Abstract
Discrepancies in self-rated and observer-rated depression severity may underlie the basis for biological heterogeneity in depressive disorders and be an important predictor of outcomes and indicators to optimize intervention strategies. However, the neural mechanisms underlying this discrepancy have been understudied. This study aimed to examine the brain networks that represent the neural basis of the discrepancy between self-rated and observer-rated depression severity using resting-state functional MRI. To examine the discrepancy between self-rated and observer-rated depression severity, self- and observer-ratings discrepancy (SOD) was defined, and the higher and lower SOD groups were selected from depressed patients as participants showing extreme deviation. Resting-state functional MRI analysis was performed to examine regions with significant differences in functional connectivity in the two groups. The results showed that, in the higher SOD group compared to the lower SOD group, there was increased functional connectivity between the frontal pole and precuneus, both of which are subregions of the default mode network that have been reported to be associated with ruminative and self-referential thinking. These results provide insight into the association of brain circuitry with discrepancies between self- and observer-rated depression severity and may lead to more treatment-oriented diagnostic reclassification in the future.
Keywords: self-rated depression, observer-rated depression, discrepancy, frontal pole, precuneus
Introduction
Depression is the leading cause of mental health–related disease, affecting an estimated 300 million people worldwide (Patel et al. 2016), with a 12-mo prevalence rate generally ~6% (Kessler and Bromet 2013), and the lifetime risk is three times higher (15% to 18%) (Bromet et al. 2011). Depression is a chronic disease, with half of the patients experience relapsing (World Health Organization 2001), and the frequency and severity of episodes tends to increase over time. It leads to a reduced quality of life (Üstün et al. 2004) and a significant burden of disease in terms of personal and economic losses (Vos et al. 2013). Depression is the third leading cause of the global burden of disease as assessed by disability-adjusted life-years, the leading cause in middle- and high-income countries, and is projected to rise to the second leading cause of the global burden of disease by 2030 (World Health Organization 2008).
There is often a discrepancy between the subjective symptoms of depression and symptoms perceived by the evaluating psychiatrist (Möller and von Zerssen 1995; Richter et al. 1998; Bagby et al. 2004). Subjective symptoms should not be disregarded as being nonobjective, as they can have a significant impact on a patient’s quality of life and are an important factor of functional and personal recovery (Demyttenaere et al. 2015; Richardson and Barkham 2020). Several demographic and personality factors have been identified as explaining the discrepancy between self-rated and observer-rated depression severity. Patients whose self-rated depressive symptoms are disproportionately severe compared with their observer-rated depression severity have been found to have higher scores for phobic anxiety (Corruble et al. 1999), characteristics of non-endogenous or neurotic depression (Rush et al. 1987; Domken et al. 1994), higher neuroticism (Enns et al. 2000; Duberstein and Heisel, 2007), lower extraversion (Enns et al. 2000), lower agreement (Corruble et al. 1999; Enns et al. 2000), lower self-esteem (Domken et al. 1994), and comorbid borderline personality disorder (Stanley and Wilson 2004). Such patients exhibit symptoms for prolonged periods, take longer to recover from depression (Rane et al. 2010; Dunlop et al. 2011), and are associated with increased risk of committing suicide (Tsujii et al. 2014). These epidemiological findings suggest that some biological heterogeneity may underlie the variation in the discrepancy between subjective and objective symptoms among patients with mood disorders. Therefore, elucidating the neural basis of the subjective–objective discrepancy is an important research topic to better understand and to develop interventions tailored for individual patients with mood disorders.
Various assessment scales have been used to quantify self-rated and observer-rated depression severity. The Beck Depression Inventory (BDI) (Beck et al. 1996), a self-rated depression severity score, and the Hamilton Depression Rating Scale (HAMD) (Hamilton 1960), an observer-rated scale, are among the most used ones to assess depression severity. Although both measures have shown to have sufficient reliability and validity (Rush 2007), the correlation between them varies widely from study to study, with Pearson’s coefficients ranging from 0.20 to 0.89 (Möller and von Zerssen 1995; Richter et al. 1998; Bagby et al. 2004). The variability in correlation coefficient may be due to the fact that they assess different components of depression, with the BDI focusing on depressive cognition (Uher et al. 2008), while the HAMD putting more stress on somatic symptoms, such as sleep and eating disturbance. Since depression is a generic label for patients with highly heterogeneous pathogenetic background, the concordance and discrepancy between self-rated and observer-rated depression severity could be useful as an indicator for subtyping depression.
However, few studies have directly investigated the neural underpinnings that lead to the discrepancy between self-rated and observer-rated depression severities. The only neuroimaging study to date on the discrepancy between self-rated and observer-rated depression severity is the near-infrared spectroscopy (NIRS) study, which reported that the task-related elevation of oxygenated hemoglobin concentration in the frontal pole (FP) and dorsolateral prefrontal cortex was higher among patients who reported disproportionately severe self-rated depression symptoms compared with those without such discrepancy (Akashi et al. 2015). Because Brodmann Area 10 (BA10), which almost overlaps FP, has been reported to be involved in self-referential processing and ruminative thinking, as well as in pondering one’s distant future (Okuda et al. 2003; D’Argembeau et al. 2008), this finding suggests subjective–objective discrepancy in depressive symptoms are partially caused by increased self-referential processing and ruminative thinking. NIRS can only record blood oxygenation changes on the cortical surface of the brain, making it difficult to investigate the function of deeper brain regions. To the best of our knowledge, no studies have been conducted on the neural basis of the discrepancy between self-rated and observer-rated depression severity using functional magnetic resonance imaging (MRI) (fMRI), which can detect the neural activity throughout the brain with higher spatial resolution.
The orbitofrontal cortex (OFC) is also a region that has been reported to be associated with ruminative thinking (Jacob et al. 2020), self-referential thinking (Wang et al. 2023), and negative affect (Tozzi et al. 2021a). However, it is so far unclear whether the OFC is related to discrepancy between self-rated and observer-rated severity of depressive disorder.
In addition, the default mode network (DMN) comprises a set of brain regions whose activity increase at rest and exhibit synchronous resting-state neural oscillation (Shulman et al. 1997; Raichle et al. 2001; Fox et al. 2005). The DMN is involved in the regulation of attention and cognition (Pearson et al. 2011; Leech and Sharp 2014). The DMN has been reported to be activated in depressed patients (Sheline et al. 2010; Veer et al. 2010). The DMN is related to an integration of the self-referential processes (Hamilton et al. 2015), and increasing levels of DMN dominance are associated with higher levels of maladaptive, depressive rumination in major depressive disorder (Hamilton et al. 2011). The medial frontal cortex (MedFC) is a hub of the DMN, and its temporal dynamics reliably predict rumination scores (Gao et al. 2023). This symptomatology is predominant among a subtype of depression whose cognitive symptoms are more severe than somatic symptoms. However, the association between the function of DMN and the discrepancy between self-rated and observer-rated depression severity remains unclear.
In this context, we hypothesized that the FP, OFC, and DMN, especially the MedFC, are associated with the subjective–objective discrepancy in depressive subjects. Specifically, given that the positive association between functional connectivity (FC) in these regions and rumination (Chou et al. 2023) and activated DMN in rumination (Zhou et al. 2020) were reported, we hypothesized a possibility that the group with higher subjective–objective discrepancy may have elevated FC in these brain areas. This study aimed to test our hypothesis to investigate the neural substrates underlying the subjective–objective discrepancy using resting-state fMRI (rsfMRI).
