Abstract
Objective
To evaluate the efficacy and safety of faricimab compared with other anti-vascular endothelial growth factor (anti-VEGF) agents in treating neovascular age-related macular degeneration (nAMD) patients.
Methods and analysis
A systematic review (SR) was conducted up to January 2023. Network meta-analyses (NMA) were performed, including sensitivity and subgroup analyses for naïve population. Outcomes included changes in visual acuity (Early Treatment of Diabetic Retinopathy Study [ETDRS] letters), anatomical changes, frequency of injections and adverse events. The Cochrane Collaboration guidelines and the Confidence in Network Meta-Analysis framework were used for the SR and the certainty of evidence, respectively.
Results
From 4128 identified records through electronic databases and complementary searches, 63 randomised controlled trials (RCTs) met the eligibility criteria, with 42 included in the NMA. Faricimab showed a significant reduction in the number of annual injections compared with most fixed and flexible anti-VEGF treatment regimens, while showing no statistically significant differences in visual acuity through ETDRS letter gain, demonstrating a comparable efficacy. Retinal thickness results showed comparable efficacy to other anti-VEGF agents, and inferior only to brolucizumab. Results also showed that more patients treated with faricimab were free from post-treatment retinal fluid compared with aflibercept every 8 weeks, and both ranibizumab and bevacizumab, in the fixed and pro re nata (PRN) assessed schedules. Faricimab showed a comparable safety profile regarding the risk of ocular adverse events and serious ocular adverse events (SOAE), except for the comparison with brolucizumab quarterly, in which faricimab showed a significant reduction for SOAE risk.
Conclusion
Faricimab showed a comparable clinical benefit in efficacy and safety outcomes, with a reduction in annual injections compared with fixed and flexible anti-VEGF drug regimens, representing a valuable treatment option for nAMD patients.
PROSPERO registration number
CRD42023394226.
Keywords: Neovascularisation, Retina, Macula, Treatment Medical
WHAT IS ALREADY KNOWN ON THIS TOPIC
There are different alternatives for treating neovascular age-related macular degeneration (nAMD) with anti-vascular endothelial growth factor (VEGF) therapy. Faricimab offers a double-bind VEGF-A and angiopoietin-2 (Ang-2), suggesting an advantage in the control of the condition. Comparison of efficacy, safety and injection frequency between the alternatives is insufficient.
WHAT THIS STUDY ADDS
Faricimab showed no statistically significant differences in visual acuity improvement, while reducing the number of injections per year, mainly compared with flexible schedules or fixed regimens with a longer injection interval. Faricimab is a safe alternative for the treatment of nAMD by offering an adequate safety profile compared with other anti-VEGF agents.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Considering the chronic nature of nAMD, the long-term extent of treatment poses a significant burden on patients, their families and the healthcare system, highlighting the importance of treatments, such as faricimab, that are effective and safe but simultaneously decrease the frequency of annual intravitreal injections.
Introduction
Age-related macular degeneration (AMD) stands as a prominent contributor to severe visual impairment and blindness, accounting for 8.7% of global blindness cases and significantly affecting patients’ well-being.1 The neovascular subtype of AMD (nAMD) has been associated with worse vision outcomes.2
Clinical trials showcasing the efficacy of vascular endothelial growth factor (anti-VEGF) inhibitors for AMD date back to 2006, transforming disease management and resulting in up to a 50% reduction in AMD-related blindness.3
The treatment landscape offers diverse options of anti-VEGF agents and the treatment regimen, which may be either fixed (at intervals of 4, 8 or 12 weeks) or flexible. Flexible regimens include PRN (pro re nata or ‘as needed’) regimens, in which patients are seen monthly and only injected according to clinical findings suggestive of disease recurrence, and the treat-and-extend regimens (T&E), in which the intervention is given at each visit, but the periodicity of the visit is adjusted according to disease activity by extending monitoring visits by 2-week intervals, up to a maximum of 10–12 weeks between doses, with the possibility of reducing them if necessary.4 Treatment also frequently includes a prior loading dose (LD) of 1 monthly injection for 3 to 4 months.4
Due to the development of different anti-VEGF agents (aflibercept (Regeneron/Bayer), ranibizumab (Genentech/Novartis), brolucizumab (Novartis) and off-label bevacizumab (Genentech/Roche)),5 along with the variability in treatment regimens, generating evidence of comparative efficacy and safety is highly valuable for decision-making and clinical practice.
Faricimab (Genentech/Roche) is a targeted antibody that binds with high affinity to both VEGF-A and angiopoietin-2 (Ang-2).6 The latter increases in some pathologies (inflammation, ischemia/hypoxia or hyperglycaemia), generating a disruptive effect on vascular stability, activating neoangiogenic and proinflammatory processes and increasing the response to VEGF; therefore, it has been suggested that dual inhibition is an advantage in controlling neovascular macular degeneration.6 7
With a lack of randomised clinical trials (RCTs) concurrently evaluating all treatment options and regimens, a comprehensive analysis incorporating direct and indirect comparisons provides valuable insights for assessing treatment evidence for nAMD. Previous network meta-analyses (NMA) have been conducted for anti-VEGF agents in nAMD. An NMA performed by NICE evaluated fixed and flexible schedules of bevacizumab, ranibizumab and aflibercept; however, the analysis conducted by NICE did not include faricimab or brolucizumab, nor aflibercept T&E due to a lack of evidence at that time.8
Subsequently, Finger et al conducted an NMA that included approved anti-VEGF agents for nAMD and off-label bevacizumab.9 While findings indicated no statistically significant difference in mean change in best-corrected visual acuity (BCVA) between brolucizumab and other anti-VEGF agents, the study did not include an adjusted comparison for injection frequency. In addition, it does not appear to have identified all the available evidence, given that a preliminary search identified that more studies could be included.10,16
This study seeks to conduct a comprehensive evaluation of the efficacy and safety of faricimab in the treatment of nAMD in comparison to other anti-VEGF agents. The assessment will encompass outcomes related to intravitreal injection frequency and integrate evidence up to 2023, enriching the current knowledge base.
Materials and methods
A systematic review (SR) and NMA were conducted according to the guidelines of the Cochrane Handbook for Systematic Reviews of Interventions.17 The protocol for the study was published in PROSPERO.
A systematic search was conducted up to January 2023 in Medline, Embase, Cochrane Central Register of Controlled Trials (CENTRAL) and LILACS. Complementary searches included ClinicalTrials.gov, academic congress, HTA databases and reviewing the lists of bibliographic references of the selected studies and similar SR previously published. Search strategies are presented in online supplemental table S1. Table 1 presents inclusion and exclusion criteria.
