Abstract
To study the source and content change of oridonin in the ice ribbons, the contents of oridonin in the ice ribbons and bleeding sap of Isodon rubescens at different times were determined with RP-HPLC. The paraffin sectioning and electron microscopy imaging were performed to study the transport channel of oridonin in the stem. The results showed that there were abundant xylem rays and perfect pit pairs in the secondary xylem of I. rubescens stems. The oridonin content in the ice ribbons of I. rubescens stems was lower than that in the stem of I. rubescens and even decreased over time. The contents of oridonin in the bleeding sap of I. rubescens stems was equal to that in second-day ice ribbons and was lower than that in first-day ice ribbons. The water in the ice ribbons of I. rubescens stems originated from water absorbed by the roots from soil. This water was transported from the roots of I. rubescens to the stem and then transferred through efficient lateral conducting tissues to the surface of the stem. The oridonin in the phloem and cortex of I. rubescens stems dissolves in water originating from the soil and freezes in the form of ice ribbons below 0 °C.
Keywords: Isodon rubescens, Ice ribbon, Bleeding sap, Oridonin, Paraffin section, Electron microscopy
Subject terms: Ecology, Physiology, Plant sciences, Ecology
Introduction
Isodon rubescens (Hemsley) H. Hara is a perennial subshrub belonging to the Lamiaceae family 1. The upper part of I. rubescens stem withers in winter. The lower lignified part of the I. rubescens stem does not wither and sprouts the next spring, although the leaves on it wither. The stem of I. rubescens forms thin feathery ice ribbons (ice sheets) when the air temperature is below 0 °C in winter. There are stripes on the ice ribbon. The ice ribbons melt when the air temperature is above 0 °C.
The dry aerial portions of Isodon rubescens are called rabdosiae rubescentis herba and are used in traditional Chinese medicine for the treatment of sore throat, inflammation and gastrointestinal problems2,3. There are some bioactive chemical components in I. rubescens, such as oridonin and ponicidin. Oridonin is a characteristic chemical composition in I. rubescens. The solubility of oridonin in water is 0.75 g/L4.
There are some reports on ice ribbons5–7. However, the mechanism of ice ribbon formation is not clear. Oridonin is dissolved in water in I. rubescens. Study the water and oridonin sources in the ice ribbons of I. rubescens can contribute to understand the mechanism of ice ribbon formation. This study analysed the contents of oridonin in ice ribbons of I. rubescens at different times and the microstructure of the secondary xylem in the stems to study the source of the characteristic chemical composition in the ice ribbons and their formation mechanism. The content of oridonin in the bleeding sap of I. rubescens was also determined.
Results
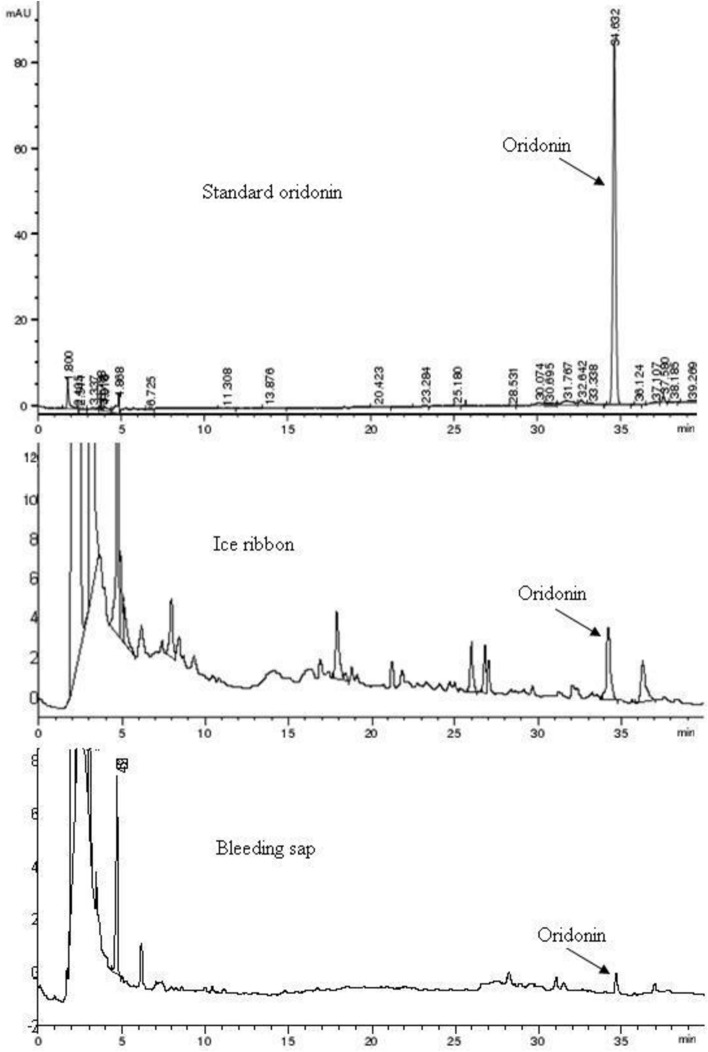
The HPLC chromatograms of the standard oridonin are shown in Fig. 1. The retention time of oridonin was 34.632 min.
