Summary
Background
The global status of women's health is underestimated, particularly the burden on women of child-bearing age (WCBA). We aim to investigate the pattern and trend of female cancers among WCBA from 1990 to 2021.
Methods
We retrieved data from the Global Burden of Disease Study (GBD) 2021 on the incidence and disability-adjusted life-years (DALYs) of four major female cancers (breast, cervical, uterine, and ovarian cancer) among WCBA (15–49 years) in 204 countries and territories from 1990 to 2021. Estimated annual percentage changes (EAPC) in the age-standardised incidence and DALY rates of female cancers, by age and socio-demographic index (SDI), were calculated to quantify the temporal trends. Spearman correlation analysis was used to examine the correlation between age-standardised rates and SDI.
Findings
In 2021, an estimated 1,013,475 new cases of overall female cancers were reported globally, with a significant increase in age-standardised incidence rate (EAPC 0.16%), and a decrease in age-standardised DALY rate (−0.73%) from 1990 to 2021. Annual increase trends of age-standardised incidence rate were observed in all cancers, except for that in cervical cancer. Contrary, the age-standardised DALY rate decreased in all cancers. Breast and cervical cancers were prevalent among WCBA worldwide, followed by ovarian and uterine cancers, with regional disparities in the burden of four female cancers. In addition, the age-standardised incidence rates of breast, ovarian, and uterine cancers basically showed a consistent upward trend with increasing SDI, while both the age-standardised incidence and DALY rates in cervical cancer exhibited downward trends with SDI. Age-specific rates of female cancers increased with age in 2021, with the most significant changes observed in younger age groups, except for uterine cancer.
Interpretation
The rising global incidence of female cancers, coupled with regional variations in DALYs, underscores the urgent need for innovative prevention and healthcare strategies to mitigate the burden among WCBA worldwide.
Funding
This study was supported by the Science Foundation for Young Scholars of Sichuan Provincial People's Hospital (NO. 2022QN44 and NO. 2022QN18); the Key R&D Projects of Sichuan Provincial Department of Science and Technology (NO. 2023YFS0196); the National Natural Science Foundation of China (No. 82303701).
Keywords: Global burden of disease study, Women of child-bearing age, Breast cancer, Cervical cancer, Ovarian cancer, Uterine cancer
Research in context.
Evidence before this study
We used the keywords “breast cancer”, “gynecologic cancer”, “cervical cancer”, “uterine cancer”, “ovarian cancer”, “global burden”, and “women of child-bearing age” to search PubMed and Web of Science from database inception to May 24th, 2024. Several recent studies have indicated that rapid socio-economic development has contributed to an increase in the incidence of female cancers and a trend towards younger ages at diagnosis, while persistent issues such as regional disparities and gender inequality have led to inequalities in the survival rates of women with cancer. To date, there has been no analysis of global burden and trends in the four major female cancers among women of child-bearing age (WCBA). The United Nations General Assembly calls for action towards the Sustainable Development Goals related to health, poverty, and gender, and aims to eliminate cervical cancer as a public health issue by 2030. One of the key actions is to provide tailored comprehensive healthcare and primary health services for WCBA. However, there is a scarcity of existing global- and regional-level female cancer among WCBA surveillance data, and the quality varies greatly.
Added value of this study
This study first analyse the global trends in the incidence and disability-adjusted life-years (DALY) of four major female cancers (breast cancer, cervical cancer, uterine cancer, ovarian cancer) among WCBA (15–49 years) in 204 countries and territories from 1990 to 2021, considering age and socio-demographic index. The findings of this study provide valuable insights for the development of evidence-based healthcare strategies and the allocation of resources aimed at mitigating the burden of female cancers among WCBA. This underscores the necessity for a comprehensive approach to prevention, screening, and care tailored specifically to this demographic group.
Implications of all the available evidence
Female cancers among WCBA pose a global public health challenge. The age-standardised incidence rate of female cancers increased worldwide from 1990 to 2021, mainly attributable to breast, ovarian, and uterine cancers. Although the age-standardised DALY rate of female cancers decreased worldwide from 1990 to 2021, there were also regional and demographic disparities for each cancer. Health-care providers should be aware of gender inequality and other societal factors on the risk of female cancers in WCBA and should develop region- and age-appropriate primary intervention, secondary intervention and health care.
Introduction
Breast cancer, cervical cancer, ovarian cancer, and uterine cancer represent significant health issues for women worldwide.1 With the global increase in the female population and rapid social development, the burden of these cancers is steadily rising, accompanied by a discernible trend towards women of child-bearing age (WCBA).2 Breast cancer is one of the most common cancers among women across the world, while cervical cancer remains a leading cause of cancer-related deaths in some developing countries.3,4 Although ovarian cancer and uterine cancer have relatively lower incidence rates, they are nonetheless significant types of malignancies affecting the female reproductive system.5
There are striking disparities across regions and countries in female cancers.6 Some regions may suffer from inadequate medical resources and insufficient healthcare services, leading to delayed cancer screening and treatment, thereby increasing the incidence and mortality rates of these diseases. An International Agency for Research on Cancer study of two million women from 81 countries found that nearly one-third of breast cancer cases were late-stage in sub-Saharan Africa, while only one-tenth in Europe and North America,7 highlighting a correlation between lower socioeconomic status and late-stage breast cancer diagnosis. According to the World Health Organization (WHO), cervical cancer is the fourth most common cancer among women globally, with approximately 94% of the 350,000 deaths occurring in low- and middle-income countries.8 There is still inadequate coverage of cervical cancer screening and human papillomavirus (HPV) vaccination in certain less developed countries, contributing to persistently high incidence rates of cervical cancer.9 Unhealthy lifestyles and environmental pollution due to rapid development in developing countries may also contribute to an increased risk of female cancers. The latest Global Cancer Statistics indicate that the mortality rates for breast and cervical cancers among women in developing countries are significantly higher than in developed countries (15.3 and 12.4 cases per 100,000 people, respectively, compared to 11.3 and 4.8 cases per 100,000 people).10 Therefore, comprehensive research and analysis of the burden of female cancers among WCBA in different regions and countries are essential for developing more targeted prevention and control strategies.
The Global Burden of Diseases, Injuries, and Risk Factors Study provided a systematic approach to assess the burden of female cancers in 204 countries and territories, offering a unique opportunity to understand the underlying burden trends across the past three decades.11 Considering that WCBA represents a crucial demographic group for reproductive health and family planning, effective interventions for female cancers during this age period can contribute to improving global women's health and population fertility issues. In this study, we focus on the four major female cancers (breast cancer, cervical cancer, ovarian cancer, and uterine cancer) that affect the female reproductive system and aim to estimate the patterns and trends of their incidence and disability-adjusted life-years (DALYs) among WCBA, to provide insights for tailored policies and strategies concerning prevention, screening, and treatment, ultimately benefiting reproductive and population fertility health.
Methods
Study population and data collection
In this study, we analysed data on female cancers from the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) 2021. Despite the diverse occurrence of cancers in women, such as breast, cervical, ovarian, uterine, vulvar, and vaginal cancers, GBD 2021 only provided estimates of the burden of four major female cancers (breast cancer, cervical cancer, ovarian cancer, and uterine cancer) (https://ghdx.healthdata.org/record/ihme-data/gbd-2021-cause-icd-code-mappings). In addition, according to the GBD definition, fallopian tube cancer is not included under ovarian cancer. The international classification of disease codes of these four cancers in GBD 2021 were defined in the supplementary appendix (Appendix 1). Moreover, according to the definition from WHO, women of child-bearing age (WCBA) was defined as 15–49 years.12
The GBD 2021, supported by over 11,500 collaborators from 164 countries, systematically assesses global health status and disease burden through extensive data provision, review, and analysis. The data sources can be found through the GBD 2021 Data Input Sources Tool (https://ghdx.healthdata.org/gbd-2021/sources) from the Institute for Health Metrics and Evaluation website. An overview of GBD data collection, modeling/analysis, and dissemination was provided in the supplementary appendix (Appendix 2). Details of the disease model of the four female cancers are presented in the GBD 2021 methods appendices (https://www.healthdata.org/gbd/methods-appendices-2021/cancers). In this study, we extracted numbers and rates on the incidence and DALYs of the four major female cancers within the age range of 15–49 years from the GBD 2021 through the GBD Results Tool (https://vizhub.healthdata.org/gbd-results/).
