ABSTRACT
Aim:
To evaluate the salivary biomarkers and plaque index after a treatment with a propolis-contained toothpaste.
Materials and Methods:
This is a longitudinal, randomized, double-blind study where 76 participants were randomized into two groups: Group I: Fluoridated Red Propolis toothpaste; Group II: Fluoridated toothpaste. The participants were selected in a municipality without fluoridated public water. All participants received standardized oral hygiene instructions from the same instructor for 3 daily brushings (after breakfast, after lunch, and before bed) for a period of 2 min; Saliva samples were collected before (D0) and after 28 days (D28) of treatment for analysis of pH and total protein, amylase, and IL-10. Saliva was collected in the initial consultation and on return, totaling two collections. All samples were collected under the same conditions, by the same operator and between 9:00 AM and 11:00 AM in order to minimize the influence of circadian rhythm on salivary flow.
Results:
On D0 and D28, the various treatments had no effect on total salivary proteins (G1: P = 0.0746; G2: P = 0.2144), and the pH stayed about the same. Additionally, there was no change in the amylase activity in G1 (P = 0.1877) or G2 (P = 0.4674). Significant decreases in G1 (P < 0.0001) and G2 (P = 0.03) were observed with IL-10. There was no statistically significant difference in the salivary flow between the BRP toothpaste-treated group (P = 0.172) and the commercial fluoridated toothpaste-treated group (P = 0.329). Compared to G2 (P = 0.03), G1 showed a superior decline in the plaque index (P = <0.0001).
Conclusions:
After 28 days of using the toothpastes, there were no changes in the amylase, pH, or total protein indicators. After 28 days, there was a decrease in the propolis group’s IL-10 dose and plaque index.
Keywords: Biofilm, Biomarker, Propolis, Saliva
INTRODUCTION
Fixed orthodontic therapy is the most appropriate therapeutic modality for treating malocclusions. Despite the proven effectiveness of orthodontic appliances, they are biofilm-retentive factors and usually cause changes in the composition of the oral microbiota and salivary markers, increasing the risk of tooth decay and gingivitis.[1,2]
The possibility of identifying microbiological, immunological, and pharmacological markers (among others) and thereby exploring the salivary components makes saliva increasingly used as oral and systemic diagnostic material.[3] Salivary analysis in clinical efficacy studies identifies biomarkers of certain diseases and can be an excellent tool for tracking progress during treatment.[4,5]
Salivary flow is another important parameter, which is an important property of saliva having an essential function in oral health. Increased salivary flow promotes the physical cleaning of saliva, increases its antimicrobial properties, and accelerates substrate elimination. Thus, changes in salivary flow can be considered a physiological response to the presence of fixed orthodontic appliances, since the introduction of such appliances alters the homeostasis of the oral environment.[6]
Studies indicate changes in biomarkers in patients undergoing orthodontic treatment, such as mucin, amylase, and other proteins, including interleukins, among other things, proteoglycans, prostaglandin E, acid and alkaline phosphatases, tumor necrosis factor-α, and transforming growth factor β1.[4,7,8]
The pharmaceutical industry has long employed the inclusion of natural materials in formulations as an alternative to conventional medications; thus, there is a constant search for safe products with biological activity.[9,10] Dentifrices or mouthwashes have been combined with fluoride and substances with active biological activity in the dental field in order to obtain antimicrobial and anti-inflammatory activities, with propolis standing out among these.[11,12]
Brazilian red propolis (BRP), whose botanical origin is Dalbergia ecastophyllum (L) Taud. (Leguminosae), is found in the Marechal Deodoro region in Alagoas, Brazil, having Geographical Indication (GI) granted by the National Institute of Industrial Property (INPI, Brazil).[13] Its derivative products are becoming more and more popular on both the domestic and global markets, where demand for product standardization and modernization of the derived items is rising.[14,15,16,17]
Propolis extract has been shown in multiple studies to have a therapeutic impact on a variety of dental biofilm microorganisms and to be a low-toxicity clinical alternative.[18] In other clinical trials with orthodontic patients, treatment obtained positive results in controlling salivary levels of Lactobacillus spp. and plaque formation[19], on salivary levels of Streptococcus mutans, Gram-negative bacteria, and gingival bleeding index,[20] and on fluoride pharmacokinetics after brushing with fluoride BRP toothpaste.[21] These dentifrices were formulated with the purpose of releasing BRP in the oral cavity in order to obtain therapeutic effects without chemical interactions with the other constituents of the pharmaceutical product, guaranteeing its effectiveness and stability.
