Abstract
Methane is an important greenhouse gas1, but the role of trees in the methane budget remains uncertain2. Although it has been shown that wetland and some upland trees can emit soil-derived methane at the stem base3,4, it has also been suggested that upland trees can serve as a net sink for atmospheric methane5,6. Here we examine in situ woody surface methane exchange of upland tropical, temperate and boreal forest trees. We find that methane uptake on woody surfaces, in particular at and above about 2 m above the forest floor, can dominate the net ecosystem contribution of trees, resulting in a net tree methane sink. Stable carbon isotope measurement of methane in woody surface chamber air and process-level investigations on extracted wood cores are consistent with methanotrophy, suggesting a microbially mediated drawdown of methane on and in tree woody surfaces and tissues. By applying terrestrial laser scanning-derived allometry to quantify global forest tree woody surface area, a preliminary first estimate suggests that trees may contribute 24.6–49.9 Tg of atmospheric methane uptake globally. Our findings indicate that the climate benefits of tropical and temperate forest protection and reforestation may be greater than previously assumed.
Subject terms: Carbon cycle, Forest ecology, Carbon cycle
Studies of in situ woody surface methane exchange in upland tropical, temperate and boreal forest trees find that methane uptake can result in a net tree methane sink that is globally significant and demonstrates an additional climate benefit provided by trees.
Main
Methane (CH4) is the most important anthropogenically enhanced greenhouse gas in the atmosphere after CO2, contributing an extra 26% of anthropogenic greenhouse warming since 1750 (ref. 1). As such, it is important that all sources and sinks are fully quantified to include the role of terrestrial ecosystems in mediating atmospheric exchange. The global CH4 budget is now unbalanced, with sources exceeding sinks, which leads to growth in the atmospheric concentration of this powerful greenhouse gas2. Soils represent an important CH4 sink in which CH4-consuming methanotrophs are ubiquitous7 but the collective CH4 uptake capacity of soils can vary, depending on a range of factors, including soil moisture and temperature7–9. At the global scale, the atmospheric CH4 sink terms (for example, hydroxyl (OH) radicals and ultraviolet-associated processes) dominate CH4 losses, with the far smaller soil sink term considered the only terrestrial sink. However, data-driven global modelling efforts tend to overestimate emission sources by about 151 Tg yr−1 when compared to smaller atmospheric ‘top-down’ derived estimates2, possibly suggesting that a substantial terrestrial CH4 sink term is either poorly quantified or missing from the global CH4 budget.
Recently, we showed that mature trees in saturated soils can emit substantial quantities of soil-produced methane10–12, which has been confirmed by others13–15. In the Amazon, for example, flooded trees are the single largest emission source from the region3. However, endophytic methane-using bacteria have been identified in temperate poplar trees16–18 and in tropical wetland tree bark, in which bark methanotrophy attenuated 36% of CH4 emissions19. Collectively, these lines of evidence raise the possibility that trees have the capacity not only to serve as an internal sink for otherwise emitted CH4 but also, where soil CH4 production is limited by low soil moisture, to serve as net sinks of atmospheric CH4.
Observations of tree CH4 uptake have primarily focused on trees in cool locations, in which flux rates in either direction tend to be low5; on trees growing in unusual and not necessarily representative landscapes6; or on wetland trees that have a known supply of soil-derived CH4, where emissions dominate19. However, some studies have reported the presence of negative CH4 fluxes on upland (non-flooded) tree stems, even though the main focus of these papers tended to be on net emissions20 (Supplementary Table 1). Indeed, measurement chamber positioning in the lowermost portions of the tree may have disproportionately identified emission fluxes over uptake, as the lowermost portions of tree stems are far closer to soil sources of CH4, which may be entrained by tree roots and then emitted to the atmosphere4 (Supplementary Table 1). Collectively, these studies do, however, present the possibility that if higher portions of upland tree stems and branches are considered, then trees on free-draining soils may be net sinks of atmospheric CH4. The net outcome of CH4 emission and uptake processes associated with tree surfaces would depend on the hydrological status of the soil and the methane-consuming capacity of the tree surface area of exchange. The large surface area of tree stems, branches and twigs21 (hereafter referred to as woody surfaces) and the relative abundance of upland (herein referring to trees on free-draining soils with low water tables) versus wetland forests means that even small fluxes, in either direction (emission and uptake), may result in large cumulative exchanges of CH4 associated with upland trees at the global scale.
To fully examine the role of upland trees in the global CH4 cycle, we examined in situ woody surface CH4 exchange spanning a latitudinal gradient of tropical, temperate and hemiboreal forest ecosystems. These measurements allowed us to quantify net exchanges of CH4 from woody surfaces across a range of biomes spanning tropical forests in Amazonia (Cuniã) and Panama (Gigante), temperate broadleaf forest in the United Kingdom (Wytham Woods) and hemiboreal coniferous forest in Sweden (Skogaryd). In addition, we made flux measurements from tree stems from three further locations in the Amazon floodplain during low water (the Negro, Solimões and Tapajós Rivers). These locations experience periodic inundation but also dry periods when the water table is many metres below the soil surface19.
Global tree stem flux measurements
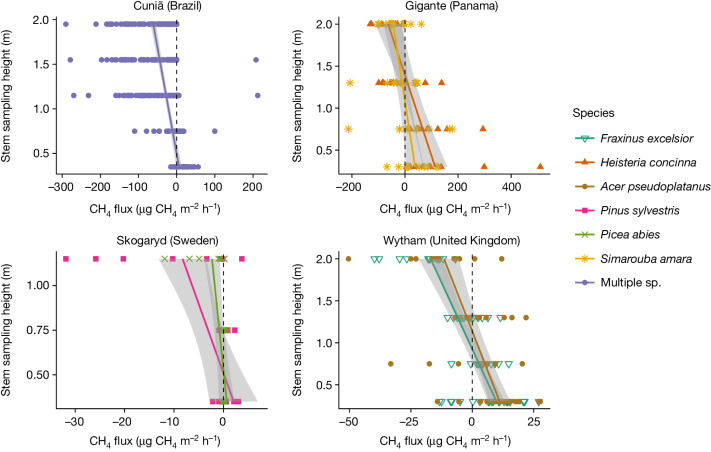
We examined CH4 fluxes, in either direction, across several tree stem heights using established methods22 that couple rapidly deployable woody surface gas exchange chambers with laser-based analysers. In addition, for two sites (Cuniã in Brazil and Skogaryd in Sweden) we collected tree stem wood cores to evaluate vertical trends in methanotrophy with height above the forest floor and assessed stable carbon isotope values of CH4 (δ13C-CH4 values) from within-chamber air. The CH4 uptake fluxes were largest in tropical forests and smallest in temperate and hemiboreal sites. Despite high flux variability, trees in each of the four measured upland sites spanning the latitudinal gradient consistently exhibited a vertical pattern of CH4 exchange. CH4 emissions were observed in the lowermost portions of the tree stem (about 30–130 cm; Fig. 1), and fluxes tended to switch from emission to CH4 uptake at and above breast height (1.3 m), although we observed uptake starting lower down the tree stem (0.7 m) in Cuniã, Brazil (Fig. 1). At our temperate site at Wytham Woods (United Kingdom), the same pattern was observed for two dominant tree species, ash and sycamore. We further measured CH4 uptake from branches in oak tree crowns at 11 m above the forest floor in Wytham Woods on a single occasion during a prolonged dry period in June 2018, where we found net CH4 uptake (−1.86 ± 1.89 µg of CH4 m−2 h−1, n = 11). Although small and highly variable from this limited sampling, it confirms, along with previous work on Swedish branches17, that uptake processes also take place on woody surfaces higher up in the tree and water limitation may influence the size of uptake processes.
Fig. 1. Regression plots of methane (CH4) fluxes against tree stem sampling position above the forest floor.