Material and methods
Participants
A total of 124 patients with mood disorders were recruited for this study. We included depressed patients who met the criteria for major depressive disorder, dysthymic disorder, bipolar I disorder, or bipolar II disorder using the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) Axis I Disorders (SCID-I) (Takahashi et al. 2010; First et al. 2012). We excluded patients with neurological diseases, including dementia, traumatic brain injury with loss of consciousness for >5 min, and low premorbid intelligence quotient (<70) as estimated using the Japanese Adult Reading Test (JART) 25-item version (Matsuoka and Kim 2006; Matsuoka et al. 2006; Hirata-Mogi et al. 2016) and manic symptoms as estimated using the Young Mania Rating Scale (Young et al. 1978; Inada et al. 2012) (score of ≥3). This study was approved by the Ethics Committee of the University of Tokyo Hospital (certification no. 630, 3150, 3202, 3349). After a thorough explanation of the purpose of the study, written informed consent was obtained from all participants, and this study was conducted following the tenets of the Declaration of Helsinki.
Clinical assessment
Observer-rated depressive symptoms were evaluated using the 17-item version of the HAMD (HAMD17) (Hamilton 1960; Tabuse et al. 2007; Williams et al. 2008), and self-rated depressive symptoms were evaluated using the BDI, 2nd edition (BDI-II) (Beck et al. 1996; Kojima et al. 2002; Kojima and Furukawa 2003) or the Center for Epidemiologic Studies Depression (CES-D) Scale (Ls 1977; Shima et al. 1985; Shima 1998). The self-rated depression scale was changed from CES-D to BDI-II during the study period to accommodate other research; the CES-D was considered a possible replacement because it is a highly correlated self-rated scale developed with BDI as a reference. In addition, the Japanese version of the modified Global Assessment of Functioning (mGAF) (Eguchi et al. 2015) was evaluated. For participants taking psychotropic medications, we recorded medication information by calculating the equivalent values of imipramine for antidepressants, chlorpromazine for antipsychotics, and diazepam for anxiolytics (Inada and Inagaki 2015; Inagaki and Inada 2017).
Definition of a discrepancy between self-rated and observer-rated depression severity
To quantify the discrepancy between self-rated and observer-rated depression severities, HAMD17 was used as an observer-rated scale, and BDI-II and CES-D as self-rated scales, and these were z-scored to make them comparable. To examine the discrepancy between self-rated and observer-rated depression severities, the self- and observer-ratings discrepancy (SOD) was defined as the difference of the z-score of the self-rated depression scale minus the z-score of the observer-rated depression scale. The self-rated symptom predominant group (higher SOD) with discrepancy > 0.2 (n = 47) and the observer-rated symptom predominant group (lower SOD) with discrepancy <−0.2 (n = 46) were selected as participants showing extreme deviation.
MRI data acquisition
T1-weighted images and rsfMRI data were acquired during the same scanning session using a GE Discovery MR750w 3.0-T scanner and a 24-channel head coil (General Electric, Waukesha, WI, USA) at the University of Tokyo Hospital. For T1-weighted images, the scanning parameters were set as follows: slice thickness, 1.2 mm; repetition time (TR), 7.7 ms; echo time (TE), 3.1 ms; voxel size, 1 mm × 1 mm × 1.2 mm; flip angle,11°; field of view (FOV), 260 mm × 260 mm × 240 mm, and the spoiled gradient recalled echo pulse sequence was used for acquisition. Resting-state functional images were acquired using a gradient-echo echo planar imaging (EPI) pulse sequence for 10 min with the following parameters: 244 volumes; slice thickness, 3.2 mm; TR/TE, 2,500/30 ms; voxel size, 3.3 mm × 3.3 mm × 3.3 mm; flip angle, 80°; FOV, 212 mm × 212 mm × 212 mm; phase-encoding direction, posterior-to-anterior (PA). During the scan, participants were instructed to open their eyes and gaze at the cross-firm viewpoint on the screen seen through the mirror without thinking about anything specific.
Resting-state functional imaging preprocessing
MRI data were preprocessed and analyzed using MATLAB 2019b (MathWorks, Natick, MA, USA), SPM12 (Wellcome Department of Cognitive Neurology, London, UK), and CONN toolbox version 19.b (Whitfield-Gabrieli and Nieto-Castanon 2012). The first ten time-point scans were removed to allow the fMRI signal to reach a steady state. Slice temporal differences were corrected based on slice order using the CONN toolbox running in MATLAB and segmented into gray matter, white matter, and cerebrospinal fluid by matching structural data with the SPM12 unified segmentation and normalization procedure (Ashburner and Friston 2005). Data were repositioned and normalized according to the standard Montreal Neurological Institute EPI template. Spatial distortion was corrected by field mapping using the CONN toolbox, minimizing physiological noise factors and motion effects using the CompCor algorithm (Behzadi et al. 2007). Scrubbing was performed using the artifact detection tool (ART), with outlier scans identified as those with framewise displacement above 2 mm and/or global signal change >9 SD. Finally, band-pass filter denoising was performed at 0.008 to 0.09 Hz, and a Gaussian filter kernel with full width at half maximum of 8 mm was applied to spatially smooth the image.
Statistical analysis
Based on our hypothesis and previous findings, as indicated in the Introduction section, seed-based rsfMRI analysis was performed using the FP, OFC, and MedFC as seed regions. The Harvard–Oxford cortical atlas of the CONN toolbox was used to set seed regions. Pearson’s correlation coefficients between the time-series blood oxygen level dependent (BOLD) signal of the seed and the time-series BOLD signal of each voxel were calculated and transformed into normally distributed z-scores by Fisher transform. Subsequently, seed-to-voxel FC maps were created. Differences in FC between the higher (n = 47) and lower (n = 46) SOD groups were examined for each seed region using t-tests including age and sex as covariates. We extracted significant cluster regions at the level of false discovery rate corrected P < 0.05 for clusters obtained by thresholding individual voxels at uncorrected P < 0.001.
Furthermore, it was necessary to explore in more detail whether such differences can be seen even across the lower SOD group, the close-to-zero SOD group, and higher SOD group. We thus examined the association in all patients (n = 124), also including those not showing extreme discrepancy, between SOD and the FC which differed between the lower and higher SOD groups, controlling for age and sex. In this analysis, we included a FC value at the peak voxel of the significant cluster found in the above analysis.
Results
Demographic and clinical characteristics of the study groups
The differences in the clinical backgrounds of the study participants are presented in Table 1. There were no significant differences in age, sex, duration of education, duration of illness, modified GAF-symptom (mGAF-S), modified GAF-functioning (mGAF-F), diagnosis of bipolar disorder, or medication use between the groups with higher SOD (n = 47) and lower SOD (n = 46). The mGAF-S and mGAF-F are indices in which the items and anchor points of the GAF are divided into “symptoms” and “social functioning” as an assessment of overall functioning in life, and the descriptions of the items are more detailed (Eguchi et al. 2015). The JART was significantly higher in the group with higher SOD levels (P = 0.03).