Thus, the objective of this study was to evaluate salivary parameters and control of dental biofilm after using toothpaste incorporated with red propolis extract in patients with orthodontic appliances.
MATERIALS AND METHODS
TYPE OF STUDY, ETHICAL ASPECTS, AND POPULATION
This study is double-blind, randomized, longitudinal, and parallel. In accordance with Brazilian resolution 466/12 and the Declaration of Helsinki on ethical principles for medical research involving human participants, this study was approved by the Ethics Committee of Human Research of the Federal University of Ceara (approval number 2.551.395). The Brazilian Registry of Clinical Trials (Rebec) has this study listed.
An active search was carried out in public elementary and high schools to select participants. After signing the informed consent of the guardians and the consent of the participants, 76 adolescents from 12 to 18 years old of both genders, caries-free (ICDAS II = 0), in good health, right-handed, users of fixed orthodontic appliances (conventional metallic brackets), and with a visible plaque index were selected. People with systemic changes or periodontitis who had undergone antimicrobial therapy up to three months prior to this study, licit/illicit drug users who had the presence of less than 10 dental elements per dental arch, or who were pregnant were excluded.
With a power of 90% (β = 0.10) and a significance level of 5% (α = 0.05), the sample for this study was created to show the statistical superiority of the toothpaste containing red propolis extract over regular toothpaste in the control of biofilm. Because the primary outcome is a quantitative variable, the sample size required to meet the aforementioned conditions was determined to be 38 people per group using the appropriate expression for studies of statistical superiority.
EXTRACT AND PREPARATION OF TOOTHPASTE
In Marechal Deodoro, Alagoas State, Brazil, the BRP extract was obtained at an altitude of 18.1 meters above sea level, in the south latitude of 9°44.555ʹ and the west latitude of 35°52.080ʹ. After extracting the BRP extract using cereal alcohol with a 96° graduation, it was diluted to a 1% concentration. In the pharmacy course’s pharmaceutics lab at the Federal University of Ceara, Brazil, this extract was added to the toothpaste that was fluoridated to a concentration of 1500 parts per million. Following the chemical identification of the ingredients by High Performance Liquid Chromatography (HPLC), which identified the primary constituents of quercetin, vestitol, and neovestitol, dentifrices were created with the same taste, color, and odor. The process of identification involved comparing the BRP samples’ chromatographic profile to standards of the extracted chemical constituents that were exposed to identical analysis circumstances. In order to determine similarity, the UV absorption spectra of the sample and reference were compared when retention times coincided.
CRITERIA AND PROCEDURES FOR SUBJECT SELECTION
Participants were selected in the municipality of Aracati-CE, Brazil, a municipality without fluoridated public water. The initial procedure consisted of clarifying the conditions under which clinical research is carried out and providing brief explanations of what it is and the procedures involved in the study.
After collecting personal and general health data, participants underwent a preliminary screening evaluation. An intraoral clinical examination was performed to assess oral health conditions and the visible plaque index.
Participants were instructed to avoid using antibiotics, anti-inflammatory drugs, anticoagulants, and anticonvulsants during this study. However, they would be withdrawn from the study in case of an emergency.
CLINICAL PHASE
Participants were randomized to one of the two groups listed below, for a total of 76 participants, 38 in each group. The sample had been previously calculated as appropriate. The treatment type applied to both investigators and participants was kept confidential. Samples were standardized for color, taste, and odor.
All participants received a toothbrush of the same brand with a straight handle, a small head, and soft bristles, as well as the treatment toothpaste. In addition, all received standardized oral hygiene instructions from the same instructor, in which the following topics were covered:
Number of brushings: 3 daily brushings (after breakfast, after lunch, and before bedtime) for a period of 2 min; and
Standardization in brushing technique, which was explained in the same way to all participants and their respective guardians.
The groups were distributed following the scheme below:
Group I (Group test): 1500 ppm fluoridated (MFP) toothpaste, incorporated with 1% BRP associated with brushing (Patent BR1020170110974).
Group II (Treatment test): 1500 ppm fluoridated (MFP) toothpaste associated with brushing.