Top left, fluxes measured from Cuniã, Brazil, from two free-draining forested plots (20 × 30 m2) in the catchment draining to the Madeira River (n = 100) during 15–25 March 2013; top right, for mature trees stems of two common tree species: Heisteria concinna (orange) and Simarouba amara (yellow) in experimental litter manipulation treatments in a tropical forest on free-draining soil in Panama, Central America, between 18 and 27 November 2015 (24 trees); bottom right, ash and sycamore in a temperate deciduous woodland on free-draining soil in Wytham Woods, United Kingdom, between October 2015 and January 2016 (24 trees); bottom left, measurements from Scots pine and Norway spruce (18 trees) in the Skogaryd Research Catchment, near Vänersborg in southwestern Sweden. Grey bands denote 95% confidence intervals, which for Cuniã (large number of trees) falls in the regression line area. In Cuniã (Brazil) and Gigante (Panama) upland forests, CH4 uptake fluxes were largest at the highest sampling points at about 1.75–2 m above the forest floor (−56.5 ± 59.4 µg of CH4 m−2 h−1 and −46.7 ± 64.7 µg of CH4 m−2 h−1, respectively). For figure clarity, some of the largest values are not included, but the highest individual measured uptake values were −290 µg of CH4 m−2 h−1 in Cuniã and −488 µg of CH4 m−2 h−1 in Gigante. At Wytham, we found mean uptake at the highest sampling position (2 m) of −18.5 ± 20.4 µg of CH4 m−2 h−1 for ash and −14.0 ± 27.4 µg of CH4 m−2 h−1 for sycamore, with the highest recorded individual uptake fluxes being −54.8 µg of CH4 m−2 h−1 on ash and −142 µg of CH4 m−2 h−1 on sycamore. General information relating to these sites is presented in Extended Data Table 5.
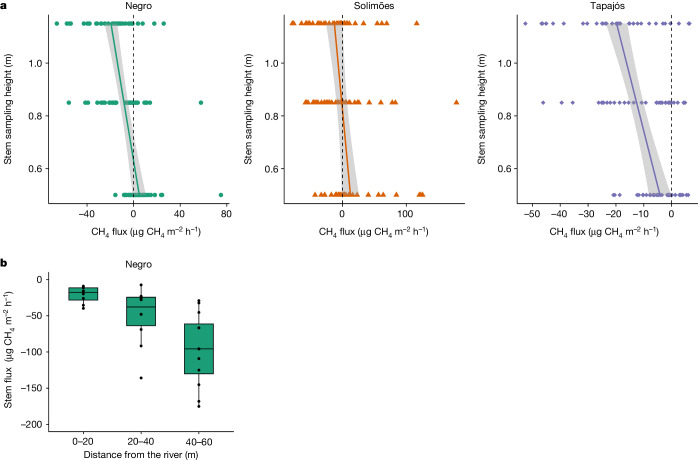
At the coolest location, hemiboreal forest in Skogaryd (Sweden), the highest sampling position was located 115 cm above the forest floor and fluxes were correspondingly small, although uptakes of −9.90 and −2.82 µg of CH4 m−2 h−1 were observed for pine and spruce tree stems, with a declining pattern of emission favouring uptake with increasing height above the soil surface. Although fluxes across all biomes tended to be highly variable, our study design, along with many trees examined in some locations (for example, Cuniã), allowed us to detect general patterns. These tree CH4 uptake patterns are further supported by observations in the three Amazonian floodplain locations. Here, trees were a significant source of CH4 during periods of inundation19. However, during low water-table conditions in the dry season (water table about 6–10 m below the soil surface), trees demonstrated an identical pattern of emission in the lowermost stem portions (Negro and Solimões Rivers) and then uptake from 60 cm (Negro River) to 90 cm (Solimões River) above the forest floor (Fig. 2a). In the location with the lowest water table, Tapajós, we observed mainly uptake at all sampling positions above the forest floor, in common with observations in Cuniã. These floodplain trees therefore demonstrate plasticity in their trace gas exchange capacity, with the hydrologically contingent capability to function as both large, yet spatially constrained point sources of atmospheric CH4 or sinks representing far more spatially extensive upland forest, depending on soil water-table level.
Fig. 2. Spatial patterns of CH4 fluxes on dry season Amazon floodplain trees.
a, Regression plots of CH4 fluxes against tree stem sampling position. Measured from above the forest floor in three floodplain locations during low water in the central Amazonian floodplain (36 trees from each plot). b, Fluxes measured at 5 m above the forest floor with increasing horizontal distance from the Negro River (n = 36 trees).
Methane oxidation potential in trees and its isotopic enrichment
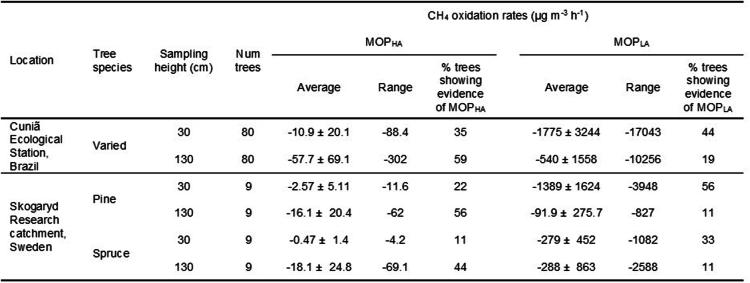
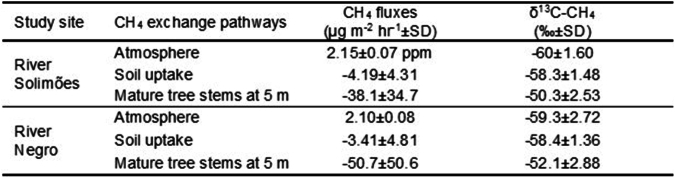
Incubations of wood cores from Cuniã in Brazil and Skogaryd in Sweden yielded a consistent capacity for CH4 uptake in response to either ambient CH4 concentrations (high-affinity methanotrophy—MOPHA) higher up the tree stem or enriched CH4 concentrations (low-affinity methanotrophy—MOPLA) at the stem base (Extended Data Table 1). We attribute this pattern to a supply of soil-derived higher-than-ambient CH4 near stem bases16, sustaining a larger population of low-affinity methanotrophs than higher up the tree. By contrast, high-affinity CH4 oxidation dominates higher up the tree stem (Extended Data Table 1), in which CH4 is supplied at ambient atmospheric concentrations. Thus, the observed pattern of CH4 emissions (or low uptake) in the lowermost portion of tree stems (less than 1 m) and substantial uptake higher up the tree stem are consistent with expected methanotroph populations occupying niches that are determined by CH4 supply. Further, δ13C-CH4 analyses from floodplain trees in Central Amazonia during an exceptional dry season demonstrate strong enrichment during chamber incubations on woody surfaces at 5 m above the forest floor. Less 13C enrichment in CH4 was found for soil uptake at those locations (Extended Data Table 2) pointing to a much stronger atmospheric CH4 oxidation potential by methanotrophic bacteria on the tree surfaces.
Extended Data Table 1.
Wood core methane oxidation potentials
High-affinity (MOPHA) and low-affinity methane (CH4) oxidation potentials (MOPLA) measured from wood cores extracted at 30 and 130 cm stem height at Cuniã, Brazil and Skogaryd, Sweden forest sites. MOPLA tended to be higher in tree stem bases than in the uppermost woody surface sampling position with higher percentages of trees demonstrating MOPLA at 30 cm (44% for Cunia and 56% and 33% for pine and spruce respectively) than at 130 cm (19% for Cunia and 11% for both pine and spruce) across all biomes and species. By contrast, the situation was reversed for MOPHA, with higher percentages of trees demonstrating MOPHA at 130 cm (59% for Cunia and 56% and 44% for pine and spruce respectively) than at 30 cm (35% for Cunia and 22% and 11% for pine and spruce respectively) and with higher MOPHA at 130 cm.
Extended Data Table 2.
Stable carbon isotope values of CH4 within flux chambers on woody and soil surface
Atmospheric CH4 concentrations in ppm were measured by drawing atmospheric air next to the tree under investigation, at 1.3 m above the soil surface, using a syringe. A second syringe sample, extracted at the same time, was used to analyse δ13C-CH4 in atmospheric air. Stem and soil CH4 flux were measured in real time using a 10-minute chamber closure time. At the end of the flux measurement, gas samples were extracted for δ13C-CH4 analysis. N = 36 for stem and soil CH4 flux and δ13C-CH4 analysis at each location.
