Abstract
The study of complex behaviors is often challenging when using manual annotation due to the absence of quantifiable behavioral definitions and the subjective nature of behavioral annotation. Integration of supervised machine learning approaches mitigates some of these issues through the inclusion of accessible and explainable model interpretation. To decrease barriers to access, and with an emphasis on accessible model explainability, we developed the open-source Simple Behavioral Analysis (SimBA) platform for behavioral neuroscientists. SimBA introduces several machine learning interpretability tools, including Shapley Additive exPlanations (SHAP) scores, that aid in creating explainable and transparent behavioral classifiers. We show how the addition of explainability metrics allows for quantifiable comparisons of aggressive social behavior across research groups and species, reconceptualizing behavior as a sharable reagent and providing an open-source framework. We provide an open-source, GUI-driven, well documented package to facilitate the movement towards improved automation and sharing of behavioral classification tools across laboratories.
INTRODUCTION
Behavioral neuroscience requires detailed behavior1, but the notoriously painstaking process of hand-annotating live or recorded assays poses a significant bottleneck preventing comprehensive behavioral analysis. The manual approach can be arduous, non-standardized, and susceptible to confounds produced by observer drift, long analysis times, and poor inter-rater-reliability 2–4. These caveats prevent the detailed study of complex social repertoires in larger datasets, and notably provide lower temporal resolution than most modern methodologies such as in vivo electrophysiological, fiber photometry, and single-cell calcium endomicroscopy recordings 5–8. Computational neuroethology4 – the marriage of traditional neuroscience techniques, ethological observation, and machine learning – is heralded as one potential solution toward deeper behavioral analysis in more ethologically relevant settings. Furthermore, these data are collected at sampling frequencies that match modern neural recording and manipulation techniques.
The recent rapid development of open-source pipelines for markerless animal pose estimation, which allow for accurate tracking of experimenter-defined body-parts in noisy and variable environments 9–12, provide a framework for automated machine-learning based behavioral analyses (Table 1). Using patterns in animal pose over sliding temporal windows, supervised algorithms are trained to find predefined behaviours of interest. These automated behavioral assessments often exceed human performance13, increase throughput and consistency14 and reduce human bias and anthropomorphism within scoring15. Therefore, open-source pipelines for pose estimation and behavioral analysis are increasingly focused on improving computational accessibility to non-specialists via graphical user interfaces (GUIs), easier installation processes, and extensive documentation and tutorials. But as more labs (and manuscript and grant reviewers) adopt these techniques as the de-facto expected standard, it is increasingly important to focus on model explainability and behavioral nuance.
Table 1:
Select open-source packages for behavioral detection.
| Year | Name | Citation | Validated species | Behavioral Classifiers validated in the original publication | Software website |
|---|---|---|---|---|---|
| Supervised | |||||
| 2009 | CADABRA74 | Dankert et al. 2009 | Fly | Lunging, tussling, wing threat/extension, circle, chase, copulation | http://www.vision.caltech.edu/cadabra/ |
| 2012 | MiceProfiler75 | de Chaumont et al. 2012 | Mouse | Head-head contact, anogenital contact, side-by-side, approach, leave, follow, chase | http://icy.bioimageanalysis.org/plugin/mice-profiler-tracker/ |
| 2013 | JAABA76 | Kabra et al. 2013 | Fly and mouse | Fly: walk, stop, crabwalk, backup, touch, chase, jump, copulation, wing flick/groom/extension, right, center pivot, tail pivot. Mouse: follow, walk. | https://sourceforge.net/projects/jaaba/files/ |
| 2013 | Unamed77 | Giancardo et al., 2013 | Mouse | Nose to body, nose to nose, nose to genitals, above, follow, stand together | |
| 2015 | Unamed78 | Hong et al. 2015 | Mouse | Attack, close investigation, mount | |
| 2020 | SimBA49 | Nilsson et al. 2020 | Mouse and rat | Attack, pursuit, lateral threat, anogenital sniff, mount, upright submissive, allogroom, flee, scramble, box, approach, avoidance, drink, clean, eat, rear, walk away, circle | https://github.com/sgoldenlab/simba |
| 2021 | MARS46 | Segalin et al. 2021 | Mouse | Attack, mount, close investigation, face-directed, genital-directed, | https://github.com/neuroethology/MARS |
| 2022 | DeepEthogram79 | Bohnslav et al. 2022 | Fly and mouse | Nose-to-nose, nose-to-body, body-to-body, chase, anogenital | https://github.com/jbohnslav/deepethogram |
| 2022 | DeepCaT-z80 | Gerós et al. 2022 | Rodent | Standstill, rear, walk, groom | https://github.com/AnaGeros/Deep-CaT-z-Software |
| 2022 | DeepAction81 | Harris et al. 2022 | Mouse | Walk, rest, rear, mover, hang, groom, eat, drink, attack, approach, sniff, and copulation | https://github.com/carlwharris/DeepAction |
| 2023 | LabGym82 | Hu et al. 2023 | Fly, larva, mouse and rat | Nest build, curl, uncoil, abdomen bend, wing extension, walk, rear, facial groom, sniff, ear groom, sit | https://github.com/umyelab/LabGym |
| Semi-supervised | |||||
| 2022 | SIPEC83 | Marks et al. 2022 | Mouse and primate | Social groom, search, object interaction | https://github.com/SIPEC-Animal-Data-Analysis/SIPEC |
| Self-supervised | |||||
| 2009 | Ctrax84 | Branson et al. 2009 | Fly | Walk, stop, sharp turn, crabwalk, backup, touch, chase | http://ctrax.sourceforge.net/ |
| 2021 | TREBA71 | Sun et al. 2021 | Fly and mouse | Sniff, mount, attack, lunge, tussle, wing extension | https://github.com/neuroethology/TREBA |
| 2022 | Selfee85 | Jia et al. 2022 | Fly and mouse | Chase, wing extension, copulation attempt, copulation, social interest, mount, intromission, ejaculation | https://github.com/EBGU/Selfee |
| Unsupervised | |||||
| 2014 | MotionMapper86 | Berman et al. 2014 | Fly | Groom: wing, leg, abdomen, head. Wing waggle, run | https://github.com/gordonberman/MotionMapper |
| 2017 | DuoMouse87 | Arakawa et al. 2017 | Mouse | Sniff, follow, indifferent | http://www.mgrl-lab.jp/DuoMouse.html |
| 2019 | LiveMouseTracker88 | de Chaumont et al. 2019 | Mouse | Rear, look up, look down, contact | https://livemousetracker.org |
| 2020 | AlphaTracker89 | Chen et al. 2020 | Mouse | Unnamed individual and social behavior clusters | https://github.com/ZexinChen/AlphaTracker |
| 2021 | Behavior Atlas90 | Huang et al, 2021 | Mouse | Clusters identified: Walk, run, rear, trot, step, sniff, up stretch, left turn, right turn, rise, fall, dive | https://behavioratlas.tech/ |
| 2022 | VAME91 | Luxem et al. 2022 | Mouse | Motifs identified: exploration, turn, stationary, walk to rear, walk, rear, unsupported rear, groom, backward | https://github.com/LINCellularNeuroscience/VAME |
| 2022 | DBscorer92 | Nandi et al. 2022 | Mouse and rat | Immobility | https://github.com/swanandlab/DBscorer |
| Heuristics | |||||
| 2022 | BehaviorDEPOT93 | Gabriel et al. 2022 | Mouse | Freeze, jump, rear, escape, locomotion, novel object exploration | https://github.com/DeNardoLab/BehaviorDEPOT |
Explainability methods in behavioral neuroscience16 aim to determine why and how machine learning models are coming to conclusions, allowing researchers to (i) standardize behavioral definitions if desired, (ii) precisely describe and report specialized, non-standard, behavioral variations, and (iii) more objectively quantify differences in unsupervised behavioral clusters and scrutinize their biological relevance17. More precisely, computing and sharing explainability metrics is an essential step in reconceptualizing behavioral classifiers as objective and shareable reagents akin to the commonly used Research Reagent Identifiers (RRIDs) system for wet lab reagents. As researchers, we are already expected to report behavioral features such as sex, time of day of testing, light cycles, and other experimental details, yet the operational definitions of behaviors themselves are often relegated to one to two sentences in the methods. Incorporation of explainability metrics allows for objective and complete reporting of behavioral classifiers, leading to enhanced reproducibility. This is not an argument for field-wide standardization of behavioral algorithms, but rather an opportunity to precisely and objectively capture and report metrics of computer-aided behavioral analyses between experiments and research groups.
Here, we present Simple Behavioral Analysis (SimBA) and introduce accessible tools for validation and explainability of supervised behavioral classifications. SimBA is an open-source, primarily GUI-based program built in a modular fashion to increase non-specialized user access to automated behavioral analysis via supervised machine learning techniques. Two parallel and integrated branches of SimBA allow (i) the generation of non-machine learning based descriptive statistics of movement and region of interest (ROI) analyses and (ii) supervised machine learning based behavioral classification. SimBA is agnostic to the choice of animal species or number of experimental subjects and has been used to classify fish18, wasp 19, moth20, mouse21–38, rat39,40, and bird41 behavior. Further, this approach promotes the wider dissemination of classifiers and associated explainability metrics between research groups, and is compatible with new or historical videos annotated by open-source packages such as BORIS42 and commercial packages like Noldus Observer or EthovisionXT43.
Importantly, SimBA introduces several machine learning interpretability tools and seamlessly incorporates the application of Shapley Additive exPlanations44 (SHAP) scores, which are one possible solution to providing explainability and transparency of behavioral classifiers. SHAP is a widely cited open-source and post-hoc explainability method which can be applied and compared across any of the current machine learning platforms regardless of pose estimation scheme. Furthermore, Shapley values are widely accepted, understood, and under continual development and scrutiny in the greater computer science and artificial intelligence fields. It is proposed that explainable AI is critical for the future of neuroscience16, and for a deeper discussion into the usefulness of explainability approaches in computational neuroethology see Goodwin, Nilsson et al., 202217.
Here, we highlight the functionality and importance of this approach in multiple datasets to demonstrate the utility of explainability metrics in capturing and describing subtle behavioral variations across sex and environment in freely moving social behavior. Specifically, we examine five behaviors (attack, anogenital sniffing, pursuit, escape, and defensive behavior) across males and females in chronic social defeat stress (CSDS) assays, and across male rodents in varied contextual environments across resident intruder or CSDS assays. To understand the relationship between such behavioral differences between laboratories, we perform SHAP analysis on aggressive social behaviors from different research groups and compare the specific timescales and feature bins characterizing behaviors. We show that this approach provides quantitative descriptions of behavior, allowing for the use of behavior as an objective shareable reagent.
We present a platform for the rapid frame-by-frame supervised analysis of animal behavior, in conjunction with explainability tools which rapidly and accessibly capture behavioral subtleties often left out of standard behavioral metrics. We propose that behavioral classifiers, in combinations explainability metrics, can be publicly shared and used in the fashion of RRIDs to increase reportability and reproducibility within behavioral neuroscience.