The socio-demographic index (SDI) is estimated to represent a comprehensive development status that exhibits a robust correlation with health outcomes. It is derived from the geometric mean of 0–1 indices of the fertility rates among females under the age of 25, average years of education for individuals aged 15 and above, and lag-distributed income per capita. For GBD 2021, final SDI values were multiplied by 100 for reporting. An SDI of 0 signifies the theoretical minimum level of development relevant to health, while an SDI of 100 represents the theoretical maximum level. A recent GBD 2021 capstone paper described how SDI is assembled and categorized the 204 countries into five quintiles (low, low-middle, middle, high-middle, and high) based on their country-level SDI estimates for the year 2021.13
Statistical analysis
We calculated age-standardised rates (ASRs) per 100,000 people of WCBA from 15 to 49 years, according to the formula11 (1):
| (1) |
In the equation, denotes the age-specific rate in the ith age group, while signifies the count of individuals within the same age group as per the GBD 2021 standard population. N is the total number of age groups. The 95% confidence interval (CI) was determined by “ageadjust.direct” function of package “epitools” within R software.14
We calculated the estimated annual percentage change (EAPC) in ASR to evaluate the average changing trends over a specified time interval.15 We assumed the natural logarithm of ASR fit the linear regressions model (2), where refers to ln (ASR), and x is the calendar year. Therefore:
| (3) |
| (4) |
We identified an ASR as indicative of an increasing or decreasing trend over time if both the EAPC and its 95% CI were above or below zero, respectively. In instances where the 95% CI encompassed zero, we deemed the change in ASR statistically insignificant.
We employed local regression smoothing models (loess) using “geom_smooth” function of package “ggplot2” to fit the correlation between the burdens of female cancers among WCBA and SDI across 21 regions and 204 countries and territories. Additionally, we used Spearman correlation analysis to compute the r indices and p values for the relationship between burdens and SDI 2021. In addition, considering the distribution of SDI across countries changed much from 1990 to 2021, we calculated the EAPC of SDI by 204 countries, and used Spearman correlation analysis to assess the relationship between EAPCs of SDI and ASRs. We regarded p < 0.05 as statistically significant. All statistical analysis and graphical representations were conducted using R software (version 4.2.2).
Ethics statement
For GBD studies, the Institutional Review Board of the University of Washington reviewed and approved a waiver of informed consent (https://www.healthdata.org/research-analysis/gbd).
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. All authors had full access to all the data in the study and accepted responsibility to submit for publication.
Results
Global, regional, and national burden of overall female cancers
In 2021, the global incidence of overall female cancers was approximately 1,013,475 cases, with an age-standardised incidence rate of 50.7 per 100,000 population. The global DALYs was approximately 12,512,451 cases, with an age-standardised DALY rate of 626.8 per 100,000 population (Table 1). Throughout 21 GBD regions and 204 countries, the highest age-standardised incidence rates were found in Central Latin America (78.0), Monaco (133.3), and the highest age-standardised DALY rates were recorded in Southern Sub-Saharan Africa (1346.4), Kiribati (2129.6), respectively (Table 1, Table S1, Fig. 1A, Fig. 2A and B).
Table 1.
Incidence and DALYs of female cancers in 1990 and 2021, and their estimated annual percentage changes from 1990 to 2021.
| Characteristics | Incidence |
DALYs |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Number of cases, 1990 | Age-standardised rate per 100,000 population, 1990 | Number of cases, 2021 | Age-standardised rate per 100,000 population, 2021 | Estimated annual percentage change, 1990–2021 | Number of cases, 1990 | Age-standardised rate per 100,000 population, 1990 | Number of cases, 2021 | Age-standardised rate per 100,000 population, 2021 | Estimated annual percentage change, 1990–2021 | |
| Global | 537,074 (500,166–576,819) | 47.1 (47.0–47.2) | 1,013,475 (929,748–1,099,212) | 50.7 (50.6–50.8) | 0.16 (0.11–0.21) | 8,594,286 (7,829,497–9,394,514) | 754.1 (753.6–754.6) | 12,512,451 (11,420,690–13,629,892) | 626.8 (626.4–627.1) | −0.73 (−0.82 to −0.64) |
| Causes | ||||||||||
| Breast cancer | 256,716 (246,082–270,224) | 23.1 (23.0–23.2) | 561,438 (523,147–602,978) | 28.1 (28.0–28.1) | 0.49 (0.44–0.55) | 3,992,794 (3,744,654–4,279,174) | 354.6 (354.3–355.0) | 6,659,460 (6,192,226–7,145,549) | 333.1 (332.9–333.4) | −0.39 (−0.49 to −0.28) |
| Cervical cancer | 204,495 (190,490–219,886) | 17.4 (17.4–17.5) | 307,428 (280,667–335,692) | 15.4 (15.4–15.5) | −0.39 (−0.48 to −0.29) | 3,509,984 (3,192,517–3,843,599) | 303.9 (303.6–304.2) | 4,184,314 (3,779,641–4,629,605) | 209.8 (209.6–210.0) | −1.25 (−1.34 to −1.16) |
| Ovarian cancer | 47,983 (40,523–55,870) | 4.1 (4.0–4.1) | 85,749 (75,169–95,090) | 4.3 (4.3–4.4) | 0.06 (0.00–0.12) | 799,648 (674,906–931,352) | 69.6 (69.5–69.8) | 1,294,996 (1,139,827–1,431,298) | 65.1 (65.0–65.2) | −0.38 (−0.45 to −0.31) |
| Uterine cancer | 27,880 (23,071–30,840) | 2.5 (2.5–2.6) | 58,860 (50,765–65,452) | 2.9 (2.9–3.0) | 0.45 (0.32–0.57) | 291,860 (217,420–340,389) | 25.9 (25.8–26.0) | 373,682 (308,997–423,441) | 18.7 (18.6–18.7) | −1.23 (−1.34 to −1.13) |
