Abstract
Frontal EEG alpha asymmetry (FAA), defined as the difference in frontal alpha power observed over the right and left frontal scalp regions, has been widely used in developmental research as a measure of multiple aspects of child behavior, such as temperament. Studies have used different equations to calculate FAA, which renders comparison of results across studies challenging. Furthermore, few studies have examined FAA’s longitudinal stability across infancy and early childhood, which is a desirable feature of a temperament measure. We investigated the cross-sectional and the longitudinal correlations of FAA values from four different equations to calculate FAA used in the literature. We used baseline EEG data from a longitudinal sample of 321 infants and 168 3-year-old children (149 of whom had data at both timepoints). Consistent with previous work, FAA values calculated using two commonly used equations were highly correlated with each other cross-sectionally but not with values from a different equation that used log-transformed relative power. The log-transformed relative power FAA values were the only values that showed significant longitudinal stability. These findings suggest that researchers interested in FAA as a trait-like measure in children should consider using the relative power equation that renders stability across ages.
Keywords: electroencephalography (EEG), frontal alpha asymmetry (FAA), infants, longitudinal stability
Introduction
Frontal EEG alpha asymmetry (FAA) in children has been associated with behavioral inhibition and regarded as a biomarker of psychopathology, such as internalizing and externalizing problems (Fox et al., 1995, 1996, 2001; Kagan et al., 1987). FAA is measured as the difference in EEG alpha power between the frontal electrodes of each hemisphere whereby left power is subtracted from right power (Allen et al., 2001). Given that brain activity is defined as reduction in alpha power, right frontal alpha asymmetry (right FAA; i.e., greater right- relative to left-sided frontal activity) refers to right power being less than left power (i.e., a negative FAA value). Correspondingly, left frontal alpha asymmetry (left FAA; i.e., greater left- relative to right-sided frontal activity) refers to left power being less than right power (i.e., a positive FAA value). As reviewed below, FAA has been associated with individual trait differences in both adults and children (for detailed reviews, see Coan & Allen, 2003, 2004).
Trait Tendencies in FAA
FAA has been associated with both affective style, defined as traits that enable people to respond to situations in different ways emotionally and behaviorally, and temperament, which refers to individual differences in affective style and biological responses to different situations (Davidson, 1998a, 1998b; Davidson et al., 1990; Davidson & Fox, 1988; Trofimova & Robbins, 2016). The term “behavioral inhibition” has been used to refer to a temperament style of extreme shyness, social vigilance, and consistent withdrawal from novelty (Kagan et al., 1987). In a landmark study, a subset of 60 infants who were classified as behaviorally inhibited at 21 or 31 months of age maintained this temperamental profile at 5 ½ years of age, showing reluctance to interact with a female examiner during a 90-minute testing session (Kagan et al., 1987). In a study by Fox et al. (2001), a longitudinal cohort of children (N = 123) who at 4 months of age displayed levels of motor activity and negative affect above the mean during a series of novel visual and auditory stimuli showed higher levels of behavioral inhibition in the lab at 14 months of age compared to infants who displayed levels of motor activity, negative affect, and positive affect below the mean and those who displayed levels of motor activity and positive affect above the mean. Children in the sample were later classified as Continuously Inhibited if they displayed scores of behavioral inhibition above the mean at 14 or 24 months of age and levels of shyness with unfamiliar peers above the mean at 4 years of age. Children were classified in the Change group if they displayed scores of behavioral inhibition above the mean at 14 or 24 months of age but showed scores below the mean in shyness at 4 years of age. Children in the Continuously Inhibited group at 9 months, 14 months, and 4 years of age showed a significantly greater degree of right FAA than children in the Change group. These results suggest that right FAA could be a biomarker of behavioral inhibition.
Similarly, in a cross-sectional study by Fox et al. (1995), it was found that children at 4 years of age who displayed right FAA were less socially competent and more inhibited compared to children who displayed left FAA. Furthermore, highly social children at 4 years of age with right FAA displayed more externalizing problems compared to highly social children with left FAA (Fox et al., 1996). In the same study, shy children with right FAA showed more internalizing problems compared to shy children displaying left FAA. Taken together, these results suggest that right FAA is associated with behavioral inhibition and may be a diathesis for psychopathology in childhood.
Stability in FAA
Baseline FAA has shown some stability within individuals across time in both adults and children. In a sample of female participants ages 17 to 21, FAA values had high internal consistency (Cronbach’s alphas from .81 to .90) and acceptable test-retest stability (intraclass correlations .53–.72 for single visits, .69–.84 for a 3-week gap between two measurements) (Tomarken et al., 1992). Past research has shown preliminary evidence for consistency in FAA values throughout infancy and childhood, although sample sizes have generally been small (<100). One study measured FAA values for 13 infants every month from 7 to 12 months of age and found that FAA values were negatively correlated from 7 to 8 months of age (r = −.51, p ≤ .05) but positively correlated from 7 to 10 months of age (r = .65, p ≤ .01) and 9 to 11 months of age (r = .83, p ≤ .01) (Fox et al., 1992). In a sample of 15 children from both depressed and non-depressed mothers, FAA values at 3 months of age were highly correlated with FAA values at 3 years of age (r = .66, p < .01) (Jones et al., 1997). Another study found significant stability between FAA values measured after 14 months of age and again at 83 months of age in a sample of 18 children (r = .64, p < .004) (Müller et al., 2015). Vuga and colleagues (2008) found low to moderate stability in FAA values during an eyes-open baseline paradigm in 87 preschool children (ages 3 to 5 years) and 38 school-age children (ages 6 to 9 years), measured across 6- to 36-month intervals (intraclass correlations 0–.48).
An exception to the historically small sample sizes is a study by Brooker and colleagues (2017), which investigated FAA stability in a longitudinal sample of 129 infant participants. In this study, infants participated in two laboratory visits that were one week apart at 6 months of age and another two laboratory visits that were one week apart at 12 months of age. Brooker et al. found significant stability in FAA values when composite scores of FAA values at each timepoint were used (r = .32, p = .01).
Given these past findings, it would be expected that FAA values in infancy and childhood should be significantly correlated across different times of measurement, although most previous sample sizes have been small and have not examined as long a time interval between assessments as used in the current study. Our sample is substantially larger than in all previous studies, with the exception of Brooker et al. (2017). The large sample size in the current study should provide greater power and increase the ability to detect statistically significant effects. Furthermore, investigating FAA from infancy to 3 years of age, which is a longer period than in most prior studies, is beneficial because temperament has shown stability across this period of time (Behrendt et al., 2019), and FAA could be an early emerging neural marker of temperament.
Calculation of FAA
Despite the widespread use of FAA in the fields of child development and psychopathology, studies have used different equations to calculate FAA, making comparison of results across studies difficult. Therefore, research is needed to determine to what extent different equations yield correlated values for FAA values in large samples. Such information would enable more informed comparisons across studies that employ different FAA equations.
Researchers use channel “F4” to denote right frontal alpha power at or around the F4 10–20 position and channel “F3” to denote left frontal alpha power at or around the F3 10–20 position in scalp regions of interest (ROIs). Equations to calculate FAA involve taking the difference between or ratio of alpha power at F3 and F4. Two equations for FAA are commonly used in the literature. Most developmental studies have used the difference in the natural log (ln) of absolute power, i.e., ln(F4) – ln(F3) (e.g., Fox et al., 1995). The second common equation takes the ratio of the difference between frontal power in the left and right hemispheres to their sum, i.e., (F4 – F3) / (F3 + F4), which is thought to normalize the difference value (Allen et al., 2004). A third, less common approach is to log-transform the ratio, resulting in: (ln(F4) – ln(F3)) / (ln(F3) + ln(F4)) (O’Reilly et al., 2017).
FAA has also been calculated with relative frontal alpha power, which is calculated as the percentage of power in the alpha band divided by total power in all frequency bands (Marshall et al., 2002). Relative power may be advantageous over absolute power in testing pediatric populations due to its improved test-retest reliability (John et al., 1980) and its ability to distinguish among changes in the frequency makeup of EEG across development (Clarke et al., 2001). A recent paper by Harrewijn et al. (2019) calculated FAA a fourth way, by computing the difference between the natural log of the relative power in both hemispheres, i.e., ln(rel(F4)) – ln(rel(F3)).
The Current Study
Given the variety of FAA equations used in the literature, we aimed to test to what extent FAA values from these four different equations are correlated cross-sectionally and longitudinally. We used baseline EEG data from a large sample of infants (5, 7, or 12 months of age; N = 321) and 3-year-old children (N = 169, of which 149 participants also had data at infancy). No previous study to our knowledge has used a longitudinal sample this large, and, as the review above indicates, most have used samples much smaller than 100.
In the current study, FAA values were calculated with the four equations, with the following shorthand variable names used to refer to the equations in this paper:
| 1) |
| 2) |
| 3) |
| 4) |
We hypothesized that FAA values using the same equation would be correlated across the infancy and 3 year timepoints given past research showing relative stability in FAA from infancy to 3 years of age (Jones et al., 1997). Past research has shown a strong correlation (r > .99) between FAA values calculated using FAAln and values calculated using FAAratio (Allen et al., 2004). Allen et al. (2004) illustrated that, because FAAln typically outputs values in the range of ±0.5, the nonlinear function that relates FAAratio and FAAln is almost perfectly linear over this range.