Bringing these lines of evidence together demonstrates a highly variable but near-ubiquitous capacity for tree woody surfaces to remove atmospheric CH4 at ambient concentrations, with vertical spatial patterns of CH4 exchange corresponding to distance from the source of any soil-produced CH4. We observed, across all biomes, a decline in CH4 emission with distance from the forest floor, followed by a switch to uptake above a certain point at or around breast height (100–130 cm above the forest floor), or lower in the Amazon region. This is further reinforced by our measurements from trees in which larger net atmospheric CH4 uptake was observed at 5 m above the forest floor with increasing horizontal distance from the river: a probable response to progressively deeper water table with increasing distance from the river and a correspondingly smaller soil CH4 source (Fig. 2b). The CH4 oxidation potential observations provide a mechanistic explanation of this spatial pattern, as supported by recent findings of methanotrophs in wetland tree bark16, in combination with declining diffusive loss of soil-derived CH4 to the atmosphere with height above the forest floor and strong δ13C-CH4 enrichment on woody surfaces at height above the forest floor (Extended Data Table 2).
Global importance of tree CH4 uptake
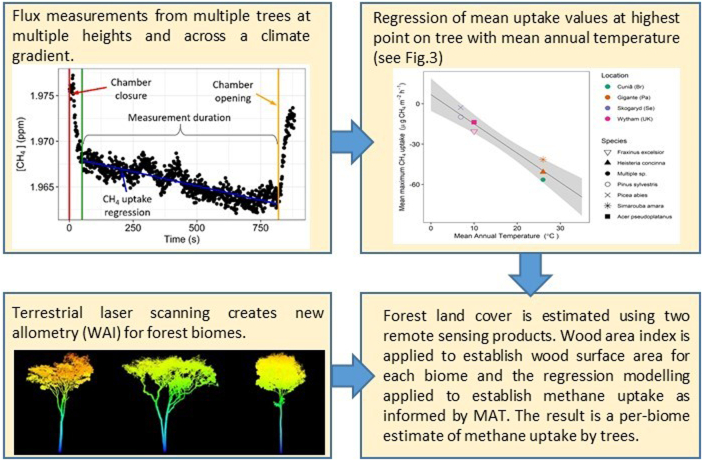
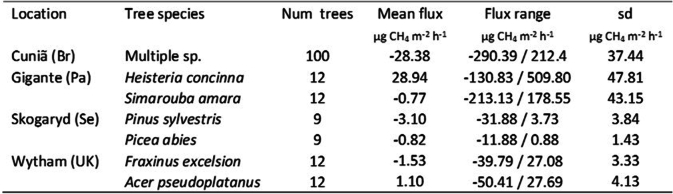
There are complex interactions between vegetation and the atmosphere with respect to CH4 exchange. Wetland trees tend to be large sources of CH4 (refs. 12,19), and these sources are well accounted for in the global CH4 budget. Although there is evidence to suggest widespread aerobic CH4 production in living organisms through reactive oxygen species23, aerobic emissions from foliage are thought to be small (less than 1 Tg yr−1)24, although uncertainties are large. They further conflict with the recent finding of uptake in some forest canopy leaves25, although this has yet to be replicated. Our findings do provide compelling evidence that woody surfaces in upland forests are net sinks in the global CH4 budget. We therefore assessed the global importance of this woody surface net atmospheric CH4 sink term in the Earth system in two steps. First, we examined how the mean CH4 uptake in the uppermost tree stem sampling positions (2 m, across our climate gradient) varied with mean annual temperature (MAT), which showed a strong relationship with larger uptake fluxes at higher MAT (Fig. 3). Given that we either sampled from large numbers of trees for which species identification was difficult (Amazonia n = 100) or from dominant tree species in established and well-monitored plots (Panama, Wytham and Skogaryd) and that we made several measurements on each tree at several positions above the forest floor, we consider our individual site data to be representative, thus permitting large-scale analysis and interpretation. We also consider our approach to be conservative because we only included the mean uptake values at the highest point that we measured on the tree stem, no higher than 2 m above the forest floor, in our global regression with mean MAT. However, our regression analysis of CH4 uptake versus tree stem sampling position suggests the probable presence of larger uptake values, on average, higher up the stem, which is supported by our measurements at 5 m above the forest floor in the Amazon floodplain during an exceptional dry season, which show twice the CH4 uptake (about 100 µg of CH4 m−2 h−1; Fig. 2b) of those measured at 2 m above the forest floor elsewhere in the tropics (Figs. 1 and 3). Second, we sought to quantify the global woody surface area. Until recently, reliable estimates of tree woody surface area have been limited. We used new terrestrial laser scanning (TLS) approaches to measure woody surface area in each of the temperate and tropical sample biomes and apply those to forests globally.
Fig. 3. Empirical model of CH4 uptake versus MAT.
Mean CH4 uptake recorded in the uppermost tree sampling position above the forest floor in each of the upland forest locations, plotted against MAT for each site. The regression equation is CH4 uptake = −2.179 MAT + 6.837. The grey shading represents the 95% confidence interval.
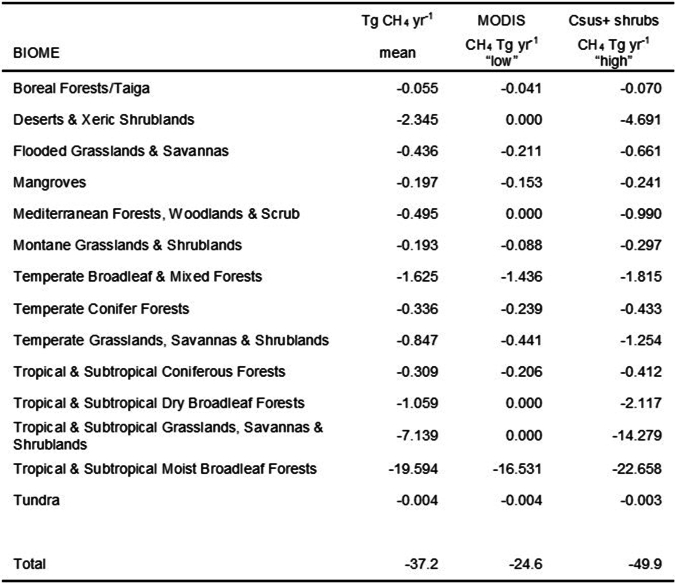
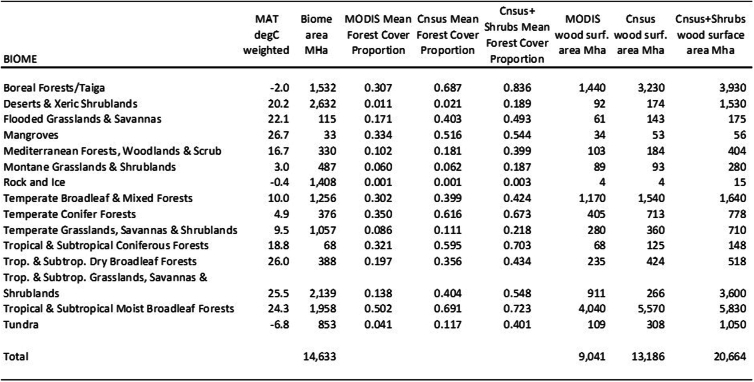
We then scaled estimated CH4 uptake across the globe based on tree canopy cover and mean climatology. Finally, we aggregated the CH4 uptake across biomes to determine per-biome values (Extended Data Table 3). A first approach that excludes any CH4 uptake from woody surfaces more than 4 m above the forest floor suggests a highly conservative uptake estimate of 1.7 Tg of CH4 yr−1. However, considering all woody tree surfaces leads to a mean total global woody surface CH4 sink estimate of 37.2 Tg of CH4 yr−1 (24.6–49.9 Tg of CH4 yr−1), with the range demonstrating uncertainty associated with the land surface products used, which offer very different global woody surface area estimates (Extended Data Table 4) or whether woody shrubs are included (‘high’ estimate, Extended Data Table 3). Despite this uncertainty, the size of this global CH4 woody surface uptake is similar to that of the global soils sink (30–40 Tg of CH4 yr−1)2, thus far the only other characterized terrestrial CH4 sink in the Earth system. We estimate the total global aboveground woody surface area to be 143 (±59) million km2 (Extended Data Table 4), approximately equal to total global land surface area (149 million km2), but, unlike the global land (soil) surface, this area of CH4 exchange will vary with changing forest cover. Hence, woody surface extent and architecture adds a poorly appreciated structural third dimension for exchange between the land biosphere and atmosphere.