| GBD regions | ||||||||||
| Andean Latin America | 3447 (2927–4083) | 46.4 (44.8–48.0) | 9542 (7181–12,366) | 56.2 (55.0–57.3) | 0.44 (0.27–0.60) | 69,628 (59,231–81,917) | 944.1 (937.0–951.2) | 120,786 (91,396–156,536) | 712.7 (708.7–716.7) | −1.12 (−1.26 to −0.97) |
| Australasia | 4117 (3787–4459) | 76.4 (74.1–78.8) | 5150 (4481–5819) | 62.8 (61.1–64.6) | −0.48 (−0.59 to −0.38) | 43,424 (40,231–46,552) | 806.7 (799.1–814.3) | 32,381 (28,891–36,249) | 395.1 (390.8–399.4) | −2.28 (−2.36 to −2.21) |
| Caribbean | 5077 (4567–5718) | 65.2 (63.4–67.0) | 8584 (6996–10,441) | 70.6 (69.1–72.1) | 0.18 (0.07–0.29) | 85,073 (74,253–98,505) | 1091.6 (1084.2–1099.0) | 125,483 (98,894–158,682) | 1032.7 (1027.0–1038.4) | −0.20 (−0.28 to −0.11) |
| Central Asia | 6549 (6128–6966) | 50.8 (49.5–52.0) | 10,345 (8909–11,837) | 41.7 (40.9–42.5) | −0.47 (−0.58 to −0.36) | 113,062 (106,285–120,264) | 880.4 (875.1–885.7) | 145,434 (125,593–167,495) | 586.8 (583.8–589.9) | −1.26 (−1.38 to −1.13) |
| Central Europe | 21,487 (20,418–22,605) | 67.1 (66.2–68.0) | 20,039 (18,032–22,218) | 60.6 (59.8–61.5) | −0.44 (−0.60 to −0.28) | 315,219 (300,526–329,775) | 984.8 (981.3–988.2) | 187,852 (168,972–206,819) | 565.0 (562.4–567.6) | −1.97 (−2.12 to −1.82) |
| Central Latin America | 20,616 (19,897–21,350) | 63.4 (62.5–64.3) | 53,502 (45,585–61,381) | 78.0 (77.3–78.7) | 0.42 (0.24–0.59) | 290,860 (281,723–300,036) | 904.1 (900.8–907.5) | 512,544 (434,425–590,436) | 747.2 (745.2–749.3) | −0.80 (−0.99 to −0.61) |
| Central Sub-Saharan Africa | 3931 (2780–5430) | 42.4 (41.1–43.8) | 12,790 (8691–17,656) | 49.9 (49.0–50.8) | 0.48 (0.33–0.62) | 102,830 (73,304–141,116) | 1117.0 (1110.1–1124.0) | 279,827 (190,316–386,599) | 1102.2 (1098.0–1106.3) | −0.09 (−0.17 to 0.00) |
| East Asia | 85,443 (64,831–107,374) | 30.9 (30.7–31.1) | 194,456 (144,961–252,143) | 47.8 (47.6–48.0) | 1.52 (1.42–1.62) | 1,548,578 (1,177,382–1,951,722) | 561.3 (560.4–562.2) | 1,676,680 (1,251,317–2,179,663) | 411.6 (410.9–412.2) | −1.11 (−1.19 to −1.02) |
| Eastern Europe | 32,735 (31,409–34,123) | 59.0 (58.4–59.7) | 40,852 (36,031–46,013) | 66.3 (65.7–67.0) | 0.17 (0.01–0.33) | 481,852 (462,015–501,828) | 867.4 (865.0–869.9) | 427,374 (374,657–489,335) | 692.4 (690.3–694.5) | −1.07 (−1.24 to −0.90) |
| Eastern Sub-Saharan Africa | 16,266 (13,103–20,294) | 51.9 (51.1–52.7) | 42,428 (33,047–55,032) | 51.1 (50.6–51.6) | −0.36 (−0.52 to −0.21) | 427,006 (342,313–534,773) | 1370.5 (1366.3–1374.7) | 913,871 (715,476–1,180,317) | 1111.8 (1109.5–1114.2) | −0.99 (−1.13 to −0.85) |
| High-income Asia Pacific | 21,286 (19,682–22,835) | 43.6 (43.0–44.2) | 31,018 (28,462–33,763) | 62.0 (61.3–62.7) | 1.20 (0.97–1.42) | 223,578 (211,032–235,945) | 458.2 (456.3–460.1) | 199,980 (186,569–214,593) | 396.9 (395.1–398.7) | −0.51 (−0.61 to −0.42) |
| High-income North America | 78,846 (76,857–80,950) | 102.9 (102.2–103.6) | 70,172 (66,809–73,688) | 76.1 (75.5–76.6) | −1.02 (−1.08 to −0.95) | 604,862 (584,040–627,992) | 792.1 (790.1–794.1) | 427,601 (404,262–452,023) | 463.2 (461.8–464.6) | −1.76 (−1.85 to −1.66) |
| North Africa and Middle East | 13,125 (11,068–16,131) | 21.9 (21.6–22.3) | 67,869 (58,559–77,753) | 43.0 (42.6–43.3) | 2.45 (2.33–2.57) | 227,702 (192,483–282,288) | 381.7 (380.1–383.3) | 669,230 (564,325–785,242) | 423.8 (422.8–424.8) | 0.56 (0.45–0.66) |
| Oceania | 720 (506–985) | 59.4 (55.1–64.0) | 1731 (1254–2473) | 55.2 (52.6–57.9) | −0.38 (−0.51 to −0.26) | 14,675 (10,254–20,143) | 1219.2 (1199.2–1239.4) | 35,638 (25,940–51,073) | 1141.7 (1129.8–1153.6) | −0.20 (−0.29 to −0.11) |
| South Asia | 68,152 (57,828–79,566) | 32.6 (32.4–32.9) | 168,616 (144,752–196,552) | 36.5 (36.3–36.7) | 0.28 (0.05–0.52) | 1,673,221 (1,422,350–1,954,078) | 804.6 (803.3–805.8) | 3,090,832 (2,651,700–3,598,857) | 670.6 (669.8–671.3) | −0.69 (−0.88 to −0.51) |
| Southeast Asia | 37,811 (31,003–46,302) | 39.5 (39.1–39.9) | 98,628 (79,648–120,356) | 52.0 (51.7–52.3) | 0.75 (0.66–0.84) | 766,793 (630,393–932,258) | 807.3 (805.5–809.2) | 1,484,056 (1,201,480–1,824,571) | 781.0 (779.8–782.3) | −0.23 (−0.31 to −0.14) |
| Southern Latin America | 7250 (6600–7887) | 62.0 (60.5–63.4) | 11,579 (10,462–12,798) | 63.1 (62.0–64.3) | 0.07 (−0.08 to 0.23) | 126,176 (116,537–135,853) | 1082.1 (1076.2–1088.1) | 138,613 (126,860–151,623) | 754.3 (750.3–758.3) | −1.13 (−1.29 to −0.96) |
| Southern Sub-Saharan Africa | 5577 (4619–6746) | 54.0 (52.5–55.4) | 15,120 (12,663–17,618) | 73.3 (72.1–74.5) | 1.92 (1.28–2.56) | 115,436 (96,221–139,157) | 1129.7 (1123.1–1136.4) | 275,920 (231,633–322,209) | 1346.4 (1341.4–1351.5) | 1.53 (0.89–2.17) |
| Tropical Latin America | 14,830 (14,196–15,524) | 44.8 (44.1–45.5) | 39,651 (37,458–42,005) | 60.6 (60.0–61.2) | 0.61 (0.46–0.76) | 270,710 (259,556–282,654) | 823.4 (820.3–826.6) | 516,560 (488,657–544,848) | 788.5 (786.3–790.7) | −0.46 (−0.58 to −0.33) |
| Western Europe | 78,530 (75,912–81,205) | 79.2 (78.7–79.8) | 74,024 (70,768–77,671) | 65.3 (64.8–65.8) | −0.52 (−0.65 to −0.39) | 809,289 (781,999–839,255) | 816.6 (814.8–818.4) | 474,240 (450,254–501,904) | 417.2 (416.0–418.4) | −2.17 (−2.24 to −2.10) |
| Western Sub-Saharan Africa | 11,278 (8914–13,937) | 34.9 (34.3–35.6) | 37,379 (27,033–48,776) | 40.0 (39.6–40.4) | 0.41 (0.33–0.48) | 284,312 (226,850–351,733) | 888.7 (885.4–892.1) | 777,549 (561,352–1,012,954) | 839.6 (837.7–841.5) | −0.22 (−0.29 to −0.15) |
Abbreviation: DALYs, disability-adjusted life-years.
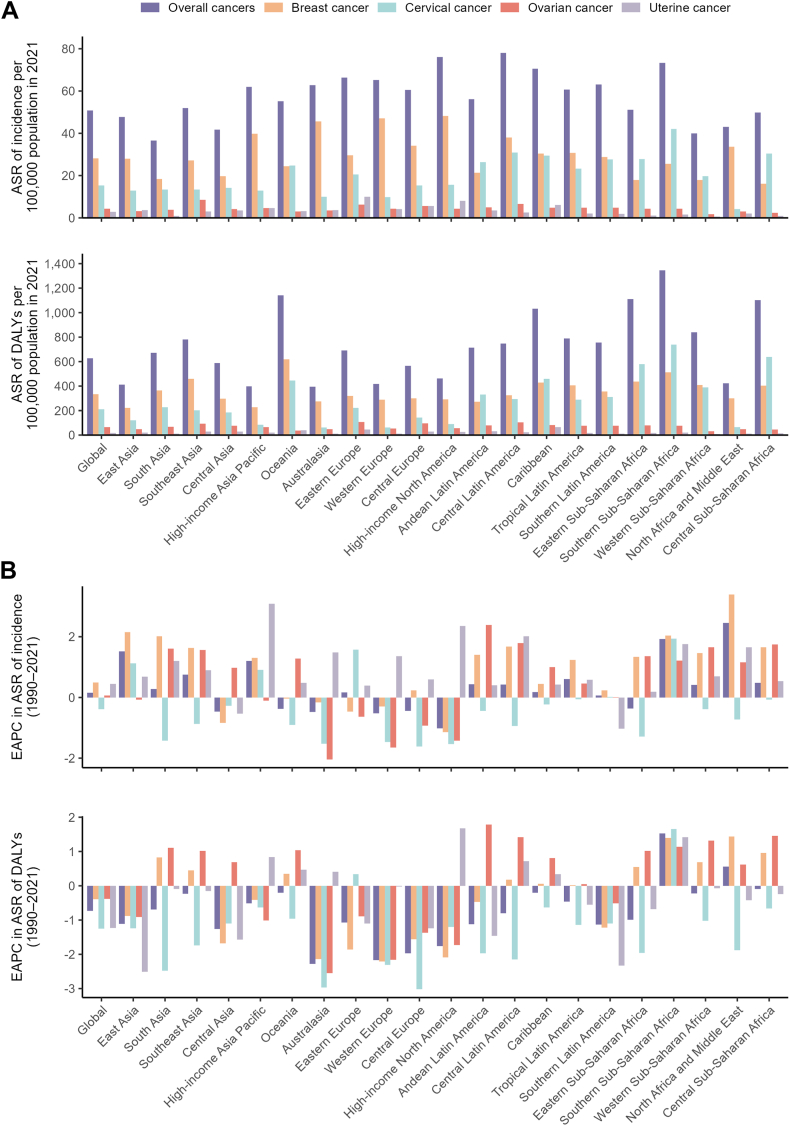
Fig. 1.
