Summary
Systemic cryptococcosis is often fatal even with the current antifungal therapy and there is no vaccine available. Induction therapy with amphotericin B (AmB) is essential for its treatment, which can be either in the form of AmB deoxycholate at 1 mg/kg/day for 7 days or a single dose of liposomal AmB (AmB-LLs) at 10 mg/kg, both in combination with flucytosine. AmB is highly toxic and it is imperative to further increase its efficacy without increasing its toxicity. Previously, we developed a targeted antifungal drug delivery system (DectiSome) that uses liposomes decorated with host-pathogen receptor dectins to target AmB to fungal cells. Here, we showed that a single dose of Dectin-2 coated liposomal AmB, relative to AmB-LLs, reduced fungal burden and prolonged animal survival in the murine model of systemic cryptococcosis. Our results demonstrate that DectiSomes are a promising antifungal delivery system that could improve cryptococcosis therapy in the future.
Subject areas: pharmacology, natural sciences, biological sciences, microbiology, medical microbiology, mycology
Graphical abstract
Highlights
-
•
Dectisomes bind cryptococcal cells more efficiently than untargeted liposomes
-
•
Dec2-AmB-LLs effectively reduced fungal burden in the systemic cryptococcosis model
-
•
Dec2-AmB-LLs prolonged animal survival in the systemic cryptococcosis model
Pharmacology; Natural sciences; Biological sciences; Microbiology; Medical Microbiology; Mycology.
Introduction
Cryptococcal meningitis is fatal without treatment. The most common perpetrator of this disease is the environmental fungus − Cryptococcus neoformans. Exposure to this fungus through the inhalation of desiccated yeasts or spores is universal.1,2,3,4,5,6 In immunocompetent individuals, this organism typically remains dormant in the lungs or gets cleared. However, individuals with chronic lung diseases or impaired pulmonary histiocytes can develop primary pulmonary cryptococcosis.7,8,9,10,11,12 Primary pulmonary cryptococcosis is predominately found in the form of granulomas or masses, and can evolve into systemic cryptococcosis.7,9,10,11,13 Systemic cryptococcosis occurs most frequently in immunocompromised individuals such as patients with AIDS or transplant recipients taking immunosuppressive drugs. Once this fungus disseminates from the lungs, it invariably penetrates the central nervous system, causing the deadly cryptococcal meningoencephalitis.5,6,14,15,16,17,18,19,20,21,22 The World Health Organization (WHO) estimated approximately 112,000 cryptococcal-related deaths each year, with C. neoformans alone responsible for 19% of AIDS-related deaths.23,24 By the time patients are diagnosed with cryptococcal meningitis, they often carry a high fungal load in the brain with a median of 104-105 yeast cells/mL in their cerebrospinal fluid (CSF).23,25 The challenges of preventing and managing this fungal disease prompted the WHO to list C. neoformans as a critical fungal pathogen that urgently needs to be controlled.
The management of cryptococcosis relies on antifungal drug therapy. Patients diagnosed with cryptococcal meningitis are recommended to receive amphotericin B (AmB) deoxycholate intravenously infused for two to 6 h per day for 7 to 14 days. AmB binds to ergosterol and disrupts the integrity of fungal cell membrane; however, it also has an affinity to the host cholesterol, leading to serious toxic effects such as anaphylaxis, anemia, hypomagnesemia, hypokalaemia, and nephrotoxicity.26 Therefore, patients receiving AmB must be hospitalized and monitored closely. Consequently, AmB is only used in induction therapy, and it is unsuitable for long-term use.27 Despite these limitations, AmB remains the most effective and the only fungicidal drug to treat cryptococcosis since the 1950s. To lessen AmB nephrotoxicity, a safer formulation, liposomal AmB (AmB-LLs, AmBisome), was developed.28 AmB-LLs have a longer plasma lifetime29,30 and are less toxic per dose of AmB,29 but they require higher doses. In 2018, the WHO recommended induction therapy using 1 mg/kg of AmB deoxycholate or 3–10 mg/kg of AmB-LLs intravenously infused for two to 6 h for 7 to 14 days. It is worth noting that patients with AIDS need to be treated for cryptococcal meningitis first before the initialization of antiretroviral therapy to prevent cryptococcal immune reconstitution inflammatory syndrome (C-IRIS).23,27 The challenges associated with the antifungal treatment and the financial burden associated with a lengthy hospital stay present a major barrier to managing this fungal disease, particularly in resource-limited settings.23,27,31 The latter motivated the recent clinical trials to shorten AmB therapy.25,32 These trials demonstrate that a single-dose AmB-LLs (10 mg/kg) with either flucytosine (100 mg/kg/day) or fluconazole (800–1200 mg/day) showed significantly fewer adverse effects without compromising treatment efficacy compared to AmB of 1 mg/kg/day coupled with flucytosine (100 mg/kg/day) for 7 days, followed by fluconazole (1,200 mg/day) for 7 days. These new results prompted the WHO to update their treatment guideline in 2022, promoting the use of single-dose AmB-LLs.26 Despite these improvements, the 10-week mortality rates of patients with cryptococcal meningitis receiving the recommended AmB-LLs treatment are still above 25%.25
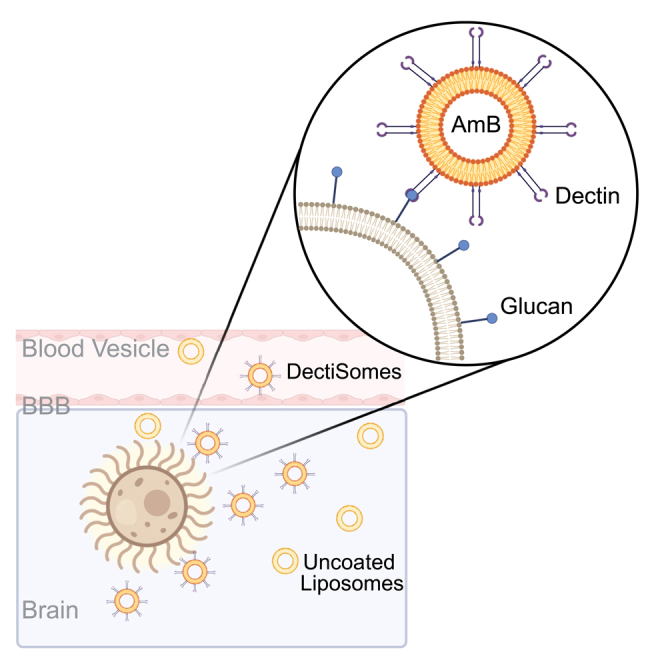
One approach to further increase AmB efficacy is targeted delivery of the drug to the fungus itself and away from the host. For that purpose, we developed dectin-decorated liposomal AmB (DectiSomes). Dectins are C-type lectin receptor proteins (CLRs) expressed primarily by dendritic cells and other lymphoid cell types.33 These host receptors recognize oligoglycans embellished on the fungal cell surface or extracellular matrix and initiate an immune response.34,35,36,37 For instance, Dectin 1 recognizes β-glucans, while Dectin 2 and Dectin 3 recognize α-mannans, both of which are highly abundant in the majority of pathogenic fungi.38,39,40 Previously, we demonstrated that relative to control liposomal amphotericin B (AmB-LLs), Dec2-AmB-LLs is effective in reducing the fungal burden and prolonging animal survival in pulmonary aspergillosis, systemic candidiasis, and pulmonary mucormycosis murine models.33,37,41,42,43,44 We have yet to test the effectiveness of DectiSomes in treating cryptococcosis as this disease presents unique challenges: 1. Cryptococcus is naturally resistant to the newer echinocandin class of antifungals. Therefore, AmB is essential for the treatment of cryptococcosis. 2. Because meningitis is the most common clinical manifestation of cryptococcosis, the antifungal drug needs to penetrate the brain in addition to other body sites. 3. Failure of the eradication of the fungus results in high relapse frequency, and approximately 90% of relapse cases are caused by the original isolates.45 Because mannans presented in the capsule are more accessible than β-glucans in the cell wall, we opted to assess if DectiSomes, specifically Dec2-AmB-LLs and Dec3-AmB-LLs, can outperform AmB-LLs in animal models of pulmonary cryptococcosis and systemic cryptococcosis.
