Abstract
Background
Previous epidemiological research has linked posttraumatic stress disorder (PTSD) with specific physical health problems, but the comprehensive landscape of medical conditions associated with PTSD remains uncharacterized. Electronic health records provide an opportunity to overcome clinical knowledge gaps and uncover associations with biological relevance that potentially vary by sex.
Methods
PTSD was defined among biobank participants (N = 145,959) in 3 major healthcare systems using 2 ICD code-based definitions: broad (≥1 PTSD or acute stress codes vs. 0; ncases = 16,706) and narrow (≥2 PTSD codes vs. 0; ncases = 3325). Using a phenome-wide association study design, we tested associations between each PTSD definition and all prevalent disease umbrella categories, i.e., phecodes. We also conducted sex-stratified phenome-wide association study analyses including a sex × diagnosis interaction term in each logistic regression.
Results
A substantial number of phecodes were significantly associated with PTSDNarrow (61%) and PTSDBroad (83%). While the strongest associations were shared between the 2 definitions, PTSDBroad captured 334 additional phecodes not significantly associated with PTSDNarrow and exhibited a wider range of significantly associated phecodes across various categories, including respiratory, genitourinary, and circulatory conditions. Sex differences were observed in that PTSDBroad was more strongly associated with osteoporosis, respiratory failure, hemorrhage, and pulmonary heart disease among male patients and with urinary tract infection, acute pharyngitis, respiratory infections, and overweight among female patients.
Conclusions
This study provides valuable insights into a diverse range of comorbidities associated with PTSD, including both known and novel associations, while highlighting the influence of sex differences and the impact of defining PTSD using electronic health records.
Keywords: Comorbidities, Electronic health record, Phenome-wide association study, PTSD, Sex specificity, Trauma
Plain Language Summary
Posttraumatic stress disorder (PTSD) is a debilitating psychiatric disorder that some people develop following a traumatic event. In addition to mental symptoms, PTSD can impact human health in ways other ways; for example, there are many known conditions that co-occur with PTSD, such as cardiovascular conditions. In this study, we set out to understand the breadth and degree to which PTSD co-occurs with medical outcomes in a sample of over 146,000 patients across 3 large medical systems. We found that both narrowly and broadly defined PTSD diagnosis co-occurred with hundreds of medical conditions, and the strongest associations were with other psychiatric disorders, respiratory conditions (asthma, GERD), sleep-related conditions, and pain. These results provide insights into future genetic studies of PTSD in large-scale biobanks and deepen our understanding of the complex needs of patients with PTSD.
Plain Language Summary
Posttraumatic stress disorder (PTSD) is a debilitating psychiatric disorder that some people develop following a traumatic event. In addition to mental symptoms, PTSD can impact human health in ways other ways; for example, there are many known conditions that co-occur with PTSD, such as cardiovascular conditions. In this study, we set out to understand the breadth and degree to which PTSD co-occurs with medical outcomes in a sample of over 146,000 patients across 3 large medical systems. We found that both narrowly and broadly defined PTSD diagnosis co-occurred with hundreds of medical conditions, and the strongest associations were with other psychiatric disorders, respiratory conditions (asthma, GERD), sleep-related conditions, and pain. These results provide insights into future genetic studies of PTSD in large-scale biobanks and deepen our understanding of the complex needs of patients with PTSD.
Posttraumatic stress disorder (PTSD) occurs following highly stressful and/or traumatic events. PTSD impairs occupational performance, social relationships, and overall quality of life and also frequently occurs with comorbidities (1), defined broadly here as co-occurring health conditions. Common PTSD comorbidities include psychiatric conditions such as depression and anxiety (2,3) and physical health problems including cardiovascular disease (4) and gastrointestinal conditions (5). However, the comprehensive landscape of PTSD comorbidities, particularly in healthcare contexts, remains incompletely characterized (6). This limits clinical insights into the complex needs of patients with PTSD (i.e., what other medical concerns to consider in formulating a treatment plan) as well as empirical insights into medical conditions that may serve as risk factors for PTSD and/or share underlying biological mechanisms with PTSD (7).
Electronic health records (EHRs) provide a unique opportunity to uncover such associations with clinical and potential biological relevance. EHRs offer large samples and longitudinal data including all diagnoses that a patient receives while active in a healthcare system, recorded using standardized diagnostic billing codes that can be examined in tandem across multiple systems. Such codes can be used in different ways to identify posttraumatic psychopathology (8). While PTSD is designated by specific billing codes, providers may choose to assign a broader code to reflect diagnostic uncertainty, higher likelihood of insurance coverage, stigma reduction, or other factors, meaning that some PTSD cases may be missed when a narrow set of codes is focused on. In addition to identifying specific codes, we can consider how frequently they are assigned to an individual. Accordingly, a broader definition might include a single diagnosis of any stress-related disorder versus a narrow definition that requires multiple diagnoses of PTSD specifically. This has implications for epidemiological (9) and genomic studies that leverage these definitions to identify PTSD cases and controls from EHRs [for example, genome-wide association study (GWAS) (10,11)], where a broader definition can inclusively capture more cases, thereby enhancing discovery power, but may also introduce nonspecific associations (12). How we identify individuals with posttraumatic psychopathology using EHRs, i.e., with more inclusive versus narrow definitions, may impact our ability to resolve relevant comorbidities and the translatability of resulting genetic findings.
In addition to a need to resolve wide-ranging PTSD comorbidities, important sex differences may also exist in such comorbidities. The burden of PTSD varies by sex (3,13), with rates of lifetime PTSD being about twice as high in females as in males (14). Understanding why PTSD prevalence differs by sex is complex; rather than simply capturing intrinsic biological sex differences in risk, differences may reflect gendered environments that include trauma exposure (e.g., interpersonal violence) (15, 16, 17, 18, 19) or available supports. Male, female, and intersex patients may also have different experiences within healthcare systems, including where and how care is received, access to care, and their diagnostic journey for PTSD. As such, the medical conditions that co-occur with PTSD may also differ (20).
In this multisite study, we sought to investigate the relationships between different PTSD phenotypes and the medical phenome using data from 145,959 patients enrolled in biobanks across 3 major EHR-based healthcare systems. Our objectives were to comprehensively identify medical comorbidities associated with PTSD and to examine whether these associations varied based on broad versus narrow definitions and by sex. Finally, we explored how each PTSD definition was related to polygenic signals to understand implications for downstream genomic and clinical research.
Methods and Materials
Samples
The current study includes 145,959 biobank research participants with available genetic and longitudinal EHR data (21) from 3 major health systems: Vanderbilt University Medical Center (VUMC; n = 73,488), Mount Sinai (MSSM; n = 42,586), and Mass General Brigham (MGB; n = 29,885). Each site employs a data floor to ensure a baseline level of data for phenome-wide analyses: MGB requires at least 3 codes >30 days apart, with at least 1 code after January 1, 2005; VUMC requires at least 3 codes; and MSSM requires at least 3 encounters >30 days apart. Sample characteristics (Table 1), phenotype category code (phecode) frequencies (Table S1 in Supplement 2), and study information (Supplement 1) for each site are provided.
Table 1.
