Abstract
Purpose
To analyze the interfering effect of plasma from COVID-19 convalescent adults vaccinated or not with intradermal Bacillus Calmette-Guérin (BCG) on human macrophages.
Methods
The BATTLE clinical trial (NCT04369794) was initiated in the 2020 SARS-CoV-2 pandemic to study the safety and efficacy of BCG revaccination of COVID-19 convalescent adults. We measured the expression induction of eleven COVID-19-related genes in human macrophages cultured in plasma taken from 22 BCG vaccinated and 17 placebo patients at baseline and 45 days post-intervention. Subgroup analysis was based on gender, age, job type (healthcare worker [HCW] vs non-HCW), and the presence of anosmia/dysgeusia.
Results
Compared to plasma from placebo counterparts, the plasma of BCG vaccinated patients increased the expression induction of interferon (IFN)β-1b (p = 0.042) in human macrophages. This increase was more pronounced in females and in healthcare workers (HCW) (p = 0.007 and 0.001, respectively). Interferon-induced transmembrane protein 3 (IFITM3) expression induction was increased by plasma from BCG vaccinated females, young age group, and HCWs (p = 0.004, 0.011, and 0.040, respectively). Interleukin (IL)-10 induction increased by the plasma of young BCG recipients (p = 0.008). Induction of IL-6 expression increased by non-HCW BCG recipients plasma but decreased by HCW BCG recipients plasma (p = 0.005). Baseline plasma of patients who presented with anosmia/dysgeusia at the time of admission induced lower angiotensin-converting enzyme 2 (ACE2) compared to those without the symptom (0.76 vs 0.97, p = 0.004). ACE2 expression induction significantly increased by plasma of BCG recipients if they had anosmia/dysgeusia on admission (p = 0.028).
Conclusion
The expressions of IFNβ-1b, IFITM3, IL-6, and IL-10 in human macrophages incubated with the plasma of COVID-19 convalescent patients were modulated by BCG. These modulations depended on subject-specific characteristics, including gender, age, clinical presentation (anosmia/dysgeusia), job type, and previous exposure to mycobacteria.
Keywords: COVID, BCG, trained immunity, gender, age, healthcare worker, IFNs, ACE2
Introduction
Bacillus Calmette-Guérin (BCG) is a modified, low-virulent Mycobacterium bovis vaccine. It is currently administered to newborns in tuberculosis-endemic countries including Brazil to protect them against severe tuberculosis. The vaccine also exhibits broad non-specific actions against non-mycobacterial infections, particularly respiratory viruses.1,2
The BATTLE randomized trial was designed in the early 2020 SARS-CoV-2 pandemic to test the safety of BCG revaccination in COVID-19 convalescent adults based on its potential cross-protection against the virus.3–6 The trial has shown improved recovery from anosmia and dysgeusia in the participants with differential responses to BCG based on individual characteristics such as job type.6 This subject-specific response to BCG in adults has been reported in the literature, showing a unpredictable nature for BCG.7–9
In the current study, the main aim is to evaluate BCG-induced trained immunity by measuring the expression levels of 11 genes in cultured macrophages incubated with plasma of select BATTLE trial participants. As the secondary aim, we investigate determinants of the subject-specific response to BCG, pairing patients by sex, age, job type (healthcare worker [HCW] vs non-HCW) and the presence of anosmia/dysgeusia at the time of injection.
Materials and Methods
The BATTLE trial was a randomized, placebo-controlled study that administered BCG to convalescent COVID-19 patients. Details of the trial have been previously described.3 Ethics approval was granted by the National Commission for Research Ethics (CONEP), University of Campinas under number 31049320.7.1001.5404. Following extensive discussion with the commission, the risk of BCG administration without testing for previous exposure was deemed negligible, given that high doses of intravesical BCG are routinely administered to bladder cancer patients. The trial was registered with ClinicalTrials.gov (NCT04369794, COVID-19 BATTLE trial).
In short, recently infected COVID-19 patients over the age of 18 who tested positive for SARS-CoV-2 via nasopharyngeal reverse transcription–quantitative polymerase chain reaction (RT-qPCR) were approached in two outpatient healthcare facilities. Volunteers who signed the consent form were randomly injected with 0.1 mL of intradermal BCG or placebo (0.9% saline) within 14 days of symptom onset. Exclusion criteria included pregnancy, history of compromised immunity, and inability or unwillingness to sign an informed consent. Blood samples were taken upon admission and 45 days post-injection.
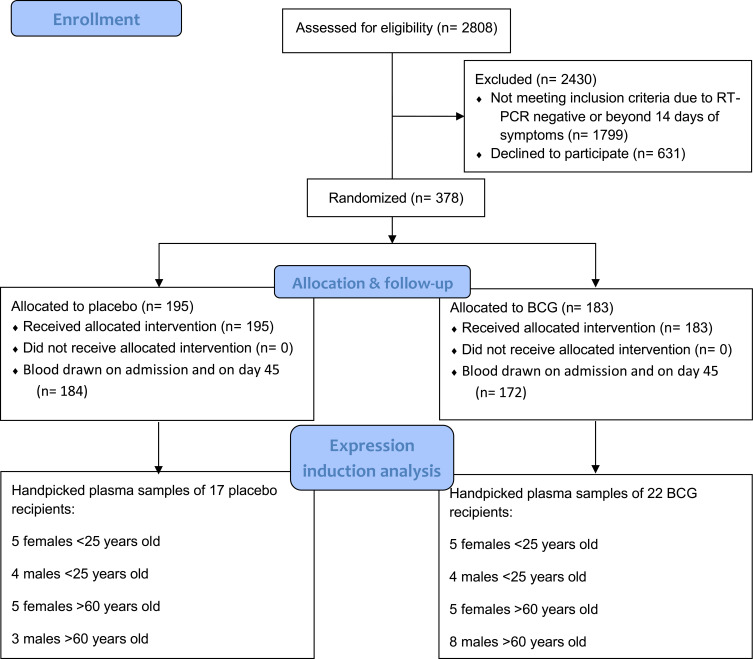
To address the primary and secondary aims of the current study, we designed a pilot study involving select BATTLE trial participants based on their sex and age. Extremes of age (below 25 and over 60) were chosen due to the pilot nature of the study. Ultimately, 22 BCG recipients and 17 placebo recipients were selected: five females younger than 25 years old, four males younger than 25 years old, and five females older than 60 years old. Eight males older than 60 were chosen from the BCG group and three were chosen from placebo (Figure 1). All selected patients had mild COVID-19 and none required hospitalization.
