Abstract
Study design
Prospective randomized placebo controlled double blind trial.
Objective
To examine the effect of ESP block after minimally invasive posterior stabilization for vertebral fractures on opioid consumption, pain, blood loss, disability level, and wound healing complications.
Methodology
Patients indicated for minimal invasive posterior stabilisation were included to the study. Our primary outcome was the opioid consumption and Visual Analogue Scale (VAS) measured during the first 48 hours. Secondary outcomes used to measure the short-term outcome included Oswestry Disability Index (ODI) and Patient Reported Outcome Spine Trauma (PROST).
Results
In total, 60 patients were included with a 93.3% follow-up. Average morphine consumption during the PACU (Post Anaesthesia Care Unit) period was 5.357 mg in ESP group and 8.607 mg in placebo group (P = .004). Average VAS during first 24 hour was 3.944 in ESP group and 5.193 in placebo group (P = .046). Blood loss was 14.8 g per screw in ESP group and 15.4 g in placebo group (P = .387). The day2 PROST value was 33.9 in ESP group and 28.8 in placebo group (P = .008) and after 4 weeks 55.2 in ESP group and 49.9 in placebo group (P = .036). No significant differences in ODI were detected.
Conclusion
The use of ESP block in minimally invasive spinal surgery for posterior fracture stabilization leads to a significant reduction of opioid consumption during PACU stay by 37.7%. Reduction of opioid consumption was accompanied with lower pain (VAS). We found positive effect of the ESP block on short term outcome scores, but no effect on perioperative blood loss and wound healing.
Keywords: regional anaesthesia, erector spinae plane block, minimal invasive spinal stabilisation, thoracic lumbar spine trauma
Introduction
Many studies show decrease of pain and blood loos with MIS stabilisation compared to classical approach.1–3 However, in spine surgery, postoperative pain control can often be challenging, especially in first 24 hours after surgery, 4 and pain after surgery strongly influence early mobilisation and hospital stay. 5
It is often difficult to achieve pain control if a one-dimensional approach is used. There have been several studies that combined different modalities, like epidural catheters, spinal and epidural morphine, catheters placed at the lateral border extra thoracic paraspinous muscles and local infiltration for analgesia after spine surgery.6–8 Lately there are studies aiming to differentiate patients before surgery, that may be prone to higher pain scores after surgery. They study many different variables that can predict difficult pain control.9,10
The erector spinae plane (ESP) block is a simple ultrasound guided block. Similar technique was first described in 2010 by surgeons as treatment option of pain associated with rib fractures. 8 In 2016 was ESP block described by Forero et al. and since then used by anaesthesiologists for acute and chronic pain management.11,12 Many articles have been published on the use of this technique for pain treatment in the abdominal wall surgery, 13 thoracic surgery, 14 and breast surgery. 15 There are only a few case reports published in the literature mentioned the use of ESP block in spinal surgery.16,17 They show that ESP block appears to be a promising technique that could be routinely used in spinal surgery.
In our study, we examined the effect of ESP block on opioid consumption in the first 48 hours after minimally invasive posterior stabilization for vertebral fracture treatment, as well as its effect on blood loss during surgery, on disability level, and wound healing complications.
Materials and Methods
The study was designed as a prospective randomized placebo controlled double blind single centre trial.
Adult patients with unstable vertebral body fracture in thoracic and lumbar region suitable for minimal invasive posterior stabilisation were included to the study. All patients were treated in a single level one trauma centre in Slovak republic. After obtaining informed consent, randomisation was performed using online software https://www.graphpad.com/quickcalcs/randomize1/ 18 by hospital pharmacy department, which was also responsible for preparation of solutions for ESP block. All patients received general anaesthesia according to the protocol. After the induction of general anaesthesia and positioning to prone position, we marked under X-ray intensifier injured vertebral body and vertebral bodies chosen for screw insertion, then the local anaesthetic was administered under ultrasound guidance under erector spinae muscles group directly on transverse process of fractured vertebra bilaterally. The solution used for the block contained 20 ml of .25% levobupivacaine (ESP group) or saline solution (placebo group) per side. Surgery was performed in standard way, using established minimal invasive spine stabilisation systems. Choose of implant, vertebral bodies suitable for screw insertion, and length of fixation was determined by fracture type, and bone quality.
