Abstract
Nonstructural glycoprotein NS1, specified by dengue virus type 1 (Den-1), is secreted from infected green monkey kidney (Vero) cells in a major soluble form characterized by biochemical and biophysical means as a unique hexameric species. This noncovalently bound oligomer is formed by three dimeric subunits and has a molecular mass of 310 kDa and a Stokes radius of 64.4 Å. During protein export, one of the two oligosaccharides of NS1 is processed into an endo-β-N-acetylglucosaminidase F-resistant complex-type sugar while the other remains of the polymannose type, protected in the dimeric subunit from the action of maturation enzymes. Complete processing of the complex-type sugar appears to be required for efficient release of soluble NS1 into the culture fluid of infected cells, as suggested by the repressive effects of the N-glycan processing inhibitors swainsonine and deoxymannojyrimicin. These results, together with observations related to the absence of secretion of NS1 from Den-infected insect cells, suggest that maturation and secretion of hexameric NS1 depend on the glycosylation status of the host cell.
Dengue (Den) is one of the most threatening mosquito-borne viral diseases of humans. It is widely spread in the tropical and subtropical areas of the world, and its incidence, in terms of morbidity and mortality, has increased dramatically over the past 20 years. The etiological agent belongs to the flavivirus genus of the family Flaviviridae, which comprises other major human pathogens such as Japanese encephalitis, yellow fever, and tick-borne encephalitis viruses (3, 35). Flaviviruses are enveloped, single-stranded, positive-sense RNA viruses formed by three structural proteins. Protein C (capsid), enclosing the genome, forms a nucleocapsid that is surrounded by a lipid bilayer in which are anchored proteins M (membrane) and E (envelope). The genome is approximately 11 kb long and contains a single open reading frame encoding a polyprotein precursor of about 3,400 amino acid residues. Individual viral proteins are generated from this precursor by the action of cellular and viral proteases (44). The three structural proteins derive from the N-terminal part of the polyprotein and are followed by seven nonstructural proteins: NS1, NS2A/2B, NS3, NS4A/4B, and NS5. The two cytosolic proteins, NS3 and NS5, have been identified as the viral protease/helicase and polymerase, respectively (44); the roles of the other nonstructural proteins remain to be determined.
Glycoprotein NS1, present in all flaviviruses, appears to be essential for virus viability. This protein contains two conserved N-glycosylation sites and 12 invariant cysteine residues (3, 35). NS1 is inserted into the lumen of the endoplasmic reticulum (ER) via a signal peptide that is cleaved cotranslationnally by the action of a cellular signalase to generate the N terminus of the protein (3). NS2A is released from the C-terminal end of NS1 by an ER resident host protease (15). Although NS1 does not contain an identifiable membrane-anchoring domain, NS1 becomes associated with membranous components upon dimerization (52, 53). Recently, intracellular NS1 was shown to be involved in the early steps of viral replication (31, 32, 36, 37, 51), in agreement with its retention in intracellular organelles of the infected cell (33, 40) and its ability to interact with membranes (8, 16, 18, 52). However, intracellular NS1 faces the lumen of the ER and may play a role only indirectly in the replication process. Dimerization is also a prerequisite for NS1 protein export along the secretory pathway to the plasma membrane (41), where it remains as the unique viral resident protein of the infected cell surface (19, 45, 50). In mammalian cells, but not in insect cell lines derived from Aedes albopictus which support flavivirus infection (33, 52), part of transported NS1 is released into the extracellular milieu. Extracellular NS1 is secreted either as a soluble protein, which may be present in a higher oligomeric form than a dimer (4, 5), or in association with microparticles but not with virions (14, 16, 29, 30, 33). In addition, NS1 has been found circulating in sera from Den virus-infected patients (13), suggesting that secretion of NS1 may be an important event in flavivirus infection in the human host.
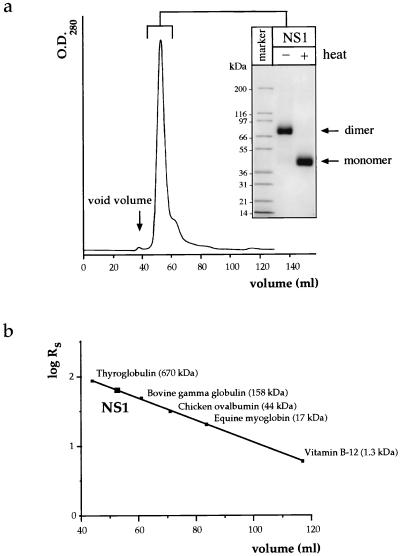