Also for mathematical reasons, we expected that FAA values calculated using FAAlnratio and FAAratio would be correlated given that the terms were identical aside from the log transformation. The correlation may not be strong, however, because raw EEG power is often non-normally distributed, and the purpose of the log transformation of EEG power is to create a more normal distribution. Therefore, we may not expect a non-normal distribution of raw data to be strongly correlated with its log transformation. By the transitive property, we expected that FAAln and FAAlnratio would be correlated. Finally, from a developmental standpoint, we hypothesized that if all the equations were capturing the same underlying construct (i.e., FAA), then FAA values from different equations would be significantly correlated within timepoints. We hypothesized that, to the extent that FAA is stable during infancy and early childhood, FAA values from the same equations across timepoints would also be significantly correlated.
Methods
Participants
Recruitment
Participants were recruited at infancy for a longitudinal project from a registry that contains local birth records and listings from more than 10,000 families. Infants were eligible for participation in the study if they were born within three weeks of their due date, had no neurological abnormalities or traumas, no prenatal, postnatal, or birth-related conditions, no developmental delays, and no uncorrected vision difficulties. The infants were either 5, 7, or 12 months of age at the infancy visit. The families were contacted to schedule a 3-year follow-up (3 YF) visit during the child’s birth month. Parents were again asked if their children had a history of neurological abnormalities or trauma, experienced any developmental delays, had a diagnosis of autism spectrum disorder (ASD), or had any uncorrected vision diagnoses. Parents were asked the same questions for 5- and 7-year follow-up timepoints, the data for which are not used here, but it should be noted that some participants were retroactively excluded if they did not meet inclusion criteria at these follow-up timepoints. The current sample contained 340 participants with acceptable EEG data for at least one timepoint (infancy and/or 3 YF). Of these participants, N = 321 had acceptable EEG data at infancy, N = 168 had acceptable EEG data at 3 YF, and N = 149 had acceptable EEG data at both timepoints.
Parents of the participants provided written informed consent before each of the child’s study visits, and ethical permission for the study was obtained from the Institutional Review Board at the hospital where the lab is located.
Demographics
Demographic data were collected before or during the infancy visit via an online or paper questionnaire. In Table 1, demographics are reported for the 340 participants with acceptable EEG data for at least one timepoint. In almost all cases (97.94%), the mother reported demographic information. The children in the sample were majority white (80.29%). There were slightly more males (53.24%) than females (46.76%). For each parent, a majority (>85%) reported a bachelor’s degree or higher as their highest level of education. Most families (63.53%) had an annual income of $100,000 or greater. Almost all parents (95.88%) were married.
Table 1:
Full sample demographics, N = 340
| Variable | n | % |
|---|---|---|
| Demographic Questionnaire Respondent’s Relationship to Child | ||
| Mother | 333 | 97.94 |
| Father | 6 | 1.76 |
| Other | 1 | 0.29 |
|
Age at Infancy Visit
(N = 321 with acceptable data) |
||
| 5 months
(Mage = 153.33 days, SD = 4.51 days, range = 142–161 days) |
110 | 34.27 |
| 7 months
(Mage = 213.54 days, SD = 4.71 days, range = 203–223 days) |
119 | 37.07 |
| 12 months
(Mage = 364.48 days, SD = 4.62 days, range = 357–374 days) |
92 | 28.66 |
|
Age at 3YF Visit (N = 168 with acceptable EEG data) |
||
| 36
months (Mage = 1158.04 days, SD = 57.58 days, range = 1089–1344 days) |
168 | 100 |
| White | 273 | 80.29 |
| Black or African American | 6 | 1.76 |
| Asian (Chinese, Japanese, Korean, Vietnamese, Indian, or Other) | 11 | 3.23 |
| Mixed Race | 48 | 14.12 |
| Did not respond | 2 | 0.59 |
| Non-Hispanic, Latino/a, or Spanish origin | 306 | 90.00 |
| Mexican, Mexican American, or Chicano/a | 4 | 1.18 |
| Puerto Rican | 4 | 1.18 |
| Cuban | 1 | 0.29 |
| Other Hispanic, Latino/a, or Spanish | 21 | 6.18 |
| Mixed Hispanic, Latino/a, or Spanish origin | 2 | 0.59 |
| Did not respond | 2 | 0.59 |
| High School/GED | 12 | 3.53 |
| Associate Degree | 6 | 1.76 |
| Bachelor’s Degree | 99 | 29.12 |
| Master’s Degree | 153 | 45.00 |
| M.D., Ph.D., J.D., or equivalent | 68 | 20.00 |
| Did not respond | 2 | 0.59 |
| Some High School | 1 | 0.29 |
| High School/GED | 26 | 7.65 |
| Associate Degree | 14 | 4.12 |
| Bachelor’s Degree | 96 | 28.24 |
| Master’s Degree | 111 | 32.65 |
| M.D., Ph.D., J.D., or equivalent | 88 | 25.88 |
| Did not respond | 4 | 1.18 |
| $16,000 through $24,999 | 4 | 1.18 |
| $25,000 through $34,999 | 3 | 0.88 |
| $35,000 through $49,999 | 12 | 3.53 |
| $50,000 through $74,999 | 33 | 9.71 |
| $75,000 through $99,999 | 50 | 14.71 |
| $100,000 or greater | 216 | 63.53 |
| Don’t know | 2 | 0.59 |
| Did not respond | 20 | 5.88 |
| Married | 326 | 95.88 |
| Cohabitating | 9 | 2.65 |
| Single | 3 | 0.88 |
| Did not respond | 2 | 0.59 |
Parent 1 was the biological mother in almost all cases (99.41%), and Parent 2 was the biological father in almost all cases (97.06%).
Note: Some percentage totals do not equal 100 due to rounding.
Although we collected data at three different timepoints in infancy, the cohort sizes were similar across the three groups of infants with acceptable EEG data (n = 110 5-month-olds, n = 119 7-month-olds, n = 92 12-month-olds). Across the three samples used here—N = 321 for the cross-sectional infancy sample, N = 168 for the cross-sectional 3 YF sample, and N = 149 for the longitudinal sample—the cohort sizes and ages at infancy were similar. Age information for the full sample (N = 340) is included in Table 1. For more information on the age distributions and demographics within each of the subsamples, see Supplementary Tables 1–6.
Baseline EEG Measurement
Acquisition
The EEG recording and analysis procedures were similar to those described in previous publications from the project (e.g., Xie et al., 2018). Continuous scalp EEG was recorded at infancy and 3 YF from a 128-channel HydroCel Geodesic Sensor Net (HCGSN; Electrical Geodesic Inc., Eugene, OR), though only 124 channels were used because the eye electrodes were removed for the sake of participant comfort. The net was connected to a NetAmps 300 amplifier (Electrical Geodesic Inc.) and referenced online to a single vertex electrode (Cz). The HydroCel Geodesic Sensor Net has an elastic tension structure and forms a tessellation of the head surface. At each vertex is a sensor pedestal housing an Ag/AgCl-coated, carbon-filled plastic electrode and a sponge soaked in electrolyte solution (6 mL of KCl per 1 L of distilled water) to facilitate electrical contact between the scalp and the respective electrode.
During the infancy visit, the child sat on their parent’s lap approximately 63 cm from a mounted screen in a dimmed room, and the parent wore a foam visor to block their eyes from the screen. At 3 YF, the child’s head was measured and marked with a washable wax pencil to ensure accurate placement of the net before it was placed on the scalp. Additionally, at 3 YF, the child was most often seated on their own in a highchair without their parent in the room, approximately 64 cm from the screen. Before data collection began, impedances for all electrodes were measured and verified using the NetStation (EGI, Inc.) recording software package. Channel impedances were kept at or below 100 kΩ, and signals were sampled at 500 Hz. During the infancy and 3 YF visits, baseline EEG was collected and recorded for 2 minutes while children watched a computer-generated video of moving infant toys to maintain engagement (Figure 1). An experimenter sat in the room next to the child at both timepoints to give instructions and provide behavioral assistance when needed. Protocols for data collection are available on the Open Science Framework (OSF): https://osf.io/6y3tj/?view_only=4e0c5622cd074e8d857e538d87e5dd09.
Figure 1.

Screen-cap of baseline EEG video
A video of computer-generated moving infant toys was displayed for a period of 2 minutes at the infancy and 3 YF visits while baseline EEG was recorded. The toys moved and emitted sounds. Image from: Rahman (2018), p. 27, figure 3.