Results
DectiSomes bind to cryptococcus cells efficiently in vitro
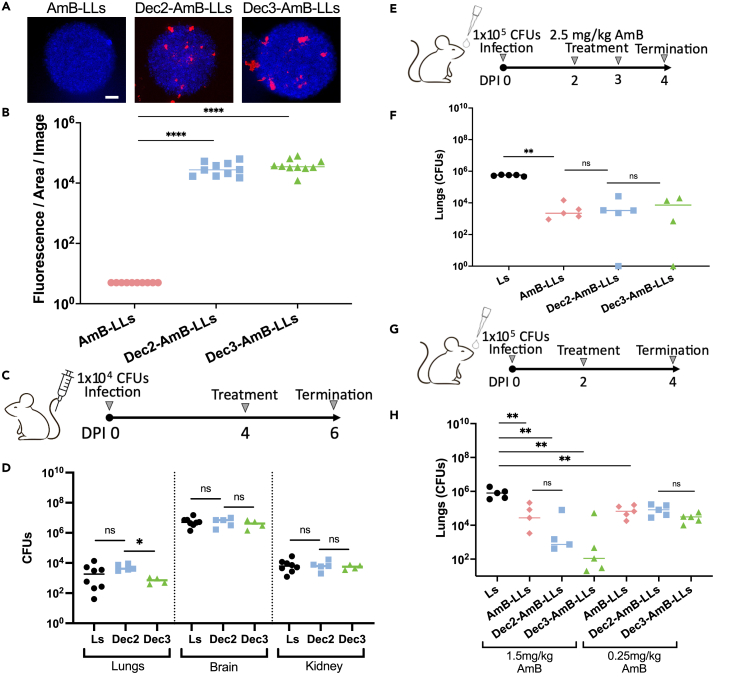
We postulate that for DectiSomes to be more effective than the control AmB-LLs against Cryptococcus, they must be able to recognize the fungus more efficiently. To that end, we measured liposomal binding affinity to cryptococcal colonies grown in vitro on the surface of agar using DectiSomes and the control AmB-LLs that carry a red fluorescent dye rhodamine (Figure 1A). Based on fluorescent images, both Dec2-AmB-LLs and Dec3-AmB-LLs bound strongly to cryptococcal colonies relative to AmB-LLs (Figure 1A), and the latter gave little if any detectable rhodamine signal. The uneven distribution of fluorescence is not unexpected given our previous findings that these DectiSomes more often bind to patches of extracellular matrix polysaccharides than to the cell wall. Further, the liposomes we use are too big (∼100 nm) to easily penetrate the fungal cell wall.42,43 Quantifying the area of fluorescent liposome binding from multiple images revealed that Dec2-AmB-LLs and Dec3-AmB-LLs bound 6,500-fold (p = 8.1 × 10−6) and 8,300-fold (p = 2.5 × 10−6) larger area than the control AmB-LLs, respectively (Figure 1B).
Figure 1.
DectiSomes display a higher affinity to Cryptococcus neoformans and are effective against pulmonary cryptococcosis
(A) All liposomes carried the fluorescent dye rhodamine (red). Cryptococcal cells in colonies grown on the surface of agar were stained with calcofluor white (blue). DectiSomes (Dec2-AmB-LLs and Dec3-AmB-LLs) bound to a larger area of the fungal colonies in comparison to AmB-LLs. Scale bar: 20 μm.
(B) Quantification of rhodamine fluorescent signals of images like panel A indicating more efficient binding of DectiSomes in comparison to undecorated liposomes (N = 10 images). Student’s t test (∗∗∗∗ p-value:<0.0001).
(C) Diagram of the intravenous infection and intravenous treatment regimen with empty liposomes for panel D.
(D) Fungal burden of the lungs, brain, and kidney for the 5 mice treated with either empty Ls, empty Dec2-LLs, or empty Dec3-LLs (i.e., lacking AmB).
(E) Diagram of the intranasal infection and two rounds of the intranasal treatment regimen for panel F.
(F) Lung fungal burdens for the 5 mice treated with either Ls, AmB-LLs, Dec2-AmB-LLs, or Dec3-AmB-LLs. There were significant differences between the negative control Ls and the AmB-containing groups, but no significant difference between AmB-containing groups.
(G) Diagram of the intranasal infection and intranasal treatment regimen for panel H.
(H) Lung fungal burdens for the 5 mice treated with either Ls, AmB-LLs, Dec2-AmB-LLs, or Dec3-AmB-LLs. There were significant differences between the negative control and the AmB-containing groups. D, F, and H: Mann-Whitney-Wilcoxon test (∗∗∗p=<0.001; ∗∗p=<0.01; ∗p=<0.05; ns: not significant).
DectiSomes without amphotericin B have no significant antifungal activity
Since dectins have the capacity to trigger an immune response, we asked if DectiSomes without the antifungal drug AmB promote the host’s antifungal activity. To answer this question, we first infected mice intravenously with cryptococcal cells at 1 × 104 cells per mouse. On day four post-infection (DPI 4), we administered plain liposomes lacking a targeting protein (Ls), Dec2-Ls, and Dec3-Ls intravenously to the infected mice. At DPI 8, we terminated the experiment, dissected the brains, lungs, and kidneys, and measured the fungal burden in these tissues (Figure 1C). In the brains, the fungal burdens for the control Ls, Dec2-Ls, and Dec3-Ls were indistinguishable (median 5.0 × 106 CFU, 7.0 × 106 CFU, and 4.2 × 106 CFU respectively with no statistically significant difference). Likewise, the fungal burdens in the kidneys were comparable among the control Ls, Dec2-Ls, and Dec3-Ls (median 6.2 × 103 CFU, 6.2 × 103 CFU, and 5.7 × 103 CFU respectively). We observed much lower and more varied fungal burdens in the lungs in this systemic infection model (median for the control Ls, Dec2-Ls, and Dec3-Ls being 1.84 × 103 CFU, 4.2 × 103 CFU, and 0.72 × 103 CFU, respectively) (Figure 1D). This result suggests that empty Dectin-coated-liposomes themselves do not induce any significant antifungal activity in the host.