Sample Characteristics by Site
| VUMC | MGB | MSSM | |
|---|---|---|---|
| Full Sample | |||
| Full Sample, n | 73,488 | 29,885 | 42,586 |
| Current Age, Years, Mean (SD) [Range] | 59.66 (19.53) [18–100] | 61.55 (16.73) [22–105] | 58.52 (17.04) [18–92] |
| Sex, Female, n (%) | 42,106 (57.3%) | 16,413 (54.9%) | 24,598 (57.76%) |
| Ancestry, n (%) | |||
| Admixed Americans | N/A | 1797 (6.0%) | 8623 (20.24%) |
| African | 11,284 (15.4%) | 1562 (5.2%) | 11,100 (26.06%) |
| East Asian | N/A | 455 (1.5%) | 557 (1.31%) |
| European | 62,204 (84.6%) | 25,787 (86.3%) | 18,449 (43.32%) |
| Native American | N/A | N/A | 1652 (3.88%) |
| South Asian | N/A | 284 (1.0%) | 1000 (2.35%) |
| Number of ICD Codes, Mean (SD) [Range] |
258.3 (375.2) [1–6937] |
416 (540) [1–15,722] |
197.13 (291.85) [3–7071] |
| PTSD Narrow Cases | |||
| PTSD Narrow, n | 1399 | 1290 | 636 |
| Current Age, Years, Mean (SD) [Range] | 47.67 (16.65) [18.1–95.3] | 52.07 (15.06) [23–94] | 56.04 (12.9) [22–91] |
| Sex, Female, n (%) | 987 (70.6%) | 914 (70.9%) | 396 (62.26%) |
| Ancestry, n (%) | |||
| Admixed Americans | N/A | 189 (14.7%) | 206 (32.39%) |
| African | 268 (19.2%) | 116 (9.0%) | 201 (31.6%) |
| East Asian | N/A | 7 (0.5%) | 4 (0.63%) |
| European | 1131 (80.8%) | 974 (75.5%) | 164 (25.79%) |
| Native American | N/A | N/A | 28 (4.4%) |
| South Asian | N/A | 4 (0.3%) | 7 (1.1%) |
| Number of ICD Codes, Mean (SD) [Range] |
524.9 (648.8) [96,937] |
855 (859) [10–11,073] |
616.19 (724.66) [6–7071] |
| PTSD Broad Cases | |||
| PTSD Broad, n | 6887 | 6616 | 3203 |
| Current Age, Years, Mean (SD) [Range] | 55.14 (18.5) [18.02–99.5] | 59.90 (16.40) [23–101] | 57.11 (16.18) [19–92] |
| Sex, Female, n (%) | 4557 (66.2%) | 4176 (63.1%) | 2106 (65.75%) |
| Ancestry, n (%) | |||
| Admixed Americans | N/A | 670 (10.1%) | 815 (25.44%) |
| African | 1091 (15.8%) | 558 (8.4%) | 1020 (31.84%) |
| East Asian | N/A | 65 (1.0%) | 18 (0.56%) |
| European | 5796 (84.2%) | 5285 (79.9%) | 1029 (32.13%) |
| Native American | N/A | N/A | 140 (4.37%) |
| South Asian | N/A | 38 (0.6%) | 65 (1.74%) |
| Number of ICD Codes, Mean (SD) [Range] | 538.2 (621.1) [4–6933] | 753 (751) [5–11,073] | 440.52 (487.27) [3–7071] |
MGB, Mass General Brigham; MSSM, Mount Sinai; N/A, not available; PTSD, posttraumatic stress disorder; VUMC, Vanderbilt University Medical Center.
PTSD Exposures
PTSD case-control phenotypes were defined using 2 ICD code-based definitions. Individuals who met broad PTSD criteria (PTSDBroad) had ≥1 PTSD code (309.81, F43.1, F43.10, F43.11, F43.12) or stress-related disorder code (310.2, F07.81, 309, 309.1, 309.2, 309.21, 309.22, 309.23, 309.24, 309.28, 309.29, 309.3, 309.4, 309.82, 309.83, 309.89, 309.9, F43, F43.0, F43.2, F43.20, F43.21, F43.22, F43.23, F43.24, F43.25, F43.29, F43.8, F43.9), while control participants had no such code; individuals who met narrow PTSD criteria (PTSDNarrow) had ≥2 PTSD codes only, and control participants had no codes for PTSD, other stress-related disorders, or postconcussive injury.
Main Phenome-Wide Association Study Analyses
We used a phenome-wide association study (PheWAS) design to test logistic regression associations between PTSDNarrow or PTSDBroad (predictor) and all phecodes (outcome) as previously defined (22). Cases for each outcome phecode had ≥2 ICD codes belonging to that phecode category, while controls had 0 codes in that category (individuals who had only 1 code were excluded). We required at least 100 cases per phecode per site. We also excluded phecodes derived from the same ICD codes used to define PTSDNarrow (phecode 300.9 [Posttraumatic Stress Disorder]) and PTSDBroad (phecodes 300.8 [Acute Reaction to Stress], 304 [Adjustment Reaction], and 300.9 [Posttraumatic Stress Disorder]) to eliminate circularity of exposure and outcome. We adjusted the models for age, genetically inferred ancestry (admixed American, African [AFR], East Asian, European [EUR], South Asian, where available at each site), sex, and the top 5 principal components (PCs) to adjust for residual differences. As sensitivity analyses, each site also tested PheWAS models in which 1) genetic PCs were not included as covariates, or 2) record density was included as a covariate. We applied a Bonferroni threshold to establish statistical significance, correcting for 1330 tests (Bonferroni-corrected p < 3.76 × 10−5; PTSDNarrow) and 1369 tests (Bonferroni-corrected p < 3.65 × 10−5; PTSDBroad), respectively. We assessed enrichment of phecode categories of associations that reached Bonferroni-level significance and the top 25% of associations using a binomial test.
For all phecodes that appeared in 2 sites in the analysis (1369 phecodes were available for ≥2 sites; 944 were available for all 3 sites), we meta-analyzed across sites using fixed effects in the PheWAS R package (23). To assess heterogeneity, we calculated I2 statistics in METAL (24) to identify associations that differed in effect size between health systems. Phecodes with I2 < 0.1 were considered significantly heterogeneous. While we observed significant heterogeneity among effect size estimates across sites for both PTSDNarrow and PTSDBroad PheWAS, directions of effect and top associations across sites were largely uniform (Table S2 in Supplement 2).
Sex-Stratified and Sex-Interaction PheWAS Analyses
We conducted sex-stratified PheWAS analyses (83,117 females, 64,839 males), adjusting for age, ancestry, and the top 5 PCs. Patient sex was categorized as sex assigned at birth for all sites; a small number of intersex individuals and individuals who declined to state their sex were excluded from this analysis. We conducted logistic regression with a sex × diagnosis interaction term (predictor) to test its association with each significant phecode (outcome) from the full and/or sex-stratified analyses. Interaction effect sizes were meta-analyzed using an inverse-variance weighted model using a STDERR scheme with METAL software (24).
