Abstract
Background
Apnea and intermittent hypoxemia (IH) are common developmental disorders in infants born earlier than 37 weeks' gestation. Caffeine administration has been shown to lower the incidence of these disorders in preterm infants. Cessation of caffeine treatment is based on different post‐menstrual ages (PMA) and resolution of symptoms. There is uncertainty about the best timing for caffeine discontinuation.
Objectives
To evaluate the effects of early versus late discontinuation of caffeine administration in preterm infants.
Search methods
We searched CENTRAL, PubMed, Embase, and three trial registries in August 2023; we applied no date limits. We checked the references of included studies and related systematic reviews.
Selection criteria
We included randomized controlled trials (RCTs) in preterm infants born earlier than 37 weeks' gestation, up to a PMA of 44 weeks and 0 days, who received caffeine for any indication for at least seven days. We compared three different strategies for caffeine cessation: 1. at different PMAs, 2. before or after five days without symptoms, and 3. at a predetermined PMA versus at the resolution of symptoms.
Data collection and analysis
We used standard Cochrane methods. Primary outcomes were: restarting caffeine therapy, intubation within one week of treatment discontinuation, and the need for non‐invasive respiratory support within one week of treatment discontinuation. Secondary outcomes were: number of episodes of apnea in the seven days after treatment discontinuation, number of infants with at least one episode of apnea in the seven days after treatment discontinuation, number of episodes of intermittent hypoxemia (IH) within seven days of treatment discontinuation, number of infants with at least one episode of IH in the seven days after of treatment discontinuation, all‐cause mortality prior to hospital discharge, major neurodevelopmental disability, number of days of respiratory support after treatment discontinuation, duration of hospital stay, and cost of neonatal care. We used GRADE to assess the certainty of evidence for each outcome.
Main results
We included three RCTs (392 preterm infants).
Discontinuation of caffeine at PMA less than 35 weeks' gestation versus PMA equal to or longer than 35 weeks' gestation
This comparison included one single completed RCT with 98 premature infants with a gestational age between 25 + 0 and 32 + 0 weeks at birth. All infants had discontinued caffeine treatment for five days at randomization. The infants received either an oral loading dose of caffeine citrate (20 mg/kg) at randomization followed by oral maintenance dosage (6 mg/kg/day) until 40 weeks PMA, or usual care (controls), during which caffeine was stopped before 37 weeks PMA.
Early cessation of caffeine administration in preterm infants at PMA less than 35 weeks' gestation may result in an increase in the number of IH episodes in the seven days after discontinuation of treatment, compared to prolonged caffeine treatment beyond 35 weeks' gestation (mean difference [MD] 4.80, 95% confidence interval [CI] 2.21 to 7.39; 1 RCT, 98 infants; low‐certainty evidence). Early cessation may result in little to no difference in all‐cause mortality prior to hospital discharge compared to late discontinuation after 35 weeks PMA (risk ratio [RR] not estimable; 98 infants; low‐certainty evidence).
No data were available for the following outcomes: restarting caffeine therapy, intubation within one week of treatment discontinuation, need for non‐invasive respiratory support within one week of treatment discontinuation, number of episodes of apnea, number of infants with at least one episode of apnea in the seven days after discontinuation of treatment, or number of infants with at least one episode of IH in the seven days after discontinuation of treatment.
Discontinuation based on PMA versus resolution of symptoms
This comparison included two RCTs with a total of 294 preterm infants.
Discontinuing caffeine at the resolution of symptoms compared to discontinuing treatment at a predetermined PMA may result in little to no difference in all‐cause mortality prior to hospital discharge (RR 1.00, 95% CI 0.14 to 7.03; 2 studies, 294 participants; low‐certainty evidence), or in the number of infants with at least one episode of apnea within the seven days after discontinuing treatment (RR 0.60, 95% CI 0.31 to 1.18; 2 studies; 294 infants; low‐certainty evidence). Discontinuing caffeine based on the resolution of symptoms probably results in more infants with IH in the seven days after discontinuation of treatment (RR 0.38, 95% CI 0.20 to 0.75; 1 study; 174 participants; moderate‐certainty evidence).
No data were available for the following outcomes: restarting caffeine therapy, intubation within one week of treatment discontinuation, need for non‐invasive respiratory support within one week of treatment discontinuation, or number of episodes of IH in the seven days after treatment discontinuation.
Adverse effects
In the Rhein 2014 study, five of the infants randomized to caffeine had the caffeine treatment discontinued at the discretion of the clinical team, because of tachycardia.
The Pradhap 2023 study reported adverse events, including recurrence of apnea of prematurity (15% in the short and 13% in the regular course caffeine therapy group), varying severities of bronchopulmonary dysplasia, hyperglycemia, extrauterine growth restriction, retinopathy of prematurity requiring laser treatment, feeding intolerance, osteopenia, and tachycardia, with no significant differences between the groups.
The Prakash 2021 study reported that adverse effects of caffeine therapy for apnea of prematurity included tachycardia, feeding intolerance, and potential neurodevelopmental impacts, though most were mild and transient.
We identified three ongoing studies.
Authors' conclusions
There may be little or no difference in the incidence of all‐cause mortality and apnea in infants who were randomized to later discontinuation of caffeine treatment. However, the number of infants with at least one episode of IH was probably reduced with later cessation.
No data were found to evaluate the benefits and harms of later caffeine discontinuation for: restarting caffeine therapy, intubation within one week of treatment discontinuation, or need for non‐invasive respiratory support within one week of treatment discontinuation.
Further studies are needed to evaluate the short‐term and long‐term effects of different caffeine cessation strategies in premature infants.
Keywords: Humans; Infant, Newborn; Apnea; Apnea/drug therapy; Bias; Caffeine; Caffeine/administration & dosage; Caffeine/adverse effects; Drug Administration Schedule; Gestational Age; Hypoxia; Infant, Premature; Infant, Premature, Diseases; Infant, Premature, Diseases/mortality; Infant, Premature, Diseases/prevention & control; Length of Stay; Randomized Controlled Trials as Topic; Time Factors; Withholding Treatment; Withholding Treatment/statistics & numerical data
Plain language summary
When is the best time to stop giving caffeine to newborns born preterm?
Key messages
• Currently, we do not know when to stop giving caffeine to preterm babies.
• If we stop the caffeine too early, babies may have brief, sometimes repetitive, episodes when they have lower levels of oxygen in their blood.
• We need to do more studies to find out the best time to stop giving caffeine to preterm babies.
Why is caffeine given to premature babies?
Caffeine is a stimulant used to prevent and treat apnea – when breathing repeatedly stops and starts – in preterm infants. Caffeine also seems to reduce episodes of intermittent hypoxia ‐ rapid moments when the blood oxygen levels drop. Caffeine reduces the risk of the baby needing mechanical ventilation (a therapy that assists or replaces spontaneous breathing) to provide enough oxygen supplementation. It improves lung development, reduces the risk of developing chronic lung damage, and improves the quality of life of preterm babies.
When is caffeine given?
Caffeine is given to preterm infants, but there is currently no clear guidance on precisely when to start it. Some studies have found the best benefits when started within the first three days of life.
There is also no clear guidance on when to stop giving caffeine. Some investigators stop it at a defined post‐menstrual age, which is the infants' age, calculated from the first day of their mother's last menstrual period. Others stop when the babies are without symptoms for five days or more.
What did we want to find out?
We wanted to find out the best time to stop caffeine. We measured this as the need to resume therapy, the need for mechanical ventilation, the number of episodes of apnea or intermittent hypoxia, survival, brain development in infancy and childhood, and the duration and cost of hospitalization.
What did we do?
We searched for studies that compared:
• the cessation of caffeine administration at different post‐menstrual ages;
• the cessation of caffeine at a definite post‐menstrual age or after a symptom‐free period;
• the cessation of caffeine after different periods free from symptoms (five days or more).
We compared and summarized the results of the studies and rated our confidence in the evidence based on factors, such as study methods and sample sizes.
What did we find?
We included three studies with 392 preterm infants, with a gestational age between 26 and 33 weeks. They were randomly divided into two groups that either stopped or continued giving caffeine.
Two studies compared stopping caffeine at a definite post‐menstrual age versus when the babies were free of symptoms. One study compared stopping caffeine at two different post‐menstrual ages. We did not find any studies that compared stopping caffeine after different symptom‐free periods.
Stopping caffeine at different post‐menstrual ages
• Stopping caffeine early (before 35 weeks of post‐menstrual age) may increase the number of intermittent hypoxemia episodes in the seven days after stopping, compared to stopping later.
• Stopping caffeine early (before 35 weeks of post‐menstrual age) may make little to no difference in the number of deaths before discharge from hospital compared to stopping later.
• The evidence is very uncertain about whether there is any difference in unwanted effects between stopping caffeine early and late.
Stopping caffeine when the babies are free of symptoms (early) or at a definite post‐menstrual age (later)
• Stopping caffeine early may make little to no difference in the number of infants with apnea in the seven days after stopping, compared to stopping later.
• Stopping caffeine early probably results in more infants with episodes of intermittent hypoxia in the seven days after stopping, compared to stopping later.
• Stopping caffeine early may make little to no difference in the number of deaths before discharge, compared to stopping later.
• The evidence is very uncertain about whether there is any difference in unwanted effects between stopping caffeine early and late.
Three studies are ongoing.
What are the limitations of the evidence?
Our confidence in the evidence is limited because the number of babies studied for each outcome was small. We did not find any studies that compared stopping caffeine during different symptom‐free periods. Finally, the evidence did not cover all the outcomes in which we were interested.
How up to date is this evidence?
The evidence is up‐to‐date until August 2023.
Summary of findings
Summary of findings 1. Summary of findings table ‐ Discontinuation of caffeine at PMA < 35 weeks? gestation vs. PMA ≥ 35 weeks? gestation for preterm infants undergoing cessation of caffeine administration.
| Discontinuation of caffeine at PMA < 35 weeks' gestation vs. PMA ≥ 35 weeks' gestation for preterm infants undergoing cessation of caffeine administration | ||||||
| Patient or population: preterm infants undergoing cessation of caffeine administration Setting: neonatal intensive care units Intervention: discontinuation of caffeine at PMA < 35 weeks' gestation Comparison: PMA ≥ 35 weeks' gestation | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with PMA ≥ 35 weeks' gestation | Risk with discontinuation of caffeine at PMA < 35 weeks' gestation | |||||
| Restarting caffeine therapy prior to hospital discharge ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No studies reported this outcome. |
| Intubation within one week of treatment discontinuation ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No studies reported this outcome. |
| Need for non‐invasive respiratory support prior to hospital discharge ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No studies reported this outcome. |
| Apnea: number of infants with at least 1 episode in the 7 days after treatment discontinued ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No studies reported this outcome. |
| Intermittent hypoxemia (IH; number of episodes [decline in SpO2 to < 80% for at least 5 seconds]) in the 7 days after treatment discontinued | The mean intermittent hypoxemia (IH; number of episodes [decline in SpO2 to < 80% for at least 5 seconds]) in the 7 days after treatment discontinued was 3.6 | MD 4.8 higher (2.21 higher to 7.39 higher) | ‐ | 98 (1 RCT) | ⊕⊕⊝⊝ Lowa | Early discontinuation (PMA < 35 weeks' gestation) may result in an increase in the number of IH episodes in the 7 days after discontinuing treatment. Our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. |
| Intermittent hypoxemia (IH; number of infants with at least 1 episode) in the 7 days after treatment discontinued ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No studies reported this outcome. |
| All‐cause mortality prior to hospital discharge | 0 per 1000 | 0 per 1000 (0 to 0) | Not estimable | 98 (1 RCT) | ⊕⊕⊝⊝ Lowa | Early discontinuation (PMA < 35 weeks' gestation) may result in little to no difference in all‐cause mortality prior to hospital discharge. Our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_444838344216019957. | ||||||
a Downgraded two levels for very serious imprecision: one small study, wide confidence intervals.
Summary of findings 2. Summary of findings table ‐ Discontinuation of caffeine based on PMA (discontinuing caffeine at a scheduled PMA) compared to discontinuation of caffeine based on resolution of symptoms (a fixed time after the infant is without respiratory support, or apneic spells).
| Discontinuation of caffeine based on PMA (discontinuing caffeine at a scheduled PMA) compared to discontinuation of caffeine based on resolution of symptoms (a fixed time after the infant is without respiratory support, or apneic spells) | ||||||
| Patient or population: preterm infants undergoing cessation of caffeine administration Setting: neonatal intensive care unit of a referral tertiary care multispecialty hospital Intervention: discontinuation of caffeine based on PMA Comparison: discontinuation of caffeine based on resolution of symptoms | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with discontinuation of caffeine based on resolution of symptoms | Risk with discontinuation of caffeine based on PMA | |||||
| Restarting caffeine therapy prior to hospital discharge ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No studies reported this outcome. |
| Intubation within one week of treatment discontinuation ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No studies reported this outcome. |
| Need for non‐invasive respiratory support prior to hospital discharge ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No studies reported this outcome. |
| Apnea: number of infants with at least 1 episode (interruption of breathing for more than 20 seconds, or as defined by the authors) in the 7 days after discontinuing treatment | 136 per 1000 | 82 per 1000 (42 to 161) | RR 0.60 (0.31 to 1.18) | 294 (2 RCTs) | ⊕⊕⊝⊝ Lowa | Discontinuation of caffeine based on resolution of symptoms may result in little to no difference in number of infants with apnea in the seven days after discontinuing treatment. |
| Intermittent hypoxemia (IH; number of episodes [decline in SpO2 to < 80% for at least 5 seconds]) in the 7 days after discontinuing treatment ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No studies reported this outcome. |
| Intermittent hypoxemia (IH; number of infants with at least 1 episode) in the 7 days after discontinuing treatment | 299 per 1000 | 114 per 1000 (60 to 224) | RR 0.38 (0.20 to 0.75) | 174 (1 RCT) | ⊕⊕⊕⊝ Moderateb | Discontinuation of caffeine based on resolution of symptoms probably results in an increase in number of infants with IH in the seven days after discontinuing treatment. |
| All‐cause mortality prior to hospital discharge | 7 per 1000 | 7 per 1000 (1 to 48) | RR 1.00 (0.14 to 7.03) | 294 (2 RCTs) | ⊕⊕⊝⊝ Lowa | Discontinuation of caffeine based on resolution of symptoms may result in little to no difference in all‐cause mortality prior to hospital discharge. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_444890037381913039. | ||||||
a Downgraded two levels for very serious imprecision: very wide confidence interval. Not downgraded for risk of bias because it is unlikely that this objective outcome might have been affected by inadequate blinding. b Downgraded one level for serious imprecision: wide confidence interval. Not downgraded for risk of bias because it is unlikely that this objective outcome might have been affected by inadequate blinding.
Background
Description of the condition
Apnea of prematurity (AOP), classified as central, obstructive, or mixed, is usually defined as a cessation of breathing in a premature infant for at least 20 seconds, or a shorter pause accompanied by bradycardia (< 100 beats per minute), cyanosis, or pallor (Eichenwald 2016). It is a common problem among preterm infants, particularly in extremely preterm infants (< 28 weeks of pregnancy [Saroha 2020]). The incidence of AOP is inversely correlated with gestational age and birth weight. Seven percent of neonates born at 34 to 35 weeks' gestation, 15% born at 32 to 33 weeks', 54% born at 30 to 31 weeks' (Martin 2011), and nearly all infants born at < 29 weeks' gestation or at < 1000 g, exhibit AOP (Robertson 2009).
