Abstract
Spatial proximity to important stimuli often induces impulsive behaviour. How we overcome impulsive tendencies is what determines behaviour to be adaptive. Here, we used virtual reality to investigate whether the spatial proximity of stimuli is causally related to the supplementary motor area (SMA) functions. In two experiments, we set out to investigate these processes using a virtual environment that recreates close and distant spaces to test the causal contributions of the SMA in spatial impulsivity. In an online first experiment (N = 93) we validated and measured the influence of distant stimuli using a go/no-go task with close (21 cm) or distant stimuli (360 cm). In experiment 2 (N = 28), we applied transcranial static magnetic stimulation (tSMS) over the SMA (double-blind, crossover, sham-controlled design) to test its computations in controlling impulsive tendencies towards close vs distant stimuli. Reaction times and error rates (omission and commission) were analysed. In addition, the EZ Model parameters (a, v, Ter and MDT) were computed. Close stimuli elicited faster responses compared to distant stimuli but also exhibited higher error rates, specifically in commission errors (experiment 1). Real stimulation over SMA slowed response latencies (experiment 2), an effect mediated by an increase in decision thresholds (a). Current findings suggest that impulsivity might be modulated by spatial proximity, resulting in accelerated actions that may lead to an increase of inaccurate responses to nearby objects. Our study also provides a first starting point on the role of the SMA in regulating spatial impulsivity.
Keywords: Spatial cognition, Impulsivity, Virtual reality, Supplementary motor area, Transcranial static magnetic stimulation
Subject terms: Attention, Cognitive control, Perception, Motor cortex, Human behaviour
Introduction
Human behaviour requires flexibility in approaching or avoiding objects in the environment1. Behavioural inhibition is essential for regulating the interaction with objects but may apply differently based on spatial information2. Spatial proximity, which refers to the physical distance between stimuli or objects, has been suggested to act as a contextual bias to influence inhibitory processes3,4. The physical proximity between an individual and environmental stimuli, shows that close distances (within 10–60 cm from the body)5,6 elicit faster actions7,8. In contrast, objects beyond this range are processed with larger caution9. The brain structures causally engaged in sub-serving a combination of spatial proximity and impulsive decisions have received little attention so far.
A fundamental structure that bridges cognition and motor systems during adaptive behaviours is the supplementary motor cortex (SMC), including the supplementary motor area (SMA) and pre-SMA10. SMA has specifically involved in a cognitive control network11,12 and motor programming functions, as described by many electroencephalography13,14 and functional imaging investigations12,15–17. Other sources of evidence have included the SMA as part of a wider control network that is responsible for decision making18, especially related to motor action planning19. Specifically, some studies have found that the SMA is activated when individuals need to inhibit an automatic response and generate a more appropriate one13, showing strong activation during settings where interactions with spatial cues are relevant. Related SMA functions to spatial adjustments include visually guided reaching movements20, spatial orienting 21 and navigation22 as well as encoding extrinsic object properties (i.e., object positioning23). Hence, the joint SMA roles in controlling and guiding actions in a given space turns this area as key when combined control within certain spaces is required.
The development of neuromodulation techniques has made possible test direct cortical roles during inhibition and adaptive behaviours. Numerous studies have used different neuromodulation protocols to test the SMA role in the initiation and inhibition of motor responses11,24–27. A novel neuromodulation option is transcranial static magnetic stimulation (tSMS), mainly offering a portable technique to inhibit neural activity in the motor cortex that outlasts the stimulation time-period28. Other neuromodulation studies using transcranial magnetic stimulation (TMS) or tSMS have used 2D visual paradigms to study inhibition and visuo-spatial decisions26,29,30. However, human space processing is executed in 3D or with depth perception31, hence the relevance of further neuromodulation studies including this modality. To the best of our knowledge, there is currently no study that has examined the impact of neuromodulation of the SMA in relation to spatial impulsivity. The SMA is an ideal cortical target to neuromodulate and test its role in spatial impulsivity given its relationship with inhibition and spatial cognitive mechanisms11,25.
The present study was aimed to investigate the role of the SMA in spatial impulsivity. To do this, we conducted two experiments: a first online experiment as to validate adequate visual stimuli in a large sample and testing the expected near and far effects32; the second experiment exploited the visual stimuli of experiment 1 to test whether SMA neuromodulation determines spatial impulsivity (experiment 2). In both settings, a go/no-go task (adapted from O’Connor et al.32) was designed in a virtual environment to simulate depth perception as a factor of distance. O’Connor’s study demonstrated that impulsive behaviours are influenced by both immediate and delayed rewards, acting as mediators through extrinsic incentives. In our approach, we eliminated the motivational impact of stimuli while promoting intrinsic motivation by encouraging subjects to enhance their performance throughout the experiment. In the experiment 1 (n = 93), an online task simulated a depth perception environment to test the possible influence of spatial proximity and validate the perceptual stimulus characteristics. In experiment 2 (n = 28), in a double-blind, crossover, sham-controlled design, we tested whether the use of a tSMS over the SMA was able to modulate spatial impulsivity using a 3D virtual environment.
Experiment 1 - method
Participants
Ninety-seven healthy subjects initially participated in the experiment. Four individuals did not complete the entire experiment, resulting a final group of ninety-three subjects (mean age 30.25 years, SD = 7.53, 57 females). They had corrected vision (38.71%), wore glasses (53.76%) or wore contact lenses (7.53%). Participant’s recruitment was carried out via public advertisements located along the School of Health Sciences of the Rey Juan Carlos University (Madrid, Spain), using a random snowball sampling procedure33. All participants gave informed consent prior to the start of the study. The experiment was conducted in accordance with the WMA Declaration of Helsinki and approved by the Research Ethics Committee of Rey Juan Carlos University (ref: 1910202018420).
Sample size calculation
The G*Power software (Version 3.1.9.6, see URL https://www.psychologie.hhu.de/arbeitsgruppen/allgemeine-psychologie-und-arbeitspsychologie/gpower)34 was utilized to determine the required number of participants for this study, resulting in a sample size of 34 subjects based on a priori statistical power of 1−β = 0.80 and medium effect size. However, to account for possible failures of the experiment in the online environment, the sample was expanded.
Procedure
The experiment was conducted using Psytoolkit35. Due to the online nature of the experiment, various equipment configurations were available across participants: screen resolution (1366 × 768, 1440 × 900, and 1920 × 1080 pixels) and screen size (< 14, 15.6 and > 15.7 inches).
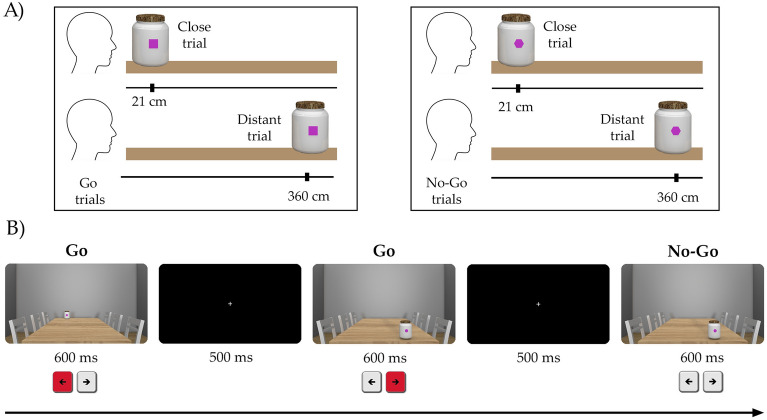
A visuo-spatial go/no-go task included two conditions: go control and go/no-go conditions (see Fig. 1A). The go control task involved 16 go trials in each block, resulting in a total of 128 trials across the 8 blocks. In this condition participants were asked to respond to every stimulus (go trials; squares or hexagons; counter-balanced) as to obtain a response initiation baseline. The go/no-go condition included 20 go trials and 8 no-go trials distributed across 8 blocks. Visual stimuli were represented by pots. Within each pot, a geometric figure (square or hexagons) was placed at the centre, which determined the response to produce initiation (go trial) or withhold of movements (no-go trial). Half of pots including a square served as go stimulus, while the no-go stimulus was represented by the hexagon. This correspondence between go/no-go stimuli and geometric figures was changed for the other half of trials (presented in a counter-balanced and randomized manner). Overall, the experimental task consisted of 224 stimuli distributed across 8 blocks, of which 160 were go trials and 64 no-go trials.
Figure 1.
Experimental paradigm used in the experiment 1: (A) Distance manipulation. Pots could appear close (21 cm) or distant (360 cm) from the subject. Go/No-Go task conditions: at the beginning of the task, the subjects were informed which geometric figure would be the go and no-go. In this example, the go trials would be the squares and the no-go trials would be the hexagons. (B) Single trial examples of the task. Example that included two go trials (distant-left and close-right), and one no-go trial (close-right). Ms milliseconds.
