatal tracers can be used to image specific binding outside of the striatum. PET and SPECT imaging are powerful imaging modalities; yet they have limitations. First, each is limited to the availability of radiotracers. If a radiotracer for a target does not yet exist, in vivo study of that target in humans is not possible. Second, few radiotracers are selective for only one receptor subtype, e.g., [11C]raclopride, binds both dopamine D2R and D3R. Third, PET and SPECT exhibit limited temporal resolution. Even with the advent of novel analytic approaches, e.g., lp-ntPET (Morris et al., 2005, 2008; Normandin and Morris, 2006), studying dopaminergic function is limited to a temporal resolution on the order of minutes. Fourth, functional dopamine studies are limited to indirect measurement of dopamine neurotransmission. A common experimental approach to measuring dopamine neurotransmission is to scan subjects before and after a pharmacologic challenge known to evoke dopamine release, e.g., amphetamine, and quantify radiotracer blocking. Dopamine release is inferred by the magnitude of radiotracer blocking due to competition with endogenous dopamine evoked by the challenge.
Table 9.1.
PET and SPECT radiotracers for imaging the dopamine system
| Ligand | Modality | Dopamine target | Bram region |
|---|---|---|---|
|
| |||
| [11C]Raclopride | PET | D2/3 receptor | Striatal |
| [11C]SCH-23390 | PET | D1/5 receptor | Striatal |
| [18F]Fallypride | PET | D2/3 receptor | Striatal/extrastriatal |
| [11C]FLB-457 | PET | D2/3 receptor | Extrastriatal |
| [18F]Fluorodopa | PET | DA synthesis capacity | Striatal/extrastriatal |
| [11C]β-CFT | PET | Dopamine transporter | Striatal |
| [123I]IBZM | SPECT | D2/3 receptor | Striatal |
| [123I]β-CIT | SPECT | Dopamine transporter | Striatal/extrastriatal |
| [123I]FP-CIT | SPECT | Dopamine transporter | Striatal/extrastriatal |
| [99mTc]TRODAT-1 | SPECT | Dopamine transporter | Striatal |
Sex differences in dopamine neurobiology
To identify prior sex differences research, we searched PubMed before March 30th, 2019 using the combination: “dopamine” and (“sex differences” or “gender differences”) and (“PET” or “SPET” or “SPECT”). Follow-up searches were conducted using Google Scholar. PET and SPECT studies that investigated sex effects in dopamine neurobiology and included healthy volunteers are summarized in the section that follows and in Tables 9.2 and 9.3.
Table 9.2.
Sex differences in dopamine neurobiology
| Dopamine target | Modality | Ligand | Sample (M/F) | ROI/s | Finding/s | Menstrual cycle | Sex hormones | Reference |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| D2/3R availability | PET | [11C]Raclopride | 33/21 | Striatum | F ~ M | No | No | Pohjalainen et al. (1998) |
| PET | [11C]Raclopride | 28/15 | Striatum | F ~ M | Yes | Yes | Munro et al. (2006) | |
| PET | [11C]Raclopride | 12/12 | Striatum, Thal | F ~ M | No | No | Haltia et al. (2007) | |
| PET | [11C]Raclopride | 11/10 | Striatum | F > M(pC) | No | No | Urban et al. (2010) | |
| PET | [11C]Raclopride | 100/81 | Striatum | F > M | No | No | Nevalainen et al. (2015) | |
| PET | [11C]Raclopride | 27/18 | Striatum | M > F (dP, dC) | Yes | Yes | Oswald et al. (2015) | |
| PET | [18F]Fallypride | 9/9 | Striatum | F ~ M | No | No | Brown et al. (2012) | |
| PET | [18F]Fallypride | 10/9 | Midbrain | F ~ M | No | No | Okita et al. (2016) | |
| PET | [18F]Fallypride | 21/21 | Striatum, IFG | F ~ M | Yes | Yes | Smith et al. (2019) | |
| PET | [11C]FLB-457 | 12/12 | FC, TC, Thal | F > M(FC) | No | No | Kaasinen et al. (2001) | |
| SPECT | [123I]epidepride | 13/7 | FC, TC, Thal | M > F (FC) | No | No | Glenthoj et al. (2006) | |
| D2/3R density | PET | [11C]Raclopride | 10/10 | Striatum | F ~ M | No | No | Farde et al. (1995) |
| PET | [11C]Raclopride | 33/21 | Striatum | F ~ M | No | No | Pohjalainen et al. (1998) | |
| PET | [11C]NMSP | 12/5 | Striatum | F ~ M | No | No | Pearlson et al. (1993) | |
| DAT availability | PET | [11C]β-CFT | 11/20 | Striatum | F>M | No | No | Wong et al. (2012) |
| SPECT | [123I]β-CIT | 14/14 | Striatum | F ~ M | No | No | van Dyck et al. (1995) | |
| SPECT | [123I]β-CIT | 23/16 | Striatum | F ~ M | No | No | Kuikka et al. (1997) | |
| SPECT | [123I]β-CIT | 70/56 | Str, DE | F ~ M | No | No | van Dyck et al. (2000) | |
| SPECT | [123I]β-CIT | 9/12 | Str, DE | F>M | No | No | Staley et al. (2001) | |
| SPECT | [123I]β-CIT | 15/8 | Str, FC, TC, PC, Thal | F ~ M | No | No | Ryding et al. (2004) | |
| SPECT | [123I]β-CIT | 70/52 | Str, DE | F ~ M | No | No | Best et al. (2005) | |
| SPECT | [123I]β-CIT | 31/48 | Str, midbrain, Thal | F ~ M | No | No | Burke et al. (2011) | |
| SPECT | [123I]FP-CIT | 23/22 | Striatum | F>M | No | No | Lavalaye et al. (2000) | |
| SPECT | [123I]FP-CIT | 74/65 | Striatum | F>M | No | No | Varrone et al. (2013) | |
| SPECT | [123I]FP-CIT | 18/33 | Str, PFC, OFC | F>M | No | No | Eusebio et al. (2012) | |
| SPECT | [123I]FP-CIT | 20/18 | Striatum | F ~ M | No | No | Mo et al. (2010) | |
| SPECT | [123I]FP-CIT | 57/46 | Str, midbrain, Thal | F > M (Thal) | No | No | Koch et al. (2014) | |
| SPECT | [123I]FP-CIT | 17/13 | Striatum | F > M | No | No | Yamamoto et al. (2017) | |
| SPECT | [99mTc]TRODAT-1 | 30/36 | Striatum | F > M (C) | No | No | Mozley et al. (2001) | |
| SPECT | [99mTc]TRODAT-1 | 10/10 | Striatum | F ~ M | No | No | Hsiao et al. (2013) | |
| DA synthesis capacity | PET | [18F]Fluorodopa | 13/10 | Str, PFC, midbrain | M > F (PFC); F > M (Str) | No | No | Ernst et al. (1998) |
| PET | [18F]Fluorodopa | 23/12 | Striatum | F>M | No | No | Laakso et al. (2002) | |
Note: “>” indicates significant sex differences (P < 0.05). “~” indicates sex differences are not significant (P > 0.05).
Abbreviations: C = caudate; d = dorsal; DE = diencephalon; F = female; FC = frontal cortex; IFG=inferior frontal gyrus; M = male; OFC = orbitofrontal cortex; p = posterior; P = putamen; PC = parietal cortex; PFC = prefrontal cortex; Ref=reference Str = striatum; TC = temporal cortex; Thal=thalamus.
Table 9.3.
Sex differences in evoked dopamine release
| Dopamine stimulus | Dose | Modality | Ligand | Sample (M/F) | ROI/s | Finding/s | Menstrual cycle | Sex hormones | Reference |
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Amphetamine | 0.3 mg/kg, IV | PET | [11C]Raclopride | 28/15 | Striatum | M>F | Yes | Yes | Munro et al. (2006) |
| Glucose | 300 mg/kg, IV | PET | [11C]Raclopride | 12/12 | Striatum, Thal | M>F | No | No | Haltia et al. (2007) |
| Glucose expectancy | N/A | PET | [11C]Raclopride | 12/12 | Striatum | M>F (VS) | No | No | Haltia et al. (2008) |
| Alcohol | 0.75g/kg water, oral | PET | [11C]Raclopride | 11/10 | Striatum | M>F | No | No | Urban et al. (2010) |
| Monetary reward | N/A | PET | [11C]Raclopride | 12/12 | Striatum | F>M (dP) | No | No | Martin-Soelch et al. (2011) |
| Amphetamine | 0.3 mg/kg, IV | PET | [11C]Raclopride | 27/18 | Striatum | M>F | Yes | Yes | Oswald et al. (2015) |
| Amphetamine | 0.43 mg/kg, oral | PET | [18F]Fallypride | 7/6 | Str, Thal, Amg, IFG | F>M (GP, IFG) | No | No | Riccardi et al. (2006) |
| Amphetamine | 0.43 mg/kg, oral | PET | [18F]Fallypride | 21/21 | Striatum, IFG | M>F (VS) | Yes | Yes | Smith et al. (2019) |
Note: “>”indicates significant sex differences (P < 0.05).
Abbreviations: Amg = amgydala; d = dorsal; F = female; GP = globus pallidus; IFG = inferior frontal gyrus; IV=intravenous; M = male; P=putamen; Ref=reference Str = striatum; Thal = thalamus; VS = ventral striatum.
Dopamine D2/3R levels
First, we identified 11 studies that reported sex effects among dopamine D2/3R availability, i.e., binding potential (BP), among healthy volunteers. Higher striatal dopamine D2/3R availability was reported among females, compared to males in two studies (Nevalainen et al., 2015; Urban et al., 2010). In the frontal cortex, one PET study reported higher dopamine D2/3R availability among females than among males (Kaasinen et al., 2001), whereas one SPECT study reported the opposite finding (Glenthoj et al., 2006). Most studies, 6 of the 11 identified, reported no significant differences in dopamine D2/3R availability between males and females across regions of interest (ROIs), including the striatum, thalamus, midbrain, and inferior frontal gyrus (IFG) (Pohjalainen et al., 1998; Munro et al., 2006; Haltia et al., 2007; Brown et al., 2012; Okita et al., 2016; Smith et al., 2019). Additionally, three studies estimated dopamine D2/3R density, i.e., Bmax, using Scatchard analyses. In theory, receptor density is a more accurate measure than receptor availability due to potential variability in endogenous dopamine levels. All three studies reported that striatal dopamine D2/3R density did not differ between males and females (Pearlson et al., 1993; Farde et al., 1995; Pohjalainen et al., 1998). Thus, the limited evidence to date suggests there are no sex differences in striatal dopamine D2/3R availability or density among healthy volunteers. Outside of the striatum, too few studies have been published to determine if sex differences exist.
Briefly, before discussing other findings, it is important to consider biologic variables that may influence, or even mediate, sex differences in dopamine neurobiology, i.e., sex hormone levels. The preclinical literature reliably indicates sex hormone levels influence dopamine neurobiology (reviewed: Becker, 1999; Becker and Hu, 2008; Becker et al., 2017) and may underpin human sex differences. Yet, sex hormone levels are often not measured or reported in clinical neuroimaging studies. Among the 14 studies described earlier, only 3 (Munro et al., 2006; Oswald et al., 2015; Smith et al., 2019) reported estradiol and/or progesterone levels. While none found linear relationships between plasma sex hormone levels and dopamine neurobiology, it is advised that future studies measure plasma sex hormone levels to explore possible relationships. If plasma sex hormone levels cannot be measured, menstrual cycle phase is a reasonable surrogate.
DAT availability
Second, we identified 16 studies that investigated sex effects in DAT availability. Of those 16 studies, 8 reported no sex differences in DAT availability across ROIs, including striatum, diencephalon, midbrain, thalamus, and frontal, temporal, and parietal cortices (van Dyck et al., 1995, 2000; Kuikka et al., 1997; Ryding et al., 2004; Best et al., 2005; Mo et al., 2010; Burke et al., 2011; Hsiao et al., 2013). These SPECT studies used several radiotracers and analytic approaches (including different reference regions) but found no sex differences in DAT availability among healthy volunteers. Three studies were especially well-powered to detect effects with more than 50 males and 50 females in each study (van Dyck et al., 2000; Best et al., 2005; Burke etal.,2011) and found no differences. The remaining eight studies (Lavalaye et al., 2000; Mozley et al., 2001; Staley et al., 2001; Eusebio et al., 2012; Wong et al., 2012; Varrone et al., 2013; Koch et al., 2014; Yamamoto et al., 2017) each found that females exhibited significantly higher DAT availability than males across several ROIs, including striatum, diencephalon, and prefrontal and orbitofrontal cortex (PFC and OFC, respectively). Yet, the same caveats described earlier also apply here, as none of these studies reported menstrual cycle phase or plasma sex hormone levels on scan day. Another caveat to consider is tracer selectivity. Most of these studies used tracers, e.g., [123I]β-CIT, known to bind to both the serotonin transporter and DATs (Brücke et al., 1993). Several investigators attempted to isolate DAT signal using a priori anatomical and temporal information (Ryding et al., 2004); yet it remains unclear the extent to which off-target binding influenced DAT findings. In summary, these studies suggest there may be sex differences in DAT availability, but more research is needed. Future studies should measure and report menstrual cycle phase and sex hormone levels, and use second-generation radiotracers that exhibit greater selectivity for the DAT.
Dopamine synthesis capacity
Third, two studies used [18F]fluorodopa to quantify an index of presynaptic dopamine synthesis capacity. Laakso et al. (2002) reported uptake (Ki) from 10–60min after tracer injection using Patlak analysis (Patlak et al., 1983; Patlak and Blasberg, 1985) and an arterial input function. Ernst et al. (1998) reported the ratio of binding in ROIs to the occipital cortex from 90–120min after tracer injection. Both studies found higher striatal [18F]fluorodopa levels in females than males, which may reflect structural and/or functional differences (Ernst et al., 1998; Laakso et al., 2002). DOPA decarboxylase is the rate-limiting enzymatic step for [18F]-labeled dopamine synthesis from [18F]fluorodopa (Gjedde et al., 1991, 1993). Thus, higher [18F]fluorodopa levels may reflect more presynaptic dopamine synapses and/or faster enzymatic activity. Again, the aforementioned findings are not without caveats. Neither study reported menstrual cycle phase or sex hormone levels—which may have contributed to the observed sex differences.
