Abstract
The membrane glycoproteins G1 and G2 of Uukuniemi virus, a member of the Bunyaviridae family, are cotranslationally cleaved from a common precursor in the endoplasmic reticulum (ER). Here, we show that newly made G1 and G2 associate transiently with calnexin and calreticulin, two lectins involved in glycoprotein folding in the ER. Stable complexes between G1-G2 and calnexin or calreticulin could be immunoprecipitated after solubilization of virus-infected BHK21 cells with the detergents digitonin or Triton X-100. In addition, G1-G2-calnexin complexes could be recovered after solubilization with CHAPS {3-[(3-cholamidopropyl)-dimethylammonio]-1-propane sulfonate}, while G1-G2-calreticulin complexes were not readily detected by using this detergent. Only endoglycosidase H-sensitive forms of G1 were found complexed with calnexin. Pulse-chase experiments showed that G1 and G2 associated with both chaperones transiently for up to 120 min. Sequential immunoprecipitations with anticalreticulin and anticalnexin antisera indicated that about 50% of newly synthesized G1 and G2 was associated with either calnexin or calreticulin. Our previous results have shown that newly synthesized G1 and G2 transiently interact also with the ER chaperone BiP and with protein disulfide isomerase (R. Persson and R. F. Pettersson, J. Cell Biol. 112:257–266, 1991). Taking all of this into consideration, we conclude that the folding of G1 and G2 in the ER is catalyzed by at least four different folding factors.
Following translation by membrane-bound ribosomes and translocation into the lumen of the endoplasmic reticulum (ER), secretory and membrane proteins undergo posttranslational modifications, folding, and in most cases assembly into ternary complexes. This process is catalyzed by a number of enzymes and chaperones, also known as folding factors (14). Only properly folded and assembled proteins, i.e., proteins that have passed the quality control mechanism, are thought to be allowed to leave the ER compartment for transport along the exocytic pathway (11). To date, several folding factors have been identified, notably, protein disulfide isomerase (PDI) and Erp57, which catalyze the formation of correct disulfide bonds, and BiP/grp78, a chaperone that prevents aggregation of folding intermediates and assists in the folding process (9, 14). In addition, two more recently identified factors, calnexin (4, 15, 25) and calreticulin (15, 17), with extensive sequence homology, are lectins that serve as ER chaperones by recognizing monoglucosylated folding intermediates and retaining them in the ER (15). Through the identification of these folding factors, which interact physically with newly synthesized proteins in the ER lumen, a picture defining the early events in the ER lumen has emerged, showing that nascent polypeptide chains are core glycosylated, folded with the help of a set of chaperones into stable conformations, and finally, in most cases, assembled into higher-order complexes. Viral spike proteins have been instrumental in dissecting these early steps in the ER (10, 12–15).
We have previously characterized some of the early events involved in the biosynthesis of the Uukuniemi (UUK) virus (a phlebovirus within the Bunyaviridae family) membrane glycoproteins G1 (Mr, 70,000; 479 amino acids) and G2 (Mr, 65,000; 495 amino acids) (26, 30). They are made from a 110,000-Da precursor, p110 (30, 33), by cotranslational, signal peptidase-mediated cleavage (1, 18). Following core glycosylation, G1 folds rapidly, while G2 folds slowly (26). G1 and G2 then heterodimerize. Due to their different folding kinetics, newly synthesized G1 is believed to heterodimerize, not with its G2 partner made from the same precursor, but rather with a properly folded G2 derived from another p110 precursor and made some 30 to 45 min earlier. During folding, G1 transiently associates with BiP less efficiently and for a shorter period than G2. Coprecipitation of PDI with anti-G1-G2 antiserum was also observed (26). G1 expressed alone is competent to exit the ER, albeit inefficiently, and transported to the Golgi. In contrast, G2 is unable to leave the ER in the absence of G1. ER-retained G2 can be rescued from the ER if it is coexpressed with G1 from a separate mRNA (23, 29). Following dimerization and transport out of the ER, the G1-G2 dimer is arrested in the Golgi complex, where virus budding occurs (19–21, 27–29). A retention signal responsible for the localization of the heterodimer to the Golgi complex was recently mapped to the cytoplasmic tail of G1 (2, 3).
