A vaccination programme against meningococcal group C infection is to start this October in the United Kingdom in a bid to halve the total number of deaths from meningitis. The United Kingdom will be the first country in the world to implement such a vaccination programme following the development of a new meningitis C vaccine.
The new vaccine, unlike the existing one, provides long term protection and can be used in babies and young children. The meningococcal surface polysaccharide is conjugated with a protein using similar technology to that used for the first time with the haemophilus influenzae type b (Hib) vaccine.
Three manufacturers have developed the vaccine. One has submitted a licence application with the Medicines Control Agency and the other two are expected to submit applications shortly.
Over the past few years the total number of cases of meningococcal infection has been increasing in the United Kingdom, and the proportion of cases caused by group C has also increased. There has also been a shift in the ages affected, with more cases in those in their late teens, among whom the death rate is the highest.
Group C infection accounts for 40%of all cases of meningococcal infection and 150 of the 260 deaths every year. The remainder of cases and deaths are due mainly to group B meningitis, for which there is still no vaccine. Research is under way, but it is likely to be at least five years before a vaccine against group B meningitis becomes available.
Dr Elizabeth Miller, an epidemiologist at the Public Health Laboratory Service (PHLS) who has been coordinating the meningococcal group C vaccine trials, said that the new vaccine had been given to 4500 children in the UK, some of whom had been followed for five years. In addition, the manufacturers’ own studies had involved 20000 individuals in the United States.
“It is a highly effective vaccine which produces an excellent antibody response,” said Dr Miller. “We expect it to provide a similar level of protection to that of the Hib vaccine at around 98%.”
As the vaccine was not a live one, the only side effect that had been seen was some local irritation at the injection site, she said. The new vaccine is immunogenic in children aged 2 months upwards. Three doses are recommended for children aged 2, 3, and 4 months; two doses for children over 4 months and under 1 year; and one dose for all others.
The health secretary, Frank Dobson, said that the new immunisation programme should start in October and expand as rapidly as the manufacturers could supply more vaccine. “Because the new vaccine is being produced for the first time it cannot be available for everyone from the start. So it will have to be targeted at babies, children, and young people at most risk,” he said.
The programme is being rolled out simultaneously in England, Scotland, Wales, and Northern Ireland. The vaccine will be given initially to babies going for their routine diphtheria, tetanus, pertussis, polio, and Hib vaccination at 2, 3, and 4 months; children receiving their first dose of MMR (measles, mumps, and rubella) vaccine at around 13 months; and teenagers aged 15, 16, and 17. Children aged over 4 months and under 12 months will then be recalled.
The second phase will involve the immunisation of children over 1 year and up to 5 years. As supplies of the vaccine become available further groups will be immunised according to their risk.
Figure.
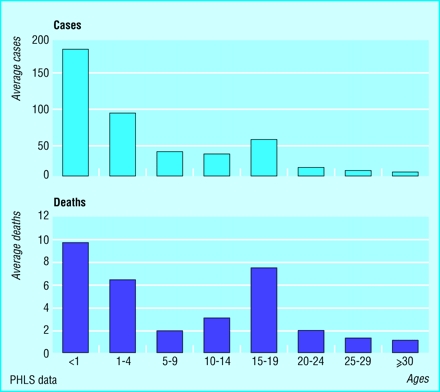
Estimated average number of cases of, and deaths from, group C meningococcal disease, by age, in England and Wales, 1998-


