Abstract
Background:
Guidelines recommend dual-energy X-ray absorptiometry (DXA) screening to assess fracture risk and benefit from antiresorptive therapy in men with metastatic hormone-sensitive prostate cancer (mHSPC) on androgen deprivation therapy (ADT). However, less than 30% of eligible patients undergo DXA screening. Biomechanical computed tomography (BCT) is a radiomic technique that measures bone mineral density (BMD) and bone strength from CT scans.
Objective:
To evaluate 1) correlations between BCT- and DXA-assessed BMD, and 2) associations between BCT-assessed metrics and subsequent fracture.
Design, Setting, and Participants:
Multi-center retrospective cohort study among patients with mHSPC between 2013 and 2020 who received CT abdomen/pelvis or PET/CT within 48 weeks before ADT initiation and during follow-up (48-96 weeks after ADT initiation).
Outcomes and Statistical Analysis:
We used univariate logistic regression to assess associations between BCT measurements and the primary outcomes of subsequent pathologic and non-pathologic fractures.
Results and Limitations:
Among 91 eligible patients, median [IQR] age was 67 years (62-75), 44 (48.4%) were White, and 41 (45.1%) were Black. During median follow-up of 82 weeks, 17 men (18.6%) developed a pathologic and 15 (16.5%) a non-pathologic fracture. BCT- and DXA-assessed femoral-neck BMD T-score were strongly correlated (R2=0.93). On baseline CTs, lower BCT-assessed BMD (Odds Ratio [OR] 1.80, 95% CI [1.10, 3.25], p=0.03) was associated with increased risk of pathologic fracture. Lower femoral strength (OR 1.63, 95% CI [0.99, 2.71], p=0.06) was marginally associated with increased risk of pathologic fracture. Neither BMD (OR 1.52, 95% CI [0.95, 2.63] p=0.11) nor strength (OR 1.14, 95% CI [0.75, 1.80] p=0.57) were associated with non-pathologic fracture. BCT identified 9 (9.9%) men eligible for antiresorptive therapy, of whom 4 (44%) were not treated. Limitations include low fracture numbers resulting in lower power to detect fracture associations.
Conclusions:
Among men diagnosed with mHSPC, BCT assessments were strongly correlated with DXA, predicted subsequent pathologic fracture, and identified additional men indicated for antiresorptive therapy.
Patient Summary:
We assess whether biomechanical computer tomography from routine CT scans can identify fracture risk among patients recently diagnosed with metastatic prostate cancer. We find that BCT and DXA-derived bone mineral density are strongly correlated and that BCT accurately identifies risk for future fracture. BCT may enable broader fracture risk assessment and facilitate timely interventions to reduce fracture-risk in metastatic prostate cancer.
INTRODUCTION
Androgen deprivation therapy (ADT), alone or with other agents, improves survival in mHSPC but accelerates bone mineral density (BMD) loss in the first year and is associated with a 20-42% risk of significant fracture at five years.1–4 Antiresorptive therapies prevent future fracture, but randomized trials have shown that routine administration to all men with mHSPC does not decrease risk of fracture and other skeletal-related events.5 Thus, guidelines recommend baseline and routine fracture risk assessment for men with mHSPC initiating ADT via dual-energy X-ray absorptiometry (DXA) to identify high-risk patients who may benefit from antiresorptive therapy. Antiresorptives are indicated in patients with prior fracture, osteoporosis (BMD T-score ≤−2.5), or osteopenia (BMD T-score between −1 and −2.5) with high-risk of fracture by Fracture Risk Assessment Tool (FRAX).6
There are several limitations with DXA-based screening for mHSPC. First, although universal DXA screening is recommended for men initiating ADT, only 8-30% of men with mHSPC initiating ADT receive baseline DXA screening.6–9 Second, DXA-based BMD measurement evaluates cortical bone density but does not capture important characteristics, including overall shape and three-dimensional geometry, the relative amount of cortical and trabecular bone, local variations in cortical thickness, and the internal spatial distribution of bone density. However, ADT primarily diminishes trabecular bone architecture.10,11 This may explain why a majority of ADT-associated fractures occur in men who do not meet BMD or FRAX thresholds for anti-resorptive therapy.12
Biomechanical Computed Tomography (BCT) is an image-based analysis technique that can be applied to CT scans obtained through routine management of mHSPC patients to assess BMD and bone strength, without need for DXA.13 BCT analyzes previously obtained imaging and can be applied to CT scans that were performed at any point in the past. BCT has already been approved by the Food and Drug Administration (FDA) for providing BMD measurements substantially equivalent to DXA, as well as bone strength measurements that independently identify those at risk of fracture.25 However, it has not been validated in patients with metastatic prostate cancer, where the presence of metastasis may theoretically alter measurements.14–20
BCT has three potential advantages for mHSPC populations. First, BCT offers a convenient fracture risk assessment by utilizing staging and surveillance CT scans gathered in routine care without need for additional DXA testing. Second, BCT facilitates longitudinal measurements of BMD and strength through a patient’s disease course, which can identify patients who newly benefit from anti-resorptive therapy during ongoing ADT exposure or during follow up after treatment cessation. Third, BCT provides a measurement of bone strength, an independent predictor of hip fracture, in addition to BMD.19 Utilizing a diverse multicenter cohort of men with newly diagnosed mHSPC beginning ADT, our overall objective was to externally validate BCT in the mHSPC population.
METHODS
Study Design
This retrospective cohort study followed the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) reporting guidelines. Our primary objectives were to: (1) Assess the robustness of BCT by correlating BCT-assessed BMD from routine CT imaging with concurrent DXA-assessed BMD, and (2) Evaluate the association between BCT-assessed BMD and strength and subsequent fractures. Secondary objectives were to: (1) Identify the number of additional patients who meet criteria for anti-resorptive therapy identified by BCT and (2) Compare longitudinal changes in BMD and bone strength between individuals receiving ADT with or without an androgen receptor signaling inhibitor (ARSi) or chemotherapy.
Ethics approval was identified for use of de-identified images by the University of Pennsylvania and the Corporal Michael J. Crescenz Department of Veterans Affairs institutional review boards (IRBs), which also waived the need for informed consent owing to the use of de-identified data.