Age-standardised incidence and DALY rates in 2021, and their estimated annual percentage changes from 1990 to 2021 for female cancers, globally and by 21 GBD regions. Age-standardised rates of incidence and DALYs (A), and estimated annual percentage changes of age-standardised rates of incidence and DALYs (B). Female cancers include breast, cervical, ovarian, and uterine cancers. DALY, disability-adjusted life-years; EAPC, estimated annual percentage change; ASR, age-standardised rate.
Fig. 2.
National age-standardised incidence and DALY rates in 2021, and their estimated annual percentage changes from 1990 to 2021 for overall female cancers. Age-standardised rates of incidence (A) and DALYs (B). Estimated annual percentage changes of age-standardised incidence rate (C) and DALY rate (D). Female cancers include breast, cervical, ovarian, and uterine cancers. DALY, disability-adjusted life-years.
From 1990 to 2021, the global age-standardised incidence rate increased with an EAPC of 0.16, while the age-standardised DALY rate decreased with an EAPC of −0.73 (Table 1). Across the regional and national levels, the most rapid increases of age-standardised incidence rate were observed in North Africa and Middle East (EAPC = 2.45) and Lesotho (4.49) (Table 1, Table S1, Figs. 1B, Fig. 2C). The age-standardised DALY rate in most regions decreased significantly except for three regions (Southern Sub-Saharan Africa (EAPC = 1.53) and North Africa and Middle East (0.56) increased significantly, and Central Sub-Saharan Africa remained stable). The most rapid increase of age-standardised DALY rate across countries was Lesotho (EAPC = 4.38) (Table 1, Table S1, Figs. 1B, Fig. 2D).
Regional disparities in the burden of four female cancers
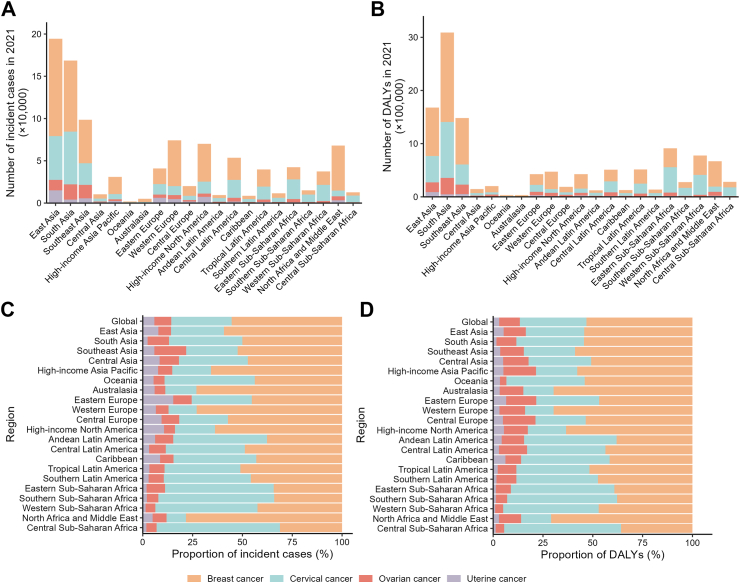
In 2021, the global numbers of new cases for breast, cervical, ovarian, and uterine cancers among WCBA were documented as 561,438, 307,428, 85,749, and 58,860, respectively. Correspondingly, their age-standardised incidence rates per 100,000 population were 28.1, 15.4, 4.3, and 2.9 cases. The global DALYs of breast, cervical, ovarian, and uterine cancers and their age-standardised DALY rates (cases per 100,000 population) reported among WCBA were 6,659,460 (333.1), 4,184,314 (209.8), 1,294,996 (65.1), and 373,682 (18.7), respectively (Table 1, Fig. 1A). In particular, the four female cancers showed higher numbers of new cases and DALYs in East Asia, South Asia, and Southeast Asia among all regions (Fig. 3A and B). Breast cancer constituted both the highest proportion of all incident cases (55.40%) and DALYs (53.22%) globally among female cancers, followed by cervical cancer (30.33% and 33.44%), ovarian cancer (8.46% and 10.35%), and uterine cancer (5.81% and 2.99%) (Fig. 3C and D). In 2021, the highest age-standardised incidence rates (per 100,000 population) were reported in High-income North America for breast cancer (48.2), in Southern Sub-Saharan Africa for cervical cancer (42.0), in Southeast Asia for ovarian cancer (8.5), and in Eastern Europe for uterine cancer (10.0). While the highest age-standardised DALY rates (per 100,000 population) of breast, cervical, ovarian, and uterine cancers were reported in Oceania (617.6), Southern Sub-Saharan Africa (737.4), Eastern Europe (106.7), and Caribbean (63.6), respectively (Tables S2–S5, Fig. 1A).
Fig. 3.
Numbers and proportions of incident cases and DALYs contributed by 21 GBD regions, for female cancers, in 2021. Numbers of incident cases (A) and DALYs (B) of each cancer. Proportions of incident cases (C) and DALYs (D) accounted for by each cancer. Female cancers include breast, cervical, ovarian, and uterine cancers. DALY, disability-adjusted life-years.
From 1990 to 2021, the global age-standardised incidence rates significantly increased for breast cancer (EAPC = 0.49), ovarian cancer (0.06), and uterine cancer (0.45), while a significantly decrease trend was observed for cervical cancer (−0.39). On the contrary, the global age-standardised DALY rate decreased for all four female cancers, including breast cancer (EAPC = −0.39), cervical cancer (−1.25), ovarian cancer (−0.38), and uterine cancer (−1.23) (Table 1, Fig. 1B). Regarding the 21 regions, the age-standardised incidence rate increased across 15, 4, 13, 18 regions for breast cancer, cervical cancer, ovarian cancer, uterine cancer, with the highest increases in North Africa and Middle East (EAPC = 3.39), Southern Sub-Saharan Africa (1.94), Andean Latin America (2.39), High-income Asia Pacific (3.09), respectively. The age-standardised DALY rate increased across 9, 2, 12, 7 regions for breast cancer, cervical cancer, ovarian cancer, uterine cancer, and the most significant increases were observed in North Africa and Middle East (EAPC = 1.44), Southern Sub-Saharan Africa (1.66), Andean Latin America (1.79), and High-income North America (1.68), respectively (Tables S2–S5, Fig. 1B).
At the national level, the highest age-standardised incidence rates of breast, cervical, ovarian, and uterine cancers were observed in Bahamas, Kiribati, Seychelles, and Cuba, while the highest age-standardised DALY rates were recorded in Nauru, Kiribati, Bahamas, and Guyana for breast, cervical, ovarian, and uterine cancers, respectively. The most significant increases of age-standardised rates of incidence and DALYs were both observed in Türkiye (EAPC = 7.42, EAPC = 4.62, respectively) for breast cancer, Lesotho (5.00, 4.92) for cervical cancer, and Ecuador (6.83, 6.32) for ovarian cancer. For uterine cancer, the fastest increasing trend in age-standardised rates of incidence and DALYs were in Italy (4.67) and Zimbabwe (4.13), respectively (Tables S6–S9, Figures S1–S4).
