Abstract
Clinical trials frequently include multiple end points that mature at different times. The initial report, typically based on the primary end point, may be published when key planned co-primary or secondary analyses are not yet available. Clinical Trial Updates provide an opportunity to disseminate additional results from studies, published in JCO or elsewhere, for which the primary end point has already been reported.
Although the CNS activity of selpercatinib in patients with RET fusion-positive non–small cell lung cancer (NSCLC) has been previously described, the ability of potent RET inhibition to prevent new CNS metastases from developing has been challenging to measure without randomized data. Serial CNS scans were studied from LIBRETTO-431, a randomized phase III trial of selpercatinib versus platinum/pemetrexed ± pembrolizumab whose primary results have been previously disclosed. Intracranial outcomes were assessed by neuroradiologic blinded independent central review in patients with baseline and ≥1 postbaseline CNS scans. Of the 192 patients within the intention-to-treat pembrolizumab population with baseline CNS scans, 150 patients were without baseline CNS metastases. The cumulative incidence of CNS progression in these patients was reduced with selpercatinib versus chemotherapy + pembrolizumab (cause-specific hazard ratio [HR], 0.17 [95% CI, 0.04 to 0.69]). The HR for intracranial progression-free survival (PFS) was 0.46 (95% CI, 0.18 to 1.18). Among the 42 patients with baseline CNS metastases, similar trends were observed in the cumulative incidence of CNS progression (cause-specific HR, 0.61 [95% CI, 0.19 to 1.92]) and intracranial PFS (HR, 0.74 [95% CI, 0.28 to 1.97]). These data demonstrate that selpercatinib effectively treats existing CNS disease and prevents or delays the formation of new CNS metastases. These results reinforce the importance of identifying RET fusions in first-line patients with NSCLC and treating with selpercatinib.
INTRODUCTION
Selpercatinib is a highly selective and potent RET-kinase inhibitor, approved in multiple geographies for the treatment of RET-driven cancers. On the basis of the marked clinical benefit observed in the single-arm LIBRETTO-001 study and randomized phase III LIBRETTO-431 study, guidelines recommend selpercatinib as a treatment option for patients with RET fusion-positive non–small cell lung cancer (NSCLC).1-4
Selpercatinib's activity against CNS metastases was first described in 20186 and more recently validated in the LIBRETTO-431 trial which demonstrated an 82% intracranial response rate in patients with measurable disease. CNS metastases are common among patients with RET fusion-positive NSCLC, who have a lifetime prevalence of up to 50%.7,8 Given the poor prognosis of patients with CNS metastases and the potential morbidity of brain radiation, the prevention of brain metastasis is an important aim but difficult to study without a randomized trial.2 Here, we further examine the CNS activity of selpercatinib with a focus on its CNS protective effect in patients without baseline CNS metastases.
METHODS
The study was conducted in accordance with the International Council for Harmonization Good Clinical Practice guidelines and principles of the Declaration of Helsinki and all applicable country and local regulations. The protocol was approved by the institutional review board or independent ethics committee at each investigator site. All patients provided written informed consent.
Detailed study design and statistical methodology are reported in the Data Supplement (online only).
RESULTS
Patients
Of 261 patients enrolled, 234 were included in the CNS-overall population (Appendix Fig A1, online only). Baseline intracranial scans were performed via magnetic resonance imaging for 69% of patients in the selpercatinib arm and 57% of patients in the control arm. The CNS-pembro population comprised 192 patients (selpercatinib: n = 120, control: n = 72), of whom 78% (n = 150) had no baseline CNS metastases per blinded independent central review assessment. In the CNS-pembro population, the selpercatinib group had a lower proportion of patients with baseline CNS metastases (mets) versus the control group (CNS-pembro-mets: 17.5% v 29.2%). In the CNS-pembro population without baseline CNS metastases (nonmets) (CNS-pembro-nonmets population), patients in the selpercatinib group were younger (median age: 58 v 62 years), more likely to have an Eastern Cooperative Oncology Group performance status of 0 (35.4% v 25.5%) and more likely to have stage IV disease (93.9% v 88.2%; Table 1).
TABLE 1.
Baseline Demographics and Disease Characteristics of CNS-Pembro Population
|
Characteristic |
CNS-Pembro Population (N = 192) | |||
|---|---|---|---|---|
| Patients Without Baseline Brain Metastases (n = 150) | Patients With Baseline Brain Metastases (n = 42) | |||
| Selpercatinib (n = 99) | Control (n = 51) | Selpercatinib (n = 21) | Control (n = 21) | |
| Age, years | ||||
| Median (range) | 58 (31-84) | 62 (31-83) | 64 (38-78) | 58 (35-79) |
| Age distribution, No. (%) | ||||
| <65 years | 64 (64.6) | 30 (58.8) | 12 (57.1) | 13 (61.9) |
| ≥65 years | 35 (35.4) | 21 (41.2) | 9 (42.9) | 8 (38.1) |
| Sex, No. (%) | ||||
| Female | 46 (46.5) | 28 (54.9) | 15 (71.4) | 13 (61.9) |
| Male | 53 (53.5) | 23 (45.1) | 6 (28.6) | 8 (38.1) |
| Race, No. (%) | ||||
| Asian | 62 (62.6) | 30 (62.5) | 12 (57.1) | 9 (45.0) |
| White | 33 (33.3) | 17 (35.4) | 9 (42.9) | 11 (55.0) |
| Othera | 4 (4.0) | 1 (2.1) | 0 | 0 |
| Region of enrollment, No. (%) | ||||
| East Asia | 62 (62.6) | 30 (58.8) | 11 (52.4) | 9 (42.9) |
| Non-East Asia | 37 (37.4) | 21 (41.2) | 10 (47.6) | 12 (57.1) |
| Smoking status, No. (%) | ||||
| Never smoked | 62 (62.6) | 35 (68.6) | 16 (76.2) | 17 (81.0) |
| Former smoker | 34 (34.3) | 14 (27.5) | 4 (19.0) | 4 (19.0) |
| Current smoker | 3 (3.0) | 2 (3.9) | 1 (4.8) | 0 |
| ECOG performance status,b No. (%) | ||||
| 0 | 35 (35.4) | 13 (25.5) | 3 (14.3) | 8 (38.1) |
| 1 | 62 (62.6) | 36 (70.6) | 17 (81.0) | 11 (52.4) |
| 2 | 2 (2.0) | 2 (3.9) | 1 (4.8) | 2 (9.5) |
| Stage of disease, No. (%) | ||||
| Stage III | 6 (6.1) | 6 (11.8) | 0 | 0 |
| Stage IVA | 40 (40.4) | 23 (45.1) | 4 (19.0) | 6 (28.6) |
| Stage IVB | 53 (53.5) | 22 (43.1) | 17 (81.0) | 15 (71.4) |
| Prior CNS radiotherapy, No. (%) | ||||
| Yes | 1.0 (1.0) | 0 | 6 (28.6) | 7 (33.3) |
| No | 98 (99.0) | 100 (100.0) | 15 (71.4) | 14 (66.7) |
| RET fusion result, No. (%) | ||||
| Positivec | 47 (47.5) | 20 (39.2) | 6 (28.6) | 4 (19.0) |
| KIF5B-RET | 40 (40.4) | 24 (47.1) | 13 (61.9) | 15 (71.4) |
| CCDC6-RET | 9 (9.1) | 6 (11.8) | 2 (9.5) | 0 |
| Otherd | 3 (3.0) | 1 (2.0) | 0 | 2 (9.6) |
NOTE. Percentages may not total 100 because of rounding.