A realistic 3D environment and images were created (software Maxon CINEMA 4D Studio Version R19.024, available at https://www.maxon.net/es/downloads), consisting of a room with a central large table and six chairs, in order to enhance the authenticity of the scene and depth (Fig. 1B). This table served as the platform where pots would appear, positioned either close (21 cm) or distant (360 cm) and on the left or right side, adding spatial variability to the stimuli presentation. Before starting the experimental task, instructions appeared in the centre of the screen explaining subjects how to respond to a series of stimuli (responding to go trials and not responding to no-go trials). Subjects were asked to respond as quickly as possible to go trials and to refrain from responding on no-go trials. At the end of each block, feedback was provided on the mean response time for go trials. Subjects were also instructed to try to beat themselves and do it better in the next block. Each trial begun with a fixation cross presented for 500 ms. Subsequently, a pot was displayed in the virtual space of the participant. To minimise differences in spatial location between table distances with respect to retinal size, the square/hexagon were created following the visual angle law36. The task (go trials of both conditions) required to use the index finger for stimuli appearing on the left side and the middle finger for stimuli appearing on the right side (by pressing the right or left keyboard keys), regardless of their spatial position (close or distant). On failed trials, error feedback indicated “too slow” for errors of omission and “error” for commission errors (duration: 500 ms). An omission error occurs when the individual fail to detect a target stimulus (not pressing the button when it should be pressed), while a commission error occurs when the individual incorrectly presses the “go” button in the absence of a real target stimulus. The pot would vanish either upon receiving a response or if no response was made within 1000 ms (omission error). Reaction times (RT), and error rates (omission and commission) were recorded. The experiment was conducted using Psytoolkit35.
Data analyses
RT for go trials and error rates (omission and commission errors) for both go and no-go conditions were analysed. Since individual variability drives adaptive behaviour in prioritizing speed vs accuracy37, these trade-offs can lead to experimental bias manifesting in either of the two measures. In order to correct for this phenomenon, the raw RT scores were corrected using a linear integrated speed-accuracy scores (LISAS)38 as follows:
RTj: mean reaction time in a given condition; σRT: overall standard deviation of RTs; σPE: overall standard deviation of percentage of errors (Pes); and PEj: mean Pes in the given condition.
In experiment 1, this rationale was used to check whether the size of the screen or resolution used by the subjects had an influence on LISAS38 and error rates (omission and commission). These control analyses were carried out by repeated measures ANOVAs, where resolution (1366, 1440 and 1920 pixels) and size screen (< 14, 15.6 and > 15.7 inches) were included as factors. No statistically significant differences were found between measures of resolution (p = 0.4) and screen size (p = 0.26) for LISAS. We found neither statistical difference for the percentage of errors omission (p = 0.94, p = 0.46) and commission (p = 0.48, p = 0.66). This allowed us to treat the sample as a single homogeneous group.
One-way ANOVA (go control, go/no-go) was performed on LISAS, comparing the go trials of the control task with the go trials of the go/no-go task. This analysis was carried out in order to determine whether the task correctly mediates the inhibition process. In addition, repeated measures ANOVAs were used to analyse LISAS and error rates (omission and commission) as a function of distance (close, distant) and condition (go, no-go). Effect sizes were reported using the partial eta squared method (η2p). Post-hoc comparisons were made with paired t-test to determine the significance of pairwise contrast, using Bonferroni tests (alpha = 0.05) to control for Type I error rate. In addition, to test whether the results obtained were due to facilitation or priming effects of the previous stimulus39, the variables of interest were analysed using the Congruency Sequence Effect (CSE)40 adapted to our experimental design. For this purpose, subjects' responses were recoded based on the previous trial (n−1). In this way, there may be two trial types: (i) complete (if the current stimulus was the same as the previous one, such as go near–go near) and (ii) partial (if the current stimulus was different from the previous one, such as go near–no-go near or go far–no-go far). A series of 2 × 2 repeated measures ANOVA with type (complete, partial) and distance (close, distant) as factors was conducted for both RT (LISAS) and error rates. All statistical analyses described in this section were performed using JASP software (Version 0.17.2)41.
Drift diffusion model: the EZ model
To better characterize the decision-making process, an analysis based on the EZ Model was carried out4,27,42–44, often used to assess binary choices. The DDM is based on the idea that decision making is a process of accumulating evidence for or against each alternative of response, with the evidence flowing in at a constant rate. The decision maker accumulates evidence until a decision threshold is reached. At which point the option with the highest accumulated evidence is chosen. The model was tested according to the following formulae:
EZ model values were extracted for different parameters: drift rate (v), decision threshold (a), mean decision time (MDT) and mean non-decision time (Ter) across conditions. The drift rate (v) represents the rate at which evidence accumulates for one decision option (e.g., “yes”) relative to the other option (e.g., “no”). The higher the diffusion value, the faster the information will accumulate, resulting in quicker decisions. Decision threshold (a) represents the level of evidence that must be reached before a decision is made. Mean decision time (MDT) represents the amount of time that it takes for a person to decide once they have begun to accumulate evidence. Finally, mean non-decision time (Ter) that controls the amount of time it takes for the decision-making process to begin once the stimulus is presented. A one-way ANOVA was employed to examine the impact of spatial proximity (close vs distant) on each of these parameters.
Experiment 1–results
LISAS
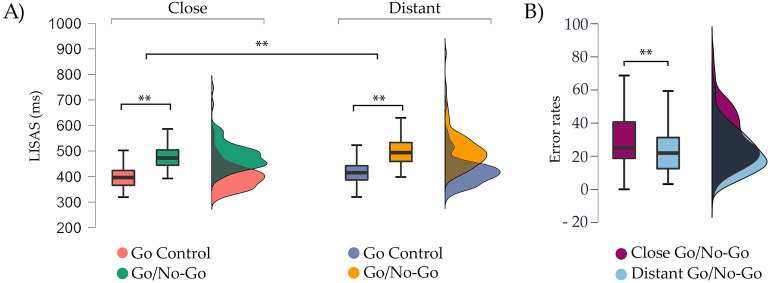
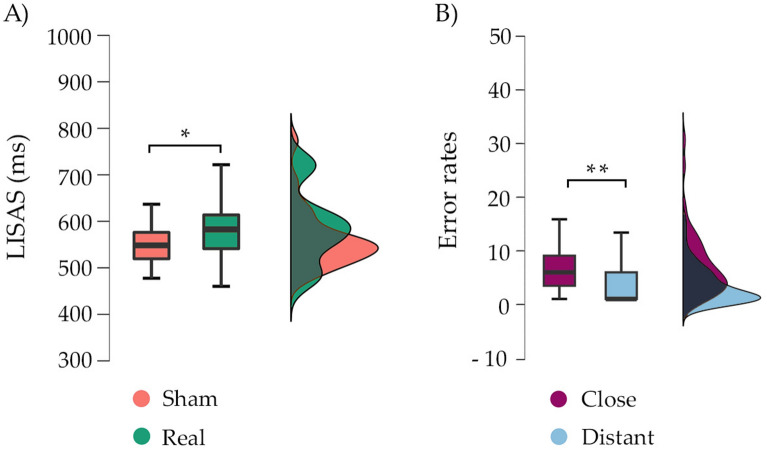
To establish the executive load of go/no-go condition, adjusted speed (LISAS) only on go trials was compared with go control condition. One-way ANOVA for LISAS showed a main effect of condition [F (1,92) = 448.25, p < 0.001, η2p = 0.83]. The go trials in the go control condition showed lower LISAS values (mean = 412.86, SD = 58.8), compared to the go trials in the go/no-go condition (mean = 481.19, SD = 54.09; p < 0.001). Repeated measures ANOVAs for LISAS with condition (go and go/no-go) and distance (close, distant) showed a main effect of condition [F (1,92) = 519.76, p < 0.001, η2p = 0.85], modulated by shorter LISAS for the go control condition (mean = 411.17, SD = 58.8) compared to go trials in the go/no-go condition (mean = 490.1, SD = 54.08; p < 0.001). An effect of distance was also found [F (1,92) = 36.62, p < 0.001, η2p = 0.28], whereby close stimuli produced faster responses (mean = 442.31, SD = 52.5) compared to distant stimuli (mean = 458.95, SD = 63.87; p < 0.001), regardless condition (Fig. 2A). The interaction condition by distance was not significant [F (1,92) = 2.18, p = 0.14, η2p = 0.02].
Figure 2.
Experiment 1 results: (A) Comparison in LISAS (ms) for close (left) and distant (right) stimuli between the go control (left) and go/no-go (right) tasks. A main effect of condition and distance is shown. (B) Comparison of the error rates (commission errors) between close and distant stimuli in the go/no-go task. A higher percentage of close errors were found compared to distant. The median is represented by the central mark within each boxplot, with the edges of the boxplot depicting the 25 and 75th percentiles. The whiskers extend to the minimum and maximum values within 1.5 times the interquartile range from the edges. Statistical significance is denoted by **p < .001.