Untargeted liposomal amphotericin B effectively treated pulmonary cryptococcosis at 2.5 mg/kg amphotericin B
The natural route of Cryptococcus infection is through inhalation, and the lungs are the initial site of infection. However, pulmonary cryptococcosis is often underdiagnosed, which can turn deadly when the fungus disseminates. A study shows that 66.7% of immunocompetent individuals with pulmonary cryptococcosis had an infection disseminated to the central nervous system at the time of diagnosis.46 To study the efficacy of DectiSomes in treating Cryptococcus pneumonia, we infected mice intranasally with cryptococcal cells at 1 × 105 CFU per animal. Given the much more rapid metabolism of mice relative to humans,47 we decided to treat the infected mice twice intranasally− once at DPI 2 and another time at DPI 3 with Ls, AmB-LLs, Dec2-AmB-LLs, or Dec3-AmB-LLs. Because the typical dose of liposomal AmB used in murine models ranges from 3 to 20 mg/kg/day,48 we decided to use a slightly lower AmB dose: 2.5 mg/kg. We terminated the experiment at DPI 4 and measured the lung fungal burden (Figure 1E). As expected, the control group receiving Ls showed a high fungal burden in the lungs, with a median fungal burden of 5.45 × 105 CFU (Figure 1F). In comparison, groups receiving AmB-LLs, Dec2-AmB-LLs, and Dec3-AmB-LLs showed over 100-fold reduction in the fungal load, with the median fungal burden 3.05 × 103 CFU, 3.35 × 103 CFU, and 8.35 × 103 CFU, respectively (Figure 1F). Larger variations were observed in these AmB-LL treatment groups where fungal burdens were relatively low. There were no statistically significant differences among the groups, suggesting that DectiSomes with AmB have at least comparable antifungal activity as AmB-LLs in this pulmonary cryptococcosis model. As we wondered if a single dose treatment was sufficient in this model, we included a small sample of one or two mice per group that were treated only once at DPI 2. We did not see much difference in fungal burden between mice treated with one dose and those treated with two doses (Figure S1). This suggests that one dose of DectiSomes or AmB-LLs is sufficient in reducing the fungal burden at this magnitude in mice. However, we could not distinguish AmB-LLs from DectiSomes using this dose and regimen.
Lowering the dose of amphotericin B revealed better efficacy of DectiSomes compared to liposomal amphotericin B in treating pulmonary cryptococcosis
Based on our previous experience in candidiasis or aspergillosis models, it was challenging to determine the superiority of DectiSomes to AmB-LLs when AmB dosage was high.41,44 Therefore, we repeated the pulmonary cryptococcosis experiment with doses of AmB lower than 2.5 mg/kg. Here, we administered Ls, AmB-LLs, Dec2-AmB-LLs, or Dec3-AmB-LLs intranasally into the cryptococcal infected mice at DPI 2 with AmB at 1.5 mg/kg or 0.25 mg/kg. We terminated the experiment at DPI 4 and measured the fungal burden (Figure 1G). In the negative control group that received Ls, the median fungal load of the lungs was 7.95 × 105 CFU, similar to the previous experiment (Figure 1H). The groups of mice receiving AmB-LLs, Dec2-AmB-LLs, and Dec3-AmB-LLs with the exceptionally low dose of AmB (0.25 mg/kg) showed a statistically significant 10-fold reduction in fungal burden compared to mice treated Ls, with median values of 6.69 × 104 CFU, 8.2 × 104 CFU, and 3.1 × 104 CFU, respectively (Figure 1H).
For the groups of mice that received AmB at 1.5 mg/kg, the median fungal load of the lungs for AmB-LLs, Dec2-AmB-LLs, and Dec3-AmB-LLs were 27 × 103 CFU, 0.74 × 103 CFU, and 0.11 × 103 CFU respectively (Figure 1H). Remarkably, the groups receiving DectiSomes containing AmB at 1.5 mg/kg showed a 300 to 500-fold or more reduction in fungal burden compared to the negative control liposomal group and a modest reduction in fungal burden compared to the AmB-LLs treatment group (Figure 1H). As DectiSomes can significantly reduce the fungal load in mice with pulmonary cryptococcosis, we then tested the efficacy of DectiSomes against systemic cryptococcosis.
Dec2-liposomal amphotericin B are more effective than liposomal amphotericin B in lowering fungal burden in all organs examined in the systemic cryptococcosis model
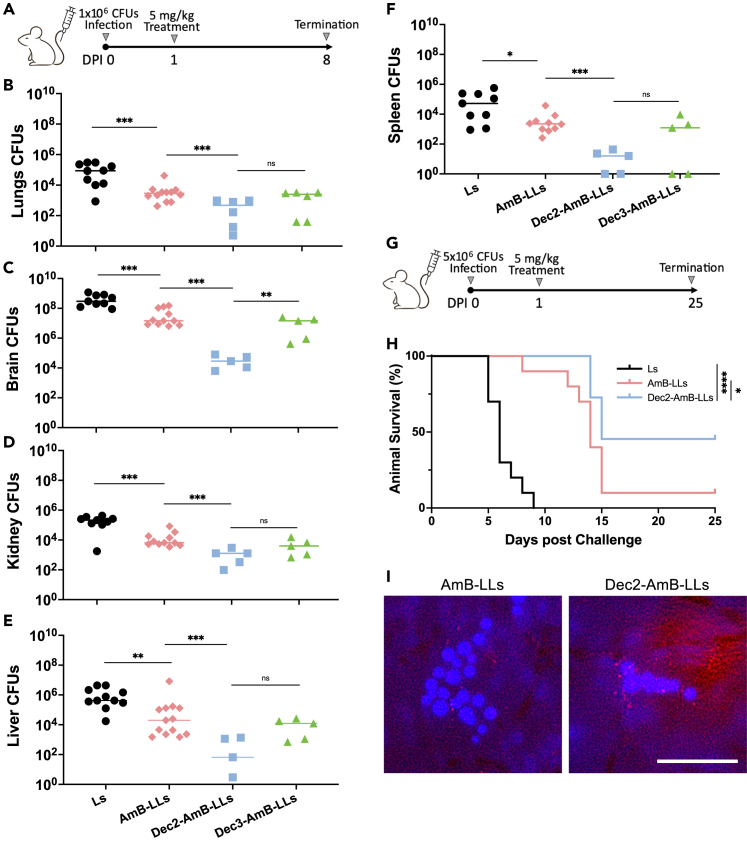
For individuals who are immunocompromised due to HIV infection or immune-suppressing therapies because of organ transplant or cancer, this fungus often disseminates extrapulmonarily through the bloodstream. C. neoformans can infect almost any organ, with a predisposition to the brain. As systemic cryptococcosis or cryptococcal meningitis has the most devastating impact on public health, we decided to examine whether DectiSomes will be effective in a systemic cryptococcal infection model. To do so, we infected mice with cryptococcal cells intravenously at the inoculum of 1 × 106 CFU/animal. At 24 h post-infection, we treated the mice with Ls, AmB-LLs, Dec2-AmB-LLs, or Dec3-AmB-LLs at the dose of 5 mg/kg of AmB intravenously. We chose 5 mg/kg because this is one of the lower doses used in previous liposomal AmB studies in mice with systemic cryptococcosis.29 The infected mice were monitored until DPI 8 (Figure 2A). As expected, mice receiving Ls became moribund prior to DPI 8, with a median survival of only 6 days (Figure 2H and data not shown). Mice in the other treatment groups survived and were terminated at DPI 8. Because different organs could accumulate different levels of AmB after AmB-LLs treatment,49 we measured the fungal burden in five organs: brains, lungs, livers, spleens, and kidneys. As expected, the negative control mice receiving Ls showed the highest fungal burden in the brains (293 × 105 CFU), followed by the liver (4.23 × 105), kidneys (2.15 × 105 CFU), lungs (0.875 × 105 CFU), and spleen (0.524 × 105) at the time of euthanization (Figures 2B–2F). Treatment with AmB-LLs provided a 10-fold reduction in fungal burden in all organs examined at DPI 8. Dec3-AmB-LLs performed similarly to AmB-LLs. Remarkably, mice treated with Dec2-AmB-LLs provided a further reduction in fungal burden in all organs examined, with another 100-fold reduction in the brains even compared to mice receiving AmB-LLs (Figures 2B–2F). This result indicates that Dec2-AmB-LLs is the most effective therapy for treating systemic cryptococcosis infections.