Results:
Accessible machine learning for behavioral neuroscientists
SimBA provides accessible machine learning tools to non-specialized users using standard computational hardware via a simple, single-line installation, a graphical user interface (GUI), and extensive documentation. Following raw video acquisition (Fig. 1), users can pre-process their videos (ex. cropping, trimming, changing resolution and contrast) in SimBA prior to performing pose estimation in their open-source program of choice such as DeepLabCut10,45, SLEAP46 DeepPoseKit9, and MARS47. Following GUI-guided import of animal tracking data (Fig. 1A), SimBA calculates relationships between body parts across static and dynamic time windows (features) that are used to train supervised random forest machine learning classifiers for behavioral predictions (Fig. 1B). SimBA – by default – computes explainable feature representations of movements, angles, paths, velocities, distances, and sizes within individual frames and as rolling time-window aggregates. To provide flexibility for advanced users, the SimBA library includes a larger battery of runtime-optimized feature calculators covering frequentist and circular statistics, anomaly scores, temporal and spectral analyses, relationships between pose-estimation and user-defined regions-of-interests, bounding-box methods and other ML distribution comparison techniques18,30,39. All of these can be deployed within user-defined time-windows in use-cases where default feature calculators are insufficient. Finally, SimBA is highly flexible and can function as a programmatic platform allowing fully customized user-composed feature extraction classes. These hundreds of features per individual video frame can then be used to either train supervised machine learning classifiers or fed to trained classifiers which create frame-by-frame predictions of the probability of a behavior of interest occurring.
Figure 1: SimBA workflow and outside integrations.
SimBA is an open-source, graphical user interface-based program built in a modular fashion to address many of the specific analysis needs of behavioral neuroscientists. SimBA contains a suite of video editing options to prepare raw experimental videos for markerless pose tracking, behavior classifications and visualizations. Once users have analyzed their videos for animal pose data via common open-source pipelines (a), the data is imported to SimBA for subsequent analysis (b). Within SimBA, users have the option to perform pose estimation outlier corrections, interpolation and smoothing methods, or use uncorrected pose data in any SimBA module. To perform supervised behavioral classification, users can download premade classifiers from our OSF repository, request classifiers from collaborators, or create classifiers by annotating new videos in the scoring interface. Users can also use historical lab annotations created in programs such as Noldus ObserverXT, Ethovision, or BORIS. A variety of tools are provided for evaluating classifier performance, including calculating standard machine learning metrics and visualization tools for easy hands-on qualitative validation. Following behavioral classification, users can perform a batch analyses’ and extract behavioral measures. To understand the decision processes of classifiers, we encourage users to calculate and report explainability metrics, including SHAP values. We provide extensive documentation, tutorials and step-by-step walkthroughs for all SimBA functionality.
Training supervised machine learning algorithms requires human annotations of a subset of video frames as either positive or negative for the behavior of interest, which algorithms learn to differentiate using the associated feature values. We have streamlined the training set construction process by building in-line behavioral annotation tools including raw video annotation, machine-assisted annotation where the user verifies annotations produced by a prior behavioral model, and methods for importing existing behavioral labels (Fig. 1B). This process is supported by video batch post-processing tools that can be used to create targeted video clips containing the behaviors of interest that maximize biological replicates and contain comparable amounts of positive and negative frames (Fig. 2C), precluding the need to annotate every frame of individual videos. Due to their ease of adoption for new users, interpretability, and robustness to overfitting48–50, our pipeline uses random forest supervised machine learning algorithms; this further allows for the calculation of classical machine learning performance metrics and supports the creation of multiple visualizations and other hands-on validation tools for individual classifiers (Fig. 1B).
Figure 2: Classifier construction workflow and classifier performance metrics.
(a) Machine learning performance metrics for the classifiers used in Figures 5–6 (See Extended Data Fig. 3 and Supplementary Figs.1-6 for in-depth classifier performance data). Left: F1 5-fold cross validation learning curves plotted against minutes of positive frames annotated (30 frames per second). Right: Precision-recall curves plotted against discrimination threshold for five classifiers, which can be used in combination with the SimBA interactive thresholding visualization tool to determine the most appropriate detection threshold for classifiers and specific datasets. (b) Extended information for the training sets for each of the five classifiers. (c) Workflow for creating high fidelity and generalizable supervised behavioral classifiers. The dotted lines indicate optional loops for iteratively improving classifier performance. Behavioral operational definitions, and classifier SHAP values, are shown in Figures S1-4.
Classifier construction and performance
In addition to accommodating shared classifiers and pooled annotation sets, pre-existing classifiers can be adapted to new experimental cohorts and conditions via GUI-assisted thresholding, with limited additional classifier training34. Random forest classifiers output a probability per frame of a behavior of interest occurring. As new cohorts of animals are screened and recording contexts are altered, the classifier certainty may decrease for positive frames, but the probability of non-event frames typically stays extremely low. As such, achieving appropriate performance on new samples is supported by dynamic control of discrimination thresholds using an interactive thresholding tool for positive versus negative behavior events (Fig. 1B, 2C). Precise thresholds can also be calculated via precision-recall curves (Fig. 2A). Most labs will eventually perform manipulations that alter behavior setups to the point of classifier failure. This is solved by the expansion and incorporation of new training sets. Using the video batch pre-processing interface, new experimental videos may be annotated, manually or with the assisted scoring platform, and easily added to prior models. Thus, new behavioral frames can be rapidly added to the training sets to improve performance, allowing groups to iteratively create updated behavioral classifiers (Fig. 2C).
Standard machine learning performance metrics for all classifiers are provided (Fig. 2A-B, Supplementary Fig. S3-4; but see Discussion for comments on data leakage). For most classifiers, performance improvement occurs within the first ~20k positively annotated frames, equivalent to ~11 minutes using standard 30fps video acquisition. Classifier performance increases with additional annotations, higher clarity of operational definitions, and further iterations for targeted misclassification correction. Rat, CRIM, and mouse classifiers achieved F1 performance on behavior present frames of > 0.91, 0.73, and 0.77 respectively (Fig. 2A-B, Fig. S3-4). Within SimBA, classifier training includes multiple checks of performance on novel videos to assess generalizability. Using the built-in visualization and validation tools, new unannotated behavioral videos can be analyzed to assess and subsequently adjust performance as necessary. Importantly, hand versus machine scoring results – comparing frame by frame classifications on independent videos – indicate high classifier performance both within labs (Attack Pearson’s R2 = 0.91 on 16 independent videos, Supplementary Fig. S6) and across labs for multiple classifiers (Pearson’s 0.936 to 0.998 R2 across 8 classifiers26; Pearson’s = 0.77, 0.94, 0.98 R2, 31; confusion matrix accuracy = 98.6%, 99.3%, 85.0%30).
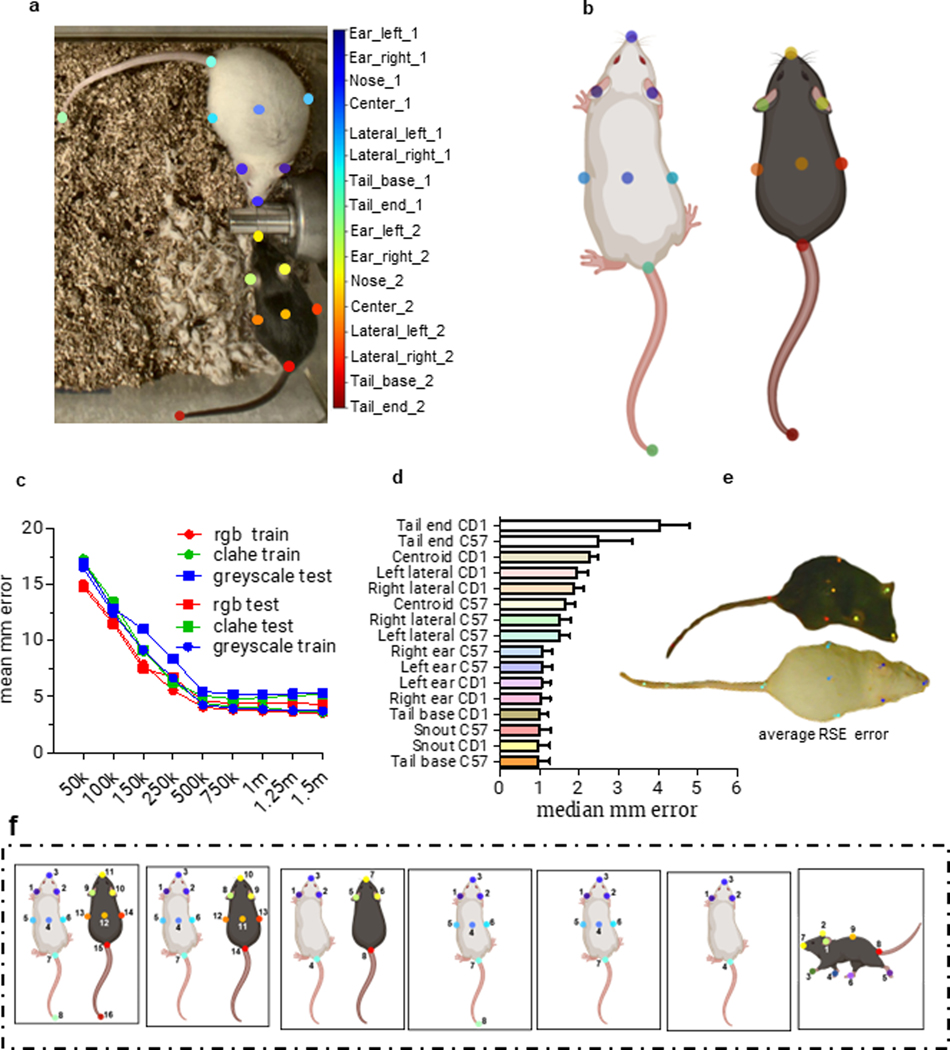
For each classifier, the maximal F1 score is impacted by pose estimation performance, behavior distinctiveness, and training set construction. For example, in the case of pose-estimation performance, a ~4mm median error in ‘tail end’ tracking results in failed classification of tail rattles, while ~1–2.5mm median error in ‘body hull’ points do not affect classifications of other behaviors at F1 > 0.74 (Extended Data Fig. 1). SimBA has several pose estimation outlier correction options available for users upon data import (Extended Data Fig. 2). For behaviors associated with distinct pose estimation signatures, such as drinking, small training sets are sufficient for high performance (<2 minutes of positive frames, F1 = 0.965, F). Conversely, to accurately classify multiple behaviors that share similar pose estimation signatures, such as attack and mounting, larger and more diverse training sets are required (attack: >30 minutes of positive frames, F1 = 0.921, Fig. 2, Extended Data Fig. 3). Comparing the ‘chase’ classifier from the CRIM datasets (~2 min of positive frames, F1 = 0.717) and the ‘pursuit’ classifier from the University of Washington (~1 minute of positive frames, F1 = 0.853) reveals the influence of training set construction on F1 score. Ultimately, users will reach an asymptotic point at which further training does not improve classification performance, which depends largely on tracking performance, the set of used features, and the distinctiveness of the behavior of interest (Fig. 2A).
SHAP reveals differences between annotators, species, and behaviors
Explanations for how machine learning models reach their decisions can help researchers communicate and compare the results of disparate classifiers and support researchers in making informed decisions for machine model implementation and use51–54. One recent influential and accessible approach for generating explainable metrics of tree-based classifiers is SHAP (Shapley Additive exPlanations)55. We chose to use SHAP because it is one of the most widely adopted open source explainability methods available, in addition to being well-documented and under active development and support44. Shapley values are just one paradigm amongst a continuously growing number of approaches for explainable artificial intelligence (XAI); alternate options include LIME52 and counterfactuals56, all with different pros and cons. While it is preferable for new pipelines to be built with inherently explainable algorithms57, we propose that post-hoc explainability metrics are highly compatible with the current state of the field due to the diversity of ML packages being developed.
