Abstract
Higher eukaryotes’ life is impossible without copper redox activity and, literally, every breath we take biochemically demonstrates this. However, this dependence comes at a considerable price to ensure target-oriented copper action. Thereto its uptake, distribution but also excretion are executed by specialized proteins with high affinity for the transition metal. Consequently, malfunction of copper enzymes/transporters, as is the case in hereditary Wilson disease that affects the intracellular copper transporter ATP7B, comes with serious cellular damage. One hallmark of this disease is the progressive copper accumulation, primarily in liver but also brain that becomes deadly if left untreated. Such excess copper toxicity may also result from accidental ingestion or attempted suicide. Recent research has shed new light into the cell-toxic mechanisms and primarily affected intracellular targets and processes of such excess copper that may even be exploited with respect to cancer therapy. Moreover, new therapies are currently under development to fight against deadly toxic copper.
Keywords: Wilson disease, Acute copper toxicity, Mitochondria
| AAS | atomic absorption spectroscopy |
| AAV | adeno associated virus |
| AMP | adenosine monophosphate |
| ANT | adenine nucleotide translocase |
| Atox1 | antioxidant protein 1 |
| ATP | adenosine triphosphate |
| ATP7A/B | P1B-type adenosine triphosphatases (ATPases) |
| CcO | cytochrome c oxidase |
| Ccs | copper chaperone for SOD1 |
| COX | cytochrome c oxidase copper chaperone |
| CP | ceruloplasmin |
| CSF | cerebrospinal fluid |
| CTR1 | copper transporter-1 |
| Cu | copper |
| CuL | low molecular weight copper ligand |
| DCYTB | duodenal cytochrome b |
| DNA | deoxy ribonucleic acid |
| DMT | divalent metal transporter |
| DPA | d-penicillamine |
| EM | electron microscopy |
| ERK | extracellular-signal regulated kinase |
| Fe | iron |
| GSH | glutathion |
| LXR | liver X receptor |
| MDA | malondialdehyde |
| MEK | mitogen-activated protein kinase |
| MPT | mitochondrial membrane permeability transition |
| MT | metallothionein |
| mtDNA | mitochondrial DNA |
| NR | nuclear receptor |
| ROS | reactive oxygen species |
| RTK | receptor tyrosine kinase |
| SCO | synthesis of cytochrome c oxidase |
| SOD | superoxide dismutase |
| Steap | Six-transmembrane epithelial antigen of prostate |
| TBARS | thiobarbituric acid-reacting substances |
| TETA | triethylenetetramine dihydrochloride |
| TGN | trans Golgi network |
| WD | Wilson disease |
| Zn | zinc |
| ΔΨm | mitochondrial membrane potential |
1. Introduction
Copper is an essential biochemical trace element, with a typical intracellular concentration of 70 μM in eukaryotic cells [1]. However, due to its protein affinity and its redox-active properties, it can become a severe cellular toxin, if not kept inert. Toxic tissue copper overload mostly occurs in the genetic disorder Wilson's disease [[2], [3], [4]], but cases of direct copper salt intoxications either by inhalation, ingestion or skin contact have been reported as well [5]. Whereas the metal is rather harmless [6], its oxidized salts can damage proteins, subcellular structures, whole cells and tissues [7]. Copper salts are typical ingredients of fungicides, ceramics, fireworks, emetics, and burn ointments [5,6]. Accidental ingestion or attempted suicide are the most common causes of acute copper poisoning in humans. In contrast, chronic copper overload diseases, e.g. Wilson's disease (WD) and Indian childhood cirrhosis, may initially manifest with milder symptoms that, however, become severe and possibly deadly when mechanisms of compensation fail. In Wilson disease, liver dysfunction is a prime consequence of excess copper deposition, but also neurologic deficits (frequently termed neurologic WD) occur in about half of the affected WD patients.
Acute and chronic copper intoxication have many symptoms in common, the main difference being the rate of copper accumulation with consequential different disease courses and organ to organ variations. This article reviews our current knowledge on deadly toxic copper. It shall provide an overview on physiological copper homeostasis in liver and brain and cellular copper handling. We review mechanisms and subcellular targets of toxic excess copper and will focus on the clinical manifestations of either acute or genetically caused chronic copper intoxication.
2. Systemic copper distribution
The recommended dietary copper intake by the Food and Nutrition Board of the Institute of Medicine, National Academics from USA and Canada in adults is 0.9–1.3 mg of Cu per day, and its intestinal daily uptake is estimated to be around 1 mg, originating from either nutrition or being recycled [[8], [9], [10]]. Dietary copper is mainly taken up in the proximal part of the small intestine and reaches the liver via the portal vein that decides its further distribution [10]. In the case of copper need, the oxidized metal is incorporated into (serum) ceruloplasmin (CP), a ferroxidase responsible for the oxidation of Fe2+ to Fe3+ [11,12]. CP was previously considered to transport 90 % of the copper present in the serum, while more recent data suggest a lower contribution, around 50–70 %. The remaining circulating copper is mainly bound to albumin, α2-macroglobulin or, to a lesser extent, to low molecular weight compounds [13,14]. From the blood, Cu can be taken up by other organs like the brain, heart and kidney [15], whereat their tissue concentrations may greatly differ (Table 1).
Table 1.
| Sample | Weight [kg] | Concentration [μg/g given in wet weight] | Amount |
|---|---|---|---|
| Blood | 5.38a | 0.08 | ∼0.43 mga |
| Brain | 1.76 | 4–5 | 8.8 mg |
| CSF | 0.15 | 0.0056 | ∼0.84 μga |
| Heart | 0.32 | 5 | 1.6 mg |
| Kidney | 0.3 each | 4–12 | 3.2 mg |
| Liver | 1.8 | 5.5 | 9.9 mg |
| Spleen | 0.15 | 1.5 | 0.23 mg |
Calculated for an average person with a body weight of 70 kg and 150 mL cerebrospinal fluid (CSF).
In cases of Cu surplus, the liver is the key organ for Cu excretion into bile and feces. Up to 1 mg of copper is daily excreted, thus balancing the daily copper uptake via nutrition [15].
3. Cellular copper turnover
Due to its chemistry, enabling a reversible switch from cuprous Cu+ to cupric Cu2+, copper is a vital cofactor for redox enzymes in metabolism. To date, 54 copper-binding or -transporting proteins have been identified, a rather small fraction of the human proteome, but comprising vital proteins, e.g., mitochondrial cytochrome c oxidase [17]. A variety of different copper enzymes and binding proteins, the latter termed Cu-chaperones, are needed to tightly bind copper and regulate copper homeostasis. This tight binding is essential, as free, i.e., aqueous, copper ions would otherwise be severely harmful to cells. In this latter case, spontaneous changes of the Cu redox state would trigger the formation of cell toxic reactive oxygen species (ROS), among them most destructive hydroxyl radicals with a lifetime of nanoseconds and thus very short reaction rates [18]. The prerequisite for cells to keep copper inertly bound throughout has been substantiated by calculations from Rae et al. The authors demonstrated that intracellular copper would have to rise by a factor of 109 for the existence of just one unbound copper atom per cell, i.e., free copper does not exist in the cell [1].
Cupric Cu2+ may be taken up by the divalent metal transporter 1 (DMT1) [19,20]. However, the large part of copper is taken up by the copper transporter-1 (CTR1) [21] for which it first needs to be reduced to cuprous Cu+ by cell surface residing reductases (DCYTB, Steap 2, 3 and 4) [22,23]. CTR1 transports Cu+ in a high-affinity manner (KD ∼ 10−14 M [24]) [25,26].