Table 1.
Demographic and clinical characteristics of the participants.
| Patients with depression (n = 93) | |||||
|---|---|---|---|---|---|
| Higher SOD | Lower SOD | ||||
| (n = 47) | (n = 46) | ||||
| Average | SD | Average | SD | P value | |
| Age (yr) | 38.0 | 10.4 | 38.0 | 13.4 | 0.67a |
| Females (N) | 27 | 18 | 0.17b | ||
| SCID-I diagnosis | 0.69b | ||||
| MDD | 29 | 29 | |||
| Dysthymic disorder | 2 | 1 | |||
| Bipolar I disorder | 10 | 7 | |||
| Bipolar II disorder | 6 | 9 | |||
| Years of education | 15.0 | 1.9 | 14.8 | 1.9 | 0.54a |
| Duration of illness (yr) | 7.9 | 7.2 | 8.1 | 5.5 | 0.98a |
| mGAF-S | 47.5 | 11.8 | 47.4 | 9.4 | 0.62a |
| mGAF-F | 48.6 | 11.0 | 47.2 | 9.1 | 0.36a |
| JART25 | 20.2 | 3.5 | 18.6 | 4.9 | 0.03a |
| HAMD17 | 9.6 | 5.3 | 13.9 | 7.0 | <0.001a |
| BDI-II (n = 58) | 29.4 | 10.2 | 21.1 | 9.6 | <0.001a |
| CES-D (n = 35) | 34.8 | 9.1 | 21.0 | 9.0 | <0.001a |
| Medication dose (mg/d) | |||||
| Antidepressants (IMP equivalent) | 96.1 | 115.1 | 130.0 | 136.2 | 0.31c |
| Antipsychotics (CP equivalent) | 99.6 | 193.4 | 63.3 | 128.3 | 0.29c |
| Anxiolytics (DZP equivalent) | 11.3 | 12.1 | 8.1 | 8.8 | 0.14c |
aStudent’s t-test
bPearson’s chi-square test
cMann–Whitney U test.
Abbreviations: SCID-I, Structured Clinical Interview for Diagnostic and Statistical Manualof Mental Disorders, Fourth Edition Axis I Disorders; MDD, major depressive disorder; mGAF-S, modified Global Assessment of Functioning-symptom; mGAF-F, modified Global Assessment of Functioning-functioning; JART, Japanese Adult Reading Test; HAMD, Hamilton Depression Rating Scale; BDI-II, Beck Depression Inventory, 2nd edition; CES-D, Center for Epidemiologic Studies Depression Scale; IMP, imipramine; CP, chlorpromazine; DZP, diazepam.
rsfMRI analysis of the discrepancy between self- and observer-rated depression severity
The brain regions that showed significantly greater FC differences in higher SOD (n = 47) than in lower SOD (n = 46) for FP, OFC, and MedFC seeds are shown in Table 2. There were no regions in which lower SODs showed a significantly greater FC than those with higher SODs.
Table 2.
Brain regions that showed significantly greater FC differences in higher SOD than in lower SOD.
| Seed | MNI coordinates (x, y, z) | Cluster size | Brain area | p-FDR | T value |
|---|---|---|---|---|---|
| Right FP | −02, −64, +36 | 254 | Precuneus | 1.84 × 10−2 | 3.97 |
| Left FP | +14, −56, +24 | 887 | Precuneus/PCC | 6.0 × 10−6 | 4.67 |
| OFC | … | ||||
| MedFC | … |
Abbreviations: MNI, Montreal Neurological Institute; FDR, false discovery rate; FP, frontal pole; OFC, orbitofrontal cortex; MedFC, medial frontal cortex; PCC, posterior cingulate cortex.
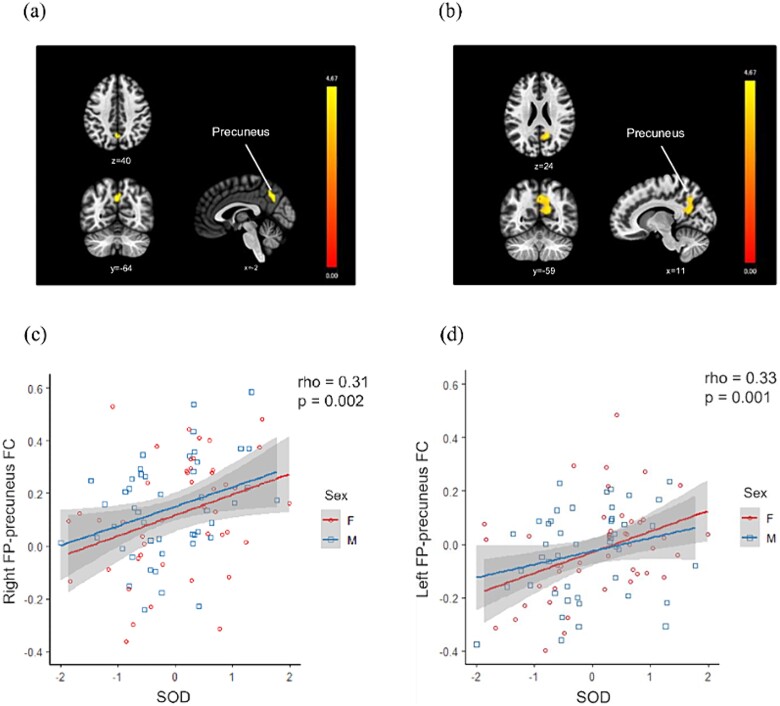
A significant region of elevated FC seeded in the right FP was found in the precuneus, and a significant region of elevated FC seeded in the left FP was found in the precuneus and posterior cingulate cortex (PCC) in the higher SOD group compared to the lower SOD group (Fig. 1a, b). There were no regions where higher SODs showed significantly more elevated OFC- or MedFC-seeded FC than regions with lower SODs.
Fig. 1.
Association of SOD with a FP-seeded FC. (a, b) Brain regions showing significantly elevated (a) right and (b) left FP-seeded FC in the higher SOD group compared to the lower SOD group are depicted. The vertical bars represent t-values. (c, d) Relationships in all patients between SOD and these FCs are described. Abbreviations: SOD, self- and observer-ratings discrepancy; FP, frontal pole; FC, functional connectivity.
The right FP–precuneus FC (rho = 0.31, P = 0.002) and left FP–precuneus FC (rho = 0.33, P = 0.001) were significantly positively correlated with SOD in all subjects (Fig. 1c, d).
Discussion
In this study, we found an elevated FC between the right FP and the precuneus and between the left FP and the precuneus in the group with higher SOD, and the SOD was correlated bilaterally with FP–precuneus FCs.
It has been reported that the discrepancy between self-rated and observer-rated depression severity is greater in non-endogenous depression and dysthymic disorder than in endogenous depression (Rush et al. 1987), associated with personality disorders and stronger anxiety symptoms (Rane et al. 2010), and tends to be less extroverted and harmonious (Enns et al. 2000). Neuroticism and introversion have also been reported to be associated with the discrepancy between self-rated and observer-rated depression severity, introverts tend to overestimate the psychological symptoms of depression (Schneibel et al. 2012), and neuroticism is associated with ruminative thinking and psychological defense (Carter et al. 2010). The group that showed a discrepancy between self-rated and observer-rated depression severity in this study may also have cognitive biases as neurotic tendencies.