The participants used the toothpaste for 28 days and returned on the last day for the final evaluation.
SALIVA COLLECTION
Unstimulated saliva from participants was collected via a pasteur pipette and stored in sterile microtubes (Eppendorfs). A protease inhibitor cocktail (Sigma, P2714) was then added, and these samples were kept and transported on ice for subsequent centrifugation at 12,000 g for 10 min at 4°C, supernatant collection, and storage at −80°C until analysis. A ratio of 5 μL (microliters) per mL (milliliter) of the following proteinase inhibitor was used: Protease Inhibitor Cocktail (Sigma Aldrich, Saint Louis, MO, USA).
Saliva was collected in the initial consultation and on return, totaling two collections. All samples were collected under the same conditions, by the same operator, and between 9:00 AM and 11:00 AM in order to minimize the influence of circadian rhythm on salivary flow.
DOSAGE OF TOTAL SALIVARY PROTEINS BY THE BICINCONINIC ACID (BCA) METHOD
Total salivary protein concentration of saliva aliquots was determined by the BCA method using a bovine serum albumin (BSA) curve as standard in Microsoft Excel 2013 (Microsoft Inc., Redmond, USA). A commercial kit (Sigma) was used following the manufacturer’s recommendations, and the solution was homogenized and read at 562 nm absorbance by a spectrophotometer (Biotec Epoch, USA). The results were calculated based on the BSA standard curve.
AMYLASE MEASUREMENT
Amylase activity was verified by aliquots of saliva using a commercial kit (Biotecnica) and following the manufacturer’s recommendations. The solution was then homogenized and read at 562 nm absorbance by a spectrophotometer (Biotec Epoch, USA). The 0.5 mL saliva sample was incubated at 37ºC for 2 min in a water bath. After a 10 mL aliquot, 0.5 mL of the working reagent and 4 mL of distilled water were added. This solution was incubated at 37ºC for exactly 7 min and 30 s, and the reading was taken.
IL-10 DOSAGE
IL-10 concentrations were determined by ELISA. Microtiter plates were coated with anti-IL-10 (Dako, 1:1000, BSA) at 1% BSA. The samples were incubated at room temperature for 30 min after washing (three times) and blocking the plates (1% BSA, 2 h). Plates were washed three times with buffer, followed by the addition of polyclonal secondary antibody (Sigma 1:1000, 1% BSA).
Following an additional 30-minute incubation period at room temperature, the plates underwent washing, and 50 µl of avidin-HRP (Abcam, 1:5000) was introduced. After 15 min, the O-phenylenediamine reagent (OPD; Biosystems, 50 µL) was added, and the plates were incubated for 30 min at 37 °C in the dark to produce IL-10. At 490 nm, absorbance was measured. The findings are presented as mean ± SEM on a typical cytokine curve and are represented in pg/mL sample.
DETERMINATION OF SALIVARY FLOW AND HYDROGEN POTENTIAL (pH)
Salivary flow was recorded in mL/min based on the total volume of saliva collected over 5 min. Salivary pH was verified by measuring tapes (Merck). The reagent strip was submerged in saliva for 5 s and the excess was removed, and readings were carried out after 15 s.
STATISTICAL METHOD
Descriptive statistics were performed for the analysis of the results, which compared the intra-group and inter-group of the two moments studied using the Mann–Whitney test (nonparametric variables). This test was designed to compare the core trends of two independent samples of equal size. A confidence index of 95% and a significance of P > 0.05 were considered.
RESULTS
The mean pH in the BRP group was 5.85 before treatment and 5.95 after treatment, while it was 6 before and after treatment in the common toothpaste group.
Graph 1 shows the variation in the concentration in µg/mL of total salivary protein in patients before and after treatment with BRP toothpaste. There was no statistically significant difference between the different groups and times in this analysis, where G1 (P = 0.0746) and G2 (P = 0.2144).
Graph 1.