Figure 1.
PRISMA flowchart of the BATTLE trial and selection of samples for the current study.
Genes Related to COVID-19 and Trained Immunity
Eleven genes were chosen as markers of enhanced antiviral immunity in response to BCG. Interferon-induced transmembrane protein 3 (IFITM3) blocks viral entry into cells and polymorphism in its gene is associated with susceptibility to COVID-19.10,11 Tumor necrosis factor (TNF)-α is a proinflammatory cytokine produced early in SARS-CoV-2 infection that may induce tissue damage and lung fibrosis.11,12 Interferons are demonstrated to have antiviral activity.13,14 Angiotensin-converting enzyme 2 (ACE2) is the key protein for SARS-CoV-2 entry into host cells and is associated with disease severity.15
Furthermore, COVID-19-specific symptoms of anosmia and dysgeusia are attributed to the virus’s attachment to ACE2-producing supportive cells in the nasal cavity.16 Tyrosine protein kinase receptor UFO (AXL) is a protein that facilitates entry of SARS-CoV-2 into human lung epithelial cells.17,18 Interleukin (IL)-1β, IL-6, IL-10, and Interleukin 2 receptor subunit alpha (IL2RA) are attributed to the COVID-19 cytokine storm and are candidates for targeted immunotherapy in severe disease.19–21 Soluble IL2RA (CD25) is the receptor for IL-2 and is linked to severe COVID-19.22 Caspase-8 activates the intracellular inflammasome in response to viral infection and has a role in pro-inflammatory cytokine maturation.23
Cell Viability Assay
To evaluate the cytotoxicity of incubation of macrophages with plasma from patients with COVID-19 before and after BCG vaccination at different concentrations, cells were incubated with plasma at concentrations 1, 2.5, and 5% in the treatment medium as described. Then, these cells were incubated in a buffer with MTT 0.5 mg/mL (M5655, Sigma Aldrich) and glucose for 2h. Then, DMSO (Sigma Aldrich) was added, and the formazan formed from the mitochondrial reaction of living cells with MTT was quantified using a spectrophotometer to read the absorbance measured at 560 λ.24
Cell Differentiation and Plasma Treatment
THP-1 lineage cells (TIB-202 – THP-1 – ATCC) were cultured according to the supplier’s cell bank specifications. Then, cells (3x105/well, 24 well plate) were differentiated into M0 macrophages with Phorbol 12-myristate 13-acetate (PMA) (P8139 – Sigma Aldrich) 1ug/mL for 24h. After resting for 4 hours in the culture medium without PMA, the THP-1 macrophages were incubated for 4 hours with patients’ plasma diluted at a concentration of 2.5% in the treatment medium, which consists of the culture medium indicated by the supplier bank with reduced concentration of fetal bovine serum (0.5%).25,26
Quantification of Gene Expression by qPCR
After 4h incubation with the plasma, the samples were resuspended in Trizol (Invitrogen Corporation, CA, USA) and had their total RNA extracted and quantified by spectrophotometry. A total of 1000 ng of RNA from each sample were reverse transcribed into complementary DNA in a thermal cycler using the High-Capacity cDNA Reverse Transcription Kit (no. 4368813; Applied Biosystems, Foster City, CA, USA) according to its instructions. Then, the expression of target genes was measured by real-time PCR. To this end, 20 ng of cDNA from each sample was added to 3 µL of master mix (TaqMan Universal no. 4369016), 0.25 µL of demineralized water, and 0.25 µL of primer (ACE2 no. 021804.1, IL2RA no. 001308242.1, IFITM3 no. 021034.2, IL-10 no. 000572.2, IL-6 no. 000600.4, TNF-α no. 000594.3, CASP8 no. 001137667.1, IL-1β no. 9759921.8, AXL no. 001699, IFNL1 no. 172140.1, IFNβ-1b no. 002176.3, Applied Biosystems), and added in duplicate to a 96-well plate and analyzed on a StepOnePlus Real-Time PCR system (Applied Biosystems). The peptidylprolyl isomerase-A (PPIA) gene expression of each sample was used as endogenous control. Primers are described in Supplementary Table 1.
Statistical Analysis
Statistical analysis was performed using R version 4.1.2 on the RStudio platform 2022.07.1 +554 using the packages tidyverse. Unpaired two-sided Wilcoxon rank-sum test was used for continuous variables [H0: true location shift = 0] and Fisher’s exact test was used for categorical variables [H0: equal distribution]. The quantified RNA expression inductions on admission were subtracted from the quantifications on day 45 post-intervention to calculate the change in these measurements. We focused on subgroup analysis to determine individual factors that influence this change. The subgroups were as follows: young age (<25) vs old age (>60), female vs male, HCW vs non-HCW, and the presence or absence of anosmia/dysgeusia at the time of admission. Since this was a pilot study, the p-values were not adjusted for multiple testing, and sensitivity analysis was not feasible due to limited sample sizes. All quantifications were described by their median ± interquartile range (IQR) for a more robust description. A p-value less than 0.05 was considered significant. For the sake of this study, we did not separate anosmia and dysgeusia; the term anosmia/dysgeusia in the results section signifies anosmia with or without concomitant dysgeusia.
Results
BCG and placebo groups were similar in all comorbidities and symptoms, except cough on admission (45.5% vs 11.8%, p = 0.036), Supplementary Table 2. IFNβ-1b expression induction by the plasma of BCG recipients significantly increased after 45 days (+0.19 vs +0.01, p = 0.042) and IL2RA was borderline reduced after 45 days in the placebo group (+0.17 vs −0.28, p=0.054), Table 1.
