Abstract
Pseudotyping can improve retroviral vector stability and transduction efficiency. Here, we describe a novel pseudotype of murine leukemia virus packaged with lymphocytic choriomeningitis virus (LCMV). This pseudotype was stable during ultracentrifugation and infected several cell lines from different species. Moreover, LCMV glycoproteins were not cell toxic.
Low titers limit in vivo applications of conventional amphotropic retroviral vectors. Virus particles cannot be concentrated to overcome this problem because of the instability of the retroviral envelope (22). In addition, transduction of some cell types, such as hematopoietic progenitors, has been inefficient (1, 11, 24, 28). Both vector stability and host range have been improved by packaging murine leukemia virus (MLV) vectors with the G protein of vesicular stomatitis virus (VSV) to generate hybrid virions called MLV(VSV) pseudotypes (13, 28, 29). However, a major drawback of these packaging systems is that the VSV G protein is cell toxic (7, 9, 17, 31).
Here we describe a retroviral vector pseudotyped with the glycoproteins of an arenavirus, the lymphocytic choriomeningitis virus (LCMV). LCMV glycoproteins are synthesized as a 74-kDa precursor protein, GP-C, and then cleaved into a 35-kDa transmembrane protein, GP-2, and an external 44-kDa protein, GP-1. In contrast to VSV G, LCMV glycoproteins are not cell toxic (25).
Initially we tested whether LCMV could rescue a retroviral vector. The env-negative packaging cell line TELCeB was infected with the LCMV WE strain. TELCeB cells are derived from the human fibroblast cell line Te671 and contain gag and pol genes as well as a retroviral lacZ vector (10). These cells lack viral envelope proteins and do not produce infectious retrovirus unless an appropriate glycoprotein is provided. After infection with LCMV, levels of production of wild-type LCMV and rescued retrovirus were measured daily and expressed as PFU and LacZ-transferring units (LTU), respectively. The titration assays have been described previously (16, 18). Additionally, production of LCMV glycoproteins in infected TELCeB cells was monitored by flow cytometric analysis with a mouse monoclonal antibody against LCMV GP-1 (6). The results of a representative experiment are shown in Fig. 1. Retroviral vector was produced for 6 days, with the maximum level of production of 5 × 104 LTU per ml, together with the highest expression of LCMV glycoprotein, occurring on day 3. The highest titer for wild-type LCMV was 3 × 108 per ml on day 2. No cytopathic effect was observed during replication of LCMV in the packaging cell line, although high levels of LCMV glycoproteins were expressed. Supernatants were then incubated with a neutralizing anti-LCMV gp44 monoclonal antibody (in mouse ascites fluid, 1:100) for 1 h (6). This led to a more than 3-log-unit reduction in vector titer. As a control, the amphotropic pseudotype of the same retroviral vector was used and was not neutralized by the anti-LCMV antibody (data not shown). These data show that the retroviral vector indeed carried on its surface LCMV glycoproteins that mediate cell entry in the absence of retroviral envelope proteins.
FIG. 1.
Rescue of the retroviral vector MFGlnsLacZ by LCMV. The retroviral env-negative packaging cell line TELCeB was infected with LCMV at a multiplicity of 0.01. Between days 1 and 7 after infection, supernatants were replaced daily and the titers of wild-type LCMV and of the LacZ vector were determined by a plaque assay on L-929 cells and by measurement of lacZ gene transfer to Sc-1 cells, respectively. Additionally, a portion of the LCMV-infected TECLeB cells was stained daily with a monoclonal antibody to the LCMV glycoprotein GP-1 and analyzed by flow cytometry (FACScalibur; Becton Dickinson, Heidelberg, Germany). The mean fluorescence is shown. ●, titer of wild-type LCMV in PFU per ml; ■, LTU per ml; ▴, mean fluorescence of LCMV glycoprotein GP expression.
