Abstract
Background
To estimate global and regional trends from 2000 to 2020 of the number of persons visually impaired by cataract and their proportion of the total number of vision-impaired individuals.
Methods
A systematic review and meta-analysis of published population studies and gray literature from 2000 to 2020 was carried out to estimate global and regional trends. We developed prevalence estimates based on modeled distance visual impairment and blindness due to cataract, producing location-, year-, age-, and sex-specific estimates of moderate to severe vision impairment (MSVI presenting visual acuity <6/18, ≥3/60) and blindness (presenting visual acuity <3/60). Estimates are age-standardized using the GBD standard population.
Results
In 2020, among overall (all ages) 43.3 million blind and 295 million with MSVI, 17.0 million (39.6%) people were blind and 83.5 million (28.3%) had MSVI due to cataract blind 60% female, MSVI 59% female. From 1990 to 2020, the count of persons blind (MSVI) due to cataract increased by 29.7%(93.1%) whereas the age-standardized global prevalence of cataract-related blindness improved by −27.5% and MSVI increased by 7.2%. The contribution of cataract to the age-standardized prevalence of blindness exceeded the global figure only in South Asia (62.9%) and Southeast Asia and Oceania (47.9%).
Conclusions
The number of people blind and with MSVI due to cataract has risen over the past 30 years, despite a decrease in the age-standardized prevalence of cataract. This indicates that cataract treatment programs have been beneficial, but population growth and aging have outpaced their impact. Growing numbers of cataract blind indicate that more, better-directed, resources are needed to increase global capacity for cataract surgery.
Subject terms: Epidemiology, Lens diseases
Introduction
For 2020, the Global Burden of Disease (GBD) Study reported that cataract remained the leading cause of blindness, with approximately 15.2 million cases [95% Uncertainty Interval (UI): 12.7–18.0) that comprised 45% of global blindness [1]. Cataract also remained the second leading cause of moderate and severe vision impairment (MSVI), with 78.8 million (95% UI: 67.2–91.4) people, which comprised 39% of global MSVI. In spite of global advocacy efforts, such as the VISION 2020 Right to Sight program undertaken by the World Health Organization and International Agency of the Prevention Blindness, and an increase in cataract surgical rates (the number of cataract surgeries per million population) reported across the world, the progress made against cataract has revealed substantial inequality and inequity, with lower-to-middle income countries (LMICs) shouldering the greater burden and having poorer visual outcomes than high-income countries [2–4].
The majority of cataracts are age-related nuclear cataracts, which typically cause vision loss in the sixth decade or later [5–7]. Cataracts, part of the spectrum of diabetic eye disease, are also increasing due to a global epidemic of diabetes, with diabetics more likely to develop cataracts and more quickly lose their vision to cataract compared to people without diabetes [5, 8, 9].
Surgery is the only treatment for cataract, during which an artificial intraocular lens replaces the damaged lens. Cataract surgery is extremely efficacious in terms of restoring sight, and multiple studies have demonstrated its cost-effectiveness, which appears to increase over time [10–12]. In 2015, the International Council of Ophthalmology estimated that globally, there were 14 ophthalmologists performing cataract surgery per million population, but that ranges from less than 1 cataract surgeon per million in low-income countries to as high as 32 in high-income countries, further revealing the global inequity in access to eye care [13]. Age-related cataract exposes another persistent inequity in universal eye health coverage–– men are 1.7 times more likely to undergo cataract surgery than women, and even in high-income countries, women are more likely to wait longer for surgery and experience poorer outcomes [14]. This gender inequity is partially due to the fact that women live longer than men, although sociocultural barriers are also at play. Based on the 2015 GBD Vision Loss Expert Group data, if women had the same access to cataract surgery as men, the blindness burden of cataract could decrease by 11%. However, women, in fact, need more access to surgery than men, to address the gender inequity [14]. Gender differences in cataract burden for 2020 have yet to be analyzed.
With the publication of 2020 GBD vision loss data, there is a need to explore further the global and regional trends in cataract burden since 1990 and better understand the regional and gender inequities of cataract burden. The objective of this article is to provide updated estimates of the global burden of vision loss due to cataract, disaggregated by sex and region, for the period from 2000 to 2020 covered by Global Vision 2020. This is done using the best available ophthalmic epidemiological database, the Global Vision Database which is a comprehensive, continuously updated, online database of ophthalmic epidemiological data curated by the Vision Loss Expert Group (VLEG) [15–17]. Additionally, we assess progress against the goals set out in ‘Towards universal eye health: global action plan 2014–2019 of the World 60 Health Assembly (2013) [18]. This Global Action Plan set a target to reduce the prevalence of avoidable blindness by 25% from 2010 to 2019.
Methods
The VLEG have maintained, and progressively updated a systematic review of population-based studies of vision impairment and blindness published between Jan 1, 1980, and Oct 1, 2018, including gray literature sources. Data from this systematic review were combined with data from the repository of Rapid Assessment of Avoidable Blindness (RAAB) studies, and data contributed by the GBD obtained from the US National Health and Nutrition Examination survey and the WHO Study on Global Ageing and Adult Health. Detailed methods are published elsewhere [17, 19], and briefly described herein.
In total, the systematic review identified 137 studies, and the VLEG extracted data from 70 studies in 2010, and a further 67 studies in 2014–18 [16]. Studies were primarily national and subnational cross-sectional surveys. The VLEG commissioned the preparation of 5-year age-disaggregated data from the RAAB repository [20]. Studies were included if they met these criteria: population-representative and visual acuity measured using a test chart that could be mapped to Snellen fractions. Studies using self-reported vision loss were excluded. We used the International Classification of Diseases 11th edition criteria for vision loss, as recommended by the WHO, which categorizes people according to presenting better-eye visual acuity. The classification defines moderate vision loss as better eye visual acuity of 6/60 or better but worse than 6/18, severe vision loss as a visual acuity of 3/60 or better but worse than 6/60, and blindness as visual acuity of worse than 3/60 or less than 10° visual field around central fixation.
Data were stratified into datasets including so-called vision-loss envelopes (as per Flaxman et al. [16]) for all-cause mild, moderate, and severe vision loss, and blindness. Data were input into a mixed-effects meta-regression tool developed by the Institute for Health Metrics and Evaluation (IHME) called MR-BRT (meta regression; Bayesian; regularized; trimmed) [21]. Presenting vision impairment defined each level of severity. Prevalence data for under-corrected refractive error were extracted where available, and otherwise calculated by subtracting best-corrected vision impairment from presenting vision impairment for each level of severity in studies that reported both measures for a given location, sex, age group, and year. Other causes were quantified as part of the best-corrected estimates of vision impairment at each level of severity. Minimum age for inclusion of data was defined as 20 years for cataract.
We generated location, year, age, and sex-specific estimates of MSVI and blindness using Disease Modeling Meta-Regression (Dismod-MR) 2.1; [19] the data processing steps are described elsewhere [17]. In brief, Dismod-MR 2.1 models were run for all vision impairment strata (moderate, severe, blindness) regardless of cause and, separately, for MSVI and blindness for each modeled cause of vision impairment. Then, models of MSVI due to cataract were split into moderate and severe estimates using the ratio of overall prevalence in the all-cause moderate presenting vision impairment and severe presenting vision impairment models. Next, prevalence estimates for cataract were stratified by severity were scaled to the models of all-cause prevalence by severity. This produced final estimates by age, sex, year, and location for cataract vision impairment stratified by severity. We age-standardized our estimates using the GBD standard population [22]. Data on blindness and MSVI due to AMD were presented by seven super-regions (Southeast Asia/East Asia/Oceania, Central Europe/Eastern Europe/Central Asia, High-income, Latin America and Caribbean, North Africa and Middle East, South Asia, and Sub-Saharan Africa) and globally. Data on other causes of vision impairment and blindness will be presented in separate publications.
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
In 2020, 17.01 million (all ages, 95% uncertainty interval (UI) 14.40–19.93) people were blind due to cataract (Table 1). This breaks down by gender as 6.78 million (95% UI 5.73–7.98) men and 10.22 million (95% UI 8.76–11.96) women blind from cataract (Table 2). The majority of these are over 50 years of age with 15.17 million (95% UI 12.70–18.00) so affected (Table 1). Of these, 5.96 million (95% UI 4.98–7.11) men and 9.22 million (95% UI 7.73–10.88) women are blind from cataract (Table 2).
Table 1.