Table 1. . Inclusion and exclusion criteria for the systematic review.
| Inclusion criteria |
|
| Exclusion criteria |
|
Note: In studies with more than two arms, where the evaluated arms included anti-VEGF combined therapies or non-anti-VEGF treatments, including PDT and intravitreal steroids, only the anti-VEGF arms monotherapies were included; the additional arms were excluded of the analysis, in accordance with the NICE technical support document.88
LDloading dosenAMDage-related macular degenerationNICENational Institute for Health and Care ExcellenceRCTrandomised controlled trialVEGFvascular endothelial growth factor
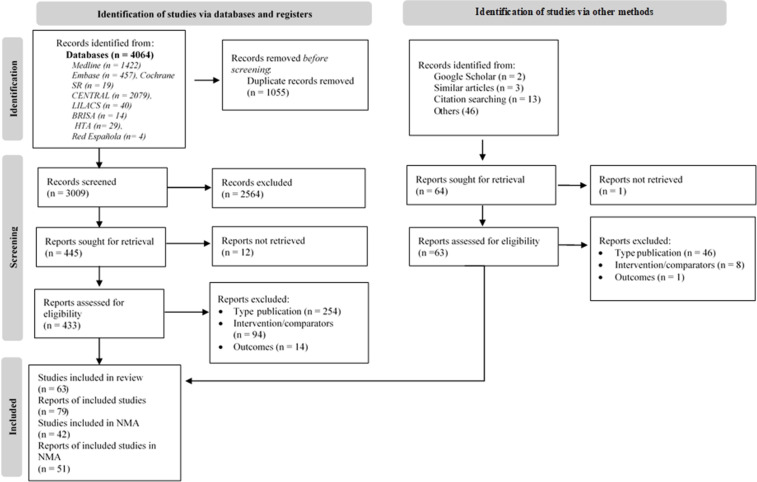
Five reviewers independently screened titles and abstracts (DS-S, CH, NG, LP-P, JK), with two reviewers (CH/NG) assessing full-text publications, resolving differences by consensus. The selection and screening was reported using the PRISMA-2020 flow diagram.18
A data extraction form was designed to record the included studies’ effect size estimates. Two reviewers (NG/CH) performed the data extraction, with quality control by a third reviewer (DS-S).
One reviewer (NG) performed the risk of bias assessment, with quality control by a second reviewer (CH), using the Cochrane Risk of Bias (RoB) tool19; disagreements were solved by consensus. The assessment was conducted for each study individually, based on the original publication and the protocol and online supplemental material, when available. The RoB tool provides a framework for evaluating the risk of bias in the findings of an RCT. The assessment is structured into five domains through which bias might be introduced into the results, including biases arising from the randomisation process, bias due to deviations from intended interventions, missing outcome data, outcome measurement and the selection of the reported results.20
Due to the absence of direct (head-to-head) comparisons between all treatments and their possible regimens (fixed, PRN, T&E), an NMA was conducted. An NMA is a statistical analysis that allows for the combination of direct evidence obtained from RCTs (faricimab vs aflibercept fixed schedule in the LUCERNE/TENAYA trial, or aflibercept fixed schedule vs aflibercept T&E in ARIES trial), and the obtaining of relative estimates of comparisons that have not yet been evaluated directly (faricimab vs aflibercept T&E (indirect estimation)). This indirect estimation is performed using common comparators in the available RCTs, that is, aflibercept fixed schedule, as the common treatment in LUCERNE/TENAYA and ARIES trial. This allows combining direct and indirect evidence in a mixed evidence analysis.
The certainty of the evidence was assessed using the Confidence in Network Meta-Analysis (CINeMA) approach, a specific framework to evaluate confidence in NMA results based on the Grading of Recommendations Assessment, Development and Evaluation (GRADE) framework.20 21 The CINeMA approach is recommended by Cochrane, especially when a large number of interventions are involved in the network.22 This approach has previously been used in NMA to evaluate treatments for retinal diseases.23
The CINeMA framework allows each evaluated intervention’s estimated effect to be expressed as a weighted sum of all available direct and indirect comparisons, assigning a weight determined by a contribution matrix.21 This approach grades the estimates of each outcome based on six domains: study bias, reporting bias, indirect evidence, imprecision, heterogeneity and inconsistency. For each domain, a judgement was assigned at three levels (no concerns, some concerns or major concerns). The cross-domain judgements are subsequently summarised at the four confidence levels, corresponding to the usual GRADE assessments: very low, low, moderate or high, which indicates how much confidence exists that the estimated effects represent the interventions’ true effects.21
Outcome measures included change in BCVA, gain or loss of 10 and 15 letters in the ETDRS system, change in central retinal thickness (CRT), absence of retinal fluid, the average number of intravitreal injections and safety outcomes. Efficacy and safety outcomes were evaluated at 1 year of treatment, including reports from week 48–56.
Serious and non-serious ocular adverse events (OAE) included the events reported in the original RCT publications. OAE included allergic keratitis, blepharitis, nAMD in the fellow eye, posterior capsule opacification, vitreous floaters, eye pain, dry eye, eye irritation and increased intraocular pressure, among others. Serious OAE (SOAE) frequently reported by the RCTs includes retinal detachment, retinal artery occlusion, retinal depigmentation, vitreous inflammation, macular hole, among others.
To conduct the analysis, the treatments were named based on each study’s description of the injection application regimens. Fixed treatments include injections every 4, 8 or 12 weeks, coded as q4w, q8w or q12w, respectively. Flexible treatments include the PRN and T&E schedules. Finally, the personalised treatment interval of faricimab includes the possibility of extending injections up to 16 weeks; hence, it was coded as faricimab up to q16w. Likewise, a distinction was made between regimens with or without LD.
Statistical analysis
The NMA was conducted using a frequentist approach, employing a random-effects model to account for population variability across different settings and disease variance, including possible differences in the clinical manifestations of the disease subtypes. The NMA bases its validity on a set of assumptions: transitivity, homogeneity (heterogeneity) and consistency.
Prior to performing the analysis, the transitivity assumption was evaluated by assessing the clinical and methodological characteristics of the studies by comparing the similarity of the distributions in the effect-modifying variables, including age, sex, visual acuity, retinal thickness and choroidal neovascularisation.
The consistency assumption reflects the agreement between the estimations obtained by direct evidence and by the simulated indirect estimates. The consistency was assessed using the Separating Indirect from Direct Evidence,24 a method based on the node-splitting approach, which compares the result obtained by direct, indirect evidence and its combination (mixed evidence).
Heterogeneity inputs, that is, the variability among the findings of the RCTs due to the inherent differences in each study, and inconsistency concerns were integrated into the confidence assessment of the CINeMA framework.21
Three statistical analyses were performed:
Base-case analysis, using the clinical evidence with complete data reporting.
Sensitivity analysis, including the base case studies and studies with imputed data or information from clinical trial registries.
Subgroup analysis: including studies with complete reporting conducted on patients without prior anti-VEGF treatment (treatment-naïve). Subgroup analysis of patients previously treated was not possible due to the small number of studies.
Only doses approved by regulatory agencies were included in the analysis (faricimab 6 mg, ranibizumab 0.5 mg, aflibercept 2 mg, brolucizumab 6 mg).25,29 Bevacizumab 1.25 mg was included (considered a relevant comparator based on the clinical expert’s opinion). Analyses and results were reported using faricimab up to q16w, with LD, as the reference.
Calculation of comparative effect sizes followed guidelines by Higgins et al17 and Harrer et al.24 The effect size estimators were inputted to the statistical program R V.4.1.2 (R-Studio interface). The ‘netmeta’ V.5.2–0 and the ‘meta’ package V.4.15.1 were used.24 30
A mean difference (MD) approach was used to estimate the treatment effect for continuous outcomes (BCVA, CRT, injection frequency). We prioritised the reporting of between-group differences (intervention A vs intervention B). For studies that did not report the between-group difference, we used the reported within-group difference of each treatment arm, that is, results of the pre-treatment and post-treatment evaluation (baseline vs follow-up); then we calculated the between-group differences (intervention A vs B). If neither were reported, the complete calculation was done using the baseline values and the follow-up assessments.
For dichotomous outcomes (patients gaining 10 or 15 letters or more, retinal fluid-free patients and adverse events), the relative risk of experiencing an event was estimated by comparing the proportion of patients with the event, for example, serious adverse events, between both treatment groups for each study.
