Abstract
The global market for alpha-hydroxy acids (AHAs) is undergoing significant expansion, propelled by increasing demand for skincare products that address aging and environmental damage. This review focuses on the dermatological applications of AHAs, particularly in cosmetic formulations like chemical peels. We have identified that AHAs, such as glycolic and lactic acids, enhance skin rejuvenation by promoting apoptosis in skin cells, boosting collagen and elastin synthesis, and improving skin texture and luminosity. Our comprehensive analysis reveals a nuanced understanding of AHAs’ effectiveness across various skin types and conditions, demonstrating their broad utility in treating conditions like acne, hyperpigmentation, and photoaging. However, the optimal concentrations for therapeutic efficacy with minimal side effects are yet to be precisely defined, necessitating further research. Regulatory compliance is underscored as essential for the safe application of AHAs in cosmetics, with international guidelines recommending specific concentrations and pH levels to minimize potential skin irritation. In Conclusion, the review highlights the effectiveness of AHAs in cosmetic dermatology, emphasizing the necessity for continued research and rigorous regulatory adherence to maximize their safe and beneficial application worldwide.
Keywords: chemical peels, alpha hydroxy acids, practice guidelines, glycolic acid
Introduction
In 2021, the total value of the global AHAs market was estimated at $1.2 billion. The global AHA is projected to reach USD 3.2 billion by 2030, at a compound annual growth rate (CAGR) of 8.5% during the forecast period 2021 to 2030.1 Global warming has boosted the sales of skincare products, including sunscreen lotions, creams, and serums, driving up demand for AHAs throughout the world.2 Preventing the onset of premature aging, boosting the synthesis of elastin and collagen, and improving circulation are major factors driving the need for AHA products.
Chemical peels are a widely used cosmetic procedure that aims to rejuvenate the skin by removing dead skin cells and promoting cell turnover.3 AHAs are a class of organic acids commonly used in chemical peels due to their ability to exfoliate the skin, increase skin turnover, and stimulate collagen and elastin synthesis.4 Among the AHAs, glycolic acid and lactic acid are the most commonly used in cosmetic formulations. These acids are available in different concentrations and formulations, making them suitable for a wide range of skin types and conditions.5,6
AHAs have been shown to induce apoptosis, or programmed cell death, in skin cells. This effect is believed to be responsible for the anti-aging and skin-rejuvenating properties of AHAs.7 However, the optimal concentration and formulation of AHAs for inducing apoptosis while minimizing skin irritation and adverse effects are still under investigation. Moreover, the effectiveness of AHAs in treating specific skin conditions, such as acne, hyperpigmentation, and photodamage, varies depending on the type and severity of the condition and the patient’s skin type and color.8 The selection of appropriate AHAs to use could be influenced by several factors, including formulation, concentration, pH, type of skin, color of skin, and the purpose of use.9
Chemical peels are widely embraced for skin rejuvenation and treating dermatological conditions. These procedures employ specific peeling agents to disrupt intercellular bonds, facilitating the removal of dead skin cells. Among AHAs, Glycolic Acid from sugar cane is commonly used due to its small size, enabling effective skin penetration.10 Lactic Acid from milk, known for its hydrating properties, is favored for dry skin.11 Mandelic Acid from almonds, with a larger molecular size, penetrates skin slowly, reducing irritation.12 Chemical peels effectively treat Acne Vulgaris by exfoliating and unclogging pores, and Melasma by reducing hyperpigmentation.13 They diminish wrinkles by stimulating collagen synthesis and treat Actinic Keratosis, a precancerous condition. Chemical peels repair Photodamage, improve skin pigmentation disorders, and reduce Acne Scars by promoting collagen production.10,13
AHAs are generally considered safe for use in cosmetic formulations when used within the recommended concentrations and formulations. However, some patients may experience skin irritation, redness, or other adverse effects, especially when using higher concentrations or when combining AHAs with other skin treatments.14 Therefore, it is important to follow the instructions for use and to consult with a qualified healthcare provider before undergoing any cosmetic procedure.
The use of AHAs in cosmetic formulations is a growing market, with many skincare brands offering products containing different AHAs and formulations. The market is expected to continue growing due to the increasing demand for safe and effective anti-aging and skin-rejuvenating products.15 However, more research is needed to fully understand the safety and efficacy of AHAs in different concentrations and formulations and to develop optimal treatment protocols for different skin types and conditions.
This review critically evaluates the legal and clinical landscapes of AHAs, primarily focusing on their dermatological applications in cosmetic formulations such as chemical peels. AHAs, notably glycolic and lactic acids, have been extensively used due to their exfoliating properties, which promote skin rejuvenation by enhancing collagen and elastin synthesis and inducing apoptosis in skin cells. The review highlights the variations in AHA formulations and concentrations, illustrating their adaptability to different skin types and conditions. Despite their widespread acceptance and reported efficacy in mitigating signs of aging and improving skin texture, the optimal concentrations that balance efficacy with minimal adverse effects remain under investigation. Furthermore, this paper underscores the importance of regulatory adherence to ensure the safe use of AHAs, reflecting on international guidelines that recommend specific concentrations and pH levels to minimize potential skin irritation. The flourishing global market for AHA-incorporated products, projected to reach significant growth by 2030, suggests a promising future for these compounds in dermatological practices and cosmetic industries alike.
Materials and Methods
A comprehensive approach taken for the review of the efficacy and safety of AHAs in dermatological practices. Structured as a narrative review, the methodology incorporates an extensive examination of a diverse array of scientific literature, extending from the earliest relevant studies to the most recent. This body of evidence encompasses randomized controlled trials, cohort studies, and case reports that critically assess the effectiveness of AHAs across various skin types and conditions, with a specific emphasis on acne, hyperpigmentation, and photoaging.
A critical aspect of the review is regulatory compliance, which entails a meticulous analysis of guidelines recommended by international regulatory bodies such as the Food and Drug Administration (FDA) and the European Medicines Agency (EMA). This analysis covers permissible AHA concentrations, pH levels, UV sensitivity, and skin barrier damage.
Additionally, the review probes the global market outlook for AHAs, employing authoritative market research reports and trend analyses. This exploration elucidates variations in AHA formulations and concentrations, highlighting their adaptability to different skin types and conditions. The methodology deployed ensures a rigorous and comprehensive analysis of both the clinical and regulatory landscapes, supported by an exhaustive synthesis of the available scientific evidence. This approach not only reinforces the findings regarding the therapeutic efficacy of AHAs but also underscores the critical nature of adhering to established regulatory standards to ensure safety and effectiveness in dermatological applications.
The inclusion and exclusion criteria for the review presented in this narrative review are presented here:
Inclusion Criteria
Type of Studies: The review includes a broad range of scientific literature encompassing randomized controlled trials, cohort studies, and case reports that assess the effectiveness of AHAs across various skin types and conditions.
Conditions Treated: The review focuses on the use of AHAs in treating specific dermatological conditions such as Acne Vulgaris, Melasma, Actinic Keratosis, Acne Scars, Hyperpigmentation, and photoaging.
Types of AHAs: Studies involving the most commonly used AHAs in cosmetic formulations, particularly glycolic acid, malic acid, mandelic acid, citric, and lactic acid, are considered. Studies examining other AHAs like trichloroacetic acid are also included based on their relevance to skin treatment outcomes.
Exclusion Criteria
Non-Dermatological Applications: Studies not related to dermatological or cosmetic use of AHAs were excluded to maintain the review’s focus on skin rejuvenation and treatment.
Insufficient Data on Safety and Efficacy: Any studies lacking robust data on the safety and efficacy of AHAs, or those not meeting the rigorous standards for inclusion such as clear methodological descriptions, were likely excluded.
Irrelevant Conditions: Research focusing on conditions not directly related to the indications of AHAs such as non-skin related diseases were not included.
Regulation of AHA Peels
For regulatory compliance, it is imperative to evaluate two primary parameters: UV sensitivity and the extent of skin barrier damage. These assessments are critical for determining the safety and effectiveness of skincare products containing AHAs.16 UV sensitivity is typically quantified using the no observed adverse effect level (NOAEL) for sunburn cells (SBC), which helps establish the optimal threshold concentration for AHA usage.17 Additionally, the minimal erythemal dose (MED) serves as a vital indicator, measuring the degree of erythema produced by UV-B radiation exposure after AHA application at varying concentrations and durations.18 Transepidermal Water Loss (TEWL) is a test that is used to measure skin barrier damage.19,20 On TEWL tests, skin penetration for the specific compound is measured following the AHA application. The penetration is measured via several methods, such as the concentration of the tested compound in urinary excretion.21 Adherence to regulatory standards set by organizations like the Food and Drug Administration (FDA) and the European Medicines Agency (EMA) ensures that skincare products undergo rigorous evaluation to guarantee consumer safety and efficacy. Compliance with these standards is essential for manufacturers seeking approval or certification to market their products. Therefore, integrating these regulatory considerations into the evaluation of AHAs is fundamental for meeting industry regulations and safeguarding public health.
International guidelines on the use of AHAs vary across regions such as the United States, Europe, and Australia. In 2005, the US FDA issued guidance on AHA concentrations, recommending that cosmetic products contain no more than 10% AHA, with a pH of 3.5 or higher for over-The-counter use.22 Besides, it should either be formulated with an SPF, or its labels contain a clear statement to use an appropriate SPF. Higher concentrations of glycolic 30% with a pH up to 3 for well-trained professionals and salons in a base of discrete use followed by a throughout rinsing and appropriate use of SPF protections.22 Basically, the mentioned recommendations were based on the Cosmetic Ingredients Review (CIR) in 1998 and the updated re-review in 2013.23
The European Union (EU) adheres to more stringent regulatory standards compared to other regions. In 2000, the EU’s Scientific Committee on Cosmetic Products and Non-Food Products Intended for Consumers (SCCS) recommended that glycolic acid be used safely at concentrations up to 4% with a pH of at least 3.8, and lactic acid up to 2.5% with a pH of at least 5. They also advised precautions such as avoiding sun exposure, using adequate sun protection factor (SPF), and preventing eye contact.24 This position was reaffirmed in 2004.25 Additionally, in 1998, the Federal Republic of Germany aligned with these precautionary measures, coinciding with the initial report from the Cosmetic Ingredient Review (CIR).26 This alignment was partly due to glycolic acid being designated a Priority Existing Chemical following numerous reports of skin irritation.26 Notably, prior to these regulations, in 1992, the US Food and Drug Administration (FDA) had received several reports concerning skin issues from the use of AHAs by non-professionals, which highlighted the absence of clear guidelines on safe concentrations at that time.26
Chemical peels, containing active acidic ingredients such as glycolic acid, trichloroacetic acid, salicylic acid, and lactic acid, often exceed concentrations of 10–15%, thus falling outside the cosmetic category under European regulations. In Spain, for instance, these products are classified as “Hygienic Products”. The categorization of chemical peels varies significantly worldwide; depending on their intended use, composition, and resultant skin effects, they may be regulated as medical devices, subject to new medical device regulations, or even classified as pharmaceuticals in various jurisdictions.27
According to Regulation (EC) No 1223/2009 by the European Parliament and Council, cosmetic products are those intended to be applied to external parts of the human body (such as the epidermis, hair, nails, lips, and external genital organs) or to the teeth and mucous membranes of the oral cavity primarily to cleanse, perfume, alter appearance, protect, keep in good condition, or correct body odors. This regulation stipulates specific guidelines on what constitutes a cosmetic, emphasizing that products not fulfilling these criteria, especially those with therapeutic claims or higher concentrations of active acids, could be subject to more stringent regulations.28
In the European Union, the classification of medical devices is determined by a risk-based system which considers the potential vulnerability of the human body to the devices and the associated risks. Products designed to perform a specific medical function, which might include certain types of peels intended for deep skin penetration or significant physiological effects, could be classified as medical devices. This classification is based on the degree of risk involved and the intended purpose of the product.29
In 2000, Australia’s National Industrial Chemicals Notification and Assessment Scheme (NICNAS) considered that the chemical is corrosive at concentrations less than 30% and only slightly irritants at 10%, and thus they suggested establishing a level of irritant and corrosive effect between 10–25%.30 They further suggested that the use of Glycolic acid at any level in cosmetics should be listed in the standard listing procedure for drugs and poisons.30 In 2005, The Australian Society of Cosmetics and Chemists (ASCC) suggested that glycolic acid should be exempted from this listing if it is formulated at a concentration of 20% or less in skin care products and 10% or less in around-The –eye products at PH of 3.5 or higher in both scenario.31 This statement is perhaps the most flexible in comparison to Europe and US. Besides, they believed that there is no strong evidence to claim that Glycolic acid with 5% or less is harmful to unborn babies. Glycolic and Lactic acids should be exempted from any regulatory issues if they are formulated in 5% or less with a PH of 4.5–7.31
Generally, the regulatory affairs recognized, perhaps, three distinct categories: daily home use, intermittent salon and professional use, and medical use by dermatologists. Accordingly, 10% for daily skin care, less than 30% for well-trained professionals and salons, and drugs if higher than this, with pH range from more than 3.5, 3, and less than 3, respectively.21 It should be noted that there is no clear distinction between categories 2 and 3 in some guidelines, as both are considered well-trained professionals.21
It is essential for consumers to be aware of these differences to ensure the safe and appropriate use of AHAs in various settings. The concentration of AHAs in both pharmaceutical and cosmetic products is important to consider when choosing a product for a particular skin condition.32 Higher concentrations may result in more effective treatment but also increase the risk of skin irritation and sensitivity. Lower concentrations may be more suitable for daily use but may not provide the desired therapeutic effect.32
Chemical Structure and Apoptosis Function of AHA
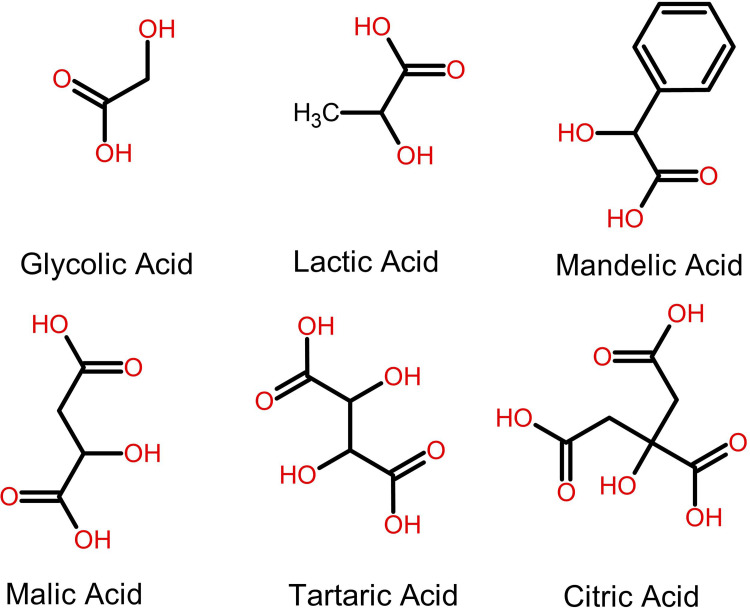
Alpha hydroxy acids are classified based on their molecular structures and the presence of hydroxy groups.31 Single AHAs, such as glycolic acid (GA) from sugar cane, lactic acid (LA) from milk, and mandelic acid from bitter almonds, are defined by their simpler structure containing a single hydroxy group.32 These AHAs are highly valued in skincare due to their effective skin penetration and exfoliation capabilities. For example, glycolic acid is noted for its small molecular size, which facilitates deep skin absorption, enhancing exfoliation and promoting cellular rejuvenation. Poly AHAs, including malic acid (MA) from apples and tartaric acid (TA) from grapes, possess multiple hydroxy groups. Tartaric acid, in particular, is utilized not only for its exfoliating properties but also for its role in stabilizing pH levels in skincare formulations, contributing to the antioxidant defense system of the skin.10 These multi-group AHAs are less commonly used individually but are essential in complex formulations for their synergistic effects, providing a balanced exfoliation that minimizes skin irritation.33 Citric acid, although typically recognized as a mono AHA, can also act as a poly AHA due to its multiple acid groups, crucial for antioxidant protection and pH adjustment in cosmetic products.34 Despite their structural differences, all AHAs work by breaking down the bonds between dead skin cells on the surface of the skin, revealing brighter, smoother, and more even-toned skin underneath. Table 1 delineates the molecular weight, natural source, and therapeutic indications of AHAs, while Figure 1 illustrates the chemical structures of various AHAs.