Apneic event frequency and duration can be reduced with respiratory interventions, including continuous positive airway pressure and pharmacologic therapies, such as methylxanthines. Caffeine represents clinicians' first choice due to its efficacy, better tolerability, wider therapeutic index, and longer half‐life (Dobson 2013).
Intermittent hypoxemia (IH) is defined as brief, repetitive episodes of decreased hemoglobin oxygen saturation from a normoxic baseline, followed by reoxygenation and a return to normoxia. IH occurs frequently in preterm infants, and may last until term‐equivalent age, and after the cessation of any clinically apparent apnea‐associated symptoms (Hunt 2011; Rhein 2014).
Bronchopulmonary dysplasia (BPD) is a chronic lung disease affecting preterm infants who require respiratory support. BPD was first described in 1967, as oxygen dependence for 28 days (NIH 1979). The most commonly used definition includes a classification of severity, and is based on gestational age (GA). For infants born at GA < 32 weeks, BPD is classified as mild (no need for oxygen), moderate (21% to 30% oxygen required), or severe (> 30% oxygen required, or positive pressure assistance), based on the required fraction of inspired oxygen at 36 weeks of corrected GA (Jobe 2001). Other BPD definitions, e.g. the physiological definition, contextualize the need for oxygen and respiratory support in the clinical condition of the infant, such as lack of episodes of desaturation for a certain amount of time (Walsh 2004). Despite the advances in neonatal care, the morbidity and mortality due to BPD remain stable (Stoll 2015). Approximately 45% of preterm infants born at a gestational age of 29 weeks develop BPD that is associated with a significantly increased risk for pulmonary and neurologic impairment, which persists into adulthood. A survey found that 18‐ to 36‐month‐old infants with severe BPD had a significantly lower quality of life than full‐term infants and preterm infants without BPD (Brady 2019). Several systematic reviews showed that within two years after birth, the readmission rate of children with BPD was as high as 50% (Resch 2011; Townsi 2018). In addition, pulmonary vascular dysplasia in children with BPD can lead to pulmonary hypertension. A systematic review revealed that pulmonary hypertension occurred in 17% of children with BPD, and up to 24% in children with severe BPD (Bui 2017).
In a retrospective economic evaluation, completed alongside the Caffeine for Apnea of Prematurity (CAP) trial, and using individual participant data, Dukhovny and colleagues found that caffeine was probably not only effective but also cost saving compared with the placebo, mainly because of the reduced number of days on mechanical ventilation (Dukhovny 2011). However, this study has limitations that may affect the precision of these results, such as the existence of retrospective analysis of cost‐effectiveness data. In addition, certain resource utilization data were not evaluated adequately in the CAP trial, such as the costs of inter‐hospital transport, postdischarge use of drugs, and other outpatient healthcare services (Abdel‐Hady 2015).
Description of the intervention
Several reviews and surveys have proposed a variety of therapeutic strategies for BPD (Arroyo 2021; Principi 2018; Sauer‐Zavala 2019; Thébaud 2019). Treatment strategies include the administration of corticosteroids (Doyle 2021), surfactants (Arroyo 2021; Isayama 2016), antioxidants (Poggi 2014), vitamin A (Darlow 2016), stem cells (Pierro 2017), and non‐invasive respiratory support (Lemyre 2017). However, these therapies are only partially beneficial. Caffeine is one of the few interventions that has been shown to be effective in targeting the symptoms observed in infants with BPD (Jensen 2015).
Cardiorespiratory benefits of caffeine that may contribute to the lower risk of BPD include: reduced exposure to positive airway pressure and supplemental oxygen, less frequent treatment for patent ductus arteriosus, improved pulmonary mechanics, and direct effects on pulmonary inflammation, alveolarization, and angiogenesis. Improvement of survival without neurodevelopmental disability at 18 to 21 months has not been shown to persist at five years (Schmidt 2007), although there is a continued reduction in the severity of motor impairment (Schmidt 2012).
Caffeine is often available as caffeine citrate, which comes in both oral and injectable formulations; the dose of caffeine base is half that of caffeine citrate (Shrestha 2017). The Food and Drug Administration (FDA)‐approved doses for caffeine citrate are 20 mg/kg/day for loading, and 5 mg/kg/day for maintenance (NDA 20‐793/S‐001). The European Medicines Agency (EMA) approved similar caffeine citrate doses for loading and maintenance (EMA 2009). The European Public Assessment Report (EPAR) of Peyona® and Gencebok® recommend higher maintenance doses (10 mg/kg/day) when there is insufficient response for the two EMA‐approved specialties that contain the active substance, caffeine citrate. This takes into account the potential for accumulation of caffeine, due to the long half‐life in preterm newborn infants, and the progressively increasing capacity to metabolize caffeine in relation to post‐menstrual age (EMA 2020). The goal is to achieve a therapeutic blood level of 5 mg/L to 25 mg/L of caffeine in preterm infants who are younger than 32 weeks' post‐menstrual age (PMA).
Despite an initial description in 1977 and widespread use of caffeine over the years, the literature recommends significantly different PMA and postnatal ages for the first administration of caffeine, different PMAs for the discontinuation of caffeine and discharge from hospital, and different total durations of caffeine use (Aranda 1977; Gentle 2018; Ji 2020; Kumar 2019).
Dobson and colleagues summarized the clinical benefits of beginning caffeine treatment before three days of age, showing that early treatment is associated with reduced incidence of BPD, death from BPD, intraventricular hemorrhage, necrotizing enterocolitis, need for treatment of PDA, retinopathy of prematurity, and reduced use of postnatal steroids, although the certainty of evidence was low (Dobson 2013).
The Cochrane review, Methylxanthine for the prevention and treatment of apnea in preterm infants, found that caffeine and aminophylline may importantly reduce the incidence of apneic events and the need for intermittent positive pressure ventilation (Marques 2023).
A prospective, multicenter randomized controlled trial that enrolled 105 infants who were born at less than 32 weeks' gestation, and were formerly treated with caffeine, found significant reductions in IH at 35 and 36 weeks' PMA with prolonged caffeine treatment (Rhein 2014). However, the optimum dosing regimen of caffeine required to alleviate IH, the long‐term effects of extended use of caffeine, and the timing of caffeine initiation and discontinuation must still be identified.
How the intervention might work
Caffeine acts as an antioxidative and anti‐inflammatory drug by reducing cell death and apoptosis‐associated factors in models of oxygen‐induced lung injury (Endesfelder 2020; Nagatomo 2016). Beneficial effects of caffeine might also be mediated through a diuretic effect, as reported in clinical (Gillot 1990), and preclinical studies (Crossley 2012). Due to differences in the maturity of hepatic and renal function among preterm infants, the response to varying doses may be considerably different, for both potential benefits and harms (Stevenson 2007).
Plasma concentrations of caffeine as low as 3 mg/L to 4 mg/L have been shown to reduce apneic spells, but optimal levels range from 8 mg/L to 20 mg/L (Aranda 1979). Typically, to maintain these caffeine plasma concentration levels, a standard regimen is used, comprising a loading dose of 20 mg/kg of caffeine citrate (10 mg/kg of caffeine base) and a maintenance dose of 5 mg/kg/day (2.5 mg/kg/day of caffeine base [Blanchard 1992]). Preterm infants can tolerate higher doses of caffeine very well, even at serum concentrations of 70 mg/L and above (Lee 1997). Higher doses have been reported to further reduce respiratory morbidity (Bruschettini 2023).
Caffeine has a long half‐life, which was shown to be present in the plasma of infants who discontinued caffeine within the last seven days prior to discharge, indicating that the level of caffeine may still be therapeutic days after stopping the drug, and carry over after discharge (Charles 2008).
The study by Doyle and colleagues showed that caffeine may persist in an infant's plasma for 11 to 12 days after cessation of therapy (Doyle 2016). For these reasons, infants should be monitored for recurrence of apnea for five to seven days, and longer, after caffeine is discontinued, and it is important to assess an infant’s functional status and stability prior to discharge, and once they are off caffeine (Ji 2020).
Managing the timing of when to discontinue caffeine and discharge from hospital also varies widely. Physician discomfort, which partially arises from a lack of scientific evidence‐based guidance, results in a wide variation of practice for apnea or bradycardia events, including a delay in discharging infants home. This increases hospital costs, and the use of home monitors. Clinicians determine that premature infants are ready to be discharged when they demonstrate functional maturation and are medically stabile. Factors contributing to a clinician’s decision to discontinue caffeine therapy include the degree of respiratory stability, and symptoms suggestive of caffeine‐induced toxicity (Butler 2014).
Although an earlier discharge has been proposed for infants on caffeine, with or without cardiorespiratory monitoring, the majority of neonatal intensive care units (NICU) keep preterm infants until they have been apnea‐free for five to seven days (Darnall 1997; Jefferies 2014; Martin 2022). It is also known that an early discontinuation increases the risk of recurrent apnea problems, and the need for increased respiratory support (Ji 2020). A safety margin of apnea‐free time before discharge has been explored, but currently, there is no consensus among physicians about how long an infant should be apnea‐free after a significant event to determine a safe discharge (Eichenwald 2016; Ji 2020).
A recent retrospective study showed that discharging stable preterm infants home on caffeine may be safe, especially for those who are waiting for the complete resolution of AOP/IH events, and are otherwise ready to go home (Ma 2020).
This was also shown in the Chimes study (Ramanathan 2001). This study confirmed that complete resolution of AOP/IH in more premature infants is variable and takes a long time. Infants discharged home on caffeine needed caffeine until an average corrected gestation age of 43 weeks, compared to 35 weeks in infants in whom it was stopped during their hospital stay.
A clear benefit of early discontinuation is related to unnecessarily prolonging hospital stays, which adds to the increased burden of health care costs (Butler 2014).
Why it is important to do this review
In recent decades, numerous clinical cross‐sectional and longitudinal studies have shown the efficacy of caffeine in treating primary apnea in premature infants by stimulating the respiratory center, and in protecting the nervous and respiratory systems (Marques 2023). However, while the benefits of caffeine therapy are well known, a consensus is missing among clinicians about the appropriate timing of discontinuation relative to the infant's discharge.
The National Institute of Child Health and Human Development Neonatal Research Network is evaluating the effectiveness and safety of continuing caffeine administration throughout hospitalization and after discharge home in moderately preterm infants with resolving AOP. This trial, which is currently enrolling participants, will help to guide decisions about the appropriate time to discontinue caffeine use in premature infants (NCT03340727).
To determine an optimal time to discontinue the use of caffeine, clinicians must weigh the risks of early discontinuation (i.e. the need to restart therapy, respiratory compromise, and the need to escalate support) with those of later discontinuation (possible prolonged hospital stay and increased costs [Butler 2014]).
Standardization of evidence‐based interventions by reducing the variability in practice is fundamental for enhancing health care.
Objectives
To evaluate the effects of early versus late discontinuation of caffeine administration in preterm infants.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs) or quasi‐RCTs (e.g. based on date of birth or hospital record number), and cluster‐RCTs.
We excluded cross‐over randomized trials. We also excluded non‐randomized cohort studies, because they are prone to bias due to confounding by indication, or by residual confounding; both of which may influence the results of the studies (Fewell 2007; Kyriacou 2016).
Types of participants
We included preterm infants born at less than 37 weeks' gestation, at a post‐menstrual age (PMA) up to 44 weeks and 0 days, who received caffeine for any indication, for at least seven days.
Types of interventions
We included studies comparing the following strategies for discontinuation of caffeine:
-
PMA
Discontinuation of caffeine at PMA less than 35 weeks' gestation versus PMA greater than or equal to 35 weeks' gestation
-
Resolution of symptoms
Discontinuation of caffeine less than five days after infants are symptom‐free (without the presence of respiratory support or apneic spells) versus discontinuation of caffeine at five days or more after infants are symptom‐free, regardless of the PMA
-
PMA versus resolution of symptoms
Discontinuation of caffeine based on PMA (with discontinuation of caffeine at a scheduled PMA) versus discontinuation based on resolution of symptoms (at a fixed time after the infant is without the presence of respiratory support, or apneic spells)
In the group where caffeine was discontinued earlier, infants would either be administered placebo or receive no intervention.
Types of outcome measures
Outcome measures of interest in the RCTs were not part of the eligibility criteria.
Primary outcomes
Restarting caffeine therapy
Intubation within one week of treatment discontinuation
Need for non‐invasive respiratory support (continuous positive airway pressure [CPAP], nasal intermittent positive pressure ventilation [NIPPV], high‐flow nasal cannulae) within one week of treatment discontinuation
Secondary outcomes
Apnea: number of episodes (defined as interruption of breathing for more than 20 seconds, or as defined by the authors) in the seven days after discontinuation of treatment
Apnea: number of infants with at least one episode (defined as interruption of breathing for more than 20 seconds) in the seven days after discontinuation of treatment
Intermittent hypoxemia (IH): number of episodes in the seven days after discontinuation of treatment
IH: number of infants with at least one episode in the seven days after discontinuation of treatment
All‐cause mortality prior to hospital discharge
Major neurodevelopmental disability: cerebral palsy; developmental delay (Bayley Mental Developmental Index [Bayley 1993; Bayley 2006], or Griffiths Mental Development Scale [Griffiths 1954]), assessed as more than two standard deviations (SDs) below the mean; intellectual impairment (intelligence quotient [IQ] more than two SDs below the mean); blindness (vision less than 6/60 in both eyes); or sensorineural deafness requiring amplification (Jacobs 2013). We planned to separately assess outcomes at 18 months to 24 months corrected age (CA), and at three to five years CA.
Each component of the composite outcome, major neurodevelopmental disability
Mortality or major neurodevelopmental disability. We planned to separately assess outcomes at age 18 to 24 months CA, and at three to five years CA for the outcome of major neurodevelopmental disability.
Number of days of respiratory support (mechanical ventilation, CPAP, high‐flow nasal cannula, NIPPV) after treatment discontinuation
Duration of hospital stay
Cost of neonatal care
Any adverse event
Search methods for identification of studies
The Cochrane Sweden Information Specialist developed a draft search strategy for PubMed in consultation with the review authors (Appendix 1). This strategy was peer‐reviewed by an information specialist using the Peer Review of Electronic Search Strategies (PRESS) Checklist (McGowan 2016a; McGowan 2016b). The PubMed strategy was then translated, using appropriate syntax, for other databases (see Electronic searches).
We used a population filter developed by Cochrane Neonatal. As recommended by Cochrane Neonatal, we adapted the RCT search filter for MEDLINE Ovid to the syntax of PubMed to identify randomized and quasi‐randomized studies. We conducted searches for eligible trials without language, publication year, publication type, or publication status restrictions.
Electronic searches
We searched the following databases:
Cochrane Central Register of Controlled Trials (CENTRAL; 2023, Issue 8), via the Cochrane Library (searched 22 August 2023);
MEDLINE via PubMed (1946 to 21 August 2023);
Embase via Elsevier (1974 to 22 August 2023).
