ABSTRACT
Bladder cancer (BC) accounts for about 4% of all malignancies. Non-muscle-invasive BC, 75% of cases, is treated with transurethral resection and adjuvant intravesical instillation, while muscle-invasive BC warrants cisplatin-based perioperative chemotherapy. Although immune-checkpoint inhibitors, antibody drug conjugates and targeted agents have provided dramatic advances, metastatic BC remains a generally incurable disease and clinical trials continue to vigorously evaluate novel molecules. Cancer vaccines aim at activating the patient’s immune system against tumor cells. Several means of delivering neoantigens have been developed, including peptides, antigen-presenting cells, virus, or nucleic acids. Various improvements are constantly being explored, such as adjuvants use and combination strategies. Nucleic acids-based vaccines are increasingly gaining attention in recent years, with promising results in other malignancies. However, despite the recent advantages, numerous obstacles persist. This review is aimed at describing the different types of cancer vaccines, their evaluations in UC patients and the more recent innovations in this field.
KEYWORDS: Bladder cancer, urothelial cancer, cancer vaccine, peptide-based vaccine, DC-based vaccine, virus-based vaccine, RNA-based vaccine, DNA-based vaccine
Introduction
Urothelial carcinoma (UC) represents the most frequent type of cancer developing in the urinary system, being the ninth most frequently diagnosed tumor. It may be classified into upper and lower urinary tract; bladder cancer (BC) constitutes 90% of the forms of UCs, with almost 613’800 new cases in 2022 (3.1% of all new cancer diagnoses) and about 220’000 deaths (2.3% of all cancer mortality).1 This is a disease of the elderly with a median age at diagnosis of 73 years, which presents challenges due to comorbidities and poor performance status. Indeed, the survival of metastatic UC is suboptimal and cure is infrequent.2 Risk factors include smoking, personal or family history of UC, occupational exposures, obesity, diabetes, chronic infection, or irritation of the urinary tract.3
Non-muscle-invasive (NMIBC) constitutes up to 75% of newly detected cases, while muscle-invasive (MIBC) and metastatic UC (mUC) form the rest. The treatment of choice of NMIBC is transurethral resection of bladder tumor (TURBT) followed by an adjuvant intravesical instillation of Bacillus Calmette Guérin (BCG) for high-risk disease.4 BCG-unresponsive or -intolerant NMIBC may be directed to a radical cystectomy (RC),4 while novel therapies have emerged including pembrolizumab5 and gene therapy with Nadofaragene firadenovec, a non-replicant recombinant adenovirus delivering the deoxyribonucleic acid (DNA) of human interferon (IFN)-alfa-2b into the bladder epithelium.6 MIBC is treated by neoadjuvant cisplatin-based combinations followed by RC.7 Adjuvant nivolumab has recently showed efficacy in high-risk patients.8 Very select patients who are ineligible for or refuse RC may receive a trimodality treatment with maximal TURBT, chemotherapy, and radiotherapy (RT).7
Approximately 10–15% of patients have a metastatic disease at diagnosis. The backbone treatment of advanced disease had been established as platinum-based chemotherapy followed by maintenance avelumab in 2020.9 However, multiple advances have occurred since then.10,11 The combination of gemcitabine-cisplatin and nivolumab improved survival, which led to the US Food and Drug Administration recent approval.12 Thereafter, antibody drug conjugates (ADCs) are increasingly emerging in the treatment landscape of patients with UC. Enfortumab vedotin, a Nectin4 binding ADC delivering a tubulin toxin payload, was approved in salvage therapy settings and more recently in combination with pembrolizumab as first-line therapy.13 Sacituzumab govitecan, a Trop2 targeting ADC that bears a topoisomerase-1 inhibitor payload, received accelerated approval in the United States for post-platinum and programmed cell death 1 (PD1)/ligand 1 (PD-L1) inhibitor treated patients with progressive disease.14 Lastly, around 20% of advanced UC expresses a mutation or fusion of fibroblast growth factor receptor (FGFR)3.15 A personalized therapy with erdafitinib, a pan-FGFR inhibitor, may be offered to these patients progressing after previous PD1/L1 inhibitor therapy.16,17 Nevertheless, the prognosis of patients affected by advanced UC remains poor, with mostly incurable disease despite recent advances. In this context, adjuvant nivolumab or pembrolizumab have prolonged the disease-free survival, but most patients destined to recur will exhibit relapse.8,18 Hence, understanding tumor biology and developing novel and tolerable agents are a high priority.
Cancer vaccines are a promising strategy against tumors. They can be classified as preventive and therapeutic cancer vaccines. The preventive vaccines aim at preventing the infection from agents known to trigger cancer development, such as Human Papillomavirus (HPV).19 In contrast, therapeutic vaccines are based on the concept of triggering the immune system against tumor cells therefore inducing tumor regression or eradicating potential minimal residual disease. They may be tailored to tumor-associated antigens, which are off-the-shelf vaccines, or tumor-specific, against the specific antigens present on the patient’s cancer cells. The ideal tumor antigen should be widely exposed by tumor cells with limited expression on normal cells. Several means have been developed with the aim of delivering tumor antigens to T lymphocytes, such as peptides, dendritic or other antigen-presenting cells, virus or nucleic acids. Recently, efforts have been put into the development of personalized vaccines, built on the spectrum of neoantigens found in the malignant cells of each specific patient. In addition, combining vaccines with other treatments such as immune checkpoint inhibitors (ICIs), chemotherapy, or RT, may enhance the overall therapeutic effect. Although numerous preclinical and clinical studies have focused on vaccine cancers, in the clinic, only sipuleucel-T, a dendritic cell (DC)-based vaccine carrying a protein fusion of the antigen prostatic acid phosphatase and granulocyte-macrophage colony stimulating factor (GM-CSF) is available in the USA and Europe, for metastatic castration-resistant prostate cancer.20 Despite the modest success, cancer vaccines have exhibited excellent tolerability and remain the focus of several experimental approaches aimed at harnessing their immunogenicity in conjunction with decreasing the immunosuppressive environment of tumor with ICIs. Several challenges remain in the development of cancer vaccines. First of all, a high heterogeneity between cancer cells may be identified, hindering the identification of universal vaccine targets21 and not every antigen is able to induce a strong immune response in the host.22 Moreover, tumors are able to downregulate antigen presentation and induce regulatory T (Treg) cells.23 Lastly, manufacturing processes should be improved in efficiency and cost-effectiveness to have a global impact. Here, we conducted a review of the main cancer vaccines strategies, their challenges and developments, and their clinical implications in UC.
Methods
The present work is a descriptive review. In June 2024, we performed a literature search on PubMed and Embase using various combinations of the following keywords: (‘peptide’ or ‘virus’ or ‘DC’ or ‘RNA’ or ‘DNA’) and (‘vaccine’ or ‘vaccine therapy’) and (‘urothelial cancer/carcinoma’ or ‘bladder cancer/carcinoma’). No restrictions were placed on the year of publication. We examined reviews, preclinical, and clinical trials, and selected the most relevant works based on their level of evidence.
Mechanism of action
The foundation of immune activation is antigen-presenting cells (APCs), particularly DCs. APCs capture antigens through phagocytosis, process and express them on major histocompatibility complex (MHC) molecules. Later, DCs migrate to draining lymph nodes and present the processed antigen epitopes through MHC I and MHC II molecules to CD8+ and CD4+ T cells, respectively24 (see Figure 1). In order to increase lymphocytes activation, costimulatory receptors are expressed and costimulatory molecules are secreted, such as interleukin (IL)-12 and IFN-γ.24 Immature DCs in the tumor microenvironment (TME) may be enhanced through Toll-like receptor (TLR) ligands.25 After the presentation of antigens, T cells differentiate into effectors and long-lived memory T cells. The former amplify and migrate to TME where they induce cytotoxicity of tumor cells.24 On the other hand, CD4+ T cells increase CD8+ T-lymphocyte proliferation and B-lymphocyte activation. B-cells produce antibodies, able to cause cellular cytotoxicity, directly or through the activation of complement.26 Lysed tumor cells release antigens able to amplify the immune response.26
Figure 1.
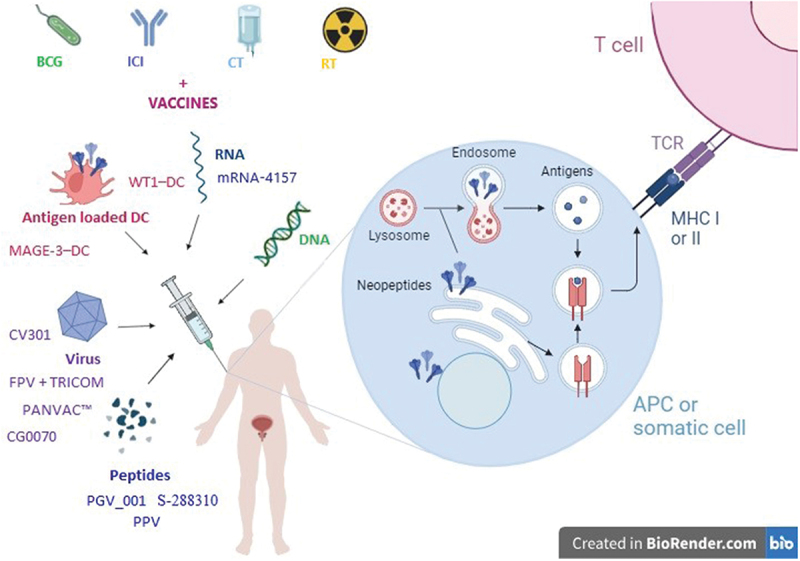
Approaches for the use of cancer vaccines in the treatment of urothelial carcinoma and their mechanism of action.
Legend: APC = antigen-presenting cell, BCG = Bacillus Calmette-Guerin, CT = chemotherapy, DC = dendritic cell, DNA = deoxyribonucleic acid, FPV = fowlpox virus, ICI = immune checkpoint inhibitor, MAGE = melanoma-associated antigens, MHC = major histocompatibility complex, PGC = peptide-based personalized genome vaccine, PPV = personalized peptide vaccination, RNA = ribonucleic acid, RT = radiotherapy, TCR = T-cell receptor, TRICOM = three immune costimulatory molecules: B7.1, ICAM-1 LFA-3, WT1 = Wilms’ tumor 1.
The backbone of the efficacy of cancer vaccination is the recognition of tumor antigens by T lymphocytes. The optimal tumor antigen should have an extensive expression on tumor cells but minimal expression on normal cells. Moreover, they should present a strong affinity to human leukocyte antigen (HLA), to be effectively presented to T cells.27 Tumor antigens are classified in tumor-associated antigen (TAA) and tumor-specific antigen (TSA). TAAs, also defined as tumor-shared, are self-antigens, generally expressed in healthy tissues but overexpressed in tumor cells, often tissue-specific. For this reason, they are usually versatile and able to address different patients. However, since they are physiologically expressed antigens, the risk of immune-tolerance, or, on the contrary, the induction of autoimmunity, is high.28 Differently, TSAs, also known as neoantigens, are only present on tumor cells, and arise as a consequence of a mutational process. They can be “shared neoantigens,” identifiable in different kind of tumors, or “personalized neoantigens,” related to a specific patient.29 They are defined based on the mutation type leading to their transcription into antigens, such as single mutation (single nucleotide variants, insertion, deletions), fusions or rearrangements, copy number duplications or loss, transcriptional abnormalities, or microsatellite instability.27 Inducing an immune reaction against these antigens could personalize the treatment, with limited autoimmunity priming, but tumor loss of their specific antigens may occur, nullifying the process; in conclusion not every TSA is able to establish an effective immune response.22 The TAAs and TSAs identification is possible through performing next generation sequencing (NGS), usually whole-exome sequencing (WES), ribonucleic acid (RNA)-sequencing and confirmed by mass spectrometry techniques.27 A neoantigen vaccine is usually composed by several different neoantigens, in order to improve its immunogenicity. Even when adequate neoantigens are identified, efforts to guarantee effective peptide procession, MHC presentation and binding and subsequent T cell recognition, should be provided. Specifically, HLA typing is a crucial step in the process. Consequently, several potent prediction algorithms have been developed.30
Cancer-based vaccines
Neoantigens may be transported and presented to T lymphocytes through several mechanisms of vaccination, based on: dendritic or other antigen-presenting cells, virus, RNA, DNA, or peptides (see Figure 1).
Administration route is also a crucial key, since different delivery methods may activate different immune-cell types. UC is a unique type of cancer, suitable for multiple administration routes beside the typical intradermal, intramuscular, subcutaneous, or intravenous, including intravesical and intratumoral. T-cells may be enhanced by intradermal and subcutaneous injection, when Langerhans and mesenchymal cells are mostly solicited,30 therefore vaccination may require lower doses, although absorption may be variable and local skin reactions might occur. A major and prompt antibody production has been reported with intravenous injection, with a spread distribution, even though a rapid liver clearance may limit its efficacy.31 Furthermore, systemic adverse events (AEs) are more frequent than with other routes. Intramuscular injection is convenient and avoids liver first-pass metabolism, although, taking into account the scarce immune cells in this tissue, adjuvants able to solicit an inflammatory response are usually needed.31,32 An oral administration is noninvasive and convenient, albeit limited by gastrointestinal barriers, gastric acid pH and digestive enzymes.31 Direct intra-tumor injection may promptly modify the TME, with limited systemic side effects showing better T cell induction, with respect to intranodal injection,33 however not every patient has accessible sites.34 Intravesical administration offers a unique route for NMIBC, with uncontested advantages of direct activity with high doses and limited or no systemic AEs. Conversely, its efficacy may be limited to bladder, not being feasible for advanced UC. Ultimately, combining different routes of administration may enhance immune responses.35 Here, we review the most relevant trials investigating cancer-based vaccines for urothelial carcinoma. The phase II trials are summarized in Table 1.
Table 1.