The FP, which was found to be related to the discrepancy between self-rated and observer-rated depression severity in this study, is a region that approximately matches BA10 and is known to play an important role in a variety of human-specific higher cognitive functions (Duncan 2010; Kovach et al. 2012; Waskom et al. 2014). The medial region of BA10 is involved in shifting attention to the future by thinking about the details of future events (Okuda et al. 2003; Addis et al. 2007; D’Argembeau et al. 2008) and may be involved in self-referential processing and ruminative thinking, leading to thought patterns specific to depression. The FP has also been suggested to be involved in cognitive functions, particularly in process, goal, and subgoal selection (Fletcher and Henson 2001), and, together with the precuneus, shown to be related to metacognitive abilities, the ability to introspect perception and memory (Fleming et al. 2010; Fleming et al. 2012; McCurdy et al. 2013; Fleming et al. 2014; Sinanaj et al. 2015).
The precuneus has been reported to play a central role in a wide range of highly integrated tasks such as visuospatial imagery, episodic memory retrieval, self-processing operations, and transformation of self-perspective (Cavanna and Trimble 2006). The precuneus, together with the PCC, anterior cingulate cortex, MedFC, and bilateral parietal junctions, constitutes the DMN (Shulman et al. 1997; Fox et al. 2005) and has been implicated in the regulation of attention and cognition (Pearson et al. 2011; Leech and Sharp 2014). The precuneus and PCC have been reported to have increased activity in a number of tasks, including autobiographical memory retrieval (Maddock et al. 2001; Lundstrom et al. 2005), reward outcome monitoring (Hayden et al. 2008), and emotional stimulus processing (Maddock et al. 2003; Cavanna and Trimble 2006). The regional homogeneity of rsfMRI of the precuneus has also been reported to be altered in patients with depression due to cognitive bias in believing that negative thoughts will actualize (Jones and Bhattacharya 2014; Peng et al. 2015), which is associated with ruminative self-referential processing (Jones and Bhattacharya 2014). In addition, severe changes in FC between the precuneus and prefrontal cortex have been reported to occur in depression (Wang et al. 2014; Peng et al. 2015). The precuneus has also been suggested to be associated with subjective well-being, and subjective well-being scores have been reported to decrease with increased activity in the precuneus (Sato et al. 2019).
Regarding the FC between the precuneus and the prefrontal cortex, it has been reported that FC between the precuneus and OFC is increased in depressed patients (Cheng et al. 2018a), while FC between the precuneus and MedFC adjacent to the FP is decreased in relation to severity in depressed patients with anhedonia (Rzepa and McCabe 2018). The PCC is considered a core component of the DMN, which is known to have close neuroanatomical connections with the FP (Mansouri et al. 2015) and involved in future planning (Addis et al. 2007), emotional decision-making (Andrews-Hanna et al. 2010), and self-referencing (Gusnard et al. 2001). It has also been reported that depressed patients have increased FC between the PCC and OFC (Cheng et al. 2018b) and elevated FC between the PCC and middle frontal gyrus (Zhang et al. 2015). In this study, we also found an elevated FC between the FP and PCC in the group with the higher subjective discrepancy, suggesting that the enhancement of anxiety and negative cognition associated with excessive self-reference and future thinking may have affected the discrepancy between self-rated and observer-rated depression severities.
The FP and precuneus, between which FC was elevated in the group with a higher subjective discrepancy in this study, are both subregions of the DMN. Increased activity in the prefrontal cortex in patients with depression has been reported to be caused by impaired cognitive processes due to poor inhibition of the DMN (Lemogne et al. 2012). The DMN is also a region reported to be activated by ruminative thinking (Berman and Jonides 2011), self-referential memory, and negative autobiographical memory (Kross et al. 2009; Nejad et al. 2013). Overall, the changes in FP–precuneus functional connectivity observed in this study suggest that they may be related with neurotic cognitive tendencies that increase anxiety through excessive self-reference and ruminative thinking.
Furthermore, we revealed that the lower SOD group was likely to exhibit a negative value of the FP–precuneus FC, while the higher SOD group tended to have a positive value. Recent meta-analytic studies have reported the lower activation in the precuneus in patients with depressive symptoms (Tozzi et al. 2021b; Xue et al. 2023). The FP is a key brain region for metacognitive judgment on non-experience (Miyamoto et al. 2018). It is suggested that patients in the lower SOD group may have impaired metacognition for their own depressive symptoms through disconnection between the FP and precuneus, while the higher SOD group may have such metacognition through the synchronized neural activity between the FP and precuneus even when the precuneus activity is decreased.
These results validate, although not completely, our initial hypothesis that the prefrontal cortex and DMN are related to the subjective–objective discrepancy in depressed patients.
Limitations
There are some limitations to this study. First, the self-rated depression scale was changed from the CES-D (n = 52, n = 17 [higher SOD], n = 18 [lower SOD]) to BDI-II (n = 72, n = 30 [higher SOD], n = 28 [lower SOD]) for consistency with other research projects. Because the CES-D was developed based on BDI-II, the similarity between the two indices is likely to be high, but a unified analysis of the indices will be considered in the future.
Second, the depressed participants in the study included multiple diagnostic groups based on the operational diagnosis: major depressive disorder, dysthymic disorder, bipolar I disorder, and bipolar II disorder. This study focused on the discrepancy between self-rated and observer-rated depression severity rather than on differences between diagnostic groups. We confirmed no significant difference in diagnostic groups between higher and lower SOD groups, which suggests that the difference in FC between diagnostic groups was, if any, negligible for our results. In this study, the participant numbers of some diagnostic groups were too small to conduct diagnosis-specific analyses for the discrepancy. However, it would be ideal to perform diagnosis-specific analyses if the number of participants is large enough.
Third, comparing the higher and lower SOD groups, both of which are abnormal, is likely to lead to difficulties in interpreting the results because it lacks a control group. We revealed a positive association in all patients, including those not showing extreme discrepancies, between the bilateral FP–precuneus FC and SOD. This implies that, compared to those with similar levels of severity in subjective and objective symptoms, this FC may be decreased in the lower SOD group and increased in the higher SOD group, respectively. However, our main results regarding the increase in this FC in the higher SOD group compared to the lower SOD group should be interpreted carefully.
Fourth, we hypothesized that the FP, OFC, and DMN, especially the MedFC, are associated with the subjective–objective discrepancy in depressive subjects because previous studies have reported these regions’ roles in rumination and self-referential processing. We thus performed seed-based rsfMRI analysis using the FP, OFC, and MedFC as seed regions. However, other brain regions may also be related to rumination and self-referential processing, possibly in connection with the FP, OFC, or MedFC. Although testing this prediction is beyond the scope of this study, future studies are expected to reveal the whole-brain network associated with the subjective–objective discrepancy.
Fifth, there was a concern about the EPI image distortion due to the phase-encoding polarity in this study. Anterior-to-posterior (AP) EPI images are likely to exhibit great signal loss in the prefrontal area (especially the orbitofrontal cortex), while PA EPI images including our data are not likely to show great signal loss in the frontal gyrus (Wang et al. 2021). In addition, spatial distortion was corrected using fieldmaps. However, our results regarding the FP-seeded FC should be cautiously interpreted.