Previous results have shown that although the glycans of G2 remain largely endoglycosidase H (endo H) sensitive during intracellular transport and in virions, G1 acquires partially endo H-resistant glycans with a half time of 30 to 45 min, resulting in slow transport of the G1-G2 dimer to the medial Golgi (18). This delayed transport could be due to slow exit from the ER as a consequence of slow folding and heterodimerization. To further elucidate this, we analyzed the role of calnexin and calreticulin in the folding process. To study whether calnexin and calreticulin interact with G1 and G2, UUK virus- or mock-infected BHK21 cells were labeled with [35S]methionine for 10 min at 14 h after infection, followed by a 10-min chase with unlabeled methionine. The cells were then lysed with either (i) 2% CHAPS {3-[(3-cholamidopropyl)-dimethylammonio]-1-propane sulfonate} in 200 mM NaCl–50 mM HEPES, pH 7.6; (ii) 1% digitonin (repurified from commercial digitonin [Sigma] by ion-exchange chromatography) in 150 mM NaCl–20 mM Tris-HCl, pH 8.0; or (iii) 1% Triton X-100 in 150 mM NaCl–20 mM Tris-HCl, pH 8.0. The solubilization buffers also contained protease inhibitors (10 IU of aprotinin, 10 μg each of antipain, chymostatin, leupeptin, pepstatin, and soybean trypsin inhibitor per ml, and 1 mM phenylmethylsulfonyl fluoride) and 10 mM N-ethylmaleimide (all from Sigma). After removal of nuclei by centrifugation, the cleared lysates were subjected to immunoprecipitation with either a polyclonal rabbit antiserum, made in our laboratory against the carboxy-terminal calnexin peptide (NH2-CEEDEILNRSPRNRKPRRE-COOH, residues 555 to 573 in reference 34), or a polyclonal rabbit anticalreticulin antiserum (Affinity BioReagents). The immunoprecipitates were analyzed on a sodium dodecyl sulfate (SDS)–10% polyacrylamide gel (22) under reducing conditions, followed by autoradiography.
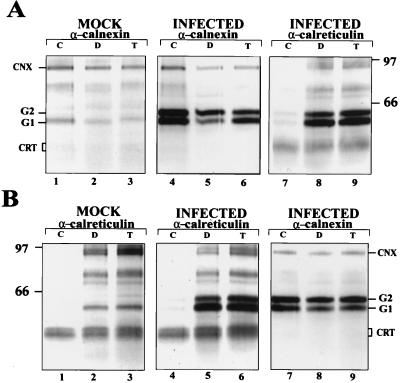
Both the anticalnexin and the anticalreticulin antisera coprecipitated G1 and G2 from infected cells after solubilization with digitonin or Triton X-100 (Fig. 1A and B, lanes 5 and 6). In contrast, G1 and G2 were readily coprecipitated from CHAPS-solubilized lysates with anticalnexin antiserum (Fig. 1A, lane 4) but very weakly coprecipitated with anticalreticulin antiserum (Fig. 1B, lane 4). The reason for this difference is unclear. It is unlikely that the G1-G2-calreticulin complexes are not solubilized selectively by CHAPS, since G1-G2-calnexin complexes were readily recovered under the same conditions. One possibility is that the G1-G2-calreticulin complexes are unstable in CHAPS.
FIG. 1.
Coprecipitation of UUK virus spike proteins G1 and G2 with calnexin and calreticulin after solubilization with different detergents. Virus- or mock-infected BHK21 cells were labeled at 14 h postinfection for 10 min with [35S]methionine and then chased for 10 min. The cells were lysed in buffers containing different detergents (2% CHAPS [C], 1% digitonin [D], 1% Triton X-100 [T]) and processed for sequential immunoprecipitation. Lanes 1 to 3, proteins coprecipitated from uninfected cells; lanes 4 to 6, coprecipitation with calnexin (A) or calreticulin (B); lanes 7 to 9, proteins reprecipitated with anticalreticulin (A) or anticalnexin (B), respectively, from the supernatants remaining after three rounds of precipitations with the antisera used for lanes 4 to 6. Proteins were analyzed on an SDS–10% polyacrylamide gel under reducing conditions, followed by fluorography. The positions of calnexin (CNX), calreticulin (CRT; migrates as a double band), G1, and G2, as well as molecular weight markers (in thousands), are indicated.