Age-group disparities in the burden of four female cancers
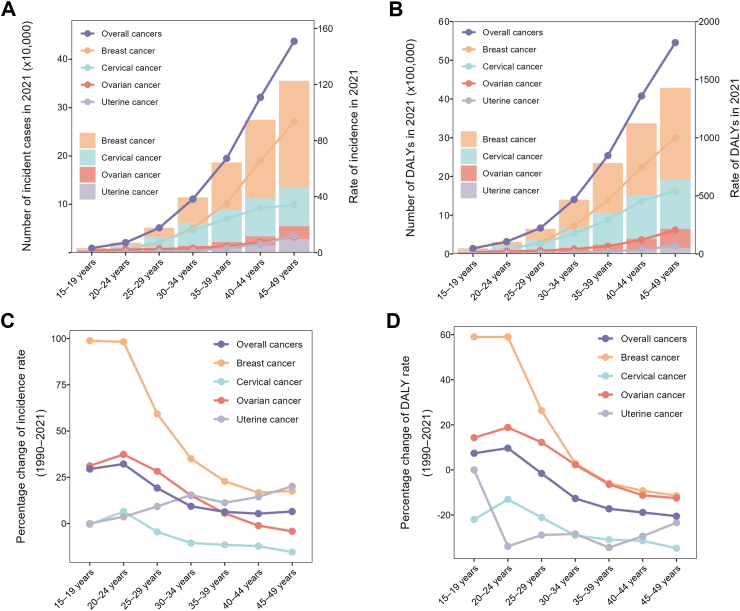
Among WCBA, the age distribution of numbers and rates in incidence and DALYs were largely consistent for the four and overall female cancers globally. Detailly, in 2021, the incidence and DALY numbers and rates of the four female cancers increased with age and reached the highest at 45–49 years (Fig. 4A and B). Additionally, in each age group, the absolute numbers and rates of incidence and DALYs were highest for breast cancer, followed by cervical cancer, ovarian cancer, and uterine cancer. The percentage changes in burdens of breast, cervical, ovarian, and overall cancers between 1990 and 2021 exhibited a declining trend. On the contrary, the percentage change of uterine cancer showed a fluctuant increase trend with female aging. In addition, the most significant increases in incidence and DALY rates of breast, cervical, and ovarian cancers were observed among those aged 15–19 years and 20–24 years (Fig. 4C and D). These patterns suggest that the burdens of these three female cancers are increasingly affecting younger women.
Fig. 4.
The cross-sectional (2021) and longitudinal trends (1990–2021) of incidence rate and DALY rate of female cancers throughout women of child-bearing age. Numbers and rates of incident cases (A) and DALYs (B) of female cancers. Percentage changes of incidence rate (C) and DALY rate (D) of female cancers. Female cancers include breast, cervical, ovarian, and uterine cancers. DALY, disability-adjusted life-years.
The association between ASR, EAPC, and SDI
From 1990 to 2021, across 21 regions, the overall age-standardised incidence rate of female cancers increased with rising SDI. The age-standardised incidence rate for breast and uterine cancers also increased, while cervical cancer decreased. The age-standardised incidence rate for ovarian cancer initially increased, then declined at an SDI of 75. The overall age-standardised DALY rate of female cancers decreased with increasing SDI, with cervical cancer showing a similar trend. However, age-standardised DALY rate for breast, ovarian, and uterine cancers initially increased with rising SDI, then declined around an SDI of 70 (Figure S5, Fig. 5).
Fig. 5.
Age-standardised rates of incidence and DALYs of each female cancer, globally and for 21 GBD regions, by SDI (2021), from 1990 to 2021. Age-standardised incidence rates of breast cancer (A), cervical cancer (B), ovarian cancer (C), and uterine cancer (D), by SDI. Age-standardised DALY rates of breast cancer (E), cervical cancer (F), ovarian cancer (G), and uterine cancer (H), by SDI. Expected values with 95% CI, based on SDI and disease rates in all locations, are shown as a solid line and shaded area; 32 points are plotted for each region and show the observed age-standardised incidence or DALY rates for each year from 1990 to 2021. Points above the solid line represent a higher-than-expected burden, and those below the line show a lower-than-expected burden. Female cancers include breast, cervical, ovarian, and uterine cancers. DALY, disability-adjusted life-years; GBD, Global Burden of Diseases, Injuries, and Risk Factors Study. SDI, socio-demographic index.
The SDI in 2021 acts as a surrogate for the level and availability of healthcare across different countries. Regarding 204 countries and territories in 2021, the overall age-standardised incidence rate of female cancers increased with rising SDI but declined after SDI reached 75. Ovarian and uterine cancers showed similar trends. Conversely, the age-standardised incidence rate for breast cancer increased with rising SDI, while cervical cancer decreased. The overall age-standardised DALY rate of female cancers decreased with rising SDI, consistent with cervical cancer. However, the age-standardised DALY rates for breast, ovarian, and uterine cancers initially increased with rising SDI and then declined around an SDI of 70 (Figures S6–S10).
From 1990 to 2021, countries and territories with low-middle and middle SDI saw faster increases in age-standardised incidence and DALY rates of overall, breast, ovarian, and uterine cancers, while those with low, low-middle, and middle SDI experienced slower decreases in rates of cervical cancer. (Figures S6–S10). In addition, positive associations were observed between the EAPCs of age-standardised rates and SDI in breast cancer and ovarian cancer, from 1990 to 2021 (Figure S11).
Discussion
This study provides a comprehensive estimation of the incidence and DALYs of female cancers among WCBA and investigates their temporal trend worldwide for the first time. The primary findings are as follows: first, the global age-standardised incidence rate of overall female cancers among WCBA increased, while the age-standardised DALY rate decreased from 1990 to 2021. Second, annual increase trends of age-standardised incidence rate were observed in all cancers, except for that in cervical cancer. Contrary, the age-standardised DALY rate decreased in all cancers. Third, breast and cervical cancers were prevalent among WCBA worldwide, followed by uterine and ovarian cancers, with regional disparities in the burden of four female cancers. Fourth, the age-standardised incidence rates of breast, ovarian, and uterine cancers basically showed a consistent upward trend with increasing SDI, while both the age-standardised incidence and DALY rates in cervical cancer exhibited downward trends with SDI. Lastly, among WCBA, the burden of female cancers increased with age groups in 2021. However, the greatest changes were observed in younger age groups from 1990 to 2021, excluding uterine cancer.
Our results indicated a global rise in the age-standardised incidence rate of female cancers among WCBA from 1990 to 2021, mainly attributable to the increases in breast, ovarian, and uterine cancers. This finding aligns with previous studies,1,6,10 indicating the need for heightened attention to the cancer burden in this population. The global rise of obesity among WCBA is likely contributing to this rise, which has been demonstrated to alter the inflammatory, metabolic, and hormonal pathways.16,17 Meanwhile, evidence strongly suggests that prolonged exposure to steroid hormones, such as estrogen replacement therapy and long-term oral contraceptive use, are implicated in breast, ovarian, and uterine cancer to different extent.18, 19, 20 Besides, researchers revealed numbers of adverse lifestyle behaviors arising from rapid social development are associated with increased female cancers through diverse mechanisms.21,22 More specific risk factors of these female cancers have been summarized in previous epidemiological studies.23, 24, 25 Given the heterogeneity and limited statistical power of these studies, additional research is warranted to gain a deeper understanding of how risk factors, particularly modifiable ones, impact female cancers,26 to achieve targeted early prevention of these cancers. In addition, socioeconomic development has led to enhanced breast cancer screening, routine gynecologic exams, and improved cancer registration, facilitating earlier detection and higher incidence rates of female cancers.27
The mentioned factors are increasingly common among women in middle SDI regions, correlating with high age-standardised incidence rates and significant increases in female cancers. A community-based research explained this phenomenon, that is cancer types were associated with different socioeconomic and lifestyle significantly.28 Rapid societal development to a middle socio-development level often leads to adverse lifestyles and improved screening, contributing to rising cancer incidence rates. Conversely, in regions that advance to a high SDI level, healthier lifestyles and effective preventive measures may reduce female cancer incidence.29 For instance, Central Latin America exhibited the highest age-standardised incidence rate, while North Africa and Middle East increased the most in our study. And the highest age-standardised incidence rate in these regions were both breast cancer, which may be associated with more risk factors.23 These findings substantiate the insufficiency of primary prevention efforts in middle SDI regions for female cancers, especially breast cancer, and emphasize the necessity for policymakers to devise and enforce easily implementable policies. This may include initiatives like promoting physical activity and healthy weight through public health centers, fostering positive lifestyle habits, and enhancing community-based public health education on female cancers.30,31
The age-standardised DALY rates of all female cancers among WCBA decreased significantly worldwide from 1990 to 2021, however, ovarian cancer experienced stable increases in many regions compared to other female cancers. According to the most recent findings of the Surveillance, Epidemiology, and End Results (SEER) Program of the National Cancer Institute (NCI), ovarian cancer ranked as the fifth leading cause of cancer-related death among women and is considered the deadliest of gynecologic cancers.32 This is primarily because early-stage ovarian cancer rarely presents symptoms, leading to delayed detection until it has spread and formed a tumor, making treatment more challenging and often resulting in a fatal outcome.33 In women deemed at high risk, the established approach continues to be risk-reducing salpingo-oophorectomy.34 Considering young women's reproductive potential, secondary prevention and healthcare measures should be actively explored to reduce the risk of ovarian dysfunction and hormonal fluctuations, thereby improving cure and survival rates.35 Meanwhile, there is currently insufficient evidence to endorse screening for the general population. The most extensive ovarian cancer screening trial conducted to date, the UK Collaborative Trial of Ovarian Cancer Screening, found that neither annual multimodal screening nor annual transvaginal ultrasound screening demonstrated a definitive reduction in ovarian or tubal cancer mortality when compared to no screening.36 Another 20-year screening trial from NCI also found no improved survival.37 Reassuringly, a clinical pilot study indicated that an 11-methylated DNA markers ovarian cancer panel can identify all five early-stage, high-grade serous ovarian cancers,38 which emphasized policies should promote larger biomarker research with more-diverse patient populations to improve early detection of ovarian cancer.