Abbreviations: ECOG, Eastern Cooperative Oncology Group; PCR, polymerase chain reaction.
Other included Black, American Indian or Alaska Native, Native Hawaiian or other Pacific Islander, and multiple races.
ECOG performance-status scores range from 0 to 5, with higher numbers reflecting greater disability.
RET fusion was indicated by PCR analysis but the RET fusion partner was not identified.
Other included NCOA4–RET, KIF13A–RET, KIAA1549L–RET, PRKAR1A–RET, or multiple fusion partners (KIF5B–RET and CDKAL1–RET in one patient and NCOA4–RET and ZNF32-AS3–RET in the other).
Cumulative Incidence Rate and Intracranial Progression-Free Survival in Patients Without CNS Metastases
In the 150 patients in the CNS-pembro-nonmets population, the 12-month cumulative incidence rate (CIR) of CNS progression was 1.1% (95% CI, 0.1 to 5.2) with selpercatinib versus 14.7% (95% CI, 5.7 to 27.6) with control (cause-specific hazard ratio [HR], 0.17 [95% CI, 0.04 to 0.69]; Fig 1A). Among selpercatinib-treated patients, 3.0% (3/99) had a first event of CNS progression versus 11.8% (6/51) of patients with control. The 12-month CIR of non-CNS progression was also lower with selpercatinib compared with control (cause-specific HR, 0.48 [95% CI, 0.27 to 0.84]; Fig 1B). In the CNS-overall-nonmets population, similar results were observed (Appendix Figs A2A and A2B) with a cause-specific HR for CNS progression of 0.16 (95% CI, 0.04 to 0.66). Patterns of progression in the CNS-overall-nonmets population are presented in Appendix Table A1.
FIG 1.

CIR and intracranial PFS in the CNS-pembro-nonmets population. (A) CIR of CNS PD; (B) CIR of non-CNS PD and death; and (C) intracranial PFS. CIR, cumulative incidence rate; CNS-pembro-nonmets, CNS-pembrolizumab population without baseline brain metastases; HR, hazard ratio; PD, progressive disease; PFS, progression-free survival.
In the CNS-pembro-nonmets population, with a median follow-up time of 15.6 months for selpercatinib and 11.1 months for control, the median intracranial progression-free survival (PFS) was not yet reached (95% CI, not evaluable [NE] to NE) with selpercatinib or control (95% CI, NE to NE). The 12-month intracranial PFS rate was 91.8% (95% CI, 83.4 to 96.0) with selpercatinib versus 74.7% (95% CI, 54.0 to 87.1) with control (HR, 0.46 [95% CI, 0.18 to 1.18]; Fig 1C). In the CNS-overall-nonmets population, similar results were observed (HR, 0.53 [95% CI, 0.22 to 1.32]; Appendix Fig A2C).
CIR and Intracranial PFS in Patients With CNS Metastases
In the 42 patients in the CNS-pembro-mets population, the 12-month CIR of CNS progression was 25.7% (95% CI, 8.8 to 46.7) with selpercatinib versus 33.3% (95% CI, 14.3 to 53.8) with control (cause-specific HR, 0.61 [95% CI, 0.19 to 1.92]; Appendix Fig A3A). Among selpercatinib-treated patients, 23.8% (5/21) had a first event of CNS progression versus 33.3% (7/21) with control. The 12-month CIR of non-CNS progression also trended lower with selpercatinib compared with control (cause-specific HR, 0.45 [95% CI, 0.15 to 1.36]; Appendix Fig A3B). In the CNS-overall-mets population, similar results were observed (Appendix Figs A4A and A4B) with a cause-specific HR for CNS progression of 0.52 (95% CI, 0.19 to 1.47). Patterns of progression in the CNS-overall-mets population are presented in Appendix Table A1.
In the CNS-pembro-mets population, with a median follow-up time of 14 months in both arms, the median intracranial PFS was not yet reached (95% CI, 9.0 to NE) with selpercatinib versus 14.6 months (95% CI, 4.8 to NE) with control. The 12-month intracranial PFS rate was 63.9% (95% CI, 38.5 to 81.1) with selpercatinib versus 56.1% (95% CI, 28.7 to 76.5) with control (HR, 0.74 [95% CI, 0.28 to 1.97]; Appendix Fig A3C). In the CNS-overall-mets population, similar results were observed (HR, 0.63 [95% CI, 0.27 to 1.48]; Appendix Fig A4C).