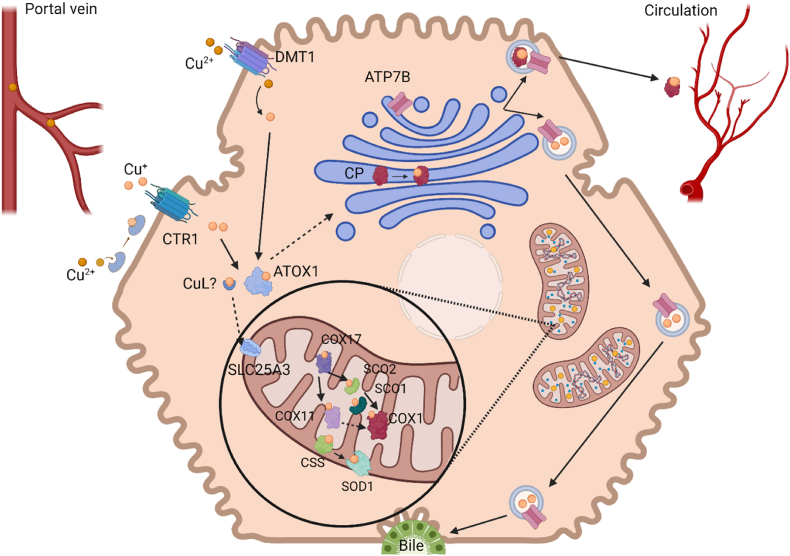
Upon cellular uptake, copper is delivered to its target sites (Fig. 1). Thereto, CTR1 delivers copper to the copper chaperone antioxidant protein 1 (Atox1) or to a low molecular weight copper ligand (CuL) [26]. It has been postulated (but not yet validated) that the latter transports copper to mitochondria. Here, solute carrier family 25 member 3 (SLC25A3), a phosphate carrier, may be responsible for copper transport into mitochondria [27]. In the mitochondrial intermembrane space, copper metalizes superoxide dismutase 1 (SOD1) with the help of the copper chaperone for SOD1 (Ccs) [28,29]. Furthermore, CuL delivers copper to cytochrome c oxidase copper chaperone 17 (COX17), which passes it on to COX11 and the synthesis of cytochrome c oxidase SCO2, which in turn deliver copper to COX1, whereas SCO1 supports the transfer of copper from SCO2 to COX1. [30,31].
Fig. 1.
Copper is taken up into hepatocytes via DMT1 or after reduction by the STEAP proteins by CTR1. Cuprous copper is then bound by ATOX1, which delivers copper to the TGN. Here, ATP7B mediates either incorporation into ceruloplasmin for further distribution in the body via circulation or excretion via the bile. Copper transport to the mitochondria occurs via an unidentified copper ligand (CuL), which delivers copper to the uptake transporter SLC25A3. Thereupon, with help of several copper chaperons, it gets either incorporated into the CuA/B sites of the cytochrome c-oxidase (COX1) or into the superoxide dismutase 1 (SOD1). Created with BioRender.com.
Atox1 may also transfer copper to the copper transporting ATPases ATP7A and ATP7B located in the membranes of the trans Golgi network (TGN) and secretory vesicles. There, Cu is incorporated into the copper-dependent enzymes ceruloplasmin, dopamine beta hydroxylase, peptidyl-glycine alpha amidating monooxygenase, tyrosinase and lysyl oxidase [32,33]. Excess copper in the cytosol is chelated by metallothioneins for storage [34].
ATP7A and ATP7B are the key players in the regulation of intracellular copper homeostasis. They belong to the family of P1B-type adenosine triphosphatases (ATPases) that hydrolyze ATP for Cu transport, e.g., across the membrane into the lumen of the TGN. ATP7A and ATP7B share 56 % of sequence identity and consist of six N-terminal metal-binding domains, eight transmembrane domains and four cytoplasmic domains [35]. Despite their similarity, the two Cu transporters fulfill different tasks in the body, as their trafficking pathways lead them from the TGN to different sites of the cellular membrane: ATP7A reaches the basolateral membrane of some polarized cells, while ATP7B can be found on the apical membrane [36]. In enterocytes, ATP7A mediates vesicular transport of Cu from the TGN to the basolateral side of the cell, from where it is released into the portal vein bound to albumin. [37]. In contrast, within hepatocytes, surplus copper is transported into the TGN via ATP7B, to be enclosed in late endosomes that may fuse with lysosomes [38]. Upon vesicular fusion with the apical membrane, copper is transported into the bile canaliculi to be excreted into feces [38,39]. In cases of copper requirements, copper gets either incorporated into mitochondria via copper chaperones or, with the help of ATP7B, into apo-ceruloplasmin, forming holo-CP that is involved in systemic copper transport [40].
ATP7A and ATP7B have a complementary expression pattern. While ATP7B is highly abundant in the liver, ATP7A can be found in the majority of other tissues, albeit a very low hepatic expression in adults. Co-expression of both is reported for the brain, kidney, lung and placenta [37,41,42].
A higher catalytic turnover of ATP7A (about six-fold faster than ATP7B [43]) ensures efficient export of excess copper. Its increased presence in several tissues, including intestine, heart and spleen, upon copper excess might indicate a potential protective mechanism against Cu overload in these tissues [37,43]. Indeed, ATP7A was reported to be augmented in duodenum of ATP7B impaired Wilson disease patients with copper overload, plausibly collaborating with a diminished CTR1 abundance as potential compensatory mechanism of the enterocytes against a high copper load [44]. A further example for protective ATP7A action is given by Kim et al., who observed elevated ATP7A in the liver upon cardiac copper deficiency in mice. The authors hypothesized, that such increased ATP7A may alleviate cardiac deficiency by elevating copper serum concentration [45].
4. Mechanisms of copper toxicity
4.1. Oxidative stress
Oxidative damage is frequently considered a prime cause of copper-induced cell damage and death [[46], [47], [48]]. This assumption is due to the propensity of dissolved copper ions to catalyze hydroxyl radical formation in the presence of peroxides via Fenton reactions [49].
Fenton Reaction:
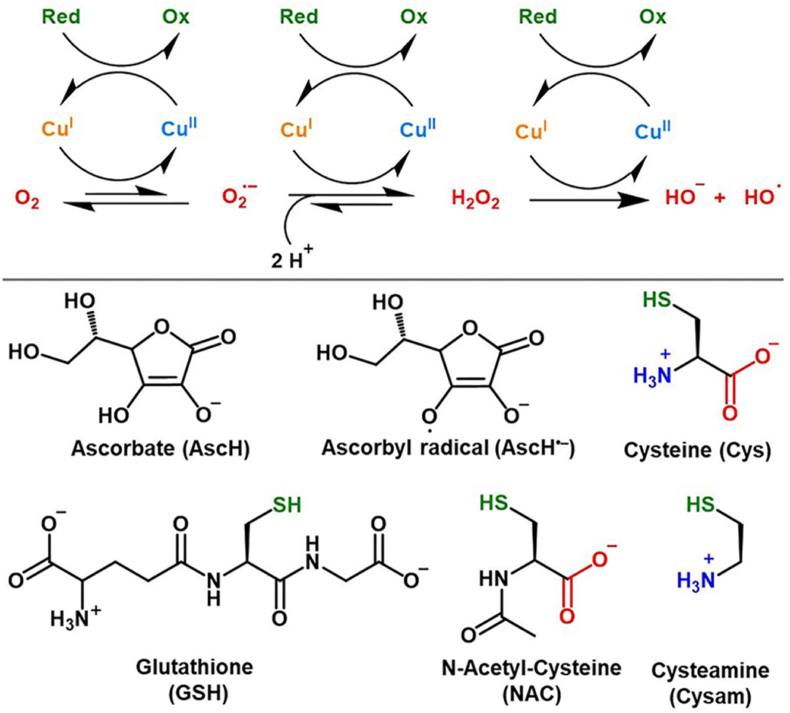
Hydroxyl radicals are oxidants of enormous potency (E0 = +2.33 V, pH 7 reduction potential) that can instantaneously react with, e.g., amino acids to deter protein function, especially tyrosine, cysteine, methionine and tryptophan [50]. Apart from the direct reaction with DNA and RNA [51], their reaction with fatty acids leads to the formation of reactive aldehydes or lipid peroxides, which in turn can form adducts with DNA or amino acids in proteins [[52], [53], [54]]. However, such formation of hydroxyl radicals by Fenton chemistry would require the presence of accessible copper ions. As mentioned, intracellular copper is not “free” in cells and its amount would have to rise several orders of magnitude to cause such reactivity [1]. This means that the cellular copper binding capacities and binding partner copper affinities are overwhelming [[55], [56], [57]] and that cells thereby compensate for excessive copper up to high levels without overt pathological findings [58,59]. Moreover, considering the reducing intracellular environment with an average potential of −0.25 V [60] and the fact that Cu2+ must be reduced by membrane reductases prior to CTR1-mediated cellular import [[61], [62], [63]], intracellular copper can be assumed to be in its cuprous state (Cu+) [64], since the oxidation potential needed to oxidize Cu + to Cu2+ lies at +0.153 V [65]. Together, this clearly questions uncontrolled copper related Fenton chemistry as prime cell-toxic mechanism of copper overload. Furthermore, a recent study by Falcone et al., 2023 demonstrated that even if hydroxyl radicals are produced via Cu-GSH/cysteine thiol cycling (Fig. 2), they are not released to induce lipid peroxidation or DNA damage but rather react with neighboring thiols of the cluster and thus preventing their release [66].