Evoked dopamine release
Fourth, we identified eight studies that evaluated sex differences in evoked dopamine release among healthy volunteers (Table 9.3). Six of the eight studies reported that men exhibited greater evoked dopamine release than women in the striatum, especially ventral striatum (Munro et al., 2006; Haltia et al., 2007; Haltia et al., 2008; Urban et al., 2010; Oswald et al., 2015; Smith et al., 2019), following amphetamine, alcohol, and glucose challenges, as well as glucose expectancy (no administration). Among the two studies that found females exhibited greater dopamine release than men, differences were observed in the dorsal putamen, globus pallidus, and IFG (Riccardi et al., 2006; Martin-Soelch et al., 2011). Unfortunately, neither study reported menstrual cycle phase or sex hormone levels. Nonetheless, the limited extant evidence suggests that men exhibit greater evoked dopamine release than women, especially in the ventral striatum. Preclinical findings (described in Section Dopamine and Sex Hormones) indicate evoked dopamine release may be modulated by estradiol, which may explain these findings. Women may exhibit greater evoked dopamine release than men in dorsal brain regions, e.g., IFG and dorsal putamen, but more research is needed to confirm these findings.
In summary, there is not compelling evidence of sex differences in dopamine D2/3R availability/density in the striatum of healthy volunteers. There is some evidence suggesting possible sex differences in DAT availability in the striatum. Findings thus far suggest females may exhibit greater DAT availability than males, but future research is needed to confirm these findings. Finally, there is consistent evidence indicating men exhibit greater evoked dopamine release in the striatum, especially ventral striatum, than women. This sex difference may have important implications for psychiatric disorders, especially SUDs. The subjective rewarding properties of abused substances are associated with dopamine release in the ventral striatum (reviewed: Volkow and Morales, 2015). Thus, if healthy, nondependent males exhibit greater evoked dopamine release in the ventral striatum than females, men may experience greater subjective effects from drug experimentation. Amplified subjective drug effects may explain, or contribute to, the higher prevalence of drug experimentation and abuse among men. We will discuss this in greater detail in Section Dopamine and Addiction.
DOPAMINE AND SEX HORMONES
Sex hormones, especially testosterone, estrogen, and progesterone, may mediate sex-specific effects of drug motivation, reward, and behavior. Along with significant changes in sex hormone levels during developmental phases, such as during puberty, major hormonal transition periods occur throughout the month and throughout the lifespan, especially for women. For example, estrogen levels rise during puberty, are high during pregnancy, rapidly fall postpartum, continue to decline during perimenopause, and remain low postmenopausal. Interestingly, the rise in estrogen levels parallels with a heightened vulnerability to drugs of abuse (Calipari et al., 2017), while progesterone reduces a drug’s rewarding effects (Lynch and Sofuoglu, 2010), both of which are mediated by the brain’s dopamine system. In the section that follows, we discuss the role of each sex hormone, individually, drawing primarily from preclinical findings, and conclude this section by describing major hormone transition periods for females: menstrual cycle phases and menopause. It is important to note that our focus is on the influence of fluctuating levels of sex hormones on addiction processes and not on physical development or maturation processes.
Testosterone
With respect to testosterone, the primary androgen steroid hormone, preclinical studies have shown mixed results. Castration of male mice around 30 days postnatal, 1 month later, decreased expression of tyrosine hydroxylase (TH)—the rate-limiting enzyme for dopamine production (Raab et al., 1995) —and thus, decreased striatal dopamine, which was recovered after administration of a testosterone metabolite. However, if adult mice are castrated at 2 or 3 months postnatal, effects on dopamine are absent (Khasnavis et al., 2013). Adolescent mice castrated at 45 days postnatal and treated with testosterone for the following 2 weeks showed an increase in gene expression of DAT, vesicular monoamine transporter-2 (VMAT-2), catechol-o-methyltransferase (COMT), and monoamine oxidase (MAO) in the substantia nigra (Purves-Tyson et al., 2012, 2014). In a study of adult male rats, striatal dopamine release increased following castration (Dluzen and Ramirez, 1989). In a nonhuman primate study, prepubescent gonadectomized vs intact male macaques did not show differences in TH expression levels; however, testosterone and TH levels in intact monkeys were correlated with subcortical dopamine levels (Morris et al., 2010). According to preclinical literature, testosterone’s effect on dopamine, if any, might be dependent on time of castration and time of hormone replacement.
Some human and animal studies suggest that testosterone may have reinforcing effects that impact the mesolimbic dopamine system. Animal studies have shown that rodents will self-administer testosterone orally (Johnson and Wood, 2001) and through direct infusion into nucleus accumbens (Packard et al., 1997) and exhibit conditioned place preference for locations associated with testosterone administration (Frye et al., 2002). Human studies have shown that high levels of testosterone are associated with reward sensitivity and risk-taking (Coates and Herbert, 2008; Sapienza et al., 2009). The widespread abuse of testosterone in body building (and structurally similar androgen steroids) suggests that testosterone may have reinforcing properties and might regulate incentive sensitivity (Frye, 2007; Wood, 2008). One study showed that females given exogenous testosterone administration relative to placebo exhibited increased ventral striatal functional magnetic resonance imaging blood oxygen level-dependent (fMRI-BOLD) response during a reward anticipation task, suggesting that testosterone acts on the mesolimbic dopamine pathway (Hermans et al., 2010). The neurobiologic mechanisms by which testosterone might modulate dopaminergic circuits remain to be established.
Estrogen
Ovarian hormones—specifically estrogen and progesterone—may also impact dopamine signaling. Estrogens, such as estradiol, have been shown to have neuroprotective effects on cells including dopamine cells. For example, estradiol increases neuronal survival after induced damage and attenuates the amount of dopamine depletion in the striatum of mice when injected before lesions (see Dluzen, 2000; Picazo et al., 2003 for review). Estrogen also appears to inhibit DAT function, an important protein in dopaminergic neurotransmission, by decreasing the affinity of the transporter. It has been postulated that this might prevent neurotoxic agents from entering dopamine nerve terminals, thereby decreasing nigrostriatal neurodegeneration (Dluzen, 2000), which might explain the gender difference in neurodegenerative disease prevalence. In studies of ovariectomized rats compared to sham-operated controls, DAT expression in the nucleus accumbens was reduced, (Chavez et al., 2010), immunoreactivity of TH positive neurons in midbrain (SN and VTA) was reduced (Johnson et al., 2010), and conditioned place preference response to amphetamine administration was reduced (Silverman and Koenig, 2007). The effects of ovariectomy on the latter two were restored with estrogen replacement.
Estrogen receptors are found throughout the dopamine circuit (Creutz and Kritzer, 2004) and are involved in motivation (Becker, 2009), reward (Justice and de Wit, 1999; Becker and Hu, 2008), and inhibitory control (Colzato et al., 2010). With respect to motivation, in vivo microdialysis studies in castrated and ovariectomized rats showed that males have two times higher basal extracellular concentrations of dopamine in striatum than females, and that this varies with estrous cycle (Xiao and Becker, 1994). However, in vivo voltammetry in intact female rats treated with cocaine or haloperidol show a greater increase in electrical stimulation evoked extracellular dopamine (Walker et al., 1999). These findings suggest that while basal dopamine levels are higher in male than female rats, stimulated dopamine release levels are estradiol-modulated in females but not in males. Becker et al. also showed that amphetamine-induced dopamine release in striatum is sex and hormone dependent (Becker and Ramirez, 1981) both in vivo using microdialysis (Becker, 1990a) and in vitro using striatal rat tissue (Becker, 1990b); ovariectomized rats showed a smaller dopamine release than castrated rats. Following estradiol treatment, amphetamine-induced dopamine response in females is restored to levels greater than castrated rats (Castner et al., 1993).
In humans, studies by Justice and de Wit have shown that subjective rewarding responses to stimulants vary by menstrual cycle (Justice and de Wit, 1999, 2000a,b) (see Section “Menstrual Cycle Phase”). Amphetamine-induced euphoria, desire, energy, and cognitive efficiency are enhanced during the follicular phase (low but rising estradiol, low progesterone levels) relative to luteal phase (moderate estradiol, high progesterone levels). Estradiol administration during follicular phase further increases these subjective effects (Justice and de Wit, 2000b), suggesting that estradiol enhances these subjective rewarding effects. With respect to inhibitory control, estradiol levels have been associated with longer reaction time in motor response inhibition (Colzato et al., 2010), poorer selective attention using the Stroop task (Hatta and Nagaya, 2009), and a bias in decision-making toward smaller, more accessible rewards (Uban et al., 2011). Taken together, these findings suggest that high estrogen levels might make women more susceptible to drugs of abuse via enhanced dopamine mesolimbic responses (Calipari et al., 2017) and diminished inhibitory control.
Progesterone
Progesterone receptors are found throughout the brain’s dopamine circuit. Progesterone’s fluctuating levels in females, and relatively constant levels in males, might explain sex differences in reward and reinforcement-related behaviors. Just as estrogen may have neuroprotective effects on cells, progesterone (and its metabolites) may induce or inhibit neoplastic changes (Baulieu and Schumacher, 2000; Mueller and Kerschbaum, 2006) and may have beneficial effects on cognitive functioning following brain injury (Ma et al., 2012). Progesterone metabolites can act as neurosteroids modulating dopaminergic neurons through activation of GABAA receptors (Frye and Walf, 2008). Because GABAA receptors exhibit inhibitory effects, progesterone’s modulatory effects on GABA have been proposed to attenuate drug reward. Most preclinical work in this area has only been conducted with cocaine administration; so it is uncertain whether progesterone’s effects on drug reward are generalizable to other drugs. Human PET studies have only provided indirect evidence that ovarian hormones may influence striatal dopamine receptor availability.
Animal studies have shown that cocaine self-administration decreased during diestrus (follicular phase; low but rising estradiol, low progesterone levels), compared to estrus (moderate progesterone, low estradiol levels) (Roberts et al., 1989). However, other studies have shown that progesterone attenuated amphetamine-induced conditioned place preference (Russo et al., 2008), cocaine-seeking was lowest when progesterone levels were highest (Feltenstein and See, 2007), and amphetamine-induced striatal dopamine release was lowest when progesterone levels were highest (Becker and Ramirez, 1981). In humans, salivary progesterone levels in women, but not in men, were negatively associated with subjective psychomotor responses to stimulant drugs (White, 2002). One PET study showed that D2R availability was lower in the putamen during the luteal phase, compared to the follicular phase (Munro et al., 2006). Progesterone administration during the follicular phase attenuated subjective response to repeated self-administration in women but not men (Sofuoglu et al., 2002; Evans and Foltin, 2005). Progesterone treatment given concurrently with estradiol treatment also counteracted the dopamine-mediated drug enhancing effects of estradiol on cocaine self-administration in rats (Jackson et al., 2005). Similar results were found with progesterone treatment in humans—positive subjective effects of cocaine were attenuated but only in female cocaine users (Evans and Foltin, 2005). Whereas estradiol is a key factor in amplifying reinforcement, progesterone is a key factor in attenuating reinforcement. Progesterone is currently being examined as a treatment for cocaine addiction in several human studies and clinical trials.
Sex hormone influence of dopamine function is difficult to disentangle as it depends on age and sex of the individual, sex hormones levels, receptor levels, interactions between sex hormones, and modulation by other neurotransmitter systems. Literature on testosterone and the dopamine system has reported mixed findings; however, high estrogen levels may increase dopamine-mediated vulnerability to drugs of abuse, while high progesterone levels may reduce rewarding drug effects.
Menstrual cycle phase
Sex hormone levels fluctuate throughout the menstrual cycle among premenopausal women, and thus, the influence of sex hormones on dopamine neurobiology may fluctuate as well. Early in the follicular phase (days 1–7 after the onset of menses), progesterone and estradiol levels are low and stable. Conversely, in the late follicular phase (days 8–13 after menses), estradiol levels increase rapidly, whereas progesterone levels remain low until ovulation. Both progesterone and estradiol levels are high throughout early luteal phase (days 15–23 after menses) but decrease gradually throughout the remainder of the cycle.
In humans, there are mixed findings regarding the influence of menstrual cycle phase on dopamine D2/3R availability (Table 9.4). Two studies reported menstrual cycle effects. Munro et al. (2006) reported higher striatal dopamine D2/3R availability among females (n = 9) scanned during their follicular phase, compared to those in their luteal phase (n = 6). Among six healthy females, Wong et al. (1988) reported faster caudate dopamine D2/3R binding rate (k3) in the follicular phase, compared to the luteal phase. Conversely, menstrual cycle phase had no effect on striatal dopamine D2/3R availability in two studies (Nordström et al., 1998; Oswald et al., 2015) and striatal DAT availability in one study (Best et al., 2005). Two studies examined the influence of the menstrual cycle phase on amphetamine-induced dopamine release, and neither found a significant relationship (Munro et al., 2006; Oswald et al., 2015). Finally, no linear relationships between sex hormone levels and dopamine D2/3R availability (Kaasinen et al., 2002; Munro et al., 2006), DAT availability (Best et al., 2005), or evoked dopamine release have been reported (Munro et al., 2006; Smith et al., 2019). In summary, there is limited evidence that menstrual cycle phase influences dopamine neurobiology in humans. However, these studies were inadequately sized and most used a between-subject design, which reduces sensitivity to detect effects. Within-subject designs are better suited to investigate potential menstrual cycle effects (Wong et al., 1988).
Table 9.4.