During the surgery blood loss was determined by weight of sterile gauze pads before and after surgery.
Postoperative analgesia was performed according to standardised protocol, was designed as patient demanded, and dosage of morphine was indicated according to actual VAS (Visual Analogue Scale) score (VAS > 3 – Morphine 5 mg, VAS > 4 – Morphine 6 mg). Opioid consumption and VAS were measured every 5 minutes after surgery on PACU (Post Anaesthesia Care Unit), at the moment of discharging from PACU, and then 3, 6, 12, 24, and 48 hours after surgery. Goal of pain management was to achieve VAS 3 or less.
The short-term outcome was measured using ODI (Oswestry Disability Index) version 2.1a 19 and Slovak version of AOSpine PROST (Patient Reported Outcome for Spine Trauma)20,21 on 2nd day, 2nd week, and 4th week after surgery. Neither ODI nor PROST are designed to evaluate short term outcome, therefore we used the modified approach with only relevant questions about pain sleeping, walking etc. to lower the bias. For ODI, we used only questions 1,2,7 on 2nd day, and questions 1,2,4,6,7 on 2nd and 4th week, respectively. For PROST we scored questions 1-9 on 2nd day and questions 1, 2, 3, 4, 6, and 9 with value 0 on 2nd and 4th week, respectively.
Data were analysed in SPSS 28 software. The normality of the distribution was evaluated continue variables using the Shapiro-Wilk test.
For comparison between 2 groups, we used a two-tailed t-test if the data met the Gaussian distribution, otherwise we used non-parametric Mann-Whitney test. Significance level was set to .05.
Results
In total, 60 patients were included in this study. Follow up finished 56 of them (93.3%), 28 in ESP group and 28 in placebo group. According to CONSORT 2010 Flow Diagram we had 81 patents eligible for enrolment. From this cohort 6 patients were not included due exclusion criteria, 5 declined to participate and 10 was not enrolled due to the lack of staff caused by the COVID pandemic.
Both groups were consistent in terms of age, gender, BMI, level of fracture, AO Spine classification of injured vertebra, length of fixation (number of screws used), and type of implant (Table 1).
Table 1.
Sociodemographic and clinical characteristics by groups.
| Placebo Group | ESP Group | Total | P value | ||
|---|---|---|---|---|---|
| Age | 54.68 (+/−13.56) | 52.68 (+/−10.31) | 53.68 (+/−11.98) | .333 | |
| Gender | Female | 12 (42.9%) | 11 (39.3%) | 23 (41.1%) | |
| Male | 16 (57.1%) | 17 (60.7%) | 33 (58.9%) | .786 | |
| BMI | 26.52 (+/−4.41) | 26.48 (+/−4.62) | 26.50 (+/−4.47) | .970 | |
| Injured vertebra | Th | 12 (42.9%) | 9 (32.1%) | 21 (37.5%) | |
| L | 16 (57.1%) | 19 (67.9%) | 35 (62.5%) | .408 | |
| AOSpine classification | A | 21 (75%) | 20 (71.4%) | 41 (73.2%) | |
| B | 7 (25.0%) | 8 (28.6%) | 15 (26.8%) | ||
| C | 0 (.0%) | 0 (.0%) | 0 (.0%) | .763 | |
| No. of screws | 4.71 (+/−1.46) | 4.93 (+/−1.39) | 4.82 (+/−1.42) | .228 | |
| Type of implant | Top load | 8 (28.6%) | 11 (39.3%) | 19 (33.9%) | |
| Side load | 20 (71.4%) | 17 (60.7%) | 37 (66.1%) | .391 | |
Morphine consumption during the PACU (Post Anaesthesia Care Unit) period was 5.357 mg in ESP group and 8.607 mg in placebo group (P = .040), whereas median of morphine consumption was 3.0 mg in ESP group and 10.0 mg in placebo group. Total morphine consumption during 48 hours follow-up was slightly lower in ESP group 8.571 mg compared to placebo group 10.154 mg, but the difference was not statistically significant (P = .253) (Table 2).
