Abstract
Study Design
Systematic review.
Objectives
The correlation between pre-operative diffusion tensor imaging (DTI) metrics and post-operative clinical outcomes in patients with degenerative cervical myelopathy (DCM) has been widely investigated with different studies reporting varied findings. We conducted a systematic review to determine the association between DTI metric and clinical outcomes after surgery.
Methods
We identified relevant articles that investigated the relationship between pre-operative DTI indices and post-operative outcome in DCM patients by searching PubMed/MEDLINE, Web of Science, Scopus, and EMBASE from inception until October 2023. In addition, quantitative synthesis and meta-analyses were performed.
Results
FA was significantly correlated with postoperative JOA or mJOA across all age and follow up subgroups, changes observed in JOA or mJOA from preoperative to postoperative stages (Δ JOA or Δ mJOA) in subgroups aged 65 and above and in those with a follow-up period of 6 months or more, as well as recovery rate in all studies pooled together and also in the under-65 age bracket. Additionally, a significant correlation was demonstrated between recovery rate and ADC across all age groups. No other significant correlations were discovered between DTI parameters (MD, AD, and ADC) and post-operative outcomes.
Conclusion
DTI is a quantitative noninvasive evaluation tool that correlates with severity of DCM. However, the current evidence is still elusive regarding whether DTI metric is a validated tool for predicting the degree of post-operative recovery, which could potentially be useful in patient selection for surgery.
Keywords: diffusion tensor imaging, degenerative cervical myelopathy, prognosis, clinical outcome
Introduction
Degenerative changes in vertebral disc, spinal bones and ligaments are common. It had been reported in 60 percent of people older than 40 years. 1 Aging process affect all the elements-discal, ligamentous, osseous, soft tissue and muscle, and neural tissue. This condition known as spondylosis. 2 Degenerative changes are common in elderly cervical spine with a linear fashion and has been seen in 75% of asymptomatic patients after 7th decade of life. 3 Spinal canal narrowing could lead to compression, blood flow limitation and ischemia. Consequently, inducing myelopathic changes in cord 4 and resulting in considerable functional impairment. Degenerative cervical myelopathy (DCM) affects approximately 60 per 100 000 persons in North America, and it is recognized as the most common cause of non-traumatic spinal cord injury. 5
Magnetic resonance imaging (MRI) is the choice of imaging for detecting DCM. Despite the ability of conventional MRI to reveal spinal cord compression or root at the level of the lesion, MRI findings in DCM does not always indicate the irreversible state of the cervical spinal cord lesion nor correlate with clinical outcomes. 6 These variations in MRI findings make it difficult to determine the relationship between image findings and disease prognosis.7,8
Diffusion tensor imaging (DTI), is a MRI technique, that is capable of depicting structural detail in the brain and spinal cord.9-13 DTI assess spinal cord microstructure by tracing water molecular diffusion at microscopic dimensions. Fractional anisotropy (FA), an assessable metric derived from DTI, varies from 0 to 1 and denotes the extent to which water diffusion is constrained to a single axis. In a healthy spinal cord, the axons predominantly travel in a single path, akin to a cluster of straws. 14 However, in DCM, this axonal integrity is impaired, leading to a predictable decrease in fractional anisotropy. Other measurable indices from DTI include the Mean Diffusivity (MD) and the Apparent Diffusion Coefficient (ADC), both of which quantify the average rate of water diffusion within a given tissue. 14
Several studies have used DTI for early detection and to improve predicting prognosis of patients with DCM.7,15,16 Hence, DTI should not merely be viewed as an auxiliary diagnostic assessment, but rather as an essential instrument in detecting DCM and a precursor in determining those patients who are most appropriate for surgical intervention. 17 Although numerous investigations have explored the application of DTI in the context of DCM, the majority of these studies are characterized by a limited sample size 14 and DTI has not been routinely incorporated into the clinical management of DCM patients. Past literature reviews have attempted to assess the relevance of DTI metrics in DCM patient populations; however, these were largely scoping or quantitative reviews, making their conclusions predominantly qualitative.14,18 Therefore, the primary aim of this study is to conduct a systematic review and, if applicable, a meta-analysis, to evaluate the correlations between preoperative DTI parameters and postoperative outcomes in DCM patients.
Methods
This review’s protocol has been registered with PROSPERO under the number CRD42023417303 and can be accessed at: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42023417303. The study adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) protocol for systematic reviews and meta-analysis. 19
Literature Search
To discover pertinent studies, a comprehensive search was conducted across multiple electronic databases including PubMed, Web of Science, Scopus, and EMBASE. This search was executed by 1 of our authors (FF) in October 2023 and was not bound by any language restrictions. The keywords utilized to guide the search included “degenerative cervical myelopathy,” “cervical spondylotic myelopathy,” “diffusion tensor imaging,” “fractional anisotropy,” “clinical outcome,” and “recovery rate.” The “recovery rate” proposed by Hirabayashi and colleagues is metric that is now broadly recognized as a key outcome indicator in DCM. 20 The selection and combination of keywords were tailored to each database individually to optimize the search yield. In addition, we manually explored the reference lists of relevant articles to identify any further potentially applicable records.
Eligibility Criteria
For inclusion in our systematic review, primary studies of any design were required to meet the following eligibility criteria: (1) Enrolment of adult patients diagnosed with DCM who have undergone any form of decompressive surgery for the condition, (2) Evaluation of the impact of DTI parameters on patient outcomes, (3) Reporting of any post-operative outcomes, and (4) Implementation of any correlation analysis between DTI parameters and surgical outcomes (5) Reporting of correlation coefficient and P value or 95% CI for it. On the contrary, studies failing to adhere to these specifications were excluded. We imposed no restrictions on the publication dates of the potential studies.
Selection Process
The process of selecting studies for inclusion was conducted in a systematic and thorough manner. Two independent reviewers, SMK and FR, initially screened all identified studies by evaluating their titles and abstracts, consequently excluding any irrelevant research. Subsequently, these reviewers scrutinized the full-text articles of the remaining studies in depth, adhering strictly to our pre-specified inclusion and exclusion criteria. In instances of discrepancies between the 2 reviewers, discussions were held to reach a consensus. If a consensus could not be reached, a third reviewer, FF, was consulted to provide additional insight and to assist in resolving the disagreement. The ultimate selection of studies for inclusion was determined based on a mutually agreed-upon decision between the reviewers.
Data Extraction
Two reviewers (FF and MM) executed the data collection process independently, employing a standardized data extraction template in MS Excel that had been pre-tested for suitability to the study requirements. Reference management was facilitated by using EndNote software. The information collected included patient demographics such as age and gender, radiographic data encompassing the DTI measures of interest, protocols used for MRI; the implementation of any other standardized clinical or radiographic measurements; specifics of surgical interventions; postoperative outcomes, and follow-up durations. The procedure to handle any discrepancies between the reviewers was structured and entailed an initial attempt to resolve the difference through discussion.
Study Risk of Bias Assessment
The Quality in Prognosis Studies (QUIPS) instrument was employed to assess the validity and potential bias present within the selected studies. 21 This tool aids in gauging the risk of bias in prognostic factor studies by examining 6 key domains: participant selection, attrition, prognostic factor measurement, accounting for confounding factors, outcome measurement, and the analysis and reporting processes. Two independent researchers conducted (FF and SK) this evaluation independently for each study, filling out their respective judgments. Upon comparison, their assessments showed full concurrence, highlighting the consistency in their evaluations.
Data Analysis
Our meta-analysis specifically calculated the pooled correlations between DTI parameters and functional or recovery status parameters if at least 3 studies reported the outcome measure. Initially, all correlations were transformed into z-scores, and the inverse variance method was used to compute the pooled effect size. Subsequently, these results were back transformed prior to reporting. Each study examined the correlation between pre-operative DTI parameters and the functional index at the longest follow-up visit. When a study indicated correlations for more than 1 spinal level, most compressed level (MC) was used in the meta-analysis. Due to possible heterogeneity across the studies, both fixed-effects and random-effects model were used for all the studies. All analytical procedures were executed using the R statistical software, version 4.2.2.
Results
Literature Search
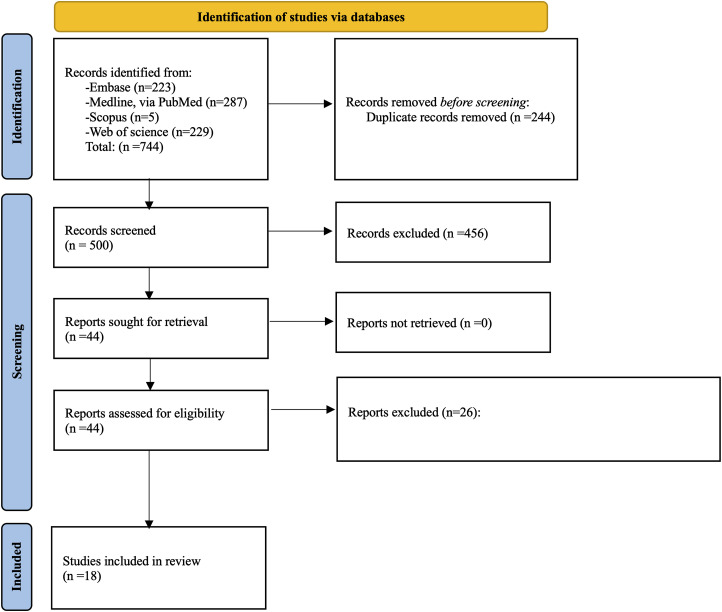
With our initial search, 744 records were identified, of which 244 duplicates were removed. Then, after screening titles and abstracts, 456 were further excluded. After reviewing full texts an additional 26 articles were excluded. Finally, 18 eligible studies were used for data extraction and quality assessment (Figure 1).