Table 1.
A Comparison of Various Alpha Hydroxy Acids Commonly Used in Skincare, Including Their Molecular Weight, Number of Hydroxyl Groups, Natural Sources, Recommended Uses, and Suitable Skin Types
| AHA | Molecular Weight | Number of Hydroxyl Groups | Source | Use | Skin Type |
|---|---|---|---|---|---|
| Glycolic Acid | 72 | Single | Sugar cane | Fine lines, wrinkles, acne, hyperpigmentation, and uneven skin tone | All except sensitive skin |
| Lactic Acid | 90 | Single | Milk, fruit | Dry skin, fine lines, wrinkles, hyperpigmentation, and uneven skin tone | Dry, dehydrated, and sensitive skin |
| Mandelic Acid | 152 | Poly | Bitter almonds | Acne, hyperpigmentation, and uneven skin tone | Sensitive, only combination acne-prone |
| Malic Acid | 134 | Single | Apples | Fine lines, wrinkles, and hyperpigmentation | All except sensitive skin |
| Tartaric Acid | 150 | Poly | Grapes | Fine lines, wrinkles, and hyperpigmentation | Dry and sensitive skin |
| Citric Acid | 192 | Poly | Citrus fruits | Fine lines, wrinkles, hyperpigmentation, and uneven skin tone | Sensitive, only combination acne-prone |
Figure 1.
Chemical Structures of Various Mono- and Poly-Alpha Hydroxy Acids.
It has been confirmed that both forms of LA are equally efficient at boosting cellular renewal activity. There were various molecular mechanisms involved in the AHA-induced apoptosis, some of which were caspase-dependent while others were caspase-independent. There are distinct functions for CA and MA in skin cells. CA was observed to stimulate the production of collagen I and procollagen II, whereas GA enhanced the epidermis and dermis, proving the efficacy of AHAs in reversing photoaged skin.35 When applied to sun-damaged skin, CA at a dosage of 20% may enhance both epidermal thickness and glycosaminoglycan levels. The skin rejuvenation rate may be boosted by CA, and it can also be used to heal sun damage.36 Inducing apoptosis in keratinocytes may be related to these activities.
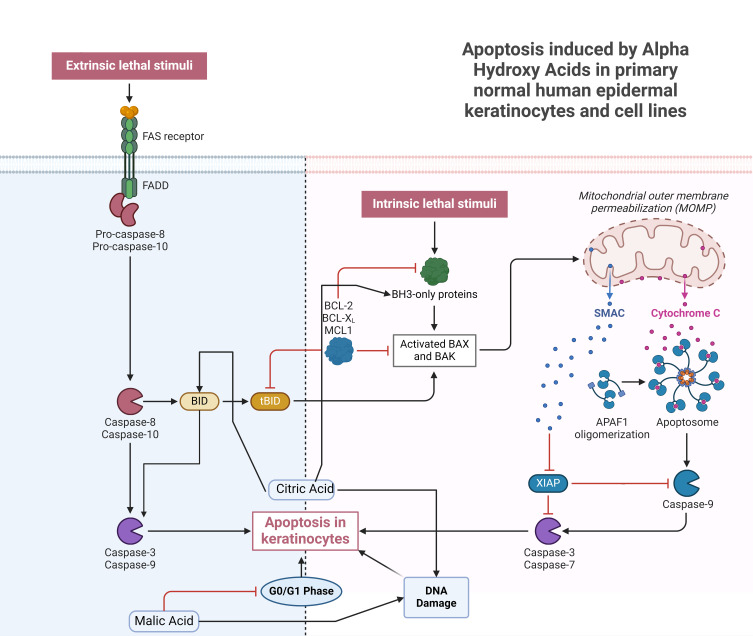
Initiation of apoptosis may occur through two major pathways: the death-receptor-dependent pathway (extrinsic pathway) and the mitochondria-dependent pathway (intrinsic pathway).37 Induction of apoptosis is regulated by several pathways in the cell, but one of the most critical involves the Fas receptor, Fas ligand, and caspase-8.38 HaCaT keratinocytes were isolated from a male melanoma patient’s normal peripheral skin biopsy.39 HaCaT cells and primary normal human epidermal keratinocytes (NHEK) vary in fundamental ways (eg, p53 mutations), but NHEKs have a shorter lifespan and can only be cultured for a few passages. For this reason, NHEK is often replaced by HaCaT since it is non-tumorigenic after being induced with the CA. CA (12.5 mM) was discovered to trigger apoptosis through the caspase-dependent pathway by activating caspase-9 and caspase-3.40,41 It was elucidated that CA triggered the BH3-interacting domain death agonist (BID) protein, upregulated caspase-8, and triggered death receptors. In HaCaT cells, CA triggers apoptosis through the mitochondrial route.42,43 The skin’s regeneration rate may accelerate as a result of these mechanisms.
In the context of malic acid, investigations have revealed its capability to impede cell cycle advancement specifically at the G0/G1 phase, thereby manifesting an inhibitory influence on the proliferation of HaCaT cells.44 When MA was given at a concentration of 15 mM, many proteins involved in endoplasmic reticulum stress (GRP78, GADD153, and ATF6) were shown to be upregulated.44 Despite their distinct chemical structures, CA and MA have a common ability to trigger apoptotic pathways. In response to CA or MA, HaCaT cells undergo caspase-3, −8, and −9 activation from mitochondria, resulting in apoptotic characteristics such as an increase in sub-G1 cells, apoptotic bodies, and DNA damage.45 Increased skin regeneration may be due to CA’s ability to induce apoptosis in keratinocytes, which would explain why CA has been linked to this effect. There is evidence that MA exhibits antibacterial properties since it has recently been studied for its potential to cure skin infections caused by Listeria monocytogenes.46 In addition, Yamamoto et al35 found that AHAs have the potential to regenerate photo-aged skin, which led them to conclude that this treatment option is worth exploring. These latest discoveries show that MA and CA serve a wider variety of biological roles in skin cells than previously thought. Refer to Figure 2 for elucidation on the mechanism underlying keratinocyte apoptosis induced by AHAs.
Figure 2.
Mechanistic Pathways of Apoptosis in Keratinocytes Influenced by Alpha Hydroxy Acids for Dermatological Cellular Renewal.
Comprehensive Chemical Peel Treatment Protocol
This comprehensive treatment protocol outlines the key steps involved in chemical peel procedures, including priming, to prepare the skin for the peeling process and the subsequent regenerative phase. To achieve optimal results, tretinoin is frequently applied one month prior to the peeling procedure. This pre-treatment step enhances the uniformity of peel penetration, leveraging tretinoin’s modulatory effects on the skin’s surface.47 In addition, tretinoin pretreatment helps to accelerate recovery time after treatment.47,48 Tretinoin concentration is adjusted based on individual skin response. Those who are allergic to tretinoin may switch to an AHA as an alternative. Sunscreens, which work by blocking UV rays from reaching the skin, are essential for preventing post-inflammatory hyperpigmentation.47 A hydroquinone-based preparation may be necessary for patients with a dark phototype. If a patient has a history of herpes infection, they should begin taking oral anti-herpes medication the day before a medium- or deep-depth peel and continue doing so for a whole week thereafter.47 Kojic acid may work in a similar but weaker action than hydroquinone.
Pretreatment, the subsequent step following priming, involves a meticulous cleansing of the treatment area to initiate every peeling procedure. This stage is crucial as it prepares the skin for the even application of the chemical peel material, which is essential for achieving the best results.47 Some investigators recommended using acetone to remove the stratum corneum of its dead tissue and fatty deposits before proceeding with a thorough cleansing of the skin.47 After that, the skin is washed and patted dry.
Treatment usually utilizes compresses, cotton, fingertips, an applicator, or a brush to apply the peeling agent to the skin. This application should be even and may be layered for enhanced effectiveness. Each layer should remain on the skin for approximately 5–10 minutes or until erythema appears. The duration of contact is dependent on the causticity of the chemical used and the depth of penetration desired.49 Generally, 4–6 week treatments are required about 3–7 days apart, depending on the depth of it. In Professional Home-kit cosmetics, twice a week is reasonable or based on your skin type.47 To neutralized, NaHCO3 or water is used once erythema is evident or once the required peel is achieved. At the end of each session, a soothing and moisturizing lotion is applied. Following a deep or medium peel treatment, some experts recommend wearing a bandage.47 The whole face or just a specific area might be treated. Facial anatomy is then broken down into four different “aesthetic units” (upper lip, both cheeks, and forehead).49,50 To reduce the visibility of the treatment boundary, we treat the whole unit at once.
Post-peeling care, the step following treatment, is essential for the recovery and maintenance of skin post-procedure. After a superficial peel, continuous hydration is sufficient.48 For a medium peel, a week of recovery is typically needed, during which a moisturizer-based regimen supports the healing process. Deep peels require more extensive postoperative care, extending up to ten days, often necessitating social isolation due to the visible effects on the patient’s appearance. Attentive daily care, utilizing moisturizers or bandages, is crucial during this period.48 Additionally, the prolonged use of sunscreen is recommended following medium and deep peels to protect the newly sensitive skin.
Following a chemical peel procedure, it is essential to prioritize post-peeling care to optimize treatment outcomes and minimize potential adverse effects. Scientific recommendations advocate several key practices to guide post-peeling skincare routines. Firstly, stringent avoidance of sun exposure is paramount, as the skin becomes more vulnerable to sunlight following a peel, thereby reducing the risk of complications. Additionally, diligent application of sunscreen containing a sufficient sun protection factor is imperative, especially during periods of heightened UV exposure, to safeguard the skin from UV-induced damage.51 Furthermore, caution should be exercised in the use of skincare products containing acids, such as AHAs and Beta Hydroxy Acids (BHAs), during sunny months, with evening application recommended and daytime sunscreen application mandated to mitigate potential adverse effect. Lastly, adopting a post-peeling skincare regimen comprising gentle, hydrating products enriched with replenishing ingredients such as squalane, ceramides, and fatty acids is advised to alleviate dryness and irritation and support skin barrier recovery.52
The Efficacy of AHA as Chemical Peels
Glycolic Acid
Chemical peels have gained popularity in recent years for treating various skin conditions, including melasma, acne, and signs of aging. The efficacy of AHAs has been investigated in numerous studies for treating these conditions.