Once the treatment effect between the interventions of each study (mean difference, relative risk) has been estimated, the NMA forms a network by combining the studies through the common treatments and using a weighted average analysis, which adjusts for the variability of the estimations, allowing to obtain the comparative results of all treatments included in the network.
SD imputation occurred only in cases where the within-group mean difference did not include a measure of dispersion, using the average of the SD of the within-group mean differences of the included studies for the same outcome. Studies with imputed data were only included in the sensitivity analyses.
Results
Description of studies
A total of 42 RCTs were included in the NMA10,1631 (figure 1). Fifty-nine per cent of the studies were conducted in the anti-VEGF treatment naïve population, while 38% included both naïve and previously treated patients with anti-VEGF agents, and only one study included only previously treated patients. Seventy-one per cent of the studies were multicentre, and 26% were multicounty. The characteristics of the included studies are detailed in online supplemental table S2.
Figure 1. Flow diagram (PRISMA 2020). NMA, network meta-analyses; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Online supplemental table S3 provides the list of included and excluded references and the reason for exclusion. Sources of information for the NMA by outcome are outlined in online supplemental table S4. Additional network geometries and forest plots are available in online supplemental tables S5–S9.
Quality and certainty of the evidence
Thirteen of the included studies were classified with a low risk of bias due to their rigorous methodology in randomisation, measurement and reporting of results. Thirty studies were classified as having an unclear risk of bias since the authors did not report sufficient information to assess the risk of bias, mainly to selection bias (generation of the random sequence) and performance bias (blinding of investigators and assessors).
The remaining 21 studies presented a high risk of bias, mainly due to the use of inadequate methods for generating the random sequence and imbalances in the losses during follow-up between the treatment arms, which does not rule out that these losses are related to the interventions.
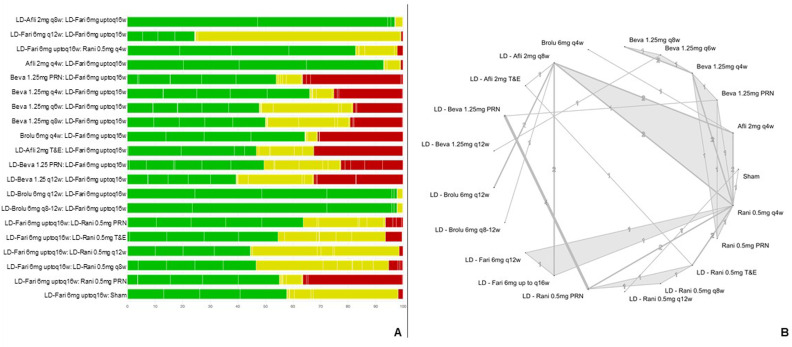
The risk of bias contribution matrix for the comparison of anti-VEGF agents for BCVA is presented in figure 2. CINeMA summary of findings is presented in online supplemental table S10–S14. Comparisons between faricimab versus aflibercept and brolucizumab had moderate to high quality. This means there is a high or moderate confidence that the true comparative effect of the interventions is close to the estimated effect in the analysis. Comparisons against ranibizumab and bevacizumab presented higher variability, with results mainly showing moderate certainty of the evidence, but for some outcomes, the quality of evidence was high or low (online supplemental table S10–S14).
Figure 2. Risk of bias contributions and network geometry for the comparison of anti-VEGF agents for age-related macular degeneration neovascular (BCVA). (A) Risk of bias contributions for the comparison for BCVA. (B) Network geometry for mean change in BCVA. Afli, aflibercept; Beva, bevacizumab; Brolu, brolucizumab; Fari, faricimab; LD, loading dose; PRN, pro re nata; Rani, ranibizumab; T&E treat and extend; VEGF, vascular endothelial growth factor; q4w, q8w, q12w, q16w, injections every 4, 8, 12 and 16 weeks, respectively.
Network meta-analyses
Network geometry for BCVA is illustrated in figure 2.
Efficacy
Visual acuity outcomes
The base case analysis for the mean change in BCVA included 28 RCTs,11,1431 encompassed 20 treatment regimens, and 11 568 patients. Comparative efficacy between faricimab with other anti-VEGF agents is reported in figure 3. The analysis found no statistically significant differences between faricimab and other anti-VEGF treatments.
Figure 3. Forest plot for efficacy outcomes at 1 year comparing faricimab 6 mg up to q16w with other anti-VEGF agents. (A) Forest plot for mean change in BCVA for faricimab 6 mg up to q16w compared with other anti-VEGF agents. (B) Forest plot for retinal thickness for faricimab 6 mg up to q16w compared with other anti-VEGF agents. (C) Forest plot for the number of injections received by each treatment regimen comparing faricimab 6 mg up to q16w to other anti-VEGF agents. (D) Forest plot for the sensibility analysis of the number of injections received by each treatment regimen comparing faricimab 6 mg up to q16w to other anti-VEGF agents. Afli, aflibercept; Beva, bevacizumab; Brolu, brolucizumab; Fari, faricimab; LD, loading dose; PRN, pro re nata; PRNX, pro re nata with possibility of interval extension; Rani, ranibizumab; T&E, treat and extend; VEGF, vascular endothelial growth factor; q4w, q8w, q12w, q16w, injections every 4, 8, 12 and 16 weeks, respectively.
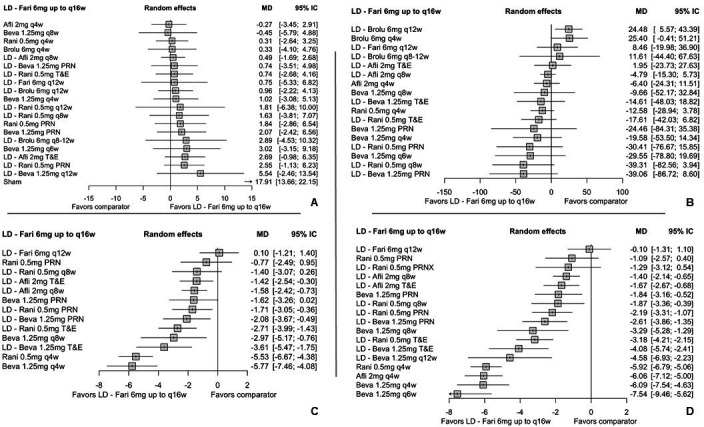
The absence of statistically significant differences was maintained even compared with fixed schedules with longer intervals between injections, such as brolucizumab 6 mg every 8–12 weeks (MD: 2.89; 95% CI −4.53 to 10.32) or ranibizumab 0.5 mg every 12 weeks (MD: 1.81; 95% CI −6.38 to 10.00) and some flexible regimens, as ranibizumab 0.5 mg PRN with LD (MD: 2.55; 95% CI −1.13 to 6.23) or bevacizumab 1.25 mg PRN without LD (MD: 2.07; 95% CI −2.42 to 6.56).
Compared with the T&E regimens, evidence was only available for comparisons against ranibizumab 0.5 mg, with LD, and aflibercept 2 mg with LD, which also found no statistically significant differences.
Results from the sensitivity analysis showed consistency with the primary analysis. The sensitivity analysis included 37 studies with 12 853 patients (online supplemental table S5). Overall, the magnitude and direction of the effect for the comparisons were maintained (online supplemental table S5). Similarly, the results of the treatment-naïve subgroup were consistent regarding the absence of differences and the direction of the effect when comparing faricimab versus fixed and flexible anti-VEGF regimens (online supplemental table S5).