Figure 1.
PRISMA flow chart for this review.
Quality Assessment
The Risk of bias for each study can be found in Table 1. Included studies showed low to moderate risk for bias in most domains of the QUIPS.
Table 1.
Bias Assessment of included Studies.
| Authors, Year | Study Participation | Study Attrition | Prognostic Factor Measurement | Outcome Measurement | Study Confounding | Statistical Analysis and Reporting |
|---|---|---|---|---|---|---|
| Nakamura et al, 2012 22 | Moderate | Moderate | Low | Low | Moderate | Low |
| Jones et al, 2013 12 | Moderate | Low | Low | Low | Low | Low |
| Arima et al, 2015 23 | Moderate | Low | Moderate | Moderate | Moderate | Low |
| Maki et al, 2017 24 | Moderate | Moderate | Low | Low | Moderate | Low |
| Rajasekaran et al, 2017 25 | Moderate | Low | Low | Low | Moderate | Low |
| Vedantam et al, 2017 26 | Moderate | Low | Moderate | Low | Moderate | Low |
| Okita et al, 2018 27 | Moderate | Low | Low | Low | Moderate | Low |
| Rao et al, 2018 28 | Moderate | Low | Low | Low | High | Low |
| Zheng et al, 2018 29 | Low | Low | Low | Low | Low | Low |
| Bhosale et al, 2019 30 | Moderate | Low | Moderate | Moderate | High | Low |
| Shabani et al, 2019 16 | Low | Moderate | Low | Low | High | Low |
| Han et al, 2020 13 | Low | Low | Low | Low | Low | Low |
| Kitamura et al, 2020 31 | Moderate | Low | Low | Low | Moderate | Low |
| Severino et al, 2020 32 | Moderate | Low | Low | Low | Low | Low |
| Tian et al, 2021 33 | Low | Low | Moderate | Low | Moderate | Low |
| Zhang et al, 2020 34 | Moderate | Moderate | Low | Low | Moderate | Low |
| Zhang et al, 2022 35 | Moderate | Low | Low | Low | Low | Low |
| Takamiya et al, 2023 36 | Moderate | Low | Low | Low | Low | Moderate |
Study Characteristics
Seventeen studies were included in this systematic review, of these 5 studies were conducted in United States of America,12,16,26,28,34 9 in Asia,13,22-24,27,29,31,33,35,36 2 in India25,30 and 1 in Switzerland. 32 The total number of patients were 653 with sample size ranging from 15 31 to 95. 33 The mean age in most studies was above 50 years. Most of studies had included cervical spondylosis myelopathic patients but there was a minority of ossification of the posterior longitudinal ligament (OPLL) 22 in other studies. Three tesla MRI were used in 12 studies12,13,23-25,27,29,31-35 and 1.5 tesla MRI was used in 5 studies.16,22,26,28,30 The commonest DTI parameter which was assessed was FA. This parameter was assessed in all studies except 1. 29 ADC25,27,29,30,33 and MD13,23,24,29 were 2 other common preoperative DTI measurements. Follow-up duration ranged from 6 weeks 30 to 24 months,16,28 but most studies had a follow-up of at least 3 months. (Table 2)
Table 2.
Study characteristics.
| Authors, Year | Country | Population (n) | Number of Cases in the Follow-Up | Follow-Up Time | Age (y), Mean (SD) | Gender (% Female) | Type of Operation |
|---|---|---|---|---|---|---|---|
| Nakamura et al, 2012 22 | Japan | 20 | 20 | 12 m | 64.6 | 25 | Post |
| Jones et al, 2013 12 | USA | 30 | 15 | mean = 181 d | 62 | 53.3 | N/S |
| Arima et al, 2015 23 | Japan | 16 | 16 | At least 3 months | 62.8 | 56 | Post, ant |
| Maki et al, 2017 24 | Japan | 26 | 26 | 6 m | 66.5 (11.5) | 30.7 | Post, ant |
| Rajasekaran et al, 2017 25 | India | 35 | 26 | 12 m | 48 | 5.7 | Post, ant |
| Vedantam et al, 2017 26 | USA | 27 | 27 | 3 m | 54.5 (1.9) | 56 | Post, ant |
| Okita et al, 2018 27 | Japan | 27 | 27 | 12 m | 70.5 (11.7) | 22.2 | Post |
| Rao et al, 2018 28 | USA | 44 | 39 | 6 m | 53.9 (9.8) | 59 | N/S |
| Zheng et al, 2018 29 | China | 61 | 61 | mean = 19.97 m | 62.4 | 36 | Post, ant |
| Bhosale et al, 2019 30 | India | 30 | 30 | 6 w | 3.3 | Post, ant | |
| Shabani et al, 2019 16 | USA | 46 | 46 | 3 m | 53.6 (9.7) | 58.6 | N/S |
| Han et al, 2020 13 | China | 55 | 44 | 3 m | 58.6 (6.8) | 38 | Post |
| Kitamura et al, 2020 31 | Japan | 15 | 15 | At least 12 m | 71.5 (6.12) | 15 | Post |
| Severino et al, 2020 32 | Switzerland | 36 | 36 | 12 m | 57.05 | 63.8 | Post, ant |
| Tian et al, 2021 33 | China | 95 | 95 | 12 m | 54.2 | 38.9 | Post, ant |
| Zhang et al, 2020 34 | USA | 42 | 42 | Mean = 22.1 m | 56.4 (8.6) | 38.1 | Post, ant |
| Zhang et al, 2022 35 | China | 48 | 48 | 3 m | 56.79 (11.26) | 18.7 | Post, ant |
| Takamiya et al, 2023 36 | Japan | 21 | 21 | 6 m | 69.6 (12.8) | 61.9 | Post, ant |
Three studies included DCM patients and healthy participants,28,34,35 2 compared outcomes between good and poor recovery groups 33 or best and normal responders, 32 1 study included surgical and non-surgical groups, 12 and 2 other studies investigated correlations between DTI parameters and outcomes among several groups.13,29 Severino et al also demonstrated that preop FA values were significantly higher in the “best responders” than the “normal responders” (.63 ± .06 vs .57 ± .08, P = .03). Also, the average FA value remained higher in the “best responders” group at both 3-months (.62 ± .08 vs .58 ± .09) and statistically significantly different at 1-year (.68 ± .07 vs .55 ± .11, P = .004). Furthermore, FA at the most stenotic level was significantly lower in the “normal responder” group preoperatively and at 1-year (P = .02 and P = .009, respectively). 32 Additionally, Tian et al observed that the preoperative FA value in the good recovery group was significantly higher than that in the poor recovery group, while the ADC value was significantly lower (both P < .001). In contrast, good recovery group had lower preoperative AD and VD than the poor recovery group, whereas there was no statistical significance (both P > .05) 33 illustrates Studies with participant groups and correlation findings Table 3.
Table 3.
Participant groups and correlation findings of included studies.