Number of people of all ages (mean [95% UI]) with blindness (presenting visual acuity <3/60) or MSVI (presenting visual acuity <6/18, ≥3/60) due to Cataract, the age-standardized prevalence (%) in people aged ≥50 years (mean [95% UI]), and the percentage of all blindness or MSVI attributed to Cataract in people aged ≥50 years (95% UI) in world regions in 2020.
| Blindness due to Cataract in 2020 | MSVI due to Cataract in 2020 | ||||||
|---|---|---|---|---|---|---|---|
| World Region | 2020, total population (‘000 s) | Number of people (‘000 s) with blindness in 2020 (all ages) | Age-standardized prevalence of Cataract blindness in 2020 (aged ≥50 years) | Percentage of all Cataract blindness in 2020 (≥50 years) | Number of people (‘000 s) with MSVI in 2020 (all ages) | Age-standardized prevalence of Cataract MSVI in people ≥50 years aged in 2020 | Percentage of Cataract MSVI in 2020 (≥50 years) |
| Global | 7,890,000 | 17,005 (14,395–19,926) | 0.84 (0.70–0.99) | 39.55 (33.48–46.34) | 83,475(71,758–95,983) | 4.34 (3.71–5.02) | 28.30 (24.32–32.54) |
| Southeast Asia, East Asia, and Oceania | 2,192,710 | 6305 (5322–7445) | 0.97 (0.81–1.15) | 41.82 (35.30–49.38) | 28,238 (24,354–32,390) | 4.71 (4.04–5.41) | 34.00 (29.32–39.00) |
| Central Europe, Eastern Europe, and Central Asia | 417,291 | 291 (234–356) | 0.19 (0.15–0.23) | 20.53 (16.50–25.15) | 3172(2620–3733) | 2.13 (1.75–2.52) | 17.64 (14.57–20.76) |
| High-income | 1,087,856 | 505(410–619) | 0.09 (0.07–0.11) | 16.82 (13.66–20.60) | 8214 (6955–9574) | 1.46 (1.22–1.71) | 26.44 (22.39–30.82) |
| Latin America and Caribbean | 601,551 | 1101 (910–1320) | 0.78 (0.64–0.95) | 30.11 (24.90–36.10) | 4658 (3935–5391) | 3.39 (2.85–3.96) | 19.03 (16.07–22.02) |
| North Africa and Middle East | 631,727 | 844 (676–1037) | 0.91 (0.71–1.14) | 27.31 (21.88–33.57) | 5399 (4573–6303) | 5.81 (4.92–6.80) | 24.73 (20.94–28.87) |
| South Asia | 1,841,435 | 6352 (5373–74169) | 2.23 (1.89–2.61) | 53.20 (45.00–62.11) | 28,744 (24,674–33,511) | 9.46 (8.11–10.93) | 29.87 (25.64–34.83) |
| Sub-Saharan Africa | 1,114,806 | 1604 (1358–1857) | 1.49 (1.24–1.78) | 31.56 (26.71–36.53) | 5047 (4342–5781) | 5.14 (4.40–5.93) | 24.69 (21.24–28.28) |
Table 2.
Number of males and females with blindness (presenting visual acuity <3/60), and the age-standardized prevalence (% [95% UI]) of blindness due to Cataract (all ages and people aged ≥50 years) in 2020.
| Total Population | Total number of Cataract blindness and aged-standardized Cataract blindness in 2020 (all ages) | Total number of Cataract blindness and aged-standardized Cataract blindness in people aged 50+ years in 2020 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| World Region | 2020, total population (‘000 s) | Number of males(‘000 s) with Cataract blindness in 2020 | Number of females (‘000 s) with Cataract blindness in 2020 | Age standardized prevalence of blindness in males in 2020 | Age standardized prevalence of blindness in females in 2020 | Number of males(‘000 s) (50+ years) with Cataract blindness in 2020 | Number of females(‘000 s) (50+ years) with Cataract blindness in 2020 | Age standardized prevalence of blindness in males in 2020 | Age standardized prevalence of blindness in females in 2020 |
| Global | 7,890,000 | 6781 (5733–7978) | 10,224 (8671–11,956) | 0.18 (0.15–0.21) | 0.23 (0.19–0.27) | 5957 (4978–7110) | 9217 (7728–10,884) | 0.73 (0.61–0.87) | 0.93 (0.78–1.09) |
| Southeast Asia, East Asia, and Oceania | 2,192,710 | 2297 (1929–2722) | 4007 (3394–4714) | 0.18 (0.16–0.22) | 0.29 (0.24–0.34) | 1966 (1626–2360) | 3577 (2990–4235) | 0.73 (0.61–0.88) | 1.16 (0.97–1.38) |
| Central Europe, Eastern Europe, and Central Asia | 417,291 | 102(81–124) | 188 (152–232) | 0.04 (0.04–0.05) | 0.05 (0.04–0.06) | 88 (70–111) | 177 (142–220) | 0.17 (0.14–0.22) | 0.20 (0.16–0.24) |
| High-income | 1,087,856 | 221(178–267) | 284 (231–351) | 0.02 (0.02–0.03) | 0.02 (0.02–0.03) | 196 (157–241) | 260 (209–325) | 0.09 (0.07–0.11) | 0.08 (0.07–0.11) |
| Latin America and Caribbean | 601,551 | 483 (397–579) | 617 (512–738) | 0.18 (0.15–0.22) | 0.19 (0.16–0.23) | 433 (353–529) | 571 (469–691) | 0.76 (0.62–0.93) | 0.80 (0.66–0.97) |
| North Africa and Middle East | 631,727 | 314 (249–389) | 529 (424–648) | 0.16 (0.13–0.20) | 0.26 (0.21–0.32) | 276 (214–350) | 478 (378–595) | 0.69 (0.54–0.86) | 1.11 (0.88–1.37) |
| South Asia | 1,841,435 | 2706 (2293–3166) | 3646 (3087–4262) | 0.45 (0.38–0.52) | 0.57 (0.48–0.66) | 2497 (2096–2939) | 3414 (2883–4026) | 1.95 (1.65–2.27) | 2.48 (2.10–2.90) |
| Sub-Saharan Africa | 1,114,806 | 655 (555–762) | 949 (801–1097) | 0.33 (0.27–0.38) | 0.40 (0.34–0.47) | 498 (415–605) | 737 (615–885) | 1.32 (1.09–1.59) | 1.63 (1.36–1.94) |
Overall, 83.48 million (95% UI 71.76–96.98) people are estimated to have MSVI from cataract (Table 1). Of these 34.59 million (95% UI 29.69–39.95) are men, and 48.89 million (95% UI 42.05–56.06) are women (Table 3). Again, the majority are over 50 years of age, 78.79 million (95% UI 67.20–91.40) people, 32.41 million (95% UI 27.55–37.74) men and 46.38 million (95% UI 39.66–53.66) women suffer from MSVI due to cataract (Tables 1 and 3).
Table 3.
Number of males and females with Cataract MSVI, and the age-standardized prevalence (% [95% uncertainty intervals (UIs)]) of Cataract MSVI (all ages and people aged ≥50 years) in 2020.
| Total population | Total number of Cataract MSVI and aged-standardized Cataract MSVI in 2020 (all ages) | Total number of Cataract MSVI and aged-standardized Cataract MSVI in people aged 50+ years in 2020 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| World Region | 2020, total population (‘000 s) | Number of males(‘000 s) with Cataract MSVI in 2020 | Number of females (‘000 s) with Cataract MSVI in 2020 | Age standardized prevalence of MSVI in males in 2020 | Age standardized prevalence of MSVI in females in 2020 | Number of males(‘000 s) (50+ years) with Cataract MSVI in 2020 | Number of females(‘000 s) (50+ years) with Cataract MSVI in 2020 | Age standardized prevalence of MSVI in males in 2020 | Age standardized prevalence of MSVI in females in 2020 |
| Global | 7,890,000 | 34,585 (29,694–39,953) | 48,889 (42,051–56,064) | 0.92 (0.79–1.05) | 1.08 (0.93–1.24) | 32,410 (27,550–37,744) | 46,375 (39,663–53,662) | 3.96 (3.38–4.59) | 4.67 (3.99–5.40) |
| Southeast Asia, East Asia, and Oceania | 2,192,710 | 11,351(9749–13,032) | 16,887 (14,577–19,432) | 0.94 (0.81–1.07) | 1.20 (1.04–1.37) | 10,676 (9,096–12,387) | 16,164 (13,880–18,651) | 4.06 (3.47–4.67) | 5.24 (4.51–6.00) |
| Central Europe, Eastern Europe, and Central Asia | 417,291 | 978 (805–1157) | 2194 (1806–2599) | 0.42 (0.35–0.49) | 0.53 (0.44–0.63) | 922(750–1101) | 2123 (1740–2524) | 1.80 (1.48–2.13) | 2.31 (1.90–2.74) |
| High-income | 1,087,856 | 2985(2525–3485) | 5228 (4431–6107) | 0.31 (0.26–0.36) | 0.38 (0.32–0.43) | 2811 (2349–3306) | 5071 (4278–5945) | 1.27 (1.06–1.48) | 1.59 (1.34–1.87) |
| Latin America and Caribbean | 601,551 | 2057 (1739–2390) | 2600 (2202–3008) | 0.78 (0.66–0.91) | 0.79 (0.67–0.92) | 1915 (1599–2242) | 2434 (2045–2847) | 3.38 (2.83–3.95) | 3.40 (2.86–3.99) |
| North Africa and Middle East | 631,727 | 2405 (2034–2811) | 2993 (2539–3482) | 1.22 (1.03–1.43) | 1.43 (1.22–1.67) | 2229(1868–2644) | 2795 (2354–3288) | 5.35 (4.52–6.30) | 6.27 (5.29–7.32) |
| South Asia | 1,841,435 | 12,732 (10,878–14,943) | 16,011 (13,766–18,590) | 1.98 (1.71–2.30) | 2.32 (2.00–2.67) | 12,017 (10,208–14,116) | 15,187 (12,989–17,698) | 8.71 (7.46–10.12) | 10.19 (8.74–11.74) |
| Sub-Saharan Africa | 1,1148,06 | 2073 (1780–2398) | 2973 (2565–3392) | 1.10 (0.95–1.27) | 1.29 (1.11–1.47) | 1837 (1562–2147) | 2599 (2219–3020) | 4.76 (4.08–5.52) | 5.47 (4.69–6.29) |
Cataract caused 39.55% (95% UI: 33.48, 46.34%) of all blindness in 2020 worldwide. Regionally, the highest proportion of cataract-related blindness was found in South Asia (53.20 [95% UI: 45.00, 62.11%]) and Southeast Asia, East Asia, and Oceania (41.82% [95% UI: 35.30, 49.38]) (Table 1). The regions with the lowest proportion of all cataract-related blindness of all blind individuals were High Income Countries (16.82% [UI: 13.66, 20.60]), and Central Europe, Eastern Europe, and Central Asia (20.53% [95% UI: 16.50, 25.15]). Cataract caused 28.30% (95% UI: 24.32, 32.54) of all cases with MSVI in 2020 worldwide. Southeast Asia, East Asia, and Oceania (34.00 (29.32-39.00)% [95% UI: 29.32, 39.00]), and South Asia (29.87% [95% UI: 25.64, 34.83]) were regions with the highest percentage of cataract-related MSVI of all visually impaired individuals (Table 1).