Fig. 2.
Mechanism of Cu-catalyzed ROS production. In presence of dioxygen and a reducing agent (Red) copper may catalyze the activation of oxygen by cycling between its cuprous and cupric redox state. Possible reducing agents are ascorbate (AscH), glutathione (GSH) and cysteine (Cys). Figure taken from “Revisiting the pro-oxidant activity of copper: interplay of ascorbate, cysteine, and glutathione” by Falcone, E. et al. Metallomics. 2023 Jul 10; 15(7): mfad040. https://doi.org/10.1093/mtomcs/mfad040 with permission (open access, distributed under the terms of the Creative Commons CC BY). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Nevertheless, there are numerous data on oxidative damage in the livers of WD patients as well as in WD animal models and in animal models with experimentally induced copper overload. Several studies using samples from WD patients have found decreased glutathione (GSH) levels, pro-mutagenic DNA adducts, and especially evidence of lipid peroxidation [[67], [68], [69], [70], [71], [72], [73]]. Interestingly, patients in all of these studies were in end-stage liver disease or had severe pathology (neurologic deterioration, acute fulminant liver disease), indicating that the observed oxidative damage rather is a late consequence due to overwhelmed defense mechanisms than the prime cause of copper driven cellular toxicity. In fact, one study in WD patients correlated a decreased antioxidant capacity and increased lipid peroxidation in serum with the severity of the patients' disease [72]. Such late appearance of oxidative stress markers upon progressive copper overload argues for a scenario in which, upon exhaustion of local copper binding capacities, catalytic copper amounts may attack intracellular sites, but it does not argue, however, for oxidative stress as main driver of copper overload toxicity. In agreement, in a recent review, we compared diverse experimental treatments in copper overload WD rats. From the therein cited reports it appeared that treatments using copper-binding compounds were therapeutically effective whereas merely anti-oxidative treatments were not, or at best of minor effect [74]. Furthermore, this concept of consequential oxidative stress, emerging upon defense exhaustion, can be challenged by scenarios of acute copper intoxication, without strongly elevated protective metallothioneins, e.g., as in copper-overload WD. Indeed, Ossola et al. found increased lipid peroxidation in rat liver homogenates as early as 3 h after a single s.c. CuSO4 injection (2 mg/kg body weight) with a peak at about 6 h [75]. In parallel, the GSH content of the tissue also decreased reaching its minimum value at 12 h. However, lipid peroxidation and GSH levels returned to control levels as early as 48 h after injection [75]. Thus, copper induced oxidative stress may be a prime mechanism of damage in acute copper poisoning scenarios, but in the copper overload WD, such damage appears late in the disease progression, when the delicate counterbalance of copper-binding partners and anti-oxidative defense is exhausted. This comes with two consequences: First, local intracellular hotspots of less tightly bound/accumulating copper are of concern and second, further/other copper-related toxicity mechanisms must be responsible for the progressive cellular damage in WD.
4.2. Cuproptosis, copper toxicity induced by a Cu2+ ionophore
The chemotherapeutic adjuvant elesclomol recently regained attention. This bis(thiohydrazide) amide compound chelates extracellular Cu2+ in a 1:1 ratio. Nagai et al. found a decade ago that elesclomol transports Cu2+ ions directly into the mitochondria of cancer cells, where it is reduced to Cu+, triggering mitochondrial oxidative stress [76,77].
Based on these findings, Tsvetkov et al. further studied the mechanism of Cu-elesclomol induced cell death [78]. They coined the term cuproptosis to indicate that it differs from other cell death modalities. In contrast to this, Gao et al. were able to block Cu-elesclomol induced cell death in colorectal cancer cells (SW480 and DLD) by the addition of ferrostatin-1, a potent inhibitor of ferroptosis [79].
Cuproptosis, as described by Tsvetkov et al., is characterized by mitochondrial copper accumulation due to the ionophore elesclomol resulting in copper-induced proteotoxic stress [78]. It occurs primarily in cells using oxidative phosphorylation as their main energy provider and inhibition of FeS-cluster synthesis was observed in their study [78]. Furthermore, lipoylation, a post-translational modification that is currently thought to be restricted to four enzymes involved in/around the TCA cycle, appears to be a key target in this process. Oligomerization of the lipoylated protein DLAT, a subunit of the pyruvate dehydrogenase complex, occurred in cells treated with Cu-loaded elesclomol [78]. Intriguingly, this protein oligomerization/aggregation was suggested by the authors to cause a gain of function of these citrate cycle associated proteins, leaving the question open of a connection to the loss of iron-sulfur proteins and how it ultimately leads to cell death [78]. Conversely, the cytotoxicity of elesclomol as a cancer drug has been the focus of research for decades. Indeed, the central hypothesis is that elesclomol delivers copper directly into the mitochondria, as also observed by Tsvetkov et al. [76,79]. There, however, the released Cu2+ appears to be reduced, mainly by ferredoxin 1 [80], and this process generates ROS in the mitochondria [79], resulting in ROS-mediated cell death [81]. Moreover, it has been shown that the presence of elesclomol-Cu in the mitochondria of cancer cells (MDA-MB-435 melanoma cell line and HL-60 leukemia cell line) can facilitate the redox cycling of copper and thus generate ROS [76].
Thus, the mode of cell death mediated by copper-containing elesclomol is still controversial, ranging from ROS-mediated cell death, protein toxicity, ferroptosis to cuproptosis and maybe a special form of copper related toxicity due to the non-physiological Cu2+ provision by elesclomol.
4.3. Protein toxicity
In 2009, Macomber and Imlay showed that copper encompasses the ability to directly inhibit bacterial enzymes – independent of oxygen, damaging thereby mainly iron-sulfur clusters of dehydratases [82]. Zischka et al. demonstrated in 2011 that proteins are direct targets of copper by binding to thiol residues and plausibly leading to cross-linkage of membrane proteins [59]. Such direct protein attack can be demonstrated by the copper elicited mitochondrial membrane permeability transition (MPT) in isolated mitochondria. Here, this event is not triggered by lipid peroxidation/oxidative damage but by interaction with thiol groups in critical mitochondrial membrane proteins, e.g., the adenine nucleotide translocase (ANT) [83,84]. Addition of thiol-containing compounds (dithiothreitol, glutathione) blocked such copper-induced MPT [83]. This latter finding was further substantiated by Borchard et al., who identified mitochondrial thiols as important targets of copper toxicity. In their study, free thiols of liver and brain mitochondria were depleted by copper in a dose-dependent manner [85]. Interestingly, the sensitivity of mitochondria to copper-induced MPT varies from organ to organ, and differences in their GSH level correlate with the sensitivity to copper-induced MPT [85]. The protective role of GSH in this scenario was further confirmed by Saporito-Margina et al., who demonstrated the independence of copper induced cell death from oxidative stress and showed protein aggregation by copper in in vitro analysis. The authors hypothesize copper to trigger a heat shock response due to protein aggregation, only to be prevented by GSH [86].
In addition, a recent study demonstrated that copper toxicity in bacteria is caused by protein aggregation and protein unfolding under anaerobic conditions, i.e., not by the production of reactive oxygen species [87]. A four-fold molar excess of Cu+ ions was able to unfold citrate synthase and aggregation appeared in vivo, despite the presence of high endogenous GSH levels. Both redox states of Cu were able to cause a loss of protein structure and function in vivo. Cuprous copper might promote protein aggregation in proteins containing thiol residues, while cupric copper preferentially affected charged proteins [87].
Thus, there is convincing evidence for direct protein-toxic copper damage that appears to especially target most vulnerable proteins in the mitochondrial compartment [59].