In essence, the Shapley value presents a game theory-based method for equitably distributing a game’s earnings among its players. To illustrate, consider a scenario where individual feature contributions need to be assessed for a binary behavioral classifier. There are two key pieces of information available beforehand. Firstly, there’s the average probability (expected value) indicating the likelihood of the behavior occurring in a video frame, such as 10% based on the number of positive annotations from the training data. Secondly, the model predicts the presence of the behavior in a new frame with 75% probability. By employing Shapley values, the disparity between these values (in this case, 65%) is attributed to the combination of feature values. This difference is then allocated to the features based on their contributions within the ensemble. The resulting Shapley value output is a vector of the same length as the number of features, with a total sum of 65 (for a detailed mathematical explanation of SHAP and its application in behavioral neuroscience, refer to Supplementary Methods).
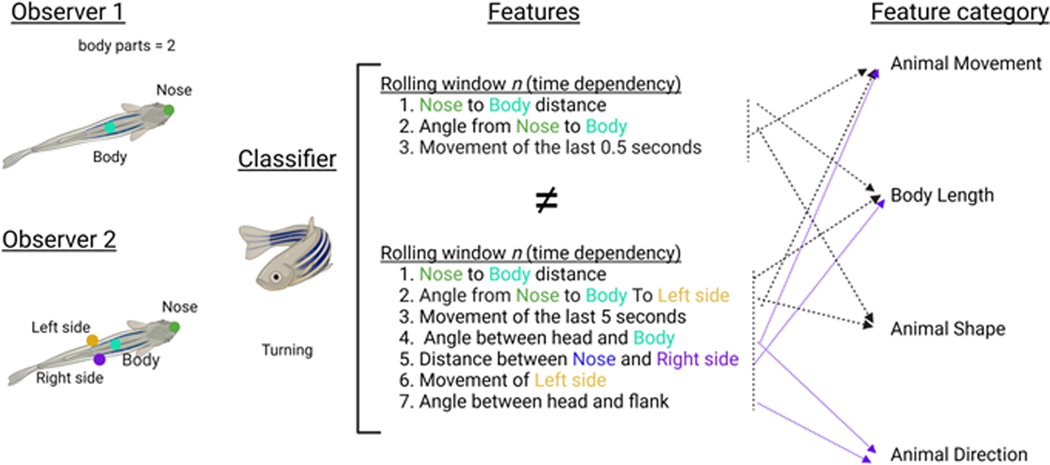
SimBA calculates many hundreds of features (Supplementary Table 1) that individually may not be very informative. While SHAP values can be calculated for each of these features if desired, the additivity axiom allows users to bin features into ethologically relevant feature categories (Supplementary Table 2) that are related to the biology of their model system and/or behaviors of interest. Another key advantage is that classifiers generated for the same behavior, but using different pose estimation schemas (e.g., one model with 5 body parts versus a model with 8 body parts), may still be directly compared by such bins, which would not be possible using individual feature importance’s (Extended Data Fig. 4). SHAP is built into SimBA classifier construction to encourage its use and accessibility, but more-so we propose that the adoption of any type of explainability paradigm is more important than the specific algorithm selected.
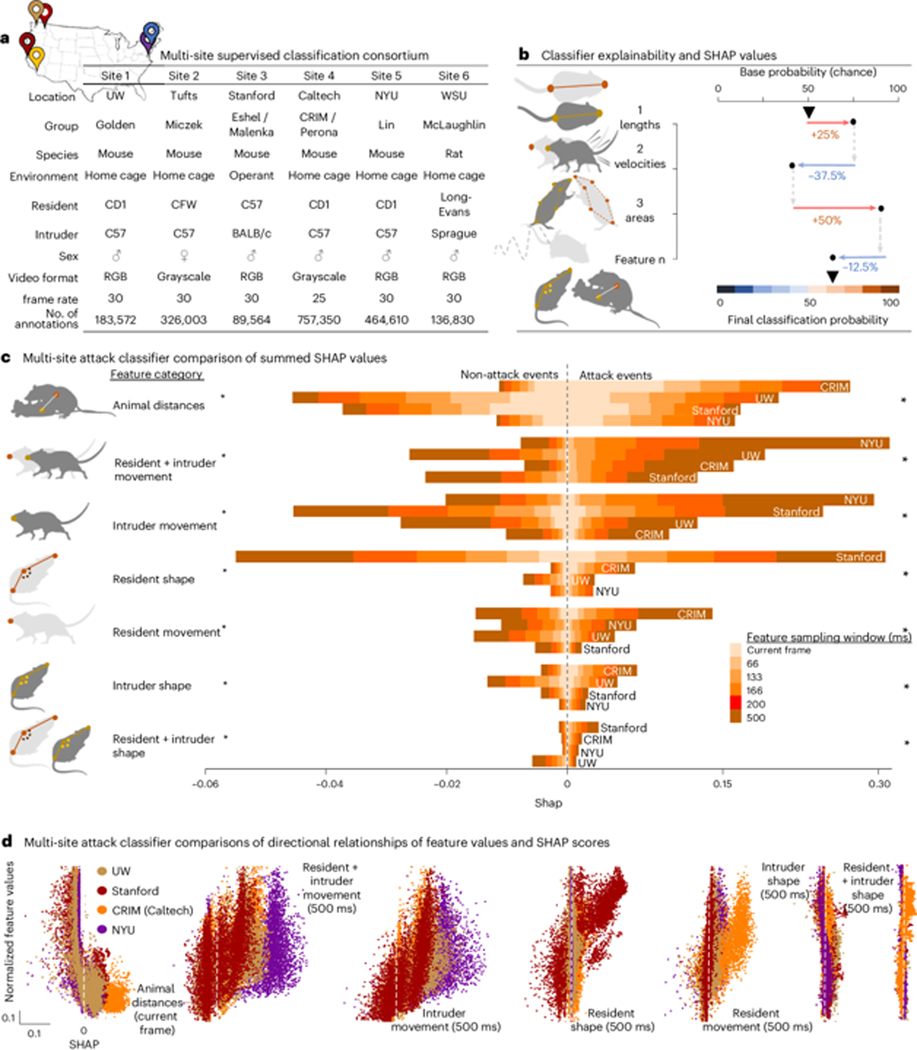
To specifically demonstrate SHAP’s potential to enhance behavioral reportability within preclinical behavioral neuroscience, we collected and compared independently created rodent attack classifiers from expert annotators at institutions across the United States. These classifiers and annotators used data from different recording environments, with different experimental protocols, strains, sex, video formats and pose-estimation models (Fig. 3A, Statistical Supplementary Note 1 — detailed statistics). The classifiers all showed high accuracy in their respective environments.
Figure 3: SHAP attack classifier consortium data.
(a) Description of the consortium dataset used for the cross-site attack classifier comparisons. (b) Schematic description of SHAP values, where the final video frame classification probability is divided among the individual features according to their contribution. (c) ANOVA comparison of summed feature SHAP values, collapsed into seven behavioral feature categories for four different mouse attack classifiers. We divided each category into six further sub-categories that represented features within the categories with different frame sampling frequencies (1 frame – 500ms) and are denoted by shaded colors. Asterisks denote significant main effect of consortium site, p < 0.0001. See Supplementary Note 1 — detailed statistics for full statistical analysis. (d) Scatter plots showing the directional relationships between normalized feature values and SHAP scores in four mouse resident-intruder attack classifiers and seven feature sub-categories. The dots represent 32k individual video frames (8k from each sites dataset), and color represents the consortium site where the annotated dataset was generated. All tests were two-sided. Bonferroni’s test was used for multiple comparisons where applicable
SHAP analysis of attack classifiers revealed the relative importance of feature category bins17 for discerning both attack and non-attack frames. Across labs, animal distances and movement were important for identifying attack, while animal shapes were least important. In addition to identifying these common patterns, a stark difference in the influence of resident shape was seen between the Stanford attack classifier and classifiers from other sites (Fig. 3C). To ensure that this was not due to incongruent operational definitions of attack behavior, we manually annotated the Standford dataset based on our operational definition of attack. Manual annotations were highly consistent between sites (Extended Data Fig. 5, R2 = 0.998; gantt plot). SHAP analysis showed that UW annotations relied on resident body shape over longer durations than Stanford. This demonstrates that while the annotators have high inter-rater reliability, SHAP determined they rely on different features for attack scoring. This observation is likely based on the biological differences between the two sites datasets, where Stanford used C57 resident mice rather than outbred strains like other research locations.
Since SHAP values do not convey directional relationships, we plotted the 32k observations within each category (8k from each site) with the normalized feature values on the y-axis and SHAP value on the x-axis (Fig. 3D, Statistical Supplementary Note 1 — detailed statistics). Here the left-most panel shows that as the distance between animals decreases, the attack classification probability increases across the sites, with shorter animal distances having a stronger positive impact on attack classification probabilities at CRIM/Caltech than the classifiers annotated at other sites.
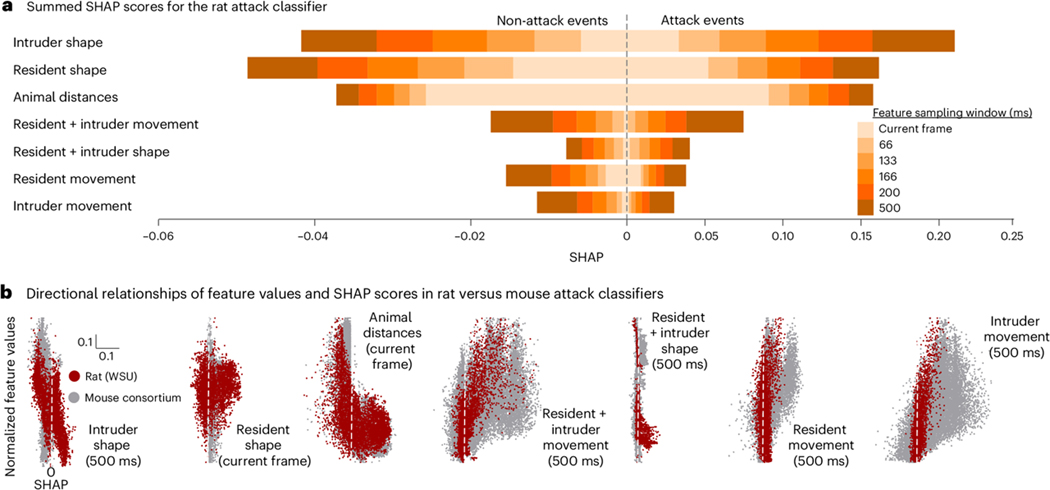
Rat versus mouse attack behavior
Next, we analyzed SHAP values for the rat resident intruder classifier and compared the values with the five classifiers generated by the mouse consortium of attack classifiers (Fig. 4A, Extended Data Fig. 6). Rat attack events were recognized primarily by the shape of the resident and the intruder, and distances between the resident and the intruder. Rat attacks differed significantly by feature category bin, but not feature sliding window size. All feature bin correlations were significant, with intruder shape being most negatively correlated with attack probability in rats and intruder movement most positively correlated in mice (Fig. 4B).
Figure 4: SHAP cross-species attack classifier data.
Explainable classification probabilities in the rat resident-intruder attack classifier using SHAP. (a) Summed SHAP values, collapsed into seven behavioral feature categories for the rat random forest attack classifier. Colors denote sliding window duration as in Figure 3. (b) Scatter plots showing the directional relationships between normalized feature values and SHAP scores in seven feature sub-categories of the rat resident intruder attack classifier. The rat attack classifier is shown in red. For comparison, the SHAP values for the mouse attack classifiers (from Figure 4), are shown in grey. Dots represent individual video frames. See Supplementary Note 1 — detailed statistics for full statistical analysis.