Phase II clinical trials evaluating cancer vaccines in patients with urothelial cancer.
| Reference | Study type | Vaccine name | Vaccine type | Vaccine target | Administration route | Schedule | Setting | Population (number of patients) | Results |
|---|---|---|---|---|---|---|---|---|---|
| Non-Muscle Invasive Bladder Cancer | |||||||||
| Obara W, et al. Cancer Immunol Immunother. 2018 | Phase II, non randomized | S288310 | Peptide-based | DEPDC1 + MPHOSPH1 | Subcutaneous | Combined with BCG | Untreated NMIBC | 127 | 2-year RFS 74% |
| Ogasawara M, et al. Ther Apher Dial. 2018 | Phase II, non randomized | NA | Dendritic cell-based | Wilms’ tumor 1 + adjuvant OK-432 | Intradermal | Monotherapy (2 NMIBC), combined with chemotherapy (UC, 1 NMIBC) or TKI (RCC) | NMIBC, mUC or metastatic RCC | 3 + 2 + 5 | NMIBC: 1 relapse at 26.8 months, 2 did not relapse with a follow-up of 84.8 months and 33.4 months mUC: ORR 0.0% |
| Packiam VT, et al. Urol Oncol Semin Orig Investig. 2018 | Phase II, open-label | CG0070 | Virus-based | GM-CSF encoding adenovirus + dodecyl matoside | Intravesical | Monotherapy | BCG-resistant NMIBC | 45 | Overall population: 6-month CR rate 47%, 6-month SD rate 27% Pure carcinoma in situ patients: 6-month CR rate 58%, 6-month SD rate 13% Pure Ta/T1 patients: 6-month CR rate 33%, 6-month SD rate 33% |
| Li R, et al. J Clin Oncol. 2022 | Phase II, open-label | CG0070 | Virus-based | GM-CSF encoding adenovirus + dodecyl matoside | Intravesical | Combined with pembrolizumab | BCG-resistant NMIBC | 35 | 3-month CR rate 87.5% |
| Saoud R, et al. J Urol. 2021 | Phase II, randomized | PANVAC™ | Virus-based | Epithelial mucin 1 and CEA encoding fowlpox + vaccinia + TRICOM | Subcutaneous | Combined with BCG vs. BCG alone | BCG-resistant NMIBC | 30 | 12-month RFS rate 44.4% (CI 24.8–79.7) vs. 42.9% (22.9–80.0), p = .971 Median TTR 9.9 vs. 11.7 months, p = .77 12-month PFS rate 78.6% (95% CI 59.8–100) vs. 79.6% (CI 56.9–100) |
| Metastatic Urothelial Carcinoma | |||||||||
| Obara W, et al. Ann Oncol. 2017 | Phase I-II, non randomized | S288310 | Peptide-based | DEPDC1 + MPHOSPH1 | Subcutaneous | Monotherapy | Pre-treated mUC | 32 | DCR 56.3% ORR 6.3% mPFS 1.9 (90% CI: 1.2–2.2) months mOS 9.4 (90% CI: 4.2–11.9) months |
| Suekane S, et al. Eur Urol Suppl. 2012 | Phase II, non randomized | NA | Peptide-based | PPV | Subcutaneous | Monotherapy | Pre-treated mUC | 25 | mOS 11.3 months |
| Noguchi M, et al. Clin Cancer Res. 2016 | Phase II, randomized, open label | NA | Peptide-based | PPV | Subcutaneous | Monotherapy vs. best supportive care | Pre-treated mUC | 80 | ORR 23.0% vs. 0.0% mPFS 2.0 (95% CI, 1.8–3.4) vs. 1.8 (95% CI, 1.3–2.3) months, HR 0.7, p = .17 mOS 7.9 (95% CI, 3.5– 12.0) vs. 4.1 (95% CI, 2.8–6.9) months HR 0.58, p = .049 |
| Suekane S, et al. Cancer Sci. 2017 | Phase II, open label | NA | Peptide-based | PPV | Subcutaneous | Monotherapy or combined with chemotherapy | Pre-treated metastatic UTUC | 48 | PPV monotherapy: mOS 4.5 (95%CI, 1.7–10.1) PPV + chemotherapy: mOS 13.0 (95% CI, 5.7–17.5) months |
| Nishiyama T, et al. Clin Cancer Res. 2001 | Phase I-II | NA | Dendritic cell-based | Melanoma Antigen − 3 | Subcutaneous | Monotherapy | mUC | 4 | DCR 75% |
| Sonpavde GP, et al. J Clin Oncol. 2022 | Phase II, single arm | CV301 | Virus-based | Epithelial mucin 1 and CEA encoding fowlpox + modified vaccinia ankara + TRICOM | Subcutaneous | Combined with atezolizumab | Cohort 1: cisplatin-ineligible mUC Cohort 2: Pre-treated mUC |
19 + 24 | Halted for futility Cohort 1: ORR 5.3%, (90%CI 0.3–22.6) Cohort 2: ORR 8.3% (90%CI 1.5–24.0). |
| Matsumoto R, et al. J Clin Oncol. 2021 | Phase I-II, single arm | TAS0313 | Peptide-based | Three long peptides and 12 CTL epitopes | Subcutaneous. | Combined with pembrolizumab | Cohort 1: cisplatin-pre-treated mUC Cohort 2: ICI-pre-treated mUC |
36 + 10 | Cohort 1: ORR 33% DCR 67% mPFS 5 months 1-year OS rate 74.3% Cohort 2: DCR 50.0% |
BCG = Bacillus Calmette-Guerin, CI = confidence interval, CR = complete response, DCR = disease control rate, DEPDC1 = DEP domain-containing 1, GM-CSF = granulocyte-macrophage colony stimulating factor; HR = hazard ratio, ICI = immune checkpoint inhibitor, mOS = median OS, mPFS = median PFS, MPHOSPH1 = M-phase phosphoprotein 1, mUC = metastatic UC, NA = not available, NMIBC = non-muscle invasive bladder cancer, ORR = objective response rate, OS = overall survival, PFS = progression-free survival, PPV = personalized peptide vaccination, RCC = renal cell carcinoma, RFS = relapse-free survival, SD = stable disease, TRICOM = three immune costimulatory molecules: B7.1, ICAM-1 LFA-3, TKI = tyrosine kinase inhibitors, TTF = time to recurrence, UC = urothelial cancer, UTUC = upper tract urothelial carcinoma.
Peptide-based vaccines
Peptide-based vaccines may be composed of TAAs or TSAs. At first, they were created as single-antigen short peptides, formed by <15 antigens, directly binding MHC class I and activating CD8+ response.36 In the absence of adequate co-stimulatory signals, they were often not effective enough to stimulate a strong T cell response and lead to tolerance.22 For this reason, synthetic long peptides (SLP) have been created. SLPs comprise about 30 antigens, that are not directly active and need to be processed by APCs and later loaded to MHC class I and II molecules, thus generating both effective CD4+ and CD8+ responses.36 Nevertheless, despite demonstrating immune-responses, clinical trials with peptide-based vaccines have led to little efficacy, probably due to the MHC restriction of epitopes.37
To enhance their activity, several improvements have been proposed. For instance, preclinical models showed how fusion with carriers proteins, i.e. keyhole limpet hemocyanin (KLH) or heat shock proteins (HSP), may enhance APC’s activation.38 Moreover, virus-like particles (VLP) or bacteria may be an effective platform for peptide-based vaccines, due to their ability to induce APCs activation and transportation to lymph nodes.30,39 In addition, modifications of the peptide structure, that may include cytotoxic T lymphocytes (CTL) and T helper peptides, could improve responses.40 Finally, adjuvants have been tested to reach the purpose, since they are able to enhance the leukocytes’ recruitment, expansion and activation and their later migration to tumor sites.41 The most evaluated adjuvants are AS04, an aluminum salt associated with a detoxified form of lipopolysaccharide, incomplete Freund’s adjuvant (IFA),42 molecules targeting TLRs and liponanomaterials.30 Recently, data supported the use of gold nanoparticles as vaccine adjuvants in preclinical mouse models. Gold nanoparticles loaded with the bacterial peptide 91–99 of the listeriolysin O toxin (GNP-LLO91–99 nanovaccines), showed a systemic T helper 1-type immune response in TME: increased percentages of CD4+ and CD8+ T cells, B cells, DCs and reduced levels of myeloid-derived suppressor cells and suppressor T cells.43 In closing, cytokines given as adjuvants, such as IL-2, GM-CSF or IFN, are gaining attention for their immunomodulatory properties.44 Peptide-based vaccines are versatile, easy to engineer with a low probability of AEs and contamination; however, the adjuvant necessity and the MHC restriction limit its immunogenicity.30,37,41
Several trials have demonstrated promising results with improved survival rates and reduced tumor recurrence in patients affected by UC, treated with peptide-based vaccines. Here, we summarized the most relevant ones. A subcutaneous peptide-based vaccine, S-288310, was developed uniting two tumor-associated antigens, identified through expression profile analysis and singularly tested to be active in patients with UC: DEP domain-containing (DEPDC1) and M-phase phosphoprotein 1 (MPHOSPH1). In a phase I-II study, S-288310 demonstrated tolerability and activity in inducing peptide-specific CTL, in 32 patients with UC, showing a disease control rate (DCR) of 56.3% and a 2 years overall survival (OS) rate of 32%.45 A subsequent non-randomized phase II trial associated subcutaneous MPHOSPH1- and DEPDC1-peptide vaccine to intravesical BCG therapy, showing a remarkable 2-year relapse-free survival (RFS) of 74%, with peptide-specific cytotoxic T lymphocyte response of 75.8–77.5%46 (see Table 1). These noteworthy results, with 2-year prolonged response, deserve further and broader evaluations to be validated.
CDX-1307, an intradermal peptide vaccine composed by a human monoclonal antibody against the mannose receptor fused to the human chorionic gonadotropin-β chain (β-HCG), a frequently expressed tumor antigen, showed safety in a phase I study; unfortunately the phase II trial in patients with UC was stopped early because of slow enrollment.47
A peptide-based vaccine against the NY-ESO-1 tumor antigen, combined with a novel delivery system, cholesteryl pullulan, together with the adjuvant MIS416, activating NOD-2 and TLR9 pathways, was tested with subcutaneous administration in a phase II trial on 16 patients, four of whom affected by UC. Although patients with prostate cancer exhibited tumor response from the treatment, the vaccine did not show activity in UC.48
Another interesting regimen of vaccination was based on the subcutaneous administration of the most reactive peptides chosen from a large cohort of candidate antigens: personalized peptide vaccination (PPV).49 In a phase I trial, a population of 10 pre-treated patients with UC received PPV, achieving one complete response (CR), one partial response (PR), two stable disease (SD) and six progressive disease (PD), with a median OS of 8.9 months, and it is noteworthy that duration of response extended to 24 months in the responders.49 Its safety and activity were confirmed in a phase II trial on 25 pre-treated patients with UC, reaching a median OS of 11.3 months50 (see Table 1). PPV was later compared to best supportive care in a randomized phase II study in 80 cisplatin-refractory patients affected by UC. PPV did not show benefit on progression-free survival (PFS), but appeared to be safe and to prolong OS with a hazard ratio (HR) of 0.58, p = .04951 (see Table 1). Successively, the PPV strategy was investigated in 48 pre-treated patients affected by metastatic upper tract urothelial cancer (UTUC), in a single arm phase II trial. PPV was well tolerated without severe AEs, and induced a specific T-cells response in 46% of the patients, related to a longer OS with respect to patients with negative response (HR 0.37; 95%CI, 0.16–0.85; p = .019). Median OS was 4.5 months, but remarkably it was extended to 13 months in patients receiving combined PPV and chemotherapy (60% of patients)52 (see Table 1). PPV is one of the most promising peptide-based vaccine strategies; the benefit was observed for only a minority of patients, but the encouraging responses were enduring. Alternative forms of personalized neoantigens peptide-vaccines are being evaluated (see Table 2). Further analyses are needed to identify the subgroup of patients who may mainly benefit.
Table 2.
Selected ongoing clinical trials evaluating cancer vaccines on patients affected by urothelial cancer (2016–2024).
| Clinicaltrials.gov Registration number | Study type | Vaccine name | Vaccine target | Regime | Population | Setting | Enrollment | Endpoints | State |
|---|---|---|---|---|---|---|---|---|---|
| NMIBC | |||||||||
| Peptide-based vaccines | |||||||||
| NCT05843448 | Phase I | IO102-IO103 | Indoleamine-pyrrole 2,3-dioxygenase + PD-L1 | Combined with pembrolizumab | NMIBC | BCG unresponsive or intolerant disease | 30 | AEs, CR, event-free survival, cystectomy-free survival, DOR | Active, recruiting |
| NCT04106115 | Phase Ib/II, multicenter | S-488210/S-488211 | 5-peptide + montanide adjuvant | Combined with durvalumab | NMIBC | BCG unresponsive or intolerant disease | 64 | DLT, DFS | Active, recruiting |
| Virus-based vaccines | |||||||||
| NCT04387461 | Phase II, single arm, open label | CG007 + n-dodecyl-B-D-maltoside adjuvant | Adenovirus, tumor-selective GM-CSF synthesis | Combined with pembrolizumab | NMIBC | BCG-unresponsive disease | 35 | CRR, AEs, DOR, OS, PFS | Active, not recruiting |
| NCT04452591 | Phase III, single arm, open label | CG007 + n-dodecyl-B-D-maltoside adjuvant | Adenovirus, tumor-selective GM-CSF synthesis | Monotherapy | NMIBC | BCG-unresponsive disease | 110 | CRR, DOR, PFS, TTP, AEs | Active, recruiting |
| MIBC | |||||||||
| Peptide-based vaccines | |||||||||
| NCT03359239 | Proof-of-concept study, single arm | PGV001 | Multipeptide personalized neoantigen + Poly-ICLC adjuvant | Combined with atezolizumab | UC | − Adjuvant therapy after surgery in localized disease - Second-line for cisplatin-resistant disease |
10 | ORR, DOR, TTP, OS | Active, recruiting |
| mRNA-based vaccines | |||||||||
| NCT03313778 | Phase I, open label, non-randomized | mRNA-4157 | Personalized neoantigens | Monotherapy or combined with pembrolizumab | Solid tumors, including UC: resected localized disease or advanced or metastatic disease | − Adjuvant monotherapy for resected disease - First-line combined with ICI in metastatic disease |
108 | AEs, DLT, change from baseline of biomarker levels, Antigen-specific T-cell responses in peripheral blood, ORR, DOR, PFS, OS |
Active, not recruiting |
| NCT06305767 | Phase II, randomized, double-blind, placebo-controlled, multicenter | V940 (mRNA-4157) | Personalized neoantigens | Combined with pembrolizumab vs pembrolizumab plus placebo | UC | Adjuvant for high-risk resected disease | 200 | DFS, OS, DMFS, AEs | Active, recruiting |
| Metastatic disease | |||||||||
| Peptide-based vaccines | |||||||||
| NCT05077709 | Phase II, multiarm (basket) | IO102-IO103 | Indoleamine-pyrrole 2,3-dioxygenase + PD-L1 | Combined with pembrolizumab | Metastatic NSCLC, metastatic SCCHN, metastatic BC | First-line | 90 | ORR, PFS, DOR, CRR, DCR, OS, TTR, safety | Active, recruiting |
| NCT03715985 | Phase I-II | NeoPepVac | 5–15 personalized neoantigens + CAF09b adjuvant | Combined with ICIs | Metastatic UC, NSCLC, melanoma | NA | 25 | AEs, OS, ORR, PFS | Active, not recruiting |
| NCT05269381 | Phase I | NeoVCA | Personalized neoantigens + GM-CSF | Combined with cyclophosphamide and pembrolizumab | Advanced or metastatic solid tumors, including UC | NA | 36 | AEs, feasibility, ORR | Active, recruiting |
| NCT03639714 | Phase I-II | GRT-C901 and GRT-R902 | Personalized neoantigens | Combined with nivolumab and ipilimumab | Metastatic NSCLC, colon cancer, UC, gastroesophageal cancer | NA | 29 | AEs, DLT, ORR, DOR, CBR, PFS, OS | Completed |
| NCT04864379 | Phase I, open label | iNeo-Vac-P01 | Personalized neoantigens + GM-CSF | Combined with ICI and radio-frequency ablation | Advanced solid tumors, including UC | NA | 30 | AEs, ORR, CD4/CD8 T lymphocyte subsets, IFN-γ T cells responses, OSS, PFS, peripheral blood T cell receptor sequencing analysis | Active, recruiting |
| NCT03502785 | Phase I/IIA, open label, multicenter | INO 5401 + INO 9012 | - INO 5401: Wilms tumor gene-1 antigene, PSMA, human telomerase reverse transcriptase - INO 9012: IL-12 |
Combined with atezolizumab | Advanced or metastatic UC | - First-line for cisplatin- ineligible disease - Second-line for cisplatin-resistant disease |
35 | AEs, Antigen-Specific Cellular Immune Response, ORR DOR, OS, PFS | Active, not recruiting |
| NCT03359239 | Proof-of-concept study, single arm | PGV001 | Multipeptide personalized neoantigen + Poly-ICLC adjuvant | Combined with atezolizumab | UC | - Second-line for cisplatin-resistant disease − Adjuvant therapy after surgery in localized disease |
10 | ORR, DOR, TTP, OS | Active, recruiting |
| DC-based vaccines | |||||||||
| NCT05749627 | Single group assignment | Neoantigen-based DC immune preparation or neoantigen peptide vaccine | Personalized tumor neoantigen DC or peptide | Monotherapy | Advanced or metastatic solid tumors, including UC | Second-line | 20 | PFS, ORR, tumor markers, OS, DCR, tumor imaging, changes in peripheral blood cytokines, ECOG-PS. |
Active, recruiting |
| Virus-based vaccines | |||||||||
| NCT02432963 | Phase I | MVA-p53 | AnkarA virus expressing p53 | Combined with pembrolizumab | Advanced or metastatic solid tumors, including UC | Second-line | 11 | AEs | Active, not recruiting |
| NCT04610671 | Phase I | CG007 + n-dodecyl-B-D-maltoside adjuvant | Adenovirus, tumor-selective GM-CSF synthesis | Combined with nivolumab | BC | Cisplatin-ineligible disease | 21 | AEs, Change in PD-L1 expression on tumor and immune cells, change in intraepithelial CD8+ T cell density | Active, not recruiting |
| mRNA-based vaccines | |||||||||
| NCT03739931 | Phase I, open label, multicenter | mRNA-2752 | Human OX40L, IL-23, IL-36γ | Monotherapy or combined with durvalumab | Advanced or metastatic solid tumors, including UC | - First-line in platinum- ineligible disease - Later lines in platinum- resistant disease |
264 | DLTs, AEs, ORR, Cmax | Active, recruiting |
| NCT03289962 | Phase Ia/Ib, openlabel, multicenter | Autogene Cevumeran (RO7198457) | Personalized neoantigens | Monotherapy or combined with atezolizumab | Advanced or metastatic solid tumors, including UC | First-line in standard therapy – ineligible − Later lines in relapsed disease |
272 | DLT, AEs, CRR, PRR, DOR, ORR, OS, PFS | Active, not recruiting |
| NCT03313778 | Phase I, open label, non-randomized | mRNA-4157 | Personalized neoantigens | Monotherapy or combined with pembrolizumab | Solid tumors, including UC: resected localized disease or advanced or metastatic disease | − Adjuvant monotherapy for resected disease - First-line combined with ICI in metastatic disease |
108 | AEs, DLT, change from baseline of biomarker levels, Antigen-specific T-cell responses in peripheral blood, ORR, DOR, PFS, OS |
Active, not recruiting |
AEs = adverse events, BC = bladder cancer, BCG = Bacillus Calmette-Guerin, CBR = Clinical benefit rate, Cmax = maximum observed concentration, CRR = complete response rate, CT = chemotherapy, DC = dendritic cell, DCR = disease control rate, DFS = disease-free survival, DLTs = dose limiting toxicities, DMFS = distant metastasis-free survival, DOR = duration of response, ECOG-PS = Eastern Cooperative Oncology Group – performance status, ICIs = immune checkpoint inhibitors, IL = interleukin, NA = not available, NMIBC = non-muscle invasive bladder cancer, NSCLC = non-small cell lung cancer, ORR = objective response rate, OS = overall survival, PD-L1 = programmed cell death ligand 1, PFS = progression-free survival, PRR = partial response rate, PSMA = prostate-specific membrane antigen, SCCHN = squamous cell carcinoma of head or neck, TTP = time to progression, TTR = time to response, UC = urothelial cancer.