Table 1.
Gene Expression Induction in Cultured Cells Exposed to Plasma of Patients Receiving Placebo and BCG (45 Days Following Intervention)
| Gene Expression | BCG | Placebo | P value |
|---|---|---|---|
| IFITM3, median change ± IQR | 0.12 ± 0.40 | −0.03 ± 0.36 | 0.11 |
| TNF-α, median change ± IQR | 0.08 ± 0.57 | −0.01 ± 0.36 | 0.38 |
| IFNB1, median change ± IQR | 0.19 ± 0.39 | 0.01 ± 0.33 | 0.042 |
| IFNL1, median change ± IQR | 0.05 ± 0.63 | −0.04 ± 0.65 | 0.51 |
| ACE2, median change ± IQR | 0.04 ± 0.57 | −0.21 ± 0.40 | 0.10 |
| AXL, median change ± IQR | −0.01 ± 0.31 | −0.02 ± 0.31 | 0.86 |
| Caspase-8, median change ± IQR | 0.08 ± 0.37 | −0.04 ± 0.28 | 0.10 |
| IL2RA, median change ± IQR | 0.17 ± 0.86 | −0.28 ± 0.80 | 0.054 |
| IL-1β, median change ± IQR | 0.01 ± 0.64 | −0.04 ± 0.35 | 0.41 |
| IL-10, median change ± IQR | −0.01 ± 0.38 | −0.13 ± 0.27 | 0.13 |
| IL-6, median change ± IQR | 0.40 ± 1.15 | −0.19 ± 0.56 | 0.48 |
Abbreviations: IFITM3, interferon-induced transmembrane protein 3; TNF-α, tumor necrosis factor α; IFN, interferon; ACE2, angiotensin-converting enzyme 2; AXL, tyrosine protein kinase receptor UFO; IL, interleukin; IL2RA, interleukin 2 receptor subunit alpha.
BCG increased IFNβ-1b quantification in females (+0.12 vs −0.01, p = 0.007, Table 2A) and in HCWs (+0.25 vs +0.01, p = 0.001, Table 2C). IFITM3 expression induction was significantly increased by BCG in females (+0.18 vs −0.10, p = 0.004, Table 2A) in the young (+0.33 vs −0.10, p = 0.011, Table 2B) and in the HCWs (+0.12 vs −0.10, p = 0.040, Table 2C). An increase in IL-10 expression induction was observed in the young BCG recipients (+0.22 in the young vs −0.20 in the old, p = 0.008, Table 2B). IL-6 expression induction was significantly increased in the non-HCW BCG recipients (+0.16 in BCG & non-HCW vs −1.20 in BCG & HCW, p = 0.005, Table 2C). Of note, male placebo recipients had borderline increase in TNF-α quantification after 45 days, whereas this quantification was borderline decreased in female placebo recipients (+0.16 vs −0.13, p = 0.055, Table 2A).
Table 2.
Subgroup Analysis of the Effect of BCG-Recipients’ Plasma on Gene Expressions in Cultured Human Monocytes.
| A | Female (n = 20) | Male (n = 19) | p-value | |||||
| Median change ± IQR | BCG (n =10) | Placebo (n =10) | p-value | BCG (n = 12) | Placebo (n = 7) | p-value | BCG vs BCG | Placebo vs Placebo |
| HCW, no. (%) | 4 (40.0) | 6 (60.0) | 0.65 | 4 (33.3) | 1 (14.3) | 0.60 | 1 | 0.13 |
| Young, no. (%) | 5 (50.0) | 5 (50.0) | 1 | 4 (33.3) | 4 (57.1) | 0.37 | 0.66 | 1 |
| Anosmia, no (%) | 5 (50.0) | 7 (70.0) | 0.65 | 4 (33.3) | 3 (42.9) | 1 | 0.67 | 0.35 |
| IFTM3 | 0.18 ± 0.19 | −0.10 ± 0.40 | 0.004 | −0.01 ± 0.48 | 0.19 ± 0.31 | 0.84 | 0.25 | 0.088 |
| TNF-α | −0.04 ± 0.37 | −0.13 ± 0.28 | 0.25 | 0.19 ± 0.88 | 0.16 ± 0.17 | 0.90 | 0.82 | 0.055 |
| IFNβ-1b | 0.12 ± 0.19 | −0.01 ± 0.26 | 0.007 | 0.24 ± 1.07 | 0.09 ± 0.37 | 0.43 | 0.45 | 0.087 |
| IFNL1 | 0.11 ± 0.24 | −0.17 ± 1.13 | 0.19 | −0.09 ± 0.86 | 0.08 ± 0.51 | 0.90 | 0.31 | 0.60 |
| ACE2 | 0.08 ± 0.56 | −0.21 ± 0.43 | 0.12 | 0.04 ± 0.53 | −0.04 ± 0.31 | 0.43 | 0.97 | 0.60 |
| AXL | 0.04 ± 0.29 | −0.06 ± 0.32 | 0.28 | −0.01 ± 0.39 | 0.19 ± 0.34 | 0.38 | 0.54 | 0.19 |
| Caspase-8 | 0.07 ± 0.2 | −0.09 ± 0.39 | 0.19 | 0.08 ± 0.38 | 0.13 ± 0.23 | 0.43 | 0.46 | 0.19 |
| IL2RA | 0.52 ± 0.76 | 0.03 ± 0.87 | 0.21 | 0.13 ± 0.95 | −0.58 ± 0.35 | 0.15 | 0.35 | 0.22 |
