Abstract
Lung cancer is a leading cause of morbidity and mortality globally, with its high mortality rate attributed mainly to non-small cell lung cancer (NSCLC). Although immunotherapy with immune checkpoint inhibitors (ICI) has revolutionized its treatment, patient response is highly variable and lacking predictive markers. We conducted a prospective study on 55 patients with NSCLC undergoing ICI therapy to identify predictive markers of both response and immune-related adverse events (IrAEs) in the airway microbiota. We also analyzed the clinical evolution and overall survival (OS) with respect to treatments that affect the integrity of the microbiota, such as antibiotics and corticosteroids. Our results demonstrated that respiratory microbiota differ significantly in ICI responders: they have higher alpha diversity values and lower abundance of the Firmicutes phylum and the Streptococcus genus. Employing a logistic regression model, the abundance of Gemella was the major predictor of non-ICI response, whereas Lachnoanaerobaculum was the best predictor of a positive response to ICI. The most relevant results were that antibiotic consumption is linked to a lower ICI response, and the use of corticosteroids correlated with poorer overall survival. Whereas previous studies have focused on gut microbiota, our findings highlight the importance of the respiratory microbiota in predicting the treatment response. Future research should explore microbiota modulation strategies to enhance immunotherapy outcomes. Understanding the impact of antibiotics, corticosteroids, and microbiota on NSCLC immunotherapy will help personalize treatment and improve patient outcomes.
Keywords: Lung cancer, Immunotherapy, Airway microbiota, Antibiotics, Corticosteroids
1. Introduction
Cancer is one of the major causes of morbidity and mortality worldwide, with lung cancer being among the most frequently diagnosed [1,2]. However, its prevalence is low due to its high mortality rate. Based on GLOBOCAN estimations, 18 % of all cancer deaths are due to lung cancer [3], and among its risk factors, tobacco is the most prevalent followed by radon gas [2]. In terms of treatment, immune-checkpoint inhibitors (ICI) have brought about a radical change, particularly in non-small cell lung cancer (NSCLC), where it has been approved in advanced/metastatic, locally advanced, and adjuvant settings; however, not all patients achieve the same results. In view of this discrepancy in response, attempts have been made to identify therapy-predictive biomarker response [4,5], with some previous studies suggesting the relevance of host-related factors, such as the microbiota, which has been directly linked to ICI effectiveness [6,7].
The microbiota has been shown to regulate immunotherapy potential by stimulating the anti-tumor immune response, although some microorganisms might metabolize these drugs, thereby inactivating them [[7], [8], [9], [10], [11]]. The lung microbiota is relatively understudied compared to the intestinal compartment, mainly because of the difficulty in obtaining representative samples, and the available evidence has recently been summarized [12]. The particularities of the gut microbiota between ICI responders and non-responders have previously been reported [[13], [14], [15]]. In addition, the impact of concomitant treatments, such as antibiotics or corticosteroids, in the microbiota composition and finally in the ICI response has been poorly evaluated, despite the current evidence suggesting their implication in the loss of gut microbiota diversity [4,[16], [17], [18], [19], [20]]. Also, a relationship between immune-related adverse events (IrAEs) and ICI response has been proposed, again without any useful marker for predicting it.
The aim of the present study was to prospectively analyze a cohort of patients with NSCLC treated with ICI to decipher microbiota-based markers for both ICI response and toxicity, as well as to estimate the impact of antibiotics and corticosteroids on survival.
2. Materials and methods
2.1. Study design, inclusion, and exclusion criteria
This was a prospective, observational study conducted at the Medical Oncology Department of the Lozano Blesa University Hospital Clinic in Zaragoza (Spain), in 55 patients diagnosed with NSCLC and ICI indication, who were consecutively included between April 2019 and October 2020.
The inclusion criteria were as follows: patients with locally advanced unresectable and metastatic NSCLC, stages III and IV, with ICI indication (Stage IV patients could receive ICI as a first line of treatment, in monotherapy or combination with chemotherapy, or in subsequent lines of treatment [1]. Patients with unresectable stage III started ICI treatment after radical treatment with concomitant chemotherapy and thoracic radiotherapy, without progressive disease after that treatment. Patients could have been diagnosed de novo or relapsed or progressed to treatment other than ICI); 18 years of age or older; and have an Eastern Cooperative Oncology Group (ECOG) performance status score lower than or equal to 2.
The exclusion criteria were as follows: contraindication to receiving ICI; histology different from NSCLC; another concomitant tumor; prior treatment with an ICI antitumor agent; or being on corticosteroid treatment with doses of prednisone ≥10 mg/24 h or equivalent.
The protocol was evaluated and approved by the Clinical Research Ethics Committee of Aragón with code (C.I. PI19/052). Prior to their inclusion in the study, all patients gave their informed consent, and baseline blood, saliva, and stool samples were collected before starting immunotherapy treatment.
Patients were followed every 2–3 weeks during the treatment administration, as performed in our routine clinical practice, collecting all relevant clinical data for the study, particularly the antibiotic data (the antibiotic cycle, the origin of the infection, and the clinical response), and the corticosteroid (prednisone >10 mg or its equivalent) consumption. The registration period covered from 2 months prior to ICI start until the end of follow-up.
Patients who achieved a response and started follow-up were evaluated every 3 months. During shadowing, tumor response was assessed every 9–12 weeks according to Response Evaluation Criteria in Solid Tumors (RECIST) (v.1.1) [21]. Treatment safety was assessed by recording adverse events and alterations in analytical parameters. IrAEs were monitored and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (v.5.0) [22].
2.2. Sampling
Prior to treatment initiation, a tumor biopsy was routinely collected for programmed death-ligand 1 (PD-L1) determination. Additionally, each patient contributed with an oral sample (saliva + sputum collected by medical staff) and peripheral blood. Samples were immediately frozen at −80 °C until recruitment was complete.
2.3. Microbiota determination
Samples were slowly defrosted at −20 °C for 24 h and 4 °C for another 24 h, to avoid bacterial death and DNA fragmentation. Total DNA was obtained by the QiaAmp kit (QIAGEN) from the saliva pellet after centrifugation. The bacterial composition was determined by polymerase chain reaction (PCR) amplification of the 16S rDNA V3–V4 region, and PCR products were pooled equally and submitted to massive sequencing (2x300 bp) on a MiSeq (Illumina, San Diego, CA, USA) platform at traslational genomics core support unit (UCAT) -Ramon y Cajal Health Research Institute (Madrid, Spain). The sequence's quality control was performed with DADA2, with a rarity of 12,000 sequences per sample. Amplicon sequencing variants were obtained by taxonomic assignment with the Silva_132 classifier. Alpha and beta diversity studies were performed, employing the q2-diversity add-on of QIIME2, after normalizing the samples by rarefaction (subsampling without replacement). In addition, a linear effect size discriminant analysis (LEfSe) was performed to assess which taxa explained the differences between groups. Sequence data were deposited in Genbank (BioProjectPRJNA1054537).