We then analyzed whether the LCMV helper function required retroviral gag and pol. 293 cells and 293gp2 cells, the latter containing MLV gag and pol, were transfected with an MLV-based retroviral vector containing the neo gene (MP1N) and were selected with G418 (12, 28). The resulting cell lines, 293MP1N and 293gp2MP1N, were then infected with LCMV. 293gpMP1N produced infectious retroviral progeny (3 × 103 G418-resistant transfer units [GTU] per ml; for the method of titration, see reference 28), but no vector could be detected in the supernatant of LCMV-infected 293MP1N cells, which did not contain retroviral gag or pol (detection limit, 1 GTU per ml). As a control, the cells were also infected with a replication-competent amphotropic helper (23). An infectious vector that transferred neo resistance was recovered from both cells lines at titers of 1 × 104 and 6 × 104 per ml for 293MP1N and 293gp2MP1N, respectively. Thus, as expected for a classical retroviral pseudotype, retroviral genomic RNA was not packaged by LCMV into infectious virions in the absence of gag and pol gene products.
To verify that MLV(LCMV) pseudotypes indeed mediated stable transduction with integration of the transgene into the target cell genome, DNA of 12 G418-resistant clones was subjected to Southern blot analysis after restriction with HindIII, a single cutter, and a neo probe (26). In 10 clones, one copy of the integrated retroviral vector genome per cell was detected, and in the remaining 2 clones, two copies were detected (data not shown). Transduction with the MLV(LCMV) pseudotype thus led to stable integration of the transgene, which also supports the conclusion that the MLV(LCMV) pseudotype indeed contained a functional retroviral core.
However, the hybrid vector particles were produced by LCMV infection of env-negative packaging cell lines, in which all LCMV proteins were expressed. Therefore, we could not completely exclude the possibility that, in addition to the glycoproteins, further LCMV proteins are required for efficient vector production. This issue is important for the generation of helper-free packaging systems and is currently addressed by expressing isolated LCMV genes in env-negative packaging cells.
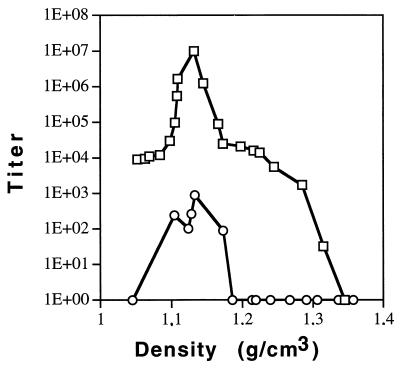
Amphotropic retroviruses lose infectivity upon ultracentrifugation, most likely because of the lability of the retroviral envelope glycoproteins (22). We tested whether the MLV(LCMV) pseudotypes are more stable. TELCeB cells were infected with LCMV or with amphotropic helper virus. Equal amounts of both virus progeny were pelleted and purified by ultracentrifugation through a 0 to 40% Urografin gradient as described previously (19). Titers of a representative gradient (in LTU per ml) are shown in Fig. 2. LCMV pseudotypes were recovered without loss of infectivity, in contrast to a >3-log-unit loss of infectious amphotropic virus. The reverse transcriptase activities in the virion peaks showed that the amounts of virus particles harvested from the gradient were similar for both pseudotypes (data not shown).
FIG. 2.
MLV(LCMV) pseudotypes retain infectivity upon ultracentrifugation. TELCeB cells were infected with LCMV or amphotropic helper virus. Supernatants were harvested and frozen. The MLV(LCMV) and amphotropic pseudotype titers were determined. Equal quantities of the infectious vector were pelleted by ultracentrifugation and then subjected to purification on a 0 to 40% Urografin gradient. Vector titers and densities in each fraction were determined. ○, amphotropic pseudotype; □, MLV(LCMV) pseudotype.
LCMV pseudotypes were also stable when they were stored at 4°C. Within the observation period of 3 days, the loss of titer was less than twofold. One cycle of freezing (−80°C) and thawing led to a twofold reduction in pseudotype titer.
The tropism of MLV(LCMV) pseudotypes was analyzed. Vector titers on different cell lines relative to the titer on the mouse fibroblast line Sc-1 are shown in Table 1. The human hematopoietic progenitor cell lines K562 and TF-1, the human hepatoma cell line HUH-7, and the human glioma cell line nce-G112 could be efficiently infected (14, 30). All these lines are derived from cell types that are relevant targets in gene therapy. Moreover, canine thymus cells (Cf2Th) and hamster cells, the latter of which are normally resistant to transduction with MLV-derived vectors, were also highly susceptible to the MLV(LCMV) pseudotypes.