Polygenic Score Analyses
We assessed genetic correlates of PTSD definitions in individuals of EUR and AFR ancestries based on PTSD polygenic scores (PGSs). We calculated PGSs inferring single nucleotide polymorphism weights from EUR PGC (Psychiatric Genomics Consortium) PTSD GWAS summary statistics (discovery ncases = 23,212, ncontrols = 151,447) (25) as implemented in PRS-CSx (26) and summing allele counts weights. We used EUR ancestry GWASs for scoring in both EUR and AFR ancestry groups because an AFR ancestry GWAS is not yet adequately powered for calculating PGSs. For each available ancestry group (AFR and EUR) within each health system, we scaled the PGS (predictor) and tested its association with PTSDNarrow or PTSDBroad (outcome) using logistic regression, including sex, age, and within-ancestry genotype-derived PCs. We estimated phenotypic variance explained by PTSD scores using Nagelkerke’s R2 (27) and meta-analyzed PGS associations with fixed effects using the metafor package (28).
Results
Sample
To examine phenome-wide comorbidities of PTSD, we conducted analyses that included 145,959 patients (3325 and 16,706 who met the PTSDNarrow vs. PTSDBroad definitions, respectively) across 3 major healthcare systems: VUMC, MGB, and MSSM (Table 1). The prevalence rates of PTSDNarrow and PTSDBroad at MGB (4.32% and 22.14%, respectively) were higher than those at the other 2 sites (VUMC: 1.9% and 9.37%, MSSM: 1.49% and 7.52%).
Narrowly and Broadly Defined PTSD Have Wide-Ranging Correlates Across the Medical Phenome
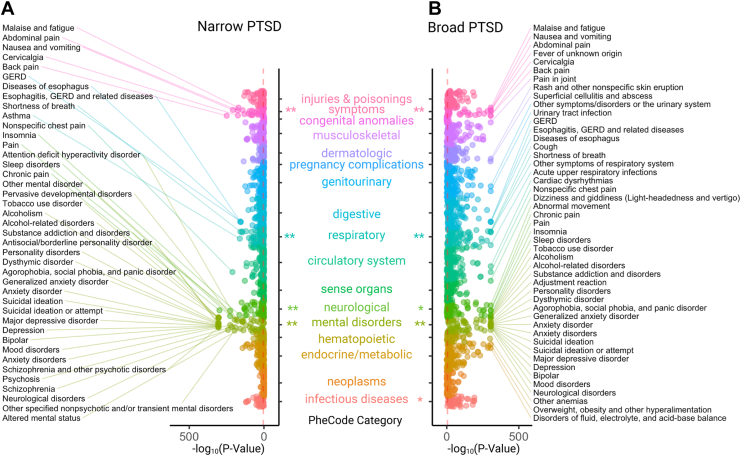
PTSDNarrow was significantly associated with 794 of 1330 (60%) phecodes, and PTSDBroad was significantly associated with 1137 of 1369 (83%) phecodes (Bonferroni-corrected p < 3.8 × 10−5, 3.7 × 10−5, respectively) (full summary statistics are provided in Table S2 in Supplement 2). Top PTSDNarrow associations included psychiatric and pain-related phecodes, whereas top PTSDBroad associations included psychiatric and pain-related phecodes as well as respiratory, genitourinary, and circulatory phecodes (Figure 1). All phecode associations with PTSD were positive: we observed an increased risk of each of these phecodes among individuals with PTSD. Overall, we observed concordance in the significant phecode associations across all 3 sites: 795 were concordant across 3 sites versus 36 that were discordant (binomial p = 0), and 270 were concordant for codes present in 2 sites only versus 3 that were discordant (binomial p = 3.9 × 10−157) for PTSDBroad; 556 were concordant across 3 sites versus 76 that were discordant (binomial p = 0), and 125 were concordant for codes present in 2 sites only versus 6 that were discordant (binomial p = 6.2 × 10−67) for PTSDNarrow.
Figure 1.
(A) Phenome-wide association meta-analysis results for the narrow posttraumatic stress disorder (PTSD) definition. (B) Phenome-wide association meta-analysis results for the broad PTSD definition. Phecodes are organized across the y-axis by their category, with the strength of association shown on the x-axis using −log10p. Red dashed line indicates the Bonferroni significance threshold. Bonferroni (∗∗) and nominally significant (∗) phecode category enrichments for the top 25% of phecode associations for PTSDNarrow (mental disorders: p < .0001, symptoms: p = .0001, neurological: p = .0019, respiratory: p = .0081) and PTSDBroad (mental disorders: p < .0001, symptoms: p < .0001, respiratory: p = .0007, neurological: p = .0269, musculoskeletal: p = .0324, endocrine/metabolic: p = .0312). GERD, gastroesophageal reflux disease.
Narrowly-Defined PTSD Shows Strong Associations With Neuropsychiatric Traits
PTSDNarrow was associated with over 88% of phecodes in the mental disorders category (Table S3 in Supplement 2). Among the top 25% of phecodes associated with PTSDNarrow, mental disorders, symptoms, neurological, and respiratory phecode categories were significantly enriched after Bonferroni correction (Table S3 in Supplement 2). The strongest associations with PTSDNarrow were in the mental disorders category, including personality disorders (antisocial/borderline personality disorder, personality disorders); mood disorders (mood disorders, depression); suicide-related phecodes (suicidal ideation, suicidal ideation or attempt); substance use disorders (substance addiction and disorders, alcohol-related disorders); and anxiety disorders (agorophobia, social phobia, panic disorder, anxiety disorder, generalized anxiety disorder). Beyond the mental disorders category, PTSDNarrow was associated with 89% of phecodes in the infectious diseases category (Table S3 in Supplement 2). Phecodes with the largest odds ratios in association with PTSDNarrow were anxiety disorders, antisocial/borderline personality disorder, personality disorder, mood disorder, suicidal ideation, suicide or self-inflicted injury, poisoning by psychotropic agents, and depression.
Additionally, PTSDNarrow showed strong associations with phecodes in the neurological and symptom categories pertaining to pain, including chronic pain, back pain, pain, and nonspecific chest pain. Several respiratory phecodes also emerged in association with PTSDNarrow, including asthma, shortness of breath, and cough.
Broadly Defined PTSD Shows Strong Associations With Neuropsychiatric Traits and Respiratory, Digestive, Genitourinary, and Cardiovascular Conditions
PTSDBroad was significantly associated with all phecodes in the mental disorders category and over 90% of phecodes in the infectious diseases, respiratory, injuries & poisonings, symptoms, and digestive categories (Table S3 in Supplement 2). Among the top 25% of significant phecodes, the mental disorders, symptoms, and respiratory phecode categories were significantly enriched after Bonferroni correction (Table S3 in Supplement 2). The top phecode associations with PTSDBroad included mood disorders (depression, suicidal ideation, anxiety disorders, bipolar disorder) and substance use disorders (substance addiction and disorders, alcohol-related disorders, alcoholism). The phecodes with the largest odds ratios in association with PTSDBroad were antisocial/borderline personality, personality, suicidal ideation, and anxiety disorders.
In addition to mental disorders, top associations with PTSDBroad were related to a wide range of medical phecode categories, including sleep (insomnia, sleep disorders, malaise and fatigue), pain (back pain, chronic pain, abdominal pain, pain, pain in joint), respiratory system (cough, other symptoms of respiratory system, acute upper respiratory infections of multiple or unspecified sites, shortness of breath, pneumonia, asthma), digestive system (gastroesophogeal reflux disease, diseases of esophagus, esophagitis), and genitourinary system (urinary tract infection, other symptoms/disorders of the urinary system, dysuria).