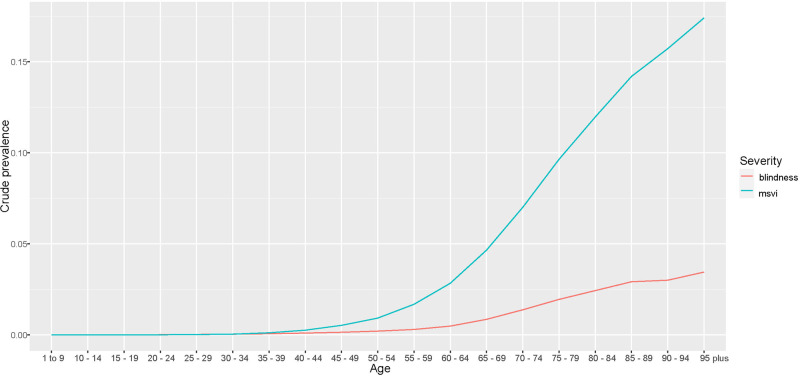
In 2020, the global age-standardized prevalence of cataract-related blindness in those aged ≥50 years was 0.84% (95% UI: 0.70, 0.99) and for cataract-related MSVI was 1.01% (95% UI: 0.87, 1.15) (Table 1). The variation of crude prevalence with age is shown in Fig. 1. The regions with the highest age-standardized prevalence of cataract-related blindness were South Asia (2.23% [95% UI: 1.89, 2.61]) and Sub-Saharan Africa (1.49% [95% UI: 1.24, 1.78]). The lowest age-standardized prevalence of cataract blindness in 2020 was in the regions of High Income Countries (0.09% [95% UI: 0.07, 0.11]) and Central Europe, Eastern Europe, and Central Asia (0.19% [95% UI: 0.15, 0.23]). The regions with the highest age-standardized prevalence of cataract-related MSVI in 2020 were South Asia (2.15% [95% UI: 1.85, 2.49]), and North Africa and the Middle East (1.33% [95% UI: 1.13, 1.55]). The lowest figures were found in high-income countries (0.35% [95% UI: 0.30, 0.40]) and Central Europe, Eastern Europe, and Central Asia (0.49% [95% UI: 0.41, 0.58]) (Table 1). The variation in these results by gender across the regions are shown in Tables 2 and 3.
Fig. 1.
Crude prevalence of Blindness and MSVI due to cataract in 2020 globally by age.
Between 2000 and 2020, the global percentage change in age-standardized prevalence of cataract-related blindness among adults ≥50 years decreased by 27.54% (95% UI: −27.68, −27.39), among males by −31.78% (95%UI −31.91, −31.64) and by 24.82% in females (95% UI: −24.97, −24.68) (Table 4). However, the absolute number of cases (unadjusted for age) increased by 29.72% (95% UI: 29.46, 29.98), in males 25.65% (95% CI 25.39, 25.92) and in females 32.49% (95% CI: 32.23, 32.75). An especially large reduction in the age-standardized prevalence of cataract-related blindness amongst adults aged ≥50 years (both sexes) was found in Southeast Asia, East Asia and Oceania (−42.99% [95% UI: −43.10, −42.88]), North Africa and Middle East (−39.97% [95% UI: −40.13, −39.81]) and South Asia (−36.53% [95% UI: −36.65, −36.41]), with a modest reduction in high-income countries (−6.86% [95% UI: −7.10, −6.62]) (Table 4). The greatest percentage increases in absolute number of cases were in Latin America and the Caribbean 71.25% (95% UI 70.86, 71.64) and in high income countries 49.30 (95% UI 48.92, 49.69). Only Central Europe, Eastern Europe, and Central Asia showed a reduction in the caseload (−4.40% [95% UI −4.66, −4.14].
Table 4.
Percentage change in crude prevalence of Cataract blindness (presenting visual acuity <3/60) in adults age 50 years and older between 2000 and 2020 by world super region.
| Crude Prevalence | Number of Cases (‘000 s) | Age standardized prevalence | |||||||
|---|---|---|---|---|---|---|---|---|---|
| World Region | Male (%, 95% UI) | Female (%, 95% UI) | Both (%, 95% UI) | Male (%, 95% UI) | Female (%, 95% UI) | Both (%, 95% UI) | Male (%, 95% UI) | Female (%, 95% UI) | Both (%, 95% UI) |
| Global | −28.75 (−28.90−28.61) | −24.58 (−24.73−24.43) | −26.30 (−26.45−26.15) | 25.65 (25.39–25.92) | 32.49 (32.23–32.75) | 29.72 (29.46–29.98) | −31.78 (−31.91−31.64) | −24.82 (−24.97−24.68) | −27.54 (−27.68−27.39) |
| High-income | 7.88 (7.61–8.16) | 2.58 (2.30–2.85) | 4.51 (4.24–4.78) | 58.76 (58.36–59.16) | 42.88 (42.50–43.26) | 49.30 (48.92–49.69) | −6.69 (−6.93−6.46) | −7.16 (−7.40−6.91) | −6.86 (−7.10−6.62) |
| Central Europe, Eastern Europe, and Central Asia | −19.82 (−20.04−19.60) | −25.74 (−25.94−25.55) | −24.23 (−24.44−24.02) | 4.00 (3.72–4.29) | −8.13 (−8.38−7.89) | −4.40 (−4.66−4.14) | −26.60 (−26.79−26.41) | −31.03 (−31.21−30.85) | −30.14 (−30.33−29.96) |
| Latin America and Caribbean | −19.70 (−19.89−19.51) | −10.77 (−10.97−10.57) | −14.83 (−15.02−14.64) | 58.24 (57.87–58.62) | 82.64 (82.23–83.05) | 71.25 (70.86–71.64) | −23.99 (−24.16−23.81) | −18.39 (−18.57−18.20) | −20.83 (−21.01−20.66) |
| North Africa and Middle East | −45.07 (−45.23−44.92) | −39.04 (−39.20−38.88) | −41.39 (−41.55−41.23) | 14.12 (13.80–14.44) | 26.84 (26.51–27.18) | 21.87 (21.54–22.19) | −41.99 (−42.15−41.83) | −39.64 (−39.80−39.49) | −39.97 (−40.13−39.81) |
| South Asia | −30.68 (−30.81−30.54) | −32.04 (−32.17−31.90) | −31.10 (−31.23−30.96) | 30.83 (30.57–31.09) | 37.40 (37.13–37.67) | 34.55 (34.28–34.81) | −34.95 (−35.06−34.83) | −38.63 (−38.74−38.52) | −36.53 (−36.65−36.41) |
| Southeast Asia, East Asia, and Oceania | −41.72 (−41.84−41.59) | −38.94 (−39.06−38.82) | −39.73 (−39.85−39.61) | 15.24 (14.99–15.49) | 24.55 (24.30–24.80) | 21.08 (20.83–21.33) | −46.86 (−46.97−46.75) | −40.55 (−40.67−40.44) | −42.99 (−43.10−42.88) |
| Sub-Saharan Africa | −29.68 (−29.84−29.52) | −28.83 (−28.98−28.68) | −28.74 (−28.90−28.59) | 24.41 (24.13–24.68) | 38.07 (37.77–38.36) | 32.21 (31.92–32.50) | −25.87 (−26.03−25.71) | −24.19 (−24.34−24.03) | −24.93 (−25.08−24.77) |
Between 2000 and 2020, the global percentage change in age-standardized prevalence of cataract MSVI among adults ( ≥ 50 years) increased (7.17% [95% UI: 6.98, 7.36]), among males (4.70% [95% UI 4.52, 4.89]) and females (8.94% [95% UI: 8.75, 9.13]) (Table 5). However, the absolute number of cases increased by 93.11% (95% UI: 92.75, 93.46), in males 93.69% (95% CI 93.32, 94.05) and in females 92.70% (95% CI: 92.36, 93.04). Sub-Saharan Africa (2.29% [95%UI 2.12, 2.47]) and Southeast Asia, East Asia and Oceania 1.96% [95%UI 1.78, 2.13]) were the only world regions where a substantial increase in the age-standardized prevalence of cataract MSVI was observed with notable decreases in South Asia (-5.53 [95% UI: -5.69, -5.37]) and Latin America and Caribbean (-4.83% [95% UI: -5.01, -4.65]). The increase in the absolute number of cataract MSVI cases was greatest in Southeast Asia, East Asia, and Oceania (115.21% [95%UI 114.83, 115.58]), and least in Central Europe, Eastern Europe, and Central Asia (38.18% [95%UI 37.87, 38.49]) (Table 5).
Table 5.