Conclusions
In this study, we found elevated FC between the FP–precuneus in the group with higher SOD. These were positively correlated with SOD, and the regions of elevation and correlation were found independently on the left and right sides. The findings of this study may provide insights into the association between the discrepancy between self-rated and observer-rated depression severity and brain circuits, which may lead to a more treatment-directed reclassification of the diagnosis in the future.
Acknowledgments
The authors would like to acknowledge the UTokyo Institute for Diversity and Adaptation of Human Mind (UTIDAHM) and the International Research Center for Neurointelligence (WPI-IRCN) at the University of Tokyo Institutes for Advanced Study (UTIAS) for the support. We would also like to thank all the participants of our study.
Contributor Information
Shintaro Kawakami, Department of Neuropsychiatry, Graduate School of Medicine, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8655, Japan.
Naohiro Okada, Department of Neuropsychiatry, Graduate School of Medicine, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8655, Japan; The International Research Center for Neurointelligence (WPI-IRCN), The University of Tokyo Institutes for Advanced Study (UTIAS), 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-0033, Japan.
Yoshihiro Satomura, Department of Neuropsychiatry, Graduate School of Medicine, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8655, Japan; Center for Diversity in Medical Education and Research (CDMER), Graduate School of Medicine and Faculty of Medicine, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8655, Japan.
Eimu Shoji, Department of Neuropsychiatry, Graduate School of Medicine, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8655, Japan.
Shunsuke Mori, Department of Neuropsychiatry, Graduate School of Medicine, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8655, Japan.
Masahiro Kiyota, Department of Neuropsychiatry, Graduate School of Medicine, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8655, Japan.
Favour Omileke, Department of Neuropsychiatry, Graduate School of Medicine, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8655, Japan.
Yu Hamamoto, Department of Neuropsychiatry, Graduate School of Medicine, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8655, Japan.
Susumu Morita, Department of Neuropsychiatry, Graduate School of Medicine, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8655, Japan.
Daisuke Koshiyama, Department of Neuropsychiatry, Graduate School of Medicine, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8655, Japan.
Mika Yamagishi, Department of Neuropsychiatry, Graduate School of Medicine, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8655, Japan.
Eisuke Sakakibara, Department of Neuropsychiatry, Graduate School of Medicine, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8655, Japan.
Shinsuke Koike, Department of Neuropsychiatry, Graduate School of Medicine, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8655, Japan; The International Research Center for Neurointelligence (WPI-IRCN), The University of Tokyo Institutes for Advanced Study (UTIAS), 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-0033, Japan; University of Tokyo Institute for Diversity & Adaptation of Human Mind (UTIDAHM), 3-8-1 Komaba, Meguro-ku, Tokyo 153-8902, Japan; Center for Evolutionary Cognitive Sciences, Graduate School of Art and Sciences, The University of Tokyo, 3-8-1 Komaba, Meguro-ku, Tokyo 153-8902, Japan.
Kiyoto Kasai, Department of Neuropsychiatry, Graduate School of Medicine, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8655, Japan; The International Research Center for Neurointelligence (WPI-IRCN), The University of Tokyo Institutes for Advanced Study (UTIAS), 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-0033, Japan.
Author contributions
Shintaro Kawakami (Conceptualization, Formal analysis, Methodology, Writing—original draft), Naohiro Okada (Conceptualization, Formal analysis, Funding acquisition, Methodology, Project administration, Investigation, Resources, Supervision, Validation, Writing—review & editing), Yoshihiro Satomura (Investigation, Resources), Eimu Shoji (Investigation, Resources), Shunsuke Mori (Investigation, Resources), Masahiro Kiyota (Investigation, Resources), Favour Omileke (Investigation, Resources), Yu Hamamoto (Investigation, Resources), Susumu Morita (Investigation, Resources), Daisuke Koshiyama (Investigation, Resources. Supervision), Mika Yamagishi (Investigation, Resources), Eisuke Sakakibara (Supervision), Shinsuke Koike (Supervision), and Kiyoto Kasai (Conceptualization, Funding acquisition, Methodology, Project administration, Investigation, Resources, Supervision, Validation, Writing—review & editing).
Funding
This study was supported by JSPS KAKENHI Grant Numbers JP20H03596 (K.K.), JP21H05171 (K.K.), JP21H05174 (K.K.), JP22H04926 (N.O.), and JP22K18419 (N.O.); AMED under Grant Numbers JP19dm0207069 (K.K.), JP18dm0307001 (K.K.), JP18dm0307004 (K.K.), and JP23wm0625001 (K.K.); and Moonshot R&D Grant Number JPMJMS2021 (K.K.).
Conflict of interest statement: None declared.
References
- Addis DR, Wong AT, Schacter DL. Remembering the past and imagining the future: common and distinct neural substrates during event construction and elaboration. Neuropsychologia. 2007:45(7):1363–1377. 10.1016/j.neuropsychologia.2006.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akashi H, Tsujii N, Mikawa W, Adachi T, Kirime E, Shirakawa O. Prefrontal cortex activation is associated with a discrepancy between self- and observer-rated depression severities of major depressive disorder: a multichannel near-infrared spectroscopy study. J Affect Disorders. 2015:174:165–172. 10.1016/j.jad.2014.11.020. [DOI] [PubMed] [Google Scholar]
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci. 2006:7(4):268–277. 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional-anatomic fractionation of the brain’s default network. Neuron. 2010:65(4):550–562. 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. NeuroImage. 2005:26(3):839–851. 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Bagby RM, Ryder AG, Schuller DR, Marshall MB. The Hamilton Depression Rating Scale: has the gold standard become a lead weight? Am J Psychiatry. 2004:161(12):2163–2177. 10.1176/appi.ajp.161.12.2163. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. BDI-II, Beck Depression Inventory: manual. San Antonio (TX): Psychological Corp; 1996. [Google Scholar]
- Behzadi Y, Restom K, Liau J, Liu TT. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. NeuroImage. 2007:37(1):90–101. 10.1016/j.neuroimage.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman MG, Jonides J. Ruminating on rumination. Biol Psychiatry. 2011:70(4):310–311. 10.1016/j.biopsych.2011.06.003. [DOI] [PubMed] [Google Scholar]