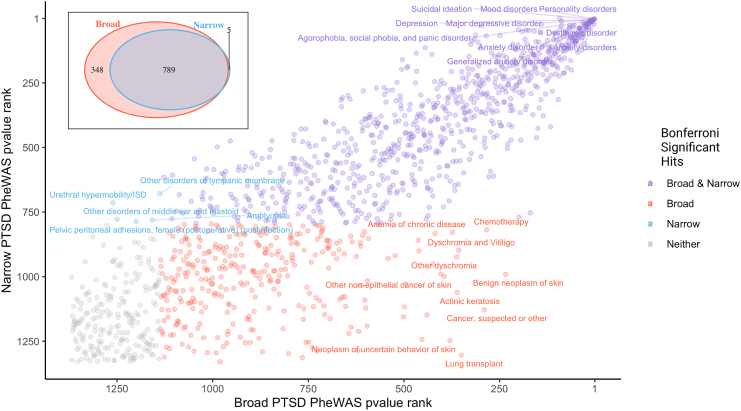
Clinical Phenome Associations Were Largely Shared but Showed Some Differences Across PTSD Definitions
Significant associations were largely shared between PTSDNarrow and PTSDBroad, especially the top-ranking associations (Figure 2). However, many more phecodes were significantly associated with PTSDBroad (1133/1369 tested) than with PTSDNarrow (794/1330), and 348 phecodes were associated with PTSDBroad but not PTSDNarrow. The strongest such associations included phecodes related to skin (neoplasms of skin, dyschromia, actinic keratosis, other nonepithelial cancer of skin), chemotherapy, lung transplant, and anemia of chronic disease; however, these phecodes were not among the strongest hits for PTSDBroad. Only 5 phecodes were significantly associated with PTSDNarrow but not PTSDBroad. These included relatively uncommon genitourinary phecodes such as urethral hypermobility/intrinsic sphincter deficiency and pelvic peritoneal adhesions, as well as amblyopia, other disorders of tympanic membrane, and other disorders of the middle ear and mastoid.
Figure 2.
Top phenome-wide association study (PheWAS) hits are shared between narrowly and broadly defined posttraumatic stress disorder (PTSD). Phecodes ranked by p value are plotted for PTSDBroad (x-axis) and PTSDNarrow (y-axis). Phecodes with a Bonferroni-significant association with PTSDNarrow (blue), PTSDBroad (red), both (purple), or neither (gray) are indicated. The number of shared and distinct phecode associations for PTSDNarrow and PTSDBroad are shown in the inset. ISD, intrinsic sphincter deficiency.
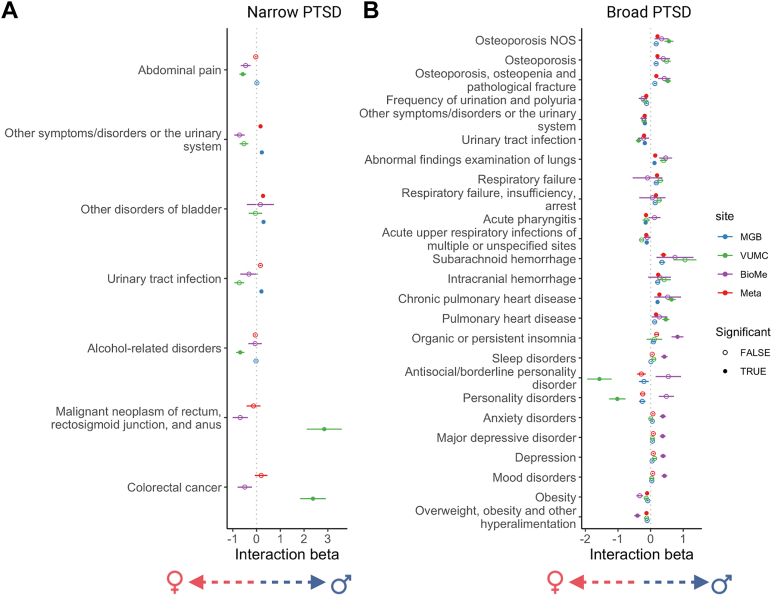
Sex Moderates Associations Between Broadly Defined PTSD and Several Other Phenotypes
We conducted sex-stratified PTSDNarrow and PTSDBroad PheWAS analyses, meta-analyzed across sites (Figures S1 and S2 in Supplement 1; Table S4 in Supplement 2), and performed a PTSD × sex interaction test that was restricted to only those phecodes with a Bonferroni-significant main effect within either sex (Figure 3). Seventeen phecodes differed significantly between males and females coded with PTSDBroad after meta-analysis. Phenotypes that were more common among male patients with PTSDBroad included osteoporosis, respiratory failure, subarachnoid and intracranial hemorrhage, and pulmonary heart disease. Phenotypes that were more common among female patients with PTSDBroad included urinary tract infection and other urinary symptoms, acute pharyngitis, acute respiratory infections, and overweight, although the directions of effect were not consistent across sites for some phecodes. For PTSDNarrow, other symptoms/disorder of the urinary system and other disorders of the bladder were the only phecodes with a significant sex interaction, driven by higher prevalence in males at MGB despite opposing associations being observed at the other 2 sites.
Figure 3.
Phecodes with a significant interaction between sex and posttraumatic stress disorder (PTSD) defined narrowly (A) and broadly (B) in at least 1 site or in meta-analysis across sites. Interaction term (PTSD × sex) coefficients and standard errors are plotted for each site independently (Mass General Brigham [MGB] = blue, Vanderbilt University Medical Center [VUMC] = green, Mount Sinai [BioMe] = purple), and those for a meta-analyzed interaction term coefficient are plotted in red. Bonferroni-significant interaction terms are indicated with a filled circle. A positive interaction term indicates a higher prevalence in males, and a negative interaction term indicates a higher prevalence in females. NOS, not otherwise specified.
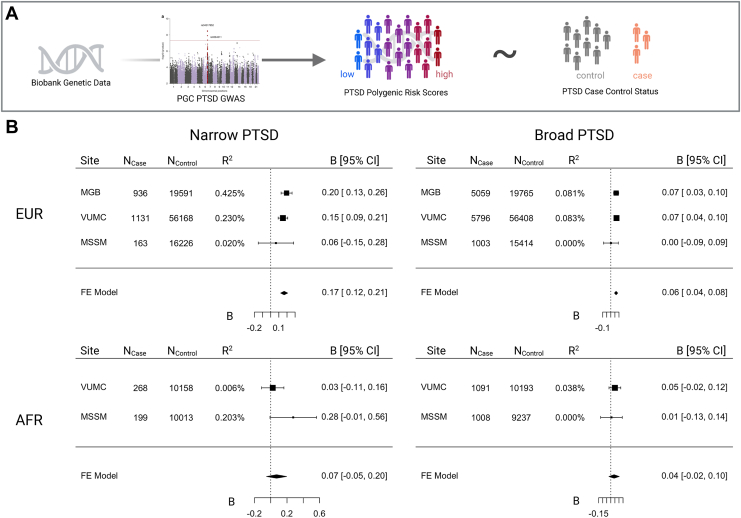
Explanatory Power of PGSs Varies According to PTSD Definition
We tested PGS associations with both PTSDNarrow and PTSDBroad (Figure 4). PTSD PGSs were significantly associated with both PTSDNarrow (meta-analytic B = 0.17, SE = 0.02, p = 8.61 × 10−14, R2 range = 0.02–0.42%; total cases = 2230) and PTSDBroad (meta-analytic B = 0.07, SE = 0.01, p = 3.4 × 10−10, R2 range = 0.00–0.08%; total cases= 11,858) in EUR subsamples, although the proportion of variance explained (R2) was low. PGSs were not significantly associated among individuals of AFR ancestry for either PTSDNarrow (B = 0.07, SE = 0.06, p = .25, R2 range = 0.006–0.203%) or PTSDBroad (B = 0.04, SE = 0.03, p = .22, R2 range = 0.00–0.04%), likely due to poor representation of AFR ancestry alleles in existing PTSD GWASs.