2.4. Programmed death-ligand 1 biomarker analysis
PD-L1 protein expression was evaluated in lung biopsies, naïve to treatment. Samples were considered suitable for PD-L1 staining if they had more than 100 valuable neoplastic cells. PD-L1 expression was assessed with PD-L1 IHC 22C3 pharmaDx, in formalin-fixed tumor samples. PD-L1 expression was confirmed when staining of the tumor cell membrane (at any intensity) was observed. The prespecified expression levels are 1 %–50 % (low expression) or more than 50 % (high expression).
2.5. Machine learning analysis
The precision of various predictive models was explored using various algorithms, such as logistic regression, random forest, k-nearest neighbors, neural networks, and support vector machines, employing the two data sets: bacterial abundance in saliva and available clinical data. Both data sets were randomly divided into training and test sets. The models’ scoring was evaluated using precision accuracy, and we paid special attention to the f1-score, which allowed us to fine-tune the precision for ICI response and/or toxicity, overall survival (OS), and progression-free survival (PFS). Our data sets are considered relatively small from a machine learning point of view, so we averaged our results over 500 experiments. All models were performed in Python 3.8.10, using the Scikit-learn package.
2.6. Statistical analysis
The normal distribution of variables was compared using the Kolmogorov–Smirnov test. For the quantitative variables, distribution normality was checked by the Anderson–Darling test to define the parametric (t-test) and non-parametric (Mann–Whitney) analyses.
The correlations between OS (months) and quantitative variables were assessed with Pearson's or Spearman's rank correlation, depending on data distribution. For the qualitative variables, Fisher's exact test was employed.
A descriptive analysis of the survival function and cumulative risk function was conducted using the Kaplan–Meier product limit estimator. The study aimed to assess whether the risk function differed based on the presence of certain factors through a bivariate analysis. The Mantel–Haenszel (log-rank) test was employed to compare the risk functions among various groups. The Cox proportional hazards model, also known as Cox regression, was utilized to estimate a model that examines how covariates collectively influence the risk of complications. Probability (p) values of <0.05 were considered significant at a 95 % confidence interval.
3. Results
3.1. Patients and treatment
Fifty-five Caucasian patients (median age 65 years, 70.9 % males) were enrolled, 65.5 % of whom had ECOG 0 at diagnosis. Regarding smoking, 3.6 % were nonsmokers, compared with 96.4 % who were smokers or former smokers. The histology included adenocarcinomas (60 %) and squamous cell carcinomas (40 %), with stage IV tumor in 70.9 % of cases. En terms of PD-L1 expression, 18.2 % of patients had an expression <1 %, with 29.1 % being high expressers (>50 %) (Table 1).
Table 1.
Descriptive variables in our cohort.
| Characteristics of the Patients at baseline | N (55) | % |
|---|---|---|
| Age (median) | 65[39,62,64–67] | |
|
39 16 |
70.9 % 29.1 % |
|
55 | 100 % |
|
53 2 |
96.4 % 3.6 % |
|
46 9 |
83.6 % 16.4 % |
|
36 19 |
65.5 % 34.5 % |
|
22 33 |
40 % 60 % |
|
16 39 |
29.1 % 70.9 % |
|
14 18 23 |
25.5 % 32,7 % 41.8 % |
|
10 21 16 8 |
18.2 % 38.2 % 29.1 % 14.5 % |
|
21 18 14 2 |
38.2 % 32.7 % 25.5 % 3.6 % |
|
10 13 12 15 5 |
18.8 % 23.6 % 21.8 % 27.3 % 9.1 % |
|
28 27 |
50.9 % 49.1 % |
|
22 33 |
40 % 60 % |
|
32 23 |
58.2 % 41.8 % |
|
9 23 |
28.1 % 71.9 % |
A total of 25.5 % of the patients received ICI and had a locally advanced stage, 32.7 % received it as first-line palliative treatment, with the remaining 41.8 % in subsequent lines of palliative treatment. Pembrolizumab was the most frequently employed ICI (38.2 %) (Table 1).
Antimicrobial therapy was required by 50.9 % of the patients. 60 % received it after the first month of immunotherapy and the other 40 % received it in the window from 60 days before to 42 after ICI starting. Although 60.7 % required only one course of antibiotic treatment, up to 7.1 % needed four courses. The indications were respiratory (71.5 %, of which 28.6 % was respiratory infection/pneumonia), urinary (14.3 %), or intra-abdominal (3.6 %) infections, and were unknown in 7.1 % of the cases. Amoxicillin/clavulanic acid, azithromycin, and levofloxacin were the most frequent antimicrobial agents.
Corticosteroids were required by 58.2 % of the patients, up to 71.9 % of them after the first ICI cycle. The reasons for their use were IrAEs (34.4 %), cerebral metastases (9.4 %), and exacerbations of chronic obstructive pulmonary disease (COPD) (9.4 %) (Table 1).
Globally, 45.5 % of patients reported IrAEs (Table 2), most of them classified as late IrAEs (>3 months of ICI); 75 % were grade 1–2, and only one patient had grade 4 toxicity (hepatitis).
Table 2.
Types and grades of immune-related adverse events described in our cohort.