Under the conditions of this study, roughly equal amounts of labeled G1 and G2 were coprecipitated with calnexin from CHAPS- and Triton X-100-solubilized lysates (Fig. 1A, lanes 4 and 6, respectively), while from digitonin-solubilized lysates, more G2 than G1 was recovered (Fig. 1A, lane 5). Somewhat more G1 was coprecipitated with calreticulin from both digitonin- and Triton X-100-solubilized lysates (Fig. 1B, lanes 5 and 6). In these precipitations, the amounts of antisera used were titrated to be as close as possible to saturation. However, reprecipitation of the supernatants two additional successive times showed that the first anticalnexin and anticalreticulin precipitations yielded only about 60 and 70%, respectively, of the total G1-G2 recovered during three rounds of precipitations. As summarized in Table 1, about 50% of the total pool of labeled G1 and G2 could be precipitated with anticalnexin and anticalreticulin antisera from Triton X-100-solubilized lysate prepared from infected cells that had been labeled for 10 min and chased for 10 min. It should be noted that a protein of unknown origin migrating at the position of G1 was coprecipitated from uninfected cells with both anticalnexin and anticalreticulin antisera (Fig. 1A, lanes 1 to 3, and 1B, lanes 2 and 3).
TABLE 1.
Recovery of G1 and G2 bound to calnexin and calreticulin after sequential immunoprecipitationa
| Protein | % of total G1 and G2 in cell lysate
|
|||
|---|---|---|---|---|
| Bound
|
Unbound | |||
| After first precipitation (antiserumb) | After second precipitation (antiserum) | Total | ||
| G1 | 25 (anti-CNX) | 23 (anti-CRT) | 48 | 52 |
| 27 (anti-CRT) | 19 (anti-CNX) | 46 | 54 | |
| G2 | 27 (anti-CNX) | 18 (anti-CRT) | 45 | 55 |
| 17 (anti-CRT) | 28 (anti-CNX) | 45 | 55 | |
At 14 h postinfection, UUK virus-infected cells were pulse-labeled for 10 min with [35S]methionine, followed by a 10-min chase. A Triton X-100-solubilized lysate was then subjected to three rounds of precipitation with the first antiserum, followed by three rounds of precipitation with the second antiserum. The combined immunoprecipitates from the three rounds were analyzed by SDS-polyacrylamide gel electrophoresis, and the bands were quantified by a PhosphorImager.
Anti-CNX, anticalnexin; anti-CRT, anticalreticulin.
To analyze whether calnexin or calreticulin interacted with separate populations of G1 and G2, we carried out sequential immunoprecipitations with the two antisera. When the supernatants remaining after three consecutive immunoprecipitations with the anticalnexin antiserum (Fig. 1A, lanes 4 to 6) were reprecipitated with anticalreticulin antiserum (Fig. 1A, lanes 7 to 9), substantial amounts of G1 and G2 were recovered from both the digitonin and Triton X-100 supernatants (Fig. 1A, lanes 8 and 9). Very little G1-G2 was recovered from the supernatant of the CHAPS-solubilized lysate (Fig. 1A, lane 7), in accordance with the observation that G1-G2 could not be effectively precipitated after solubilization with CHAPS (Fig. 1B, lane 4). From all three supernatants remaining after anticalreticulin precipitation (Fig. 1B, lanes 4 to 6), G1 and G2 were likewise readily recovered with the anticalnexin antiserum (Fig. 1B, lanes 7 to 9). Table 1 summarizes the quantification of G1 and G2 precipitated from a Triton X-100-solubilized lysate. Two protein species roughly comigrating with calnexin were coprecipitated with the anticalreticulin antiserum (Fig. 1A, lanes 8 and 9, Fig. 1B, lanes 2, 3, 5, and 6, and Fig. 2C). A more careful analysis revealed that calnexin migrated at a position between the two bands. To exclude the possibility that either of the bands represented calnexin, we analyzed the anticalreticulin precipitates by immunoblotting with anticalnexin. Since no calnexin was detected, we conclude that the doublet does not represent calnexin. Thus, we have not been able to demonstrate G1-G2 bound simultaneously to calnexin and calreticulin. Instead, it seems that shortly after synthesis, substantial portions of G1 and G2 associate respectively with one chaperone or the other.
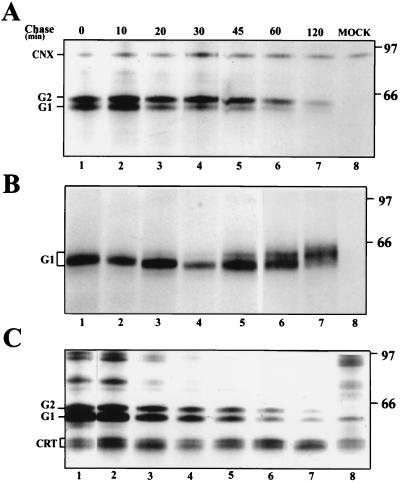
FIG. 2.