Searching other resources
We identified trial registration records using CENTRAL, and by independent searches of:
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (https://clinicaltrials.gov/);
ICTRP — World Health Organization International Clinical Trials Registry Platform (https://trialsearch.who.int/Default.aspx);
ISRCTN registry (https://www.isrctn.com/).
We searched the trial registries on 23 August 2023.
We screened the reference lists of included studies and related systematic reviews for studies not identified by the database searches.
We searched for errata or retractions for included studies, published in PubMed (https://pubmed.ncbi.nlm.nih.gov/) and the Retraction Watch database.
Data collection and analysis
We used the standard methods of Cochrane Neonatal, as described below.
Selection of studies
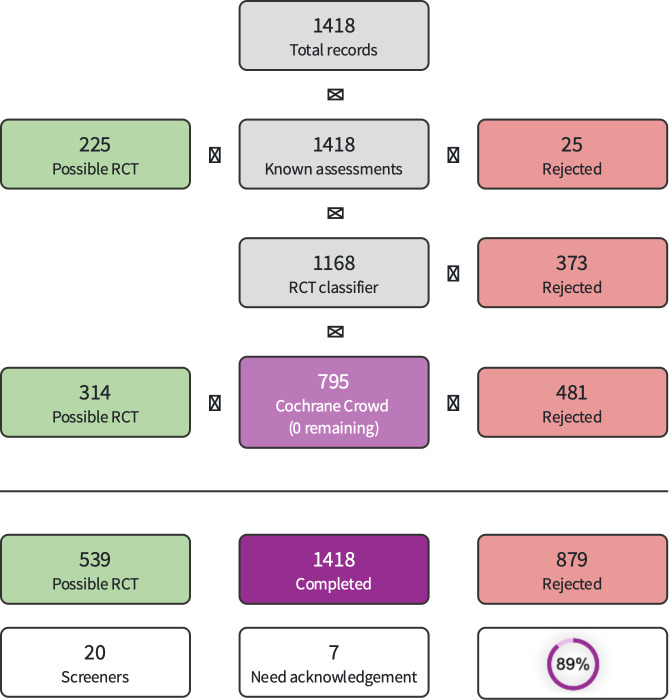
We downloaded all titles and abstracts retrieved by electronic searching to reference management software and removed duplicates. We used Cochrane's Screen4Me to reduce screening activities by the authors (Marshall 2018; Noel‐Storr 2020; Noel‐Storr 2021; Thomas 2021). Screen4Me comprises three components:
Known assessments (a service that matches records in the search results to records that have been screened by Cochrane Crowd and labeled as 'RCT' or 'not an RCT');
The RCT classifier (a machine‐learning model that distinguishes RCTs from non‐RCTs);
Cochrane Crowd (Cochrane’s crowdsourcing platform, through which contributors from around the world help to identify randomized trials, and other types of healthcare‐related research).
Any references categorized as non‐RCTs through the known assessments and the RCT classifier were added to the irrelevant segment of Covidence. This approach means that references are available for the purposes of deduplication when the review is updated; and for verification purposes should questions arise about the accuracy of Screen4Me categorization. The results of Screen4Me are presented in Figure 1, and the disposition of references is incorporated into Figure 2 (Liberati 2009).
1.
Screen4Me summary diagram
2.
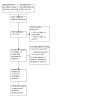
PRISMA study flow diagram
Two review authors (EB, MB, MG, RC, SU) independently screened the remaining titles and abstracts. Two other review authors (EB, MB, MG, RC) independently assessed the full text of references remaining after the title and abstract screening. At any point in the screening process, disagreements were resolved between review authors by discussion. We documented the reasons for exclusion in the Characteristics of excluded studies table. We excluded studies if one or more PICO‐S elements were absent; if a study omitted more than one PICO‐S element, we documented only one.
We collated multiple references from the same study so that each study, rather than each reference, represented the unit of interest in the review. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram (Liberati 2009).
Data extraction and management
Two review authors, (either RC, EB, or MG), independently extracted data by using a data extraction form integrated with a modified version of the Cochrane Effective Practice and Organisation of Care Group data collection checklist (EPOC 2017). We piloted the form within the review team, using a sample of included studies.
Three review authors (EB, MG, RC) independently extracted the following characteristics from each included study:
Administrative details: study author(s), published or unpublished, year of publication, year in which study was conducted, presence of vested interest, details of other relevant papers cited;
Study characteristics: study registration, study design type, study setting, number of study centers and location, informed consent, ethics approval, completeness of follow‐up (e.g. greater than 80%);
Participants: number randomized, number lost to follow‐up/withdrawn, number analyzed, mean gestational age (GA), GA age range, mean CA or CA age range, inclusion criteria, and exclusion criteria;
Interventions: initiation, dose, and duration of caffeine administration;
Outcomes: outlined under Types of outcome measures.
Any disagreements were resolved by discussion.
Ongoing studies identified by our search were described, and available information was documented, such as the primary author, research question(s), methods, and outcome measures, together with an estimate of the anticipated reporting date in Characteristics of ongoing studies.
When queries arose, or we required additional data, we contacted study investigators/authors via e‐mail, for clarification. Two review authors used Cochrane software for data entry (RevMan 2024). We then replaced any standard error of the mean (SEM) with the corresponding SD.
Assessment of risk of bias in included studies
Two different review authors (RC, EB, or MG) used the Cochrane RoB 1 tool to independently assess the risk of bias (low, high, or unclear) of all included studies for the following domains (Higgins 2017):
Sequence generation (selection bias);
Allocation concealment (selection bias);
Blinding of participants and personnel (performance bias);
Blinding of outcome assessment (detection bias);
Incomplete outcome data (attrition bias);
Selective reporting (reporting bias);
Any other bias.
Any disagreements were resolved by discussion and consultation with a third review author (MB). A more detailed description of the risk of bias for each domain is given in Appendix 2.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results using risk ratios (RR) and risk differences (RD) with 95% confidence intervals (CIs). If there was a statistically significant reduction (or increase) in RD, we calculated the number needed to treat for an additional beneficial outcome (NNTB), or the number needed to treat for an additional harmful outcome (NNTH) with 95% CIs.
Continuous data
For continuous data, we used the mean difference (MD) when outcomes were measured in the same way between trials. We used the standardized mean difference (SMD) to combine data from trials that measured the same outcome but used different methods. When trials reported continuous data as median and interquartile range (IQR), and the data passed the test of skewness, we converted the median to the mean, and estimated the standard deviation as IQR/1.35.
Unit of analysis issues
The unit of analysis was the participating infant in individually randomized trials; an infant was considered only once in the analysis. The participating neonatal unit or section of a neonatal unit or hospital was the unit of analysis in cluster‐randomized trials.
We did not include any cluster‐randomized trials. If we do in future updates, we will abstract information on the study design and unit of analysis for each study, indicating whether clustering of observations was presented due to allocation to the intervention at the group level, or clustering of individually randomized observations (e.g. infants within clinics). We will extract available statistical information needed to account for the implications of clustering on the estimation of outcome variances, such as design effects or intra‐cluster correlations (ICCs), and whether the study adjusted results for the correlations in the data. If the study does not account for clustering, we will make appropriate adjustments to the effective sample size, following Cochrane guidance (Higgins 2023). Where possible, we will derive the ICC for these adjustments from the trial itself, or from a similar trial. If an appropriate ICC is unavailable, we will conduct a sensitivity analysis to investigate the potential effect of clustering, by imputing a range of values of ICC.
In future updates, if we include trials with multiple arms compared against the same controls that are included in the same meta‐analysis, we will either combine groups to create a single pair‐wise comparison, or select the pair of interventions that most closely matches the definitions given in Types of interventions, and exclude the others. We will acknowledge this potential selective bias of data used for analysis in the Discussion.
Dealing with missing data
We carried out the analysis on an intention‐to‐treat (ITT) basis for all included outcomes. Whenever possible, we analyzed all participants in the treatment group to which they were randomized, regardless of the actual treatment received. When we identified important missing data (in the outcomes) or unclear data, we contacted the original investigators via e‐mail to request them. We were explicit about our assumptions of any methods used to deal with missing data.
For missing dichotomous outcomes, we included participants with incomplete or missing data in the sensitivity analysis by imputing them according to the following scenarios:
Extreme‐case analysis favoring the experimental intervention (best‐worst case scenario): none of the dropouts/participants lost from the experimental arm, but all the dropouts/participants lost from the control arm experienced the outcome, including all randomized participants in the denominator;
Extreme‐case analysis favoring the control (worst‐best case scenario): all dropouts/participants lost from the experimental arm, but none from the control arm experienced the outcome, including all randomized participants in the denominator.
For continuous outcomes, we calculated missing standard deviations using reported P values or CIs (Higgins 2023). If a calculation was not possible, we imputed a standard deviation as the highest standard deviation reported in the other trials for the corresponding treatment group and outcome.
We addressed the potential impact of missing data on the findings of the review in the Discussion.
Assessment of heterogeneity
We described the clinical diversity and methodological variability of the evidence narratively and in tables. Tables included data on study characteristics, such as design features, population characteristics, and intervention details.
To assess statistical heterogeneity, we visually inspected forest plots and described the direction and magnitude of effects and the degree of overlap between confidence intervals. We also considered the statistics generated in forest plots that measured statistical heterogeneity. We used the I² statistic to quantify inconsistency among the trials in each analysis. We also considered the P value from the Chi² test to assess if this heterogeneity was significant (P < 0.1). When we identified substantial heterogeneity, we reported the finding and explored possible explanatory factors using prespecified subgroup analysis.
We classified the degree of heterogeneity as follows:
0% to 40% might not represent important heterogeneity;
30% to 60% may represent moderate heterogeneity;
50% to 90% may represent substantial heterogeneity;
More than 75% may represent considerable heterogeneity.
A rough guideline was used to interpret the I2 value rather than a simple threshold, and our interpretation took into account the understanding that measures of heterogeneity (I2 and Tau) were estimated with high uncertainty when the number of studies was small (Deeks 2023).
Assessment of reporting biases
We assessed reporting bias by comparing the stated primary and secondary outcomes, and reported outcomes. When study protocols were available, we compared these to the full publications to determine the likelihood of reporting bias. We documented studies that used interventions in a potentially eligible infant population, but did not report on any of the primary and secondary outcomes that were included in the Characteristics of included studies table.
Since our review included fewer than 10 studies eligible for meta‐analysis, the ability to detect publication bias was largely diminished, and we noted our inability to rule out possible publication bias or small study effects.
Data synthesis
When we identified multiple studies that we considered to be sufficiently similar, we performed a meta‐analysis using Review Manager software (RevMan 2024). For categorical outcomes, we calculated the typical estimates of RR and RD, each with its 95% CI; for continuous outcomes, we calculated the MD or the SMD, each with its 95% CI. We used a fixed‐effect model to combine data when it was reasonable to assume that studies were estimating the same underlying treatment effect. When we judged meta‐analysis to be inappropriate, we analyzed and interpreted individual trials separately. When there was evidence of clinical heterogeneity, we tried to explain this based on the different study characteristics and subgroup analyses.
Subgroup analysis and investigation of heterogeneity
We interpreted tests for subgroup differences in effects with caution, given the potential for confounding with other study characteristics and the observational nature of the comparisons (Higgins 2023). In particular, subgroup analyses with fewer than five studies per category are unlikely to be adequate to ascertain valid differences in effects, and we did not highlight them in our results. When subgroup comparisons were possible, we conducted stratified meta‐analysis and a formal statistical test for interaction to examine subgroup differences that could account for effect heterogeneity (e.g. Cochran’s Q test, meta‐regression [Borenstein 2013; Deeks 2023]).
Given the potential differences in the intervention effectiveness related to gestational age and duration of caffeine treatment, as discussed in the Background, we conducted subgroup comparisons to see if the intervention was more effective.
We carried out the following subgroup analyses of factors that may contribute to heterogeneity in the effects of the intervention:
Gestational age: extremely preterm (less than 28 weeks); very preterm (less than 32 weeks); 32 weeks or more;
Duration of caffeine treatment before randomization to discontinuation: less than one week; one to four weeks; more than four weeks;
Indication for initial treatment: prevention of apnea; treatment of apnea; peri‐extubation management.
We used the main outcomes (those specified in the Table 1; Table 2) in subgroup analyses when there were enough studies reporting the outcomes to support valid subgroup comparisons (at least five studies per subgroup).
Sensitivity analysis
We planned to conduct sensitivity analysis for studies with a high risk of bias in at least two domains, to ascertain whether these studies overestimated the effect of treatment.
Because the type of randomization can affect the findings in systematic reviews, we planned to conduct sensitivity analyses to compare the effects of caffeine in randomized versus quasi‐randomized trials. However, we did not include any quasi‐randomized trials.
Summary of findings and assessment of the certainty of the evidence
We used the GRADE approach, as outlined in the GRADE Handbook, to assess the certainty of evidence for the following (clinically relevant) outcomes (Schünemann 2013);
Restarting caffeine therapy;
Intubation within one week of treatment discontinuation;
Need for non‐invasive respiratory support (CPAP, NIPPV, high‐flow nasal cannulae) within one week of treatment discontinuation;
Apnea: number of infants with at least one episode (defined as interruption of breathing for more than 20 seconds, or as defined by the authors) in the seven days after discontinuation of treatment;
Intermittent hypoxemia (IH): number of episodes in the seven days after discontinuation of treatment;
IH: number of infants with at least one episode in the seven days after discontinuation of treatment;
All‐cause mortality prior to hospital discharge.
Two review authors (SU, MB) independently assessed the certainty of the evidence for each of the outcomes above, for each comparison where at least one study was included. We considered evidence from randomized controlled trials as high certainty, downgrading the evidence by one level for serious (or two levels for very serious) limitations, based upon the following: design (risk of bias), consistency across studies, directness of the evidence, precision of estimates, and presence of publication bias. We used GRADEpro GDT software to create Table 1 and Table 2 to report the certainty of evidence.
The GRADE approach resulted in an assessment of the certainty of a body of evidence at one of the following four grades:
High certainty: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect.
Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect.
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies; Characteristics of ongoing studies for study details.
Results of the search
The search identified a total of 1910 references through database searching (N = 1845) and trial registries (N = 65). Among these, we identified 1411 potentially eligible references. After removing 53 duplicates, 1358 references remained. We excluded 1345 references; 879 were excluded by Screen4Me, and the other 466 were excluded by the review authors, based on title/abstract.
We reviewed 13 full‐text articles (7 studies, 13 references). We excluded one full‐text article (1 study, 1 reference) because of an inappropriate intervention. We classified five full‐text articles (3 studies, 5 references) as ongoing. We included three studies (7 references) in the qualitative synthesis and three studies in the quantitative synthesis. Details of study selection are presented in Figure 1 and Figure 2.
Included studies
We included three randomized controlled trials (RCTs) enrolling 392 preterm infants (Pradhap 2023; Prakash 2021; Rhein 2014). Details of the trials are described in the Characteristics of included studies table.
Two studies compared post‐menstrual age (PMA; with discontinuation of caffeine at a scheduled PMA) versus the resolution of symptoms (at a fixed time after the infant was without the presence of respiratory support, or apneic spells), and were conducted for the prevention of apnea (Pradhap 2023; Prakash 2021). One study compared the intervention type of PMA, meaning PMA less than 35 weeks' gestation versus PMA greater than or equal to 35 weeks' gestation, within the two intervention groups; it was conducted for the prevention of intermittent hypoxia episodes (Rhein 2014).