Table 2.
Morphine consumption.
| Placebo Group | ESP Group | Total | P value | ||
|---|---|---|---|---|---|
| Morphine during PACU | N | 28 | 28 | 56 | |
| Mean | 8.607 | 5.357 | 6.982 | ||
| Std. Deviation | 6.500 | 5.794 | 6.317 | ||
| 95% CI | 2.520 | 2.247 | |||
| Median | 10.0 | 3.0 | 8.0 | .040 | |
| Minimum | 0.0 | 0.0 | 0.0 | ||
| Maximum | 20.0 | 20.0 | 20.0 | ||
| Morphine in 48 hours | N | 28 | 28 | 56 | |
| Mean | 10.154 | 8.571 | 9.363 | ||
| Std. Deviation | 8.508 | 10.005 | 9.237 | ||
| 95% CI | 2.520 | 2.247 | |||
| Median | 10.0 | 8.0 | 10.0 | .253 | |
| Minimum | 0.0 | 0.0 | 0.0 | ||
| Maximum | 34.0 | 47.0 | 47.0 | ||
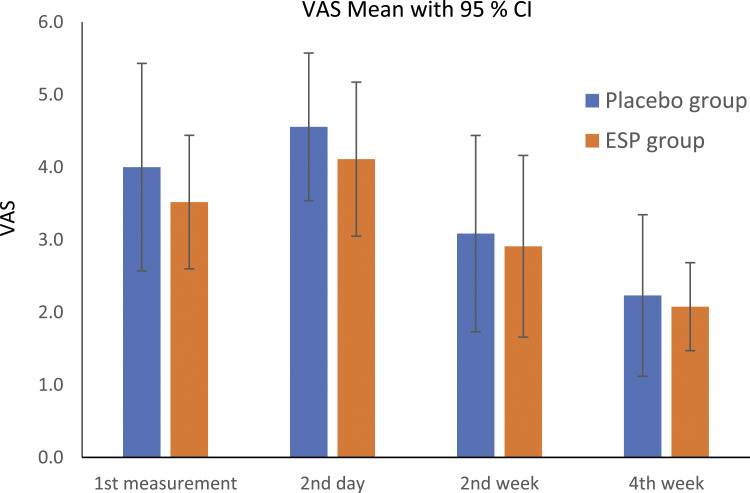
An individual measurement of VAS according to the protocol of the study was not statistically significant. Average VAS for each patient during first 24 hour was in ESP group 3.944 and in placebo group 5.193 (P = .046) (Graph 1).
Graph 1.
Visual Analogue scale.
Blood loss per screw was 14.8 g in ESP group and 21.2 g in placebo group (P = .198). There were two higher values both in placebo group. After exclusion of these two higher values, due to the technical error during the weight measurement in the OR, blood loss per screw was 14.8 g in ESP group and 15.4 g in placebo group (P = .387).
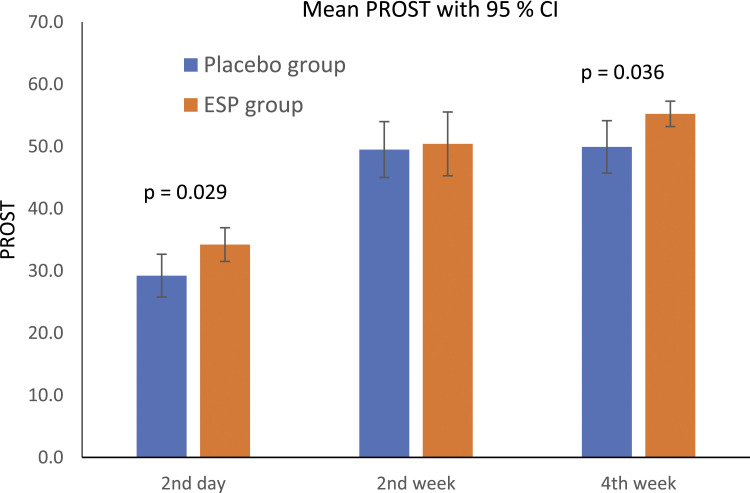
The average value of the AO Spine PROST at 2nd day after surgery was 34.2 in ESP group and 29.2 in placebo group (P = .029). At 2nd week was 50.4 in ESP group and 49.5 in placebo group (P = .550). At 4th week was 55.2 in ESP group and 49.9 in placebo group (P = .036) (Graph 2).
