Abstract
Purpose
This study aims to investigate the potential role of Caprini risk assessment model (RAM) in predicting the risk of venous thromboembolism (VTE) in patients undergoing total hip or knee arthroplasty (THA/TKA). No national study has investigated the role of Caprini RAM after primary THA/TKA.
Methods
Data from The National Sample of Healthcare Cost and Utilization Project (HCUP) in 2019 were utilized for this study. The dataset consisted of 229,134 patients who underwent primary THA/TKA. Deep vein thrombosis (DVT) and pulmonary embolism (PE) were considered as VTE. The incidence of thrombosis was calculated based on different Caprini scores, and the risk of the Caprini indicator for VTE events was evaluated using a forest plot.
Results
The prevalence of VTE after primary THA/TKA in the U.S. population in 2019 was found to be 4.7 cases per 1000 patients. Age, body mass index (BMI), and Caprini score showed a positive association with the risk of VTE (P < 0.05). The receiver operating characteristic (ROC) curve analysis indicated that a Caprini score of 9.5 had a sensitivity of 47.2% and a specificity of 82.7%, with an area under the curve (AUC) of 0.693 (95% CI, 0.677−0.710). The highest Youden index was 0.299. Multivariate logistic regression analysis revealed that malignancy, varicose vein, positive blood test for thrombophilia, history of thrombosis, COPD, hip fracture, blood transfusion, and age were significant risk factors for VTE. Based on these findings, a new risk stratification system incorporating the Caprini score was proposed.
Conclusions
Although the Caprini score does not seem to be a good predictive model for VTE after primary THA/TKA, new risk stratification for the Caprini score is proposed to increase its usefulness.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12959-024-00633-4.
Keywords: Caprini risk assessment model, Total hip arthroplasty, Total knee arthroplasty, Venous thromboembolism, Healthcare Cost and utilization project (HCUP)
Introduction
With the aging of the population and the demand for a high quality of life, the incidence of joint replacement is increasing year by year. But for the postoperative complications of joint replacement, Venous thromboembolism (VTE), including deep vein thrombosis (DVT) and pulmonary embolism (PE), is a life-threatening complication of joint replacement [1]. In Europe and America, the incidence of DVT ranges from 2.22 to 3.29%, PE ranges from 0.87 to 1.99%, and fatal PE is 0.30% [2, 3]. In Asia, DVT is 1.4%, and PE is 1.1% [4]. In the US alone, VTE causes 100,000 to 180,000 deaths annually [5] and poses a significant burden on the healthcare system [6]. Major orthopedic surgeries are recognized to be at high risk for VTE, including hip fracture repair, total hip arthroplasty (THA), and knee arthroplasty (TKA) [7]. However, there are still many controversies in choosing preventive measures and assessing VTE risk. Both the American College of Chest Physicians (ACCP) and the American Association of Orthopaedic Surgeons (AAOS) agreed with the use of pharmacological prophylaxis for VTE after THA/TKA, but there is no consensus on the choice or dosage of the drug [8, 9]. Despite the routine use of anticoagulants or antiplatelets after surgery, VTE events still occur.
At present, the commonly used thrombosis risk assessment models are the Wells rule, Padua prediction score, and Caprini risk assessment model (Caprini RAM). However, there are questions about the validity of these models. One clinical trial indicated that the Wells score is more applicable to outpatients than inpatients [10] and Antonia Perez-Martin et al. reported the Wells score performed poorly for discrimination of risk for proximal DVT in hospitalized patients [11]. Using the Padua score to classify VTE risk was shown to be suboptimal [12], with inferior ability to identify medical patients who were not critically ill for risk of VTE [13].
The Caprini score not only integrated the general condition, past medical history, and perioperative conditions of the patients but also included relevant preventive measures. Caprini RAM was originally developed in 1991, and updated in 2013, 2019 [14, 15]. The Caprini score has been validated in assessing the VTE risk of critically ill surgical patients, general surgery patients, and urologic surgery patients [14, 16]. However, there is still a lack of large-scale studies in orthopedics especially with primary joint replacement, to analyze the efficacy of the Caprini score in predicting VTE risk. Therefore, the purpose of this study was to evaluate the effectiveness of the Caprini RAM in primary THA/TKA patients during hospitalizations.