Figure 2.
Dec2-AmB-LLs lower fungal burden and extend the life expectancy in mice with systemic cryptococcosis compared to AmB-LLs
(A) Diagram of the intravenous infection and intravenous treatment regimen for panels B-F.
(B–F) Fungal burden of the lungs, brain, kidney, liver, and spleen for the 11 mice treated with Ls or AmB-LLs, and the 5 mice treated with Dec2-AmB-LLs or Dec3-AmB-LLs. Fungal burdens for the 11 mice in the negative control group were obtained at the time of euthanization when infected mice became moribund. The data for all the other groups were obtained from surviving mice at DPI 8.
(G) Diagram of the intravenous infection and intravenous treatment regimen for animal survival experiment in panel H.
(H) Survival curve for mice with systemic cryptococcosis when treated with Ls, AmB-LLs, Dec2-AmB-LLs, and Dec3-AmB-LLs intravenously (10 mice per group).
(I) Coronal sections of the brain revealed red fluorescent Dec2-AmB-LLs concentrated in the vicinity of calcofluor white stained fungal cells, while AmB-LLs were only rarely observed near fungal cells. A scale bar (50 microns) indicates the degree of magnification. (B–F) Mann-Whitney-Wilcoxon test (∗∗∗p=<0.001; ∗∗p=<0.01; ∗p=<0.05; ns: not significant). (H) Log-rank test (∗∗∗p=<0.001; ∗∗p=<0.01; ∗p=<0.05; ns: not significant).
Dec2-liposomal amphotericin B prolonged animal survival
To determine if the dramatic effect of Dec2-AmB-LLs on fungal burden reduction translates into improved animal survival, we performed a survival experiment comparing the efficacy of Dec2-AmB-LLs to AmB-LLs. Here, we infected mice intravenously with C. neoformans at the inoculum of 5 × 106 CFU/animal. At 24 h post-infection, we treated the mice intravenously with Ls, AmB-LLs, or Dec2-AmB-LLs at the dose of 5 mg/kg of AmB. The infected mice were monitored for 25 days (Figure 2G). Consistent with our earlier data, the negative control mice had a median survival of 6 days (Figure 2H). The AmB-LLs group had a median survival of 14 days. Mice treated with Dec2-AmB-LLs had a prolonged median survival of 18.5 days (Figure 2H). Additionally, more mice survived at DPI 25 when treated with Dec2-AmB-LLs in comparison to AmB-LLs (Figure 2H).
Previously we had observed that relative to untargeted AmB-LLs, more DectiSomes accumulated in association with Aspergillus fumigatus colonies in the lung sections44 and Candida albicans colonies in the kidneys.41 When we examined fresh sections of brains of C. neoformans infected mice at 24 h post treatment with either AmB-LLs or Dec2-AmB-LLs, we observed more red fluorescent Dec2-AmB-LLs accumulating in association with cryptococcal cells than the AmB-LLs (Figure 2I).
Collectively, our data indicate that Dec2-AmB-LLs are more effective than AmB-LLs in both pulmonary and systemic cryptococcosis in animal models. We hope that this promising drug delivery platform of using Dec2-AmB-LLs can translate to shortened hospital stays, improved fungal clearance, and reduced mortality rates in patients with cryptococcal meningitis in the future.
Discussion
Cryptococcosis is a deadly disease without treatment, therefore it is pertinent to find a successful antifungal therapy with limited side effects. Currently, the first line of treatment against this deadly disease is with the use of amphotericin B. AmB is effective, but is also highly toxic as it targets cholesterol to a lesser extent. To minimize the host toxicity of AmB, liposomal amphotericin B (AmB-LLs, AmBisome) was developed. However, even with AmB-LLs, the efficacy of the drug is still unsatisfactory. Therefore, our group has developed a targeted drug delivery platform using host dectin-coated liposomes loaded with amphotericin B, known as DectiSomes. To test the efficacy of this targeted drug delivery against cryptococcal infection, we turned our attention to the pulmonary and systemic cryptococcosis murine models. Unlike aspergillosis and candidiasis, cryptococcosis is difficult to treat with AmB in mouse models.
Previously, we have shown that DectiSomes most often bind to extracellular matrix materials than to the cell wall in this and other fungal species. In Figure 1A, the agar plugs were 8 mm in diameter and contained many colonies, each of ∼100 μm, and the various DectiSomes bind to the somewhat randomly distributed exopolysaccharide matrix as we expected. Nevertheless, our in vitro and in vivo data showed that DectiSomes bind more efficiently to regions where fungal cells are than naked liposomal AmB. We found infected mice treated with empty liposomes or with empty DectiSomes succumbed to cryptococcosis similarly as infected mice without any treatment based on our previous work,50 with similar fungal burdens in the lungs, brains, and kidneys. Thus, it is reasonable to conclude that empty liposomes or empty dectin-coated liposomes do not provide any significant antifungal activity. When mice with pulmonary cryptococcosis were treated with DectiSomes, we saw at least a 100-fold reduction in the fungal burden compared to untreated mice, and a higher reduction compared to AmB-LLs treated mice. Interestingly, when treated mice with two doses within a close interval, we did not observe an obvious increase in antifungal efficacy compared to the one dose regimen. This was surprising to us at that time but it is not unexpected in retrospect. In the clinical setting, it has been shown that one week of liposomal AmB treatment is not inferior to two weeks of liposomal AmB treatment.49 That result led to the revised treatment guidelines to shorten the duration of AmB treatment. Now one high dose of liposomal AmB used in the clinical trial has been promoted as the preferred treatment with benefits of reduced host toxicity, reduced hospital stay, and increased treatment feasibility for many patients. It is possible that DectiSomes could further improve the treatment outcome in the future.
Delivering drugs across BBB has always been a challenge. Although liposomes are more flexible than other types of nanoparticles because they could deform and potentially squeeze through the intercellular space, liposomal delivery of AmB to the brain is at one or two orders of magnitude lower levels than other organs such as liver, kidney, or lung.29,51,52,53,54,55 Consequently, AmB at 5 mg/kg or higher (10 or 20 mg/kg) are typically used in mouse models to treat systemic cryptococcosis.29,49 Here, we used AmB at 5 mg/kg. We found that mice treated with Dec2-AmB-LLs at this dose had a significantly lower fungal burden (10–100-fold) compared to those treated with AmB-LLs. It is particularly exciting that DectiSomes are more effective in reducing fungal burden in the brain. Moreover, mice treated with Dec2-AmB-LLs had longer survival. Collectively, our findings indicate that DectiSomes are not inferior to AmB-LLs in the pulmonary cryptococcosis model and they are superior to AmB-LLs against systemic cryptococcosis. Given that meningitis is the fatal clinical manifestation of systemic cryptococcosis, DectiSomes can be a promising method of targeted drug delivery against cryptococcal meningitis to improve the outcome of antifungal therapy.