| No. (%) | GRADE (%) 1 2 3 4 |
||||
|---|---|---|---|---|---|
| Infusion reaction | 1 (1.8) | 100.0 | – | – | – |
| Endocrine | 5 (9.1) | 40.0 | 60.0 | – | – |
| Pneumonitis | 6 (10.9) | 16.7 | 66.7 | 16.7 | |
| Colitis | 2 (3.6) | – | 100.0 | – | – |
| Hepatitis | 3 (5.5) | 33.3 | 33.3 | – | 33.3 |
| Nephritis | 4 (7.3) | 25.0 | 25.0 | 50.0 | – |
| Neurologic | 1 (1.8) | – | – | 100.0 | – |
| Musculoskeletal | 5 (9.1) | 40.0 | 20.0 | 40.0 | – |
| Skin | 6 (10.9) | 50.0 | 33.3 | 16.7 | – |
| Cardiovascular | 1 (1.8) | – | – | 100.0 | – |
| Ocular | 1 (1.8) | 100.0 | – | – | – |
| Other | 1 (1.8) | – | 100.0 | – | – |
3.2. Microbiota analysis
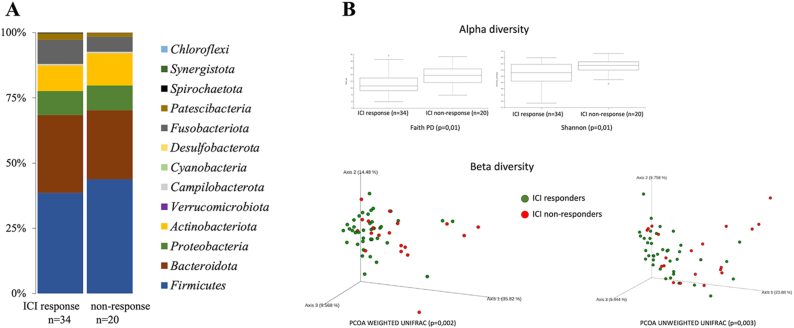
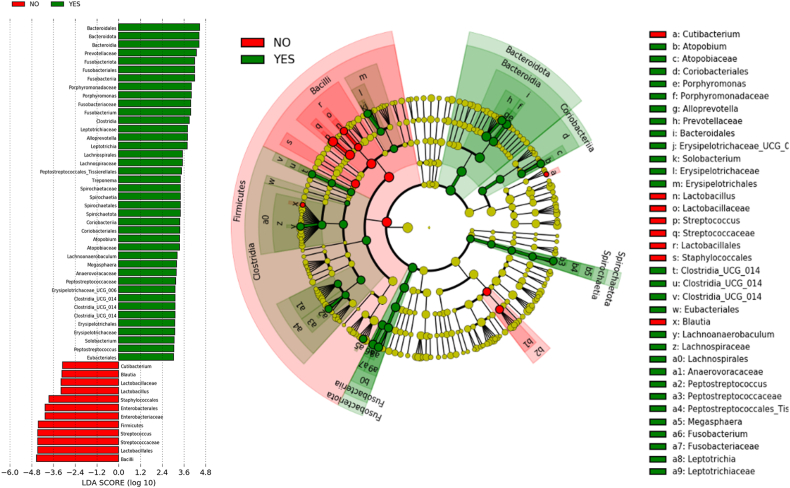
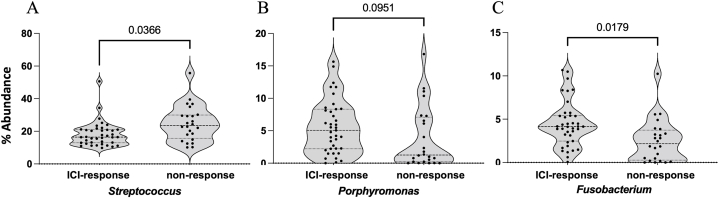
After quality control, only one saliva sample was eliminated by the low quantity of reads. Significant differences were observed in the respiratory microbiota between ICI responders and ICI non-responders [Fig. 1(A-B) − 3(A-C)]. ICI responders presented a significantly greater abundance of Fusobacteria and Porphyromonas, whereas in ICI non-responders the Streptococcus genera was significantly more abundant [Fig. 2, Fig. 3].
Fig. 1.
Oral microbiota. A: Bacterial phyla distribution for ICI responders and non- responders. B: Alfa and beta diversity statistical análisis among ICI responders and non- responders.
Fig. 2.
LEfSe analysis of the oral microbiota between ICI responders (in green) and non-responders (in red). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Fig. 3.
Percentage of the most relevant genera with statistical differences between ICI responders and non-responders. A: Streptococcus; B: Porphyromonas; C: Fusobacterium.
3.3. Survival analysis
The median overall survival (mOS) was 19 months (95 % CI, 11.13 to 26.87), while the median PFS was 10 months (95 % CI, 2.81 to 17.19). At the 24-month mark, 36 % of the patients were still alive, and 30 % had not experienced disease progression.
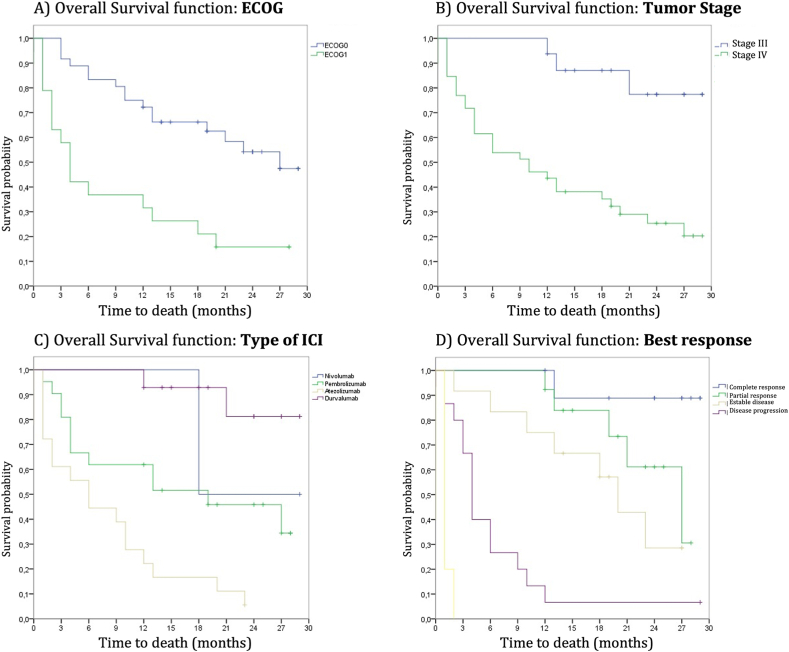
We detected no differences in OS according to sex, smoking status, body mass index, or tumor histology. There were also no significant differences based on age (cut-off 75 years), although there was a tendency toward a shorter OS in older patients. Regarding ECOG, patients with ECOG 0 had a longer OS (27 months) compared with those with ECOG 1 (4 months), and these differences were statistically significant (p < 0.001) [Fig. 4(A-D)].
Fig. 4.
Kaplan–Meier survival curve graphs. A) Survival curves based on Eastern Cooperative Oncology Group scale. B) Survival curves based on tumor stage. C) Survival curves based on the type of immunotherapy. D) Survival curves based on the best-achieved response. The numerical data and p-values are specified in the text above.