Error rates
A 3 × 2 repeated measures ANOVAs (go control omission errors, nogo omission errors and nogo commission errors) by distance (close, distant) was computed. A significant main effect of condition was observed, indicating higher commission errors in the go/no-go task compared to both the go control task and omission errors [F (1,92) = 228.5, p < 0.001, η2p = 0.71]. A main effect of distance was also found [F (1,92) = 21.19, p < 0.001, η2p = 0.18], explained by higher error rates in close stimuli (mean = 12.8%, SD = 6.14) compared to distant stimuli (mean = 10.57%, SD = 5.38; p < 0.001). Interestingly, a condition by distance interaction effect was found [F (1,92) = 29.99, p < 0.001, η2p = 0.24]. Since commission errors reflect the cognitive control component, post-hoc analyses revealed greater percentage of commission errors in close stimuli (mean = 30.37%, SD = 15.57) compared to distant ones (mean = 22.78%, SD = 12.56; p < 0.001) (Fig. 2B).
Congruency sequence effect (CSE)
For LISAS, a main effect was found as a function of trial type [F (1,92) = 262.86, p < 0.001, η2p = 0.74], showing lower LISAS values for complete trials (mean = 451.85, SD = 38.3) compared to partial trials (mean = 490.61, SD = 31.42). No main effects were found as a function of distance [F (1,92) = 0.62, p = 0.43, η2p = 0.007] or trial type x distance interaction [F (1,92) = 0.1, p = 0.75, η2p = 0.001]. For error rates, a main effect was found as a function of trial type [F (1,92) = 229.23, p < 0.001, η2p = 0.71]. A lower percentage of commission errors was observed for complete trials (mean = 1.37%, SD = 1.06) compared to partial trials (mean = 14.57%, SD = 8.5). Similarly, a main effect of distance was also found [F (1,92) = 20.37, p < 0.001, η2p = 0.18], indicating a lower percentage of commission errors for distant trials (mean = 5.88%, SD = 3.69) as compared to close trials (mean = 7.68%, SD = 4.01). In addition, a trial type x distance interaction effect was found [F (1,92) = 18.55, p < 0.001, η2p = 0.17]. Post-hoc comparisons are shown in Table 1.
Table 1.
Results obtained from the CSE analysis.
| Post hoc comparisons–trial type ✻ distance (error rates) | ||||||
|---|---|---|---|---|---|---|
| Mean difference | SE | t | pbonf | |||
| Complete, close | Partial, close | −15.66 | 0.96 | −16.30 | < .001 | ** |
| Complete, distant | 0.05 | 0.64 | 0.08 | 1.000 | ||
| Partial, distant | −11.66 | 0.95 | −12.19 | < .001 | ** | |
| Partial, close | Complete, distant | 15.71 | 0.95 | 16.43 | < .001 | ** |
| Partial, distant | 3.99 | 0.64 | 6.23 | < .001 | ** | |
| Complete, distant | Partial, distant | −11.71 | 0.96 | −12.19 | < .001 | ** |
Drift diffusion model: the EZ model
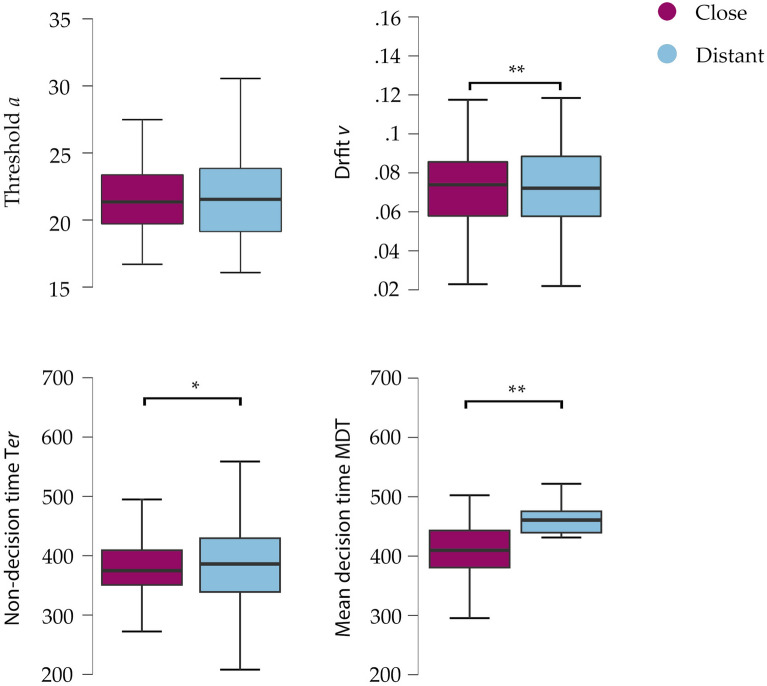
EZ Model was used to analyze the parameters a, v, Ter and MDT with stimulus distance set as factor. Repeated measures ANOVAs on drift (v) revealed a main effect on distance [F (1,92) = 38.08, p < 0.001, η2p = 0.29], with a higher value for distant stimuli (mean = 0.085, SD = 0.024) compared to close stimuli (near = 0.072, SD = 0.022). A main effect was also found for Ter [F (1,92) = 6.31, p = 0.014, η2p = 0.064], where distant stimuli showed higher values (mean = 401.55, SD = 78.55) compared to close (mean = 380.19, SD = 52.31). Finally, a main effect was also found for the parameter MDT [F (1,92) = 83.69, p < 0.001, η2p = 0.47], where close stimuli showed lower values (mean = 397.1, SD = 50.12) compared to distant (mean = 423.31, SD = 63.59). No statistically significant differences were found for a [F (1,92) = 0.029, p = 0.87, η2p < 0.001] (Fig. 3).
Figure 3.
Main effects of stimulation on the EZ-diffusion parameters. Higher values were found for distant stimuli in the parameters v, Ter and MDT. No differences were found for the parameter a. The median is represented by the central mark within each boxplot, with the edges of the boxplot depicting the 25 and 75th percentiles. The whiskers extend to the minimum and maximum values within 1.5 times the interquartile range from the edges. Statistical significance is denoted by *p < .05.
Experiment 2 - method
Participants
A total of thirty healthy subjects (mean age 24.59 years, SD = 3.22, 19 females and 11 males) were included in experiment 2. Two participants were excluded from the analysis because they cancelled their participation in the second session. As in the experiment 1, subjects who participated in the experiment had correct vision (53.57%), wore glasses (28.57%), or wore contact lenses (17.86%). Participants were recruited from the staff and students of the HM-CINAC and students from the Universidad Rey Juan Carlos (Madrid, Spain). All participants gave informed consent before study commencement. The study was conducted in accordance with the WMA Declaration of Helsinki and approved by the Research Ethics Committee of HM Hospitales (ref: 1286E1-GHM). The sessions were conducted at HM-CINAC (Madrid, Spain).
Sample size calculation
The G*Power software (Version 3.1.9.6, see URL https://www.psychologie.hhu.de/arbeitsgruppen/allgemeine-psychologie-und-arbeitspsychologie/gpower)34 was employed to calculate the necessary participant count, resulting in a sample size of 28 subjects to achieve a desired statistical power of 1−β = 0.80 and medium effect size. Adjustments were made for potential experimental losses, leading to the final sample size of 30 subjects.