Survivin-2B80–88 is a HLA-A24 restricted antigenic apoptosis inhibitor, recognized by CTLs. After demonstrating its safety and activity in a phase I trial,53 the subcutaneous vaccine was tested in association with IFA and IFNα in 12 patients with metastatic UC, achieving a significantly longer OS with respect to a control group (p = .0009).42 Subsequent evaluations are likely anticipated, ideally confirming these results.
Promising results were reported combining peptide-based vaccines and BCG. The association of NY-ESO-1 intradermal peptide-based vaccine with GM-CSF and BCG, demonstrated safety and ability to induce the generation of antibodies and a predominant CD4+ T-cell response in a cohort of six patients with UC.54 In a non-randomized phase I open-label exploratory study, a recombinant melanoma-associated antigens (MAGE)-A3 protein vaccine was tested with the adjuvant AS15, alone or added to BCG instillations, in 24 patients with NMIBC. The treatment was safe and lead to the identification in blood of vaccine-specific T cells, in half of the patients, although no oncological outcomes were available.55 Unfortunately the following phase II trial, the MAGNOLIA trial, that was aiming to assess the activity of adjuvant recombinant MAGE-A3 and AS15 after RC for MIBC, was stopped prematurely because of negative outcomes in parallel phase III trials in other cancer types.56
Apart from NGS, neoantigens’ identification is feasible through platforms such as ATLAS™, an autologous immune assay that uses ex vivo screening of all patient-specific mutations to select neoantigens. For instance, this technique showed promising results identifying up to 20 TSAs, leading to a subcutaneous peptide vaccine, GEN-009, that was successfully tested in combination with PD1 inhibition in a phase 1 trial in patients affected by solid tumors, including UC. Preliminarily, broad neoantigen-specific immune responses and epitope spreading was observed.57
Recently, a subcutaneous and intradermal peptide-based personalized genome vaccine (PGV_001) platform was developed employing the OpenVax computational pipeline applied to genomic and transcriptomic sequencing of tumor aimed at generating personalized neoantigen vaccines.58 In patients with various solid tumors, including UC, its safety and efficacy as a monotherapy or in combination with atezolizumab in the adjuvant or metastatic setting was evaluated, finding a neoantigen-specific immune response in all patients.59,60 Further trials are ongoing (see Table 2).
These trials and additional ones in other malignancies are demonstrating the induction of persistent memory T cell responses and epitopes, spreading showcase the promise of personalized neoantigen-based peptide vaccines patients.59,60
Cell-based vaccines
A cellular vaccine is a method of delivering TAAs, based on tumor cells or patient-derived immune cells loaded with antigens. Different types of whole cells or fragments have been tested, such as tumor cells and modified autologous cancer or immune cells. Tumor cell vaccines are killed tumor cells injected into the patient in order to stimulate a response against numerous TAAs. Nevertheless, their immunogenicity is limited, probably due to the concurrence of immunosuppressor factors.61 Various ways of improving it have been tried, facilitating cell death, such as adding IFN genes-activating nanoparticles,30 or utilizing emulsions, liposomes, or polymeric particulate as carriers.62 Moreover, cells can be engineered to secrete co-stimulatory molecules or receptors, such as IL-21, IL-7 and chemokine receptor-7, to enhance lymphoid-homing and antigen’s presentation and generate a more efficacious immune response.63,64
Usually, the immune cell vaccines of choice are DCs-based. Monocytes and hematopoietic stem cells are isolated from patient’s peripheral blood or from umbilical cord blood, they are activated in monocyte-derived DCs, loaded with TAAs and injected into the patient, intravenously, subcutaneously or intradermally. The loading may be by direct pulsing,65 messenger RNA (mRNA) electroporation,66 viral transduction,67 fusion with tumor cells68 or incubation with tumor lysate.69 DC vaccines are able to present TAAs to T cells and may also be able to transfer antigens to endogenous cross-presenting DCs.70 Their efficacy may depend on the DC subset. In fact, it has been shown that conventional DCs and myeloid/plasmacytoid DCs act on different T-activation pathways,71 and their combined use may improve the vaccine’s immunogenicity.72
Due to the interactions between DCs and the different types of T cells in lymph nodes involved at different sites, the administration route may influence the type of immune response and combining different routes of DC administration may enhance immune responses.35 Several potential adjuvants have been evaluated to improve DC-based vaccines efficacy. The most widely promoted have been IL-2,73 GM-CSF,47 IL-4-secreting autologous fibroblasts,74 and KLH.75
An emerging strategy of DCs vaccination is in situ vaccine. The rationale is based on an attempt of enhancing DCs intratumoral infiltration and activation causing a local extensive antigen release. In detail, it consists of injection of FMS-like tyrosine kinase 3 ligand and TLR agonist into the tumor lesion, aimed at causing infiltration of DCs in the tumor and their activation, respectively; a local irradiation is provided to generate the TAAs release. A phase I trial on patients affected by lymphoma reported promising anti-tumor T cell responses and cancer remission.76
So far, DCs have had the greatest success in efficacy, among the different types of cancer vaccines, being safe and able to generate strong immune responses, although they may be limited by the complexity and the cost of the implementation process, since the vaccine needs to be customized and engineered specifically for each patient.77 In order to overcome this limit, recently DCs have been developed from induced pluripotent stem cells, with promising results.78
Described below are the most interesting clinical trials on cell-based cancer vaccines.
A phase I-II trial, assessed a DCs-based vaccine loaded with Wilms’ tumor 1 (WT1), in association with the adjuvant OK-432, a penicillin-killed and lyophilized preparation of Streptococcus pyogenes, able to enhance DC’s activity through the TLR-4 link.65 Patients affected by advanced UC (n = 2), NMIBC (n = 3), or renal cell carcinoma (RCC) (n = 5) received the adjuvant and the intradermal vaccine in addition to standard of care therapy, either chemotherapy or tyrosine kinase inhibitors (TKI). The treatment was tolerable and active. Seventy percent of patients with RCC reached SD, no patient with advanced UC had benefits, although all three patients with NMIBC achieved long-term RFS, two of whom avoiding cystectomy (RFS of the three patients: 26.8, 84.8, and 33.4 months after the initiation of DC)65 (see Table 1). Albeit limited by the small sample size, these results are promising and hopefully will be validated in additional and wider trials in patients with NMIBC.
A DC-based intradermal vaccine loaded with human epidermal growth factor receptor 2 (HER-2) was developed. DCs were transduced with an adenovirus targeting HER-2.67 A phase I study was held, evaluating 21 patients with advanced pre-treated UC expressing HER-2. Preliminary results showed safety and promising activity with a clinical benefit rate of 33.3%.79
The efficacy of a subcutaneous DC-vaccine loaded with MAGE-3 was evaluated in preclinical studies80 and in a phase I/II trial on four patients with advanced UC. Three PR were noted, without significant toxicity81 (see Table 1). To our knowledge, no subsequent evaluations are currently ongoing, hopefully further trials will affirm these data.
A DC-based intradermal or intracutaneous vaccine pulsed with CDX-1307 was evaluated in a phase I trial in combination with several adjuvants: GM-CSF, the TLR3 agonist Poly-ICLC and/or the TLR7/8 agonist resiquimod. The experimental treatment was well tolerated, generating a significant hCG-β–specific cellular and promising results.47 Unfortunately, a randomized phase II trial on patients with newly diagnosed, resectable UC, was ended early due to scarce accrual.82
To our knowledge, the only allogeneic whole cell intradermal vaccine being tested in UC is Vesigenurtacel-L. It uses HSP technology to chaperone TSAs for presentation to T-cells. A phase I-II trial assessed Vesigenurtacel-L as an adjuvant treatment for 10 patients affected by NMIBC, considered at high risk or relapse (see Table 1). The 1-year RFS rate was 70%. Unluckily, the following phase II trial comparing Vesigenurtacel-L in combination with BCG vs BCG alone, was terminated early because of accrual inability due to evolution of the treatment landscape.83 Allogeneic natural killer (NK) cell-based vaccines and chimeric antigen receptor (CAR)-T cell therapies are being vigorously developed for solid tumors but have not been evaluated in UC yet.84
Virus-based vaccines
The virus-based vaccine is composed of a virus whose encoded genes are modified to include TAA sequences. Viruses have a high efficiency of cell infection and replication in the cytoplasm. They are able to transport a large amount of recombinant genetic material without losing infectivity and may activate both cellular and humoral immune responses.26 In addition, oncolytic virus can directly lyse the tumor, enhancing antigens and virus release.85 The virus may be delivered in live attenuated or inactivated, although, more frequently, VLP are used.39 The most used virus species are adenovirus,86 poxvirus,87 HSV,88 measles,89 reovirus,90 and coxsackievirus.91 Particularly, adenovirus is considered one of the best vectors, because of its stability, its broad tropism, the facility of its production and the manipulability of its genic material.92 As well, poxviruses are able to carry large antigens and have a strong immunogenicity mediated by TLR-dependent and -independent cytokines.93 To reduce the replication in normal cells viruses are usually genetically modified to be tumor cell selective.94
A major limitation of virus-based vaccines is the host’s natural immune response, which is able to neutralize the viral vector. In order to avoid this effect, different types of viral vectors with the same tumor antigen may be sequential injected.94 In addition, the TME inhibitory pathways may limit efficacy; hence various strategies have been proposed. First of all, combining the vaccine with ICI,95 RT,96 or chemotherapy97 showed promising results. Secondly, other promising approaches include targeting immunosuppressor molecules such as yes-associated protein, a coactivator of the immunosuppressive environment secreted by Tregs,98 or transforming growth factor (TGF)-β.99 In conclusion, virus’s genetic code may be modified with immune-regulating genes, such as human mucin-1, IL-12, and GM-CSF.86
We now summarize the most relevant results of trials on virus-based vaccines in patients with UC.
An adenovirus-based vaccine encoding for GM-CSF, CG0070, was developed.86 It is an oncolytic virus selectively replicating in retinoblastoma-deficient cells of UC. Moreover, the transcription of GM-CSF is controlled by the E2F–1 promoter, selectively active in UC cells with a retinoblastoma pathway defect. Therefore, the GM-CSF production is tumor-selective. In a phase I trial, the BOND trial, intravesical CG0070 was safe and active with a CR rate of 48.6%.86 In the following phase II trial, BONDII, CG0070 was administered intravesically, after dodecyl maltoside, an agent used to improve transduction, on 45 patients with NMIBC relapsed after BCG. It is noteworthy that the 6-month CR rate was 47% in the overall population, 58% in patients with pure carcinoma in situ (CIS), 33% in pure Ta/T1 forms and 50% in patients with both100 (see Table 1). This promising results led to the design of a phase II trial in patients with NMIBC, that tested CG0070 associated with pembrolizumab, with preliminary interesting results, with a durable CR rate of 87.5%101 (see Table 1). This encouraging strategy is being evaluated in further ongoing trials (see Table 2).
A Coxsackievirus A21 (CVA21)-based vaccine, targeting an intercellular adhesion molecule-1 (ICAM-1), was evaluated in a phase I trial, the CANON trial, on 15 patients with NMIBC; CVA21 was administered intravesically alone or in combination with mitomycin-C, prior to surgery. The treatment was well tolerated and active, with 1 CR and evidence of marked inflammatory changes in tissue biopsies, toward a more “immunological hot.”91 Intravenous CVA21-based vaccine was tested in combination with pembrolizumab in a phase I-II on patients with metastatic non-small cell lung cancer (NSCLC) or UC. The 43 patients affected by UC showed an objective response rate (ORR) of 20%, similar to the efficacy of pembrolizumab alone102 (see Table 1). Consequently, this strategy was not subsequently investigated in patients with UC.
Following the positive results of the vaccination with vaccinia virus in patients with melanoma metastases,103 a phase I trial was held, on four patients with NMIBC. They received a neoadjuvant treatment with intravesically Dryvax vaccinia virus before RC. The histological report described significant mucosal and submucosal inflammatory infiltration and evidence of viral infection. The treatment showed no severe systemic side effects. At a median follow-up of 4 years, three patients out of four were free of recurrence.104 Expectantly, deeper and broader trials will probably assess the vaccine, with the hope of confirming these encouraging findings.