According to the best achieved response, differences were observed, which were confirmed to be significant. The mOS was 4 months for progressive disease (PR), 20 months for stable disease (EE), 27 months for partial response (RP), and not reached (NR) for complete response (RC).
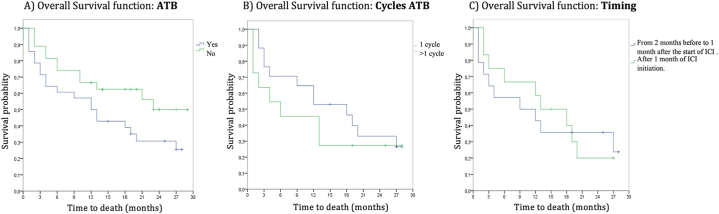
Although no statistically significant differences were found in the analysis of survival and antibiotic exposure, there was a clear trend toward longer survival in patients who did not require antibiotic treatment (mOS 23 months for non-use vs 12 months for use of antibiotics; p = 0.078). Within the cohort of responders, it was observed that 60 % did not receive antibiotics, whereas among non-responders, 74 % required antibiotic treatment. Statistically significant differences were not found when analyzing this item based on the number of cycles or on the timing of its use (from 2 months before to 1 month after the start of ICI vs. 1 month after ICI initiation) [Fig. 5(A-C)]. Neither of the differences was found when the relationship with PFS was studied.
Fig. 5.
Kaplan–Meier overall survival curve graphs. A) Survival curves based on use or non-use of antibiotics. B) Survival curves based on cycles of antibiotics. C) Survival curves based on timing of use.
No association between antibiotic use and OS was found; however, non-responders to ICI were exposed to more antibiotics, whereas a higher number of patients without antibiotic use was observed among responders (p = 0.0439).
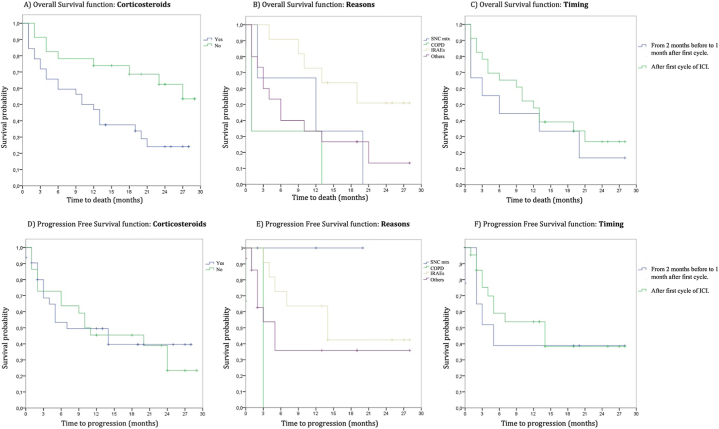
When analyzing the relationship between OS and corticosteroid use, statistically significant differences were found (p = 0.011). However, when performing the subgroup analysis based on the reasons for use (COPD, IrAEs, brain metastasis, and others) or timing of use (from 2 months before to 1 month after the first cycle vs. after the first cycle of ICI), these differences did not persist [Fig. 6(A-C)].
Fig. 6.
Kaplan–Meier overall survival and progression-free survival curve graphs. A) Survival curves based on the use or non-use of corticosteroids. B) Survival curves based on reasons for its use. C) Survival curves based on timing. D) Progression-free survival based on use or non-use of corticosteroids. E) Progression-free survival based on reasons for its use. F) Progression free survival based on timing.
When analyzing associations between PFS and corticosteroid use, we found no statistically significant differences (p = 0.846). The same occurred when analyzing the previously mentioned subtypes [Fig. 6(D-F)].
The occurrence of IrAEs had a positive correlation with extended OS (p = 0.051). The median OS of patients with IrAEs was 21 months, whereas in those without such events it was 6 months. However, when we analyzed PFS, the differences observed in the graphs were confirmed in the survival analysis, and these differences were statistically significant (p = 0.03). No significant differences were found in OS, nor in PFS, based on the type or grade of toxicity.
As mentioned earlier, blood samples were also collected. We attempted to find an association between the presence of IrAEs and variation in the percentage of various lymphocyte populations. We analyzed CD8 T lymphocytes (LAG3+, TIM3+), CD4 T lymphocytes, and natural killer cells. No significant differences were found with any of these lymphocyte subpopulations.
3.4. Markers for immune checkpoint inhibitor response
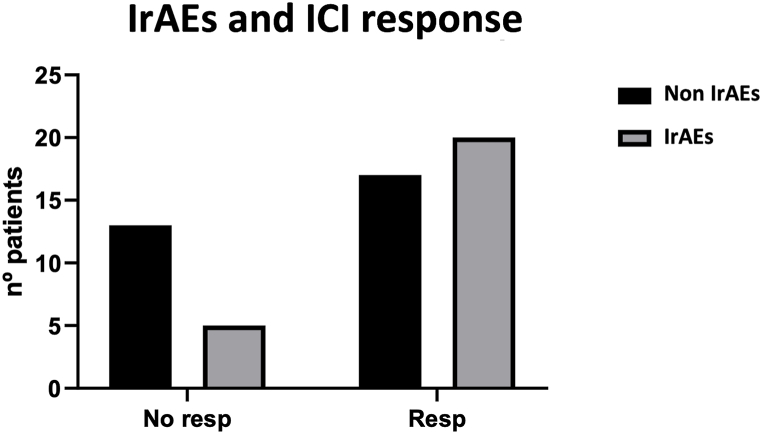
Thirty-five (63.6 %) patients were considered as ICI responders, with a statistically significant association with longer OS and PFS. A correlation was also found between the presence of IrAEs and the response to immunotherapy. Specifically, there was a higher percentage of patients without immune-related toxicity among non-responders to ICI (Fig. 7).
Fig. 7.
Correlation between immune checkpoint inhibitor response and immune-related adverse events. Chi-square test p = 0.06.
ICI responders presented greater abundance of Bacteroidota to the detriment of Firmicutes, with a fourfold increase in Fusobacterium and Porphyromonas, whereas the abundance of Streptococcus was up to 4.5 times lower [Fig. 2, Fig. 3].