Extended Data Table 3.
Mean and high/low estimates of total annual CH4 uptake per biome
The low estimate uses the MODIS VCF product and the high estimate uses the 1 km global consensus land cover project which includes woody shrubs. We zeroed uptake in probable water-limited biomes in our low estimate in keeping with our conservative approach. Parameters used for the different estimates are presented in Extended Data Table 4. See Methods.
Extended Data Table 4.
Parameters employed to estimate total annual forest biome methane uptake
MAT weighted is mean annual temperature across the biome weighted by forest cover in each pixel. Biome area is the total land area in that biome. We applied a woody area index (WAI) of 4.12 for tropical and subtropical moist broad-leaved forest and 3.07 for remaining biomes from our revised forest allometry. Forest cover proportion is derived from the two land cover products we use. The consensus land cover product includes shrub cover as a separate category, so we calculate woody surface area with and without them. We assign the mean tropical forest woody surface area per ground area value to shrubs.
Implications and conclusions
We show that woody surfaces of most trees constitute a new sink term for CH4, which presents opportunities to close the now unbalanced global CH4 budget. In this budget, fully integrative top-down estimates of atmospheric CH4 removal have been smaller than bottom-up estimates, which have neglected the process we now uncover2. Further, our findings indicate that there may be substantial CH4-associated climate benefit from the presence of trees, which is not now accounted for in natural climate solutions frameworks. Indeed, as the net annual C sequestration of tropical rainforests declines26, the contribution of this upland tree woody surface CH4 sink term may increase in relative importance because it probably responds to increasing temperature and atmospheric CH4 concentration5.
Further, the woody surface tree uptake mechanism shows opportunities for enhanced mitigation of atmospheric CH4 growth. The largest biomes contributing to this total woody methane sink are tropical and subtropical moist broadleaf forests (16.5–22.7 Tg of CH4 yr−1 for our ‘low’ and ‘high’ estimates; Extended Data Table 3) and tropical and subtropical grasslands, savannas and shrublands (0–14.3 Tg CH4 yr−1 for our low and high estimates; Extended Data Table 3). To first estimate the methane-associated climate benefit of long-term intact forests, we equate a 1 Mg of CH4 yr−1 constant methane sink with removal of 8 Mg of CO2-warming equivalent (we) yr−1 (ref. 27). For a mature Amazonian forest, we therefore estimate that its tree stem surface CH4 sink is equivalent (in terms of the equivalent amount of C in CO2) to 0.037 Mg of CO2-C ha−1 yr−1, approximately 15% the value of the mean biomass carbon sink in Amazonian forests (about 0.24 Mg of C ha−1 yr−1; ref. 26), thus pointing to a significant extra climate benefit of intact tropical forest biomes and probable benefit to large-scale forest regrowth and restoration
We consider the CH4 sink consequences of removing trees to be small relative to total biomass C loss; however, the impact of reforestation may be more significant. Despite the lower biomass of secondary forests, their large numbers of small trees mean that they often have high woody surface area, similar to or higher than that of old growth forests28,29. We estimate an extra greenhouse gas mitigation value from CH4 uptake as equivalent to 0.131 and 0.586 Mg of C ha−1 yr−1 in temperate and tropical forests, respectively, corresponding to a 7% and 12% extra climate benefit of new trees in these respective biomes. This suggests a possible global extra climate benefit, through the enlarged tree CH4 sink, equivalent to up to 0.3 Pg of C yr−1 or 1.1 Pg of CO2-we yr−1. This is equivalent to a 10% extra mitigation potential over benefits already estimated for expansion of temperate and tropical forests30,31. Hence, the tree CH4 sink may have a particularly important climate mitigation role in the context of reforestation, though this prediction needs to be tested through field studies in regrowing forests.
Our findings demonstrate high spatial and taxonomic variability in the CH4 uptake capacity of trees. Identifying tree species with the largest capacity for CH4 uptake offers opportunities to further address the growth in atmospheric CH4. Measurements along the full vertical profile of trees may find a yet stronger CH4 sink in tree branches.
Methods
Individual tree CH4 flux observations were made as follows in each of the following sites. Chambers used at all sites were constructed from gas-impermeable polyethylene terephthalate or polycarbonate plastic sheet. The chamber characteristics, accuracy and precision of the method when used either in manual syringe mode for later analysis on Los Gatos analysers or in continuous mode (with in situ analysis by means of field portable microportable and ultraportable greenhouse gas Los Gatos analysers (MGGA and UGGA, respectively)) are detailed in ref. 22. To summarize, and unless specified below, two ‘sleeve’ chamber sizes were used, small (25 × 16 × 1.5 cm3) and large (30 × 24 × 1.5 cm3), depending on the dimensions of the woody surface being measured. All fluxes were measured in the middle part of the day (10:00–15:00) or at times between 09:00 and 18:00. Fluxes measured in continuous flow mode had variable deployment and measurement periods, depending on the rate of concentration change, which was observed in real time. A photograph of a typical chamber deployment is presented in Extended Data Fig. 1. The minimum flux that could be detected using the modified fast methane analyser (FMA) analysis method32 based on instrument sensitivity and chamber volume was 0.4–3.5 µg of CH4 m2 h−1. The minimum flux that could be detected using the LGR UGGA and the LGR MGGA in real-time measurements based, respectively, on instrument sensitivities of 4 ppb with 1 s precision and 2 ppb with 1 s precision, as well as on chamber volume, is less than 1 µg of CH4 m2 h−1.
Extended Data Fig. 1. Photograph of a typical tree woody surface flux measurement chamber.

Each chamber has an inlet and an outlet that join to a laser methane analyser. See methods. For a fuller description of the method see Siegenthaler et al.22. Photograph by Josep Barba.
Cuniã Nature Reserve, Amazonia, Brazil (63° 5′ W, 8° 1′ S)
Stem CH4 emissions from mature trees (equal to or more than 10 cm diameter) were measured from two free-draining forested plots. The two plots (20 × 30 m2) were located in the Madeira River catchment, a white-water river system and one of the largest tributaries of the Amazon. The Madeira drains the Andean area upstream, which results in high suspended and dissolved solids concentrations in water, with neutral to alkaline pH33,34. The MAT is 26 °C and mean annual rainfall is 2,500 mm (ref. 35).
Methane flux measurements from mature trees stems (n = 50 per plot) at five stem heights (20, 60, 100, 140 and 180 cm above the soil surface) were performed during a period of transition towards the dry season when the water tables in both the plots were more than 10 m below the soil surface. Measurements were carried out between 15 and 25 March 2013. Methane fluxes were measured using static chambers as described in refs. 3,22, with air samples from the static flux chambers drawn using 30 ml syringes and immediately transferred to a 12 ml exetainer (Exetainer) for later analysis of CH4 using the modified LGR CH4 laser-based analyser3 (LGR UGGA).
Gigante Peninsula, Barro Colorado Nature Monument, Panama (9° 6′ N, 79° 54′ W)
Measurements in semi-evergreen tropical forest were carried out between 18 and 27 November 2015 in the five control plots of the Gigante Litter Manipulation Project, approximately 5 km south of Barro Colorado Island, Panama, Central America. A full description of the litter manipulation experiment is given in refs. 36,37. The MAT at the weather station on Barro Colorado Island is 26 °C, mean annual rainfall is 2,600 mm and there is a strong dry season from mid-December to mid-April38.