| Studies | Participant groups | Additional findings |
|---|---|---|
| Jones et al. 2013 | 15 CSM patients who underwent surgery vs. 15 CSM patients without surgery | Baseline SF-36 (MCS) was statistically higher in the surgery cohort (12.64 ± 13.2 (n = 13)) than non-surgery cohort (1.49 ± 11.16 (n = 14), P = .03. Baseline mJOA was statistically higher in the non-surgery cohort (14.93 ± 1.98 (n = 15)) than the surgery cohort (12.4±3.04 (n = 15)), P =.02 FA at the C2-C3 level was statistically higher in the non-surgery cohort (.69 ± .05 (n = 15)) than the surgery cohort (0.6±0.07 (n = 15)), P < .01. FA at the stenosis level was statistically higher in the non-surgery cohort (.55 ± .1 (n = 15)) than the surgery cohort (.47±0.09 (n = 15)), P = .05 |
| Rao et al. 2018 | Forty-four patients presenting with CSM and the control group consisted of 24 healthy subjects with normal MRI of the cervical spine | -Preoperative FA at C1-2 was also lower in the patient group compared to the controls (.56 ± .05 vs .61 ± .04, P < .001). However, FA at C1-2 did not correlate with changes in mJOA in follow-up in this group of patients. -FA calculated values were lower in the patient group, compared to the controls, at the LMC (.51 ± .06 vs .57 ± .04, P < .001). In the 22 CSM patients whose FA values were less than .55, postoperative mJOA scores improved by an average of 1.45 points. The 12 CSM patients with FA values above 0.55 declined, on average, by −.92 points, although the absolute mJOA values at 12 months varied from +6 to −5. The difference in improvement between these 2 groups was statistically significant (P = .042). |
| Zheng et al. 2018 | All the patients were further categorized into 4 groups according to JOA recovery rate. Group A: consisted of CSM patients whose JOA recovery rate was below 25%. Group B: consisted of CSM patients whose JOA recovery rate ranged between 25% and 50%. Group C: consisted of CSM patients whose JOA recovery rate ranged between 50% and 75%. Group D: consisted of CSM patients whose JOA recovery rate ranged between 75% and 100%. |
-The ADC, MD, AD, and RD values increased when JOA recovery rate decreased in the 4 subgroups. -The ADC value in groups C and D were significantly higher than those in groups A and B (P < .0001), but no significant differences in ADC value were found between groups A and B (P = .1277). -ADC value was not significantly different between groups C and D (P = .1898). - The MD values in groups C and D were significantly higher than those in groups A and B (P < .05). -No significant difference in MD value were found between groups A and B (P ¼ .1275), and MD value was not significantly different between groups C and D (P = .1947). -No significant differences of AD value were found among the 4 subgroups (P > .05). -The RD values in groups C and D were significantly higher than those in groups A and B (P < .0001). -No significant differences in RD value were found between groups A and B (P ¼ .0601), and RD value was not significantly different between groups C and D (P = .2633). |
| Han et al. 2020 | Authors divided patients into 3 subgroups based on the signal changes in the T1-weighted and T2-weighted images: Group 1: patients with no signal changes Group 2: patients with signal changes in only T2-weighted images. Group 3: patients with signal changes in both T1-weighted and T2-weighted images. |
-For the correlation analysis in each subgroup, in group 1 with no signal changes, the AD value at the C2 level was correlated with the preoperative mJOA (r = .596, P = .041), and the FA value at the C2 level was correlated with the mJOA recovery rate (r = .634, P = .027). -In group 2 with T2-weighted signal changes only, the FA value of the C2 level was correlated with the mJOA recovery rate (r = .484, P = .042). -In group 3 with both T1- weighted and T2-weighted signal changes, the AD values of AC levels and the AD, FA, and RD values of the C2 level were correlated with the mJOA recovery rate (r = .462, .469, .457, and -.446; P = .03, .028, .033, and .037, respectively). -The ROC analysis suggests that for the MC or average of AC levels, no DTI metrics were predictive of the mJOA recovery rate. For the C2 level, FA showed predictive capability with an area under the curve = 0.68, P = .04, sensitivity = .56, specificity = .81. |
| Severino et al. 2020 | Participants divided into “best responders” and “normal responders. | Concerning the preoperative DTI parameters, the preoperative FA values were significantly higher in the “best responders” than the “normal responders” (.63 ± .06 vs. .57 ± .08, P = .03). -Six patients were excluded from the postoperative analysis because they presented with artefacts on their MRIs related to implanted metallic devices. In the remaining 30 patients, the average FA value remained higher in the “best responders” group at both 3-months (.62 ± .08 vs. .58 ± .09) and statistically significantly different at 1-year (.68 ± .07 vs. .55 ± .11, P = .004,). - FA at the most stenotic level was significantly lower in the “normal responder” group preoperatively and at 1-year (P = .02 and P = .009, respectively). |
| Tian et al. 2021 | Patients were divided into a good recovery group (JOA recovery rate ≥60%, n = 47) and a poor recovery group (JOA recovery rate <60%, n = 48) | -The preoperative FA value in the good recovery group was significantly higher than that in the poor recovery group, while the ADC value was significantly lower (both P < .001). -The good recovery group had lower preoperative AD and VD than the poor recovery group, whereas there was no statistical significance (both P > .05). -The CR, TA, MSCC and MCC values measured before surgery in the good recovery group were significantly lower than those in the poor recovery group (all P < .001). |
| Zhang et al 2022 | 48 patients with CSM were enrolled in the analysis, and 36 healthy volunteers with no history of neck injuries/surgeries, neurologic disorders, or abnormalities on routine cervical MRI, were included as controls. |
-DTI FA and DKI FA decreased significantly in patients with CSM. Although DTI MD had no statistical difference between the two groups (P = .897), DKI MD was significantly higher in patients with CSM. -NODDI ODI and NODDI ISOVF were significantly higher in patients with CSM. However, there was no significant difference in NODDI ICVF or DKI MK between groups (P = .914 and .586, respectively; Figure 3). -Regarding DWI metrics, DTI FA, DKI FA, and NODDI ISOVF (r = .31, .41, and −.34, respectively) showed a significant correlation with the 3-month follow-up RR. However, DKI MD and NODDI ODI were not associated with short-term recovery (P = .090 and .105, respectively). -Multivariate analysis demonstrated that DTI FA, DKI FA, and NODDI ISOVF contributed to RR significantly after adjusting for age (std. coef = .13, .16, and −.14, respectively). |
| Zhang et al. 2022 | Fifty CSM and 20 healthy control patients were enrolled in this study. Three patients found not to meet inclusion criteria and five CSM patients having poor quality MRI data were excluded from all analyses, yielding 23 (55%) mild (mJOA 15–17), 9 (24%) moderate (mJOA 12–14), and 10 (21%) severe CSM (mJOA <11) patients. |
-Although there were no demographic differences between CSM and control patients, baseline mJOA and patient-reported outcome measures were worse in CSM patients (Table 1, all P < .001). Specifically, CSM patients had lower mJOA and higher MDI and DASH scores, reflecting worse neurofunctional status. - CSM patients scored lower on the SF-36 PCS and SF-36 MCS, corresponding to worse quality-of-life. The CSM cohort also possessed higher scores on the NDI, demonstrating greater pain. |
VD; lateral dispersion rate, CR; spinal cord compression ratio, TA; spinal cord cross-sectional area, MSCC; maximum spinal cord compression, MCC; maximum canal compromise, MD: mean diffusivity, FA: fractional anisotropy, ADC: apparent diffusion coefficient, AD: axial diffusivity, RD: radial diffusivity, NODDI; neurite orientation dispersion and density imaging, ISOVF; isotropic volume fraction, ODI; orientation dispersion index, DWI; Diffusion-weighted imaging, CSM; cervical spondylotic myelopathy, MC, maximum compression, AC; average compression.
Outcome Assessment
In 2 studies, recovery rate of mJOA was investigates13,35 whiles in 7 other studies, recovery rate of JOA was used for outcome assessment.22-24,27,29,31,33 Delta mJOA16,26,28,32,34 and mJOA12,13,30,32 were 2 other common tools in studies for evaluation of outcome. Nurick scale in 2 studies,12,25 delta JOA in 2 studies,24,31 and JOA, 29 NDI, 12 delta NDI, 26 SF36 12 and delta SF36 26 each were assessed in specific included studies. For correlation analysis between DTI parameters and outcomes, 8 studies utilized Pearson correlation coefficient,25-27,32-36 6 studies Spearman correlation coefficient12,13,22-24,31 and 4 studies Regression analysis.16,28-30
Correlation Findings
Correlation Between Preoperative DTI Parameters and Postoperative JOA or mJOA
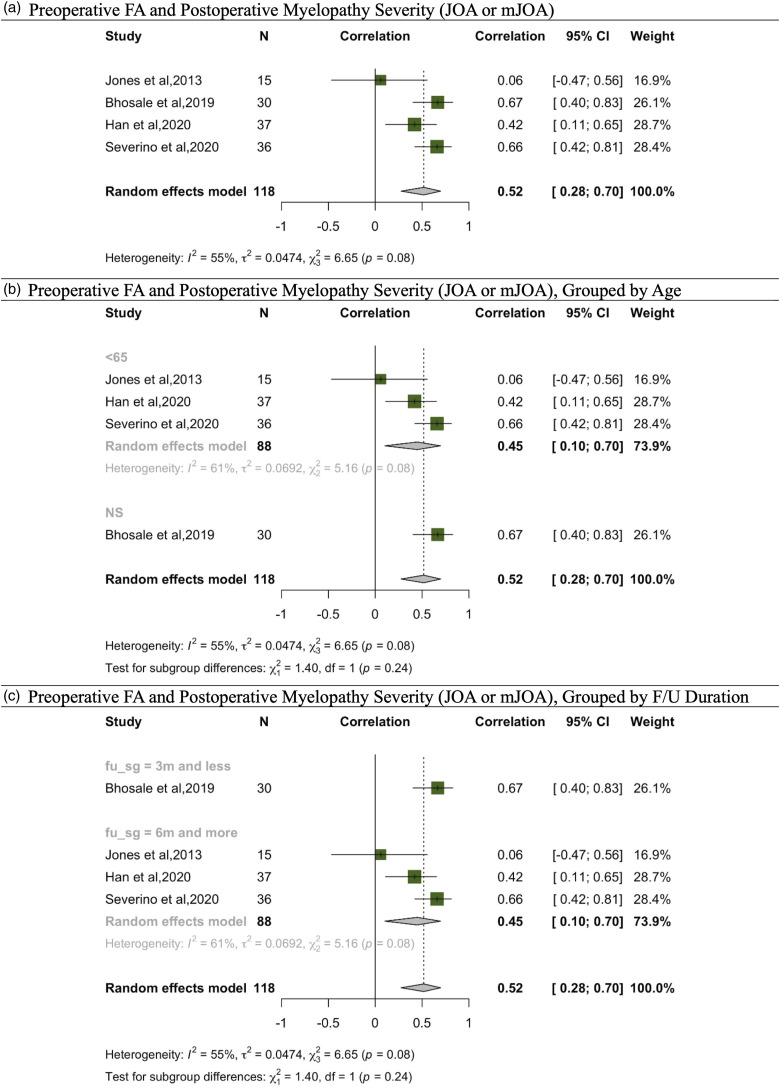
In assessing post-operative outcomes based on JOA or mJOA, 4 studies explored the correlation with FA.12,13,30,32 Additionally, 2 studies probed into the relationship between this outcome and MD, ADC, and RD,13,29 while another 2 examined the correlation with ADC alone.29,30 Given these, our meta-analysis was confined to the 4 studies addressing the correlation between FA and post-operative myelopathy severity gauged via JOA or mJOA (Figure 2). The aggregated findings revealed a pooled correlation coefficient of .52 (95% CI: .28 to .70) for a total sample size of 118, reflecting heterogeneity values of I^2 = 55% and τ^2 = .0474. Within the subgroup aged 65 and above, the coefficient stood at .45 (95% CI: .10 to .70) for N = 88, accompanied by heterogeneity metrics of I^2 = 61% and τ^2 = .0692. Considering follow-up durations of 3 months or less, data from Bhosale et al (2019) for N = 30 showed a correlation of .67 (95% CI: .40 to .83), contributing 26.1% weight. For follow-ups extending beyond 6 months, the accumulated correlation across relevant studies for N = 88 was .45 (95% CI: .10 to .70), with consistent heterogeneity markers at I^2 = 61% and τ^2 = .0692.