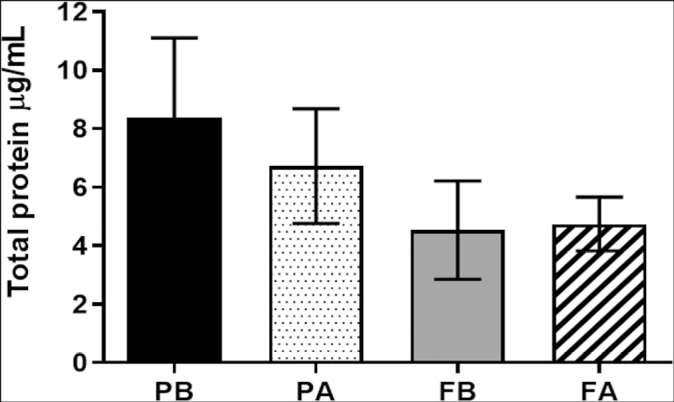
Concentration of total proteins in the groups. Caption: PB corresponds to the value found before the start of treatment (D0) and PA to the value found after the end of treatment (D28) with BRP dentifrice. FB corresponds to the value found before the beginning of treatment (D0) and FA to the value found after the end of treatment (D28) with common dentifrice.
Graph 2 shows the U/L amylase activity in the saliva of the patients before and after treatment with the toothpaste. There was no statistically significant difference between the different groups and times in this analysis, G1 (P = 0.1877) and G2 (P = 0.4674).
Graph 2.
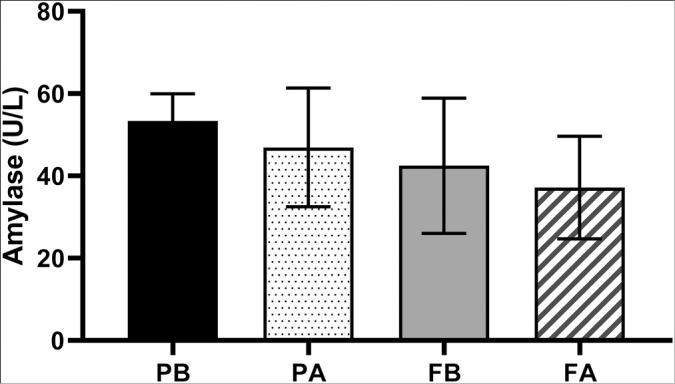
Amylase activity in the groups studied at different times. Caption: PB corresponds to the value found before the start of treatment (D0) and PA to the value found after the end of treatment (D28) with BRP dentifrice. FB corresponds to the value found before the beginning of treatment (D0) and FA to the value found after the end of treatment (D28) with common dentifrice.
Graph 3 shows the dosage of interleukin 10 (IL-10) in the saliva of patients at the end of different treatments. There was a statistical difference with a significant reduction in this analysis, G1 (P < 0.0001) and G2 (P = 0.03).
Graph 3.
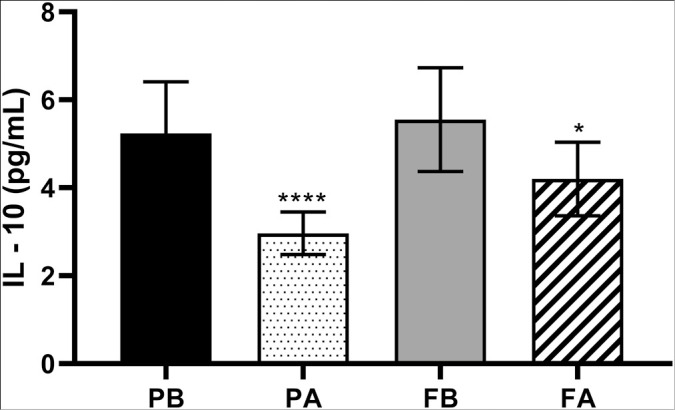
Dosage of IL-10 in the groups studied at different times. Caption: PB corresponds to the value found before the start of treatment (D0) and PA to the value found after the end of treatment (D28) with BRP dentifrice. FA corresponds to the value found before the beginning of treatment (D0) and FB to the value found after the end of treatment (D28) with common dentifrice.
Table 1 shows the salivary flow measurements of the participants in the different groups and times. There was an increase in flow in the group treated with BRP toothpaste, but without statistical significance (P = 0.172). There was also an increase in the group treated with common toothpaste, but without statistical significance (P = 0.329).
Table 1.