We measured tree stem CH4 fluxes at 30, 75, 130 and 200 cm height from two common tree species: the fast-growing canopy tree Simarouba amara (Aubl.) and the shade-tolerant subcanopy tree Heisteria concinna (Standl.) (12 trees per species). Tree stem gas fluxes were measured using a flexible chamber (45 cm × 30 cm × 19 mm polycarbonate) as described in refs. 4,20. Gas samples were taken by syringe from a septum in the middle of the chamber at 0, 5, 10 and 15 min and injected into pre-evacuated 12 ml borosilicate vials. All samples were analysed in the United Kingdom using off-axis integrated cavity output spectroscopy (FMA-200 fast methane analyser).
Wytham Woods, Oxfordshire, United Kingdom (51° 46′ 42″ N, 1° 19′ 42″ W)
Measurements at the temperate site were conducted in four control plots of a litter manipulation experiment39,40 at Wytham Woods, an old growth (about 120 yr) mixed deciduous woodland in Oxfordshire, United Kingdom. The canopy at the study site is dominated by ash (Fraxinus excelsior L.), beech (Fagus sylvatica L.), sycamore (Acer pseudoplatanus L.) and oak (Quercus robur L.)41. MAT was 10 °C (ref. 40). In each 25 × 25 m2 plot, three individuals each of ash and sycamore were randomly selected, making a total of 24 trees. Tree stem CH4 fluxes were sampled using the same chamber design, sampling heights and procedure as described for Gigante above, except that gas samples were collected at 0, 3, 6 and 10 min. All samples were analysed in the United Kingdom using off-axis integrated cavity output spectroscopy (FMA-200 fast methane analyser).
Skogaryd, Sweden
The stem and soil flux measurements were conducted in the Skogaryd Research Catchment, near Vänersborg in southwestern Sweden, on three occasions in spring 2014 (2–3 April, 14–16 April and 28–30 April). Tree stem CH4 fluxes were measured from Norway spruce (Picea abies (L.) Karst.) and Scots pine (Pinus sylvestris (L.)), based on the dominance of these tree species in the European boreal forest42 and at the study site. Methane fluxes from tree stems were measured in real time using static chambers connected to a laser-based CH4 analyser (LGR UGGA) as described in ref. 3. Stem CH4 emissions were measured from mature tree stems (equal to or more than 10 cm, n = 9 per species) from three stem heights (20, 60 and 100 cm above the soil surface). The water table was more than 5 m below the surface in the plot and soil was characterized as a histosol. The mean air temperatures during the three sampling occasions were 13.1, 14.2 and 18.1 °C, respectively, with all measurements carried out during daytime (09:00–18:00). MAT is 6.9 °C.
Following stem flux measurements at the Skogaryd and Cuniã study sites, we assessed the CH4 oxidation potentials (both high affinity and low affinity) in the tree stems by extracting wood cores at 30 and 130 cm stem height from a subset of the trees (Cuniã, 80 trees; Skogaryd, 9 trees per species).
Wood cores were extracted at four cross-sections radially from bark to pith at the same location on the stem where CH4 flux measurement were performed using a 5.1 mm increment corer. These cores were immediately transferred into a 50 ml vial and incubated on the same day of sampling to quantify the potential rates of CH4 production and oxidation. Incubations were carried out onsite in the dark, at field temperature of 25–27 °C and 11–15 °C, respectively. An initial headspace CH4 concentration of 6.5 and 750 ppm was maintained for high- and low-affinity CH4 oxidation potentials, respectively, and the cores were incubated aerobically for 48 h. Headspace samples from all incubations were extracted at 4, 24 and 48 h and CH4 concentrations analysed using methods described in refs. 3,12.
Further measurements made in the Amazon floodplain
Sampling design and measurement protocols for fluxes presented in Fig. 2 are detailed in refs. 19,20. In summary, we established three temporary plots (60 × 60 m2) in the floodplains of three principal rivers of the Amazon, the Negro (black water), Solimões (white water) and Tapajós (clear water). The CH4 fluxes were measured from a total of 108 trees (36 across each plot) at vertical intervals above the forest floor during low water in January 2018. We returned in the exceptional dry season of 2021 (October) to make measurements from a subset of trees at 5 m above the forest floor and also to sample for methane isotopes.
Chamber CH4 isotopes in the Amazon
For δ13C-CH4 analysis, 30 ml gas samples were collected from tree woody surfaces and soil surfaces at the Negro and Solimões floodplain forests during the dry season of 2021. Samples were taken from air and from flux chambers on the soils surface and tree stem surface at 5 m above the forest floor using gas-tight syringes and then transferred to pre-evacuated 12 ml borosilicate vials fitted with double wadded caps (Exetainer). Vials were over-pressurized to prevent ingress of air from pressure or temperature changes during transport to the laboratory. The δ13C values of CH4 were analysed using a cavity ring-down spectrometer (model G2201-i, Picarro) coupled with a custom-built auto-sampler and are reported relative to the Vienna Pee Dee Belemnite standard. The instrument was calibrated for δ13C-CH4 using isotopic reference gases with isotope ratios of −23.9‰, −54.5‰ and −66.5‰ (Isometric Instruments). The overall analytical precision based on replicate measurements of reference gases was ±0.4‰.
CH4 uptake global estimate methods
Our study leveraged TLS technology to develop a new surface area allometry43. TLS provides high-resolution, three-dimensional representations of tree structures, enabling precise surface area estimations. We scanned a total of 2,161 trees across 22 plots in tropical forests, temperate conifer forests, temperate broadleaf forests, temperate dry eucalypt forests and tropical savannahs to capture a wide range of tree morphologies. We built two woody area index (WAI) allometries (one for tropical forests, one for the rest of the world) to predict woody surface area from tree stem diameter (diameter at breast height, DBH) based on three-dimensional models of trees43. Each tree was scanned from several angles to ensure comprehensive coverage and cylindrical models were fit with TreeQSM44. These models were analysed using the treestruct R package45, which resulted in woody surface area and DBH for each tree. We then applied hierarchical generalized additive models46 to build the surface area allometric equations. These models were designed to capture the complex, nonlinear relationships between tree surface areas and their respective DBH (Extended Data Fig. 3).
Extended Data Fig. 3. Woody surface allometry.
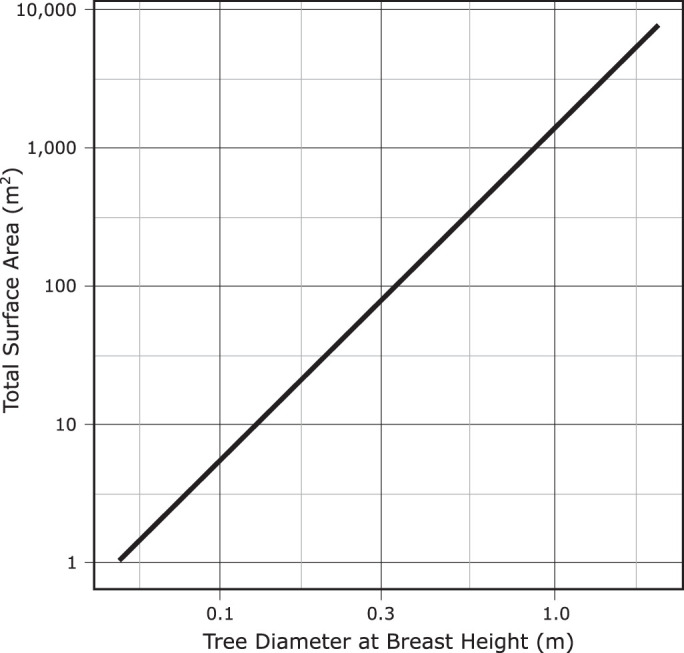
Allometric relationship between tree woody surface area and diameter at breast height.