Percentage change in crude prevalence of Cataract MSVI (presenting visual acuity <6/18, ≥3/60) and in adults aged 50 years and older between 2000 and 2020 by world super region.
| Crude prevalence | Number of cases (‘000 s) | Age standardized prevalence | |||||||
|---|---|---|---|---|---|---|---|---|---|
| World Region | Male (%, 95% UI) | Female (%, 95% UI) | Both (%, 95% UI) | Male (%, 95% UI) | Female (%, 95% UI) | Both (%, 95% UI) | Male (%, 95% UI) | Female (%, 95% UI) | Both (%, 95% UI) |
| Global | 9.82 (9.61–10.03) | 9.70 (9.50–9.89) | 9.72 (9.52–9.92) | 93.69 (93.32–94.05) | 92.70 (92.36–93.04) | 93.11 (92.75–93.46) | 4.70 (4.52–4.89) | 8.94 (8.75–9.13) | 7.17 (6.98–7.36) |
| High-income | 20.98 (20.74–21.23) | 9.66 (9.44–9.88) | 12.62 (12.40–12.84) | 78.04 (77.67–78.40) | 52.75 (52.45–53.05) | 60.90 (60.58–61.21) | 1.56 (1.36–1.76) | 0.02 (−0.18–0.21) | −0.48 (−0.67–−0.28) |
| Central Europe, Eastern Europe, and Central Asia | 12.39 (12.13–12.66) | 9.21 (8.96–9.46) | 9.52 (9.27–9.77) | 45.79 (45.45–46.13) | 35.12 (34.81–35.42) | 38.18 (37.87–38.49) | 0.96 (0.74–1.17) | 1.33 (1.11–1.55) | 0.46 (0.24–0.68) |
| Latin America and Caribbean | −0.64 (−0.83–−0.44) | 2.41 (2.22–2.61) | 1.11 (0.91–1.31) | 95.81 (95.42–96.19) | 109.62 (109.21–110.02) | 103.30 (102.91–103.70) | −5.59 (−5.77–−5.41) | −4.22 (−4.40–−4.04) | −4.83 (−5.01–−4.65) |
| North Africa and Middle East | −6.92 (−7.11–−6.73) | −3.43 (−3.62–−3.24) | −5.00 (−5.19–−4.81) | 93.39 (92.99–93.78) | 100.96 (100.57–101.35) | 97.53 (97.14–97.92) | −1.03 (−1.22–−0.84) | −2.89 (−3.07–−2.71) | −1.92 (−2.10–−1.73) |
| South Asia | 0.83 (0.64–1.02) | −3.41 (−3.59–−3.23) | −1.14 (−1.33–−0.96) | 90.29 (89.93–90.65) | 95.27 (94.91–95.63) | 93.04 (92.68–93.40) | −3.46 (−3.63–−3.29) | −7.64 (−7.80–−7.48) | −5.53 (−5.69–−5.37) |
| Southeast Asia, East Asia, and Oceania | 8.65 (8.45–8.85) | 5.62 (5.43–5.80) | 7.12 (6.93–7.31) | 114.84 (114.44–115.24) | 115.45 (115.07–115.83) | 115.21 (114.83–115.58) | −0.70 (−0.87–−0.53) | 3.53 (3.36–3.70) | 1.96 (1.78–2.13) |
| Sub-Saharan Africa | −6.04 (−6.22–−5.87) | −0.15 (−0.33–0.03) | −2.29 (−2.47–−2.11) | 66.22 (65.91–66.53) | 93.70 (93.35–94.04) | 81.29 (80.96–81.62) | −0.60 (−0.78–−0.43) | 4.70 (4.52–4.88) | 2.29 (2.12–2.47) |
Discussion
Cataract, the world’s leading cause of blindness, remains one of the greatest opportunities in global health to make impactful and cost-effective contributions. Cataract surgery is safe and highly effective with both higher and lower technology approaches (e.g., phacoemulsification or extracapsular techniques respectively), and can be provided relatively inexpensively [23]. As a surgical condition, it requires a system able to provide one-at-a-time clinical care, like most causes of blindness and visual impairment. There are various eye service delivery models that can be used to address the cataract burden. However, it makes sense to combine it in a system with other ophthalmic services, ethically addressing other issues that will come to the attention of the service as well as providing a more professional-friendly work environment to retain capable eye care professionals (ophthalmologists, optometrists, eye nurses and others).
As an endemic condition, the ideal approach to the problem is to develop sufficient capacity and health system functionality to make ophthalmic surgery widely available worldwide. Given the relatively low level of infrastructure and consumables required for quality surgery, government health systems are well positioned to address this issue for the economically poorest persons. While funding limitations may constrain their systems’ scale [24], cataract surgery has considerable economic and quality of life benefits compared to its cost [10], which can offset the investment. Moreover, several health systems in different locations have demonstrated that self-sustaining services can be provided at costs most patients are willing to pay while also generating surpluses to provide service to the very poor [25, 26]. Such “cross-subsidizing” systems have made a large contribution to alleviating cataract blindness in much of the world, although these require a dominant service provider e.g. Aravind Eye Care System in South India. Systems for eye care should contemplate the value of ”patient financial contribution” for cataract surgery as much as possible; offering universal free or highly subsidized surgery may unnecessarily leave that health care financing resource at the table. In addition, surgical campaigns have been used extensively to deal with “backlogs” in cataract blindness; these are ideal for unreached/remote areas where development is unlikely to reach the cataract blind on a reasonable time scale without interfering with the ultimate solution of local capacity development. Our data demonstrate that these sorts of efforts have been fruitful in reducing the per capita levels of cataract blindness over the last 20 years over much of the world. Indeed, the World Health Assembly Global Action Plan target of a 25% reduction from 2010 to 2019 in avoidable vision impairment (WHA 66.3 24/5/2013) was met for cataract blindness (from an age-adjusted prevalence perspective) [18].
However, the successes have not kept pace with the impact of population growth and aging, with the result that the number of cataract blind is substantially increasing. Cataract also remains the leading cause of blindness despite these improvements and its favorable treatability. Thus, further investment in sustainable health systems able to provide quality cataract surgeries is likely to provide very substantial societal and economic net benefits. Because development is a long-term proposition, sustained commitment will be needed, whether through committed funders (e.g., government or charity programs) or self-sustaining organizations (private non-profit or social enterprise systems, or government systems allowing cost recovery).
While our data demonstrate a notable improvement in blindness (worse than 20/400 visual acuity), we did not see a similar decrease in MSVI (worse than 20/60 to 20/400) which also is associated with substantial disability/economic impact [27, 28]. Indeed, MSVI became more prevalent and nearly doubled in the number of cases. This pattern suggests successful targeting of the most severely impaired cases, albeit at the neglect of the less severely impaired. However, MSVI also needs to be targeted to alleviate visual disability and its socioeconomic impacts [27, 28]. Indeed, research into willingness to pay for cataract surgery suggests that people in the MSVI range (e.g., younger people otherwise capable of employment) may be more willing to pay for cataract surgery than more severe “blind” persons [26]. Expansion of the indications for cataract surgery may be needed to accomplish improvements in cataract MSVI also [29].
The WHO criteria score blindness and visual impairment based on the vision in the better eye. Following this logic, it would seem sensible in an economically constrained environment to focus on operating one eye. However, second eye surgeries also have important benefits to vision, visual ability and well-being [30, 31], and has been shown to have very high cost-effectiveness (cost per quality-adjusted life year gained) and a favorable cost-effectiveness in an evidence-based review [32, 33]. In addition, second eye surgery provides insurance that vision could continue in the event something happened to the first eye for persons in locations with poor service access. Binocular vision is important for activities requiring depth perception, falls prevention, increases contrast sensitivity and provides better binocular visual acuity than single eye surgery alone [31]. Because case finding of second eye cataracts and second eye operations have less marginal cost for bilateral cases than first eye cataracts [34], it is desirable to operate second eyes as well. Persons also may be more willing to pay for a cataract surgery after seeing the result of first eye cataract surgery [33]. Second eye cataract surgeries generally should be made available to patients in cataract programs, especially if patients are willing to pay some or all of the cost.
While improvements in cataract blindness were observed over the last 20 years, huge disparities in the prevalence remain between low- and high-income regions. South Asia has the highest number of cataract blind and by far the highest prevalence, a significant focus in this super region has the greatest potential for improvement. However, other poor regions (e.g., Sub-Saharan Africa) which are expected to see a growth in the elderly population in coming years and have a very high prevalence of cataract blindness amongst the elderly needing aggressive efforts to develop an eye care system capable of handling the volume of cataract surgery and other eye care services which can be forecast to be needed. Given the very low number of ophthalmologists and other eye care professionals in these areas, the time is now to strengthen and expand both training and systems for eye care delivery [34].
Our results demonstrated again that women are disproportionately represented amongst the cataract blind and visually impaired, and that the inequity is widening. The extent of this difference varies across the globe, but is generally consistent. The difference might reflect differences in family willingness to pay for male and female surgery [35]. Differences in acceptance of surgery between males and females could be another explanation. However, acceptance of clinical services tends to be higher among women than men in high income settings. Notably, female survival is generally longer than male survival which might be associated with a higher burden of age-related cataract even if service utilization were equal. Baruwa et al found that five years’ access to free cataract screening and low-cost high quality cataract surgery was associated with equalization in willingness to pay for cataract surgery across males and females [36]. Improving cataract surgery quality, community knowledge of the benefits of cataract surgery, and reducing barriers to surgical access likely are among the core strategies that need to be implemented in order to overcome the male-female gap in cataract surgery utilization. Without foregoing the promotion of cataract surgery among males, who also need to increase cataract surgery utilization, female surgery promoters and other strategies to increase female use of cataract surgery also could be helpful to reduce the disproportionately higher female cataract blindness and visual impairment burden.
The impact of the COVID-19 pandemic on cataract blindness is unclear at this time. Emerging evidence that service delivery was adversely affected during the emergency phase of the pandemic may drive the cataract burden up [37]. This may be offset by global decreases in life expectance from the disease and its sequelae [37]. These impacts may not be visible for several years, but are likely to be overwhelmed by existing trajectories of population growth and ageing.