TRICOM is the combination of three immune costimulatory molecules: B7.1, ICAM-1, and leukocyte function-associated antigen-3 (LFA-3). It has been evaluated in multiple trials. A virus-based vaccine encoding GM-CSF or TRICOM, using the viral vector fowlpox (FPV), a recombinant poxvirus, was administered intravesically in 20 patients affected by BCG unresponsive NMIBC, scheduled for surgery. The histological reports of the cystectomies showed proof of effective infection/transfection. The treatment was safe and able to generate immune responses, testified by the detection of serum antibodies.87
PANVAC™ is a vaccine composed by an association of two viral vectors, vaccinia and FPV, encoding epithelial mucin 1 (MUC1) and carcinoembryonic antigen (CEA), administered with TRICOM. In a randomized phase II trial, subcutaneous PANVAC™ in association with BCG was compared to BCG alone, in 30 patients affected by NMIBC relapsed after BCG. The treatment was safe, with comparable outcomes to those with BCG alone105 (see Table 1). A second generation of PANVAC, CV301, was then developed. CV301 contains two recombinant poxviruses, Modified Vaccinia Ankara (MVA), used for priming, and FPV, for booster doses; the viruses encode CEA and MUC1, along with TRICOM. The association of CV301 and ICI showed potential activity in a phase I trial106; unfortunately the following phase II trial testing the combination of subcutaneous CV301 and intravenous atezolizumab on 43 cisplatin-refractory or cisplatin-unfit patients with advanced UC, was halted at the interim analysis for poor efficacy95 (see Table 1).
Nucleic acids-based vaccines
The rationale of generating a vaccine based on nucleic acids, lies on the ubiquity and sharing of the transcriptional and translational systems. The host is able to translate the information encoded into the nucleic acids-based vaccine generating antigens able to induce immune responses. Both mRNA and DNA vaccines have been developed.
mRNA-based vaccines
mRNA is formed by an exogenous plasmid which is translated directly inside the cytoplasm, instantly generating peptides. The TAAs are then expressed by MHC molecules to APCs.26 mRNA vaccines usually contain several TAAs able to induce a wide cellular immunity.26 Humoral immunity is frequently increased by the co-administration of adjuvants.107 Moreover mRNA itself has immunogenic features.108
mRNA-based vaccines are efficient, easy to produce and safe, without the risk of insertion in the host’s genome, although the molecule is not very stable and the delivery efficiency may be challenging.109 In addition, when the required mRNA sequence information is determined, rapid and large-scale production can be achieved. Ultimately, the molecule is intracellular degraded by natural pathways.109
Two types of mRNA-based vaccines exist: non-replicating mRNA and self-amplifying RNA (SAM). The structure of the former comprises 7-methylguanosine 5′ cap, 5’-untranslated region, open reading frame (OFR), 3’-untranslated region, and 3’poly(A) tail.110,111 SAMs contain two OFRs, one encoding TAAs and one including genes necessary for viral replication and amplification, thus producing numerous copies of TAAs.112 Both are complexed with protamine, able to link with TLR and increase immune response.113
SAM-based vaccines on cancer have not yet reached trials on human, non-replicating mRNA-vaccines are the most studied. Both types of mRNA-based vaccines are synthesized through in vitro transcription, a method that uses bacteriophage RNA polymerase and DNA template.114 This method is also able to enhance innate immunity due to short RNA contaminants, with consequent high levels of IFN secretion; the subsequent purification process by high-performance liquid chromatography or cellulose, decreases the excessive immune activation.115 New production techniques enhancing stability and nanotechnologies improving delivery, together with acting on the untranslated regions of mRNA, are the key to increasing vaccine efficacy. For instance, crucial elements include regulating the GC content, modifying the transcript with alternative nucleotides,116 correcting hairpin loops, replacing rare codons117 and encapsulating mRNA into positively charged liposomal particles.30,118 Moreover, epigenomic modifications of post-transcriptional RNA may regulate translation, efficiency, and immunogenicity.119 5’ cap may be engineered to link translation factors120 and the poly(A) sequence is crucial to stability, translation start, and molecule degradation.121 To conclude, the liponanoparticles platform can be induced to target specific cells.122
mRNA-4157, a lipid-encapsulated personalized mRNA-based intramuscular vaccine that codes for up to 34 neoantigens, was assessed in a phase I dose-escalation trial enrolling patients affected by various solid tumors. The vaccine was administered alone as an adjuvant therapy (n = 16) or in combination with pembrolizumab (n = 63), in patients with advanced disease. The treatment showed no grade 3 AEs. At a median follow-up of 8 months, 92% of patients who received adjuvant therapy was free from recurrence. Whereas among the 20 patients who were treated with the combination (6 UC), 3 CR and 2 PR were reported. The mRNA-4157-based vaccine showed good anti-tumor activity.122 Adjuvant intramuscular mRNA-4157 plus intravenous pembrolizumab prolonged RFS versus pembrolizumab monotherapy (HR for recurrence or death, 0.561 [95%CI 0.309–1.017]; two-sided p = .053) in a randomized phase II trial of 157 patients with resected high-risk melanoma. The safety profile was excellent with grade ≥3 treatment-related AE in 25% of patients in the combination group and 18% of patients in the monotherapy group, with no mRNA-4157-related grade 4–5 events and similar rates of immune-mediated adverse events.123 These promising data have led to a phase III trial ongoing in the melanoma setting (NCT05933577). A randomized phase II trial (InterPath-005, NCT06305767) is evaluating adjuvant mRNA-4157 combined to pembrolizumab in high-risk resected UC (see Table 2). Thus, the customized mRNA-based neoantigen platform appears highly promising preliminarily.
DNA-based vaccines
DNA-based vaccines derive from bacterial plasmids engineered to encode TAAs.124 They are more stable and durable in the human body than mRNA. The DNA plasmid needs to enter inside the cell nucleus to be transcripted and translated into peptides; from a single DNA plasmid numerous mRNA copies and peptides may derive. The vaccine may be activated inside a somatic cell, where peptides may be directly presented by MHC-I, or they may be released and subsequently processed by APCs. The double-stranded DNA structure is also able to directly improve responses, activating innate immunity.125 In addition, DNA may be encoded inside APCs, through intradermal delivery. Thus, DNA-based vaccines are able to activate both humoral and cellular immune responses.126 Moreover, they may prime the direct activation of signaling pathways leading to GM-CSF and cytokines production,30 or they may be modified to express immune agonists in the form of CpG motifs, polymers, liposomes, or small molecules.127 Nano-carriers protect DNA vaccines from degradation, they may be based on lipids, proteins, inorganic materials, or polymeric nano-carriers.128 DNA-based vaccines are selective and safe. The main concern is the rare but potential risk of DNA insertion into the genome, with the consequent possible activation of oncogenes.129
In order to improve the DNA-based vaccine efficacy, powerful promoter sequences may be added. The Kozak sequence130 and cytomegalovirus (CMV) promoters131 were proven to be effective. Multi-antigens DNA vaccines are constructed to diversify the responses,132 while nanodevice vaccines are able to assemble antigen and adjuvants inside tumor cells.30 In order to ease the delivery through extra and intracellular barriers, electroporation, gene gun, and sonoporation strategies have been developed.30 Ultimately, chimeric antigens, such as xenoantigens, including homologous and heterologous antigens, are able to bypass immune tolerance.133
A DNA vaccine (pTVG-HP [MVI-816]) encoding prostatic acid phosphatase (PAP) appeared to improve outcomes in a subgroup of non-metastatic prostate cancer with rapidly progressive disease.134 Additional trials using pTVG-HP in combination with PD-1 blockade are under way.
To our knowledge, there are no data on DNA-based vaccines trials focused on patients with UC. Here, we reported the most promising developments from preclinical studies. It has been proved that antisense oligonucleotide-based therapy encoding TGF-beta may be able to arrest tumor cells growth in vitro and in UC mouse models.135 A recombinant BCG DNA-based vaccine proved the ability of generating immune responses, CR and prolonging survival in preclinical models of UC.136 The association of BCG DNA-based and murine IL-12-based vaccines showed promising preclinical activity as well.137
Histone deacetylases (HDAC) are enzymes that catalyze the removal of acetyl groups from histone, inhibiting DNA transcription. Adding an HDAC inhibitor could improve DNA-based vaccine efficacy. The combination of an HDAC inhibitor with a CMV promoter driven-DNA vaccine against HER-2 was able to increase lymphocyte infiltration and specific CTL enhancing the antitumor effects.131 DNA-based vaccines encoding Mycobacterium tuberculosis’s antigens Ag85A and Ag85B showed anti-tumor effects in UC mice models.138
A DNA-based vaccine encoding Flk-1 extracellular domain and the complement 3d component was developed and assessed in UC mice models, with tumor growth inhibition and survival improvements.139 The combination of a tumor cell-based vaccine secreting IL-2, IL-4 and GM-CSF and a DNA vaccine against HER-2 showed antitumor activity and an increase of CD4+ T lymphocytes and anti-HER-2 antibodies, in UC mice models.140
Combination strategies
The promising results of cancer vaccines appear to be related to a limited group of patients. Several mechanisms of resistance have been pointed out. Firstly, TAAs expression varies in the tissue because of tumor heterogeneity. The immunogenicity of TAAs may be not effective enough to induce a strong immune response. Moreover, the tumor may generate immune escape strategies.141 In detail the prolonged exposure to antigens lead to the increase of inhibitory molecules on T-cells, such as programmed cell death protein 1, cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), and lymphocyte-activation gene (LAG)-3. This leads to T-cells exhaustion.141 Based on these findings, the combination of cancer vaccination and immune-checkpoint inhibitors (ICIs) may overcome resistance mechanisms.142 In addition, cancer vaccines seem to increase TME susceptibility to ICIs.143 Other combination strategies include treatment with chemotherapy, RT or CAR-T cell therapy. Chemotherapy may enhance responses to cancer vaccines increasing TAAs release, reducing or weakening Treg and modulating DCs and T cells activity.30 Occasionally, a systemic response after stereotactic RT has been reported, in addition to local response. This finding, defined abscopal effect, firstly suggested a relationship between response to RT and host’s immune system. Subsequent research confirmed the RT ability of enhancing immune activation, to the point of being considered as an in situ vaccine.144 Additionally, RT is able to improve cytokines release, APC activity and MHC expression.145 Therefore, the combination of cancer vaccines and RT was tested in pre-clinical models, showing promising activity.144 Ultimately, a mRNA-based vaccine targeting CLDN6 was proved to enhance the outcomes of claudin-CAR-T cell therapy, in hematological and solid tumors.146
To our knowledge, no clinical trials evaluating chemotherapy or RT combined with cancer vaccines have been conducted in patients with UC. Therefore, we discuss the major evidence on ICIs and cancer vaccines combination strategies.
The treatment with NEO-PV-01, a personalized neoantigen peptide subcutaneous vaccine, combined with nivolumab, was shown to be safe and effective in a phase Ib clinical trial, that evaluated 60 patients with melanoma, NSCLC and UC. At a median follow-up of 12 months, the following results were reported in the cohort of patients with UC: ORR 27%, response conversion rate 7%, median PFS 5.8 months, median OS 20.7 months and 1-year OS rates 67%.147 Results in UC patients were similar to the benefit conferred by ICI alone, consequently further studies focused on melanoma and NSCLC.
A phase I/II study evaluated 46 patients with chemotherapy-resistant UC treated with an association of TAS0313 and pembrolizumab. TAS0313 is a subcutaneous vaccine formed by three long peptides and 12 CTL epitopes. The combination was safe and active, with an ORR of 33%, a DCR of 67%, a median PFS of 5 months and 1-year OS rate of 74.3%, in ICI-naïve patients, while in ICI-resistant patients the DCR was 50%.148 Given the promising results in extending survival, larger studies in ICI-naïve patients are expected.
A fascinating vaccine strategy against immunosuppressive molecules has been developed. IO102-IO103 is an immune-modulatory subcutaneous peptide vaccine targeting indoleamine-pyrrole 2,3-dioxygenase (IDO) and PD-L1. T-cells against tumor or immune cells expressing IDO and PD-L1 in TME, may enhance ICIs efficacy.149 Trials evaluating the combination of IO102-IO103 vaccine and ICI are ongoing (see Table 2).
Safety
Safety and feasibility are critical aspects of drug development. Cancer vaccines appear extremely safe in general, with injection site reaction being the most common AE. Almost every clinical trial described in this review reported injection site reactions, including pain, erythema, swelling, and induration, typically mild and self-limiting. Sometimes, influenza-like illness, fever, myalgia, or fatigue were reported with a full recovery within few days42,45,93,100,102,147,49,51–53,55,56,65,83. In addition, gastrointestinal alterations were described, such as nausea, diarrhea, abdominal pain, and appetite loss.83,93,147 In the event of intravesical administration, irritation symptoms may occur like dysuria, frequency, and hematuria.55,100 Grade 3 or higher AEs are rare, as confirmed in an analysis that described only six severe AEs in 500 patients receiving subcutaneous peptide-based cancer vaccines (1%).150 These included cellulitis or ulceration around the injection site, edemas of the head and neck regions, diarrhea and rectal bleeding, ulcerative colitis, and bladder-vaginal fistulae.150 Infrequently, allergic reactions have been reported, mainly mild (rash, itching), exceptionally severe (anaphylaxis).83 While vaccines are generally perceived as safe, their combination with other therapies, may increase toxicity: rarely, myelotoxicity, increased liver enzymes, acute kidney injury, or urological AEs have been reported, mainly in combination with chemotherapy or ICIs.51,52,55,65,93
Future perspectives and conclusions
Several preclinical and clinical trials on cancer vaccines are ongoing, exploring novel strategies to overcome the major obstacles to vaccination response. Particularly new antigens, adjuvants, platforms, and combination approaches are under investigation.
The novel technologies of genetic sequencing, advanced genomics and proteomics, artificial intelligence and computational assessment of the mutations, may help us to better define the immunogenicity and expression of TAAs, identifying the ideal candidate.57 Furthermore, a crucial element of efficacious vaccine production is the prediction of HLA typing and neoantigen-MHC binding and modern tools like OptiType151 and HLAscan,152 NetMHCpan-4.0,153 and MHCflurry 2.0,154 may be useful. In addition, beyond TAAs, cancer vaccine may encode cytokines and molecules able to stimulate APCs and T cells activity.40
Adjuvants are an important component of the immunogenicity of a vaccine and developments are rapidly increasing the potentiality of these means.41 Furthermore, novel materials are being explored, able to enhance APCs’ activity, antigens presentation, and immune cells’ activation. For instance, photothermal nanomaterials are gaining interest, for their direct photothermal effects and their synergic immune activation. Consequently, a photothermal nano-vaccine was developed, showing potent antitumor activity in melanoma mice models.155
While UC treatment is rapidly evolving, most cancer vaccines struggle to gain their spot in the current therapeutic algorithm.
Positive preclinical studies on UC cells or UC mice models focused on new antigens such as B7-H1,156 antigen 85A and α-syn,157 an element of the p53/p21 signaling pathway.158 Promising responses were detected in UC models treated with autologous DCs loaded with an allogeneic UC cell line;159 also irradiated allogeneic whole apoptotic tumor cells or autologous patient-derived tumor cells were able to induce antitumor immune responses.160
A promising approach is to utilize different kinds of vectors targeting the same TAA: one vector as a priming and the following as a boosting.94 A synergistic activity of vaccine and ICI or cytokine therapy may be a successful strategy to overcome the immune suppressive environment of tumor.93 Moreover, vaccines targeting immune receptors or molecules may induce a change in TME.149
The neoantigen coding mRNA agents appear highly promising preliminarily and might be evaluated for the adjuvant therapy of high-risk UC. This customized neoantigen-based approach incorporates the principles of precision medicine. Additionally, the adjuvant setting might be well suited to develop vaccines in the context of low burden of microscopic disease. Precision medicine needs to be developed in concert to select patients with molecular residual disease.