Automated behavioral analysis of reactive aggression by sex
Female assays for the study of aggression in mice have historically focused on maternal aggression due to the stated low propensity for these mice to display aggression during reactive aggression tasks (resident intruder or chronic social defeat stress tests) typically used for males58. Recently, studies have revisited reactive aggression in females and have found that a subset of female Swiss Webster (CFW) mice attack same-sex intruders when either virgin and singly housed59, or cohoused with a castrated male60. While CFW females do not appear to show aggression reward by standard measures including aggression conditioned place preference and aggression self-administration61, this still presents an opportunity to directly compare male and female reactive aggression behavior.
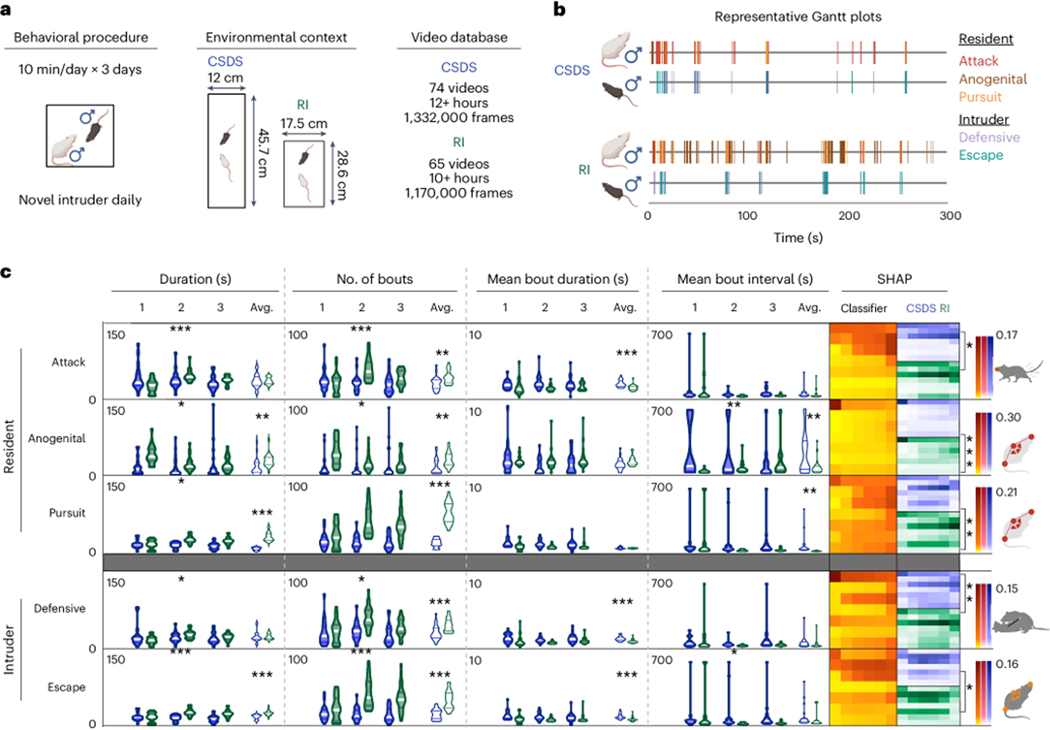
Following screening females for resident-intruder aggression, we conducted female60,61 and male62 chronic social defeat stress (CSDS) assays (see Supplemental Methods). We calculated the total duration, number of bouts, mean bout duration and interval for attack, pursuit, anogenital sniffing, escape, and defensive behaviors (SHAP values, Extended Data Fig. 7), both per day of testing and averaged across all five testing days. We analyzed attack, pursuit, and anogenital sniffing for the residents, and defensive and escape behavior for the intruders. Males and females showed significant differences in all five assayed behaviors (Fig. 5D), with females showing higher average total durations and number of bouts in attack, pursuit, and escape behaviors (p < 0.001 for all), while males had higher levels of anogenital sniffing and defensive behaviors (duration: p = 0.0461, 0.0374; bouts: p = 0.0298, 0.0146). There were no differences for any of the behaviors in mean bout interval or in attack or anogenital mean bout duration. Females showed longer behavioral bouts for pursuit, defensive, and escape behaviors (p < 0.001 for all). Only three metrics were significantly affected by day: escape duration and bouts (duration: interaction p = 0.0122, day p = 0.3700, sex p < 0.001; bouts: interaction p = 0.0415, day p = 0.3947, sex p < 0.001) and number of pursuit bouts (interaction p = 0.0404, day p = 0.1724, sex p < 0.001).
Figure 5: Social stress experience influences aggression and coping behaviors differently in males and females.
(a) Schematic representation of the mouse chronic social defeat (CSDS) behavioral protocol and the analysis pipeline for supervised machine learning behavioral classification. (b) Representative Gantt charts of classified male (top) and female (bottom) resident and intruder behaviors. (c) Key for the SHAP analysis and feature bin comparisons. (d) Supervised behavioral data and SHAP comparisons for five behavioral classifiers. We analyzed attack, pursuit, and anogenital sniffing for the residents, and defensive and escape behavior for the intruders. Male data are represented in blue, and female in pink. For each classifier, SimBA provided the total duration (s), number of classified bouts, mean bout duration (s), and mean bout interval (s) across individual testing days (n = 21 males for all classifiers, 11 female residents for attack, anogenital sniffing, pursuit and classifiers, and 10 female intruders for defensive and escape classifiers). Males and females showed significant differences in all five assayed behaviors, with females showing higher average total durations and number of bouts in attack, pursuit, and escape behaviors (p < 0.001 for all), while males had higher levels of anogenital sniffing and defensive behaviors (duration: p = 0.0461, 0.0374; bouts: p = 0.0298, 0.0146). Only three metrics were significantly affected by day: escape duration and bouts (duration: interaction p = 0.0122, day p = 0.3700, sex p < 0.001; bouts: interaction p = 0.0415, day p = 0.3947, sex p < 0.001) and number of pursuit bouts (interaction p = 0.0404, day p = 0.1724, sex p < 0.001). Average SHAP values are reported in Figure S2. The color intensity for all three SHAP datasets per classifier are on the same scale, as indicated by the scales on the right. For each behavior, comparisons with the lowest p-value per category are highlighted via a comparison bracket and the feature bin mouse icon. Asterisks denote significance levels * < 0.05, ** < 0.01, *** < 0.001. See Supplementary Note 1 — detailed statistics for full statistical analysis.
To understand how males and females performed behaviors predicted by the same supervised behavioral classes, we calculated SHAP values for 10k positive behavioral frames per behavior and sex (Fig. 5D, right). There were significant differences in SHAP values across sexes for all behaviors (Supplementary Note 1 — detailed statistics), with attack and pursuit behaviors differing most significantly in intruder shape, anogenital sniffing in intruder movement, defensive behavior in combined animal movement, and escape behavior in resident shape (Supplementary Note 1 — detailed statistics).
Automated analysis of male aggression by environment
Resident-intruder assays are typically conducted in an animal’s home cage to measure the reactive or territorial aggression of the resident animal. Frequently, these assays examine resident males across their first exposures to established subordinate intruders. Alternately, CSDS testing is typically performed in thinner subdivided hamster cages, where the “resident” animal is an established aggressor62 and the intruder is a smaller mouse that experiences up to 10 consecutive days of defeat. Directly comparing RI and CSDS datasets allows us to gain a preliminary understanding of the intersection of aggression experience and testing environment for driving social behaviors.
RI and CSDS male behavior differed significantly by day, total duration across all five behaviors, number of bouts in attack, anogenital, defensive, and escape behaviors, and in the mean bout interval between anogenital and escape events (Fig. 6C, Supplementary Note 1 — detailed statistics). RI males showed a marked decrease in anogenital sniffing duration across days (interaction p = 0.0123, day p = 0.0478, environment p < 0.0071), with concomitant increases in attack (interaction p < 0.001, day p = 0.0204, environment p = 0.9408) and pursuit behaviors (interaction p < 0.0258, day p = 0.0295, environment p < 0.001), indicating a shift from exploratory to aggressive behaviors as males gained aggression experience. SHAP comparisons of classifiers revealed differing behavioral motifs between environments. Attack differed most significantly in intruder movement, while anogenital and pursuit behaviors differed in resident shape, defensive behavior by animal distances, and escape by intruder shape (Fig. 6C, Supplementary Note 1 — detailed statistics, Extended Data Fig.8).
Figure 6: Environment and experience influence male aggression and coping behaviors.
(a) Schematic representation of the mouse chronic social defeat (CSDS) and resident intruder (RI) behavioral design. (b) Representative Gantt charts of classified CSDS (top) and RI (bottom) resident and intruder behaviors. (c) Supervised behavioral data and SHAP comparisons for five behavioral classifiers. CSDS data are represented in blue, while RI data are shown in green. For each classifier, SimBA provided the total duration (s), number of bouts, mean bout duration (s), and mean bout interval (s) across individual testing days (n = 21 CSDS, 24 RI). RI males showed a marked decrease in anogenital sniffing duration across days (interaction p = 0.0123, day p = 0.0478, environment p < 0.0071), with concomitant increases in attack (interaction p < 0.001, day p = 0.0204, environment p = 0.9408) and pursuit behaviors (interaction p < 0.0258, day p = 0.0295, environment p < 0.001). The color intensity for all three SHAP datasets per classifier are on the same scale, as indicated by the scales on the right. For each behavior, comparisons with the lowest p-value per category are highlighted via a comparison bracket and the feature bin mouse icon. Asterisks denote significance levels * < 0.05, ** < 0.01, *** < 0.001. See Supplementary Note 1 — detailed statistics for full statistical analysis.
Discussion
There is a vibrant and growing ecosystem of computational behavioral tools designed specifically for the behavioral neuroscience community, that have directly impacted scientific directions and methods63 (see Table 1 for examples). SimBA has been heavily influenced by many of these packages9,10,46,64, and now influences others39,65,66. Since its release, numerous independent labs have used SimBA to address varied scientific questions across diverse model systems, providing critical feedback on SimBA and its ongoing development. The key feedback has consistently focused on introducing easily accessible explainability metrics that are useful to non-specialized users and allow for generalizable use of classifiers and compatibility of disparate datasets.
Here, we used SimBA to analyze data including male and female chronic social defeat stress (CSDS) behavior, and male CSDS and resident intruder (RI) behaviors and describe the use of explainability metrics. Between sexes we found that male and female intruders differ significantly in their coping strategies, with females defending themselves less than males. These differences may partially explain differences in resilience to social stress between males and females. Between CSDS and RI assays, which are performed in differently sized arenas, we found differences in resident aggressive and intruder coping behaviors between males. This demonstrates clear cross-protocol behavioral differences and emphasizes a need for standardization and quantification of experimental variation within social behavior research in general and aggression research specifically, and we provide a tool to do this.
We demonstrate the utility of SHAP values for uncovering differences within supervised machine learning scenarios. We quantify significant differences in features and time bins defining female and male aggression as well as aggressive behaviors across distinct testing environments. For example, SHAP values revealed and quantified potential cross-lab classifier confounds, and aided comparisons between male and female attack events.