The proportion of patients with a gain of 10 letters in the ETDRS chart was not statistically different between treatments. For the proportion of patients gaining at least 15 letters, the results showed no differences, except for the comparison against ranibizumab 0.5 mg every 12 weeks, with an LD, where faricimab 6 mg showed a significant effect in increasing the probability of achieving a gain of 15 letters or more (RR: 5.20: 95% CI 1.50 to 18.01) (online supplemental table S5).
The certainty of the evidence supporting these findings was mostly low and moderate.
Anatomical outcomes
The results of the CRT analysis are presented in figure 3. The analysis included 25 studies with 18 treatment regimens and 8730 patients (online supplemental table S4). The analysis showed no statistical effect for most comparisons, except when compared with the fixed 12-week dosing regimen of brolucizumab 6 mg. However, the extended dosing regimen of brolicuzumab 6 mg (8 to 12 weeks) showed no significant difference in the main analysis. Sensitivity analysis for CRT was consistent for most of the comparisons; nevertheless, when compared against aflibercept 2 mg PRN, ranibizumab 0.5 mg fixed and flexible, and bevacizumab 1.25 mg PRN, the results showed statistical differences where faricimab 6 mg produced a greater reduction in CRT (online supplemental table S6).
The analysis for the absence of fluid in the retina included 10 studies with 7060 patients (online supplemental table S7).1136,40 51 52 Results showed a significant effect favouring faricimab 6 mg when compared with fixed and flexible PRN schedules, such as aflibercept 2 mg every 8 weeks, ranibizumab 0.5 mg and bevacizumab 1.25 mg, with or without an LD.
The certainty of the evidence supporting these findings was primarily moderate.
Frequency of injections
The NMA of the frequency of injections included 17 studies, 13 treatment regimens and 5948 patients.13,1531 The base case and the sensitivity analysis (9050 patients) are presented in figure 3. Both analyses were similar in their findings; still, the sensitivity analysis had a larger number of comparisons. The results showed that, in 14 of 17 comparisons, faricimab showed a significant reduction in the number of injections per year, with reductions ranging from more than one (compared with aflibercept 2 mg every 8 weeks and T&E), to reductions between 2 and 4 injections against PRN and T&E regimens of both ranibizumab 0.5 mg and bevacizumab 1.25 mg.
No results on the average number of injections were identified in the original publications of the brolucizumab studies40 61; therefore, it was not included in the analysis.
Results were consistent when evaluating the treatment-naïve population (online supplemental table S8).
The certainty of the evidence supporting these findings was mostly moderate.
Safety
Ocular adverse events
The NMA for OAE included 12 studies,3134 38,40 50 nine treatment regimens and 6265 patients (figure 4).
Figure 4. Network geometry and forest plot for ocular adverse events and serious ocular adverse events comparing faricimab 6 mg up to q16w with other anti-VEGF agents. (A) Network geometry for ocular adverse events. (B) Forest plot for ocular adverse events comparing faricimab 6 mg up to q16w to other anti-VEGF agents. (C) Network geometry for serious ocular adverse events. (D) Forest plot for serious ocular adverse events comparing faricimab 6 mg up to q16w to other anti-VEGF agents. Afli, aflibercept; Beva, bevacizumab; Fari, faricimab; LD, loading dose; PRN, pro re nata; PRNX, pro re nata with possibility of interval extension; Rani, ranibizumab; T&E, treat and extend; VEGF, vascular endothelial growth factor; q4w, q8w, q12w, q16w, injections every 4, 8, 12 and 16 weeks, respectively.
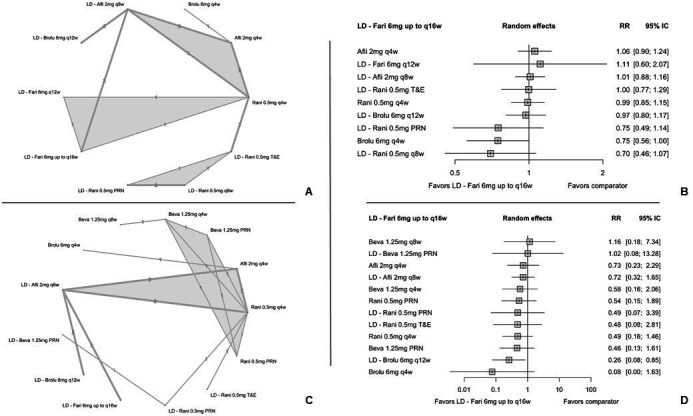
Faricimab 6 mg showed no statistically significant differences in the risk of OAE when compared with monthly treatment regimens, including aflibercept 2 mg, ranibizumab 0.5mg and brolucizumab 6 mg, bimonthly regimens as ranibizumab 0.5 mg and aflibercept 2 mg, and flexible regimens, such as ranibizumab 0.5 mg PRN and T&E.
The certainty of the evidence supporting these findings was mostly moderate.
Serious ocular adverse events
The NMA for OSAE included 12 studies,1431 32 36,40 51 52 12 treatment regimens and 8047 patients (figure 4). Faricimab 6 mg was associated with a 74% reduction of serious OAE risk compared with brolucizumab 6 mg every 12 weeks with an LD (RR: 0.26; CI 95% 0.08 to 0.85). Compared with other anti-VEGF agents, faricimab 6 mg showed an absence of statistically significant differences in the risk of serious OAE.
The certainty of the evidence supporting these findings was mostly moderate.
Discussion
This SR and network meta-analysis represent a comprehensive evaluation of nAMD therapies, incorporating data from 63 RCTs and pooling information from up to 42 studies. With a focus on efficacy, safety and intravitreal injection frequency outcomes, and with analyses including up to 12 853 patients, this study provides an extensive and inclusive analysis of nAMD therapies to date.
In terms of the certainty of the evidence, the majority of comparisons presented a moderate level of certainty, meaning that the results of our analysis are likely to be close to the interventions’ true effects. The robustness of the findings was reinforced by consistent results across base case, sensitivity and subgroup analyses, especially in the treatment-naïve population.
The evaluation of the BCVA identified that the personalised treatment interval of faricimab is comparable to other anti-VEGF agents, including fixed or flexible treatment schedules used in clinical practice.
These findings align with previous research by Finger et al9 and a Bayesian NMA conducted by Ye et al, that included 29 RCTs, which found a similar effect for most comparisons, specifically for the gain of 15 or more letters.75 Although Ye et al did not include faricimab, it showed a more significant effect for the T&E regimen over other PRN. This could indicate the clinical relevance of opting for schedules that enhance visual acuity with fewer treatment frequency.
In addition to building on prior research, our review included an increased number of studies and recent data on therapies approved for clinical use in nAMD patients.76,79 The analysis covered various treatment strategies, including LDs, and specifically explored data for the treatment-naïve population, providing clinicians with comprehensive evidence to guide nAMD management.
Our analyses for anatomic outcomes showed favourable results for faricimab compared with other treatments, including an increased probability of retinal fluid-free post-treatment versus flexible (PRN) or fixed (monthly) treatment schedules at 1 year of follow-up. Similarly, sensitivity analysis for CRT showed a favourable significant effect of faricimab in comparison with aflibercept PRN, fixed and flexible ranibizumab schedules, and bevacizumab PRN.