Graph 2.
Patient reported outcome for spinal trauma.
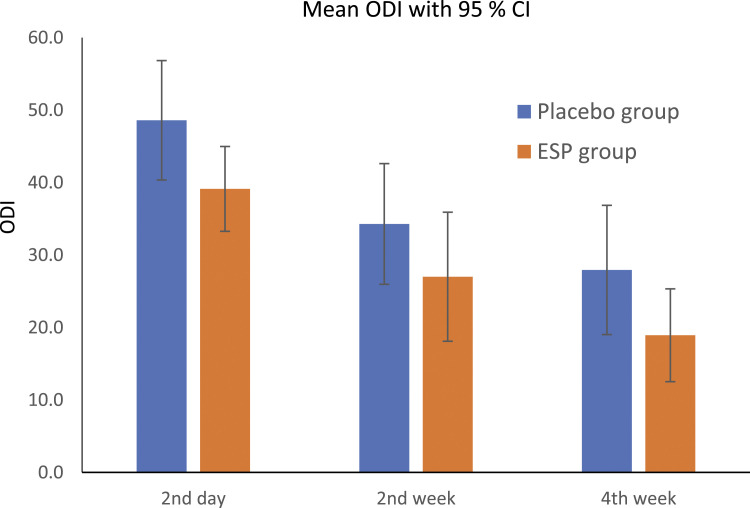
The ODI score at 2nd day after surgery was 39.1 in ESP group and 48.6 in placebo group (P = .071). At 2nd week was 27.0 in ESP group and 34.3 in placebo group (P = .212). At 4th week was 18.9 in ESP group and 27.9 in placebo group (P = .122) (Graph 3).
Graph 3.
Oswestry disability index.
We had one deep surgical site infection which required surgical revision and negative pressure wound healing therapy in placebo group (1.79% of all patients) and we had 2 local haematomas with spontaneous resorption, one in each group (3.58% of all patients).
Discussion
The goal of this prospective, randomized, single-centre study was to examine the effect of ESP block after minimally invasive posterior stabilization for vertebral fractures on opioid consumption, blood loss during surgery, disability level, and wound healing complications. Sixty patients were included and the short-term follow-up rate after 4 weeks was 93%.
ESP block reduced the opioid consumption in first hours after surgery by 37.7%. This was similar to findings of systematic review and meta-analysis of analgesic efficacy of erector spinae plane block in lumbar spine surgery. 22 However, after 24 and 48 hours there was no statistically significant opioid consumption difference.
In the literature minimal invasive spinal surgery reduce the pain after surgery compared to the classical approach.1–3 However, the authors evaluated the pain only first day after surgery. In our study, average opioid consumption in both groups after fist 24 hours was .39 and only 3 patients needed any morphine at all.
Patients in ESP group had significantly lower average pain scores in first 24 hours compared to placebo group, however individual measurements of VAS on predefined time according to study protocol were slightly better in ESP group but were statistically insignificant. It is unclear how these findings can be interpreted in clinical practice.
There were two patients with higher blood losses both in the placebo group. After analysing these cases we found technical error during the weight measurement in the OR. After excluding these values of blood loss per screw were 14.8 g in ESP group and 15.4 g in placebo group (P = .387). We had expected higher blood loss in ESP group due to the vasodilatation effect of levobupivacaine, but this was not confirmed in our study.