Recent human trials evaluating patients with UC are displayed in Table 2. The most frequently assessed strategy is a combination therapy with ICI; personalized neoantigens associated with various adjuvants are often the target of choice and peptide- or adenovirus-based the most tested platforms. To our knowledge, only one phase III trial is currently ongoing on patients with UC, evaluating CG007, in BCG-unresponsive NMIBC.
Of all the ongoing strategies to improve vaccines’ efficacy, the combination with common cancer treatment seems the most appealing. Both ICIs and vaccines may influence TME, decreasing the immune-suppressive features and thus enhancing host’s immune responses.142,143 Chemotherapy and RT can directly kill tumor cells, increasing the neoantigen burden released in the bloodstream. Moreover, chemotherapy might alter the bone marrow cell composition, ultimately enhancing antigen-specific T-cell responses, when combined to SLP vaccine.161 In contrast, RT is sometimes able to elicit systemic immune responses, and the abscopal effect is considered a form of in situ vaccination. Its combination with cancer vaccine is thought to be capable of improving immune activation.144 Intriguingly, the intravesical administration of cancer vaccine, may be combined to BCG, with the dual aim of directly targeting UC cells and synergizing the immune responses.46 Lastly, cancer vaccines may both prime the immune system and later sustain the activity and persistence of CAR-T cells, leading to enduring responses.146
Current UC treatment comprehends both local therapies, such as intravesical instillation and RT, and systemic treatments, including ICIs, chemotherapy, and target therapy. Hence, combining cancer vaccines with current UC therapies offers a promising approach to improve efficacy, overcoming resistance and ultimately increasing patients’ outcomes.
In conclusion, a deeper understanding of the interactions between vaccines, tumor and immune system, combined with innovative manufacturing strategies and combination with current treatments, will help us define the role of vaccines in the UC treatment.
Abbreviations
- ADC
antibody drug conjugate
- APC
antigen-presenting cell
- AE
adverse event
- β-HCG
human chorionic gonadotropin-β
- BC
bladder cancer
- BCG
Bacillus Calmette Guérin
- CAR
chimeric antigen receptor
- CIS
carcinoma in situ
- CEA
carcinoembryonic antigen
- CMV
cytomegalovirus
- CR
complete response
- CTL
cytotoxic T lymphocytes
- CTLA4
cytotoxic T-lymphocyte-associated protein 4
- CVA21
Coxsackievirus A21
- DC
dendritic cell
- DCR
disease control rate
- DEPDC1
DEP domain-containing 1
- DNA
deoxyribonucleic acid
- FGFR
fibroblast growth factor receptor
- FPV
fowlpox virus
- GM-CSF
granulocyte-macrophage colony stimulating factor
- HDAC
histone deacetylases
- HER-2
human epidermal growth factor receptor 2
- HLA
human leukocyte antigen
- HR
hazard ratio
- HSP
heat shock proteins
- HSV
herpes simplex virus
- ICAM-1
intercellular adhesion molecule-1
- ICI
immune checkpoint inhibitors
- IDO
indoleamine-pyrrole 2,3-dioxygenase
- IFA
incomplete Freund’s adjuvant
- IFN
interferon
- IL
interleukin
- KLH
keyhole limpet hemocyanin
- LAG
lymphocyte-activation gene
- LFA-3
leukocyte function-associated antigen-3
- MAGE
melanoma-associated antigens
- MHC
major histocompatibility complex
- MIBC
muscle-invasive BC
- MPHOSPH1
M-phase phosphoprotein 1
- Mrna
messenger RNA
- mUC
metastatic UC
- MUC1
epithelial mucin 1
- MVA
modified vaccinia ankara
- NGS
next generation sequencing
- NK
natural killer
- NMIBC
non-muscle-invasive BC
- NSCLC
non-small cell lung cancer
- OFR
open reading frame
- ORR
objective response rate
- OS
overall survival
- PAP
prostatic acid phosphatase
- PD
progressive disease
- PD1
programmed cell death protein 1
- PD-L1
programmed cell death ligand 1
- PFS
progression-free survival
- PGV
peptide-based personalized genome vaccine
- PPV
personalized peptide vaccination
- PR
partial response
- RC
radical cystectomy
- RCC
renal cell carcinoma
- RFS
relapse free survival
- RNA
ribonucleic acid
- RT
radiotherapy
- SAM
self-amplifying RNA
- SD
stable disease
- SLP
synthetic long peptides
- TAA
tumor-associated antigen
- TGF
tissue growth factor
- TLR
toll like receptor
- TKI
tyrosin kinase inhibitor
- TME
tumor microenvironment
- TRICOM
three immune costimulatory molecules
- Treg
regulatory T cells
- TSA
tumor-specific antigen
- TURBT
transurethral resection of bladder tumorUC: urothelial cancer
- UTUC
upper tract urothelial cancer
- VLP
virus-like particles
- WT1
Wilms’ tumor 1
Funding Statement
The authors reported there is no funding associated with the work featured in this article.
Disclosure statement
Guru Sonpavde, MD:
• Advisory Board: BMS, Genentech, EMD Serono, Merck, AstraZeneca, Sanofi, Seattle Genetics/Astellas, AstraZeneca, Exelixis, Janssen, Bicycle Therapeutics, Pfizer, Gilead, Scholar Rock, G1 Therapeutics, Eli Lilly/Loxo Oncology, Infinity Pharmaceuticals, Lucence Health, IMV, Vial, Syapse, Tempus, Ellipses Pharma, PrecisCa, Primum
• Consultant/Scientific Advisory Board (SAB): Suba Therapeutics, Syapse, Servier, Merck, Syncorp
• Research Support to institution: Sanofi, AstraZeneca, Gilead, Helsinn, Lucence, BMS, EMD Serono, Jazz Therapeutics
• Speaker: BIO – INFORMAÇÃO BRASILEIRA DE ONCOLOGIA Ltda, OLE Forum (Mexico), Seagen, Gilead, Natera, Exelixis, Janssen, Bayer, Aveo
• Data safety monitoring committee honorarium: Mereo
• Employment: Spouse employed by Myriad
Author contribution
Concept and Study design: Sonpavde; Methods and experimental work: Giudice, Sonpavde; Results analysis and conclusions: Giudice, Sonpavde; Manuscript preparation: Giudice, Sonpavde; Supervision: Sonpavde; Data collection: Giudice, Sonpavde; Manuscript drafting: Giudice, Sonpavde; Critical revision: Sonpavde; Approval of the manuscript: Sonpavde, Giudice.
References
- 1.Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A.. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74(3):229–21. doi: 10.3322/caac.21834. [DOI] [PubMed] [Google Scholar]
- 2.Bladder Cancer — Cancer Stat Facts . [accessed 2024 June 24]. https://seer.cancer.gov/statfacts/html/urinb.html.
- 3.Burger M, Catto JWF, Dalbagni G, Grossman HB, Herr H, Karakiewicz P, Kassouf W, Kiemeney LA, La Vecchia C, Shariat S, et al. Epidemiology and risk factors of urothelial bladder cancer. Eur Urol. 2013;63(2):234–41. doi: 10.1016/j.eururo.2012.07.033. [DOI] [PubMed] [Google Scholar]
- 4.Jin YH, Zeng XT, Liu TZ, Bai ZM, Dou ZL, Ding DG, Fan ZL, Han P, Huang YR, Huang X, et al. Treatment and surveillance for non-muscle-invasive bladder cancer: a clinical practice guideline (2021 edition). Mil Med Res. 2022;9(1):44. doi: 10.1186/s40779-022-00406-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balar AV, Kamat AM, Kulkarni GS, Uchio EM, Boormans JL, Roumiguié M, Krieger LEM, Singer EA, Bajorin DF, Grivas P, et al. Pembrolizumab monotherapy for the treatment of high-risk non-muscle-invasive bladder cancer unresponsive to BCG (KEYNOTE-057): an open-label, single-arm, multicentre, phase 2 study. Lancet Oncol. 2021;22(7):919–30. doi: 10.1016/S1470-2045(21)00147-9. [DOI] [PubMed] [Google Scholar]
- 6.Lee A. Nadofaragene firadenovec: first approval. Drugs. 2023;83(4):353–7. doi: 10.1007/s40265-023-01846-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flaig TW, Spiess PE, Abern M, Agarwal N, Bangs R, Buyyounouski MK, Chan K, Chang SS, Chang P, Friedlander T, et al. NCCN Guidelines® Insights: Bladder Cancer, Version 3.2024. J Natl Compr Canc Netw. 2024;22(4):216–225. doi: 10.6004/jnccn.2024.0024. [DOI] [PubMed] [Google Scholar]
- 8.Bajorin DF, Witjes JA, Gschwend JE, Schenker M, Valderrama BP, Tomita Y, Bamias A, Lebret T, Shariat SF, Park SH, et al. Adjuvant Nivolumab versus Placebo in Muscle-Invasive Urothelial Carcinoma. N Engl J Med. 2021;384(22):2102–14. doi: 10.1056/NEJMoa2034442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Powles T, Park SH, Voog E, Caserta C, Valderrama BP, Gurney H, Kalofonos H, Radulović S, Demey W, Ullén A, et al. Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N Engl J Med. 2020;383(13):1218–30. doi: 10.1056/NEJMoa2002788. [DOI] [PubMed] [Google Scholar]
- 10.Balar AV, Castellano D, O’Donnell PH, Grivas P, Vuky J, Powles T, Plimack ER, Hahn NM, de Wit R, Pang L, et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. Lancet Oncol. 2017;18(11):1483–92. doi: 10.1016/S1470-2045(17)30616-2. [DOI] [PubMed] [Google Scholar]
- 11.Bellmunt J, de Wit R, Vaughn DJ, Fradet Y, Lee J-L, Fong L, Vogelzang NJ, Climent MA, Petrylak DP, Choueiri TK, et al. Pembrolizumab as Second-line therapy for advanced urothelial carcinoma. N Engl J Med. 2017;376(11):1015–26. doi: 10.1056/NEJMoa1613683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van der Heijden Ms, Sonpavde G, Powles T, van der Heijden MS, Necchi A, Burotto M, Schenker M, Sade JP, Bamias A, Beuzeboc P, et al. Nivolumab plus gemcitabine–cisplatin in advanced urothelial carcinoma. N Engl J Med. 2023;389(19):1778–89. doi: 10.1056/nejmoa2309863. [DOI] [PubMed] [Google Scholar]
- 13.Powles T, Valderrama BP, Gupta S, Bedke J, Kikuchi E, Hoffman-Censits J, Iyer G, Vulsteke C, Park SH, Shin SJ, et al. Enfortumab vedotin and pembrolizumab in untreated advanced urothelial cancer. N Engl J Med. 2024;390(10):875–88. doi: 10.1056/NEJMoa2312117. [DOI] [PubMed] [Google Scholar]
- 14.Tagawa ST, Balar AV, Petrylak DP, Kalebasty AR, Loriot Y, Fléchon A, Jain RK, Agarwal N, Bupathi M, Barthelemy P, et al. TROPHY-U-01: a phase II open-label study of sacituzumab govitecan in patients with metastatic urothelial carcinoma progressing after platinum-based chemotherapy and checkpoint inhibitors. J Clin Oncol. 2021;39(22):2474–85. doi: 10.1200/JCO.20.03489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ross JS, Wang K, Khaira D, Ali SM, Fisher HAG, Mian B, Nazeer T, Elvin JA, Palma N, Yelensky R, et al. Comprehensive genomic profiling of 295 cases of clinically advanced urothelial carcinoma of the urinary bladder reveals a high frequency of clinically relevant genomic alterations. Cancer. 2016;122(5):702–11. doi: 10.1002/cncr.29826. [DOI] [PubMed] [Google Scholar]
- 16.Loriot Y, Matsubara N, Park SH, Huddart RA, Burgess EF, Houede N, Banek S, Guadalupi V, Ku JH, Valderrama BP, et al. Erdafitinib or chemotherapy in advanced or metastatic urothelial carcinoma. N Engl J Med. 2023;389(21):1961–71. doi: 10.1056/NEJMoa2308849. [DOI] [PubMed] [Google Scholar]
- 17.Siefker-Radtke AO, Matsubara N, Park SH, Huddart RA, Burgess EF, Özgüroğlu M, Valderrama BP, Laguerre B, Basso U, Triantos S, et al. Erdafitinib versus pembrolizumab in pretreated patients with advanced or metastatic urothelial cancer with select FGFR alterations: cohort 2 of the randomized phase III THOR trial. Ann Oncol. 2024;35(1):107–17. doi: 10.1016/j.annonc.2023.10.003. [DOI] [PubMed] [Google Scholar]
- 18.Apolo AB, Ballman KV, Sonpavde GP, Berg SA, Kim WY, Parikh RA, Teo MY, Sweis RF, Geynisman DM, Grivas P, et al. AMBASSADOR alliance A031501: phase III randomized adjuvant study of pembrolizumab in muscle-invasive and locally advanced urothelial carcinoma (MIUC) vs observation. J Clin Oncol. 2024;42(4_suppl):531–531. doi: 10.1200/JCO.2024.42.4_suppl.LBA531. [DOI] [Google Scholar]
- 19.Wang R, Pan W, Jin L, Huang W, Li Y, Wu D, Gao C, Ma D, Liao S. Human papillomavirus vaccine against cervical cancer: opportunity and challenge. Cancer Lett. 2020;471:88–102. doi: 10.1016/j.canlet.2019.11.039. [DOI] [PubMed] [Google Scholar]
- 20.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363(5):411–22. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 21.Gerlinger M, Rowan AJ, Horswell S, Larkin J, Endesfelder D, Gronroos E, Martinez P, Matthews N, Stewart A, Tarpey P, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366(10):883–92. doi: 10.1056/NEJMoa1113205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bijker MS, van den Eeden Sjf, Franken KL, Melief CJM, Offringa R, van der Burg SH, van den Eeden SJF. CD8+ CTL priming by exact peptide epitopes in incomplete freund’s adjuvant induces a vanishing CTL response, whereas long peptides induce sustained CTL reactivity. J Immunol. 2007;179(8):5033–40. doi: 10.4049/jimmunol.179.8.5033. [DOI] [PubMed] [Google Scholar]
- 23.Kallingal A, Olszewski M, Maciejewska N, Brankiewicz W, Baginski M. Cancer immune escape: the role of antigen presentation machinery. J Cancer Res Clin Oncol. 2023;149(10):8131–41. doi: 10.1007/s00432-023-04737-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wculek SK, Cueto FJ, Mujal AM, Melero I, Krummel MF, Sancho D. Dendritic cells in cancer immunology and immunotherapy. Nat Rev Immunol. 2020;20(1):7–24. doi: 10.1038/s41577-019-0210-z. [DOI] [PubMed] [Google Scholar]
- 25.West MA, Wallin RPA, Matthews SP, Svensson HG, Zaru R, Ljunggren H-G, Prescott AR, Watts C. Enhanced dendritic cell antigen capture via toll-like receptor-induced actin remodeling. Science. 2004;305(5687):1153–7. doi: 10.1126/science.1099153. [DOI] [PubMed] [Google Scholar]