Intracranial Tumor Response
We previously reported response data in the CNS-pembro-mets population with measurable disease at baseline (n = 29). In the CNS-pembro-mets population with measurable or nonmeasurable disease at baseline (n = 42), the complete response rate was 42.9% (9/21) with selpercatinib versus 33.3% (7/21) with control (Appendix Table A2). In the CNS-pembro-mets population without prior CNS radiotherapy, the intracranial response rate was 93.3% (14/15) with selpercatinib versus 64.3% (9/14) with control (Appendix Table A3). Intracranial responses to selpercatinib were more common in patients without prior CNS radiotherapy (93%) than with prior CNS radiotherapy (50%). In the CNS-overall-mets population, intracranial responses were consistent with the CNS-pembro-mets population and listed in Appendix Table A4.
Time to Intracranial Response and Duration of Intracranial Response
Time to intracranial response, duration of response (DOR), and time to intracranial progression were also assessed in the CNS-pembro-mets population with measurable or nonmeasurable disease at baseline (n = 42). The median time to intracranial response was 1.4 months with selpercatinib and 2.2 months with control (Appendix Table A2). With a median follow-up time of 12.4 months in the selpercatinib group and 9.5 months in the control group, the median intracranial DOR was not yet reached with selpercatinib (95% CI, 14.8 to NE) or control (95% CI, 8.7 to NE). The 12-month intracranial DOR rate was 80.7% (95% CI, 51.1 to 93.4) with selpercatinib and 75.8% (95% CI, 30.5 to 93.7) with control (Appendix Table A2). Overall, seven of nine patients on selpercatinib and three of seven patients on control achieving intracranial CR remained on treatment with no intracranial PD at the time of data cutoff (Fig 2).
FIG 2.
Duration of treatment and time to intracranial response and progression by blinded independent central review assessment in the CNS-pembro-mets population for (A) selpercatinib and (B) control. CNS-pembro-mets, CNS-pembrolizumab population with baseline brain metastases.
DISCUSSION
Selpercatinib has previously demonstrated robust CNS activity in single-arm studies of patients with advanced RET fusion-positive NSCLC.1,9 To our knowledge, LIBRETTO-431 is the first randomized trial to evaluate the intracranial efficacy of first-line selpercatinib compared with chemotherapy ± pembrolizumab. This report focuses on intracranial efficacy in the 150 patients in the CNS-pembro-nonmets population (CNS-overall-nonmets population, n = 183). In this population, we observed a pronounced difference in the 12-month CIR of CNS progression between selpercatinib and control (1.1% v 14.7%), indicating a delay in CNS progression among patients without CNS metastases (cause-specific HR, 0.17 [95% CI, 0.04 to 0.69]). Although the data have yet to fully mature, the 12-month intracranial PFS rate was 91.8% with selpercatinib versus 74.7% with control (HR, 0.46 [95% CI, 0.18 to 1.18]). The intracranial overall response rate of 93.3% among patients that were not previously irradiated to the CNS may be a more accurate estimation of the intracranial efficacy of selpercatinib in patients harboring RET rearrangements. These data supplement the primary LIBRETTO-431 report to further demonstrate that selpercatinib may prevent the formation of new CNS metastases in addition to treating existing CNS metastases.
Limitations of our study include short follow-up time, small number of events, and low number of evaluable patients in certain subgroups, particularly the population of patients with baseline CNS metastases. Another potential limitation of the study was that serial CNS imaging was added as a requirement for all patients with a protocol amendment and not at the outset of the trial. Despite this, the majority of study patients were evaluable for CNS outcomes.
Longer follow-up will be required to confirm the intracranial efficacy signal seen at this interim analysis. Despite this, LIBRETTO-431 intracranial response data were consistent with that of LIBRETTO-001. Intracranial responses to selpercatinib observed among patients with measurable baseline CNS metastases in LIBRETTO-431 (n = 21) were 82% and 85% in LIBRETTO-001 (n = 26), and in both studies responses were observed regardless of whether patients received prior CNS radiotherapy. Although the median intracranial PFS was not yet reached in LIBRETTO-431 patients with baseline CNS metastases (n = 99), in LIBRETTO-001, the median intracranial PFS was 19.4 months, at a median follow-up of 22.1 months. One retrospective report with patients treated at a single institution examined patients without CNS metastases (n = 31) and suggested that selpercatinib may play a CNS-protective role, as the 12-month CIR of CNS progression was 0%.10 Maturing data from LIBRETTO-431, as well as other ongoing studies such as LIBRETTO-432, which is evaluating selpercatinib in the adjuvant setting, will continue to build on the CNS efficacy signal first observed in LIBRETTO-001.
Advancements in available therapies for oncogene-addicted NSCLC have reinforced the importance of testing for and using potent treatments up-front. Emerging data suggest the order in which the most potent therapies are given may also play an important and clinically relevant role for CNS disease.11 Furthermore, the occurrence of a CNS metastasis is always a critical event, with a major impact on quality of life. Indeed, despite advances, stereotactic CNS radiotherapy carries a non-negligible risk of radionecrosis, which is often difficult to differentiate from tumor progression. Therefore, delaying or preventing the development of new CNS metastases is a critical treatment goal in patients with RET-driven NSCLC. The CNS outcomes data presented here are consistent with our primary report2 and further reinforce the importance of identifying RET fusions in patients with newly diagnosed NSCLC and treating with first-line selpercatinib.
ACKNOWLEDGMENT
We thank the investigators and site personnel for their participation in the study, as well as the clinical trial participants and their families and caregivers, without whom this work would not be possible. We thank Geoffrey Oxnard, Aimee Bence Lin, and Collin Churchill for their insights, guidance, and critical revisions of the manuscript files; Hongmei Han for statistical oversight and review; and Sara Broncano and Ian Towler for critical operational and data review support.
APPENDIX
FIG A1.