Melasma is a common pigmentation disorder that has been effectively treated with various AHA chemical peels, including glycolic acid (GA) and salicylic-mandelic acid (SM) combinations. Sarkar et al53 conducted a randomized controlled trial (RCT) on 90 Indian patients with melasma and observed a significant reduction in melasma area and severity index (MASI) scores following treatment with these peels. Additional studies have shown that GA peels are effective in reducing MASI scores in patients with melasma.54,55 Furthermore, GA peels combined with modified Kligman’s formula resulted in rapid and better outcomes for melasma treatment in dark-skinned patients.56 When compared with 2% hydroquinone, GA peels and 0.025% tretinoin showed a significant reduction in the MASI after 6 and 9 months of treatment (p<0.001). However, they showed that adding 2% hydroquinone to GA peels was associated with the risk of post-peel post-inflammatory hyperpigmentation compared to 0.025% tretinoin.57 Another RCT performed by the same researchers revealed that among 30 melasma patients, a 35% GA full-face peel significantly reduced the MASI. There was no significant difference between a 35% GA full-face peel performed alone and a 35% GA full-face peel followed by a 10% or 20% TCA spot peel, indicating that combining the peels in the same sitting does not appear to have any synergistic or additive impacts but may rise the adverse reactions.58 Cotellessa et al59 investigated the difference between the combination of 50% GA and 10% kojic acid vs 15–25% TCA in terms of disease status. Their findings showed that complete regression of diffuse melasma was observed in 30%, partial regression in 60%, and no regression in 10% treated with the combination group. Complete regression of localized hyperpigmentations was observed in 40%, partial regression in 50%, and no regression in 10% treated with 15–25% TCA. In patients with recalcitrant melasma, an RCT compared between GA 20–70% peel with and without topical azelaic acid 20% cream and adapalene 0.1% gel. Adding GA 20–70% peel was associated with better outcomes (p=0.048). However, three patients in the GA-containing group developed mild-degree postinflammatory hyperpigmentation, which resolved spontaneously.60
Acne vulgaris, a prevalent skin condition, has also been treated with AHAs. Studies comparing GA with other peels, such as salicylic acid and amino fruit acid (AFA), have found significant improvements in acne lesion count and skin texture. Kessler et al61 conducted a split-face RCT on 20 patients with mild to moderately severe facial acne vulgaris. The patients were assigned to GA 30% on one side and 30% salicylic acid on the other side. Both treatments showed a significant improvement in the patient’s condition; however, 30% salicylic acid was associated with more persistent outcomes and lower adverse events.61 In a similar study, Ilknur et al revealed that the administration of GA 20–70% peel in patients with mild to moderate acne was associated with a significant reduction in the number of inflamed lesions. Moreover, GA was associated with a rapid reduction in the non-inflamed lesions at the first month of treatment, compared to AFA (20–60%), which exerted its effect at the end of the second month.62 In an Egyptian cohort with mild to very severe acne, El Refaei et al showed that both groups experienced substantial improvements; however, 35% GA peel was less effective than SM in improving the condition of the following lesions: boxcar scars (25.8% vs 29.3%), icepick scars (11.9% vs 17.85%), post-acne hyperpigmentation (46.88% vs 66.13%), total acne score (68.50% vs 85.29%), pustules (75.65% vs 85.38%), papules (77.78% vs 81.72%), and comedones (35.87% vs 90.2%), respectively.63 Additionally, they highlighted that both peeling agents were associated with acne flare, desquamation, dryness, and a burning sensation.63 When compared with a placebo, 40% GA peel demonstrated a significant reduction in acne lesions in patients with moderate to severe acne, with a better response for noninflammatory lesions than for inflammatory lesions. Additionally, after 8 and 10 weeks of treatment, sebum levels decreased statistically considerably, although there were no significant differences between the groups.64 Regarding patients with atrophic acne scars, 35% GA peel was found to be better in terms of improving skin texture compared to 15% TCA peeling.65 To determine the concentration-related safety of GA, Perić et al66 investigated three concentrations of GA (20%, 30%, and 50%) in 90 patients with acne.66 The vast majority of patients had a positive experience throughout the treatment. Patients who received 50% GA were associated with a significantly higher rate of moderate to severe erythema, desquamation, and sensation of pulling than other concentrations. The aforementioned adverse events were easily treatable and did not prevent participants from participating in the study. No patients showed signs of post-inflammatory hyper or hypopigmentation in the affected area or in the unaffected skin around it.66 In a split-face RCT, Kim et al evaluated the efficacy of 70% GA peel versus Jessner’s solution in patients with mild to moderate acne. After three sessions, both treatments showed an improvement in disease activity. However, neither treatment approach yielded noticeably different results. In terms of adverse effects, Jessner’s solution resulted in much more exfoliation than glycolic acid (p<0.01).67 Another research compared Jessner’s solution to a combination of 50% GA (pH 3.0) + 0.5% salicylic acid (SA) for the treatment of acne vulgaris. The total lesion count decreased for both the GA and Jessner’s solution groups (P<0.001). However, there was no significant difference in the subjective effectiveness rating, acne severity, or overall lesion count between the different sides (P > 0.05). The GA side experienced fewer side effects than Jessner’s solution side.68 Overall, the efficacy of GA peels varied depending on the concentration and type of acne lesions treated, with generally positive experiences reported by patients. While some adverse events were observed, they were typically treatable and did not hinder participation in the studies.
Patients with acanthosis nigricans were investigated in a retrospective study by Zeeshan et al,69 who compared salicylic-mandelic acid and maintenance with GA-urea combination cream. One hundred percent of patients saw a reduction in lesion thickness and an increase in pigmentation. Forty-one percent had very excellent improvement in skin thickening, while 29% experienced moderate improvement. There was a very good or moderate improvement in pigmentation for 35% of the population. Most patients had some redness and burning after a peel; however, this generally lasted 1–2 days at most. No relapses occurred throughout the following nine months of patient follow-up.69 In patients with pseudo-acanthosis nigricans, Zaki et al70 showed that GA 70% peel was significantly more effective than fractional CO2 in terms of reducing the Acanthosis Nigricans Area and Severity Index.
In order to assess the anti-aging effect of GA, Rouvrais et al71 conducted an RCT on 57 elderly subjects who were assigned to GA peels (20%, 50%, and 70%) or 0.1% RAL associated with Pre-tocopheryl and Glycylglycine Oleamide. Both treatments were equally effective in lowering the total number of fine lines and wrinkles at the treatment’s end, crow’s feet and periorbital wrinkle length, and crow’s feet wrinkle depth (P=0.30). When compared to peels, the cream was much more effective in improving the appearance of the skin’s texture (p=0.025). The anti-aging cream therapy, which lasted eight weeks, was well tolerated, with fewer and less severe side effects than those associated with the peels (Table 2).71
Table 2.
Safety and Efficacy of AHAs Peel in Patients with Acne
| Study ID | Design | Sample size | Patients | Interventions | Main Outcome | Findings |
|---|---|---|---|---|---|---|
| Kessler et al, 200861 | Split-face, double-blind, randomized, controlled study | 20 | Mild to moderately severe facial acne vulgaris | 30% GA | Quantitative assessment of papules and pustules | The glycolic acid and salicylic acid peels were similarly effective. The salicylic acid peel had sustained effectiveness and fewer side effects. |
| 30% salicylic acid | ||||||
| Ilknur et al, 201062 | Single-blind, randomized, right-left comparison study | 24 | Mild to moderate Acne | GA (from 20% to 70%) peels | Cutaneous tolerability assessments | Both GA and AFA peels are efficacious for comedonal acne. And, compared to a GA peel, an AFA peel is less irritating and better tolerated. |
| AFA (from 20% to 60%) | ||||||
| EI Refaei et al 201563 | Single-center, open-label RCT. | 40 | Mild to very severe acne | 35% GA (in distilled water) | Objective and subjective assessment/ Patients’ safety and satisfaction | Both peeling agents were effective. However, SMP proved to have a higher efficacy than the more commonly used GAP. |
| 20% SA+10% MA (in ethyl alcohol vehicle) | ||||||
| Kaminaka et al 201464 | Single-center, double-blind, split-face RCT | 25 | Moderate to severe Acne | 40% GA | Reduction in acne lesions | Forty percent of GA peels significantly improved moderate acne in this study. It is effective and safe in Asians. |
| Placebo (hydrochloric acid in polyethylene glycol vehicle) | ||||||
| Kim et al 199967 | Single-center, single-blind, split-face RCT. | 26 | Mild to moderate Acne | 70% GA | Reduction in acne lesions and safety profile | Considering the equal treatment effect and a lesser degree of exfoliation in GA, it was recommended to use GA over Jessner’s solution for acne patients. |
| Jessner’s solution (resorcinol, salicylic acid, lactic acid in ethanol) | ||||||
| Dayal et al, 202265 | RCT | 20 | Atrophic Acne Scars | 35% GA | Goodman and Baron’s qualitative and quantitative global acne scar grading systems, physician’s global assessment, and VAS | Both combinations were equally efficacious in treating acne scars. Glycolic acid peel delivered additional advantage of improvement in skin texture. |
| 20 | 15% TCA peeling | |||||
| Dayal et al, 201972 | Comparative study | 25 | Mild-to-moderate acne vulgaris | 45% mandelic acid | Reduction in acne lesions and safety profile | About 45% MA peel was found to be equally effective as 30% SA peel in mild-to-moderate facial AV. However, safety and tolerability of MA peel were better than SA peel. |
| 25 | 30% salicylic acid | |||||
| Bs et al, 202173 | Comparative, retrospective analysis | 22 | Acne and Acne-Induced Pigmentation | Salicylic acid-mandelic acid peel | Complication rate and patients’ satisfaction | The use of isotretinoin did not result in any complications; hence, they encouraged combination therapy to achieve enhanced and faster resolution of acne. |
| 18 | GA peels (20%, 50%, and 70%) | |||||
| 7 | Modified Jessner’s peel | |||||
| Dixit et al, 202274 | Double-blind randomized single-center interventional open-label study | 28 | Acne tarda | Low-dose isotretinoin alone | Reduction in acne lesions and safety profile | The combination of oral isotretinoin with SM peeling was highly effective and could be used as newer therapy against AT without any serious side effects. |
| 30 | Low-dose isotretinoin and combination with salicylic acid and mandelic peel | |||||
| Nofal et al, 201875 | Comparative study | 15 | Mild-to-moderate acne | Modified Jessner’s solution followed by TCA 20% on the right (Rt) side of the face vs TCA 30% on the left (Lt) | Reduction in acne lesions and safety profile | Combination peels achieved a higher and earlier therapeutic response with a reasonable cost that is maintained for a relatively long periods than single peel. Combination sequential peels gave the best results. |
| 15 | Combination peels of salicylic (20%) mandelic (10%) acids (SM) mixture on the Rt half vs salicylic acid 30% on the Lt half. | |||||
| 15 | Combination sequential peeling of MJ and TCA on the Rt side vs SM combination peels on the Lt side. | |||||
| Dayal et al, 201676 | RCT | 20 | Mild-to-moderate facial acne in Indian patients | 30% SA | Michaelsson acne scores (MAS) and clinical photographs | 30% SA peels were more effective than JS peels in the treatment of noninflammatory lesions, that is, comedones and in overall improvement of mild-to-moderate facial acne vulgaris. |
| 20 | Jessner’s solution peels | |||||
| How et al, 202077 | A randomized, double-blinded, split-face, controlled trial | 36 | Acne vulgaris and postacne hyperpigmentation in patients with colored skin | Salicylic acid (SA) 30% | Michaelsson acne score (MAS), photographs, and postacne hyperpigmentation index (PAHPI) | Both JS and SA were equally effective in the treatment of acne vulgaris and reducing postacne hyperpigmentation in patients with colored skin. |
| Jessner’s solution | ||||||
| Jae et al, 201868 | Prospective, randomized, evaluator-blind, split-face clinical trial | 20 | Acne vulgaris | Jessner’s solution | Reduction in acne lesions and safety profile | Chemical peeling using the 50% GA (pH 3.0) + 0.5% SA solution can be as effective and convenient as the conventional peeling using Jessner’s solution in the treatment of acne vulgaris and may show fewer adverse events than the conventional peeling. |
| 50% GA (pH 3.0) + 0.5% salicylic acid (SA) | ||||||
| Perić et al, 201166 | Comparative study | 30 | Acne | 20% GA | Safety profile | Chemical peeling with glycolic acid is a well-tolerated and safe treatment modality in acne type I. |
| 30 | 35% GA | |||||
| 30 | 50% GA |
Abbreviations: RCT, Randomized Controlled Trial; GA, Glycolic Acid; AFA, amino fruit acid; JS, Jessner’s solution; VAS, Visual Analog Scale; AT, Acne tarda.