Figure 2.
Meta-analysis for correlation between preoperative FA and post operative myelopathy severity (JOA, mJOA). (A) Preoperative FA and postoperative myelopathy severity (JOA or mJOA). (B) Preoperative FA and postoperative myelopathy severity (JOA or mJOA), grouped by age. (C) Preoperative FA and postoperative myelopathy severity (JOA or mJOA), grouped by F/U duration.
Correlation Between Preoperative DTI Parameters and Delta JOA or mJOA
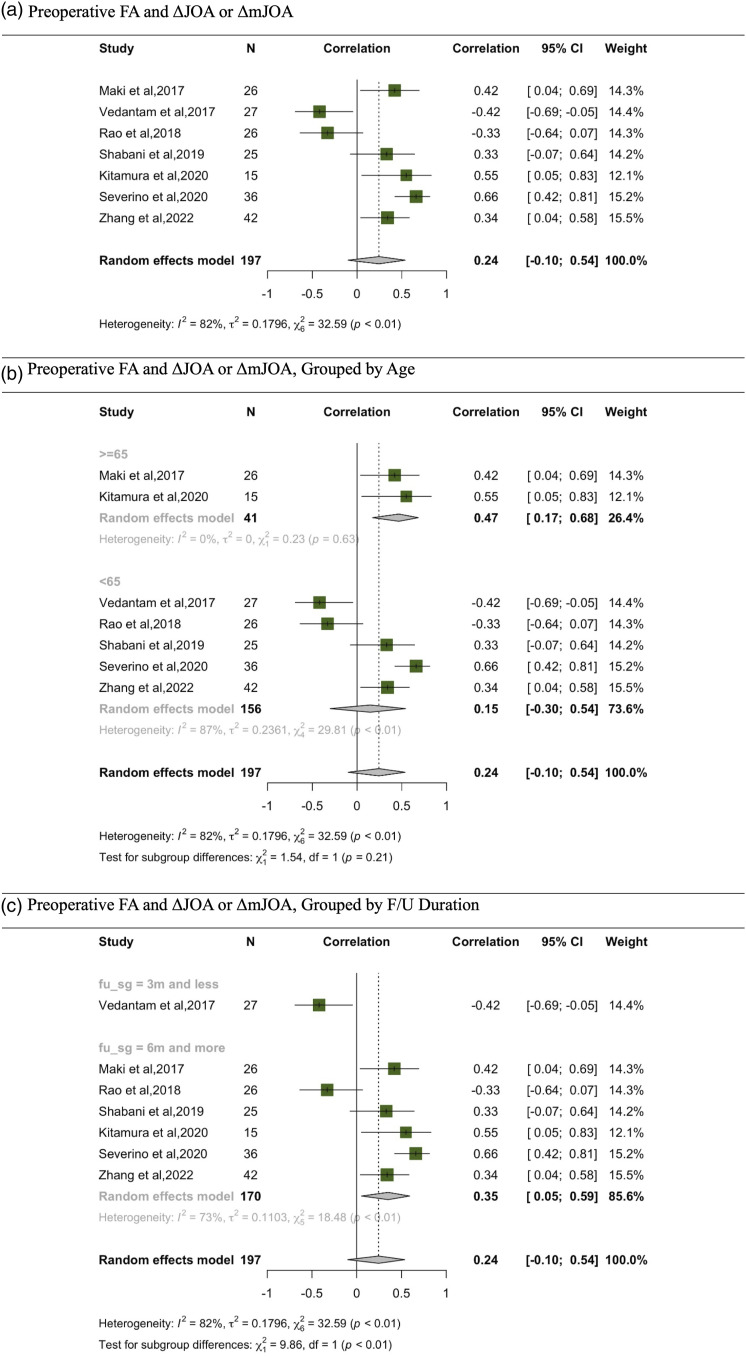
A total of 7 studies explored the correlation between preoperative FA and the changes observed in JOA or mJOA from preoperative to postoperative stages, denoted as ΔJOA or ΔmJOA.16,24,26,28,31,32,34 Other metrics, AD, 34 and MD, 24 were also examined in relation to ΔJOA or ΔmJOA. However, the meta-analysis was exclusively conducted for FA (Figure 3).
Figure 3.
Meta-analysis for preoperative FA and ΔJOA or ΔmJOA. (A) Preoperative FA and ΔJOA or ΔmJOA. (B) Preoperative FA and ΔJOA or ΔmJOA, grouped by age. (C) Preoperative FA and ΔJOA or ΔmJOA, grouped by F/U duration.
From this comprehensive analysis, using a random-effects model, a pooled correlation coefficient of .24 was deduced, with a 95% CI ranging from −.10 to .54. A significant heterogeneity was identified, evidenced by an I^2 value of 82% and τ^2 of .1796. Diving deeper, age-based subgroup analysis revealed differing correlations. For individuals aged 65 and above (N = 41), a significant correlation of .47 was identified [95% CI: .17, .68]. Conversely, for participants below 65 years old of age (N = 156), the correlation dropped to a non-significant .15 [95% CI: −.30, .54]. Furthermore, when segmenting the data by follow-up duration, a significant correlation of .35 [95% CI: .05, .59] emerged for those with a follow-up of 6 months or more.
Correlation Between Preoperative DTI Parameters and Recovery Rate
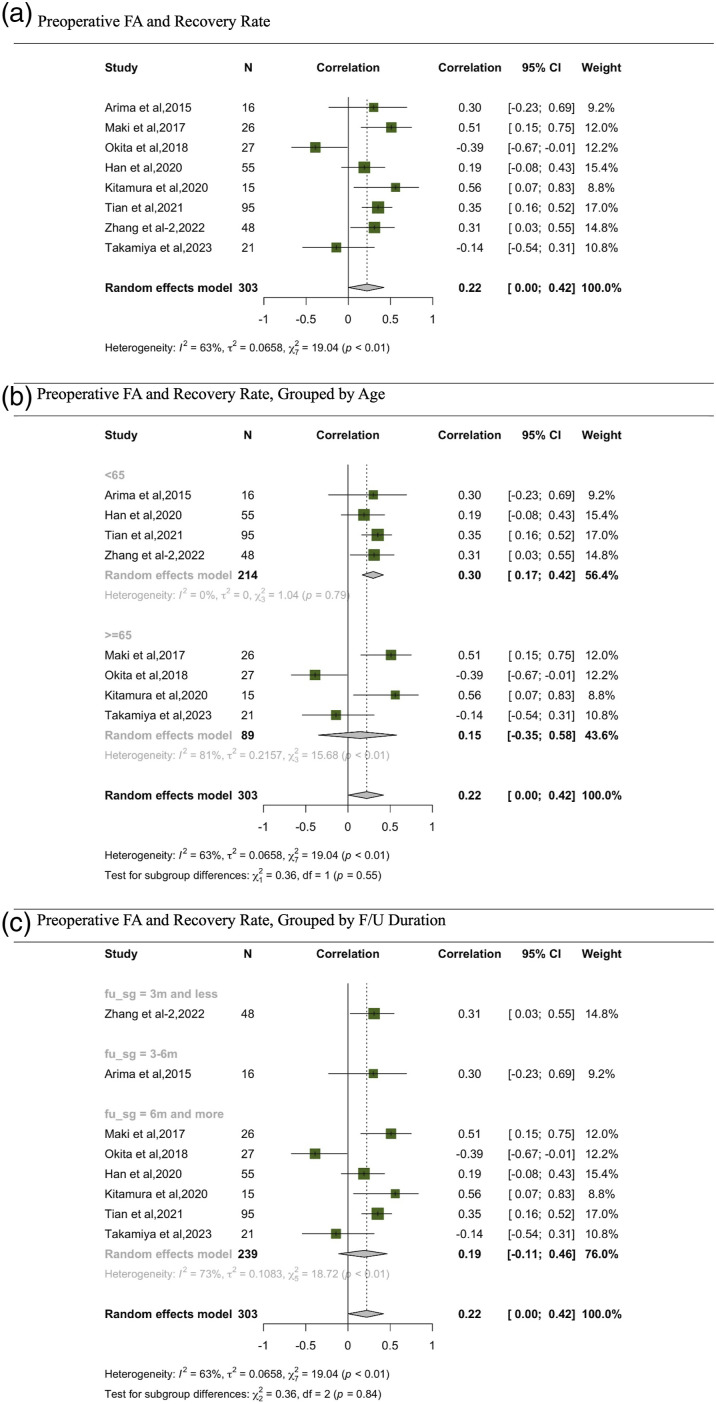
Correlations between recovery rate and DTI parameters was the most commonly studied. 8 studies investigated the correlation between FA and recovery rate.13,23,24,27,31,33,35,36 In the meta-analysis exploring the relationship between FA and recovery rate, a discernible correlation emerges. The comprehensive random effects model, consolidating data from 303 participants across various studies, pinpointed a correlation of .22, with a 95% CI from .00 to .42, indicating a positive marginally significant correlation (Figure 4). Delving into subgroup analyses based on age offers intriguing insights. Specifically, studies focusing on participants below 65 years manifested a stronger correlation of .30, coupled with a tighter 95% CI of .17 to .42. This enhanced correlation underscores a potentially more pronounced relationship between FA and recovery rate in this age bracket. In stark contrast, the subgroup with participants aged 65 and above presented a correlation of .15, with a notably broader 95% CI of −.35 to .58, rendering the correlation insignificant. Parsing the data by follow-up duration, studies with a period of 3 months or less reflected a correlation of .31 (95% CI: .03, .55). Those spanning a follow-up of 3-6 months and beyond 6 months registered correlations of .30 (95% CI: .23, .69) and .19 (95% CI: .11, .46) respectively.