One deep infection in placebo group (1.79% of all patients) occurred and 2 patients, one in each group, developed haematomas with spontaneous resorption (3.58% of all patients). This is comparable to wound healing complications reported in the literature.23,24
To evaluate short term outcome we used two tools: ODI in all measurement showed no significant difference; PROST on 2nd day and 4th week after surgery showed significantly better results in ESP group. We can not compare our results with literature since PROST is a new tool and has not been widely used yet. However, PROST was designed to evaluate results after trauma to the spine and therefore compares actual status to the status before trauma.
The limitations of our study are the relatively small sample size and the short-term follow-up regarding the opioid consumption. However, the results clearly point out that ESP block could be an important technique for postoperative pain management protocols. Similar studies with larger sample size are necessary to confirm this hypothesis.
Conclusion
The ESP block in minimal invasive spinal surgery after trauma leads to the reduction of opioid consumption in first hours after surgery by 37.7%., but not in 24 and 48 hours after surgery. This reduction of opioid consumption was accompanied with lower value of VAS. ESP block improves short term outcome but has no effect on perioperative blood loss and wound healing.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This project no: AOSDIA2019-067 was supported by AO Foundation, AOSpine. AOSpine is a clinical division of the AO Foundation
Ethical Approval: This study was approved by the institutional ethics committee F. D. Roosevelt University General Hospital, Banska Bystrica, Slovakia (No. 5/2019; from 19. March 2019). The study was performed in accordance with the Declaration of Helsinki and Good Clinical Practice (GCP) guidelines. All enrolled patients provided written and signed informed consent.
Data Availability: All data generated and analysed during this study are included in this published article (and its supplementary information files).
ORCID iDs
Martin Holas https://orcid.org/0000-0002-3763-8767
Peter Merjavy https://orcid.org/0000-0001-6813-3641
Robert Nagypal https://orcid.org/0000-0002-1401-8135
References
- 1.Merom L, Raz N, Hamud C, et al. Minimally invasive burst fracture fixation in the thoracolumbar region. Orthopedics. 2009;32(4):11. [DOI] [PubMed] [Google Scholar]
- 2.Kantelhardt SR, Martinez R, Baerwinkel S, et al. Perioperative course and accuracy of screw positioning in conventional, open robotic-guided and percutaneous robotic-guided, pedicle screw placement. Eur Spine J. 2011;20(6):860-868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vanek P, Bradac O, Konopkova R, et al. Treatment of thoracolumbar trauma by short-segment percutaneous transpedicular screw instrumentation: prospective comparative study with a minimum 2-year follow-up. J Neurosurg Spine. 2014;20(2):150-156. [DOI] [PubMed] [Google Scholar]
- 4.Nielsen RV, Fomsgaard JS, Dahl JB, et al. Insufficient pain management after spine surgery. Dan Med J. 2014;61:A4835. [PubMed] [Google Scholar]
- 5.Bajwa SJ, Haldar R. Pain management following spinal surgeries: an appraisal of the available options. J Craniovertebr Junction Spine. 2015;6(3):105-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benyahia NM, Verster A, Saldien V, et al. Regional anaesthesia and postoperative analgesia techniques for spine surgery - a review. Rom J Anaesth Intensive Care. 2015;22(1):25-33. [PMC free article] [PubMed] [Google Scholar]
- 7.Perez-Roman RJ, Govindarajan V, Bryant JP, et al. Spinal anesthesia in awake surgical procedures of the lumbar spine: a systematic review and meta-analysis of 3709 patients. Neurosurg Focus. 2021;51(6):E7. [DOI] [PubMed] [Google Scholar]