Although strong arguments remain for using inherently explainable models in machine learning57, many maturing behavioral neuroscience tools still rely on black-box methods. The post-hoc nature of SHAP allows independent labs an algorithmic freedom while maintaining options for cross-model explainability comparisons through game theory and the additivity axiom. A significant drawback of SHAP, however, is the computational and runtime cost associated with analyzing larger datasets. SimBA depends on the TreeSHAP67 algorithm to calculate SHAP values. A challenge when calculating SHAP values through TreeSHAP is the computational complexity and run-times that scale polynomially with the number of estimators and features, and can interfere with the analyses of larger datasets68. To negate this, SimBA includes multiprocessing options that allows users to linearly reduce their run-times with the count of their available CPU-cores. SHAP also represents a post-hoc approach when inherently explainable models (e.g., using a few carefully selected relevant features joined with low algorithm complexity) may suffice for low-complexity behaviors17. Furthermore, as a correlative approach, users should avoid interpreting causal relationships between SHAP-inferred feature importance’s and classification probabilities. Ongoing efforts in SimBA involve parallelization and just-in-time compilation making the full catalogue of methods available on standard behavioral neuroscience hardware at the maximum speeds allowed by the machine. Notably, the SimBA platform has additional in-built access to other prevalent model interpretability methods (e.g., partial dependencies, permutation importance’s and gini/entropy-based measures) when needed.
Manual behavioral analyses typically depend on nondescript qualitative operational definitions, posing challenges for standardization across individuals and laboratories, and potentially contributing to issues with reproducibility downstream. In contrast, as demonstrated here, the incorporation of ML-based behavioral analysis coupled with explainability metrics produce comprehensive quantitative operational definitions of behavior. This allows us to re-conceptualize behavioral analysis though precise and verbalizable statistical component rules that are applied when scoring behaviors of interest. These quantified definitions can be shared as resources, akin to RRID-like reagents, enhancing transparency and reportability and facilitating cross-study comparisons and reproducibility.
As a supervised learning tool, a weakness of our approach is the cost of collecting the human behavioral annotations required for fitting reliable downstream models. Other prominent platforms may circumvent such costs through active learning, task-programming, or semi-supervised statistical techniques69–72. Despite our publicly available classifiers and annotations, users will typically have to add a few short, varied, and representative annotations to their classifier training sets due to the inherent variation in individual laboratory experimental setups. However, the behavioral neuroscience community has an exceptionally rich historical bounty of carefully documented animal behaviors in video recordings. To lessen user burden, SimBA includes several tools to accommodate use of such historical annotations for supervised machine learning purposes. Further parallel initiatives, including MABe73,74, the OpenBehavior project75, and Jackson Laboratory Mouse Phenome Database76, may also address these limitations through the distribution of larger repositories encompassing diverse video recordings with associated human annotations.
A further challenge for the field is appreciating how to accurately judge the performance of machine learning models, which can be biased towards subsets of animals and recording environment and that depend on time-series data that is vulnerable to leakage77. Maintaining true independence across training and hold-out sets is a standing challenge within machine learning which can be particularly difficult in behavioral neuroscience use-cases where insufficient amounts of fully independent annotations are available. Fundamentally, although low performance metrics are indicative of an inadequate classifier, high metrics may not be sufficiently indicative of reliable and generalizable out-of-sample performance. We recommend that users include smaller held-out and hand-scored representative validation sets for validation purposes.
In conclusion, SimBA provides a user-centric, modular, and accessible platform for machine learning analysis of behavior with the primary aim of promoting an understanding of the behavioral nuances that we value as neuroethologists. Here, we demonstrate the utility of SimBA as a behavioral analysis tool by consolidating cross-site behavioral datasets with machine learning methods to quantify and describe complex behaviors across experimental contexts.
Extended Data
Extended Data Table 1.
A list of open-source programs for machine-learning based automated behavioral detection.
Extended Data Figure 1.
These data were calculated from 12,686 images from n=101 mice. (a) The 16 body-parts labeled. (b) Schematic depiction of the location of each of the 16 body part labels. (c) Evaluations of three models (rgb, clahe, greyscale) using the DeepLabCut evaluation tool. Pixel distances were converted to millimeter by using the lowest resolution images in the dataset (1000×1544px; 4.6px/millimeter). (d) Median millimeter error per body part. (e) Image representing the relative standard error (RSE) of the median millimeter error across all test images. The labelled images and DeepLabCut generated weights are available to download on the Open Science Framework, osf.io/mutws. (f) SimBA supports a range of alternative body-part settings for single animals and dyadic protocols through the File-> Create Project menu. Note: tail end tracking performance was insufficient for a tail rattle classifier, and the tail end body parts were dropped for all analysis in the main figures. Data are presented as mean +/− SEM.
Extended Data Figure 2.
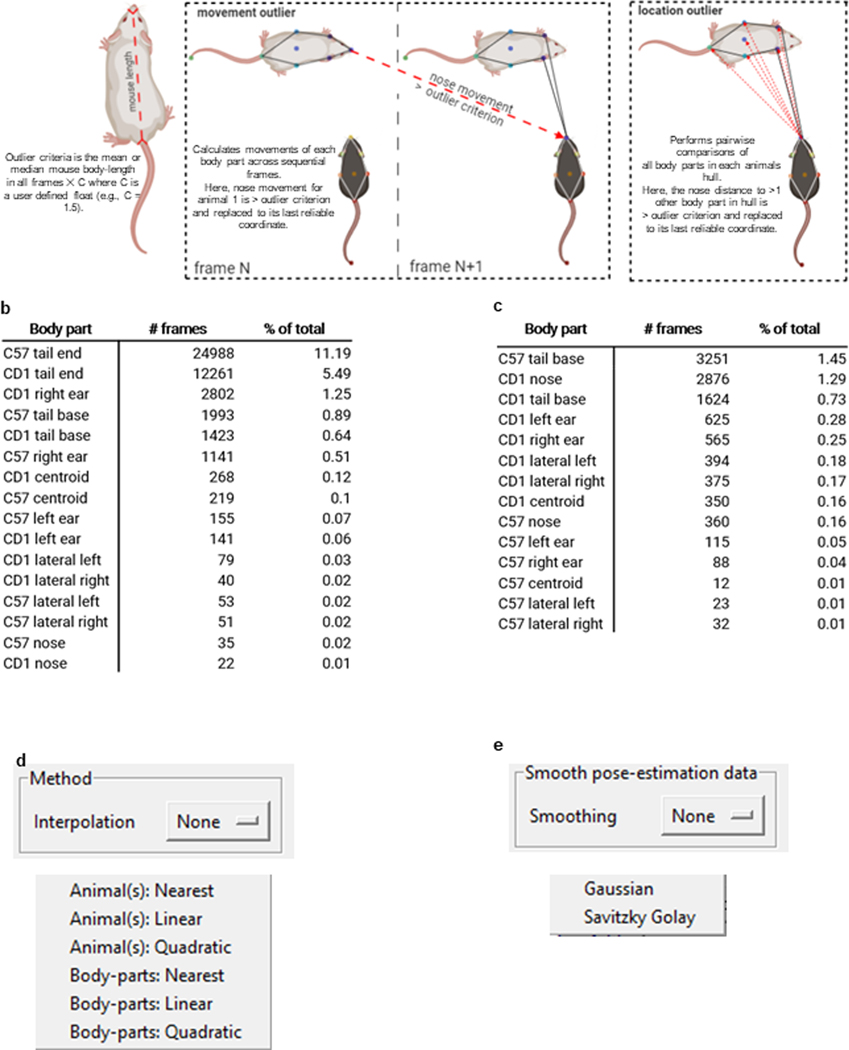
(a) SimBA calculates the mean or median distance between two user-defined body-parts across the frames of each video. We set the user-defined body-parts to be the nose and the tail-base of each animal. The user also defines a movement criterion value, and a location criterion value. We set the movement criterion to 0.7, and location criterion to 1.5. Two different outlier criteria are then calculated by SimBA. These criteria are the mean length between the two user-defined body parts in all frames of the video, multiplied by the either user-defined movement criterion value or location criterion value. SimBA corrects movement outliers prior to correcting location outliers. (b) Schematic representations of a pose-estimation body-part ‘movement outlier’ (top) and a ‘location outlier’ (bottom). A body-part violates the movement criterion when the movement of the body-part across sequential frames is greater than the movement outlier criterion. A body-part violates the location criteria when its distance to more than one other body-part in the animals’ hull (except the tail-end) is greater than the location outlier criterion. Any body part that violates either the movement or location criterion is corrected by placing the body-part at its last reliable coordinate. (c) The ratio of body-part movements (top) and body-part locations (bottom) detected as outliers and corrected by SimBA in the RGB-format mouse resident-intruder data-set. For the outlier corrected in rat and the CRIM13 datasets, see the SimBA GitHub repository. We also offer (d) interpolation options for frames with missing body parts and (3) smoothing options to reduce frame-to-frame jitter.
Extended Data Figure 3.
Training set information for mouse, rat, and CRIM13 mouse resident intruder behavioral classifiers.
Extended Data Figure 4.
Classifiers for the same behavior using different pose estimation schemes will have different feature lists, but can be directly compared via feature binning through the SHAP additivity axiom.
Extended Data Figure 5.
UW and Stanford manual scoring of the same dataset for attack behavior. (a) Manual annotations (n=9 videos) were highly correlated (R2 = 0.998). (b) Gantt plot of UW versus Stanford scores for a high-attack video. (c) SHAP scores for UW positive or Stanford positive attack frames. UW scores rely more on longer rolling windows of behavior than Stanford does.
Extended Data Figure 6.
SHAP values across feature bins and rolling windows for rat attack classifier.
Extended Data Figure 7.
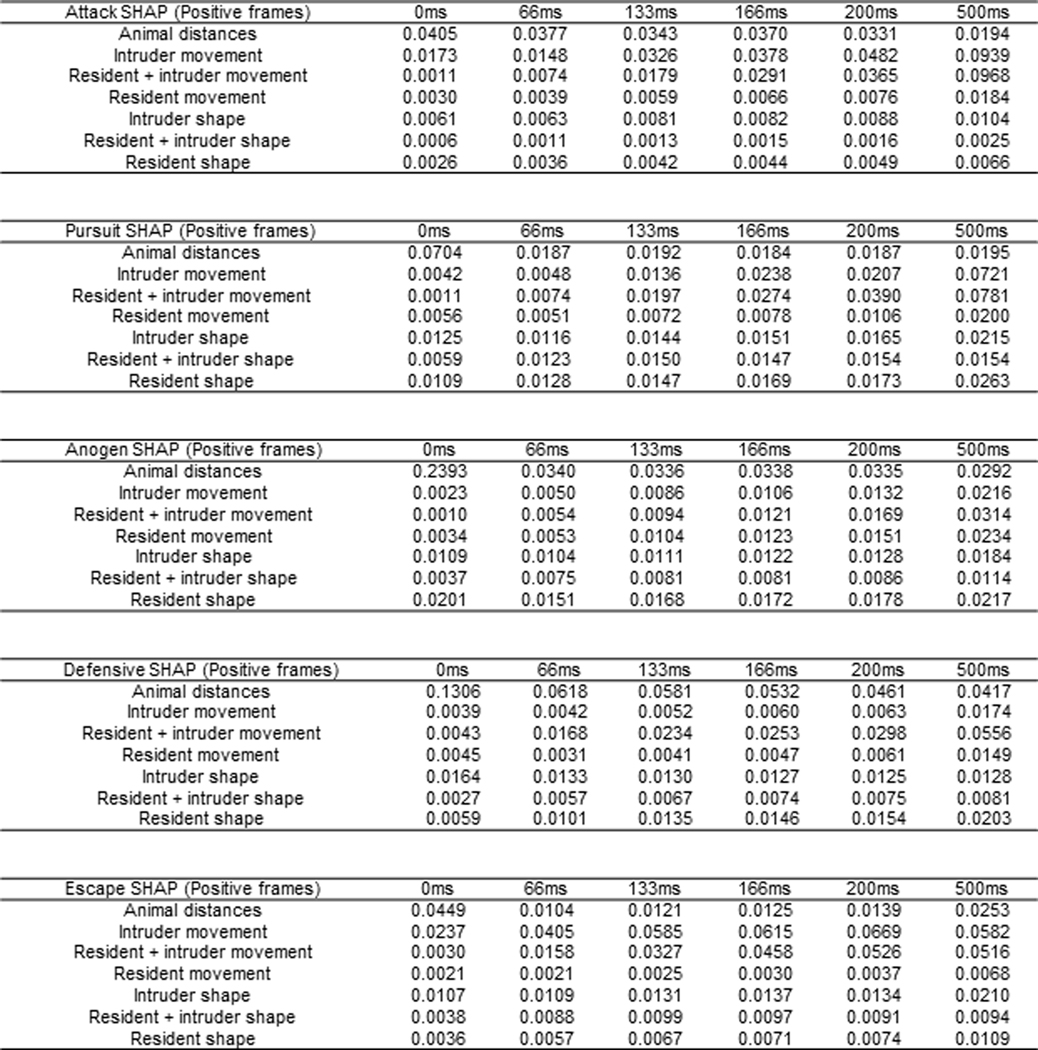
SHAP values for attack, pursuit, anogenital sniffing, defensive, and escape behavioral classifiers used in figures 5–6.