Procedure
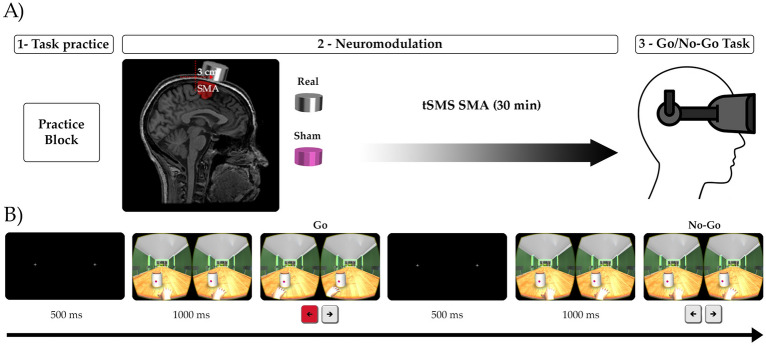
Virtual reality go/no-go task
After each stimulation protocol finished (described below), participants first performed the go control task and then the go/no-go task (Fig. 4B). The environment, stimuli and different spatial locations of the objects were similar to experiment 1. To investigate the possible influence of SMA in spatial impulsivity, in a more controlled and ecological way, a 3D virtual environment, was developed in experiment 2. Unity software (Version 4.7.0, available at https://unity.com/es/releases/editor/archive) was used for task design. A computer with an Intel Core I7-7700HQ processor and NVIDIA GTX 1060 graphics card was used to run the task, projected on a cell phone with virtual reality glasses system (Samsung Gear VR). An immersive 3D environment was presented to the participants, with a fixpoint that remained fixed throughout. As in experiment 1, a room environment with a centrally positioned table and six surrounding chairs was shown, alongside the same geometric shapes (squares or hexagons) and distances (close/distant and left/right). Similar to experiment 1, the task consisted of 4 consecutive blocks composed of 2 conditions: a go-control, including 10 trials to respond only with go trials; and a go/no-go (20 go trials and 8 no-go trials) where geometric figures was presented in a counter-balanced and randomized manner. A total of 40 go-control stimuli, 160 go stimuli and 64 no-go stimuli (264 stimuli in total) were presented. Prior to the start of the experiment, participants completed a practice block of 18 trials (8 go-control, 7 go and 3 no-go) to ensure full comprehension of task instructions, as well as to promote proper familiarization with the virtual reality system and control. The instructions given to participants were similar to experiment 1. Subjects were told to respond as quickly as possible to go trials and not to respond to no-go trials. They also received feedback on their average reaction time and were encouraged to try to do better in the next block.
Figure 4.
Paradigm design and neuromodulation procedure in experiment 2. (A) The image depicts a T1-weighted magnetic resonance image (MRI) in standard space, with a magnet positioned precisely over the average SMA target. The SMA target is located 3 cm anterior to Cz. Participants received double-blind cross-over stimulation (real or sham) during 30 min over the SMA. Once completed, they performed the go/no-go task using a virtual reality. (B) Example of the go/no-go task as seen in the virtual reality device. This image is perceived as unique and with depth thanks to the virtual reality glasses. The participant was required to respond to the pot by pressing either the left or right bottoms or abstaining from responding altogether. For this experiment, the task was executed with a peripheral control using a joystick and button pad (DualShock 4 controller for PlayStation 4).
The response to the stimuli was made by pointing the joystick to the right or left and pressing a button to “grab” the can. The pot would disappear either if a response was made or after 1000 ms (omission error). RT, omission, and commission errors were recorded.
Transcranial static magnetic stimulation (tSMS)
In a double-blind, crossover, sham-controlled design, each participant attended 2 neuromodulation sessions. The target was initially determined from the vertex, located with measurements from the nasion to inion and between the preauricular points (marked with a pen). The stimulation target was then placed 3 cm anterior to the vertex, specifically targeting the SMA bilaterally (Fig. 4A). The helmet incorporated a magnet cylinder of nickel-plated neodymium, measuring 45 mm in diameter and 30 mm in thickness, weighing 360 g (MAG45r from Neurek). This was securely attached to the helmet through multiple fixation points, attached safely to participant’s head. There were two experimental conditions: real stimulation and sham stimulation. The sham condition followed the same procedure as real stimulation, but with the magnet replaced by a visually indistinguishable nickel-plated steel cylinder of equal size, weight, and appearance (Neurek MAG45s). Two colour coded helmets (black and white) were blinded to the experimenter conducting the neuromodulation, containing real or sham magnets. Only one experimenter (IO) was aware of the colour code, revealed after ending the experiment. The experimenter who carried out the recordings (AC and DG) did not know the correspondence between the colour of the helmet and the type of stimulation. From the beginning of the experiment, participants knew that there were two possible helmets or types of stimulation (real or sham). However, at no point in the procedure did the subjects know which type of stimulation they were going to receive. At the end of each session, they were asked about their subjective perception regarding the type of helmet stimulation (real or sham). Both the real and sham conditions involved seating the participants comfortably in the experimental room for a duration of 30 min, during which they were instructed to maintain silence and refrain from engaging in any motor or verbal activities. Once the neuromodulation session was over, participants performed the experimental go/no-go task.
Data analyses
Repeated measures ANOVAs on LISAS were used to analyse RT as a function of distance (close, distant) and condition (go control, go/no-go). Similarly, repeated measures ANOVAs were performed with two factors (distance and stimulation) and two levels (close-distant and real-sham) on error rates. Effect sizes were reported using the partial eta squared method (η2p). Post-hoc comparisons were made with paired t-test to determine the significance of pairwise contrast, using the Bonferroni test (alpha = 0.05) for controlling the Type I error rate. To verify whether the potential effects of neuromodulation were genuine and not caused by facilitation or priming effects from the previous stimulus, the variables of interest were analysed using the Congruency Sequence Effect (CSE)40. A 2 × 2 × 2 repeated measures ANOVA including trial type (complete, partial), distance (close, distant), and stimulation (real, sham) was conducted for both RT (LISAS) and error rates.
Drift diffusion model: the EZ model
Similarly Experiment 1, to characterise the decision-making process after stimulation, the EZ Model was carried out. In the current design, using DDM to characterise behavioural changes after neuromodulation is of added value as to decipher the causal computations of SMA in the accumulation of decision mechanisms. Thus, a 2 × 2 repeated measures ANOVAs was employed to examine the impact of two factors: spatial proximity (close vs. distant objects) and stimulation type (real vs. sham).
Experiment 2–results
The task took less than 30 min to be completed by each participant (mean sham = 23’, SD = 3.06’; mean real = 22.96’, SD = 3.75’), which ensured the stimulation effects covered the behavioural performance. Following the session, participants were asked to indicate their perception of real or sham stimulation to test our blindness procedures. No statistically significant differences were found between the type of stimulation applied and the subjects’ perception [χ2 (1) = 0.64, p = 0.42].
LISAS
Within the go control task, the influence of the type of stimulation on distance was tested. A 2 × 2 repeated measure ANOVAs with stimulation (real, sham) and condition (close, distant) on LISAS showed a main effect of the distance [F (1,27) = 41.36, p < 0.001, η2p = 0.6], revealing that the close condition (mean = 415.71, SD = 50.26) generated shorter go RT compared to the distant condition (mean = 468.44, SD = 70.13; p < 0.001). As in experiment 1, the shorter RT on close stimuli reflect faster processing. No main effects were found for stimulation [F (1,27) = 1.06, p = 0.41, η2p = 0.03] or for distance x stimulation interaction [F (1,27) = 0.68, p = 0.31, η2p = 0.02]. Regarding the go/no-go task, a main effect of distance on LISAS was found [F (1,27) = 6.43, p = 0.01, η2p = 0.19], but in this case revealing that close condition (mean = 582.3, SD = 56.3) generated a significantly higher go RT than distant condition (mean = 562.35, SD = 78.64; p = 0.017). When analysing real vs. sham stimulation (independent of distance), an effect of stimulation was found [F (1,27) = 5.44, p = 0.02, η2p = 0.17], revealing that real stimulation showed higher LISAS (mean = 584.99, SD = 78.65) than sham stimulation (mean = 559.66, SD = 62.85; p = 0.027) (Fig. 5A). No interaction effect of distance x stimulation was found [F (1,27) = 0.03, p = 0.95, η2p = 0.01].
Figure 5.
Behavioural findings of experiment 2. (A) A general increment in LISAS (ms) for real compared to sham is found; (B) Comparison of the percentage of commission errors generated by close vs distant stimuli, showing a higher percentage of errors in the close condition compared to distant. The median is represented by the central mark within each boxplot, with the edges of the boxplot depicting the 25 and 75th percentiles. The whiskers extend to the minimum and maximum values within 1.5 times the interquartile range from the edges. Statistical significance is denoted by *p < .05, **p < .001.
Error rates
In errors of omission of the task go control, a main effect of distance was also found on error rates [F (1,27) = 13.07, p < 0.001, η2p = 0.32], where the close condition (mean = 0.98, SD = 1.57) generated fewer omission errors than the distant condition (mean = 3.3, SD = 3.79; p < 0.001). No main effects were found for stimulation type [F (1,27) = 0.05, p = 0.81, η2p = 0.002] or distance x stimulation interaction on omission errors [F (1,27) = 0.37, p = 0.58, η2p = 0.01]. Regarding omission errors of the go/no-go task, there were no significant differences observed based on distance [F (1,27) = 1.17, p = 0.29, η2p = 0.04], type of stimulation [F (1,27) = 1.58, p = 0.22, η2p = 0.05], or the interaction between distance and stimulation [F (1,27) = 1.71, p = 0.2, η2p = 0.06]. Finally, a main effect of distance was found on commission errors in the go/no-go task [F (1,27) = 30.03, p < 0.001, η2p = 0.53], regardless of the stimulation type, showing higher commission errors percentage on close objects (mean = 7.64%, SD = 6.51) compared to distant ones (mean = 3.12%, SD = 3.53; p < 0.001; Fig. 5B). No main effect of the stimulation type on the percentage of commission errors was found [F (1,27) = 2.44, p = 0.13, η2p = 0.08]. An effect close to significance was found for the interaction between distance and type of stimulation [F (1,27) = 3.8, p = 0.06, η2p = 0.12].