In summary, as the population grows and ages while coverage of cataract surgery remains incomplete, immense numbers of people remain blind and vision impaired from cataract. These numbers are expected to continue growing markedly as the population increases and ages worldwide, especially in the least developed countries with young but rapidly aging populations and high cataract blindness/MSVI prevalence. While much has been achieved by initiatives to tackle cataract blindness, much more needs to be done to provide cataract surgery to those in need. Programs for delivering cataract to the vision impaired should not only target the blind, but also those with MSVI who also substantially benefit from treatment and appear to be under-targeted. High quality service provision is essential for inciting demand for cataract surgery, and thus is a key issue along with increasing the number of surgeries. Ophthalmologist training, which takes a long time, needs to be developed urgently in areas of insufficient coverage. Eye care systems in which ophthalmologists can operate successfully and other eye care professionals can work successful also are very important. While all regions with substantial numbers of cataract blind need increased services, females especially need to access cataract surgery more. Culturally appropriate efforts to promote female cataract surgery are an important piece of what needs to be done. Globally, immense increases in resource mobilization for treating cataract are required. All sources of healthcare financing need to be tapped to develop sustainable eye care systems able to tackle the cataract problem with high quality surgery.
Summary
What was known before
Globally, in 2020, 17.0 million people were blind and nearly 83.5 million were visually impaired by cataract.
What this study adds
The contribution of cataract to blindness and moderate and severe vision impairment (MSVI) by region and the change in this contribution between 2000 and 2020. The change in global age-standardized prevalence of cataract-related blindness and MSVI between 2000 and 2020 and the differences by sex and region.
Supplementary information
Author contributions
Please see Appendix for more detailed information about individual author contributions to the research, divided into the following categories: managing the overall research enterprise; writing the first draft of the manuscript; primary responsibility for applying analytical methods to produce estimates; primary responsibility for seeking, cataloguing, extracting, or cleaning data; designing or coding figures and tables; providing data or critical feedback on data sources; developing methods or computational machinery; providing critical feedback on methods or results; drafting the manuscript or revising it critically for important intellectual content; and managing the estimation or publications process.
Funding
This study was funded by Brien Holden Vision Institute, Fondation Thea, Fred Hollows Foundation, Bill & Melinda Gates Foundation, Lions Clubs International Foundation (LCIF), Sightsavers International, and University of Heidelberg. Open Access funding enabled and organized by CAUL and its Member Institutions.
Data availability
Data sources for the Global Vision Database are listed at the following weblink http://www.anglia.ac.uk/verigbd. Fully disaggregated data is not available publicly due to data sharing agreements with some principal investigators yet requests for summary data can be made to the corresponding author.
Competing interests
Vision Loss Expert Group of the Global Burden of Disease Study A Bron reports payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Théa. M A Del Monte reports support for attending meetings and/or travel from the University of Michigan, and leadership or fiduciary roles in board, society, committee or advocacy groups, paid or unpaid as past president of Costenbader Society. D Friedman reports leadership or fiduciary role in other board, society, committee or advocacy group paid or unpaid with Orbis International as member of board of governors (no payment). J M Furtado reports consulting fees from Pan American Health Organization and from Lions Club International Foundation. M E Hartnett reports support for the present manuscript (e.g., funding, provision of study materials, medical writing, article processing charges, etc.) from Michael F. Marmor, M.D. Professor of Retinal Science and Disease as endowment to support salary, grants or contracts from any entity (from National Eye Institute R01 EY017011 and National Eye Institute R01 EY015130) as partial salary support, patents planned, issued or pending (WO2015123561A2 and WO2021062169A1), and leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid with Jack McGovern Coats’ Disease Foundation and as director of Women’s Eye Health and Macular Society Grant Review Chair. J H Kempen reports support for the present manuscript (e.g., funding, provision of study materials, medical writing, article processing charges, etc.) from Sight for Souls and from Mass Eye and Ear Global Surgery Program (both as general support of salary), and leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid with Sight for Souls (as president). J E Kim reports consulting fees from Genentech/Roche, DORC, Notal Vision and Outlook Therapeutics (all as payment to J E Kim); participation on a Data Safety Monitoring Board or Advisory Board with Allergan, Amgen, Apellis, Bausch & Lomb, Clearside, Coherus, Novartis and Regeneron (all as participation on advisory board); leadership or fiduciary role in other borad, society, committee or advocacy group, paid or unpaid, with AAO, APRIS, ASRS, Macular Society and NAEVR/AEVR (all unpaid); and receipt of equipment, materials, drugs, medical writing, gifts or other services from Clearside and Genentech/Roche (both for medical writing). V C Lansingh reports consulting fees from HelpMeSee (as an employee), and support for attending meetings and/or travel from HelpMeSee (pay airfare and hotel). J Leasher reports leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid with National Eye Institute (as a member) and National Eye Health Education Program planning committee (unpaid). M Nowak reports participation on a Data Safety Monitoring Board or Advisory Board with Vision Express Co. Poland as the chairman of medical advisory board of Vision Express Co. Poland. P Ramulu reports grants or contracts from National Institute of Health and Perfuse Therapeutics, and consulting fees from Alcon and W. L. Gore. F Topouzis reports grants or contracts from Théa, Omikron, Pfizer, Alcon, Abbvie and Bayer (all paid to Institution), consulting fees from Omikron, Théa and Bausch & Lomb (all paid to Topouzis), payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Omikron (paid to Topouzis), Abbvie and Roche (both paid to Institute), and leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid with European Glaucoma Society (as president), Greek Glaucoma Society (as president) and Board of Governors, World Glaucoma Association (all unpaid). GBD 2019 Blindness and Vision Impairment Collaborators N S Bayileyegn reports participation on a Data Safety Monitoring Board or Advisory Board with Jimma University, and leadership or fiduciary roles in board, society, committee or advocacy groups, paid or unpaid with Jimma University as a discipline committee member; outside the submitted work. S Bhaskar reports grants or contracts from the Japan Society for the Promotion of Science (JSPS), JSPS International Fellowship, Japanese Ministry of Education, Culture, Sports, Science and Technology (MEXT), the Australian Academy of Science, Grant-in-Aid for Scientific Research (KAKENHI); leadership or fiduciary roles in board, society, committee or advocacy groups, paid or unpaid with Rotary District 9675 as the District Chair of Diversity, Equity, and Inclusion; the Global Health & Migration Hub Community and the Global Health Hub Germany (Berlin, Germany) as the Chair and Manager; PLOS One, BMC Neurology, Frontiers In Neurology, Frontiers in Stroke, Frontiers in Public Health and BMC Medical Research Methodology as an Editorial Board Member; and with the College of Reviewers, Canadian Institutes of Health Research (CIHR), and the Government of Canada as a Member; outside the submitted work. X Dai reports support for the present manuscript from the Institute for Health Metrics and Evaluation and the University of Washington. M Cenderadewi reports grants or contracts from James Cook University (International Research Training Program Scholarship for doctoral study), and support for attending meetings and travel from James Cook University; all outside the submitted