Figure 4.
Posttraumatic stress disorder (PTSD) polygenic score meta-analysis association with PTSD definitions. (A) Biobank genetic data was used to estimate polygenic scores for each individual based on the PGC (Psychiatric Genomics Consortium) PTSD genome-wide association study (GWAS). Polygenic scores were then tested for association with PTSD case-control status in each biobank and then meta-analyzed with a fixed-effects model. (B) Meta-analysis of association beta estimates for narrow (left) and broad (right) PTSD definitions in individuals of European (EUR) (top) and African (AFR) (bottom) ancestry. Number of cases, number of controls, and Nagelkerke’s R2 for each site, definition, and ancestry are listed. FE, finite element; MGB, Mass General Brigham; MSSM, Mount Sinai; VUMC, Vanderbilt University Medical Center.
Discussion
In this study, we examined phenome-wide relationships between EHR-based PTSD and over 1300 medical phecodes among over 145,000 participants across 3 health systems, including 16,706 and 3325 individuals who met broad and narrow definitions for PTSD, respectively. We identified known and novel PTSD comorbidities that replicated across multiple health systems and contextualized the relative strength of PTSD-related associations across the medical phenome. Additionally, we identified several phenotypes for which males and females exhibited differing relationships with PTSD. These results characterize a wide range of PTSD-related diagnostic comorbidities in a health system setting and provide a useful catalog of phenotype-phenotype relationships for interpreting future biobank-based genetic association studies of PTSD.
Contextualizing Known and Novel PTSD Comorbidities
Our results build upon known psychiatric (29, 30, 31) and nonpsychiatric PTSD comorbidities. Psychiatric disorders were consistently identified as the strongest comorbidities for both broad and narrow PTSD definitions; among nonpsychiatric disorders, we observed a strong association of both PTSD definitions with pain phenotypes, which encompass physical sensory components, as well as cognitive and emotional components (32, 33, 34, 35, 36). Given inflammatory/immune links to PTSD (37), we were surprised to find that autoimmune disorders such as rheumatoid arthritis and multiple sclerosis were not significantly associated with PTSD. This suggests that these comorbidities may be less common on a health system scale or that these comorbidities present with specific PTSD subtypes (for example, those with a specific immune component or etiopathology) that are not well represented or captured in our biobanks. We identified novel associations of genitourinary phenotypes with PTSD, with a particularly strong association with PTSDBroad, with significance levels comparable to respiratory and cardiovascular comorbidities. It is possible that these associations stem from shared risk factors, i.e., that sexual assault or related traumatic experiences lead to both genitourinary and psychiatric outcomes (38), or are artifactual associations resulting from cross-disorder correlations, where conditions that appear significant are not directly associated with the exposure but rather linked to other diseases. It is also possible these associations reflect healthcare pleiotropy, i.e., the association stems from an artifact of the clinical settings where traumatic exposures are more commonly/routinely discussed (i.e., obstetrics and gynecology clinics).
Sex Differences in PTSD Comorbidities
Epidemiological evidence indicates that females exposed to traumatic events are at higher risk for developing PTSD compared with trauma-exposed males. We identified several phenotypes for which associations with PTSDBroad differed between males and females. For females, obesity had a stronger association with PTSD than males. This relationship has been previously reported for some studies of PTSD (39,40) and other psychiatric phenotypes (41). We also found stronger association of urinary symptoms with PTSD in females. This may be due to genitourinary symptoms that occur at higher rates following exposure to abuse (42); for example, interstitial cystitis/bladder pain syndrome (43), vulvodynia (44), and chronic pelvic pain (45,46), coupled with a greater prevalence of sexual trauma among females (17,47) or perhaps due to the clinical setting where trauma and genitourinary symptoms are assessed. Respiratory failure, cranial hemorrhage, and pulmonary heart disease were more prevalent among male PTSDBroad cases. Together these results may reflect differential patterns of diagnosis of these disorders or PTSD, differences in the care received between sexes (for example, in medical fields with sex specificity like gynecology) or sex differences in PTSD pathology, and the downstream system-wide consequences of having PTSD.
Scope of PTSD Phenotyping and Implications for Genetic Studies
This study explored the role of phenotypic specificity in resolving comorbid phenotypes. A narrow definition requiring multiple PTSD codes likely captures individuals with more severe and specific symptomatology. In contrast, the broad definition, consisting of a single code inclusive of stress-related reactions, may include individuals with a broader range of symptom severity. Psychiatric genetic studies increasingly tend to include broader definitions that facilitate greater sample sizes but at the potential cost of specificity (12). Both PTSD definitions showed strikingly high levels of comorbidities. However, we found that top PTSDNarrow associations were concentrated in mental disorders and pain-related symptoms, whereas PTSDBroad associations were spread across more phecode categories such as respiratory, genitourinary, and circulatory conditions. Generally, the top phecodes were consistent across both definitions, with the broader definition associated with 300 additional phenotypes.
These association patterns have significant implications for our understanding of broad versus narrow definitions of psychiatric disorders and may shape future analytical and inclusion/exclusion criteria, as well as how we should interpret associations specific to PTSDBroad. The most significant of these were skin conditions (dyschromia, actinic keratosis, etc.), which may reflect racial differences in PTSD prevalence, trauma exposure, or diagnostic rates (15). To assess relevance for future studies, we consider each definition. If PTSDBroad captures both full PTSD and subthreshold PTSD cases (or, if an accurate diagnosis is obtained using only one ICD code, PTSDBroad may capture primarily full PTSD cases), then we might conceptualize these associations as true PTSD associations, discoverable only thanks to this increase in power. However, if the broad category involves decreased diagnostic accuracy, PTSDBroad may simply add noise to the analysis rather than identifying subthreshold or otherwise missed cases, and these additional associations may not contribute meaningfully to our understanding of PTSD and its comorbidities. At the genetic level, we observed that a PTSD PGS [trained on a GWAS based mainly on studies of clinically ascertained PTSD (25)] was significantly associated with both PTSDBroad and PTSDNarrow status, although more strongly with PTSDNarrow than with PTSDBroad. In the future, as sample sizes for PTSD GWASs increase, focusing on more strictly defined cases may become more feasible and may lead to increased predictive power of PGSs.