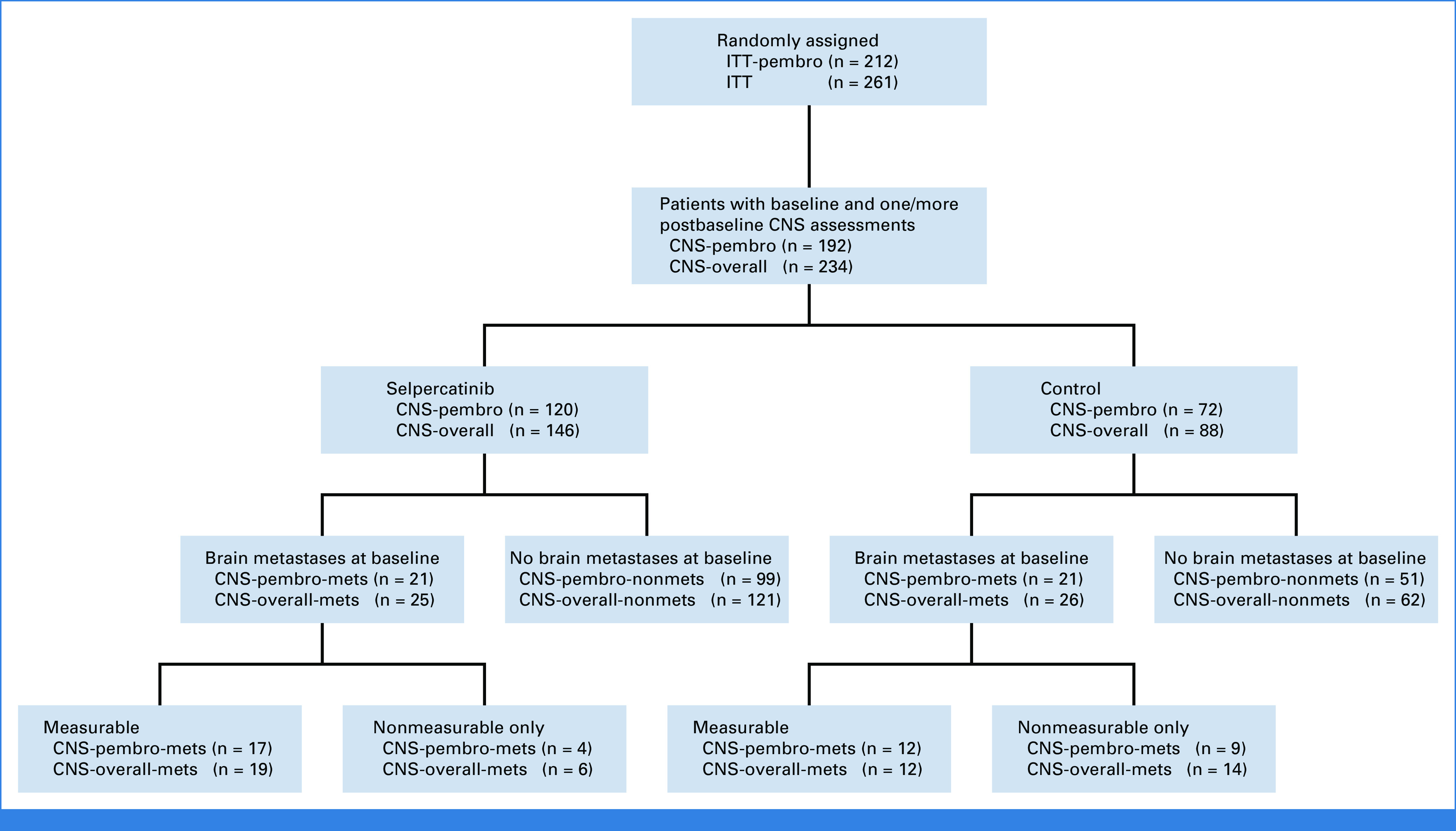
CONSORT diagram. CNS-overall, CNS-overall population; CNS-pembro, CNS-pembrolizumab population; ITT, intent-to-treat.
TABLE A1.
Patterns of Progression in the CNS-Overall Population With CNS Progressive Disease
| Patterns of CNS Progression | CNS Progression on Selpercatinib Arm (n = 10) | CNS Progression on Control Arm (n = 17) |
|---|---|---|
| CNS PD, No. | ||
| In patients with baseline metastasesa | 7 | 11 |
| In patients with no baseline metastases | 3 | 6 |
| Concurrent CNS and systemic PD, No. | ||
| Yes | 7 | 13 |
| No | 3 | 4 |
| CNS progression pattern, No. | ||
| In patients with baseline metastases | 7 | 11 |
| New lesion(s) only | 2 | 6 |
| Existing CNS PD only | 3 | 4 |
| New lesion and existing CNS PD | 2 | 1 |
| In patients with no baseline metastases | 3 | 6 |
| New lesion(s) only | 3 | 6 |
| CNS radiotherapy following CNS PD, No. | ||
| Yes | 4 | 5 |
| While continuing study treatment | 2 | 0 |
| After discontinuing study treatment | 2 | 5 |
| No | 6 | 12 |
Abbreviation: PD, progressive disease.
One selpercatinib patient and two control patients had CNS PD after systemic PD.
TABLE A2.
Intracranial Responses in the CNS-Pembro-Mets Population
| Intracranial Response | CNS-Pembro-Mets (N = 42) | |
|---|---|---|
| Selpercatinib (n = 21) | Control (n = 21) | |
| Measurable disease, No. (%) | 17 (81.0) | 12 (57.1) |
| Nonmeasurable disease, No. (%) | 4 (19.0) | 9 (42.9) |
| Intracranial BOR, No. (%) | ||
| CR | 9 (42.9)a | 7 (33.3)b |
| PR | 8 (38.1) | 5 (23.8) |
| SDc | 2 (9.5) | 5 (23.8) |
| PD | 0 | 2 (9.5) |
| NE | 2 (9.5) | 2 (9.5) |
| Intracranial overall response rate, % (95% CI) | 81.0 (58.1 to 94.6) | 57.1 (34.0 to 78.2) |
| Median time to intracranial response, months (range) | 1.4 (1.2-3.0) | 2.2 (1.2-11.0) |
| Median intracranial DOR, months (95% CI) | NE (14.8 to NE) | NE (8.7 to NE) |
| Intracranial DOR rate, % (95% CI) | ||
| At 6 months | 94.1 (65.0 to 99.1) | 90.9 (50.8 to 98.7) |
| At 12 months | 80.7 (51.1 to 93.4) | 75.8 (30.5 to 93.7) |
Abbreviations: BOR, best overall response; CNS-pembrolizumab-mets, CNS-pembrolizumab population with baseline brain metastases; CR, complete response; CT, computed tomography; DOR, duration of response; MRI, magnetic resonance imaging; NE, not evaluable; PD, progressive disease; PR, partial response; SD, stable disease.