4.4. Nuclear receptor (NR) signaling
Transcriptomic studies in various WD animal models enabled the identification of key biological processes affected by copper overload. Among them, activation of the cell cycle and cell division and inhibition of lipid metabolism were most prevalent [88]. Further analysis of the signaling pathways affected by copper overload revealed significant depletion of the nuclear receptors (liver X receptor LXR, retinoid X receptor and farnesoid X receptor) [88] all three of which are involved in transcriptional regulation of genes associated with lipid metabolism [[89], [90], [91]]. Excess copper reduced the expression of NRs and binding to their target sequences in vitro [92,93]. The sensitivity of NRs to excess copper is based on their specific DNA-binding motif - the NR zinc-finger. Here, Zn ions are coordinated by four sulfhydryl groups provided by two C-X-X-C motifs. Similar motifs are responsible for the specific linear Cu+ coordination in the copper chaperone ATOX-1 and also in the copper transporters ATP7A and ATP7B [94,95]. The interference of copper with NR-mediated signaling networks was confirmed in a recent transcriptional network analysis that identified four specific clusters of NRs affected in Atp7b knockout mice, an important WD animal model [96]. This study revealed four additional transcriptional activator clusters that include non-NR zinc finger transcription factors also influenced by chronic copper overload [96]. Thus, copper excess affects global cellular transcription by interfering with zinc finger-based transcriptional regulators, particularly NRs. In the Atp7b knockout mice, restoration of NR signalling ameliorated the pathological phenotype albeit still having significant liver copper overload [97], highlighting the importance of impaired NR signalling in chronic copper overload toxicity.
4.5. Interference with intracellular signaling
The importance of copper as an essential intracellular signaling modulator is increasingly recognized [98,99]. Disturbed cellular signal transduction must therefore be considered as a further potential mechanism of copper overload toxicity. Copper can affect a variety of cellular signaling pathways at nearly every level of the signaling cascade: In neurons, copper has been shown to modulate brain-derived neurotrophic factor signaling by directly binding to this factor, affecting its conformation, and thereby binding to its cognate receptor [100]. Also, a direct binding of copper to SH residues in the epidermal growth factor receptor has been discussed, possibly leading to structural changes and dimerization of the receptor with activation of its receptor kinase activity [101].
In addition, studies in various cell lines showed, that the epidermal growth factor receptor is phosphorylated by cellular copper treatment, leading to downstream activation of mitogen-activated protein kinase (MEK) and extracellular-signal regulated kinase (ERK) [[101], [102], [103]]. One suggested mechanism may be the inactivation of receptor tyrosine phosphatases by direct copper binding, as all tyrosine phosphatases contain vulnerable SH residues [[101], [102], [103], [104]]. More recent data indicate that copper may interfere with receptor tyrosine kinase (RTK) mediated signaling: deletion of copper transporter 1 resulted in reduced ERK activation after cellular exposure to various RTK ligands [105]. It also has been shown that deficiency of CTR1 greatly reduces SOD1 levels (up to 17-fold), thereby reducing H2O2 formation, which is known to act as a cofactor in ligand-induced RTK activation [105]. Similar data were reported in another study for CTR1-deficient Drosophila and mice, which both exhibited decreased MEK-1 activity with reduced ERK phosphorylation [106]. The authors demonstrated direct binding of copper to MEK-1, resulting in enhanced MEK-1-driven ERK phosphorylation in a dose-dependent manner [106]. Copper-induced MEK-1 activation promotes tumorigenesis in certain cancers [107,108]. Interestingly, 60 % of Long-Evans Cinnamon (LEC) rats, a model of Wilson's disease, survive copper overload-induced acute hepatitis and develop chronic (long-lasting) hepatitis with subsequent liver cancer. In Atp7b knockout mice, the development of cholangiocarcinoma at older ages has been reported [109,110]. Furthermore, pharmacological copper depletion might serve as cancer therapy in triple negative breast cancer cells [111,112]. Inactivation of complex IV by copper deficiency and thus reduction of oxidative phosphorylation triggers AMP activated protein kinase activation, which further leads to a reduced invasion. In addition, copper serves as a cofactor for lysyl oxidase 2, which is secreted by some breast tumors to create a stiffer extracellular matrix to form “pre-metastatic niches,” and the lack of copper also leads to decreased secretion of lysyl oxidase 2, further reducing metastasis [112].
Thus, chronic copper overload appears to be a high risk factor for cancer, indirectly by causing chronic inflammation in the liver, but also directly, via affecting several cellular signaling pathways.
4.6. Zinc-dependent enzymes
Zinc is a critical cofactor in more than 300 different enzymes encompassing all seven known enzyme classes [113]. The provision of Zn for metal loading to these enzymes depends on its distribution through binding to metallothioneins (MT). Copper is capable of displacing bound Zn from Zn-MT (EC50 (Cu) = 1.93 mM, EC50 (Zn) = 8.06 mM) [114], excess copper therefore leads to a high ratio of bound copper to zinc in MT, which limits the Zn provision to Zn-dependent proteins such as sorbitol dehydrogenase, since this enzyme receives Zn from MT [96,115,116]. Phenotypic similarities between Atp7b knockout mice with copper overload and Zip14 Zn deficient mice, in terms of abnormalities in hepatic glucose metabolism, for example impaired gluconeogenesis, further validate that copper overload may inhibit Zn-dependent enzymes [92,117]. For a detailed review, see Barber et al. [118].
4.7. Interference with iron homeostasis
Metabolic pathways of copper and iron are closely intertwined. Dietary copper and iron are absorbed in the duodenum. Iron deficiency increases enterocytic ATP7A expression (increased copper efflux to the portal vein), whereas copper deficiency leads to decreased hephaestin activity, a copper-dependent ferroxidase that blocks nutritional iron uptake [[119], [120], [121], [122]]. Dietary copper excess, on the other hand, was shown to inhibit iron absorption in the intestines of rats and pigs [123,124].
In the liver, the copper-dependent ferroxidase ceruloplasmin (CP) mobilizes iron via ferroportin and thereby facilitates its incorporation into transferrin by oxidation of Fe2+ to Fe3+ [125]. Loss of CP function therefore leads to iron accumulation in the liver and other organs, accompanied by decreased circulating iron, which manifests itself in various clinical symptoms such as diabetes mellitus, liver damage, mild anemia and neurodegeneration [[126], [127], [128]]. Such secondary iron accumulation can be found in copper burdened livers of patients with untreated Wilson disease, which may worsen the clinical course of the liver disease by accelerating the progression of fibrosis and scarring [129].
5. Targets of copper toxicity
5.1. Mitochondrial copper toxicity
Intracellular copper is essential for cellular bioenergetics and survival as it is a cofactor of the mitochondrial enzyme cytochrome c oxidase (CcO). The metal loading of CcO occurs within mitochondria [130]. Therefore, a constant mitochondrial copper supply is ensured by copper chaperones delivering the metal to CcO, driven by increasing copper affinity [55]. Indeed, based on studies in yeast, mitochondria are thought to be the intracellular copper store [131,132]. Moreover, analysis of rat liver tissue by atomic absorption spectroscopy showed that the copper binding capacity of mitochondria was second only to the cytosolic protein fraction [133]. When cellular copper load is elevated, mitochondrial copper levels in particular increase to cope with such excess [59,131]. By treating rats for six weeks with a daily intraperitoneal injection of 1.25 mg copper/kg body weight, i.e., a six-fold physiological excess for an average size rat (intake of ca. 0.2 mg/kg b.w. copper), the mitochondrial fraction (including lysosomes) of the liver showed the highest increase in copper (three-fold increase, whereas in other compartments copper did not significantly increase) [134]. Mitochondria are therefore an early target for increased copper intake, while the nuclear fraction is primarily affected upon longer treatment periods. [134].