Pre-Processing and Frequency Analysis
The EEG recordings were preprocessed using the EEGLAB (Delorme & Makeig, 2004) and ERPLAB (Lopez-Calderon & Luck, 2014) toolboxes in MATLAB (R2015b, the Mathworks, Inc.). The continuous EEG data were filtered with an 8th order Butterworth band-pass filter with a pass band of 1–50 Hz. The filtered EEG data were segmented into 1s epochs. It is worth noting that signal loss due to the shape of the Hanning window can be prevented by creating overlapped epochs (Bell, 2002; Thorpe et al., 2016). Since the current study only included participants with sufficient data points (i.e., > 60s) in the final analysis, no overlapped epochs were used. Independent component analysis (ICA) was performed to remove components related to eye movements, blinks, and focal activity using SASICA (Chaumon et al., 2015) and part of the functions of the MADE pipeline (Debnath et al., 2020). The EEG epochs were further inspected for artifacts using both absolute and stepwise algorithms (EEG > 100 μV, EEG < −100 μV, or ΔEEG > 100 μV within 100 ms with a 50 ms window step). Channel interpolation was conducted using the five closest channels if there were fewer than 18 (15%) electrodes that were missing or had bad data consistent with previous work from this lab (Xie et al., 2019, 2021). Each participant had to have at least 60 clean trials for the data to be included for further analyses.
Frequency analysis was conducted to assess the power spectral density (PSD, i.e., spectral power) with the Fieldtrip toolbox (Oostenveld et al., 2011). Fast Fourier Transform (FFT) was applied on EEG epochs with a 1s-width Hanning window. The PSD was calculated for all frequency bins from 1–50 Hz. The absolute alpha power was calculated as the average PSD in age-appropriate alpha bands (infants: 6–9 Hz; 3-year-olds: 7–10 Hz) (Thorpe et al., 2016; Xie et al., 2018). The relative alpha power was calculated as the proportion of alpha PSD relative to the total PSD from the beginning of the child theta band to 50 Hz (i.e., 3–50 Hz).
Although the infants in the current sample were at different ages (5 months, 7 months, or 12 months), the 6–9 Hz frequency range has shown some consistency from 5 to 10 months of age and stronger consistency from 10 to 14 months (see Table 2 in Marshall et al., 2002 for more detail on stability metrics). Five-month-olds may or may not have a clear alpha peak between 6–9 Hz; that is, their alpha rhythm may not be fully developed yet, but studies have examined alpha power in infants at 5 months of age (Marshall et al., 2002). Therefore, infancy data were collapsed across age groups.
Table 2:
Within-time correlations of FAA values
| Infancy | Three Year Follow-up | |||||
|---|---|---|---|---|---|---|
| Variable | FAAratio | FAAlnratio | FAAlnrel | FAAratio | FAAlnratio | FAAlnrel |
| FAAln |
r = 1.00** p < .001 N = 321 |
r = .22** p < .001 N = 321 |
r = .13* p = .020 N = 321 |
r = 1.00** p < .001 N = 168 |
r = .62** p < .001 N = 168 |
r = .27** p < .001 N = 168 |
| FAAratio |
r = .22** p < .001 N = 321 |
r = .13* p = .021 N = 321 |
r = .61** p < .001 N = 168 |
r = .27** p < .001 N = 168 |
||
| FAAlnratio |
r =
.00 p = .958 N = 321 |
r = .17* p = .028 N = 168 |
||||
Correlation is significant at the 0.01 level (2-tailed).
Correlation is significant at the 0.05 level (2-tailed).
FAA Calculations
The frontal absolute and relative alpha power was averaged over the HGSN electrodes including and surrounding the F3 (19, 20, 23, 24 (F3), 27, and 28) and F4 (3, 4, 117, 118, 123, and 124 (F4)) 10–20 positions as shown in Figure 2. The ROIs were chosen based on existing literature (Coan & Allen, 2004). Given that brain activity is defined as reduction in alpha power, a negative asymmetry value indicates right frontal asymmetry, and a positive asymmetry value indicates left frontal asymmetry.
Figure 2:
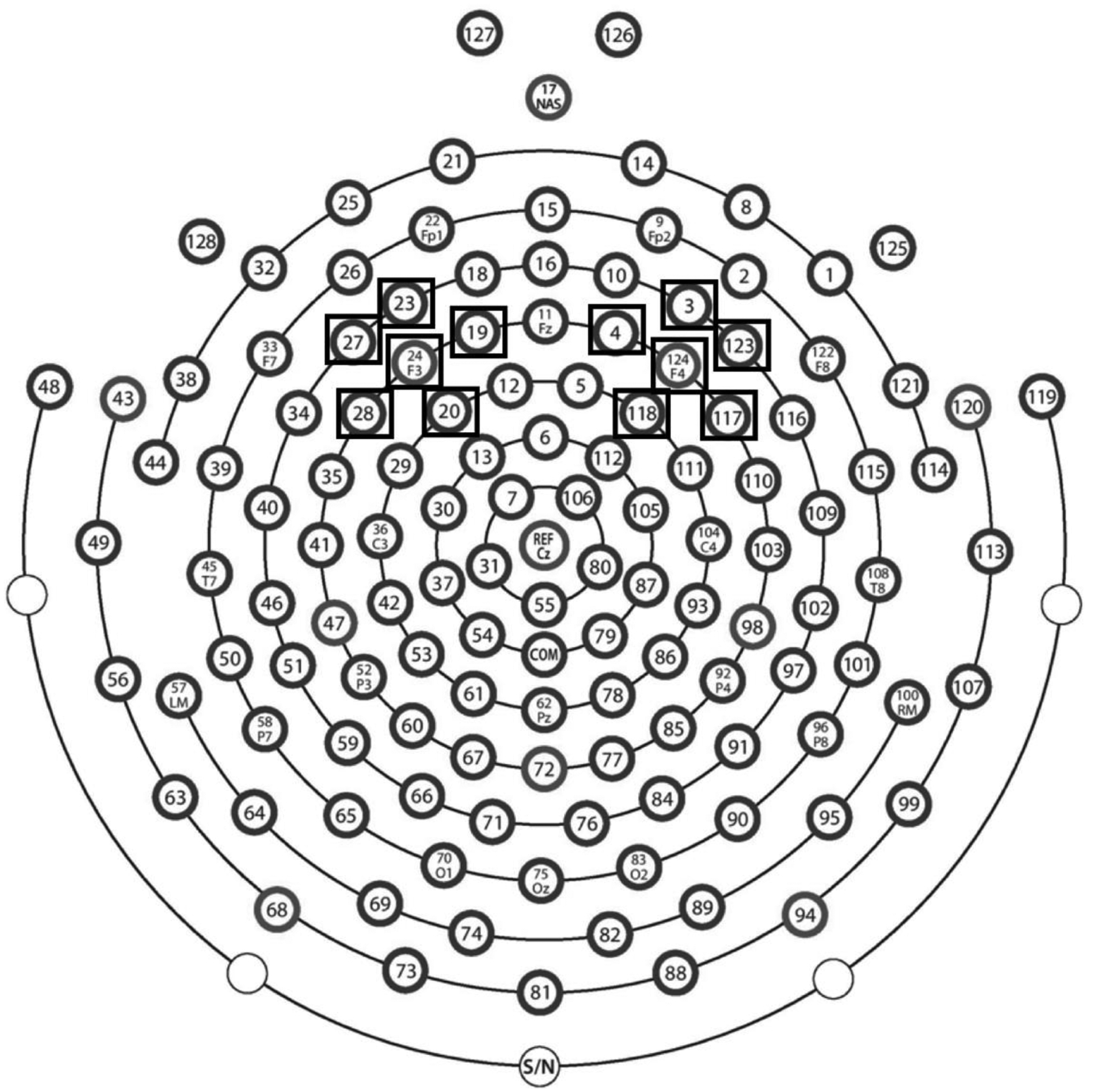
EEG map and ROIs for FAA
Schematic of a 128-channel HydroCel Geodesic Sensor Net (HGSN; Electrical Geodesic Inc., Eugene, OR) from Electrical Geodesics, Inc. (EGI) map: https://drive.google.com/file/d/0B388xdH0Vxl2T3U4aS1oWDdPdTg/view. Electrodes used for the right and left regions of interest (ROIs) to assess frontal alpha asymmetry (FAA) are in black boxes.
Data Analysis and Availability
Excel formulas were employed to calculate FAA values using the four equations with relative and absolute power in the right and left frontal ROIs. SPSS (Version 27) was used to calculate correlations within and across timepoints between FAA values, and correlation values were rounded to two decimal places for consistency with previous studies. Fisher’s r-to-z transformations were performed using Excel template spreadsheets from Kane (2013) based on Steiger (1980) for overlapping correlations and Raghunathan, Rosenthal, and Rubin (1996) for nonoverlapping correlations.
Processed EEG data and a spreadsheet of FAA values along with ages at the infancy and 3 YF visits are available on the Open Science Framework: https://osf.io/6y3tj/?view_only=4e0c5622cd074e8d857e538d87e5dd09. Raw data are available from the corresponding author upon reasonable request.
Results
Distributions of FAA Values
As displayed in Supplementary Figures 1–8, FAA values at infancy and 3 YF calculated using the four equations were all approximately normally distributed. The distributions of FAA values at infancy and 3 YF calculated using FAAlnratio had outliers resulting from large values in the numerators and values close to 0 in the denominators, but most values clustered around the mean.