Methods
Patient population
The study population was extracted from the National Inpatient Sample of Healthcare Cost and Utilization Project (HCUP) in 2019 compiled by the Agency for Healthcare Research and Quality, which includes the largest collection of longitudinal hospital care data in the United States. The inclusion criteria were age ≥ 18 years and primary hip or knee arthroplasty, while exclusion criteria were arthroplasty revision and age < 18 years. Data were queried with procedure codes for primary THA/TKA International Classification of Diseases, Tenth Revision Clinical Modification (ICD − 10-CM). In 2019, a total of 229,134 patients out of 7,083,805 patients met inclusion and exclusion criteria.
VTE determination
VTE defined as patients with DVT or PE was confirmed by duplex ultrasonography, venography, computed tomography pulmonary angiography or other methods. Deep vein thrombosis included acute thrombosis of lower-extremity veins including iliac, femoral, popliteal, or calf veins. A total of 1,077 patients out of 229,134 patients were diagnosed with VTE. The patients were divided into the VTE (n = 1,077) and non-VTE groups (n = 228,057).
Caprini score
The Caprini score is calculated based on VTE risk factors according to Caprini RAM (2013 Version, Supplementary Table 1). Points are weighted according to their risk factors to calculate the Caprini score.
Data analysis
Independent t-tests and Chi-square test were used to compare baseline conditions between VTE (both DVT and PE) and non-VTE group. The ability of caprini to identify VTE patients was evaluated by plotting ROC curves and calculating AUC values through sensitivity and specificity. Integrate Bootstrap with the above analysis to enhance the reliability of the results. A logistic regression model was used to calculate the odds ratio for factors including Caprini RAM and presented in the form of a forest graph. All analyses were performed using statistical software R version 4.2 and a result was considered statistically significant at the P < 0.05 level of significance.
Result
In our study, we analyzed a total of 229,134 patients who underwent primary joint replacement. Of these, 1,077 patients were included in the VTE group, while 228,057 patients were included in the non-VTE group. When comparing the VTE group with the non-VTE group, we found that the VTE group had a higher average age (P < 0.001) and a higher proportion of patients with a BMI ≥ 25 (P < 0.05). There are no significant differences between the two groups when it came to gender and BMI ≥ 40 (Table 1).
Table 1.
Demographics. Abbreviations: BMI, body mass index (calculated as the weight in kilograms divided by height in meters squared)
| Criteria | non-VTE | VTE | P Value | |
|---|---|---|---|---|
| n=228,057 | n=1,077 | |||
| Age, years | 68(57.2-78.8) | 72(60.6-83.4) | <0.0001 | |
| BMI(Kg/m²) | ||||
| BMI≥25 | 58,151(25.50%) | 243(22.56%) | 0.022 | |
| BMI≥40 | 15,504(6.8%) | 71(6.59%) | 0.693 | |
| Gender, N(%) | F=137,050(60.09%) | F=665(61.75%) | 0.270 | |
| M=91,007(39.91%) | M=412(38.25%) | |||
| THA | 115,249 | 577 | - | |
| TKA | 112,808 | 500 | - |
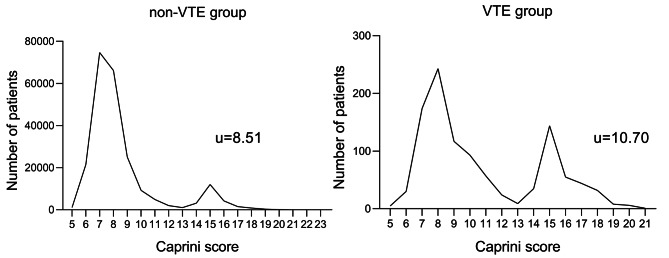
Two groups of patients’ Caprini scores were counted and drew their distribution as shown in Fig. 1, separately. The mean Caprini score was 10.70 in the VTE group, 8.51 in the non-VTE group, and a statistically significant difference was found between the two groups (P < 0.0001). There were two peaks observed in the distribution, with one peak at 7 in the non-VTE group and 8 in the VTE group, and another peak at 15 points in both groups (Fig. 1). The second peak at 15 points was mainly attributable to patients with hip fractures, who accounted for 30% of VTE patients in 10% of the total population (322 out of 1,077 VTE patients and 22,909 out of 229,134 population). The incidence of VTE was significantly higher in the hip fractures group (14‰) compared to the non-hip fractures group (3.7‰), and statistical analysis confirmed that the hip fractures group had a higher risk of thrombosis (P < 0.0001, Table 2).