work. M Foschi reports consulting fees from Roche; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Sanofi, Merck, and Novartis; support for attending meetings and travel from Novartis and Roche; leadership or fiduciary roles in board, society, committee or advocacy groups, paid or unpaid with MSBase as a scientific leadership board member, and Cochrane Review Group for Multiple Sclerosis and other rate diseases of the CNS as a member; all outside the submitted work. F Ghassemi reports support for the present manuscript from medical writing. B N G Goulart reports stock or stock options with Bristo Myers-Squibb and Pfizer; outside the submitted work. V B Gupta reports grants or contracts from the National Health and Medical Research Council (NHMRC); outside the submitted work. S Hallaj reports support for the present manuscript from the National Institute of Health, Bridge to AI common fund (grant number: OT2 OD032644). I M Ilic reports support for the present manuscript from the Ministry of Education, Science and Technological development, Republic of Serbia (project No 175042, 2011-2023). S Islam reports support for the present manuscript from the National Health and Medical Research Council (NHMRC) Investigator Grant and the Heart Foundation Vanguard Grant. J H Kempen reports support for the present manuscript from Sight for Souls and Mass Eye and Ear Global Surgery Program; and leadership or fiduciary roles in board, society, committee or advocacy groups, paid or unpaid with Sight for Souls as the President. K Krishan reports other non-financial support from the UGC Centre of Advanced Study, CAS II, awarded to the Department of Anthropology, Panjab University (Chandigarh, India); outside the submitted work. O P Kurmi reports grants or contracts from the British Council India paid to Coventry University; outside the submitted work. V C Lansingh reports consulting fees from HelpMeSee, and financial support for attending meetings and travel from HelpMeSee; outside the submitted work. J L Leasher leadership or fiduciary roles in board, society, committee or advocacy groups, unpaid with the National Eye Institute as a member and the National Eye Health Education Program as a planning committee member; outside the submitted work. M Lee reports support for the present manuscript from the Ministry of Education of the Republic of Korea, and the National Research Foundation of Korea (NRF-2021R1I1A4A01057428) and Bio-convergence Technology Education Program through the Korea Institute for Advancement Technology (KIAT) funded by the Ministry of Trade, Industry and Energy (No. P0017805). C McAlinden reports grants or contracts from the Welsh Government on the following study: Feasibility of an alternative pathway for hospital referrals from Diabetic Eye Screening Wales (DESW) for people suspected with sight-threatening diabetic eye disease (diabetic maculopathy). No funds will be received from the author’s institution or personally related to this study. Any work conducted as part of this study is as an unpaid collaborator; consulting fees from Acufocus (Irvine, California, USA), Atia Vision (Campbell, California, USA), Bausch and Lomb (Bridgewater, New Jersey, USA), BVI / PhysIOL (Liège, Belgium), Coopervision (Pleasanton, California, USA), Cutting Edge (Labége, France), Fudan University (Fudan, China), Hoya (Frankfurt, Germany), Knowledge Gate Group (Copenhagen, Denmark), Johnson & Johnson Surgical Vision (Santa Ana, California, USA), Keio University (Tokyo, Japan), Ludwig-Maximilians-University (München, Germany), Medevise Consulting SAS (Strasbourg, France), Novartis (Basel, Switzerland), Ophtec BV (Groningen, The Netherlands), Sun Yat-sen University (Guangzhou, China), SightGlass vision (Menlo Park, California, USA), Science in Vision (Bend, Oregan, USA), SpyGlass (Aliso Viejo, California, USA), Targomed GmbH (Bruchsal, Germany), University of São Paulo (São Paulo, Brazil), and Vold Vision (Arkansas, USA); payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Scope (Crawley, UK), Bausch and Lomb (Bridgewater, New Jersey, USA), and Thea pharmaceuticals (Clemont-Ferrand, France); support for attending meetings and/or travel from Royal College of Ophthalmologists (London, UK), Scope (Crawley, UK), Portuguese Society of Ophthalmology (Portugal), British Society of Refractive surgery (BSRS), Thea pharmaceuticals (Clemont-Ferrand, France), Bausch and Lomb (Bridgewater, New Jersey, USA); leadership or fiduciary roles in board, society, committee or advocacy groups, paid or unpaid with the British Society of Refractive Surgery (BSRS) as an unpaid council member, and the Royal College of Ophthalmologists (London, UK) as an unpaid PROM advisor; other financial interests from the Quality of Vision (QoV) Questionnaire tool, the Orthokeratology and Contact Lens Quality of Life Questionnaire (OCL-QoL), and paid peer reviews for Research Square; outside of the submitted work. S Nargus reports receipt of equipment, materials, drugs, medical writing, gifts or other services from Medical writing services; outside the submitted work. Y L Samodra and J H V Ticoalu report other financial or non-financial interests as co-founders of Benang Merah Research Center; outside the submitted work. J A Singh reports consulting fees from AstraZeneca, Crealta/Horizon, Medisys, Fidia, PK Med, Two labs Inc., Adept Field Solutions, Clinical Care options, Clearview healthcare partners, Putnam associates, Focus forward, Navigant consulting, Spherix, MedIQ, Jupiter Life Science, UBM LLC, Trio Health, Medscape, WebMD, Practice Point communications, and the National Institutes of Health and the American College of Rheumatology; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from the speaker’s bureau of Simply Speaking; support for attending meetings from OMERACT as a member of the steering committee; participation on an Advisory Committee with the FDA Arthritis Advisory Committee; leadership or fiduciary roles in board, society, committee or advocacy groups, paid or unpaid as a past steering committee member of the OMERACT, an international organization that develops measures for clinical trials and receives arms length funding from 12 pharmaceutical companies, Co-Chair of the Veterans Affairs Rheumatology Field Advisory Committee, and the editor and Director of the UAB Cochrane Musculoskeletal Group Satellite Center on Network Meta-analysis; stock or stock options in Atai Life Sciences, Kintara Therapeutics, Intelligent Biosolutions, Acumen Pharmaceutical, TPT Global Tech, Vaxart Pharmaceuticals, Atyu Biopharma, Adaptimmune Therapeutics, GeoVax Labs, Pieris Pharmaceuticals, Enzolytics Inc., Seres Therapeutics, Tonix Pharmaceuticals Holding Corp., and Charlotte’s Web Holdings, Inc, as well as previously owned stock options in Amarin, Viking and Moderna Pharmaceuticals; outside the submitted work. E Skiadaresi reports consulting fees from Bayer (Leverkusen, Germany), Novartis (Basel, Switzerland), Roche ((Basel, Switzerland), Medevise Consulting SAS (Strasbourg, France); payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Bayer (Leverkusen, Germany), Novartis (Basel, Switzerland), Roche ((Basel, Switzerland); support for attending meetings and travel from Bayer (Leverkusen, Germany), Novartis (Basel, Switzerland), Roche ((Basel, Switzerland); leadership or fiduciary roles in board, society, committee or advocacy groups, unpaid with the ATHENA Trial Steering Committee as the Chair; all outside the submitted work. J D Steinmetz reports support for the present manuscript from the Bill and Melinda Gates Foundation (IHME funding for GBD analyses). M Zielińska reports other financial interests as an AstraZeneca employee; outside the submitted work. The authors declare no competing interests.
Footnotes
The original online version of this article was revised. The author list has been corrected from “Konrad Pesudovs, Van Charles Lansingh, John H. Kempen, Ian Tapply, Arthur G. Fernandes, Maria V. Cicinelli, Alessandro Arrigo, Nicolas Leveziel, Paul Svitil Briant, Theo Vos, Serge Resnikoff, Hugh R. Taylor, Tabassom Sedighi, Seth Flaxman, Jaimie Steinmetz, Rupert Bourne, Vision Loss Expert Group of the Global Burden of Disease Study and the GBD 2019 Blindness and Vision Impairment Collaborators” to include only the following institutional authors “Vision Loss Expert Group of the Global Burden of Disease Study and the GBD 2019 Blindness and Vision Impairment Collaborators”. The list of the individual authors and affiliations now appear at the end of the original paper. Furthermore, the following Article Note has been added: “These authors contributed equally: Jaimie Steinmetz and Rupert Bourne. These authors share the last authorship: Seth Flaxman and Jaimie Steinmetz.” The ‘Funding’, ‘Competing Interests’ and ‘Author Contributions’ sections have also been updated.