Influence of sex hormones and menstrual cycle phase
| Dopamine target | Modality | Ligand | Sample | ROI/s | Finding/s | Reference |
|---|---|---|---|---|---|---|
|
| ||||||
| D2/3R binding rate | PET | [11C]NMSP | 6F | Caudate | Follicular > luteal | Wong et al. (1988) |
| D2/3R availability | PET | [11C]Raclopride | 5F | Putamen | Follicular ~ luteal | Nordstrom et al. (1998) |
| PET | [11C]FLB-457 | 37F | FC, TC, Thal | No correlation with sex hormone levels | Kaasinen et al. (2002) | |
| PET | [11C]Raclopride | 15F | Striatum | Follicular > luteal (P); No correlation with sex hormone levels | Munro et al. (2006) | |
| PET | [11C]Raclopride | 18F | Striatum | Follicular ~ luteal | Oswald et al. (2015) | |
| DAT availability | SPECT | [123I]β-CIT | 10F | Str, DE | Follicular ~ luteal; no correlation with sex hormone levels | Best et al. (2005) |
| AMPH-evoked DA release | PET | [11C]Raclopride | 15F | Striatum | Follicular ~ luteal; no correlation with sex hormone levels | Munro et al. (2006) |
| PET | [11C]Raclopride | 18F | Striatum | Follicular ~ luteal | Oswald et al. (2015) | |
| PET | [18F]Fallypride | 21F | Str, IFG | No correlation with sex hormone levels | Smith et al. (2019) | |
Note: “>”indicates significant sex differences (P <0.05). “~” indicates sex differences are not significant (P >0.05).
Abbreviations: DE = diencephalon; F = female; FC = frontal cortex; IFG = inferior frontal gyrus; P = putamen; Ref=reference; Str = striatum; TC=temporal cortex; Thal = thalamus.
Menopause
Menopause is often defined as the absence of menses for ≥12months in women (with a uterus) who are not pregnant or lactating and typically occurs during the fifth decade of life. Menopause is associated with significantly lower and less variable estradiol and progesterone levels. Thus, premenopausal women may exhibit different dopamine neurobiology than postmenopausal women. Indeed, there is evidence to suggest this may be the case. Yamamoto et al. (2017) found significantly higher DAT availability among Japanese females aged 60–79 years relative to Japanese males aged 60–79 years. Menopausal status was not described in this study, but it is reasonable to assume most, if not all, females examined were postmenopausal. These findings indicate postmenopausal women exhibit higher DAT availability than age- and ethnicity-matched men, suggesting possible menopause effects. Menopausal status should be considered in future studies.
DOPAMINE AND GENETICS
Sex chromosomes and gene expression influence sex differences in dopamine synthesis, catabolism/degradation (MAO and COMT), neurotransmission (VMAT2), and function/regulation (DRDs and DAT). Emerging literature has shown that sex differences in the dopamine system stem from the X and Y chromosomes—the male-determining gene called Sry found only on the Y chromosome is expressed in dopamine neurons in substantia nigra. Specifically, the Sry gene regulates the rate-limiting dopamine synthesis enzyme TH (Levitt et al., 1965), which is not found in females (see Ngun et al., 2011 for review). Preclinical studies have shown that TH is regulated by estrogen in females (see Section Dopamine and Sex Hormones), suggesting that dopamine synthesis regulation is sexually dimorphic. Females also have 20% fewer dopaminergic neurons in the substantia nigra, which has been attributed to the Sry gene (Carruth et al., 2002; Dewing et al., 2006). Independent of sex, COMT (dopamine degradation) activity in PFC is influenced by a single nucleotide polymorphism (SNP) at codon 158 that makes COMT more thermolabile (in Met158 vs Val158), and therefore, extracellular dopamine in PFC is ~40% higher in Met158 homozygotes than Val158 homozygotes (Chen et al., 2004). Independent of genetic SNP, women have lower COMT activity than men in dorsolateral (dl)PFC postmortem human tissue (Chen et al., 2004).
Hormonal differences between males and females also inform our understanding of the relationship between dopamine function and genetics. In the hypothalamus, TH expression increases when progesterone receptors are blocked in rats (González-Flores et al., 2011), whereas in VTA, TH expression is repressed in progesterone receptor knockout mice, compared to wild type (Woolley et al., 2006). In ovariectomized rats compared to intact rats, D2R density is increased by ~25% in the nucleus accumbens and caudate nucleus, and DAT expression is reduced by 44% in the nucleus accumbens, both of which are reversed by estrogen (Chavez et al., 2010). DAT expression is reduced in hypothalamic dopaminergic neurons of ovariectomized rats. While MAO levels vary throughout the estrous cycle in rats, progesterone can also reduce the expression of MAO isoforms (Gundlah et al., 2002). In substantia nigra and the nucleus accumbens, VMAT2 expression decreases in response to progesterone treatment in ovariectomized rats (Rehavi et al., 1998).
Genetic differences can influence sex differences in symptomatology and disease progression via altered cognitive functioning. Preclinical literature has shown that the Sry gene affects the development of habit-driven behavior in mice. Independent of sex and circulating sex steroid hormone, XX mice developed habitual behavior more rapidly than XY mice (Quinn et al., 2007), suggesting a potential explanation for the “telescoping effect” in females. Estradiol also interacts with COMT in Val158 carriers to modulate delay discounting (monetary temporal reward choice) performance (Smith et al., 2014). A recent study assessed the relationship between risk performance and a composite gene score of dopamine signaling function calculated as the sum of risk alleles at functional polymorphic loci of the following genes: DRD2, DRD3, DRD4, DAT1, and COMT. This study showed that the negative association between risky decision-making in the dlPFC and composite “dopamine gene functioning” score is stronger in males than females (Kohno et al., 2015). More research on sex differences in dopamine neurobiology and cognitive function is needed (see Table 9.5 for summary).
Table 9.5.
Sex differences in dopamine-related genes. Biologic and psychologic/behavioral effect of dopamine-related genetic sex difference
| Biologic effect |
Psychologic effect |
||||
|---|---|---|---|---|---|
| Gene | Function | Males | Females | Males | Females |
|
| |||||
| Sry | Y-chromosome male-determining gene | Regulates TH (dopamine synthesis) | Slower development of habit-forming behavior | ||
| COMT | Dopamine regulation | ↓ Activity in dlPFC | Composite DRD, DAT, COMT “gene score” function related to dlFPC modulation during risky decision-making | Estrogen/estradiol interact with COMT SNPs to effect working memory/delay, discounting, respectively | |
| DRDs | Dopamine receptor function | ↑ D2/3R density | |||
| DAT1 | Dopamine reuptake | Estrogen ↓ DAT1 expression | |||
| MAO | Dopamine catabolism | Progesterone ↓ MAO expression | |||
| VMAT2 | Dopamine neurotransmission | Progesterone ↓ VMAT2 expression | |||
DOPAMINE AND COGNITION
Experimental evidence of dopamine’s role in cognitive function dates back to at least the 1970s. Brozoski et al. (1979) experimentally depleted dopamine in nonhuman primates and observed significant working memory deficits. Dopamine agonists reversed these deficits which implicated dopamine in working memory processes (Brozoski et al., 1979). From that seminal work, we now know dopamine neurotransmission is involved in numerous cognitive functions, including perception of painful stimuli (Hagelberg et al., 2002; Potvin et al., 2009), attention (Nieoullon, 2002; Dang et al., 2012), incentive motivation (Volkow et al., 2007; Volkow et al., 2009; Volkow et al., 2017), working memory (Sawaguchi and Goldman-Rakic, 1991; Luciana and Collins, 1997; Cools et al., 2008; Cools and D’Esposito, 2011), episodic memory (Schott et al., 2006; Lisman et al., 2011; Chowdhury et al., 2012), executive control (Montague et al., 2004; Volkow et al., 2011; Sofuoglu et al., 2013), and decision-making (van Gaalen et al., 2006; St Onge and Floresco, 2009; Linnet et al., 2011a,b; Oswald et al., 2015). A comprehensive review of the role of dopamine in cognitive function is beyond the scope of this chapter but other excellent reviews have been published (Nieoullon, 2002; Pillon et al., 2003; Cools, 2008; Volkow et al., 2017). Herein, we focus on PET/SPECT imaging studies that have examined sex differences in dopamine neurobiology and cognitive function among healthy volunteers.
To identify prior research, we searched PubMed before April 5th, 2019 using the combination: “dopamine” and (“sex differences” or “sex effects” or “gender differences” or “gender effects”) and (“PET” or “SPET” or “SPECT”) and (“cognitive” or “decision-making” or “learning” or “memory” or “inhibit*” or “attention” or “impulsiv*”). Follow-up searches were conducted using Google Scholar. Only PET or SPECT studies that investigated sex effects in dopamine neurobiology and cognitive function among healthy volunteers are summarized in Table 9.6.
Table 9.6.
Sex differences in dopamine and cognitive function
| Reference | Sample (M/F) | ROI/s | Dopamine target | DA challenge | Cognitive process | Sex and cognition | DA and cognition |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Riccardi et al. (2011) | 7/6 | Str, Thal, Amg, IFG | DA release | AMPH (0.43 mg/kg oral) | Executive control | Baseline: F > M. Post-AMPH: F~M | Among M, AMPH-enhanced executive control associated with less Thai and TC DA release |
| Riccardi et al. (2006) | 7/6 | Str, Thal, Amg, IFG | DA release | AMPH (0.43 mg/kg oral) | Working memory | None noted | Among M, AMPH-impaired working memory associated with less P DA release |
| Attention | None noted | Among F, AMPH-enhanced attention associated with VS DA release. Among M, AMPH-impaired attention associated with VS and HPC DA release | |||||
| Oswald et al. (2015) | 27/18 | Striatum | DA release | AMPH (0.3mg/kg IV) | Decision-making | F~M | Worse decision-making associated with greater VS and dC DA release |
| Mozley et al. (2001) | 30/36 | Striatum | DAT availability | N/A | Verbal memory | F>M | Memory performance associated with striatal DAT availability |
| Executive control | F~M | Among F, faster cognitive processing associated with striatal DAT availability and greater executive control associated with P DAT availability | |||||
| Fine motor speed | M>F | Among F, faster fine motor speed associated with striatal DAT availability | |||||
| Burke et al. (2011) | 31/48 | Str, midbrain, Thal | DAT availability | N/A | Mental rotation | M>F | No significant relationships |
Note: “>” indicates significant sex differences (P <0.05). indicates sex differences are not significant (P >0.05). DA and cognition findings: “associated with” indicates significant bivariate correlation.
Abbreviations: Amg = amgydala; AMPH = amphetamine; F = female; IFG = inferior frontal gyrus; IV = intravenous; M = male; Ref=reference; Str = striatum; Thai = thalamus.
Working memory
Among the most widely studied cognitive processes associated with dopamine is working memory. Working memory is the flexible neural representation of stimuli over a brief time period (Goldman-Rakic, 1995) and is associated with excitatory microcircuits in the PFC, especially the dlPFC (Goldman-Rakic, 1995; Arnsten, 2009; Wang et al., 2013). Nonhuman primate research indicates an inverted “U”-shaped relationship with extrasynaptic dopamine levels and spatial working memory performance (reviewed: Arnsten, 2009; Arnsten et al., 2012). Too little and too much dopamine D1R stimulation impairs working memory via disruption of PFC excitatory microcircuits (Sawaguchi and Goldman-Rakic, 1991; Cai and Arnsten, 1997; Zahrt et al., 1997; Vijayraghavan et al., 2007). Thus, individual differences in homeostatic dopaminergic “set point,” i.e., basal dopaminergic neurotransmission, will influence working memory function. For example, individuals with lower dopaminergic tone, e.g., COMT Val158Met genotype (Cai and Arnsten, 1997, exhibit lower working memory capacity (Egan et al., 2001; Frias De et al., 2005) and dopamine agonists enhance working memory function in those individuals (Kimberg et al., 1997; Luciana and Collins, 1997; Kimberg and D’Esposito, 2003; Jacobs and D’Esposito, 2011). Conversely, high basal working memory capacity (and presumably, optimal dopamine neurotransmission) is impaired by either dopamine agonists or antagonists (Mattay et al., 2003; Jacobs and D’Esposito, 2011), as those pharmacologic challenges may have shifted subjects down the inverted “U” curve. Mesocortical dopaminergic projections are sparse, yet influential for working memory. For example, Cools et al. (2008) reported striatal dopamine synthesis capacity predicted working memory function, which suggests a central role for mesocortical dopaminergic PFC projections in working memory processes.
There are numerous studies indicating sex differences in working memory performance. A recent meta-analysis of 98 samples found a small, but significant, sex effect on working memory performance, indicating males perform modestly better than females (Voyer et al., 2017). However, sex hormone levels and menstrual cycle phase may influence working memory proficiency (Postma et al., 1999; Janowsky et al., 2000; Cherrier et al., 2001) but were largely ignored in those studies. The extent to which biologic sex and sex hormone levels influence working memory via dopamine modulation is not fully understood in humans. A pharmacogenomic study found an interaction between circulating estradiol levels and COMT Val158Met genotype (Cai and Arnsten, 1997) on working memory performance and neural activation (Jacobs and D’Esposito, 2011). Specifically, females with lower basal dopaminergic tone (val/val genotype) performed better on a working memory task when estradiol levels were higher (i.e., late follicular phase), compared to lower (i.e., early follicular phase) (Jacobs and D’Esposito, 2011), presumably due to estradiol’s facilitatory effect on dopamine neurotransmission (Becker, 1990a,b; Becker et al., 2017). Conversely, females with higher basal dopaminergic tone (met/met genotype) exhibited the opposite interaction with estradiol levels, suggesting that estradiol levels bidirectionally influence dopamine neurotransmission. Finally, Riccardi et al. (2006) found that among men, but not women, greater amphetamine-induced dopamine release in the putamen was correlated with a smaller amphetamine-induced decrement in working memory performance. Unfortunately, neither raw scores nor sex differences in working memory performance were reported at baseline or post amphetamine in that study (Riccardi et al., 2006).
Strictly speaking, working memory likely does not directly influence SUDs. However, working memory proficiency, as a proxy for PFC function, has numerous implications for SUDs and has been shown to predict cigarette smoking behavior (Patterson et al., 2010; Loughead et al., 2015). The PFC is the central executive hub of the brain and is implicated in self-control, decision-making, problem-solving, inhibitory control, and delayed gratification—cognitive processes with obvious implications for addiction. Diminished working memory capacity may be a cognitive phenotype that predicts addiction vulnerability and propensity to relapse.