Notably, while Finger et al reported a reduction in CRT favouring brolucizumab versus faricimab,9 our main analysis found these results only in fixed brolucizumab regimens and not in flexible 8–12-week brolucizumab schedules. Our sensitivity analysis showed that brolucizumab had a greater CRT reduction than faricimab, demonstrating consistency with the Finger et al findings. However, our sensitivity analysis showed a higher CRT reduction with faricimab compared with other anti-VEGF agents in flexible regimes such as aflibercept PRN, ranibizumab PRN and bevecizumab PRN. CRT has been considered a surrogate outcome for macular diseases with a low correlation score on the variance of visual acuity results,80 meaning its reduction does not necessarily correlate with visual gains; however, CRT reduction should be considered in clinical practice due to its importance in assessing the control of disease activity.81
Patients with nAMD undergo regular intravitreal injections as part of their treatment regimen, ranging from monthly to longer intervals. Given the chronic nature of nAMD, this prolonged treatment duration poses an increased burden on patients, their families and healthcare providers.82
A patient-reported outcome study revealed that over half of nAMD, patients receive between 5 and 12 injections annually, necessitating substantial time between 1 and 15 hours and support from a caregiver, causing discomfort, anxiety and stress.83 Therefore, prioritising treatments that provide clinical benefits while reducing the frequency of intravitreal injections is essential for patients with nAMD.
This review is the first to conduct a mixed comparison analysis for intravitreal injection frequency. Results showed that the personalised treatment interval of faricimab requires a frequency of 6.3 injections per year and consistently reported a significant reduction in injection frequency against aflibercept, ranibizumab and bevacizumab by up to four fewer injections per year, in both flexible PRN and T&E schedules. No comparison was made with brolucizumab due to lack of data on number of brolucizumab injections in the clinical trials.
Regarding safety outcomes, faricimab showed an adequate safety profile, a similar risk in ocular events. On the other hand, a statistically significant reduction in SOAEs favoured faricimab compared with brolucizumab 6 mg every 12 weeks, suggesting a better safety profile for faricimab among anti-VEGF agents.
Real-world studies of faricimab have shown consistent results with its pivotal clinical trials. The FARWIDE-AMD and the FARETINA-AMD studies found that visual acuity remained stable after three injections in previously treated patients with anti-VEGF and showed improvement in naïve patients.84 85 This is also consistent with the reported improvement after three injections of clinical outcomes in the TRUKEE study, where the improvements were mainly in the previously untreated patients, with gains of 8.1 letters in BCVA and reductions in the retinal thickness of −80.1 µm.86 Currently, real-world studies support our results on maintaining functional visual acuity outcomes and improving anatomical outcomes with the use of faricimab. It is important to continue evaluating the long-term effects of faricimab, which will complement the evidence for the use of this new therapeutic alternative for treating nAMD.87
Our study provides a comprehensive analysis of a large number of studies, providing robust evidence for evaluating anti-VEGF treatments with different regimens. The inclusion of over 12 000 patients for visual acuity outcomes and 9000 for injection frequency analyses adds to the study’s strength. Sensitivity analyses were performed to ensure result robustness by considering the quality of the included studies and deriving valuable subgroup insights. Furthermore, this study unveils previously unexplored evidence, including injection load-adjusted comparative analyses.
An additional strength of this study lies in the meticulous assessment of evidence certainty. While conventional meta-analyses typically incorporate a risk of bias assessment, it is important to consider other crucial domains such as heterogeneity, imprecision, indirect evidence and inconsistency within the network21; these aspects seem to have been overlooked in previously published analyses of nAMD. The complexity of evaluating the certainty of evidence becomes apparent, particularly in network analyses involving up to 20 comparisons. To address this challenge and adhere to recommended practices by Cochrane22 and other NMA publications in ophthalmology,23 the study employed the CINeMA software. This decision facilitated a more objective approach in establishing the certainty of the evidence.
The results of this study should be interpreted within the limitations inherent to an indirect comparison analysis. The estimators obtained are subjected to transitivity and consistency assumptions, and while efforts were made to assess transitivity and that the studies were sufficiently similar to be included in a network, inherent variability in population, treatment protocols or methodology, including eligibility criteria, could impact the consistency assumption. These possible limitations were addressed by using the CINeMA framework, providing an objective assessment of evidence certainty for each comparison in the analysis, adjusting for study variability and network consistency.
Reporting data limitations from included studies, such as missing measures of dispersion or measurement scale discrepancies, limited our possibilities for aggregated analysis. These issues were addressed through sensitivity analyses, further enhancing the study’s methodological rigour and strengthening the certainty of our results.
Conclusion
Faricimab showed comparable efficacy to most anti-VEGF agents for best-corrected functional outcomes, including non-significant differences in BCVA and the proportion of patients gaining 10 and 15 or more letters in the ETDRS system. However, faricimab significantly increased the probability of achieving a gain of 15 letters or more compared with ranibizumab quarterly.
Anatomical outcomes support faricimab’s efficacy, showing a higher likelihood of achieving a fluid-free retina compared with frequent injections or flexible schedules. The reductions in CRT also demonstrated faricimab’s comparable efficacy against other anti-VEGF agents, except for brolucizumab, which showed a higher reduction of CRT. However, the sensitivity analysis showed a higher CRT reduction with faricimab compared with PRN regimens of aflibercept, ranibizumab and bevacizumab.
Faricimab significantly reduces the number of annual injections compared with aflibercept, ranibizumab and bevacizumab, even against PRN or T&E regimens; no comparison was made with brolucizumab due to lack of report on the number injections. This suggests faricimab’s potential to alleviate treatment-related challenges potentially reducing injection frequency for nAMD patients.
In terms of safety, faricimab exhibits a similar profile to other anti-VEGF agents based on the absence of statistical differences in the risk of ocular adverse events. Particularly, faricimab showed a lower risk of SOAEs compared with quarterly brolucizumab.
supplementary material
Acknowledgements
Ana Parra and Nicole Gil (IQVIA consultant and analyst, respectively) provided technical and administrative support for the article's publication.
Footnotes
Funding: This study was funded by Roche, Colombia.
Provenance and peer review: Not commissioned; externally peer-reviewed.
Patient consent for publication: Not applicable.
Ethics approval: Not applicable.
Contributor Information
Daniel Samacá-Samacá, Email: daniel.samaca@roche.com.
Claudia Hernández-Castillo, Email: claudia.hernandez2@iqvia.com.
Laura Prieto-Pinto, Email: laura_catalina.prieto_pinto@roche.com.
Francisco Rodríguez, Email: fjrodriguez@fon.org.co.
Carolina Sardi, Email: csardi@inio.com.co.
Hugo Ocampo, Email: hhocampo@gmail.com.
Joshua Kock, Email: joshua.kock_sierra@roche.com.
Fabián Hernández, Email: faahernandezta@unal.edu.co.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
References
- 1.World Health Organization (WHO) Vision 2020 the right to sight. Global iniciative for the elimination of avoidable blindness. action plan 2006-2011. 2007:1–89.