Figure 1.
HPLC Chromatograms of ice ribbon and bleeding sap of I. rubescens compared to that of standard oridonin solution.
The standard curve of oridonin was established according to oridonin contents and corresponding peak areas (Fig. 2). The equation for the standard curve of oridonin was y = 20040x–2.0022 (x: Concentration, y: Peak area, R2 = 0.9987).
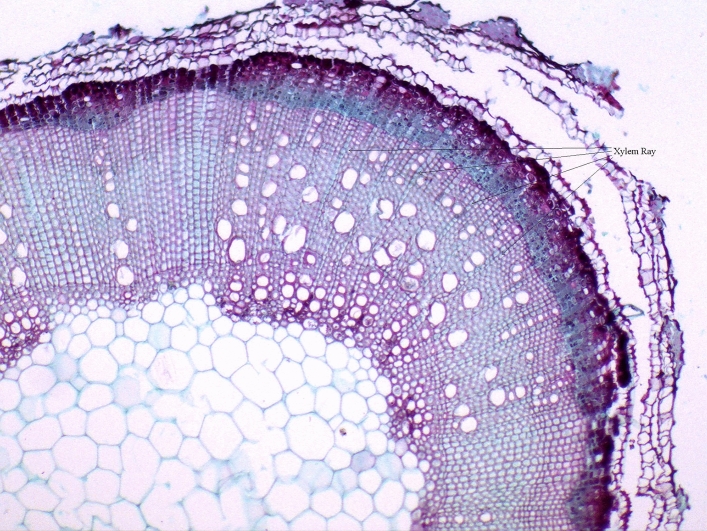
Figure 2.
Paraffin section of I. rubescens stem.
The peaks of oridonin in the chromatograms of ice ribbons, bleeding sap and extracts were identified according to their retention time in HPLC (Fig. 1).
The concentrations of oridonin in these liquids were analysed according to their peak areas and the standard curve (Table 1). The contents of oridonin in I. rubescens stems were analysed according to the methods for preparing the extracts.
Table 1.
Contents of oridonin in ice ribbon and bleeding sap of I. rubescens.
| Source | Peak area | Concentration (mg/ml) | Multiple comparisons * |
|---|---|---|---|
| First day ice ribbon | 66.9 | 0.003438 | 0.003059b |
| 58.2 | 0.003004 | ||
| 52.8 | 0.002735 | ||
| Second day ice ribbon | 14.094 | 0.000803 | 0.000895c |
| 13.627 | 0.00078 | ||
| 20.094 | 0.001103 | ||
| Third day ice ribbon | 2.51 | 0.000225 | 0.000245d |
| 2.67 | 0.000233 | ||
| 3.55 | 0.000277 | ||
| Bleeding sap | 22.626 | 0.001229 | 0.000984c |
| 17.672 | 0.000982 | ||
| 12.82 | 0.00074 | ||
| Stem | 235.2 | 0.1398 (mg/g) | 0.139918a(mg/g) |
| 233.9 | 0.139034 (mg/g) | ||
| 237.1 | 0.14092 (mg/g) |
*The mean difference is significant at the 0.01 level. The different letters indicates there is obvious difference between these means.