Lactic Acid
LA can help remove dead skin cells, smooth rough areas, and moisturize the skin. Moreover, it has been shown to improve signs of aging by stimulating collagen renewal, which helps keep the skin firm. Lactic acid can also fade sun spots or age spots and smooth and soften fine lines and wrinkles, although it is not effective in treating deeper lines. One of the applications of lactic acid in skincare is in the treatment of eczema.78 Over-The-counter eczema creams containing lactic acid can help alleviate the symptoms of this skin condition. Additionally, lactic acid peels can be performed by professionals in day spas (superficial peels) or medical settings (deeper peels) to help manage eczema.79 Most of the previously published studies investigated the role of LA as a part of Jessner’s solution, which contains 14 mL of LA, 14 g of salicylic acid, 14 g of resorcinol, and 100 mg of 95% ethanol.80 Ejaz et al compared Jessner’s solution and 30% salicylic acid in 60 patients with melasma. Both groups induced a significant reduction in the MASI score from the baseline; however, there was no statistically significant difference between the two groups (p=0.55). The adverse effects were mild and similar in both groups.81 On the other hand, Sharquie et al80 conducted a clinical trial on 20 patients with melasma who received pure LA (92%; pH 3.5). They showed that all patients experienced a significant decline in the MASI score with no recorded adverse effects (Table 3).
Table 3.
An Overview of Studies Investigating the Efficacy of Various Chemical Peels for the Treatment of Melasma. Studies Include Details Such as Study Design, Sample Size, Patient Demographics, Interventions, Main Outcomes, and Key Findings
| Study ID | Design | Sample size | Patients | Interventions | Main Outcome | Findings |
|---|---|---|---|---|---|---|
| Sarkar et al, 201653 | RCT | 30 | Indian Patients with Melasma | GA-35% peel | Melasma area and severity index | GA (35%) and SM acid peels are both equally efficacious and a safe treatment modality for melasma in Indian skin, and are more effective than phytic acid peels |
| 30 | Salicylic-mandelic (SM) acid (20% salicylic/10% mandelic acid) | |||||
| 30 | Phytic combination peels | |||||
| Erbil et al, 200760 | RCT | 16 | Recalcitrant melasma | GA peels (20–70%) in combination with topical azelaic acid 20% cream (b.i.d). and adapalene 0.1% gel | Melasma area and severity index | Combined treatment with serial glycolic acid peels, azelaic acid cream and adapalene gel should be considered as an effective and safe therapy in recalcitrant melasma |
| 12 | Topical treatment including topical azelaic acid and adapalene | |||||
| Kumari et al, 201054 | Comparative study | 40 | Indian Patients with Melasma | GA 20–35% vs Trichloroacetic acid (TCA 10–20%) |
Melasma area and severity index | Objective response to treatment evaluated by reduction in MASI scoring after 12 weeks was by 79% reduction in GA group and by 73% reduction in TCA group but this difference was not significant. Subjective response, as graded by the patient, showed good or very good response in 75% in GA group and 65% in TCA group. |
| Ilknur et al, 201055 | Single-blind, randomized right-left comparison study | 31 | Melasma | GA (from 20% to 70%) peels and amino fruit acid (AFA; 20% to 60%) | Modified Melasma Area and Severity Index | GA and AFA peels for melasma therapy were efficacious, but the AFA peel was found to be less irritating and was better tolerated. |
| Garg et al, 200857 | RCT | 20 | Melasma | GA peels | Modified Melasma Area and Severity Index | Results are better with hydroquinone as priming agent compared to tretinoin in enhancing the results with glycolic acid peels in melasma and in decreasing postpeel postinflammatory hyperpigmentation. |
| 20 | 0.025% tretinoin | |||||
| 20 | 2% hydroquinone | |||||
| Garg et al, 201958 | RCT | 10 | Facial melasma | 35% GA full-face peel | Melasma area and severity index and safety outcomes | Chemical peels with GA alone or in combination with TCA do result in a significant improvement in melasma, but the combination of the peels in the same sitting does not seem to have any additive or synergistic effect while they may increase the side effects. |
| 10 | 35% GA full-face peel followed by a 10%TCA spot peel | |||||
| 10 | 35% GA full-face peel followed by a 20% TCA spot peel | |||||
| Sarkar et al, 202256 | Comparative study | 20 | Melasma in dark-skinned patients | GA peel combined with Kligman’s formula (hydroquinone 5%, tretinoin 0.05%, hydrocortisone acetate 1% in a cream base). | Melasma area and severity index | Serial GA peels provide an additional effect to a topical regimen which is a modification of the time-tested Kligman’s regimen for treating melasma in dark-complexioned individuals if used judiciously and under supervision. It demonstrates that superficial chemical peels are beneficial in the treatment of melasma. |
| 20 | Kligman’s formula | |||||
| Chaudhary and Dayal 201382 | RCT | 20 | Melasma | Topical regimen (2% hydroquinone, 1% hydrocortisone and 0.05% tretinoin) with serial GA peeling | Melasma area and severity index | Topical regimen with serial GA peeling significantly enhances the therapeutic efficacy of GA peeling. The combination of GA peeling with the topical regimen is a highly effective, safe and promising therapeutic option in treatment of melasma. |
| 20 | Control group received topical regimen (2% hydroquinone, 1% hydrocortisone, 0.05% tretinoin). | |||||
| Cotellessa et al, 199959 | Comparative study | 20 | Melasma | 50% GA and 10% kojic acid | Disease status | Both peelings can be considered effective in the treatment of cutaneous hyperpigmentations. |
| 20 | 15–25% TCA | |||||
| Ejaz et al, 200881 | Double blind, randomized, interventional comparative study | 30 | Melasma | Jessner’s solution | Melasma area and severity index | Jessner’s solution and 30% salicylic acid are equally effective and safe peeling agents for use in epidermal melasma in Asian skin. |
| 30 | 30% salicylic acid | |||||
| Sharquie et al, 200580 | Clinical trial | 20 | Melasma | Pure lactic acid, full strength (92%; pH 3.5) | Melasma area and severity index | Lactic acid was found to be a new effective and safe peeling agent in the treatment of melasma. |
| Dayal et al, 201683 | Comparative study | 30 | Periorbital melanosis | 20% GA peel | POM grading | Glycolic peel was best among the three modalities, although it was associated with increased rate of side effects. |
| 30 | 15% lactic peel | |||||
| 30 | Topical 20% vitamin C |
Abbreviations: RCT, Randomized Controlled Trial; GA, Glycolic Acid; TCA, Trichloroacetic Acid; MASI scoring, Melasma Area and Severity Index.
Ellabban et al84 conducted an RCT to investigate the safety and efficacy of trichloroacetic acid and LA vs PRP injection in patients with periorbital hyperpigmentation. Chemical peels outperformed platelet-rich plasma by a wide margin. Comparatively, 47.6% of those in the LA group showed good improvement, whereas just 4.8% of those in the PRP group did (p<0.001). Regarding excellent improvement, it was achieved in 38% in the LA group vs 0% in the PRP group. In contrast to PRP, where 52.5% were just satisfied, the majority of LA patients reported being very pleased (47.6%). The rate of itching and redness was comparable in both groups (p= 0.07).84
Mandelic Acid
Mandelic acid’s efficacy as a chemical peel agent for treating various skin concerns is supported by numerous studies. Dayal et al compared 45% mandelic acid to 30% salicylic acid in individuals with mild to severe acne vulgaris. Mild to severe acne vulgaris symptoms were similarly alleviated by both treatments.72 Noninflammatory lesions responded better to salicylic acid, whereas inflammatory lesions responded better to MA. When comparing the two peels for their ability to improve MAS and reduce MAS by percentage, there was no discernible winner. In contrast, MA peels have fewer adverse effects.72 In a study conducted by Taylor (3), the use of mandelic acid peels was examined in the treatment of acne and post-inflammatory hyperpigmentation (PIH) in patients with darker skin tones. The results demonstrated significant improvement in the participants’ acne and PIH with minimal side effects, indicating that mandelic acid peels are both safe and effective for these populations.85 In addition to its effectiveness, mandelic acid is well-tolerated and associated with minimal side effects. This makes it an attractive option for a broad range of patients, including those with sensitive skin or darker skin tones who may be at higher risk for post-inflammatory hyperpigmentation.86 Overall, mandelic acid has demonstrated its potential as a safe and effective AHA for chemical peels, providing a valuable treatment option for individuals seeking to improve their skin’s appearance and health. While more research may further elucidate its full range of applications, current evidence supports mandelic acid’s use as a chemical peel agent for various skin concerns, from acne and hyperpigmentation to mild photoaging, as shown in Table 2.
Malic Acid
Malic Acid is commonly used in cosmetic formulations as a pH adjuster, while Sodium Malate functions as a skin conditioning agent and humectant. Past product formulation data from 1998 revealed that Malic Acid was present in 47 cosmetic product formulations, while Sodium Malate was only present in one cosmetic formulation.87 However, the data regarding the current concentration of use are not available. A study conducted in 1996 showed that Malic Acid could increase cell renewal rates by 18%, 10%, and 5% at pH levels of 3, 5, and 7, respectively.88 Malic Acid has also been used in clinical settings to treat ichthyosiform dermatoses, providing significant improvement for patients except for one with epidermolytic hyperkeratosis.87 A test conducted in 1995 evaluated the efficacy and safety of a tablet containing 200mg of Malic Acid and 50mg of magnesium on patients with primary fibromyalgia syndrome. The study showed some patients experienced mild side effects, such as diarrhea and nausea.89 In a study conducted in 1979, 34 patients with atopic dermatitis were tested for sensitivity to foods containing Malic and citric acid. Results revealed that immediate and delayed reactions occurred in 18 patients reacting to both Malic and citric acid and six patients reacting to only Malic Acid.89 The skin irritation potential of Malic Acid was evaluated by applying it to the nasal fold area of subjects, and results showed average irritation scores of 39.4, 37.1, and 23.1 for pH levels of 3, 5, and 7, respectively.88 The Expert Panel for Cosmetic Ingredient Safety reassessed the safety of Malic Acid and Sodium Malate in cosmetics. Malic Acid is used as a fragrance ingredient and pH adjuster, while Sodium Malate acts as a skin-conditioning agent and humectant. After reviewing available data, the Panel concluded that both Malic Acid and Sodium Malate are safe for use in cosmetics at the current practices and concentrations outlined in the safety assessment.90
Citric Acid
Citric acid has been shown to have various beneficial effects in treating skin diseases. Its effectiveness as an exfoliant and antioxidant has been investigated in a very limited number of studies. In a study conducted by Ditre et al,34 17 participants with moderate to severe photoaged skin used a lotion containing 25% alpha hydroxy acid (AHA) on one forearm and a placebo on the other. The AHAs included glycolic (5 patients), lactic (5 patients), and citric acids (7 patients). The authors assessed skin conditions through clinical, histological, and ultrastructural methods. Results demonstrated a 25% increase in skin thickness after AHA treatment, with the epidermis and dermis being significantly thicker compared to the placebo side. Additionally, the treatment led to improvements in skin cell structure, a reduction in basal cell atypia, more even melanin distribution, and a return to normal skin patterns. However, the study did not differentiate between the specific effects of each AHA used.34 Citric acid has been shown to be useful in the treatment of acne and melasma.91,92 The CIR Expert Panel assessed the safety of citric acid, 12 inorganic citrate salts, and 20 alkyl citrate esters in cosmetics and concluded that they are safe for use at current concentrations and practices. Citric acid and some salts serve as pH adjusters, chelating agents, or fragrance ingredients, while several citrates function as skin-conditioning agents, among other uses.14 Although the Panel examined animal and clinical data, the focus was on dermal exposure for ingredients that are recognized as safe, direct food additives, such as citric acid and several citrate salts. They determined that citric acid, inorganic citrate salts, and alkyl citrate esters are safe in their current uses and concentrations in cosmetics,14 as shown in Table 4.
Table 4.