6 of 9 were detected via MRI and 3 of 9 were detected via CT scan.
3 of 7 were detected via MRI and 4 of 7 were detected via CT scan.
Includes non-CR/non-PD in patients with baseline nonmeasurable lesions only.
TABLE A3.
Intracranial Responses in the CNS-Pembro-Mets Population With or Without Prior CNS Radiotherapy
| Intracranial Response | CNS-Pembro-Mets (N = 42) | |||
|---|---|---|---|---|
| With Prior CNS Radiotherapy (n = 13) | Without Prior CNS Radiotherapy (n = 29) | |||
| Selpercatinib (n = 6) | Control (n = 7) | Selpercatinib (n = 15) | Control (n = 14) | |
| Intracranial BOR, No. (%) | ||||
| CR | 1 (16.7) | 0 | 8 (53.3) | 7 (50.0) |
| PR | 2 (33.3) | 3 (42.9) | 6 (40.0) | 2 (14.3) |
| SDa | 2 (33.3) | 2 (28.6) | 0 | 3 (21.4) |
| PD | 0 | 1 (14.3) | 0 | 1 (7.1) |
| NE | 1 (16.7) | 1 (14.3) | 1 (6.7) | 1 (7.1) |
| Intracranial overall response rate, % (95% CI) | 50.0 (11.8 to 88.2) | 42.9 (9.9 to 81.6) | 93.3 (68.1 to 99.8) | 64.3 (35.1 to 87.2) |
| Median intracranial DOR, months (95% CI) | 14.75 (NE to NE) | 13.40 (8.74 to NE) | NE (7.62 to NE) | NE (3.45 to NE) |
| Intracranial DOR rate, % (95% CI) | ||||
| At 6 months | 100 (100 to 100) | 100 (100 to 100) | 92.9 (59.1 to 99.0) | 87.5 (38.7 to 98.1) |
| At 12 months | 100 (100 to 100) | 66.7 (5.4 to 94.5) | 77.4 (44.9 to 92.1) | 87.5 (38.7 to 98.1) |
Abbreviations: BOR, best overall response; CNS-pembrolizumab-mets, CNS-pembrolizumab population with baseline brain metastases; CR, complete response; DOR, duration of response; NE, not evaluable; PD, progressive disease; PR, partial response; SD, stable disease.
Includes non-CR/non-PD in patients with baseline nonmeasurable lesions only.
TABLE A4.
Intracranial Responses in the CNS-Overall-Mets Population
| Intracranial Response | CNS-Overall-Mets (N = 51) | |
|---|---|---|
| Selpercatinib (n = 25) | Control (n = 26) | |
| Measurable disease, No. (%) | 19 (76.0) | 12 (46.2) |
| Nonmeasurable disease, No. (%) | 6 (24.0) | 14 (53.8) |
| Intracranial BOR, No. (%) | ||
| CR | 11 (44.0)a | 8 (30.8)b |
| PR | 10 (40.0) | 5 (19.2) |
| SDc | 2 (8.0) | 9 (34.6) |
| PD | 0 | 2 (7.7) |
| NE | 2 (8.0) | 2 (7.7) |
| Intracranial overall response rate, % (95% CI) | 84.0 (63.9 to 95.5) | 50.0 (29.9 to 70.1) |
| Median time to intracranial response, months (range) | 1.5 (1.2-11.4) | 2.8 (1.2-11.0) |
| Median intracranial DOR, months (95% CI) | NE (9.5 to NE) | 13.4 (4.2 to NE) |
| Intracranial DOR rate, % (95% CI) | ||
| At 6 months | 89.9 (65.3 to 97.4) | 82.5 (46.1 to 95.3) |
| At 12 months | 72.8 (45.9 to 87.8) | 68.8 (29.1 to 89.3) |
Abbreviations: BOR, best overall response; CNS-overall-mets, CNS-overall population with baseline brain metastases; CR, complete; CT, computed tomography; DOR, duration of response; MRI, magnetic resonance imaging; NE, not evaluable; PD, progressive disease; PR, partial response; SD, stable disease.
8 of 11 were detected via MRI and 3 of 11 were detected via CT scan.
4 of 8 were detected via MRI and 4 of 8 were detected via CT scan.
Includes non-CR/non-PD in patients with baseline nonmeasurable lesions only.
FIG A2.

CIR and intracranial PFS in the CNS-overall-nonmets population. (A) CIR of CNS PD; (B) CIR of non-CNS PD and death; and (C) intracranial PFS. CIR, cumulative incidence rate; CNS-overall-nonmets, CNS-overall population without baseline brain metastases; HR, hazard ratio; PD, progressive disease; PFS, progression-free survival.
FIG A3.

CIR and intracranial PFS in the CNS-pembrolizumab-mets population. (A) CIR of CNS PD; (B) CIR of non-CNS PD and death; and (C) intracranial PFS. CIR, cumulative incidence rate; CNS-pembrolizumab-mets, CNS-pembrolizumab population with baseline brain metastases; HR, hazard ratio; NE, not evaluable; PD, progressive disease; PFS, progression-free survival.
FIG A4.