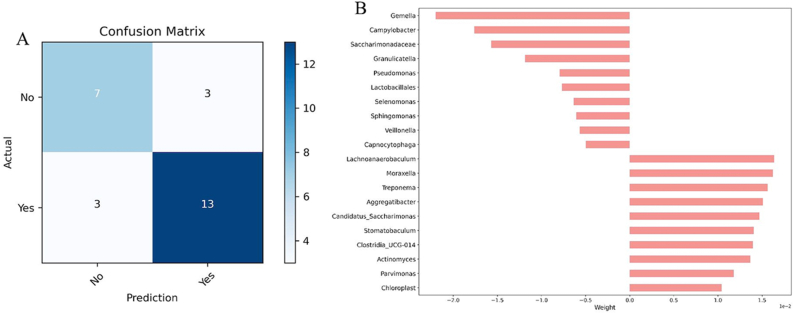
Considering the possibility of predicting response to ICI using information arising from the saliva microbiota, we employed a logistic regression model with parameters c = 1 and a Ridge penalty. Based on our analysis, the abundance of Gemella was the best predictor of no response, whereas Lachnoanaerobaculum was the best predictor of a positive response to ICI [Fig. 8(A-B)].
Fig. 8.
Machine learning results for ICI response prediction. A: Confusion matrix, B: Linear discriminant analysis (LDA) representing the significant differences in the abundance of each species.
4. Discussion
Our study examined a prospective cohort of 55 patients diagnosed with advanced or metastatic lung cancer who underwent ICI treatment, aiming to evaluate the effect of antibiotics and corticosteroids on the efficacy of ICI and on IrAEs occurrence. We also considered respiratory microbiota as a possible predictive marker of both response to ICI and the occurrence of IrAEs.
The baseline characteristics of our patients were similar to those found in the literature [[23], [24], [25], [26]]. It is worth mentioning that in some of the referenced trials, there was a lower proportion of patients with ECOG 0 and a higher proportion of non-smokers [27,28]. The second difference could be explained by the presence of more actionable alterations found in non-smoking patients, making them ineligible for ICI treatment [23,26,[29], [30], [31]].
With respect to survival and response rate, in our study, we had a median PFS of 10 months, an mOS of 19 months, and an overall response rate of 42.4 %. These data are similar to those previously reported in clinical trials with comparable samples performed with ICI [26,30,[32], [33], [34], [35]]. Not all patients respond in the same way to ICI. Just as the response is not homogeneous, neither is the development of IrAEs [28,36]. IrAEs are reported in up to 80 % of patients receiving ICI in monotherapy and in up to 95 % of those receiving combinations with ICI.
Regarding concomitant treatments, several studies have examined the influence of the use of proton pump inhibitors, antibiotics, or corticosteroids, among others, on the efficacy of immunotherapy. Most associate the use of these treatments with shorter survival and a poorer treatment response [19,26,[37], [38], [39], [40], [41]]. An illustrative instance of this phenomenon is the retrospective review conducted by Tinsley et al. In their cohort of 291 patients, a subset of 92 received antibiotics, and they exhibited both a reduced PFS and OS in comparison to those who did not receive it (median OS 10.4 months vs. 21.7 months) [42]. These results are closely parallel to those observed within our cohort, with an mOS of 12 months among individuals who used antibiotics, in contrast to 23 months in those who did not.
Our findings reveal a disparity in PFS and OS greater than that reported in the meta-analysis conducted by Lurienne et al. In their study, antibiotic use yielded a reduction of 1.2 months in PFS (not statistically significant) and 6.7 months in OS. However, in contrast to our study, the differences in OS were statistically significant. This disparity can be potentially elucidated by the inclusion of ECOG 2 patients (an attribute not encompassed within our cohort) and by the ICI indication. In this meta-analysis, the majority of patients received ICI in second and subsequent lines of therapy, in contrast to our dataset, in which the majority of patients underwent administration of ICI as first-line or adjuvant therapy [43].
Regarding the relationship between corticosteroids and OS, our results are consistent with those published in the literature [[44], [45], [46]]. However, our study did not reach statistical significance in PFS or in the different subgroups, as other studies such as Arbour et al., among others, have achieved [44].
It has been suggested that de-structuring of the microbiota, particularly those produced by the antibiotics, could have a negative impact on ICI's efficacy [37,42,43]. Insights from retrospective studies have taught us that antibiotics and corticosteroids produce dysbiosis in the gut microbiota when used during immunotherapy. This could cause poorer outcomes in these cases. However, there are still many aspects that deserve further investigation [40], including the impact of each family of antimicrobials.
Previous studies have shown that exposure to antibiotics in the month preceding the start of ICI therapy is associated with shorter OS and a poorer response. This aligns with research suggesting that the microbiota takes approximately 4–6 weeks to restore after exposure. However, a recent study has been published suggesting that even antibiotic exposure in the year prior to the initiation of ICI could have deleterious effects [38]. In the same way, the use of corticosteroids was related to a higher amount of Actinomyces in saliva.
Similar results were published by Georgiou in 2022, which showed a tendency to a higher proportion of Actinobacteria in the salivary microbiota of those patients diagnosed with lung cancer, without the use of concomitant medication that could condition the results [8]. In a systematic review published in 2021, patients receiving corticosteroids for respiratory tract diseases found a significant increase in the salivary microbiota of Actinobacteria, mainly at the expense of the family Microbacteriaceae, but without highlighting differences with respect to the species Actinomyces [39].
Our cohort study also assessed differences in microbiota composition between ICI responders and non-responders. It is important to emphasize that compositional studies are not comparable to functionality studies, we should check if these bacteria are the real responsible for the lack of response by degradation of the drug, but these microorganisms are extremely difficult to handle in the laboratory. Following the same trend as the results presented by McCulloch et al. (patients with melanoma treated with anti-PD-1), our study also observed the enrichment of the genera Streptococcus in non-responders’ microbiota [15].
As previously demonstrated in other studies, ICI have been associated with a reduction in Bacteroidetes and Firmicutes within the gut microbiota. However, within our cohort, we observed an increase in Firmicutes abundance among individuals exhibiting a subpar response to ICI [47]. These findings appear to contradict the literature suggesting that supplementation with Bifidobacterium could enhance ICI response. However, some studies have reported results consistent with ours regarding the increase in Bacteroidetes among individuals with a poor ICI response and, consequently, a shorter OS [48].
We are aware of the detrimental effects of antibiotics and corticosteroids on the efficacy of ICI. The composition of salivary microbiota might have been influenced by concurrent medication use, potentially contributing to differences between responders and non-responders. A critical question is determining which microbiota composition is more favorable for ICI effectiveness, and how it can be restored to a normal composition following antibiotic or corticosteroid treatment [49]. Some trials have demonstrated that certain commensal bacteria, such as Akkermansia muciniphila, are more abundant in stool samples from responders [47,50]. However, this pattern did not hold true in our cohort.