TABLE 1.
Host range of MLV(LCMV) pseudotypes
| Cell line (no. of expts) | Cell type | Mean vector titer relative to titer on SC-1 cells (range) |
|---|---|---|
| 293 (5) | Epithelial, human | 5.1 (1.2–16.7) |
| Te671 (5) | Fibroblast, Human | 16.5 (3.5–25) |
| K562 (5) | Myeloid progenitor, human | 0.26 (0.06–0.5) |
| TF-1 (4) | Myeloid progenitor, human | 0.14 (0.03–0.27) |
| HUH-7 (5) | Hepatoma, human | 0.08 (0.02–0.19) |
| nce-G112 (3) | Glioma, human | 3.44 (0.1–10) |
| CHO (4) | Epithelial, hamster | 0.7 (0.02–2.5) |
| Cf2Th (4) | Thymus stroma, dog | 15.3 (6–20) |
| Sc-1a | Fibroblast, mouse | 1 |
The titers on Sc-1 cells of the different virus stocks used here (non concentrated supernatants) ranged from 0.2 × 104 to 5 × 104 LTU per ml.
Wild-type LCMV has been shown to infect several cell types from different tissues and species (2, 3, 5, 15, 20, 27). Alpha-dystroglycan, which is widely expressed in different tissues, was recently found to be a receptor for LCMV (4, 8). It was, therefore, not surprising that in our analysis MLV(LCMV) pseudotypes also infected several different cell lines, including hamster cells, which are resistant to amphotropic retroviral vectors (21). An interesting property of LCMV is that single amino acid changes in the glycoprotein can alter cell tropism, indicating that the LCMV glycoproteins may use different closely related receptors (or one receptor with different posttranslational modifications) on different cell types (20, 27). This flexibility of the glycoprotein may be of considerable advantage for the design of vectors with preferential tropism for specific tissues and cell types.
In conclusion, although several questions remain open, especially regarding the feasibility of a helper-free packaging system, MLV(LCMV) pseudotypes are a promising alternative to current retroviral pseudotypes and may allow stable production of broad-host-range retroviral vectors which can be concentrated by ultracentrifugation.
Acknowledgments
We thank Carol Stocking for critical reading of the manuscript.
This work was supported by Deutsche Forschungsgemeinschaft grant LA 1135/3-1 and by Bundesministerium für Bildung und Forschung grant 01KV9811.4.
REFERENCES
- 1.Beck-Engeser G, Stocking C, Just U, Albritton L, Dexter M, Spooncer E, Ostertag W. Retroviral vectors related to the myeloproliferative sarcoma virus allow efficient expression in hematopoietic stem and precursor cell lines, but retroviral infection is reduced in more primitive cells. Hum Gene Ther. 1991;2:61–70. doi: 10.1089/hum.1991.2.1-61. [DOI] [PubMed] [Google Scholar]
- 2.Binder D, Fehr J, Hengartner H, Zinkernagel R M. Virus-induced transient bone marrow aplasia: major role of interferon-alpha/beta during acute infection with the noncytopathic lymphocytic choriomeningitis virus. J Exp Med. 1997;185:517–530. doi: 10.1084/jem.185.3.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borrow P, Evans C F, Oldstone M B A. Virus-induced immunosuppression: immune system-mediated destruction of virus-infected dendritic cells results in generalized immune suppression. J Virol. 1995;69:1059–1070. doi: 10.1128/jvi.69.2.1059-1070.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borrow P, Oldstone M B A. Characterization of lymphocytic choriomeningitis virus-binding proteins: a candidate cellular receptor for the virus. J Virol. 1992;66:7270–7281. doi: 10.1128/jvi.66.12.7270-7281.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borrow P, Tishon A, Oldstone M B A. Infection of lymphocytes by a virus that aborts cytotoxic T lymphocyte activity and establishes persistent infection. J Exp Med. 1991;174:203–212. doi: 10.1084/jem.174.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruns M, Cihak J, Müller G, Lehmann-Grube F. Lymphcytic choriomeningitis virus. VI. isolation of a glycoprotein mediating neutralization. Virology. 1983;130:247–251. doi: 10.1016/0042-6822(83)90135-6. [DOI] [PubMed] [Google Scholar]