- 2.Keenan TD, Cukras CA, Chew EY. Age-related Macular Degeneration. vol 1256. Springer, Cham; 2021. Age-related macular degeneration: epidemiology and clinical aspects. [DOI] [PubMed] [Google Scholar]
- 3.Jonas JB, Cheung CMG, Panda-Jonas S. Updates on the epidemiology of age-related macular degeneration. Asia Pac J Ophthalmol (Phila) 2017;6:493–7. doi: 10.22608/APO.2017251. [DOI] [PubMed] [Google Scholar]
- 4.Lanzetta P, Loewenstein A, Vision Academy Steering C. Fundamental principles of an anti-VEGF treatment regimen: optimal application of intravitreal anti-vascular endothelial growth factor therapy of macular diseases. Graefes Arch Clin Exp Ophthalmol. 2017;255:1259–73. doi: 10.1007/s00417-017-3647-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fabre M, Mateo L, Lamaa D, et al. Recent advances in age-related macular degeneration therapies. Molecules. 2022;27:5089. doi: 10.3390/molecules27165089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferro Desideri L, Traverso CE, Nicolò M, et al. Faricimab for the treatment of diabetic macular edema and neovascular age-related macular degeneration. Pharmaceutics. 2023;15:1413. doi: 10.3390/pharmaceutics15051413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rush RB. One year results of faricimab for aflibercept-resistant diabetic macular edema. Clin Ophthalmol. 2023;17:2397–403. doi: 10.2147/OPTH.S424314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Institute for Health and Care Excellence (NICE) Age-related macular degeneration: diagnosis and management. 2018 [PubMed]
- 9.Finger RP, Dennis N, Freitas R, et al. Comparative efficacy of brolucizumab in the treatment of neovascular age-related macular degeneration: a systematic literature review and network meta-analysis. Adv Ther. 2022;39:3425–48. doi: 10.1007/s12325-022-02193-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clinical trial Efficacy and safety of two different aflibercept regimens in subjects with neovascular age-related macular degeneration (nAMD) 2015
- 11.Li X, Zhu Q, Egger A, et al. Two different treatment regimens of ranibizumab 0.5mg for neovascular age-related macular degeneration with or without polypoidal choroidal vasculopathy in chinese patients: results from the phase IV, randomized, DRAGON study. Acta Ophthalmol. 2021;99:e336–45. doi: 10.1111/aos.14588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Subramanian ML, Abedi G, Ness S, et al. Bevacizumab vs ranibizumab for age-related macular degeneration: 1-year outcomes of a prospective, double-masked randomised clinical trial. Eye (Lond) 2010;24:1708–15. doi: 10.1038/eye.2010.147. [DOI] [PubMed] [Google Scholar]
- 13.Mitchell P, Holz FG, Hykin P, et al. Efficacy and safety of intravitreal aflibercept using a treat-and-extend regimen for neovascular age-related macular degeneration: the aries study: a randomized clinical trial. RETINA. 2021;41:1911–20. doi: 10.1097/IAE.0000000000003128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amarakoon S, Martinez-Ciriano JP, van den Born LI, et al. Bevacizumab in age-related macular degeneration: a randomized controlled trial on the effect of on-demand therapy every 4 or 8 weeks. Acta Ophthalmol. 2019;97:107–12. doi: 10.1111/aos.13774. [DOI] [PubMed] [Google Scholar]
- 15.El-Mollayess GM, Mahfoud Z, Schakal AR, et al. Fixed-interval versus OCT-guided variable dosing of intravitreal bevacizumab in the management of neovascular age-related macular degeneration: a 12-month randomized prospective study. Am J Ophthalmol. 2012;153:481–9. doi: 10.1016/j.ajo.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 16.Mori R, Tanaka K, Haruyama M, et al. Comparison of pro re nata versus bimonthly injection of intravitreal aflibercept for typical neovascular age-related macular degeneration. Ophthalmologica. 2017;238:17–22. doi: 10.1159/000468950. [DOI] [PubMed] [Google Scholar]
- 17.Higgins JP, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions. John Wiley & Sons; 2019. [Google Scholar]
- 18.Page MJ, McKenzie JE, Bossuyt PM, et al. Declaración PRISMA 2020: una guía actualizada para la publicación de revisiones sistemáticas. Rev Esp Cardiol. 2021;74:790–9. doi: 10.1016/j.recesp.2021.06.016. [DOI] [PubMed] [Google Scholar]
- 19.Higgins JP, Savović J, Page MJ, et al. Cochrane Handbook for Systematic Reviews of Interventions. 2019. Assessing risk of bias in a randomized trial; pp. 205–28. [Google Scholar]
- 20.Papakonstantinou T, Nikolakopoulou A, Higgins JPT, et al. CINeMA: software for semiautomated assessment of the confidence in the results of network meta-analysis. Campbell Syst Rev . 2020;16:e1080. doi: 10.1002/cl2.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nikolakopoulou A, Higgins JPT, Papakonstantinou T, et al. CINeMA: an approach for assessing confidence in the results of a network meta-analysis. PLOS Med. 2020;17:e1003082. doi: 10.1371/journal.pmed.1003082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chaimani A, Caldwell DM, Li T, et al. Cochrane Handbook for Systematic Reviews of Interventions. 2019. Undertaking network meta-analyses; pp. 285–320. [Google Scholar]
- 23.You EL, Hébert M, Jin TS, et al. Comparing interventions for chronic central serous chorioretinopathy: a network meta-analysis. Surv Ophthalmol. 2023;68:601–14. doi: 10.1016/j.survophthal.2023.03.001. [DOI] [PubMed] [Google Scholar]
- 24.Harrer M, Cuijpers P, Furukawa TA. Boca Raton: Chapman and Hall/CRC; 2021. Doing meta-analysis with R: a hands-on guide .https://www.taylorfrancis.com/books/9781003107347 Available. [DOI] [Google Scholar]
- 25.Instituto Nacional de Vigilancia de Medicamentos y Alimentos (INVIMA) Registros viegentes. http://consultaregistro.invima.gov.co:8082/Consultas/consultas/consreg_encabcum.jsp n.d. Available.