Decision-making
Decision-making is a broad construct measured via numerous behavioral tasks. For our purposes, we focus on the Iowa Gambling task (IGT), as it is widely used in neuroimaging studies (Brevers et al., 2013). The IGT involves probabilistic learning via monetary rewards and punishments (Bechara et al., 1994; Brevers et al., 2013). Participants select cards from four decks with different probabilistic monetary risk/reward profiles, wherein it is advantageous to select smaller, longer-term rewards rather than large, immediate rewards to avoid large losses in the end (Brevers et al., 2013). Optimal strategy necessitates self-control and goal-directed decision-making to achieve maximal monetary reward (Buelow and Suhr, 2009). In this way, poor IGT performance has been used as a behavioral index of risky or impulsive decision-making (Buelow and Suhr, 2009). Neuroimaging studies indicate advantageous IGT decision-making is associated with the amygdala and PFC activation, especially OFC and dlPFC (Bolla et al., 2004; Li et al., 2010).
There are sex differences in IGT performance: men tend to perform better than women (Reavis and Overman, 2001; Bolla et al., 2004; Overman, 2004; van den Bos et al., 2013). A more detailed analysis suggests women exhibit a more flexible approach to the IGT, whereas men tend to focus more on the long-term payoff (Overman, 2004; van den Bos et al., 2009; van den Bos et al., 2013). Neural sex differences indicate men exhibited greater neural activation in the dlPFC during IGT than women (Bolla et al., 2004). The investigation of sex hormone levels and menstrual cycle phase effects has been limited. No effect of menstrual cycle phase was found in one study (Reavis and Overman, 2001), whereas another study reported possible effects (van den Bos et al., 2009). Interestingly, higher saliva testosterone levels were associated with worse IGT performance in both men and women (Stanton et al., 2011).
The role of dopamine in IGT performance has been widely discussed. Preclinical IGT analogues indicate links between decision-making and dopamine neurobiology (reviewed: de Visser et al., 2011). In humans, pharmacologic depletion of dopamine levels impaired IGT performance among healthy volunteers (Sevy et al., 2006). In two PET studies, Linnet et al. (2011a,b) reported that more advantageous IGT performance among healthy men was associated with dopamine release in the ventral striatum. Unfortunately, no women were included in either study. Oswald et al. (2015) reported less advantageous IGT performance was correlated with greater amphetamine-evoked dopamine release in the ventral striatum and dorsal caudate among both male and female healthy volunteers. No sex differences in task performance or task-dopamine correlations were observed (Oswald et al., 2015). However, a limitation of that study was IGT performance was measured ~2).months prior to PET scanning (Oswald et al., 2015
In summary, men reliably exhibit better IGT performance than women, yet little is known about the dopaminergic underpinnings of this difference. Future research is needed to examine the extent to which advantageous decision-making is associated with PFC dopamine release and possible sex differences therein. Advantageous decision-making on the IGT requires self-control and delayed gratification to maximize long-term monetary reward. These cognitive processes are clearly applicable to SUDs, especially treatment response and long-term abstinence. Indeed, substance dependent individuals reliably exhibit worse IGT performance than healthy controls (Petry et al., 1998; Grant et al., 2000; Bechara et al., 2001; Whitlow et al., 2004), indicative of impaired decision-making and consistent with the shortened time horizon exemplified in SUDs. IGT task performance among substance dependent individuals may serve as an endophenotypic marker predictive of treatment response (Passetti et al., 2008; Stevens et al., 2013, 2015).
Executive Control
Executive control refers to processes that facilitate adaptive and flexible integration of contextual information such that complex tasks can be completed accurately (often involving the inhibition of habitual responses) (Norman and Shallice, 1986; Egner and Hirsch, 2005). One common way to quantify executive control is via the Stroop task (Stroop, 1935). There are numerous variations of the Stroop task, but the original and most widely used is the word–color version (Stroop, 1935). The names of common colors are presented serially using different font colors. Incongruent trials, i.e., the name of the color and its font don’t match, are associated with more errors and longer reaction times than congruent or neutral trials, i.e., the Stroop effect. Incongruent trials invoke cognitive conflict; competing neural signals that must be parsed by the executive control network to execute the correct behavioral response. Neuroimaging studies of Stroop performance indicate the dorsal anterior cingulate is involved in conflict and error monitoring (Botvinick et al., 1999; Barch et al., 2001; Milham et al., 2001, 2003; Durston et al., 2003; Fan et al., 2003; Hazeltine et al., 2003; Kerns et al., 2004) and the dlPFC is involved in directed attention and cognitive control (Milham et al., 2001; Durston et al., 2003; Milham et al., 2003; Kerns et al., 2004).
Sex differences in Stroop task performance are present in some (Golden, 1974; Sarmány, 1977; Mekarski et al., 1996; Baroun and Alansari, 2006; Van der Elst et al., 2006), but not all, studies (Swerdlow et al., 1995; Klein et al., 1997; Daniel et al., 2000; Alansari and Baroun, 2004). In general, if sex differences are reported, females tend to outperform males (Golden, 1974; Sarmány, 1977; Mekarski et al., 1996; Baroun and Alansari, 2006; Van der Elst et al., 2006). Further, menstrual cycle phase may influence Stroop task performance (Hatta and Nagaya, 2009; Hoyer et al., 2013; DeVito et al., 2014), though more research is needed to confirm these findings.
Dopamine levels influence Stroop performance in humans. Acute pharmacologic depletion of dopamine was associated with improved Stroop performance (Scholes et al., 2007). Interestingly, both dopamine antagonists and agonists have been shown to improve Stroop performance (Williams et al., 1996; Roesch-Ely et al., 2005). PET imaging studies indicate significant linear relationships between in vivo dopamine neurobiology and Stroop task performance metrics. For example, [18F]fluorodopa uptake, i.e., an index of dopamine synthesis capacity, was associated with Stroop interference in healthy subjects (McGowan et al., 2004). Further, PET imaging studies report sex-specific relationships. Riccardi et al. (2011) reported that females performed better than males on the Stroop task at baseline, but those differences disappeared after amphetamine administration. Amphetamine improved Stroop task performance among males but impaired performance among females (Riccardi et al., 2011). Among males (but not females), greater amphetamine-induced dopamine release in the thalamus and temporal cortex was associated with less amphetamine-induced improvement in Stroop performance (Riccardi et al., 2011). Mozley et al. (2001) did not find sex differences in Stroop performance. However, among females (but not males), faster and more accurate Stroop responding was correlated with greater striatal DAT availability (Mozley et al., 2001). Taken together, these studies suggest lower levels of both tonic (Mozley et al., 2001; Scholes et al., 2007) and phasic (Riccardi et al., 2011) dopaminergic neurotransmission are associated with improved executive control as measured by Stroop task performance. Further, these studies indicate sex-specific relationships between dopamine neurobiology and executive control, though more research is needed to confirm these findings. Executive control is composed of subprocesses, e.g., attention and inhibitory control. Sex differences in attention and inhibitory control (and associated dopamine neurobiology) may also play an important role in addiction, as discussed in the next section.
DOPAMINE AND ADDICTION
Sex differences exist throughout the addiction life cycle, and dopamine plays an important role in the development and persistence of addiction. Dopamine signaling is crucial in the reinforcing effects of drugs, motivational learning, mood regulation, impulsivity, executive control, inhibitory control, and decision-making and may, therefore, play a primary role in understanding sex differences in addiction. While men use drugs more frequently and at higher doses than women (SAMHSA, 2015), women progress from casual drug-taking to addiction faster than men, experience worse withdrawal symptoms, and are more likely to relapse after quitting (see Becker and Hu, 2008; Zakiniaeiz and Potenza, 2018 for review). Numerous PET studies have demonstrated that individuals with addictive disorders, including tobacco smokers (Christoph Fehr et al., 2008; Brown et al., 2012) and methamphetamine (Volkow et al., 2001), alcohol (Martinez et al., 2005), cocaine (Martinez et al., 2004; Martinez et al., 2011), and heroin (Martinez et al., 2012) use disorders, have significantly lower striatal D2/3R availability than comparison groups. However, some of these studies failed to include women or investigate the effect of sex on D2R availability compared to controls. Drug challenge studies using PET have also allowed us to probe the function of the dopamine system. Low dopamine transmission following a methylphenidate challenge in cocaine dependents has been associated with treatment failure (Martinez et al., 2011), but again, these findings were not stratified by sex.
Thanks to recent advancements in this research area, tobacco smoking is an excellent example for studying dopamine-mediated sex differences in motivation, reinforcement, inhibitory control, and decision-making. Sex differences have been well-documented in the reinforcing effects of nicotine and in tobacco smoking treatment (Perkins et al., 1999). For example, men experience greater nicotine-induced reinforcement than women (Perkins, 1996; Perkins et al., 1999) are better able to detect nicotine from de-nicotinized cigarettes than (Perkins et al., 2001, 2002) women, and respond better to nicotine replacement therapies (NRTs) than women (McKee et al., 2016). Women metabolize nicotine and cotinine (a nicotine metabolite) faster than men (in part due to estrogen) (Johnstone et al., 2006), and report shorter intervals between cigarettes (Perkins et al., 1999), which may explain why they typically experience more adverse effects related to nicotine (Sofuoglu and Mooney, 2009) and worse treatment outcomes than men. Women are more reinforced by smoking cues (McBride et al., 2006), tend to relapse to smoking in response to stress (Xu et al., 2008; Cofta-Woerpel et al., 2011), and have a harder time quitting (Smith et al., 2016). These sex differences in nicotine sensitivity and smoking-related behaviors presumably reflect underlying neurobiologic differences that might, in part, be explained by dopamine.
Neuroimaging studies from our group, as well as other groups, have begun to disentangle the molecular mechanisms underlying sex/gender-based behavioral differences to understand and treat female tobacco smokers more effectively. Nicotine binds to and activates nicotinic acetylcholine receptors, which, in turn, facilitates dopamine release in striatal and cortical brain regions (Benowitz, 2010; Cosgrove et al., 2015) via the mesolimbic and mesocortical pathways, respectively. Similar to other drug and alcohol use disorders, tobacco smoking has significantly lower striatal D2/3R availability compared to healthy controls (Volkow et al., 2001; Martinez et al., 2004, 2005, 2011, 2012; Christoph Fehr et al., 2008; Worhunsky et al., 2017). However, lower striatal D2/3R availability has only been shown in male (Christoph Fehr et al., 2008; Brown et al., 2012), not female, tobacco smokers (Brown et al., 2012). Sex differences in D2/3R availability have also been found in the midbrain, where dopamine neurons originate—female smokers have higher D2/3R availability in the midbrain than female nonsmokers, whereas male smokers and nonsmokers are not different (Okita et al., 2016). Midbrain D2/3Rs are predominantly inhibitory, and it has been postulated that higher midbrain D2/3R availability may lead to a suppression of ventral striatal smoking-induced dopamine release in female smokers, compared to nonsmokers. Following a cigarette smoking challenge in the scanner, our laboratory found that male smokers have a robust smoking-induced dopaminergic response in the right ventral striatum, but female smokers do not (Cosgrove et al., 2014). We recently conducted a study examining the mesocortical dopamine pathway in smokers and found that tobacco smokers had significantly lower D2/3R availability in the dlPFC, compared to nonsmokers, which was driven by differences between male smokers and male nonsmokers but not female smokers compared to female nonsmokers (consistent with striatal findings) (Zakiniaeiz et al., 2019). Following an amphetamine-challenge, we found that female smokers had significantly less amphetamine-induced dopamine release in the dlPFC, compared to male smokers and compared to female nonsmokers (Zakiniaeiz et al., 2019).
While lower D2/3R availability is generally interpreted as D2/3R downregulation, our findings can also be interpreted as higher basal dopamine levels or a combination of both (Laruelle et al., 1997). Although we did not directly measure basal dopamine levels, there is prior evidence from in vivo microdialysis studies in animals that acute drug exposure increases basal dopamine levels in a time-locked manner (Imperato and Di Chiara, 1986; Di Chiara and Imperato, 1988; Carboni et al., 1989; Jianping et al., 1990; Zocchi et al., 2003) and chronic drug exposure increases tonic dopamine levels (Diana, 2011; Volkow et al., 2011). Taken together, our results could be interpreted as: [1] male smokers have higher basal dopamine levels than male nonsmokers, and dopamine neurotransmission remains intact—chronic smoking does not impact dlPFC dopamine transmission in men and, [2] female smokers have comparable basal dopamine levels to female nonsmokers, but chronic tobacco smoking leads to blunted dopamine neurotransmission (see Fig. 9.3 for schematic interpretation). These results may be explained by a tonic/phasic model of the dopamine system (Grace, 2000); tonic firing results in basal dopamine levels in the synaptic cleft (Parsons and Justice, 1992); and phasic firing results in greater release of dopamine into the synaptic cleft (Schultz, 1998). When basal dopamine levels are relatively low (tonic firing), postsynaptic dopamine levels may not be high enough to produce a dopamine response but may be sufficient to stimulate inhibitory D2-type auto-receptors on dopamine terminals (Grace, 2000; Okita et al., 2016) that regulate release of dopamine (Grace, 2000), thereby decreasing dopamine release in the mesocortical pathway. We hypothesize that female smokers have lower basal dopamine levels than male smokers, likely making dopamine firing thresholds more difficult to reach, autoregulation more feasible, and an inhibited dopamine response more likely. More work is needed to fully understand the dopamine-mediated neurobiologic underpinnings of sex differences in tobacco smokers.
Fig. 9.3.