Limitations of the study
-
(1)
In this study, 10 or fewer mice were used each group for survival experiments and five mice in general were used for fungal burden experiments. Given the variations that we observed among individual mice, increasing the number of mice per group would improve our power in decerning the differences between treatment groups. This will demand a scale up of our production of DectiSomes and AmB-LLs, a goal of our future endeavors.
-
(2)
The liposomes used here and in previous studies are approximately 100 μm in diameter, which may not be ideal to deliver AmB across the blood-brain barrier. Several studies have shown that after the intravenous administration of liposomal AmB, the AmB concentrations in the brain are 100-fold lower compared to other organs. In some cases, the brain AmB levels are even below detection.29 In future studies, we would like to examine if decreasing the size of liposomes/DectiSomes would enable more efficient BBB crossing and further improve treatment outcome.
-
(3)
Due to the limited materials, we only tested few concentrations of AmB in the pulmonary cryptococcosis model and only one dose of AmB in the systemic cryptococcosis model. We would like to test a range of AmB concentrations to find the lowest drug concentration of DectiSomes that provide the best therapeutic outcome.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Biological samples | ||
| Female CD-1 mice | Charles Rivers Laboratory | N/A |
| Cryptococcus neoformans (H99) | Duke University | N/A |
Resource availability
Lead contact
Further information and requests should be directed to the lead contact, Xiaorong Lin (Xiaorong.Lin@uga.edu).
Materials availability
All reagents are commercially available. Procedures are described in the methods.
Data and code availability
-
•
All of the data supporting this study are presented herein.
-
•
No code was generated for this study.
-
•
Any additional information required is available from the lead contact upon request.
Experimental model and study participant details
Fungal strain and growth conditions
The highly virulent clinical Cryptococcus neoformans isolate, H99, was used in all experiments in this study. The fungal strain was stored in 15% glycerol at −80°C. It was streaked out and cultured on yeast peptone D-glucose (YPD) medium at 30°C prior to use. For the in vitro binding experiment, cells were plated onto RPMI + MOPS plates or grown in liquid YPD medium shaking at 200 rpm and incubated at 30°C.
Mice
Female CD-1 mice of 19–21 g in body weight and approximately 2 months old in age were purchased from the Charles River laboratories. Mice were acclimated to the UGA central animal facility prior to infection. This study was performed according to the guidelines of NIH and the University of Georgia Institutional Animal Care and Use Committee (IACUC). The animal models and procedures used have been approved by the IACUC (AUP protocol numbers: A2020 06–015 and A2023 03–033).
Methods details
Construction of DectiSomes
Sterile pegylated 100-nm diameter liposomes were obtained from FormuMax Sci. Inc. (DSPC–CHOL–mPEG2000-DSPE, 53:47:5 mol ratio, FormuMax F10203A). Amphotericin B was remotely loaded into liposomes (AmB-LLs) with 11 mol percent AmB intercalated into the outer membrane relative to moles of membrane lipid. Loading was quantified by the substrative method, making use of the strong absorption peak of AmB at A407 and standard AmB solutions as we described in detail previously.43 The compositions of AmB-LLs are similar to the commercial formulation of AmBisome except that AmB-LLs contain pegylated lipids. Pegylation stabilizes liposomes (a.k.a., stealth liposomes) and extends their half-life.56 Relative to moles of lipid, 1 mol percent of the CRD (carbohydrate recognition domain) domains of mouse Dectin-2 or Dectin-3 proteins were coupled to the lipid carrier DSPE-PEG-3400-NHS (Nanosoft Polymers, 1544–3400) and integrated into AmB-LLs via their DSPE moiety42 to make Dec2-AmB-LLs and Dec3-AmB-LLs.41,44 DectiSomes and AmB-LLs for fluorescence quantification contain 2 mol percent rhodamine tethered to a lipid carrier integrated into the liposomal membrane.42,43 The remote loading of the dectins and rhodamine is quantitative, approaching 100%. Liposomes were dialyzed and stored in TAS2 buffer (pH 7.5, 20 mM Tris base, 8 mM acetic acid, 1 mM β-mercaptoethanol, and 9% w/v sucrose) at 4°C. β-mercaptoethanol is replenished at 0.5 mM every month. DectiSomes are stable and fully active in binding their ligands after 6 months of storage at 4°C.
In vitro liposome binding assay
Agar plugs (8 mm diameter) with C. neoformans colonies were removed from the RPMI medium plates with a cork borer, washed in PBS, fixed with 4% formalin, and washed three more times with PBS prior to staining. DectiSomes tagged with rhodamine B were diluted in liposome dilution buffer (20 mM HEPES, 10 mM triethanolamine, 150 mM NaCl, 10 mM CaCl2, 1 mM β-mercaptoethanol, pH 8.0) to reach the final concentration of dectin proteins at 1:200 w/v. DectiSomes and control AmB-LLs (at the same relative dilution) were incubated with the agar plugs for 1 h at 22°C with mild agitation. Agar plugs were washed four times with PBS. Cells were co-stained with calcofluor white to reveal chitin in the fungal cell wall.
Brain tissue liposome binding assay
Brain tissue liposome binding assay: At 24 h post-infection (DPI 1), mice were treated intravenously with Dec2-AmB-LLs or control AmB-LLs, and were euthanized at day 2 post-infection (DPI 2). Brains were dissected, hand sectioned in the coronal plane with a razor blade, stained with calcofluor white (20 μM) for 10 min, and viewed top down under a glass coverslip. We examined coronal sections of the frontal cortex and round cryptococcal cells were identified by their blue fluorescence visualized through the DAPI channel while Dec2-AmB-LLs or control AmB-LLs were visualized in the Texas Red channel using a 10× NA 0.35 lens.
Microscopy
The agar plugs (8 mm in diameter) containing cryptococcal colonies were transferred to a 24-well microtiter plate, treated with rhodamine B tagged DectiSome (1:200 w:v, protein:buffer) or control an equivalent amount of AmB-LLs, stained with calcofluor white (20 μM), and examined under a REVOLVE R4 (Upright & Inverted) epifluorescence microscope with a 20× NA 0.8 objective lens. Images were acquired using DAPI (Ex380/Em450) filters for the calcofluor white stained chitin and Texas RED (Ex560/Em630) filters for rhodamine-tagged liposomes. Quantification of area of red fluorescence (pixels) from batches of microscopic images in the Texas RED channel was standardized and carried out using our recently developed Cell Profiler program, AreaPipe.57
Murine models of cryptococcosis
Infection
The clinical Cryptococcus neoformans H99 strain was cultured in 3 mL of liquid YPD medium at an initial inoculum of ∼106 cells per mL, shaking at 220 rpm at 30°C for 15 h. Cells were washed three times with sterile saline and adjusted to the appropriate concentrations. For the pulmonary cryptococcosis model, mice were sedated with Ketamine and Xylazine via intraperitoneal injection.50,58,59,60,61 Sedated mice hung on a cotton line by their incisors were inoculated slowly with 50 μL of fungal cell suspension to one of their nares.50,58,59,60,61 For the systemic cryptococcosis model, mice were sedated with isoflurane in an inhalation chamber and the sedated mice were immediately inoculated intravenously via the retro-orbital route with 100 μL of fungal cell suspension.50,59,60 Mice were monitored and euthanized at the pre-designed time point for fungal burden experiments. For the survival experiment, mice were monitored and terminated when they reached clinical endpoints as defined in our animal protocols.