| IL-1β | −0.03 ± 0.34 | −0.08 ± 0.43 | 0.85 | 0.12 ± 0.74 | 0.04 ± 0.21 | 0.48 | 0.20 | 0.47 |
| IL-10 | 0.05 ± 0.25 | −0.22 ± 0.39 | 0.11 | −0.02 ± 0.52 | −0.08 ± 0.14 | 0.71 | 0.50 | 0.60 |
| IL-6 | −0.46 ± 1.18 | −0.27 ± 1.55 | 0.80 | 0.14 ± 0.51 | 0.07 ± 0.45 | 0.28 | 0.27 | 0.62 |
| B | Age < 25 (n =18) | Age > 60 (n =21) | p-value | |||||
| Median change ± IQR | BCG (n =9) | Placebo (n = 9) | p-value | BCG (n = 13) | Placebo (n = 8) | p-value | BCG vs BCG | Placebo vs Placebo |
| Female, no. (%) | 5 (55.6) | 5 (55.6) | 1 | 5 (38.5) | 5 (62.5) | 0.39 | 0.67 | 1 |
| HCW, no (%) | 2 (22.2) | 3 (33.3) | 1 | 6 (46.2) | 4 (50.0) | 1 | 0.38 | 0.64 |
| Anosmia, no (%) | 4 (44.4) | 7 (77.8) | 0.33 | 5 (38.5) | 3 (37.5) | 1 | 1 | 0.15 |
| IFTM3 | 0.33 ± 0.38 | −0.10 ± 0.15 | 0.011 | 0.00 ± 0.29 | 0.11 ± 0.41 | 0.70 | 0.036 | 0.32 |
| TNF-α | −0.06 ± 0.40 | −0.04 ± 0.43 | 0.60 | 0.19 ± 0.64 | 0.08 ± 0.37 | 0.59 | 0.89 | 0.61 |
| IFNβ-1b | 0.17 ± 0.34 | 0.01 ± 0.39 | 0.08 | 0.21 ± 0.51 | 0.02 ± 1.13 | 0.30 | 0.51 | 0.96 |
| IFNL1 | 0.12 ± 0.96 | −0.21 ± 0.73 | 0.49 | −0.09 ± 0.54 | −0.02 ± 0.56 | 0.86 | 0.39 | 0.74 |
| ACE2 | 0.06 ± 0.55 | −0.11 ± 0.36 | 0.22 | 0.01 ±0.44 | −0.23 ± 0.45 | 0.34 | 0.56 | 0.96 |
| AXL | −0.04 ± 0.25 | −0.08 ± 0.18 | 0.44 | 0.00 ± 0. 32 | 0.08 ± 0.40 | 0.86 | 0.84 | 0.61 |
| Caspase-8 | 0.03 ± 0.31 | −0.07 ± 0.10 | 0.19 | 0.08 ± 0.36 | 0.10 ± 0.31 | 0.60 | 0.29 | 0.24 |
| IL2RA | 0.04 ± 0.25 | −0.30 ± 0.63 | 0.16 | 0.68 ± 1.03 | −0.28 ± 1.6 | 0.23 | 0.082 | 0.95 |
| IL-1β | −0.12 ± 0.88 | −0.04 ± 0.34 | 0.93 | 0.03 ± 0.32 | −0.04 ± 0.42 | 0.41 | 0.64 | 0.67 |
| IL-10 | 0.22 ± 0.35 | −0.20 ± 0.26 | 0.008 | −0.02 ± 0.29 | −0.10 ± 0.43 | 0.97 | 0.051 | 0.67 |
| IL-6 | 0.07 ± 0.61 | −0.27 ± 0.72 | 0.28 | −0.04 ± 1.12 | 0.16 ± 0.51 | 0.79 | 1 | 0.22 |
| C | HCW (n = 15) | Non-HCW (n =24) | p-value | |||||
| BCG (n = 8) | Placebo (n =7) | p-value | BCG (n = 14) | Placebo (n = 10) | p-value | BCG vs BCG | Placebo vs Placebo | |
| Female, no. (%) | 4 (50.0) | 6 (85.7) | 0.28 | 6 (42.9) | 4 (40.0) | 1 | 1 | 0.13 |
| Young, no. (%) | 2 (25.0) | 3 (42.9) | 0.60 | 7 (50.0) | 6 (60.0) | 0.70 | 0.38 | 0.64 |
| Anosmia, no (%) | 3 (37.5) | 5 (71.4) | 0.31 | 6 (42.9) | 5 (50.0) | 1 | 1 | 0.62 |
| IFTM3 | 0.12 ± 0.43 | −0.10 ± 0.25 | 0.040 | 0.11 ± 0.39 | 0.05 ± 0.39 | 0.71 | 0.62 | 0.19 |
| TNF-α | 0.2 ± 0.5 | −0.01 ± 0.33 | 0.09 | −0.08 ± 0.55 | 0.04 ± 0.36 | 0.98 | 0.27 | 0.67 |
| IFNβ-1b | 0.25 ± 0.32 | 0.01 ± 0.08 | 0.001 | 0.01 ± 0.46 | 0.00 ± 0. 39 | 0.47 | 0.069 | 0.88 |
| IFNL1 | 0.07 ± 0.57 | −0.04 ± 1.37 | 0.61 | −0.01 ± 0.76 | −0.11 ± 0.61 | 0.75 | 0.62 | 1 |
| ACE2 | −0.16 ± 0.75 | −0.22 ± 0.52 | 0.34 | 0.08 ± 0.35 | −0.13 ± 0.33 | 0.21 | 0.76 | 0.60 |
| AXL | 0.08 ± 0.24 | 0.02 ± 0.47 | 0.69 | −0.05 ± 0.30 | −0.05 ± 0.29 | 0.84 | 0.33 | 0.96 |
| Caspase-8 | 0.06 ± 0.15 | −0.06 ± 0.27 | 0.15 | 0.10 ± 0.37 | 0.09 ± 0.17 | 0.28 | 0.87 | 0.47 |
| IL2RA | 0.45 ± 0.76 | −0.04 ± 1.58 | 0.46 | 0.12 ± 0.77 | −0.42 ± 0.69 | 0.10 | 0.70 | 0.53 |
| IL-1β | 0.12 ± 0.38 | −0.11 ± 0.39 | 0.28 | 0.01 ± 0.40 | 0.00 ± 0.27 | 0.98 | 0.71 | 0.47 |
| IL-10 | −0.02 ± 0.10 | −0.21 ± 0.26 | 0.15 | 0.05 ± 0.53 | −0.08 ± 0.24 | 0.34 | 0.61 | 0.47 |
| IL-6 | −1.20 ± 0.97 | −0.34 ± 1.35 | 0.42 | 0.16 ± 0.64 | −0.04 ± 0.50 | 0.18 | 0.005 | 0.62 |
| D | Anosmia/dysgeusia on admission (n = 19) | No anosmia/dysgeusia on admission (n =20) | p-value | |||||