Extended Data Figure 8.
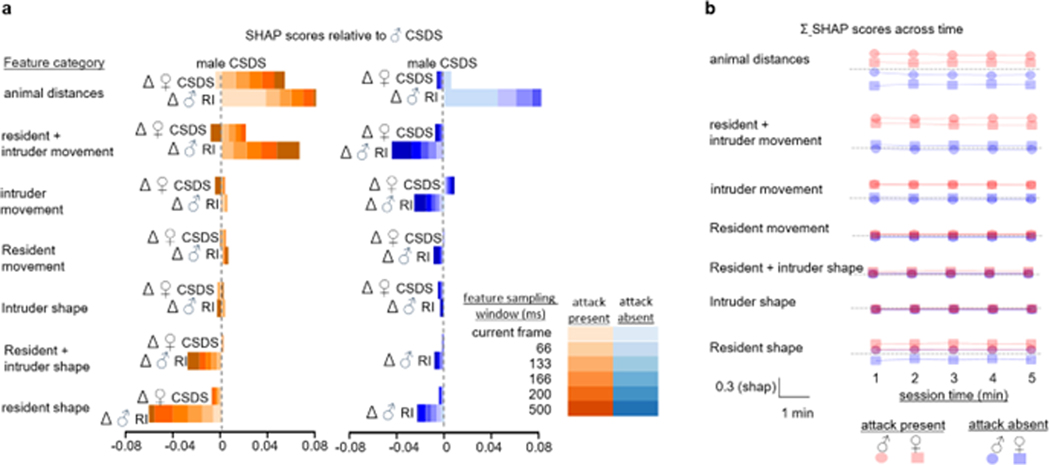
We calculated SHAP values for 1250 attack frames and 1250 non-attack frames within each experimental protocol. (a) We used these values to calculate delta shap values, where we evaluated the female CSDS and male RI SHAP values against male CSDS SHAP value baseline. The SHAP analyses revealed large similarities in how feature values affected attack classification probabilities in the three experiments (all feature sub-category delta shap < 0.044). The most notable experiment difference was the importance of animal distance features within the current frame, which was associated with higher attack classification probabilities in the RI experiment than in the male CSDS experiment. Attack classification probabilities in the RI experiments were also less affected by features of the resident shape than in the males CSDS experiment. These differences may relate to the different attack strategies and experimental setup used in the experimental protocols. (b) Next, we analyzed SHAP vales for classifying attack and non-attack events in the male and female CSDS experiments within 1min bins and showed that SHAP values are not affected by time of session.
Supplementary Material
Acknowledgements:
The research was supported by NIDA R00DA045662 (SAG), NIDA R01DA059374 (SAG), NIDA P30DA048736 (MH & SAG), NARSAD Young Investigator Award 27082 (SAG), NIMH 1F31MH125587 (NLG), F31AA025827 (ELN), F32MH125634 (ELN), NIGMS R35GM146751 (MH), National Institutes of Health grant K08MH123791 (NE), Burroughs Wellcome Fund Career Award for Medical Scientists (NE), Simons Foundation Bridge to Independence Award (NE), and the Washington Research Foundation Postdoctoral Fellowship (ERS). We thank Valerie Tsai, Roël Vrooman, and Cindy Xu for their skillful technical contributions. We thank Brandon Bentzley, and Dayu Lin for contributing to aggression consortium data. SRON has continued development and maintenance of SimBA independent of other funding sources. Figures were created with Biorender.com.
Footnotes
Competing Interests Statement:
The authors have no competing interests.
Data availability statement
The data used in this manuscript is available as a branch of the SimBA github repository. https://github.com/sgoldenlab/simba/tree/simba-data/data
The CRIM13 database is publicly available at https://paperswithcode.com/dataset/crim13.
Code availability statement
Code and documentation are available at https://github.com/sgoldenlab/simba. SimBA was built with Python version 3.6.
References47,64,72,78–94
- 1.Krakauer JW, Ghazanfar AA, Gomez-Marin A, MacIver MA, and Poeppel D. (2017). Neuroscience Needs Behavior: Correcting a Reductionist Bias. Neuron 93, 480–490. 10.1016/j.neuron.2016.12.041. [DOI] [PubMed] [Google Scholar]
- 2.Anderson DJ, and Perona P. (2014). Toward a Science of Computational Ethology. Neuron 84, 18–31. 10.1016/j.neuron.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 3.Egnor SER, and Branson K. (2016). Computational Analysis of Behavior. Annu. Rev. Neurosci. 39, 217–236. 10.1146/annurev-neuro-070815-013845. [DOI] [PubMed] [Google Scholar]
- 4.Datta SR, Anderson DJ, Branson K, Perona P, and Leifer A. (2019). Computational Neuroethology: A Call to Action. Neuron 104, 11–24. 10.1016/j.neuron.2019.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Falkner AL, Grosenick L, Davidson TJ, Deisseroth K, and Lin D. (2016). Hypothalamic control of male aggression-seeking behavior. Nat Neurosci 19, 596–604. 10.1038/nn.4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferenczi EA, Zalocusky KA, Liston C, Grosenick L, Warden MR, Amatya D, Katovich K, Mehta H, Patenaude B, Ramakrishnan C, et al. (2016). Prefrontal cortical regulation of brainwide circuit dynamics and reward-related behavior. Science 351, aac9698–aac9698. 10.1126/science.aac9698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim Y, Venkataraju KU, Pradhan K, Mende C, Taranda J, Turaga SC, Arganda-Carreras I, Ng L, Hawrylycz MJ, Rockland KS, et al. (2015). Mapping Social Behavior-Induced Brain Activation at Cellular Resolution in the Mouse. Cell Reports 10, 292–305. 10.1016/j.celrep.2014.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gunaydin LA, Grosenick L, Finkelstein JC, Kauvar IV, Fenno LE, Adhikari A, Lammel S, Mirzabekov JJ, Airan RD, Zalocusky KA, et al. (2014). Natural Neural Projection Dynamics Underlying Social Behavior. Cell 157, 1535–1551. 10.1016/j.cell.2014.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Graving JM, Chae D, Naik H, Li L, Costelloe BR, and Couzin ID. (2019). DeepPoseKit, a software toolkit for fast and robust animal pose estimation using deep learning. eLife, 8:e47994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mathis A, Mamidanna P, Cury KM, Abe T, Murthy VN, Mathis MW, and Bethge M. (2018). DeepLabCut: markerless pose estimation of user-defined body parts with deep learning. Nat Neurosci 21, 1281–1289. 10.1038/s41593-018-0209-y. [DOI] [PubMed] [Google Scholar]
- 11.Pereira TD, Aldarondo DE, Willmore L, Kislin M, Wang SS-H, Murthy M, and Shaevitz JW. (2019). Fast animal pose estimation using deep neural networks. Nat Methods 16, 117–125. 10.1038/s41592-018-0234-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geuther BQ, Deats SP, Fox KJ, Murray SA, Braun RE, White JK, Chesler EJ, Lutz CM, and Kumar V. (2019). Robust mouse tracking in complex environments using neural networks. Communications Biology 2, 1–11. 10.1038/s42003-019-0362-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gris KV, Coutu J-P, and Gris D. (2017). Supervised and Unsupervised Learning Technology in the Study of Rodent Behavior. Front Behav Neurosci 11. 10.3389/fnbeh.2017.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schaefer AT, and Claridge-Chang A. (2012). The surveillance state of behavioral automation. Current Opinion in Neurobiology 22, 170–176. 10.1016/j.conb.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robie AA, Seagraves KM, Egnor SER, and Branson K. (2017). Machine vision methods for analyzing social interactions. J Exp Biol 220, 25–34. 10.1242/jeb.142281. [DOI] [PubMed] [Google Scholar]
- 16.Vu M-AT, Adalı T, Ba D, Buzsáki G, Carlson D, Heller K, Liston C, Rudin C, Sohal VS, Widge AS, et al. (2018). A Shared Vision for Machine Learning in Neuroscience. J. Neurosci. 38, 1601–1607. 10.1523/JNEUROSCI.0508-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goodwin NL, Nilsson SRO, Choong JJ, and Golden SA. (2022). Toward the explainability, transparency, and universality of machine learning for behavioral classification in neuroscience. Current Opinion in Neurobiology 73, 102544. 10.1016/j.conb.2022.102544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Newton KC, Kacev D, Nilsson SRO, Golden SA, and Sheets L. (2021). Lateral Line Ablation by Toxins Results in Distinct Rheotaxis Profiles in Fish. bioRxiv, 2021.11.15.468723. 10.1101/2021.11.15.468723. [DOI]
- 19.Jernigan CM, Stafstrom JA, Zaba NC, Vogt CC, and Sheehan MJ. (2022). Color is necessary for face discrimination in the Northern paper wasp, Polistes fuscatus. Anim Cogn. 10.1007/s10071-022-01691-9. [DOI] [PMC free article] [PubMed]
- 20.Dahake A, Jain P, Vogt C, Kandalaft W, Stroock A, and Raguso RA. (2022). Floral humidity as a signal – not a cue – in a nocturnal pollination system. bioRxiv, 2022.04.27.489805. 10.1101/2022.04.27.489805. [DOI] [PMC free article] [PubMed]
- 21.Dawson M, Terstege DJ, Jamani N, Pavlov D, Tsutsui M, Bugescu R, Epp JR, Leinninger GM, and Sargin D. (2022). Sex-dependent role of hypocretin/orexin neurons in social behavior. bioRxiv, 2022.08.19.504565. 10.1101/2022.08.19.504565. [DOI]
- 22.Baleisyte A, Schneggenburger R, and Kochubey O. (2022). Stimulation of medial amygdala GABA neurons with kinetically different channelrhodopsins yields opposite behavioral outcomes. Cell Reports 39, 110850. 10.1016/j.celrep.2022.110850. [DOI] [PubMed] [Google Scholar]