The oridonin content in the first-day ice ribbons of the I. rubescens stem was highest of all the oridonin contents of ice ribbons but lower than that of oridonin in the stem. According to the above data, the oridonin content in ice ribbons of I. rubescens stems decreased over time. The oridonin content in the bleeding sap of I. rubescens stems, which was equal to that of the second-day ice ribbons, was lower than that in the first-day ice ribbons.
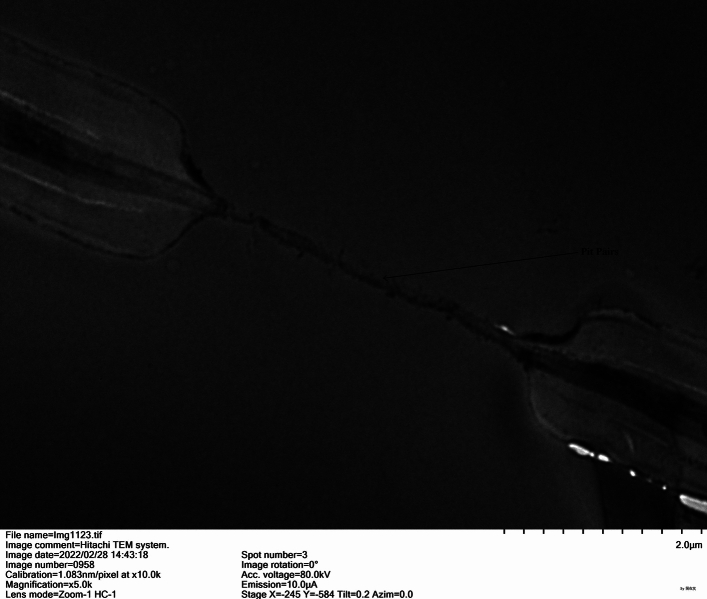
The paraffin section of I. rubescens stems is shown in Fig. 2. The vessels in the secondary xylem at the corners of stems were much more abundant than those on the plane side. There were many xylem rays in the secondary xylem of I. rubescens stems. The xylem rays consisted of 1–3 cells in a row and extended from the inside of the secondary xylem to the outside. The xylem rays were linked with the phloem rays at the cambium. A photograph of the xylem ray taken with a transmission electron microscope is shown in Fig. 3. There were many perfect pit pairs between adjacent cells of xylem rays. The pit membranes were very thin.
Figure 3.
Photograph of pit pairs in I. rubescens stem taken with transmission electron microscope.
Discussion and Conclusions
The content of oridonin in the stem of I. rubescens is quite high8–10. In this study, the oridonin content in the ice ribbons of I. rubescens stems was lower than that in the stem of I. rubescens and even decreased over time. The oridonin content in the bleeding sap of I. rubescens stems was lower than that in the first-day ice ribbons. There were abundant xylem rays and perfect pit pairs in the secondary xylem of I. rubescens stems. The water and the oridonin dissolved in the water could be efficiently transported from the inside of the secondary xylem to the outside. The ultimate sources of water in I. rubescens was soil moisture except rain. Therefore, the water in the ice ribbons of I. rubescens stems originated from water absorbed by the roots from soil. Water was transported from the root of I. rubescens to the stem and then transferred through the phloem and cortex to the surface of the stem. The oridonin in the I. rubescens stems was dissolved in water from the soil and frozen into ice ribbons below 0 °C. However, the oridonin content in the ice ribbons on the third day was obviously lower than that in the bleeding sap of I. rubescens stems. Therefore, only a small proportion of oridonin in the ice ribbons came from the I. rubescens stems. Most of the oridonin in the ice ribbons came from the root of I. rubescens. There were efficient lateral conducting tissues in the secondary xylem of I. rubescens stems. Some oridonin dissolved in the water in the roots and was transported to the stem and then froze in the form of ice ribbons below 0 °C. The oridonin content in the ice ribbons decreased over time because the oridonin content in the stem and roots decreased and the synthesis of oridonin takes time.