Congruency sequence effect (CSE)
For LISAS, no main effect was found as a function of trial type [F (1,27) = 0.03, p = 0.86, η2p = 0.001], distance [F (1,27) = 0.008, p = 0.93, η2p < 0.001], or stimulation [F (1,27) = 0.002, p = 0.97, η2p < 0.001]. We found no effects for any of interactions: trial type x distance [F (1,27) = 0.48, p = 0.49, η2p = 0.017], trial type x stimulation type [F (1,27) = 0.22, p = 0.64, η2p = 0.008], distance x stimulation type [F (1,27) = 1.4, p = 0.25, η2p = 0.05] or trial type x distance x stimulation type [F (1,27) = 0.2, p = 0.66, η2p = 0.007]. Likewise, for error rates, no main effects were found as a function of trial type [F (1,27) = 0.01, p = 0.91, η2p < 0.001], distance type [F (1,27) = 0.1, p = 0.75, η2p = 0.004], or stimulation type [F (1,27) = 2.77, p = 0.11, η2p = 0.09]. Error rates were neither unaffected trial type x distance [F (1,27) = 0.1, p = 0.74, η2p = 0.004], trial type x stimulation type [F (1,27) = 0.14, p = 0.71, η2p = 0.005], distance x stimulation type [F (1,27) = 2.23, p = 0.15, η2p = 0.076] or trial type x distance x stimulation type [F (1,27) = 0.27, p = 0.6, η2p = 0.01] interactions.
Drift diffusion model: the EZ model
To deepen on the cognitive computations associated with SMA neuromodulation in spatial impulsivity, we used DDM-EZ to characterize speed changes and reduced errors associated to spatial locations. Repeated measures ANOVAs yielded significant differences in parameter v as a function of distance [F (1,27) = 7.91, p < 0.01, η2p = 0.22], where distant stimuli had a higher drift (mean = 0.13, SD = 0.02) compared to close stimuli (mean = 0.11, SD = 0.01; p = 0.009). No differences were found on type of stimulation [F (1,27) = 2.95, p = 0.09, η2p = 0.09] or in the interaction distance by stimulation [F (1,27) = < 0.001, p = 0.09, η2p < 0.001]. In the Ter parameter, the repeated measures ANOVAs showed a main effect of the distance [F (1,27) = 5.97, p = 0.02, η2p = 0.18], where close stimuli had higher non-decision times (mean = 473.35, SD = 54.32) compared to distant (mean = 456.41, SD = 69.08; p = 0.021). No differences were found by type of stimulation [F (1,27) = 1.48, p = 0.23, η2p = 0.05] or in the interaction distance by type [F (1,27) < 0.001, p = 1, η2p < 0.001]. In the MDT parameter, no differences were found as a function of distance [F (1,27) = 0.95, p = 0.34, η2p = 0.03], stimulation [F (1,27) < 0.001, p = 1, η2p < 0.001] or distance by stimulation interaction [F (1,27) = 0.99, p = 0.33, η2p = 0.03].
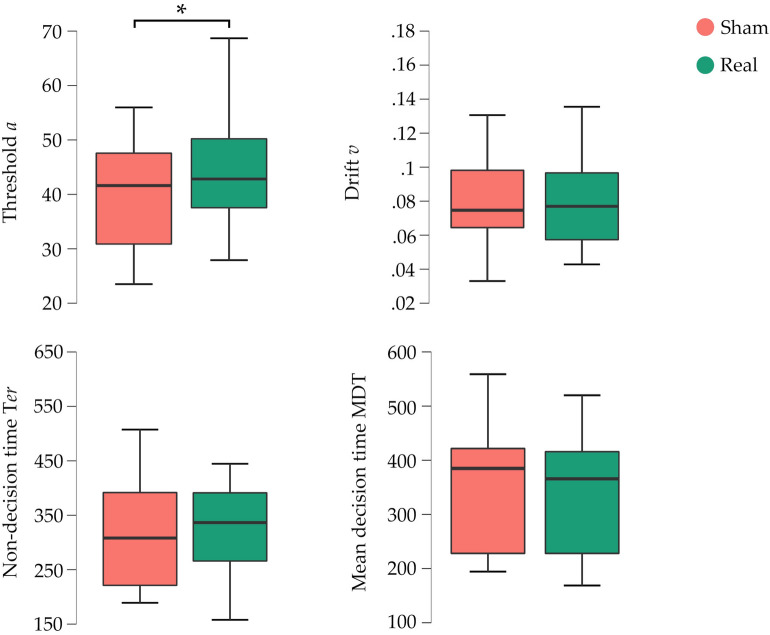
Regarding threshold separation (parameter a), a main effect of the type of stimulation was found [F (1,27) = 4.49, p = 0.04, η2p = 0.14]. This effect was marked by a higher threshold in the real stimulation (mean = 43.28, SD = 12.19) compared to sham stimulation (mean = 39.79, SD = 10.32: p = 0.04; Fig. 6). No differences were found by distance [F (1,27) = 2.84, p = 0.1, η2p = 0.09] or in the interaction distance by stimulation [F (1,27) < 0.001, p = 0.1, η2p = < 0.001].
Figure 6.
Main effects of stimulation on the EZ-diffusion parameters. A main effect of threshold (a) was found showing a higher value for real stimulation compared to sham. The median is represented by the central mark within each boxplot, with the edges of the boxplot depicting the 25 and 75th percentiles. The whiskers extend to the minimum and maximum values within 1.5 times the interquartile range from the edges. Statistical significance is denoted by *p < .05.
Discussion
We combined virtual 3D environments with inhibitory responses and neuromodulation as to investigate the role of SMA in spatial impulsivity. Our results show promising views on (i) novel insights on how to impact on impulsivity in naturalistic spatial settings and (ii) a possible relationship of SMA in spatial impulsive contexts. First, our study replicates a previous finding32 showing that close stimuli generate impulsive behaviours. Second, there was a significant main effect of SMA stimulation, where actions became slower after real stimulation than sham. Given response inhibition can be primed with automatic processing45–47, we also analysed the influence of facilitation or priming effects. We performed a (CSE) analysis40 showing a main effect depending on the type of previous trial. Lower LISAS values for complete trials were found compared to partial trials (Experiment 1). Activation of the SMA has been found to correlate with the effects of reverse priming on reaction times, making the response on the next trial slower39. The use of neuromodulation (tSMS) elicited a behavioural slowing (experiment 2), explained by raising decision thresholds during task performance. Moreover, no statistically significant differences were found for stimuli type or interactions nor for the priming analysis, suggesting that the observed increase in reaction times during the real stimulation is likely attributable to a general stimulation effect. In this sense, although mild or moderate, the tSMS stimulation seems to be exerting a significant and measurable effect on behaviour.
Previous literature shows an enhanced ability in humans to detect and respond to close stimuli compared to distant ones32. Yet, this biological constrain does not imply that we are able to inhibit an approach-avoidance balance to an immediate object in a biologically adaptive manner. Indeed, objects presented close generate shorter latencies, demonstrating the influence and priority of nearby spatial locations7,32,48. Facing more challenging tasks or accumulating ambiguous evidence might lead to prolonged decision times, potentially favouring adaptive but also slower decisions. Hence, as humans we have been sufficiently trained to interact with far-distant spaces, whereby behavioural inhibition plays a major role2. A higher drift (v) value was observed for distant stimuli, which may indicate the need to process more details of the stimulus. This is supported by the increase in reaction times (LISAS) and Ter that would represent prior motor preparation (Experiment 1). We reveal increments in response thresholds after inhibitory SMA neuromodulation that relates its computational mechanisms when spatial impulsivity is needed (Experiment 2).
Close proximity to stimuli of interest may trigger automatic and reflexive responses, while stimuli further away require more deliberate and effortful processing49. In line with this view, previous accounts suggest a prominent role of attentional sources that bias the space location guidance with impulsive behaviour50,51. When objects are placed close in personal proximity, they may capture our attention more easily, which may lead faster and more impulsive responses. This can be due to the fact that objects that are closer to us occupy a larger portion of our visual field52 and therefore require less cognitive effort. However, this reduced effort in attention may come at the cost of accuracy, as we report in experiment 1. Hence, the biological and natural response of delaying in order to succeed comes at a cost for close stimuli, possibly meaning that such close by condition makes our decision more vulnerable to fail. On the other hand, stimuli that are placed further away require greater effort to allocate our attention53, which may provide time for deliberation and result in more accurate responses. Therefore, we might hypothesise that adaptive control constrained by a given spatial context could be influenced by distance (both for 2D and 3D stimuli).