In this study, we were interested in the biochemical characterization of the major extracellular form of NS1 released from Den virus type 1 (Den-1)-infected Vero cells. Vero cells were infected at a multiplicity of infection (m.o.i) of 1 focus forming unit of Den-1, strain FGA/89 (7), per cell. The supernatant was harvested at 5 days postinfection, clarified by centrifugation to eliminate cell debris, and treated with 7.5% polyethylene glycol (PEG) 6000 to precipitate particles, such as virions and any remaining membraneous components. The PEG-precipitable fraction contained less than 5% of the total amount of NS1 released in the extracellular fluid. NS1 found in this fraction could represent a microparticulate form of NS1, as has been noted for Japanese encephalitis virus infection of Vero cells (33). Soluble NS1, accumulating at concentrations of 5 to 10 μg/ml (as determined by enzyme-linked immunosorbent assay [data not shown]), was purified from the PEG-clarified supernatant by immunoaffinity chromatography with a column prepared with the anti-NS1 Den virus-specific monoclonal antibody (MAb) 3D1.4, kindly provided by A. Falconar. For this purpose, antibodies contained in a MAb-enriched ascitic fluid were recovered by ammonium sulfate precipitation and ion-exchange chromatography (DEAE-Trisacryl M matrix; Sepracor) and further coupled to CNBr-activated Sepharose 4B (Pharmacia), according to the manufacturer’s instructions. The PEG-treated supernatant was passed over the immunoadsorbant at a low flow rate, and bound protein was subsequently eluted at a basic pH of 11.2, conditions that were previously shown to preserve the dimeric state of the protein (14). The eluted protein was concentrated by ultrafiltration, the buffer was exchanged for phosphate-buffered saline (PBS), and the sample was subjected to size exclusion chromatography (SEC) to determine both the apparent molecular mass and the degree of homogeneity of the purified product (Fig. 1). One major peak that solely contains the NS1 protein is apparent on the chromatogram, as detected by Coomassie blue staining after sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (Fig. 1a). NS1 exhibits a Stokes radius (RS) of 64.4 Å (Fig. 1b), corresponding to an apparent molecular mass of about 300 kDa. In SDS-PAGE, however, only the SDS-resistant dimer of NS1, migrating with an apparent molecular mass of 80 kDa, can be visualized (Fig. 1a), and it is converted into the monomer (50 kDa) by heat denaturation. No species with an apparent molecular mass of 300 kDa can be detected on the gel (Fig. 1a), suggesting that if a higher oligomeric species is initially present, it dissociates into its dimeric subunits in SDS-PAGE. We used analytical ultracentrifugation to determine unequivocally the molecular mass of purified soluble NS1 (data not shown), since a high RS value could be due to an elongated form of the molecule with a smaller mass than expected. The sedimentation coefficient obtained by this method for NS1 recovered from the SEC column is 11.2S (data not shown). This, together with an RS value of 64.4 Å, yields a molecular mass for NS1 of 310 kDa, consistent with the presence of a hexamer. If a hexamer, the molecular mass of NS1, estimated from its amino acid sequence, would be 240.9 kDa. Since each monomer is glycosylated at two positions, we can roughly add 8 to 10 kDa per monomer (see Fig. 4a), reaching a value of about 300 kDa as an upper limit. Given that hydration effects usually tend to increase the molecular mass determined experimentally, the results obtained are concordant with the existence of a hexamer of NS1, as was suggested previously for NS1 from tick-borne encephalitis virus (4, 5).
FIG. 1.
Analysis of immunoaffinity-purified soluble extracellular NS1 by SEC. (a) Immunoaffinity-purified NS1 was submitted to SEC on an S300 gel filtration column, and the single peak was concentrated by ultrafiltration and analyzed on a 4 to 20% gradient SDS-polyacrylamide gel stained by Coomassie blue. The protein was either unheated or heated to 95°C for 3 min prior to electrophoresis. (b) By comparison with protein standards used to calibrate the SEC column, the NS1 protein exhibited a Stokes radius (RS) of 64.4 Å, corresponding to an apparent molecular mass of 300 kDa.
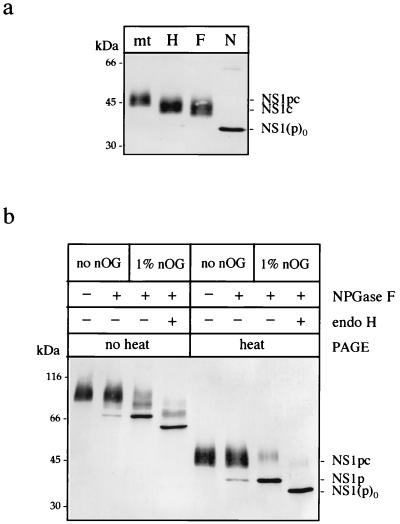
FIG. 4.
Analysis of the nature and accessibility of the two oligosaccharides of extracellular NS1. (a) Extracellular NS1 was mock treated (mt) or treated for 1 h at 37°C with endo H (H), endo F (F), or NPGase F (N) in its monomeric form. Samples were placed in nonreducing Laemmli sample buffer and separated by SDS–12% PAGE, and the resulting products were detected by immunoblotting after transfer to nitrocellulose. “p” indicates the presence of a polymannose-type oligosaccharide; “c” indicates a complex-type oligosaccharide on the protein. NS1(p)0 is the deglycosylated form of the protein. (b) Purified NS1 was either mock treated or treated with NPGase F for 1 h at 37°C in 10 mM sodium phosphate buffer, pH 7.5. One sample was further treated by endo H after the pH was readjusted to 5.5 with 50 mM citrate buffer. All samples were subjected to SDS–12% PAGE with or without preliminary heat denaturation in nonreducing Laemmli sample buffer.
Purified NS1 is a unique hexameric species.