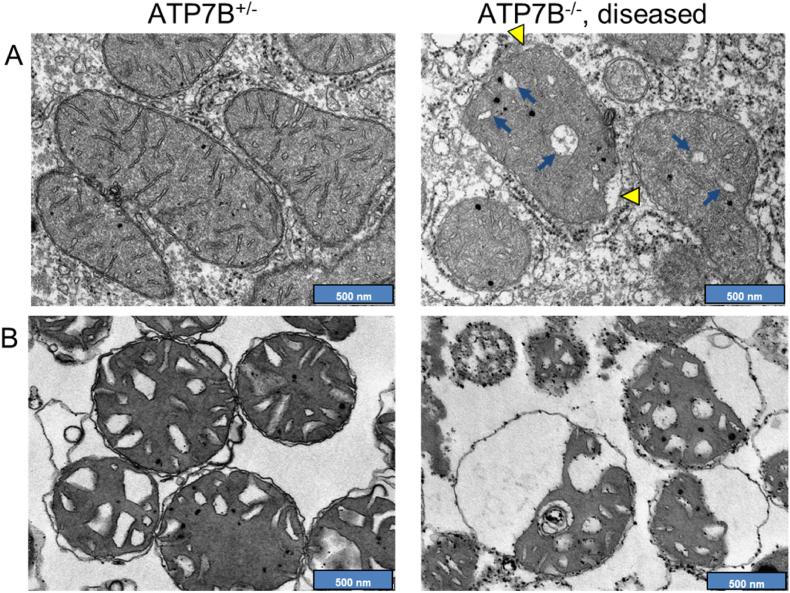
As repeatedly observed in liver samples from WD patients, the increased copper load has a destructive effect on the mitochondrial ultrastructure, provoking elongations, deformations, vacuolar and crystalline inclusions, and cristae dilatations (Fig. 3) [[135], [136], [137], [138]]. Abnormal hepatic mitochondria were found in early disease stages of animal models of WD as well, but also in copper-intoxicated animals [39,58,59,110,[139], [140], [141]]. The observed functional and morphological changes in mitochondria correlated with the level of mitochondrial, but not general liver copper content, and were reversible by reducing mitochondrial copper load using copper chelators [58,59,136,137], again marking mitochondria as a prime target of copper toxicity.
Fig. 3.
Copper induced mitochondrial structure alterations in WD rat livers. A Electron micrographs of liver in situ. Diseased WD rats have altered liver mitochondria with electron translucent widened cristae (arrows) and detached outer membranes (arrowheads). B Electron micrographs of isolated rat liver mitochondria from the same animals. Mitochondria from the diseased animal are structurally altered compared to mitochondria from heterozygous control animals. Membrane detachments, membranous inclusions and dilated cristae are apparent. Pictures adapted from Einer, C. et al. Gastroenterology. 2023 Jul; 165(1):187–200.e7. https://doi.org/10.1053/j.gastro.2023.03.216 with permission.
Brain mitochondria appear to be especially susceptible to elevated copper [142]. Compared to isolated mitochondria from liver, kidney and heart, lower copper doses initiate a loss of the mitochondrial membrane potential and ultrastructural changes in isolated brain mitochondria [85]. Furthermore, a dose-dependently decreased ability to produce ATP was observed for mitochondria of the SHSY5Y cell line (neuroblastoma), already at comparatively low copper challenges, but not for mitochondria from the U87MG cell line (astroglioma) [85].
Ultrastructural defects of copper-loaded mitochondria are associated with functional deficits: WD patients with end-stage liver disease were found to have reduced activity of the electron transport chain complexes in liver homogenates [67]. Animal models of WD and experimental copper overload also showed progressive and significant impairment of ATP production capacity and electron transport chain complex activity [58,59,141,143]. Interestingly, these structural and functional mitochondrial defects have been found without evidence of severe oxidative damage already at early disease stages and were rather attributed to the protein-toxic property of copper [58,59,143].
5.2. Inhibition of autophagy and mitophagy
In one study focused on WD patients, 12 out of 22 patients had no, or only mild, mitochondrial abnormalities despite symptoms of liver disease [137], arguing for further intracellular copper targets. Indeed, such absence of abnormal mitochondria was attributed to the occurrence of lysosomal copper sequestration [137]. Goldfischer and Sternlieb detected copper to be diffuse in the cytoplasm of hepatocytes at early stages of WD, along with abnormal mitochondria. However, when fibrosis and cirrhosis were the predominant symptoms of WD liver damage, copper was mainly found in the lysosomes of the hepatocytes [144]. In several animal models of copper overload, an increased number of pleiomorphic lysosomes was observed [39,139,140,145,146]. One of these studies found additional electron dense material consistent with copper in lysosomes [140]. Myers et al. demonstrated that in rat liver upon a 10-fold increase in copper, hepatic lysosomes exhibited various morphological abnormalities, increased in number, and were filled with copper-containing material [145]. Lysosomal copper storage was also observed in the Atp7b-deficient LPP rat and LEC rat, both models of WD, and hepatocytes from LPP rats, as well as ATP7B-deficient HepG2 cells, showed morphological changes in lysosomes [146,147]. Thus, chronic copper overload targets the lysosomal compartment, especially in later stages of WD.
Polishchuk et al. reported sequestration of damaged mitochondria containing high levels of copper as a major source of lysosomal copper, indicating autophagic degradation of copper loaded mitochondria (mitophagy). The authors further showed that copper treatment of ATP7B-deficient cells induced mitophagy and general autophagy. Inhibition of autophagy by the specific inhibitor spautin-1 increased apoptosis in vitro in ATP7B-deficient cells and liver injury animals [146]. This indicates an intertwined targeting of mitochondria and lysosomes by excess copper, either by increased elimination of mitochondria in the lysosomal compartment or, vice versa, by copper dependent blocked lysosomal activity avoiding mitochondrial renewal.
Induction of autophagy or an increase in autophagic flux by copper can also be detected in wild-type liver cells and non-liver tissues and cell lines [146,[148], [149], [150]]. Recently, it has been shown that binding of copper to the pro-autophagic kinases Unc-51-like autophagy-activating kinase 1 and 2 dose-dependently increases their kinase activity in vitro and can be blocked by copper depletion at the cellular level [151]. Thus, copper directly induces autophagy via activation of pro-autophagy intracellular signaling pathways, which may balance copper overload to some extent. However, upon excess, such a protective mechanism may be limited, as a common feature in WD patients and in WD animal models is the appearance of highly copper-loaded, defective mitochondria [58,59,67,138,141]. Interestingly, mitochondrial ANT is a critical regulator of mitophagy induction [152] and is required for inhibition of the presequence translocase TIM23, which leads to stabilization of PINK1 in response to mitochondrial membrane depolarization. The rat ANT has four cysteine residues, three of which are located in loops facing the matrix side where interaction with TIM23 occurs [153]. These cysteine residues appear to be sensitive to copper-catalyzed formation of intermolecular disulfide bridges, which could affect interactions with TIM23 and thus PINK1-induced mitophagy [84,153]. Thus, on a hypothetical level, excess copper might target the cysteine residues of the ANT, thereby hampering mitochondrial renewal and explaining the omnipresence of damaged organelles in livers of WD patients and WD animal models [58,59,67,138,141].
The appearance of damaged, copper-laden mitochondria in lysosomes further suggests that copper may also impair cellular autophagic flux by damaging the lysosomal compartment as well. Indeed, lysosomal membranes from copper-stressed animals exhibited reduced fluidity, high levels of lipid peroxidation, and altered lipid composition, similar to membrane damage in copper-stressed mitochondria [145]. Indeed, parallel to such membrane damage, cells and animals lacking ATP7B or loaded with high copper concentrations exhibit lysosomal dysfunction, e.g., decreased lysosomal acidification and enzyme activities [145,146]. Such an increased lysosomal pH could be a direct consequence of copper accumulation, as the vacuolar H+-ATPase, responsible for lysosomal acidification, can be blocked in vitro by the formation of intramolecular disulfide bonds, e.g., by copper induced thiol-oxidative protein damage [154].
5.3. Target sites of copper toxicity in the central nervous system
Compared to liver, the mechanisms of copper toxicity in the brain are much less understood, in part because there is no animal model for acute copper toxicity in the brain, and only a few for chronic copper toxicity. In the brain, astrocytes appear to be somewhat equivalent to hepatocytes since they store copper and are remarkably resistant to copper toxicity because of high levels of MT 3 and 4, as well as high levels of GSH, that even increase upon copper elevation [[155], [156], [157]]. Astrocytes are the first parenchymal cells to be exposed to various metals via the blood brain barrier and an increased metal exposure can induce the expression of protective GSH and MTs [158,159].