Treatment
Ls, AmB-LLs, Dec2-AmB-LLs, and Dec3-AmB-LLs were prepared to the final concentration required.33,41,42,43,44 For inhalation treatments in the pulmonary infection model, mice were sedated with Ketamine and Xylazine via intraperitoneal injection.6,58,59,60 Sedated mice were then administered intranasally with 50 μL of the different treatments as indicated in the texts. For intravenous administration of the antifungals in the systemic infection model, mice were sedated with isoflurane via an inhalation chamber and the infected mice were inoculated immediately via retro-orbital with 100 μL of the drugs.
Fungal burden analysis
At the indicated time of euthanization, specific organs were obtained based on the experimental design. The organs (lungs, kidneys, spleens, livers, or brains) were dissected from euthanized mice and homogenized in 2 mL of cold PBS using an IKA-T18 homogenizer with the same setting for each type of organ.21,50,58,59,60,61 The homogenized tissue suspensions were serially diluted (10x), plated onto yeast nitrogen base (YNB) agar medium, and incubated at 30°C for 2 days such that the colonies became visible to count colony forming units (CFUs).
Quantification and statistical analysis
The fluorescent image data were initially processed in Microsoft Excel (version 16.76) and moved into GraphPad Prism version 9.0 for graphic presentation. Statistical data for DectiSome and AmB-LLs fluorescence binding assay were analyzed using Student’s t test. Statistical data for fungal burdens measured in colonies forming units were analyzed using Mann-Whitney nonparametric t-test and Student’s t test. The significance of survival data between each treatment was measured using the log rank Mantel-Cox test. Any p-values lower than 0.05 were considered statistically significant. All statistical analyses were performed in GraphPad Prism.
Acknowledgments
We thank Dr. Zachary Lewis and Lin lab members for their comments and suggestions. The work is funded by the University of Georgia Research Foundation, Inc. (UGARF to SA and RBM), the National Institute of Allergy and Infectious Diseases (R01AI162989 to RBM, SA, and XL), and the Georgia Research Alliance Ventures (to RBM). The funders had no role in study design, data collection, and interpretation, or the decision to submit the work for publication. They are not responsible for the content of this article. Graphical Abstract was created with BioRender.
Author contributions
Conceived and designed the experiments: T.P., R.S., S.A., R.M., and X.L. Performed the experiments: T.P., R.S., S.A., R.M., and X.L. Analyzed the data: T.P., R.S., S.A., R.M., and X.L. Wrote the article: T.P., R.S., S.A., R.M., and X.L. Edited the article: T.P., R.S., S.A., R.M., and X.L.
Declaration of interests
The authors have applied for patents on this technology.
Published: June 22, 2024
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2024.110349.
Contributor Information
Richard Meagher, Email: meagher@uga.edu.
Xiaorong Lin, Email: xiaorong.lin@uga.edu.
Supplemental information
References
- 1.Mitchell A.P. Updated view of Cryptococcus neoformans mating type and virulence. Infect. Immun. 2003;71:4829–4830. doi: 10.1128/IAI.71.9.4829-4830.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wickes B.L. The role of mating type and morphology in Cryptococcus neoformans pathogenesis. Int. J. Med. Microbiol. 2002;292:313–329. doi: 10.1078/1438-4221-00216. [DOI] [PubMed] [Google Scholar]
- 3.Shapiro R.S., Robbins N., Cowen L.E. Regulatory circuitry governing fungal development, drug resistance, and disease. Microbiol. Mol. Biol. Rev. 2011;75:213–267. doi: 10.1128/MMBR.00045-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.London R., Orozco B.S., Mylonakis E. The pursuit of cryptococcal pathogenesis: heterologous hosts and the study of cryptococcal host-pathogen interactions. FEMS Yeast Res. 2006;6:567–573. doi: 10.1111/j.1567-1364.2006.00056.x. [DOI] [PubMed] [Google Scholar]
- 5.Shokouhi S., Hakamifard A. Meningitis Caused by Cryptococcus neoformans in an Apparently Immunocompetent Patient. J. Investig. Med. High Impact Case Rep. 2022;10 doi: 10.1177/23247096221111779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang L., Zhai B., Lin X. The link between morphotype transition and virulence in Cryptococcus neoformans. PLoS Pathog. 2012;8 doi: 10.1371/journal.ppat.1002765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ye F., Xie J.X., Zeng Q.S., Chen G.Q., Zhong S.Q., Zhong N.S. Retrospective analysis of 76 immunocompetent patients with primary pulmonary cryptococcosis. Lung. 2012;190:339–346. doi: 10.1007/s00408-011-9362-8. [DOI] [PubMed] [Google Scholar]
- 8.Setianingrum F., Rautemaa-Richardson R., Denning D.W. Pulmonary cryptococcosis: A review of pathobiology and clinical aspects. Med. Mycol. 2019;57:133–150. doi: 10.1093/mmy/myy086. [DOI] [PubMed] [Google Scholar]
- 9.Kanjanapradit K., Kosjerina Z., Tanomkiat W., Keeratichananont W., Panthuwong S. Pulmonary Cryptococcosis Presenting With Lung Mass: Report of 7 Cases and Review of Literature. Clin. Med. Insights Pathol. 2017;10 doi: 10.1177/1179555717722962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aberg J.A., Mundy L.M., Powderly W.G. Pulmonary cryptococcosis in patients without HIV infection. Chest. 1999;115:734–740. doi: 10.1378/chest.115.3.734. [DOI] [PubMed] [Google Scholar]
- 11.Wu B., Liu H., Huang J., Zhang W., Zhang T. Pulmonary cryptococcosis in non-AIDS patients. Clin. Invest. Med. 2009;32:E70–E77. doi: 10.25011/cim.v32i1.5090. [DOI] [PubMed] [Google Scholar]
- 12.Campbell G.D. Primary pulmonary cryptococcosis. Am. Rev. Respir. Dis. 1966;94:236–243. doi: 10.1164/arrd.1966.94.2.236. [DOI] [PubMed] [Google Scholar]