The inclusion of a heterogeneous patient population, who received ICI treatment in both adjuvant and metastatic settings, across first and subsequent lines of treatment, poses a notable limitation. Patients in subsequent lines often undergo multiple courses of antibiotics and corticosteroids, which may attenuate the true impact of the medications on the microbiota and outcomes. This could potentially account for the lack of statistical significance observed in the results.
Taxonomic features associated with improved response during ICI treatment should be further characterized in larger prospective trials. The characterization of salivary microbiota in patients with lung cancer (given its close association) warrants investigation [49]. Additional studies are needed to identify microbiome-modulating therapies, such as fecal transplantation and dietary or supplement interventions, to reverse the dysbiosis induced by administered treatments, thereby enhancing the ICI response.
5. Conclusions
In our cohort, the use of corticosteroids was significantly associated with a poorer OS, whereas exposition to antibiotics is linked to a diminished response to ICI, although we did not observe a significant association with OS. The absence of a statistically significant difference in OS between patients who received or did not receive antibiotics suggests that their use does not directly impact long-term survival outcomes. Nevertheless, the efficacy of ICI could be influenced by the specific antibiotic and their specific impact on the bacterial populations. Possible mechanisms underlying this relationship include alterations in the microbiota, including a decreased abundance of commensal microorganisms and changes in the taxonomic characteristics of the salivary microbiota, as observed in our cohort.
Regarding IrAEs, we did not find a significant difference in survival based on their presence or absence, although there is a trend toward improved PFS in the absence of IrAEs. However, similar to what occurs with antibiotics, we observed a better response to ICIs in those who have not experienced IrAEs.
Concordant with that previously reported for the gut microbiota, the airway microbiota appears to play a substantial role in the response to ICI. Its composition significantly differs between responders and non-responders. Responders have a greater abundance of the phyla Bacteroidota and the genera Fusobacterium and Porphyromonas, and a lower population of the phyla Firmicutes and Streptococcus. Specifically, reversing the alterations caused by corticosteroids and antibiotics to achieve taxonomic characteristics of the microbiota similar to those of responders could lead to improved responses to ICI and enhanced survival outcomes in patients with NSCLC.
Ethics statement
This study was conducted in accordance with the Declaration of Helsinki and was approved by the Clinical Research Ethics Committee of Aragón (CEICA) with the code C.I. PI19/052.
Informed Consent Statement: All patients signed the informed consent.
Funding
This research was supported by CIBER - Consorcio Centro de Investigación Biomédica en Red - (CB 2021), Instituto de Salud Carlos III, Ministerio de Ciencia e Innovación and Unión Europea – NextGenerationEU, FEDER (Fondo Europeo de Desarrollo Regional), Gobierno de Aragón (Group B29_23R), Ministerio de Ciencia, Innovación e Universidades (MCNU), Agencia Estatal de Investigación (PID2020-113963RB-I00). It was also supported by a Personalized and Precision Medicine grant from the Instituto de Salud Carlos III (MePRAM Project, PMP22/00092), Instituto de Salud Carlos III, Ministerio de Ciencia e Innovación, funded by NextGenerationEU funds from the European Union that finance the actions of the Resilience and Recovery Facility.
Data availability statement
Sequence data were deposited in Genbank (BioProject PRJNA1054537).
CRediT authorship contribution statement
María Zapata-García: Writing – original draft, Validation, Methodology, Investigation, Formal analysis. Alba Moratiel-Pellitero: Visualization, Formal analysis. Dolores Isla: Conceptualization. Eva Gálvez: Writing – review & editing, Conceptualization. Marta Gascón-Ruiz: Visualization. Andrea Sesma: Visualization. Raquel Barbero: Methodology, Conceptualization. Javier Galeano: Methodology. Rosa del Campo: Writing – review & editing, Resources, Formal analysis. Maitane Ocáriz: Visualization. Elisa Quílez: Validation. Mara Cruellas: Validation. Ariel Remírez-Labrada: Resources. Julián Pardo: Methodology. Luis Martínez-Lostao: Investigation. María Pilar Domingo: Investigation. Patricia Esteban: Investigation. Irene Torres-Ramón: Investigation. Alfonso Yubero: Writing – original draft. José Ramón Paño: Supervision. Rodrigo Lastra: Writing – original draft, Validation, Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We would like to acknowledge the patients and the Biobank of the Aragon Health System integrated in the Spanish National Biobanks Network (PT20/00112) for their collaboration.
References
- 1.Hendriks L.E., Kerr K.M., Menis J., Mok T.S., Nestle U., Passaro A., et al. Non-oncogene-addicted metastatic non-small-cell lung cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2023;34(4):358–376. doi: 10.1016/j.annonc.2022.12.013. [DOI] [PubMed] [Google Scholar]
- 2.Leiter A., Veluswamy R.R., Wisnivesky J.P. The global burden of lung cancer: current status and future trends. Nat. Rev. Clin. Oncol. 2023;20:624–639. doi: 10.1038/s41571-023-00798-3. [DOI] [PubMed] [Google Scholar]
- 3.Siegel R.L., Miller K.D., Wagle N.S., Jemal A. Cancer statistics, 2023. CA A Cancer J. Clin. 2023;73(1):17–48. doi: 10.3322/caac.21763. [DOI] [PubMed] [Google Scholar]
- 4.Huang X.-Z., Gao P., Song Y.-X., Xu Y., Sun J.-X., Chen X.-W., et al. Antibiotic use and the efficacy of immune checkpoint inhibitors in cancer patients: a pooled analysis of 2740 cancer patients. OncoImmunology. 2019;00(00) doi: 10.1080/2162402X.2019.1665973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yi M., Yu S., Qin S., Liu Q., Xu H., Zhao W., et al. Gut microbiome modulates efficacy of immune checkpoint inhibitors. J. Hematol. Oncol. 2018;11(1):1–10. doi: 10.1186/s13045-018-0592-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Derosa L., Routy B., Thomas A.M., Iebba V., Zalcman G., Friard S., et al. Intestinal Akkermansia muciniphila predicts clinical response to PD-1 blockade in patients with advanced non-small-cell lung cancer. Nat Med. 2022;28(2):315–324. doi: 10.1038/s41591-021-01655-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belloumini L, Caldart A, Avancini A, Dodi A, Trestini I, Kadrija D, et al. Infections and immunotherapy in lung cancer: a bad relationship? Int. J. Mol. Sci. 22(1):42. [DOI] [PMC free article] [PubMed]