Cohort
Ninety-nine patients were identified from internal registries of patients receiving primary oncologic care and CT imaging at the University of Pennsylvania, a large academic medical center, or the Corporal Michael J. Crescenz VA Medical Center (VA), a tertiary VA referral center. Eligibility criteria were determined via chart review and included (1) age ≥21 years; (2) biopsy-proven prostate cancer; (3) biopsy or radiographic confirmation of recurrent or de novo metastatic hormone sensitive PC between 2013 and 2020; (4) no ADT exposure in the year prior to metastatic diagnosis; and (5) eligible CT scans. We used 1:1:1 purposive sampling to achieve relatively equal distributions in therapies (ADT, ADT+chemotherapy, and ADT+ARSi).
Subjects were scanned with CT at two time-points: 1) Baseline CT: within 48 weeks prior to ADT initiation and 2) follow-up CT: between 48 and 96 weeks after ADT initiation. To enable accurate comparisons over time, only baseline and follow-up CTs that used the same contrast protocol and scan manufacturer were included. Eligible CT protocols included CT abdomen/pelvis (with or without contrast), CT Urogram, and PET/CT scans. The scans were acquired across four different CT scanner manufacturers (GE = 29, Philips = 22, Siemens = 128, Toshiba = 19), with kVp ranging from 100–130, and reconstructed with a standard (or equivalent) kernel, with slice thickness ranging from 0.625–5 mm.
Overview of BCT Analysis
BCT13 or “biomechanical computed tomography,” is an image-based analysis technique that processes a patient’s previously obtained CT scan to measure a BMD T-score (equivalent to that from DXA) and a non-invasive assessment of the breaking strength of the patient’s femur.14,15,17,19 The FDA-cleared implementation of BCT in the USA is by the VirtuOst® software (O.N. Diagnostics, Berkeley, CA), and is a regulated class-II medical device. The analysis uses highly automated image processing algorithms, and is guided by trained BCT technologists. For this study, one technologist performed the analyses while blinded to DXA measurements and clinical outcomes (i.e., pathologic fracture, non-pathologic fracture, and non-fracture skeletal related events).
To measure a BMD T-score using BCT, the femur in the CT image is first virtually removed from the surrounding tissue. Then, the 3D femur is calibrated into units of volumetric BMD using the patient’s internal tissues as calibrating references, oriented into a standard DXA-like orientation, and the standard DXA regions of interest are placed on the 2D projection to measure areal BMD. For consistency with the DXA data, T-scores were calculated from BMD using young reference values from White men, and can be used also with FRAX.
In addition to bone density, BCT measures bone strength. This is done via a virtual stress test that utilizes the engineering technique called finite element analysis, and the process accounts for biomechanically important characteristics not captured in a BMD measurement.13 For this analysis, like the BMD component of BCT, the femur is virtually removed from the surrounding tissue and calibrated into units of volumetric bone mineral density. Then, the femur is registered into a sideways-fall orientation and converted into a finite element model by resampling the image voxels into 1 mm-sized cube-shaped finite elements (Figure 1). For each finite element, elastic and failure material properties for the bone are assigned based on the average volumetric BMD value of each element. To estimate the breaking strength (“bone strength”, in units of newtons, N), the femurs are virtually loaded to failure, defined from the resulting nonlinear force-deformation curves as the force at 4.0% overall deformation.
Figure 1: BCT analysis for two men at follow-up.
Left images show the DXA-equivalent assessment and right images show cut-out views of from the virtual stress testing, depicting the distribution of BMD (black and white) and bone failure (colors) after a simulated sideways fall. For BMD, both men had the same a femoral neck T-score of −1.8. For strength, Patient A had a femoral strength value of 4,150 N (above the fragile bone strength threshold of 3,500 N) and did not have a non-pathological fracture, while Patient B had femoral strength value of 3,400 N (indicating fragile bone) and had a non-pathological fracture.
For analyses of concordance of femoral neck BMD with DXA measurements, we analyzed DXA and CT measurements from the same side. For analyses measuring association with fracture risk, the left femur was preferentially analyzed for BCT analysis (as is standard with DXA). If there was a deformity, metastasis, or artifact preventing analysis of the left femur, the right femur was preferentially analyzed.
Overview of DXA Analysis
Results from concurrent DXA imaging (closest to CT date) were extracted from medical records. The two medical centers each utilized a different DXA scanner manufacturer: GE Lunar at the University of Pennsylvania, and Hologic at the VA. These original DXA data were not reanalyzed for the purposes of this study. However, since some DXA T-scores were calculated with young reference values from White women, the T-scores were recalculated using manufacturer-specific young reference values for White men, for consistency across the cohort.
Statistical Analyses
All outcomes and covariates were assessed via chart review by a trained clinical research coordinator (CH). All covariates and outcomes were double-coded between the research coordinator and Principal Investigator (RBP) and the codebook was refined until complete agreement was achieved; all coding was performed prior to BCT analysis.
Correlations Between BCT and DXA Femoral Neck BMD T-Score
Because we anticipated that BMD would change over time due to ADT, we only analyzed correlations with concurrent DXAs performed within 90 days before or after a baseline or follow-up CT scan. In a sensitivity analysis, we analyzed correlations using DXAs performed regardless of the time difference between CT and DXA. All T-scores were standardized. We followed the International Society for Clinical Densitometry guidelines to standardize BMD measurements,21 with the exception of using the male reference for sex-specific strength measurements. Because correlation does not necessarily imply agreement, we also conducted a linear regression with DXA-assessed BMD T-score as the dependent variable and BCT-assessed BMD T-score as the independent variable.
Association Between BCT-assessed BMD and Strength and Subsequent Fractures
We used univariate logistic regression to assess associations between BCT measurements and subsequent fracture. Pathologic and non-pathologic fracture designation were determined from either 1) coded assessments in chart problem lists, or 2) radiology reports from CT or X-rays and defined as occurring within 3 years following mHSPC diagnosis. Pathologic fractures were determined when radiology reports explicitly described the fracture as such. All other fractures were considered “non-pathologic”. We compared means for baseline BCT T-score and strength for patients that experienced vs. did not experience pathologic or non-pathologic fracture. Individuals with fractures that occurred prior to the CT were excluded in relevant analyses. We controlled for baseline antiresorptive therapy use.
In a sensitivity analysis, we assessed associations between BCT measurements and other skeletal related events, defined as presence of one of the following: symptomatic bone metastasis, pathologic or non-pathologic fracture, orthopedic surgical intervention of bone, bone irradiation, spinal cord compression, and death.4 Pathologic fractures were ascertained from radiologist reports explicitly mentioning “pathologic fracture” or a fracture at the site of a prior metastasis. All other fractures were considered non-pathologic.