Measurement of salivary flow in the group treated with BRP dentifrice and common dentifrice at different times
| Salivary flow (mL/min) | ||||
|---|---|---|---|---|
| G1 before | G1 after | G2 before | G2 after | |
| Mean ± SD | 0.787 ± 0.126 | 0.846 ± 0.128 | 0.799 ± 0.154 | 0.851 ± 0.153 |
| p | 0.172 | 0.329 | ||
G1 Before: BRP dentifrice before the treatment; G1 After: BRP dentifrice after the treatment; G2 Before: Common dentifrice before the treatment; G2 After: Common dentifrice after the treatment
Table 2 shows the plaque index of the participants in the different groups and times. In the group treated with BRP toothpaste, after the treatment, we can see a better decrease of plaque (P < 0.0001) when compared with the decrease of the common toothpaste (P = 0.03).
Table 2.
Changes in plaque index in the group treated with BRP dentifrice and common dentifrice at different times
| Plaque index | ||||
|---|---|---|---|---|
| G1 before | G1 after | G2 before | G2 after | |
| Mean ± SD | 38.10 ± 17.95 | 20.60 ± 16.44 | 38.38 ± 19.65 | 27.40 ± 14.63 |
| p | <0.0001 | 0.03 | ||
G1 Before: BRP dentifrice before the treatment; G1 After: BRP dentifrice after the treatment; G2 Before: Common dentifrice before the treatment; G2 After: Common dentifrice after the treatment
DISCUSSION
The present study evaluated changes in salivary biomarkers after 28 days of use with a new proposed toothpaste with proven antimicrobial activity.[19,20] Studies in the literature show that biofilm and the prevalence of active caries and gingivitis lesions are higher during orthodontic treatment.[22,23] In this study, all individuals were orthodontic patients with gingivitis. Saliva was used because it is a fluid that reflects oral and systemic changes and is an excellent biomarker.[4] In this case, unstimulated saliva was chosen as it was suitable for this type of study.
Analysis of changes in proteins has been studied in orthodontic patients. Zogakis et al.[24] found changes in pH and protein in patients treated with fixed orthodontic appliances. A similar result was also found by Bilgic et al.[1]
Henskens et al.[25] investigated changes in salivary proteins in patients with gingivitis and periodontitis, also using the BCA method. Salivary protein levels increased considerably in individuals with periodontal disease. In our study, we used the same method and there was no statistically significant intra-group difference in the comparison of different treatments at D0 and D28.
Individuals with gingivitis have already shown changes in amylase activity, although less than those with periodontitis.[5] Amylase activity also showed no change between the different groups before and after treatment, G1 (P = 0.1877) and G2 (P = 0.4674). These findings are similar to Teixeira et al.[26]
There is strong evidence for the relationship between orthodontic appliance treatment and biofilm increase of gingival bleeding and pH decrease, being common changes on the salivary parameters in these patients.[24,27,28] In this study, there were no significant differences between the pH and salivary flow at the different times before and after treatment in both groups. However, the plaque index decreased, especially in the group of BRP toothpaste that had a greater reduction, findings similar to Lotif et al.[19]
In addition to changes in the microbiota, orthodontic appliances can alter salivary flow and viscosity.[29] Arab et al.[6] also evaluated changes in salivary parameters in patients undergoing orthodontic treatment. Both groups in our study showed increased salivary flow, but without significance (G1: P = 0.172; G2: P = 0.329).
Teixeira et al.[26] also evaluated possible changes in salivary parameters after beginning orthodontic treatment. Salivary flow, pH, buffer capacity, amylase activity, total protein concentrations, calcium, and glucose were measured in all salivary samples. Their study showed a lower pH in the saliva and an increase in the total protein and amylase when compared to individuals without orthodontic appliances. Different from this present study, they did not have an intervention to evaluate changes in parameters. However, the authors cite the importance of additional oral care procedures for these orthodontic patients.
The literature relates a strong relationship between interleukin-10 (IL-10) and periodontal disease. Increased interleukin-10 (IL-10) levels are a potential risk factor for periodontal disease.[30] Geng et al.[31] quantified IL-10 production in patients with periodontal disease, finding a higher concentration in patients with periodontal disease. At the beginning of treatment, individuals had higher levels of IL-10, which is in line with the study cited. After the treatment, the IL-10 analysis showed a statistical difference with a significant reduction in G1 (P < 0.0001) and G2 (P = 0.03) at the end of the treatment. The greatest reduction in the group treated with BRP is due to its anti-inflammatory activity, a fact also evidenced by Furtado et al.[20]
It is known that adequate daily control of mechanical biofilm is the most important prevention strategy for periodontal diseases; however, it is not enough in the case of some orthodontic patients, which makes this group look for alternatives such as mouthwash. Although chlorhexidine has antibiofilm and antimicrobial results, it should not have continuous use and is not indicated for long-term periods.[32,33] The reduction in plaque index is supported by the fact that mechanical control was carried out in both groups, but with the addition of an active antimicrobial ingredient such as BRP, this reduction can be enhanced, as shown in the findings.