- Bromet E, Andrade L, Hwang I, Sampson NA, Alonso J, de Girolamo G, de Graaf R, Demyttenaere K, Hu C, Iwata N, et al. Cross-national epidemiology of DSM-IV major depressive episode. BMC Med. 2011:9(1):90. 10.1186/1741-7015-9-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter JD, Frampton CM, Mulder RT, Luty SE, Joyce PR. The relationship of demographic, clinical, cognitive and personality variables to the discrepancy between self and clinician rated depression. J Affect Disord. 2010:124(1–2):202–206. 10.1016/j.jad.2009.11.011. [DOI] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006:129(3):564–583. 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Cheng W, Rolls ET, Qiu J, Yang D, Ruan H, Wei D, Zhao L, Meng J, Xie P, Feng J. Functional connectivity of the precuneus in unmedicated patients with depression. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018a:3(12):1040–1049. 10.1016/j.bpsc.2018.07.008. [DOI] [PubMed] [Google Scholar]
- Cheng W, Rolls ET, Qiu J, Xie X, Wei D, Huang C-C, Yang AC, Tsai S-J, Li Q, Meng J, et al. Increased functional connectivity of the posterior cingulate cortex with the lateral orbitofrontal cortex in depression. Transl Psychiatry. 2018b:8(1):90. 10.1038/s41398-018-0139-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou T, Deckersbach T, Dougherty DD, Hooley JM. The default mode network and rumination in individuals at risk for depression. Soc Cogn Affect Neurosci. 2023:18(1):nsad032. 10.1093/scan/nsad032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corruble E, Legrand JM, Zvenigorowski H, Duret C, Guelfi JD. Concordance between self-report and clinician’s assessment of depression. J Psychiatry Res. 1999:33(5):457–465. 10.1016/S0022-3956(99)00011-4. [DOI] [PubMed] [Google Scholar]
- D’Argembeau A, Xue G, Lu Z-L, Linden MV, Bechara A. Neural correlates of envisioning emotional events in the near and far future. NeuroImage. 2008:40(1):398–407. 10.1016/j.neuroimage.2007.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demyttenaere K, Donneau AF, Albert A, Ansseau M, Constant E, Heeringen KV. What is important in being cured from depression? Discordance between physicians and patients (1). J Affect Disord. 2015:174:390–396. 10.1016/j.jad.2014.12.004. [DOI] [PubMed] [Google Scholar]
- Domken M, Scott J, Kelly P. What factors predict discrepancies between self and observer ratings of depression? J Affect Disord. 1994:31(4):253–259. 10.1016/0165-0327(94)90101-5. [DOI] [PubMed] [Google Scholar]
- Duberstein PR, Heisel MJ. Personality traits and the reporting of affective disorder symptoms in depressed patients. J Affect Disord. 2007:103(1–3):165–171. 10.1016/j.jad.2007.01.025. [DOI] [PubMed] [Google Scholar]
- Duncan J. The multiple-demand (MD) system of the primate brain: mental programs for intelligent behaviour. Trends Cogn Sci. 2010:14(4):172–179. 10.1016/j.tics.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Dunlop BW, Li T, Kornstein SG, Friedman ES, Rothschild AJ, Pedersen R, Ninan P, Keller M, Trivedi MH. Concordance between clinician and patient ratings as predictors of response, remission, and recurrence in major depressive disorder. J Psychiatry Res. 2011:45(1):96–103. 10.1016/j.jpsychires.2010.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eguchi S, Koike S, Suga M, Takizawa R, Kasai K. Psychological symptom and social functioning subscales of the modified global assessment of functioning scale: reliability and validity of the Japanese version. Psychiatry Clin Neurosci. 2015:69(2):126–127. 10.1111/pcn.12250. [DOI] [PubMed] [Google Scholar]
- Enns MW, Larsen DK, Cox BJ. Discrepancies between self and observer ratings of depression. The relationship to demographic, clinical and personality variables. J Affect Disord. 2000:60(1):33–41. 10.1016/S0165-0327(99)00156-1. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV Axis I disorders (SCID-I), clinician version, administration booklet. Washington DC: American Psychiatric Publishing; 2012. [Google Scholar]
- Fleming SM, Weil RS, Nagy Z, Dolan RJ, Rees G. Relating introspective accuracy to individual differences in brain structure. Science. 2010:329(5998):1541–1543. 10.1126/science.1191883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming SM, Huijgen J, Dolan RJ. Prefrontal contributions to metacognition in perceptual decision making. J Neurosci. 2012:32(18):6117–6125. 10.1523/JNEUROSCI.6489-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming SM, Ryu J, Golfinos JG, Blackmon KE. Domain-specific impairment in metacognitive accuracy following anterior prefrontal lesions. Brain. 2014:137(10):2811–2822. 10.1093/brain/awu221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher PC, Henson RNA. Frontal lobes and human memory: insights from functional neuroimaging. Brain. 2001:124(5):849–881. 10.1093/brain/124.5.849. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Essen DCV, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. 2005:102(27):9673–9678. 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Biswal B, Yang J, Li S, Wang YQ, Chen S, Yuan JJ. Temporal dynamic patterns of the ventromedial prefrontal cortex underlie the association between rumination and depression. Cereb Cortex. 2023:33(4):969–982. 10.1093/cercor/bhac115. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci USA. 2001:98(7):4259–4264. 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton MA. Rating scale for depression. J Neurol Neurosurg Psychiatry. 1960:23(1):56–62. 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JP, Furman DJ, Chang C, Thomason ME, Dennis E, Gotlib IH. Default-mode and task-positive network activity in major depressive disorder: implications for adaptive and maladaptive rumination. Biol Psychiatry. 2011:70(4):327–333. 10.1016/j.biopsych.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JP, Farmer M, Fogelman P, Gotlib IH. Depressive rumination, the default-mode network, and the dark matter of clinical neuroscience. Biol Psychiatry. 2015:78(4):224–230. 10.1016/j.biopsych.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden BY, Nair AC, McCoy AN, Platt ML. Posterior cingulate cortex mediates outcome-contingent allocation of behavior. Neuron. 2008:60(1):19–25. 10.1016/j.neuron.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata-Mogi S, Koike S, Toriyama R, Matsuoka K, Kim Y, Kasai K. Reliability of a paper-and-pencil version of the Japanese Adult Reading Test short version (JART25). Psychiatry Clin Neurosci. 2016:70(8):362. 10.1111/pcn.12400. [DOI] [PubMed] [Google Scholar]
- Inada T, Inagaki A. Psychotropic dose equivalence in Japan. Psychiatry Clin Neurosci. 2015:69(8):440–447. 10.1111/pcn.12275. [DOI] [PubMed] [Google Scholar]
- Inada T, Inagaki A, Iwamoto K, Nakatani M, Hori H, Higuchi T. Clinical assessment of mania using the Young Mania Rating Scale-J (YMRS-J). Jihou: Revised Edition; 2012.