Fig. 1.
Describes the distribution of the population’s Caprini score
Table 2.
VTE events statistics for hip fractures and non-hip fractures groups
| Criteria | non-hip fractrue | hip fracture | P Value |
|---|---|---|---|
| n=206,225 | n=22,909 | ||
| VTE Event | 755 | 322 | |
| VTE Rate | 3.7‰ | 14‰ | <0.0001 |
To further assess these findings, the ROC curve was drawn between the VTE and the non-VTE groups and calculating the area under the curve (AUC). According to ROC analysis (Fig. 2; Table 3), the optimal Caprini score cut-off value for predicting VTE was 9.5, with an AUC of 0.693 (95% CI, 0.677–0.710). This cut-off value had the highest Youden index (0.299), a sensitivity of 47.2% and a specificity of 82.7%.
Fig. 2.
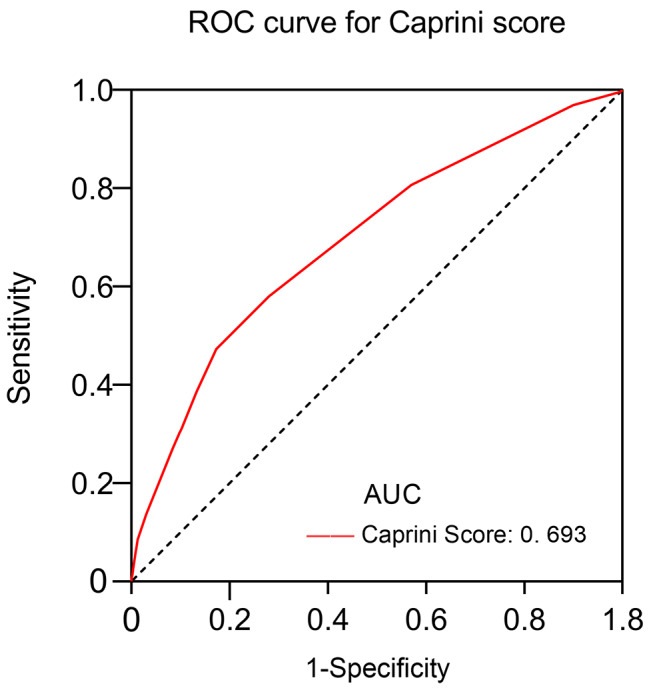
ROC curve analysis of Caprini score for the prediction of VTE. ROC, receiver operating characteristic curve; AUC, area under the curve
Table 3.
ROC curve of Caprini score-related evaluation parameters in the prediction of VTE.