Two studies were conducted in India (Pradhap 2023; Prakash 2021), and one in the USA (Rhein 2014).
All participants were preterm infants with a gestational age between 26 and 33 weeks. Sample sizes varied, from 98 (Rhein 2014), to 120 (Prakash 2021), to 174 (Pradhap 2023). In all studies, participants were divided into two groups and randomized to either discontinue or continue caffeine. Caffeine was administered orally or intravenously. The loading dose of caffeine citrate was 20 mg/kg; the maintenance dose ranged from 5 mg/kg (Pradhap 2023; Prakash 2021), to 6 mg/kg (Rhein 2014). Randomization took place when they were either free of apnea and respiratory support for seven consecutive days (Pradhap 2023; Prakash 2021), or when caffeine had been discontinued for five days, and their PMA was 34 to 37 weeks (Rhein 2014).
Ongoing studies
We included three ongoing RCTs enrolling 1280 preterm infants (NCT03321734; NCT03340727; NCT04868565).
Two ongoing trials compare caffeine discontinuation at early PMA (PMA less than 35 weeks' gestation) versus late PMA (PMA greater than or equal to 35 weeks' gestation), and are conducted to prevent intermittent hypoxia episodes (NCT03321734), and apnea (NCT03340727; NCT04868565).
Two ongoing trials are conducted in the USA (NCT03321734; NCT03340727), and one in China (NCT04868565).
All participants are preterm infants with a gestational age between 29 and 36 weeks. Estimated sample sizes vary from 170 (NCT03321734), to 310 (NCT04868565), to 800 (NCT03340727). In all three trials, participants are divided into two groups and randomized to either discontinue or continue caffeine. Caffeine is administered orally (NCT03321734; NCT03340727), or intravenously (NCT04868565). The total daily dose of caffeine citrate is 10 mg/kg (NCT03321734; NCT03340727; NCT04868565). Two trials used placebo in the group of early caffeine discontinuation (NCT03321734; NCT03340727); one trial continued giving oxygen supplement to those infants ending caffeine treatment (NCT04868565).
Excluded studies
Following full‐text screening, we excluded one ongoing study, which enrolled late preterm infants; it did not compare different strategies for cessation of caffeine administration (ACTRN12622001344785).
Risk of bias in included studies
Figure 3 and Figure 4 represent the risk of bias assessment for the included RCTs.
3.

Review authors' judgements about each risk of bias item presented as percentages across all included studies
4.

Review authors' judgements about each risk of bias item for each included study
Allocation
We deemed the risk of selection bias to be low in all included studies.
In Rhein 2014, the groups were randomized using computer generated random numbers, with blocks; sealed opaque envelopes provided by the Data Coordinating Center contained each randomization, to extend caffeine use or usual care. In Prakash 2021, the groups were randomized using a computer generated random number sequence; once assigned, group allocation concealment was accomplished using sequentially numbered, opaque, sealed envelopes. Pradhap 2023 used a computer generated random number sequence to randomize the infants, with block randomization into blocks of variable sizes (2, 4, 6, and 8). Allocation concealment was accomplished with sequentially numbered, opaque, sealed envelopes.
Blinding
We classified Rhein 2014 as having a high risk of performance bias due to the lack of blinding of the clinical team. However, the risk of detection bias was low, since all analysis of outcome measures were to be performed by persons masked to treatment assignment.
The Prakash 2021 study raised serious concerns regarding the risk of both performance and detection bias owing to the lack of blinding of personnel, which also, in accordance to the study protocol, were the primary outcome assessors.
We deemed Pradhap 2023 to have a high risk of performance bias due to the lack of blinding. They did not specify whether the outcome assessors were made aware of the different treatment groups or not. Therefore, we classified the risk of detection bias as unclear.
Incomplete outcome data
We deemed the risk of attrition biases to be low in all three studies since none of them reported any missing data between the point of randomization and the outcome analyses.
Selective reporting
We assessed the overall risk of reporting bias in all three studies to be low, since the predetermined PICO‐model for the study protocol matched the published full text in all three cases.
Other potential sources of bias
No other biases were identified in all three studies. Therefore, we determined that all studies had a low risk of other potential sources of bias.
Effects of interventions
Discontinuation of caffeine at post‐menstrual age (PMA) less than 35 weeks' gestation versus PMA greater than or equal to 35 weeks' gestation
One study was included in this comparison (Rhein 2014). A summary of key results is in Table 1.
Primary outcomes
Restarting caffeine therapy
No studies reported this outcome.
Intubation within one week of treatment discontinuation
No studies reported this outcome.
Need for non‐invasive respiratory support (continuous positive airway pressure [CPAP], nasal intermittent positive pressure ventilation [NIPPV], high‐flow nasal cannulae) within one week of treatment discontinuation
No studies reported this outcome.
Secondary outcomes
Apnea: number of episodes (defined as interruption of breathing for more than 20 seconds, or as defined by the authors) in the seven days after discontinuation of treatment
No studies reported this outcome.
Apnea: number of infants with at least one episode (defined as interruption of breathing for more than 20 seconds, or as defined by the authors) in the seven days after discontinuation of treatment
No studies reported this outcome.
Intermittent hypoxemia (IH): number of episodes in the seven days after discontinuation of treatment
Early discontinuation (PMA < 35 weeks at discontinuation of caffeine treatment) may result in an increase in the number of IH episodes in the seven days after discontinuation of treatment (mean difference [MD] 4.8, 95% confidence interval [CI] 2.21 to 7.39; I2 not applicable; 1 RCT, 98 infants; low‐certainty evidence; Analysis 1.1). The true effect may be substantially different from the estimate of the effect.
1.1. Analysis.

Comparison 1: Discontinuation of caffeine at PMA < 35 weeks' gestation versus PMA ≥ 35 weeks' gestation, Outcome 1: Intermittent hypoxemia (IH): number of episodes in the 7 days after treatment discontinued
IH: number of infants with at least one episode in the seven days after discontinuation of treatment
No studies reported this outcome.
All‐cause mortality prior to hospital discharge
Early discontinuation (PMA < 35 weeks' gestation) may result in little to no difference in all‐cause mortality prior to hospital discharge (risk ratio [RR] not estimable [no events], RD 0.00, 95% CI ‐0.04 to 0.04; I2 not applicable; low‐certainty evidence [personal communication with study authors]; Analysis 1.2). The true effect may be substantially different from the estimate of the effect.
1.2. Analysis.

Comparison 1: Discontinuation of caffeine at PMA < 35 weeks' gestation versus PMA ≥ 35 weeks' gestation, Outcome 2: All‐cause mortality prior to hospital discharge
Major neurodevelopmental disability
No studies reported this outcome.
Each component of the composite outcome, major neurodevelopmental disability
No studies reported any of the components of the composite outcome, major neurodevelopmental disability.
Mortality or major neurodevelopmental disability
Only mortality was reported, as stated under all‐cause mortality prior to hospital discharge.
Number of days of respiratory support (mechanical ventilation, CPAP, high‐flow nasal cannula, NIPPV) after treatment discontinuation
No studies reported this outcome.
Duration of hospital stay
No studies reported this outcome.
Cost of neonatal care
No studies reported this outcome.
Adverse effects
Five of the infants randomized to caffeine had the caffeine treatment discontinued at the discretion of the clinical team, because of tachycardia.
Discontinuation of caffeine less than five days after infants are symptom‐free (without the presence of respiratory support or apneic spells) versus discontinuation of caffeine at five days or more after infants are symptom‐free, regardless of the PMA
No studies were included in this comparison.
Discontinuation of caffeine based on PMA (with discontinuation of caffeine at a scheduled PMA) versus discontinuation based on resolution of symptoms (at a fixed time after the infant is without the presence of respiratory support or apneic spells)
Two studies were included in this comparison (Pradhap 2023; Prakash 2021). A summary of key results is in Table 2.
Primary outcomes
Restarting caffeine therapy
No studies reported this outcome.
Intubation within one week of treatment discontinuation
No studies reported this outcome.
Need for non‐invasive respiratory support (continuous positive airway pressure [CPAP], nasal intermittent positive pressure ventilation [NIPPV], high‐flow nasal cannulae) within one week of treatment discontinuation
No studies reported this outcome.
Secondary outcomes
Apnea: number of episodes (defined as interruption of breathing for more than 20 seconds, or as defined by the authors) in the seven days after discontinuation of treatment
No studies reported this outcome.
Apnea: number of infants with at least one episode (defined as interruption of breathing for more than 20 seconds, or as defined by the authors) in the seven days after discontinuation of treatment
Discontinuation of caffeine based on the resolution of symptoms may result in little to no difference in the number of infants with apnea in the seven days after discontinuation of treatment (RR 0.60, 95% CI 0.31 to 1.18; I2 0%; 2 RCTs, 394 infants; low‐certainty evidence; Analysis 2.1).
2.1. Analysis.

Comparison 2: Discontinuation of caffeine based on PMA (caffeine discontinued at a scheduled PMA) versus discontinuation based on resolution of symptoms (a fixed time after the infant is without respiratory support, or apneic spells) , Outcome 1: Apnea: number of infants with at least 1 episode (interruption of breathing for more than 20 seconds, or as defined by the authors) in the 7 days after treatment discontinued
Intermittent hypoxemia (IH): number of episodes in the seven days after discontinuation of treatment
No studies reported this outcome.
IH: number of infants with at least one episode in the seven days after discontinuation of treatment
Discontinuation of caffeine based on the resolution of symptoms probably results in an increase in the number of infants with IH in the seven days after discontinuation of treatment (RR 0.38, 95% CI 0.20 to 0.75; I2 not applicable; 1 RCT, 174 infants; moderate‐certainty evidence; Analysis 2.2).
2.2. Analysis.

Comparison 2: Discontinuation of caffeine based on PMA (caffeine discontinued at a scheduled PMA) versus discontinuation based on resolution of symptoms (a fixed time after the infant is without respiratory support, or apneic spells) , Outcome 2: IH: number of infants with at least 1 episode in the 7 days after treatment discontinued
All‐cause mortality prior to hospital discharge
Early discontinuation (at resolution of symptoms) versus late discontinuation (at a scheduled PMA) may result in little to no difference in the all‐cause mortality rate prior to hospital discharge (RR 1.00, 95% CI 0.14 to 7.03; I2 0%; 2 RCTs, 294 infants; low‐certainty evidence; Analysis 2.3).
2.3. Analysis.

Comparison 2: Discontinuation of caffeine based on PMA (caffeine discontinued at a scheduled PMA) versus discontinuation based on resolution of symptoms (a fixed time after the infant is without respiratory support, or apneic spells) , Outcome 3: All‐cause mortality prior to hospital discharge
Major neurodevelopmental disability
No studies reported this outcome.
Each component of the composite outcome, major neurodevelopmental disability
No studies reported this outcome.
Mortality or major neurodevelopmental disability.
One case of mortality was reported in each group, as stated under all‐cause mortality prior to hospital discharge.
Number of days of respiratory support (mechanical ventilation, CPAP, high‐flow nasal cannula, NIPPV) after treatment discontinuation
No studies reported this outcome.
Duration of hospital stay
No studies reported this outcome.
Cost of neonatal care
No studies reported this outcome.
Adverse effects
The Pradhap 2023 study reported adverse events, including recurrence of apnea of prematurity (15% in the short and 13% in the regular course caffeine therapy groups), varying severities of bronchopulmonary dysplasia, hyperglycemia, extrauterine growth restriction, retinopathy of prematurity requiring laser treatment, feeding intolerance, osteopenia, and tachycardia, with no significant differences between the groups.
The Prakash 2021 study reported that adverse effects of caffeine therapy for apnea of prematurity included tachycardia, feeding intolerance, and potential neurodevelopmental impacts, though most were mild and transient.
Discussion
Summary of main results
The review included three studies (392 infants), which we pooled into two different strategies for discontinuation of caffeine administration: one study compared discontinuation based on different post‐menstrual age (PMA) thresholds (Rhein 2014), and two studies compared discontinuation based on PMA versus the resolution of symptoms (Pradhap 2023; Prakash 2021). None of the included studies reported any primary outcomes of interest for this review. Three of the secondary outcomes of this review were reported: apnea, intermittent hypoxemia (IH), and mortality.
Early cessation of caffeine administration in preterm infants at a PMA less than 35 weeks' of gestation may result in an increase in the number of IH episodes in the seven days after discontinuation of treatment compared to prolonged caffeine treatment beyond 35 weeks of gestation. Early cessation may result in little to no difference in all‐cause mortality prior to hospital discharge compared to late discontinuation after 35 weeks of PMA. We classified the certainty of evidence in both outcomes as low.
Discontinuation of caffeine based on the resolution of symptoms may result in little to no difference in the number of infants with apnea in the seven days after discontinuation of treatment, or all‐cause mortality rate prior to hospital discharge. We deemed the certainty of the evidence in both outcomes to be low, meaning that the results are likely to differ significantly from the true outcome. Discontinuation of caffeine based on the resolution of symptoms probably results in an increase in the number of infants with IH in the seven days after discontinuation of treatment. We classified the certainty of evidence as moderate, meaning the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Overall completeness and applicability of evidence
Study design
All included studies were randomized controlled trials (RCTs).
Types of participants
In all three included studies, preterm infants were at a gestational age between 26 and 33 weeks. They all matched the review's inclusion criteria and were randomized into two groups to either discontinue or continue caffeine. All infants received caffeine for at least seven days. In Pradhap 2023, they received caffeine for exactly seven days; in Prakash 2021 and Rhein 2014, they received it for longer than seven days. The loading dose of caffeine citrate was 20 mg/kg in all three studies; the maintenance dose ranged from 5 mg/kg (Pradhap 2023; Prakash 2021), to 6 mg/kg (Rhein 2014).
Intervention and comparison
Randomization took place when the infants were either free of apnea and respiratory support for seven consecutive days (Pradhap 2023; Prakash 2021), or when caffeine had been discontinued for five days and their PMA was 34 to 37 weeks (Rhein 2014). Thus, the participants in the included studies represented the participants specified in our inclusion criteria.
Outcome measures
In the protocol, we decided to assess the certainty of evidence and display those results in the Table 1 and Table 2 only for the following (clinically relevant) four outcomes: restarting caffeine therapy, intubation, need for non‐invasive respiratory support, and major neurodevelopmental disability. However, in the review, we added the outcomes, all‐cause mortality prior to hospital discharge, number of episodes of intermittent hypoxemia in the seven days after discontinuation of treatment, and number of infants with at least one episode of apnea the seven days after discontinuation of treatment, due to their relevance and presentation of results by the included studies.
Certainty of the evidence
Study limitations
We did not downgrade any studies for risk of bias in the outcomes listed in Table 1 or Table 2.
Indirectness
In general, the included studies closely matched the eligibility criteria we specified, particularly for population, intervention, and comparison. For some outcomes, studies measured the outcome at different post‐menstrual ages. However, we did not consider this clinically significant because of the matching intervention strategies.