Kinetics of the association of G1 and G2 with calnexin and calreticulin. At 14 h postinfection, BHK21 cells were labeled for 10 min with [35S]methionine and chased for the indicated times. The cells were lysed in 2% CHAPS (A and B) or 1% digitonin (C) and processed for immunoprecipitation with polyclonal anticalnexin (A), anti-G1 (B), or anticalreticulin (C) antisera. The samples in panels A and B are from the same lysate. Immunoprecipitates were analyzed on an SDS–10% polyacrylamide gel under reducing conditions, followed by fluorography. The positions of calnexin (CNX), calreticulin (CRT), G1, and G2, as well as molecular weight markers (in thousands), are indicated. MOCK, immunoprecipitated proteins from uninfected cells.
We next analyzed the kinetics of G1-G2 association with the two chaperones. Virus- or mock-infected cells were labeled at 14 h after infection for 10 min followed by chase periods of up to 120 min (Fig. 2). Cells were lysed with either 2% CHAPS (Fig. 2A and B), or 1% digitonin (Fig. 2C) followed by immunoprecipitation with either anticalnexin, anti-G1, or anticalreticulin antisera (Fig. 2A, B, and C, respectively). Decreasing amounts of G1 and G2 were coprecipitated with both calnexin and calreticulin during the chase. G2 was associated for a longer time with calnexin than G1, reminiscent of the association with BiP (26). The approximate half time for association with calnexin was about 30 min for G2 and 10 to 20 min for G1. Association of G1 and G2 with calreticulin followed about the same kinetics, the half time being about 20 to 30 min for both proteins. A separate experiment, in which cells were labeled for only 3 min followed by 0-, 5-, 10-, and 30-min chases, showed that G1 and G2 were readily bound to both chaperones after a 5-min chase (data not shown).
During biosynthesis, the four N-linked glycans of G1 and G2 are trimmed in the ER and in the Golgi complex. As shown in Fig. 2B, this trimming, exemplified with G1, can be followed during a chase period as a gradual shift in mobility on an SDS gel (see also reference 26). Removal of the glucose and some of the mannose residues increases the mobility (Fig. 2, lanes 3 to 5), while terminal glycosylation retards migration (Fig. 2, lanes 5 to 7). The addition of sialic acid residues results in smearing of the G1 band (Fig. 2, lanes 6 and 7) (26). From Fig. 2A and C, it is evident that the mobility of the G1 species associated with calnexin and calreticulin remains unaltered during the chase. Only the slowly migrating, presumably monoglucosylated, form was bound to the two chaperones.
Castanospermine (CST), which inhibits the action of glucosidases I and II, has been shown to prevent the binding of calnexin and calreticulin to folding intermediates (12). When UUK virus-infected cells were pretreated with CST (1 mM) for 1 h and then pulse-labeled for 10 min followed by a 20-min chase in the presence of the drug, a distinct reduction in the mobility of G1 and G2 was observed, indicating a lack of trimming of the glucose residues. Under these conditions, binding of calnexin and calreticulin to G1-G2 was reduced by only about 30 to 50% (data not shown). Similar results have recently been observed for the hepatitis C virus (HCV) E1 and E2 glycoproteins. CST reduced binding of E1 to calnexin by only 34%, while binding to calreticulin was increased to 232% (6). The reason for the moderate reducing effect of CST treatment on binding of UUK virus G1-G2 to calnexin and calreticulin is unclear. It is possible that G1 and G2 are coprecipitated with the chaperones as part of mixed detergent micelles or larger aggregates. Alternatively, coprecipitation could be due to protein-protein interactions between the glycoproteins and the chaperones.