Group Information: A list of the members of the Vision Loss Expert Group of the Global Burden of Disease Study can be found by accessing this site: HYPERLINK “https://urldefense.com/v3/__http:/www.anglia.ac.uk/verigbd__;!!NLFGqXoFfo8MMQ!uk9hazJ83udxE3YQ0PLyOxFNdlc_Flr5EqBuJG6wDrS-RMdepA4rGs2taZa52wqB53Y5VhGz-b60YdKdbKNo2Z8IafW1kw$” http://www.anglia.ac.uk/verigbd.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Jaimie D. Steinmetz, Rupert Bourne.
These authors jointly supervised this work: Seth Flaxman, Jaimie D. Steinmetz.
Lists of authors and their affiliations appear at the end of the paper.
Change history
7/16/2024
A Correction to this paper has been published: 10.1038/s41433-024-03161-7
Contributor Information
Vision Loss Expert Group of the Global Burden of Disease Study:
Konrad Pesudovs, Van Charles Lansingh, John H. Kempen, Ian Tapply, Arthur G. Fernandes, Maria Vittoria Cicinelli, Alessandro Arrigo, Nicolas Leveziel, Serge Resnikoff, Hugh R. Taylor, Tabassom Sedighi, Seth Flaxman, Mukkharram M. Bikbov, Tasanee Braithwaite, Alain Bron, Ching-Yu Cheng, Monte A. Del Monte, Joshua R. Ehrlich, Leon B. Ellwein, David Friedman, João M. Furtado, Gus Gazzard, Ronnie George, M. Elizabeth Hartnett, Jost B. Jonas, Rim Kahloun, Moncef Khairallah, Rohit C. Khanna, Janet Leasher, Julie-Anne Little, Vinay Nangia, Michal Nowak, Tunde Peto, Pradeep Ramulu, Fotis Topouzis, Mitiadis Tsilimbaris, Ya Xing Wang, Ningli Wang, and Rupert Bourne
GBD 2019 Blindness and Vision Impairment Collaborators:
Konrad Pesudovs, Van Charles Lansingh, John H. Kempen, Ian Tapply, Arthur G. Fernandes, Maria Vittoria Cicinelli, Alessandro Arrigo, Nicolas Leveziel, Paul Svitil Briant, Theo Vos, Serge Resnikoff, Seth Flaxman, Yohannes Habtegiorgis Abate, Mohammad Abdollahi, Mozhan Abdollahi, Ayele Mamo Abebe, Olumide Abiodun, Richard Gyan Aboagye, Woldu Aberhe Abrha, Hasan Abualruz, Hiwa Abubaker Ali, Eman Abu-Gharbieh, Salahdein Aburuz, Tadele Girum Girum Adal, Mesafint Molla Adane, Isaac Yeboah Addo, Qorinah Estiningtyas Sakilah Adnani, Muhammad Sohail Afzal, Shahin Aghamiri, Bright Opoku Ahinkorah, Aqeel Ahmad, Sajjad Ahmad, Ali Ahmadi, Ayman Ahmed, Haroon Ahmed, Ahmad Samir Alfaar, Abid Ali, Syed Shujait Shujait Ali, Awais Altaf, Hubert Amu, Sofia Androudi, Rodrigo Anguita, Abhishek Anil, Saeid Anvari, Anayochukwu Edward Anyasodor, Francis Appiah, Jalal Arabloo, Mosab Arafat, Damelash Areda, Reza Arefnezhad, Brhane Berhe Aregawi, Akeza Awealom Asgedom, Tahira Ashraf, Seyyed Shamsadin Athari, Bantalem Tilaye Tilaye Atinafu, Maha Moh’d Wahbi Atout, Alok Atreya, Haleh Ayatollahi, Ahmed Y. Azzam, Hassan Babamohamadi, Sara Bagherieh, Yogesh Bahurupi, Atif Amin Baig, Biswajit Banik, Mainak Bardhan, Saurav Basu, Kavita Batra, Nebiyou Simegnew Bayileyegn, Fatemeh Bazvand, Addisu Shunu Beyene, Devidas S. Bhagat, Akshaya Srikanth Bhagavathula, Pankaj Bhardwaj, Sonu Bhaskar, Jasvinder Singh Bhatti, Mukharram Bikbov, Niloufar Bineshfar, Marina G. Birck, Veera R. Bitra, Tasanee Braithwaite, Katrin Burkart, Yasser Bustanji, Zahid A. Butt, Florentino Luciano Caetano dos Santos, Luis Alberto Cámera, Vera L. A. Carneiro, Muthia Cenderadewi, Eeshwar K. Chandrasekar, Vijay Kumar Chattu, Nitin Chitranshi, Hitesh Chopra, Dinh-Toi Chu, Kaleb Coberly, João M. Coelho, Natália Cruz-Martins, Omid Dadras, Xiaochen Dai, Subasish Das, Ana Maria Dascalu, Mohsen Dashti, Maedeh Dastmardi, Berecha Hundessa Demessa, Biniyam Demisse, Diriba Dereje, Awoke Masrie Asrat Derese, Nikolaos Dervenis, Vinoth Gnana Chellaiyan Devanbu, Thanh Chi Do, Thao Huynh Phuong Do, Francisco Winter dos Santos Figueiredo, Arkadiusz Marian Dziedzic, Hisham Atan Edinur, Ferry Efendi, Joshua R. Ehrlich, Michael Ekholuenetale, Temitope Cyrus Ekundayo, Iman El Sayed, Muhammed Elhadi, Mohammad Hassan Emamian, Mehdi Emamverdi, Azin Etemadimanesh, Adeniyi Francis Fagbamigbe, Ayesha Fahim, Hossein Farrokhpour, Ali Fatehizadeh, Alireza Feizkhah, Lorenzo Ferro Desideri, Getahun Fetensa, Florian Fischer, Ali Forouhari, Matteo Foschi, Kayode Raphael Fowobaje, Abhay Motiramji Gaidhane, Aravind P. Gandhi, Miglas W. W. Gebregergis, Mesfin Gebrehiwot, Brhane Gebremariam, Urge Gerema, Fariba Ghassemi, Sherief Ghozy, Mahaveer Golechha, Pouya Goleij, Bárbara Niegia Garcia Goulart, Shi-Yang Guan, Zewdie Gudisa, Sapna Gupta, Veer Bala Gupta, Vivek Kumar Gupta, Arvin Haj-Mirzaian, Aram Halimi, Shahin Hallaj, Samer Hamidi, Mehdi Harorani, Hamidreza Hasani, Demisu Zenbaba Heyi, Nguyen Quoc Hoan, Ramesh Holla, Sung Hwi Hong, Mehdi Hosseinzadeh, Chengxi Hu, John J. Huang, Hong-Han Huynh, Segun Emmanuel Ibitoye, Irena M. Ilic, Mustapha Immurana, Md. Rabiul Islam, Sheikh Mohammed Shariful Islam, Chidozie C. D. Iwu, Louis Jacob, Ammar Abdulrahman Jairoun, Manthan Dilipkumar Janodia, Shubha Jayaram, Har Ashish Jindal, Mohammad Jokar, Nitin Joseph, Charity Ehimwenma Joshua, Vidya Kadashetti, Laleh R. Kalankesh, Rohollah Kalhor, Sagarika Kamath, Himal Kandel, Rami S. Kantar, Ibraheem M. Karaye, Hengameh Kasraei, Soujanya Kaup, Navjot Kaur, Rimple Jeet Kaur, Gbenga A. Kayode, Yousef Saleh Khader, Himanshu Khajuria, Rovshan Khalilov, Mahalaqua Nazli Khatib, Adnan Kisa, Soewarta Kosen, Ai Koyanagi, Kewal Krishan, Mukhtar Kulimbet, Nithin Kumar, Om P. Kurmi, Chandrakant Lahariya, Tuo Lan, Iván Landires, Janet L. Leasher, Munjae Lee, Seung Won Lee, Wei-Chen Lee, Stephen S. Lim, Julie-Anne Little, Preetam Bhalchandra Mahajan, Sandeep B. Maharaj, Alireza Mahmoudi, Razzagh Mahmoudi, Kashish Malhotra, Tauqeer Hussain Mallhi, Vahid Mansouri, Emmanuel Manu, Roy Rillera Marzo, Andrea Maugeri, Colm McAlinden, Wondwosen Mebratu, Tesfahun Mekene Meto, Yang Meng, Abera M. Mersha, Tomislav Mestrovic, Le Huu Nhat Minh, Awoke Misganaw, Manish Mishra, Sanjeev Misra, Nouh Saad Mohamed, Soheil Mohammadi, Mustapha Mohammed, Hoda Mojiri-forushani, Ali H. Mokdad, Hossein Molavi Vardanjani, Mohammad Ali Moni, Fateme Montazeri, Maryam Moradi, Rohith Motappa, Parsa Mousavi, Admir Mulita, Christopher J. L. Murray, Ganesh R. Naik, Gurudatta Naik, Shumaila Nargus, Zuhair S. Natto, Biswa Prakash Nayak, Mohammad Negaresh, Hadush Negash, Dang H. Nguyen, Phat Tuan Nguyen, Van Thanh Nguyen, Robina Khan Niazi, Osaretin Christabel Okonji, Andrew T. Olagunju, Matthew Idowu Olatubi, Michal Ordak, Uchechukwu Levi Osuagwu, Nikita Otstavnov, Mayowa O. Owolabi, Jagadish Rao Padubidri, Ashok Pandey, Georgios D. Panos, Shahina Pardhan, Seoyeon Park, Jay Patel, Shrikant Pawar, Prince Peprah, Ionela-Roxana Petcu, Alireza Peyman, Hoang Tran Pham, Mohsen Pourazizi, Nguyen Khoi Quan, Fakher Rahim, Vafa Rahimi-Movaghar, Mohammad Hifz Ur Rahman, Sathish Rajaa, Shakthi Kumaran Ramasamy, Premkumar Ramasubramani, Shubham Ranjan, Mohammad-Mahdi Rashidi, Rama Shankar Rath, Annisa Utami Rauf, Salman Rawaf, Amirmasoud Rayati Damavandi, Elrashdy Moustafa Mohamed Redwan, Priyanka Roy, Koushik Roy Pramanik, Zahra Saadatian, Siamak Sabour, Basema Saddik, Umar Saeed, Sare Safi, Sher Zaman Safi, Amene Saghazadeh, Fatemeh Saheb Sharif-Askari, Amirhossein Sahebkar, Mohammad Ali Sahraian, Joseph W. Sakshaug, Mohamed A. Saleh, Sara Samadzadeh, Yoseph Leonardo Samodra, Vijaya Paul Samuel, Abdallah M. Samy, Aswini Saravanan, Siddharthan Selvaraj, Farbod Semnani, Sabyasachi Senapati, Yashendra Sethi, Seyed Arsalan Seyedi, Allen Seylani, Amira A. Shaheen, Samiah Shahid, Moyad Jamal Shahwan, Masood Ali Shaikh, Sunder Sham, Muhammad Aaqib Shamim, Mohammed Shannawaz, Bereket Beyene Shashamo, Maryam Shayan, Aminu Shittu, Ivy Shiue, K. M. Shivakumar, Seyed Afshin Shorofi, Migbar Mekonnen Sibhat, Emmanuel Edwar Siddig, Juan Carlos Silva, Jasvinder A. Singh, Paramdeep Singh, Eirini Skiadaresi, Yonatan Solomon, Raúl A. R. C. Sousa, Chandrashekhar T. Sreeramareddy, Vladimir I. Starodubov, Mohana Devi Subramaniam, Sri Susanty, Seyyed Mohammad Tabatabaei, Birhan Tsegaw Taye, Gebrehiwot Teklay, Mohamad-Hani Temsah, Dufera Rikitu Terefa, Jansje Henny Vera Ticoalu, Temesgen Mohammed Toma, Aristidis Tsatsakis, Guesh Mebrahtom Tsegay, Munkhtuya Tumurkhuu, Biruk Shalmeno Tusa, Sree Sudha Ty, Chukwudi S. Ubah, Muhammad Umair, Tungki Pratama Umar, Rohollah Valizadeh, Jef Van den Eynde, Stephanie Louise Watson Watson, Tewodros Eshete Wonde, Guadie Sharew Wondimagegn, Hong Xiao, Yao Yao, Iman Yazdani Nia, Arzu Yiğit, Yazachew Yismaw, Dong Keon Yon, Naohiro Yonemoto, Yuyi You, Chuanhua Yu, Mikhail Sergeevich Zastrozhin, Hanqing Zhao, Makan Ziafati, Magdalena Zielińska, Yossef Teshome Zikarg, Mohammad Zoladl, and Jaimie D. Steinmetz
Supplementary information
The online version contains supplementary material available at 10.1038/s41433-024-02961-1.