Inconsistency
Heterogeneity was not a factor in the comparison, Discontinuation of caffeine at post‐menstrual age (PMA) less than 35 weeks' gestation versus PMA greater than or equal to 35 weeks' gestation, because only one RCT fell into this category (Rhein 2014). It ended up not being a factor in the comparison, Discontinuation of caffeine based on PMA (with discontinuation of caffeine at a scheduled PMA) versus discontinuation based on resolution of symptoms (at a fixed time after the infant is without the presence of respiratory support or apneic spells) either, since the I2 was 0% for each measured outcome, with a good overlap of the confidence intervals (Pradhap 2023; Prakash 2021).
Imprecision
We downgraded the certainty of the evidence one level for imprecision due to either wide confidence intervals, few infants, or few events. We downgraded two levels when there was a combination of these factors. Most analyses were based on a relatively small number of preterm infants and events. Many results had wide confidence intervals, sometimes crossing the line of no effect, and including the possibility that early cessation was both more beneficial and more harmful than late discontinuation.
Other considerations
We did not find any other reasons to downgrade evidence certainty. We did not strongly suspect publication bias, which, however, is difficult to rule out.
Potential biases in the review process
At every step of the way, our review was conducted according to Cochrane methodological expectations. Data collection, assessment of risk of bias, rating the certainty of evidence, and interpreting data followed Cochrane guidelines systematically and appropriately. The possibility that some relevant trials were missed was minimized by performing study selection, data extraction, and quality assessment in duplicate, and with additional discussion by all authors when disagreements arose. Therefore, we minimized bias in the review process.
We rephrased the definitions of interventions in the full review to improve their clarity from that set out in the protocol. In the protocol, we set out three different strategies for caffeine cessation: (1) discontinuation at different PMAs, (2) discontinuation before or after five days without symptoms, and (3) discontinuation at a predetermined PMA versus at resolution of symptoms. We included results for only two strategies due to the lack of studies comparing early versus late resolution of symptoms. None of the included studies reported any primary outcomes of interest, and only reported three of the secondary outcomes of interest.
Agreements and disagreements with other studies or reviews
Former Cochrane reviews focused on appropriate doses of caffeine, comparing high versus standard dose (Brattström 2019; Bruschettini 2023), and caffeine therapy versus other methylxanthines or placebo (Marques 2023; Moresco 2023).
We were unable to find any primary studies that explicitly compared caffeine cessation strategies in preterm infants besides those included in this review. Previous studies have focused more on dosing regimens (Chavez 2022; Rostas 2019), short‐term outcomes in infants receiving either caffeine or placebo (Schmidt 2006), and the impact of caffeine discontinuation on caffeine levels, apnea, and hypoxemia (Chung 2022; Tabacaru 2017).
However, other trial designs have reported results of caffeine discontinuation. An American cohort study, which compared discontinuation at different PMAs (as in our Table 1), reported that infants who discontinued caffeine prior to the last week of hospitalization were more likely to experience a higher incidence of BPD than those who discontinued caffeine within the last week of hospitalization. That said, when caffeine was discontinued, the PMA in the two groups differed by one week (34 versus 35 weeks), and the total duration of caffeine differed by three days (37 versus 40 days). However, the sample sizes varied widely (75,404 versus 5706 [Ji 2020]). Therefore, the results should be viewed with great caution, and are not comparable to the results and outcomes in our review.
A retrospective chart review found that caffeine can be discontinued at 33 to 35 weeks of PMA, with a failure rate of 10% (Haddad 2015), which does not align with the results in our review, which were much lower, at 1% (Rhein 2014).
We found no studies that compared the discontinuation of caffeine before or after the resolution of symptoms (as in our Table 2).
Authors' conclusions
Implications for practice.
Early cessation of caffeine administration in preterm infants at a post‐menstrual age less than 35 weeks' gestation may result in an increased number of episodes of intermittent hypoxemia in the seven days after discontinuing treatment compared with prolonged caffeine treatment beyond 35 weeks' gestation. Early cessation may result in little to no difference in all‐cause mortality prior to hospital discharge compared to late discontinuation, after 35 weeks post‐menstrual age.
Discontinuation of caffeine based on the resolution of symptoms may result in little to no difference in the number of infants with apnea in the seven days after discontinuing treatment and all‐cause mortality prior to hospital discharge. Discontinuation of caffeine based on the resolution of symptoms probably results in an increased number of infants with intermittent hypoxemia in the seven days after discontinuation of treatment.
Implications for research.
Further studies should evaluate the effects of different caffeine cessation strategies on premature infants, as planned in the three ongoing studies identified in this Cochrane review. Studies evaluating the effects of caffeine withdrawal based on different symptom‐free periods of time are also needed.
Another relevant research gap to be addressed is the role of gestational age at birth in different cessation strategies.
Finally, future studies should evaluate long‐term effects, such as neuropsychomotor development in preterm infants who received different caffeine cessation strategies.
History
Protocol first published: Issue 8, 2023
Acknowledgements
The Methods section of this protocol is based on a standard template used by Cochrane Neonatal.
We thank Matthias Bank (Library and ICT services, Lund University) for designing the search strategy.
We thank Carl E Hunt (Professor of Pediatrics Emeritus, F Edward Hébert School of Medicine – 'America’s Medical School', Uniformed Services University, Bethesda, MD, USA) for providing additional information on Rhein 2014 and NCT03321734.
We would like to acknowledge and thank the following people for their help in assessing the search results for this review via Cochrane’s Screen4Me workflow: Igor Svintsitskyi, Nicole Askin, Therese Dalsbø, Susanna Wisniewski, Shammas Mohammed, Gustavo José Padovezi Neves Cruz, Brisa Magdalena Calvillo Ruiz.
Editorial and peer‐reviewer contributions
Cochrane Neonatal (Jane Cracknell and Michelle Fiander, Managing Editors; and Roger Soll and Bill McGuire, Co‐co‐ordinating Editors) supported the authors in the development of this review.
The following people conducted the editorial process for this review.
• Sign‐off Editor (final editorial decision): Nai Ming Lai, Professor of Paediatrics, Taylor's University, Malaysia
• Managing Editor (selected peer reviewers, provided editorial guidance to authors, edited the article): Leanne Jones, Central Editorial Service
• Editorial Assistant (conducted editorial policy checks, collated peer‐reviewer comments and supported editorial team): Addie‐Ann Smyth, Central Editorial Service
• Copy Editor (copy editing and production): Victoria Pennick, Cochrane Central Production Service
• Peer‐reviewers (provided comments and recommended an editorial decision): Viral G Jain, MD. University of Alabama at Birmingham (clinical/content review), Jane Alsweiler, School of Medicine, University of Auckland (clinical/content review), Brian Duncan (consumer review), Nuala Livingstone, PhD, Cochrane Evidence Production and Methods Directorate (methods review), Jo Platt, Central Editorial Information Specialist (search review). One additional peer reviewer provided clinical/content peer review but chose not to be publicly acknowledged.
Appendices
Appendix 1. Search strategies
Information specialist: Matthias Bank
Affiliation: Lund University, Faculty of Medicine, Library & ICT, Sweden
PubMed (National Library of Medicine)
Date of search: 21 August 2023
No publication date limitations or language limitations were used.
Search filters: the Cochrane Neonatal search filter for RCTs in Ovid Medline was used and converted to the PubMed syntax; the Cochrane Neonatal filter for neonatal populations in Ovid Medline was used and converted to the PubMed syntax.
#1 "Caffeine"[Mesh] OR caffeine*[tw] OR caffedrine*[tw] OR coffein*[tw] OR caffeine*[tw] OR methylxanthin*[tw] OR trimethylxanthin*[tw] 42,675
CAFFEINE
#2 (infant, newborn[Mesh]) OR (intensive care, neonatal[Mesh]) OR (intensive care units, neonatal[Mesh]) OR (gestational age[Mesh]) 724,785
#3 babe[TW] OR babes[TW] baby*[TW] OR babies[TW] OR gestational age[TW] OR gestational ages[TW] OR infant[TW] OR infants[TW] OR infant s[TW] OR infant's[TW] OR infantile[TW] OR infancy[TW] OR low birth weight[TW] OR low birthweight[TW] OR neonat*[TW] OR neo‐nat*[TW] OR newborn*[TW] OR new born[TW] OR new borns[TW] OR newly born[TW] OR premature[TW] OR prematures[TW] OR pre‐mature*[TW] OR prematurity[TW] OR pre‐maturity[TW] OR preterm[TW] OR preterms[TW] OR pre term[TW] OR pre terms[TW] OR preemie[TW] OR preemies[TW] OR premies[TW] OR premie[TW] OR VLBW[TW] OR VLBWI[TW] OR VLBW‐I[TW] OR VLBWs[TW] OR LBW[TW] OR LBWI[TW] OR LBWs[TW] OR ELBW[TW] OR ELBWI[TW] OR ELBWs[TW] OR NICU[TW] OR NICUs[TW] 1,812,826
#4 #2 OR #3 1,812,826
NEONATES
#5 randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized [tiab] OR placebo [tiab] OR drug therapy [sh] OR randomly [tiab] OR trial [tiab] OR groups [tiab] 5,813,560 #6 quasirandom*[tw] or quasi‐random*[tw] or randomi*[tw] or randomly[tw] 1,335,506
#7 control*[tw] AND (group[tw] OR groups[tw] OR random[tw] OR trial[tw] OR trials[tw] OR study[tw]) 3,929,669
#8 #5 OR #6 OR #7 7,725,861
#9 (animals [mh] NOT humans [mh]) 5,146,857
#10 #8 NOT #9 6,636,178
RANDOMIZED AND QUASI‐RANDOMIZED CONTROLLED TRIALS
#11 #1 AND #4 AND #10 888
CENTRAL via Cochrane Library Online (Issue 8 of 12, August 2023)
Date of search: 22 August 2023
No publication date limitations or language limitations were used.
Search filters: The Cochrane Neonatal filter for Cochrane Library, neonatal populations, was used.
#1 MeSH descriptor: [Caffeine] explode all trees 2,512
#2 (caffeine* OR caffedrine* OR coffein* OR caffeine* OR methylxanthin* OR trimethylxanthin*):ti,ab,kw (Word variations have been searched) 5,476
#3 #1 OR #2 5,476
CAFFEINE
#4 MeSH descriptor: [Infant, Newborn] explode all trees 20,627
#5 MeSH descriptor: [Intensive Care, Neonatal] explode all trees 377
#6 MeSH descriptor: [Intensive Care Units, Neonatal] explode all trees 1,036
#7 MeSH descriptor: [Gestational Age] explode all trees 3,970
#8 (("babe" or "babes" or baby* or "babies" or "gestational age" or "gestational ages" or infant? or "infantile" or infancy or "low birth weight" OR "low birth weights" or "low birthweight" or "low birthweights" or neonat* or "neo‐nate" or “neo‐nates” or newborn* or "new born" or “new borns” or “newly born” or "premature" or "pre‐mature" or "pre‐matures" or prematures or prematurity or "pre‐maturity" or "preterm" or "preterms" or "pre term" or “pre terms” or "preemie" or "preemies" or "premies" or "premie" or "VLBW" or "VLBWI" or "VLBW‐I" or "VLBWs" or "LBW" or "LBWI" or "LBWs" or "ELBW" or "ELBWI" or "ELBWs" or "NICU" or "NICUs")):ti,ab,kw (Word variations have been searched) 110,145
#9 #4 OR #5 OR #6 OR #7 OR #8 110,145
NEONATES
#10 #3 AND #9 425
Embase.com (Elsevier, 1947‐present)
Date of search: 22 August 2023
No publication date limitations or language limitations were used.
Search filters: the Cochrane Neonatal filter for RCTs for OVID Embase was adapted to the syntax of Embase.com; the Cochrane Neonatal filter for neonatal populations for OVID Embase was adapted to the syntax of Embase.com.
#1. 'caffeine'/exp OR 'methylxanthine'/exp 46,222
#2. caffeine*:ti,ab,kw OR caffedrine*:ti,ab,kw OR coffein*:ti,ab,kw OR caffeine*:ti,ab,kw OR methylxanthin*:ti,ab,kw OR trimethylxanthin*:ti,ab,kw 56,218
#3. #1 OR #2 68,014
CAFFEINE
#4. 'newborn'/de OR 'prematurity'/de OR 'newborn intensive care'/de OR 'newborn care'/de OR 'gestational age'/de 873,629
#5. babe:ti,ab,kw OR babes:ti,ab,kw OR baby*:ti,ab,kw OR babies:ti,ab,kw OR ‘gestational age$’:ti,ab,kw OR infant$:ti,ab,kw OR infantile:ti,ab,kw OR infancy:ti,ab,kw OR 'low birth weight':ti,ab,kw OR 'low birthweight':ti,ab,kw OR neonat*:ti,ab,kw OR 'neo‐nat*':ti,ab,kw OR newborn*:ti,ab,kw OR ‘new born$’:ti,ab,kw OR 'newly born':ti,ab,kw OR premature:ti,ab,kw OR 'pre mature':ti,ab,kw OR 'pre matures':ti,ab,kw OR prematures:ti,ab,kw OR prematurity:ti,ab,kw OR 'pre maturity':ti,ab,kw OR preterm:ti,ab,kw OR preterms:ti,ab,kw OR ‘pre term$’:ti,ab,kw OR preemie:ti,ab,kw OR preemies:ti,ab,kw OR premies:ti,ab,kw OR premie:ti,ab,kw OR vlbw:ti,ab,kw OR vlbwi:ti,ab,kw OR 'vlbw i':ti,ab,kw OR vlbws:ti,ab,kw OR lbw:ti,ab,kw OR lbwi:ti,ab,kw OR lbws:ti,ab,kw OR elbw:ti,ab,kw OR elbwi:ti,ab,kw OR elbws:ti,ab,kw OR nicu:ti,ab,kw OR nicus:ti,ab,kw 1,322,214
#6. #4 OR #5 1,643,573
NEONATES
#7. 'randomized controlled trial'/de OR 'controlled clinical trial'/de 959,795
#8. random*:ti,ab,kw 1,967,444
#9. 'randomization'/de 98,027
#10. placebo:ti,ab,kw 364,949
#11. ((double OR single OR doubly OR singly) NEAR/2 (blind OR blinded OR blindly)):ti,ab,kw 276,251
#12. 'double blind procedure'/de 210,057
#13. (controlled NEAR/7 (study OR design OR trial)):ti,ab,kw 458,023
#14. 'parallel group$':ti,ab 31,900
#15. crossover:ti,ab OR 'cross over':ti,ab 124,259
#16. ((assign* OR match OR matched OR allocation) NEAR/5 (alternate OR group$ OR intervention$ OR patient$ OR subject$ OR participant$)):ti,ab 411,867
#17. (open NEAR/2 label):ti,ab 107,930
#18. quasirandom*:ti,ab,kw OR 'quasi random*':ti,ab,kw OR randomi*:ti,ab,kw OR randomly:ti,ab,kw 1,599,033
#19. (control* NEAR/2 (group$ OR random*)):ti,ab,kw 1,306,615
#20. #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR 19 3,331,383
#21. ('animal'/exp OR 'invertebrate'/exp OR 'animal experiment'/de OR 'animal model'/de OR 'animal tissue'/de OR 'animal cell'/de OR 'nonhuman'/de) AND ('human'/de OR 'normal human'/de OR 'human cell'/de) 26,569,269
#22. 'animal'/exp OR 'invertebrate'/exp OR 'animal experiment'/de OR 'animal model'/de OR 'animal tissue'/de OR 'animal cell'/de OR 'nonhuman'/de 34,386,124
#23. #22 NOT #21 7,816,855
#24. #20 NOT #23 2,864,953
RANDOMIZED AND QUASI‐RANDOMIZED CONTROLLED TRIALS
#25. #3 AND #6 AND #24 532
Number of records from literature databases: 1845
Search strategies in clinical trial registries
ClinicalTrials.gov (US National Library of Medicine)
Date of search: 23 August 2023
Advanced search
Other terms: premature OR prematurity OR preterms OR preterm OR “very low birth” OR “low birth weight” OR “low birthweight” OR newborn OR newborns OR neonate OR neonates OR infant OR infants
Intervention/treatment: caffeine OR methylxanthin* OR caffein* OR caffedrine* OR coffein* OR trimethylxanthin*
Filters: Child (birth‐17) 47 records
International Clinical Trials Registries Platform Search Portal, ICTRP (World Health Organization)
Date of search: 23 August 2023
Advanced search Title: premature OR prematurity OR preterms OR preterm OR “very low birth” OR “low birth weight” OR “low birthweight” OR newborn OR newborns OR neonate OR neonates OR infant OR infants Intervention: caffeine OR methylxanthin* OR caffein* OR caffedrine* OR coffein* OR trimethylxanthin* Filters: clinical trials in children 18 records
Number of records from trial registries: 65
Total number from literature databases and trial registries: 1910
Number of duplicates automatically removed in EndNote: 446
Total number after automatic deduplication in EndNote and S4M: 1418
Appendix 2. Risk of bias domains (RoB 1)
We will use the standard methods of Cochrane and Cochrane Neonatal to assess the ris of bias of the trials. For each trial, we will seek information regarding the method of randomization, blinding, and reporting of all outcomes of all the infants enrolled in the trial. We will assess each criterion as being at a low, high, or unclear risk of bias. Two review authors will separately assess each study. We will resolve any disagreements by discussion. We will add this information to the characteristics of included studies table. We will evaluate the following issues and enter the findings into the risk of bias table.