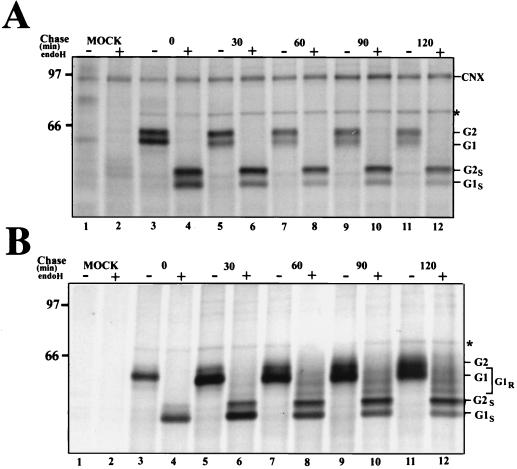
As mentioned above, a portion of G1 acquires endo H-resistant glycans, while G2 remains endo H-sensitive throughout a 120-min chase (18). We therefore performed an analysis to identify the processing forms of G1 with which calnexin was associated. Cells were pulse-labeled for 10 min as described above, followed by chase periods of up to 120 min. Samples from digitonin-solubilized lysates were first precipitated with anticalnexin antiserum (Fig. 3A). The immunocomplexes were collected, and the supernatants were reprecipitated with a mixture of a monoclonal antibody (26) and a polyclonal anti-G1 antiserum (35) (Fig. 3B). For each sample, half of the precipitate was treated with endo H and the other half was left untreated. The anticalnexin antiserum precipitated only G1 possessing endo H-sensitive glycans (Fig. 3A). In contrast, the anti-G1 antibodies precipitated increasing amounts of a set of partially endo H-resistant forms of G1 during the chase (Fig. 3B). Under the nondenaturing conditions used here, the mixture of G1 antisera also coprecipitated G2 during the chase. These results confirm that calnexin is associated with only immature forms of G1 possessing high-mannose, endo H-sensitive glycans. BiP, identified by immunoblotting with a polyclonal anti-BiP antiserum (StressGen Biotechnologies Corp., Victoria, British Columbia, Canada) (data not shown), was also coprecipitated both with the anticalnexin antiserum and the anti-G1 antibodies (Fig. 3A and B). Since BiP did not coprecipitate with the anticalnexin antiserum from the mock-infected lysate (Fig. 3A, lanes 1 and 2), this suggests that a portion of G1-G2 was associated with BiP and calnexin simultaneously.
FIG. 3.
Calnexin associates with the endo H-sensitive form of G1. Virus-infected BHK21 cells were labeled with [35S]methionine for 10 min at 14 h postinfection and chased for the indicated times. Cells were lysed with 1% digitonin and first precipitated with anticalnexin antiserum (A). Proteins remaining in the supernatants were reprecipitated with a mixture of poly- and monoclonal anti-G1 antibodies (B). Half of the samples were treated with endo H, and half were left untreated. Proteins were analyzed on an SDS–10% polyacrylamide gel under reducing conditions, followed by fluorography. The positions of calnexin (CNX), G1, G2, and BiP (asterisk), as well as molecular weight markers (in thousands), are indicated. MOCK, immunoprecipitated proteins from mock-infected cells; G1S and G2S, endo H-sensitive forms of the spike proteins G1 and G2, respectively; G1R, an endo H-resistant form of G1.
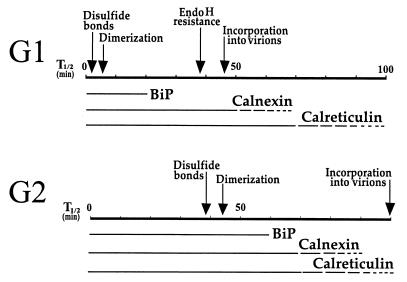
Taken together, the results reported here establish that newly synthesized G1 and G2 of UUK virus transiently associate in the ER with both calnexin and calreticulin over an extended period. A portion of G1-G2 coprecipitated with anticalnexin and anticalreticulin antisera for up to 120 min. Combined with our previous results showing transient association with BiP, and coprecipitation with PDI, as well as the kinetics of G1-G2 heterodimerization (26), our current view on the early events taking place in the ER is summarized in Fig. 4. The four folding factors BiP, PDI, calnexin, and calreticulin could associate with both monomeric and heterodimeric proteins and with larger aggregates. The fact that substantial amounts of newly synthesized G1 coprecipitate with both chaperones and yet heterodimerize rapidly with properly folded G2 (26) suggests that calnexin and calreticulin are likely to associate also with heterodimers. G2 folds slowly and apparently exists as a monomer for an extended period, implying that the chaperones are likely to interact also with monomeric G2 during the folding process. The half time for arrival of G1-G2 at the medial Golgi as assayed by acquisition of endo H-resistant glycans on G1 is about 45 min. In light of the present results, this slow transport can now be explained by the slow maturation of the G1-G2 complex and, hence, its slow acquisition of transport competence for exit from the ER. Since BiP, PDI, calnexin, and calreticulin all contain ER retention or Golgi-to-ER retrieval signals, it seems likely that the G1-G2 complexes are retained in the ER through interaction with these chaperones.