| Caprini Score | |
|---|---|
| Area under the curve | 0.693 |
| (0.677-0.710) | |
| Optimal threshold | 9.5 |
| Specificity | 0.827 |
| Sensitivity | 0.472 |
| Accuracy | 0.826 |
| Diagnose odds ratio | 4.29 |
| Positive predictive value | 0.013 |
| Negative predictive value | 0.997 |
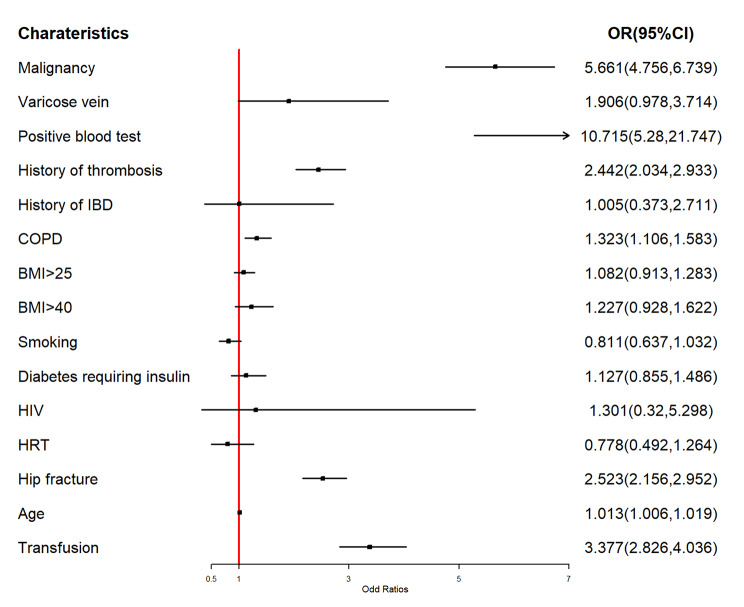
To elucidate the individual contributions of indicators including Caprini RAM versus VTE, multivariate logistic regression was used, and the results were plotted in a forest graph (Fig. 3). In this analysis, we found that several factors, including malignancy, varicose vein, positive blood test for thrombophilia, history of thrombosis, chronic obstructive pulmonary disease (COPD), hip fracture, transfusion, and age, were considered risk factors for VTE. Among these factors, positive blood test for thrombophilia (OR:10.715), malignancy (OR:5.661), and transfusion (OR:3.377) were found to be the three most important risk factors for VTE (Fig. 3).
Fig. 3.
Multivariable logistic regression model showing the effect of Caprini indicators on VTE event after primary THA/TKA. Abbreviations: IBD, inflammatory bowel disease; COPD, chronic obstructive pulmonary disease; HIV, Human immunodeficiency virus; HRT, hormone replacement therapy
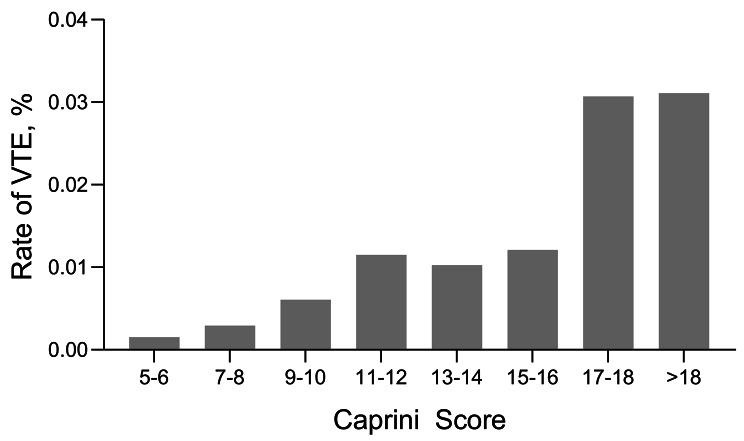
Furthermore, we observed that the risk of thrombosis increased with an increase in the Caprini score (Fig. 4). A Caprini score greater than 16 was significantly associated with a higher risk of developing VTE events compared to a score of 15–16 (odds ratio [OR], 2.59; 95%CI, 1.98–3.38; P < 0.001). Similarly, a score of 11–12 was significantly associated with a higher risk compared to a score of 9–10 (odds ratio [OR], 1.91; 95%CI, 1.47–2.47; P < 0.001), and a score of 9–10 was associated with a higher risk compared to a score of 7–8 (odds ratio [OR], 2.06; 95%CI, 1.75–2.44; P < 0.001). Moreover, a score of 7–8 was significantly associated with a higher risk compared to a score of 5–6 (OR, 1.91; 95%CI, 1.36–2.70; P < 0.001) (Table 4, other odds ratios are also visible).