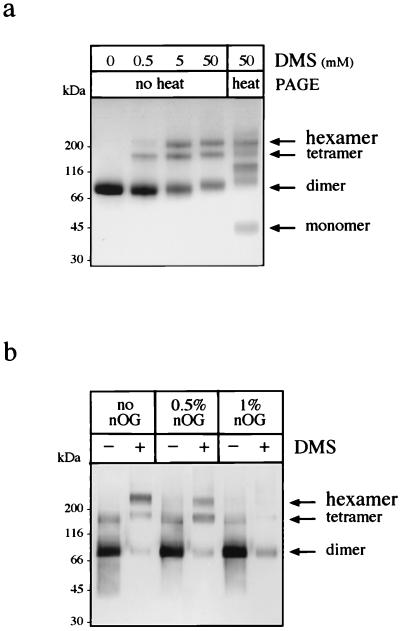
To further characterize the hexameric form of NS1, we carried out chemical cross-linking on purified NS1 (Fig. 2a) with the bifunctional cross-linker dimethylsuberimidate (DMS) (Pierce). DMS, solubilized in 200 mM triethanolamine, was added to a cold solution of NS1 in 50 mM triethanolamine (pH 8.5)–100 mM NaCl, and the reaction was carried out for 1 h at room temperature. The resulting products were subjected to SDS-PAGE (4 to 20% gradient gel) and stained with Coomassie blue (Fig. 2a). At 0.5 mM DMS, we observed the formation of tetrameric intermediates and cross-linked hexamers, the latter species accumulating with increasing concentrations of DMS, as would be expected for a hexameric species formed by dimeric building blocks. Boiling the sample treated with the highest concentration of DMS prior to electrophoresis generates species ranging from monomers to hexamers. Hexamers, as opposed to dimeric subunits, have been shown to be sensitive to very low concentrations of SDS (5). We analyzed the nature of the interfaces between dimers by subjecting hexameric NS1 to the action of the nonionic detergent n-octylglucoside (nOG) (Fig. 2b). Purified NS1 was incubated overnight at 37°C in the absence or presence of nOG (0.5 or 1%) and subsequently treated with 25 mM DMS. The resulting products were separated on a 4 to 20% acrylamide gradient gel, transferred to a nitrocellulose membrane, and revealed with MAb 3D1.4. In the samples that did not contain nOG, bands corresponding to dimers, tetramers, and hexamers were produced after DMS treatment, although it can be noted that the DMS-treated dimer was poorly recognized by MAb 3D1.4 compared to the cross-linked hexamer or to the noncovalently bound dimer. The hexamer was found to be partially dissociated by 0.5% nOG, as shown by the decrease in hexameric species and the concomitant increase in the amount of tetrameric intermediates. Levels of tetramers and hexamers were undetectable after treatment of the purified product with 1% nOG (Fig. 2b), suggesting that the protein is converted into dimers under these conditions. This suggests that the interfaces stabilizing the dimeric subunits are essentially weak hydrophobic interactions.
FIG. 2.
Purified extracellular NS1 is a hexamer that can be converted to its dimeric subunit in the presence of the nonionic detergent nOG, as demonstrated by chemical cross-linking. (a) Subsequent to SEC, the protein was concentrated to 0.5 mg/ml by ultrafiltration and treated with final concentrations of 0, 0.5, 5, and 50 mM DMS. The resulting products were placed in nonreducing Laemmli sample buffer, separated on a 4 to 20% gradient acrylamide gel, and stained with Coomassie blue. One sample, treated with 50 mM DMS, was treated for 3 min at 95°C prior to electrophoresis to dissociate noncovalently linked oligomers. (b) Purified NS1 was treated overnight at 37°C with 0, 0.5, or 1% nOG and submitted or not submitted to 25 mM DMS for 1 h. Proteins were separated without heat denaturation on a 4 to 20% gradient acrylamide gel and detected by immunoblotting with MAb 3D1.4.
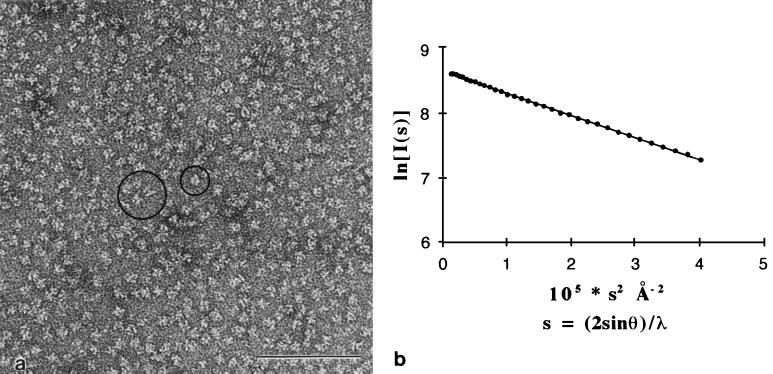
We examined the homogeneity of our purified NS1 preparation by electron microscopy, both by negative staining (Fig. 3a) and by rotary shadowing (data not shown). In both cases, we observed a homogeneous distribution of isometric particles of a diameter of 11 ± 2 nm. Most of the particles did not display any identifiable symmetry. A few of them, however, seemed to show a twofold rotational symmetry (large circle in Fig. 3a) or a threefold rotational symmetry (small circle). The fact that the electron micrographs show twofold and threefold rotational symmetries for some of the particles suggests that the NS1 hexamer has 32-point symmetry. Since the particles are oriented randomly on the electron microscope grid, it is expected that symmetry will be apparent only for those particles that happen to lie with one symmetry axis normal to the plane of the grid. There is evidence of a space between subunits in the hexamer, in particular in particles seen along the twofold rotational axis.
FIG. 3.
Hexameric NS1 appears as a unique species by electron microscopy and SAXS analysis. (a) Negatively stained sample of purified NS1 at 0.2 mg/ml observed by electron microscopy at 80 kV. The image shows a field containing NS1 particles in random orientations. Dimensions are quite homogeneous, with a diameter of roughly 11 nm. Occasionally, particles lying with a twofold (large circle) or a threefold (small circle) axis of symmetry perpendicular to the plane of the figure are seen. Bar, 0.1 μm. (b) Guinier plot of purified NS1 at a protein concentration of 6.2 mg/ml. The radius of gyration (see text) calculated from the slope, Rg, is 5 ± 0.1 nm. Note that the curve is linear from very small angles (lower s values), showing that there is a single form of NS1 protein in solution. The molecular mass estimated from extrapolation to s = 0 is 300 ± 50 kDa.