Several factors suggest that the former, rather than the latter, conceptualization is correct. First, addition of truly random cases (i.e., introduction of noise) should reduce PheWAS discovery power (48) rather than identify additional significant associations. Second, both PTSDBroad and PTSDNarrow have significant psychiatric associations; it does not appear that one group is obviously more accurately ascertained than the other. Third, we note that a primary difference between the 2 groups reflects frequency of diagnosis; a single ICD code for PTSD does not necessarily imply an incorrect diagnosis, subclinical presentation, or less severe symptoms (this could be examined through in-depth chart reviews in the future); rather, these patients may leave the medical system or be referred elsewhere for treatment. In particular, we note that MSSM is associated with the Department of Veteran Affairs, where veterans experiencing combat-related PTSD may pursue PTSD care instead of the initial diagnostic site.
Strengths and Limitations
The current study has several limitations. First, while studying the full clinical phenome using EHR is a strength of our study, our biobank-based patient populations may not be fully representative of the general population. To some extent, we mitigate this concern by comparing effect sizes and meta-analyzing (48) across 3 healthcare settings that represent distinct geographical areas (Boston, New York, Nashville) with differing demographics and healthcare needs (e.g., the proportion of veterans seeking care at these sites differs significantly) and differing proportions of individuals with PTSD. In particular, one site (MGB) has a much higher rate of PTSD diagnosis (PTSDNarrow: 4.3% vs. 1.9%, 1.5% at VUMC, MSSM, respectively). Consequently, we observe some heterogeneity in the effect size of associations; however, effect directions are largely consistent across all 3 sites, which is highly unlikely to be due to chance.
Given the fact that most individuals seek care in a hospital setting for some symptom or condition, EHR-based analyses likely underestimate the proportion of healthy individuals and will not identify subthreshold PTSD or patients with well-managed PTSD receiving care in a different system. In our main analyses, healthcare utilization rates were not adjusted for to avoid potential collider bias (see the Supplement 1), but they likely inflated the type I error rate. Individuals in our study may have uneven access to healthcare or to certain specialists within a healthcare system; psychiatric or mental health services in particular may be unevenly accessed due to differences in insurance coverage or stigma or immigration status. Even when healthcare is accessed, patients may receive differing levels of care, and stereotyping bias or institutionalized racism in healthcare may lead to under- or overdiagnosis (48, 49, 50). These issues may be particularly exacerbated in psychiatry, where certain presentations of PTSD may be more readily diagnosed, potentially biasing our results toward that subtype of PTSD.
Another limitation is the lack of temporal resolution. Understanding whether these associated phecodes occur before or after PTSD onset would allow us to delineate associations into antecedents versus potential consequences of PTSD diagnosis. However, this is difficult in practice because patients may report existing diagnoses to a clinician upon enrollment or may experience long diagnostic journeys. In the latter case, a patient may experience PTSD symptoms for several years before an accurate diagnosis of PTSD is made, and other diagnoses received may either be comorbid with PTSD or represent inaccurate diagnoses given to explain initial symptoms (for example, major depressive disorder). Difficulty pinpointing disease onset precludes analysis of temporal relationships. Informed by this comprehensive map of associations, studies should be repeated in longitudinal cohorts with detailed and repeated psychiatric questionnaires that provide nuanced temporal resolution. Further exploration of the genetic factors that contribute to PTSD and its comorbidities is also warranted.
Conclusions and Future Directions
This multisite study investigated relationships between PTSD phenotypes and medical comorbidities using data from over 145,000 patients from 3 major EHR-based healthcare systems. Our findings provide insights into the diagnostic complexity of PTSD and its associations with the clinical phenome. By considering both broad and narrow definitions of PTSD, we comprehensively identified known and novel diagnostic comorbidities, particularly in the domains of mental disorders, pain, respiratory, genitourinary, and cardiovascular conditions. These associations were observed across multiple health systems, highlighting their generalizability. Importantly, we observed sex differences in the associations between PTSD and certain medical phenotypes, which underscores the need to consider sex-specific factors. Furthermore, our genetic analyses revealed significant associations between PGSs derived from GWASs and both broad and narrow definitions of PTSD. Future research should include longitudinal analyses to examine temporal relationships between PTSD and medical comorbidities, thereby providing insights into the directionality of these associations. Investigating sex-specific factors, such as hormonal influences, trauma response differences, or care-seeking patterns, will enhance our understanding of sex disparities in PTSD and associated medical conditions, while mechanistic studies could explore underlying biological pathways and shared genetic factors that contribute to the observed associations. Overall, this study deepens our understanding of the diagnostic complexity of PTSD obtained using EHRs and provides insights for research toward integrated treatment approaches that address both PTSD diagnosis and its comorbidities.
Acknowledgments and Disclosures
This work was supported in part by PsycheMERGE (Grant No. MH118233 [to JWS and LKD]) and administrative supplement to Psychiatric Genomics Consortium PTSD R01 (R01MH106595). KWC was supported in part by funding from the National Institute of Mental Health (NIMH) (Grant No. K08MH127413) and a NARSAD Young Investigator Award from the Brain & Behavior Foundation. EMH, JC, and LMH acknowledge funding from NIMH (Grant Nos. R01MH124839 and R01MH118278) and National Institute of Environmental Health Sciences (Grant No. R01ES033630). LKD was supported by NIMH (Grant No. R01MH118223); the National Human Genome Research Institute, and the Office of Research on Women's Health (Grant No. RM1HG009034). MSSM: This work was supported in part through computational resources and staff expertise provided by Scientific Computing at the Icahn School of Medicine at Mount Sinai under Grant No. S10OD018522 from the Office of Research Infrastructure of the National Institutes of Health. This work was supported through the resources and staff expertise provided by the Charles Bronfman Institute for Personalized Medicine and the BioMe Biobank Program at the Icahn School of Medicine at Mount Sinai. BioVU: The dataset(s) used for the analyses described were obtained from Vanderbilt University Medical Center’s BioVU, which is supported by numerous sources including institutional funding, private agencies, and federal grants. These include National Institutes of Health–funded Shared Instrumentation Grant No. S10RR025141 and Clinical and Translational Science Award (Grant Nos. UL1TR002243, UL1TR000445, and UL1RR024975). Genomic data are also supported by investigator-led projects that include Grant Nos. U01HG004798, R01NS032830, RC2GM092618, P50GM115305, U01HG006378, U19HL065962, R01HD074711; additional funding sources are listed at https://victr.vumc.org/biovu-funding/. The MGB Biobank provided samples, genomic data, and health information data. This publication was supported by Grant No. R01 HG011405, partially funded by the Office of Research on Women's Health, Office of the Director, National Institutes of Health, and by the National Human Genome Research Institute. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the Office of Research on Women’s Health or the National Human Genome Research Institute.
A previous version of this article was published as a preprint on medRxiv: https://doi.org/10.1101/2023.08.25.23294572
JWS is a member of the Scientific Advisory Board of Sensorium Therapeutics (with equity) and has received grant support from Biogen, Inc. He is the principal investigator of a collaborative study of the genetics of depression and bipolar disorder sponsored by 23andMe for which 23andMe provides analysis time as in-kind support but no payments. KCK has been a paid consultant for Baker Hostetler, Discovery Vitality, the U.S. Department of Justice, and Covington & Burling, LLP. She receives royalties from Guilford Press and Oxford University Press. All other authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Supplementary material cited in this article is available online at https://doi.org/10.1016/j.bpsgos.2024.100337.