Fig. 4.
Caprini Scores and Venous Thromboembolism (VTE) Rates
Table 4.
Odds for venous thromboembolism stratified by Caprini score. CS: Caprini score, data are presented as odds ratios (95% CI), NS = no significant difference (p > 0.05)
| CS 7-8 | CS 9-10 | CS 11-12 | CS 13-14 | CS 15-16 | CS 17-18 | CS >18 | |
|---|---|---|---|---|---|---|---|
| CS 5-6 | 1.91(1.36-2.70) | 3.95(2.76-5.65) | 7.53(5.06-12.21) | 6.71(4.30-10.48) | 7.92(5.23-11.35) | 20.49(13.70-30.65) | 20.76(11.26-38.27) |
| P value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| CS 7-8 | 2.06(1.75-2.44) | 3.94(3.10-5.00) | 3.51(2.57-4.79) | 4.14(3.49-4.90) | 10.71(8.36-13.72) | 10.85(6.43-18.30) | |
| P value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| CS 9-10 | 1.91(1.47-2.47) | 1.70(1.23-2.36) | 2.00(1.65-2.44) | 5.19(3.98-6.76) | 5.25(3.09-8.94) | ||
| P value | <0.001 | <0.002 | <0.001 | <0.001 | <0.001 | ||
| CS 11-12 | 0.89(0.62-1.69) | 1.05(0.81-1.36) | 2.72(1.98-3.73) | 2.76(1.58-4.82) | |||
| P value | 0.54(NS) | 0.705(NS) | <0.001 | <0.001 | |||
| CS 13-14 | 1.18(0.85-1.64) | 3.05(2.10-4.44) | 3.09(1.78-5.60) | ||||
| P value | 0.322(NS) | <0.001 | <0.001 | ||||
| CS 15-16 | 2.59(1.98-3.38) | 2.62(1.54-4.46) | |||||
| P value | <0.001 | <0.001 | |||||
| CS 17-18 | 1.01(0.58-1.78) | ||||||
| P value | 0.964(NS) |
Discussion
It is estimated that 100,000 premature deaths are caused by VTE every year in the United States [6]. VTE has become one of the important complications of orthopedic surgery, especially in THA/TKA [9, 17]. Known factors that contribute to thrombosis, include venous stasis, vascular injury, and hypercoagulability [18]. In addition, hematological changes, tourniquet use, and reduced perioperative mobility resulting from THA and TKA surgery further increases the risk of VTE. The development of VTE can significantly increase the 30-day mortality rate in postoperative patients [19]. As a result, orthopedic surgeons are highly concerned about the risk of thrombosis in these patients [20]. While there are various measures available to prevent thrombosis, ranging from intermittent pneumatic compressive devices to pharmaceutical agents like low molecular weight heparin, aspirin, warfarin, and factor Xa inhibitors, an effective thrombosis risk assessment system is still lacking. Thromboprophylaxis is recommended for up to 35 days from the day of surgery rather than for only 10 to 14 days [9]. Symptoms associated with thrombosis formation may include lower limb pain and swelling, as well as chest pain and hemoptysis. Therefore, it is crucial to identify risk factors that can predict the likelihood of VTE.
To our knowledge, this is the largest study to focus on this topic. The results showed that the VTE group had significantly older age and higher BMI (Table 1), which is consistent with previous knowledge that older age and obesity are risk factors for thrombosis [13, 21]. The peak of Caprini score showed a bimodal distribution (Fig. 1), with the second peak caused by hip fracture leading to prolonged bed rest and venous stasis before THA. Additionally, patients with hip fractures have a 3.78-fold higher risk of VTE as compared to those without hip fractures (Table 2). It is obvious that hip fracture is one of the important risk factors for the development of VTE. Similarly, numerous studies have pointed out that hip fracture is an important risk factor for VTE [22, 23].