To confirm the monodispersity of the protein in solution, we tested our purified NS1 preparation by small-angle X-ray scattering (SAXS) on a synchrotron source (Fig. 3b). The detection instrument (6), the data acquisition system (2) and the thermostated cell under vacuum (10) have been described previously. This method provides a very sensitive test to check the homogeneity of a given macromolecule in solution. In addition, it provides the radius of gyration, Rg, of the molecule (which is the second moment of the electron density distribution of the particle), as well as the molecular weight. At small angles, the scattering pattern of a monodisperse solution can be approximated by a Gaussian curve the width of which yields the radius of gyration of the macromolecule (21). A plot of intensity as a function of the square of the scattering vector s, s = (2sinθ)/λ (where 2θ is the scattering angle and λ is the wavelength of the incident beam), called “Guinier plot,” should thus give a straight line if the solution is monodisperse. The slope provides the Rg, and extrapolation of this straight line to s = 0 provides the total intensity, I(0), scattered in the direction of the incident beam. This value is proportional to the molecular mass of the protein and to the concentration of the protein in solution. The data were collected at different protein concentrations by using synchrotron radiation at a wavelength of 1.488 Å and a sample-to-detector distance of 2,580 mm. Figure 3b shows the Guinier plot of NS1 at a concentration of 6.2 mg/ml in solution. The scattering curve is indeed Gaussian, even at very small angles. This plot proves that purified NS1 is a unique species and does not form any oligomeric structures larger than a hexamer. The value we obtained for Rg, 5 ± 0.1 nm, is in agreement with the diameter found by electron microscopy and with the Stokes radius, RS, obtained by SEC. The molecular mass of NS1 was estimated by using a reference solution of rotavirus protein VP6 (which forms a trimer of 120 kDa) at defined concentrations and yielded a value of roughly 300 kDa, in agreement with the data obtained from analytical centrifugation and SEC. The fairly large radius of gyration of hexameric NS1 (Rg of 5 nm) indicates that the electron distribution is away from the center of the particle. A compact spherical molecule with a uniform electron density distribution and an identical Rg would have a molecular mass of 106 Da, more than three times the actual mass of NS1. All of this is in agreement with electron micrographs that show a space between subunits down the twofold symmetry axis of the particle, as explained above. This case is reminiscent of the hexameric enzyme aspartyl transcarbamylase, which has a molecular mass of 300 kDa and 32-point symmetry. The relaxed state of this allosteric enzyme has an Rg of 5 nm (22) and contains a big cavity in the center, as observed by X-ray crystallography (23). The presence of such cavities in the structure of hexameric NS1 would thus explain the discrepancy we find between the second moment of electron density distribution and the molecular weight of the hexamer.
Maturation of NS1 carbohydrates is site dependent and may be required in the secretion process.
The glycosylation status of extracellular NS1 was determined by using the endoglycosidases endo-β-N-acetylglucosaminidase H (endo H), endo-β-N-acetylglucosaminidase F (endo F), and peptide-N-(acetyl-β-glucosaminyl)asparagine amidase (NPGase F) (Fig. 4a). Purified NS1 was incubated for 3 min at 95°C in the presence of 1% SDS prior to digestion to convert dimeric subunits into monomers. It was then necessary to add 1% Nonidet P-40 to the sample before adding endoglycosidase in order to avoid inactivation of the enzyme by SDS. At the end of the 1-h incubation period at 37°C, samples were subjected to SDS-PAGE in nonreducing Laemmli sample buffer and detected by immunoblotting with anti-NS1 MAb 3D1.4 (Fig. 4a). Although NPGase F is able to cleave both sugars, yielding the unglycosylated form of NS1 [NS1(p)0] (Fig. 4a), endo H and endo F cleave only one of the two glycans, leaving the complex-type sugar (NS1c) attached to the polypeptide chain (Fig. 4a). The experiment with endo F was also carried out at an acidic pH of 5.5, which was reported to be more favorable to the action of the enzyme, but no further digestion was observed (data not shown). These digestion profiles show that the observed microheterogeneity of extracellular NS1 on SDS-PAGE is due exclusively to the complex-type carbohydrate moiety. More importantly, they suggest that only one sugar is modified into a multibranched—at least triantennary—complex chain (47), the second glycan being maintained as a high-mannose type. This indicates that the latter is protected from the action of enzymes of N-glycan biosynthesis during transport of the protein to the cell surface. This particular feature has been described for the NS1 glycoprotein of several flaviviruses (8, 33, 42, 53). Examples of site-specific glycosylation, in which maturation of the glycan depends on the particular environment in the three-dimensional structure of the protein or in the quaternary structure of a protein complex, have been reported (25, 26, 34, 38).
Accessibility of the carbohydrate side chains on the oligomeric structures of NS1 was assessed in vitro by NPGase F, the reactivity of which appears to be highly dependent on the free exposure of its substrate (47, 48). Purified NS1 was treated with NPGase F in its native state or in the presence of 1% nOG, known from the experiment reported in Fig. 2b to disrupt interactions between the dimeric subunits. At the end of the 1-h incubation period, samples were placed in nonreducing Laemmli buffer and either boiled or not boiled prior to analysis by Western blotting (Fig. 4b). The polymannose-type sugar (designated p) is not accessible to the enzymatic cleavage of NPGase F when the protein is present in a dimeric form (NS1p) (Fig. 4b) and is hydrolyzed only when the protein is subsequently subjected to endo H, which still recognizes its polymannose-rich substrate under these conditions [NS1(p)0] (Fig. 4b). We observed a similar profile for the intracellular form of the protein (data not shown), suggesting that the dimeric association may by itself protect one of the N-glycans against further maturation in the Golgi. It corroborates previous studies showing that a mutation at the second glycosylation site on the polypeptide chain, accordingly lacking the polymannose-type sugar, significantly reduced dimer stability and secretion of the protein (42). Interestingly, NPGase F appeared to be unable to reach either of its two substrates on native hexameric NS1 (NS1pc) (Fig. 4b). The fact that the complex-type carbohydrate moiety has acquired endo F resistance during transport of NS1 along the secretory pathway favors the view of prolonged accessibility of this particular oligosaccharide to the specific enzymes of glycan biosynthesis, as opposed to the polymannose-type glycan. When processing of the complex glycan is completed, it could become somewhat cryptic after a change in protein conformation. This would suggest that the complex-type sugar may be involved in triggering or stabilizing a mature form of the hexamer, a role similar to that of the polymannose-type sugar in the dimeric association (42). In favor of this hypothesis are the results obtained with the N-glycosylation inhibitors swainsonine and deoxymannojyrimicin (DMJ).