CIR and intracranial PFS in the CNS-overall-mets population. (A) CIR of CNS PD; (B) CIR of non-CNS PD and death; and (C) intracranial PFS. CIR, cumulative incidence rate; CNS-overall-mets, CNS-overall population with baseline brain metastases; HR, hazard ratio; NE, not evaluable; PD, progressive disease; PFS, progression-free survival.
Maurice Pérol
This author is a member of the Journal of Clinical Oncology Clinical Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Consulting or Advisory Role: Lilly, Roche/Genentech, Pfizer, AstraZeneca, Merck Sharp & Dohme, Bristol Myers Squibb, Novartis, Amgen, Takeda, Sanofi, GlaxoSmithKline, Janssen Oncology, Ipsen, Eisai, Novocure, Daiichi Sankyo, Gilead Sciences
Research Funding: Takeda (Inst)
Travel, Accommodations, Expenses: AstraZeneca, Roche, Bristol Myers Squibb, Merck Sharp & Dohme, Pfizer, Takeda, Chugai Pharma
Benjamin J. Solomon
Honoraria: AstraZeneca, Merck Sharp & Dohme (Inst), Roche/Genentech, Pfizer (Inst), Amgen (Inst)
Consulting or Advisory Role: Bristol Myers Squibb (Inst), Merck Sharp & Dohme, AstraZeneca (Inst), Pfizer (Inst), Roche/Genentech (Inst), Amgen, Lilly, BeiGene, Takeda, GlaxoSmithKline (Inst), Janssen (Inst), GlaxoSmithKline
Research Funding: Sanofi (Inst)
Patents, Royalties, Other Intellectual Property: UpToDate
Koichi Goto
Honoraria: Amgen, Amoy Diagnostics, AstraZeneca Japan, Bristol Myers Squibb K.K., Chugai Pharma, Daiichi Sankyo Co, Ltd, Eisai, Lilly Japan, Guardant Health, Janssen, Merck, Novartis, Ono Pharmaceutical, Taiho Pharmaceutical, Takeda, iTeos Therapeutics Inc, Thermo Fisher Scientific, Nippon Kayaku, Pharma Mar, S.A., Riken Genesis Co, Ltd, Bayer
Consulting or Advisory Role: Bayer, Lilly Japan, Amgen, Janssen, AstraZeneca Japan, Bristol Myers Squibb K.K., Daiichi Sankyo Co, Ltd, Syneos Health, GlaxoSmithKline K.K., Haihe Biopharma Co, Ltd
Research Funding: Medical & Biological Laboratories Co, Ltd (Inst), Kyowa Kirin Co, Ltd (Inst), Merck (Inst), Merus (Inst), Spectrum Pharmaceuticals (Inst), MSD K.K. (Inst), AstraZeneca Japan (Inst), Taiho Pharmaceutical (Inst), Chugai Pharma (Inst), Boehringer Ingelheim (Inst), Ono Pharmaceutical (Inst), Takeda (Inst), Eisai (Inst), Lilly Japan (Inst), Bristol Myers Squibb K.K. (Inst), Ignyta (Inst), Janssen (Inst), Loxo (Inst), Sysmex (Inst), Amgen (Inst), Daiichi Sankyo Co, Ltd (Inst), Turning Point Therapeutics (Inst), Novartis (Inst), Blueprint Medicines (Inst), Bayer Yakuhin (Inst), Amgen (Inst), HaiHe Biopharma Co, Ltd (Inst), Sumitomo Pharma Co, Ltd (Inst), Pfizer (Inst), Life Technologies (Inst), CRAIF Inc (Inst), Precision Medicine Asia Co, Ltd (Inst), Riken Genesis Co, LTD (Inst), AbbVie (Inst), AnHeart Therapeutics Inc (Inst), Guardant Health Asia, Middle East & Africa, Inc (Inst), Lunit (Inst)
Keunchil Park
Honoraria: Incyte, BeiGene
Consulting or Advisory Role: AstraZeneca, Daiichi Sankyo, JNJ, IMBdx, Geninus, Abion
Speakers' Bureau: Boehringer Ingelheim
Ernest Nadal
Honoraria: Apollomics, Roche/Genentech, Transgene
Consulting or Advisory Role: MSD, Bristol Myers Squibb, Roche, Boehringer Ingelheim, Pfizer, Takeda, AstraZeneca, Lilly, Amgen, Bayer, Sanofi, Merck Serono, Janssen Oncology, Qiagen, Pierre Fabre
Research Funding: Roche (Inst), Pfizer (Inst), Bristol Myers Squibb (Inst), Merck Serono (Inst)
Travel, Accommodations, Expenses: MSD, Bristol Myers Squibb, Pfizer, Roche, Lilly
Emilio Bria
Consulting or Advisory Role: MSD Oncology, AstraZeneca, Pfizer, Lilly, Bristol Myers Squibb, Roche, Takeda, Celltrion
Research Funding: AstraZeneca (Inst), Roche (Inst)
Travel, Accommodations, Expenses: Roche, Bristol Myers Squibb, MSD
Claudio Martin
Consulting or Advisory Role: MSD Oncology, Boehringer Ingelheim, AstraZeneca, Bristol Myers Squibb, Pfizer, Roche, Takeda
Speakers' Bureau: Bristol Myers Squibb, Roche, Boehringer Ingelheim, Pfizer, MSD Oncology, AstraZeneca
Jair Bar
Stock and Other Ownership Interests: Causalis
Consulting or Advisory Role: Pfizer, Bristol Myers Squibb, AstraZeneca, AbbVie, MSD, Takeda, Bayer, Novartis, Roche, Merck Serono
Research Funding: AstraZeneca/MedImmune (Inst), MSD (Inst), Pfizer (Inst), Roche (Inst), AbbVie (Inst), Novartis (Inst), Takeda (Inst), Oncohost (Inst), Immunai (Inst)
Justin N. Williams
Employment: Lilly
Stock and Other Ownership Interests: Lilly
Tarun Puri
Employment: Lilly
Stock and Other Ownership Interests: Lilly
Jian Li
Employment: Lilly
Stock and Other Ownership Interests: Lilly
Minji K. Uh
Employment: Lilly
Stock and Other Ownership Interests: Lilly
Boris K. Lin
Employment: Lilly
Stock and Other Ownership Interests: Lilly
Travel, Accommodations, Expenses: Lilly
Caicun Zhou
Leadership: IASLC BOD
Honoraria: Lilly, Roche, Boehringer Ingelheim, MSD, Hengrui Therapeutics, QiLu Pharmaceutical, Innovent Biologics, Sanofi, Merck Sharpe and Dohme, Alice, CStone Pharmaceuticals, Luye Pharma, TopAlliance BioSciences Inc, Amoy Diagnostics, Anheart Therapeutics
Consulting or Advisory Role: Amoy Diagnostics, Hengrui Therapeutics, Innovent Biologics, QiLu Pharmaceutical, Top Alliance BioScience
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented in part at the European Society for Medical Oncology 2023 Congress, Madrid, Spain, October 20-24, 2023.