Contributor Information
Laura M. Huckins, Email: laura.huckins@yale.edu.
Karmel W. Choi, Email: KWCHOI@mgh.harvard.edu.
Supplementary Material
References
- 1.Sareen J., Cox B.J., Stein M.B., Afifi T.O., Fleet C., Asmundson G.J.G. Physical and mental comorbidity, disability, and suicidal behavior associated with posttraumatic stress disorder in a large community sample. Psychosom Med. 2007;69:242–248. doi: 10.1097/PSY.0b013e31803146d8. [DOI] [PubMed] [Google Scholar]
- 2.Flory J.D., Yehuda R. Comorbidity between post-traumatic stress disorder and major depressive disorder: Alternative explanations and treatment considerations. Dialogues Clin Neurosci. 2015;17:141–150. doi: 10.31887/DCNS.2015.17.2/jflory. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qassem T., Aly-ElGabry D., Alzarouni A., Abdel-Aziz K., Arnone D. Psychiatric co-morbidities in post-traumatic stress disorder: Detailed findings from the adult psychiatric morbidity survey in the English population. Psychiatr Q. 2021;92:321–330. doi: 10.1007/s11126-020-09797-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pietrzak R.H., Goldstein R.B., Southwick S.M., Grant B.F. Physical health conditions associated with posttraumatic stress disorder in U.S. Older adults: Results from wave 2 of the national epidemiologic survey on alcohol and related conditions. J Am Geriatr Soc. 2012;60:296–303. doi: 10.1111/j.1532-5415.2011.03788.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pacella M.L., Hruska B., Delahanty D.L. The physical health consequences of PTSD and PTSD symptoms: A meta-analytic review. J Anxiety Disord. 2013;27:33–46. doi: 10.1016/j.janxdis.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 6.Qureshi S.U., Pyne J.M., Magruder K.M., Schulz P.E., Kunik M.E. The link between post-traumatic stress disorder and physical comorbidities: A systematic review. Psychiatr Q. 2009;80:87–97. doi: 10.1007/s11126-009-9096-4. [DOI] [PubMed] [Google Scholar]
- 7.Johnston K.J.A., Huckins L.M. Chronic pain and psychiatric conditions. Complex Psychiatry. 2023;9:24–43. doi: 10.1159/000527041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harrington K.M., Quaden R., Stein M.B., Honerlaw J.P., Cissell S., Pietrzak R.H., et al. Validation of an electronic medical record-based algorithm for identifying posttraumatic stress disorder in U.S. veterans. J Trauma Stress. 2019;32:226–237. doi: 10.1002/jts.22399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Livingston N.A., Lynch K.E., Hinds Z., Gatsby E., DuVall S.L., Shipherd J.C. Identifying posttraumatic stress disorder and disparity among transgender veterans using nationwide Veterans Health Administration electronic health record data. LGBT Health. 2022;9:94–102. doi: 10.1089/lgbt.2021.0246. [DOI] [PubMed] [Google Scholar]
- 10.Smoller J.W. The use of electronic health records for psychiatric phenotyping and genomics. Am J Med Genet B Neuropsychiatr Genet. 2018;177:601–612. doi: 10.1002/ajmg.b.32548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stein M.B., Levey D.F., Cheng Z., Wendt F.R., Harrington K., Pathak G.A., et al. Genome-wide association analyses of post-traumatic stress disorder and its symptom subdomains in the Million Veteran Program. Nat Genet. 2021;53:174–184. doi: 10.1038/s41588-020-00767-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cai N., Revez J.A., Adams M.J., Andlauer T.F.M., Breen G., Byrne E.M., et al. Minimal phenotyping yields genome-wide association signals of low specificity for major depression. Nat Genet. 2020;52:437–447. doi: 10.1038/s41588-020-0594-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duncan L.E., Ratanatharathorn A., Aiello A.E., Almli L.M., Amstadter A.B., Ashley-Koch A.E., et al. Largest GWAS of PTSD (N=20 070) yields genetic overlap with schizophrenia and sex differences in heritability. Mol Psychiatry. 2018;23:666–673. doi: 10.1038/mp.2017.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olff M. Sex and gender differences in post-traumatic stress disorder: An update. Eur J Psychotraumatol. 2017;8(sup4) [Google Scholar]
- 15.Marchese S., Huckins L.M. Trauma matters: Integrating genetic and environmental components of PTSD. Adv Genet (Hoboken) 2023;4 doi: 10.1002/ggn2.202200017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim G.S., Uddin M. Sex-specific and shared expression profiles of vulnerability and resilience to trauma in brain and blood. Biol Sex Differ. 2020;11:13. doi: 10.1186/s13293-020-00288-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elliott D.M., Mok D.S., Briere J. Adult sexual assault: Prevalence, symptomatology, and sex differences in the general population. J Trauma Stress. 2004;17:203–211. doi: 10.1023/B:JOTS.0000029263.11104.23. [DOI] [PubMed] [Google Scholar]
- 18.Kucharska J. Sex differences in the appraisal of traumatic events and psychopathology. Psychol Trauma. 2017;9:575–582. doi: 10.1037/tra0000244. [DOI] [PubMed] [Google Scholar]
- 19.Mundy S.S., Foss S.L.W., Poulsen S., Hjorthøj C., Carlsson J. Sex differences in trauma exposure and symptomatology in trauma-affected refugees. Psychiatry Res. 2020;293 doi: 10.1016/j.psychres.2020.113445. [DOI] [PubMed] [Google Scholar]
- 20.Muniz Carvalho C., Wendt F.R., Pathak G.A., Maihofer A.X., Stein D.J., Sumner J.A., et al. Disentangling sex differences in the shared genetic architecture of posttraumatic stress disorder, traumatic experiences, and social support with body size and composition. Neurobiol Stress. 2021;15 doi: 10.1016/j.ynstr.2021.100400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kohane I.S., Aronow B.J., Avillach P., Beaulieu-Jones B.K., Bellazzi R., Bradford R.L., et al. What every reader should know about studies using electronic health record data but may be afraid to ask. J Med Internet Res. 2021;23 doi: 10.2196/22219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu P., Gifford A., Meng X., Li X., Campbell H., Varley T., et al. Mapping ICD-10 and ICD-10-CM codes to phecodes: Workflow development and initial evaluation. JMIR Med Inform. 2019;7 doi: 10.2196/14325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carroll R.J., Bastarache L., Denny J.C. R PheWAS: Data analysis and plotting tools for phenome-wide association studies in the R environment. Bioinformatics. 2014;30:2375–2376. doi: 10.1093/bioinformatics/btu197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Willer C.J., Li Y., Abecasis G.R. METAL: Fast and efficient meta-analysis of genomewide association scans. Bioinformatics. 2010;26:2190–2191. doi: 10.1093/bioinformatics/btq340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nievergelt C.M., Maihofer A.X., Klengel T., Atkinson E.G., Chen C.-Y., Choi K.W., et al. International meta-analysis of PTSD genome-wide association studies identifies sex- and ancestry-specific genetic risk loci. Nat Commun. 2019;10:4558. doi: 10.1038/s41467-019-12576-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ruan Y., Lin Y.-F., Feng Y.A., Chen C.-Y., Lam M., Guo Z., et al. Improving polygenic prediction in ancestrally diverse populations. Nat Genet. 2022;54:573–580. doi: 10.1038/s41588-022-01054-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nagelkerke N.J.D. A note on a general definition of the coefficient of determination. Biometrika. 1991;78:691–692. [Google Scholar]