- Inagaki A, Inada T. Dose equivalence of psychotropic drugs (part 26) dose equivalence of novel antipsychotics: asenapine. Jpn J Clin Psychopharmacol. 2017:20(1):89–97. [Google Scholar]
- Jacob Y, Morris LS, Huang KH, Schneider M, Rutter S, Verma G, Murrough JW, Balchandani P. Neural correlates of rumination in major depressive disorder: a brain network analysis. Neuroimage Clin. 2020:25:102142. 10.1016/j.nicl.2019.102142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R, Bhattacharya J. A role for the precuneus in thought-action fusion: evidence from participants with significant obsessive-compulsive symptoms. Neuroimage Clin. 2014:4:112–121. 10.1016/j.nicl.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Bromet EJ. The epidemiology of depression across cultures. Annu Rev Publ Health. 2013:34(1):119–138. 10.1146/annurev-publhealth-031912-114409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima M, Furukawa T. BDI-II - Beck Depression Inventory. Tokyo, Japan: Nihon Bunka Kagakusha; 2003. [Google Scholar]
- Kojima M, Furukawa T, Takahashi H, Kawai M, Nagaya T, Tokudome S. Cross-cultural validation of the Beck Depression Inventory-II in Japan. Psychiatry Res. 2002:110(3):291–299. 10.1016/S0165-1781(02)00106-3. [DOI] [PubMed] [Google Scholar]
- Kovach CK, Daw ND, Rudrauf D, Tranel D, O’Doherty JP, Adolphs R. Anterior prefrontal cortex contributes to action selection through tracking of recent reward trends. J Neurosci. 2012:32(25):8434–8442. 10.1523/JNEUROSCI.5468-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kross E, Davidson M, Weber J, Ochsner K. Coping with emotions past: the neural bases of regulating affect associated with negative autobiographical memories. Biol Psychiatry. 2009:65(5):361–366. 10.1016/j.biopsych.2008.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech R, Sharp DJ. The role of the posterior cingulate cortex in cognition and disease. Brain. 2014:137(1):12–32. 10.1093/brain/awt162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemogne C, Delaveau P, Freton M, Guionnet S, Fossati P. Medial prefrontal cortex and the self in major depression. J Affect Disord. 2012:136(1–2):e1–e11. 10.1016/j.jad.2010.11.034. [DOI] [PubMed] [Google Scholar]
- Ls R. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977:1(3):385–401. 10.1177/014662167700100306. [DOI] [Google Scholar]
- Lundstrom BN, Ingvar M, Petersson KM. The role of precuneus and left inferior frontal cortex during source memory episodic retrieval. NeuroImage. 2005:27(4):824–834. 10.1016/j.neuroimage.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Maddock RJ, Garrett AS, Buonocore MH. Remembering familiar people: the posterior cingulate cortex and autobiographical memory retrieval. Neuroscience. 2001:104(3):667–676. 10.1016/S0306-4522(01)00108-7. [DOI] [PubMed] [Google Scholar]
- Maddock RJ, Garrett AS, Buonocore MH. Posterior cingulate cortex activation by emotional words: fMRI evidence from a valence decision task. Hum Brain Mapp. 2003:18(1):30–41. 10.1002/hbm.10075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansouri FA, Buckley MJ, Mahboubi M, Tanaka K. Behavioral consequences of selective damage to frontal pole and posterior cingulate cortices. Proc Natl Acad Sci USA. 2015:112(29):E3940–E3949. 10.1073/pnas.1422629112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka K, Kim Y. Brief assessment of intellectual function: Japanese Adult Reading Test (JART). Tokyo, Japan: Shinkoh Igaku Shuppansha, 2006. [Google Scholar]
- Matsuoka K, Uno M, Kasai K, Koyama K, Kim Y. Estimation of premorbid IQ in individuals with Alzheimer's disease using Japanese ideographic script (kanji) compound words: Japanese version of National Adult Reading Test. Psychiatry Clin Neurosci. 2006:60(3):332–339. 10.1111/j.1440-1819.2006.01510.x. [DOI] [PubMed] [Google Scholar]
- McCurdy LY, Maniscalco B, Metcalfe J, Liu KY, Lange FP, Lau H. Anatomical coupling between distinct metacognitive systems for memory and visual perception. J Neurosci. 2013:33(5):1897–1906. 10.1523/JNEUROSCI.1890-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto K, Setsuie R, Osada T, Miyashita Y. Reversible silencing of the frontopolar cortex selectively impairs metacognitive judgment on non-experience in primates. Neuron. 2018:97(4):980–989.e6. 10.1016/j.neuron.2017.12.040. [DOI] [PubMed] [Google Scholar]
- Möller HJ, Zerssen D. Self-rating procedures in the evaluation of antidepressants. Psychopathology. 1995:28(6):291–306. 10.1159/000284941. [DOI] [PubMed] [Google Scholar]
- Nejad AB, Fossati P, Lemogne C. Self-referential processing, rumination, and cortical midline structures in major depression. Front Hum Neurosci. 2013:7:666. 10.3389/fnhum.2013.00666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci. 2005:9(5):242–249. 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Okuda J, Fujii T, Ohtake H, Tsukiura T, Tanji K, Suzuki K, Kawashima R, Fukuda H, Itoh M, Yamadori A. Thinking of the future and past: the roles of the frontal pole and the medial temporal lobes. NeuroImage. 2003:19(4):1369–1380. 10.1016/S1053-8119(03)00179-4. [DOI] [PubMed] [Google Scholar]
- Patel V, Chisholm D, Parikh R, Charlson FJ, Degenhardt L, Dua T, Ferrari AJ, Hyman S, Laxminarayan R, Levin C, et al. Addressing the burden of mental, neurological, and substance use disorders: key messages from disease control priorities, 3rd edition. Lancet. 2016:387(10028):1672–1685. 10.1016/S0140-6736(15)00390-6. [DOI] [PubMed] [Google Scholar]
- Pearson JM, Heilbronner SR, Barack DL, Hayden BY, Platt ML. Posterior cingulate cortex: adapting behavior to a changing world. Trends Cogn Sci. 2011:15(4):143–151. 10.1016/j.tics.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng D, Liddle EB, Iwabuchi SJ, Zhang C, Wu Z, Liu J, Jiang K, Xu L, Liddle PF, Palaniyappan L, et al. Dissociated large-scale functional connectivity networks of the precuneus in medication-naïve first-episode depression. Psychiatry Res Neuroimaging. 2015:232(3):250–256. 10.1016/j.pscychresns.2015.03.003. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci USA. 2001:98(2):676–682. 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rane LJ, Fekadu A, Wooderson S, Poon L, Markopoulou K, Cleare AJ. Discrepancy between subjective and objective severity in treatment-resistant depression: prediction of treatment outcome. J Psychiatry Res. 2010:44(15):1082–1087. 10.1016/j.jpsychires.2010.03.020. [DOI] [PubMed] [Google Scholar]
- Richardson K, Barkham M. Recovery from depression: a systematic review of perceptions and associated factors. J Ment Health. 2020:29(1):103–115. 10.1080/09638237.2017.1370629. [DOI] [PubMed] [Google Scholar]
- Richter P, Werner J, Heerlein A, Kraus A, Sauer H. On the validity of the Beck Depression Inventory. A review. Psychopathology. 1998:31(3):160–168. 10.1159/000066239. [DOI] [PubMed] [Google Scholar]
- Rush AJ. The varied clinical presentations of major depressive disorder. J Clin Psychiatry. 2007:68(10):1617–1610. 10.4088/JCP.v68n1023a. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Hiser W, Giles DE. A comparison of self-reported versus clinician-related symptoms in depression. J Clin Psychiatry. 1987:48(6):246–248. [PubMed] [Google Scholar]