- 13.Poley M., Koubek R., Walsh L., McGillen B. Cryptococcal Meningitis in an Apparent Immunocompetent Patient. J. Investig. Med. High Impact Case Rep. 2019;7 doi: 10.1177/2324709619834578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rajasingham R., Smith R.M., Park B.J., Jarvis J.N., Govender N.P., Chiller T.M., Denning D.W., Loyse A., Boulware D.R. Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. Lancet Infect. Dis. 2017;17:873–881. doi: 10.1016/S1473-3099(17)30243-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woo Y.H., Martinez L.R. Cryptococcus neoformans-astrocyte interactions: effect on fungal blood brain barrier disruption, brain invasion, and meningitis progression. Crit. Rev. Microbiol. 2021;47:206–223. doi: 10.1080/1040841X.2020.1869178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen J., Varma A., Diaz M.R., Litvintseva A.P., Wollenberg K.K., Kwon-Chung K.J. Cryptococcus neoformans strains and infection in apparently immunocompetent patients, China. Emerg. Infect. Dis. 2008;14:755–762. doi: 10.3201/eid1405.071312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nielsen K., Cox G.M., Litvintseva A.P., Mylonakis E., Malliaris S.D., Benjamin D.K., Jr., Giles S.S., Mitchell T.G., Casadevall A., Perfect J.R., Heitman J. Cryptococcus neoformans {alpha} strains preferentially disseminate to the central nervous system during coinfection. Infect. Immun. 2005;73:4922–4933. doi: 10.1128/IAI.73.8.4922-4933.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jain D., Najjar M., Azher Q., Bachuwa G. Cryptococcal sternal osteomyelitis in a healthy woman: a review of Cryptococcus neoformans. BMJ Case Rep. 2013;2013 doi: 10.1136/bcr-2013-009129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zahra L.V., Azzopardi C.M., Scott G. Cryptococcal meningitis in two apparently immunocompetent Maltese patients. Mycoses. 2004;47:168–173. doi: 10.1111/j.1439-0507.2004.00963.x. [DOI] [PubMed] [Google Scholar]
- 20.Braun D.K., Janssen D.A., Marcus J.R., Kauffman C.A. Cryptococcal infection of a prosthetic dialysis fistula. Am. J. Kidney Dis. 1994;24:864–867. doi: 10.1016/s0272-6386(12)80683-4. [DOI] [PubMed] [Google Scholar]
- 21.Zhai B., Zhu P., Foyle D., Upadhyay S., Idnurm A., Lin X. Congenic strains of the filamentous form of Cryptococcus neoformans for studies of fungal morphogenesis and virulence. Infect. Immun. 2013;81:2626–2637. doi: 10.1128/IAI.00259-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang D., Wu C., Gao J., Zhao S., Ma X., Wei B., Feng L., Wang Y., Xue X. Comparative study of primary pulmonary cryptococcosis with multiple nodules or masses by CT and pathology. Exp. Ther. Med. 2018;16:4437–4444. doi: 10.3892/etm.2018.6745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Molloy S.F., Kanyama C., Heyderman R.S., Loyse A., Kouanfack C., Chanda D., Mfinanga S., Temfack E., Lakhi S., Lesikari S., et al. Antifungal Combinations for Treatment of Cryptococcal Meningitis in Africa. N. Engl. J. Med. 2018;378:1004–1017. doi: 10.1056/NEJMoa1710922. [DOI] [PubMed] [Google Scholar]
- 24.Rajasingham R., Govender N.P., Jordan A., Loyse A., Shroufi A., Denning D.W., Meya D.B., Chiller T.M., Boulware D.R. The global burden of HIV-associated cryptococcal infection in adults in 2020: a modelling analysis. Lancet Infect. Dis. 2022;22:1748–1755. doi: 10.1016/S1473-3099(22)00499-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jarvis J.N., Lawrence D.S., Meya D.B., Kagimu E., Kasibante J., Mpoza E., Rutakingirwa M.K., Ssebambulidde K., Tugume L., Rhein J., et al. Single-Dose Liposomal Amphotericin B Treatment for Cryptococcal Meningitis. N. Engl. J. Med. 2022;386:1109–1120. doi: 10.1056/NEJMoa2111904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Supplement to the 2016 Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection. WHO; 2018. Guidelines for The Diagnosis, Prevention and Management of Cryptococcal Disease in HIV-Infected Adults, Adolescents and Children. [PubMed] [Google Scholar]
- 27.Noor A., Preuss C.V. StatPearls; 2023. Amphotericin B. [PubMed] [Google Scholar]
- 28.Stone N.R.H., Bicanic T., Salim R., Hope W. Liposomal Amphotericin B (AmBisome(®)): A Review of the Pharmacokinetics, Pharmacodynamics, Clinical Experience and Future Directions. Drugs. 2016;76:485–500. doi: 10.1007/s40265-016-0538-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adler-Moore J.P., Proffitt R.T., Olson J.A., Jensen G.M. Tissue pharmacokinetics and pharmacodynamics of AmBisome® (L-AmBis) in uninfected and infected animals and their effects on dosing regimens. J. Liposome Res. 2017;27:195–209. doi: 10.1080/08982104.2017.1327543. [DOI] [PubMed] [Google Scholar]
- 30.Adler-Moore J. AmBisome targeting to fungal infections. Bone Marrow Transplant. 1994;14:S3–S7. [PubMed] [Google Scholar]
- 31.Perfect J.R., Dismukes W.E., Dromer F., Goldman D.L., Graybill J.R., Hamill R.J., Harrison T.S., Larsen R.A., Lortholary O., Nguyen M.H., et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the infectious diseases society of america. Clin. Infect. Dis. 2010;50:291–322. doi: 10.1086/649858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jarvis J.N., Leeme T.B., Molefi M., Chofle A.A., Bidwell G., Tsholo K., Tlhako N., Mawoko N., Patel R.K.K., Tenforde M.W., et al. Short-course High-dose Liposomal Amphotericin B for Human Immunodeficiency Virus-associated Cryptococcal Meningitis: A Phase 2 Randomized Controlled Trial. Clin. Infect. Dis. 2019;68:393–401. doi: 10.1093/cid/ciy515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meagher R.B., Lewis Z.A., Ambati S., Lin X. DectiSomes: C-type lectin receptor-targeted liposomes as pan-antifungal drugs. Adv. Drug Deliv. Rev. 2023;196 doi: 10.1016/j.addr.2023.114776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reid D.M., Gow N.A.R., Brown G.D. Pattern recognition: recent insights from Dectin-1. Curr. Opin. Immunol. 2009;21:30–37. doi: 10.1016/j.coi.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haider M., Dambuza I.M., Asamaphan P., Stappers M., Reid D., Yamasaki S., Brown G.D., Gow N.A.R., Erwig L.P. The pattern recognition receptors dectin-2, mincle, and FcRγ impact the dynamics of phagocytosis of Candida, Saccharomyces, Malassezia, and Mucor species. PLoS One. 2019;14 doi: 10.1371/journal.pone.0220867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stoppelkamp S., Reid D.M., Yeoh J., Taylor J., McKenzie E.J., Brown G.D., Gordon S., Forrester J.V., Wong S.Y.C. Murine pattern recognition receptor dectin-1 is essential in the development of experimental autoimmune uveoretinitis. Mol. Immunol. 2015;67:398–406. doi: 10.1016/j.molimm.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 37.Meagher R.B., Lewis Z.A., Ambati S., Lin X. Aiming for a bull's-eye: Targeting antifungals to fungi with dectin-decorated liposomes. PLoS Pathog. 2021;17 doi: 10.1371/journal.ppat.1009699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schenten D., Medzhitov R. The control of adaptive immune responses by the innate immune system. Adv. Immunol. 2011;109:87–124. doi: 10.1016/B978-0-12-387664-5.00003-0. [DOI] [PubMed] [Google Scholar]