- 26.European Medicines Agency (EMA) Eylea: EPAR - product infomation. Annex 1. Summary of products characteristics. 2023
- 27.European Medicines Agency (EMA) Vabysmo: EPAR - product infomation. Annex 1. Summary of products characteristics. 2023
- 28.European Medicines Agency (EMA) Lucentis: EPAR - product infomation. Annex 1. Summary of products characteristics. 2023
- 29.European Medicines Agency (EMA) Beovu: EPAR - Product infomation. Annex 1. Summary of products characteristics. 2023
- 30.Rücker G, Krahn U, König J, et al. netmeta: Network meta-analysis using frequentist methods. 2020
- 31.Silva R, Berta A, Larsen M, et al. Treat-and-extend versus monthly regimen in neovascular age-related macular degeneration: results with ranibizumab from the TREND study. Ophthalmology. 2018;125:57–65. doi: 10.1016/j.ophtha.2017.07.014. [DOI] [PubMed] [Google Scholar]
- 32.Busbee BG, Ho AC, Brown DM, et al. Twelve-month efficacy and safety of 0.5 mg or 2.0 mg ranibizumab in patients with subfoveal neovascular age-related macular degeneration. Ophthalmology. 2013;120:1046–56. doi: 10.1016/j.ophtha.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 33.Scholler A, Richter-Mueksch S, Weingessel B, et al. Differences of frequency in administration of ranibizumab and bevacizumab in patients with neovascular AMD. Wien Klin Wochenschr. 2014;126:355–9. doi: 10.1007/s00508-014-0539-z. [DOI] [PubMed] [Google Scholar]
- 34.López Gálvez MI, Arias Barquet L, S Figueroa M, et al. Bimonthly, treat-and-extend and as-needed ranibizumab in naïve neovascular age-related macular degeneration patients: 12-month outcomes of a randomized study. Acta Ophthalmol. 2020;98:e820–9. doi: 10.1111/aos.14399. [DOI] [PubMed] [Google Scholar]
- 35.Barikian A, Mahfoud Z, Abdulaal M, et al. Induction with intravitreal bevacizumab every two weeks in the management of neovascular age-related macular degeneration. Am J Ophthalmol. 2015;159:131–7. doi: 10.1016/j.ajo.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 36.CATT Research Group. Martin DF, Maguire MG, et al. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364:1897–908. doi: 10.1056/NEJMoa1102673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kodjikian L, Souied EH, Mimoun G, et al. Ranibizumab versus bevacizumab for neovascular age-related macular degeneration: results from the GEFAL noninferiority randomized trial. Ophthalmology. 2013;120:2300–9. doi: 10.1016/j.ophtha.2013.06.020. [DOI] [PubMed] [Google Scholar]
- 38.Heier JS, Khanani AM, Quezada Ruiz C, et al. Efficacy, durability, and safety of intravitreal faricimab up to every 16 weeks for neovascular age-related macular degeneration (TENAYA and LUCERNE): two randomised, double-masked, phase 3, non-inferiority trials. Lancet. 2022;399:729–40. doi: 10.1016/S0140-6736(22)00010-1. [DOI] [PubMed] [Google Scholar]
- 39.Khanani AM, Brown DM, Jaffe GJ, et al. MERLIN: phase 3a, multicenter, randomized, double-masked trial of brolucizumab in participants with neovascular age-related macular degeneration and persistent retinal fluid. Ophthalmology. 2022;129:974–85. doi: 10.1016/j.ophtha.2022.04.028. [DOI] [PubMed] [Google Scholar]
- 40.Dugel PU, Koh A, Ogura Y, et al. HAWK and HARRIER: phase 3, multicenter, randomized, double-masked trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology. 2020;127:72–84. doi: 10.1016/j.ophtha.2019.04.017. [DOI] [PubMed] [Google Scholar]
- 41.Schauwvlieghe AME, Dijkman G, Hooymans JM, et al. Comparing the effectiveness of bevacizumab to ranibizumab in patients with exudative age-related macular degeneration. the BRAMD study. PLoS One. 2016;11:e0153052. doi: 10.1371/journal.pone.0153052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kertes PJ, Galic IJ, Greve M, et al. Canadian treat-and-extend analysis trial with ranibizumab in patients with neovascular age-related macular disease: one-year results of the randomized Canadian treat-and-extend analysis trial with ranibizumab study. Ophthalmology. 2019;126:841–8. doi: 10.1016/j.ophtha.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 43.Ehlers JP. The MANTA 1-year results: the anti-VEGF debate continues: table1. Br J Ophthalmol. 2013;97:248–50. doi: 10.1136/bjophthalmol-2012-302489. [DOI] [PubMed] [Google Scholar]
- 44.Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–31. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 45.Li X, Hu Y, Sun X, et al. Neovascular age-related macular degeneration treatment trial using B. bevacizumab for neovascular age-related macular degeneration in China. Ophthalmology. 2012;119:2087–93. doi: 10.1016/j.ophtha.2012.05.016. [DOI] [PubMed] [Google Scholar]
- 46.Lushchyk T, Amarakoon S, Martinez-Ciriano JP, et al. Bevacizumab in age-related macular degeneration: a randomized controlled trial on the effect of injections every 4 weeks, 6 weeks and 8 weeks. Acta Ophthalmol. 2013;91:e456–61. doi: 10.1111/aos.12119. [DOI] [PubMed] [Google Scholar]
- 47.Dugel PU, Jaffe GJ, Sallstig P, et al. Brolucizumab versus aflibercept in participants with neovascular age-related macular degeneration: a randomized trial. Ophthalmology. 2017;124:1296–304. doi: 10.1016/j.ophtha.2017.03.057. [DOI] [PubMed] [Google Scholar]
- 48.Regillo CD, Brown DM, Abraham P, et al. Randomized, double-masked, sham-controlled trial of ranibizumab for neovascular age-related macular degeneration: PIER study year 1. Am J Ophthalmol. 2008;145:239–48. doi: 10.1016/j.ajo.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 49.Gillies MC, Hunyor AP, Arnold JJ, et al. Effect of ranibizumab and aflibercept on best-corrected visual acuity in treat-and-extend for neovascular age-related macular degeneration: a randomized clinical trial. JAMA Ophthalmol. 2019;137:372–9. doi: 10.1001/jamaophthalmol.2018.6776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Khanani AM, Patel SS, Ferrone PJ, et al. Efficacy of every four monthly and quarterly dosing of faricimab vs ranibizumab in neovascular age-related macular degeneration: the STAIRWAY phase 2 randomized clinical trial. JAMA Ophthalmol. 2020;138:964–72. doi: 10.1001/jamaophthalmol.2020.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Heier JS, Brown DM, Chong V, et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119:2537–48. doi: 10.1016/j.ophtha.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 52.Yuzawa M, Fujita K, Wittrup-Jensen KU, et al. Improvement in vision-related function with intravitreal aflibercept: data from phase 3 studies in wet age-related macular degeneration. Ophthalmology. 2015;122:571–8. doi: 10.1016/j.ophtha.2014.09.024. [DOI] [PubMed] [Google Scholar]
- 53.Feltgen N, Bertelmann T, Bretag M, et al. Efficacy and safety of a fixed bimonthly ranibizumab treatment regimen in eyes with neovascular age-related macular degeneration: results from the RABIMO trial. Graefes Arch Clin Exp Ophthalmol. 2017;255:923–34. doi: 10.1007/s00417-017-3589-x. [DOI] [PubMed] [Google Scholar]
- 54.Wykoff CC, Croft DE, Brown DM, et al. Prospective trial of treat-and-extend versus monthly dosing for neovascular age-related macular degeneration: TREX-AMD 1-year results. Ophthalmology. 2015;122:2514–22. doi: 10.1016/j.ophtha.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 55.Menon G, Chandran M, Sivaprasad S, et al. Is it necessary to use three mandatory loading doses when commencing therapy for neovascular age-related macular degeneration using bevacizumab? (BeMOc trial) Eye (Lond) 2013;27:959–63. doi: 10.1038/eye.2013.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Biswas P, Sengupta S, Choudhary R, et al. Comparative role of intravitreal ranibizumab versus bevacizumab in choroidal neovascular membrane in age-related macular degeneration. Indian J Ophthalmol. 2011;59:191–6. doi: 10.4103/0301-4738.81023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kertes PJ, Galic IJ, Greve M, et al. Efficacy of a treat-and-extend regimen with ranibizumab in patients with neovascular age-related macular disease: a randomized clinical trial. JAMA Ophthalmol. 2020;138:244–50. doi: 10.1001/jamaophthalmol.2019.5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Research Group. Martin DF, Maguire MG, et al. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology. 2012;119:1388–98. doi: 10.1016/j.ophtha.2012.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mishra SK, Kumar P, Khullar S, et al. Efficacy and safety of brolucizumab versus aflibercept in patients with neovascular age-related macular degeneration: a randomized trial in indian patients. Int J Retina Vitreous. 2022;8:51. doi: 10.1186/s40942-022-00401-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ho AC, Busbee BG, Regillo CD, et al. Twenty-four-month efficacy and safety of 0.5 mg or 2.0 mg ranibizumab in patients with subfoveal neovascular age-related macular degeneration. Ophthalmology. 2014;121:2181–92. doi: 10.1016/j.ophtha.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 61.Dugel PU, Singh RP, Koh A, et al. HAWK and HARRIER: ninety-six-week outcomes from the phase 3 trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology. 2021;128:89–99. doi: 10.1016/j.ophtha.2020.06.028. [DOI] [PubMed] [Google Scholar]
- 62.Berg K, Hadzalic E, Gjertsen I, et al. Ranibizumab or bevacizumab for neovascular age-related macular degeneration according to the lucentis compared to avastin study treat-and-extend protocol. Ophthalmology. 2016;123:51–9. doi: 10.1016/j.ophtha.2015.09.018. [DOI] [PubMed] [Google Scholar]
- 63.Chang TS, Bressler NM, Fine JT, et al. Improved vision-related function after ranibizumab treatment of neovascular age-related macular degeneration: results of a randomized clinical trial. Arch Ophthalmol . 2007;125:1460–9. doi: 10.1001/archopht.125.11.1460. [DOI] [PubMed] [Google Scholar]
- 64.Clinical Trial Treating neovascular age-related macular degeneration with aflibercept: a multi-centre randomized controlled trial comparing standard care with an individualised treat and extend regimen. 2022.