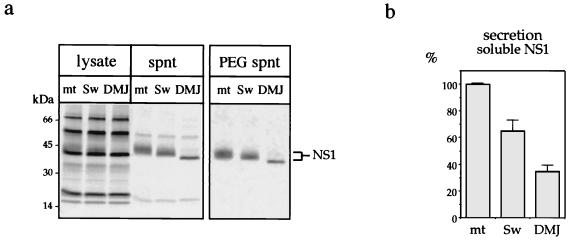
To evaluate the importance of maturation of the complex sugar chain on the processing and secretion of NS1, infected cells were treated with glycosylation inhibitors that specifically block steps in the mannose trimming of N-glycans in the ER or Golgi (11, 12, 24) but that do not interfere with the preceding glucose trimming required for chaperone-mediated protein folding (49). Swainsonine blocks Golgi mannosidase II and leads to the generation of hybrid glycans instead of complex-type sugars; DMJ inhibits mannosidase I and gives rise to polymannose-rich carbohydrates. After treatment with the glycosylation inhibitors, the presence of radiolabeled extracellular NS1 was analyzed by immunoprecipitation of proteins from total culture fluids or from supernatants recovered after 7.5% PEG precipitation and containing only the soluble form of NS1 (Fig. 5a). Experiments were carried out with polyclonal ascitic fluid (PAb) directed against Den virus antigens, MAb 13A1 (not shown), or MAb 3D1.4 (not shown), and immunoprecipitated products were boiled prior to electrophoresis. When the PAb was used on total supernatants, two proteins other than NS1 were precipitated (Fig. 5a). These proteins migrate at molecular masses of about 20 and 60 kDa and are neither present in the MAb immunoprecipitates nor in the PEG supernatants precipitated with the PAb. The natures of the two proteins are unknown, but they could be structural proteins C (or prM) and E. The molecular weight of NS1 released from swainsonine-treated cells is slightly lower than that of NS1 in mock-treated supernatants, according to a hybrid-type structure replacing the complex-type sugar. In DMJ-treated supernatants, NS1 migrates with a significantly lower molecular weight and is a homogeneous species in SDS-PAGE, in agreement with the presence of polymannose-rich glycans only.
FIG. 5.
Altered secretion of NS1 from infected cells treated with N-glycan processing inhibitors. (a) Cells were infected with Den virus at an m.o.i. of 3. At 7 h postinfection, swainsonine (Sw) and DMJ were added for 20 h at concentrations of 5 μM and 1 mM, respectively. Proteins were then radiolabeled for 3.5 h; immunoprecipitated with Den virus-specific PAb from cell lysates, total supernatants (spnt), or PEG-treated supernatants; separated by SDS-PAGE; and analyzed by autoradiography. (b) NS1-related radioactive signals from PEG-treated supernatants immunoprecipitated with the PAb were quantified directly from the gels with a PhophorImager. Average values of at least three independent experiments and corresponding standard deviations are reported on the graph. mt, mock treatment.
Both glycosylation inhibitors appear to have a down-regulating effect on secretion of NS1 but not of the two others proteins recognized by the PAb. This effect was quantified by measuring radioactive signals with a PhosphorImager. The average value of at least three independent experiments, in which the amount of soluble NS1 released from mock-treated Den virus-infected cells was defined as 100%, is reported in Fig. 5b. If secretion of soluble NS1 is quantified from supernatants recovered after PEG precipitation and is immunoprecipitated by the PAb (Fig. 5b), the intensity of the signals is reduced by 35% in swainsonine-treated samples and by 65% in DMJ-treated samples. Similar results can be obtained after immunoprecipitations of the same samples with the two MAbs (data not shown). Thus, both inhibitors seem to repress the secretion of soluble NS1 from Den virus-infected cells (Fig. 5), the inhibitory effect being the highest when N-glycans are maintained in a polymannose-type structure in the presence of DMJ. The variation in the amount of NS1 secreted under the different conditions of cell treatment does not seem to be due to a difference in the level of protein expression, as radioactive signals that are measured from cell lysates immunoprecipitated either with the PAb (Fig. 5a) or with MAbs (not shown) vary in a range lower than 5%. The smaller amount of extracellular soluble NS1 in the supernatants of swainsonine- or DMJ-treated cells may be a direct effect on the cell secretory machinery, but the proteins P20 and P60, recognized by the PAb (Fig. 5a), appear not to be affected by the inhibitors; examples where the secretion of viral or cellular proteins was not impaired by inhibition with swainsonine or DMJ have been reported (1, 20, 43, 46, 54). This down-regulation could be the result of specific targeting of intracellular NS1 to the degradation pathway or reduced stability of the protein secreted in the presence of inhibitors. Alternatively, the oligosaccharides of NS1 could be important for the protein to reach a mature quaternary conformation (26, 38) and/or in regulating protein transport along the secretory pathway (9, 17).