Unlike all the studies found on salivary parameters in orthodontic patients, the present study evaluated a change after an intervention, which is actually a relevant scientific contribution. As limitations, we can cite the short age range evaluated and the fact that it is not a multicentric study.
Thus, we seek alternatives for chemical and mechanical biofilm control for these patients. A toothpaste having antimicrobial activity is an advantage in these situations.
From the above, it can be seen that the BRP toothpaste did not change salivary parameters, and it was possible to denote anti-inflammatory activity due to the reduction in its parameters. Future studies will be necessary with a larger, more heterogeneous population and a longer follow-up period.
Through this study, it was possible to verify the beneficial effects in vivo of a toothpaste incorporated with natural products that already have antibiofilm activity against microorganisms that participate in pathogenic processes in the oral cavity, such as caries and gingivitis.
CONCLUSION
In this study, there were no differences between the total protein, pH, and amylase markers when comparing the BRP and common toothpastes after 4 weeks of use by participants. The IL-10 dosage and plaque index were reduced in the BRP group after the period of use.
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest.
FINANCIAL SUPPORT AND SPONSORSHIP
This study has been supported by the National Council for Scientific and Technological Development.
AUTHOR CONTRIBUTIONS
Conceptualization, Methodology, and Writing—Original Draft Preparation (Silva MA); Investigation (Silva MA, Valadas LAR, Rodrigues Neto EM, and Dantas TCFB); Data curation and Formal Analysis (Oliveira GAL); Writing—Review and Editing, Visualization, Supervision, Project Administration, and Funding Acquisition (Bandeira MAM, Fonteles MMF, and Baptista GR).
ETHICAL POLICY AND INSTITUTIONAL REVIEW BOARD STATEMENT
This research was approved by ethical committees of the Federal University of Ceará.
PATIENT DECLARATION OF CONSENT
All patients and parents declared informed consent.
DATA AVAILABILITY STATEMENT
Data available within the article or its supplementary materials
ACKNOWLEDGEMENT
We acknowledge all the participants and their parents (National Council for Scientific and Technological Development—CNPQ) for supporting this study.
REFERENCES
- 1.Bilgic F, Akinci Sozer O, Ozcan O, Gurpinar AB, Yilmaz H, Ay Y. Evaluation of inflammation during fixed orthodontic treatment. Arch Oral Biol. 2016;71:54–8. doi: 10.1016/j.archoralbio.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 2.Pitts NB, Zero DT, Marsh PD, et al. Dental caries. Nat Rev Dis Prim. 2017;3:17030. doi: 10.1038/nrdp.2017.30. [DOI] [PubMed] [Google Scholar]
- 3.Fraga E, Rodrigues Neto EM, Campos FM, da Silva Cavalcante MP. Evaluation of chewing gums containing natural products on salivary concentrations of streptococcus mutans in Children. J Young Pharm. 2022;15:182–8. [Google Scholar]
- 4.Siqueira EC, Campos FM, Mendes JC, De Lima LN. Evaluation of the effects of propolis and xylitol chewable tablets on the salivary concentrations of oral micro-organisms in orthodontic patients: A pilot study. J Young Pharm. 2021;13:68. [Google Scholar]
- 5.Carolina DN, Rusyanti Y, Susanto A. Comparison of salivary alpha-amylase levels in gingivitis and periodontitis. Dent J Majalah Kedokteran Gigi. 2017;50:216–9. [Google Scholar]
- 6.Arab S, Nouhzadeh Malekshah S, Abouei Mehrizi E, Ebrahimi Khanghah A, Naseh R, Imani MM. Effect of fixed orthodontic treatment on salivary flow, pH and microbial count. J Dent (Tehran) 2016;13:18–22. [PMC free article] [PubMed] [Google Scholar]