The ROC curves analysis revealed that the optimal cut-off value for the Caprini score in predicting VTE was 9.5, with an area under the curve (AUC) of 0.693 (Fig. 2; Table 3). This result is consistent with recently reported findings by Krauss et al [24]. Thus, patient with Caprini scores greater than 9.5 were classified as high-risk, whereas others were classified as low-risk. In our study, 66,871 (29.18%) were identified as high-risk groups. Thus, the Caprini RAM showed a certain predictive power with an AUC of 0.693. Although relatively lower sensitivity of 47.2%, high specificity of 82.7% appears to be more important for VTE prediction.
Although age contribution is limited (OR:1.013, Fig. 3), it is still considered a risk factor for VTE due to its age-cumulative effect. It cannot be ignored that some indicators were excluded, which seems to indicate that these indicators may not contribute to the VTE event. In this context, it is necessary to re-verify the effect of these indicators on promoting VTE and explore new indicators to enhance the risk assessment model in predicting VTE.
In the meantime, the study found that an increased Caprini score was associated with increased risk of VTE (Fig. 4; Table 4). Logistic regression results show that for every 2-point increase in the Caprini score within the range of 5–12, the risk approximately doubled. However, there was no significant increase in risk for scores between 11–12 and 13–16 (P > 0.05). Similar findings were observed for scores between 17–18 and greater than 18 points (Table 4), meaning patients within the same score range had similar VTE risk. Early in its inception a Caprini score greater than 5 was considered as high risk for general surgery patients [25]. Since total joint replacement alone adds 5 points to the score, this cutoff was not useful. Combined with these findings, we propose a new risk stratification for the Caprini score in primary THA/TKA patients: very low risk (5–6), low risk (7–8), intermediate risk (9–10), high-risk (11–16) and very high risk (> 16). Although the Caprini score may not be an ideal predictive model for VTE after primary THA/TKA, this new risk stratification enhances its usefulness. However, this study has several limitations. First, it was a retrospective study conducted in a single country and method of thromboprophylaxis was not mentioned. Second, the database does not provide information regarding the timing or other details about the occurrence of VTE events. Third, due to the diverse range of hospitals contributing data to the HCUP database, ensuring accuracy and consistency in ICD coding practices and variable identification across institutions poses a challenge. These factors may lead to potential bias in research findings. As a result, it is crucial to approach the conclusions of this study with caution, acknowledging the need for ongoing verification and refinement in future clinical research. Additionally, further research should involve multiple countries and cities and verify the effectiveness of the new risk stratification model under different VTE preventive measures.
Conclusions
In summary, this study demonstrated that Caprini RAM has moderate predictive power for VTE risk in patients with primary THA/TKA. Nevertheless, some indicators within the Caprini score seem to have limited efficacy in predicting thrombosis. Therefore, it is crucial to identify novel methods or indicators that can accurately predict VTE events after primary THA/TKA. However, this new risk stratification proposed gives us a better understanding of the Caprini score, allowing us to more clearly stratify patients into different risk categories after THA/TKA. This makes it possible to utilize patient-specific VTE prevention measures and ultimately achieving the purpose of reducing the incidence of VTE.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Not applicable.
Abbreviations
- HCUP
Healthcare Cost and Utilization Project
- RAM
Risk assessment model
- VTE
Venous Thromboembolism
- THA
Total hip arthroplasty
- TKA
Total knee arthroplasty
- DVT
Deep vein thrombosis
- PE
Pulmonary embolism
- BMI
Body mass index
- ROC
Receiver operating characteristic
- AUC
Area under the ROC curve
- ACCP
American College of Chest Physicians
- AAOS
American Association of Orthopaedic Surgeons
- ICD − 10-CM
International Classification of Diseases, Tenth Revision Clinical Modification
- IRB
institutional review board
- OR
Odds ratio
- CS
Caprini score
- IBD
Inflammatory bowel disease
- COPD
Chronic obstructive pulmonary disease
- HIV
Human immunodeficiency virus
- HRT
Hormone replacement therapy
Author contributions
Zhencan Lin Conception and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript. Hao Sun, Meiyi Chen Conception and design, acquisition of data, critical revision of the manuscript. Deng Li, Zhiqing Cai, Yimin Wang Conception and design. Fangzhou Liu, Zhencheng Huang Acquisition and curation of data. Jie Xu, Ruofan Ma Final approval of the version, agree to be accountable for all aspects of the work. All authors have read and approved the manuscript.