Differences Depending on Infancy Group
Due to the difference in age groups at infancy, we tested whether FAA values differed significantly by the age group to which the infant belonged. One-way independent ANOVAs revealed no significant differences between FAA values by infancy age group for FAAratio, FAAln, and FAAlnratio. There were significant differences in FAA values by age group for FAAlnrel (F (2, 318) = 6.19, p = .002). Tukey’s HSD post-hoc tests revealed that there were significant differences between FAA values for 5- and 7-month-olds and 5- and 12-month-olds, but not 7- and 12-month-olds using FAAlnrel. Five-month-olds showed significantly greater FAA values (that is, greater relative right-sided activity) compared to 7-month-olds by .15 units (95% CI [.01, .29], p = .030). Five-month-olds also showed significantly greater FAA values compared to 12-month-olds by .21 units (95% CI [.06, .36], p = .003).
Within-Time Correlations at Infancy
The left side of Table 2 and Figures 3 and 4 show the within-time correlations between FAA values at infancy. Consistent with past work, FAA values calculated using FAAln and FAAratio were strongly correlated at infancy (r = 1.00, p < .001, N = 321). As expected, there were significant within-time correlations between FAA values in infancy calculated using equations with identical or similar terms. That is, FAA values calculated using FAAlnratio and FAAratio were significantly correlated, though the correlation was small, likely due to the aforementioned purpose of the log transformation to normalize raw EEG power (r = .22, p < .001, N = 321). As expected, due to the transitive property, FAAlnratio and FAAln were significantly correlated (r = .22, p < .001, N = 321).
Figure 3:
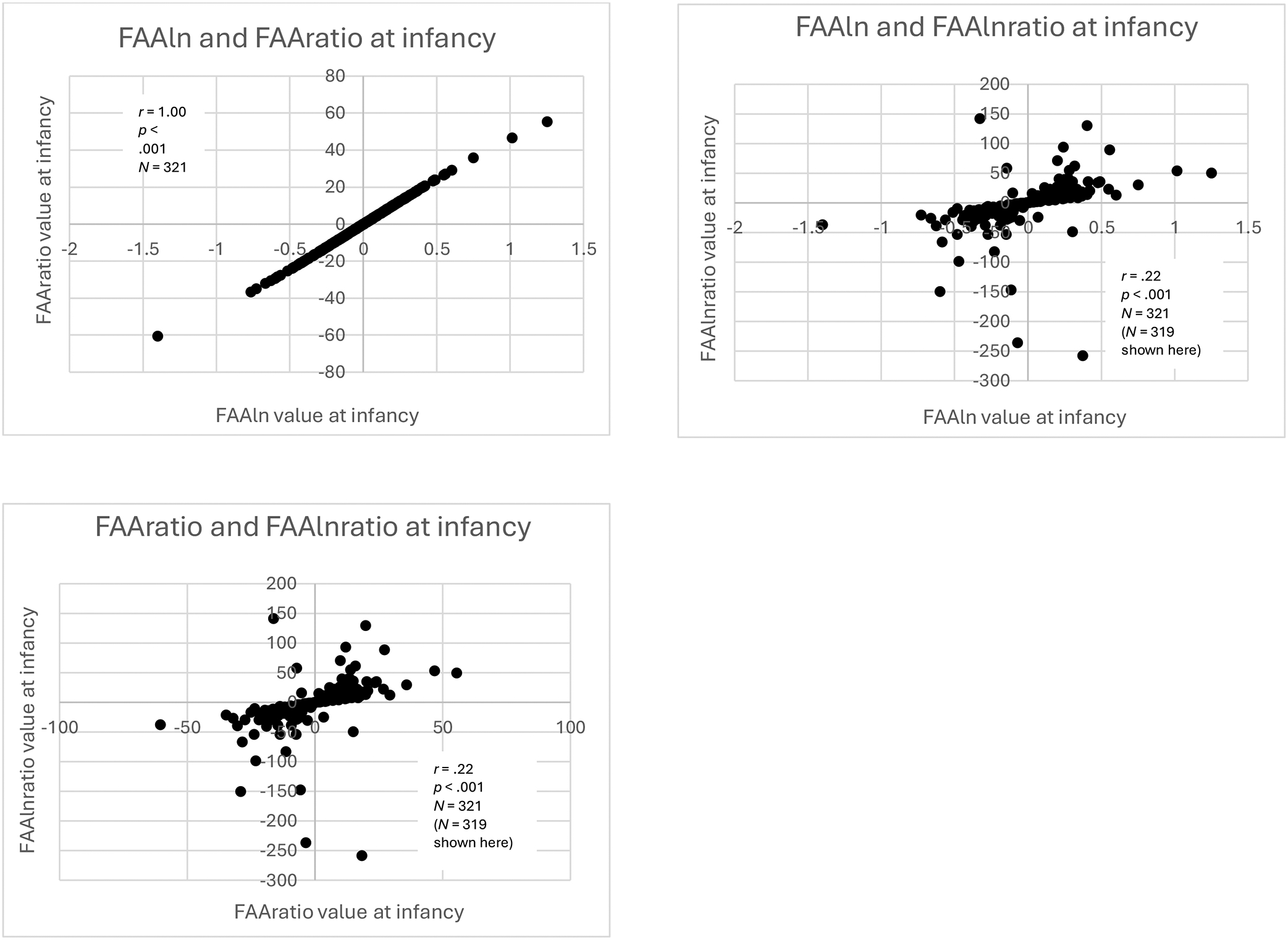
Scatterplots of infancy within-time correlations of FAAln, FAAratio, and FAAlnratio
Scatterplots of within-time correlations of FAAln, FAAratio, and FAAlnratio values at infancy. For the purpose of visualization, the most extreme outliers on either end of the distribution of FAAlnratio values were omitted from the original sample of N = 321, but correlation analysis was performed on the full sample.
Figure 4:
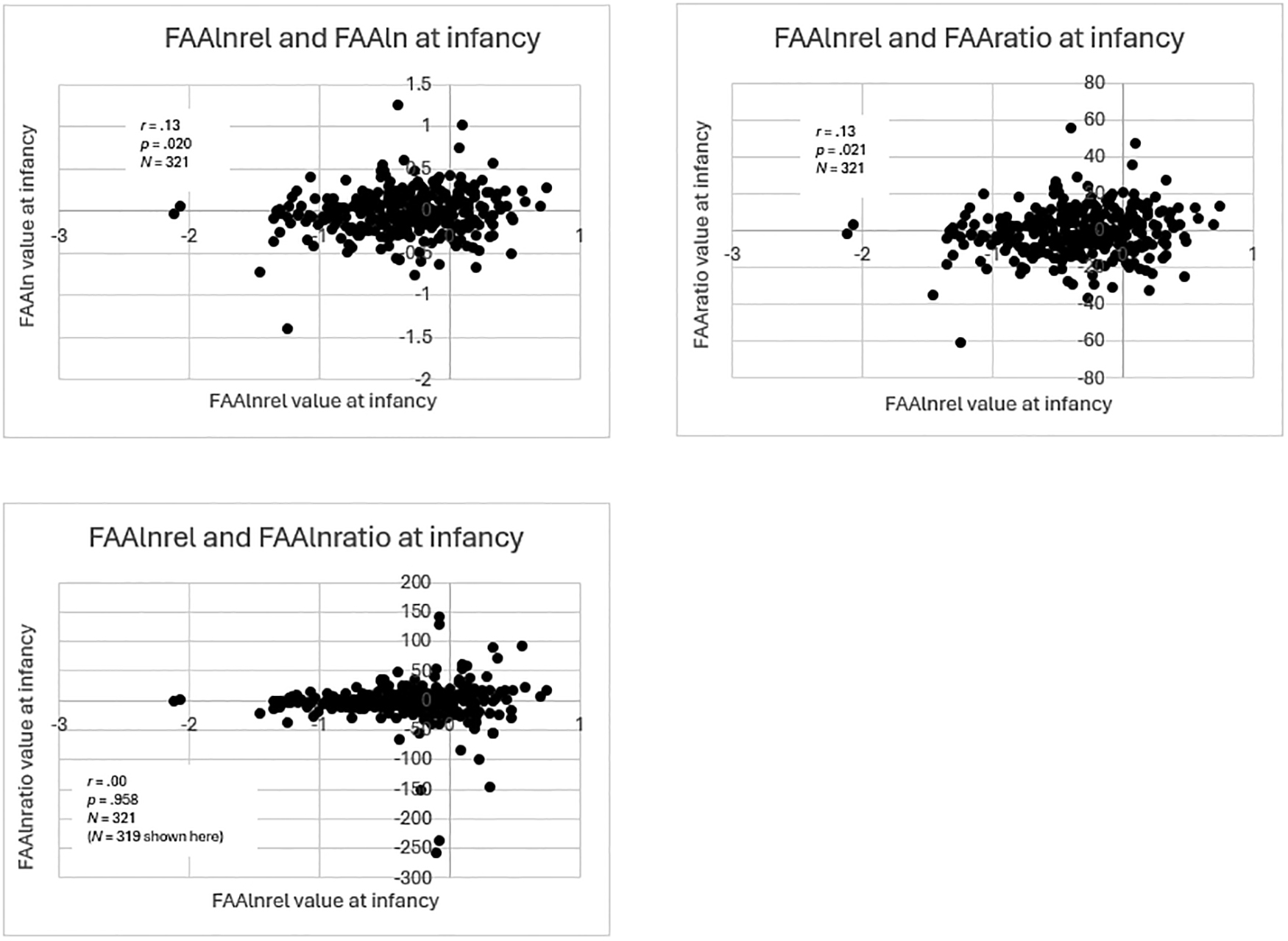
Scatterplots of infancy within-time correlations of FAAlnrel with other equations
Scatterplots of within-time correlations of FAAlnrel values at infancy with values from other equations. For the purpose of visualization, the most extreme outliers on either end of the distribution of FAAlnratio values were omitted from the original sample of N = 321, but correlation analysis was performed on the full sample.