FIG. 4.
Schematic representation of the time course of the early biosynthetic events of the UUK virus spike proteins G1 and G2. The half times (T1/2) for disulfide bond formation, heterodimerization, acquisition of endo H resistance, and incorporation into virions are marked by arrows. The transient association of G1 and G2 with the ER chaperones BiP, calnexin, and calreticulin is indicated by bars.
Due to the complexity of G1 and G2 folding and heterodimerization, we have not yet been able to unambiguously resolve the question of to what extent the chaperones bind sequentially or to separate molecules (i.e., to what extent they represent alternative pathways) or to what extent overlapping binding occurs. The data indicate that BiP, calnexin, and calreticulin are associated transiently with G1-G2 with about the same kinetics. Sequential precipitation with anticalnexin and anticalreticulin antisera showed that calnexin and calreticulin may partly be associated with different pools of G1-G2. Coprecipitation of BiP with anticalnexin in virus-infected but not mock-infected cells points to the possibility that G1-G2 could associate with calnexin and BiP simultaneously in a ternary complex. Data collected with influenza virus hemagglutinin as a model suggest that a collection of chaperones may form a complex matrix to which early folding and assembly intermediates are associated prior to exit from the ER (32).
With respect to these questions, analyses of cellular and viral glycoproteins have yielded different results depending on the protein under study. In the case of the vesicular stomatitis virus G protein, BiP was found to bind to early folding intermediates, while calnexin bound after a short lag to more-folded molecules and calreticulin did not bind at all (11). In contrast, calnexin and BiP bind sequentially (in the reverse order) to folding thyroglobulin (16). Influenza virus hemagglutinin initially associates with both calnexin and calreticulin, but the latter dissociates from the complex earlier (5, 13). Calnexin binds to free human class I heavy chains but is replaced by calreticulin when the heavy chain oligomerizes with β2-microglobulin (31). Yet another situation is exemplified by human immunodeficiency virus gp160, which binds to calnexin and calreticulin with similar kinetics: most of the gp160 bound to calreticulin was also bound to calnexin, while only a portion of gp160 associated with calnexin was also bound to calreticulin (24). The rapidly folding herpesvirus 1 glycoproteins gC and gD were found to associate with calnexin for a rather short period (half times, 25 and 30 min, respectively), while the more slowly folding gB was associated for a longer period (half time, 70 min) (37). These and other examples (8, 25, 36) indicate that the time of association of glycoproteins with calnexin-calreticulin correlates with the folding kinetics.
The situation described for HCV E1 and E2 spike proteins seems quite analogous to that of UUK virus. As in the case of UUK virus G1 and G2, HCV E2 folds rapidly, while E1 folds slowly (7). Folding of HCV E1 is apparently rate limiting for heterodimerization. Both HCV proteins associate with similar kinetics to calnexin and calreticulin and somewhat more slowly to BiP. Calreticulin and BiP associate preferentially with E1-E2 aggregates, while calnexin was found to be preferentially associated with monomeric and dimeric forms of E1 and E2 (6, 7). From the data published so far, it seems justified to draw the conclusion that the relative role and importance, as well as the place in the folding sequence, of each ER folding chaperone depend largely on the protein in question. The ER chaperones are likely to have redundant or complementing activities. In the case of UUK virus G1 and G2, at least four chaperones or folding factors seem to operate in a complicated and concerted manner to form a fully folded and assembled spike protein complex competent for export to the Golgi complex.
Acknowledgments
We thank Anita Bergström for excellent technical assistance and Agneta Andersson for the calnexin antiserum.