| BCG (n = 9) | Placebo (n =10) | p-value | BCG (n = 13) | Placebo (n = 7) | p-value | BCG vs BCG | Placebo vs Placebo | |
| Female, no. (%) | 5 (55.6) | 7 (70.0) | 0.65 | 5 (38.5) | 3 (42.9) | 1 | 0.67 | 0.35 |
| Young, no. (%) | 4 (44.4) | 7 (70.0) | 0.37 | 5 (38.5) | 2 (28.6) | 1 | 1 | 0.15 |
| HCW, no. (%) | 3 (33.3) | 5 (50.0) | 0.65 | 5 (38.5) | 2 (28.6) | 1 | 0.62 | 1 |
| IFTM3 | 0.00 ± 0.44 | −0.03 ± 0.17 | 0.60 | 0.14 ± 0.32 | −0.02 ± 0.73 | 0.27 | 0.65 | 0.96 |
| TNF-α | −0.02 ± 0.42 | 0.01 ± 0.33 | 0.78 | 0.22 ± 0.79 | −0.04 ± 0.41 | 0.39 | 0.47 | 0.74 |
| IFNβ-1b | 0.17 ± 0.25 | 0.01 ± 0.10 | 0.09 | 0.21 ± 0.96 | 0.04 ± 0.50 | 0.18 | 0.65 | 0.67 |
| IFNL1 | −0.09 ± 0.20 | −0.12 ± 0.63 | 0.78 | 0.33 ± 0.98 | 0.08 ± 1.13 | 0.35 | 0.47 | 0.81 |
| ACE2 | 0.21 ± 0.36 | −0.05 ± 0.39 | 0.028 | −0.15 ± 0.74 | −0.24 ± 0.47 | 0.39 | 0.096 | 0.31 |
| AXL | 0.00 ± 0.25 | 0.04 ± 0.26 | 0.84 | −0.01 ± 0.31 | −0.12 ± 0.56 | 0.35 | 0.79 | 0.16 |
| Caspase-8 | 0.04 ± 0.11 | −0.05 ± 0.17 | 0.45 | 0.13 ± 0.37 | 0.13 ± 0.65 | 0.18 | 0.21 | 1 |
| IL2RA | 0.21 ± 1.56 | 0.03 ± 0.71 | 0.42 | 0.17 ± 0.59 | −0.59 ± 0.34 | 0.067 | 0.97 | 0.32 |
| IL-1β | 0.03 ± 0.38 | −0.05 ± 0.44 | 0.60 | −0.02 ± 0.47 | −0.03 ± 0.23 | 0.59 | 0.84 | 1 |
| IL-10 | −0.07 ± 0.31 | −0.1 ± 0.27 | 0.90 | 0.00 ± 0.32 | −0.13 ± 0.35 | 0.056 | 0.35 | 0.31 |
| IL-6 | −0.46 ± 1.13 | 0.07 ± 0.47 | 0.78 | 0.09 ± 0.50 | −0.31 ± 0.68 | 0.24 | 0.43 | 0.83 |
Notes: Subgroups are Based on (A) Gender, (B) Age, (C) Job Type, and (D) Presence or Absence of Anosmia/Dysgeusia on Admission. All Quantifications are Described as Median Change (Day 45 − Day 0) ± Interquartile Range (IQR)
Abbreviations: HCW, healthcare worker; Young, patients less than 25 years old (the other age group is patients older than 60 years); IFTM3, interferon-induced transmembrane protein 3; TNF-α, tumor necrosis factor α; IFN, interferon; ACE2, angiotensin-converting enzyme 2; AXL, tyrosine protein kinase receptor UFO; IL, interleukin; IL2RA, interleukin 2 receptor subunit alpha.
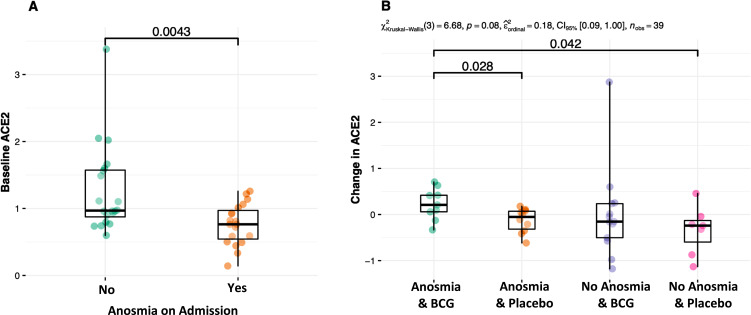
At baseline, patients with anosmia/dysgeusia had significantly lower ACE2 expression induction compared to those without the symptoms (+0.76 vs +0.97, p = 0.004, Figure 2). BCG significantly increased ACE2 expression induction in patients with anosmia/dysgeusia (+0.21 vs −0.05, p = 0.028, Table 2D). There was no significant difference in the other quantifications based on this subgroup analysis except a borderline decrease in IL-10 in placebo recipients without anosmia/dysgeusia (−0.13 vs 0.00, p = 0.056).
Figure 2.
(A) Baseline expression induction of the ACE2 gene was lower in patients with anosmia/ dysgeusia. (B) BCG increased expression induction of ACE2 only in those with anosmia/dysgeusia.
Discussion
The BATTLE trial is unique among BCG clinical trials in that it injected the vaccine as a potential treatment to SARS-CoV-2 infected patients rather than as prophylaxis to healthy individuals. This gave us the distinctive opportunity to observe BCG’s interference in active antiviral responses.27–29 BCG vaccine contributes a non-specific type of immunity in the human body called “trained immunity”.30 In this study, we quantified BCG’s “trained immunity” by measuring the induction of 11 genes with non-specific anti-viral properties in human macrophage culture.