Funding
This study was funded by the National Natural Science Foundation of China (82272443, 82372435), Guangdong Basic and Applied Basic Research Foundation (Grant No. 2019A1515011647, 2022A1515010256, 2023A1515010501), Science andTechnology Planning Project of Guangzhou City, China (Grant No. 202201020495, 202201020481).
Data availability
All other data can be obtained from the authors upon reasonable request.
Declarations
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This study was ruled exempt from formal review by the Ethical Committee of Sun Yat-sen Memorial Hospital, given the use of deidentifed, previously collected data.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zhencan Lin, Hao Sun and Meiyi Chen contributed equally to this work.
Contributor Information
Jie Xu, Email: lplllpfe@163.com.
Ruofan Ma, Email: maruofan@mail.sysu.edu.cn.
References
- 1.Wen S, Duan Q, Yang F, Li G, Wang L. Early diagnosis of venous thromboembolism as a clinical primary symptom of occult cancer: core proteins of a venous thrombus. Oncol Lett. 2017;14(1):491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dixon J, Ahn E, Zhou L, Lim R, Simpson D, Merriman EG. Venous thromboembolism rates in patients undergoing major hip and knee joint surgery at Waitemata District Health Board: a retrospective audit. Intern Med J. 2015;45(4):416. [DOI] [PubMed] [Google Scholar]
- 3.Akpinar EE, Hosgün D, Akan B, Ates C, Gülhan M. Does Thromboprophylaxis prevent venous thromboembolism after major orthopedic surgery? J Bras Pneumol. 2013;39(3):280–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cha SI, Lee SY, Kim CH, Park JY, Jung TH, Yi JH, Lee J, Huh S, Lee HJ, Kim SY. Venous thromboembolism in Korean patients undergoing major orthopedic surgery: a prospective observational study using computed tomographic (CT) pulmonary angiography and indirect CT venography. J Korean Med Sci. 2010;25(1):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldhaber SZ. Risk factors for venous thromboembolism. J Am Coll Cardiol. 2010;56(1):1. [DOI] [PubMed] [Google Scholar]
- 6.MacDougall DA, Feliu AL, Boccuzzi SJ, Lin J. Economic burden of deep-vein thrombosis, pulmonary embolism, and post-thrombotic syndrome. Am J Health Syst Pharm. 2006;63(20 Suppl 6):S5. [DOI] [PubMed] [Google Scholar]
- 7.Geerts WH, Pineo GF, Heit JA, Bergqvist D, Lassen MR, Colwell CW, Ray JG. Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and thrombolytic therapy. Chest. 2004;126(3 Suppl):338S. [DOI] [PubMed] [Google Scholar]
- 8.Michael A, Mont M, Joshua J, Jacobs M, Nilay Patel M, Patrick Sluka M. (2011) Preventing Venous Thromboembolic Disease in Patients Undergoing Elective Hip and Knee Arthroplasty. J Am Acad Orthop Surg.19(12):768 – 76. [DOI] [PubMed]
- 9.Falck-Ytter Y, Francis CW, Johanson NA, Curley C, Dahl OE, Schulman S, Ortel TL, Pauker SG, Colwell CW Jr. (2012) Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 141(2 Suppl): e278S. [DOI] [PMC free article] [PubMed]
- 10.Silveira PC, Ip IK, Goldhaber SZ, Piazza G, Benson CB, Khorasani R. Performance of Wells score for deep vein thrombosis in the Inpatient setting. JAMA Intern Med. 2015;175(7):1112. [DOI] [PubMed] [Google Scholar]
- 11.Trihan JE, Adam M, Jidal S, Aichoun I, Coudray S, Laurent J, Chaussavoine L, Chausserie S, Guillaumat J, Laneelle D, Perez-Martin A. Performance of the Wells score in predicting deep vein thrombosis in medical and surgical hospitalized patients with or without thromboprophylaxis: the R-WITT study. Vasc Med. 2021;26(3):288. [DOI] [PubMed] [Google Scholar]