- Rzepa E, McCabe C. Anhedonia and depression severity dissociated by dmPFC resting-state functional connectivity in adolescents. J Psychopharmacol. 2018:32(10):1067–1074. 10.1177/0269881118799935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato W, Kochiyama T, Uono S, Sawada R, Kubota Y, Yoshimura S, Toichi M. Resting-state neural activity and connectivity associated with subjective happiness. Sci Rep. 2019:9(1):12098. 10.1038/s41598-019-48510-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneibel R, Brakemeier E-L, Wilbertz G, Dykierek P, Zobel I, Schramm E. Sensitivity to detect change and the correlation of clinical factors with the Hamilton Depression Rating Scale and the Beck Depression Inventory in depressed inpatients. Psychiatry Res. 2012:198(1):62–67. 10.1016/j.psychres.2011.11.014. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Price JL, Yan Z, Mintun MA. Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proc Natl Acad Sci USA. 2010:107(24):11020–11025. 10.1073/pnas.1000446107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shima S. CES-D scale. Tokyo: Chiba Test Center; 1998 [Google Scholar]
- Shima S, Shikano T, Kitamura T. A new self-report depression scale. Psychiatry. 1985:27:717–723. [Google Scholar]
- Shulman GL, Fiez JA, Corbetta M, Buckner RL, Miezin FM, Raichle ME, Petersen SE. Common blood flow changes across visual tasks: II. Decreases in cerebral cortex. J Cogn Neurosci. 1997:9(5):648–663. 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- Sinanaj I, Cojan Y, Vuilleumier P. Inter-individual variability in metacognitive ability for visuomotor performance and underlying brain structures. Conscious Cogn. 2015:36:327–337. 10.1016/j.concog.2015.07.012. [DOI] [PubMed] [Google Scholar]
- Stanley B, Wilson ST. Heightened subjective experience of depression in borderline personality disorder. J Personal Disord. 2004:20(4):307–318. 10.1521/pedi.2006.20.4.307. [DOI] [PubMed] [Google Scholar]
- Tabuse H, Kalali A, Azuma H, Ozaki N, Iwata N, Naitoh H, Higuchi T, Kanba S, Shioe K, Akechi T, et al. The new GRID Hamilton Rating Scale for depression demonstrates excellent inter-rater reliability for inexperienced and experienced raters before and after training. Psychiatry Res. 2007:153(1):61–67. 10.1016/j.psychres.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Kitamura T, Okano T. Seishinka Shindan Mensetsu Manual SCID [Japanese Version of the Structured Clinical Interview for DSM-VI-TR Axis I Disorders], 2nd edition. Tokyo, Japan: Nihonhyouronsha; 2010. [Google Scholar]
- Tozzi L, Tuzhilina E, Glasser MF, Hastie TJ, Williams LM. Relating whole-brain functional connectivity to self-reported negative emotion in a large sample of young adults using group regularized canonical correlation analysis. NeuroImage. 2021a:237:118137. 10.1016/j.neuroimage.2021.118137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozzi L, Zhang X, Chesnut M, Holt-Gosselin B, Ramirez CA, Williams LM. Reduced functional connectivity of default mode network subsystems in depression: meta-analytic evidence and relationship with trait rumination. Neuroimage Clin. 2021b:30:102570. 10.1016/j.nicl.2021.102570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujii N, Akashi H, Mikawa W, Tsujimoto E, Niwa A, Adachi T, Shirakawa O. Discrepancy between self- and observer-rated depression severities as a predictor of vulnerability to suicide in patients with mild depression. J Affect Disord. 2014:161:144–149. 10.1016/j.jad.2014.03.014. [DOI] [PubMed] [Google Scholar]
- Uher R, Farmer A, Maier W, Rietschel M, Hauser J, Marusic A, Mors O, Elkin A, Williamson RJ, Schmael C, et al. Measuring depression: comparison and integration of three scales in the GENDEP study. Psychol Med. 2008:38(2):289–300. 10.1017/S0033291707001730. [DOI] [PubMed] [Google Scholar]
- Üstün TB, Ayuso-Mateos JL, Chatterji S, Mathers C, Murray CJL. Global burden of depressive disorders in the year 2000. Br J Psychiatry. 2004:184(5):386–392. 10.1192/bjp.184.5.386. [DOI] [PubMed] [Google Scholar]
- Veer IM, Beckmann CF, Tol MJ, Ferrarini L, Milles J, Veltman DJ, Aleman A, Van BMA, Vander WNJ, Rombouts SARB. Whole brain resting-state analysis reveals decreased functional connectivity in major depression. Front Syst Neurosci. 2010:4:41. 10.3389/fnsys.2010.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S, Aboyans V, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the global burden of disease study 2010. Lancet. 2013:380(9859):2163–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Li K, Zhang Q, Zeng Y, Dai W, Su Y, Wang G, Tan Y, Jin Z, Yu X, et al. Short-term effects of escitalopram on regional brain function in first-episode drug-naive patients with major depressive disorder assessed by resting-state functional magnetic resonance imaging. Psychol Med. 2014:44(7):1417–1426. 10.1017/S0033291713002031. [DOI] [PubMed] [Google Scholar]
- Wang Y, Chen X, Liu R, Zhang Z, Zhou J, Feng Y, Jiang C, Zuo XN, Zhou Y, Wang G. Effect of phase-encoding direction on gender differences: a resting-state functional magnetic resonance imaging study. Front Neurosci. 2021:15:748080. 10.3389/fnins.2021.748080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Zeng M, Li J, Liu Y, Wei D, Long Z, Chen H, Zang X, Yang J. Neural representation of collective self-esteem in resting-state functional connectivity and its validation in task-dependent modality. Neuroscience. 2023:530:66–78. 10.1016/j.neuroscience.2023.08.017. [DOI] [PubMed] [Google Scholar]
- Waskom ML, Kumaran D, Gordon AM, Rissman J, Wagner AD. Frontoparietal representations of task context support the flexible control of goal-directed cognition. J Neurosci. 2014:34(32):10743–10755. 10.1523/JNEUROSCI.5282-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012:2(3):125–141. 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- Williams J, Kobak K, Bech P, Engelhardt N, Evans K, Lipsitz J, Olin J, Pearson J, Kalali A. The GRID-HAMD: standardization of the Hamilton Depression Rating Scale. Int Clin Psychopharmacol. 2008:23(3):120–129. 10.1097/YIC.0b013e3282f948f5. [DOI] [PubMed] [Google Scholar]
- World Health Organization . The World Health Report 2001 - mental health: new understanding, new hope. Geneva, Switzerland: World Health Organization; 2001. [Google Scholar]
- World Health Organization . The global burden of disease: 2004 update. Geneva, Switzerland: World Health Organization; 2008.
- Xue C, Zhang X, Cao P, Yuan Q, Liang X, Zhang D, Qi W, Hu J, Xiao C. Evidence of functional abnormalities in the default mode network in bipolar depression: a coordinate-based activation likelihood estimation meta-analysis. J Affect Disord. 2023:326:96–104. 10.1016/j.jad.2023.01.088. [DOI] [PubMed] [Google Scholar]
- Young R, Biggs J, Ziegler V, Meyer D. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978:133(5):429–435. 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Han Y, Wang Y, Zhang Y, Jia H, Jin E, Deng L, Li L. Characterization of resting-state fMRI-derived functional connectivity in patients with deficiency versus excess pattern of major depression. Complement Ther Med. 2015:23(1):7–13. 10.1016/j.ctim.2014.12.010. [DOI] [PubMed] [Google Scholar]
- Zhou HX, Chen X, Shen YQ, Li L, Chen NX, Zhu ZC, Castellanos FX, Yan CG. Rumination and the default mode network: meta-analysis of brain imaging studies and implications for depression. NeuroImage. 2020:206:116287. 10.1016/j.neuroimage.2019.116287. [DOI] [PubMed] [Google Scholar]