In general, compared to placebo, BCG significantly increased IFNβ-1b and borderline increased IL2RA expression induction in COVID-19 convalescent patients. IFNβ-1 is secreted by many cells and belongs to the type I family of interferons with demonstrated anti-inflammatory effects. Both fibroblasts and epithelial cells can produce IFNβ in response to viral infections to reduce viral propagation.31,32 Treatment of severe COVID-19 with recombinant IFNβ-1b has shown promising results.33
Subgroup analysis showed that IFNβ-1b expression induction was robustly increased in females as well as HCW BCG recipients. A differential change in the IFITM3 was also observed in females and young participants. IFITM3 codifies a membrane protein considered an “innate immune effector”. It is produced by many cells in response to various viruses and reduces viral propagation. The protein is linked to susceptibility to SARS-CoV-2.10,11,33 IFITM3 has not previously been studied in BCG vaccination and could be a future candidate in understanding the mechanism of BCG’s protection against viral diseases. Gender difference in immunity is not a novel subject, though it has been neglected.34 In our study, the difference in the male versus female placebo recipients in TNF-α expression induction after 45 days reflects published literature.35,36 Gender difference in response to BCG is also previously studied, showing involvement of sex hormone and their receptors plus other factors.37–39
Anosmia and dysgeusia were extensively studied in the BATTLE trial. Our group has shown that these symptoms were recovered faster in BCG recipients.3,4,6 In the current study, we showed that baseline ACE2 expression induction was lower in patients with anosmia/dysgeusia. BCG increased this quantification only in those with the symptom. We postulate that increased ACE2 expression induction after BCG injection could be the result of reduced inflammation in the paranasal cavities, allowing the supportive olfactory cells to heal.
The plasma of mild COVID patients with anosmia on admission induced lower ACE2 expression in macrophages while BCG vaccination increased it to normal expression level. Although high ACE2 expression and anti-ACE2 IgM antibody are both linked to severe COVID-19,14,40 no BCG trial has shown any worsening of COVID-19 respiratory complications.4,27
Previous exposure to mycobacterial species greatly changes the response to BCG. This interesting property of BCG is extensively gaining attention; while immune response might fade with time, mainly for the BCG vaccine which is offered in the first days of life, some researchers recommend the exclusion of patients with latent tuberculosis from all future BCG-related clinical trials.7–9 In our study, we did not perform any test to determine recent exposure or latent mycobacterial infection, based on safety of high BCG dose re-exposure in the bladder cancer treatment.41 However, our subgroup analysis of HCW vs non-HCW is largely based on occupational exposure to these bacteria. Table 2C shows a more pronounced increase in IFITM3 and IFNβ-1b in HCW BCG recipients while IL-6 only increased in non-HCW BCG recipients. In our previously published analysis of the BATTLE trial, we showed that HCWs had more rapid and slightly more severe local skin reactions to BCG, suggesting recent exposure in this subgroup.6 On the other hand, anosmia/dysgeusia was mainly healed in non-HCW BCG recipients; this suggests that BCG is less likely to benefit those with previous mycobacterial exposure.6,8 The differential response to BCG based on exposure can explain why BCG in newborns is more predictable, making BCG the number one vaccine used worldwide;1 in newborns BCG is injected within a few hours of birth, to an individual that has not yet been exposed to any environmental pathogen, prompting a more predictable response.42
Studies have shown that IL-10 cytokine release is indirectly stimulated by BCG through dendritic cell mediation. This cytokine has anti-inflammatory properties.43,44 In our study, IL-10 expression was significantly influenced by BCG in young patients. IL-6 gene in mononuclear cells is shown to be regulated by promoters that directly interact with mycobacterial components.45 In our analysis, this gene’s expression was induced by BCG in non-HCWs. Both young age and working outside healthcare reduce an individual’s chance of exposure to mycobacterial components. The promoter of IL-6-coding gene may become less sensitive to mycobacterial components after frequent exposures.
Study limitations: this was a pilot study on samples acquired from a clinical trial with a different main aim. Numerous statistical tests were performed on relatively small data, the p-values were not adjusted for multiple testing, and sensitivity analysis was not feasible. Participants were not tested for latent TB.
Conclusion
The expressions of IFNβ-1b, IFITM3, IL-6, and IL-10 in human macrophages incubated with the plasma of COVID-19 convalescent patients were modulated by BCG. These modulations depended on subject-specific characteristics, including gender, age, clinical presentation (anosmia/dysgeusia), job type, and previous exposure to mycobacteria. Our study provides new insights into BCG’s influence on active antiviral reactions. We strongly urge future BCG trials to aim beyond preventive scopes and specifically design their studies for detailed subgroup analysis.
Acknowledgments
Keini Buosi and Mehrsa Jalalizadeh are co-first authors for this study. To our volunteer patients. The BATTLE trial was supported by Community Health Center (CeCom-UNICAMP, Campinas-SP-Brazil) and Paulínia Municipal Hospital (HMP-Paulínia-SP-Brazil).
Funding Statement
Coordination for the Improvement of Higher Education Personnel, CAPES, Federal Government, Brazil, grants number: 88887.506617/2020-00 and 88887.657670/2021-00 (Reis LO). General Coordination of the National Immunization Program - CGPNI/DEIDT/SVS/MS, Ministry of Health, Brazil, official letter number 465/2020 (Reis LO). “National Council for Scientific and Technological Development” – CNPq, Research Productivity, grant number: 310135/2022-2 (Reis LO).
Data Sharing Statement
The availability of collected non-personal patient data and material transfer of this study are available after communication and agreement of the Lead Contact.