- 12.Pavon JM, Sloane RJ, Pieper CF, Colon-Emeric CS, Cohen HJ, Gallagher D, Morey MC, McCarty M, Ortel TL, Hastings SN. Automated versus Manual Data extraction of the Padua Prediction score for venous thromboembolism risk in hospitalized older adults. Appl Clin Inf. 2018;9(3):743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heit JA. Epidemiology of venous thromboembolism. Nat Rev Cardiol. 2015;12(8):464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.JOSEPH A.CAORINI M, F.A.C.S. JIA MD, JAMES H.HASTY PD, PD AJITC, TAMHANE, PD, FRANCESC FABREGA MD. (1991) Clinical assessment of venous thromboembolic risk in surgical patients. Semin Thromb Hemost. 17 Suppl 3:304 – 12. [PubMed]
- 15.Cronin M, Dengler N, Krauss ES, Segal A, Wei N, Daly M, Mota F, Caprini JA. Completion of the updated Caprini Risk Assessment Model (2013 version). Clin Appl Thromb Hemost. 2019;25:1076029619838052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bahl V, Hu HM, Henke PK, Wakefield TW, Campbell DA Jr., Caprini JA. A validation study of a retrospective venous thromboembolism risk scoring method. Ann Surg. 2010;251(2):344. [DOI] [PubMed] [Google Scholar]
- 17.Mitani G, Takagaki T, Hamahashi K, Serigano K, Nakamura Y, Sato M, Mochida J. Associations between venous thromboembolism onset, D-dimer, and soluble fibrin monomer complex after total knee arthroplasty. J Orthop Surg Res. 2015;10:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu JX, Qing JH, Yao Y, Chen DY, Jiang Q. Performance of age-adjusted D-dimer values for predicting DVT before the knee and hip arthroplasty. J Orthop Surg Res. 2021;16(1):82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gangireddy C, Rectenwald JR, Upchurch GR, Wakefield TW, Khuri S, Henderson WG, Henke PK. Risk factors and clinical impact of postoperative symptomatic venous thromboembolism. J Vasc Surg. 2007;45(2):335. [DOI] [PubMed] [Google Scholar]
- 20.Lasse J, Lapidus S, Ponzer, Bri Ed HP. Symptomatic venous thromboembolism and mortality in orthopaedic surgery – an observational study of 45 968 consecutive procedures. BMC Musculoskelet Disord. 2013;14:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gregson J, Kaptoge S, Bolton T, Danesh J, Di Angelantonio E, Meade T, Emerging Risk Factors C. Cardiovascular Risk factors Associated with venous thromboembolism. JAMA Cardiol. 2019;4(2):163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pedersen AB, Ehrenstein V, Szepligeti SK, Sorensen HT. Excess risk of venous thromboembolism in hip fracture patients and the prognostic impact of comorbidity. Osteoporos Int. 2017;28(12):3421. [DOI] [PubMed] [Google Scholar]
- 23.Marsland D, Mears SC, Kates SL. Venous thromboembolic prophylaxis for hip fractures. Osteoporos Int. 2010;21(Suppl 4):S593. [DOI] [PubMed] [Google Scholar]
- 24.Krauss ES, Segal A, Cronin M, Dengler N, Lesser ML, Ahn S, Caprini JA. Implementation and validation of the 2013 Caprini score for risk stratification of Arthroplasty patients in the Prevention of venous thrombosis. Clin Appl Thromb Hemost. 2019;25:1076029619838066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gould MK, Garcia DA, Wren SM, Karanicolas PJ, Arcelus JI, Heit JA, Samama CM. Prevention of VTE in nonorthopedic surgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 Suppl):e227S. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All other data can be obtained from the authors upon reasonable request.