- 8.Georgiou K., Marinov B., Farooqi A.A., Gazouli M. Gut microbiota in lung cancer: where do we stand? Int. J. Mol. Sci. 2021;22(19):1–17. doi: 10.3390/ijms221910429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Georgiou K. In: The Microbiomes of Humans, Animals, Plants, and the Environment. Gazouli M., Theodoropoulos G., editors. Springer I; Switzerland: 2021. Gut microbiota in obesity and bariatric surgery: where do we stand? In gut microbiome-related diseases and therapies; pp. 183–227. [Google Scholar]
- 10.Peterson S.N., Bradley L.M., Ronai Z.A. The gut microbiome: an unexpected player in cancer immunity. Curr. Opin. Neurobiol. 2020;62:48–52. doi: 10.1016/j.conb.2019.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pitt J.M., Vétizou M., Waldschmitt N., Kroemer G., Chamaillard M., Boneca I.G., et al. Fine-tuning cancer immunotherapy: optimizing the gut microbiome. Cancer Res. 2016;76:4602–4607. doi: 10.1158/0008-5472.CAN-16-0448. [DOI] [PubMed] [Google Scholar]
- 12.Perrone F, Belluomini L, Mazzota M, Bianconi M, Di Noia V, Meacci F, et al. Exploring the role of respiratory microbiome in lung cancer: a systematic review. Crit. Rev. Oncol. Hematol. 164(103404). [DOI] [PubMed]
- 13.Routy B., Derosa L., Daille R., Roberti P.M., Zitvogel L. The impact of the intestinal microbiota in therapeutic Impact du microbiote intestinal dans les re le cancer. C R Biol. 2020;341(2018):284–289. doi: 10.1016/j.crvi.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 14.Routy B., Le Chatelier E., Derosa L., Duong C.P.M., Alou M.T., Daillère R., et al. Gut microbiome influences efficacy of PD-1–based immunotherapy against epithelial tumors. Science. 2018;359:91–97. doi: 10.1126/science.aan3706. [DOI] [PubMed] [Google Scholar]
- 15.McCulloch J., Davar D., Rodrigues R., Badger J., Fang J., Cole A., et al. Intestinal microbiota signatures of clinical response and immune-related adverse events in melanoma patients treated with anti-PD-1. Nat Med. 2022;28(3):545–556. doi: 10.1038/s41591-022-01698-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Langdon A., Crook N., Dantas G. The effects of antibiotics on the microbiome throughout development and alternative approaches for therapeutic modulation. Genome Med [Internet] 2016;8(1) doi: 10.1186/s13073-016-0294-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giles A., Hutchinson M.-K., Sonnermann H., Jung J., Fecci P. Dexamethasone-induced immunosuppression: mechanisms and implications for immunotherapy. J Immunother Cancer. 2018;6(1) doi: 10.1186/s40425-018-0371-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fucà G., Galli G., Poggi M., Lo Russo G., Proto C., Imbimbo M., et al. Modulation of peripheral blood immune cells by early use of steroids and its association with clinical outcomes in patients with metastatic non-small cell lung cancer treated with immune checkpoint inhibitors. ESMO Open. 2019;4(1):1–8. doi: 10.1136/esmoopen-2018-000457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cruellas M., Yubero A., Zapata M., Galvez E.M., Gascón M., Isla D., et al. How could antibiotics, probiotics, and corticoids modify microbiota and its influence in cancer immune checkpoint inhibitors: a review. Infect. Immun. 2021;89(9):1–7. doi: 10.1128/IAI.00665-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gori S., Inno A., Belluomini L., Bocus P., Bisoffi Z., Russo A., et al. Gut microbiota and cancer: how gut microbiota modulates activity, efficacy and toxicity of antitumoral therapy. Crit. Rev. Oncol. Hematol. 2019;143:139–147. doi: 10.1016/j.critrevonc.2019.09.003. [DOI] [PubMed] [Google Scholar]
- 21.Eisenhauer E., Therasse P., Bogaerts J., Schwartz L., Sargent D., Ford R., et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur. J. Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 22.Brahmer J.R., Lacchetti C., Schneider B.J., Atkins M.B., Brassil K.J., Caterino J.M., et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American society of clinical oncology clinical practice guideline. J. Clin. Oncol. 2018;36(17):1714–1768. doi: 10.1200/JCO.2017.77.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paz-Ares L.G., Ramalingam S.S., Ciuleanu T.E., Lee J.S., Urban L., Caro R.B., et al. First-line nivolumab plus ipilimumab in advanced NSCLC: 4-year outcomes from the randomized, open-label, phase 3 CheckMate 227 Part 1 trial. J Thorac Oncol [Internet] 2022;17(2):289–308. doi: 10.1016/j.jtho.2021.09.010. [DOI] [PubMed] [Google Scholar]
- 24.Paz-Ares L., Luft A., Vicente D., Tafreshi A., Gümüş M., Mazières J., et al. Pembrolizumab plus chemotherapy for squamous non–small-cell lung cancer. N. Engl. J. Med. 2018;379(21):2040–2051. doi: 10.1056/NEJMoa1810865. [DOI] [PubMed] [Google Scholar]
- 25.Gandhi L., Rodríguez-Abreu D., Gadgeel S., Esteban E., Felip E., De Angelis F., et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N. Engl. J. Med. 2018;378(22):2078–2092. doi: 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
- 26.Rittmeyer A., Barlesi F., Waterkamp D., Park K., Ciardiello F., von Pawel J. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (oak): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389(10066):255–265. doi: 10.1016/S0140-6736(16)32517-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ettinger D.S., Wood D.E., Aisner D.L., Akerley W., Bauman J.R., Ankit B. NCCN Guidel; 2022. Non-Small Cell Lung Cancer.https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf version 3. Available from: [Google Scholar]
- 28.Schneider B.J., Naidoo J., Santomasso B.D., Lacchetti C., Adkins S., Anadkat M., et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: ASCO guideline update. J. Clin. Oncol. 2021;39(36):4073–4126. doi: 10.1200/JCO.21.01440. [DOI] [PubMed] [Google Scholar]