Ethical Approval
National Research Ethics Commission (CONEP) approval number 4.173.0694 and ClinicalTrials.gov register number NCT04369794. Informed consent obtained per protocol.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Jalalizadeh M, Dal Col LSB, Yadollahvandmiandoab R, Reis LO. BCG Immunotherapy: old Tool and New Concepts. In: Rezaei N, editor. Handbook of Cancer and Immunology. Cham: Springer International Publishing; 2022:1–23. [Google Scholar]
- 2.Covián C, Fernández-Fierro A, Retamal-Díaz A, et al. BCG-induced cross-protection and development of trained immunity: implication for vaccine design. Front Immunol. 2019;10:2806. doi: 10.3389/fimmu.2019.02806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jalalizadeh M, Buosi K, Dionato FAV, et al. Randomized clinical trial of BCG vaccine in patients with convalescent COVID-19: clinical evolution, adverse events, and humoral immune response. J Intern Med. 2022;292:654–666. doi: 10.1111/joim.13523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dionato FAV, Jalalizadeh M, Buosi K, et al. BCG vaccine safety in COVID-19 convalescent adults: BATTLE a randomized controlled trial. Vaccine. 2022;40:4603–4608. doi: 10.1016/j.vaccine.2022.06.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jalalizadeh M, Giacomelli CF, Leme PA, et al. Comparing Bacillus Calmette-Guérin (BCG) strains in convalescent COVID-19 patients. Immunotherapy. 2023;15:9–15. doi: 10.2217/imt-2022-0048 [DOI] [PubMed] [Google Scholar]
- 6.Jalalizadeh M, Leme PAF, Buosi K, et al. Healthcare Workers (HCWs) and non-HCWs reaction to Bacillus Calmette-Guérin (BCG) in the BATTLE trial. Vaccine. 2023;41:6599–6606. doi: 10.1016/j.vaccine.2023.09.031 [DOI] [PubMed] [Google Scholar]
- 7.Andersen P, Doherty TM. The success and failure of BCG - implications for a novel tuberculosis vaccine. Nat Rev Microbiol. 2005;3:656–662. doi: 10.1038/nrmicro1211 [DOI] [PubMed] [Google Scholar]
- 8.Barreto ML, Pilger D, Pereira SM, et al. Causes of variation in BCG vaccine efficacy: examining evidence from the BCG REVAC cluster randomized trial to explore the masking and the blocking hypotheses. Vaccine. 2014;32:3759–3764. doi: 10.1016/j.vaccine.2014.05.042 [DOI] [PubMed] [Google Scholar]
- 9.Gong W, Du J. Excluding participants with mycobacteria infections from clinical trials: a critical consideration in evaluating the efficacy of BCG Against COVID-19. J Korean Med Sci. 2023;38:e343. doi: 10.3346/jkms.2023.38.e343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu F, Wang G, Zhao F, et al. IFITM3 Inhibits SARS-CoV-2 Infection and Is Associated with COVID-19 Susceptibility. Viruses. 2022;14:2553. doi: 10.3390/v14112553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pecoraro V, Cuccorese M, Trenti T. Genetic polymorphisms of ACE1, ACE2, IFITM3, TMPRSS2 and TNFα genes associated with susceptibility and severity of SARS-CoV-2 infection: a systematic review and meta-analysis. Clin Exp Med. 2023;23:3251–3264. doi: 10.1007/s10238-023-01038-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mohd Zawawi Z, Kalyanasundram J, Mohd Zain R, Thayan R, Basri DF, Yap WB. Prospective roles of tumor necrosis factor-alpha (TNF-α) in COVID-19: prognosis, therapeutic and management. Int J Mol Sci. 2023;24:6142. doi: 10.3390/ijms24076142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McNab F, Mayer-Barber K, Sher A, Wack A, O’Garra A. Type I interferons in infectious disease. Nat Rev Immunol. 2015;15:87–103. doi: 10.1038/nri3787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park A, Iwasaki A. Type I and type iii interferons - induction, signaling, evasion, and application to combat COVID-19. Cell Host Microbe. 2020;27(6):870–878. doi: 10.1016/j.chom.2020.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gheware A, Ray A, Rana D, et al. ACE2 protein expression in lung tissues of severe COVID-19 infection. Sci Rep. 2022;12:4058. doi: 10.1038/s41598-022-07918-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nasr MJ, Alizadeh Khatir A, Babazadeh A, Ebrahimpour S. The Role of ACE2 receptors of the olfactory system in anosmia in COVID-19: an overview. Neurol Res Int. 2021;2021:5776801. doi: 10.1155/2021/5776801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang S, Qiu Z, Hou Y, et al. AXL is a candidate receptor for SARS-CoV-2 that promotes infection of pulmonary and bronchial epithelial cells. Cell Res. 2021;31:126–140. doi: 10.1038/s41422-020-00460-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacob AN, Kalapriya J, Davidson WR, et al. A receptor tyrosine kinase, UFO/Axl, and other genes isolated by a modified differential display PCR are overexpressed in metastatic prostatic carcinoma cell line DU145. Cancer Detect Prev. 1999;23:325–332. doi: 10.1046/j.1525-1500.1999.99034.x [DOI] [PubMed] [Google Scholar]
- 19.Cron RQ. No perfect therapy for the imperfect COVID-19 cytokine storm. Lancet Rheumatol. 2022;4:e308–10. doi: 10.1016/S2665-9913(22)00068-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dhar SK, V K, Damodar S, Gujar S, Das M. IL-6 and IL-10 as predictors of disease severity in COVID-19 patients: results from meta-analysis and regression. Heliyon. 2021;7:e06155. doi: 10.1016/j.heliyon.2021.e06155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henderson LA, Canna SW, Schulert GS, et al. On the alert for cytokine storm: immunopathology in COVID-19. Arthritis Rheumatol. 2020;72:1059–1063. doi: 10.1002/art.41285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shi H, Wang W, Yin J, et al. The inhibition of IL-2/IL-2R gives rise to CD8+ T cell and lymphocyte decrease through JAK1-STAT5 in critical patients with COVID-19 pneumonia. Cell Death Dis. 2020;11:429. doi: 10.1038/s41419-020-2636-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Acat M, Gülhan P Y, Eröz R, Ertınmaz Özkan A, Koca O, Çınar C. Evaluation of both expression and serum protein levels of caspase-8 and mitogen-activated protein kinase 1 genes in patients with different severities of COVID-19 infection. Mol Biol Rep. 2023;50:3241–3248. doi: 10.1007/s11033-023-08244-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumar P, Nagarajan A, Uchil PD. Analysis of Cell Viability by the MTT Assay. Cold Spring Harb Protoc. 2018;2018:6. [DOI] [PubMed] [Google Scholar]