References
- 1.GBD 2019 Blindness and Vision Impairment Collaborators; Vision Loss Expert Group of the Global Burden of Disease Study. Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: the Right to Sight: an analysis for the Global Burden of Disease Study. Lancet Glob Health. 2021;9:e144–e160. 10.1016/S2214-109X(20)30489-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang W, Yan W, Fotis K, Prasad NM, Lansingh VC, Taylor HR, et al. Cataract surgical rate and socioeconomics: a global study. Invest Ophthalmol Vis Sci. 2016;57:5872–81. 10.1167/iovs.16-19894 [DOI] [PubMed] [Google Scholar]
- 3.Lou L, Wang J, Xu P, Ye X, Ye J. Socioeconomic disparity in global burden of cataract: an analysis for 2013 with time trends since 1990. Am J Ophthalmol. 2017;180:91–96. 10.1016/j.ajo.2017.04.008 [DOI] [PubMed] [Google Scholar]
- 4.Wang W, Yan W, Müller A, He M. A global view on output and outcomes of cataract surgery with national indices of socioeconomic development. Invest Ophthalmol Vis Sci. 2017;58:3669–76. [DOI] [PubMed] [Google Scholar]
- 5.Lim JC, Caballero Arredondo M, Braakhuis AJ, Donaldson PJ. Vitamin C and the lens: new insights into delaying the onset of cataract. Nutrients. 2020;12:3142. 10.3390/nu12103142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hodge WG, Whitcher JP, Satariano W. Risk factors for age-related cataracts. Epidemiol Rev. 1995;17:336–46. 10.1093/oxfordjournals.epirev.a036197 [DOI] [PubMed] [Google Scholar]
- 7.Age Related Eye Disease Group. Risk factors associated with age-related nuclear and cortical cataract: a case-control study in the Age-Related Eye Disease Study, AREDS Report No. Ophthalmology. 2001;108:1400–8. 10.1016/S0161-6420(01)00626-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klein BE, Klein RE, Moss SE. Incidence of cataract surgery in the Wisconsin Epidemiologic Study of Diabetic Retinopathy. Am J Ophthalmol. 1995;119:295–300. 10.1016/S0002-9394(14)71170-5 [DOI] [PubMed] [Google Scholar]
- 9.Caird FI, Garrett CJ. Progression and regression of diabetic retinopathy. Proc R Soc Med. 1962;55:477–9. [PMC free article] [PubMed] [Google Scholar]
- 10.Lansingh VC, Carter MJ, Martens M. Global cost-effectiveness of cataract surgery. Ophthalmology. 2007;114:1670–8. 10.1016/j.ophtha.2006.12.013 [DOI] [PubMed] [Google Scholar]
- 11.Lansingh VC, Carter MJ. Use of global visual acuity data in a time trade-off approach to calculate the cost utility of cataract surgery. Arch Ophthalmol. 2009;127:1183–93. 10.1001/archophthalmol.2009.113 [DOI] [PubMed] [Google Scholar]
- 12.Brown GC, Brown MM, Busbee BG. Cost-utility analysis of cataract surgery in the United States for the year 2018. J Cataract Refract Surg. 2019;45:927–38. 10.1016/j.jcrs.2019.02.006 [DOI] [PubMed] [Google Scholar]
- 13.Resnikoff S, Lansingh VC, Washburn L, Felch W, Gauthier TM, Taylor HR, et al. Estimated number of ophthalmologists worldwide (International Council of Ophthalmology update): will we meet the needs? Br J Ophthalmol. 2020;104:588–92. 10.1136/bjophthalmol-2019-314336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lou L, Ye X, Xu P, Wang J, Xu Y, Jin K, et al. Association of sex with the global burden of cataract. JAMA Ophthalmol. 2018;136:116–21. 10.1001/jamaophthalmol.2017.5668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bourne RRA, Flaxman SR, Braithwaite T. Magnitude, temporal trends, and projections of the global prevalence of blindness and distance and near vision impairment: a systematic review and meta-analysis. Lancet Glob Health. 2017;5:e888–e897. 10.1016/S2214-109X(17)30293-0 [DOI] [PubMed] [Google Scholar]
- 16.Flaxman SR, Bourne RRA, Resnikoff S. Global causes of blindness and distance vision impairment 1990-2020: a systematic review and meta-analysis. Lancet Glob Health. 2017;5:e1221–e1234. 10.1016/S2214-109X(17)30393-5 [DOI] [PubMed] [Google Scholar]
- 17.Steinmetz JD, Bourne RA, Briant PS, Flaxman SR, Taylor HRB, Jonas JB, et al. GBD 2019 Blindness and Vision Impairment Collaborators; Vision Loss Expert Group of the Global Burden of Disease Study. Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: the Right to Sight: an analysis for the Global Burden of Disease Study. Lancet Glob Health. 2021;9:e144–e160. 10.1016/S2214-109X(20)30489-7. 10.1016/S2214-109X(20)30489-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.WHO. Universal eye health: a global action plan 2014–2019. World Health Organization; Geneva: 2013. https://www.who.int/publications/i/item/universal-eye-health-a-global-action-plan-2014-2019.
- 19.James SL, Abate D, Hassan Abate K, Abay SM, Abbafati C, Abbasi N, et al. GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789–858. 10.1016/S0140-4906736(18)32279-7 10.1016/S0140-4906736(18)32279-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.RAAB Repository. 2023. http://raabdata.info. accessed 1 February 2023.
- 21.Vos T, Lim SS, Abbafati C, Abbas KM, Abbasi M, Abbasifard M, et al. GBD 2019 Diseases, Injuries, and Impairments Collaborators. Global burden of 359 diseases, injuries, and impairments, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1204–22. 10.1016/S0140-6736(20)30925-9. 10.1016/S0140-6736(20)30925-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vollset SE, Goren E, Yuan C-W, Cao J, Smith A, Hsiao T, et al. Fertility, mortality, migration, and population scenarios for 195 countries and territories from 2017 to 2100: a forecasting analysis for the Global Burden of Disease Study. Lancet. 2020;396:1285–306. 10.1016/S0140-4956736(20)30677-2 10.1016/S0140-4956736(20)30677-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Powe NR, Schein OD, Gieser SC, Tielsch JM, Luthra R, Javitt J, et al. Synthesis of the literature on visual acuity and complications following cataract extraction with intraocular lens implantation. Cataract Patient Outcome Research Team. Arch Ophthalmol. 1994;112:239–52. 10.1001/archopht.1994.01090140115033. 10.1001/archopht.1994.01090140115033 [DOI] [PubMed] [Google Scholar]
- 24.Muralikrishnan R, Venkatesh R, Prajna NV, Frick KD. Economic cost of cataract surgery procedures in an established eye care centre in Southern India. Ophthalmic Epidemiol. 2004;11:369–80. 10.1080/09286580490888762. 10.1080/09286580490888762 [DOI] [PubMed] [Google Scholar]
- 25.Le HG, Ehrlich JR, Venkatesh R, Srinivasan A, Kolli A, Haripriya A, et al. A Sustainable Model For Delivering High-Quality, Efficient Cataract Surgery In Southern India. Health Aff. 2016;35:1783–90. 10.1377/hlthaff.2016.0562. 10.1377/hlthaff.2016.0562 [DOI] [PubMed] [Google Scholar]
- 26.He M, Chan V, Baruwa E, Gilbert D, Frick KD, Congdon N. Willingness to pay for cataract surgery in rural Southern China. Ophthalmology. 2007;114:411–6. 10.1016/j.ophtha.2006.09.012. 10.1016/j.ophtha.2006.09.012 [DOI] [PubMed] [Google Scholar]
- 27.Lundstrom M, Behndig A, Kugelberg M, Montan P, Stenevi U, Pesudovs K. The outcome of cataract surgery measured with the Catquest-9SF. Acta Ophthalmol. 2011;89:718–23. 10.1111/j.1755-3768.2009.01801.x. 10.1111/j.1755-3768.2009.01801.x [DOI] [PubMed] [Google Scholar]
- 28.Marques AP, Ramke J, Cairns J, Butt T, Zhang JH, Jones I, et al. The economics of vision impairment and its leading causes: A systematic review. EClinicalMedicine. 2022;46:101354. 10.1016/j.eclinm.2022.101354. 10.1016/j.eclinm.2022.101354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vashist P, Senjam SS, Gupta V, Gupta N, Kumar A. Definition of blindness under National Programme for Control of Blindness: Do we need to revise it? Indian J Ophthalmol. 2017;65:92–96. 10.4103/ijo.IJO_869_16. 10.4103/ijo.IJO_869_16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shekhawat NS, Stock MV, Baze EF, Daly MK, Vollman DE, Lawrence MG, et al. Impact of First Eye versus Second Eye Cataract Surgery on Visual Function and Quality of Life. Ophthalmology. 2017;124:1496–503. 10.1016/j.ophtha.2017.04.014. 10.1016/j.ophtha.2017.04.014 [DOI] [PubMed] [Google Scholar]
- 31.Gothwal VK, Wright TA, Lamoureux EL, Khadka J, McAlinden C, Pesudovs K. Improvements in visual ability with first-eye, second-eye, and bilateral cataract surgery measured with the visual symptoms and quality of life questionnaire. J Cataract Refract Surg. 2011;37:1208–16. 10.1016/j.jcrs.2011.01.028. 10.1016/j.jcrs.2011.01.028 [DOI] [PubMed] [Google Scholar]
- 32.Busbee BG, Brown MM, Brown GC, Sharma S. Cost-utility analysis of cataract surgery in the second eye. Ophthalmology. 2003;110:2310–7. 10.1016/S0161-6420(03)00796-6. 10.1016/S0161-6420(03)00796-6 [DOI] [PubMed] [Google Scholar]
- 33.Frampton G, Harris P, Cooper K, Lotery A, Shepherd J. The clinical effectiveness and cost-effectiveness of second-eye cataract surgery: a systematic review and economic evaluation. Health Technol Assess. 2014;18:1–205. 10.3310/hta18680. 10.3310/hta18680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dean WH, Buchan JC, Gichuhi S, Faal H, Mpyet C, Resnikoff S, et al. Ophthalmology training in sub-Saharan Africa: a scoping review. Eye. 2021;35:1066–83. 10.1038/s41433-020-01335-7. 10.1038/s41433-020-01335-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ibrahim N, Ramke J, Pozo-Martin F, Gilbert CE. Willingness to pay for cataract surgery is much lower than actual costs in Zamfara state, northern Nigeria. Ophthalmic Epidemiol. 2018;25:227–33. 10.1080/09286586.2017.1408845. 10.1080/09286586.2017.1408845 [DOI] [PubMed] [Google Scholar]
- 36.Baruwa E, Tzu J, Congdon N, He M, Frick KD. Reversal in gender valuations of cataract surgery after the implementation of free screening and low-priced high-quality surgery in a rural population of southern China. Ophthalmic Epidemiol. 2008;15:99–104. 10.1080/09286580801999118. 10.1080/09286580801999118 [DOI] [PubMed] [Google Scholar]
- 37.Ung L, Jonas JB, Lietman TM, Chodosh J. COVID-19 and the Unfinished Agenda of VISION 2020. Am J Ophthalmol. 2021;224:30–35. 10.1016/j.ajo.2020.11.016. 10.1016/j.ajo.2020.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sources for the Global Vision Database are listed at the following weblink http://www.anglia.ac.uk/verigbd. Fully disaggregated data is not available publicly due to data sharing agreements with some principal investigators yet requests for summary data can be made to the corresponding author.