- 23.Cruz-Pereira JS, Moloney GM, Bastiaanssen TFS, Boscaini S, Tofani G, Borras-Bisa J, van de Wouw M, Fitzgerald P, Dinan TG, Clarke G, et al. (2022). Prebiotic supplementation modulates selective effects of stress on behavior and brain metabolome in aged mice. Neurobiology of Stress 21, 100501. 10.1016/j.ynstr.2022.100501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Linders LE, Patrikiou L, Soiza-Reilly M, Schut EHS, van Schaffelaar BF, Böger L, Wolterink-Donselaar IG, Luijendijk MCM, Adan RAH, and Meye FJ. (2022). Stress-driven potentiation of lateral hypothalamic synapses onto ventral tegmental area dopamine neurons causes increased consumption of palatable food. Nat Commun 13, 6898. 10.1038/s41467-022-34625-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Slivicki RA, Earnest T, Chang Y-H, Pareta R, Casey E, Li J-N, Tooley J, Abiraman K, Vachez YM, Wolf DK, et al. (2023). Oral oxycodone self-administration leads to features of opioid misuse in male and female mice. Addiction Biology 28, e13253. 10.1111/adb.13253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miczek KA, Akdilek N, Ferreira VMM, Kenneally E, Leonard MZ, and Covington HE. (2022). Excessive alcohol consumption after exposure to two types of chronic social stress: intermittent episodes vs. continuous exposure in C57BL/6J mice with a history of drinking. Psychopharmacology. 10.1007/s00213-022-06211-8. [DOI] [PubMed]
- 27.Cui Q, Du X, Chang IYM, Pamukcu A, Lilascharoen V, Berceau BL, García D, Hong D, Chon U, Narayanan A, et al. (2021). Striatal Direct Pathway Targets Npas1+ Pallidal Neurons. J. Neurosci. 41, 3966–3987. 10.1523/JNEUROSCI.2306-20.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen J, Lambo ME, Ge X, Dearborn JT, Liu Y, McCullough KB, Swift RG, Tabachnick DR, Tian L, Noguchi K, et al. (2021). A MYT1L syndrome mouse model recapitulates patient phenotypes and reveals altered brain development due to disrupted neuronal maturation. Neuron 109, 3775–3792.e14. 10.1016/j.neuron.2021.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rigney N, Zbib A, de Vries GJ, and Petrulis A. (2021). Knockdown of sexually differentiated vasopressin expression in the bed nucleus of the stria terminalis reduces social and sexual behaviour in male, but not female, mice. Journal of Neuroendocrinology n/a, e13083. 10.1111/jne.13083. [DOI] [PMC free article] [PubMed]
- 30.Winters C, Gorssen W, Ossorio-Salazar VA, Nilsson S, Golden S, and D’Hooge R. (2022). Automated procedure to assess pup retrieval in laboratory mice. Sci Rep 12, 1663. 10.1038/s41598-022-05641-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neira S, Hassanein LA, Stanhope CM, Buccini MC, D’Ambrosio SL, Flanigan ME, Haun HL, Boyt KM, Bains JS, and Kash TL. (2022). Chronic Alcohol Consumption Alters Home-Cage Behaviors and Responses to Ethologically Relevant Predator Tasks in Mice. bioRxiv. 10.1101/2022.02.04.479122. [DOI] [PMC free article] [PubMed]
- 32.Kwiatkowski CC, Akaeze H, Ndlebe I, Goodwin N, Eagle AL, Moon K, Bender AR, Golden SA, and Robison AJ. (2021). Quantitative standardization of resident mouse behavior for studies of aggression and social defeat. Neuropsychopharmacology, 1–10. 10.1038/s41386-021-01018-1. [DOI] [PMC free article] [PubMed]
- 33.Yamaguchi T, Wei D, Song SC, Lim B, Tritsch NX, and Lin D. (2020). Posterior amygdala regulates sexual and aggressive behaviors in male mice. Nat Neurosci. 10.1038/s41593-020-0675-x. [DOI] [PMC free article] [PubMed]
- 34.Nygaard KR, Maloney SE, Swift RG, McCullough KB, Wagner RE, Fass SB, Garbett K, Mirnics K, Veenstra-VanderWeele J, and Dougherty JD. (2023). Extensive characterization of a Williams Syndrome murine model shows Gtf2ird1 -mediated rescue of select sensorimotor tasks, but no effect on enhanced social behavior. Preprint at Animal Behavior and Cognition, 10.1101/2023.01.18.523029 10.1101/2023.01.18.523029. [DOI] [PMC free article] [PubMed]
- 35.Ojanen S, Kuznetsova T, Kharybina Z, Voikar V, Lauri SE, and Taira T. (2023). Interneuronal GluK1 kainate receptors control maturation of GABAergic transmission and network synchrony in the hippocampus. Molecular Brain 16, 43. 10.1186/s13041-023-01035-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hon OJ, DiBerto JF, Mazzone CM, Sugam J, Bloodgood DW, Hardaway JA, Husain M, Kendra A, McCall NM, Lopez AJ, et al. (2022). Serotonin modulates an inhibitory input to the central amygdala from the ventral periaqueductal gray. Neuropsychopharmacol. 47, 2194–2204. 10.1038/s41386-022-01392-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murphy CA, Chang Y-H, Pareta R, Li J-N, Slivicki RA, Earnest T, Tooley J, Abiraman K, Vachez YM, Gereau RW, et al. (2021). Modeling features of addiction with an oral oxycodone self-administration paradigm (Neuroscience) 10.1101/2021.02.08.430180. [DOI]
- 38.Neira S, Lee S, Hassanein LA, Sides T, D’Ambrosio SL, Boyt KM, Bains JS, and Kash TL. (2023). Impact and role of hypothalamic corticotropin releasing hormone neurons in withdrawal from chronic alcohol consumption in female and male mice. Preprint at bioRxiv, 10.1101/2023.05.30.542746 10.1101/2023.05.30.542746. [DOI] [PMC free article] [PubMed]
- 39.Lapp HE, Salazar MG, and Champagne FA. (2023). Automated Maternal Behavior during Early life in Rodents (AMBER) pipeline. Preprint at bioRxiv, 10.1101/2023.09.15.557946 10.1101/2023.09.15.557946. [DOI] [PMC free article] [PubMed]
- 40.Barnard IL, Onofrychuk TJ, Toderash AD, Patel VN, Glass AE, Adrian JC, Laprairie RB, and Howland JG. (2023). High-THC Cannabis smoke impairs working memory capacity in spontaneous tests of novelty preference for objects and odors in rats. Preprint at bioRxiv, 10.1101/2023.04.06.535880 10.1101/2023.04.06.535880. [DOI] [PMC free article] [PubMed]
- 41.Ausra J, Munger SJ, Azami A, Burton A, Peralta R, Miller JE, and Gutruf P. (2021). Wireless battery free fully implantable multimodal recording and neuromodulation tools for songbirds. Nat Commun 12, 1968. 10.1038/s41467-021-22138-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Friard O, and Gamba M. (2016). BORIS: a free, versatile open-source event-logging software for video/audio coding and live observations. Methods in Ecology and Evolution 7, 1325–1330. 10.1111/2041-210X.12584. [DOI] [Google Scholar]
- 43.Spink AJ, Tegelenbosch RAJ, Buma MOS, and Noldus LPJJ. (2001). The EthoVision video tracking system—A tool for behavioral phenotyping of transgenic mice. Physiology & Behavior 73, 731–744. 10.1016/S0031-9384(01)00530-3. [DOI] [PubMed] [Google Scholar]
- 44.Lundberg S. (2022). slundberg/shap.
- 45.Lauer J, Zhou M, Ye S, Menegas W, Nath T, Rahman MM, Di Santo V, Soberanes D, Feng G, Murthy VN, et al. (2021). Multi-animal pose estimation and tracking with DeepLabCut (Animal Behavior and Cognition) 10.1101/2021.04.30.442096. [DOI] [PMC free article] [PubMed]
- 46.Pereira TD, Tabris N, Matsliah A, Turner DM, Li J, Ravindranath S, Papadoyannis ES, Normand E, Deutsch DS, Wang ZY, et al. (2022). SLEAP: A deep learning system for multi-animal pose tracking. Nat Methods, 1–10. 10.1038/s41592-022-01426-1. [DOI] [PMC free article] [PubMed]
- 47.Segalin C, Williams J, Karigo T, Hui M, Zelikowsky M, Sun JJ, Perona P, Anderson DJ, and Kennedy A. (2021). The Mouse Action Recognition System (MARS) software pipeline for automated analysis of social behaviors in mice. eLife 10, e63720. 10.7554/eLife.63720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Breiman L. (2001). Random Forests. Machine Learning 45, 5–32. 10.1023/A:1010933404324. [DOI] [Google Scholar]
- 49.Liaw A, and Wiener M. (2002). Classification and Regression by randomForest. R news 2, 6. [Google Scholar]
- 50.Goodwin NL, Nilsson SRO, and Golden SA. (2020). Rage Against the Machine: Advancing the study of aggression ethology via machine learning. Psychopharmacology 237, 2569–2588. 10.1007/s00213-020-05577-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lundberg SM, Erion G, Chen H, DeGrave A, Prutkin JM, Nair B, Katz R, Himmelfarb J, Bansal N, and Lee S-I. (2020). From Local Explanations to Global Understanding with Explainable AI for Trees. Nat Mach Intell 2, 56–67. 10.1038/s42256-019-0138-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ribeiro MT, Singh S, and Guestrin C. (2016). “Why Should I Trust You?”: Explaining the Predictions of Any Classifier. Preprint at arXiv, 10.48550/arXiv.1602.04938 10.48550/arXiv.1602.04938. [DOI]
- 53.Sundararajan M, Taly A, and Yan Q. (2017). Axiomatic Attribution for Deep Networks. In Proceedings of the 34th International Conference on Machine Learning (PMLR), pp. 3319–3328. [Google Scholar]
- 54.Hatwell J, Gaber MM, and Azad RMA. (2020). CHIRPS: Explaining random forest classification. Artif Intell Rev 53, 5747–5788. 10.1007/s10462-020-09833-6. [DOI] [Google Scholar]
- 55.Lundberg S, and Lee S-I. (2017). A Unified Approach to Interpreting Model Predictions. arXiv:1705.07874 [cs, stat].
- 56.Verma S, Dickerson J, and Hines K. (2020). Counterfactual Explanations for Machine Learning: A Review. arXiv:2010.10596 [cs, stat].