Values from the FAAln, FAAratio, and FAAlnratio equations showed lower correlations with values from the relative power equation (FAAlnrel). FAA values calculated using FAAlnratio and FAAlnrel were not significantly correlated (r = .00, p = .958, N = 321). Although the correlations of FAAratio and FAAln with FAAlnrel were significant, they were small: FAAlnrel and FAAratio (r = .13, p = .021, N = 321), and FAAln and FAAlnrel (r = .13, p = .020, N = 321).
Fisher’s r-to-z transformations revealed that the large differences between the correlation of FAAln and FAAratio compared to all the other correlations were statistically significant (p < .001 in all cases). The only other statistically significant differences (both p = .003) were between the correlations of FAAlnratio and FAAlnrel with: 1) FAAlnratio and FAAln; and 2) FAAlnratio and FAAratio.
Given the differences in ages within infancy, we also calculated within-time correlations of FAA values for each of the three infancy age groups individually (Supplementary Tables 7–9). Compared to the results from the combined infancy group, two main differences were: 1) the correlations of FAAlnratio with FAAratio and FAAln were greater; and 2) the correlations of FAAlnrel with FAAln and FAAratio were significant for the 7-month-olds but not for the 5-month-olds or 12-month-olds.
We also calculated within-time correlations of FAA values for the infancy cohort without the 5-month-olds (Supplementary Table 10). We found that the correlation values were greater, and the p values were smaller compared to analyses with all three infancy groups combined.
Within-Time Correlations at 3 YF
The right side of Table 2 and Figures 5 and 6 shows the within-time correlations between FAA values at 3 YF. Consistent with the infancy findings, FAA values calculated using FAAln and FAAratio were highly correlated (r = 1.00, p < .001, N = 168). Additionally, as at infancy, FAA values calculated using FAAlnratio and FAAratio were significantly correlated, although more strongly compared to infancy (r = .61, p < .001, N = 168). The same was true for FAA values calculated using FAAlnratio and FAAln (r = .62, p < .001, N = 168).
Figure 5:
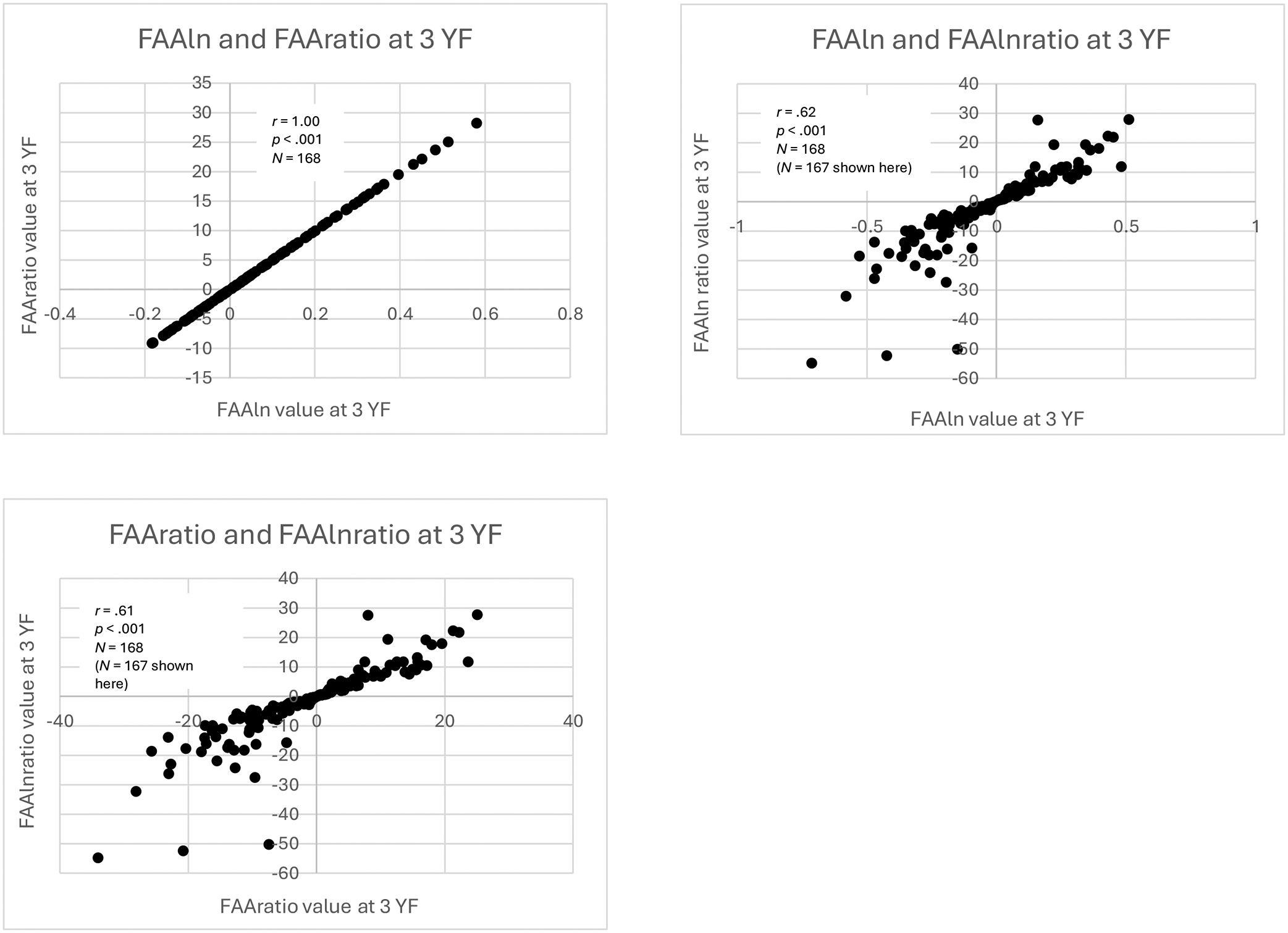
Scatterplots of three-year (3 YF) within-time correlations of FAAln, FAAratio, and FAAlnratio
Scatterplots of within-time correlations of FAAln, FAAratio, and FAAlnratio values at 3 YF. For the purpose of visualization, the most extreme maximum outlier on the distribution of FAAlnratio values was omitted from the original sample of N = 168, but correlation analysis was performed on the full sample.
Figure 6:
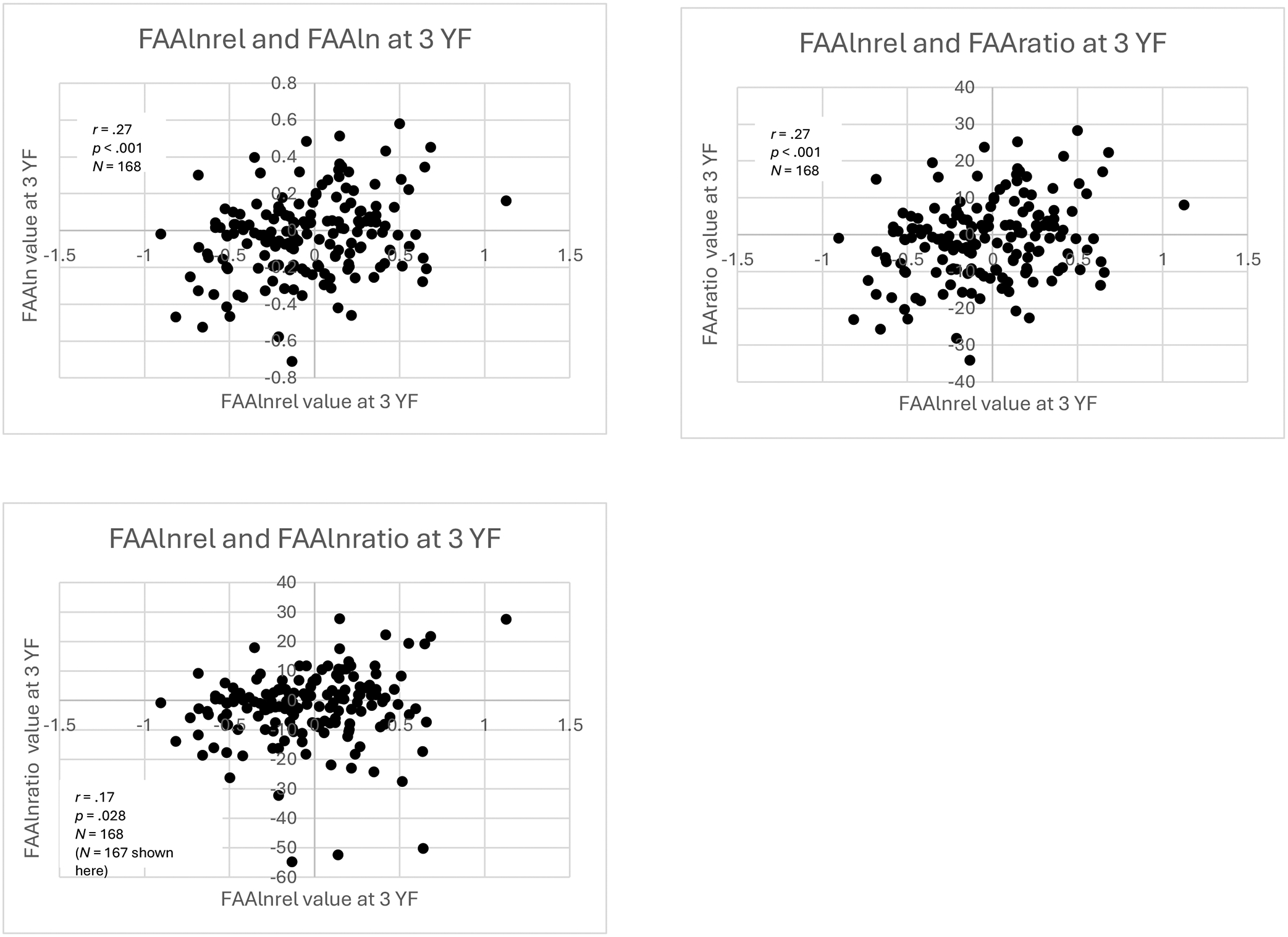
Scatterplots of three-year (3 YF) within-time
Scatterplots of within-time correlations of FAAlnrel values at 3 YF with values from other equations. For the purpose of visualization, the most extreme maximum outlier on the distribution of FAAlnratio values was omitted from the original sample of N = 168, but correlation analysis was performed on the full sample.