Sequence generation (checking for possible selection bias). Was the allocation sequence adequately generated?
For each included study, we will categorize the method used to generate the allocation sequence as:
low risk (any truly random process, e.g. random number table; computer random number generator);
high risk (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number); or
unclear risk.
Allocation concealment (checking for possible selection bias). Was allocation adequately concealed?
For each included study, we will categorize the method used to conceal the allocation sequence as:
low risk (e.g. telephone or central randomization; consecutively numbered, sealed, opaque envelopes);
high risk (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth); or
unclear risk
Blinding of participants and personnel (checking for possible performance bias). Was knowledge of the allocated intervention adequately prevented during the study?
For each included study, we will categorize the methods used to blind study participants and personnel from knowledge of which intervention a participant received. We will assess blinding separately for different outcomes or class of outcomes. We will categorize the methods as:
low risk, high risk, or unclear risk for participants; and
low risk, high risk, or unclear risk for personnel.
Blinding of outcome assessment (checking for possible detection bias). Was knowledge of the allocated intervention adequately prevented at the time of outcome assessment?
For each included study, we will categorize the methods used to blind outcome assessment. We will assess blinding separately for different outcomes or class of outcomes. We will categorize the methods as:
low risk for outcome assessors;
high risk for outcome assessors; or
unclear risk for outcome assessors
Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations). Were incomplete outcome data adequately addressed?
For each included study and for each outcome, we will describe the completeness of data, including attrition and exclusions from the analysis. We will note whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomized participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information is reported or supplied by the trial authors, we will re‐include missing data in the analyses. We will categorize the methods as:
low risk (< 20% missing data);
high risk (≥ 20% missing data); or
unclear risk.
Selective reporting bias. Are reports of the study free of the suggestion of selective outcome reporting?
For each included study, we will describe how we investigated the possibility of selective outcome reporting bias and what we found. For studies in which study protocols were published in advance, we will compare prespecified outcomes versus outcomes eventually reported in the published results. If the study protocol was not published in advance, we will contact study authors to gain access to the study protocol. We will assess the methods as:
low risk (where it is clear that all of the study's prespecified outcomes and all expected outcomes of interest to the review have been reported);
high risk (where not all the study's prespecified outcomes have been reported; one or more reported primary outcomes were not prespecified outcomes of interest and are reported incompletely and so cannot be used; the study fails to include results of a key outcome that would have been expected to have been reported); or
unclear risk.
Other sources of bias. Was the study apparently free of other problems that could put it at high risk of bias?
For each included study, we will describe any important concerns we had about other possible sources of bias (e.g. whether there was a potential source of bias related to the specific study design or whether the trial was stopped early due to some data‐dependent process). We will assess whether each study was free of other problems that could put it at risk of bias as:
low risk;
high risk;
unclear risk.
If needed, we plan to explore the impact of the level of bias by undertaking sensitivity analyses.
Data and analyses
Comparison 1. Discontinuation of caffeine at PMA < 35 weeks' gestation versus PMA ≥ 35 weeks' gestation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Intermittent hypoxemia (IH): number of episodes in the 7 days after treatment discontinued | 1 | 98 | Mean Difference (IV, Fixed, 95% CI) | 4.80 [2.21, 7.39] |
| 1.2 All‐cause mortality prior to hospital discharge | 1 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
Comparison 2. Discontinuation of caffeine based on PMA (caffeine discontinued at a scheduled PMA) versus discontinuation based on resolution of symptoms (a fixed time after the infant is without respiratory support, or apneic spells).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Apnea: number of infants with at least 1 episode (interruption of breathing for more than 20 seconds, or as defined by the authors) in the 7 days after treatment discontinued | 2 | 294 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.31, 1.18] |
| 2.2 IH: number of infants with at least 1 episode in the 7 days after treatment discontinued | 1 | 174 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.20, 0.75] |
| 2.3 All‐cause mortality prior to hospital discharge | 2 | 294 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.14, 7.03] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Pradhap 2023.
| Study characteristics | ||
| Methods | Randomized, parallel‐group trial | |
| Participants | All preterm neonates with birth gestation 26 to 32 weeks and on caffeine therapy Exclusion criteria:
Characteristics:
|
|
| Interventions | Intervention 1: Short course caffeine therapy: caffeine was stopped directly when babies were off respiratory support and apnea‐free for 7 consecutive days. Intervention 2: Regular course caffeine therapy: caffeine was continued until 34 weeks PMA and stopped only when babies were off respiratory support and apnea‐free for 7 consecutive days. |
|
| Outcomes | Primary outcome:
Secondary outcomes:
|
|
| Funding and declaration of interest | Funding: The authors received no financial support. Declaration of interest: Pradhap reports being a senior resident of the Department of Neonatology of the Chengalpattu Government Medical College. The authors declared no potential conflicts of interest. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer generated random number sequence was used to randomize with block randomization of variable blocks of sizes 2, 4, 6 and 8. |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, opaque, sealed envelopes were used for allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not done. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All infants who were included in the randomization received the intended intervention; none of the infants were excluded from the analysis. Sample size 174 babies; intervention group 87; control group 87; Analyzed: 174. |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes in the study protocol match the outcomes reported in the published results. |
| Other bias | Low risk | No other potential sources of bias were identified in the quality assessment. |
Prakash 2021.
| Study characteristics | ||
| Methods | Prospective, randomized controlled parallel‐group trial, conducted in India | |
| Participants | Inclusion criteria:
Exclusion criteria:
Characteristics:
|
|
| Interventions | Once these babies (already on therapy) were free of apnea and RS for 7 consecutive days (irrespective of indication for commencing therapy), they were randomized to 2 groups. Group 1 ‐ early discontinuation: caffeine was discontinued straightaway when they were apnea‐free; and not on RS (no oxygen, HFNC, CPAP or MV) for seven days consecutively Group 2 ‐ late discontinuation: caffeine was continued for a prefixed period till at least 34 weeks' PMA, and then ceased only after baby was free of apnea and RS for minimum 7 days |
|
| Outcomes | Primary outcome:
Secondary outcomes:
|
|
| Funding and declaration of interest | Primary funding: Kerala Institute of Medical Science Secondary funding: Dr Naveen Jain Declaration of interest: no conflict of interest according to the authors |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated random number sequence |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered opaque sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding. However, the primary outcome of recurrence of apnea always requires a drop in saturation below a certain number (80%), which leaves little room for interpretation, and thus minimizes the need for blinding. The secondary outcomes are overall well‐defined and any risk of bias would most likely be due to the unblinded clinicians' judgment (blinding of personnel). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Exclusion before randomization is defined. All infants who were randomized received allocated intervention except for one infant in group 1 who expired. |
| Selective reporting (reporting bias) | Low risk | PICO of protocol and full text match |
| Other bias | Unclear risk | The study reports results obtained when 2/3 of the pre‐specified required sample size was attained. It was calculated using and expected 10% absolute difference of primary outcome for clinical significance. The authors are currently in the process of validating results based on a smaller obtained difference (2%). |
Rhein 2014.
| Study characteristics | ||
| Methods | Prospective, randomized controlled parallel‐group trial onducted in the USA | |
| Participants | Inclusion criteria:
Exclusion criteria:
Characteristics:
|
|
| Interventions | As soon as clinical caffeine treatment had been discontinued for 5 days and their PMA was 34 to 37 weeks, infants were randomized to either receive the study caffeine protocol or to continue with usual care (controls). Group 1 ‐ late discontinuation: oral loading dose of caffeine citrate (20 mg/kg) at randomization followed by oral maintenance dosage (6mg/kg/die) until 40 weeks PMA Group 2 ‐ early discontinuation: no caffeine (usual care) until 40 weeks PMA |
|
| Outcomes | Primary outcomes:
|
|
| Funding and declaration of interest | Funding: American SIDS Institute Declaration of interest: Dr McEntire reports being the executive director of the American SIDS Institute, and Dr Hunt reports being a member of the American SIDS Institute board of directors. No other disclosures were reported. | |
| Notes | Mail from C. Hunt: Enrollment ended 31 July 2023, and the last subject will complete the study protocol this week. We will not be ready to unblind the study until late October at the earliest, but we nevertheless hope to submit a PAS abstract with our primary results for the Spring 2024 meeting in Toronto. We ended the study with 170 enrolled subjects. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Personal communication with study authors: computer generated random numbers, with blocks. |
| Allocation concealment (selection bias) | Low risk | Personal communication with study authors: sealed opaque envelopes provided by the Data Coordinating Center were used for each randomization to extend caffeine use or usual care |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The control group did not receive a placebo. The decision to discontinue routine caffeine treatment and the exact timing of discontinuation was completely at the discretion of the clinical team and not dictated by the study protocol. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All analyses of outcome measures were to be performed by persons masked to treatment assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Sample size: 98; intervention group: 45; control group: 53; analysed: 95. 3 infants in intervention group dropped out because of insufficient data analysed |
| Selective reporting (reporting bias) | Low risk | PICO of protocol and full text match |
| Other bias | Low risk | No comments |
AED: antiepileptic drugs; AOP: apnea of prematurity; BPD: bronchopulmonary dysplasia; BW: birth weight; CPAP: continuous positive airway pressure; EUGR: Extrauterine growth restriction; GA: gestational age; HFNC: High flow nasal cannula; IH: intermittent hypoxia; MV: mechanical ventilation; NEC: necrotizing enterocolitis; PMA: postmenstrual age; PNA: Postnatal age; RAP: recurrence of apnea of prematurity; RDS: respiratory distress syndrome; ROP: retinopathy of prematurity; RS: respiratory support; SIDS: Sudden infant death syndrome
* Mean (SD); ° Median (interquartile range).
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| ACTRN12622001344785 | Study in late preterm infants; not about early vs late discontinuation |
Characteristics of ongoing studies [ordered by study ID]
NCT03321734.
| Study name | Intermittent hypoxia and caffeine in infants born preterm |
| Methods | Randomized, placebo‐controlled, double‐blinded clinical trial |
| Participants | Inclusion criteria:
Exclusion Criteria:
|
| Interventions | Infants in the extended caffeine treatment arm will, beginning the next day after stopping routine caffeine treatment, receive 5 mg/kg/day of caffeine base and increase to 5 mg/kg/twice a day (BID) of caffeine base beginning at 36 weeks + 0 days PMA and continuing the BID doses through 42 weeks + 6 days PMA Infants in the placebo arm will, beginning the next day after stopping routine caffeine treatment, receive the equivalent (to study drug) volume of placebo daily and increase to the equivalent (to study drug) volume placebo BID through 42 weeks + 6 days PMA |
| Outcomes | Primary outcomes:
Secondary outcomes:
|
| Starting date | 18 January 2019 |
| Contact information | Principal Investigator: Carl E Hunt, MD, Children's Research Institute |
| Comparison | |
| Notes | Enrollment ended 31 July 2023. The study will be unblinded at the end of October at the earliest. Primary results are scheduled to be released in spring 2024. |
NCT03340727.
| Study name | Moderately preterm infants with caffeine at home for apnea (MoCHA) trial |
| Methods | Randomized controlled trial. Parallel assignment |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Group 1: the study intervention is caffeine citrate given once daily at 10 mg/kg/day. It is given orally, before hospital discharge and 28 days after discharge Group 2: the study intervention is placebo given once daily at a volume equivalent to 10 mg/kg of caffeine citrate. It is given orally, before hospital discharge and 28 days after discharge |
| Outcomes | Primary outcome:
Secondary outcomes:
|
| Starting date | 27 February 2019 |
| Contact information | Principal Investigator: Waldemar Carlo, MD, University of Alabama at Birmingham |
| Comparison | |
| Notes | Primary completion (estimated) March 2023 according to protocol last edited 10 February 2023 |
NCT04868565.
| Study name | Target weaning oxygen to determine caffeine duration for AOP (DCAP) |
| Methods | Multicenter, prospective, randomized controlled trial |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Samples assigned to the "ongoing caffeine with oxygen supplement (group 1)" will continue caffeine administration combined with oxygen supplement until the patients are weaned from oxygen. Samples assigned to the "discontinuing caffeine with oxygen supplement (group 2)" will discontinue caffeine immediately after randomization, while oxygen supplement is going on |
| Outcomes | Primary outcomes:
Secondary outcomes:
|
| Starting date | 5 January 2021 |
| Contact information |
Name: Jianhui Wang Phone Number: +86‐13678428167 Email: wangjh@cqmu.edu.cn |
| Comparison | |
| Notes | Estimated primary completion date 31 October 2023 |
AOP: apnea of prematurity; BPD: Bronchopulmonary dysplasia; BID: twice a day; CPAP: continuous positive airway pressure; IH: intermittent hypoxemia; MRI: Magnetic Resonance Imaging; NICHD: National Institute of Child Health and Human Development; NICU: Neonatal intensive care unit; NPO: nothing per mouth; NRN: Neonatal Research Network; PMA: postmenstrual age; RAP: recurrence of apnea of prematurity.
Differences between protocol and review
We changed the definitions of the comparisons to improve clarity (Types of interventions). For the same reason, we changed the title of the review from 'Early versus late discontinuation of caffeine administration in preterm infants' to 'Strategies for cessation of caffeine administration in preterm infants'.
We added the outcome, all‐cause mortality prior to hospital discharge, to the summary of findings tables due to its relevance.