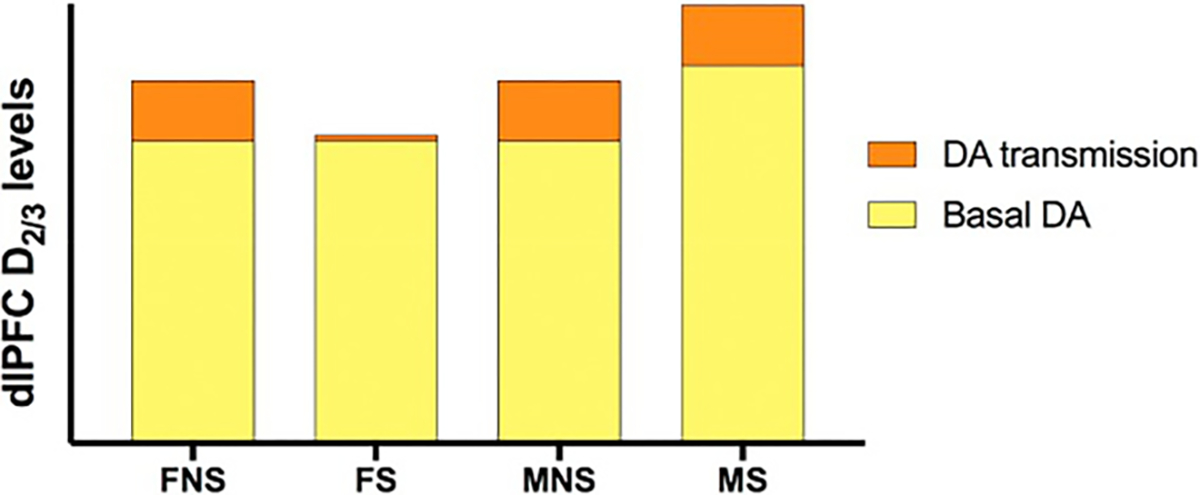
Schematic interpretation of primary findings. In the dlPFC, male smokers (MS) had higher basal dopamine (D2/3) levels than male nonsmokers (MNS) but dopamine neurotransmission remained intact; whereas, female smokers (FS) have comparable basal dopamine levels to female nonsmokers (FNS); but dopamine neurotransmission was dysfunctional. No error bars are shown as this is an interpretation of the data being used as visual aid. Abbreviations: S = smokers, NS = nonsmokers, M = males, F = females
DISCUSSION
The investigation of sex differences in biologic systems and psychiatric disorders is a relatively young field, yet seminal findings have highlighted the clinical importance of sex as a biologic variable. In this chapter, we summarized the limited yet meaningful body of literature describing sex differences throughout the dopaminergic system and SUDs. Specifically, we described sex differences in dopamine neurobiology, the role of sex hormones in dopamine functioning, genetic underpinnings of sex differences in dopamine, and how sex differences in cognitive function and addiction might be mediated by dopamine.
Among healthy individuals, to date, there is no convincing evidence of sex differences in dopamine D2/3R or DAT availability in the striatum using PET imaging. Conversely, healthy men reliably exhibit greater evoked dopamine release in the striatum than their female counterparts. Phasic dopamine release in the striatum is known to mediate drug reward in animals. Although phasic dopamine cannot be directly tested in humans, we hypothesize that greater drug-induced dopamine release may explain why men are more likely to use drugs, and at higher doses, than women. Future research is needed to determine if sex differences in dopamine receptor availability exist outside of the striatum.
Sex hormone levels likely mediate the influence of biologic sex on dopamine neurobiology and addiction processes. Human PET imaging research has been limited in this area, and many studies do not measure plasma sex hormone levels or report/control for menstrual cycle phase on scan day. However, preclinical evidence suggests that estradiol may facilitate, whereas progesterone may inhibit, dopamine neurotransmission. Also, high estradiol levels have been shown to enhance subjective drug responses and degrade self-control. Thus, periods of high estrogen levels, i.e., during late follicular or luteal phase, may increase susceptibility to drugs of abuse. Conversely, high progesterone levels have been shown to attenuate drug reinforcement, and progesterone is currently being investigated as a treatment for cocaine dependence. Preclinical manipulations of testosterone levels have yielded mixed findings regarding dopamine neurobiology. Yet, there is some evidence that testosterone, on its own, might be reinforcing—likely via dopaminergic mechanisms.
The genetic influence of sex differences in dopamine neurobiology may stem from X and Y chromosomes. The male-determining gene, Sry, regulates the rate-limiting dopamine synthesis enzyme TH and is found only in males. Conversely, in females, evidence suggests estrogen levels regulate TH. Indeed, these sex differences in dopamine synthesis regulation may explain why men reliably exhibit greater evoked dopamine release than females. Further, SNPs influence dopamine neurobiology. For example, the common polymorphism, Val158Met, is associated with diminished COMT activity, which results in higher extrasynaptic dopamine levels. This genetic variant is associated with altered working memory function and impulsive decision-making and has been linked to addiction.
Dopamine’s role in cognition is extensive and beyond the scope of this review. Briefly, we highlighted sex differences in three cognitive processes associated with dopamine and addiction: working memory, decision-making, and executive control. While there is evidence of sex differences in proficiency among these cognitive processes, the role of dopamine in these sex differences is not yet known. For example, men reliably exhibit better decision-making during the IGT than women. Further, men exhibit greater neural activation in the dlPFC during the task performance than women. However, human PET studies found opposing relationships between striatal dopamine release and task performance, and only one study included female subjects. Thus, it remains unclear the extent to which dopamine may mediate sex differences in IGT performance. Dopamine’s role in cognition is not disputed, but dopamine’s role in sex differences in cognition is not yet clear.
There are sex differences throughout the addiction lifecycle—from drug experimentation to treatment response—highlighting the importance of considering sex in addiction research. Females initiate substance use later, progress to substance dependence more quickly, and exhibit a more severe clinical phenotype than men. Further, male smokers exhibit greater nicotine-induced reinforcement and respond better to nicotine replacement therapy than female smokers. These findings highlight the tremendous need for sex-specific treatments in SUDs. PET imaging research has shown that male smokers exhibit greater evoked dopamine release in the striatum than their female counterparts. Also, there is some evidence that male smokers exhibit lower dopamine D2/3R availability in the striatum and prefrontal cortex than male nonsmokers, whereas female smokers and nonsmokers did not differ. Finally, female smokers exhibited less amphetamine-induced dopamine release in the prefrontal cortex than male smokers and female nonsmokers.
In conclusion, there is clear evidence demonstrating sex differences in dopamine neurobiology and addiction-related processes. However, we have only just begun to parse the genetic and hormonal contributions to sexual dimorphism in dopamine neurobiology, cognition, and addiction. Future research, using prospective designs that consider sex throughout the research pipeline including the recruitment plan, study methodology, and analysis strategy, are needed to disentangle the complex relationships between sex, dopamine, and addiction.
FUNDING
Funding generously provided by the NIH: T32 DA022975, K99 DA048125, R01 DA038832, R01 DA038709, R01 DA045465, and P01 AA027473. Funding sources were not involved in the interpretation of data described in this manuscript.
Footnotes
CONFLICTS OF INTEREST
The authors have no financial or ethical conflicts of interests to disclose.
REFERENCES
- Alansari B, Baroun K (2004). Gender and cultural performance differences on the Stroop color and word test: a comparative study. Soc Behav Personal Int J 32: 235–245. [Google Scholar]
- American Psychological Association (2012). Guidelines for psychological practice with lesbian, gay, and bisexual clients. Am Psychol 67: 10–42. [DOI] [PubMed] [Google Scholar]
- Arnsten AF (2009). Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci 10: 410–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF,Wang MJ, Paspalas CD (2012). Neuromodulation of thought: flexibilities and vulnerabilities in prefrontal cortical network synapses. Neuron 76: 223–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, Braver TS, Akbudak E et al. (2001). Anterior cingulate cortex and response conflict: effects of response modality and processing domain. Cereb Cortex 11: 837–848. [DOI] [PubMed] [Google Scholar]
- Baroun K, Alansari B (2006). Gender differences in performance on the Stroop test. Soc Behav Personal Int J 34: 309–318. [Google Scholar]
- Baulieu E-E, Schumacher M (2000). Progesterone as a neuroactive neurosteroid, with special reference to the effect of progesterone on myelination. Steroids 65: 605–612. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio AR, Damasio H et al. (1994). Insensitivity to future consequences following damage to human prefrontal cortex. Cognition 50: 7–15. [DOI] [PubMed] [Google Scholar]
- Bechara A, Dolan S, Denburg N et al. (2001). Decision-making deficits, linked to a dysfunctional ventromedial prefrontal cortex, revealed in alcohol and stimulant abusers. Neuropsychologia 39: 376–389. [DOI] [PubMed] [Google Scholar]
- Becker JB (1990a). Estrogen rapidly potentiates amphetamine-induced striatal dopamine release and rotational behavior during microdialysis. Neurosci Lett 118: 169–171. [DOI] [PubMed] [Google Scholar]
- Becker JB (1990b). Direct effect of 17b-estradiol on striatum: sex differences in dopamine release. Synapse 5: 157–164. [DOI] [PubMed] [Google Scholar]
- Becker JB (1999). Gender differences in dopaminergic function in striatum and nucleus accumbens. Pharmacol Biochem Behav 64: 803–812. [DOI] [PubMed] [Google Scholar]
- Becker JB (2009). Sexual differentiation of motivation: a novel mechanism? Horm Behav 55: 646–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Hu M (2008). Sex differences in drug abuse. Front Neuroendocrinol 29: 36–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Ramirez VD (1981). Sex differences in the amphetamine stimulated release of catecholamines from rat striatal tissue in vitro. Brain Res 204: 361–372. [DOI] [PubMed] [Google Scholar]
- Becker JB, McClellan ML, Reed BG (2017). Sex differences, gender and addiction. J Neurosci Res 95: 136–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL (2010). Nicotine addiction. N Engl J Med 362: 2295–2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best SE, Sarrel PM, Malison RT et al. (2005). Striatal dopamine transporter availability with [123 I] β-CIT SPECT is unrelated to gender or menstrual cycle. Psychopharmacology (Berl) 183: 181–189. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Eldreth D, Matochik J et al. (2004). Sex-related differences in a gambling task and its neurological correlates. Cereb Cortex 14: 1226–1232. [DOI] [PubMed] [Google Scholar]
- Botvinick M, Nystrom LE, Fissell K et al. (1999). Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature 402: 179. [DOI] [PubMed] [Google Scholar]
- Brevers D, Bechara A, Cleeremans A et al. (2013). Iowa gambling task (IGT): twenty years after–gambling disorder and IGT. Front Psychol 4: 665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg-Martin ES, Matsumoto M, Hikosaka O (2010). Dopamine in motivational control: rewarding, aversive, and alerting. Neuron 68: 815–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AK, Mandelkern MA, Farahi J et al. (2012). Sex differences in striatal dopamine D2/D3 receptor availability in smokers and non-smokers. Int J Neuropsychopharmacol 15: 989–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brozoski TJ, Brown RM, Rosvold H et al. (1979). Cognitive deficit caused by regional depletion of dopamine in prefrontal cortex of rhesus monkey. Science 205: 929–932. [DOI] [PubMed] [Google Scholar]
- Brücke T, Kornhuber J, Angelberger P et al. (1993). SPECT imaging of dopamine and serotonin transporters with [123 I] β-CIT. Binding kinetics in the human brain. J Neural Transm Gen Sect 94: 137–146. [DOI] [PubMed] [Google Scholar]
- Buelow MT, Suhr JA (2009). Construct validity of the Iowa gambling task. Neuropsychol Rev 19: 102–114. [DOI] [PubMed] [Google Scholar]
- Burke SM, van de Giessen E, de Win M et al. (2011). Serotonin and dopamine transporters in relation to neuropsychological functioning, personality traits and mood in young adult healthy subjects. Psychol Med 41: 419–429. [DOI] [PubMed] [Google Scholar]
- Cai J, Arnsten A (1997). Dose-dependent effects of the dopamine D1 receptor agonists A77636 or SKF81297 on spatial working memory in aged monkeys. J Pharmacol Exp Ther 283: 183–189. [PubMed] [Google Scholar]
- Calipari ES, Juarez B, Morel C et al. (2017). Dopaminergic dynamics underlying sex-specific cocaine reward. Nat Commun 8: 13877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carboni E, Imperato A, Perezzani L et al. (1989). Amphetamine, cocaine, phencyclidine and nomifensine increase extracellular dopamine concentrations preferentially in the nucleus accumbens of freely moving rats. Neuroscience 28: 653–661. [DOI] [PubMed] [Google Scholar]
- Carlsson A, Lindqvist M, Magnusson T et al. (1958). On the presence of 3-hydroxytyramine in brain. Science 127: 471. [DOI] [PubMed] [Google Scholar]
- Carruth LL, Reisert I, Arnold AP (2002). Sex chromosome genes directly affect brain sexual differentiation. Nat Neurosci 5: 933–934. [DOI] [PubMed] [Google Scholar]
- Castner SA, Xiao L, Becker JB (1993). Sex differences in striatal dopamine: in vivo microdialysis and behavioral studies. Brain Res 610: 127–134. [DOI] [PubMed] [Google Scholar]
- Chavez C, Hollaus M, Scarr E et al. (2010). The effect of estrogen on dopamine and serotonin receptor and transporter levels in the brain: an autoradiography study. Brain Res 1321: 51–59. [DOI] [PubMed] [Google Scholar]
- Chen J, Lipska BK, Halim N et al. (2004). Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): effects on mRNA, protein, and enzyme activity in postmortem human brain. Am J Hum Genet 75: 807–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherrier M, Asthana S, Plymate S et al. (2001). Testosterone supplementation improves spatial and verbal memory in healthy older men. Neurology 57: 80–88. [DOI] [PubMed] [Google Scholar]
- Chowdhury R, Guitart-Masip M, Bunzeck N et al. (2012). Dopamine modulates episodic memory persistence in old age. J Neurosci 32: 14193–14204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoph Fehr MD, Igor Yakushev MD, Nina Hohmann DP et al. (2008). Association of low striatal dopamine D 2 receptor availability with nicotine dependence similar to that seen with other drugs of abuse. Am J Psychiatry 165: 507–514. [DOI] [PubMed] [Google Scholar]
- Coates JM, Herbert J (2008). Endogenous steroids and financial risk taking on a London trading floor. Proc Natl Acad Sci U S A 105: 6167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cofta-Woerpel L, McClure JB, Li Y et al. (2011). Early cessation success or failure among women attempting to quit smoking: trajectories and volatility of urge and negative mood during the first post-cessation week. J Abnorm Psychol 120: 596–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colzato LS, Hertsig G, van den Wildenberg WPM et al. (2010). Estrogen modulates inhibitory control in healthy human females: evidence from the stop-signal paradigm. Neuroscience 167: 709–715. [DOI] [PubMed] [Google Scholar]