Materials and methods
Instruments: Agilent 1260 HPLC instrument, Shimadzu (C18 reverse-phase column, 5 µm, 250 × 4.6 mm), electronic analytical balance (precision: 0.0001), ultrasonicator, rotary evaporator, transmission electron microscope (Hitachi HT7700, Japan).
Reagents: Methanol (AR), ethanol (AR), and acetonitrile (HPLC grade). Standard oridonin (99.8%) was purchased from Sichuan Weikeqi Biotechnology Co. Ltd. in China in June 2017.
A Shimadzu C18 reverse-phase column (5 µm, 250 × 4.6 mm) was used as the HPLC column. The temperature of the HPLC column was 35 °C. The volume of extract injected was 10 µl. The mobile phase consisted of acetonitrile and water. The content (v/v) of acetonitrile in the mobile phase varied from 11 to 15% over 0–10 min, 15 to 37% over 10–40 min and 37 to 45% over 40–55 min. The flow rate of the mobile phase was 0.8 ml/min. A variable wavelength analyzer was set to 238 nm to detect ingredients eluted from the column.
Standard oridonin solutions were prepared at 0.001 mg/mL, 0.002 mg/mL, 0.005 mg/mL, 0.007 mg/mL and 0.01 mg/mL. Standard oridonin solutions were analysed according to this HPLC method.
Materials: The applied plant materials were identified as I. rubescens by JIAN Zaiyou, who is an expert in botany. The voucher specimens were deposited in the publicly available herbarium at the Henan Institute of Science and Technology. These wild I. rubescens from the Taihang Mountain of China are not privately owned or protected in any way. I. rubescens is not an endangered or protected species in China. Currently, no specific permits are needed to harvest plants from these locations. It was permitted by the local government to collect the wild I. rubescens plants as experimental materials. The methods for the collection of plant materials and the performance of experimental research on I. rubescens plants complied with the national guidelines of China.
Approximately 30 wild I. rubescens plants were dug out of the soil from Guanshan in Henan Province, China, and evenly planted in 3 flowerpots in March 2019. These flowerpots were moved close a freezer at 18:00 on August 2, 2019. Then, 3 stems of live I. rubescens were bent, and their upper parts were placed in the freezer. The temperature in the freezer was − 3 °C. The ice ribbons formed on I. rubescens stems in the freezer were collected as first-day ice ribbons at 8:00 on August 3, 2019. The stems were placed in the freezer again after the ice ribbons were collected. The ice ribbons were collected once again as second-day ice ribbons at 8:00 on August 4, 2019, and then the stems were placed in the freezer in the same way. The third-day ice ribbons were obtained at 8:00 on August 5, 2019. These ice ribbons were thawed and analysed according to the above HPLC method immediately after they were collected.
Another 3 stems of live I. rubescens were cut from the base of the plants at 18:00 on August 2, 2019. The stem connected to the root was inserted in a syringe. The plunger of the syringe was drawn out to induce low pressure in the syringe. The bleeding sap from the stems of I. rubescens was collected in the syringe at 18:00 on August 3, 2019, and was immediately analysed according to the above HPLC method.
The stems of the remaining 10 plants of I. rubescens were cut. The leaves were removed from the stems. These fresh stems were weighed to 2.54 g. These fresh stems were ground with quartz sand in a mortar for 30 min and then extracted with 30 ml ethanol (75%) in an ultrasonic bath for 30 min. Then, the mixture was filtered with filter paper. The filtrate was added to 30 ml and taken as the extract. The extract was filtered with a 0.22 µm membrane filter. The extraction was repeated three times. These extracts were analysed by the above HPLC method.
The chromatographic peak areas of oridonin in all chromatograms were recorded. A standard curve for oridonin was plotted according to the peak areas in chromatograms and the corresponding contents. The contents of oridonin in the samples were analysed according to the chromatography peak areas and the standard curve. All of the data were analysed with SPSS (Statistical Product and Service Solutions).