We planned to use funnel plots to screen for publication bias if there were more than 10 studies reporting the same outcome. If publication bias was suggested by a significant asymmetry of the funnel plot on visual assessment, we planned to incorporate this in our assessment of certainty of evidence (Egger 1997). However, since our review only included three studies, the ability to detect publication bias was largely diminished, and we simply noted our inability to rule out possible publication bias or small study effects.
Contributions of authors
For the protocol
SU reviewed the literature, wrote the background section of the protocol, reviewed and approved the final version of the protocol.
MB identified the topic of the review and wrote the methods section of the protocol, reviewed and approved the final version of the protocol.
For the full 2024 review:
All the authors (EB, MB, MG, RC, SU) worked on title and abstract screening.
The full‐text review on potentially relevant titles was carried out by EB, MB, MG, RC, and SU.
Risk of bias assessment and data extraction of the included studies were independently conducted by two authors each, who were labeled as first and second reviewer (for Pradhap 2023: RC, EB; for Prakash 2021: RC, MG; for Rhein 2014: EB, MG).
The consensus on data extraction was reached in a common discussion between all authors, led by MB.
References of studies were transferred from Covidence to RevMan by MB.
When additional data for included and ongoing studies were required, MB contacted study investigators/authors by e‐mail for clarification.
All authors (EB, MB, MG, RC, SU) reviewed and approved the final version of the review.
Sources of support
Internal sources
-
Forska Utan Djurförsök, Sweden
Funding for this review was provided by Forska Utan Djurförsök
-
Institute for Clinical Sciences, Lund University, Lund, Sweden
MB is employed by this organization
-
ALF grant, Sweden
Funding for this review was provided by an ALF grant (non‐profit; Lund University)
External sources
-
Region Skåne, Skåne University Hospital, Lund University, and Region Västra Götaland, Sweden, Sweden
Cochrane Sweden received support from Region Skåne, Skåne University Hospital Lund University, and Region Västra Götaland
-
Vermont Oxford Network, USA
Cochrane Neonatal Reviews are produced with support from the Vermont Oxford Network, a worldwide collaboration of health professionals dedicated to providing evidence‐based care of the highest quality for newborn infants and their families.
Declarations of interest
EB, MG, RC, and SU have no interests to declare.
MB is an Associate Editor for the Cochrane Neonatal Group. However, he had no involvement in the editorial processing of this protocol.
New
References
References to studies included in this review
Pradhap 2023 {published data only}
- CTRI/2021/09/036141. How long is caffeine duration enough for preterm infants? https://trialsearch.who.int/Trial2.aspx?TrialID=CTRI/2021/09/036141 (registered 02 September 2021).
- Pradhap K, Solaiappan M, Ramya S, Muthukumaran N. Effect of short course caffeine on recurrenceof apnea of prematurity in preterm infants lesst han 32 weeks: a randomized controlled trial. Journal of Neonatology 2023;0(0):1-7. [DOI: 10.1177/09732179231198302] [DOI] [Google Scholar]
Prakash 2021 {published data only}
- CTRI/2016/12/007559. A clinical trial to evaluate the duration of caffeine therapy for apnea of prematurity. https://trialsearch.who.int/Trial2.aspx?TrialID=CTRI/2016/12/007559 (registered 9 December 2016).
- Prakash R, Pournami F, Prabhakar J, Nandakumar A, Nair PM, Jain N. Duration of caffeine for apnea of prematurity- a randomized controlled trial. Indian Journal of Pediatrics 2021;88(12):1174-9. [DOI: 10.1007/s12098-021-03659-y] [PMID: ] [DOI] [PubMed] [Google Scholar]
Rhein 2014 {published data only}
- NCT01875159. Effects of caffeine on intermittent hypoxia in infants born preterm. https://clinicaltrials.gov/show/NCT01875159 (first submitted 06 March 2013).
- Rhein LM, Dobson NR, Darnall RA, Corwin MJ, Heeren TC, Poets CF, et al. Effects of caffeine on intermittent hypoxia in infants born prematurely: a randomized clinical trial. JAMA Pediatrics 2014;168(3):250-7. [DOI: 10.1001/jamapediatrics.2013.4371] [PMID: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
ACTRN12622001344785 {published data only}
- ACTRN12622001344785. Investigating the effect of caffeine to improve neurodevelopmental outcomes in babies born late preterm: a randomised controlled trial. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=384799 (first submitted 11 October 2022).
References to ongoing studies
NCT03321734 {published data only}
- NCT03321734. Intermittent hypoxia and caffeine in infants born preterm. https://classic.clinicaltrials.gov/ct2/show/NCT03321734 (first submitted 23 October 2017).
NCT03340727 {published data only}
- NCT03340727. Moderately preterm infants with caffeine at home for apnea (MoCHA) trial. https://clinicaltrials.gov/study/NCT03340727 (first submitted 08 Novmeber 2017).
NCT04868565 {published data only}
- NCT04868565. Target weaning oxygen to determine cafffeine duration for AOP. https://clinicaltrials.gov/study/NCT04868565?tab=table (first submitted 27 April 2021).
Additional references
Abdel‐Hady 2015
- Abdel-Hady H, Nasef N, Shabaan AE, Nour I. Caffeine therapy in preterm infants. World Journal of Clinical Pediatrics 2015;4(4):81-93. [DOI: 10.5409/wjcp.v4.i4.81] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Aranda 1977
- Aranda JV, Gorman W, Bergsteinsson H, Gunn T. Efficacy of caffeine in treatment of apnea in the low-birth-weight infant. Journal of Pediatrics 1977;90(3):467-72. [DOI: 10.1016/s0022-3476(77)80718-x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Aranda 1979
- Aranda JV, Cook CE, Gorman W, Collinge JM, Loughnan PM, Outerbridge EW, et al. Pharmacokinetic profile of caffeine in the premature newborn infant with apnea. Journal of Pediatrics 1979;94(4):663. [DOI: 10.1016/s0022-3476(79)80047-5] [PMID: ] [DOI] [PubMed] [Google Scholar]
Arroyo 2021
- Arroyo R, Kingma P. Surfactant protein D and bronchopulmonary dysplasia: a new way to approach an old problem. Respiratory Research 2021;22(141):1-13. [DOI: 10.1186/s12931-021-01738-4] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bayley 1993
- Bayley N. Bayley Scales of Infant Development-II. San Antonio (TX): Psychological Corporation, 1993. [Google Scholar]
Bayley 2006
- Bayley N. Bayley Scales of Infant and Toddler Development. 3rd edition. San Antonio (TX): Harcourt Assessment, 2006. [Google Scholar]
Blanchard 1992
- Blanchard J, Weber CW, Shearer LE. Methylxanthine levels in breast milk of lactating women of different ethnic and socioeconomic classes. Biopharmaceutics & Drug Disposition Apr;13(3):187-96. [DOI: 10.1002/bdd.2510130305] [PMID: ] [DOI] [PubMed] [Google Scholar]
Borenstein 2013
- Borenstein M, Higgins JP. Meta-analysis and subgroups. Prevention Science 2013;14(2):134-43. [DOI: 10.1007/s11121-013-0377-7] [PMID: ] [DOI] [PubMed] [Google Scholar]
Brady 2019
- Brady JM, Zhang H, Kirpalani H, DeMauro SB. Living with severe bronchopulmonary dysplasia-parental views of their child's quality of life. Journal of Pediatrics 2019;207:117-22. [DOI: 10.1016/j.jpeds.2018.10.001] [PMID: ] [DOI] [PubMed] [Google Scholar]
Brattström 2019
- Brattström P, Russo C, Ley D, Bruschettini M. High-versus low-dose caffeine in preterm infants: a systematic review and meta-analysis. Acta Paediatrica 2019;108(3):401-10. [DOI: 10.1111/apa.14586] [DOI] [PubMed] [Google Scholar]
Bruschettini 2023
- Bruschettini M, Brattström P, Russo C, Onland W, Davis PG, Soll R. Caffeine dosing regimens in preterm infants with or at risk for apnea of prematurity. Cochrane Database of Systematic Reviews 2023, Issue 4. Art. No: CD013873. [DOI: 10.1002/14651858.CD013873.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bui 2017
- Bui CB, Pang MA, Sehgal A, Theda C, Lao JC, Berger PJ, et al. Pulmonary hypertension associated with bronchopulmonary dysplasia in preterm infants. Journal of Reproductive Immunology 2017;124:21-9. [DOI: 10.1016/j.jri.2017.09.013] [PMID: ] [DOI] [PubMed] [Google Scholar]
Butler 2014
- Butler TJ, Firestone KS, Grow JL, Kantak AD. Standardizing documentation and the clinical approach to apnea of prematurity reduces length of stay, improves staff satisfaction, and decreases hospital cost. Joint Commission Journal on Quality and Patient Safety 2014;40(6):263-9. [DOI: 10.1016/s1553-7250(14)40035-7] [PMID: ] [DOI] [PubMed] [Google Scholar]
Charles 2008
- Charles BG, Townsend SR, Steer PA, Flenady VJ, Gray PH, Shearman A. Caffeine citrate treatment for extremely premature infants with apnea: population pharmacokinetics, absolute bioavailability, and implications for therapeutic drug monitoring. Therapeutic Drug Monitoring 2008;30(6):709-16. [DOI: 10.1097/FTD.0b013e3181898b6f] [PMID: ] [DOI] [PubMed] [Google Scholar]
Chavez 2022
- Chavez L, Bancalari E. Caffeine: some of the evidence behind its use and abuse in the preterm infant. Neonatology 2022;119(4):428-32. [DOI: 10.1159/000525267] [DOI] [PubMed] [Google Scholar]
Chung 2022
- Chung J, Tran Lopez K, Amendolia B, Bhat V, Nakhla T, Slater-Myer L, et al. Stopping caffeine in premature neonates: how long does it take for the level of caffeine to fall below the therapeutic range? Journal of Maternal-Fetal & Neonatal Medicine 2022;35(3):551-5. [DOI: 10.1080/14767058.2020.1729117] [DOI] [PubMed] [Google Scholar]
Covidence [Computer program]
- Covidence. Version accessed 13 February 2023. Melbourne, Australia: Veritas Health Innovation, 2023. Available at https://www.covidence.org.
Crossley 2012
- Crossley KJ, Allison BJ, Polglase GR, Morley CJ, Harding R, Davis PG, et al. Effects of caffeine on renal and pulmonary function in preterm newborn lambs. Pediatric Research 2012;72(1):19-25. [DOI: 10.1038/pr.2012.49] [PMID: ] [DOI] [PubMed] [Google Scholar]
Darlow 2016
- Darlow BA, Graham PJ, Rojas-Reyes MX. Vitamin A supplementation to prevent mortality and short- and long-term morbidity in very low birth weight infants. Cochrane Database of Systematic Reviews 2016, Issue 8. Art. No: CD000501. [DOI: 10.1002/14651858.CD000501.pub4] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Darnall 1997
- Darnall RA, Kattwinkel J, Nattie C, Robinson M. Margin of safety for discharge after apnea in preterm infants. Pediatrics 1997;100(5):795–801. [DOI: 10.1542/peds.100.5.795] [PMID: ] [DOI] [PubMed] [Google Scholar]
Deeks 2023
- Deeks JJ, Higgins JP, Altman DG, editor(s). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.4 (updated August 2023). Cochrane, 2023. Available from https://training.cochrane.org/handbook.
Dobson 2013
- Dobson NR, Hunt CE. Pharmacology review: caffeine use in neonates: indications, pharmacokinetics. NeoReviews 2013;14(11):e540–50. [DOI: 10.1542/neo.14-11-e540] [DOI] [Google Scholar]
Doyle 2016
- Doyle J, Davidson D, Katz S, Varela M, Demeglio D, DeCristofaro J. Apnea of prematurity and caffeine pharmacokinetics: potential impact on hospital discharge. Journal of Perinatology 2016;36(2):141-4. [DOI: 10.1038/jp.2015.167] [PMID: ] [DOI] [PubMed] [Google Scholar]
Doyle 2021
- Doyle LW, Cheong JL, Hay S, Manley BJ, Halliday HL, Soll R. Early (< 7 days) systemic postnatal corticosteroids for prevention of bronchopulmonary dysplasia in preterm infants. Cochrane Database of Systematic Reviews 2021, Issue 10. Art. No: CD001146. [DOI: 10.1002/14651858.CD001146.pub6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dukhovny 2011
- Dukhovny D, Lorch SA, Schmidt B, Doyle LW, Kok JH, Roberts RS, et al, Caffeine for Apnea of Prematurity Trial Group. Economic evaluation of caffeine for apnea of prematurity. Pediatrics 2011;127(1):e146-55. [DOI: 10.1542/peds.2010-1014] [PMID: ] [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clinical Research Ed.) 1997;315(7109):629-34. [DOI: 10.1136/bmj.315.7109.629] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Eichenwald 2016
- Eichenwald EC, Committee on Fetus, Newborn American Academy of Pediatrics. Apnea of prematurity. Pediatrics 2016;137(1):e20153757. [DOI: 10.1542/peds.2015-3757] [PMID: ] [DOI] [PubMed] [Google Scholar]
EMA 2009
- Chiesi Farmaceutici SpA. European Public Assessment Report Nymusa/Peyona. Doc.Ref.: EMEA/CHMP/207328/2009. https://www.ema.europa.eu/en/medicines/human/EPAR/peyona-previously-nymusa#overview (accessed 1 February 2023).
EMA 2020
- EMA. European Public Assessment Report Gencebok. EMA/353540/2020 27/08/2020. https://www.ema.europa.eu/en/medicines/human/EPAR/gencebok (accessed 1 February 2023).
Endesfelder 2020
- Endesfelder S, Strauß E, Bendix I, Schmitz T, Bührer C. Prevention of oxygen-induced inflammatory lung injury by caffeine in neonatal rats. Oxidative Medicine and Cellular Longevity 2020;3840124:1-19. [DOI: 10.1155/2020/3840124] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
EPOC 2017
- Cochrane Effective Practice and Organisation of Care Review Group. Data collection checklist. Available at https://methods.cochrane.org/sites/methods.cochrane.org.bias/files/uploads/EPOC%20Data%20Collection%20Checklist.pdf (accessed 12 July 2023).
Fewell 2007
- Fewell Z, Davey Smith G, Sterne JA. The impact of residual and unmeasured confounding in epidemiologic studies: a simulation study. American Journal of Epidemiology 2007;166(6):646-55. [DOI: 10.1093/aje/kwm165] [PMID: ] [DOI] [PubMed] [Google Scholar]
Gentle 2018
- Gentle SJ, Travers CP, Carlo WA. Caffeine controversies. Current Opinion in Pediatrics 2018;30(2):177-81. [DOI: 10.1097/MOP.0000000000000588] [PMID: ] [DOI] [PubMed] [Google Scholar]
Gillot 1990
- Gillot I, Gouyon JB, Guignard JP. Renal effects of caffeine in preterm infants. Biology of the Neonate 1990;58(3):133-6. [DOI: 10.1159/000243252] [PMID: ] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Version accessed 15 February 2023. Hamilton (ON): McMaster University (developed by Evidence Prime), 2023. Available at https://www.gradepro.org/.