SUPPORT
Supported by Loxo@Lilly, a wholly owned subsidiary of Eli Lilly and Company.
CLINICAL TRIAL INFORMATION
NCT04194944 (LIBRETTO-431)
DATA SHARING STATEMENT
A data sharing statement provided by the authors is available with this article at DOI https://doi.org/10.1200/JCO.24.00724.
AUTHOR CONTRIBUTIONS
Conception and design: Maurice Pérol, Koichi Goto, Keunchil Park, Boris K. Lin, Caicun Zhou
Provision of study materials or patients: Maurice Pérol, Benjamin J. Solomon, Koichi Goto, Keunchil Park, Ernest Nadal, Emilio Bria, Jair Bar, Caicun Zhou
Collection and assembly of data: Maurice Pérol, Benjamin J. Solomon, Koichi Goto, Keunchil Park, Ernest Nadal, Emilio Bria, Claudio Martin, Jair Bar, Minji K. Uh, Caicun Zhou
Data analysis and interpretation: Maurice Pérol, Benjamin J. Solomon, Koichi Goto, Keunchil Park, Ernest Nadal, Emilio Bria, Justin N. Williams, Tarun Puri, Jian Li, Minji K. Uh, Boris K. Lin, Caicun Zhou
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
CNS Protective Effect of Selpercatinib in First-Line RET Fusion-Positive Advanced Non–Small Cell Lung Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Maurice Pérol
This author is a member of the Journal of Clinical Oncology Clinical Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Consulting or Advisory Role: Lilly, Roche/Genentech, Pfizer, AstraZeneca, Merck Sharp & Dohme, Bristol Myers Squibb, Novartis, Amgen, Takeda, Sanofi, GlaxoSmithKline, Janssen Oncology, Ipsen, Eisai, Novocure, Daiichi Sankyo, Gilead Sciences
Research Funding: Takeda (Inst)
Travel, Accommodations, Expenses: AstraZeneca, Roche, Bristol Myers Squibb, Merck Sharp & Dohme, Pfizer, Takeda, Chugai Pharma
Benjamin J. Solomon
Honoraria: AstraZeneca, Merck Sharp & Dohme (Inst), Roche/Genentech, Pfizer (Inst), Amgen (Inst)
Consulting or Advisory Role: Bristol Myers Squibb (Inst), Merck Sharp & Dohme, AstraZeneca (Inst), Pfizer (Inst), Roche/Genentech (Inst), Amgen, Lilly, BeiGene, Takeda, GlaxoSmithKline (Inst), Janssen (Inst), GlaxoSmithKline
Research Funding: Sanofi (Inst)
Patents, Royalties, Other Intellectual Property: UpToDate
Koichi Goto
Honoraria: Amgen, Amoy Diagnostics, AstraZeneca Japan, Bristol Myers Squibb K.K., Chugai Pharma, Daiichi Sankyo Co, Ltd, Eisai, Lilly Japan, Guardant Health, Janssen, Merck, Novartis, Ono Pharmaceutical, Taiho Pharmaceutical, Takeda, iTeos Therapeutics Inc, Thermo Fisher Scientific, Nippon Kayaku, Pharma Mar, S.A., Riken Genesis Co, Ltd, Bayer
Consulting or Advisory Role: Bayer, Lilly Japan, Amgen, Janssen, AstraZeneca Japan, Bristol Myers Squibb K.K., Daiichi Sankyo Co, Ltd, Syneos Health, GlaxoSmithKline K.K., Haihe Biopharma Co, Ltd
Research Funding: Medical & Biological Laboratories Co, Ltd (Inst), Kyowa Kirin Co, Ltd (Inst), Merck (Inst), Merus (Inst), Spectrum Pharmaceuticals (Inst), MSD K.K. (Inst), AstraZeneca Japan (Inst), Taiho Pharmaceutical (Inst), Chugai Pharma (Inst), Boehringer Ingelheim (Inst), Ono Pharmaceutical (Inst), Takeda (Inst), Eisai (Inst), Lilly Japan (Inst), Bristol Myers Squibb K.K. (Inst), Ignyta (Inst), Janssen (Inst), Loxo (Inst), Sysmex (Inst), Amgen (Inst), Daiichi Sankyo Co, Ltd (Inst), Turning Point Therapeutics (Inst), Novartis (Inst), Blueprint Medicines (Inst), Bayer Yakuhin (Inst), Amgen (Inst), HaiHe Biopharma Co, Ltd (Inst), Sumitomo Pharma Co, Ltd (Inst), Pfizer (Inst), Life Technologies (Inst), CRAIF Inc (Inst), Precision Medicine Asia Co, Ltd (Inst), Riken Genesis Co, LTD (Inst), AbbVie (Inst), AnHeart Therapeutics Inc (Inst), Guardant Health Asia, Middle East & Africa, Inc (Inst), Lunit (Inst)
Keunchil Park
Honoraria: Incyte, BeiGene
Consulting or Advisory Role: AstraZeneca, Daiichi Sankyo, JNJ, IMBdx, Geninus, Abion
Speakers' Bureau: Boehringer Ingelheim
Ernest Nadal
Honoraria: Apollomics, Roche/Genentech, Transgene
Consulting or Advisory Role: MSD, Bristol Myers Squibb, Roche, Boehringer Ingelheim, Pfizer, Takeda, AstraZeneca, Lilly, Amgen, Bayer, Sanofi, Merck Serono, Janssen Oncology, Qiagen, Pierre Fabre
Research Funding: Roche (Inst), Pfizer (Inst), Bristol Myers Squibb (Inst), Merck Serono (Inst)
Travel, Accommodations, Expenses: MSD, Bristol Myers Squibb, Pfizer, Roche, Lilly
Emilio Bria
Consulting or Advisory Role: MSD Oncology, AstraZeneca, Pfizer, Lilly, Bristol Myers Squibb, Roche, Takeda, Celltrion
Research Funding: AstraZeneca (Inst), Roche (Inst)