In our study, using a linear integration model of speed-accuracy, SMA stimulation made participants more conservative showing slower responses. This was achieved by regulating decision trade-offs mediated by response thresholds. In a similar vein, the study by Pineda-Pardo (2019) found a decrease in errors but also an increase in RTs during a 4-choice RT task. Indeed, a parallel finding to our diffusion parameters was the increased decision thresholds modulated during real SMA stimulation. Consequently, the distance dimension included in our design may be exerting an influence on both the linear increase of LISAS (which includes both RTs and accuracy plus deviation parameters) and decision thresholds, making the results not directly comparable to those of earlier studies. However, a general effect on slower RTs may require a joint view of the specific process tested in our experiments. The increment seen after real stimulation on LISAS holds jointly for error, variability (SD) and speed. Thus, the general slower effects on the sample for closer stimuli may be confounded, at least, by either (i) parallel cognitive changes in improving accuracy following real stimulation (such as raising decision thresholds) or (ii) due to the neuromodulation protocol itself (30 min of withholding the real/sham tSMS helmet may contribute to a prolonged RTs in the sample, maybe linked to fatigue or attentional lapses). Yet, is difficult to disentangle them as the significant improvement on error rates during real tSMS can influence the increment in LISAS as well. A possible neurobiological explanation links our protocol to previous studies where tSMS influenced selection of actions in a choice reaction time (CRT) task when applied to the motor association cortex54. In their study, tSMS over bilateral motor cortex increased reaction times. Similarly, tSMS applied to the SMA appears to modulate impulsive behaviours by improving anticipation of movements, by reducing errors and prolonging RTs27. It is possible to suggest that we have modulated classical SMA functions. Being part of the cognitive control system10, the SMA is needed when inhibition prepotent actions11,12,15,16,55, movement anticipation13,14 and explorative decisions56. Hence, the underlying SMA cognitive operations shall be considered in our findings, where a neuromodulation perturbation modified some of its related functions when introduced distance as factor, possibly by modulating the choice quality, rather than its movement vigour and speed. In conclusion, the application of tSMS seems to influence impulsive performance, where distance implicates a qualitatively different choice relevant to SMA and its subcortical functional connectivity that may have been influenced by our stimulation.
Our results raise a first approach to the idea that the SMA could serve as a potential hub to integrate perceptual spatial information with inhibitory cortical commands. Neuromodulation of the SMA with tSMS could provide a neural marker to modify spatial impulsivity and probably part of a cortical system that constructs the best possible action. However, our tSMS protocol lacks a control stimulation site to compare possible related effects in related areas (i.e. inferior frontal gyrus, motor cortex), thus may not be sufficiently reflecting the essential influence of the SMA on spatial impulsivity. Yet, the possible SMA functions contributing to our results are multiple. Of note, the multi-functional SMA roles including planning and initiating movements57, response inhibition (Albares et al.13; Obeso et al.17), switching58, learning59 or spatial cognition10 could have been modulated within task performance. Moreover, previous neuroimaging evidence identifies the SMA role in detection of both object size and location in the context of grasping actions23. In this investigation, researchers aimed to examine brain activation patterns using fMRI while participants were tasked with grasping objects that exhibited variations in both size and location. The findings imply that the SMA, along with regions like the primary motor cortex and the dorsal premotor cortex, play a role in sophisticated adaptive processes when dealing with information related to both object size and location within the framework of grasping movements. Here, SMA stimulation puts the individual in a more adaptive decision moment, by significantly increasing the decision thresholds and modulate action selection when faced with spatial moves. Our hypothesis is that the application of stimulation would influence performance by jointly increasing slower reaction times, without necessarily improving the selection of the correct moment to respond. This neuromodulator effect appears to manifest primarily in slower reaction times, while it does not seem to have a significant impact on errors committed.
Based on the mixed functions assessed with our virtual reality environment (i.e., inhibition and spatial functions), the behavioural changes after tSMS can be interpreted from two possible neurobiological viewpoints. First, based on prior works on brain connectivity changes after tSMS over the SMA26,27, other interconnected brain areas may take control of the spatial decisions made during our task. Indeed, inferior-frontal gyrus was enhanced after 30 min tSMS procedure27, which could provide a warning signal close or distant objects to withhold actions. This is plausible given its object detection and inhibitory roles60 that could have been boosted following SMA neuromodulation. An alternative (non-exclusive) explanation of our findings is directly linked to local physiological SMA changes after neuromodulation. With similar inhibitory protocols using TMS, the physiological effects in humans can vary individually61 and have been described as modifying the levels of neural “noise” after stimulation62. tSMS over the SMA could possibly create a similar adjustment of neural noise and distant neural connectivity, resulting in ‘paradoxically’ improvement of spatial processing and/or impulsive outcomes. Together with the neural noise change, the mechanics of tSMS possibly relate to reductions in the accumulation of noisy evidence until a certain threshold is reached and decisions made63,64.
The present study counts with some methodological limitations. In experiment 1, spatial perception was evaluated using a two-dimensional environment. While assessments of spatial perception have traditionally been conducted in two dimensions7,65,66, the advancement toward constructing three-dimensional virtual environments offers a significant improvement. These 3D environments not only enhance ecological validity67 but also provide greater control over experimental conditions68. They closely mimic real-world settings, fostering a heightened sense of immersion and sustaining participants' attention more effectively. Regarding experiment 2, the absence of another stimulation target could reframe the inference of our findings. The application of tSMS to other cortical areas related to spatial learning and/or control over impulsive tendencies could show related effects as the ones here reported, which would relate as well other possible cortical hubs. We could anticipate the possible scenario where other areas show related findings as those here obtained, given that the system level functioning of both learning69 and inhibition70 might involve implies other regions also relevant in the process. On the other hand, the reliability of the stimulation results could be a problem due to the large number of measures analysed in the study. We used from the most basic (RTs, errors) to speed-accuracy models (LISAS, DDM) to account for different understanding levels of SMA functions, which added considerable statistical tests to our design. Hence, a potential reliability problem arises due to the increased likelihood of false positives when several measures are examined simultaneously. Finally, although previous studies using tSMS70,71 selected relatively small sample sizes, the absence of interaction effects (close to significance in Experiment 2) could have been influenced by increasing the sample size. Future research shall be conducted on larger samples with approval of local committees.
Overall, our study might suggest that SMA computes the integration of spatial information and inhibitory functions, where SMA shall play a role in accommodating decision thresholds. Current results show promising effects of neuromodulation on spatial impulsivity but should be taken with caution as we did not find significant distance x stimulation interaction. However, our findings place the SMA as a possible cortical driver in charge of favouring a response threshold. Alternatively, regions interconnected with the SMA could have driven a more conservative and adaptive response by signalling spatial cues to favour optimal behaviour. These findings could provide valuable information to better understand the organization of adaptive responses in various contexts where spatial cues drive lost control over behaviour, including those associated with conditions such as OCD, ADHD, or addictions.
Author contributions
Conceptualization, A.C., J.D, F.M. and I.O; methodology, A.C., F.M. and I.O.; software, A.C.; validation, D.F. and D.G.; formal analysis, A.C., F.M. and I.O.; investigation, A.C., D.F. and D.G; re-sources, F.M. and I.O.; data curation, A.C.; writing original draft preparation, A.C., F.M. and I.O.; writing review and editing, J.D.; visualization, F.M. and I.O; supervision, F.M. and I.O.; project administration, F.M. and I.O.; funding acquisition, F.M.. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants PID2020-115463RB-I00 from the Ministerio de Ciencia e Innovación of Spain and SAPIENTIA-CM H2019/HUM-5705 of the Comunidad de Madrid. Alberto Carpio Moreno was supported by a Pre-Doctoral Grant from the Universidad Rey Juan Carlos.
Data availability
The data supporting the findings of this study are available on: https://osf.io/e5mpx/?view_only=a6b31e4deb6f457db200da0bb96d6636.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Francisco Mercado, Email: francisco.mercado@urjc.es.
Ignacio Obeso, Email: iobeso@csic.es.