Furthermore, in the context of the global decline in age-standardised DALY rates of female cancers among WCBA from 1990 to 2021, significant negative correlations were observed between the age-standardised DALY rate and its EAPC with SDI. A systematic review summarized delays and barriers to cancer care are common in low- and middle-income regions.39 Additionally, socioeconomic disparities exist in these regions and financial resources are proportional to health status.40 Moreover, among women in Africa and Middle East, sociocultural factors have been shown to impact early screening and effective treatment of female cancers.41, 42, 43 This is underscored by our study, which identified regions with middle SDI levels, such as Southern Sub-Saharan Africa and North Africa and Middle East demonstrated significant increases in age-standardised DALY rate among WCBA. These increases were driven by both ovarian and breast cancers. Besides, these two cancers exhibited growth in most low, low-middle, and middle SDI regions, aligning with other epidemiologic studies.44,45 A review found significant disparities in ovarian cancer outcomes influenced by factors such as race and ethnicity, insurance coverage, socioeconomic status, and geographic location.46 Similarly, a report about breast cancer screening from the Centers for Disease Control and Prevention (CDC) summarized that women with low income and education were less likely to have had a mammogram.47 Researchers also observed that compared to women with high socioeconomic status (SES), those with low SES are less adherent to screening and have a two-fold risk of late-stage breast cancer.48,49 Reassuringly, the establishment of the Africa CDC in 2017 signifies an important stride in enhancing capacity and preparedness across the continent. However, to effectively tackle regional disparities in female cancers, urgent policy reforms are needed to reduce poverty and inequality through improving governance quality, economic growth, revenue distribution, and health education.50 Furthermore, given the limited and fragmented nature of female cancer research in these regions, strengthening population-based registry systems for monitoring is pressing.
Leaving aside regional differences, breast cancer was the most prevalent both in incidence and DALYs among WCBA worldwide of the female cancers included in this study, followed by cervical cancer. With giant strides in WHO, our study showed that the global age-standardised DALY rate of breast cancer had declined significantly over the past 30 years, consistent with another study.51 However, there is room for substantial improvement. In 2023, the WHO introduced the Global Breast Cancer Initiative Framework, aimed at reducing breast cancer mortality by 2.5% annually and preventing 2.5 million breast cancer deaths worldwide by 2040.52 Early detection, timely diagnosis, and complete treatment are the fundamental strategies. Nevertheless, the persistently increasing global and regional age-standardised incidence rate of breast cancer indicated that current efforts in disease prevention are significantly inadequate. Pan American Health Organization has published a Breast Health Global Initiative, suggesting lifestyle modifications (diet, exercise, alcohol), chemoprevention drugs (tamoxifen) for moderate to high-risk women, and prophylactic surgery (mastectomy or oophorectomy) for high-risk women with appropriate testing and counseling.53 Updates to this guideline with new research should guide clinical practice and benefit a broader global population, particularly in low- and middle-income countries.
Regarding cervical cancer, while it remains a significant health concern among younger women, there is reassurance in the effectiveness of scaled-up strategies such as prophylactic vaccination against HPV, along with timely screening and treatment of precancerous lesions.9 Cervical cancer has emerged as one of the most successfully prevented and treated female cancers worldwide. Also, in our study, the age-standardised rates of incidence and DALYs of cervical cancer both declined significantly from 1990 to 2021 globally. Nonetheless, the illness proves fatal for women living in lower-income nations, as they frequently encounter advanced and incurable stages due to resource constraints.4 Recent research has unveiled a surge in the incidence rate of distant-stage cervical cancer among White women in low-income counties, escalating at an annual rate of 4.4% since 2007.54 These findings align with our study results, indicating higher incidence and DALY rates of cervical cancer occurred in regions with middle and low SDI levels. Overall survival at five years in women with late-stage cervical cancer was below 19%.55 Hence, it is imperative for all nations, especially those with lower SDI, to support the resolution adopted by the World Health Assembly in 2020, which advocates for the “Elimination of Cervical Cancer” by 2030, achieved through attaining targets of immunizing 90% of girls by age 15 with the HPV vaccine, screening 70% of women at ages 35–45 using high-performance tests, and treating 90% of precancerous lesions and managing 90% of invasive cancer cases.56 Notably, despite a high false positivity rate, visual inspection with acetic acid (VIA) could be considered as an alternative screening tool in primary care in low-income countries,57 with attention given to the specificity of VIA.
Overall, the age-standardised incidence rates of breast, ovarian, and uterine cancers among WCBA basically increased with SDI, and these cancers had the highest age-standardised DALY rates in middle and high-middle SDI regions. Although breast, ovarian, and uterine cancers originate from different tissues, they share common epidemiological and hormonal risk factors, such as current age, age at menarche, and parity. A population-based cohort study developed models to predict the absolute risk of these cancers, aiding informed decisions on prevention and treatment.58 However, broader studies are needed to refine the risk factor model to support prevention and treatment strategies for WCBA. Furthermore, the global age-standardised incidence rate of overall female cancers showed a positive correlation with SDI, whereas the age-standardised DALY rate demonstrated a negative correlation, with the most rapid increases in lower SDI regions. This suggests that regions and countries should adapt their preventing or treating strategies based on their specific incidence and DALY rates. In particular, lower SDI countries should focus on primary prevention as a cost-effective strategy for long-term cancer control, as evidenced by the decline in global cervical cancer rates.
Our study also revealed that the age-standardised rates of incidence and DALYs of female cancers among WCBA increased with age groups in 2021. However, the greatest changes were observed in younger age groups from 1990 to 2021, excluding uterine cancer. This disproportionately affects women in their prime years, who are the primary caregivers for children, managing household responsibilities, and simultaneously engaging in critical professional or agricultural activities. Moreover, findings from clinical cancer trials indicated that women experience more severe symptomatic and hematologic adverse events across various treatment modalities, including immunotherapy, targeted therapy, and chemotherapy,59 suggesting significant sex differences exist. Governments worldwide must prioritize cancer control among WCBA within their development agendas and integrate gender considerations into personalized cancer medicine. Adequate resources should be allocated to investigate gender disparities and develop less toxic and women-specific therapies. Notably, a qualitative analysis found that 66.7% of younger women with gynecologic cancers reported unmet care needs, and 28% cited organizational difficulties within healthcare systems.60 Additionally, survivors often face psychological and social-sexual issues, leading to negative life changes.61 These experiences underscore the importance of high-quality women's care through psychological and pharmacological interventions.
Our study has several limitations. First, the estimation of the burden of female cancers relies heavily on the availability and quality of data from the GBD 2021. There may be a lack of access to the raw/original data for some countries, particularly those with low and middle incomes, which can hinder GBD researchers from producing their estimates. Second, our study exclusively focuses on describing the burden of four common female cancers: breast, cervical, uterine, and ovarian cancers, excluding other types of female cancers. Third, variations in the diagnosis and detection protocols for these female cancers across countries and over time may potentially impact the comparability of results. Given the uncertainties associated with the raw data, caution is warranted in interpreting the trends in the burden of female cancers among WCBA identified in this study. Fourth, a narrow focus on significance testing may overlook the clinical relevance of the findings. To mitigate this limitation, we advocate for the development and implementation of diverse analytical approaches to broaden and validate the results of this study.