Figure 4.
Meta-analysis for correlation between preoperative FA and recovery rate. (A) Preoperative FA and recovery rate. (B) Preoperative FA and recovery rate, grouped by age. (C) Preoperative FA and recovery rate, grouped by F/U duration.
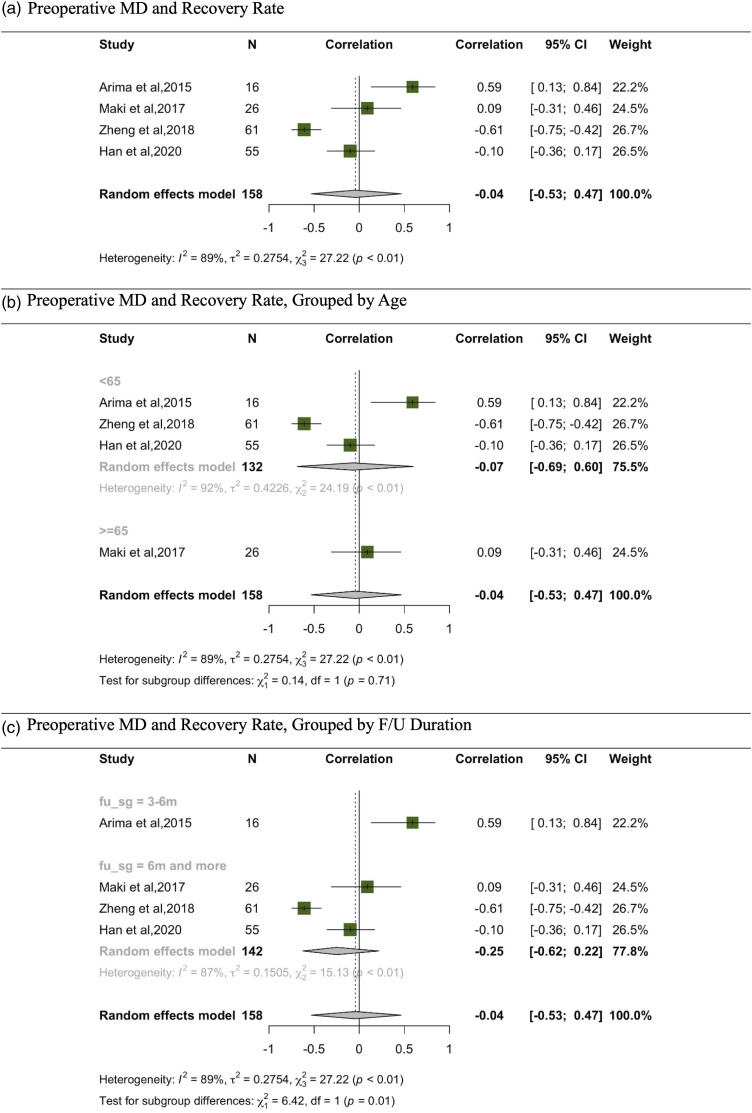
In the meta-analysis evaluating the relationship between MD and recovery rate13,23,24,29 the random effects model, which aggregates data from 158 participants across various studies, indicates a correlation of −.04 (Figure 5). This is accompanied by a 95% CI ranging from −.53 to .47, suggesting a minimal, non-significant negative correlation (see Fig.). Additionally, subgroup analyses based on age and follow-up duration did not reveal any significant correlations within the respective subgroups.
Figure 5.
Meta-analysis for correlation between preoperative MD and recovery rate. (A) Preoperative MD and recovery rate. (B) Preoperative MD and recovery rate, grouped by age. (C) Preoperative MD and recovery rate, grouped by F/U duration.
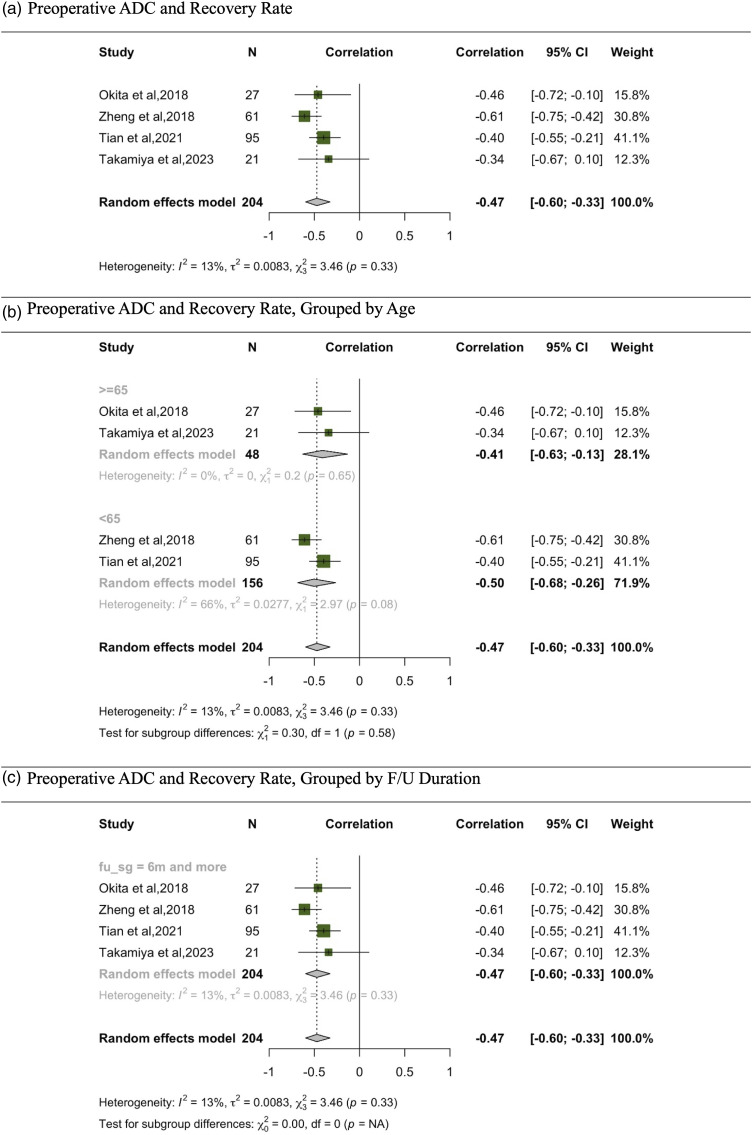
In the meta-analysis examining the correlation between recovery rate and ADC, data were consolidated from 4 studies (Figure 6).27,29,33,36 The random effects model, which collates findings from 204 participants, illustrates a correlation coefficient of −.47. This correlation is supported by a 95% CI that spans from −.60 to −.33, indicating a significant negative correlation. When stratifying data based on age, both age subgroups (<65 and ≥65) demonstrated significant moderate negative correlations. Notably, heterogeneity was minimal across the studies, as evidenced by an I2 value of 13% and a τ2 of .0083. Importantly, all the studies included in this analysis had a follow-up duration of 6 months or more; no studies with a shorter follow-up period were part of this analysis.
Figure 6.
Meta-analysis for correlation between preoperative ADC and recovery rate. (A) Preoperative ADC and recovery rate. (B) Preoperative ADC and recovery rate, grouped by age. (C) Preoperative ADC and recovery rate, grouped by F/U duration.