- 26.Liu J, Fu M, Wang M, Wan D, Wei Y, Wei X. Cancer vaccines as promising immuno-therapeutics: platforms and current progress. Vol 2022. Biomed Central Ltd. 2022;15(1):28. doi: 10.1186/s13045-022-01247-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lang F, Schrörs B, Löwer M, Türeci Ö, Sahin U. Identification of neoantigens for individualized therapeutic cancer vaccines. Nat Rev Drug Discov. 2022;21(4):261–82. doi: 10.1038/s41573-021-00387-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Addeo A, Friedlaender A, Giovannetti E, Russo A, de Miguel-Perez D, Arrieta O, Cardona AF, Rolfo C. A new generation of vaccines in the age of immunotherapy. Curr Oncol Rep. 2021;23(12):137. doi: 10.1007/s11912-021-01130-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Srivastava PK. Neoepitopes of cancers: looking back, looking ahead. Cancer Immunol Res. 2015;3(9):969–77. doi: 10.1158/2326-6066.CIR-15-0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fan T, Zhang M, Yang J, Zhu Z, Cao W, Dong C. Therapeutic cancer vaccines: advancements, challenges, and prospects. Signal Transduct Target Ther. 2023;8(1):450. doi: 10.1038/s41392-023-01674-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yuan M, Han Z, Liang Y, Sun Y, He B, Chen W, Li F. mRNA nanodelivery systems: targeting strategies and administration routes. Biomater Res. 2023;27(1). doi: 10.1186/s40824-023-00425-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen X. Emerging adjuvants for intradermal vaccination. Int J Pharm. 2023;632:122559. doi: 10.1016/j.ijpharm.2022.122559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hato L, Vizcay A, Eguren I, Pérez-Gracia JL, Rodríguez J, Gállego Pérez-Larraya J, Sarobe P, Inogés S, Díaz de Cerio AL, Santisteban M, et al. Dendritic cells in cancer immunology and immunotherapy. Cancers (Basel). 2024;16(5):981. doi: 10.3390/cancers16050981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bedrosian I, Mick R, Xu S, Nisenbaum H, Faries M, Zhang P, Cohen PA, Koski G, Czerniecki BJ. Intranodal administration of peptide-pulsed mature dendritic cell vaccines results in superior CD8 + T-cell function in melanoma patients. J Clin Oncol. 2003;21(20):3826–35. doi: 10.1200/JCO.2003.04.042. [DOI] [PubMed] [Google Scholar]
- 35.Fong L, Brockstedt D, Benike C, Wu L, Engleman EG. Dendritic cells injected via different routes induce immunity in cancer patients. J Immunol. 2001;166(6):4254–9. doi: 10.4049/jimmunol.166.6.4254. [DOI] [PubMed] [Google Scholar]
- 36.Zhang H, Hong H, Li D, Ma S, Di Y, Stoten A, Haig N, Di Gleria K, Yu Z, Xu X-N, et al. Comparing pooled peptides with intact protein for accessing cross-presentation pathways for protective CD8+ and CD4+ T cells. J Biol Chem. 2009;284(14):9184–91. doi: 10.1074/jbc.M809456200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kreiter S, Vormehr M, van de Roemer N, Diken M, Löwer M, Diekmann J, Boegel S, Schrörs B, Vascotto F, Castle JC, et al. Mutant MHC class II epitopes drive therapeutic immune responses to cancer. Nature. 2015;520(7549):692–6. doi: 10.1038/nature14426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jiang J, Xie D, Zhang W, Xiao G, Wen J. Fusion of Hsp70 to Mage-a1 enhances the potency of vaccine-specific immune responses. J Transl Med. 2013;11(1):300. doi: 10.1186/1479-5876-11-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mohsen MO, Zha L, Cabral-Miranda G, Bachmann MF. Major findings and recent advances in virus–like particle (VLP)-based vaccines. Semin Immunol. 2017;34:123–32. doi: 10.1016/j.smim.2017.08.014. [DOI] [PubMed] [Google Scholar]
- 40.Tine JA, Firat H, Payne A, Russo G, Davis SW, Tartaglia J, Lemonnier FA, Demoyen PL, Moingeon P. Enhanced multiepitope-based vaccines elicit CD8+ cytotoxic T cells against both immunodominant and cryptic epitopes. Vaccine. 2005;23(8):1085–91. doi: 10.1016/j.vaccine.2003.01.001. [DOI] [PubMed] [Google Scholar]
- 41.Khong H, Overwijk WW. Adjuvants for peptide-based cancer vaccines. J Immunother Cancer. 2016;4(1):56. doi: 10.1186/s40425-016-0160-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tanaka T, Kitamura H, Inoue R, Nishida S, Takahashi-Takaya A, Kawami S, Torigoe T, Hirohashi Y, Tsukamoto T, Sato N, et al. Potential survival benefit of anti-apoptosis protein: survivin-derived peptide vaccine with and without interferon alpha therapy for patients with advanced or recurrent urothelial cancer—results from phase I clinical trials. Clin Dev Immunol. 2013;2013:1–9. doi: 10.1155/2013/262967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Terán-Navarro H, Zeoli A, Salines-Cuevas D, Marradi M, Montoya N, Gonzalez-Lopez E, Ocejo-Vinyals JG, Dominguez-Esteban M, Gutierrez-Baños JL, Campos-Juanatey F, et al. Gold glyconanoparticles combined with 91–99 peptide of the bacterial toxin, listeriolysin O, are efficient immunotherapies in experimental bladder tumors. Cancers (Basel). 2022;14(10):2413. doi: 10.3390/cancers14102413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Petrina M, Martin J, Basta S. Granulocyte macrophage colony-stimulating factor has come of age: from a vaccine adjuvant to antiviral immunotherapy. Cytokine Growth Factor Rev. 2021;59:101–10. doi: 10.1016/j.cytogfr.2021.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Obara W, Eto M, Mimata H, Kohri K, Mitsuhata N, Miura I, Shuin T, Miki T, Koie T, Fujimoto H, et al. A phase I/II study of cancer peptide vaccine S-288310 in patients with advanced urothelial carcinoma of the bladder. Ann Oncol. 2017;28(4):798–803. doi: 10.1093/annonc/mdw675. [DOI] [PubMed] [Google Scholar]
- 46.Obara W, Hara I, Kato Y, Kato R, Inoue K, Sato F, Mimata H, Nakamura Y, Fujioka T. Immunotherapy with cancer peptides in combination with intravesical Bacillus Calmette–Guerin for patients with non-muscle invasive bladder cancer. Cancer Immunol Immunother. 2018;67(9):1371–80. doi: 10.1007/s00262-018-2197-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morse MA, Bradley DA, Keler T, Laliberte RJ, Green JA, Davis TA, Inman BA. CDX-1307: a novel vaccine under study as treatment for muscle-invasive bladder cancer. Expert Rev Vaccines. 2011;10(6):733–42. doi: 10.1586/erv.11.20. [DOI] [PubMed] [Google Scholar]
- 48.Sasaki T, Ishihara M, Kanda H, Hori Y, Soga N, Harada N, Miyahara Y, Kageyama S, Shiku H, Sugimura Y, et al. PD32-05 phase I clinical study on the combination therapy of CHP-NY-ESO-1 cancer vaccine and MIS416 for the treatment of patients with NY-ESO-1 expressing refractory urothelial cancer or castration-resistant prostate cancer. J Urol. 2016;195(4S). doi: 10.1016/j.juro.2016.02.681. [DOI] [Google Scholar]
- 49.Matsumoto K, Noguchi M, Satoh T, Tabata K-I, Fujita T, Iwamura M, Yamada A, Komatsu N, Baba S, Itoh K, et al. A phase i study of personalized peptide vaccination for advanced urothelial carcinoma patients who failed treatment with methotrexate, vinblastine, adriamycin and cisplatin. BJU Int. 2011;108(6):831–8. doi: 10.1111/j.1464-410X.2010.09933.x. [DOI] [PubMed] [Google Scholar]
- 50.Suekane S, Noguchi M, Moriya F, Sasada T, Matsueda S, Itoh K, Matsuoka K. 863 Phase II study of personalized peptide vaccination for standard therapy-resistant urothelial carcinoma patients. Eur Urol Suppl. 2012;11(1):863–863. doi: 10.1016/S1569-9056(12)60860-4. [DOI] [Google Scholar]
- 51.Noguchi M, Matsumoto K, Uemura H, Arai G, Eto M, Naito S, Ohyama C, Nasu Y, Tanaka M, Moriya F, et al. An open-label, randomized phase II trial of personalized peptide vaccination in patients with bladder cancer that progressed after platinum-based chemotherapy. Clin Cancer Res. 2016;22(1):54–60. doi: 10.1158/1078-0432.CCR-15-1265. [DOI] [PubMed] [Google Scholar]
- 52.Suekane S, Ueda K, Nishihara K, Sasada T, Yamashita T, Koga N, Yutani S, Shichijo S, Itoh K, Igawa T, et al. Personalized peptide vaccination as second-line treatment for metastatic upper tract urothelial carcinoma. Cancer Sci. 2017;108(12):2430–7. doi: 10.1111/cas.13404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Honma I, Kitamura H, Torigoe T, Takahashi A, Tanaka T, Sato E, Hirohashi Y, Masumori N, Tsukamoto T, Sato N, et al. Phase I clinical study of anti-apoptosis protein survivin-derived peptide vaccination for patients with advanced or recurrent urothelial cancer. Cancer Immunol Immunother. 2009;58(11):1801–7. doi: 10.1007/s00262-009-0691-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sharma P, Bajorin DF, Jungbluth AA, Herr H, Old LJ, Gnjatic S. Immune responses detected in urothelial carcinoma patients after vaccination with NY-ESO-1 protein plus BCG and GM-CSF. J Immunother. 2008;31(9):849–57. doi: 10.1097/CJI.0b013e3181891574. [DOI] [PubMed] [Google Scholar]
- 55.Derré L, Cesson V, Lucca I, Cerantola Y, Valerio M, Fritschi U, Vlamopoulos Y, Burruni R, Legris A-S, Dartiguenave F, et al. Intravesical Bacillus Calmette Guerin combined with a cancer vaccine increases local T-Cell Responses in Non-muscle–Invasive Bladder Cancer Patients. Clin Cancer Res. 2017;23(3):717–25. doi: 10.1158/1078-0432.CCR-16-1189. [DOI] [PubMed] [Google Scholar]
- 56.Mulders PFA, Martínez-Piñeiro L, Heidenreich A, Babjuk M, Colombel M, Colombo R, Radziszewski P, Korneyev I, Surcel C, Yakovlev P, et al. Adjuvant RecMAGE-A3 immunotherapy after cystectomy for muscle-invasive bladder cancer: lessons learned from the phase 2 Magnolia clinical trial. Eur Urology Focus. 2019;5(5):849–52. Elsevier B.V. doi: 10.1016/j.euf.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 57.Gillison M, Awad M, Twardowski P, Sukari A, Johnson ML, Stein MN, Hernandez R, Price J, Mancini KJ, Shainheit M, et al. 485 Long term results from a phase 1 trial of GEN-009, a personalized neoantigen vaccine, combined with PD-1 inhibition in advanced solid tumors. J Immunother Cancer. 2021;9(Suppl 2):515–515. doi: 10.1136/jitc-2021-SITC2021.485. [DOI] [Google Scholar]
- 58.Saxena M, Marron T, Kodysh J, Rubinsteyn A, Finnigan J, Blasquez A, Meseck M, O’Donnell T, Delbeau D, Galsky M, et al. Abstract CT270: immunogenicity of PGV_001 neoantigen vaccine in a phase-I clinical trial, across various types of cancers in adjuvant setting. Cancer Res. 2023;83(8_Supplement):270–270. doi: 10.1158/1538-7445.AM2023-CT270. [DOI] [Google Scholar]
- 59.Hu Z, Leet DE, Allesøe RL, Oliveira G, Li S, Luoma AM, Liu J, Forman J, Huang T, Iorgulescu JB, et al. Personal neoantigen vaccines induce persistent memory T cell responses and epitope spreading in patients with melanoma. Nat Med. 2021;27(3):515–25. doi: 10.1038/s41591-020-01206-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Anker JF, Saxena M, Kodysh J, O’Donnell T, Meseck M, Hapanowicz O, Niglio SA, Shah HR, Kinoshita Y, Brody R, et al. Atezolizumab plus personalized neoantigen vaccination (PGV001) in patients with urothelial cancer. J Clin Oncol. 2024;42(4_suppl):597–597. doi: 10.1200/JCO.2024.42.4_suppl.597. [DOI] [Google Scholar]
- 61.Demoulin S, Herfs M, Delvenne P, Hubert P. Tumor microenvironment converts plasmacytoid dendritic cells into immunosuppressive/tolerogenic cells: insight into the molecular mechanisms. J Leukoc Biol. 2012;93(3):343–52. doi: 10.1189/jlb.0812397. [DOI] [PubMed] [Google Scholar]
- 62.Najafi S, Mortezaee K. Advances in dendritic cell vaccination therapy of cancer. Biomed Pharmacother. 2023;164:114954. doi: 10.1016/j.biopha.2023.114954. [DOI] [PubMed] [Google Scholar]
- 63.Gu YZ, Fan CW, Lu R, Shao B, Sang Y-X, Huang Q-R, Li X, Meng W-T, Mo X-M, Wei Y-Q, et al. Forced co-expression of IL-21 and IL-7 in whole-cell cancer vaccines promotes antitumor immunity. Sci Rep. 2016;6(1):32351. doi: 10.1038/srep32351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Förster R, Davalos-Misslitz AC, Rot A. CCR7 and its ligands: balancing immunity and tolerance. Nat Rev Immunol. 2008;8(5):362–71. doi: 10.1038/nri2297. [DOI] [PubMed] [Google Scholar]
- 65.Ogasawara M, Miyashita M, Ota S. Vaccination of urological cancer patients with WT1 peptide-pulsed dendritic cells in combination with molecular targeted therapy or conventional chemotherapy induces immunological and clinical responses. Ther Apher Dial. 2018;22(3):266–77. doi: 10.1111/1744-9987.12694. [DOI] [PubMed] [Google Scholar]
- 66.Sullenger BA, Gilboa E. Emerging clinical applications of RNA. Nature. 2002;418(6894):252–8. doi: 10.1038/418252a. [DOI] [PubMed] [Google Scholar]
- 67.Maeng HM, Wood LV, Moore B, Bagheri MH, Webb S, England L, Martinez G, Steinberg SM, Pack S, Stroncek D, et al. Preliminary results of a phase I clinical trial using an autologous dendritic cell cancer vaccine targeting HER2 in patients with metastatic cancer or operated high-risk bladder cancer (NCT01730118). J Clin Oncol. 2019;37(15_suppl):2639–2639. doi: 10.1200/JCO.2019.37.15_suppl.2639. [DOI] [Google Scholar]
- 68.Lindner M, Schirrmacher V. Tumour cell-dendritic cell fusion for cancer immunotherapy: comparison of therapeutic efficiency of polyethylene-glycol versus electro-fusion protocols. Eur J Clin Invest. 2002;32(3):207–17. doi: 10.1046/j.1365-2362.2002.00968.x. [DOI] [PubMed] [Google Scholar]
- 69.Nestle FO, Alijagic S, Gilliet M, Sun Y, Grabbe S, Dummer R, Burg G, Schadendorf D. Vaccination of melanoma patients with peptide- or tumorlysate-pulsed dendritic cells. Nat Med. 1998;4(3):328–32. doi: 10.1038/nm0398-328. [DOI] [PubMed] [Google Scholar]