- 8.Truitt MS, Mooty RC, Amos J, et al. Out with the old, in with the new: a novel approach to treating pain associated with rib fractures. World J Surg. 2010;34(10):2359-2362. [DOI] [PubMed] [Google Scholar]
- 9.Johnson ZD, Connors SW, Christian Z, et al. Development and Internal Validation of the Postoperative Analgesic Intake Needs Score: A Predictive Model for Post-Operative Narcotic Requirement after Spine Surgery. Global Spine J. 2022;2022:21925682211072490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barrie U, Montgomery EY, Ogwumike E, et al. Household Income as a Predictor for Surgical Outcomes and Opioid Use After Spine Surgery in the United States. Global Spine J. 2022;2022:21925682211070823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Forero M, Adhikary SD, Lopez H, et al. The erector spinae plane block: a novel analgesic technique in thoracic neuropathic pain. Reg Anesth Pain Med. 2016;41(5):621-627. [DOI] [PubMed] [Google Scholar]
- 12.Uppal V, Ip VHI. Curb your enthusiasm: erector spinae plane block - ‘because it is easy’ is not a good reason to do it! ASRA Pain Med. https://www.asra.com/asra-news/article/213/curb-your-enthusiasm-erector-spinae-plan#r23. https://www.asra.com/asra-news/article/213/curb-your-enthusiasm-erector-spinae-plan#r23. Accessed November 1, 2019. [Google Scholar]
- 13.Chin KJ, Malhas L, Perlas A. The erector spinae plane block provides visceral abdominal analgesia in bariatric surgery: a report of 3 cases. Reg Anesth Pain Med. 2017;42(3):372-376. [DOI] [PubMed] [Google Scholar]
- 14.Taketa Y, Irisawa Y, Fujitani T. Comparison of ultrasound-guided erector spinae plane block and thoracic paravertebral block for postoperative analgesia after video-assisted thoracic surgery: a randomized controlled non-inferiority clinical trial. Reg Anesth Pain Med. 2020;45(1):10-15. [DOI] [PubMed] [Google Scholar]
- 15.Gürkan Y, Aksu C, Kuş A, et al. Ultrasound guided erector spinae plane block reduces postoperative opioid consumption following breast surgery: a randomized controlled study. J Clin Anesth. 2018;50:65-68. [DOI] [PubMed] [Google Scholar]
- 16.Singh S, Choudhary NK, Lalin D, et al. Bilateral ultrasound-guided erector spinae plane block for postoperative analgesia in lumbar spine surgery: a randomized control trial. J Neurosurg Anesth. 2020;32(4):330-334. [DOI] [PubMed] [Google Scholar]
- 17.Ueshima H, Inagaki M, Toyone T, et al. Efficacy of the erector spinae plane block for lumbar spinal surgery: A retrospective study. Asian Spine J. 2019;13(2):254-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Randomize online was performed using GraphPad Prism version 8.0.0 for Windows, San Diego, California USA: GraphPad Software, 2022. Accessed August 20, 2022. www.graphpad.com. [Google Scholar]
- 19.Fairbank JC, Couper J, Davies JB, et al. The Oswestry low back pain disability questionnaire. Physiotherapy. 1980;66(8):271-273. [PubMed] [Google Scholar]
- 20.Sadiqi S, Post MW, Hosman AJ, et al. Reliability, validity and responsiveness of the Dutch version of the AOSpine PROST (Patient Reported Outcome Spine Trauma). Eur Spine J. 2021;30(9):2631-2644. [DOI] [PubMed] [Google Scholar]
- 21.Sadiqi S, Dvorak MF, Vaccaro AR, et al. Reliability and Validity of the English Version of the AOSpine PROST (Patient Reported Outcome Spine Trauma). Spine. 2020;45(17):E1111-E1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oh SK, Lim BG, Won YJ, et al. Analgesic efficacy of erector spinae plane block in lumbar spine surgery: A systematic review and meta-analysis. J Clin Anesth. 2022;78:110647. [DOI] [PubMed] [Google Scholar]
- 23.Gu W, Tu L, Liang Z, et al. Incidence and risk factors for infection in spine surgery: a prospective multicenter study of 1764 instrumented spinal procedures. Am J Infect Control. 2018;46(1):8-13. [DOI] [PubMed] [Google Scholar]
- 24.Weinstein MA, McCabe JP, Cammisa FP, Jr. Postoperative spinal wound infection: a review of 2,391 consecutive index procedures. J Spinal Disord. 2000;13(5):422-426. [DOI] [PubMed] [Google Scholar]