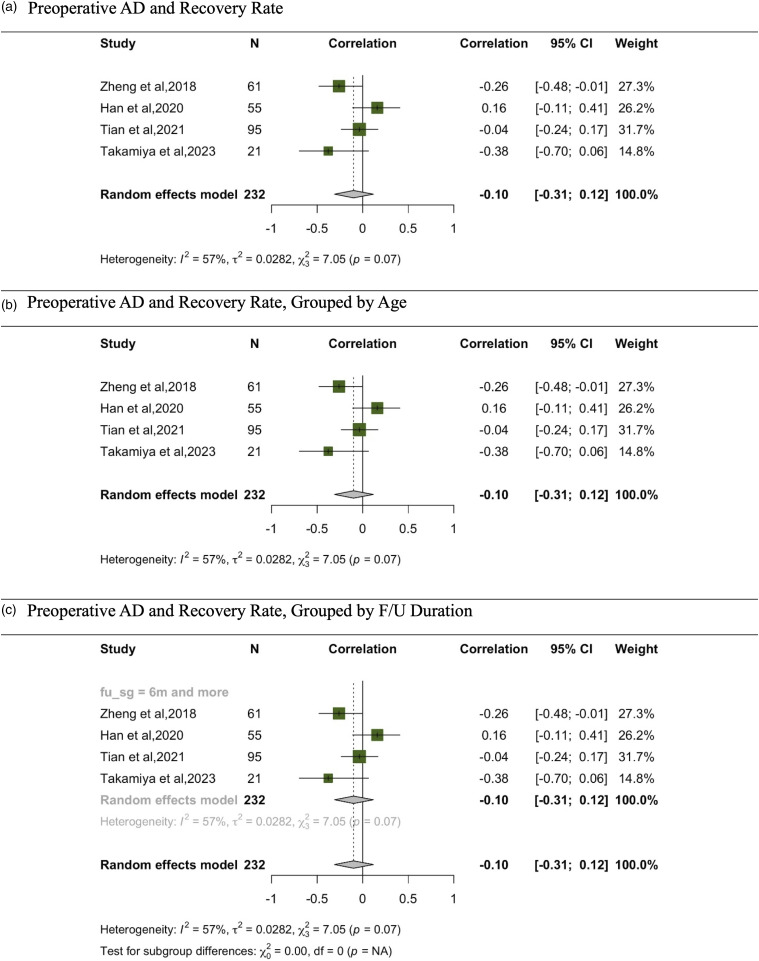
In the meta-analysis evaluating the correlation between AD and recovery rate, data has been synthesized from 4 studies (Figure 7).13,29,33,36 The overall random effects model, comprising findings from 232 participants, demonstrates a correlation coefficient of −.10 with a 95% CI spanning from −.31 to .12, suggesting no significant correlation. Upon analyzing the data stratified by age, the subgroup with participants aged 65 and above produced a correlation of −.05 with a 95% CI of −.27 to .18, reiterating the absence of a significant correlation. Similarly, in the subgroup with participants aged below 65, represented solely by the study from Takamiya et al, 2023, the correlation coefficient was −.38, with a CI spanning from −.70 to .06, also indicating no significant correlation. All studies in this meta-analysis had a follow-up period of 6 months or more.
Figure 7.
Meta-analysis for correlation between preoperative AD and recovery rate. (A) Preoperative AD and recovery rate. (B) Preoperative AD and recovery rate, grouped by age. (C) Preoperative AD and recovery rate, grouped by F/U duration.
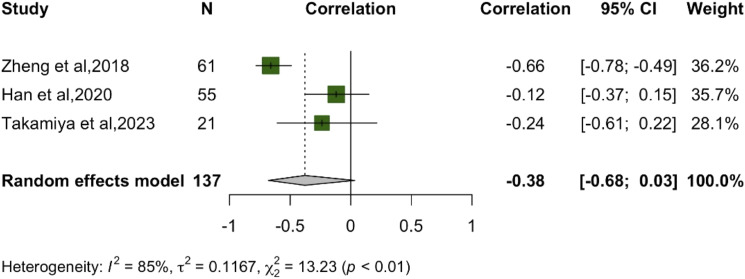
The meta-analysis depicted in the Figure 8 demonstrates results from 3 different studies evaluating the correlation between RD and recovery rate.13,29,36 The combined result under the random effects model for these studies yielded a non-significant correlation of −.38. It’s worth noting the significant heterogeneity among the studies with I2 at 85%. All the studies included in this meta-analysis had a follow-up duration of 6 months or more. Out of these, 2 studies involved participants aged 65 or above, while 1 study focused on participants below this age. Due to the limited number of studies (only 3), no subgroup analysis is reported in our paper for this particular meta-analysis.
Figure 8.
Meta-analysis for correlation between preoperative RD and recovery rate.
Correlation Between Pre-Operative DTI Indices and Other Postoperative Clinical Scores
We were not able to perform a meta-analysis for other clinical outcomes than myelopathy. Although 1 study found no correlation between FA and change NDI at MC and C1-C2 at 3 months F/U, 26 Jones et al showed a significant correlation between FA and NDI at the MC level after mean follow up duration of 181 days. 12 On the other hand, no correlation was observed between FA and Nurick12,25 at MC was found at 12 months F/U. Also, FA and SF-36 at MC, C7-T1, C2-C3 12 and change in SF-36 26 at MC and C1-C2 levels at 3 months did not correlated. Enough data was not available to perform meta-analysis for this outcome.
Discussion
This systematic review aimed to illuminate the relationship between pre-operative DTI parameters and surgical outcomes in patients with DCM. Pinpointing this relationship could refine surgical decision-making and facilitate determining the optimal timing for intervention. Intriguingly, our meta-analysis showed that FA was significantly correlated with postoperative JOA or mJOA across all age and follow up subgroups, change in Δ JOA or Δ mJOA in subgroups aged 65 and above and in those with a follow-up period of 6 months or more, as well as recovery rate in all studies pooled together and also in the under-65 age bracket. Additionally, a significant correlation was demonstrated between recovery rate and ADC across all age groups. No other significant correlations were discovered between DTI parameters (MD, AD, and ADC) and post-operative outcomes.
Our findings yield several compelling insights. Firstly, the review reveals that FA might be a more reliable predictor of outcomes for patients under 65 years old compared to those aged 65 and older—the latter being categorized as the elderly population. This is evidenced by FA’s significant correlation with both myelopathy severity post-operatively and recovery rate in the younger age group but not the elderly aged subgroup of studies. Conversely, while FA was not significantly correlated with recovery rate in the subgroups aged 65 and above (which corresponds to elderly patients), preoperative ADC was significantly correlated with the recovery rate in both age cohorts, suggesting that while FA may not be a useful marker of postoperative myelopathy outcomes in the elderly, ADC might still retain utility in this populations. Although our study stands as the first and largest to systematically and quantitatively highlight these capabilities of different DTI parameters, these findings must be interpreted cautiously. Even in this substantially pooled analysis, the largest pooled sample size only approximates 300 patients, underscoring the necessity for future investigations.
Postoperative FA was correlated with post-operative myelopathy severity in the entirety of the sample and in subgroup analyses. Coupling this with the previous discovery that FA was correlated with recovery or alterations in myelopathy severity post-operation only in certain subgroups, it may suggest that FA is also related to preoperative myelopathy severity. This is due to its correlation with postoperative JOA or mJOA in all groups, but only correlated with recovery rate or changes in myelopathy severity in some age brackets. These latter measures might more effectively gauge the surgical benefit for patients with varied baseline myelopathy severity, hinting at a potential relationship between both pre and post-operative myelopathy severity and FA. Discerning the role of preoperative FA values in surgical patient selection is invaluable, particularly regarding its widespread clinical applicability. A lower preoperative FA value, in contrast to a higher 1, might be more instrumental for surgical patient selection as it largely represents acute spinal cord pathology with no prospect of improvement if left untreated. 37 Nevertheless, if preoperative FA only correlates with myelopathy severity and not the recovery rate in certain age groups, an in-depth comprehension of how this relationship might exist or alter preoperatively—a matter beyond the scope of this review—is warranted. While we posit that patients with mild myelopathy and higher preoperative FA values are more likely to benefit from either conservative management or surgery, the absence of a definitive relationship between pre-operative DTI parameters and baseline clinical and myelopathy measures still requires addressing.
Moreover, our review uncovered that FA was correlated with ΔJOA or ΔmJOA in studies with follow-up periods exceeding 6 months, but not in shorter follow-ups. Despite the fewer number and smaller sample size of short follow-up studies, this observation intimates that certain effects of decompression for DCM materialize over more extended post-operative periods. Hence, while FA might not forecast myelopathy alterations in the short term, they remain beneficial in gauging longer, or potentially final, surgical outcomes in these patient cohorts.
In previous studies, no correlation was identified between canal diameter and surgical outcomes using CT scans, nor was any relationship found between post-operative measurements and clinical outcomes. 38 Moreover, while MRI stands as the gold standard for investigating degenerative changes in the cervical spine, its limitations include a lack of correlation between imaging findings and symptom severity. 39 Thus, the present study is the largest study that signals DTI as a promising alternative for improved imaging predictors of surgical outcome. Notably, even though studies from as early as 2012 pointed toward this correlation, the collective sample size remains constrained for this prevalent condition of DCM, thereby allowing only a few DTI parameters to be explored in a quantitative meta-analysis.
The employment of DWI to assess the white matter of the brain and spinal cord has garnered increased traction, leveraging the movement of water molecules as the primary contrast mechanism. 15 DTI, exploiting DWI in a multi-directional approach to gauge both the directionality and diffusivity of water molecules, has evidenced efficacy in predicting patient symptom severity and surgical outcomes.28-30,32,33,40 ADC, FA, and MD emerge as the most frequently assessed parameters in DTI for evaluating the integrity of the cord and nerves, with our research providing robust evidence supporting this assertion.