- 39.Garcia-Rubio R., de Oliveira H.C., Rivera J., Trevijano-Contador N. The Fungal Cell Wall: Candida, Cryptococcus, and Aspergillus Species. Front. Microbiol. 2019;10:2993. doi: 10.3389/fmicb.2019.02993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu L.L., Zhao X.Q., Jiang C., You Y., Chen X.P., Jiang Y.Y., Jia X.M., Lin X. C-type lectin receptors Dectin-3 and Dectin-2 form a heterodimeric pattern-recognition receptor for host defense against fungal infection. Immunity. 2013;39:324–334. doi: 10.1016/j.immuni.2013.05.017. [DOI] [PubMed] [Google Scholar]
- 41.Ambati S., Pham T., Lewis Z.A., Lin X., Meagher R.B. DectiSomes: Glycan Targeting of Liposomal Drugs Improves the Treatment of Disseminated Candidiasis. Antimicrob. Agents Chemother. 2022;66 doi: 10.1128/AAC.01467-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ambati S., Ellis E.C., Lin J., Lin X., Lewis Z.A., Meagher R.B. Dectin-2-Targeted Antifungal Liposomes Exhibit Enhanced Efficacy. mSphere. 2019;4 doi: 10.1128/mSphere.00715-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ambati S., Ferarro A.R., Kang S.E., Lin J., Lin X., Momany M., Lewis Z.A., Meagher R.B. Dectin-1-Targeted Antifungal Liposomes Exhibit Enhanced Efficacy. mSphere. 2019;4 doi: 10.1128/mSphere.00025-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ambati S., Ellis E.C., Pham T., Lewis Z.A., Lin X., Meagher R.B. Antifungal Liposomes Directed by Dectin-2 Offer a Promising Therapeutic Option for Pulmonary Aspergillosis. mBio. 2021;12 doi: 10.1128/mBio.00030-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van Wyk M., Govender N.P., Mitchell T.G., Litvintseva A.P., GERMS-SA Multilocus sequence typing of serially collected isolates of Cryptococcus from HIV-infected patients in South Africa. J. Clin. Microbiol. 2014;52:1921–1931. doi: 10.1128/JCM.03177-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Suwatanapongched T., Sangsatra W., Boonsarngsuk V., Watcharananan S.P., Incharoen P. Clinical and radiologic manifestations of pulmonary cryptococcosis in immunocompetent patients and their outcomes after treatment. Diagn. Interv. Radiol. 2013;19:438–446. doi: 10.5152/dir.2013.13049. [DOI] [PubMed] [Google Scholar]
- 47.Kim H., Loebenberg D., Marco A., Symchowicz S., Lin C. Comparative pharmacokinetics of Sch 28191 and amphotericin B in mice, rats, dogs, and cynomolgus monkeys. Antimicrob. Agents Chemother. 1984;26:446–449. doi: 10.1128/AAC.26.4.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu N., Gu J., Zhu Y., Wen H., Ren Q., Chen J. Efficacy of intravenous amphotericin B-polybutylcyanoacrylate nanoparticles against cryptococcal meningitis in mice. Int. J. Nanomedicine. 2011;6:905–913. doi: 10.2147/IJN.S17503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chang T., Olson J.A., Proffitt R.T., Adler-Moore J.P. Differences in tissue drug concentrations following intravenous versus intraperitoneal treatment with amphotericin B deoxycholate or liposomal amphotericin B. Med. Mycol. 2010;48:430–435. doi: 10.3109/13693780903208249. [DOI] [PubMed] [Google Scholar]
- 50.Chadwick B.J., Pham T., Xie X., Ristow L.C., Krysan D.J., Lin X. The RAM signaling pathway links morphology, thermotolerance, and CO(2) tolerance in the global fungal pathogen Cryptococcus neoformans. Elife. 2022;11 doi: 10.7554/eLife.82563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Proffitt R.T., Satorius A., Chiang S.M., Sullivan L., Adler-Moore J.P. Pharmacology and toxicology of a liposomal formulation of amphotericin B (AmBisome) in rodents. J. Antimicrob. Chemother. 1991;28:49–61. doi: 10.1093/jac/28.suppl_b.49. [DOI] [PubMed] [Google Scholar]
- 52.Ibrahim A.S., Gebremariam T., Husseiny M.I., Stevens D.A., Fu Y., Edwards J.E., Jr., Spellberg B. Comparison of lipid amphotericin B preparations in treating murine zygomycosis. Antimicrob. Agents Chemother. 2008;52:1573–1576. doi: 10.1128/AAC.01488-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bekersky I., Boswell G.W., Hiles R., Fielding R.M., Buell D., Walsh T.J. Safety and toxicokinetics of intravenous liposomal amphotericin B (AmBisome) in beagle dogs. Pharm. Res. (N. Y.) 1999;16:1694–1701. doi: 10.1023/a:1018997730462. [DOI] [PubMed] [Google Scholar]
- 54.Wasan K.M., Sivak O., Rosland M., Risovic V., Bartlett K. Assessing the antifungal activity, pharmacokinetics, and tissue distribution of amphotericin B following the administration of Abelcet and AmBisome in combination with caspofungin to rats infected with Aspergillus fumigatus. J. Pharm. Sci. 2007;96:1737–1747. doi: 10.1002/jps.20801. [DOI] [PubMed] [Google Scholar]
- 55.Groll A.H., Giri N., Petraitis V., Petraitiene R., Candelario M., Bacher J.S., Piscitelli S.C., Walsh T.J. Comparative efficacy and distribution of lipid formulations of amphotericin B in experimental Candida albicans infection of the central nervous system. J. Infect. Dis. 2000;182:274–282. doi: 10.1086/315643. [DOI] [PubMed] [Google Scholar]
- 56.Immordino M.L., Dosio F., Cattel L. Stealth liposomes: review of the basic science, rationale, and clinical applications, existing and potential. Int. J. Nanomedicine. 2006;1:297–315. [PMC free article] [PubMed] [Google Scholar]
- 57.Choudhury Q.J., Ambati S., Link C.D., Lin X., Lewis Z.A., Meagher R.B. Dectin-3-targeted antifungal liposomes efficiently bind and kill diverse fungal pathogens. Mol. Microbiol. 2023;120:723–739. doi: 10.1111/mmi.15174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lin J., Pham T., Hipsher K., Glueck N., Fan Y., Lin X. Immunoprotection against Cryptococcosis Offered by Znf2 Depends on Capsule and the Hyphal Morphology. mBio. 2022;13 doi: 10.1128/mbio.02785-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhai B., Wozniak K.L., Masso-Silva J., Upadhyay S., Hole C., Rivera A., Wormley F.L., Jr., Lin X. Development of protective inflammation and cell-mediated immunity against Cryptococcus neoformans after exposure to hyphal mutants. mBio. 2015;6 doi: 10.1128/mBio.01433-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhai B., Wu C., Wang L., Sachs M.S., Lin X. The antidepressant sertraline provides a promising therapeutic option for neurotropic cryptococcal infections. Antimicrob. Agents Chemother. 2012;56:3758–3766. doi: 10.1128/AAC.00212-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhao Y., Wang Y., Upadhyay S., Xue C., Lin X. Activation of Meiotic Genes Mediates Ploidy Reduction during Cryptococcal Infection. Curr. Biol. 2020;30:1387–1396.e5. doi: 10.1016/j.cub.2020.01.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
All of the data supporting this study are presented herein.
-
•
No code was generated for this study.
-
•
Any additional information required is available from the lead contact upon request.