Whatever the mechanism involved, proper processing of N-glycans appears to be essential for the NS1 protein to be efficiently matured and released from Den virus-infected cells. Concordant with these observations, we (data not shown) and others (33, 52) have noted the absence of NS1 secretion in insect cell lines derived from A. albopictus. As oligosaccharides synthesized in insect cells may accumulate as polymannose-rich structures (27, 28), similar to those produced in DMJ-treated cells, we propose that the discrepancy in glycoconjugate biosynthesis between mammalian and invertebrate cells may be one of the host-specific factors finely tuning the maturation, transport, and secretion of NS1. Interestingly, previous experiments showed that ablation of the complex-type oligosaccharide correlated with a decrease in mouse neurovirulence (36, 39), emphasizing the fact that the complex-type sugar may have a specific role to play in the formation of a mature extracellular form of NS1 which could specifically contribute to virulence. Identifying the site of NS1 hexamer formation, the signals triggering cell membrane association or release, and at which steps the carbohydrate moieties may be involved remains a prerequisite for the more general concern of the biological relevance of the NS1 protein in flavivirus infection and pathology.
Acknowledgments
We thank Andrew Falconar for the generous gift of 3D1.4 hybridoma cells, Jean-Claude Mazié for preparing 3D1.4 MAb-containing ascitic fluid, Patrice Vachette for the SAXS experiment (beam line D24; LURE, Orsay, France), Gérard Batelier for the analytical ultracentrifugation study, Hany Goudran-Botros for early help in characterizing NS1 by SEC, Robert Putnak for providing MAb 13A1, Franz Heinz for advice on the DMS cross-linking experiments, Friedrich Piller for advice on the use of glycosylation inhibitors, and Michele Wien for helpful comments on the manuscript.
This work was supported in part by grant RG-509 from the Human Frontiers Science Program (RG-509) to F.A.R. M.M. was the recipient of an EMBO long-term postdoctoral fellowship during this work.
REFERENCES
- 1.Bergh M, Cepko C, Wolf D, Robbins P. Expression of the Saccharomyces cerevisiae glycoprotein invertase in mouse fibroblasts: glycosylation, secretion, and enzymatic activity. Proc Natl Acad Sci USA. 1987;84:3570–3574. doi: 10.1073/pnas.84.11.3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boulin C, Kempf R, Koch M H J, Mclaughlin S M. Data appraisal, evaluation and display for synchrotron radiation experiments: hardware and software. Nucl Instrum Methods. 1986;A249:399–407. [Google Scholar]
- 3.Chambers T J, Hahn C S, Galler R, Rice C M. Flavivirus genome organization, expression, and replication. Annu Rev Microbiol. 1990;44:649–688. doi: 10.1146/annurev.mi.44.100190.003245. [DOI] [PubMed] [Google Scholar]
- 4.Crooks A J, Lee J M, Dowsett A B, Stephenson J R. Purification and analysis of infectious virions and native non-structural antigens from cells infected with tick-borne encephalitis virus. J Chromatogr. 1990;502:59–68. doi: 10.1016/s0021-9673(01)89563-7. [DOI] [PubMed] [Google Scholar]
- 5.Crooks A J, Lee J M, Easterbrook L M, Timofeev A V, Stephenson J R. The NS1 protein of tick-borne encephalitis virus forms multimeric species upon secretion from the host cell. J Gen Virol. 1994;75:3453–3460. doi: 10.1099/0022-1317-75-12-3453. [DOI] [PubMed] [Google Scholar]
- 6.Depautex C, Desvignes C, Leboucher P, Lemonnier M, Dagneaux D, Benoit J P, Vachette P. The small angle X-ray scattering instrument D24. Annual report 75. Orsay, France: LURE; 1987. [Google Scholar]
- 7.Desprès P, Frenkiel M-P, Deubel V. Differences between cell membrane fusion activities of two dengue type-1 isolates reflect modifications of viral structure. Virology. 1993;196:209–219. doi: 10.1006/viro.1993.1469. [DOI] [PubMed] [Google Scholar]
- 8.Desprès P, Girard M, Bouloy M. Characterization of yellow fever virus proteins E and NS1 expressed in Vero and Spodoptera frugiperda cells. J Gen Virol. 1991;72:1331–1342. doi: 10.1099/0022-1317-72-6-1331. [DOI] [PubMed] [Google Scholar]
- 9.Doms R W, Lamb R A, Rose J K, Helenius A. Folding and assembly of viral membrane proteins. Virology. 1993;193:545–562. doi: 10.1006/viro.1993.1164. [DOI] [PubMed] [Google Scholar]
- 10.Dubuisson J M, Decamps T, Vachette P. Improved signal-to-background ratio in small-angle X-ray scattering experiments with synchrotron radiation using an evacuated cell for solutions. J Appl Crystalogr. 1997;30:49–54. [Google Scholar]
- 11.Elbein A D. Glycosidase inhibitors: inhibitors of N-linked oligosaccharide processing. FASEB J. 1991;5:3055–3063. doi: 10.1096/fasebj.5.15.1743438. [DOI] [PubMed] [Google Scholar]
- 12.Elbein A D. Glycosylation inhibitors for N-linked glycoproteins. Methods Enzymol. 1987;138:661–709. doi: 10.1016/0076-6879(87)38060-7. [DOI] [PubMed] [Google Scholar]
- 13.Falconar A K I. The dengue virus nonstructural-1 protein (NS1) generates antibodies to common epitopes on human blood clotting, integrin/adhesin proteins and binds to human endothelial cells: potential implications in haemorrhagic fever pathogenesis. Arch Virol. 1997;142:897–916. doi: 10.1007/s007050050127. [DOI] [PubMed] [Google Scholar]