There is some evidence to suggest that higher preoperative FA values and lower ADC values are prevalent among patients exhibiting optimal responses to surgery.32,33 Nevertheless, assessing these parameters poses notable challenges, particularly when the exact region of interest (ROI) in the compressed spinal cord is difficult to pinpoint. 22 Consequently, 1 study identified the fiber tract (FT) ratio—calculated as (the number of fibers at the compressed level)/(number of fibers at the C-2 level) × 100%—as a superior alternative in terms of consistency and reproducibility. 22 Regrettably, although a limited number of studies have measured FT or regions other than the maximal compression level (MC), they could not be utilized in the meta-analysis, underlining that these ROIs necessitate further examination in subsequent studies.
The utility of preoperative DTI values as a predictor is getting more apparent, but It’s Imperative to discern the extent of signal loss due to reversible vs irreversible spinal cord injury and to further investigate the hypothesis that certain FA thresholds, alongside baseline functions, dictate recovery potential post-surgery. 12 Furthermore, it’s crucial to recognize that cut-off values of FA for predicting good surgical outcomes and recovery rates can vary. 24 However, previous studies found a significant association between preoperative FT ratio and JOA and mJOA recovery rates,22,26,35 of which 1 proposed an FT ratio below 60% could be equated to a poor recovery rate. 22 In a long follow-up study, the authors found no significant correlation between FA value and ∆mJOA between 2- and 3-years follow-up. 22 This suggest that 2 years after surgery, patients may likely reach their maximum clinical recovery. 40 Also, at follow-up, the FA value may slightly decrease and ADC increase, and natural aging and degeneration in the cervical cord has proposed to be cause of these changes.40,41 The strength of prediction for FA value was estimated to 66.7% and for ADC value 28.7%, 30 indicating that only in 66.7% of cases, postoperative mJOA scores could be accurately predicted based on preoperative FA values. 30
A notable finding in the existing literature is a discord between baseline clinical severity, as demarcated by the mJOA score, and DTI metrics.12,31 Severino et al identified that the correlation between preoperative FA value and postoperative mJOA alterations was only statistically significant at a 1-year follow-up. 32 While preoperative FA values have been routinely linked with predicting surgical outcomes24,32,42 the correlation between postoperative DTI metrics, including FA and Mean Diffusivity (MD) values, and postoperative spinal cord neural status remains elusive. Various studies indicate a correlation25,40,43 while others contest it. Discrepancies might be attributed to postoperative neurological improvements, notwithstanding observable alterations in the pathological spinal cord tissue status visualized via DTI. Alternatively, neurological status recovery post-decompression surgery may be ascribed to electrophysiological improvements.31,44 The systematic review and meta-analysis conducted herein aims to elucidate this multifaceted relationship, offering refined insights that amplify the existing literature. Our findings augment understanding of the interaction between preoperative DTI parameters and surgical outcomes, bolstering the foundation for clinical decision-making in managing DCM patients.
Although FA is vital in predicting outcomes, other clinical assessment scores, such as the Neck Disability Index (NDI) and SF36, exhibit variable correlations with DTI metrics in certain studies12,26 Importantly, the NDI is not a myelopathy scale. Zhang et al found no significant correlation between FA value and NDI either preoperatively or postoperatively. 40 Conversely, Arima et al correlated DTI metrics with the Neurosurgical Cervical Spine Scale (NCSS) and found a significant correlation between MD-z and preoperative NCSS scores; higher MD-z correlated well with higher NCSS scores, suggesting MD-z may decrease concomitantly with the severity of cervical myelopathy. However, no significant correlation was found between FA-z and preoperative NCSS scores. Exploring other advanced MRI techniques, such as magnetization transfer, myelin water fraction, magnetic resonance spectroscopy, functional MRI, and cerebrospinal fluid analysis using cine MRI, may unearth further insights into measuring spinal cord neurodegeneration at both microstructural and functional levels.24,45
This systematic review illuminates pivotal insights into the correlation between pre-operative DTI parameters and subsequent surgical outcomes in DCM) patients. Significantly, FA and ADC emerge as crucial markers, particularly implicating FA’s substantial correlation with postoperative outcomes, especially in patients below 65 years. Though our findings carve a pathway toward better predictive surgical models and nuanced patient-specific intervention strategies, the constrained sample sizes and complexity of existing studies necessitate further, robust investigations. Future endeavors should delve deeper, harnessing additional advanced MRI techniques, to comprehensively unravel the intricate relationship between spinal cord neurodegeneration, DTI parameters, and surgical outcomes, thereby refining and fortifying the predictive capabilities of pre-operative evaluations in DCM management.
Limitation
This systematic review experienced several restrictions, warranting careful interpretation of the findings. Firstly, our quantitative pooling and meta-analysis were solely reliant on FA, ADC or MD, despite the existence of additional DTI indices. While these additional indices were evaluated, the paucity of studies precluded the formation of robust quantitative conclusions.
A significant limitation within the existing literature is the lack of research using multivariate analysis to determine if FA, or comparable metrics, can predict outcomes such as alterations in mJOA/JOA or the recovery rate, even after accounting for baseline neurological conditions. While there’s a robust association between FA and pre-operative JOA/mJOA, patients with initial lower JOA/mJOA scores frequently display marked post-operative improvement, yet may still have comparatively lower JOA/mJOA scores post-surgery. This suggests that the meta-analyses discussed in this review could be affected by baseline JOA/mJOA values. Secondly, the meta-analysis exclusively incorporated JOA, mJOA, and recovery rate as outcome measures due to the sufficient research available for these specific metrics. Even though these are validated metrics for appraising myelopathy, embracing other patient-reported outcomes, such as quality-of-life measures, may provide a more holistic view of patient experiences post-surgery. Thus, a palpable gap exists, signaling the necessity for further exploration into the correlation between DTI parameters and quality-of-life outcomes.
Additionally, the review highlighted that studies have employed various levels and ratios in measuring DTI indices, yet there remains an absence of a definitive recommendation regarding the optimal level for measurement. This inconsistency in measurement levels across studies introduces variability that could influence the comparative and pooled analyses, necessitating a standardized approach in future research.
Furthermore, there is an extant hypothesis suggesting a potential reversal in the correlation between DTI parameters and clinical severity at certain junctures following the initial pathology in the CNS. 37 This review, however, was unable to substantiate or refute this hypothesis, albeit an attempt to mitigate this limitation was made by conducting the meta-analysis predicated on follow-up duration. This underscores an imperative for future research to delve deeper into this hypothesis, offering clarity and additional dimensions to the understanding of DTI parameters in the context of CNS pathology.
Conclusion
DTI is a useful noninvasive evaluation tool that is quantifiable and easy to measure and can be employed in patients with DCM. DTI shows promise for becoming an integral part of the diagnostic imaging work-up for DCM. Although DCM remains a clinical diagnosis, DTI may add value in assessing disease severity and influence the treatment plan. The advantage of this imaging is that it gives objective data, which removes interobserver variability in interpretation of the conventional MRI.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD
Farzin Farahbakhsh https://orcid.org/0000-0003-4435-9034
References
- 1.Boden SD, McCowin PR, Davis DO, Dina TS, Mark AS, Wiesel S. Abnormal magnetic-resonance scans of the cervical spine in asymptomatic subjects. A prospective investigation. JBJS. 1990;72(8):1178-1184. [PubMed] [Google Scholar]
- 2.Modic M, Masaryk TJ, Mulopulos GP, Bundschuh C, Han JS, Bohlman H. Cervical radiculopathy: prospective evaluation with surface coil MR imaging, CT with metrizamide, and metrizamide myelography. Radiology. 1986;161(3):753-759. [DOI] [PubMed] [Google Scholar]
- 3.Friedenberg Z, Miller W. Degenerative disc disease of the cervical spine: a comparative study of asymptomatic and symptomatic patients. JBJS. 1963;45(6):1171-1178. [PubMed] [Google Scholar]
- 4.Levine DN. Pathogenesis of cervical spondylotic myelopathy. J Neurol Neurosurg Psychiatry. 1997;62(4):334-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nouri A, Tetreault L, Singh A, Karadimas SK, Fehlings MG. Degenerative cervical myelopathy: epidemiology, genetics, and pathogenesis. Spine. 2015;40(12):E675-E693. [DOI] [PubMed] [Google Scholar]