To scale our TLS-derived allometry to forest plots, we integrated tree census data from 70 global ecosystem monitoring plots that were chosen to have close to 100% canopy cover and be generally greater than 5 m tall. Six Centre for Tropical Forest Science plots across the tropics were also used, which, in addition to their greater than 10 cm census, included trees down to 1 cm diameter. In most plots, trees of more than 10 cm DBH were measured, and we used plots for which trees were measured down to more than 1 cm DBH (six ForestGeo plots47) to estimate the percentage contribution of trees with 1–10 cm DBH to total woody surface area. We applied our allometry to each tree in these plots, thereby estimating the total woody surface area for each plot. We then performed a weighted average, based on plot size, across all plots to determine mean surface area for tropical forests.
The integration of TLS data with forest plot census data not only validated our allometric model but also allowed the extrapolation of surface area estimates across different biomes and forest structures.
Global scaling using satellite remote sensing data
For global-scale extrapolation, we used a combination of satellite remote sensing datasets. This approach allowed us to estimate woody surface areas across the world’s ecosystems, integrating our allometric model with global forest cover data.
We used The Nature Conservancy’s Ecoregions map to determine biome extents and both the 1 km global consensus land cover project48 and the MODIS MOD44B Version 6 Vegetation Continuous Fields (VCF49) 2019 product, as well as the 1 km consensus land cover map, to determine per-pixel forest cover. This dataset provided a global view of vegetation cover, quantified on a continuous scale for each pixel.
Combining these datasets, we were able to scale our allometric model from individual trees to a global scale. For each pixel, we determine the per-hectare woody surface area based on the undisturbed forest plots in that ecoregion and applying our allometry-derived WAI. We then scaled (multiplied) that potential value by the proportion of that pixel covered by forest, according to the 1 km consensus map and MODIS VCF.
To account for climatic variations in our model, we used the ERA5 monthly climate dataset50. This dataset provides mean monthly temperatures, which we converted to MAT. With the woody surface area in each pixel known (Extended Data Table 4), we then applied the CH4 uptake versus MAT regression across the globe (Extended Data Table 4).
Finally, we aggregate the CH4 uptake across biomes to determine per-biome figures (Extended Data Table 3). The entire approach is summarized in Extended Data Fig. 2.
Extended Data Fig. 2. Schematic workflow diagram.
Diagram of approach to deriving global, per-biome woody surface methane uptake estimates.
Global upscaling uncertainty analysis
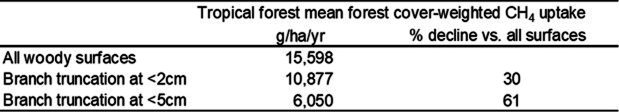
We examined how woody surface area was influenced by various parameters, in particular the branch size fraction. Small branches and twigs carry greater uncertainty in our scaling because of challenges in estimation of their area from TLS measurements. Exclusion of twigs smaller than 2 cm in diameter from our analysis resulted in a 30% reduction in calculated uptake at the biome level and exclusion of branches smaller than 5 cm in diameter resulted in a 61% reduction in uptake (Extended Data Table 6). This represents uncertainty in our biome and global-scaled estimates, which may be reduced in future with more measurements in these smaller branch size fractions and better estimation of their surface area.
Extended Data Table 6.
Branch size contribution to woody surface uptake in tropical forests
Table shows how different branch sizes contribute to total woody surface methane uptake in tropical forests.
A second source of uncertainty is the relatively low uptake flux estimates derived from tropical trees at 2 m above the forest floor for our upscaling estimates, as opposed to fluxes that were twice as large when measured at 5 m (Fig. 2b), which is likely to counterbalance any such reductions from excluding small branch area size fractions. Because of the limited size of our dataset at 5 m height, we chose to use only the smaller 2 m flux values for our scaling. If the 5 m values are more typical of the whole tree, which seems plausible as most of the tree surface area is above 5 m and further away from any soil-generated methane carried through and lost from lower portions of the tree trunk, then our biome-scaled fluxes would increase by up to 100%. Hence the possible biases in small branch fluxes and flux sampling height probably work in opposite directions and cancel each other out to some extent. These uncertainties can only be reduced by a greatly expanded series of measurements of woody surface methane measurements in tropical trees at a range of heights and branch sizes, coupled with fine-scale assessment of small branch surface area.
We further considered forest structure uncertainty introduced through the WAI allometry. We considered variability in TLS-derived WAI across the 60 census plots spanning the tropics, which were used to inform the metric. The weighted mean of the surface area per hectare is 41,176 m2 (the WAI of 4.12 we applied to our estimates). Using Cochrane’s51 formula for variance of a weighted mean, we identify an s.e. of 221 m2 with the 95% confidence interval (1.96 × s.e.) of 433 m2 on either side of the mean, which, when propagated across our upscaling approach, falls well within the broader uncertainties already detailed.
A further uncertainty concerns local hydrological or humidity control of woody surface methanotrophy functioning. We have therefore eliminated water-limited biomes from our low estimates (Extended Data Table 3) and so provide a representative estimate spread that takes into account this uncertainty. Finally, there is some variability in the CH4 exchange behaviour of floodplain trees with respect to hydrology. They act as large point sources of CH4 when inundated, contributing to the comparatively well-known global wetland CH4 source, but our data show they also take up CH4 during the dry season, albeit with orders of magnitude smaller fluxes. Given the small area of tropical floodplain forests versus all tropical and subtropical moist broad-leaved forest (less than 1.5%), their contribution to global CH4 uptake is negligible.
Estimating the CO2 equivalence of methane uptake and comparisons with ecosystem C dynamics
To examine the relative importance of CH4 uptake, we compared it to the C fluxes and stocks of forests. Although metrics such as global warming potential (GWP) have long been used, a recent consensus has developed that expressing CH4 emissions as CO2 equivalent emissions using GWP-100 overstates the effect of constant CH4 emissions on global surface temperature by a factor of 3–4 (ref. 52) while understating the effect of any new CH4 source or sink by a factor of 4–5 over the 20 years following the introduction of the new source (IPCC AR6). A more accurate indication of CO2-we emissions is to equate a constant 1 t yr−1 of CH4 source that is more than 20 years old with 8 t of CO2-we yr−1 but to account for the warming (or cooling) impact of a 1 t of CH4 yr−1 step-change in CH4 emission rate by adding (or subtracting) 120 t of CO2-we yr−1 over the 20 years following the change. Hence, a new constant source (or sink) of CH4 introduced in year 1 is equated with 128 t of CO2-we for years 1–20 and 8 t of CO2-we thereafter53. For direct comparisons with ecosystem C stocks and fluxes, we converted all CO2-we values to C only.
Effects of changing forest area analysis
Tropical deforestation entails the sudden loss of this woody surface CH4 sink, corresponding to a net loss of 128 t of CO2-we per t of CH4 for the first 20 years following deforestation (Methods) or a total ‘stock loss’ of 2,560(20 × 128) t of CO2-we per t of CH4 yr−1 of the CH4 sink. Tropical forest was lost at a rate of 10.3 million ha yr−1 over the period 2002–2018 (ref. 54). This results in 0.59 Mg of CO2-we-C ha−1 yr−1 sink reduction (2.15 Mg of CO2-we ha−1 yr−1) or a total of 6.04 Tg of CO2-we-C from the act of deforestation, a small extra climate impact, dwarfed by the release of biomass C stocks (1 Pg of C yr−1). It remains unknown how quickly a methanotrophic community equivalent to that of a mature forest takes to develop, but, if it develops quickly, the CH4 sink in young secondary forests is likely to be similar to that in mature forests. Hence, the CH4 sink benefits of new forest could manifest much more quickly than the C storage benefits. Assuming a similar woody surface area and CH4 sink per hectare as for mature forests and a CO2-we of 128 for the first 20 year timeframe of interest, the tree CH4 sink would add an extra greenhouse gas mitigation value of 0.131 and 0.586 Mg of CO2-we-C ha−1 yr−1 in temperate and tropical forests, respectively, corresponding to a 7% and 12% extra climate benefit of new trees in these respective biomes.
Online content
Any methods, additional references, Nature Portfolio reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at 10.1038/s41586-024-07592-w.
Supplementary information
Supplementary Table 1, acknowledgements and references.