- 14.Falconar A K I, Young P R. Immunoaffinity purification of native dimer forms of the flavivirus nonstructural glycoprotein NS1. J Virol Methods. 1990;30:323–332. doi: 10.1016/0166-0934(90)90075-q. [DOI] [PubMed] [Google Scholar]
- 15.Falgout B, Markoff L. Evidence that flavivirus NS1-NS2a cleavage is mediated by a membrane-bound host protease in the endoplasmic reticulum. J Virol. 1995;69:7232–7243. doi: 10.1128/jvi.69.11.7232-7243.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fan W, Mason P W. Membrane association and secretion of the Japanese encephalitis virus NS1 protein from cells expressing NS1 cDNA. Virology. 1990;177:470–476. doi: 10.1016/0042-6822(90)90511-o. [DOI] [PubMed] [Google Scholar]
- 17.Fiedler K, Simons K. The role of N-glycans in the secretory pathway. Cell. 1995;81:309–312. doi: 10.1016/0092-8674(95)90380-1. [DOI] [PubMed] [Google Scholar]
- 18.Flamand M, Deubel V, Girard M. Expression and secretion of Japanese encephalitis virus nonstructural protein NS1 by insect cells using a recombinant baculovirus. Virology. 1992;191:826–836. doi: 10.1016/0042-6822(92)90258-q. [DOI] [PubMed] [Google Scholar]
- 19.Gould E A, Buckley A, Cammack N, Barrett A D T, Clegg J C S, Ishak R, Varma M G R. Examination of the immunological relationships between flaviviruses using yellow fever virus monoclonal antibodies. J Gen Virol. 1985;66:1369–1382. doi: 10.1099/0022-1317-66-7-1369. [DOI] [PubMed] [Google Scholar]
- 20.Gross V, Steube K, Tran-Thi T-A, McDowell W, Schwarz R, Decker K, Gerok W, Heinrich P. Secretion of high-mannose-type α1-proteinase inhibitor and α1-acid glycoprotein by primary cultures of rat hepatocytes in the presence of the mannosidase I inhibitor 1-deoxymannojirimycin. Eur J Biochem. 1985;150:41–46. doi: 10.1111/j.1432-1033.1985.tb08985.x. [DOI] [PubMed] [Google Scholar]
- 21.Guinier A, Fournet G. Small angle scattering of X-rays. New York, N.Y: Wiley; 1955. [Google Scholar]
- 22.Herve G, Moody M F, Tauc P, Vachette P, Jones P T. Quaternary structure changes in aspartate transcarbamylase studied by X-ray solution scattering. Signal transmission following effector binding. J Mol Biol. 1985;185:189–199. doi: 10.1016/0022-2836(85)90190-1. [DOI] [PubMed] [Google Scholar]
- 23.Honzatko R B, Crawford J L, Monaco H L, Ladner J E, Ewards B F, Evans D R, Warren S G, Wiley D C, Ladner R C, Lipscomb W N. Crystal and molecular structures of native and CTP-liganded aspartate carbamoyltransferase from Escherichia coli. J Mol Biol. 1982;160:219–263. doi: 10.1016/0022-2836(82)90175-9. [DOI] [PubMed] [Google Scholar]
- 24.Kaushal G P, Elbein A D. Glycosidase inhibitors in study of glycoconjugates. Methods Enzymol. 1994;230:316–329. doi: 10.1016/0076-6879(94)30021-6. [DOI] [PubMed] [Google Scholar]
- 25.Keil W, Geyer R, Dabrowski J, Dabrowski U, Niemann H, Stirm S, Klenk H-D. Carbohydrates of influenza virus. Structural elucidation of the individual glycans of the FPV hemagglutinin by two-dimensional 1H n.m.r. and methylation analysis. EMBO J. 1985;4:2711–2720. doi: 10.1002/j.1460-2075.1985.tb03991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kobata A. Structures and functions of the sugar chains of glycoproteins. Eur J Biochem. 1992;209:483–501. doi: 10.1111/j.1432-1033.1992.tb17313.x. [DOI] [PubMed] [Google Scholar]
- 27.Kulakosky P C, Shuler M L, Wood H A. N-Glycosylation of a baculovirus-expressed recombinant glycoprotein in three insect cell lines. In Vitro Cell Dev Biol. 1998;34:101–108. doi: 10.1007/s11626-998-0091-0. [DOI] [PubMed] [Google Scholar]
- 28.Kuroda K, Geyer H, Geyer R, Doerfler W, Klenk H-D. The oligosaccharides of influenza virus hemagglutinin expressed in insect cells by a baculovirus vector. Virology. 1990;174:418–429. doi: 10.1016/0042-6822(90)90095-9. [DOI] [PubMed] [Google Scholar]
- 29.Lee J M, Crooks A J, Stephenson J R. The synthesis and maturation of a non-structural extracellular antigen from tick-borne encephalitis virus and its relationship to the intracellular NS1 protein. J Gen Virol. 1989;70:335–343. doi: 10.1099/0022-1317-70-2-335. [DOI] [PubMed] [Google Scholar]
- 30.Lee T, Watanabe K, Aizawa C, Nomoto A, Hashimoto H. Preparation of Japanese encephalitis virus nonstructural protein NS1 obtained from culture fluid of JEV-infected Vero cells. Arch Virol. 1991;116:253–260. doi: 10.1007/BF01319246. [DOI] [PubMed] [Google Scholar]
- 31.Lindenbach B D, Rice C M. trans-Complementation of yellow fever virus NS1 reveals a role in early RNA replication. J Virol. 1997;71:9608–9617. doi: 10.1128/jvi.71.12.9608-9617.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mackenzie J M, Jones M K, Young P R. Immunolocalization of the dengue virus nonstructural glycoprotein NS1 suggests a role in viral RNA replication. Virology. 1996;220:232–240. doi: 10.1006/viro.1996.0307. [DOI] [PubMed] [Google Scholar]