Safety and Efficacy of AHAs in Patients with Other Dermatological Diseases
| Study ID | Design | Sample size | Patients | Interventions | Main Outcome | Findings |
|---|---|---|---|---|---|---|
| Liu et al, 202293 | Randomized, split-face, evaluator-blinded trial | 30 | Disseminated facial verruca plana | 35% GA | The clearance rate | Chemical peeling with 35% GA could be a safe and effective option for treating disseminated facial verruca plana, especially for those who desire skin improvement. |
| Adapalene gel | ||||||
| Oresajo et al, 200847 | Split-face study RCT | 44 | Mild to moderate facial hyperpigmentation and fine lines/wrinkles | 5–10% LHA | Reduction of hyperpigmentation | Five percent to 10% of LHA peel is generally safe and as effective as 20–50% GA peel in reducing facial hyperpigmentation and fine lines/wrinkles. |
| 20–50% GA | ||||||
| Rouvrais et al, 201871 | RCT | 30 | Aging | GA peels (20%, 50%, and 70%) | Reducing crow’s feet wrinkles depth and volume, crow’s feet and periorbital wrinkle length, and number of fine lines and wrinkles at end of treatment | A dermo-cosmetic cream containing 0.1% RAL, GGO (Relastide®) and Pre-tocopheryl® is as effective as 3 sequential GA peels, better tolerated, and is an alternative in the management of photoaged skin. |
| 27 | 0.1% RAL associated with Glycylglycine Oleamide and Pre-tocopheryl® | |||||
| Zaki et al, 201870 | Comparative split neck study | 20 | Pseudo-acanthosis nigricans | GA peel 70% | Acanthosis Nigricans Area and Severity Index (ANASI) | Glycolic acid peel shows superior results to fractional CO2 due to accelerated induced exfoliation, yet still fractional CO2 results are promising due to a presumably long-term improvement of skin texture. |
| Fractional CO2 | ||||||
| Ellabban et al, 201984 | RCT | 21 | Periorbital hyperpigmentation | TCA and lactic acid | Patients’ satisfaction and objective response | Both PRP and chemical peeling are effective for treatment of POH; however, chemical peeling is much more effective, tolerable, and satisfying procedure than PRP. |
| 21 | PRP injection | |||||
| Zeeshan et al, 202269 | Retrospective pilot study | 17 | Acanthosis nigricans | Salicylic-mandelic acid | Objective response and safety profile | Combination of keratolytic chemical peels and topical mild keratolytic application ensures better therapeutic outcome in patients of AN with long lasting effect. |
| Maintenance with a glycolic acid-urea combination cream |
Tartaric Acid
Tartaric acid has been shown to have the potential as a chemical peel in skincare treatments. As an AHA, tartaric acid works by exfoliating the skin’s surface, stimulating cell renewal, and improving skin texture and tone.8 Though other AHAs like glycolic and lactic acids are more commonly used, tartaric acid has demonstrated the potential to provide similar benefits.34 A study conducted by Fiume et al94 has indicated the safety of tartaric acid when used in cosmetic formulations, making it a promising alternative to other chemical peels. The efficacy of tartaric acid as a chemical peel has been investigated in various studies, with promising results. A study by Kornhauser et al5 examined the use of tartaric acid in combination with other AHAs in a chemical peel and found that it effectively improved skin appearance by reducing fine lines, wrinkles, and hyperpigmentation. The authors also noted that tartaric acid could enhance the effects of other AHAs in the formulation, thus contributing to its overall effectiveness. However, it is essential to consider the safety of tartaric acid in chemical peels. Like other AHAs, tartaric acid can cause skin irritation and sensitivity, particularly when used at higher concentrations or when applied improperly.95 Therefore, it is crucial to follow the recommended usage guidelines and concentrations to minimize the risk of adverse effects. In conclusion, tartaric acid shows potential as a chemical peel agent, but further research is needed to establish its efficacy and safety profile more conclusively.
Formulation
The popularity of AHAs in cosmetic Formulations has grown significantly over the past 15 years. In the original 1998 safety assessment, glycolic acid was reported to be included in 42 formulations, while lactic acid was used in 342 formulations. As of 2013, according to the Voluntary Cosmetic Registration Program (VCRP) data, glycolic acid is now incorporated in 337 formulations and lactic acid in 1042 formulations. Myristyl lactate is reported to be used in 215 formulations, with the highest maximum reported use concentration of 13.2% in lipsticks.96
When selecting an AHA for use in a formulation, several factors must be considered, including the size of the molecule and its solubility. One advantage of small molecule AHAs, such as glycolic and lactic acid, is their ability to penetrate the skin more deeply.95 This makes them more effective at treating fine lines, wrinkles, and acne. On the other hand, larger molecules like mandelic and tartaric acid are less likely to cause skin irritation or sensitivity, making them better suited for those with sensitive skin. In addition to molecule size, lipophilic (oil-soluble) AHAs, like mandelic acid, have been shown to penetrate the skin more easily than hydrophilic (water-soluble) AHAs, like lactic acid.72,85 This property makes them more effective at treating acne and hyperpigmentation. Another important factor to consider when formulating with AHAs is the chemical structure of the chosen AHA. Glycolic acid can be derived from both natural and synthetic sources and is available in several different salt forms, each with unique properties.97 For example, sodium glycolate is a water-soluble salt of glycolic acid and is often used in cleansers and toners, while glycolic acid polymers are larger molecules that are more suitable for use in leave-on products like creams and lotions. Finally, the chosen formulation type can also affect the efficacy of the chosen AHA. For example, solutions are typically more effective at exfoliating the skin than creams or lotions. Emulsions, on the other hand, can provide both exfoliation and moisturization and are often used in leave-on products like serums.34
The increased use of these AHAs can be attributed to their versatile applications in different cosmetic products. Glycolic and lactic acids are commonly found in formulations such as facial cleansers, exfoliants, moisturizers, and anti-aging products. Some AHAs are present in formulations that could potentially be inhaled. For instance, glycolic acid is reported to be used in aerosol and pump hair sprays at concentrations of 0.0005% and 0.05%, respectively, while lactic acid is found in aerosol propellant hair spray formulations at a concentration of 0.0002% and in tonic, dressing, and other hair grooming aids pump spray formulations at a concentration of 5.8%.98 In practice, 95% to 99% of the droplets or particles released from cosmetic sprays have equivalent aerodynamic diameters greater than 10 µm. Propellant sprays produce a higher fraction of droplets or particles smaller than 10 µm compared to pump sprays. As a result, most droplets or particles that are incidentally inhaled from cosmetic sprays would be deposited in the nasopharyngeal and thoracic regions of the respiratory tract and would not be respirable (ie, they would not enter the lungs) to any significant extent.99,100 These formulations of AHAs offer various benefits, including promoting skin cell turnover, improving skin texture and tone, reducing the appearance of fine lines and wrinkles, and helping to combat acne. As a result, the cosmetic industry continues to develop and introduce new formulations containing glycolic and lactic acids to meet the growing demand for effective skincare products.
Combination Therapy
GA peels have often been used in conjunction with other treatments to enhance results. In a study conducted by Sharad J. et al101 35% GA peel was combined with micro-needling to address acne scars in patients with skin types III–IV. Microneedling sessions took place every six weeks, and the GA peel was applied three weeks after each micro-needling session. This combination led to significant improvements in superficial and moderately deep atrophic box scars, rolling scars, skin texture, and post-acne pigmentation. In cases of post-inflammatory hyperpigmentation, post-acne pigmentation, and melasma, GA and trichloroacetic acid (TCA) peels have been used together. This combination has been shown to produce a more profound and uniform peel than using TCA alone.102 Additionally, combining Jessner’s Solution with GA to treat photoaged skin, actinic keratoses, and wrinkles resulted in a uniform GA peel. However, there is an increased risk of over-peeling and scarring, particularly in individuals with darker skin.103–105 GA has also been combined with 5-fluorouracil for treating actinic keratosis. Pre-treating the skin with 5% 5-fluorouracil enhances the effectiveness of the treatment and reduces the healing time.106 GA peeling has also been used in combination with microdermabrasion for the treatment of acne vulgaris and superficial acne scars in order to increase treatment efficacy and achieve treatment goals within a shorter time. Alpha-hydroxy acid peels decrease corneocyte cohesion, making the abrasion more efficient.107 However, combining GA peels with microdermabrasion during the same session could lead to postinflammatory hyperpigmentation in skin types III–VI. Therefore, care should be taken with darker skin types.108 Briden et al109 reported good patient satisfaction when using superficial GA peels with microdermabrasion for photoaging. A study investigating photodamaged skin utilized an intense light combining narrow-band blue light (405–420 nm, anti-inflammatory) and near-infrared (850–890 nm) emissions alongside glycolic peels and daily Vitamin C cream application. The results demonstrated significant improvements in pore size, wrinkles, and skin radiance.110 Glycolic acid peeling has also been combined with Vitamin C in cases of melasma and post-inflammatory hyperpigmentation. In a melasma patient, one side of the face received 70% GA peeling with iontophoresis and nanosome Vitamin C, while the other side underwent 70% GA peeling alone. Both sides improved, but the side treated with iontophoresis and nanosome Vitamin C showed better results.111 Superficial glycolic acid peels can be used in conjunction with botulinum toxin and dermal fillers to achieve overall enhancements in wrinkles, skin tone, texture, radiance, and clarity. In one study, the interval between peels and fillers was one week. The peel was administered after injecting botulinum toxin during the same visit, or the procedures were separated by one or more days to minimize potential side effects.112,113 Another study by Bs et al73 showed that combining salicylic acid with mandelic acid peel in patients with acne was associated with a significant improvement in the patient’s condition; however, this effect was comparable to the effect achieved by GA peels (20%, 50%, and 70%) and modified Jessner’s peel.
Market Analysis
The top companies that manufacture AHA products are Tokyo Chemical Industry, H Plus Limited, Lotion Crafter, Sculptra Aesthetics, Chemours Company, Airedale Chemical Company Limited, Mehul Dye Chem Industries, Ava Chemicals, Crosschem, and Cargill. Regionally, Due to the region’s focus on skin health and the existence of significant manufacturers of personal care products, North America has the largest market for AHA products. Europe, the Asia-Pacific region, the Middle East and Africa, and Latin America are included after North America. The demand for alpha hydroxyl acid is increasing as a result of research on active ingredients in the European skincare market. During the projected period, it is expected that the market in Asia Pacific will grow at a rapid CAGR, driven by the increased attention being paid to skin care because of pollution, particularly in light of the smog case in China. Alpha hydroxyl acid, which helps to minimize wrinkles, is also in high demand in Japan due to the country’s aging population.
Customers’ rising expenditures on cosmetics and personal care items are among the factors driving global demand. Since the regulation varies among countries, someone can easily find glycolic with up to 70% to be delivered to his home. In Amazon, Noon, and eBay, for example, the price range for GA 30% and 50% is $19-22, and the rate of customers ranges between 4.5 and 5. It is available in several formulations, especially serums and solutions, especially higher concentrations of 30–70%. Some of which have no clear labels to advise for professional use only or salon products. Professional grade or dermatologist grade is used instead of professional use, which is not as per the regulation as it imposes a misleading statement. Many products contain no clear recommendations for UV protection or the SPF minimum requirements. For LA, it has the same price range with customer satisfaction between 4 and 4.5. In Amazon EU, the price range for GA 10%, 30%, and 50% is €12-30, and the rate of customers ranges between 4.5 and 5. For LA, it has the same price range with a customer satisfaction between 4 and 4.5. Besides Amazon and eBay, Hundreds or thousands of mini-e-shops or boutiques around the world are selling different brands by the same practices direct from their websites or factories to customers in any part of the world. This makes the practices very difficult to regulate or monitor.
The major concern is that these products might be used by non-professionals and are applied directly without either a pre-treatment step or even without adequate neutralization. Thus, some companies have started formulating a proper home kit to include all the required steps with all the professional details and are sold as an OTC medication. Another term coined recently by the market is professional home use, in which the kit is sold with all the required steps and details as a cosmetic product. This huge variability is perhaps caused by the ambiguity of the regulations in some countries.
Discussion
The synthesized data from various studies illustrate that AHAs, especially glycolic acid and lactic acid, effectively promote skin rejuvenation through mechanisms such as exfoliation, increased skin turnover, and the stimulation of collagen and elastin synthesis. These actions enhance skin texture and treat conditions like acne, hyperpigmentation, and photoaging, although the effectiveness of AHAs varies based on their type, concentration, and the individual’s skin type. Despite their prevalent use, the optimal concentrations for maximizing therapeutic benefits while minimizing side effects are not well-defined, highlighting the need for customized treatments to balance efficacy and skin sensitivity.
The importance of regulatory compliance is emphasized, with guidelines from bodies like the FDA and EMA critical in setting safe usage parameters for AHAs in cosmetics. Adhering to these guidelines ensures patient safety and enhances product credibility and marketability, though ongoing vigilance is necessary, especially when AHAs are used at higher concentrations or in combination with other treatments.
The increasing global demand for AHA-based products calls for continuous innovation in formulations, alongside efforts to ensure consumer safety, particularly in less regulated markets. There is a pressing need for further research to refine AHA formulations and treatment protocols, focusing on understanding their mechanistic actions and long-term safety to develop more targeted and effective treatments that minimize risks and optimize patient outcomes.
Several contemporary treatment approaches for skin anti-aging and rejuvenation effectively mitigate and reverse signs of aging, employing both invasive and non-invasive methods. Microdermabrasion physically exfoliates the skin to remove dead cells and stimulate collagen production, enhancing skin texture.114 Dermal fillers and Botox injections restore volume and reduce wrinkles by filling in soft tissue or relaxing facial muscles.115 Laser skin resurfacing and Intense Pulsed Light (IPL) treatments target deeper layers, addressing wrinkles, pigmentation, and texture issues.116 Mesotherapy injects mixtures of vitamins and enzymes directly into the skin, providing nourishment and promoting collagen and elastin production.117 These modalities offer various degrees of invasiveness from non-invasive laser treatments to surgical options, each catering to specific aging signs and skin types.
AHAs, including glycolic and lactic acids, are widely used in skin rejuvenation treatments due to their exfoliating properties that promote cell turnover and reveal newer, healthier skin layers. This process not only improves skin texture and reduces fine lines but also enhances overall brightness and increases collagen production, thereby aiding in hydration and reducing hyperpigmentation.6 However, the use of AHAs is not without disadvantages; they can increase skin’s sensitivity to sunlight, necessitating the consistent application of sunscreen, and can cause side effects like swelling, burning, and itching when used in higher concentrations for professional peels.9
In comparison, other skin rejuvenation treatments like laser therapy and IPL provide deeper skin rejuvenation and address more significant issues such as deep wrinkles and texture concerns with longer-lasting results, albeit with potentially more downtime and a higher risk of side effects. Treatments like microneedling and dermal fillers offer benefits beyond the reach of AHAs by adding substantial volume and reducing deep wrinkles,118,119 while Botox and other neurotoxins target muscle contractions to smooth out wrinkles,120 particularly in the upper face. Thus, while AHAs offer excellent superficial rejuvenation suitable for a broad range of skin types, more invasive treatments may be necessary for more pronounced signs of aging or specific skin conditions. Each treatment option should be selected based on individual skin concerns, desired outcomes, and tolerance for potential side effects.