A recent report found that copper rarely accumulates in neurons in WD patients, but does accumulate in large amounts in oligodendrocytes [160]. The latter appear to be sensitive to elevated copper levels, responding with the formation of edema and demyelination [142,161]. Nevertheless, neurons are also very sensitive to copper toxicity because they synthesize only small amounts of GSH and rely on protective copper storage in astrocytes. Moreover, co-culturing of astrocytes and neurons increases GSH production in the latter in vitro and in vivo, the former provide a GSH precursor to neurons, supporting neuronal GSH synthesis. [162]. Astrocytes thereby help neurons to resist against copper stress, but also mediate neuronal mitochondrial repair by transfer of healthy mitochondria to them [[163], [164], [165]]. This may further protect neurons from copper overload, as their mitochondria have been demonstrated to be exceptionally vulnerable to toxic copper overload [85]. This vulnerability may especially affect dopaminergic neurons in the substantia nigra as they have extremely high energy requirements. Therefore, an energy crisis due to mitochondrial dysfunction could result in extensive damage to these cells [166], which might also become relevant in chronic or acute copper intoxication, since mitochondria are a prime target of copper.
Of particular interest in the brain is protein-toxic copper, demonstrated to interact with the prion protein [167], amyloid precursor protein and huntingtin, and therefore possibly being involved in pathologies like spongiform encephalopathy, Alzheimer's disease [168,169] and Huntington's disease [170]. Copper dyshomeostasis has also been suggested as determining contributor to Parkinson disease by causing a decrease of specific binding sites of dopamine D2 receptors, which was shown by an in vitro binding assay (40–60 % reduced binding of the dopamine antagonist [3H]spiperone) [171,172]. This reduction in binding D2 receptor binding sites might be one trigger of the observed symptoms [171].
Thus, while mitochondria and lysosomes are prime targets for copper toxicity, especially in the brain, its protein toxicity may be of major concern as well.
6. Acute copper intoxication
Being essential, the recommended amount of copper for adults is 0.9–1.3 mg of copper per day and varies according to food choices and dietary habits [9]. Acute copper toxicity results from the accumulation of excess amounts of copper (∼8–10 mg Cu /kg body weight) [173]. Accidental ingestion or attempted suicide are typical for rare cases of acute copper toxicity in human patients reported. In addition, copper salts can also be inhaled or absorbed through the skin, as they are widely used in a variety of fungicides, ceramics, fireworks, emetics, and burn ointments [5]. Acute poisoning with copper salts provokes symptoms such as vomiting, gastrointestinal bleeding, cardiac arrhythmia, hypotension, acute kidney damage and acute hepatitis [174]. The intoxication with copper causes haemolytic anemia within the first 24–48 h upon ingestion by binding of copper to erythrocyte haemoglobin with oxidation of the heme group and the appearance of methaemoglobin [175]. On the second to third day, the liver starts to fail due to mitochondrial dysfunction, which is followed by kidney failure on the third to fourth day [175]. Kidney damage is triggered by a combination of direct copper toxicity on the proximal tube, inducing necrosis and pre-renal damage due to dehydration (caused by vomiting and diarrhea before) and haemolysis [5,176]. In addition, liver damage may lead to the hepatorenal syndrome, that has a high mortality rate if not diagnosed early [177].
6.1. Acute copper toxicity in humans
Excessive copper consumption in suicidal attempts has been described in the literature (Table 2). Other case reports have described exposure to high copper concentrations as a result of tap water contamination, which led to irreversible liver failure in exposed patients (Table 2).
Table 2.
Cases of acute copper toxicity in humans.
| Model | Sex, Age | Dose, Route | Analysis | Clinical manifestations, | Treatment and Outcome | Reference |
|---|---|---|---|---|---|---|
| Copper toxicity in human | Male, 26 yr-old | Ingestion of approximately 30 g of copper sulfate | Blood tests, pulse rate, ultrasonography of organs, neurological examination | Several complications such as haemolysis, acute kidney injury, erosive gastritis with upper gastrointestinal bleeding, methaemoglobinaemia and hepatitis | The patient treated with d-penicillamine (DPA) (500 mg 6 hourly) until serum copper had normalized, hemodialysis for 4 weeks, started at day 4, omeprazole and red blood cell transfusion due to erosive gastropathy, IV methylene blue 2.5 mg/kg for methaemoglobinaenemia, treated for acute pancreatitis. Good clinical recovery after 38 days, no further complication | Gamakaranage et al., 2011 [174] |
| Male, 45 yr-old | Ingestion of 50 g of highly concentrated copper sulfate, in a suicidal attempt | Respiratory, abdominal and nervous system examination, pulse rate | Developed severe epigastric pain with loose stools and melaena, intravascular haemolysis, severe aspiration pneumonia, acute kidney injury. | The patient was treated with supportive care and chelation therapy (DPA 500 mg, 6 hourly) until serum copper had normalized. Hemodialysis from day 5 on for 10 days, mechanical ventilation for 8 days. Omeprazol infusion for erosive gastropathy, meropenem 1g 8 h for aspiration pneunomia. Gradual convalescence, discharged after 25 days with no further complication | Gamakaranage et al., 2011 [174] | |
| Male, 42 yr-old | Ingestion of ≈250 g of CuSO4 (≈100 g Cu) in attempted suicide | Blood tests, pulse rate, neurological examination | Protracted vomiting, transient jaundice and rhapdomyolysis. | The patient was initially treated with a single injection of BAL (dimercaprol), 4 mg/kg. Activated charcoal and magnesium sulfate were given in addition to oral DPAe 250 mg every 6 h. Patient was discharged still under DPA therapy, he recovered well, no further information | Jantsch et al., 1984 [178] | |
| Male, female 1 yr-old | Two infant siblings consumed for >9 months Cu-containing tap water (Cu at 2.2–3.4 mg/L); the Cu was derived from Cu pipes | Liver biopsy, quantitative copper determination | Micronodular cirrhosis with hepatic Cu storage, hepatosplenomegaly, jaundice, and hypertransaminasemia; no information about therapy | One died with 13 months due to liver failure, the other one survived, but no further information | Müller-Höcker et al., 1988 [179] | |
| Female, 41 yr-old | Oral ingestion of CuSO4 with suicidal intent | Physical examination, pulse rate | Vomiting, diarrhea, hepatorenal failure, and gram-negative septicemia | Gastric lavage after arrival with sodium bicarbonate and EDTA i.v.for 1 h. Red blood cell transfusion and hemodialysis were performed. Died after the 6th day in hospital due to haemolysis, severe hepatocellular and renal failure. Autopsy showed Cu deposits in brain, heart, liver, kidney | Agarwal et al., 1975 [180] |
6.2. Rodent models of copper toxicosis
In animal studies, the consequences of excessive copper intake by feeding, oral administration or injection into small rodents, mainly rats (Table 3) and mice (Table 4), were investigated.
Table 3.
Summary of rat models for acute copper toxicity.