- 70.Hanlon D, Saluja S, Sharp F, Hong E, Khalil D, Tigelaar R, Fahmy T, Edelson R, Robinson E. Targeting human dendritic cells via DEC-205 using PLGA nanoparticles leads to enhanced cross-presentation of a melanoma-associated antigen. Int J Nanomed. 2014. Nov;5231. doi: 10.2147/IJN.S66639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Osugi Y, Vuckovic S, Hart DNJ. Myeloid blood CD11c+ dendritic cells and monocyte-derived dendritic cells differ in their ability to stimulate T lymphocytes. Blood. 2002;100(8):2858–66. doi: 10.1182/blood.V100.8.2858. [DOI] [PubMed] [Google Scholar]
- 72.Chen H, Tan J, Li X, Li H, Wu W, Wu Y, Zhang J, Gu L. Myeloid and plasmacytoid dendritic cell combined vaccines loaded with heat‑treated tumor cell lysates enhance antitumor activity in murine lung cancer. Oncol Lett. 2020;21(2):90. doi: 10.3892/ol.2020.12351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stift A, Friedl J, Dubsky P, Bachleitner-Hofmann T, Schueller G, Zontsich T, Benkoe T, Radelbauer K, Brostjan C, Jakesz R, et al. Dendritic cell-based vaccination in solid cancer. J Clin Oncol. 2003;21(1):135–42. doi: 10.1200/JCO.2003.02.135. [DOI] [PubMed] [Google Scholar]
- 74.Okada H. Gene therapy of malignant gliomas: a pilot study of vaccination with irradiated autologous glioma and dendritic cells admixed with IL-4 transduced fibroblasts to elicit an immune response. Hum Gene Ther. 2001;12(5):575–95. doi: 10.1089/104303401300042528. [DOI] [PubMed] [Google Scholar]
- 75.Timmerman JM, Levy R. Linkage of foreign carrier protein to a self-tumor antigen enhances the immunogenicity of a pulsed dendritic cell vaccine. J Immunol. 2000;164(9):4797–803. doi: 10.4049/jimmunol.164.9.4797. [DOI] [PubMed] [Google Scholar]
- 76.Hammerich L, Marron TU, Upadhyay R, Svensson-Arvelund J, Dhainaut M, Hussein S, Zhan Y, Ostrowski D, Yellin M, Marsh H, et al. Systemic clinical tumor regressions and potentiation of PD1 blockade with in situ vaccination. Nat Med. 2019;25(5):814–24. doi: 10.1038/s41591-019-0410-x. [DOI] [PubMed] [Google Scholar]
- 77.Tacken PJ, Figdor CG. Targeted antigen delivery and activation of dendritic cells in vivo: Steps towards cost effective vaccines. Semin Immunol. 2011;23(1):12–20. doi: 10.1016/j.smim.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 78.Tominaga S, Ojima T, Miyazawa M, Iwamoto H, Kitadani J, Maruoka S, Hayata K, Yamaue H. Induced pluripotent stem cell-derived dendritic cell vaccine therapy genetically modified on the ubiquitin-proteasome system. Gene Ther. 2023;30(7–8):552–9. doi: 10.1038/s41434-023-00388-z. [DOI] [PubMed] [Google Scholar]
- 79.Maeng HM, Moore BN, Bagheri H, Steinberg SM, Inglefield J, Dunham K, Wei W-Z, Morris JC, Terabe M, England LC, et al. Phase I clinical trial of an autologous dendritic cell vaccine against HER2 shows safety and preliminary clinical efficacy. Front Oncol. 2021;11:11. doi: 10.3389/fonc.2021.789078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li XZ, Han Y, Tian J, Ren X, Ma X, Ma M. Enhancement of dendritic cells with melanoma-associated antigen 3 for inducing cytotoxicity by cytotoxic T lymphocytes on bladder cancer BIU-87 cells. Genet Mol Res. 2016;15(3). doi: 10.4238/gmr.15039001. [DOI] [PubMed] [Google Scholar]
- 81.Nishiyama T, Tachibana M, Horiguchi Y, Nakamura K, Ikeda Y, Takesako K, Murai M. Immunotherapy of bladder cancer using autologous dendritic cells pulsed with human lymphocyte antigen-A24-specific MAGE-3 peptide. Clin Cancer Res. 2001;7(1):23–31. [PubMed] [Google Scholar]
- 82.Bradley DA, Morse M, Keler T, Green JA, Davis TA, Inman BA. A randomized phase II study of a novel antigen-presenting cell-targeted hCG-β vaccine (the CDX-1307 regimen) in muscle-invasive bladder cancer. J Clin Oncol. 2010;28(15_suppl):228–228. doi: 10.1200/jco.2010.28.15_suppl.tps228. [DOI] [Google Scholar]
- 83.Keehn A, Gartrell B, Schoenberg MP. Vesigenurtacel-L (HS-410) in the management of high-grade nonmuscle invasive bladder cancer. Futur Oncol. 2016;12(23):2673–82. doi: 10.2217/fon-2016-0284. [DOI] [PubMed] [Google Scholar]
- 84.Cui Y, Luo M, Gu C, He Y, Yao Y, Li P. CAR designs for solid tumors: overcoming hurdles and paving the way for effective immunotherapy. Biophys Rep. 2023;9(5):279. doi: 10.52601/bpr.2023.230020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kaufman HL, Kohlhapp FJ, Zloza A. Oncolytic viruses: a new class of immunotherapy drugs. Nat Rev Drug Discov. 2015;14(9):642–62. doi: 10.1038/nrd4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Burke JM, Lamm DL, Meng MV, Nemunaitis JJ, Stephenson JJ, Arseneau JC, Aimi J, Lerner S, Yeung AW, Kazarian T, et al. A first in human phase 1 study of CG0070, a GM-CSF expressing oncolytic adenovirus, for the treatment of nonmuscle invasive bladder cancer. J Urol. 2012;188(6):2391–7. doi: 10.1016/j.juro.2012.07.097. [DOI] [PubMed] [Google Scholar]
- 87.Portal DE, Weiss RE, Wojtowicz M, Mansour A, Monken C, Mehnert JM, Aisner JA, Kane M, Nishioka J, Aisner S, et al. Phase I neoadjuvant study of intravesical recombinant fowlpox-GM-CSF (rF-GM-CSF) or fowlpox-TRICOM (rF-TRICOM) in patients with bladder carcinoma. Cancer Gene Ther. 2020;27(6):438–47. doi: 10.1038/s41417-019-0112-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Benencia F, Courrèges MC, Fraser NW, Coukos G. Herpes virus oncolytic therapy reverses tumor immune dysfunction and facilitates tumor antigen presentation. Cancer Biol Ther. 2008;7(8):1194–205. doi: 10.4161/cbt.7.8.6216. [DOI] [PubMed] [Google Scholar]
- 89.Msaouel P, Opyrchal M, Domingo Musibay E, Galanis E. Oncolytic measles virus strains as novel anticancer agents. Expert Opin Biol Ther. 2013;13(4):483–502. doi: 10.1517/14712598.2013.749851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vidal L, Pandha HS, Yap TA, White CL, Twigger K, Vile RG, Melcher A, Coffey M, Harrington KJ, DeBono JS, et al. A phase I study of intravenous oncolytic reovirus type 3 dearing in patients with advanced cancer. Clin Cancer Res. 2008;14(21):7127–37. doi: 10.1158/1078-0432.CCR-08-0524. [DOI] [PubMed] [Google Scholar]
- 91.Annels NE, Mansfield D, Arif M, Ballesteros-Merino C, Simpson GR, Denyer M, Sandhu SS, Melcher AA, Harrington KJ, Davies B, et al. Phase I trial of an ICAM-1-Targeted immunotherapeutic-coxsackievirus A21 (CVA21) as an oncolytic agent against non muscle-invasive bladder cancer. Clin Cancer Res. 2019;25(19):5818–31. doi: 10.1158/1078-0432.CCR-18-4022. [DOI] [PubMed] [Google Scholar]
- 92.Chang J. Adenovirus vectors: excellent tools for vaccine development. Immune Netw. 2021;21(1). doi: 10.4110/in.2021.21.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sonpavde GP, Maughan BL, McGregor BA, Wei XX, Kilbridge KL, Lee RJ, Yu EY, Schweizer MT, Montgomery RB, Cheng HH, et al. Phase II trial of CV301 vaccine combined with atezolizumab in advanced urothelial carcinoma. Cancer Immunol Immunother. 2023;72(3):775–82. doi: 10.1007/s00262-022-03274-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hollingsworth RE, Jansen K. Turning the corner on therapeutic cancer vaccines. npj Vaccines. 2019;4(1):7. doi: 10.1038/s41541-019-0103-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sonpavde GP, Maughan BL, McGregor BA, Wei XX, Kilbridge KL, Lee RJ, Yu EY, Schweizer MT, Montgomery RB, Cheng HH, et al. Phase 2 trial of CV301 vaccine plus atezolizumab (Atezo) in advanced urothelial carcinoma (aUC). J Clin Oncol. 2022;40(6_suppl):511–511. doi: 10.1200/JCO.2022.40.6_suppl.511. [DOI] [Google Scholar]
- 96.Freytag SO, Stricker H, Lu M, Elshaikh M, Aref I, Pradhan D, Levin K, Kim JH, Peabody J, Siddiqui F, et al. Prospective randomized phase 2 trial of intensity modulated radiation therapy with or without oncolytic adenovirus-mediated cytotoxic gene therapy in intermediate-risk prostate cancer. Int J Radiat Oncol. 2014;89(2):268–76. doi: 10.1016/j.ijrobp.2014.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Aghi M, Rabkin S, Martuza RL. Effect of chemotherapy-induced DNA repair on oncolytic herpes simplex viral replication. JNCI J Natl Cancer Inst. 2006;98(1):38–50. doi: 10.1093/jnci/djj003. [DOI] [PubMed] [Google Scholar]
- 98.Ni X, Tao J, Barbi J, Chen Q, Park BV, Li Z, Zhang N, Lebid A, Ramaswamy A, Wei P, et al. YAP is essential for treg-mediated suppression of antitumor immunity. Cancer Discov. 2018;8(8):1026–43. doi: 10.1158/2159-8290.CD-17-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Travis MA, Sheppard D. TGF-β activation and function in immunity. Annu Rev Immunol. 2014;32(1):51–82. doi: 10.1146/annurev-immunol-032713-120257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Packiam VT, Lamm DL, Barocas DA, Trainer A, Fand B, Davis RL, Clark W, Kroeger M, Dumbadze I, Chamie K, et al. An open label, single-arm, phase II multicenter study of the safety and efficacy of CG0070 oncolytic vector regimen in patients with BCG-unresponsive non–muscle-invasive bladder cancer: interim results. Urol Oncol Semin Orig Investig. 2018;36(10):440–7. doi: 10.1016/j.urolonc.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 101.Li R, Steinberg GD, Uchio EM, Lamm DL, Shah P, Kamat AM, Bivalacqua T, Packiam VT, Chisamore MJ, McAdory J, et al. CORE1: phase 2, single-arm study of CG0070 combined with pembrolizumab in patients with nonmuscle-invasive bladder cancer (NMIBC) unresponsive to Bacillus Calmette-Guerin (BCG). J Clin Oncol. 2022;40(16_suppl):4597–4597. doi: 10.1200/JCO.2022.40.16_suppl.4597. [DOI] [Google Scholar]
- 102.Rudin CM, Pandha HS, Zibelman M, Akerley WL, Harrington KJ, Day D, Hill AG, O’Day SJ, Clay TD, Wright GM, et al. Phase 1, open-label, dose-escalation study on the safety, pharmacokinetics, and preliminary efficacy of intravenous Coxsackievirus A21 (V937), with or without pembrolizumab, in patients with advanced solid tumors. J Immunother Cancer. 2023;11(1):e005007. doi: 10.1136/jitc-2022-005007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mastrangelo MJ, Maguire HC, McCue P. A pilot study demonstrating the feasibility of using intratumoral vaccinia injections as a vector for gene transfer. Vaccine Res. 1995;4:55–69. [Google Scholar]
- 104.Gomella LG, Mastrangelo MJ, McCue PA, Maguire HJ, Mulholland SG, Lattime EC. Phase i study of intravesical vaccinia virus as a vector for gene therapy of bladder cancer. J Urol. 2001;166(4):1291–5. doi: 10.1016/S0022-5347(05)65755-2. [DOI] [PubMed] [Google Scholar]
- 105.Saoud R, Telfer S, Maruf M, Singer E, Weiss R, Jang T, Elsamra S, Marino M, Valera V, Rodriguez BW, et al. MP16-14 clinical outcomes of a randomized, prospective, phase II study to determine the efficacy of Bacillus Calmette-Guerin (BCG) given in combination with panvac versus BCG given alone in adults with high grade BCG-refractory non-muscle invasive bladder cancer. J Urol. 2021;206(Supplement 3). doi: 10.1097/JU.0000000000002001.14. [DOI] [Google Scholar]
- 106.Foy SP, Mandl SJ, Dela Cruz T, Cote JJ, Gordon EJ, Trent E, Delcayre A, Breitmeyer J, Franzusoff A, Rountree RB, et al. Poxvirus-based active immunotherapy synergizes with CTLA-4 blockade to increase survival in a murine tumor model by improving the magnitude and quality of cytotoxic T cells. Cancer Immunol Immunother. 2016;65(5):537–49. doi: 10.1007/s00262-016-1816-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Luchner M, Reinke S, Milicic A. TLR agonists as vaccine adjuvants targeting cancer and infectious diseases. Pharmaceutics. 2021;13(2):142. doi: 10.3390/pharmaceutics13020142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mu X, Hur S. Immunogenicity of in vitro -Transcribed RNA. Acc Chem Res. 2021;54(21):4012–23. doi: 10.1021/acs.accounts.1c00521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yuan Y, Gao F, Chang Y, Zhao Q, He X. Advances of mRNA vaccine in tumor: a maze of opportunities and challenges. Biomark Res. 2023;11(1). doi: 10.1186/s40364-023-00449-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kim J, Eygeris Y, Gupta M, Sahay G. Self-assembled mRNA vaccines. Adv Drug Deliv Rev. 2021;170:83–112. doi: 10.1016/j.addr.2020.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Passmore LA, Coller J. Roles of mRNA poly(A) tails in regulation of eukaryotic gene expression. Nat Rev Mol Cell Biol. 2022;23(2):93–106. doi: 10.1038/s41580-021-00417-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bloom K, van den Berg F, Arbuthnot P. Self-amplifying RNA vaccines for infectious diseases. Gene Ther. 2021;28(3–4):117–29. doi: 10.1038/s41434-020-00204-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kallen KJ, Heidenreich R, Schnee M, Petsch B, Schlake T, Thess A, Baumhof P, Scheel B, Koch SD, Fotin-Mleczek M, et al. A novel, disruptive vaccination technology. Hum Vaccin Immunother. 2013;9(10):2263–76. doi: 10.4161/hv.25181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pardi N, Muramatsu H, Weissman D, Karikó K. In vitro transcription of long RNA containing modified nucleosides. 2013:29–42. doi: 10.1007/978-1-62703-260-5_2. [DOI] [PubMed] [Google Scholar]