Identifying the number of additional patients who meet criteria for anti-resorptive therapy identified by BCT
We defined qualification for bone antiresorptive therapy using two criteria: (1) “standard criteria” by BMD and/or FRAX: osteoporosis or osteopenia with high-risk of fracture by FRAX and (2) “enhanced criteria” by BMD, bone strength, and/or FRAX: osteoporosis, fragile bone strength, osteopenia with high-risk of fracture, or low bone strength with high-risk of fracture. Per World Health Organization criteria, we defined osteoporosis as BMD T-score ≤−2.5 and osteopenia as BMD T-score between −1 and −2.5. Using the FRAX tool with BCT BMD measurements and elements from chart-review, we identified patients with 10-year probability of hip fracture ≥3% or major osteoporosis-related fracture ≥20% as high-risk. Per prior studies using BCT to predict fracture risk, we defined “fragile bone strength” as ≤3,500 N and “low bone strength” as between 3,500 and 5,000 N; fragile bone strength is a validated independent criteria for antiresorptive therapy in the general population.13,16,22
Comparing longitudinal changes in BMD and bone strength between individuals receiving ADT with or without an androgen receptor signaling inhibitor (ARSi) or chemotherapy
We calculated change in BMD T-score and bone strength from baseline to follow-up scan. Results were stratified for the following treatment groups: 1) receipt of antiresorptive therapy (bisphosphonate or denosumab) vs. no receipt of antiresorptive therapy and 2) ADT alone vs. ADT+chemotherapy vs. ADT+ARSi. We used analysis of covariance (ANCOVA) to determine whether there any significant differences existed between treatment groups, when controlling for antiresorptive therapy.
Statistical significance was set at P > 0.05, with all tests being 2-tailed. Data were analyzed using R statistical software, version 4.2.1 (R Project for Statistical Computing).
RESULTS
Of 99 patients, 91 were eligible for BCT analysis; for eight subjects, BCT analysis of the femur was not analyzed due to insufficient coverage of the femur by the scan (7) or image artifact from metal implant (1) (Supplemental Figure 1). Among 91 eligible patients, median (interquartile range) age was 67 years (61-75), 44 (48.4%) were White, and 41 (45.1%) were Black (Table 1). For first-line therapy of mHSPC, 28 (30.8%) received ADT alone, 31 (34.1%) received ADT + ARSi, and 32 (35.2%) received ADT + chemotherapy.
Table 1:
Patient Characteristics
| Full Cohort | University of Pennsylvania Cohort | VA Cohort | |
|---|---|---|---|
| N=91 | N=65 | N=26 | |
| Demographics | |||
| Age – median (IQR) | 67 (61, 75) | 66 (61, 74) | 69 (63, 75) |
| Self-Reported Race, no. (%) | |||
| White | 44 (48.4%) | 36 (55.4%) | 8 (30.8%) |
| Black | 41 (45.1%) | 23 (35.4%) | 18 (69.2%) |
| Asian | 2 (2.2%) | 2 (3.1%) | 0 (0%) |
| Other | 3 (3.3%) | 3 (4.6%) | 0 (0%) |
| Prefer not to answer | 1 (1.1%) | 1 (1.5%) | 0 (0%) |
| Baseline Clinical Characteristics at mHSPC diagnosis, no. (%) | |||
| Site(s) of metastatic disease | |||