Generally, it is advised to use a broad-spectrum sunscreen that provides both UVA and UVB protection following chemical peeling.121 Given the heightened sensitivity of the skin after such treatments, physical sunscreens containing mineral-based ingredients such as titanium dioxide or zinc oxide are often recommended due to their lower potential for irritation. These physical blockers provide a barrier that reflects and scatters ultraviolet radiation, offering immediate protection upon application, which is particularly beneficial for post-procedure skin.122 Nonetheless, for comprehensive coverage, formulations that combine physical blockers with chemical filters can also be considered, provided they are well-tolerated by the individual’s skin. It would be prudent to select sunscreens that are also fragrance-free and non-comedogenic to minimize any potential irritation and avoid clogging pores.
In a comparative analysis of AHAs based on effectiveness and suitability, each AHA exhibits distinct characteristics. Glycolic acid, with its small molecular size, penetrates deeply into the skin, making it highly effective for enhancing collagen production, improving texture, and reducing wrinkles. It is best suited for normal to oily skin types, although its deep penetration might cause irritation in sensitive skin.123 Lactic acid, with a larger molecular size, penetrates less deeply, which reduces the risk of irritation, making it ideal for dry to sensitive skin types. It effectively stimulates collagen renewal and increases skin hydration.124 Mandelic acid’s larger molecular structure results in slower skin absorption, making it less irritating and particularly effective for sensitive skin, including those prone to hyperpigmentation and redness. It is renowned for its antibacterial properties, which are beneficial for acne-prone skin.125 Malic acid, typically used in combination with other AHAs, is known for improving skin smoothness and tone, and is suitable for all skin types as part of a combined AHA formulation. Tartaric acid, often used to stabilize pH levels in skincare products, acts as an antioxidant and is suitable for most skin types when used in conjunction with other AHAs.126
Study Limitations
In the domain of dermatological research, this review presents several notable limitations that compromise the breadth and depth of current scientific findings. To enhance the reliability and applicability of results, we indicate those limitations. Broadening the scope of study designs beyond randomized controlled trials, cohort studies, and case reports is essential. Incorporating systematic reviews and meta-analyses would elevate the level of evidence and offer broader insights into the effects of AHAs, thereby enriching the research landscape. There is a critical need to standardize AHA treatment protocols across studies. This includes precise definitions of AHA concentrations, application methods, treatment durations, and follow-up periods to ensure consistency and reproducibility of outcomes, reducing variability and enhancing the quality of evidence. Long-term follow-up studies are crucial for assessing the sustainability of AHA treatment effects and monitoring potential long-term adverse effects. Such studies will help delineate the long-term safety and efficacy profiles of AHAs, providing deeper insights into their prolonged use.
Detailed documentation of regulatory compliance, including adherence to international guidelines from bodies like the FDA and EMA, is paramount. Comparative analyses of AHA regulations across different countries could highlight best practices and identify areas for regulatory harmonization. Ensuring diverse demographic representation in study populations—including variations in age, skin type, ethnicity, and underlying skin conditions, is vital for generalizing findings to a broader population and understanding the differential effects of AHAs across diverse groups.
Enhancing the monitoring of adverse effects in clinical trials is necessary. This involves detailed recording and analysis of side effects, their severity, and the circumstances under which they arise, alongside establishing standardized approaches for managing these effects. Research should also focus on the interactions between AHAs and other dermatological treatments. Understanding these interactions can inform integrated treatment approaches and prevent potential negative interactions, optimizing therapeutic outcomes.
Conclusion
This comprehensive review investigates about the multifaceted role of AHAs within dermatological practices, particularly emphasizing their application in cosmetic formulations like chemical peels. AHAs, including glycolic and lactic acids, have demonstrated significant efficacy in skin rejuvenation, harnessing their ability to induce apoptosis in skin cells and foster the synthesis of collagen and elastin. These mechanisms contribute markedly to improving skin texture and mitigating signs of aging and photodamage.
Despite the broad utility and acceptance of AHAs in cosmetic dermatology, challenges persist, particularly regarding the optimization of concentration levels to balance efficacy with minimal adverse effects. The nuances of AHA formulations are critical, highlighting the need for tailored treatments according to individual skin types and conditions. Furthermore, the regulatory landscape presents an ongoing challenge, necessitating strict compliance with international guidelines to ensure the safe use of AHAs. These regulations dictate permissible concentrations and pH levels to minimize potential skin irritation.
The global market outlook for AHAs remains robust, with projections indicating significant growth driven by an increasing demand for effective anti-aging and skin rejuvenation solutions. This growth underscores the importance of continued research and innovation in the development of AHA-based treatments, ensuring they meet safety standards while fulfilling consumer expectations for efficacy and minimal side effects.
Future research directions should focus on defining the optimal formulations and treatment protocols for AHAs, enhancing our understanding of their mechanistic pathways in skin rejuvenation, and further evaluating their long-term safety profiles. Such efforts will be essential for advancing the use of AHAs in clinical dermatology, ensuring they remain a cornerstone of non-invasive cosmetic treatments.
Acknowledgment
I would like to thank the Deanship of Scientific Research, Qassim University for funding publication of this project.
Disclosure
The author declares no competing interests in this work.
References
- 1.Spherical Insights. Alpha hydroxy acid market size, trend during forecast 2030; 2022.
- 2.Williams ML. Global warming, heat-related illnesses, and the dermatologist. Int J Women’s Dermatology. 2021;7(1):70–84. doi: 10.1016/j.ijwd.2020.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Samargandy S, Raggio BS. Skin Resurfacing Chemical Peels. Treasure Island (FL); 2022. [PubMed] [Google Scholar]
- 4.Egli C, Min M, Afzal N, Sivamani RK. The hydroxy acids: where have we been and what’s new? Dermatological Rev. 2023;4(6):260–267. doi: 10.1002/der2.217 [DOI] [Google Scholar]
- 5.Kornhauser A, Coelho SG, Hearing VJ. Applications of hydroxy acids: classification, mechanisms, and photoactivity. Clin Cosmet Invest Dermatol. 2010;3:135–142. doi: 10.2147/CCID.S9042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karwal K, Mukovozov I. Topical AHA in Dermatology: formulations, Mechanisms of Action, Efficacy, and Future Perspectives. Cosmetics. 2023;10(5):131. doi: 10.3390/cosmetics10050131 [DOI] [Google Scholar]
- 7.Justynski O, Bridges K, Krause W, et al. Apoptosis recognition receptors regulate skin tissue repair in mice. Elife. 2023;12:e86269. doi: 10.7554/eLife.86269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharad J. Glycolic acid peel therapy - a current review. Clin Cosmet Invest Dermatol. 2013;6:281–288. doi: 10.2147/CCID.S34029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang S-C, Yang J-H. Dual Effects of Alpha-Hydroxy Acids on the Skin. Molecules. 2018;23(4):863. doi: 10.3390/molecules23040863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rendon MI, Berson DS, Cohen JL, Roberts WE, Starker I, Wang B. Evidence and considerations in the application of chemical peels in skin disorders and aesthetic resurfacing. J Clin Aesthet Dermatol. 2010;3(7):32–43. [PMC free article] [PubMed] [Google Scholar]
- 11.Bernstein EF. Chemical peels. Atlas Cosmet Surg with DVD. 2008;2008:117–134. [Google Scholar]
- 12.Tören E, Mazari AA, Buzgo M. Exploring the efficacy of AHA–BHA infused nanofiber skin masks as a topical treatment for acne vulgaris. J Appl Polym Sci. 2024;141(14):e55203. doi: 10.1002/app.55203 [DOI] [Google Scholar]
- 13.Samargandy S, Raggio BS. Chemical Peels for Skin Resurfacing. Treasure Island (FL),: StatPearls Publishing; 2023. [PubMed] [Google Scholar]
- 14.Fiume MM, Heldreth BA, Bergfeld WF, et al. Safety Assessment of Citric Acid, Inorganic Citrate Salts, and Alkyl Citrate Esters as Used in Cosmetics. Int J Toxicol. 2014;33(2 suppl):16S–46S. doi: 10.1177/1091581814526891 [DOI] [PubMed] [Google Scholar]
- 15.Liu J-K. Natural products in cosmetics. Nat Products Bioprospect. 2022;12(1):40. doi: 10.1007/s13659-022-00363-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim MA, Jung YC, Suh B-F, Lee HN, Kim E. Skin biophysical properties including impaired skin barrier function determine ultraviolet sensitivity. J Cosmet Dermatol. 2022;21(10):5066–5072. doi: 10.1111/jocd.14964 [DOI] [PubMed] [Google Scholar]
- 17.Kornhauser A, Wei -R-R, Yamaguchi Y, et al. The effects of topically applied glycolic acid and salicylic acid on ultraviolet radiation-induced erythema, DNA damage and sunburn cell formation in human skin. J Dermatol Sci. 2009;55(1):10–17. doi: 10.1016/j.jdermsci.2009.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heckman CJ, Chandler R, Kloss JD, et al. Minimal Erythema Dose (MED) testing. J Vis Exp. 2013;(75):e50175. doi: 10.3791/50175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alexander H, Brown S, Danby S, Flohr C. Research techniques made simple: transepidermal water loss measurement as a research tool. J Invest Dermatol. 2018;138(11):2295–2300.e1. doi: 10.1016/j.jid.2018.09.001 [DOI] [PubMed] [Google Scholar]
- 20.Green M, Kashetsky N, Feschuk A, Maibach HI. Transepidermal water loss (TEWL): environment and pollution-A systematic review. Ski Heal Dis. 2022;2(2):e104. doi: 10.1002/ski2.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.The Scientific Committee On Cosmetic Products And Non-Food Products Intended For Consumers. Consumer safety of alpha-hydroxy acids: updated position paper; 2004.
- 22.US Food and Drug Administration. Alpha Hydroxy Acids; 2022.
- 23.Cosmetic Ingredient Review. Safety assessment of alpha hydroxy acids as used in cosmetics; 2013.
- 24.SCCNFP. The safety of alpha-hydroxy acids; 2000.
- 25.SCCNFP. Consumer safety of alpha-hydroxy acids; 2004.
- 26.B. für Risikobewertung. BgVV rät zur Vorsicht bei der Anwendung von kosmetischen Mitteln mit Alphahydroxysäuren; 1998.
- 27.García JÁG. Chemical peels: cosmetic, medical device, drug…or what? Medical devices group; 2018. https://www.medicaldevicesgroup.net/medical-devices/chemical-peels-cosmetic-medical-device/. Accessed July 06, 2024.
- 28.C. of the E. U. European Parliament. Regulation (EC) No 1223/2009 of the European Parliament; 2009. Available from: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex%3A32009R1223. Accessed July 06, 2024.
- 29.European and Commission. Guidance on classification of medical devices; 2021. Available from: https://health.ec.europa.eu/system/files/2021-10/mdcg_2021-24_en_0.pdf. Accessed July 06, 2024.
- 30.NICNAS. Glycolic acid - priority existing chemical assessment report no. 12; 2000:1–23.
- 31.Williams RJE. The use and safety of hydroxy acids in cosmetics; 2005.