- 7.Burns J C, Friedmann T, Driever W, Burrascano M, Yee J-K. Vesicular stomatitis virus G glycoprotein pseudotyped retorviral vectors: concentration to very high titer and efficient gene transfer into mammalian and nonmammalian cells. Proc Natl Acad Sci USA. 1993;90:8033–8037. doi: 10.1073/pnas.90.17.8033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cao W, Henry M D, Borrow P, Yamada H, Elder J H, Ravkov E V, Nichol S T, Compans R W, Campbell K P, Oldstone M B. Identification of alpha-dystroglycan as a receptor for lymphocytic choriomeningitis virus and Lassa fever virus. Science. 1998;282:2079–2081. doi: 10.1126/science.282.5396.2079. [DOI] [PubMed] [Google Scholar]
- 9.Chen S-T, Iida A, Guo L, Friedmann T, Yee J-K. Generation of packaging cell lines for pseudotyped retroviral vectors of the G protein of vesicular stomatitis virus by using a modified tetracycline inducible system. Proc Natl Acad Sci USA. 1996;93:10057–10062. doi: 10.1073/pnas.93.19.10057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cosset F L, Takeuchi Y, Battini J L, Weiss R A, Collins M K. High-titer packaging cells producing recombinant retroviruses resistant to human serum. J Virol. 1995;69:7430–7436. doi: 10.1128/jvi.69.12.7430-7436.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crooks G M, Kohn D B. Growth factors increase amphotropic retrovirus binding to human CD34+ bone marrow progenitor cells. Blood. 1993;82:3290–3297. [PubMed] [Google Scholar]
- 12.Eckert H-G, Stockschläger M, Just U, Hegewisch-Becker S, Grez M, Uhde A, Zander A, Ostertag W, Baum C. High-dose multidrug resistance in primary human hematopoietic progenitor cells transduced with optimized retroviral vectors. Blood. 1996;88:3407–3415. [PubMed] [Google Scholar]
- 13.Friedmann T, Yee J-K. Pseudotyped retroviral vectors for studies of human gene therapy. Nat Med. 1995;1:275–277. doi: 10.1038/nm0395-275. [DOI] [PubMed] [Google Scholar]
- 14.Heberlein C, Friel J, Laker C, von Laer D, Bergholz U, Bögel M, Ashman L K, Klingler K, Ostertag W. Downregulation of c-kit in transformed hematopoietic precursor cells by stroma cells. Blood. 1999;93:554–563. [PubMed] [Google Scholar]
- 15.Klavinskis L S, Oldstone M B A. Lymphocytic choriomeningitis virus can persistently infect thyroid epithelial cells and perturb thyroid hormone production. J Gen Virol. 1987;68:1867–1873. doi: 10.1099/0022-1317-68-7-1867. [DOI] [PubMed] [Google Scholar]
- 16.Lehmann-Grube F, Ambrassat J. A new method to detect lymphocytic choriomeningitis virus-specific antibody in human sera. J Gen Virol. 1977;37:85–92. doi: 10.1099/0022-1317-37-1-85. [DOI] [PubMed] [Google Scholar]
- 17.Liu M-L, Winther B L, Kay M A. Pseudotransduction of hepatocytes by using concentrated pseudotyped vesicular stomatitis virus G glycoprotein (VSV-G)–Moloney murine leukemia virus-derived retrovirus vector: comparison of VSV-G and amphotropic vectors for hepatic gene transfer. J Virol. 1996;70:2497–2502. doi: 10.1128/jvi.70.4.2497-2502.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.MacGregor G R. Use of E. coli lacZ as a reporter gene. Methods Mol Biol. 1989;7:1–19. doi: 10.1385/0-89603-178-0:217. [DOI] [PubMed] [Google Scholar]