Travel, Accommodations, Expenses: Roche, Bristol Myers Squibb, MSD
Claudio Martin
Consulting or Advisory Role: MSD Oncology, Boehringer Ingelheim, AstraZeneca, Bristol Myers Squibb, Pfizer, Roche, Takeda
Speakers' Bureau: Bristol Myers Squibb, Roche, Boehringer Ingelheim, Pfizer, MSD Oncology, AstraZeneca
Jair Bar
Stock and Other Ownership Interests: Causalis
Consulting or Advisory Role: Pfizer, Bristol Myers Squibb, AstraZeneca, AbbVie, MSD, Takeda, Bayer, Novartis, Roche, Merck Serono
Research Funding: AstraZeneca/MedImmune (Inst), MSD (Inst), Pfizer (Inst), Roche (Inst), AbbVie (Inst), Novartis (Inst), Takeda (Inst), Oncohost (Inst), Immunai (Inst)
Justin N. Williams
Employment: Lilly
Stock and Other Ownership Interests: Lilly
Tarun Puri
Employment: Lilly
Stock and Other Ownership Interests: Lilly
Jian Li
Employment: Lilly
Stock and Other Ownership Interests: Lilly
Minji K. Uh
Employment: Lilly
Stock and Other Ownership Interests: Lilly
Boris K. Lin
Employment: Lilly
Stock and Other Ownership Interests: Lilly
Travel, Accommodations, Expenses: Lilly
Caicun Zhou
Leadership: IASLC BOD
Honoraria: Lilly, Roche, Boehringer Ingelheim, MSD, Hengrui Therapeutics, QiLu Pharmaceutical, Innovent Biologics, Sanofi, Merck Sharpe and Dohme, Alice, CStone Pharmaceuticals, Luye Pharma, TopAlliance BioSciences Inc, Amoy Diagnostics, Anheart Therapeutics
Consulting or Advisory Role: Amoy Diagnostics, Hengrui Therapeutics, Innovent Biologics, QiLu Pharmaceutical, Top Alliance BioScience
No other potential conflicts of interest were reported.
REFERENCES
- 1. Drilon A, Subbiah V, Gautschi O, et al. Selpercatinib in patients with RET fusion-positive non-small-cell lung cancer: Updated safety and efficacy from the registrational LIBRETTO-001 phase I/II trial. J Clin Oncol. 2023;41:385–394. doi: 10.1200/JCO.22.00393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhou C, Solomon B, Loong HH, et al. First-line selpercatinib or chemotherapy and pembrolizumab in RET fusion-positive NSCLC. N Engl J Med. 2023;389:1839–1850. doi: 10.1056/NEJMoa2309457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hendriks LE, Kerr KM, Menis J, et al. Oncogene-addicted metastatic non-small-cell lung cancer: ESMO clinical Practice guideline for diagnosis, treatment and follow-up. Ann Oncol. 2023;34:339–357. doi: 10.1016/j.annonc.2022.12.009. [DOI] [PubMed] [Google Scholar]
- 4. Jaiyesimi IA, Leighl NB, Ismaila N, et al. Therapy for stage IV non–small cell lung cancer with driver alterations: ASCO living guideline, version 2023.3. J Clin Oncol. 2024;42:e1–e22. doi: 10.1200/JCO.23.02744. [DOI] [PubMed] [Google Scholar]
- 5.Reference deleted. [Google Scholar]
- 6. Subbiah V, Velcheti V, Tuch BB, et al. Selective RET kinase inhibition for patients with RET-altered cancers. Ann Oncol. 2018;29:1869–1876. doi: 10.1093/annonc/mdy137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Drilon A, Lin JJ, Filleron T, et al. Frequency of brain metastases and multikinase inhibitor outcomes in patients with RET-rearranged lung cancers. J Thorac Oncol. 2018;13:1595–1601. doi: 10.1016/j.jtho.2018.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Remon J, Besse B. Brain metastases in oncogene-addicted non-small cell lung cancer patients: Incidence and treatment. Front Oncol. 2018;8:88. doi: 10.3389/fonc.2018.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Subbiah V, Gainor JF, Oxnard GR, et al. Intracranial efficacy of selpercatinib in RET fusion-positive non-small cell lung cancers on the LIBRETTO-001 trial. Clin Cancer Res. 2021;27:4160–4167. doi: 10.1158/1078-0432.CCR-21-0800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Murciano-Goroff YR, Falcon CJ, Lin ST, et al. Central nervous system disease in patients with RET fusion-positive NSCLC treated with selpercatinib. J Thorac Oncol. 2023;18:620–627. doi: 10.1016/j.jtho.2023.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Remon J, Besse B, Aix SP, et al. Overall survival from the EORTC LCG-1613 APPLE trial of osimertinib versus gefitinib followed by osimertinib in advanced EGFR-mutant non-small-cell lung cancer. J Clin Oncol. 2024;42:1350–1356. doi: 10.1200/JCO.23.01521. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
A data sharing statement provided by the authors is available with this article at DOI https://doi.org/10.1200/JCO.24.00724.