- 57.Rudin C. (2019). Stop explaining black box machine learning models for high stakes decisions and use interpretable models instead. Nat Mach Intell 1, 206–215. 10.1038/s42256-019-0048-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Takahashi A, Chung J-R, Zhang S, Zhang H, Grossman Y, Aleyasin H, Flanigan ME, Pfau ML, Menard C, Dumitriu D, et al. (2017). Establishment of a repeated social defeat stress model in female mice. Sci Rep 7, 12838. 10.1038/s41598-017-12811-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hashikawa K, Hashikawa Y, Tremblay R, Zhang J, Feng JE, Sabol A, Piper WT, Lee H, Rudy B, and Lin D. (2017). Esr1+ cells in the ventromedial hypothalamus control female aggression. Nat. Neurosci. 20, 1580–1590. 10.1038/nn.4644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Newman EL, Covington HE, Suh J, Bicakci MB, Ressler KJ, DeBold JF, and Miczek KA. (2019). Fighting Females: Neural and Behavioral Consequences of Social Defeat Stress in Female Mice. Biological Psychiatry 86, 657–668. 10.1016/j.biopsych.2019.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aubry AV, Joseph Burnett C, Goodwin NL, Li L, Navarrete J, Zhang Y, Tsai V, Durand-de Cuttoli R, Golden SA, and Russo SJ. (2022). Sex differences in appetitive and reactive aggression. Neuropsychopharmacol. 10.1038/s41386-022-01375-5. [DOI] [PMC free article] [PubMed]
- 62.Golden SA, Covington HE, Berton O, and Russo SJ. (2011). A standardized protocol for repeated social defeat stress in mice. Nat Protoc 6, 1183–1191. 10.1038/nprot.2011.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shemesh Y, and Chen A. (2023). A paradigm shift in translational psychiatry through rodent neuroethology. Mol Psychiatry 28, 993–1003. 10.1038/s41380-022-01913-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kabra M, Robie AA, Rivera-Alba M, Branson S, and Branson K. (2013). JAABA: interactive machine learning for automatic annotation of animal behavior. Nat Methods 10, 64–67. 10.1038/nmeth.2281. [DOI] [PubMed] [Google Scholar]
- 65.Bordes J, Miranda L, Reinhardt M, Brix LM, Doeselaar L van Engelhardt C, Pütz B, Agakov F, Müller-Myhsok B, and Schmidt MV. (2022). Automatically annotated motion tracking identifies a distinct social behavioral profile following chronic social defeat stress. Preprint at bioRxiv, 10.1101/2022.06.23.497350 10.1101/2022.06.23.497350. [DOI] [PMC free article] [PubMed]
- 66.Winters C, Gorssen W, Wöhr M, and D’Hooge R. (2023). BAMBI: A new method for automated assessment of bidirectional early-life interaction between maternal behavior and pup vocalization in mouse dam-pup dyads. Frontiers in Behavioral Neuroscience 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lundberg SM, Erion GG, and Lee S-I. (2019). Consistent Individualized Feature Attribution for Tree Ensembles. arXiv:1802.03888 [cs, stat].
- 68.Covert IC, Lundberg S, and Lee S-I. (2021). Explaining by Removing: A Unified Framework for Model Explanation. Journal of Machine Learning Research, 90. [Google Scholar]
- 69.Lorbach M, Poppe R, and Veltkamp RC. (2019). Interactive rodent behavior annotation in video using active learning. Multimed Tools Appl 78, 19787–19806. 10.1007/s11042-019-7169-4. [DOI] [Google Scholar]
- 70.Schweihoff JF, Hsu AI, Schwarz MK, and Yttri EA. (2022). A-SOiD, an active learning platform for expert-guided, data efficient discovery of behavior. Preprint at bioRxiv, 10.1101/2022.11.04.515138 10.1101/2022.11.04.515138. [DOI] [PubMed]
- 71.Whiteway MR, Biderman D, Friedman Y, Dipoppa M, Buchanan EK, Wu A, Zhou J, Bonacchi N, Miska NJ, Noel J-P, et al. (2021). Partitioning variability in animal behavioral videos using semi-supervised variational autoencoders. PLOS Computational Biology 17, e1009439. 10.1371/journal.pcbi.1009439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sun JJ, Kennedy A, Zhan E, Anderson DJ, Yue Y, and Perona P. (2021). Task Programming: Learning Data Efficient Behavior Representations. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.MABe. 2022. https://sites.google.com/view/mabe22/home.
- 74.Sun JJ, Karigo T, Chakraborty D, Mohanty SP, Wild B, Sun Q, Chen C, Anderson DJ, Perona P, Yue Y, et al. (2021). The Multi-Agent Behavior Dataset: Mouse Dyadic Social Interactions. Preprint at arXiv. [PMC free article] [PubMed]
- 75.About OpenBehavior (2016). OpenBehavior. https://edspace.american.edu/openbehavior/.
- 76.MPD: About the Mouse Phenome Database. https://phenome.jax.org/about.
- 77.Kapoor S, and Narayanan A. (2022). Leakage and the Reproducibility Crisis in ML-based Science. Preprint at arXiv, 10.48550/arXiv.2207.07048 10.48550/arXiv.2207.07048. [DOI]
Methods only references:
- 78.Dankert H, Wang L, Hoopfer ED, Anderson DJ, and Perona P. (2009). Automated monitoring and analysis of social behavior in Drosophila. Nat Methods 6, 297–303. 10.1038/nmeth.1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.de Chaumont F, Ey E, Torquet N, Lagache T, Dallongeville S, Imbert A, Legou T, Le Sourd A-M, Faure P, Bourgeron T, et al. (2019). Real-time analysis of the behaviour of groups of mice via a depth-sensing camera and machine learning. Nat Biomed Eng. 10.1038/s41551-019-0396-1. [DOI] [PubMed]
- 80.Giancardo L, Sona D, Huang H, Sannino S, Managò F, Scheggia D, Papaleo F, and Murino V. (2013). Automatic Visual Tracking and Social Behaviour Analysis with Multiple Mice. PLOS ONE 8, e74557. 10.1371/journal.pone.0074557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hong W, Kennedy A, Burgos-Artizzu XP, Zelikowsky M, Navonne SG, Perona P, and Anderson DJ. (2015). Automated measurement of mouse social behaviors using depth sensing, video tracking, and machine learning. Proc Natl Acad Sci USA 112, E5351–E5360. 10.1073/pnas.1515982112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bohnslav JP, Wimalasena NK, Clausing KJ, Dai YY, Yarmolinsky DA, Cruz T, Kashlan AD, Chiappe ME, Orefice LL, Woolf CJ, et al. (2021). DeepEthogram, a machine learning pipeline for supervised behavior classification from raw pixels. eLife 10, e63377. 10.7554/eLife.63377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gerós A, Magalhães A, and Aguiar P. (2020). Improved 3D tracking and automated classification of rodents’ behavioral activity using depth-sensing cameras. Behav Res. 10.3758/s13428-020-01381-9. [DOI] [PubMed]
- 84.Harris C, Finn KR, Kieseler M-L, Maechler MR, and Tse PU. (2023). DeepAction: a MATLAB toolbox for automated classification of animal behavior in video. Sci Rep 13, 2688. 10.1038/s41598-023-29574-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hu Y, Ferrario CR, Maitland AD, Ionides RB, Ghimire A, Watson B, Iwasaki K, White H, Xi Y, Zhou J, et al. (2023). LabGym: Quantification of user-defined animal behaviors using learning-based holistic assessment. Cell Reports Methods 3. 10.1016/j.crmeth.2023.100415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Marks M, Qiuhan J, Sturman O, Ziegler L von Kollmorgen S, Behrens W von der Mante V, Bohacek J, and Yanik MF. (2022). Deep-learning based identification, tracking, pose estimation, and behavior classification of interacting primates and mice in complex environments. Preprint at bioRxiv, 10.1101/2020.10.26.355115 10.1101/2020.10.26.355115. [DOI] [PMC free article] [PubMed]
- 87.Branson K, Robie AA, Bender J, Perona P, and Dickinson MH. (2009). High-throughput ethomics in large groups of Drosophila. Nat Methods 6, 451–457. 10.1038/nmeth.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Berman GJ, Choi DM, Bialek W, and Shaevitz JW. (2014). Mapping the stereotyped behaviour of freely moving fruit flies. J. R. Soc. Interface 11, 20140672. 10.1098/rsif.2014.0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Arakawa T, Tanave A, Takahashi A, Kakihara S, Koide T, and Tsuchiya T. (2017). Automated Estimation of Mouse Social Behaviors Based on a Hidden Markov Model. In Hidden Markov Models: Methods and Protocols Methods in Biology Molecular., Westhead DR and Vijayabaskar MS, eds. (Springer; New York: ), pp. 185–197. 10.1007/978-1-4939-6753-7_14. [DOI] [PubMed] [Google Scholar]
- 90.Chen Z, Zhang R, Eva Zhang Y, Zhou H, Fang H-S, Rock RR, Bal A, Padilla-Coreano N, Keyes L, Tye KM, et al. (2020). AlphaTracker: A Multi-Animal Tracking and Behavioral Analysis Tool (Animal Behavior and Cognition) 10.1101/2020.12.04.405159. [DOI] [PMC free article] [PubMed]
- 91.Huang K, Han Y, Chen K, Pan H, Zhao G, Yi W, Li X, Liu S, Wei P, and Wang L. (2021). A hierarchical 3D-motion learning framework for animal spontaneous behavior mapping. Nat Commun 12, 2784. 10.1038/s41467-021-22970-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Luxem K, Mocellin P, Fuhrmann F, Kürsch J, Remy S, and Bauer P. (2020). Identifying Behavioral Structure from Deep Variational Embeddings of Animal Motion (Neuroscience) 10.1101/2020.05.14.095430. [DOI] [PMC free article] [PubMed]
- 93.Nandi A, Virmani G, Barve A, and Marathe S. (2021). DBscorer: An Open-Source Software for Automated Accurate Analysis of Rodent Behavior in Forced Swim Test and Tail Suspension Test. eNeuro 8. 10.1523/ENEURO.0305-21.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gabriel CJ, Zeidler Z, Jin B, Guo C, Goodpaster CM, Kashay AQ, Wu A, Delaney M, Cheung J, DiFazio LE, et al. (2022). BehaviorDEPOT is a simple, flexible tool for automated behavioral detection based on markerless pose tracking. eLife 11, e74314. 10.7554/eLife.74314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Golden SA, Christoffel DJ, Heshmati M, Hodes GE, Magida J, Davis K, Cahill ME, Dias C, Ribeiro E, Ables JL, et al. (2013). Epigenetic regulation of RAC1 induces synaptic remodeling in stress disorders and depression. Nat Med 19, 337–344. 10.1038/nm.3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Karashchuk P, Tuthill JC, and Brunton BW. (2021). The DANNCE of the rats: a new toolkit for 3D tracking of animal behavior. Nat Methods 18, 460–462. 10.1038/s41592-021-01110-w. [DOI] [PubMed] [Google Scholar]
- 97.Branson K. APT. GitHub. https://github.com/kristinbranson/APT.
- 98.Nilsson SRO, Goodwin NL, Choong JJ, Hwang S, Wright HR, Norville Z, Tong X, Lin D, Bentzley BS, Eshel N, et al. (2020). Simple Behavioral Analysis (SimBA): an open source toolkit for computer classification of complex social behaviors in experimental animals. Preprint at Cold Spring Harbor Laboratory, 10.1101/2020.04.19.049452 10.1101/2020.04.19.049452. [DOI]
- 99.Xavier P Burgos-Artizzu, o Piotr, Lin Dayu, Anderson David J., and Perona Pietro (2021). CRIM13 (Caltech Resident-Intruder Mouse 13). Version 1.0 (CaltechDATA). 10.22002/D1.1892 10.22002/D1.1892. [DOI]
- 100.Lee W, Fu J, Bouwman N, Farago P, and Curley JP. (2019). Temporal microstructure of dyadic social behavior during relationship formation in mice. PLoS ONE 14, e0220596. 10.1371/journal.pone.0220596. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used in this manuscript is available as a branch of the SimBA github repository. https://github.com/sgoldenlab/simba/tree/simba-data/data
The CRIM13 database is publicly available at https://paperswithcode.com/dataset/crim13.
Code and documentation are available at https://github.com/sgoldenlab/simba. SimBA was built with Python version 3.6.