- Cools R(2008). Roleof dopamine in the motivational and cognitive control of behavior. Neuroscientist 14: 381–395. [DOI] [PubMed] [Google Scholar]
- Cools R, D’Esposito M (2011). Inverted-U–shaped dopamine actions on human working memory and cognitive control. Biol Psychiatry 69: e113–e125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, Gibbs SE, Miyakawa A et al. (2008). Working memory capacity predicts dopamine synthesis capacity in the human striatum. J Neurosci 28: 1208–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove KP, Wang S, Kim SJ et al. (2014). Sex differences in the brain’s dopamine signature of cigarette smoking. J Neurosci 34: 16851–16855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove KP, Esterlis I, Sandiego C et al. (2015). Imaging tobacco smoking with PET and SPECT. In: Balfour JKD, Munafò RM (Eds.), The neuropharmacology of nicotine dependence. vol. 24. Springer International Publishing, Cham, pp. 1–17. [DOI] [PubMed] [Google Scholar]
- Creutz LM, Kritzer MF (2004). Mesostriatal and mesolimbic projections of midbrain neurons immunoreactive for estrogen receptor beta or androgen receptors in rats. J Comp Neurol 476: 348–362. [DOI] [PubMed] [Google Scholar]
- Dang LC, O’Neil JP, Jagust WJ (2012). Dopamine supports coupling of attention-related networks. J Neurosci 32: 9582–9587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel DB, Pelotte M, Lewis J (2000). Lack of sex differences on the Stroop color-word test across three age groups. Percept Mot Skills 90: 483–484. [DOI] [PubMed] [Google Scholar]
- Danjo T,Yoshimi K,Funabiki K et al.(2014).Aversive behavior induced by optogenetic inactivation of ventral tegmental area dopamine neurons is mediated by dopamine D2 receptors in the nucleus accumbens. Proc Natl Acad Sci U S A 111: 6455–6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Visser L, Homberg J, Mitsogiannis M et al. (2011). Rodent versions of the Iowa gambling task: opportunities and challenges for the understanding of decision-making. Front Neurosci 5: 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVito EE, Herman AI, Waters AJ et al. (2014). Subjective, physiological, and cognitive responses to intravenous nicotine: effects of sex and menstrual cycle phase. Neuropsychopharmacology 39: 1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewing P, Chiang CWK, Sinchak K et al. (2006). Direct regulation of adult brain function by the male-specific factor SRY. Curr Biol 16: 415–420. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A (1988). Opposite effects of mu and kappa opiate agonists on dopamine release in the nucleus accumbens and in the dorsal caudate of freely moving rats. J Pharmacol Exp Ther 244: 1067. [PubMed] [Google Scholar]
- Diana M (2011). The dopamine hypothesis of drug addiction and its potential therapeutic value. Front Psychol 2: 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dluzen DE (2000). Neuroprotective effects of estrogen upon the nigrostriatal dopaminergic system. J Neurocytol 29: 387–399. [DOI] [PubMed] [Google Scholar]
- Dluzen DE, Ramirez VD (1989). Effects of orchidectomy on nigro-striatal dopaminergic function: behavioral and physiological evidence. J Neuroendocrinol 1: 285–290. [DOI] [PubMed] [Google Scholar]
- Dreyer JK, Herrik KF, Berg RW et al. (2010). Influence of phasic and tonic dopamine release on receptor activation. J Neurosci 30: 14273–14283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durston S, Davidson M, Thomas K et al. (2003). Parametric manipulation of conflict and response competition using rapid mixed-trial event-related fMRI. Neuroimage 20: 2135–2141. [DOI] [PubMed] [Google Scholar]
- Egan MF, Goldberg TE, Kolachana BS et al. (2001). Effect of COMT Val108/158 met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci U S A 98: 6917–6922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egner T, Hirsch J (2005). The neural correlates and functional integration of cognitive control in a Stroop task. Neuroimage 24: 539–547. [DOI] [PubMed] [Google Scholar]
- Ernst M, Zametkin AJ, Matochik JA et al. (1998). DOPA decarboxylase activity in attention deficit hyperactivity disorder adults. A [fluorine-18] fluorodopa positron emission tomographic study. J Neurosci 18: 5901–5907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eusebio A, Azulay J-P, Ceccaldi M et al. (2012). Voxel-based analysis of whole-brain effects of age and gender on dopamine transporter SPECT imaging in healthy subjects. Eur J Nucl Med Mol Imaging 39: 1778–1783. [DOI] [PubMed] [Google Scholar]
- Evans SM, Foltin RW (2005). Exogenous progesterone attenuates the subjective effects of smoked cocaine in women, but not in men. Neuropsychopharmacology 31: 659. [DOI] [PubMed] [Google Scholar]
- Fahn S (2008). The history of dopamine and levodopa in the treatment of Parkinson’s disease. Mov Disord 23: S497–S508. [DOI] [PubMed] [Google Scholar]
- Fallon JH (1988). Topographic organization of ascending dopaminergic projections. Ann N Y Acad Sci 537: 1–9. [DOI] [PubMed] [Google Scholar]
- Fan J, Flombaum JI, McCandliss BD et al. (2003). Cognitive and brain consequences of conflict. Neuroimage 18: 42–57. [DOI] [PubMed] [Google Scholar]
- Farde L, Hall H, Pauli S et al. (1995). Variability in D2-dopamine receptor density and affinity: a PET study with [11C] raclopride in man. Synapse 20: 200–208. [DOI] [PubMed] [Google Scholar]
- Feltenstein MW, See RE (2007). Plasma progesterone levels and cocaine-seeking in freely cycling female rats across the estrous cycle. Drug Alcohol Depend 89: 183–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frias De CM, Annerbrink K, Westberg L et al. (2005). Catechol O-methyltransferase Val158Met polymorphism is associated with cognitive performance in nondemented adults. J Cogn Neurosci 17: 1018–1025. [DOI] [PubMed] [Google Scholar]
- Frye CA (2007). Some rewarding effects of androgens may be mediated by actions of its 5a-reduced metabolite 3aandrostanediol. Pharmacol Biochem Behav 86: 354–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Walf AA (2008). Membrane actions of progestins at dopamine type 1-like and GABAA receptors involve downstream signal transduction pathways. Steroids 73: 906–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Rhodes ME, Rosellini R et al. (2002). The nucleus accumbens as a site of action for rewarding properties of testosterone and its 5α-reduced metabolites. Pharmacol Biochem Behav 74: 119–127. [DOI] [PubMed] [Google Scholar]
- Gjedde A, Reith J, Dyve S et al. (1991). Dopa decarboxylase activity of the living human brain. Proc Natl Acad Sci USA 88: 2721–2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjedde A, Léger GC, Cumming P et al. (1993). Striatal L-dopa decarboxylase activity in Parkinson’s disease in vivo: implications for the regulation of dopamine synthesis. J Neurochem 61: 1538–1541. [DOI] [PubMed] [Google Scholar]
- Glenthoj BY, Mackeprang T, Svarer C et al. (2006). Frontal dopamine D2/3 receptor binding in drug-naive first-episode schizophrenic patients correlates with positive psychotic symptoms and gender. Biol Psychiatry 60: 621–629. [DOI] [PubMed] [Google Scholar]
- Golden CJ (1974). Sex differences in performance on the Stroop color and word test. Percept Mot Skills 39: 1067–1070. [Google Scholar]
- Goldman-Rakic P (1995). Cellular basis of working memory. Neuron 14: 477–485. [DOI] [PubMed] [Google Scholar]
- González-Flores O, Gómora-Arrati P, García-Juárez M et al. (2011). Progesterone receptor isoforms differentially regulate the expression of tryptophan and tyrosine hydroxylase and glutamic acid decarboxylase in the rat hypothalamus. Neurochem Int 59: 671–676. [DOI] [PubMed] [Google Scholar]
- Grace AA (2000). The tonic/phase model of dopamine system regulation and its implications for understanding alcohol and psychostimulant craving. Addiction 95: S119–S128. [DOI] [PubMed] [Google Scholar]
- Grant S, Contoreggi C, London ED (2000). Drug abusers show impaired performance in a laboratory test of decision making. Neuropsychologia 38: 1180–1187. [DOI] [PubMed] [Google Scholar]
- Greenfield SF, Back SE, Lawson K et al. (2010). Substance abuse in women. Psychiatr Clin 33: 339–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greengard P (2001). The neurobiology of dopamine signaling. Biosci Rep 21: 247–269. [DOI] [PubMed] [Google Scholar]
- Gundlah C, Lu NZ, Bethea CL (2002). Ovarian steroid regulation of monoamine oxidase-A and B mRNAs in the macaque dorsal raphe and hypothalamic nuclei. Psychopharmacology (Berl) 160: 271–282. [DOI] [PubMed] [Google Scholar]
- Hagelberg N, Martikainen IK, Mansikka H et al. (2002). Dopamine D2 receptor binding in the human brain is associated with the response to painful stimulation and pain modulatory capacity. Pain 99: 273–279. [DOI] [PubMed] [Google Scholar]
- Haltia LT, Rinne JO, Merisaari H et al. (2007). Effects of intravenous glucose on dopaminergic function in the human brain in vivo. Synapse 61: 748–756. [DOI] [PubMed] [Google Scholar]
- Haltia LT, Rinne JO, Helin S et al. (2008). Effects of intravenous placebo with glucose expectation on human basal ganglia dopaminergic function. Synapse 62: 682–688. [DOI] [PubMed] [Google Scholar]
- Hatta T, Nagaya K (2009). Menstrual cycle phase effects on memory and Stroop task performance. Arch Sex Behav 38: 821. [DOI] [PubMed] [Google Scholar]
- Hazeltine E, Bunge SA, Scanlon MD et al. (2003). Material dependent and material-independent selection processes in the frontal and parietal lobes: an event-related fMRI investigation of response competition. Neuropsychologia 41: 1208–1217. [DOI] [PubMed] [Google Scholar]
- Hermans EJ, Bos PA, Ossewaarde L et al. (2010). Effects of exogenous testosterone on the ventral striatal BOLD response during reward anticipation in healthy women. Neuroimage 52: 277–283. [DOI] [PubMed] [Google Scholar]
- Hernandez-Avila CA, Rounsaville BJ, Kranzler HR (2004). Opioid-, cannabis-and alcohol-dependent women show more rapid progression to substance abuse treatment. Drug Alcohol Depend 74: 265–272. [DOI] [PubMed] [Google Scholar]
- Hoyer J, Burmann I, Kieseler M-L et al. (2013). Menstrual cycle phase modulates emotional conflict processing in women with and without premenstrual syndrome (PMS)—a pilot study. PLoS One 8: e59780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao M-C, Lin K-J, Liu C-Y et al. (2013). The interaction between dopamine transporter function, gender differences, and possible laterality in depression. Psychiatry Res 211: 72–77. [DOI] [PubMed] [Google Scholar]
- Imperato A, Di Chiara G (1986). Preferential stimulation of dopamine release in the nucleus accumbens of freely moving rats by ethanol. J Pharmacol Exp Ther 239: 219. [PubMed] [Google Scholar]
- Jackson LR, Robinson TE, Becker JB (2005). Sex differences and hormonal influences on acquisition of cocaine self-administration in rats. Neuropsychopharmacology 31: 129. [DOI] [PubMed] [Google Scholar]
- Jacobs E, D’Esposito M (2011). Estrogen shapes dopamine-dependent cognitive processes: implications for women’s health. J Neurosci 31: 5286–5293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janowsky JS, Chavez B, Orwoll E (2000). Sex steroids modify working memory. J Cogn Neurosci 12: 407–414. [DOI] [PubMed] [Google Scholar]
- Jianping C, Paredes W, Lowinson JH et al. (1990). Δ9-Tetrahydroeannabinol enhances presynaptic dopamine efflux in medial prefrontal cortex. Eur J Pharmacol 190: 259–262. [DOI] [PubMed] [Google Scholar]
- Johnson LR, Wood RI (2001). Oral testosterone self-administration in male hamsters. Neuroendocrinology 73: 285–292. [DOI] [PubMed] [Google Scholar]
- Johnson ML, Ho CC, Day AE et al. (2010). Oestrogen receptors enhance dopamine neurone survival in rat midbrain. J Neuroendocrinol 22: 226–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone E, Benowitz N, Cargill A et al. (2006). Determinants of the rate of nicotine metabolism and effects on smoking behavior. Clin Pharmacol Ther 80: 319–330. [DOI] [PubMed] [Google Scholar]
- Justice AJH, de Wit H (1999). Acute effects of d-amphetamine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacology (Berl) 145: 67–75. [DOI] [PubMed] [Google Scholar]
- Justice AJH, de Wit H (2000a). Acute effects of estradiol pretreatment on the response to d-amphetamine in women. Neuroendocrinology 71: 51–59. [DOI] [PubMed] [Google Scholar]
- Justice AJH, de Wit H (2000b). Acute effects of d-amphetamine during the early and late follicular phases of the menstrual cycle in women. Pharmacol Biochem Behav 66: 509–515. [DOI] [PubMed] [Google Scholar]
- Kaasinen V, Någren K, Hietala J et al. (2001). Sex differences in extrastriatal dopamine D2-like receptors in the human brain. Am J Psychiatry 158: 308–311. [DOI] [PubMed] [Google Scholar]
- Kaasinen V, Kemppainen N, Någren K et al. (2002). Age-related loss of extrastriatal dopamine D2-like receptors in women. J Neurochem 81: 1005–1010. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW et al. (2004). Anterior cingulate conflict monitoring and adjustments in control. Science 303: 1023–1026. [DOI] [PubMed] [Google Scholar]
- Khasnavis S, Ghosh A, Roy A et al. (2013). Castration induces Parkinson disease pathologies in young male mice via inducible nitric-oxide synthase. J Biol Chem 288: 20843–20855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimberg DY, D’Esposito M (2003). Cognitive effects of the dopamine receptor agonist pergolide. Neuropsychologia 41: 1020–1027. [DOI] [PubMed] [Google Scholar]
- Kimberg DY, D’esposito M, Farah MJ (1997). Effects of bromocriptine on human subjects depend on working memory capacity. Neuroreport 8: 3581–3585. [DOI] [PubMed] [Google Scholar]
- Klein M, Ponds RW, Houx PJ et al. (1997). Effect of test duration on age-related differences in Stroop interference. J Clin Exp Neuropsychol 19: 77–82. [DOI] [PubMed] [Google Scholar]