| Model | Dose, Route | Analysis | Main findings | Reference | |
|---|---|---|---|---|---|
| Copper toxicity in rats | Sprague-Dawley rat | Up to 3.75 mg Cu/kg b.w., i.p. daily for 6, 12 or 18 weeks | Spectrophotometric determination of copper in subcellular fractions of the liver | Depression in body weight, dose-dependent increase in mitochondrial/lysosomal copper content, indication of large, but not unlimited, copper storage capacity | Lal and Sourkes, 1971 [134] |
| Sprague-Dawley rat | Copper-enriched diet (1000 ppm Cu for 4 weeks + 2000 ppm Cu for 4 weeks) | Light microscopy and electron microscopy (EM), determination of in vivo lipid peroxidation by TBARS (thiobarbituric acid-reacting substances) determination of copper by atomic absorbance, lipid-conjugated dienes in isolated liver mitochondria | Hepatocyte necrosis, dilated cristae, peroxidation of mitochondrial lipids, abnormally dilated cristae of mitochondria, electron dense material in hepatic lysosomes | Sokol et al., 1990 [140] |
|
| Sprague-Dawley rat | Copper-enriched diet (1000 ppm Cu for 4 weeks + 2000 ppm Cu for 4 weeks) | TBARS, Clarke electrode, enzyme activity assays in isolated liver mitochondria | Lipid peroxidation increased, significantly increased mitochondrial copper content, state 3 respiration (OXPHOS) decreased, reduced complex IV activity | Sokol et al., 1993 [141] |
|
| Sprague-Dawley rat | Up to 20 mg CuSO4 /kg b.w, daily by oral gavage for 45 or up to 90 days | ΔΨm by Rh123 fluorescence, AAS, TBARS, ROS determination by DHE or DCFDA fluorescence, ATP bioluminescence assay absorbance (A540 nm) in primary hepatocytes and isolated liver mitochondria | Reduced ΔΨm, increased mitochondrial copper content, increased superoxide levels, reduced ATP production, increased mitochondrial swelling | Roy et al., 2009 [181] |
|
| Sprague-Dawley rat | Single i.p. injection of 3, 10 and 30 mg/kg b.w. of cupric sulfate (CuSO4 .5H2O), animals sacrificed after 48 h | Liver Cu content (AAS), liver oxidative stress (TBARS) and damage in liver tissue | Dose- and time-dependent metal accumulation in the liver, enhanced rate of free-radical mediated lipid peroxidation with increased liver chemiluminescence | Boveris et al., 2012 [173] |
|
| Sprague-Dawley rat | 200 mg/kg b.w. CuCl2·2H2O, 100 mg/kg b. w. nano-copper 50, 100, 200 mg/kg b. w., for 28 days as continuous gavage | Measurements of cytokines and oxidative stress, gene expression analyses, histopathology of liver tissue and homogenates | Liver sinus congestion caused by copper ions, dose-dependent vacuolar degeneration, inflammation and oxidative stress caused only by nano-copper increased levels of the inflammatory cytokines IL-1β, TNF-α, IL-6, and MIP-1 | Tang et al., 2019 [182] |
|
| Wistar rat | Copper-enriched diet (1500 ppm Cu for up to 16 weeks) | EM and AAS of copper content in liver tissue | Swelling of mitochondria and electron dense lysosomes in early phase of copper overload, hypertrophy and dilatation of SER | Fuentealba and Haywood, 1989 [39] |
|
| Wistar rat | One time intraperitoneal (i.p.) injections of Cu2+ doses 2.5, 5, and 10 mg/kg b.w. | Mitochondrial ROS level, ΔΨm, ATP assay, TBARS, in isolated liver mitochondria | Increased ALT and AST levels, ROS production, decreased ΔΨm, reduced mitochondrial ATP levels, lipid peroxidation, decrease in mitochondrial GSH content | Hosseini et al., 2014 [183] |
|
| Wistar rat | Copper sulfate (CuSO4.5H2O) 100 mg/kg b.w./daily orally for 4 weeks | Spontaneous locomotor activity, rotating rod and grip strength test, AAS, GSH, malondialdehyde and total antioxidant capacity determination, immunohisto-chemistry in brain tissue | Increased copper levels in corpus striatum of the Cu-exposed rats, cell death in corpus striatum likely as a result of Cu-mediated oxidative stress leading to apoptosis and glial dysfunction | Kalita et al., 2020 [184] |
Table 4.
Summary of mice models for acute copper toxicity.
| Model | Dose, Route | Analysis | Main findings | Reference | |
|---|---|---|---|---|---|
| Copper toxicity in mice | ICR mice | Single oral gavage 500–5000 mg/kg b.w. CuCl2·2H2O | Morphological and pathological examination of the organs, blood biochemical assay | Pathological examinations revealed that kidney, liver and spleen are target organs for nano-copper particles, blood biochemical indexes reflect the renal and hepatic functions of experimental mice | Chen et al., 2006 [119] |
| ICR mice | Ad libitum, diet group received 6 ppm, 15 ppm and 30 ppm copper, water group drank copper solution 6 ppm, 15 ppm and 30 ppm supplied as CuSO4·5H2O for 3 months | Copper concentration in blood and organs, GSH and SOD enzyme activity in liver | Hepatic GSH decreased with increased concentrations of copper in the water group, water group showed a lower SOD activity than the diet group. Significant copper deposition in liver and serum | Wu et al., 2016 [185] |
|
| ICR mice | Intragastric doses of 4, 8, or 16 mg/kg b.w. Cu (Cu2+-CuSO4) for 21 and 42 days | Hepatic Cu concentrations, pathological assessment, ΔΨm, ROS production in liver tissue | Cu accumulation in the liver, increased hepatic cells with granular and vacuolar degeneration by Cu exposure in a time-dependent manner, increased ROS production | Liu et al., 2020 [186] |
|
| CFI mice | Gavage with a daily dose of 8.25 mg/kg b.w. (CuSO4) for 6 consecutive days | Comet assay, micronucleus test, PIXE analysis in liver tissue and blood | Copper induced DNA damage shown by comet assay, genotoxicity and mutagenicity of copper evaluated by comet and micronucleus assay | Pra et al., 2008 [187] |
|
| Kunming mice | Ad libitum in the diet as 15.93, 31.86, 63.72, and 127.44 mg/kg dry matter in high-copper group for 95 days | Colorimetric examination for alkaline phosphatase, SOD, and CP in plasma, histopathology of the liver, Cu content by FAAS, EM in liver tissue and blood | Increased Cu content in the liver. Pathological changes such as swelling, necrosis, and cytoplasm crack of liver cells in Cu-supplemented group. Extensive liver tissue damage shown by EM. | Wang et al., 2014 [188] |
In copper-loaded rats, a rapid accumulation of copper in the liver was observed. As a result, severe mitochondrial damage was documented, manifested by increased mitochondrial copper, dilated mitochondrial cristae and reduced ATP production (Table 3).
In murine models of copper toxicosis, mice were either fed a copper-containing diet, administered via gavage or ingested copper via water. The copper exposure experiments provided similar results to the rat models: Copper-induced damage was observed in the livers of mice treated with excess copper. In these studies, enlarged liver cells, pathological changes in the liver and copper-induced DNA damage were documented. In addition, extensive morphological and functional changes in the mitochondria were detected (Table 4).
6.3. Treatment of acute copper intoxication in humans
Few data on acute copper poisoning in a clinical setting exist, and there are no specific recommendations for the treatment of acute copper poisoning. In the reported cases, poisoning was treated symptomatically: the occurrence of haemolytic anemia and renal failure was treated with blood transfusions and hemodialysis [174,175,189,190]. Treatment with the copper chelator d-penicillamine (DPA) was also reported, although some authors doubt the clinical efficacy of DPA in acute poisoning [174,175]. Moreover, in patients with acute copper-induced renal failure, DPA may be contraindicated due to its potential nephrotoxicity [5,175,191].
7. Genetically induced chronic copper overload – Wilson disease
In contrast to acute copper toxicity, significantly more data exist for chronically induced copper overload, mostly referring to Wilson disease (WD). Here, we will briefly review this disease, focusing on pathognomonic liver and brain symptoms.
WD is a genetic disease, which affects systemic copper homeostasis. A mutation in chromosome 13 in the locus of the ATP7B gene, resulting in its dysfunction, leads to systemic copper overload, profoundly in the liver but also brain [194,195]. This results in hepatic, neurological and/or psychiatric symptoms, with the latter sometimes conflated as neuropsychiatric symptoms. While hepatic symptoms mostly occur in the first decade of life, the other two mainly occur in the second decade of life [196]. Other organs, e.g. heart or kidney, may be affected as well, but typically to a much lower frequency [197].
7.1. The hepatic manifestation of Wilson disease
Hepatic WD is characterized by the development of cirrhosis, chronic hepatitis, and, in advanced stages, acute liver failure. Liver pathology can be manifold and progressive as depicted in Table 5.
Table 5.