There were significant, small correlations between FAA values calculated using FAAlnrel and FAAratio (r = .27, p < .001, N = 168) and FAAln and FAAlnrel (r = .27, p < .001, N = 168). Unlike at infancy, FAA values calculated using FAAlnratio and FAAlnrel were significantly correlated (r = .17, p = .028, N = 168).
Fisher’s r-to-z transformations revealed that all the correlations were significantly different from each other except for three pairs of correlation values: 1) FAAln and FAAlnrel with FAAlnrel and FAAratio (p = .347); 2) FAAln and FAAlnrel with FAAlnratio and FAAlnrel (p = .140); and 3) FAAlnrel and FAAratio with FAAlnratio and FAAlnrel (p = .145).
Longitudinal Correlations
Table 3 and Figure 7 show the longitudinal correlations between FAA values calculated using the same equation at both timepoints. There was significant, moderate stability in FAA values from infancy to 3 YF calculated using FAAlnrel (r = .36, p < .001, N = 149). There was not significant stability in FAA values using FAAln, FAAratio, or FAAlnratio. Additionally, we calculated the longitudinal correlations between FAA values excluding the 5-month-olds, and results were nearly identical compared to longitudinal correlations including all three infancy groups (Supplementary Table 11).
Table 3:
Longitudinal correlations of FAA values
| Variable | |
|---|---|
| FAAln |
r =
−.02 p = .789 N = 149 |
| FAAratio |
r =
−.02 p = .804 N = 149 |
| FAAlnratio |
r =
−.07 p = .395 N = 149 |
| FAAlnrel |
r = .36** p < .001 N = 149 |
Correlation is significant at the 0.01 level (2-tailed).
Correlation is significant at the 0.05 level (2-tailed).
Figure 7:
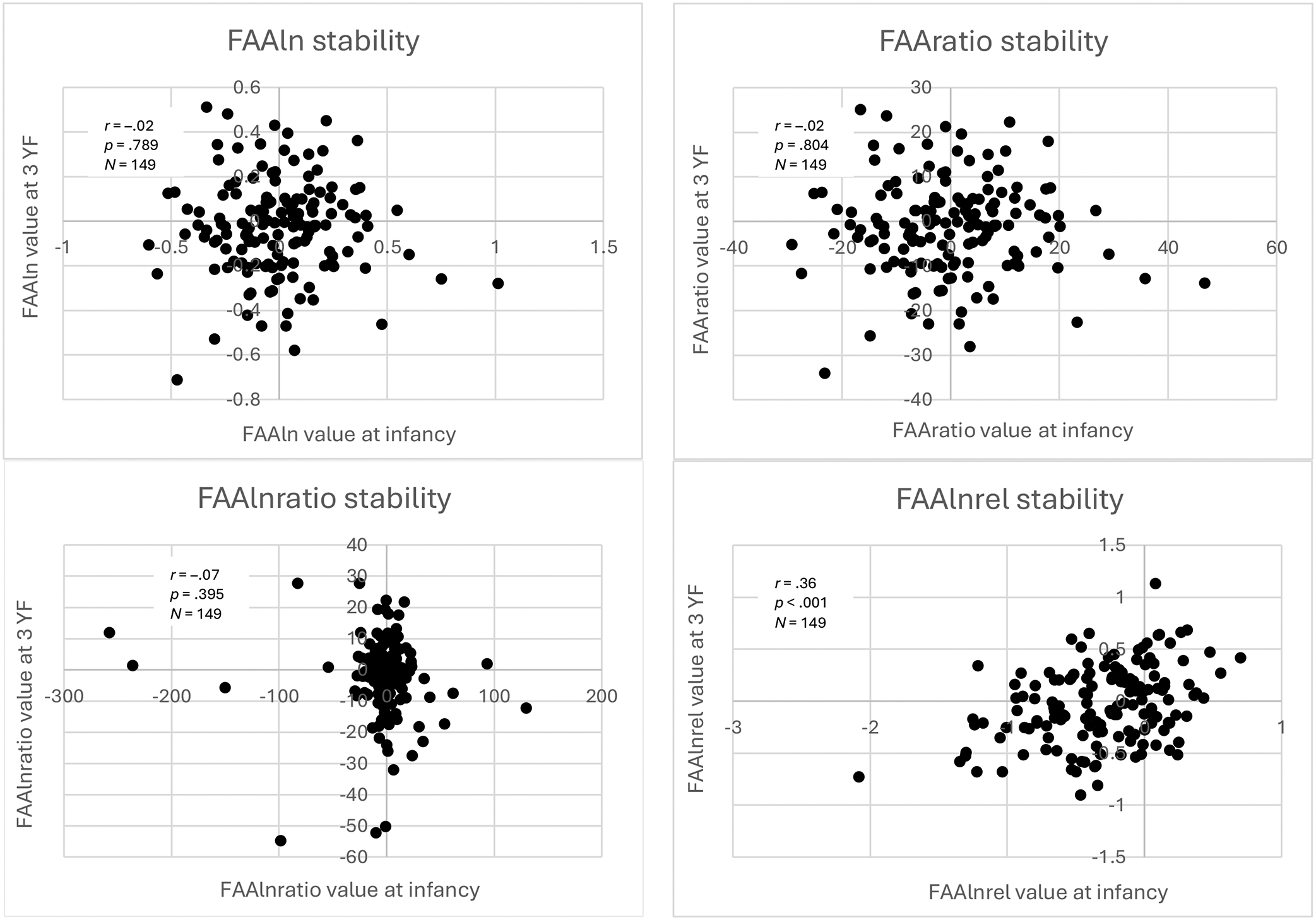
Scatterplots of longitudinal correlations of FAA values
Scatterplots of longitudinal correlations of FAA values at infancy and 3 YF for each equation.
Analyses Without Outliers
To investigate the potential impact of outliers on the results, we removed FAA values with z-scores > and reran the analyses. Results are shown in Supplementary Tables 12 and 13, respectively for within-time and longitudinal correlations. Removal of outliers did not substantially change the longitudinal correlations. In the case of the within-time correlations, two main changes occurred: 1) correlations of FAAlnratio with FAAln and FAAratio increased for both infancy and 3YF; and 2) correlations of FAAlnrel with FAAln and FAAratio became marginally non-significant.
Discussion
We examined the degree to which different calculations of FAA values were correlated within and across timepoints using different FAA equations in the literature and a much larger longitudinal sample of infants and children than any previous study to our knowledge. We found that values from the most common equations used to calculate FAA, i.e., FAAln and FAAratio, were highly correlated. This finding has been previously established in the literature due to the fundamental mathematical similarity of these equations within typical ranges of asymmetry values (Allen et al., 2004).
We found that FAA values were not strongly correlated within timepoints for the equations other than FAAln and FAAratio, especially for the infancy cohort. Furthermore, it is notable that the significant within-time correlation values for infancy were smaller than the within-time correlations for 3YF. The difference in the size of the correlations between the two timepoints is likely due to greater variability in infancy EEG data compared to data from 3-year-old children in this sample, which was also observed in a recent study utilizing the same sample (Xie et al., 2021).