- 7.Alikhani M, Alyami B, Lee IS, Almoammar S, Vongthongleur T, Alikhani M, et al. Saturation of the biological response to orthodontic forces and its effect on the rate of tooth movement. Orthod Craniofac Res. 2015;18:8–17. doi: 10.1111/ocr.12090. [DOI] [PubMed] [Google Scholar]
- 8.Kaczor-Urbanowicz KE, Deutsch O, Zaks B, Krief G, Chaushu S, Palmon A. Identification of salivary protein biomarkers for orthodontically induced inflammatory root resorption. Proteomics Clin Appl. 2017;11:1600119. doi: 10.1002/prca.201600119. [DOI] [PubMed] [Google Scholar]
- 9.George DRM, Kasliwal DAV. Effectiveness of propolis, probiotics and chlorhexidine on streptococcus mutans and candida albicans: An in-vitro study. IOSR J Dent Med Sci. 2017;16:15–8. [Google Scholar]
- 10.Valadas LAR, Gurgel MF, Mororó JM, Fonseca SGC, Fonteles CSR, de Carvalho CBM, et al. Dose-response evaluation of a copaiba-containing varnish against streptococcus mutans in vivo. Saudi Pharm J. 2019;27:363–7. doi: 10.1016/j.jsps.2018.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lobo PLD, Fonteles CSR, Marques LARV, Jamacaru FVF, Fonseca SGC, de Carvalho CBM, et al. The efficacy of three formulations of Lippia sidoides Cham. essential oil in the reduction of salivary Streptococcus mutans in children with caries: A randomized, double-blind, controlled study. Phytomedicine. 2014;21:1043–7. doi: 10.1016/j.phymed.2014.04.021. [DOI] [PubMed] [Google Scholar]
- 12.de Carvalho Furtado JH, Jr, Rocha Valadas LA, Mendonça KS, de Oliveira Filho RD, Gadelha LMU, de Mello Fiallos N, et al. Propolis and its dental applications: A technological prospection. Recent Pat Biotechnol. 2018;12:288–96. doi: 10.2174/2211550107666180815114855. [DOI] [PubMed] [Google Scholar]
- 13.Freires IA, de Alencar SM, Rosalen PL. A pharmacological perspective on the use of Brazilian Red Propolis and its isolated compounds against human diseases. Eur J Med Chem. 2016;110:267–79. doi: 10.1016/j.ejmech.2016.01.033. [DOI] [PubMed] [Google Scholar]
- 14.do Nascimento TG, da Silva PF, Azevedo LF, da Rocha LG, de Moraes Porto ICC, Lima E Moura TFA, et al. Polymeric Nanoparticles of Brazilian Red Propolis Extract: Preparation, Characterization, Antioxidant and Leishmanicidal Activity. Nanoscale Res Lett. 2016;11:301. doi: 10.1186/s11671-016-1517-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farias Azevedo L, da Fonseca Silva P, Porfírio Brandão M, Guerra da Rocha L, Aragão CFS, da Silva SAS, et al. Polymeric nanoparticle systems loaded with red propolis extract: A comparative study of the encapsulating systems, PCL-Pluronic versus Eudragit® E100-Pluronic. J Api Res. 2018;57:255–70. [Google Scholar]
- 16.Salatino A. Brazilian red propolis: Legitimate name of the plant resin source. MOJ Food Process Technol. 2018;6 [Google Scholar]
- 17.Barros KBNT, Neto EMR, de França Fonteles MM. Propolis and its cosmetic applications: A technological prospection. J Young Pharm. 2019;11:350–2. [Google Scholar]
- 18.Bueno-Silva B, Alencar SM, Koo H, Ikegaki M, Silva GVJ, Napimoga MH, et al. Anti-Inflammatory and Antimicrobial Evaluation of Neovestitol and Vestitol Isolated from Brazilian Red Propolis. J Agric Food Chem. 2013;61:4546–50. doi: 10.1021/jf305468f. [DOI] [PubMed] [Google Scholar]
- 19.Lotif MAL, Valadas LAR, Fechine FV, Fonseca SGC, Bandeira MAM, Dantas TCFB, et al. A double-blind randomized clinical trial of Brazilian red propolis dentifrice efficacy in orthodontic patients. J Oral Sci. 2022;64:28–32. doi: 10.2334/josnusd.21-0270. [DOI] [PubMed] [Google Scholar]