- Koch W, Unterrainer M, Xiong G et al. (2014). Extrastriatal binding of [123 I] FP-CIT in the thalamus and pons: gender and age dependencies assessed in a European multicentre database of healthy controls. Eur J Nucl Med Mol Imaging 41: 1938–1946. [DOI] [PubMed] [Google Scholar]
- Kohno M, Nurmi EL, Laughlin CP et al. (2015). Functional genetic variation in dopamine signaling moderates prefrontal cortical activity during risky decision making. Neuropsychopharmacology 41: 695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuikka JT, Tiihonen J, Karhu J et al. (1997). Fractal analysis of striatal dopamine re-uptake sites. Eur J Nucl Med 24: 1085–1090. [DOI] [PubMed] [Google Scholar]
- Laakso A, Vilkman H, Örgen Bergman J et al. (2002). Sex differences in striatal presynaptic dopamine synthesis capacity in healthy subjects. Biol Psychiatry 52: 759–763. [DOI] [PubMed] [Google Scholar]
- Laruelle M, D’Souza CD, Baldwin RM et al. (1997). Imaging D2 receptor occupancy by endogenous dopamine in humans. Neuropsychopharmacology 17: 162. [DOI] [PubMed] [Google Scholar]
- Lavalaye J, Booij J, Reneman L et al. (2000). Effect of age and gender on dopamine transporter imaging with [123 I] FP-CIT SPET in healthy volunteers. Eur J Nucl Med 27: 867–869. [DOI] [PubMed] [Google Scholar]
- Levitt M, Spector S, Sjoerdsma A et al. (1965). Elucidation of the rate-limiting step in norepinephrine biosynthesis in the perfused Guinea-pig heart. J Pharmacol Exp Ther 148: 1. [PubMed] [Google Scholar]
- Li X, Lu ZL, D’argembeau A et al. (2010). The Iowa gambling task in fMRI images. Hum Brain Mapp 31: 410–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnet J, Møller A, Peterson E et al. (2011a). Dopamine release in ventral striatum during Iowa gambling task performance is associated with increased excitement levels in pathological gambling. Addiction 106: 383–390. [DOI] [PubMed] [Google Scholar]
- Linnet J, Møller A, Peterson E et al. (2011b). Inverse association between dopaminergic neurotransmission and Iowa gambling task performance in pathological gamblers and healthy controls. Scand J Psychol 52: 28–34. [DOI] [PubMed] [Google Scholar]
- Lisman J, Grace AA, Duzel E (2011). A neoHebbian framework for episodic memory; role of dopamine-dependent late LTP. Trends Neurosci 34: 536–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loughead J, Wileyto EP, Ruparel K et al. (2015). Working memory-related neural activity predicts future smoking relapse. Neuropsychopharmacology 40: 1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loughlin SE, Fallon JH (1982). Mesostriatal projections from ventral tegmentum and dorsal raphe: cells project ipsilaterally or contralaterally but not bilaterally. Neurosci Lett 32: 11–16. [DOI] [PubMed] [Google Scholar]
- Luciana M, Collins PF (1997). Dopaminergic modulation of working memory for spatial but not object cues in normal humans. J Cogn Neurosci 9: 330–347. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Sofuoglu M (2010). Role of progesterone in nicotine addiction: evidence from initiation to relapse. Exp Clin Psychopharmacol 18: 451–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z, Chang S-M, Huang Y-G (2012). Re: predictive factors for cutting-out in femoral intramedullary nailing [injury 2010:41(December (12));1312–16]. Injury 43: 1222–1223. [DOI] [PubMed] [Google Scholar]
- Martinez D, Broft A, Foltin RW et al. (2004). Cocaine dependence and D2 receptor availability in the functional subdivisions of the striatum: relationship with cocaine-seeking behavior. Neuropsychopharmacology 29: 1190. [DOI] [PubMed] [Google Scholar]
- Martinez D, Gil R, Slifstein M et al. (2005). Alcohol dependence is associated with blunted dopamine transmission in the ventral striatum. Biol Psychiatry 58: 779–786. [DOI] [PubMed] [Google Scholar]
- Martinez D, Carpenter KM, Liu F et al. (2011). Imaging dopamine transmission in cocaine dependence: link between neurochemistry and response to treatment. Am J Psychiatry 168: 634–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez D, Saccone PA, Liu F et al. (2012). Deficits in dopamine D2 receptors and presynaptic dopamine in heroin dependence: commonalities and differences with other types of addiction. Biol Psychiatry 71: 192–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Soelch C, Szczepanik J, Nugent A et al. (2011). Lateralization and gender differences in the dopaminergic response to unpredictable reward in the human ventral striatum. Eur J Neurosci 33: 1706–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattay VS, Goldberg TE, Fera F et al. (2003). Catechol O-methyltransferase val158-met genotype and individual variation in the brain response to amphetamine. Proc Natl Acad Sci U S A 100: 6186–6191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride D, Barrett SP, Kelly JT et al. (2006). Effects of expectancy and abstinence on the neural response to smoking cues in cigarette smokers: an fMRI study. Neuropsychopharmacology 31: 2728. [DOI] [PubMed] [Google Scholar]
- McGowan S, Lawrence AD, Sales T et al. (2004). Presynaptic dopaminergic dysfunction in schizophrenia: a positron emission tomographic [18F] fluorodopa study. Arch Gen Psychiatry 61: 134–142. [DOI] [PubMed] [Google Scholar]
- McKee SA, Smith PH, Kaufman M et al. (2016). Sex differences in varenicline efficacy for smoking cessation: a meta-analysis. Nicotine Tob Res 18: 1002–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekarski J, Cutmore T, Suboski W (1996). Gender differences during processing of the Stroop task. Percept Mot Skills 83: 563–568. [DOI] [PubMed] [Google Scholar]
- Mental AS, Health Services Administration (2017). Results from the 2016 National Survey on drug use and health: detailed tables, Center for Behavioral Health Statistics and Quality, Rockville, MD2017. [Google Scholar]
- Milham M, Banich M, Webb A et al. (2001). The relative involvement of anterior cingulate and prefrontal cortex in attentional control depends on nature of conflict. Cogn Brain Res 12: 467–473. [DOI] [PubMed] [Google Scholar]
- Milham M, Banich M, Claus E et al. (2003). Practice-related effects demonstrate complementary roles of anterior cingulate and prefrontal cortices in attentional control. Neuroimage 18: 483–493. [DOI] [PubMed] [Google Scholar]
- Mo SJ, Linder J, Forsgren L et al. (2010). Pre-and postsynaptic dopamine SPECT in the early phase of idiopathic parkinsonism: a population-based study. Eur J Nucl Med Mol Imaging 37: 2154–2164. [DOI] [PubMed] [Google Scholar]
- Montagu K (1957). Catechol compounds in rat tissues and in brains of different animals. Nature 180: 244. [DOI] [PubMed] [Google Scholar]
- Montague PR, Hyman SE, Cohen JD (2004). Computational roles for dopamine in behavioural control. Nature 431: 760. [DOI] [PubMed] [Google Scholar]
- Morris ED, Yoder KK, Wang C et al. (2005). ntPET: a new application of PET imaging for characterizing the kinetics of endogenous neurotransmitter release. Molecular Imaging 4: 473–489. [DOI] [PubMed] [Google Scholar]
- Morris ED, Normandin MD, Schiffer WK (2008). Initial comparison of ntPET with microdialysis measurements of methamphetamine-induced dopamine release in rats: support for estimation of dopamine curves from PET data. Mol Imaging Biol 10: 67–73. [DOI] [PubMed] [Google Scholar]
- Morris RW, Fung SJ, Rothmond DA et al. (2010). The effect of gonadectomy on prepulse inhibition and fear-potentiated startle in adolescent rhesus macaques. Psychoneuroendocrinology 35: 896–905. [DOI] [PubMed] [Google Scholar]
- Morris ED, Lucas MV, Cosgrove KP (2013). How to study smoking and drinking with PET, positron emission tomography. In: Sandro Misciagna I (Ed.), Positron emission tomography—recent developments in instrumentation, research and clinical oncological practice. InTech; 103–150. [Google Scholar]
- Mozley LH, Gur RC, Mozley PD et al. (2001). Striatal dopamine transporters and cognitive functioning in healthy men and women. Am J Psychiatry 158: 1492–1499. [DOI] [PubMed] [Google Scholar]
- Mueller E, Kerschbaum H (2006). Progesterone and its metabolites 5-dihydroprogesterone and 5–3-tetrahydropr-ogesterone decrease LPS-induced NO release in the murine microglial cell line, BV-2. Neuro Endocrinol Lett 27: 675–678. Vol 272006. [PubMed] [Google Scholar]
- Munro CA, McCaul ME, Wong DF et al. (2006). Sex differences in striatal dopamine release in healthy adults. Biol Psychiatry 59: 966–974. [DOI] [PubMed] [Google Scholar]
- Nevalainen N, Riklund K, Andersson M et al. (2015). COBRA: a prospective multimodal imaging study of dopamine, brain structure and function, and cognition. Brain Res 1612: 83–103. [DOI] [PubMed] [Google Scholar]
- Ngun TC, Ghahramani N, Sánchez FJ et al. (2011). The genetics of sex differences in brain and behavior. Front Neuroendocrinol 32: 227–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieoullon A (2002). Dopamine and the regulation of cognition and attention. Prog Neurobiol 67: 53–83. [DOI] [PubMed] [Google Scholar]
- Nordström A-L, Olsson H, Halldin C (1998). A PET study of D2 dopamine receptor density at different phases of the menstrual cycle. Psychiatry Res Neuroimaging 83: 1–6. [DOI] [PubMed] [Google Scholar]
- Norman DA, Shallice T (1986). Attention to action. In: Consciousness and self-regulation, Springer; 1–18. [Google Scholar]
- Normandin MD, Morris ED (2006). Temporal resolution of ntPET using either arterial or reference region-derived plasma input functions. Paper presented at: 2006 international conference of the IEEE engineering in medicine and biology society. [DOI] [PubMed] [Google Scholar]
- Okita K, Petersen N, Robertson CL et al. (2016). Sex differences in midbrain dopamine D2-type receptor availability and association with nicotine dependence. Neuropsychopharmacology 41: 2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswald LM, Wand GS, Wong DF et al. (2015). Risky decision-making and ventral striatal dopamine responses to amphetamine: a positron emission tomography [11C] raclopride study in healthy adults. Neuroimage 113: 26–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overman WH (2004). Sex differences in early childhood, adolescence, and adulthood on cognitive tasks that rely on orbital prefrontal cortex. Brain Cogn 55: 134–147. [DOI] [PubMed] [Google Scholar]
- Packard MG, Cornell AH, Alexander GM (1997). Rewarding affective properties of intra-nucleus accumbens injections of testosterone. Behav Neurosci 111: 219–224. [DOI] [PubMed] [Google Scholar]
- Parsons LH, Justice JB (1992). Extracellular concentration and in vivo recovery of dopamine in the nucleus accumbens using microdialysis. J Neurochem 58: 212–218. [DOI] [PubMed] [Google Scholar]
- Passetti F, Clark L, Mehta M et al. (2008). Neuropsychological predictors of clinical outcome in opiate addiction. Drug Alcohol Depend 94: 82–91. [DOI] [PubMed] [Google Scholar]
- Patlak CS, Blasberg RG (1985). Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. Generalizations. J Cereb Blood Flow Metab 5: 584–590. [DOI] [PubMed] [Google Scholar]
- Patlak CS, Blasberg RG, Fenstermacher JD (1983). Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. J Cereb Blood Flow Metab 3: 1–7. [DOI] [PubMed] [Google Scholar]
- Patterson F, Jepson C, Loughead J et al. (2010). Working memory deficits predict short-term smoking resumption following brief abstinence. Drug Alcohol Depend 106: 61–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlson GD, Tune LE, Wong DF et al. (1993). Quantitative D2 dopamine receptor PET and structural MRI changes in late-onset schizophrenia. Schizophr Bull 19: 783–795. [DOI] [PubMed] [Google Scholar]
- Perkins KA (1996). Sex differences in nicotine versus nonnicotine reinforcement as determinants of tobacco smoking. Exp Clin Psychopharmacol 4: 166–177. [Google Scholar]
- Perkins KA, Donny E, Caggiula AR (1999). Sex differences in nicotine effects and self-administration: review of human and animal evidence. Nicotine Tob Res 1: 301–315. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Gerlach D, Vender J et al. (2001). Sex differences in the subjective and reinforcing effects of visual and olfactory cigarette smoke stimuli. Nicotine Tob Res 3: 141–150. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Jacobs L, Sanders M et al. (2002). Sex differences in the subjective and reinforcing effects of cigarette nicotine dose. Psychopharmacology (Berl) 163: 194–201. [DOI] [PubMed] [Google Scholar]
- Petry NM, Bickel WK, Arnett M (1998). Shortened time horizons and insensitivity to future consequences in heroin addicts. Addiction 93: 729–738. [DOI] [PubMed] [Google Scholar]
- Picazo O, Azcoitia I, Garcia-Segura LM (2003). Neuroprotective and neurotoxic effects of estrogens. Brain Res 990: 20–27. [DOI] [PubMed] [Google Scholar]
- Pillon B, Czernecki V, Dubois B (2003). Dopamine and cognitive function. Curr Opin Neurol 16: S17–S22. [DOI] [PubMed] [Google Scholar]
- Pohjalainen T, Rinne JO, Någren K et al. (1998). Sex differences in the striatal dopamine D2 receptor binding characteristics in vivo. Am J Psychiatry 155: 768–773. [DOI] [PubMed] [Google Scholar]
- Postma A,Winkel J,Tuiten A et al.(1999).Sex differences and menstrual cycle effects in human spatial memory. Psychoneuroendocrinology 24: 175–192. [DOI] [PubMed] [Google Scholar]
- Potvin S, Grignon S, Marchand S (2009). Human evidence of a supra-spinal modulating role of dopamine on pain perception. Synapse 63: 390–402. [DOI] [PubMed] [Google Scholar]
- Purves-Tyson TD, Handelsman DJ, Double KL et al. (2012). Testosterone regulation of sex steroid-related mRNAs and dopamine-related mRNAs in adolescent male rat substantia nigra. BMC Neurosci 13: 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purves-Tyson TD, Owens SJ, Double KL et al. (2014). Testosterone induces molecular changes in dopamine signaling pathway molecules in the adolescent male rat nigrostriatal pathway. PLoS One 9: e91151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn JJ, Hitchcott PK, Umeda EA et al. (2007). Sex chromosome complement regulates habit formation. Nat Neurosci 10: 1398. [DOI] [PubMed] [Google Scholar]