| Bone | 85 (93.4%) | 61 (93.8%) | 24 (92.3%) |
| Lymph node (retroperitoneal, non-pelvic) | 22 (24.2%) | 15 (23.1%) | 7 (26.9%) |
| Visceral (liver, lung, soft tissue) | 23 (25.3%) | 18 (27.7%) | 5 (19.2%) |
| Other | 3 (3.0% | 1 (1.5%) | 2 (7.7%) |
| Bone Health Related Medical Problems | |||
| Chronic kidney disease | 6 (6.6%) | 2 (3.1%) | 4 (15.4%) |
| Smoking history | |||
| Current smoker | 21 (23.1%) | 12 (18.5%) | 9 (34.6%) |
| Former smoker | 36 (39.6%) | 25 (38.5%) | 11 (42.3%) |
| Never smoker | 31 (34.1%) | 26 (40.0%) | 5 (19.2%) |
| Not reported | 3 (3.3%) | 2 (3.1%) | 1 (3.8%) |
| Alcohol history | |||
| Current alcohol use | 45 (49.5%) | 31 (47.7%) | 14 (53.8%) |
| Former alcohol use | 10 (11.0%) | 5 (7.7%) | 5 (19.2%) |
| Never alcohol use | 25 (27.5%) | 19 (29.2%) | 6 (23.1%) |
| Not reported | 11 (12.1%) | 10 (15.4%) | 1 (3.8%) |
| Baseline lab values, median (interquartile range) | |||
| Prostate Specific Antigen (ng/mL) | 94.39 (20.8, 582.5) | 85.8 (21.5, 338.0) | 119.1 (27.7, 1084.5) |
| Calcium (mg/dL) | 9.3 (9.0, 9.7) | 9.2 (8.9, 9.6) | 9.4 (9.2, 9.95) |
| Vitamin D (ng/mL) | 36 (26.7, 45.0) | 37.0 (36.0, 42.4) | 23.9 (12.4, 36.5) |
| Total alkaline phosphatase (U/L) | 90.5 (66.8, 211.5) | 99.0 (68.0, 256.5) | 80.0 (60.0, 144.0) |
| Radiology Characteristics, no. (%) | |||
| DXA at baseline | 55 (60.4%) | 39 (60%) | 16 (61.5%) |
| T-score of femoral neck based on DXA at baseline | |||
| <−2.5 | 4 (7.3%) | 4 (10.2%) | 0 (0.0%) |
| −2.5 to −1 | 19 (34.5%) | 13 (33.3%) | 6 (37.5%) |
| >−1 | 32 (59.0%) | 22 (56.4%) | 10 (62.5%) |
| Baseline scan type used for BCT measurement | |||
| CT abdomen/pelvis with contrast | 64 (70.3%) | 49 (75.4%) | 15 (57.7%) |
| CT abdomen/pelvis without contrast | 15 (16.5%) | 13 (20%) | 2 (7.7%) |
| PET/CT | 10 (11.1%) | 1 (1.5%) | 9 (34.6%) |
| CT urogram with contrast | 2 (2.2%) | 2 (3.1%) | 0 (0.0%) |
| Therapies, no. (%) | |||
| Systemic therapy at baseline | |||
| ADT alone (no other therapy) | 28 (30.8%) | 19 (29.2%) | 9 (34.6%) |
| ADT with Docetaxel | 32 (35.2%) | 26 (40.0%) | 6 (20.7%) |
| ADT with Abiraterone | 28 (30.8%) | 19 (29.2%) | 9 (34.6%) |
| ADT with Enzalutamide | 3 (3.3%) | 1 (1.5%) | 2 (7.7%) |
| Bone antiresorptive therapy | |||
| Denosumab | 20 (22.0%) | 21 (30.0%) | 1 (3.8%) |
| Zoledronic Acid | 3 (3.3%) | 2 (2.9%) | 1 (3.8%) |
| None | 68 (74.7%) | 44 (77.7%) | 24 (92.3%) |
| Vitamin Supplementation | |||
| Calcium supplementation administered within a year prior to or after mHSPC onset | 42 (46.2%) | 27 (41.5%) | 15 (57.7%) |
| Vitamin D supplementation administered within a year prior to or after mHSPC onset | 43 (47.3%) | 28 (43.1%) | 15 (57.7%) |
| Clinical Outcomes during follow-up | |||
| Pathologic fracture | 17 (18.7%) | 14 (21.5%) | 3 (11.5%) |
| Non-pathologic fracture | 15 (16.5%) | 13 (20.0%) | 2 (7.7%) |
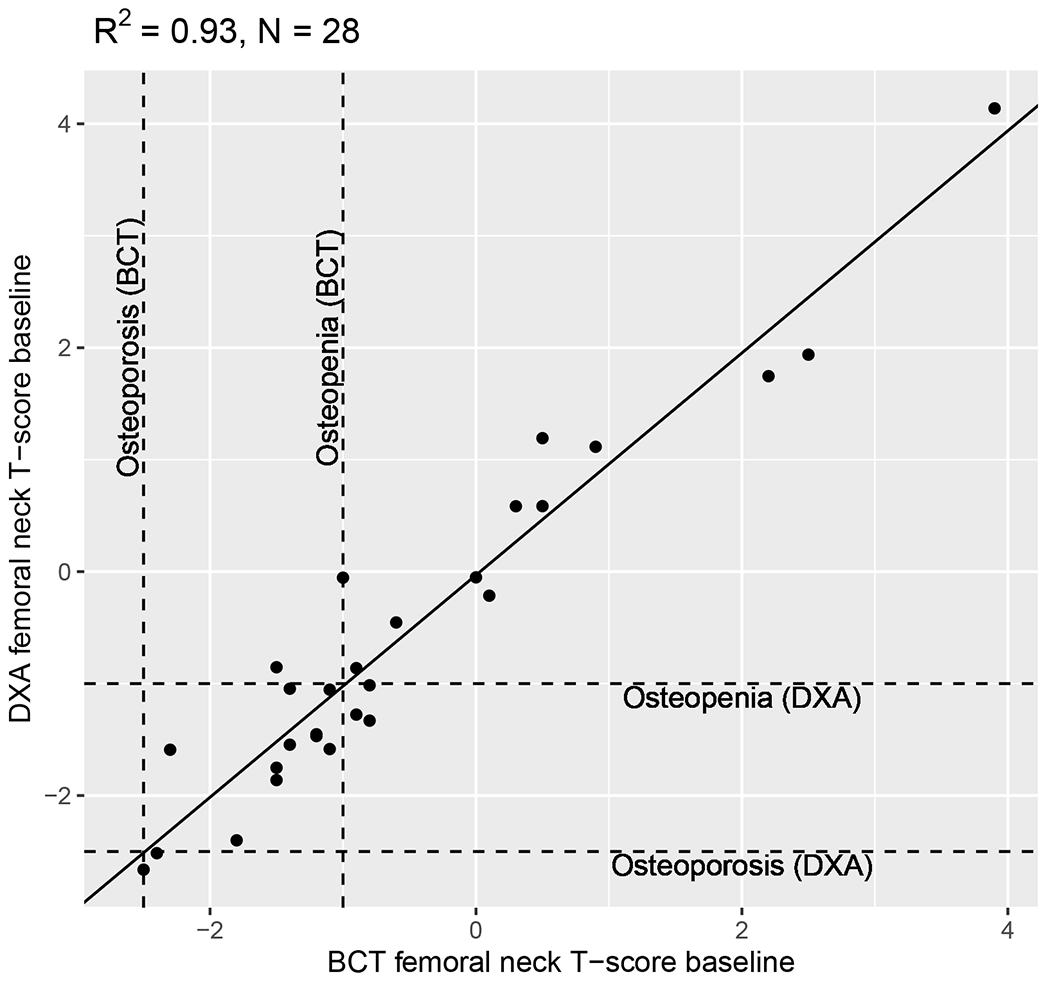
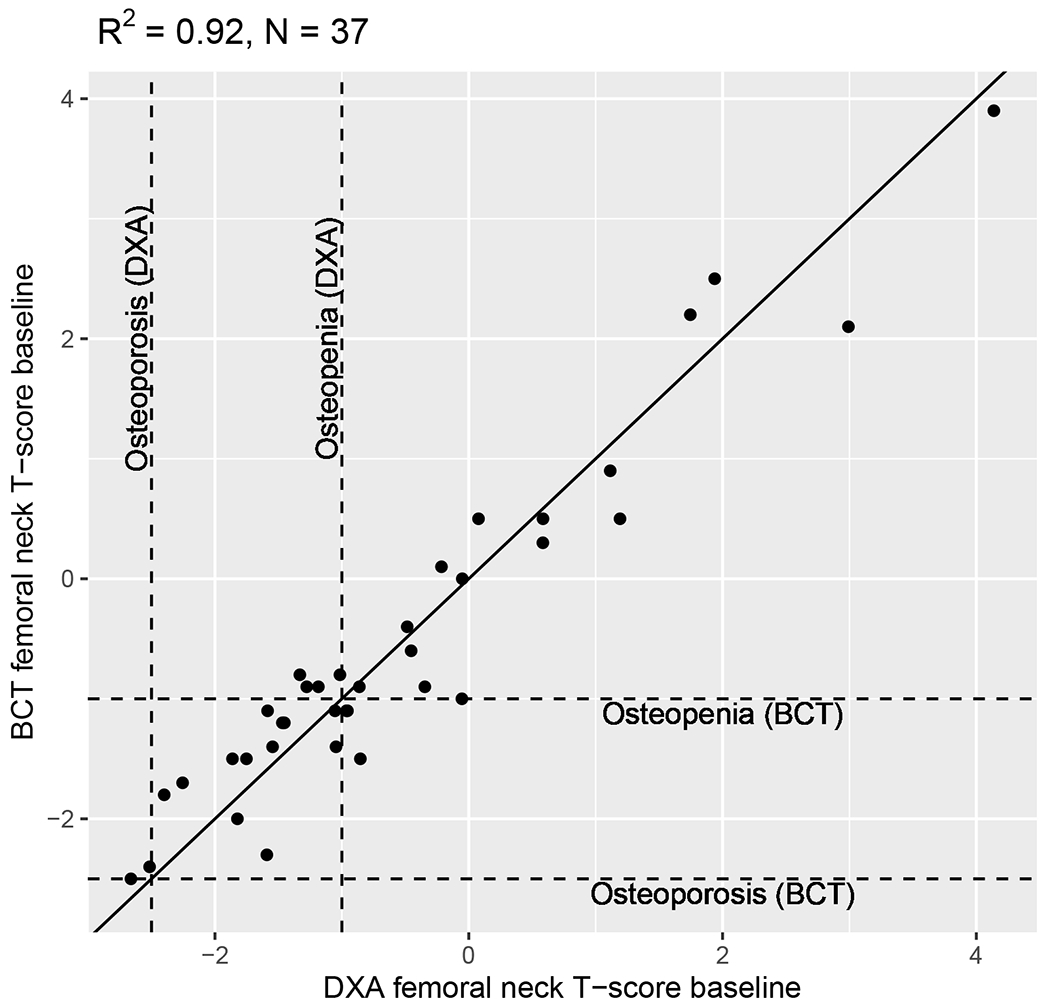