- 25.Barhoumi T, Alghanem B, Shaibah H, et al. SARS-CoV-2 coronavirus spike protein-induced apoptosis, inflammatory, and oxidative stress responses in THP-1-like-macrophages: potential role of angiotensin-converting enzyme inhibitor (Perindopril). Front Immunol. 2021;12:728896. doi: 10.3389/fimmu.2021.728896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Genin M, Clement F, Fattaccioli A, Raes M, Michiels C. M1 and M2 macrophages derived from THP-1 cells differentially modulate the response of cancer cells to etoposide. BMC Cancer. 2015;15:577. doi: 10.1186/s12885-015-1546-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.da Costa CG, Jalalizadeh M, Yadollahvandmiandoab R, Buosi K, Reis LO. Effect of BCG on respiratory complications caused by COVID-19: a scoping review. Int J Gen Med. 2022;15:8727–8741. doi: 10.2147/IJGM.S393861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Noble CCA, Messina NL, Pittet LF, Curtis N. Interpreting the results of trials of BCG vaccination for protection against COVID-19. J Infect Dis. 2023;228:1467–1478. doi: 10.1093/infdis/jiad316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Du J, Su Y, Wang R, et al. Research progress on specific and non-specific immune effects of BCG and the possibility of BCG protection against COVID-19. Front Immunol. 2023;14:1118378. doi: 10.3389/fimmu.2023.1118378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arts RJW, Moorlag SJ, Novakovic B, et al. BCG vaccination protects against experimental viral infection in humans through the induction of cytokines associated with trained immunity. Cell Host Microbe. 2018;23:89–100.e5. doi: 10.1016/j.chom.2017.12.010 [DOI] [PubMed] [Google Scholar]
- 31.Seo Y-J, Hahm B. Type I interferon modulates the battle of host immune system against viruses. Adv Appl Microbiol. 2010;73:83–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nagarajan U. Induction and function of IFNβ during viral and bacterial infection. Crit Rev Immunol. 2011;31:459–474. doi: 10.1615/CritRevImmunol.v31.i6.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shalhoub S. Interferon beta-1b for COVID-19. Lancet. 2020;395:1670–1671. doi: 10.1016/S0140-6736(20)31101-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arnegard ME, Whitten LA, Hunter C, Clayton JA. Sex as a biological variable: a 5-year progress report and call to action. J Womens Health. 2020;29:858–864. doi: 10.1089/jwh.2019.8247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spence JS, He R, Hoffmann -H-H, et al. IFITM3 directly engages and shuttles incoming virus particles to lysosomes. Nat Chem Biol. 2019;15:259–268. doi: 10.1038/s41589-018-0213-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aomatsu M, Kato T, Kasahara E, Kitagawa S. Gender difference in tumor necrosis factor-α production in human neutrophils stimulated by lipopolysaccharide and interferon-γ. Biochem Biophys Res Commun. 2013;441:220–225. doi: 10.1016/j.bbrc.2013.10.042 [DOI] [PubMed] [Google Scholar]
- 37.Rhodes SJ, Knight GM, Fielding K, et al. Individual-level factors associated with variation in mycobacterial-specific immune response: gender and previous BCG vaccination status. Tuberculosis. 2016;96:37–43. doi: 10.1016/j.tube.2015.10.002 [DOI] [PubMed] [Google Scholar]
- 38.Aaby P, Benn CS, Flanagan KL, et al. The non-specific and sex-differential effects of vaccines. Nat Rev Immunol. 2020;20:464–470. doi: 10.1038/s41577-020-0338-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Bree LCJ, Janssen R, Aaby P, et al. The impact of sex hormones on BCG-induced trained immunity. J Leukoc Biol. 2018;104:573–578. doi: 10.1002/JLB.5MA0118-027R [DOI] [PubMed] [Google Scholar]
- 40.Casciola-Rosen L, Thiemann DR, Andrade F, et al. IgM anti-ACE2 autoantibodies in severe COVID-19 activate complement and perturb vascular endothelial function. JCI Insight. 2022;7:e158362. doi: 10.1172/jci.insight.158362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carneiro BDB, Sanches BCF, Andrade DL, Voris BRI, Reis LO. Moreau strain bacillus Calmette-Guérin low versus standard dose in the treatment of high-grade T1 bladder cancer: a retrospective observational cohort study. Clin Genitourin Cancer. 2019;17:e779–e783. doi: 10.1016/j.clgc.2019.04.003 [DOI] [PubMed] [Google Scholar]
- 42.Bhatt K, Verma S, Ellner JJ, Salgame P. Quest for correlates of protection against tuberculosis. Clin Vaccine Immunol. 2015;22:258–266. doi: 10.1128/CVI.00721-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gagliardi MC, Teloni R, Giannoni F, et al. Mycobacterium bovis Bacillus Calmette-Guerin infects DC-SIGN- dendritic cell and causes the inhibition of IL-12 and the enhancement of IL-10 production. J Leukoc Biol. 2005;78:106–113. doi: 10.1189/jlb.0105037 [DOI] [PubMed] [Google Scholar]
- 44.Madura Larsen J, Benn CS, Fillie Y, van der Kleij D, Aaby P, Yazdanbakhsh M. BCG stimulated dendritic cells induce an interleukin-10 producing T-cell population with no T helper 1 or T helper 2 bias in vitro. Immunology. 2007;121:276–282. doi: 10.1111/j.1365-2567.2007.02575.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang Y, Broser M, Rom WN. Activation of the interleukin 6 gene by Mycobacterium tuberculosis or lipopolysaccharide is mediated by nuclear factors NF-IL6 and NF-kappa B. Proc Natl Acad Sci USA. 1994;91:2225–2229. doi: 10.1073/pnas.91.6.2225 [DOI] [PMC free article] [PubMed] [Google Scholar]