Although FAA values calculated using FAAln and FAAratio were highly correlated with each other cross-sectionally (i.e., within infancy and 3 YF), they did not show significant stability across time. Given that FAA is thought to be a stable, trait-like measure, it would be expected that FAA values would be correlated across timepoints. In the current sample, FAA values were not stable using FAAln, in contrast with previous research that has demonstrated significant stability in FAA using FAAln. As discussed earlier, Jones et al. (1997) found that, in a sample of 15 children from both depressed and non-depressed mothers, FAA scores calculated using FAAln at 3 months of age were highly correlated with FAA values at 3 years of age (r = .66, p < .01). The discrepancy could be due to the much larger sample, larger number of electrodes, and different frequency band used in the current study. Our longitudinal sample included 149 participants, compared to 15 participants in Jones et al. (1997). Furthermore, Jones et al. (1997) used only two electrodes (F4 and F3) and the 6–9 Hz frequency band for alpha power at 3 YF, while the current study used a larger cluster of electrodes around F4 and F3 and the 7–10 Hz frequency band at 3 YF given findings from the seminal Marshall et al. (2002) paper.
Brooker et al. (2017) also found significant stability in FAA values using FAAln from 6 to 12 months of age but used the 5–9 Hz frequency band for alpha power and only the F4 and F3 electrodes. Similarly, although Müller et al. (2015) found significant stability in FAA values using FAAln in 3- to 9-year-old children, the sample size (N = 18) was much smaller than the longitudinal sample employed here (N = 149) and used older children. It is also important to note that different electrode clusters have been used for calculating FAA, which introduces another source of variability into the calculation of FAA that we did not explore here but could also impact comparison of results.
Another explanation for the lack of stability in FAAln and FAAratio is that FAA may not be a highly stable biomarker, especially given results from some studies showing developmental change and weak rank-order stability in FAA (Fox et al., 2001; Gabard-Durnam et al., 2015; Howarth et al., 2016). However, in contrast to the lack of stability in FAA values from the other three equations, we found that FAA values were stable across timepoints using FAAlnrel, a relatively new equation in the FAA literature (Harrewijn et al., 2019). This finding suggests that using relative power and a log transformation could better capture underlying stability in FAA across infancy and early childhood. Relative power is more sensitive to age-related changes in EEG bands and normalizes individual differences in skull thickness that may impact absolute power (Marshall et al., 2002). Skull thickness is a main non-functional source of variance that may significantly contribute to individual differences in the magnitude of EEG amplitudes and power (Forssell et al., 2021). EEG power spectrum density decreases as the skull thickness increases with age. Thus, when comparing EEG metrics (e.g., FAA scores) derived from absolute or log-transformed EEG power between two different ages, the result can be driven by the differences in skull thickness between the two age groups rather than differences in intracranial sources (e.g., alpha-band activation in the anterior cingulate cortex). Using (log-transformed) relative power can effectively alleviate the impact of skull thickness, as it is calculated by taking the ratio of the power at each frequency bin to the total (or average) power across the entire frequency band, such as 1–50 Hz. Future work should investigate whether FAA values calculated using FAAlnrel continue to show stability into mid-childhood.
The current study is novel in its investigation of the cross-sectional correlations of FAA values and the longitudinal correlations among FAA values from the same equations for a larger number of equations (four) and in a larger sample of participants than previous studies have considered. Nevertheless, this study has several limitations. First, infancy data were collected across different ages for participants (5, 7, or 12 months of age). As previously mentioned, 5-month-olds may or may not have a clear alpha peak between 6–9 Hz; that is, their alpha rhythm may not be fully developed yet (Marshall et al., 2002). This could be a cause of the differences in asymmetry values using FAAlnrel at this timepoint compared to later points in infancy. Furthermore, the within-time correlations between FAA values without the 5-month-old group appeared to be larger, but it should be noted that there was a difference in sample size of 110 participants compared to the combined infancy group.
Second, we collected only baseline, not task-based, FAA at only two timepoints, with a long period of time between assessments. FAA may have shown increased stability had we collected data at more than two timepoints between infancy and 3 years of age, especially because this time period includes many developmentally sensitive windows for changes in the EEG power spectra (Marshall et al., 2002). We also collected baseline, not task-based, FAA. A recent study by Goodman et al. (2020) found that 12-month-old infants with depressed mothers showed small to moderate correlations of FAA values across five different contexts, although this study was cross-sectional. Most studies to our knowledge have compared FAA longitudinally at baseline and not during tasks. Future designs should employ more repeated measures timepoints with both baseline and event-related FAA longitudinally to explore in greater detail the extent to which FAA is stable across infancy into early childhood in large (>100) sample sizes.
Third, the sample comes from a predominately white, high socioeconomic status (SES) population, which means that the results might not generalize to children growing up in more racially, ethnically, and socioeconomically diverse households. Given past research linking SES to differential patterns of brain development, including EEG power (Brito et al., 2016), future studies should employ more diverse samples to investigate whether FAA might be influenced by SES.
Overall, the current study offers promising results for the stability of FAA using the FAAlnrel equation, suggesting that future researchers might be best served by using this equation when investigating FAA as a trait-like measure. These results also suggest that the traditional equations used to calculate FAA, i.e., FAAln and FAAratio, may not serve as appropriate indices of FAA as a trait-like measure. Additional research is needed to determine which equations best index relative differences in electrical activity across the left and right hemispheres at various stages of development. More generally, researchers should use caution when comparing their results to previous work given the range of equations and methods used for calculating FAA historically and the weak correlations cross-sectionally among FAA equations reported here.
Supplementary Material
Acknowledgements
The work was supported by the National Institute of Mental Health (R01 MH078829 awarded to CAN). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health of the National Institutes of Health. Study data were collected and managed using Research Electronic Data Capture (REDCap) tools hosted at Boston Children’s Hospital (Harris et al., 2009). REDCap (Research Electronic Data Capture) is a secure, web-based application designed to support data capture for research studies, providing: 1) an intuitive interface for validated data entry; 2) audit trails for tracking data manipulation and export procedures; 3) automated export procedures for seamless data downloads to common statistical packages; and 4) procedures for importing data from external sources.
The authors would like to thank the research assistants, students, and postdoctoral fellows who helped collect and code data, Rachel Kwon for data management, Jebediah Taylor for assistance with the supplementary diagrams, and the parents and children who participated in the research.
Footnotes
The authors declare no conflicts of interest.
The data that support the findings of this study are available on the Open Science Framework: https://osf.io/6y3tj/?view_only=4e0c5622cd074e8d857e538d87e5dd09.
References
- Allen JJB, Coan JA, & Nazarian M (2004). Issues and assumptions on the road from raw signals to metrics of frontal EEG asymmetry in emotion. Biological Psychology, 67(1), 183–218. 10.1016/j.biopsycho.2004.03.007 [DOI] [PubMed] [Google Scholar]
- Allen JJB, Harmon-Jones EA, & Cavender JH (2001). Manipulation of frontal EEG asymmetry through biofeedback alters self‐reported emotional responses and facial EMG. Psychophysiology, 38(4), 685–693. 10.1111/1469-8986.3860912 [DOI] [PubMed] [Google Scholar]
- Behrendt HF, Wade M, Bayet L, Nelson CA, & Bosquet Enlow M (2020). Pathways to social-emotional functioning in the preschool period: The role of child temperament and maternal anxiety in boys and girls. Development and Psychopathology, 32(3), 961–974. 10.1017/S0954579419000853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell MA (2002). Power changes in infant EEG frequency bands during a spatial working memory task. Psychophysiology, 39(4), 450–458. 10.1017/S0048577201393174 [DOI] [PubMed] [Google Scholar]
- Brito NH, Fifer WP, Myers MM, Elliott AJ, & Noble KG (2016). Associations among family socioeconomic status, EEG power at birth, and cognitive skills during infancy. Developmental Cognitive Neuroscience, 19, 144–151. 10.1016/j.dcn.2016.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooker RJ, Canen MJ, Davidson RJ, & Hill Goldsmith H (2017). Short‐ and long‐term stability of alpha asymmetry in infants: Baseline and affective measures. Psychophysiology, 54(8), 1100–1109. 10.1111/psyp.12866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaumon M, Bishop DVM, & Busch NA (2015). A practical guide to the selection of independent components of the electroencephalogram for artifact correction. Journal of Neuroscience Methods, 250, 47–63. 10.1016/j.jneumeth.2015.02.025 [DOI] [PubMed] [Google Scholar]