Griffiths 1954
- Griffiths R. Abilities of Babies: A Study of Mental Measurement. London (UK): University of London Press, 1954. [Google Scholar]
Haddad 2015
- Haddad W, Sajous C, Hummel P, Guo R. Discontinuing caffeine in preterm infants at 33-35 weeks corrected gestational age: Failure rate and predictive factors. Journal of Neonatal-Perinatal Medicine 2015;8(1):41-5. [DOI: 10.3233/NPM-15814071] [DOI] [PubMed] [Google Scholar]
Higgins 2017
- Higgins JP, Altman DG, Sterne JA, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). Cochrane, 2017. Available from https://training.cochrane.org/handbook/archive/v5.2.
Higgins 2023
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated August 2023). Cochrane, 2023. Available from https://training.cochrane.org/handbook.
Hunt 2011
- Hunt CE, Corwin MJ, Weese-Mayer DE, Ward SL, Ramanathan R, Lister G, et al, Collaborative Home Infant Monitoring Evaluation (CHIME) Study Group. Longitudinal assessment of hemoglobin oxygen saturation in preterm and term infants in the first six months of life. Journal of Pediatrics 2011;159(3):377-83.e1. [DOI: 10.1016/j.jpeds.2011.02.011] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Isayama 2016
- Isayama T, Iwami H, McDonald S, Beyene J. Association of noninvasive ventilation strategies with mortality and bronchopulmonary dysplasia among preterm infants: a systematic review and meta-analysis. JAMA 2016;316(6):611-24. [DOI: 10.1001/jama.2016.10708] [PMID: ] [DOI] [PubMed] [Google Scholar]
Jacobs 2013
- Jacobs SE, Berg M, Hunt R, Tarnow-Mordi WO, Inder TE, Davis PG. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database of Systematic Reviews 2013, Issue 1. Art. No: CD003311. [DOI: 10.1002/14651858.CD003311.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jefferies 2014
- Jefferies AL, Canadian Paediatric Society Fetus, Newborn Committee. Going home: facilitating discharge of the preterm infant. Paediatrics Child Health 2014;19(1):31-42. [DOI: 10.1093/pch/19.1.31] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jensen 2015
- Jensen EA, Foglia EE, Schmidt B. Evidence-based pharmacologic therapies for prevention of bronchopulmonary dysplasia: application of the grading of recommendations assessment, development, and evaluation methodology. Clinics in Perinatology 2015;42(4):755-79. [DOI: 10.1016/j.clp.2015.08.005] [PMID: ] [DOI] [PubMed] [Google Scholar]
Ji 2020
- Ji D, Smith PB, Clark RH, Zimmerman KO, Laughon M, Ku L, et al. Wide variation in caffeine discontinuation timing in premature infants. Journal of Perinatology 2020;40(2):288-93. [DOI: 10.1038/s41372-019-0561-0] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jobe 2001
- Jobe AH, Bancalari E. Bronchopulmonary dysplasia. American Journal of Respiratory and Critical Care Medicine 2001;163(7):1723-9. [DOI: 10.1164/ajrccm.163.7.2011060] [PMID: ] [DOI] [PubMed] [Google Scholar]
Kumar 2019
- Kumar VH, Lipshultz SE. Caffeine and clinical outcomes in premature neonates. Children (Basel, Switzerland) 2019;6(11):118. [DOI: 10.3390/children6110118] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kyriacou 2016
- Kyriacou DN, Lewis RJ. Confounding by indication in clinical research. JAMA 2016;316(7):1818-9. [DOI: 10.1001/jama.2016.16435] [PMID: ] [DOI] [PubMed] [Google Scholar]
Lee 1997
- Lee TC, Charles B, Steer P, Flenady V, Shearman A. Population pharmacokinetics of intravenous caffeine in neonates with apnea of prematurity. Clinical Pharmacology and Therapeutics 1997;61(6):628-40. [DOI: 10.1016/S0009-9236(97)90097-7] [PMID: ] [DOI] [PubMed] [Google Scholar]
Lemyre 2017
- Lemyre B, Davis PG, De Paoli AG, Kirpalani H. Nasal intermittent positive pressure ventilation (NIPPV) versus nasal continuous positive airway pressure (NCPAP) for preterm neonates after extubation. Cochrane Database of Systematic Reviews 2017, Issue 2. Art. No: CD003212. [DOI: 10.1002/14651858.CD003212.pub3] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Medicine 2009;6(7):e1000100. [DOI: 10.1136/bmj.b2700] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ma 2020
- Ma C, Broadbent D, Levin G, Panda S, Sambalingam D, Garcia N, et al. Discharging preterm infants home on caffeine, a single center experience. Children (Basel, Switzerland) 2020;7(9):114. [DOI: 10.3390/children7090114] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Marques 2023
- Marques K, Bruschettini M, Roehr CC, Davis PG, Fiander M, Soll R. Methylxanthine for the prevention and treatment of apnea in preterm infants. Cochrane Database of Systematic Reviews 2023, Issue 10. Art. No: CD013830. [DOI: 10.1002/14651858.CD013830.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Marshall 2018
- Marshall IJ, Noel-Storr AH, Kuiper J, Thomas J, Wallace BC. Machine learning for identifying randomized controlled trials: an evaluation and practitioner’s guide. Research Synthesis Methods 2018;9(4):602-14. [DOI: 10.1002/jrsm.1287] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Martin 2011
- Martin RJ, Wang K, Köroğlu Ö, Di Fiore J, Kc P. Intermittent hypoxic episodes in preterm infants: do they matter? Neonatology 2011;100(3):303-10. [DOI: 10.1159/000329922] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Martin 2022
- Martin R. Management of apnea of prematurity. Available at https://www.uptodate.com/contents/management-of-apnea-of-prematurity (accessed 12 July 2023).
McGowan 2016a
- McGowan J, Sampson M, Salzwedel D, Cogo E, Foerster V, Lefebvre C. PRESS Peer Review of Electronic Search Strategies: 2015 Guideline Explanation and Elaboration (PRESS E&E). Ottawa (ON): CADTH, 2016. [DOI] [PubMed] [Google Scholar]
McGowan 2016b
- McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS peer review of electronic search strategies: 2015 guideline statement. Journal of Clinical Epidemiology 2016;75:40-6. [DOI: 10.1016/j.jclinepi.2016.01.021] [PMID: ] [DOI] [PubMed] [Google Scholar]
Moresco 2023
- Moresco L, Sjögren A, Marques K, Soll R, Bruschettini M. Caffeine versus other methylxanthines for the prevention and treatment of apnea in preterm infants. Cochrane Database of Systematic Reviews 2023, Issue 10. Art. No: CD015462. [DOI: 10.1002/14651858.CD015462.pub2] [PMCID: PMC10548499] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nagatomo 2016
- Nagatomo T, Jiménez J, Richter J, De Baere S, Vanoirbeek J, Naulaers G, et al. Caffeine prevents hyperoxia-induced functional and structural lung damage in preterm rabbits. Neonatology 2016;109(4):274–81. [DOI: 10.1159/000442937] [PMID: ] [DOI] [PubMed] [Google Scholar]
NDA 20‐793/S‐001
- NDA 20-793/S-001 (2000). CAFCIT (caffeine citrate) Injection CAFCIT® (caffeine citrate) oral solution. accessdata.fda.gov/drugsatfda_docs/label/2000/20793s1lbl.pdf (accessed 1 February 2023).
NIH 1979
- National Institutes of Health (NIH). Report of Workshop on Bronchopulmonary Dysplasia; NIH Publication No. 80-1660. Washington (DC): National Institutes of Health, 1979. [Google Scholar]
Noel‐Storr 2020
- Noel-Storr AH, Dooley G, Wisniewski S, Glanville J, Thomas J, Cox S, et al. Cochrane Centralised Search Service showed high sensitivity identifying randomised controlled trials: a retrospective analysis. Journal of Clinical Epidemiology 2020;127:142-50. [DOI: 10.1016/j.jclinepi.2020.08.008] [PMID: ] [DOI] [PubMed] [Google Scholar]
Noel‐Storr 2021
- Noel-Storr AH, Dooley G, Elliott J, Steele E, Shemilt I, Mavergames C, et al. An evaluation of Cochrane Crowd found that crowdsourcing produced accurate results in identifying randomised trials. Journal of Clinical Epidemiology 2021;4356(21):00008-1. [DOI: 10.1016/j.jclinepi.2021.01.006] [PMID: ] [DOI] [PubMed] [Google Scholar]
Pierro 2017
- Pierro M, Thébaud B, Soll R. Mesenchymal stem cells for the prevention and treatment of bronchopulmonary dysplasia in preterm infants. Cochrane Database of Systematic Reviews 2017, Issue 11. Art. No: CD011932. [DOI: 10.1002/14651858.CD011932.pub2] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Poggi 2014
- Poggi C, Dani C. Antioxidant strategies and respiratory disease of the preterm newborn: an update. Oxidative Medicine and Cellular Longevity 2014;721043:1-11. [DOI: 10.1155/2014/721043] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Principi 2018
- Principi N, Di Pietro GM, Esposito S. Bronchopulmonary dysplasia: clinical aspects and preventive and therapeutic strategies. Journal of Translational Medicine 2018;16(1):36. [DOI: 10.1186/s12967-018-1417-7] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ramanathan 2001
- Ramanathan R, Corwin MJ, Hunt CE, Lister G, Tinsley LR, Baird T, et al, Collaborative Home Infant Monitoring Evaluation (CHIME) Study Group. Cardiorespiratory events recorded on home monitors: comparison of healthy infants with those at increased risk for SIDS. JAMA 2001;285(17):2199-207. [DOI: 10.1001/jama.285.17.2199] [DOI] [PubMed] [Google Scholar]
Resch 2011
- Resch B, Kurath S, Manzoni P. Epidemiology of respiratory syncytial virus infection in preterm infants. Open Microbiology Journal 2011;5:135-43. [DOI: 10.2174/1874285801105010135] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2024 [Computer program]
- Review Manager (RevMan). Version 7.12. The Cochrane Collaboration, 2024. Available at https://revman.cochrane.org.
Rhein 2014
- Rhein LM, Dobson NR, Darnall RA, Corwin MJ, Heeren TC, Poets CF, et al, Caffeine Pilot Study Group. Caffeine pilot study group. Effects of caffeine on intermittent hypoxia in infants born prematurely: a randomized clinical trial. JAMA Pediatrics 2014;168(3):250-7. [DOI: 10.1001/jamapediatrics.2013.4371] [PMID: ] [DOI] [PubMed] [Google Scholar]
Robertson 2009
- Robertson CM, Watt MJ, Dinu IA. Outcomes for the extremely premature infant: what is new? And where are we going? Pediatric Neurology 2009;40(3):189-96. [DOI: 10.1016/j.pediatrneurol.2008.09.017] [PMID: ] [DOI] [PubMed] [Google Scholar]
Rostas 2019
- Rostas SE, McPherson C. Caffeine therapy in preterm infants: the dose (and timing) make the medicine. Neonatal Network 2019;38(6):365-74. [DOI: 10.1891/0730-0832.38.6.365] [DOI] [PubMed] [Google Scholar]
Saroha 2020
- Saroha V, Patel RM. Caffeine for preterm infants: fixed standard dose, adjustments for age or high dose? Seminars in Fetal & Neonatal Medicine 2020;25(6):101178. [DOI: 10.1016/j.siny.2020.101178] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sauer‐Zavala 2019
- Sauer-Zavala S, Wilner J, Cassiello-Robbins C, Saraff P, Pagan D. Isolating the effect of opposite action in borderline personality disorder: a laboratory-based alternating treatment design. Behaviour Research and Therapy 2019;117:79-86. [DOI: 10.1016/j.brat.2018.10.006] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schmidt 2006
- Schmidt B, Roberts RS, Davis P, Doyle LW, Barrington KJ, Ohlsson A, et al. Caffeine therapy for apnea of prematurity. New England Journal of Medicine 2006;354(20):2112-21. [DOI: 10.1056/NEJMoa054065] [DOI] [PubMed] [Google Scholar]
Schmidt 2007
- Schmidt B, Roberts RS, Davis P, Doyle LW, Barrington KJ, Ohlsson A, et al, Caffeine for Apnea of Prematurity Trial Group. Long-term effects of caffeine therapy for apnea of prematurity. New England Journal of Medicine 2007;8(357):1893-902. [DOI: 10.1056/NEJMoa073679] [PMID: ] [DOI] [PubMed] [Google Scholar]
Schmidt 2012
- Schmidt B, Anderson PJ, Doyle LW, Dewey D, Grunau RE, Asztalos EV, et al, Caffeine for Apnea of Prematurity Trial Investigators. Survival without disability to age 5 years after neonatal caffeine therapy for apnea of prematurity. JAMA 2012;307(3):275-82. [DOI: 10.1001/jama.2011.2024] [PMID: ] [DOI] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from https://gdt.gradepro.org/app/handbook/handbook.html.
Shrestha 2017
- Shrestha B, Jawa G. Caffeine citrate – Is it a silver bullet in neonatology? Pediatrics and Neonatology 2017;58(5):391-7. [DOI: 10.1016/j.pedneo.2016.10.003] [PMID: ] [DOI] [PubMed] [Google Scholar]
Stevenson 2007
- Stevenson DK. On the caffeination of prematurity. New England Journal of Medicine 2007;357(19):1967-8. [DOI: 10.1056/NEJMe078200] [PMID: ] [DOI] [PubMed] [Google Scholar]
Stoll 2015
- Stoll BJ, Hansen NI, Bell EF, Walsh MC, Carlo WA, Shankaran S, et al, Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993–2012. JAMA 2015;314(10):1039–51. [DOI: 10.1001/jama.2015.10244] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tabacaru 2017
- Tabacaru CR, Jang SY, Patel M, Davalian F, Zanelli S, Fairchild KD. Impact of caffeine boluses and caffeine discontinuation on apnea and hypoxemia in preterm infants. Journal of Caffeine Research 2017;7(3):103-10. [DOI: 10.1089/jcr.2017.0002] [DOI] [PMC free article] [PubMed] [Google Scholar]
Thomas 2021
- Thomas J, McDonald S, Noel-Storr AH, Shemilt I, Elliott J, Mavergames C, et al. Machine learning reduces workload with minimal risk of missing studies: development and evaluation of an RCT classifier for Cochrane Reviews. Journal of Clinical Epidemiology 2021;133:140-51. [DOI: 10.1016/j.jclinepi.2020.11.003] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Thébaud 2019
- Thébaud B, Goss KN, Laughon M, Whitsett JA, Abman SH, Steinhorn RH, et al. Bronchopulmonary dysplasia. Nature Reviews Disease Primers 2019;5(1):78. [DOI: 10.1038/s41572-019-0127-7] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Townsi 2018
- Townsi N, Laing IA, Hall GL, Simpson SJ. The impact of respiratory viruses on lung health after preterm birth. European Clinical Respiratory Journal 2018;5(1):1-10. [DOI: 10.1080/20018525.2018.1487214] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Walsh 2004
- Walsh MC, Yao Q, Gettner P, Hale E, Collins M, Hensman A, et al, National Institute of Child Health and Human Development Neonatal Research Network. Impact of a physiologic definition on bronchopulmonary dysplasia rates. Pediatrics 2004;114(5):1305-11. [DOI: 10.1542/peds.2004-0204] [PMID: ] [DOI] [PubMed] [Google Scholar]