Among patients with concurrent CT and DXA scans, BCT-assessed and DXA-assessed femoral-neck BMD T-score were strongly correlated in primary (R2=0.93, N=28, Figure 2) analyses (i.e., scans conducted within 90 days) and sensitivity (R2=0.85, N=55, Supplemental Figure 2) analyses (i.e., all scans included regardless of the time between CT and DXA). Concordance between categorizations of normal bone density vs. osteopenia vs. osteoporosis was high. Because correlation does not necessarily imply agreement, we conducted a linear regression with DXA-assessed BMD T-score as the dependent variable and BCT-assessed BMD T-score as the independent variable. The beta coefficient for the slope was 0.992 (P<0.001) indicating strong agreement.
Figure 2: Correlation between BMD T-scores assessed from BCT and DXA scans.
Correlation between BMD T-scores assessed from BCT and DXA scans taken within 90 days of each other.
Among all patients, 45 (49.4%) had a DXA scan ever performed, including 28 (58.3%) of those with osteopenia, osteoporosis, low bone strength, or fragile bone strength and 17 (39.5%) with normal BMD and strength (as assessed by BCT) (Figure 3A). Of the 4 (4.4%) patients who qualified for antiresorptive therapy by standard criteria, 3 (75%) received antiresorptive therapy (Figure 3B). Of the 9 (9.9%) patients who qualified antiresorptive therapy by enhanced criteria, 5 (55.5%) received antiresorptive therapy. Of these 9 patients identified as having high fracture risk by BCT, a future fracture developed in 40% of the patients (2 of 5) who received antiresorptive therapy and 25% (1 of 4) who never received antiresorptive therapy (Figures 3C, 3D).
Figure 3: Associations between Baseline BCT metrics and presence of DXA, bone antiresorptive agent use, and subsequent fracture.
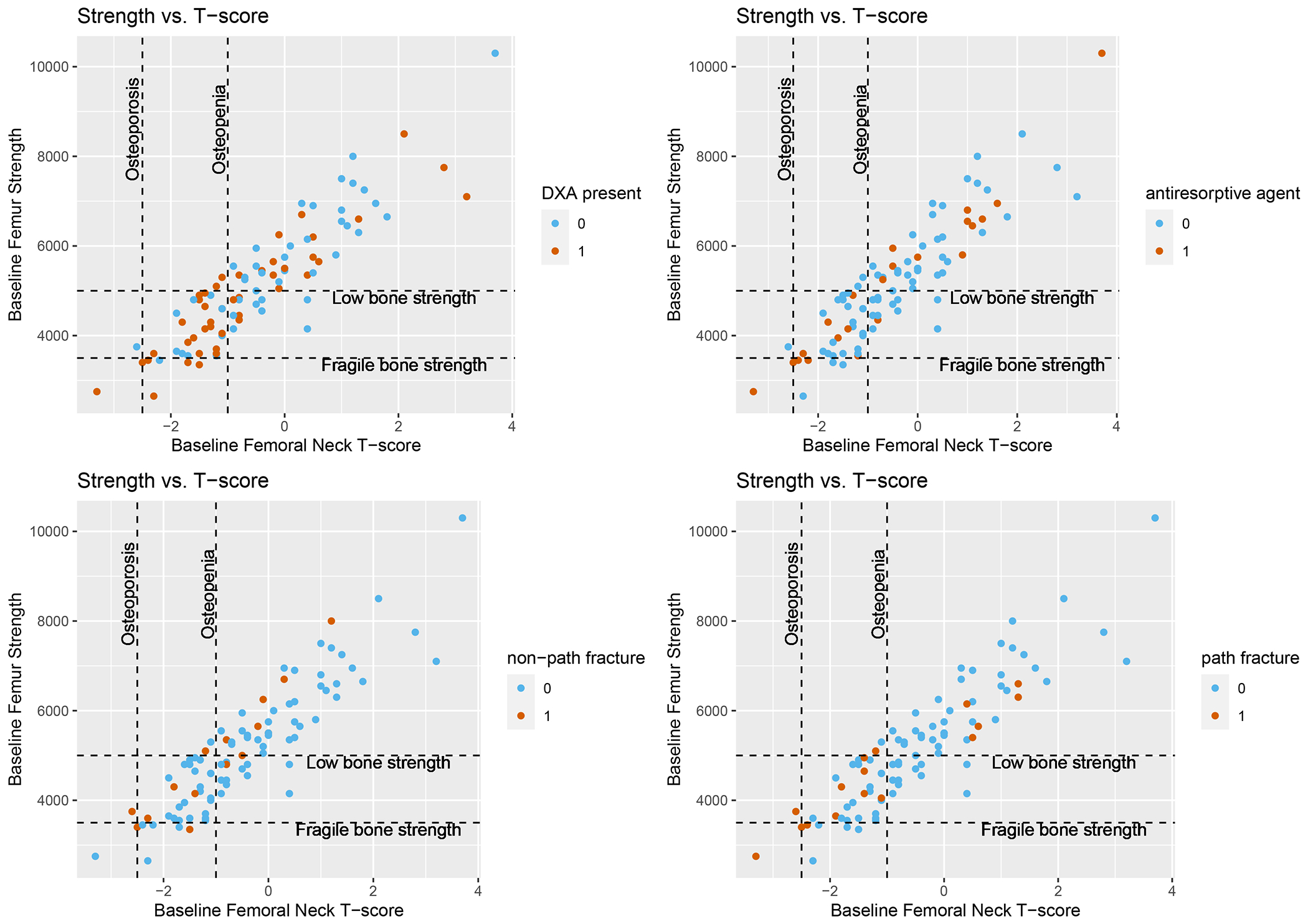
Plots show correlations between baseline bone mineral density and baseline bone strength; dotted lines indicate clinically meaningful thresholds for bone strength (low bone strength, fragile bone strength) and BMD (osteopenia and osteoporosis). Plots are color coded to reflect patients who had A) Presence of baseline DXA testing; B) Baseline bone antiresorptive agent use; C) Subsequent non-pathologic fracture after baseline scan; and D) Subsequent pathologic fracture after baseline scan
During a median follow-up of 82 weeks, 17 (18.7%) had a pathologic fracture and 15 (16.5%) had a non-pathologic fracture (Figure 3C, 3D). Among individuals with fracture, the average time from ADT initiation to pathologic or non-pathologic fracture was 14.2 months and 15.5 months, respectively. Lower baseline BMD (Odds Ratio [OR] 1.80 [95% CI 1.10, 3.25], p=0.03) was significantly associated with increased subsequent pathologic fracture when controlling for antiresorptive therapy (Table 2). Lower femoral strength (OR 1.63, [95% CI 0.99, 2.71], p=0.06) was associated with pathologic fracture with similar magnitude but did not reach statistical significance. Neither lower BMD (OR 1.52, [95% CI 0.95, 2.63], p=0.11) nor lower femoral strength (OR 1.14, [95% CI 0.75, 1.80], p=0.57) were significantly associated with subsequent non-pathologic fracture. They were also not associated with non-fracture skeletal related events (Supplemental Tables 1–2).