Source data
Acknowledgements
V.G. acknowledges support from the UK NERC (grant nos. NE/J010928/1 and NE/N015606/1 as part of The Global Methane Budget MOYA consortium) the AXA Research Fund and the Royal Society. S.R.P. acknowledges support from the Royal Society Dorothy Hodgkin Research fellowship (DH160111). A.E.-P. acknowledges support from the Brazilian funding agencies CNPq, CAPES and FAPERJ grants that supported part of the work and the Swedish Research agency Formas (grant number 2021-02429). Y.M. is supported by the Frank Jackson Foundation and the Leverhulme Trust. D.B. acknowledges the European Research Council (ERC; H2020 grant no. 725546), the Swedish Research Council and Formas. Work at the Skogaryd Research station was supported by L. Klemedsson and the Swedish Infrastructure for Ecosystem Science (SITES), funded by the Swedish Research Council (2017-00635). J.B was supported by the Severo Ochoa Program (CEX-2018–000828-S) by the Spanish Ministry of Sciences. E.J.S. was supported by the European Research Council under the European Union’s Seventh Framework Programme (FP/2007-2013)/ ERC grant agreement no. 307888. M.R.A. was supported by the FORCeS and NEGEM projects (H2020 grant nos. 821205 and 869192).
Extended data figures and tables
Extended Data Table 5.
General information of study sites
Mean flux and standard deviation (sd) are calculated from the mean fluxes from each tree after averaging fluxes from different sampling positions above the forest floor. Flux ranges are the maximum and minimum fluxes from any stem height for each location and species.
Author contributions
V.G. conceived the overall study and, together with A.S. and Y.M., integrated the various elements. The Brazil expeditions were planned and organized by S.R.P., V.F., T.S., V.G., D.B. and A.E.-P. B.W. performed the Panamanian and Wytham measurements with input from E.S. and supplementary measurements on oak at Wytham made by C.G. S.R.P. performed the flux measurements in Sweden and the process investigations of methanotrophy with local field planning assistance by D.B. D.E. and N.M. analysed and interpreted the methane isotopes. J.B. performed statistical analyses and synthesis of past studies. A.S. made the tree surface area and CH4 uptake estimates globally and per biome. M.A. made the CO2-we estimates and guided their interpretation. V.G. wrote the manuscript with contributions from S.R.P., B.W., A.S., M.A. and Y.M. and further contributions from the remaining authors. All authors commented on earlier versions of this manuscript.
Peer review
Peer review information
Nature thanks Frank Keppler, Pasi Raumonen and Patrik Vestin for their contribution to the peer review of this work.
Data availability
Methane flux data from all sites included in our analysis are included both in the data files associated with each figure as well as on the UBIRA e-Data repository at 10.25500/edata.bham.00001060. Source data are provided with this paper.
Competing interests
A.S. has formed a company, SelvaFlux, Inc., that intends to commercialize products that benefit from insights and is a named inventor in a pending US patent application on inventions derived from this research. The remaining authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
is available for this paper at 10.1038/s41586-024-07592-w.
Supplementary information
The online version contains supplementary material available at 10.1038/s41586-024-07592-w.
References
- 1.Etminan, M., Myhre, G., Highwood, E. J. & Shine, K. P. Radiative forcing of carbon dioxide, methane and nitrous oxide: a significant revision of the methane radiative forcing. Geophys. Res. Lett.43, 12614–12623 (2016). 10.1002/2016GL071930 [DOI] [Google Scholar]
- 2.Saunois, M. et al. The global methane budget 2000–2017. Earth Syst. Sci. Data12, 1561–1623 (2020). 10.5194/essd-12-1561-2020 [DOI] [Google Scholar]
- 3.Pangala, S. R. et al. Large emissions from floodplain trees close the Amazon methane budget. Nature552, 230 (2017). 10.1038/nature24639 [DOI] [PubMed] [Google Scholar]
- 4.Welch, B., Gauci, V. & Sayer, E. J. Tree stem bases are sources of CH4 and N2O in a tropical forest on upland soil during the dry to wet season transition. Glob. Change Biol.25, 361–372 (2019). 10.1111/gcb.14498 [DOI] [PubMed] [Google Scholar]
- 5.Sundqvist, E., Crill, P., Mölder, M., Vestin, P. & Lindroth, A. Atmospheric methane removal by boreal plants. Geophys. Res. Lett.10.1029/2012GL053592 (2012).
- 6.Machacova, K. et al. Trees as net sinks for methane (CH4) and nitrous oxide (N2O) in the lowland tropical rain forest on volcanic Réunion Island. New Phytol.229, 1983–1994 (2021). 10.1111/nph.17002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Le Mer, J. & Roger, P. Production, oxidation, emission and consumption of methane by soils: a review. Eur. J. Soil Biol.37, 25–50 (2001). 10.1016/S1164-5563(01)01067-6 [DOI] [Google Scholar]
- 8.Lohila, A. et al. Large contribution of boreal upland forest soils to a catchment-scale CH4 balance in a wet year. Geophys. Res. Lett.43, 2946–2953 (2016). 10.1002/2016GL067718 [DOI] [Google Scholar]
- 9.Murguia-Flores, F., Ganesan, A. L., Arndt, S. & Hornibrook, E. R. C. Global uptake of atmospheric methane by soil from 1900 to 2100. Glob. Biogeochem. Cycles35, e2020GB006774 (2021).
- 10.Gauci, V., Gowing, D. J. G., Hornibrook, E. R. C., Davis, J. M. & Dise, N. B. Woody stem methane emission in mature wetland alder trees. Atmos. Environ.44, 2157–2160 (2010). 10.1016/j.atmosenv.2010.02.034 [DOI] [Google Scholar]
- 11.Pangala, S. R., Moore, S., Hornibrook, E. R. C. & Gauci, V. Trees are major conduits for methane egress from tropical forested wetlands. New Phytol.197, 524–531 (2013). 10.1111/nph.12031 [DOI] [PubMed] [Google Scholar]
- 12.Pangala, S. R., Hornibrook, E. R. C., Gowing, D. J. & Gauci, V. The contribution of trees to ecosystem methane emissions in a temperate forested wetland. Glob. Change Biol.21, 2642–2654 (2015). 10.1111/gcb.12891 [DOI] [PubMed] [Google Scholar]
- 13.Jeffrey, L. C., Maher, D. T., Tait, D. R., Euler, S. & Johnston, S. G. Tree stem methane emissions from subtropical lowland forest (Melaleuca quinquenervia) regulated by local and seasonal hydrology. Biogeochemistry151, 273–290 (2020). 10.1007/s10533-020-00726-y [DOI] [Google Scholar]
- 14.Sjögersten, S. et al. Methane emissions from tree stems in neotropical peatlands. New Phytol.225, 769–781 (2020). 10.1111/nph.16178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Terazawa, K., Takeshi, T., Tadashi, S., Kenji, Y. & Ishizuka, S. Seasonal and weather-related controls on methane emissions from the stems of mature trees in a cool-temperate forested wetland. Biogeochemistry156, 211–230 (2021). 10.1007/s10533-021-00841-4 [DOI] [Google Scholar]
- 16.Putkinen, A. et al. New insight to the role of microbes in the methane exchange in trees: evidence from metagenomic sequencing. New Phytol.231, 524–536 (2021). 10.1111/nph.17365 [DOI] [PubMed] [Google Scholar]
- 17.Feng, H. et al. Methane emissions may be driven by hydrogenotrophic methanogens inhabiting the stem tissues of poplar. New Phytol.233, 182–193 (2022). 10.1111/nph.17778 [DOI] [PubMed] [Google Scholar]
- 18.Van Aken, B., Peres, C. M., Doty, S. L., Yoon, J. M. & Schnoor, J. L. Methylobacterium populi sp. nov., a novel aerobic, pink-pigmented, facultatively methylotrophic, methane-utilizing bacterium isolated from poplar trees (Populus deltoides x nigra DN34). Int. J. Syst. Evol. Microbiol.54, 1191–1196 (2004). 10.1099/ijs.0.02796-0 [DOI] [PubMed] [Google Scholar]
- 19.Jeffrey, L. C. et al. Bark-dwelling methanotrophic bacteria decrease methane emissions from trees. Nat. Commun.12, 2127 (2021). [DOI] [PMC free article] [PubMed]
- 20.Gauci, V. et al. Non-flooded riparian Amazon trees are a regionally significant methane source. Philos. Trans. R. Soc. A380, 20200446 (2021). 10.1098/rsta.2020.0446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meir, P. et al. in Plant Respiration: Metabolic Fluxes and Carbon Balance (eds Tcherkez, G. & Ghashghaie, J.) 89–105 (Springer, 2017).