In conclusion, female cancers among WCBA pose a global public health challenge. From 1990 to 2021, the incidence of female cancers worldwide has continued to rise. Despite a downward trend in the global DALY rate for female cancers from 1990 to 2021, regional disparities persist. Healthcare providers should recognise that social factors associated with globalization may contribute to an increasing number of WCBA being at risk for female cancers. Moreover, tailored primary prevention, secondary prevention and healthcare strategies should be optimized to address the needs of WCBA based on age, region, and disease type, particularly in aging societies.
Contributors
ZWW conceived the study. PS designed the protocol. PS, CY, LMY, and YC analysed the GBD data. CY, ZCS, TTZ, PS, KHZ, XQY, JYC, and YPL contributed to the statistical analysis and interpretation of data. PS and ZWW drafted the manuscript, and other authors critically revised the manuscript. PS and ZWW accessed and verified the underlying data. All authors have read and approved the final version of the manuscript.
Data sharing statement
Data used for the analyses are publicly available from the Institute of Health Metrics and Evaluation (http://www.healthdata.org/; http://ghdx.healthdata.org/gbd-results-tool).
Editor note
The Lancet Group takes a neutral position with respect to territorial claims in published maps and institutional affiliations.
Declaration of interests
All authors declare no competing interests.
Acknowledgements
This study was supported by the Science Foundation for Young Scholars of Sichuan Provincial People's Hospital (NO. 2022QN44 and NO. 2022QN18); the Key R&D Projects of Sichuan Provincial Department of Science and Technology (NO. 2023YFS0196); the National Natural Science Foundation of China (No. 82303701). We acknowledge the Institute for Health Metrics and Evaluation (University of Washington), the GBD Collaborators, and all staff who provided the data necessary for this study. The opinions expressed here are those of the authors and do not necessarily represent the official position of the organizations with which they are affiliated.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2024.102713.
Contributor Information
Yuping Liu, Email: 18981838972@163.com.
Zhengwei Wan, Email: 18715799366@163.com.
Appendix A. Supplementary data
References
- 1.Yi M., Li T., Niu M., Luo S., Chu Q., Wu K. Epidemiological trends of women's cancers from 1990 to 2019 at the global, regional, and national levels: a population-based study. Biomark Res. 2021;9(1):55. doi: 10.1186/s40364-021-00310-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ugai T., Sasamoto N., Lee H.Y., et al. Is early-onset cancer an emerging global epidemic? Current evidence and future implications. Nat Rev Clin Oncol. 2022;19(10):656–673. doi: 10.1038/s41571-022-00672-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li N., Deng Y., Zhou L., et al. Global burden of breast cancer and attributable risk factors in 195 countries and territories, from 1990 to 2017: results from the global burden of disease study 2017. J Hematol Oncol. 2019;12(1):140. doi: 10.1186/s13045-019-0828-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Canfell K., Kim J.J., Brisson M., et al. Mortality impact of achieving WHO cervical cancer elimination targets: a comparative modelling analysis in 78 low-income and lower-middle-income countries. Lancet. 2020;395(10224):591–603. doi: 10.1016/S0140-6736(20)30157-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koh B., Tan D.J.H., Ng C.H., et al. Patterns in cancer incidence among people younger than 50 years in the US, 2010 to 2019. JAMA Netw Open. 2023;6(8) doi: 10.1001/jamanetworkopen.2023.28171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ginsburg O., Bray F., Coleman M.P., et al. The global burden of women's cancers: a grand challenge in global health. Lancet. 2017;389(10071):847–860. doi: 10.1016/S0140-6736(16)31392-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benitez Fuentes J.D., Morgan E., de Luna Aguilar A., et al. Global stage distribution of breast cancer at diagnosis: a systematic review and meta-analysis. JAMA Oncol. 2024;10(1):71–78. doi: 10.1001/jamaoncol.2023.4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization Fact sheets: cervical cancer. https://www.who.int/news-room/fact-sheets/detail/cervical-cancer
- 9.Singh D., Vignat J., Lorenzoni V., et al. Global estimates of incidence and mortality of cervical cancer in 2020: a baseline analysis of the WHO global cervical cancer elimination initiative. Lancet Glob Health. 2023;11(2):e197–e206. doi: 10.1016/S2214-109X(22)00501-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bray F., Laversanne M., Sung H., et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74(3):229–263. doi: 10.3322/caac.21834. [DOI] [PubMed] [Google Scholar]
- 11.Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the global burden of disease study 2019. Lancet. 2020;396(10258):1204–1222. doi: 10.1016/S0140-6736(20)30925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization Women of reproductive age (15-49 years) population (thousands) https://www.who.int/data/gho/indicator-metadata-registry/imr-details/women-of-reproductive-age-(15-49-years)-population-(thousands)
- 13.Global incidence, prevalence, years lived with disability (YLDs), disability-adjusted life-years (DALYs), and healthy life expectancy (HALE) for 371 diseases and injuries in 204 countries and territories and 811 subnational locations, 1990-2021: a systematic analysis for the global burden of disease study 2021. Lancet. 2024;403(10440):2133–2161. doi: 10.1016/S0140-6736(24)00757-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fay M.P., Feuer E.J. Confidence intervals for directly standardized rates: a method based on the gamma distribution. Stat Med. 1997;16(7):791–801. doi: 10.1002/(sici)1097-0258(19970415)16:7<791::aid-sim500>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 15.Hankey B.F., Ries L.A., Kosary C.L., et al. Partitioning linear trends in age-adjusted rates. Cancer Causes Control. 2000;11(1):31–35. doi: 10.1023/a:1008953201688. [DOI] [PubMed] [Google Scholar]
- 16.Wichmann I.A., Cuello M.A. Obesity and gynecological cancers: a toxic relationship. Int J Gynaecol Obstet. 2021;155 Suppl 1(Suppl 1):123–134. doi: 10.1002/ijgo.13870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Picon-Ruiz M., Morata-Tarifa C., Valle-Goffin J.J., Friedman E.R., Slingerland J.M. Obesity and adverse breast cancer risk and outcome: mechanistic insights and strategies for intervention. CA Cancer J Clin. 2017;67(5):378–397. doi: 10.3322/caac.21405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Travis R.C., Key T.J. Oestrogen exposure and breast cancer risk. Breast Cancer Res. 2003;5(5):239–247. doi: 10.1186/bcr628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ho S.M. Estrogen, progesterone and epithelial ovarian cancer. Reprod Biol Endocrinol. 2003;1:73. doi: 10.1186/1477-7827-1-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liang Y., Jiao H., Qu L., Liu H. Association between hormone replacement therapy and development of endometrial cancer: results from a prospective US cohort study. Front Med (Lausanne) 2021;8 doi: 10.3389/fmed.2021.802959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghosn B., Benisi-Kohansal S., Ebrahimpour-Koujan S., Azadbakht L., Esmaillzadeh A. Association between healthy lifestyle score and breast cancer. Nutr J. 2020;19(1):4. doi: 10.1186/s12937-020-0520-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rieck G., Fiander A. The effect of lifestyle factors on gynaecological cancer. Best Pract Res Clin Obstet Gynaecol. 2006;20(2):227–251. doi: 10.1016/j.bpobgyn.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 23.Łukasiewicz S., Czeczelewski M., Forma A., Baj J., Sitarz R., Stanisławek A. Breast cancer-epidemiology, risk factors, classification, prognostic markers, and current treatment strategies-an updated review. Cancers (Basel) 2021;13(17):4287. doi: 10.3390/cancers13174287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reid B.M., Permuth J.B., Sellers T.A. Epidemiology of ovarian cancer: a review. Cancer Biol Med. 2017;14(1):9–32. doi: 10.20892/j.issn.2095-3941.2016.0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Felix A.S., Brinton L.A. Cancer progress and priorities: uterine cancer. Cancer Epidemiol Biomarkers Prev. 2018;27(9):985–994. doi: 10.1158/1055-9965.EPI-18-0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anand P., Kunnumakkara A.B., Sundaram C., et al. Cancer is a preventable disease that requires major lifestyle changes. Pharm Res. 2008;25(9):2097–2116. doi: 10.1007/s11095-008-9661-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu C.H., Grodzinski P. Nanotechnology for cancer imaging: advances, challenges, and clinical opportunities. Radiol Imaging Cancer. 