The slide specimens of I. rubescens stems were prepared with a paraffin section method at Henan Yulin Education Engineering Co., Ltd.11,12. The prepared slide specimens were photographed with a microcamera.
Imaging with transmission electron microscopy: The I. rubescens stems were stabilized in 3% glutaraldehyde (prepared with 0.1 mol/l phosphate buffer) at 4 °C for 10 h. The stabilized stems were rinsed in phosphate buffer 3 times for 15 min each time. These stems were dehydrated in 50%, 70%, 80%, 90%, 95%, 100% and 100% ethanol for 30 min for each gradient. The dehydrated stems were soaked in iso-amyl acetate 2 times for 30 min each time and then freeze-dried in vacuum. The dried stems were stabilized in 2% osmium tetroxide solution for 2 h and then embedded in epoxy resin. The embedded stems were laterally sectioned (8–9 μm in thickness) with a microtome. These sections were examined and imaged on a transmission electron microscope.
Author contributions
J.Z. designed the study, implemented the experiment and wrote the manuscript. T.X. performed the experiment. H.G. participated in the data analysis.
Funding
This work was supported by Natural Science Foundation of Henan province (232300420025).
Data availability
Data is provided within the manuscript or supplementary information files.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Flora of China Committee. Flora of China (volume 66) 457–458 (Science Press, 1979). [Google Scholar]
- 2.Chinese Pharmacopoeia Committee. Pharmacopoeia of the People’s Republic of China, Part I 2015th edn, 106–107 (Chemical Industry Press, Beijing, 2015). [Google Scholar]
- 3.Sun, H. D., Huang, S. X. & Han, Q. B. Diterpenoids from Isodon species and their biological activities. Nat. Prod. Rep.23, 673–698 (2006). 10.1039/b604174d [DOI] [PubMed] [Google Scholar]
- 4.Wen, X. et al. Determination of equilibrium solubility of oridonin and its apparent oil/water partition coefficient by HPLC. J. Shenyang Pharm. Univ.24(4), 220–222 (2007). [Google Scholar]
- 5.Carter, J. R. Unusual ice formations studying the natural growths of ice from soils, tems, branches, and rocks. Weatherwise62, 34–40 (2009). 10.3200/WEWI.62.1.34-40 [DOI] [Google Scholar]
- 6.Carter, J. R. Flowers and Ribbons of Ice. American Scientist101(5), 360–369 (2013). 10.1511/2013.104.360 [DOI] [Google Scholar]
- 7.Hofmann, D., Preuss, G. & Mätzler, C. Evidence for biological shaping of hair ice. Biogeosciences12, 4261–4273 (2015). 10.5194/bg-12-4261-2015 [DOI] [Google Scholar]
- 8.Yanhao, L., Guojun, L., Meirong, L., Dachao, L. & Xiaoli, Y. Determination of Oridonin in Leaves and Stems of Blushred Rabdosia by HPLC. Chin. J. Pharm. Anal.20(6), 390–392 (2000). [Google Scholar]
- 9.Huijun, J. I. N. G., Suiqing, C. H. E. N., Weisheng, F. E. N. G., Li, W. A. N. G. & Sanli, D. O. N. G. Determination of oridonin and rosemarinic acid in different parts of rabdosia rubescens. J. Chin. Med. Mater.27(6), 413–414 (2004). [Google Scholar]
- 10.Xiuhong, S., Xueju, L., Chengming, D. & Weili, T. Study on the dynamics of spatial accumulation of oridonin and rosmarinic acid of rabdosia rubesens (Henmsl.) Hara. China Med. Herald6(21), 5–6 (2009). [Google Scholar]
- 11.Liu, T. et al. The paraffin section making conditions of chrysanthemum different tissues in different period. J. Nanjing Agric. Univ.5, 739–746 (2016). [Google Scholar]
- 12.Zaiyou, J., Xiaomin, T. & Lei, D. Study on the cause of peeling and withering in Isodon rubescens stems in winter. J. King Saud Univ. Sci.34, 1–4 (2022). 10.1016/j.jksus.2022.101987 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is provided within the manuscript or supplementary information files.