- 32.Panico A, Serio F, Bagordo F, et al. Skin safety and health prevention: an overview of chemicals in cosmetic products. J Prev Med Hyg. 2019;60(1):E50–E57. doi: 10.15167/2421-4248/jpmh2019.60.1.1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bernstein EF, Lee J, Brown DB, Yu R, Van Scott E. Glycolic acid treatment increases type I collagen mRNA and hyaluronic acid content of human skin. Dermatologic Surg off Publ Am Soc Dermatologic Surg. 2001;27(5):429–433. doi: 10.1046/j.1524-4725.2001.00234.x [DOI] [PubMed] [Google Scholar]
- 34.Ditre CM, Griffin TD, Murphy GF, et al. Effects of alpha-hydroxy acids on photoaged skin: a pilot clinical, histologic, and ultrastructural study. J Am Acad Dermatol. 1996;34(2 Pt 1):187–195. doi: 10.1016/s0190-9622(96)80110-1 [DOI] [PubMed] [Google Scholar]
- 35.Yamamoto Y, Uede K, Yonei N, Kishioka A, Ohtani T, Furukawa F. Effects of alpha-hydroxy acids on the human skin of Japanese subjects: the rationale for chemical peeling. J Dermatol. 2006;33(1):16–22. doi: 10.1111/j.1346-8138.2006.00003.x [DOI] [PubMed] [Google Scholar]
- 36.Yu RJ, Van Scott EJ. Alpha-hydroxyacids and carboxylic acids. J Cosmet Dermatol. 2004;3(2):76–87. doi: 10.1111/j.1473-2130.2004.00059.x [DOI] [PubMed] [Google Scholar]
- 37.Eberle J, Fecker LF, Forschner T, Ulrich C, Röwert-Huber J, Stockfleth E. Apoptosis pathways as promising targets for skin cancer therapy. Br J Dermatol. 2007;156:18–24. doi: 10.1111/j.1365-2133.2007.07855.x [DOI] [PubMed] [Google Scholar]
- 38.de Sousa LQ, Machado KDC, Oliveira SFDC, et al. Bufadienolides from amphibians: a promising source of anticancer prototypes for radical innovation, apoptosis triggering and Na(+)/K(+)-ATPase inhibition. Toxicon. 2017;127:63–76. doi: 10.1016/j.toxicon.2017.01.004 [DOI] [PubMed] [Google Scholar]
- 39.Wilson LZ, Davis G, Illum SS. Human Keratinocyte Cell Lines. In: Pharmaceutical Applications of Cell and Tissue Culture to Drug Transport. New York, NY, USA: Springer; 1991:283–284. [Google Scholar]
- 40.Spörl F, Schellenberg K, Blatt T, et al. A circadian clock in HaCaT keratinocytes. J Invest Dermatol. 2011;131(2):338–348. doi: 10.1038/jid.2010.315 [DOI] [PubMed] [Google Scholar]
- 41.Shaik Mohamed Sayed UF, Moshawih S, Goh HP, et al. Natural products as novel anti-obesity agents: insights into mechanisms of action and potential for therapeutic management. Front Pharmacol. 2023;14:1182937. doi: 10.3389/fphar.2023.1182937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ying T-H, Chen C-W, Hsiao Y-P, Hung S-J, Chung J-G, Yang J-H. Citric acid induces cell-cycle arrest and apoptosis of human immortalized keratinocyte cell line (HaCaT) via caspase- and mitochondrial-dependent signaling pathways. Anticancer Res. 2013;33(10):4411–4420. [PubMed] [Google Scholar]
- 43.Moshawih S, Goh HP, Kifli N, et al. Synergy between machine learning and natural products cheminformatics: application to the lead discovery of anthraquinone derivatives. Chem Biol Drug Des. 2022;100(2):185–217. doi: 10.1111/cbdd.14062 [DOI] [PubMed] [Google Scholar]
- 44.Hsiao Y-P, Lai -W-W, Wu S-B, et al. Triggering apoptotic death of human epidermal keratinocytes by malic Acid: involvement of endoplasmic reticulum stress- and mitochondria-dependent signaling pathways. Toxins (Basel). 2015;7(1):81–96. doi: 10.3390/toxins7010081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang T-H, Lin C-F, Alalaiwe A, Yang S-C, Fang J-Y. Apoptotic or Antiproliferative Activity of Natural Products against Keratinocytes for the Treatment of Psoriasis. Int J Mol Sci. 2019;20(10). doi: 10.3390/ijms20102558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.González-Fandos E, Herrera B. Efficacy of malic acid against Listeria monocytogenes attached to poultry skin during refrigerated storage. Poult Sci. 2013;92(7):1936–1941. doi: 10.3382/ps.2012-02968 [DOI] [PubMed] [Google Scholar]
- 47.Oresajo C, Yatskayer M, Hansenne I. Clinical tolerance and efficacy of capryloyl salicylic acid peel compared to a glycolic acid peel in subjects with fine lines/wrinkles and hyperpigmented skin. J Cosmet Dermatol. 2008;7(4):259–262. doi: 10.1111/j.1473-2165.2008.00403.x [DOI] [PubMed] [Google Scholar]
- 48.Monheit GD, Chastain MA. Chemical peels. Facial Plast Surg Clin North Am. 2001;9(2):239–255. doi: 10.1016/S1064-7406(23)00399-1 [DOI] [PubMed] [Google Scholar]
- 49.Fattahi TT. An overview of facial aesthetic units. J Oral Maxillofac Surg off J Am Assoc Oral Maxillofac Surg. 2003;61(10):1207–1211. doi: 10.1016/s0278-2391(03)00684-0 [DOI] [PubMed] [Google Scholar]
- 50.Hevia O, Nemeth AJ, Taylor JR. Tretinoin accelerates healing after trichloroacetic acid chemical peel. Arch Dermatol. 1991;127(5):678–682. doi: 10.1001/archderm.1991.01680040086008 [DOI] [PubMed] [Google Scholar]
- 51.Gerber PA, Kukova G, Bölke E, Homey B, Diedrichson E. Severe hyperpigmentation and scarring following glycolic acid peel treatment in combination with low-dose isotretinoin. Eur J Med Res. 2014;19:1–4. doi: 10.1186/s40001-014-0060-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Amore R, Deriu F, Sbarbati A, et al. Facial anti-aging treatments with soft peeling and microneedling technique. Appl Sci. 2021;11(13):6068. doi: 10.3390/app11136068 [DOI] [Google Scholar]
- 53.Sarkar R, Garg V, Bansal S, Sethi S, Gupta C. Comparative evaluation of efficacy and tolerability of glycolic acid, salicylic mandelic acid, and phytic acid combination peels in melasma. Dermatologic Surg. 2016;42(3):384–391. doi: 10.1097/DSS.0000000000000642 [DOI] [PubMed] [Google Scholar]
- 54.Thappa D, Kumari R. Comparative study of trichloroacetic acid versus glycolic acid chemical peels in the treatment of melasma. Indian J Dermatol Venereol Leprol. 2010;76(4):447. doi: 10.4103/0378-6323.66602 [DOI] [PubMed] [Google Scholar]
- 55.İlknur T, Biçak MÜ, Demirtaşoğlu M, Özkan Ş. Glycolic acid peels versus amino fruit acid peels in the treatment of melasma. Dermatologic Surg. 2010;36(4):490–495. doi: 10.1111/j.1524-4725.2010.01481.x [DOI] [PubMed] [Google Scholar]
- 56.Sarkar R, Kaur C, Bhalla M, Kanwar AJ. The combination of glycolic acid peels with a topical regimen in the treatment of melasma in dark-skinned patients: a comparative study. Dermatologic Surg. 2002;28(9):828–832. doi: 10.1046/j.1524-4725.2002.02034.x [DOI] [PubMed] [Google Scholar]
- 57.Garg VK, Sarkar R, Agarwal R. Comparative evaluation of beneficiary effects of priming agents (2% hydroquinone and 0.025% retinoic acid) in the treatment of melasma with glycolic acid peels. Dermatologic Surg. 2008;34(8):1032–1040. doi: 10.1111/j.1524-4725.2008.34202.x [DOI] [PubMed] [Google Scholar]
- 58.Garg S, Thami GP, Bhalla M, Kaur J, Kumar A. Comparative efficacy of a 35% glycolic acid peel alone or in combination with a 10% and 20% trichloroacetic acid spot peel for melasma: a randomized control trial. Dermatologic Surg. 2019;45(11):1394–1400. doi: 10.1097/DSS.0000000000001964 [DOI] [PubMed] [Google Scholar]
- 59.Cotellessa C, Peris K, Onorati MT, Fargnoli MC, Chimenti S. The use of chemical peelings in the treatment of different cutaneous hyperpigmentations. Dermatologic Surg. 1999;25(6):450–454. doi: 10.1046/j.1524-4725.1999.08217.x [DOI] [PubMed] [Google Scholar]
- 60.Erbil H, Sezer E, Taştan B, Arca E, Kurumlu Z. Efficacy and safety of serial glycolic acid peels and a topical regimen in the treatment of recalcitrant melasma. J Dermatol. 2007;34(1):25–30. doi: 10.1111/j.1346-8138.2007.00211.x [DOI] [PubMed] [Google Scholar]
- 61.Kessler E, Flanagan K, Chia C, Rogers C, Anna Glaser D. Comparison of α- and β-Hydroxy Acid Chemical Peels In The Treatment Of Mild To Moderately Severe Facial Acne Vulgaris. Dermatologic Surg. 2007;34(1):45–51. doi: 10.1111/j.1524-4725.2007.34007.x [DOI] [PubMed] [Google Scholar]
- 62.İlknur T, Demirtaşoğlu M, Ü.biçak M, Özkan Ş. Glycolic acid peels versus amino fruit acid peels for acne. J Cosmet Laser Ther. 2010;12(5):242–245. doi: 10.3109/14764172.2010.514919 [DOI] [PubMed] [Google Scholar]
- 63.El Refaei AM, Abdel Salam HA, Sorour NE. Salicylic–mandelic acid versus glycolic acid peels in Egyptian patients with acne vulgaris. J Egypt Women’s Dermatologic Soc. 2015;12(3):196–202. doi: 10.1097/01.EWX.0000464740.18592.42 [DOI] [Google Scholar]
- 64.Kaminaka C, Uede M, Matsunaka H, Furukawa F, Yamomoto Y. Clinical evaluation of glycolic acid chemical peeling in patients with acne vulgaris: a randomized, double-blind, placebo-controlled, split-face comparative study. Dermatologic Surg. 2014;40(3):314–322. doi: 10.1111/dsu.12417 [DOI] [PubMed] [Google Scholar]
- 65.Dayal S, Kaur R, Sahu P. Efficacy of microneedling with 35% glycolic acid peels versus microneedling with 15% trichloroacetic acid peels in treatment of atrophic acne scars: a randomized controlled trial. Dermatologic Surg. 2022;48(11):1203–1209. doi: 10.1097/DSS.0000000000003556 [DOI] [PubMed] [Google Scholar]
- 66.Perić S, Bubanj M, Bubanj S, Jančić S. Side effects assessment in glicolyc acid peelings in patients with acne type I. Bosn J Basic Med Sci. 2011;11(1):52. doi: 10.17305/bjbms.2011.2624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim SW, Moon SE, Kim JA, Eun HC. Glycolic acid versus jessner’s solution: which is better for facial acne patients? Dermatologic Surg. 1999;25(4):270–273. doi: 10.1046/j.1524-4725.1999.08251.x [DOI] [PubMed] [Google Scholar]
- 68.In Jae J, Dong Ju H, Dong Hyun K, Yoon MS, Lee HJ. Comparative study of buffered 50% glycolic acid (pH 3.0) + 0.5% salicylic acid solution vs Jessner’s solution in patients with acne vulgaris. J Cosmet Dermatol. 2018;17(5):797–801. doi: 10.1111/jocd.12445 [DOI] [PubMed] [Google Scholar]
- 69.Zeeshan M, Arfeen N, Sonthalia S, Singh A, Roy PK. Treatment of acanthosis nigricans with sequential salicylic acid‐mandelic acid combination peel and maintenance with glycolic acid‐urea combination cream: a retrospective pilot study. J Cosmet Dermatol. 2022;21(9):3905–3909. doi: 10.1111/jocd.14731 [DOI] [PubMed] [Google Scholar]
- 70.Zaki NS, Hilal RF, Essam RM. Comparative study using fractional carbon dioxide laser versus glycolic acid peel in treatment of pseudo-acanthosis nigricans. Lasers Med Sci. 2018;33(7):1485–1491. doi: 10.1007/s10103-018-2505-x [DOI] [PubMed] [Google Scholar]
- 71.Rouvrais C, Baspeyras M, Mengeaud V, Rossi AB. Antiaging efficacy of a retinaldehyde‐based cream compared with glycolic acid peel sessions: a randomized controlled study. J Cosmet Dermatol. 2018;17(6):1136–1143. doi: 10.1111/jocd.12511 [DOI] [PubMed] [Google Scholar]
- 72.Dayal S, Kalra KD, Sahu P. Comparative study of efficacy and safety of 45% mandelic acid versus 30% salicylic acid peels in mild‐to‐moderate acne vulgaris. J Cosmet Dermatol. 2020;19(2):393–399. doi: 10.1111/jocd.13168 [DOI] [PubMed] [Google Scholar]
- 73.Bs C, Vadlamudi SL, Shenoy C. Safety of performing superficial chemical peels in patients on oral isotretinoin for acne and acne-induced pigmentation. J Clin Aesthet Dermatol. 2021;14(11):41–43. [PMC free article] [PubMed] [Google Scholar]
- 74.Dixit N, Jena A, Panda M, Debasmita B, Ipsita D. Randomized prospective study of low‐dose isotretinoin alone and combination with salicylic acid and mandelic peel against acne tarda. J Cosmet Dermatol. 2022;21(10):4398–4404. doi: 10.1111/jocd.14973 [DOI] [PubMed] [Google Scholar]
- 75.Nofal E, Nofal A, Gharib K, Nasr M, Abdelshafy A, Elsaid E. Combination chemical peels are more effective than single chemical peel in treatment of mild-to-moderate acne vulgaris: a split face comparative clinical trial. J Cosmet Dermatol. 2018;17(5):802–810. doi: 10.1111/jocd.12763 [DOI] [PubMed] [Google Scholar]
- 76.Dayal S, Amrani A, Sahu P, Jain VK. Jessner’s solution vs. 30% salicylic acid peels: a comparative study of the efficacy and safety in mild-to-moderate acne vulgaris. J Cosmet Dermatol. 2017;16(1):43–51. doi: 10.1111/jocd.12266 [DOI] [PubMed] [Google Scholar]
- 77.How KN, Lim PY, Wan Ahmad Kammal WSL, Shamsudin N. Efficacy and safety of Jessner’s solution peel in comparison with salicylic acid 30% peel in the management of patients with acne vulgaris and postacne hyperpigmentation with skin of color: a randomized, double‐blinded, split‐face, controlled trial. Int J Dermatol. 2020;59(7):804–812. doi: 10.1111/ijd.14948 [DOI] [PubMed] [Google Scholar]
- 78.Palmer A. Lactic acid for skin care: benefits and side effects: everything you need to know about this over-the-counter chemical exfoliant. Verywell health; 2023.