2021;3(3) doi: 10.1148/rycan.2021200052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sandström N.A.-O.X., Johansson M.A.-O., Jekunen A.A.-O., Andersén H.A.-O. Socioeconomic status and lifestyle patterns in the most common cancer types-community-based research. BMC Public Health. 2023;23(1):1722. doi: 10.1186/s12889-023-16677-6. (Electronic)) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stewart D.W., Cano M.A., Correa-Fernández V., et al. Lower health literacy predicts smoking relapse among racially/ethnically diverse smokers with low socioeconomic status. BMC Public Health. 2014;14:716. doi: 10.1186/1471-2458-14-716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yin X., Zhang T., Zhang Y., Man J., Yang X., Lu M. The global, regional, and national disease burden of breast cancer attributable to low physical activity from 1990 to 2019: an analysis of the Global Burden of Disease Study 2019. Int J Behav Nutr Phys Act. 2022;19(1):42. doi: 10.1186/s12966-022-01283-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guo Q., Lu Y., Liu W., Lan G., Lan T. The global, regional, and national disease burden of breast cancer attributable to tobacco from 1990 to 2019: a global burden of disease study. BMC Public Health. 2024;24(1):107. doi: 10.1186/s12889-023-17405-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.National Cancer Institute SEER cancer statistics factsheets: ovarian cancer. https://seer.cancer.gov/statfacts/html/ovary.html
- 33.Malvezzi M., Carioli G., Rodriguez T., Negri E., La Vecchia C. Global trends and predictions in ovarian cancer mortality. Ann Oncol. 2016;27(11):2017–2025. doi: 10.1093/annonc/mdw306. [DOI] [PubMed] [Google Scholar]
- 34.Manchanda R., Gaba F., Talaulikar V., et al. Risk-reducing salpingo-oophorectomy and the use of hormone replacement therapy below the age of natural menopause: scientific impact paper No. 66 October 2021: scientific impact paper No. 66. BJOG. 2022;129(1):e16–e34. doi: 10.1111/1471-0528.16896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reynolds A.C., McKenzie L.J. Cancer treatment-related ovarian dysfunction in women of childbearing potential: management and fertility preservation options. J Clin Oncol. 2023;41(12):2281–2292. doi: 10.1200/JCO.22.01885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Menon U., Gentry-Maharaj A., Burnell M., et al. Ovarian cancer population screening and mortality after long-term follow-up in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): a randomised controlled trial. Lancet. 2021;397(10290):2182–2193. doi: 10.1016/S0140-6736(21)00731-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.National Cancer Institute Division of cancer prevention. Prostate, lung, colorectal, and ovarian cancer screening trial (PLCO) https://prevention.cancer.gov/major-programs/prostate-lung-colorectal-and-ovarian-cancer-screening-trial-plco
- 38.Marinelli L.M., Kisiel J.B., Slettedahl S.W., et al. Methylated DNA markers for plasma detection of ovarian cancer: discovery, validation, and clinical feasibility. Gynecol Oncol. 2022;165(3):568–576. doi: 10.1016/j.ygyno.2022.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brand N.R., Qu L.G., Chao A., Ilbawi A.M. Delays and barriers to cancer care in low- and middle-income countries: a systematic review. Oncol. 2019;24(12):e1371–e1380. doi: 10.1634/theoncologist.2019-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Demakakos P., Nazroo J., Breeze E., Marmot M. Socioeconomic status and health: the role of subjective social status. Soc Sci Med. 2008;67(2):330–340. doi: 10.1016/j.socscimed.2008.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sarmah N., Sibiya M.N., Khoza T.E. The sociocultural influences on breast cancer screening among rural african women in South Africa. Int J Environ Res Public Health. 2023;20(21):7005. doi: 10.3390/ijerph20217005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mantula F., Toefy Y., Sewram V. Barriers to cervical cancer screening in Africa: a systematic review. BMC Public Health. 2024;24(1):525. doi: 10.1186/s12889-024-17842-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bowser D., Marqusee H., El Koussa M., Atun R. Health system barriers and enablers to early access to breast cancer screening, detection, and diagnosis: a global analysis applied to the MENA region. Public Health. 2017;152:58–74. doi: 10.1016/j.puhe.2017.07.020. [DOI] [PubMed] [Google Scholar]
- 44.Cabasag C.J., Fagan P.J., Ferlay J., et al. Ovarian cancer today and tomorrow: a global assessment by world region and human development index using GLOBOCAN 2020. Int J Cancer. 2022;151(9):1535–1541. doi: 10.1002/ijc.34002. [DOI] [PubMed] [Google Scholar]
- 45.Xu Y., Gong M., Wang Y., Yang Y., Liu S., Zeng Q. Global trends and forecasts of breast cancer incidence and deaths. Sci Data. 2023;10(1):334. doi: 10.1038/s41597-023-02253-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mei S., Chelmow D., Gecsi K., et al. Health disparities in ovarian cancer: report from the ovarian cancer evidence review conference. Obstet Gynecol. 2023;142(1):196–210. doi: 10.1097/AOG.0000000000005210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Breast cancer screening and socioeconomic status--35 metropolitan areas, 2000 and 2002. MMWR Morb Mortal Wkly Rep. 2005;54(39):981–985. [PubMed] [Google Scholar]
- 48.Kasper G., Momen M., Sorice K.A., et al. Effect of neighborhood and individual-level socioeconomic factors on breast cancer screening adherence in a multi-ethnic study. BMC Public Health. 2024;24(1):63. doi: 10.1186/s12889-023-17252-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Orsini M., Trétarre B., Daurès J.P., Bessaoud F. Individual socioeconomic status and breast cancer diagnostic stages: a French case-control study. Eur J Public Health. 2016;26(3):445–450. doi: 10.1093/eurpub/ckv233. [DOI] [PubMed] [Google Scholar]
- 50.Gallifant J., Kistler E.A., Nakayama L.F., et al. Disparity dashboards: an evaluation of the literature and framework for health equity improvement. Lancet Digit Health. 2023;5(11):e831–e839. doi: 10.1016/S2589-7500(23)00150-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.World Health Organization Fact sheets: breast cancer. https://www.who.int/news-room/fact-sheets/detail/breast-cancer
- 52.World Health Organization Global breast cancer initiative implementation framework: assessing, strengthening and scaling up of services for the early detection and management of breast cancer: executive summary. https://www.who.int/publications/i/item/9789240067134
- 53.Pan American Health Organization Prevention: breast cancer risk factors and prevention. https://www.paho.org/en/documents/prevention-breast-cancer-risk-factors-and-prevention
- 54.Amboree T.L., Damgacioglu H., Sonawane K., Adsul P., Montealegre J.R., Deshmukh A.A. Recent trends in cervical cancer incidence, stage at diagnosis, and mortality according to county-level income in the United States, 2000-2019. Int J Cancer. 2024;154(9):1549–1555. doi: 10.1002/ijc.34860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Centers for Disease Control and Prevention United States cancer statistics: data visualizations. https://gis.cdc.gov/Cancer/USCS
- 56.World Health Organization Initiatives: cervical cancer elimination initiative. https://www.who.int/initiatives/cervical-cancer-elimination-initiative
- 57.Namale G., Mayanja Y., Kamacooko O., et al. Visual inspection with acetic acid (VIA) positivity among female sex workers: a cross-sectional study highlighting one-year experiences in early detection of pre-cancerous and cancerous cervical lesions in Kampala, Uganda. Infect Agent Cancer. 2021;16(1):31. doi: 10.1186/s13027-021-00373-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pfeiffer R.M., Park Y., Kreimer A.R., et al. Risk prediction for breast, endometrial, and ovarian cancer in white women aged 50 y or older: derivation and validation from population-based cohort studies. PLoS Med. 2013;10(7) doi: 10.1371/journal.pmed.1001492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Unger J.M., Vaidya R., Albain K.S., et al. Sex differences in risk of severe adverse events in patients receiving immunotherapy, targeted therapy, or chemotherapy in cancer clinical trials. J Clin Oncol. 2022;40(13):1474–1486. doi: 10.1200/JCO.21.02377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mattsson E., Ljungman L., Einhorn K., Sundström Poromaa I., Stålberg K., Wikman A. Perceptions of care after end-of-treatment among younger women with different gynecologic cancer diagnoses - a qualitative analysis of written responses submitted via a survey. BMC Womens Health. 2020;20(1):276. doi: 10.1186/s12905-020-01133-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Abbott-Anderson K., Kwekkeboom K.L. A systematic review of sexual concerns reported by gynecological cancer survivors. Gynecol Oncol. 2012;124(3):477–489. doi: 10.1016/j.ygyno.2011.11.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.