- 29.Papadimitrakopoulou V., Cobo M., Bordon R., Al E. IASLC 19th World Conference on Lung Cancer. 2018; abstr. 2018. IMPOWER132: PFS and safety results with 1L atezolizumab + carboplatin/cisplatin + pemetrexed in stage IV non-squamous NSCLC. OA05.07. [Google Scholar]
- 30.Herbst R., Baas P., Kim D., Felip E., Perez-Gracia J., Han J. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 31.De Castro G.J., Kudaba I., Wu Y., Lopes G., Kowalski D.M. Five-year outcomes with pembrolizumab versus chemotherapy as first-line therapy in patients with non – small-cell lung cancer and programmed death ligand-1 tumor proportion score ‡ 1 % in the KEYNOTE-042 study clinical trial updates abstract. J. Clin. Oncol. 2022;0(0):1. doi: 10.1200/JCO.21.02885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Borghaei H., Paz-Ares L., Horn L., Spigel D., Steins M., Ready N., et al. Nivolumab versus docetaxel in advancer nonsquamous non-small-cell lung caner. N. Engl. J. Med. 2015;373(17):1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herbst R., Giaccone G., de Marinis F., Reinmuth N., Vergnenegre A., Barrios C., et al. Atezolizumab for first-line treatment of PD-L1-selected patients with NSCLC. N. Engl. J. Med. 2020;383(14):1328–1339. doi: 10.1056/NEJMoa1917346. [DOI] [PubMed] [Google Scholar]
- 34.Reck M., Rodriguez-Abreu D., Robinson A., Hui R., Csoszi T., Fulop A., et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N. Engl. J. Med. 2016;375(19):1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 35.Gray J.E., Villegas A., Daniel D., Vicente D., Murakami S., Hui R., et al. Three-year overall survival with durvalumab after chemoradiotherapy in stage III NSCLC—update from PACIFIC. J. Thorac. Oncol. 2020;15(2):288–293. doi: 10.1016/j.jtho.2019.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Socinski M.A., Jotte R.M., Cappuzzo F., Nishio M., Mok T., Reck M., et al. 2021. Pooled Analyses of Immune-Related Adverse Events and Efficacy from the Phase 3 Trials IMpower130, IMpower132 and IMpower150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zalcman G., Crespin A., Cervesi J., Al E. ESMO Immuno-Oncology Virtual Congres. 2020. Update of systematic reviews and meta-analyses studying the association between antibiotic use and clinical outcomes of cancer patients treated with immune checkpoint inhibitors; p. 50P. [Google Scholar]
- 38.Eng L., Sutradhar R., Niu Y., Liu N., Liu Y., Kaliwal Y., et al. Impact of antibiotic exposure before immune checkpoint inhibitor treatment on overall survival in older adults with cancer: a population-based study. J. Clin. Oncol. 2023;41(17):3122–3134. doi: 10.1200/JCO.22.00074. [DOI] [PubMed] [Google Scholar]
- 39.Hartmann J.E., Albrich W.C., Dmitrijeva M., Kahlert C.R. The effects of corticosteroids on the respiratory microbiome: a systematic review. Front. Med. 2021;8(March) doi: 10.3389/fmed.2021.588584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meriggi F., Zaniboni A. Antibiotics and steroids, the double enemies of anticancer immunotherapy: a review of the literature. Cancer Immunol. Immunother. 2021;70(6):1511–1517. doi: 10.1007/s00262-020-02786-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chalabi M., Cardona A., Nagarkar D.R., Dhawahir Scala A., Gandara D.R., Rittmeyer A., et al. Efficacy of chemotherapy and atezolizumab in patients with non-small-cell lung cancer receiving antibiotics and proton pump inhibitors: pooled post hoc analyses of the OAK and POPLAR trials. Ann. Oncol. 2020;31(4):525–531. doi: 10.1016/j.annonc.2020.01.006. [DOI] [PubMed] [Google Scholar]
- 42.Tinsley N., Zhou C., Tan G., Rack S., Lorigan P., Blackhall F., et al. Cumulative antibiotic use significantly decreases efficacy of checkpoint inhibitors in patients with advanced cancer. Oncol. 2020;25(1):55–63. doi: 10.1634/theoncologist.2019-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lurienne L., Cervesi J., Duhalde L., de Gunzburg J., Andremont A., Zalcman G., et al. NSCLC immunotherapy efficacy and antibiotic use: a systematic review and meta-analysis. J. Thorac. Oncol. 2020;15(7):1147–1159. doi: 10.1016/j.jtho.2020.03.002. [DOI] [PubMed] [Google Scholar]
- 44.Arbour K.C., Mezquita L., Long N., Rizvi H., Auclin E., Ni A., et al. Impact of baseline steroids on efficacy of programmed cell death-1 and programmed death-ligand 1 blockade in patients with non–small-cell lung cancer. J. Clin. Oncol. 2018;36(28):2872–2878. doi: 10.1200/JCO.2018.79.0006. [DOI] [PubMed] [Google Scholar]
- 45.De Giglio A., Mezquita L., Auclin E., Blanc-Durand F., El-Amarti L., Caramella C., et al. 2020. Impact of Early Introduction of Steroids on Immune- Checkpoint Inhibitors (ICI) in Patients with Advanced Non- Small-Cell Lung Cancer Disclosure Information Laura Mezquita. Paris. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Drakaki A., Luhn P., Wakelee H., Dhillon P.K., Kent M., Shim J., et al. Association of systemic corticosteroids with overall survival in patients receiving cancer immunotherapy for advanced melanoma, non-small cell lung cancer or urothelial cancer in routine clinical practice. Ann. Oncol. 2019;30(Supplement 11):xi16–x17. [Google Scholar]
- 47.Sevcikova A., Izoldova N., Stevurkova V., Kasperova B., Chovanec M., Ciernikova S., et al. The impact of the microbiome on resistance to cancer treatment with chemotherapeutic agents and immunotherapy. Int. J. Mol. Sci. 2022;23(1) doi: 10.3390/ijms23010488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gopalakrishnan V., Spencer C.N., Nezi L., Reuben A., Andrews M. Gut microbiome modulates response to anti–PD-1 immunotherapy in melanoma patients. Science. 2018;359:97–103. doi: 10.1126/science.aan4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alkan G., Senturk Oztas N., Turna Z.H., Ozguroglu M. Effect of antibiotic treatment on immune checkpoint inhibitor efficacy in patients with advanced non-small cell lung cancer. Lung Cancer. 2023:184. doi: 10.1016/j.lungcan.2023.107347. [DOI] [PubMed] [Google Scholar]
- 50.Derosa L., Hellmann M., Spaziano M., Halpenny D., Fidelle M., Rizvi H., et al. Negative association of antibiotics on clinical activity of immune checkpoint inhibitors in patients with advanced renal cell and non-small-cell lung cancer. Ann. Oncol. 2018;29(6):1437–1444. doi: 10.1093/annonc/mdy103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Sequence data were deposited in Genbank (BioProject PRJNA1054537).