REFERENCES
- 1.Andersson A M, Melin L, Persson R, Raschperger E, Wikström L, Pettersson R F. Processing and membrane topology of the spike proteins G1 and G2 of Uukuniemi virus. J Virol. 1997;71:218–225. doi: 10.1128/jvi.71.1.218-225.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersson A M, Melin L, Bean A, Pettersson R F. A retention signal necessary and sufficient for Golgi localization maps to the cytoplasmic tail of a Bunyaviridae (Uukuniemi virus) membrane glycoprotein. J Virol. 1997;71:4717–4727. doi: 10.1128/jvi.71.6.4717-4727.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersson A M, Pettersson R F. Targeting of a short peptide derived from the cytoplasmic tail of the G1 membrane glycoprotein of Uukuniemi virus (Bunyaviridae) to the Golgi complex. J Virol. 1998;72:9585–9596. doi: 10.1128/jvi.72.12.9585-9596.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergeron J J M, Brenner M B, Thomas D Y, Williams D B. Calnexin: a membrane-bound chaperone of the endoplasmic reticulum. Trends Biochem Sci. 1994;19:124–128. doi: 10.1016/0968-0004(94)90205-4. [DOI] [PubMed] [Google Scholar]
- 5.Chen W, Helenius J, Braakman I, Helenius A. Cotranslational folding and calnexin binding during glycoprotein synthesis. Proc Natl Acad Sci USA. 1995;92:6229–6233. doi: 10.1073/pnas.92.14.6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choukhi A, Ung S, Wychowski C, Dubuisson J. Involvement of endoplasmic reticulum chaperones in the folding of hepatitis C virus glycoproteins. J Virol. 1998;72:3851–3858. doi: 10.1128/jvi.72.5.3851-3858.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dubuisson J, Rice C M. Hepatitis C virus glycoprotein folding: disulfide bond formation and association with calnexin. J Virol. 1996;70:778–786. doi: 10.1128/jvi.70.2.778-786.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaudin Y. Folding of rabies virus glycoprotein: epitope acquisition and interaction with endoplasmic reticulum chaperones. J Virol. 1997;71:3742–3750. doi: 10.1128/jvi.71.5.3742-3750.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gething M J, Sambrook J. Protein folding in the cell. Nature (London) 1992;355:33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- 10.Hammond C, Helenius A. Folding of VSV G protein: sequential interaction with BiP and calnexin. Science. 1994;266:456–458. doi: 10.1126/science.7939687. [DOI] [PubMed] [Google Scholar]
- 11.Hammond C, Helenius A. Quality control in the secretory pathway. Curr Opin Cell Biol. 1995;7:523–529. doi: 10.1016/0955-0674(95)80009-3. [DOI] [PubMed] [Google Scholar]
- 12.Hammond C, Braakman I, Helenius A. Role of N-linked oligosaccharide recognition, glucose trimming, and calnexin in glycoprotein folding and quality control. Proc Natl Acad Sci USA. 1994;91:913–917. doi: 10.1073/pnas.91.3.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hebert D N, Foellmer B, Helenius A. Calnexin and calreticulin promote folding, delay oligomerization and suppress degradation of influenza virus hemagglutinin in microsomes. EMBO J. 1996;15:2961–2968. [PMC free article] [PubMed] [Google Scholar]
- 14.Helenius A, Marquardt T, Braakman I. The endoplasmic reticulum as a protein-folding compartment. Trends Cell Biol. 1992;2:227–231. doi: 10.1016/0962-8924(92)90309-b. [DOI] [PubMed] [Google Scholar]
- 15.Helenius A, Trombetta E S, Hebert D N, Simons J F. Calnexin, calreticulin and folding of glycoproteins. Trends Cell Biol. 1997;7:193–200. doi: 10.1016/S0962-8924(97)01032-5. [DOI] [PubMed] [Google Scholar]
- 16.Kim P S, Arvan P. Calnexin and BiP act as sequential molecular chaperones during thyroglobulin folding in the endoplasmic reticulum. J Cell Biol. 1995;128:29–38. doi: 10.1083/jcb.128.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krause K-H, Michalak M. Calreticulin. Cell. 1997;88:439–443. doi: 10.1016/s0092-8674(00)81884-x. [DOI] [PubMed] [Google Scholar]