- 65.Abraham P, Yue H, Wilson L. Randomized, double-masked, sham-controlled trial of ranibizumab for neovascular age-related macular degeneration: PIER study year 2. Am J Ophthalmol. 2010;150:315–24. doi: 10.1016/j.ajo.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 66.Clinical Trial Efficacy of ranibizumab compared to aflibercept bimonthly intravitreal injections on retinal thickness stability in in patients with wet AMD A 12-month, phase IV, randomized, open label, multicenter study to compare efficacy of 0.5 mg ranibizumab PRN versus 2 mg aflibercept bimonthly intravitreal injections on retinal thickness stability till month 6 of treatment and explore functional outcomes up to month 12 in patients with neovascular (wet) age-related macular degeneration (AMD) - SALT. 2014
- 67.Wykoff CC, Ou WC, Brown DM, et al. Randomized trial of treat-and-extend versus monthly dosing for neovascular age-related macular degeneration. Ophthalmol Retina. 2017;1:314–21. doi: 10.1016/j.oret.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 68.Haga A, Kawaji T, Ideta R, et al. Treat‐and‐extend versus every‐other‐month regimens with aflibercept in age‐related macular degeneration. Acta Ophthalmol (Copenh) 2018;96:e393–8. doi: 10.1111/aos.13607. [DOI] [PubMed] [Google Scholar]
- 69.Eldem BM, Muftuoglu G, Topbaş S, et al. A randomized trial to compare the safety and efficacy of two ranibizumab dosing regimens in a Turkish cohort of patients with choroidal neovascularization secondary to AMD. Acta Ophthalmol. 2015;93:e458–64. doi: 10.1111/aos.12540. [DOI] [PubMed] [Google Scholar]
- 70.Gillies MC, Hunyor AP, Arnold JJ, et al. Macular atrophy in neovascular age-related macular degeneration: a randomized clinical trial comparing ranibizumab and aflibercept (RIVAL study) Ophthalmology. 2020;127:198–210. doi: 10.1016/j.ophtha.2019.08.023. [DOI] [PubMed] [Google Scholar]
- 71.Berg K, Pedersen TR, Sandvik L, et al. Comparison of ranibizumab and bevacizumab for neovascular age-related macular degeneration according to LUCAS treat-and-extend protocol. Ophthalmology. 2015;122:146–52. doi: 10.1016/j.ophtha.2014.07.041. [DOI] [PubMed] [Google Scholar]
- 72.Nunes RP, Hirai FE, Barroso LF, et al. Effectiveness of monthly and fortnightly anti-VEGF treatments for age-related macular degeneration. Arq Bras Oftalmol. 2019;82:225–32. doi: 10.5935/0004-2749.20190043. [DOI] [PubMed] [Google Scholar]
- 73.Schmidt-Erfurth U, Kaiser PK, Korobelnik J-F, et al. Intravitreal aflibercept injection for neovascular age-related macular degeneration: ninety-six-week results of the VIEW studies. Ophthalmology. 2014;121:193–201. doi: 10.1016/j.ophtha.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 74.Abdelfattah NS, Al-Sheikh M, Pitetta S, et al. Macular atrophy in neovascular age-related macular degeneration with monthly versus treat-and-extend ranibizumab. Ophthalmology. 2017;124:215–23. doi: 10.1016/j.ophtha.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 75.Ye L, Jiaqi Z, Jianchao W, et al. Comparative efficacy and safety of anti-vascular endothelial growth factor regimens for neovascular age-related macular degeneration: systematic review and Bayesian network meta-analysis. Ther Adv Chronic Dis. 2020;11:2040622320953349. doi: 10.1177/2040622320953349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.NICE Age-related macular degeneration (NG82) 2018
- 77.NICE Faricimab for treating wet age-related macular degeneration (TA800) 2022
- 78.NICE Brolucizumab for treating wet age-related macular degeneration (TA672) 2021
- 79.NICE Ranibizumab and pegaptanib for the treatment of agerelated macular degeneration (TA155) 2008
- 80.Diabetic Retinopathy Clinical Research N, Browning DJ, Glassman AR, et al. Relationship between optical coherence tomography–measured central retinal thickness and visual acuity in diabetic macular edema. Ophthalmology. 2007;114:525–36. doi: 10.1016/j.ophtha.2006.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kaiser PK, Wykoff CC, Singh RP, et al. RETINAL fluid and thickness as measures of disease activity in neovascular age-related macular degeneration. Retina (Philadelphia, Pa) 2021;41:1579–86. doi: 10.1097/IAE.0000000000003194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jørstad ØK, Steffensen LA, Eriksen K, et al. Thirteen years of intravitreal anti‐vascular endothelial growth factor therapy: the promises and burdens of a paradigm shift told from the perspective of the largest retina service in Norway. Acta Ophthalmol (Copenh) 2020;98:774–9. doi: 10.1111/aos.14177. [DOI] [PubMed] [Google Scholar]
- 83.Reitan G, Kjellevold Haugen IB, Andersen K, et al. Through the eyes of patients: understanding treatment burden of intravitreal anti-VEGF injections for namd patients in Norway. Clin Ophthalmol. 2023;17:1465–74. doi: 10.2147/OPTH.S409103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Penha FM, Masud M, Khanani ZA, et al. Review of real-world evidence of dual inhibition of VEGF-A and ANG-2 with faricimab in NAMD and DME. Int J Retin Vitr. 2024;10:5. doi: 10.1186/s40942-024-00525-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Borkar D, Tabano D, Garmo V, et al. FARETINA-AMD-early treatment patterns and outcomes in patients with neovascular age-related macular degeneration initiating faricimab: an IRIS registrytm analysis. ARVO Annual Meeting Abstr; 2023. [Google Scholar]
- 86.Khanani AM, Aziz AA, Khan H, et al. The real-world efficacy and safety of faricimab in neovascular age-related macular degeneration: the TRUCKEE study - 6 month results. Eye (Lond) 2023;37:3574–81. doi: 10.1038/s41433-023-02553-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Agostini H, Abreu F, Baumal CR, et al. Faricimab for neovascular age-related macular degeneration and diabetic macular edema: from preclinical studies to phase 3 outcomes. Graefes Arch Clin Exp Ophthalmol. 2024:1–15. doi: 10.1007/s00417-024-06531-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dias S, Welton NJ, Sutton AJ, et al. NICE DSU technical support document 2: a generalised linear modelling framework for pairwise and network meta-analysis of randomised controlled trials. 2011 [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.