- 115.Karikó K, Muramatsu H, Ludwig J, Weissman D. Generating the optimal mRNA for therapy: HPLC purification eliminates immune activation and improves translation of nucleoside-modified, protein-encoding mRNA. Nucleic Acids Res. 2011;39(21):142–142. doi: 10.1093/nar/gkr695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Karikó K, Buckstein M, Ni H, Weissman D. Suppression of RNA recognition by toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA. Immunity. 2005;23(2):165–75. doi: 10.1016/j.immuni.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 117.Chen SL, Lee W, Hottes AK, Shapiro L, McAdams HH. Codon usage between genomes is constrained by genome-wide mutational processes. Proc Natl Acad Sci. 2004;101(10):3480–5. doi: 10.1073/pnas.0307827100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Linares-Fernández S, Lacroix C, Exposito JY, Verrier B. Tailoring mRNA vaccine to balance innate/adaptive immune response. Trends Mol Med. 2020;26(3):311–23. doi: 10.1016/j.molmed.2019.10.002. [DOI] [PubMed] [Google Scholar]
- 119.Arango D, Sturgill D, Alhusaini N, Dillman AA, Sweet TJ, Hanson G, Hosogane M, Sinclair WR, Nanan KK, Mandler MD, et al. Acetylation of cytidine in mRNA promotes translation efficiency. Cell. 2018;175(7):1872–86.e24. doi: 10.1016/j.cell.2018.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cao J, He L, Lin G, Hu C, Dong R, Zhang J, Zhu H, Hu Y, Wagner CR, He Q, et al. Cap-dependent translation initiation factor, eIF4E, is the target for Ouabain-mediated inhibition of HIF-1α. Biochem Pharmacol. 2014;89(1):20–30. doi: 10.1016/j.bcp.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 121.Mangus DA, Evans MC, Jacobson A. Poly(A)-binding proteins: multifunctional scaffolds for the post-transcriptional control of gene expression. Genome Biol. 2003;4(7):223. doi: 10.1186/gb-2003-4-7-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bauman J, Burris H, Clarke J, Patel M, Cho D, Gutierrez M, Julian R, Scott A, Cohen P, Frederick J, Robert-Tissot C. 798 Safety, tolerability, and immunogenicity of mRNA-4157 in combination with pembrolizumab in subjects with unresectable solid tumors (KEYNOTE-603): an update. In: Late-breaking abstracts. BMJ Publishing Group Ltd; 2020. p. A477.1–A477. doi: 10.1136/jitc-2020-SITC2020.0798. [DOI] [Google Scholar]
- 123.Weber JS, Carlino MS, Khattak A, Meniawy T, Ansstas G, Taylor MH, Kim KB, McKean M, Long GV, Sullivan RJ, et al. Individualised neoantigen therapy mRNA-4157 (V940) plus pembrolizumab versus pembrolizumab monotherapy in resected melanoma (KEYNOTE-942): a randomised, phase 2b study. The Lancet. 2024;403(10427):632–44. doi: 10.1016/S0140-6736(23)02268-7. [DOI] [PubMed] [Google Scholar]
- 124.Liu MA. DNA vaccines: an historical perspective and view to the future. Immunol Rev. 2011;239(1):62–84. doi: 10.1111/j.1600-065X.2010.00980.x. [DOI] [PubMed] [Google Scholar]
- 125.Ori D, Murase M, Kawai T. Cytosolic nucleic acid sensors and innate immune regulation. Int Rev Immunol. 2017;36(2):74–88. doi: 10.1080/08830185.2017.1298749. [DOI] [PubMed] [Google Scholar]
- 126.Tiptiri-Kourpeti A, Spyridopoulou K, Pappa A, Chlichlia K. DNA vaccines to attack cancer: strategies for improving immunogenicity and efficacy. Pharmacol Ther. 2016;165:32–49. doi: 10.1016/j.pharmthera.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 127.Nigar S, Shimosato T. Cooperation of oligodeoxynucleotides and synthetic molecules as enhanced immune modulators. Front Nutr. 2019;6:6. doi: 10.3389/fnut.2019.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Cai P, Zhang X, Wang M, Wu YL, Chen X. Combinatorial nano–bio interfaces. ACS Nano. 2018;12(6):5078–84. doi: 10.1021/acsnano.8b03285. [DOI] [PubMed] [Google Scholar]
- 129.Wang Z, Troilo PJ, Wang X, Griffiths TG, Pacchione SJ, Barnum AB, Harper LB, Pauley CJ, Niu Z, Denisova L, et al. Detection of integration of plasmid DNA into host genomic DNA following intramuscular injection and electroporation. Gene Ther. 2004;11(8):711–21. doi: 10.1038/sj.gt.3302213. [DOI] [PubMed] [Google Scholar]
- 130.Kozak M. Recognition of AUG and alternative initiator codons is augmented by G in position +4 but is not generally affected by the nucleotides in positions +5 and +6. Embo J. 1997;16(9):2482–92. doi: 10.1093/emboj/16.9.2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lai MD, Chen CS, Yang CR, Yuan SY, Tsai JJ, Tu CF, Wang CC, Yen MC, Lin CC. An HDAC inhibitor enhances the antitumor activity of a CMV promoter-driven DNA vaccine. Cancer Gene Ther. 2010;17(3):203–11. doi: 10.1038/cgt.2009.65. [DOI] [PubMed] [Google Scholar]
- 132.Duperret EK, Perales-Puchalt A, Stoltz RGH, Mandloi N, Barlow J, Chaudhuri A, Sardesai NY, Weiner DB. A synthetic DNA, multi-neoantigen vaccine drives predominately MHC class I CD8+ T-cell responses, impacting tumor challenge. Cancer Immunol Res. 2019;7(2):174–82. doi: 10.1158/2326-6066.CIR-18-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Riccardo F, Bolli E, Macagno M, Arigoni M, Cavallo F, Quaglino E. Chimeric DNA vaccines: an effective way to overcome immune tolerance. 2014:99–122. doi: 10.1007/82_2014_426. [DOI] [PubMed] [Google Scholar]
- 134.McNeel DG, Eickhoff JC, Johnson LE, Roth AR, Perk TG, Fong L, Antonarakis ES, Wargowski E, Jeraj R, Liu G, et al. Phase II trial of a DNA vaccine encoding prostatic acid phosphatase (pTVG-HP [MVI-816]) in patients with progressive, nonmetastatic, castration-sensitive prostate cancer. J Clin Oncol. 2019;37(36):3507–17. doi: 10.1200/JCO.19.01701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tzai TS, Lin CI, Shiau AL, Wu CL. Antisense oligonucleotide specific for transforming growth factor-beta 1 inhibit both in vitro and in vivo growth of MBT-2 murine bladder cancer. Anticancer Res. 1998;18(3A):1585–9. [PubMed] [Google Scholar]
- 136.Lee CF, Chang SY, Hsieh DS, Yu DS. Treatment of bladder carcinomas using recombinant BCG DNA vaccines and electroporative gene immunotherapy. Cancer Gene Ther. 2004;11(3):194–207. doi: 10.1038/sj.cgt.7700658. [DOI] [PubMed] [Google Scholar]
- 137.Cf LEE, Chang SY, Hsieh DS, Yu DS. Immunotherapy for bladder cancer using recombinant Bacillus Calmette-Guerin DNA vaccines and interleukin-12 DNA vaccine. J Urol. 2004;171(3):1343–7. doi: 10.1097/01.ju.0000103924.93206.93. [DOI] [PubMed] [Google Scholar]
- 138.Liu J, Han GL, Yang XF, Tian PG, Wang P, Zhang XJ. Immunotherapeutic effect of Ag85A DNA vaccine and Ag85B DNA vaccine on bladder tumor in rats. Chin J Cancer Biother. 2008;15(2):144–9. https://www.embase.com/search/results?subaction=viewrecord&id=L351689473&from=export. [Google Scholar]
- 139.Liang P, Zhang K-Q, Xu G-L, Li Y, Wang L-F, Nie Z-L, Ye J, Wu G, Ge C, Jin F-S. Construction of a DNA vaccine encoding Flk-1 extracellular domain and C3d fusion gene and investigation of its suppressing e V ect on tumor growth. Cancer Immunol, Immunother. 2010;59(1):93–101. Published online. doi: 10.1007/s00262-009-0727-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chen SA, Tsai MH, Wu FT, Hsiang A, Chen YL, Lei HY, Tzai TS, Leung HW, Jin YT, Hsieh CL, et al. Induction of antitumor immunity with combination of HER2/neu DNA vaccine and interleukin 2 gene-modified tumor vaccine. Clin Cancer Res. 2000;6(11):4381–8. [PubMed] [Google Scholar]
- 141.Drake CG, Jaffee E, Pardoll DM. Mechanisms of immune evasion by tumors. 2006:51–81. doi: 10.1016/S0065-2776(06)90002-9. [DOI] [PubMed] [Google Scholar]
- 142.Grivas P, Koshkin VS, Pal SK. Cancer vaccines at the age of immune checkpoint inhibitors: reasonable approach as combination therapy in advanced urothelial carcinoma? Ann Oncol. 2017;28(4):680–2. doi: 10.1093/annonc/mdx063. [DOI] [PubMed] [Google Scholar]
- 143.Funt S, Snyder Charen A, Yusko E, Vignali M, Benzeno S, Boyd ME, Moran MM, Kania BE, Cipolla CK, Regazzi AM, et al. Correlation of peripheral and intratumoral T-cell receptor (TCR) clonality with clinical outcomes in patients with metastatic urothelial cancer (mUC) treated with atezolizumab. J Clin Oncol. 2016;34(15_suppl):3005–3005. doi: 10.1200/JCO.2016.34.15_suppl.3005.27400947 [DOI] [Google Scholar]
- 144.Cadena A, Cushman T, Anderson C, Barsoumian H, Welsh J, Cortez M. Radiation and anti-cancer vaccines: a winning combination. Vaccines. 2018;6(1):9. doi: 10.3390/vaccines6010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chakraborty M, Abrams SI, Coleman CN, Camphausen K, Schlom J, Hodge JW. External beam radiation of tumors alters phenotype of tumor cells to render them susceptible to vaccine-mediated T-cell killing. Cancer Res. 2004;64(12):4328–37. doi: 10.1158/0008-5472.CAN-04-0073. [DOI] [PubMed] [Google Scholar]
- 146.Reinhard K, Rengstl B, Oehm P, Michel K, Billmeier A, Hayduk N, Klein O, Kuna K, Ouchan Y, Wöll S, et al. An RNA vaccine drives expansion and efficacy of claudin-CAR-T cells against solid tumors. Science. 2020;367(6476):446–53. doi: 10.1126/science.aay5967. [DOI] [PubMed] [Google Scholar]
- 147.Ott PA, Hu-Lieskovan S, Chmielowski B, Govindan R, Naing A, Bhardwaj N, Margolin K, Awad MM, Hellmann MD, Lin JJ, et al. A phase Ib trial of personalized neoantigen therapy plus anti-PD-1 in patients with advanced melanoma, non-small cell lung cancer, or bladder cancer. Cell. 2020;183(2):347–62.e24. doi: 10.1016/j.cell.2020.08.053. [DOI] [PubMed] [Google Scholar]
- 148.Matsumoto R, Yonese J, Kawahara T, Miyake H, Matsubara N, Uemura H, Eto M, Azuma H, Obara W, Terai A, et al. Phase I/II study to evaluate the efficacy of TAS0313, a cancer peptide vaccine, combined with pembrolizumab for locally advanced or metastatic urothelial carcinoma. J Clin Oncol. 2021;39(15_suppl):4522–4522. doi: 10.1200/JCO.2021.39.15_suppl.4522. [DOI] [Google Scholar]
- 149.Riess JW, Shaw P, Srinivasan D, Garrido P, Vuky J, Chaney MF, O’Neill S, Alavi A, McDowell DO, Ehrnrooth E, et al. Phase 2 study of the IDO/PD-L1-targeted immune-modulatory vaccine, IO102-IO103, plus pembrolizumab as first-line treatment for metastatic non–small cell lung cancer (NSCLC), squamous cell carcinoma of the head and neck (SCCHN), or urothelial bladder cancer (UBC). J Clin Oncol. 2022;40(16_suppl):2699–2699. doi: 10.1200/JCO.2022.40.16_suppl.TPS2699. [DOI] [Google Scholar]
- 150.Yamada, Y. Characteristics of severe adverse events after peptide vaccination for advanced cancer patients: analysis of 500 cases. Oncol Rep. 2010;25(1). doi: 10.3892/or_00001041. [DOI] [PubMed] [Google Scholar]
- 151.Szolek A, Schubert B, Mohr C, Sturm M, Feldhahn M, Kohlbacher O. OptiType: precision HLA typing from next-generation sequencing data. Bioinformatics. 2014;30(23):3310–6. doi: 10.1093/bioinformatics/btu548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ka S, Lee S, Hong J, Cho Y, Sung J, Kim H-N, Kim H-L, Jung J. HLAscan: genotyping of the HLA region using next-generation sequencing data. BMC Bioinf. 2017;18(1):258. doi: 10.1186/s12859-017-1671-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Jurtz V, Paul S, Andreatta M, Marcatili P, Peters B, Nielsen M. NetMHCpan-4.0: Improved Peptide–MHC class I interaction predictions integrating eluted ligand and peptide binding affinity data. J Immunol. 2017;199(9):3360–8. doi: 10.4049/jimmunol.1700893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.O’Donnell TJ, Rubinsteyn A, Laserson U. MHCflurry 2.0: improved pan-allele prediction of MHC class I-Presented peptides by incorporating antigen processing. Cell Syst. 2020;11(1):42–8.e7. doi: 10.1016/j.cels.2020.06.010. [DOI] [PubMed] [Google Scholar]
- 155.Zhu J, Chang R, Wei B, Fu Y, Chen X, Liu H, Zhou W. Photothermal nano-vaccine promoting antigen presentation and dendritic cells infiltration for enhanced immunotherapy of melanoma via transdermal microneedles delivery. Research. 2022;2022. doi: 10.34133/2022/9816272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wang S, Liu J, Shao S, Li X, Gao J, Niu H, Wang X, Wang Y. Silencing B7-H1 enhances the anti-tumor effect of bladder cancer antigen-loaded dendritic cell vaccine in vitro. Onco Targets Ther. 2014. Aug. 1389. Published online. doi: 10.2147/OTT.S65367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zhang P, Wang J, Wang D, Wang H, Shan F, Chen L, Hou Y, Wang E, Lu C-L. Dendritic cell vaccine modified by Ag85A gene enhances anti-tumor immunity against bladder cancer. Int Immunopharmacol. 2012;14(3):252–60. doi: 10.1016/j.intimp.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 158.Wu Z, Xia C, Zhang C, Tang D, Liu F, Ou Y, Gao J, Yi H, Yang D, Ma K, et al. Adeno-associated virus-delivered alpha synuclein inhibits bladder cancer growth via the p53/p21 signaling pathway. Cancer Gene Ther. 2022;29(8–9):1193–206. doi: 10.1038/s41417-022-00425-w. [DOI] [PubMed] [Google Scholar]
- 159.Hwang EC, Jung SI, Lee HJ, Lee JJ, Kwon DD. Generation of potent cytotoxic T lymphocytes against in male patients with non-muscle invasive bladder cancer by dendritic cells loaded with dying T24 bladder cancer cells. Int braz j urol. 2017;43(4):615–27. doi: 10.1590/s1677-5538.ibju.2016.0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Xie XF, Ding Q, Hou JG, Chen G. Inhibitory effects of a dendritic cell vaccine loaded with radiation-induced apoptotic tumor cells on tumor cell antigens in mouse bladder cancer. Genet Mol Res. 2015;14(3):7548–55. doi: 10.4238/2015.July.3.30. [DOI] [PubMed] [Google Scholar]
- 161.Melief CJM, Welters MJP, Vergote I, Kroep JR, Kenter GG, Ottevanger PB, Tjalma WAA, Denys H, van Poelgeest MIE, Nijman HW, et al. Strong vaccine responses during chemotherapy are associated with prolonged cancer survival. Sci Transl Med. 2020;12(535). doi: 10.1126/scitranslmed.aaz8235. [DOI] [PubMed] [Google Scholar]