- 22.Siegenthaler, A. et al. Technical note: semi-rigid chambers for methane gas flux measurements on tree stems. Biogeosciences13, 1197–1207 (2016). 10.5194/bg-13-1197-2016 [DOI] [Google Scholar]
- 23.Ernst, L. et al. Methane formation driven by reactive oxygen species across all living organisms. Nature603, 482–487 (2022). 10.1038/s41586-022-04511-9 [DOI] [PubMed] [Google Scholar]
- 24.Kohl, L. et al. Radiation and temperature drive diurnal variation of aerobic methane emissions from Scots pine canopy. Proc. Natl Acad. Sci. USA120, e2308516120 (2023). 10.1073/pnas.2308516120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gorgolewski, A. S., Caspersen, J. P., Vantellingen, J. & Thomas, S. C. Tree foliage is a methane sink in upland temperate forests. Ecosystems26, 174–186 (2023). 10.1007/s10021-022-00751-y [DOI] [Google Scholar]
- 26.Hubau, W. et al. Asynchronous carbon sink saturation in African and Amazonian tropical forests. Nature579, 80–87 (2020). 10.1038/s41586-020-2035-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith, M. A., Cain, M. & Allen, M. R. Further improvement of warming-equivalent emissions calculation. Clim. Atmos. Sci.4, 19 (2021). 10.1038/s41612-021-00169-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Inoue, A., Koyama, R., Koshikawa, K. & Yamamoto, K. Comparison of models for estimating stem surface area of coniferous trees grown in old-growth natural forests. J. For. Res.26, 1–6 (2020). 10.1080/13416979.2020.1847818 [DOI] [Google Scholar]
- 29.Tíscar, P. A. & Lucas-Borja, M. E. Structure of old-growth and managed stands and growth of old trees in a Mediterranean Pinus nigra forest in southern Spain. For. Int. J. For. Res.89, 201–207 (2016). [Google Scholar]
- 30.Griscom, B. W. et al. Natural climate solutions. Proc. Natl Acad. Sci. USA114, 11645–11650 (2017). 10.1073/pnas.1710465114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cook-Patton, S. C. et al. Mapping carbon accumulation potential from global natural forest regrowth. Nature585, 545 (2020). 10.1038/s41586-020-2686-x [DOI] [PubMed] [Google Scholar]
- 32.Baird, A. J. et al. CH4 flux from peatlands: a new measurement method. Ecohydrology3, 360–367 (2010). 10.1002/eco.109 [DOI] [Google Scholar]
- 33.Sioli, H. The effects of deforestation in the Amazonia. Geogr. J.151, 197–203 (1985). 10.2307/633533 [DOI] [Google Scholar]
- 34.Mayorga, E. & Aufdenkampe, A. in The Ecohydrology of South American Rivers and Wetlands (ed. McClain, M. E.) Ch. 1 (IAHS AISH, 2002).
- 35.Alvares, C. A. et al. Köppen’s climate classification map for Brazil. Meteorol. Z.22, 711–728 (2013). 10.1127/0941-2948/2013/0507 [DOI] [Google Scholar]
- 36.Sayer, E. J., Tanner, E. V. J. & Lacey, A. L. Effects of litter manipulation on early-stage decomposition and meso-arthropod abundance in a tropical moist forest. For. Ecol. Manag.229, 285–293 (2006). 10.1016/j.foreco.2006.04.007 [DOI] [Google Scholar]
- 37.Sayer, E. J. & Tanner, E. V. J. Experimental investigation of the importance of litterfall in lowland semi-evergreen tropical forest nutrient cycling. J. Ecol.98, 1052–1062 (2010). 10.1111/j.1365-2745.2010.01680.x [DOI] [Google Scholar]
- 38.Leight, E. G. Tropical Forest Ecology: A View from Barro Colorado Island (Oxford Univ. Press, 1999).
- 39.Lopez-Sangil, L. et al. The automated root exudate system (ARES): a method to apply solutes at regular intervals to soils in the field. Methods Ecol. Evol.8, 1042–1050 (2017). 10.1111/2041-210X.12764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bréchet, L. M. et al. Distinct responses of soil respiration to experimental litter manipulation in temperate woodland and tropical forest. Ecol. Evol.8, 3787–3796 (2018). 10.1002/ece3.3945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fenn, K. M., Malhi, Y. & Morecroft, M. D. Soil CO2 efflux in a temperate deciduous forest: environmental drivers and component contributions. Soil Biol. Biochem.42, 1685–1693 (2010). 10.1016/j.soilbio.2010.05.028 [DOI] [Google Scholar]
- 42.Lindroth, A. & Crill, P. in Forest Hydrology and Biogeochemistry (eds Levia, D. F. et al.) 321–339 (Springer, 2011).
- 43.Shenkin, A. et al. Tree surface area allometry. Preprint at bioRxiv10.1101/2024.04.23.590783 (2024).
- 44.Raumonen, P. et al. Fast automatic precision tree models from terrestrial laser scanner data. Remote Sens.5, 491–520 (2013). 10.3390/rs5020491 [DOI] [Google Scholar]
- 45.Shenkin, A. treestruct: R package for analysis and manipulation of tree structure models. GitHub www.github.com/ashenkin/treestruct (2020).
- 46.Pedersen, E. J., Miller, D. L., Simpson, G. L. & Ross, N. Hierarchical generalized additive models in ecology: an introduction with mgcv. PeerJ7, e6876 (2019). 10.7717/peerj.6876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Anderson‐Teixeira, K. J. et al. CTFS‐Forest GEO: a worldwide network monitoring forests in an era of global change. Glob. Change Biol.21, 528–549 (2015). 10.1111/gcb.12712 [DOI] [PubMed] [Google Scholar]
- 48.Tuanmu, M. N. & Jetz, W. A global 1-km consensus land-cover product for biodiversity and ecosystem modelling. Glob. Ecol. Biogeogr.23, 1031–1045 (2014). 10.1111/geb.12182 [DOI] [Google Scholar]
- 49.DiMiceli, C. et al. MOD44B MODIS/Terra Vegetation Continuous Fields Yearly L3 Global 250 m SIN Grid V006. NASA EOSDIS Land Processes DAAC (USGS), (2015); 10.5067/MODIS/MOD44B.006.
- 50.Hersbach, H. et al. The ERA5 global reanalysis. Q. J. R. Meteorol. Soc.146, 1999–2049 (2020). 10.1002/qj.3803 [DOI] [Google Scholar]
- 51.Cochrane, W. G. Sampling Techniques (John Wiley and Sons, 1977).
- 52.Lynch, J., Cain, M., Pierrehumbert, R. & Allen, M. Demonstrating GWP*: a means of reporting warming-equivalent emissions that captures the contrasting impacts of short- and long-lived climate pollutants. Environ. Res. Lett.15, 044023 (2020). 10.1088/1748-9326/ab6d7e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cain, M. et al. Comment on ‘Unintentional unfairness when applying new greenhouse gas emissions metrics at country level’. Environ. Res. Lett. 16, 068001 (2021).
- 54.Hansen, A. et al. Global humid tropics forest structural condition and forest structural integrity maps. Sci. Data6, 232 (2019). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1, acknowledgements and references.
Data Availability Statement
Methane flux data from all sites included in our analysis are included both in the data files associated with each figure as well as on the UBIRA e-Data repository at 10.25500/edata.bham.00001060. Source data are provided with this paper.