References
- 1.Botvinick, M. & Plaut, D. C. Doing without schema hierarchies: A recurrent connectionist approach to normal and impaired routine sequential action. Psychol. Rev.111, 395–429 (2004). 10.1037/0033-295X.111.2.395 [DOI] [PubMed] [Google Scholar]
- 2.Stein, M., Fey, W., Koenig, T., Oehy, J. & Moggi, F. Context-specific inhibition is related to craving in alcohol use disorders: A dangerous imbalance. Alcohol. Clin. Exp. Res.42, 69–80 (2018). 10.1111/acer.13532 [DOI] [PubMed] [Google Scholar]
- 3.Lloyd, D., Morrison, I. & Roberts, N. Role for human posterior parietal cortex in visual processing of aversive objects in peripersonal space. J. Neurophysiol.95, 205–214 (2006). 10.1152/jn.00614.2005 [DOI] [PubMed] [Google Scholar]
- 4.Westbrook, A. & Frank, M. Dopamine and proximity in motivation and cognitive control. Curr. Opin. Behav. Sci.22, 28–34 (2018). 10.1016/j.cobeha.2017.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bassolino, M., Finisguerra, A., Canzoneri, E., Serino, A. & Pozzo, T. Dissociating effect of upper limb non-use and overuse on space and body representations. Neuropsychologia70, 385–392 (2015). 10.1016/j.neuropsychologia.2014.11.028 [DOI] [PubMed] [Google Scholar]
- 6.Bremmer, F. et al. Polymodal motion processing in posterior parietal and premotor cortex. Neuron29, 287–296 (2001). 10.1016/S0896-6273(01)00198-2 [DOI] [PubMed] [Google Scholar]
- 7.Valdés-Conroy, B., Sebastián, M., Hinojosa, J. A., Román, F. J. & Santaniello, G. A close look into the near/far space division: A real-distance ERP study. Neuropsychologia59, 27–34 (2014). 10.1016/j.neuropsychologia.2014.04.009 [DOI] [PubMed] [Google Scholar]
- 8.Li, T., Watter, S. & Sun, H. J. Differential visual processing for equivalent retinal information from near versus far space. Neuropsychologia49, 3863–3869 (2011). 10.1016/j.neuropsychologia.2011.10.002 [DOI] [PubMed] [Google Scholar]
- 9.Costantini, M., Ambrosini, E., Scorolli, C. & Borghi, A. M. When objects are close to me: Affordances in the peripersonal space. Psychon. Bull. Rev.18, 302–308 (2011). 10.3758/s13423-011-0054-4 [DOI] [PubMed] [Google Scholar]
- 10.Nachev, P., Kennard, C. & Husain, M. Functional role of the supplementary and pre-supplementary motor areas. Nat. Rev. Neurosci.9, 856–869 (2008). 10.1038/nrn2478 [DOI] [PubMed] [Google Scholar]
- 11.Aron, A. R. & Poldrack, R. A. Cortical and subcortical contributions to stop signal response inhibition: Role of the subthalamic nucleus. J. Neurosci.26, 2424–2433 (2006). 10.1523/JNEUROSCI.4682-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aron, A. R., Behrens, T. E., Smith, S., Frank, M. J. & Poldrack, R. A. Triangulating a cognitive control network using diffusion-weighted magnetic resonance imaging (MRI) and functional MRI. J. Neurosci.27, 3743–3752 (2007). 10.1523/JNEUROSCI.0519-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Albares, M. et al. The dorsal medial frontal cortex mediates automatic motor inhibition in uncertain contexts: Evidence from combined fMRI and EEG studies. Hum. Brain Mapp.35, 5517–5531 (2014). 10.1002/hbm.22567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Albert, J., López-Martín, S., Hinojosa, J. A. & Carretié, L. Spatiotemporal characterization of response inhibition. Neuroimage76, 272–281 (2013). 10.1016/j.neuroimage.2013.03.011 [DOI] [PubMed] [Google Scholar]
- 15.Jahfari, S. et al. Effective connectivity reveals important roles for both the hyperdirect (fronto-subthalamic) and the indirect (fronto-striatal-pallidal) fronto-basal ganglia pathways during response inhibition. J. Neurosci.31, 6891–6899 (2011). 10.1523/JNEUROSCI.5253-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rubia, K. et al. Mapping motor inhibition: Conjunctive brain activations across different versions of go/no-go and stop tasks. Neuroimage13, 250–261 (2001). 10.1006/nimg.2000.0685 [DOI] [PubMed] [Google Scholar]
- 17.Obeso, I. et al. Stimulation of the pre-SMA influences cerebral blood flow in frontal areas involved with inhibitory control of action. Brain Stimul.6, 769–776 (2013). 10.1016/j.brs.2013.02.002 [DOI] [PubMed] [Google Scholar]
- 18.Mostofsky, S. H. & Simmonds, D. J. Response inhibition and response selection: Two sides of the same coin. J. Cogn. Neurosci.20, 751–761 (2008). 10.1162/jocn.2008.20500 [DOI] [PubMed] [Google Scholar]
- 19.Hupfeld, K. E., Ketcham, C. J. & Schneider, H. D. Transcranial direct current stimulation (tDCS) to the supplementary motor area (SMA) influences performance on motor tasks. Exp. Brain Res.235, 851–859 (2017). 10.1007/s00221-016-4848-5 [DOI] [PubMed] [Google Scholar]
- 20.Picard, N. Activation of the supplementary motor area (SMA) during performance of visually guided movements. Cereb. Cortex13, 977–986 (2003). 10.1093/cercor/13.9.977 [DOI] [PubMed] [Google Scholar]
- 21.Cristescu, T. C., Devlin, J. T. & Nobre, A. C. Orienting attention to semantic categories. Neuroimage33, 1178–1187 (2006). 10.1016/j.neuroimage.2006.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tau, G. Z. et al. Neural correlates of reward-based spatial learning in persons with cocaine dependence. Neuropsychopharmacology39, 545–555 (2014). 10.1038/npp.2013.189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Monaco, S., Sedda, A., Cavina-Pratesi, C. & Culham, J. C. Neural correlates of object size and object location during grasping actions. Eur. J. Neurosci.41, 454–465 (2015). 10.1111/ejn.12786 [DOI] [PubMed] [Google Scholar]
- 24.Makoshi, Z., Kroliczak, G. & Van Donkelaar, P. Human supplementary motor area contribution to predictive motor planning. J. Mot. Behav.43, 303–309 (2011). 10.1080/00222895.2011.584085 [DOI] [PubMed] [Google Scholar]
- 25.Shirota, Y. et al. Supplementary motor area plays a causal role in automatic inhibition of motor responses. Brain Stimul.12, 1020–1026 (2019). 10.1016/j.brs.2019.03.002 [DOI] [PubMed] [Google Scholar]
- 26.Guida, P., Foffani, G. & Obeso, I. The supplementary motor area and automatic cognitive control: Lack of evidence from two neuromodulation techniques. J. Cogn. Neurosci.35, 439–451 (2023). 10.1162/jocn_a_01954 [DOI] [PubMed] [Google Scholar]
- 27.Pineda-Pardo, J. A. et al. Static magnetic field stimulation of the supplementary motor area modulates resting-state activity and motor behavior. Commun. Biol.10.1038/s42003-019-0643-8 (2019). 10.1038/s42003-019-0643-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oliviero, A. et al. Transcranial static magnetic field stimulation of the human motor cortex. J. Physiol.589, 4949–4958 (2011). 10.1113/jphysiol.2011.211953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chica, A. B., Bartolomeo, P. & Valero-Cabré, A. Dorsal and ventral parietal contributions to spatial orienting in the human brain. J. Neurosci.31, 8143–8149 (2011). 10.1523/JNEUROSCI.5463-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sack, A. T. et al. Imaging the brain activity changes underlying impaired visuospatial judgments: Simultaneous fMRI, TMS, and behavioral studies. Cereb. Cortex17, 2841–2852 (2007). 10.1093/cercor/bhm013 [DOI] [PubMed] [Google Scholar]
- 31.Welchman, A. E., Deubelius, A., Conrad, V., Bülthoff, H. H. & Kourtzi, Z. 3D shape perception from combined depth cues in human visual cortex. Nat. Neurosci.8, 820–827 (2005). 10.1038/nn1461 [DOI] [PubMed] [Google Scholar]
- 32.O’Connor, D. A. et al. Rewards that are near increase impulsive action. iScience24, 102292 (2021). 10.1016/j.isci.2021.102292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goodman, L. A. Snowball sampling. Ann. Math. Stat.32, 148–170 (1961). 10.1214/aoms/1177705148 [DOI] [Google Scholar]
- 34.Faul, F., Erdfelder, E., Lang, A.-G. & Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods39, 175–191 (2007). 10.3758/BF03193146 [DOI] [PubMed] [Google Scholar]
- 35.Stoet, G. PsyToolkit: A novel web-based method for running online questionnaires and reaction-time experiments. Teach. Psychol.44, 24–31 (2017). 10.1177/0098628316677643 [DOI] [Google Scholar]
- 36.McCready, D. On size, distance, and visual angle perception. Percept. Psychophys.37, 323–334 (1985). 10.3758/BF03211355 [DOI] [PubMed] [Google Scholar]
- 37.Liesefeld, H. R. & Janczyk, M. Combining speed and accuracy to control for speed-accuracy trade-offs(?). Behav. Res. Methods51, 40–60 (2019). 10.3758/s13428-018-1076-x [DOI] [PubMed] [Google Scholar]
- 38.Vandierendonck, A. A comparison of methods to combine speed and accuracy measures of performance: A rejoinder on the binning procedure. Behav. Res. Methods49, 653–673 (2017). 10.3758/s13428-016-0721-5 [DOI] [PubMed] [Google Scholar]
- 39.Krüger, D., Klapötke, S., Bode, S. & Mattler, U. Neural correlates of control operations in inverse priming with relevant and irrelevant masks. Neuroimage64, 197–208 (2013). 10.1016/j.neuroimage.2012.09.018 [DOI] [PubMed] [Google Scholar]
- 40.Egner, T. Congruency sequence effects and cognitive control. Cogn. Affect. Behav. Neurosci.7, 380–390 (2007). 10.3758/CABN.7.4.380 [DOI] [PubMed] [Google Scholar]
- 41.JASP Team. JASP team. https://jasp-stats.org/ (2023).