Table 2:
Association between biomechanical computed tomography assessments and subsequent fracture
| Overall fracture | Pathologic fracture | Non-pathologic fracture | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Fracture present (n=26) | Fracture absent (n=62) | Odds ratio (95% CI)* | Fracture present (n=16) | Fracture absent (n=70) | Odds ratio (95% CI)* | Fracture present (n=15) | Fracture absent (n=76) | Odds ratio (95% CI)* | |
| Femoral Neck BMD (T-Score) (mean) | −0.83 | −0.28 | 1.43 (0.98, 2.21) p=0.07 |
−1.06 | −0.26 | 1.80 (1.10, 3.25) p = 0.03 |
−0.94 | −0.33 | 1.52 (0.95, 2.63) p = 0.11 |
| Femur Strength (kN) (mean) | 4.94 | 5.30 | 1.23 (0.87. 1.81) p=0.26 |
4.64 | 5.37 | 1.63 (0.99 2.71) p = 0.055 |
4.99 | 5.22 | 1.14 (0.75, 1.80) p = 0.57 |
Odds Ratios reflect odds of subsequent fracture for lower BMD or strength, relative to higher BMD or strength, and are controlled for use of antiresorptive therapy.
NOTE: BMD = Bone mineral density.
Patients who received ADT without antiresorptive therapy experienced declines in BMD from baseline to follow-up (mean baseline 0.78 g/cm2, mean follow-up 0.75 g/cm2, percentage change −4.2%) and femoral strength (mean baseline 5.18 kN, mean follow-up 4.84 kN, percentage change −6.6%) (Supplemental Table 3). After controlling for receipt of antiresorptive therapy, first-line mHSPC treatment with ADT+chemotherapy or ADT+ARSi were not associated with longitudinal changes in BMD (chemotherapy p=0.25, ARSi p=0.85) or bone strength (chemotherapy p=0.49; ARSi p=0.93), relative to ADT alone (Supplemental Table 4). For instance, among patients without antiresorptive therapy, relative changes in BMD were similar for those who had ADT alone (−7.1%), ADT+chemo (−0.2%), and ADT+ARSi (−10.7%). Patients who received ADT plus ARSi had lower PSA and were more likely to have a T-score <−2.5 at baseline (Supplemental Table 5)
DISCUSSION
Fracture risk assessment is essential for patients with mHSPC as antiresorptive treatment can reduce the risk of fragility fracture in this population. However real-world utilization of DXA for fracture risk screening has been low, in part due to reliance on DXA as a separate scan that must be ordered, completed, and acted upon in a population of older adults that may be frail, dependent on caregivers, or have multiple comorbidities to manage. In this study, we show that BCT-assessed BMD T-score calculated using scans performed in routine clinical management of this population was strongly correlated with DXA-measured femoral-neck BMD T-score (R2=0.92). Baseline BCT-assessed BMD T-score was associated with subsequent pathologic fracture. The association between BCT-assessed femoral strength and subsequent pathologic fracture was similar in magnitude but did not reach statistical significance. Neither BMD nor bone strength were associated with non-pathologic fracture. Among men identified as high-risk by BCT and indicated for antiresorptive therapy, 45% did not receive it. There are three points worth emphasis.