- 28.Viechtbauer W. Conducting Meta-Analyses in R with the metafor Package. J Stat Soft. 2010;36:1–48. [Google Scholar]
- 29.Kang B., Xu H., McConnell E.S. Neurocognitive and psychiatric comorbidities of posttraumatic stress disorder among older veterans: A systematic review. Int J Geriatr Psychiatry. 2019;34:522–538. doi: 10.1002/gps.5055. [DOI] [PubMed] [Google Scholar]
- 30.Facer-Irwin E., Karatzias T., Bird A., Blackwood N., MacManus D. PTSD and complex PTSD in sentenced male prisoners in the UK: Prevalence, trauma antecedents, and psychiatric comorbidities. Psychol Med. 2022;52:2794–2804. doi: 10.1017/S0033291720004936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pietrzak R.H., Goldstein R.B., Southwick S.M., Grant B.F. Psychiatric comorbidity of full and partial posttraumatic stress disorder among older adults in the United States: Results from wave 2 of the national epidemiologic survey on alcohol and related conditions. Am J Geriatr Psychiatry. 2012;20:380–390. doi: 10.1097/JGP.0b013e31820d92e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Asmundson G.J.G., Coons M.J., Taylor S., Katz J. PTSD and the experience of pain: Research and clinical implications of shared vulnerability and mutual maintenance models. Can J Psychiatry. 2002;47:930–937. doi: 10.1177/070674370204701004. [DOI] [PubMed] [Google Scholar]
- 33.Sharp T.J., Harvey A.G. Chronic pain and posttraumatic stress disorder: Mutual maintenance? Clin Psychol Rev. 2001;21:857–877. doi: 10.1016/s0272-7358(00)00071-4. [DOI] [PubMed] [Google Scholar]
- 34.Brennstuhl M.-J., Tarquinio C., Montel S. Chronic Pain and PTSD: Evolving Views on Their Comorbidity. Perspect Psychiatr Care. 2015;51:295–304. doi: 10.1111/ppc.12093. [DOI] [PubMed] [Google Scholar]
- 35.Giordano N.A., Richmond T.S., Farrar J.T., ‘Trip’ Buckenmaier C.C., Gallagher R.M., Polomano R.C. Differential pain presentations observed across post-traumatic stress disorder symptom trajectories after combat injury. Pain Med. 2021;22:2638–2647. doi: 10.1093/pm/pnab204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gasperi M., Afari N., Goldberg J., Suri P., Panizzon M.S. Pain and trauma: The role of criterion A trauma and stressful life events in the pain and PTSD relationship. J Pain. 2021;22:1506–1517. doi: 10.1016/j.jpain.2021.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Katrinli S., Oliveira N.C.S., Felger J.C., Michopoulos V., Smith A.K. The role of the immune system in posttraumatic stress disorder. Transl Psychiatry. 2022;12:313. doi: 10.1038/s41398-022-02094-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vézina-Gagnon P., Bergeron S., Hébert M., McDuff P., Guérin V., Daigneault I. Childhood sexual abuse, girls’ genitourinary diseases, and psychiatric comorbidity: A matched-cohort study. Health Psychol. 2021;40:104–112. doi: 10.1037/hea0000994. [DOI] [PubMed] [Google Scholar]
- 39.Perkonigg A., Owashi T., Stein M.B., Kirschbaum C., Wittchen H.-U. Posttraumatic stress disorder and obesity: Evidence for a risk association. Am J Prev Med. 2009;36:1–8. doi: 10.1016/j.amepre.2008.09.026. [DOI] [PubMed] [Google Scholar]
- 40.Tronieri J.S., Wurst C.M., Pearl R.L., Allison K.C. Sex differences in obesity and mental health. Curr Psychiatry Rep. 2017;19:29. doi: 10.1007/s11920-017-0784-8. [DOI] [PubMed] [Google Scholar]
- 41.Lopresti A.L., Drummond P.D. Obesity and psychiatric disorders: Commonalities in dysregulated biological pathways and their implications for treatment. Prog Neuropsychopharmacol Biol Psychiatry. 2013;45:92–99. doi: 10.1016/j.pnpbp.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 42.Yehuda R., Lehrner A., Rosenbaum T.Y. PTSD and Sexual Dysfunction in Men and Women. J Sex Med. 2015;12:1107–1119. doi: 10.1111/jsm.12856. [DOI] [PubMed] [Google Scholar]
- 43.McKernan L.C., Johnson B.N., Reynolds W.S., Williams D.A., Cheavens J.S., Dmochowski R.R., Crofford L.J. Posttraumatic stress disorder in interstitial cystitis/bladder pain syndrome: Relationship to patient phenotype and clinical practice implications. Neurourol Urodyn. 2019;38:353–362. doi: 10.1002/nau.23861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Iglesias-Rios L., Harlow S.D., Reed B.D. Depression and posttraumatic stress disorder among women with vulvodynia: Evidence from the population-based woman to woman health study. J Womens Health (Larchmt) 2015;24:557–562. doi: 10.1089/jwh.2014.5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lamvu G., Carrillo J., Ouyang C., Rapkin A. Chronic pelvic pain in women: A review. JAMA. 2021;325:2381–2391. doi: 10.1001/jama.2021.2631. [DOI] [PubMed] [Google Scholar]
- 46.Meltzer-Brody S., Leserman J., Zolnoun D., Steege J., Green E., Teich A. Trauma and posttraumatic stress disorder in women with chronic pelvic pain. Obstet Gynecol. 2007;109:902–908. doi: 10.1097/01.AOG.0000258296.35538.88. [DOI] [PubMed] [Google Scholar]
- 47.Tolin D.F., Foa E.B. Sex differences in trauma and posttraumatic stress disorder: A quantitative review of 25 years of research. Psychol Bull. 2006;132:959–992. doi: 10.1037/0033-2909.132.6.959. [DOI] [PubMed] [Google Scholar]
- 48.Dueñas H.R., Seah C., Johnson J.S., Huckins L.M. Implicit bias of encoded variables: Frameworks for addressing structured bias in EHR–GWAS data. Hum Mol Genet. 2020;29:R33–R41. doi: 10.1093/hmg/ddaa192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huckins L.M. Thoughtful phenotype definitions empower participants and power studies. Complex Psychiatry. 2023;8:57–62. doi: 10.1159/000527022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huckins L.M., Signer R., Johnson J., Wu Y.-K., Mitchell K.S., Bulik C.M. What next for eating disorder genetics? Replacing myths with facts to sharpen our understanding. Mol Psychiatry. 2022;27:3929–3938. doi: 10.1038/s41380-022-01601-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.