- 20.Furtado Júnior JHDC, Valadas LAR, Fonseca SGDC, Lobo PLD, Calixto LHM, Lima AGF, et al. Clinical and microbiological evaluation of Brazilian red propolis containing-dentifrice in orthodontic patients: A randomized clinical trial. EvidBased Complement Alternat Med. 2020;2020:8532701. doi: 10.1155/2020/8532701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Girão Júnior FJ, Valadas LAR, Bottenberg P, Lotif MAL, Rodrigues Neto EM, Fonseca SGC, et al. Salivary fluoride bioavailability after brushing with Brazilian red propolis dentifrice. Evid Based Complement Alternat Med. 2022;2022:1–7. doi: 10.1155/2022/6148137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahmed MAA, Mohammed-Salih HS, Yassir YA, Al-Judy HJ. Time-related salivary cathepsin B levels and periodontal status in different orthodontic force magnitudes. J Bagh Coll Dent. 2015;27:115–22. [Google Scholar]
- 23.Zogakis IP, Koren E, Gorelik S, Ginsburg I, Shalish M. Effect of fixed orthodontic appliances on nonmicrobial salivary parameters. Angle Orthod. 2018;88:806–11. doi: 10.2319/111317-773.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cardoso AA, Lopes LM, Rodrigues LP, Teixeira JJ, Steiner-Oliveira C, Nobre-dos-Santos M. Influence of salivary parameters in the caries development in orthodontic patients-an observational clinical study. Int J Paediatr Dent. 2017;27:540–50. doi: 10.1111/ipd.12295. [DOI] [PubMed] [Google Scholar]
- 25.Henskens YMC, Velden U, Veerman ECI, Amerongen AVN. Protein, albumin and cystatin concentrations in saliva of healthy subjects and of patients with gingivitis periodonitis. J Periodontal Res. 1993;28:43–8. doi: 10.1111/j.1600-0765.1993.tb01049.x. [DOI] [PubMed] [Google Scholar]
- 26.Teixeira HS, Kaulfuss SMO, Ribeiro JS, Pereira B do R, Brancher JA, Camargo ES. Calcium, amylase, glucose, total protein concentrations, flow rate, pH and buffering capacity of saliva in patients undergoing orthodontic treatment with fixed appliances. Dental Press J Orthod. 2012;17:157–61. [Google Scholar]
- 27.Rego RO, Oliveira CA, dos Santos-Pinto A, Jordan SF, Zambon JJ, Cirelli JA, et al. Clinical and microbiological studies of children and adolescents receiving orthodontic treatment. Am J Dent. 2010;23:317–23. [PubMed] [Google Scholar]
- 28.Ahmed Moussa S. Dental biofilm and saliva biochemical composition changes in young orthodontic patients. J Dent Oral Disord Ther. 2017;5:1–5. [Google Scholar]
- 29.Sánchez ERB, Honores MJC. Efecto de la aparatología ortodóntica fi ja sobre el flujo y la viscosidad salival. Rev Mex Ortod. 2015;3:186–90. [Google Scholar]
- 30.Moudi B, Heidari Z, Mahmoudzadeh-Sagheb H, Moudi M. Analysis of interleukin-10 gene polymorphisms in patients with chronic periodontitis and healthy controls. Dent Res J (Isfahan) 2018;15:71–9. doi: 10.4103/1735-3327.223614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Geng Y, Li L, Wang X, He F, Zhou Y, Yang M, et al. Interleukin-10 polymorphisms affect the key periodontal pathogens in Chinese periodontitis patients. Sci Rep. 2018;8:9068. doi: 10.1038/s41598-018-26236-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haas AN, Pannuti CM, Andrade AKP de, et al. Mouthwashes for the control of supragingival biofilm and gingivitis in orthodontic patients: Evidence-based recommendations for clinicians. Braz Oral Res. 2014;28:1–8. doi: 10.1590/1807-3107bor-2014.vol28.0021. [DOI] [PubMed] [Google Scholar]
- 33.Goes P, Dutra CS, Lisboa MRP, Gondim DV, Leitão R, Brito GAC, et al. Clinical efficacy of a 1% Matricaria chamomile L. mouthwash and 0.12% chlorhexidine for gingivitis control in patients undergoing orthodontic treatment with fixed appliances. J Oral Sci. 2016;58:569–74. doi: 10.2334/josnusd.16-0280. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available within the article or its supplementary materials