- 79.Sharquie KE, Al-Tikreety MM, Al-Mashhadani SA. Lactic acid as a new therapeutic peeling agent in melasma. Dermatologic Surg off Publ Am Soc Dermatologic Surg. 2005;31(2):149–154. doi: 10.1111/j.1524-4725.2005.31035 [DOI] [PubMed] [Google Scholar]
- 80.Sharquie KE, Al-Tikreety MM, Al-Mashhadani SA. Lactic acid chemical peels as a new therapeutic modality in melasma in comparison to Jessner’s solution chemical peels. Dermatologic Surg off Publ Am Soc Dermatologic Surg. 2006;32(12):1429–1436. doi: 10.1111/j.1524-4725.2006.32352.x [DOI] [PubMed] [Google Scholar]
- 81.Ejaz A, Raza N, Iftikhar N, Muzzafar F. Comparison of 30% salicylic acid with Jessner’s solution for superficial chemical peeling in epidermal melasma. J Coll Physicians Surg Pak. 2008;18(4):205–208. [PubMed] [Google Scholar]
- 82.Chaudhary S, Dayal S. Efficacy of combination of glycolic acid peeling with topical regimen in treatment of melasma. J Drugs Dermatol. 2013;12(10):1149–1153. [PubMed] [Google Scholar]
- 83.Dayal S, Sahu P, Jain VK, Khetri S. Clinical efficacy and safety of 20% glycolic peel, 15% lactic peel, and topical 20% vitamin C in constitutional type of periorbital melanosis: a comparative study. J Cosmet Dermatol. 2016;15(4):367–373. doi: 10.1111/jocd.12255 [DOI] [PubMed] [Google Scholar]
- 84.Ellabban NF, Eyada M, Nada H, Kamel N. Efficacy and tolerability of using platelet‐rich plasma versus chemical peeling in periorbital hyperpigmentation. J Cosmet Dermatol. 2019;18(6):1680–1685. doi: 10.1111/jocd.12964 [DOI] [PubMed] [Google Scholar]
- 85.Taylor MB. Summary of mandelic acid for the improvement of skin conditions. Cosmet Dermatol. 1999;12(June):26–28. [Google Scholar]
- 86.Garg V, Sinha S, Sarkar R. Glycolic acid peels versus salicylic-mandelic acid peels in active acne vulgaris and post-acne scarring and hyperpigmentation: a comparative study. Dermatol Surg. 2008;35:59–65. doi: 10.1111/j.1524-4725.2008.34383.x [DOI] [PubMed] [Google Scholar]
- 87.Fiume Z. Final report on the safety assessment of Malic Acid and Sodium Malate. Int J Toxicol. 2001;20(1):47–55. doi: 10.1080/109158101750300946 [DOI] [PubMed] [Google Scholar]
- 88.Smith WP. Comparative effectiveness of alpha-hydroxy acids on skin properties. Int J Cosmet Sci. 1996;18(2):75–83. doi: 10.1111/j.1467-2494.1996.tb00137.x [DOI] [PubMed] [Google Scholar]
- 89.Russell IJ, Michalek JE, Flechas JD, Abraham GE. Treatment of fibromyalgia syndrome with Super Malic: a randomized, double blind, placebo controlled, crossover pilot study. J Rheumatol. 1995;22(5):953–958. [PubMed] [Google Scholar]
- 90.Burnett CL, Bergfeld WF, Belsito DV, et al. Amended safety assessment of malic acid and sodium malate as used in cosmetics. Int J Toxicol. 2022;41(3_suppl):69–76. doi: 10.1177/10915818221117535 [DOI] [PubMed] [Google Scholar]
- 91.Campos V, Pitassi L, Kalil C, Gonçalves Júnior JE, Sant’Anna B, Correia P. Clinical evaluation of the efficacy of a facial serum containing dioic acid, glycolic acid, salicylic acid, LHA, citric acid, and HEPES in treating post-inflammatory hyperchromia and controlling oily skin in patients with acne vulgaris. J Cosmet Dermatol. 2021;20(6):1766–1773. doi: 10.1111/jocd.14016 [DOI] [PubMed] [Google Scholar]
- 92.Baumann LS, Oresajo C, Yatskayer M, Dahl A, Figueras K. Comparison of clindamycin 1% and benzoyl peroxide 5% gel to a novel composition containing salicylic acid, capryloyl salicylic acid, HEPES, glycolic acid, citric acid, and dioic acid in the treatment of acne vulgaris. J Drugs Dermatol. 2013;12(3):266–269. [PubMed] [Google Scholar]
- 93.Liu Z, Ai J, Zhu Z, et al. Chemical peeling with 35% glycolic acid for the treatment of disseminated facial verruca plana: a randomized, split‐face, evaluator‐blinded trial. Dermatol Ther. 2022;35(8). doi: 10.1111/dth.15594 [DOI] [PubMed] [Google Scholar]
- 94.Fiume MM, Bergfeld WF, Belsito DV, et al. Safety assessment of Vitis vinifera (grape)-derived ingredients as used in cosmetics. Int J Toxicol. 2014;33(3 Suppl):48S–83S. doi: 10.1177/1091581814545247 [DOI] [PubMed] [Google Scholar]
- 95.Smith WP. Epidermal and dermal effects of topical lactic acid. J Am Acad Dermatol. 1996;35(3 Pt 1):388–391. doi: 10.1016/s0190-9622(96)90602-7 [DOI] [PubMed] [Google Scholar]
- 96.Bergfeld WF. Safety assessment of alpha hydroxy acids as used in cosmetics. Cosmet Ingred Rev. 2013;2013:1–498. [Google Scholar]
- 97.Obagi S. Overview of skin care and chemical peels. J Oral Maxillofac Surg. 2006;64(9):21. doi: 10.1016/j.joms.2006.06.064 [DOI] [Google Scholar]
- 98.Johnsen MA. The influence of particle size. Spray technology and marketing. Sci Educ. 2004;3:1. [Google Scholar]
- 99.Rothe H, Fautz R, Gerber E, et al. Special aspects of cosmetic spray safety evaluations: principles on inhalation risk assessment. Toxicol Lett. 2011;205(2):97–104. doi: 10.1016/j.toxlet.2011.05.1038 [DOI] [PubMed] [Google Scholar]
- 100.Bremmer HJ, Assessment IR, Products C. Exposure to compounds in Cosmetics; 2006:1–77.
- 101.Sharad J. Combination of microneedling and glycolic acid peels for the treatment of acne scars in dark skin. J Cosmet Dermatol. 2011;10(4):317–323. doi: 10.1111/j.1473-2165.2011.00583.x [DOI] [PubMed] [Google Scholar]
- 102.Coleman WP, Futrell JM. The glycolic acid trichloroacetic acid peel. J Dermatol Surg Oncol. 1994;20(1):76–80. doi: 10.1111/j.1524-4725.1994.tb03753.x [DOI] [PubMed] [Google Scholar]
- 103.Moy LS. Superficial chemical peels with alpha-hydroxy acids. In: Robinson JK, Arndt KA, Le Boit PE, editors. Atlas of Cutaneous Surgery. Philadelphia, PA: WB Saunders; 1996:345–350. [Google Scholar]
- 104.Monheit GD. The Jessner’s + TCA peel: a medium-depth chemical peel. J Dermatol Surg Oncol. 1989;15(9):945–950. doi: 10.1111/j.1524-4725.1989.tb03181.x [DOI] [PubMed] [Google Scholar]
- 105.Monheit GD. How to select the appropriate peel for each patient. In: Rubin TR, editor. Procedures in Cosmetic Dermatology Series: Chemical Peels. St Louis, MO: Elsevier; 2006:115–136. [Google Scholar]
- 106.Marrero GM, Katz BE. The new fluor-hydroxy pulse peel. A combination of 5-fluorouracil and glycolic acid. Dermatologic Surg off Publ Am Soc Dermatologic Surg. 1998;24(9):973–978. doi: 10.1111/j.1524-4725.1998.tb04290.x [DOI] [PubMed] [Google Scholar]
- 107.Landau M. Chemical peels. Clin Dermatol. 2008;26(2):200–208. doi: 10.1016/j.clindermatol.2007.09.012 [DOI] [PubMed] [Google Scholar]
- 108.Kempiak SJ, Uebelhoer N. Superficial chemical peels and microdermabrasion for acne vulgaris. Semin Cutan Med Surg. 2008;27(3):212–220. doi: 10.1016/j.sder.2008.06.003 [DOI] [PubMed] [Google Scholar]
- 109.Briden E, Jacobsen E, Johnson C. Combining superficial glycolic acid (alpha-hydroxy acid) peels with microdermabrasion to maximize treatment results and patient satisfaction. Cutis. 2007;79(1):13–16. [PubMed] [Google Scholar]
- 110.Fournier N, Fritz K, Mordon S. Use of nonthermal blue (405- to 420-nm) and near-infrared light (850- to 900-nm) dual-wavelength system in combination with glycolic acid peels and topical vitamin C for skin photorejuvenation. Dermatologic Surg off Publ Am Soc Dermatologic Surg. 2006;32(9):1140–1146. doi: 10.1111/j.1524-4725.2006.32251.x [DOI] [PubMed] [Google Scholar]
- 111.Sobhi RM, Sobhi AM. A single-blinded comparative study between the use of glycolic acid 70% peel and the use of topical nanosome vitamin C iontophoresis in the treatment of melasma. J Cosmet Dermatol. 2012;11(1):65–71. doi: 10.1111/j.1473-2165.2011.00599.x [DOI] [PubMed] [Google Scholar]
- 112.Rendon MI, Effron C, Edison BL. The use of fillers and botulinum toxin type A in combination with superficial glycolic acid (alpha-hydroxy acid) peels: optimizing injection therapy with the skin-smoothing properties of peels. Cutis. 2007;79(1):9–12. [PubMed] [Google Scholar]
- 113.Landau M. Combination of chemical peelings with botulinum toxin injections and dermal fillers. J Cosmet Dermatol. 2006;5(2):121–126. doi: 10.1111/j.1473-2165.2006.00237.x [DOI] [PubMed] [Google Scholar]
- 114.Mattia D. Microdermabrasion: what it is, benefits, before and afters, side effects, cost. Dermcollection; 2019. Available from: https://dermcollective.com/microdermabrasion/. Accessed July 06, 2024.
- 115.Wang F, Do TT, Smith N, et al. Implications for cumulative and prolonged clinical improvement induced by cross-linked hyaluronic acid: an in vivo biochemical/microscopic study in humans. Exp Dermatol. 2024;33(1):e14998. doi: 10.1111/exd.14998 [DOI] [PubMed] [Google Scholar]
- 116.Goldberg DJ. Current trends in intense pulsed light. J Clin Aesthet Dermatol. 2012;5(6):45. [PMC free article] [PubMed] [Google Scholar]
- 117.El‐Domyati M, El‐Ammawi TS, Moawad O, et al. Efficacy of mesotherapy in facial rejuvenation: a histological and immunohistochemical evaluation. Int J Dermatol. 2012;51(8):913–919. doi: 10.1111/j.1365-4632.2011.05184.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ahn GS, Ting M, Liu CY, Korn BS, Kikkawa DO. Delayed hypersensitivity reaction from microneedling twenty years after silicone fillers. Orbit. 2023;42(4):455–458. doi: 10.1080/01676830.2022.2037141 [DOI] [PubMed] [Google Scholar]
- 119.Zduńska K, Kołodziejczak A, Rotsztejn H. Is skin microneedling a good alternative method of various skin defects removal. Dermatol Ther. 2018;31(6):e12714. doi: 10.1111/dth.12714 [DOI] [PubMed] [Google Scholar]
- 120.Wang J, Rieder EA. A systematic review of patient-reported outcomes for cosmetic indications of botulinum toxin treatment. Dermatologic Surg. 2019;45(5):668–688. doi: 10.1097/DSS.0000000000001878 [DOI] [PubMed] [Google Scholar]
- 121.Khunger N, Chanana C. Chemical peels. In: Essentials for Aesthetic Dermatology in Ethnic Skin. CRC Press; 2023:171–176. [Google Scholar]
- 122.Mahto A. The Skincare Bible: Your No-Nonsense Guide to Great Skin. Penguin UK; 2018. [Google Scholar]
- 123.Steeb T, Koch EAT, Wessely A, et al. Chemical peelings for the treatment of actinic keratosis: a systematic review and meta‐analysis. J Eur Acad Dermatol Venereol. 2021;35(3):641–649. doi: 10.1111/jdv.16844 [DOI] [PubMed] [Google Scholar]
- 124.Lindh JD, Bradley M. Clinical effectiveness of moisturizers in atopic dermatitis and related disorders: a systematic review. Am J Clin Dermatol. 2015;16:341–359. doi: 10.1007/s40257-015-0146-4 [DOI] [PubMed] [Google Scholar]
- 125.Măgerușan ȘE, Hancu G, Rusu A. A comprehensive bibliographic review concerning the efficacy of organic acids for chemical peels treating acne vulgaris. Molecules. 2023;28(20):7219. doi: 10.3390/molecules28207219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sheraz MA, Ahmed S, Ahmad I, Shaikh RH, Vaid FHM, Iqbal K. Formulation and stability of ascorbic acid in topical preparations. Surg Neurol Int. 2011;2(2):1.21297923 [Google Scholar]