- 33.Mason P W. Maturation of Japanese encephalitis virus glycoproteins produced by infected mammalian and mosquito cells. Virology. 1989;169:354–364. doi: 10.1016/0042-6822(89)90161-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mir-Sheraki S Y, Ashford D A, Harvey D J, Dwek R A, Schulze I T. The glycosylation of the influenza A virus hemagglutinin by mammalian cells—a site-specific study. J Biol Chem. 1997;272:4027–4036. doi: 10.1074/jbc.272.7.4027. [DOI] [PubMed] [Google Scholar]
- 35.Monath T P, Heinz F X. Flaviviruses. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. Vol. 1. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 961–1034. [Google Scholar]
- 36.Muylaert I R, Chambers T J, Galler R, Rice C M. Mutagenesis of the N-linked glycosylation sites of the yellow fever virus NS1 protein: Effects on virus replication and mouse neurovirulence. Virology. 1996;222:159–168. doi: 10.1006/viro.1996.0406. [DOI] [PubMed] [Google Scholar]
- 37.Muylaert I R, Galler R, Rice C M. Genetic analysis of yellow fever virus NS1 protein: identification of a temperature-sensitive mutation which blocks RNA accumulation. J Virol. 1997;71:291–298. doi: 10.1128/jvi.71.1.291-298.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Opdenakker G, Rudd P M, Ponting C P, Dwek R A. Concepts and principles of glycobiology. FASEB J. 1993;7:1330–1337. doi: 10.1096/fasebj.7.14.8224606. [DOI] [PubMed] [Google Scholar]
- 39.Pletnev A G, Bray M, Lai C-J. Chimeric tick-borne encephalitis and dengue type 4 viruses: effects of mutations on neurovirulence in mice. J Virol. 1993;67:4956–4963. doi: 10.1128/jvi.67.8.4956-4963.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Post P R, Carvalho R, Galler R. Glycosylation and secretion of yellow fever virus nonstructural protein NS1. Virus Res. 1990;18:291–302. doi: 10.1016/0168-1702(91)90025-q. [DOI] [PubMed] [Google Scholar]
- 41.Pryor M J, Wright P J. The effects of site-directed mutagenesis on the dimerization and secretion of the NS1 protein specified by dengue virus. Virology. 1993;194:769–780. doi: 10.1006/viro.1993.1318. [DOI] [PubMed] [Google Scholar]
- 42.Pryor M J, Wright P J. Glycosylation mutants of dengue virus NS1 protein. J Gen Virol. 1994;75:1183–1187. doi: 10.1099/0022-1317-75-5-1183. [DOI] [PubMed] [Google Scholar]
- 43.Rabouille C, Spiro R. Nonselective utilization of the endomannosidase pathway for processing glycoproteins by human hepatoma (HepG2) cells. J Biol Chem. 1992;267:11573–11578. [PubMed] [Google Scholar]
- 44.Rice C M. Flaviviridae: the viruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. Vol. 1. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 931–959. [Google Scholar]
- 45.Schlesinger J J, Brandriss M W, Putnak J R, Walsh E E. Cell surface expression of yellow fever virus nonstructural glycoprotein NS1: consequences of interaction with antibody. J Gen Virol. 1990;71:593–599. doi: 10.1099/0022-1317-71-3-593. [DOI] [PubMed] [Google Scholar]
- 46.Simsolo R, Ong J, Kern P. Characterization of lipoprotein lipase activity, secretion, and degradation at different sites of post-translational processing in primary cultures of rat adipocytes. J Lipid Res. 1992;33:1777–1784. [PubMed] [Google Scholar]
- 47.Tarentino A L, Plummer T H. Enzymatic deglycosylation of asparagine-linked glycans: purification, properties, and specificity of oligosaccharide-cleaving enzymes from Flavobacterium meningosepticum. Methods Enzymol. 1994;230:44–57. doi: 10.1016/0076-6879(94)30006-2. [DOI] [PubMed] [Google Scholar]
- 48.Tarentino A L, Plummer T H. Peptide-N-(N-acetyl-β-glucosaminyl) asparagine amidase and endo-N-acetylglucosaminidase from Flavobacterium meningosepticum. Methods Enzymol. 1987;138:770–778. doi: 10.1016/0076-6879(87)38065-6. [DOI] [PubMed] [Google Scholar]
- 49.Trombetta E, Helenius A. Lectins as chaperones in glycoprotein folding. Curr Opin Struct Biol. 1998;8:587–592. doi: 10.1016/s0959-440x(98)80148-6. [DOI] [PubMed] [Google Scholar]
- 50.Westaway E G, Goodman M R. Variation in distribution of the three flavivirus-specified glycoproteins detected by immunofluorescence in infected Vero cells. Arch Virol. 1987;94:215–228. doi: 10.1007/BF01310715. [DOI] [PubMed] [Google Scholar]
- 51.Westaway E G, Mackenzie J M, Kenney M T, Jones M K, Khromykh A A. Ultrastructure of Kunjin virus-infected cells: colocalization of NS1 and NS3 with double-stranded RNA, and of NS2B with NS3, in virus-induced membrane structures. J Virol. 1997;71:6650–6661. doi: 10.1128/jvi.71.9.6650-6661.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Winkler G, Maxwell S E, Ruemmler C, Stollar V. Newly synthesized dengue-2 virus nonstructural protein NS1 is a soluble protein but becomes partially hydrophobic and membrane-associated after dimerization. Virology. 1989;171:302–305. doi: 10.1016/0042-6822(89)90544-8. [DOI] [PubMed] [Google Scholar]
- 53.Winkler G, Randolph V B, Cleaves G R, Ryan T E, Stollar V. Evidence that the mature form of the flavivirus nonstructural protein NS1 is a dimer. Virology. 1988;162:187–196. doi: 10.1016/0042-6822(88)90408-4. [DOI] [PubMed] [Google Scholar]
- 54.Wojczyk B, Stwora-Wojczyk M, Shakin-Eshelman S, Wunner W, Spitalnik S. The role of site-specific N-glycosylation in secretion of soluble forms of rabies virus glycoprotein. Glycobiology. 1998;8:121–130. doi: 10.1093/glycob/8.2.121. [DOI] [PubMed] [Google Scholar]