- 6.Ellingson BM, Salamon N, Hardy AJ, Holly LT. Prediction of neurological impairment in cervical spondylotic myelopathy using a combination of diffusion MRI and proton MR spectroscopy. PLoS One. 2015;10(10):e0139451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iwasaki M, Yokohama T, Oura D, Furuya S, Niiya Y, Okuaki T. Decreased value of highly accurate fractional anisotropy using 3-tesla zoom diffusion tensor imaging after decompressive surgery in patients with cervical spondylotic myelopathy: aligned fibers effect. World Neurosurg X. 2019;4:100056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muhle C, Metzner J, Weinert D, et al. Classification system based on kinematic MR imaging in cervical spondylitic myelopathy. AJNR Am J Neuroradiol. 1998;19(9):1763-1771. [PMC free article] [PubMed] [Google Scholar]
- 9.Assaf Y, Pasternak O. Diffusion tensor imaging (DTI)-based white matter mapping in brain research: a review. J Mol Neurosci. 2008;34:51-61. [DOI] [PubMed] [Google Scholar]
- 10.Berman JI, Berger MS, Chung SW, Nagarajan SS, Henry RG. Accuracy of diffusion tensor magnetic resonance imaging tractography assessed using intraoperative subcortical stimulation mapping and magnetic source imaging. J Neurosurg. 2007;107(3):488-494. [DOI] [PubMed] [Google Scholar]
- 11.Ducreux D, Fillard P, Facon D, et al. Diffusion tensor magnetic resonance imaging and fiber tracking in spinal cord lesions: current and future indications. Neuroimaging Clin N Am. 2007;17(1):137-147. [DOI] [PubMed] [Google Scholar]
- 12.Jones JG, Cen SY, Lebel RM, Hsieh PC, Law M. Diffusion tensor imaging correlates with the clinical assessment of disease severity in cervical spondylotic myelopathy and predicts outcome following surgery. AJNR Am J Neuroradiol. 2013;34(2):471-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han X, Ma X, Li D, et al. The evaluation and prediction of laminoplasty surgery outcome in patients with degenerative cervical myelopathy using diffusion tensor MRI. AJNR Am J Neuroradiol. 2020;41(9):1745-1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ellingson BM, Cohen-Adad J. Diffusion-weighted imaging of the spinal cord. In: Quantitative MRI of the Spinal Cord. Amsterdam, Netherlands: Elsevier; 2014:123-145. [Google Scholar]
- 15.Kerkovský M, Bednarík J, Dušek L, et al. Magnetic resonance diffusion tensor imaging in patients with cervical spondylotic spinal cord compression: correlations between clinical and electrophysiological findings. Spine. 2012;37(1):48-56. [DOI] [PubMed] [Google Scholar]
- 16.Shabani S, Kaushal M, Budde M, Schmit B, Wang MC, Kurpad S. Comparison between quantitative measurements of diffusion tensor imaging and T2 signal intensity in a large series of cervical spondylotic myelopathy patients for assessment of disease severity and prognostication of recovery. J Neurosurg Spine. 2019;31(4):473-479. [DOI] [PubMed] [Google Scholar]
- 17.He B, Sheldrick K, Das A, Diwan A. Clinical and research MRI techniques for assessing spinal cord integrity in degenerative cervical myelopathy-a scoping review. Biomedicines. 2022;10(10):2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martin AR, Tetreault L, Nouri A, et al. Imaging and electrophysiology for degenerative cervical myelopathy [AO spine RECODE-DCM research priority number 9]. Global Spine J. 2022;12(1_suppl):130s-146s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hirabayashi K, Miyakawa J, Satomi K, Maruyama T, Wakano K. Operative results and postoperative progression of ossification among patients with ossification of cervical posterior longitudinal ligament. Spine. 1981;6(4):354-364. [DOI] [PubMed] [Google Scholar]
- 21.Hayden JA, van der Windt DA, Cartwright JL, Côté P, Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med. 2013;158(4):280-286. [DOI] [PubMed] [Google Scholar]
- 22.Nakamura M, Fujiyoshi K, Tsuji O, et al. Clinical significance of diffusion tensor tractography as a predictor of functional recovery after laminoplasty in patients with cervical compressive myelopathy. J Neurosurg Spine. 2012;17(2):147-152. [DOI] [PubMed] [Google Scholar]
- 23.Arima H, Sakamoto S, Naito K, et al. Prediction of the efficacy of surgical intervention in patients with cervical myelopathy by using diffusion tensor 3T-magnetic resonance imaging parameters. J Craniovertebral Junction Spine. 2015;6(3):120-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maki S, Koda M, Kitamura M, et al. Diffusion tensor imaging can predict surgical outcomes of patients with cervical compression myelopathy. Eur Spine J. 2017;26:2459-2466. [DOI] [PubMed] [Google Scholar]
- 25.Rajasekaran S, Kanna RM, Chittode VS, Maheswaran A, Aiyer SN, Shetty AP. Efficacy of diffusion tensor imaging indices in assessing postoperative neural recovery in cervical spondylotic myelopathy. Spine. 2017;42(1):8-13. [DOI] [PubMed] [Google Scholar]
- 26.Vedantam A, Rao A, Kurpad SN, et al. Diffusion tensor imaging correlates with short-term myelopathy outcome in patients with cervical spondylotic myelopathy. World Neurosurg. 2017;97:489-494. [DOI] [PubMed] [Google Scholar]
- 27.Okita G, Ohba T, Takamura T, et al. Application of neurite orientation dispersion and density imaging or diffusion tensor imaging to quantify the severity of cervical spondylotic myelopathy and to assess postoperative neurologic recovery. Spine J. 2018;18(2):268-275. [DOI] [PubMed] [Google Scholar]
- 28.Rao A, Soliman H, Kaushal M, et al. Diffusion tensor imaging in a large longitudinal series of patients with cervical spondylotic myelopathy correlated with long-term functional outcome. Neurosurgery. 2018;83(4):753-760. [DOI] [PubMed] [Google Scholar]
- 29.Zheng W, Chen H, Wang N, et al. Application of diffusion tensor imaging cutoff value to evaluate the severity and postoperative neurologic recovery of cervical spondylotic myelopathy. World Neurosurg. 2018;118:e849-e855. [DOI] [PubMed] [Google Scholar]
- 30.Bhosale S, Ingale P, Srivastava S, Marathe N, Bhide P. Diffusion tensor imaging as an additional postoperative prognostic predictor factor in cervical myelopathy patients: an observational study. J Craniovertebral Junction Spine. 2019;10(1):10-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kitamura M, Maki S, Koda M, et al. Longitudinal diffusion tensor imaging of patients with degenerative cervical myelopathy following decompression surgery. J Clin Neurosci. 2020;74:194-198. [DOI] [PubMed] [Google Scholar]
- 32.Severino R, Nouri A, Tessitore E. Degenerative cervical myelopathy: how to identify the best responders to surgery? J Clin Med. 2020;9(3):759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tian X, Zhang L, Zhang X, Meng L, Li X. Correlations between preoperative diffusion tensor imaging and surgical outcome in patients with cervical spondylotic myelopathy. Am J Transl Res. 2021;13(10):11461-11471. [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang JK, Sun P, Jayasekera D, et al. Utility of diffusion basis spectrum imaging in quantifying baseline disease severity and prognosis of cervical spondylotic myelopathy. Spine. 2022;47(24):1687-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang MZ, Ou-Yang HQ, Liu JF, et al. Utility of advanced DWI in the detection of spinal cord microstructural alterations and assessment of neurologic function in cervical spondylotic myelopathy patients. J Magn Reson Imag. 2022;55(3):930-940. [DOI] [PubMed] [Google Scholar]
- 36.Takamiya S, Iwasaki M, Yokohama T, Oura D, Niiya Y, Fujimura M. The prediction of neurological prognosis for cervical spondylotic myelopathy using diffusion tensor imaging. Neurospine. 2023;20(1):248-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Farahbakhsh F. Letter to the editor on “imaging and electrophysiology for degenerative cervical myelopathy [AO spine RECODE-DCM research priority number 9]” by Allan R. Martin et al. Global Spine J 2023;21925682231188629. [DOI] [PubMed] [Google Scholar]
- 38.Hamburger C, Büttner A, Uhl E. The cross-sectional area of the cervical spinal canal in patients with cervical spondylotic myelopathy. Correlation of preoperative and postoperative area with clinical symptoms. Spine. 1997;22(17):1990-1994. [DOI] [PubMed] [Google Scholar]
- 39.Mink JH, Gordon RE, Deutsch AL. The cervical spine: radiologist’s perspective. Phys Med Rehabil Clin. 2003;14(3):493-548. [DOI] [PubMed] [Google Scholar]
- 40.Zhang H, Guan L, Hai Y, Liu Y, Ding H, Chen X. Multi-shot echo-planar diffusion tensor imaging in cervical spondylotic myelopathy: a longitudinal study. Bone Joint Lett J. 2020;102-B(9):1210-1218. [DOI] [PubMed] [Google Scholar]
- 41.Mamata H, Jolesz FA, Maier SE. Apparent diffusion coefficient and fractional anisotropy in spinal cord: age and cervical spondylosis–related changes. J Magn Reson Imag. 2005;22(1):38-43. [DOI] [PubMed] [Google Scholar]
- 42.Rindler RS, Chokshi FH, Malcolm JG, et al. Spinal diffusion tensor imaging in evaluation of preoperative and postoperative severity of cervical spondylotic myelopathy: systematic review of literature. World Neurosurg. 2017;99:150-158. [DOI] [PubMed] [Google Scholar]
- 43.Wang K, Idowu O, Thompson CB, et al. Tract-specific diffusion tensor imaging in cervical spondylotic myelopathy before and after decompressive spinal surgery: preliminary results. Clin Neuroradiol. 2017;27:61-69. [DOI] [PubMed] [Google Scholar]
- 44.Wang S, Tian Y, Wang C, et al. Prognostic value of intraoperative MEP signal improvement during surgical treatment of cervical compressive myelopathy. Eur Spine J. 2016;25:1875-1880. [DOI] [PubMed] [Google Scholar]
- 45.Martin AR, Aleksanderek I, Cohen-Adad J, et al. Translating state-of-the-art spinal cord MRI techniques to clinical use: a systematic review of clinical studies utilizing DTI, MT, MWF, MRS, and fMRI. Neuroimage Clin. 2016;10:192-238. [DOI] [PMC free article] [PubMed] [Google Scholar]