- 18.Kuismanen E. Posttranslational processing of Uukuniemi virus glycoproteins G1 and G2. J Virol. 1984;51:806–812. doi: 10.1128/jvi.51.3.806-812.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuismanen E, Hedman K, Saraste J, Pettersson R F. Uukuniemi virus maturation: accumulation of virus particles and viral antigens in the Golgi complex. Mol Cell Biol. 1982;2:1444–1458. doi: 10.1128/mcb.2.11.1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuismanen E, Bång B, Hurme M, Pettersson R F. Uukuniemi virus maturation: immunofluorescence microscopy with monoclonal glycoprotein-specific antibodies. J Virol. 1984;51:137–146. doi: 10.1128/jvi.51.1.137-146.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuismanen E, Saraste J, Pettersson R F. Effect of monensin on the assembly of Uukuniemi virus in the Golgi complex. J Virol. 1985;55:813–822. doi: 10.1128/jvi.55.3.813-822.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maizel J V., Jr Polyacrylamide gel electrophoresis of viral proteins. Methods Virol. 1971;5:179–246. [Google Scholar]
- 23.Melin L, Persson R, Andersson A, Bergström A, Rönnholm R, Pettersson R F. The membrane glycoprotein G1 of Uukuniemi virus contains a signal for localization to the Golgi complex. Virus Res. 1995;36:49–66. doi: 10.1016/0168-1702(95)00006-C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Otteken A, Moss B. Calreticulin interacts with newly synthesized human immunodeficiency virus type 1 envelope glycoprotein, suggesting a chaperone function similar to that of calnexin. J Biol Chem. 1996;271:97–103. doi: 10.1074/jbc.271.1.97. [DOI] [PubMed] [Google Scholar]
- 25.Ou W-J, Cameron P H, Thomas D Y, Bergeron J J M. Association of folding intermediates of glycoproteins with calnexin during protein maturation. Nature (London) 1993;364:771–776. doi: 10.1038/364771a0. [DOI] [PubMed] [Google Scholar]
- 26.Persson R, Pettersson R F. Formation and intracellular transport of a heterodimeric viral spike protein complex. J Cell Biol. 1991;112:257–266. doi: 10.1083/jcb.112.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pettersson R F. Protein localization and viral assembly at intracellular membranes. Curr Top Microbiol Immunol. 1991;170:67–104. doi: 10.1007/978-3-642-76389-2_3. [DOI] [PubMed] [Google Scholar]
- 28.Pettersson R F, Melin L. Synthesis, assembly, and intracellular transport of Bunyaviridae membrane proteins. In: Elliott R M, editor. The Bunyaviridae. New York, N.Y: Plenum Press; 1996. pp. 159–188. [Google Scholar]
- 29.Rönnholm R. Localization to the Golgi complex of Uukuniemi virus glycoproteins G1 and G2 expressed from cloned cDNAs. J Virol. 1992;66:4525–4531. doi: 10.1128/jvi.66.7.4525-4531.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rönnholm R, Pettersson R F. Complete nucleotide sequence of the M RNA segment of Uukuniemi virus encoding the membrane glycoproteins G1 and G2. Virology. 1987;160:191–202. doi: 10.1016/0042-6822(87)90060-2. [DOI] [PubMed] [Google Scholar]
- 31.Sadasivan B, Lehner P J, Ortmann B, Spies T, Cresswell P. Roles for calreticulin and a novel glycoprotein, tapasin, in the interaction of MHC class I molecules with TAP. Immunity. 1996;5:103–114. doi: 10.1016/s1074-7613(00)80487-2. [DOI] [PubMed] [Google Scholar]
- 32.Tatu U, Helenius A. Interactions between newly synthesized glycoproteins, calnexin and a network of resident chaperones in the endoplasmic reticulum. J Cell Biol. 1997;136:555–565. doi: 10.1083/jcb.136.3.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ulmanen I, Seppälä P, Pettersson R F. In vitro translation of Uukuniemi virus-specific RNAs: identification of a nonstructural protein and a precursor to the membrane glycoproteins. J Virol. 1981;37:72–79. doi: 10.1128/jvi.37.1.72-79.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wada I, Rindress D, Cameron P H, Ou W-J, Doherty II J J, Louvard D, Bell A W, Dignard D, Thomas D Y, Bergeron J J M. SSRα and associated calnexin are major calcium binding proteins of the endoplasmic reticulum. J Biol Chem. 1991;266:19599–19610. [PubMed] [Google Scholar]
- 35.Wikström L, Persson R, Pettersson R F. Intracellular transport of the G1 and G2 membrane glycoproteins of Uukuniemi virus. In: Kolakofsky D, Mahy B W J, editors. Genetics and pathogenicity of negative-strand viruses. New York, N.Y: Elsevier; 1989. pp. 33–41. [Google Scholar]
- 36.Yamashita Y, Shimokata K, Mizuno S, Daikoku T, Tsurumi T, Nishiyama Y. Calnexin acts as a molecular chaperone during the folding of glycoprotein B of human cytomegalovirus. J Virol. 1996;70:2237–2246. doi: 10.1128/jvi.70.4.2237-2246.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamashita Y, Yamada M, Daikoku T, Yamada H, Tadauchi A, Tsurumi T, Nishiyama Y. Calnexin associates with the precursors of glycoprotein B, C, and D of herpes simplex virus type 1. Virology. 1996;225:216–222. doi: 10.1006/viro.1996.0590. [DOI] [PubMed] [Google Scholar]