- Clarke AR, Barry RJ, McCarthy R, & Selikowitz M (2001). Age and sex effects in the EEG: Development of the normal child. Clinical Neurophysiology, 112(5), 806–814. 10.1016/S1388-2457(01)00488-6 [DOI] [PubMed] [Google Scholar]
- Coan JA, & Allen JJB (2003). The state and trait nature of frontal EEG asymmetry in emotion. In Davidson RJ & Hugdahl K (Eds.), The Asymmetrical Brain (pp. 566–615). The MIT Press. [Google Scholar]
- Coan JA, & Allen JJB (2004). Frontal EEG asymmetry as a moderator and mediator of emotion. Biological Psychology, 67(1), 7–49. 10.1016/j.biopsycho.2004.03.002 [DOI] [PubMed] [Google Scholar]
- Davidson RJ (1998a). Affective style and affective disorders: Perspectives from affective neuroscience. Cognition & Emotion, 12(3), 307–330. 10.1080/026999398379628 [DOI] [Google Scholar]
- Davidson RJ (1998b). Anterior electrophysiological asymmetries, emotion, and depression: Conceptual and methodological conundrums. Psychophysiology, 35(5), 607–614. 10.1017/S0048577298000134 [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Ekman P, Saron CD, Senulis JA, & Friesen WV (1990). Approach–Withdrawal and Cerebral Asymmetry: Emotional Expression and Brain Physiology I. Journal of Personality and Social Psychology, 58(2), 330–341. 10.1037/0022-3514.58.2.330 [DOI] [PubMed] [Google Scholar]
- Davidson RJ, & Fox NA (1988). Cerebral asymmetry and emotion: Developmental and individual differences. In Molfese DL & Segalowitz SJ (Eds.), Brain Lateralization in children: Developmental implications (pp. 191–206). Guilford. [Google Scholar]
- Debnath R, Buzzell GA, Morales S, Bowers ME, Leach SC, & Fox NA (2020). The Maryland analysis of developmental EEG (MADE) pipeline. Psychophysiology, 57(6), e13580–n/a. 10.1111/psyp.13580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme A, & Makeig S (2004). EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods, 134(1), 9–21. 10.1016/j.jneumeth.2003.10.009 [DOI] [PubMed] [Google Scholar]
- Forssell M, Goswami C, Krishnan A, Chamanzar M, & Grover P (2021). Effect of skull thickness and conductivity on current propagation for noninvasively injected currents. Journal of Neural Engineering, 18(4), 46042. 10.1088/1741-2552/abebc3 [DOI] [PubMed] [Google Scholar]
- Fox NA, Bell MA, & Jones NA (1992). Individual differences in response to stress and cerebral asymmetry. Developmental Neuropsychology, 8(2–3), 161–184. 10.1080/87565649209540523 [DOI] [Google Scholar]
- Fox NA, Henderson HA, Rubin KH, Calkins SD, & Schmidt LA (2001). Continuity and discontinuity of behavioral inhibition and exuberance: Psychophysiological and behavioral influences across the first four years of life. Child Development, 72(1), 1–21. 10.1111/1467-8624.00262 [DOI] [PubMed] [Google Scholar]
- Fox NA, Rubin KH, Calkins SD, Marshall TR, Coplan RJ, Porges SW, Long JM, & Stewart S (1995). Frontal activation asymmetry and social competence at four years of age. Child Development, 66(6), 1770–1784. 10.1111/j.1467-8624.1995.tb00964.x [DOI] [PubMed] [Google Scholar]
- Fox NA, Schmidt LA, Calkins SD, Rubin KH, & Coplan RJ (1996). The role of frontal activation in the regulation and dysregulation of social behavior during the preschool years. Development and Psychopathology, 8(1), 89–102. 10.1017/S0954579400006982 [DOI] [Google Scholar]
- Gabard-Durnam L, Tierney AL, Vogel-Farley V, Tager-Flusberg H, & Nelson CA (2015). Alpha asymmetry in infants at risk for autism spectrum disorders. Journal of Autism and Developmental Disorders, 45(2), 473–480. 10.1007/s10803-013-1926-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman SH, Liu R, Lusby CM, Park JS, Bell MA, Newport DJ, & Stowe ZN (2020). Consistency of EEG asymmetry patterns in infants of depressed mothers. Developmental Psychobiology, 63, 768–781. 10.1002/dev.22046 [DOI] [PubMed] [Google Scholar]
- Harrewijn A, Buzzell GA, Debnath R, Leibenluft E, Pine DS, & Fox NA (2019). Frontal alpha asymmetry moderates the relations between behavioral inhibition and social-effect ERN. Biological Psychology, 141, 10–16. 10.1016/j.biopsycho.2018.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, & Conde JG (2009). Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics, 42(2), 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howarth GZ, Fettig NB, Curby TW, & Bell MA (2016). Frontal electroencephalogram asymmetry and temperament across infancy and early childhood: An exploration of stability and bidirectional relations. Child Development, 87(2), 465–476. 10.1111/cdev.12466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- John ER, Ahn H, Prichep L, Trepetin M, Brown D, & Kaye H (1980). Developmental equations for the electroencephalogram. Science, 210(4475), 1255–1258. 10.1126/science.7434026 [DOI] [PubMed] [Google Scholar]
- Jones NA, Field T, Davalos M, & Pickens J (1997). EEG stability in infants/children of depressed mothers. Child Psychiatry and Human Development, 28(2), 59–70. 10.1023/A:1025197101496 [DOI] [PubMed] [Google Scholar]
- Kagan J, Reznick JS, & Snidman N (1987). The physiology and psychology of behavioral inhibition in children. Child Development, 58, 1459–1473. 10.1111/j.1467-8624.1987.tb03858.x [DOI] [PubMed] [Google Scholar]
- Kane J (2021). An Excel Tool for Testing Differences between Correlations. Professional Statistical Services. https://www.prostatservices.com/blog/an-excel-tool-for-testing-differences-between-correlations [Google Scholar]
- Lopez-Calderon J, & Luck SJ (2014). ERPLAB: an open-source toolbox for the analysis of event related potentials. Frontiers in Human Neuroscience, 8, 213. 10.3389/fnhum.2014.00213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall PJ, Bar-Haim Y, & Fox NA (2002). Development of the EEG from 5 months to 4 years of age. Clinical Neurophysiology, 113(8), 1199–1208. 10.1016/S1388-2457(02)00163-3 [DOI] [PubMed] [Google Scholar]
- Müller BCN, Kühn-Popp N, Meinhardt J, Sodian B, & Paulus M (2015). Long-term stability in children’s frontal EEG alpha asymmetry between 14-months and 83-months. International Journal of Developmental Neuroscience, 41, 110–114. 10.1016/j.ijdevneu.2015.01.002 [DOI] [PubMed] [Google Scholar]
- Oostenveld R, Fries P, Maris E, & Schoffelen J (2011). FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Computational Intelligence and Neuroscience, 2011, Article ID 156869. 10.1155/2011/156869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly MA, Bathelt J, Sakkalou E, Sakki H, Salt A, Dale NJ, & de Haan M (2017). Frontal EEG asymmetry and later behavior vulnerability in infants with congenital visual impairment. Clinical Neurophysiology, 128(11), 2191–2199. 10.1016/j.clinph.2017.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghunathan TE, Rosenthal R, & Rubin DB (1996). Comparing correlated but nonoverlapping correlations. Psychological Methods, 1(2), 178–183. 10.1037/1082-989X.1.2.178 [DOI] [Google Scholar]
- Rahman ZI The impact of maternal depression on the relationship between EEG and behavioral inhibition through the first three years of life [Unpublished undergraduate thesis]. Harvard University. [Google Scholar]
- Steiger JH (1980). Tests for comparing elements of a correlation matrix. Psychological Bulletin, 87(2), 245–251. 10.1037/0033-2909.87.2.245 [DOI] [Google Scholar]
- Thorpe SG, Cannon EN, & Fox NA (2016). Spectral and source structural development of mu and alpha rhythms from infancy through adulthood. Clinical Neurophysiology, 127(1), 254–269. 10.1016/j.clinph.2015.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomarken AJ, Davidson RJ, Wheeler RE, & Kinney L (1992). Psychometric properties of resting anterior EEG asymmetry: Temporal stability and internal consistency. Psychophysiology, 29(5), 576–592. 10.1111/j.1469-8986.1992.tb02034.x [DOI] [PubMed] [Google Scholar]
- Trofimova I, & Robbins TW (2016). Temperament and arousal systems: A new synthesis of differential psychology and functional neurochemistry. Neuroscience and Biobehavioral Reviews, 64, 382–402. 10.1016/j.neubiorev.2016.03.008 [DOI] [PubMed] [Google Scholar]
- Vincent KM, Xie W, & Nelson CA (2021). Using different methods for calculating frontal alpha asymmetry (FAA) to study its development from infancy to 3 years of age in a large longitudinal sample. Retrieved from https://osf.io/6y3tj/?view_only=4e0c5622cd074e8d857e538d87e5dd09 [DOI] [PMC free article] [PubMed]
- Vuga M, Fox NA, Cohn JF, Kovacs M, & George CJ (2008). Long-term stability of electroencephalographic asymmetry and power in 3 to 9 year-old children. International Journal of Psychophysiology, 67(1), 70–77. 10.1016/j.ijpsycho.2007.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W, Jensen SKG, Wade M, Kumar S, Westerlund A, Kakon SH, Haque R, Petri WA, & Nelson CA (2019). Growth faltering is associated with altered brain functional connectivity and cognitive outcomes in urban Bangladeshi children exposed to early adversity. BMC Medicine, 17(1), 199–199. 10.1186/s12916-019-1431-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W, Leppänen JM, Kane-Grade FE, & Nelson CA (2021). Converging neural and behavioral evidence for a rapid, generalized response to threat-related facial expressions in 3-year-old children. NeuroImage (Orlando, Fla.), 117732–117732. 10.1016/j.neuroimage.2021.117732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W, McCormick SA, Westerlund A, Bowman LC, & Nelson CA (2018). Neural correlates of facial emotion processing in infancy. Developmental Science, e12758. 10.1111/desc.12758 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