First, this is the first validation of BCT in patients with mHSPC, and BCT-assessed BMD was concordant with DXA-measured femoral neck BMD T-score. Although this has previously been demonstrated in general non-cancer populations, it had not been validated in patients with prostate cancer, where the presence of bone metastases and routine use of IV contrast could theoretically affect a BCT-assessed BMD measure. Despite this, we found strong agreement between BCT-assessed BMD T-score and DXA-assessed femoral neck BMD T-score among men with mHSPC. Due to the ease of obtaining BCT-assessed BMD and convenience to patients who have already had a CT scan for staging or surveillance, the comparability of BCT-assessed BMD to DXA in this study should motivate future research to investigate whether BCT can be used in lieu of DXA for routine fracture risk assessment.
Second, BCT-based BMD and strength were associated with subsequent pathologic fracture. The magnitude of associations between BCT assessments and non-pathologic fracture were similar but did not achieve statistical significance. This may be related to the lower prevalence of non-pathologic fracture in our cohort, underpowering this analysis. Hence, the lack of observed statistical association should not be interpreted as meaning that no difference exists; larger studies should assess associations between BCT measures and non-pathologic fracture. These results are the first to validate BCT-assessed measures as predictive of clinical outcomes in mHSPC patients. This is important considering prior studies that have shown that administering bone anti-resorptive therapy to all patients with mHSPC does not reduce future fracture.5 It is possible that better tools to predict fractures may allow us to risk stratify patients and identify sub-populations at particularly high risk of fracture that will benefit from targeted use of anti-resorptive therapy.
Third, both BCT-assessed BMD and strength declined over time in patients receiving ADT. This aligns with literature demonstrating early DXA-assessed declines in bone mineral density while on treatment with ADT. Although not the focus of this analysis, we also found that declines in BMD were similar among patients who received ADT alone (or with chemotherapy) versus those who received ADT along with androgen receptor signaling inhibitors. While we caveat that our analysis may have been underpowered to detect differences, we note they also align with those in a secondary analysis of the randomized controlled trial PEACE-1, in which patients who received ADT along with abiraterone and docetaxel had similar declines in BMD compared to those who received ADT and docetaxel alone during the 2-year follow-up period.23 This is an important area of research, as ADT + ARSI is now the dominant first-line systemic regimen in mHSPC, and overall exposure to ADT and ARSi is increasing as the combination becomes indicated in earlier treatment settings.24 Declines in BMD as well as bone strength were attenuated or even reversed in patients who were taking anti-resorptive agents, which has been suggested in prior DXA-based literature.
BCT—which has already been approved by the FDA—shows promise in addressing inadequate bone health screening among patients with mHSPC, who face high risk of fracture..25 Although uptake has not been high in the general population, where it is not necessarily more convenient than DXA, our study validates its use in patients with mHSPC, where CT scans are routinely obtained (as either conventional CTs or PET/CTs). Moreover, BCT can also be embedded into picture archiving and communication systems (PACS), which facilitates its use in routine clinical care. In our cohort, 50% of patients with BCT-measured osteoporosis (which we show is strongly correlated with DXA-measured BMD) were not receiving bone antiresorptive agents. This suggests that BCT measurements obtained through routine care can also be used to identify patients that may benefit from antiresorptive agents, and potentially even enable equitable assessment of BMD and address disparities in DXA screening by patient race and age that have previously been described.7
There are several limitations to this study. First, our populations came from two institutions, one of which was a major academic medical center, and the other of which was a VA that is its academic affiliate. Future work should include patients from multiple centers, where practice patterns may vary more widely. A key strength of this study was racial diversity – 45.4% of the patients in our cohort were Black. Second, as a retrospective review, it is also possible that other confounding factors, such as access to DXA screening, played a role in the decision to obtain DXA and treatment. Nevertheless, these confounders would not affect our main analyses, which were to correlate BCT-assessed BMD with DXA-assessed BMD and to evaluate whether BCT-assessed BMD and strength were associated with subsequent fracture. Third, the number of eligible scans was low owing to the need for longitudinal CT scans within a specific time period with matching protocols, and thus our analyses may have been underpowered for detection of certain outcomes, particularly non-pathologic fracture. Fourth, while PET/CTs were included in this cohort, because of the retrospective nature of this study, next generation PET/CTs (e.g., PSMA PET/CT) may be underrepresented in this cohort compared to current practice. However, BCT can be used on the CT portion of all PET/CTs (which is common in all PET/CTs, including PSMA PET/CTs), and thus is applicable to those scans. BCT cannot be used in MRIs, including whole-body MRI). Finally, the use of BCT to improve diagnostic screening for osteoporosis longitudinally has the limitation of requiring serial cross-sectional imaging to be performed as routine clinical care. In patients without suspected disease progression, clinicians will need to perform CT scans routinely. In practice, to screen for bone density or strength, any routine CT could be used for BCT, and if no CT is available, a DEXA scan could be ordered.
Future prospective studies should assess whether early administration of bone antiresorptive agents to patients with elevated BCT-assessed fracture risk (by BMD or bone strength) augments BMD and prevents fractures. This is important. as administration of bone antiresorptive agents to unselected patients with mHSPC does not prevent downstream SREs, fractures, or death.5
Conclusions
Given low use of guideline-directed DXA screening in the real-world, BCT of routinely-collected CT scans may offer an accurate and convenient means to screen for fracture risk among patients with mHSPC.
Supplementary Material
Funding/Support:
Prostate Cancer Foundation, Department of Defense, NIH/NCI (P30-CA016520)
Role of the Sponsors:
The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Financial Disclosure of the Authors: Drs. Keaveny and Lee are employed at O.N. Diagnostics, LLC, where they also hold intellectual property interests and stock. Other authors have no conflicts of interest relevant to this work.