- 42.Bogacz, R., Wagenmakers, E. J., Forstmann, B. U. & Nieuwenhuis, S. The neural basis of the speed-accuracy tradeoff. Trends Neurosci.33, 10–16 (2010). 10.1016/j.tins.2009.09.002 [DOI] [PubMed] [Google Scholar]
- 43.Ratcliff, R., Smith, P. L., Brown, S. D. & McKoon, G. Diffusion decision model: Current issues and history. Trends Cogn. Sci.20, 260–281 (2016). 10.1016/j.tics.2016.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wagenmakers, E. J., Van Der Maas, H. L. J. & Grasman, R. P. P. P. An EZ-diffusion model for response time and accuracy. Psychon. Bull. Rev.14, 3–22 (2007). 10.3758/BF03194023 [DOI] [PubMed] [Google Scholar]
- 45.Jacob, L. P. L., Potter, K. W. & Huber, D. E. A neural habituation account of the negative compatibility effect. J. Exp. Psychol. Gen.150, 2567–2590 (2021). 10.1037/xge0001055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Verbruggen, F. & Logan, G. D. Automaticity of cognitive control: goal priming in response-inhibition paradigms. J. Exp. Psychol. Learn. Mem. Cogn.35, 1381–1388 (2009). 10.1037/a0016645 [DOI] [PubMed] [Google Scholar]
- 47.Wadsley, C. G., Cirillo, J., Nieuwenhuys, A. & Byblow, W. D. Stopping interference in response inhibition: Behavioral and neural signatures of selective stopping. J. Neurosci.42, 156–165 (2022). 10.1523/JNEUROSCI.0668-21.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O’Connor, D. A., Meade, B., Carter, O., Rossiter, S. & Hester, R. Behavioral sensitivity to reward is reduced for far objects. Psychol. Sci.25, 271–277 (2014). 10.1177/0956797613503663 [DOI] [PubMed] [Google Scholar]
- 49.West, G. L., Stevens, S. A., Pun, C. & Pratt, J. Visuospatial experience modulates attentional capture: Evidence from action video game players. J. Vis.8, 1–9 (2008). 10.1167/8.16.13 [DOI] [PubMed] [Google Scholar]
- 50.Dewitte, M., De Houwer, J., Buysse, A. & Koster, E. H. W. Proximity seeking in adult attachment: Examining the role of automatic approach-avoidance tendencies. Br. J. Soc. Psychol.47, 557–573 (2008). 10.1348/014466607X265148 [DOI] [PubMed] [Google Scholar]
- 51.Amengual, J. L., Di Bello, F., Ben Hadj Hassen, S. & Ben Hamed, S. Distractibility and impulsivity neural states are distinct from selective attention and modulate the implementation of spatial attention. Nat. Commun.10.1038/s41467-022-32385-y (2022). 10.1038/s41467-022-32385-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Murray, S. O., Boyaci, H. & Kersten, D. The representation of perceived angular size in human primary visual cortex. Nat. Neurosci.9, 429–434 (2006). 10.1038/nn1641 [DOI] [PubMed] [Google Scholar]
- 53.Klatt, S., Noël, B. & Brocher, A. Pupil size in the evaluation of static and dynamic stimuli in peripheral vision. PLoS ONE16, 1–12 (2021). 10.1371/journal.pone.0250027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Matsumoto, T. et al. Effect of transcranial static magnetic stimulation over unilateral or bilateral motor association cortex on performance of simple and choice reaction time tasks. Front. Hum. Neurosci.10.3389/fnhum.2023.1298761 (2023). 10.3389/fnhum.2023.1298761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Obeso, I., Robles, N., Marrón, E. M. & Redolar-Ripoll, D. Dissociating the role of the pre-SMA in response inhibition and switching: A combined online and offline TMS approach. Front. Hum. Neurosci.7, 1–9 (2013). 10.3389/fnhum.2013.00150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chakroun, K., Mathar, D., Wiehler, A., Ganzer, F. & Peters, J. Dopaminergic modulation of the exploration/exploitation trade-off in human decision-making. Elife9, 1–44 (2020). 10.7554/eLife.51260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thickbroom, G. W. et al. The role of the supplementary motor area in externally timed movement: The influence of predictability of movement timing. Brain Res.874, 233–241 (2000). 10.1016/S0006-8993(00)02588-9 [DOI] [PubMed] [Google Scholar]
- 58.Roberts, R. E. & Husain, M. A dissociation between stopping and switching actions following a lesion of the pre-supplementary motor area. Cortex63, 184–195 (2015). 10.1016/j.cortex.2014.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vollmann, H. et al. Anodal transcranial direct current stimulation (tDCS) over supplementary motor area (SMA) but not pre-SMA promotes short-term visuomotor learning. Brain Stimul.6, 101–107 (2013). 10.1016/j.brs.2012.03.018 [DOI] [PubMed] [Google Scholar]
- 60.Sharp, D. J. et al. Distinct frontal systems for response inhibition, attentional capture, and error processing. Proc. Natl. Acad. Sci. USA107, 6106–6111 (2010). 10.1073/pnas.1000175107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vallence, A. M. et al. Inter- and intra-subject variability of motor cortex plasticity following continuous theta-burst stimulation. Neuroscience304, 266–278 (2015). 10.1016/j.neuroscience.2015.07.043 [DOI] [PubMed] [Google Scholar]
- 62.Rocchi, L., Casula, E., Tocco, P., Berardelli, A. & Rothwell, J. Somatosensory temporal discrimination threshold involves inhibitory mechanisms in the primary somatosensory area. J. Neurosci.36, 325–335 (2016). 10.1523/JNEUROSCI.2008-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van Ravenzwaaij, D., Donkin, C. & Vandekerckhove, J. The EZ diffusion model provides a powerful test of simple empirical effects. Psychon. Bull. Rev.24, 547–556 (2017). 10.3758/s13423-016-1081-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Voss, A., Nagler, M. & Lerche, V. Diffusion models in experimental psychology: A practical introduction. Exp. Psychol.60, 385–402 (2013). 10.1027/1618-3169/a000218 [DOI] [PubMed] [Google Scholar]
- 65.Renier, L. et al. Cross-modal activation of visual cortex during depth perception using auditory substitution of vision. Neuroimage26, 573–580 (2005). 10.1016/j.neuroimage.2005.01.047 [DOI] [PubMed] [Google Scholar]
- 66.Saito, D. N., Okada, T., Honda, M., Yonekura, Y. & Sadato, N. Practice makes perfect: The neural substrates of tactile discrimination by mah-jong experts include the primary visual cortex. BMC Neurosci.7, 1–10 (2006). 10.1186/1471-2202-7-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Parsons, T. D. Virtual reality for enhanced ecological validity and experimental control in the clinical, affective and social neurosciences. Front. Hum. Neurosci.9, 1–19 (2015). 10.3389/fnhum.2015.00660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Smith, S. P. & Burd, E. L. Response activation and inhibition after exposure to virtual reality. Array3–4, 100010 (2019). 10.1016/j.array.2019.100010 [DOI] [Google Scholar]
- 69.Nojima, I. et al. Transcranial static magnetic stimulation over the primary motor cortex alters sequential implicit motor learning. Neurosci. Lett.696, 33–37 (2019). 10.1016/j.neulet.2018.12.010 [DOI] [PubMed] [Google Scholar]
- 70.Watanabe, T. et al. Null effect of transcranial static magnetic field stimulation over the dorsolateral prefrontal cortex on behavioral performance in a go/nogo task. Brain Sci.11(4), 483 (2021). 10.3390/brainsci11040483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Oliviero, A. et al. Safety study of transcranial static magnetic field stimulation (tSMS) of the human cortex. Brain Stimul.8, 481–485 (2015). 10.1016/j.brs.2014.12.002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting the findings of this study are available on: https://osf.io/e5mpx/?view_only=a6b31e4deb6f457db200da0bb96d6636.