- Raab H, Pilgrim C, Reisert I (1995). Effects of sex and estrogen on tyrosine hydroxylase mRNA in cultured embryonic rat mesencephalon. Mol Brain Res 33: 157–164. [DOI] [PubMed] [Google Scholar]
- Reavis R, Overman WH (2001). Adult sex differences on a decision-making task previously shown to depend on the orbital prefrontal cortex. Behav Neurosci 115: 196. [DOI] [PubMed] [Google Scholar]
- Rehavi M, Goldin M, Roz N et al. (1998). Regulation of rat brain vesicular monoamine transporter by chronic treatment with ovarian hormones. Mol Brain Res 57: 31–37. [DOI] [PubMed] [Google Scholar]
- Riccardi P, Li R, Ansari MS et al. (2006). Amphetamine-induced displacement of [18 F] fallypride in striatum and extrastriatal regions in humans. Neuropsychopharmacology 31: 1016. [DOI] [PubMed] [Google Scholar]
- Riccardi P, Park S, Anderson S et al. (2011). Sex differences in the relationship of regional dopamine release to affect and cognitive function in striatal and extrastriatal regions using positron emission tomography and [18F] fallypride. Synapse 65: 99–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DC, Bennett SA, Vickers GJ (1989). The estrous cycle affects cocaine self-administration on a progressive ratio schedule in rats. Psychopharmacology (Berl) 98: 408–411. [DOI] [PubMed] [Google Scholar]
- Roesch-Ely D, Scheffel H, Weiland S et al. (2005). Differential dopaminergic modulation of executive control in healthy subjects. Psychopharmacology (Berl) 178: 420–430. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Sun WL, Minerly ACE et al. (2008). Progesterone attenuates cocaine-induced conditioned place preference in female rats. Brain Res 1189: 229–235. [DOI] [PubMed] [Google Scholar]
- Ryding E, Lindström M, Brådvik B et al. (2004). A new model for separation between brain dopamine and serotonin transporters in 123 I-β-CIT SPECT measurements: normal values and sex and age dependence. Eur J Nucl Med Mol Imaging 31: 1114–1118. [DOI] [PubMed] [Google Scholar]
- SAMHSA (2015). Results from the 2014 national survey on drug use and health, Substance Abuse and Mental Health Services Administration, Rockville, MD, USA. [Google Scholar]
- Sapienza P, Zingales L, Maestripieri D (2009). Gender differences in financial risk aversion and career choices are affected by testosterone. Proc Natl Acad Sci U S A 106: 15268–15273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarmány I (1977). Different performance in Stroop’s interference test from the aspect of personality and sex. Stud. Psychol. 19: 60–67. [Google Scholar]
- Sawaguchi T, Goldman-Rakic PS (1991). D1 dopamine receptors in prefrontal cortex: involvement in working memory. Science 251: 947–950. [DOI] [PubMed] [Google Scholar]
- Scholes KE, Harrison BJ, O’Neill BV et al. (2007). Acute serotonin and dopamine depletion improves attentional control: findings from the stroop task. Neuropsychopharmacology 32: 1600. [DOI] [PubMed] [Google Scholar]
- Schott BH, Seidenbecher CI, Fenker DB et al. (2006). The dopaminergic midbrain participates in human episodic memory formation: evidence from genetic imaging. J Neurosci 26: 1407–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W (1998). Predictive reward signal of dopamine neurons. J Neurophysiol 80: 1–27. [DOI] [PubMed] [Google Scholar]
- Sevy S, Hassoun Y, Bechara A et al. (2006). Emotion-based decision-making in healthy subjects: short-term effects of reducing dopamine levels. Psychopharmacology (Berl) 188: 228–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JL, Koenig JI (2007). Evidence for the involvement of ERb and RGS9–2 in 17-β estradiol enhancement of amphetamine-induced place preference behavior. Horm Behav 52: 146–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CT, Sierra Y, Oppler SH et al. (2014). Ovarian cycle effects on immediate reward selection bias in humans: a role for estradiol. J Neurosci 34: 5468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PH, Bessette AJ, Weinberger AH et al. (2016). Sex/gender differences in smoking cessation: a review. Prev Med 92: 135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CT, Dang LC, Burgess LL et al. (2019). Lack of consistent sex differences in D-amphetamine-induced dopamine release measured with [18 F] fallypride PET. Psychopharmacology (Berl) 236: 581–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu M, Mooney M (2009). Subjective responses to intravenous nicotine: greater sensitivity in women than in men. Exp Clin Psychopharmacol 17: 63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu M, Babb DA, Hatsukami DK (2002). Effects of progesterone treatment on smoked cocaine response in women. Pharmacol Biochem Behav 72: 431–435. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, DeVito EE, Waters AJ et al. (2013). Cognitive enhancement as a treatment for drug addictions. Neuropharmacology 64: 452–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Onge JR, Floresco SB (2009). Dopaminergic modulation of risk-based decision making. Neuropsychopharmacology 34: 681. [DOI] [PubMed] [Google Scholar]
- Staley JK, Krishnan-Sarin S, Zoghbi S et al. (2001). Sex differences in [123I] β-CIT SPECT measures of dopamine and serotonin transporter availability in healthy smokers and nonsmokers. Synapse 41: 275–284. [DOI] [PubMed] [Google Scholar]
- Stanton SJ, Liening SH, Schultheiss OC (2011). Testosterone is positively associated with risk taking in the Iowa gambling task. Horm Behav 59: 252–256. [DOI] [PubMed] [Google Scholar]
- Stevens L, Betanzos-Espinosa P, Crunelle CL et al. (2013). Disadvantageous decision-making as a predictor of dropout among cocaine-dependent individuals in long-term residential treatment. Front Psych 4: 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens L, Goudriaan A, Verdejo-Garcia A et al. (2015). Impulsive choice predicts short-term relapse in substance-dependent individuals attending an in-patient detoxification programme. Psychol Med 45: 2083–2093. [DOI] [PubMed] [Google Scholar]
- Stroop JR (1935). Studies of interference in serial verbal reactions. J Exp Psychol 18: 643. [Google Scholar]
- Swerdlow NR, Filion D, Geyer MA et al. (1995). “Normal” personality correlates of sensorimotor, cognitive, and visuospatial gating. Biol Psychiatry 37: 286–299. [DOI] [PubMed] [Google Scholar]
- Tuchman E (2010). Women and addiction: the importance of gender issues in substance abuse research. J Addict Dis 29: 127–138. [DOI] [PubMed] [Google Scholar]
- Uban KA, Rummel J, Floresco SB et al. (2011). Estradiol modulates effort-based decision making in female rats. Neuropsychopharmacology 37: 390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban NB, Kegeles LS, Slifstein M et al. (2010). Sex differences in striatal dopamine release in young adults after oral alcohol challenge: a positron emission tomography imaging study with [11C] raclopride. Biol Psychiatry 68: 689–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bos R, den Heijer E, Vlaar S et al. (2009). Exploring gender differences in decision-making using the Iowa Gambling Task. In: Encyclopedia of psychology of decision making, Nova Science Publishers, Inc., New York, pp. 1115–1134. [Google Scholar]
- van den Bos R, Homberg J, de Visser L (2013). A critical review of sex differences in decision-making tasks: focus on the Iowa gambling task. Behav Brain Res 238: 95–108. [DOI] [PubMed] [Google Scholar]
- Van der Elst W, Van Boxtel MP, Van Breukelen GJ et al. (2006). The Stroop color-word test: influence of age, sex, and education; and normative data for a large sample across the adult age range. Assessment 13: 62–79. [DOI] [PubMed] [Google Scholar]
- Van Dyck CH, Seibyl JP, Malison RT et al. (1995). Age-related decline in striatal dopamine transporter binding with iodine-123-b-CITSPECT. J Nucl Med 36: 1175–1181. [PubMed] [Google Scholar]
- Van Dyck CH, Malison RT, Seibyl JP et al. (2000). Age-related decline in central serotonin transporter availability with [123I] β-CIT SPECT. Neurobiol Aging 21: 497–501. [DOI] [PubMed] [Google Scholar]
- van Gaalen MM, van Koten R, Schoffelmeer AN et al. (2006). Critical involvement of dopaminergic neurotransmission in impulsive decision making. Biol Psychiatry 60: 66–73. [DOI] [PubMed] [Google Scholar]
- Varrone A, Dickson JC, Tossici-Bolt L et al. (2013). European multicentre database of healthy controls for [123 I] FP-CIT SPECT (ENC-DAT): age-related effects, gender differences and evaluation of different methods of analysis. Eur J Nucl Med Mol Imaging 40: 213–227. [DOI] [PubMed] [Google Scholar]
- Vijayraghavan S, Wang M, Birnbaum SG et al. (2007). Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat Neurosci 10: 376. [DOI] [PubMed] [Google Scholar]
- Volkow N, Morales M (2015). The brain on drugs: from reward to addiction. Cell 162: 712–725. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang G-J et al. (2001). Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Am J Psychiatry 158: 2015–2021. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang G-J et al. (2007). Dopamine in drug abuse and addiction: results of imaging studies and treatment implications. Arch Neurol 64: 1575–1579. [DOI] [PubMed] [Google Scholar]
- Volkow N, Fowler J, Wang G et al. (2009). Imaging dopamine’s role in drug abuse and addiction. Neuropharmacology 56: 3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang G-J, Fowler JS et al. (2011). Addiction: beyond dopamine reward circuitry. Proc Natl Acad Sci U S A 108: 15037–15042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wise RA, Baler R (2017). The dopamine motive system: implications for drug and food addiction. Nat Rev Neurosci 18: 741. [DOI] [PubMed] [Google Scholar]
- Voyer D, Voyer SD, Saint-Aubin J (2017). Sex differences in visual-spatial working memory: a meta-analysis. Psychon Bull Rev 24: 307–334. [DOI] [PubMed] [Google Scholar]
- Walker QD, Rooney MB, Wightman RM et al. (1999). Dopamine release and uptake are greater in female than male rat striatum as measured by fast cyclic voltammetry. Neuroscience 95: 1061–1070. [DOI] [PubMed] [Google Scholar]
- Wang M, Yang Y, Wang C-J et al. (2013). NMDA receptors subserve persistent neuronal firing during working memory in dorsolateral prefrontal cortex. Neuron 77: 736–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil-Malherbe H, Bone A (1957). Intracellular distribution of catecholamines in the brain. Nature 180: 1050. [DOI] [PubMed] [Google Scholar]
- White FJ (2002). A behavioral/systems approach to the neuroscience of drug addiction. J Neurosci 22: 3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlow CT, Liguori A, Livengood LB et al. (2004). Long-term heavy marijuana users make costly decisions on a gambling task. Drug Alcohol Depend 76: 107–111. [DOI] [PubMed] [Google Scholar]
- Williams J, Wellman N, Geaney D et al. (1996). Haloperidol reduces stroop interference and increases negative priming in healthy people. Schizophr Res 2: 223. [Google Scholar]
- Wise RA (2004). Dopamine, learning and motivation. Nat Rev Neurosci 5: 483. [DOI] [PubMed] [Google Scholar]
- Wise RA (2009). Roles for nigrostriatal—not just mesocorticolimbic—dopamine in reward and addiction. Trends Neurosci 32: 517–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong DF, Broussolle EP, Wand G et al. (1988). In vivo measurement of dopamine receptors in human brain by positron emission tomography age and sex differences a. Ann N Y Acad Sci 515: 203–214. [DOI] [PubMed] [Google Scholar]
- Wong KK, Müller ML, Kuwabara H et al. (2012). Gender differences in nigrostriatal dopaminergic innervation are present at young-to-middle but not at older age in normal adults. J Clin Neurosci 19: 183–184. [DOI] [PubMed] [Google Scholar]
- Wood RI (2008). Anabolic–androgenic steroid dependence? Insights from animals and humans. Front Neuroendocrinol 29: 490–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley SC, O’Malley B, Lydon J et al. (2006). Genotype differences in behavior and tyrosine hydroxylase expression between wild-type and progesterone receptor knockout mice. Behav Brain Res 167: 197–204. [DOI] [PubMed] [Google Scholar]
- Worhunsky PD, Matuskey D, Gallezot J-D et al. (2017). Regional and source-based patterns of [(11)C]-(+)-PHNO binding potential reveal concurrent alterations in dopamine D(2) and D(3) receptor availability in cocaine-use disorder. Neuroimage 148: 343–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L, Becker JB (1994). Quantitative microdialysis determination of extracellular striatal dopamine concentration in male and female rats: effects of estrous cycle and gonadectomy. Neurosci Lett 180: 155–158. [DOI] [PubMed] [Google Scholar]
- Xu J, Azizian A, Monterosso J et al. (2008). Gender effects on mood and cigarette craving during early abstinence and resumption of smoking. Nicotine Tob Res 10: 1653–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H, Arimura S, Nakanishi A et al. (2017). Age-related effects and gender differences in Japanese healthy controls for [123 I] FP-CIT SPECT. Ann Nucl Med 31: 407–412. [DOI] [PubMed] [Google Scholar]
- Zahrt J, Taylor JR, Mathew RG et al. (1997). Supranormal stimulation of D1 dopamine receptors in the rodent prefrontal cortex impairs spatial working memory performance. J Neurosci 17: 8528–8535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakiniaeiz Y, Potenza MN (2018). Gender-related differences in addiction: a review of human studies. Curr Opin Behav Sci 23: 171–175. [Google Scholar]
- Zakiniaeiz Y, Cosgrove KP, Potenza MN et al. (2016). Balance of the sexes: addressing sex differences in preclinical research. Yale J Biol Med 89: 255–259. [PMC free article] [PubMed] [Google Scholar]
- Zakiniaeiz Y, Hillmer AT, Matuskey D et al. (2019). Sex differences in amphetamine-induced dopamine release in the dorsolateral prefrontal cortex of tobacco smokers. Neuropsychopharmacology 44: 2205–2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zocchi A, Varnier G, Sartori I et al. (2003). Dopamine response to drugs of abuse in the nucleus accumbens of the mouse: a shell-core investigation. Eur Neuropsychopharmacol 13: S31–S32. [DOI] [PubMed] [Google Scholar]