REFERENCES
- 1.Shahinian VB, Kuo YF, Freeman JL, Goodwin JS. Risk of Fracture after Androgen Deprivation for Prostate Cancer. New England Journal of Medicine. 2005;352(2):154–164. doi: 10.1056/NEJMoa041943 [DOI] [PubMed] [Google Scholar]
- 2.Nguyen PL, Alibhai SMH, Basaria S, et al. Adverse effects of androgen deprivation therapy and strategies to mitigate them. European Urology. 2015;67(5):825–836. doi: 10.1016/j.eururo.2014.07.010 [DOI] [PubMed] [Google Scholar]
- 3.Wadhwa VK, Weston R, Mistry R, Parr NJ. Long-term changes in bone mineral density and predicted fracture risk in patients receiving androgen-deprivation therapy for prostate cancer, with stratification of treatment based on presenting values. BJU International. 2009;104(6):800–805. doi: 10.1111/j.1464-410X.2009.08483.x [DOI] [PubMed] [Google Scholar]
- 4.Saylor PJ, Lee RJ, Smith MR. Emerging Therapies to Prevent Skeletal Morbidity in Men With Prostate Cancer. JCO. 2011;29(27):3705–3714. doi: 10.1200/JCO.2010.34.4994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith MR, Halabi S, Ryan CJ, et al. Randomized controlled trial of early zoledronic acid in men with castration-sensitive prostate cancer and bone metastases: Results of CALGB 90202 (Alliance). Journal of Clinical Oncology. 2014;32(11):1143–1150. doi: 10.1200/JCO.2013.51.6500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colón-Emeric CS, Pieper CF, Van Houtven CH, et al. Limited Osteoporosis Screening Effectiveness Due to Low Treatment Rates in a National Sample of Older Men. Mayo Clinic Proceedings. 2018;93(12):1749–1759. doi: 10.1016/j.mayocp.2018.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morgans AK, Smith MR, O’Malley AJ, Keating NL. Bone density testing among prostate cancer survivors treated with androgen-deprivation therapy. Cancer. 2013;119(4):863–870. doi: 10.1002/cncr.27830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Skolarus TA, Wolf AMD, Erb NL, et al. American Cancer Society prostate cancer survivorship care guidelines. CA: A Cancer Journal for Clinicians. 2014;64(4):225–249. doi: 10.3322/caac.21234 [DOI] [PubMed] [Google Scholar]
- 9.Suarez-Almazor ME, Pundole X, Cabanillas G, et al. Association of Bone Mineral Density Testing With Risk of Major Osteoporotic Fractures Among Older Men Receiving Androgen Deprivation Therapy to Treat Localized or Regional Prostate Cancer. JAMA Netw Open. 2022;5(4):e225432. doi: 10.1001/jamanetworkopen.2022.5432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greenspan SL, Wagner J, Nelson JB, Perera S, Britton C, Resnick NM. Vertebral fractures and trabecular microstructure in men with prostate cancer on androgen deprivation therapy. Journal of Bone and Mineral Research. 2013;28(2):325–332. doi: 10.1002/jbmr.1771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sullivan S, Wagner J, Resnick NM, Nelson J, Perera SK, Greenspan SL. Vertebral Fractures and the Misclassification of Osteoporosis in Men With Prostate Cancer. Journal of Clinical Densitometry. 2011;14(3):348–353. doi: 10.1016/j.jocd.2011.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neubecker K, Adams-Huet B, Farukhi IM, Delapena RC, Gruntmanis U. Predictors of Fracture Risk and Bone Mineral Density in Men with Prostate Cancer on Androgen Deprivation Therapy. Journal of Osteoporosis. 2011;2011:1–6. doi: 10.4061/2011/924595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keaveny TM, Clarke BL, Cosman F, et al. Biomechanical Computed Tomography analysis (BCT) for clinical assessment of osteoporosis. Osteoporosis International. 2020;31(6):1025–1048. doi: 10.1007/s00198-020-05384-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Orwoll ES, Marshall LM, Nielson CM, et al. Finite element analysis of the proximal femur and hip fracture risk in older men. Journal of Bone and Mineral Research. 2009;24(3):475–483. doi: 10.1359/jbmr.081201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang X, Sanyal A, Cawthon PM, et al. Prediction of new clinical vertebral fractures in elderly men using finite element analysis of CT scans. J Bone Miner Res. 2012;27(4):808–816. doi: 10.1002/jbmr.1539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kopperdahl DL, Aspelund T, Hoffmann PF, et al. Assessment of incident spine and hip fractures in women and men using finite element analysis of CT scans: INCIDENT FRACTURE ASSESSMENT USING FEA OF CT SCANS. J Bone Miner Res. 2014;29(3):570–580. doi: 10.1002/jbmr.2069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwaiger BJ, Facchetti L, Gersing AS, et al. Vertebral and femoral bone mineral density and bone strength in prostate cancer patients assessed in phantomless PET/CT examinations. Bone. 2017;101:62–69. doi: 10.1016/j.bone.2017.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Snyder PJ, Kopperdahl DL, Stephens-Shields AJ, et al. Effect of testosterone treatment on volumetric bone density and strength in older men with low testosterone a controlled clinical trial. JAMA Internal Medicine. 2017;177(4):471–479. doi: 10.1001/jamainternmed.2016.9539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adams AL, Fischer H, Kopperdahl DL, et al. Osteoporosis and Hip Fracture Risk From Routine Computed Tomography Scans: The Fracture, Osteoporosis, and CT Utilization Study (FOCUS). Journal of Bone and Mineral Research. 2018;33(7):1291–1301. doi: 10.1002/jbmr.3423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Teng PF, Chiang JM, Schafer AL, Sukerkar PA, Keaveny TM, Bikle D. Prevalence of osteoporosis in older male veterans receiving hip-containing computed tomography scans: opportunistic use of biomechanical computed tomography analysis (BCT). Osteoporos Int. Published online December 29, 2022. doi: 10.1007/s00198-022-06624-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.The International Society for Clinical Densitometry. 2019. Official Positions.
- 22.Zysset P, Qin L, Lang T, et al. Clinical Use of Quantitative Computed Tomography–Based Finite Element Analysis of the Hip and Spine in the Management of Osteoporosis in Adults: the 2015 ISCD Official Positions—Part II. Journal of Clinical Densitometry. 2015;18(3):359–392. doi: 10.1016/j.jocd.2015.06.011 [DOI] [PubMed] [Google Scholar]
- 23.Roubaud G, Kostine M, McDermott RS, et al. Bone mineral density in men with de novo metastatic castration-sensitive prostate cancer treated with or without abiraterone plus prednisone in the PEACE-1 phase 3 trial. JCO. 2022;40(6_suppl):19–19. doi: 10.1200/JCO.2022.40.6_suppl.019 [DOI] [Google Scholar]
- 24.Schaeffer EM, Srinivas S, Adra N, et al. NCCN Guidelines® Insights: Prostate Cancer, Version 1.2023. J Natl Compr Canc Netw. 2022;20(12):1288–1298. doi: 10.6004/jnccn.2022.0063 [DOI] [PubMed] [Google Scholar]
- 25.U.S. Food and Drug Administration. Device ID K113725 Premarket Notification (Virtuost). Accessed February 23, 2023. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMN/pmn.cfm?ID=K113725
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