- 19.Martínez Peralta L, Bruns M, Lehmann-Grube F. Biochemical composition of lymphocytic choriomeningitis virus interfering particles. J Gen Virol. 1981;55:475–479. doi: 10.1099/0022-1317-55-2-475. [DOI] [PubMed] [Google Scholar]
- 20.Matloubian M, Kolhekar S R, Somasundaram T, Ahmed R. Molecular determinants of macrophage tropism and viral persistence: importance of single amino acid changes in the polymerase and glycoprotein of lymphocytic choriomeningitis virus. J Virol. 1993;67:7340–7349. doi: 10.1128/jvi.67.12.7340-7349.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller D G, Miller A D. Tunicamycin treatment of CHO cells abrogates multiple blocks to retrovirus infection, one of which is due to a secreted inhibitor. J Virol. 1992;66:78–84. doi: 10.1128/jvi.66.1.78-84.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moennig V, Frank H, Hunsmann G, Schneider I, Schäfer W. Properties of mouse leukemia viruses. VII. The major glycoprotein of friend leukemia virus. Isolation and physicochemical properties. Virology. 1974;61:100–111. doi: 10.1016/0042-6822(74)90245-1. [DOI] [PubMed] [Google Scholar]
- 23.Münk C, Löhler J, Prassolov V, Just U, Stockschläger M, Stocking C. Amphotropic murine leukemia viruses induce spongiform encephalopathy. Proc Natl Acad Sci USA. 1997;94:5837–5842. doi: 10.1073/pnas.94.11.5837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Orlic D, Girard L J, Jordan C T, Andersen S M, Cline A P, Bodine D M. The level of mRNA encoding the amphotropic retrovirus receptor in mouse and human hematopoietic stem cells is low and correlates with the efficiency of retrovirus transduction. Proc Natl Acad Sci USA. 1996;93:11097–11102. doi: 10.1073/pnas.93.20.11097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Southern P. Arenaviruses; the viruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 1505–1519. [Google Scholar]
- 26.Stocking C, Löliger C, Kawai M, Suciu S, Gough N, Ostertag W. Identification of genes involved in growth autonomy of hematopoietic cells by analysis of factor-independent mutants. Cell. 1988;53:869–879. doi: 10.1016/s0092-8674(88)90329-7. [DOI] [PubMed] [Google Scholar]
- 27.Teng M N, Borrow P, Oldstone M B, de la Torre J C. A single amino acid change in the glycoprotein of lymphocytic choriomeningitis virus is associated with the ability to cause growth hormone deficiency syndrome. J Virol. 1996;70:8438–8443. doi: 10.1128/jvi.70.12.8438-8443.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.von Laer D, Thomsen S, Vogt B, Donath M, Kruppa J, Rein A, Ostertag W, Stocking C. Entry of amphotropic and 10A1 pseudotyped murine retroviruses is restricted in hematopoietic stem cell lines. J Virol. 1998;72:1424–1430. doi: 10.1128/jvi.72.2.1424-1430.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weiss R A. Pseudotyped viruses and envelope composition. In: Levy J A, editor. The Retroviridae. New York, N.Y: Plenum Press; 1993. pp. 5–8. [Google Scholar]
- 30.Westphal M, Meima L, Szonyi E, Lofgren J, Meissner H, Hamel W, Nikolics K, Sliwkowski M X. Heregulins and the ErbB-2/3/4 receptors in gliomas. J Neurooncol. 1997;35:335–346. doi: 10.1023/a:1005837122181. [DOI] [PubMed] [Google Scholar]
- 31.Yang Y P, Vanin E F, Whitt M A, Fornerod M, Zwart R, Schneiderman R D, Grosveld G, Nienhuis A W. Inducible, high-level production of infectious murine leukemia retroviral vector particles pseudotyped with vesicular stomatitis virus G envelope protein. Hum Gene Ther. 1995;6:1203–1213. doi: 10.1089/hum.1995.6.9-1203. [DOI] [PubMed] [Google Scholar]