Hepatic manifestations in human Wilson disease patients.
| No of WD patients | Analysis | Findings in the liver | Mitochondria | Reference |
|---|---|---|---|---|
| 8 (6 untreated, 2 treated with DPA) | Biopsy of liver tissue, EM | Excessive hepatic copper concentration, (ranging from 500 to 1300 μg/g dry weight) fatty metamorphosis of hepatocytes | Increased matrix density and intermembrane space, varying frequency of abnormally shaped mitochondria, electron-transparent inclusions, impaired fatty acid catabolism | Sternlieb, 1968 [135] |
| 7 (before and after DPA treatment) | Biopsy of liver tissue, EM | Hepatic copper concentration between 821 and 117 μg/g dry weight | Increased matrix density, presence of granular inclusions, partial separation of inner and outer membrane of mitochondria DPA treatment reversed structural alterations of the mitochondria |
Sternlieb and Feldmann, 1976 [136] |
| 64 (untreated) | Biopsy liver tissue, EM | Increased hepatic copper content (500–1100 μg/g dry weight), fibrosis and cirrhosis in 9 of 22 patients | Mitochondria were found to be enlarged, pleomorphic, partially containing large granular vacuoles not related to patient age, hepatic copper level or AST | Sternlieb, 1992 [137] |
| 3 (treated with DPA) | Isolated liver mitochondria, TBARS | Hepatic copper content in WD patients ∼400 μg/g dry weight, liver cirrhosis in all cases | 33-fold increased copper level, increased lipid peroxidation | Sokol et al., 1994 [138] |
| 16 (7 untreated, 9 treated with chelating therapy) | Biopsy of liver tissue, mtDNA sequencing | 6 patients with cirrhosis, 14 patients with fibrosis | High prevalence of large mtDNA deletions, appearing in early stages of WD | Mansouri et al., 1997 [192] |
| 3 (2 treated with DPA) | Liver homogenate, enzyme activity assays | All three patients presented cirrhosis, contained 300–700 μg/g dry weight of copper. | Significantly decreased activity of mitochondrial aconitase and respiratory complexes | Gu et al., 2000 [67] |
| 11 (untreated) | Biopsy of liver tissue, EM | Portal tract fibrosis in 9, liver cirrhosis in 6 | Pleomorphic mitochondria with enlarged, electron-dense granules, loss of cristae structure, increased intermembrane space | Shawky et al., 2010 [193] |
7.2. The neuropsychiatric manifestation of wilson disease
Psychiatric symptoms are determined by the presence of cognitive impairment, affective disorders, and psychosis [195], while the neurological phenotype (Table 6) is mainly characterized by movement disorders, including dystonia, tremors, rigidity and parkinsonism, and speech disorders, characterized by dysarthria, dysphagia and drooling [2,198]. In his thesis, S.A.K. Wilson described a characteristic softening in the “lenticular nucleus” (putamen and globus pallidus) [199]. Today, it is known that pathologic alterations may extend to the thalamus, brainstem, pons and frontal cortex and may include the complete basal ganglia as well [2,200]. The most severe abnormalities can be found in the putamen, which appears brownish and shrunken [200]. Brain atrophy and ventricular dilatation are further frequent macroscopic findings and, in more severe cases, white matter degeneration and neuronal loss are observed [195,[200], [201], [202]].
Table 6.
Neurologic manifestations in human Wilson disease patients.
| No of patients | Main neurological symptoms | Sample, analysis | Findings in the liver | Findings in the brain | Reference |
|---|---|---|---|---|---|
| 4 patients examined, 2 from literature | Tremor, dysphagia, dysarthria and Risus sardonicus | Post mortem analysis of 3 patients | Liver cirrhosis | Lenticular degeneration especially dentate nucleus and putamen, liver cirrhosis | Wilson 1912 [199] |
| 2 WD patients | In one patient: Torsion spasm, tremors, dysarthria, rigidity, | Autopsy only possible in one (no description of symptoms) | Liver cirrhosis, marked excess of copper in liver: Cu in liver 4.6 mg/100 g wet weight (15.3 mg/100 g dry weighta) | Marked copper excess in basal ganglia and to a lesser extent in cortex, basal ganglia 1.275 mg/100 g (6.36 mg/100 g dry weighta); cortex 0.781 mg/100 g wet weight (3.9 mg/100 g dry weighta). | Glazebrook 1945 [208] |
| 3 WD patients | Not given | Post mortem analysis of liver and brain, copper determination via sodium diethyldithio-carbamate method | Increased hepatic copper content, liver control: 10.7 mg/100 g dry weight, WD: 83.6 mg/100 g dry weight | Increased copper content in the brain, especially globus pallidus and putamen, but also cortical white and grey matter. | Cumings et al., 1948 [3] |
| 89 WD patients | Not given | Liver biopsy, computed tomography of the brain | Liver cirrhosis, steatosis and fibrosis | In the brain: Basal ganglia lesions, generalized atrophy and ventricular dilatation most common. | Brewer and Yusbastan Gurkitan 1992 [195] |
| 2 WD patients, confirmed by PCR | Dysarthria, dystonia, dysphagia and tremors | Autopsy, copper determination via ICP-AES in post mortem samples of liver and brain from both patients | Incomplete liver cirrhosis, enlarged mitochondria with pseudo-crystalline inclusions; in liver Cu: 23.25 mg/100 g dry weight (Control <2.5 mg/100 g dry weight). | Brain lesions in superior frontal lobe, lenticular nuclei, base of pons and cerebellum. Neuronal loss in basal ganglia with Alzheimer type II cells, oligodendrocytes rare in caviations. Presence of Opalski cells most abundant in white matter (only in neuro WD). In brain, the white matter frontal lobe (23.96 mg/100 g dry weight) putamen (20.08 mg/100 g dry weight). Controls (1.84 and 0.7 mg/100 g dry weight) for the frontal lobe and the putamen, |
Mikol et al., 2005 [202] |
| 8 WD patients, (2 hepatic, 1 neuropsychiatric, 4 neurologic, 1 mixed) | Tremors (4), dysarthria (5), bradykinesia (2), dystonia (3), rigidity (4) | Autopsy, immunohistochemistry of the brain and liver | Liver involvement in all patients: Cirrhosis (6), steatosis (4), chronic active hepatitis in 2. | In the brain central pontine myelinolysis (5), subcortical white matter cavitations (5), putaminal softening (4), variable ventricular dilatation (6), widespread myelin loss was found. Presence of Opalski cells and Alzheimer type I in brain stem and basal ganglia, Alzheimer type II cells in cerebral white matter more diffusively distributed | Meenaksi Sundaram 2008 [206] |
| 12 WD patients, 5 control | Not given | Autopsy brain tissue samples from four regions of the brain: frontal cortex, putamen, pons, and nucleus dentatus, Cu, Mn and Zn determined via ICP MS, Fe via AAS | – | Copper accumulation in all analyzed brain structures (41 +- 18, compared to 5.4 +- 1.8 μg/g dry weight in control) and higher iron levels in the dentate nucleus of WD patients 56.8 ± 14.1 control: 32.6 ± 6.0⁎. | Litwin et al., 2013 [209] |
Estimated under the assumption that the brain contains roughly 80 % water and the liver 70 % water (Canadian B Campus Human Anatomy and Physiology textbook).
Microscopic examination reveals the presence of swollen astrocytes, with pale nuclei and little cytoplasm (also known as Alzheimer type II cells) in the deep grey matter. Rarely Alzheimer type I cells can be found and are characterized as enlarged and multinucleated cells deriving probably also from astrocytes [203]. These two cell forms are named after a drawing of enlarged and multinucleated cells in the brain of a WD patient, that was published by von Hösslin and Alzheimer in 1912, which Spielmeyer later referred to as Alzheimer glia cells [204]. Reactive astrocytes, enlarged cells containing glial fibrillary acidic protein can also be found [205]. Opalski cells are the most characteristic cells for WD. These are large cells with a foamy cytoplasm and irregularly shaped nucleus, somewhat characteristic for neurologic WD, originating from degenerated astrocytes [206]. Furthermore, a low number of oligodendrocytes can be detected [202,206] that respond with swelling of myelin layers and demyelination to an increased copper burden [160,207].
7.3. Treatments of Wilson disease
Upon treatment and if diagnosed early, WD prognosis is very good and a normal life expectancy can be achieved [210]. However, this is especially true for patients with hepatic symptoms. In patients who also have neurologic symptoms, therapy may be threatening and inadequate due to neurologic worsening and neurologic symptoms may persist despite treatment with anti-copper drugs [211].
An obvious, typically advised therapeutic approach for WD patients would be to avoid foods with high copper content, like liver (15 mg/100 g), oysters (5.7 mg/100 g) and nuts (2.2 mg/100 g) [212]. However, excess copper accumulation in WD cannot solely be treated by a low-copper diet alone due to its omnipresence in nutrition and since a reduction of daily copper intake to below 2 or 3 mg is hardly achievable [213]. Thus, copper chelators or zinc salts are typically therapeutically administered, but also new WD treatments are under current development.
7.3.1. Treatment with copper chelators
British Anti Lewisite BAL (2,3-dimercaptopropanol) was the first metal chelator described in the therapy of Wilson Disease [214]. Due to its parenteral administration and severe adverse advents, it is only rarely used in this disease [215,216].
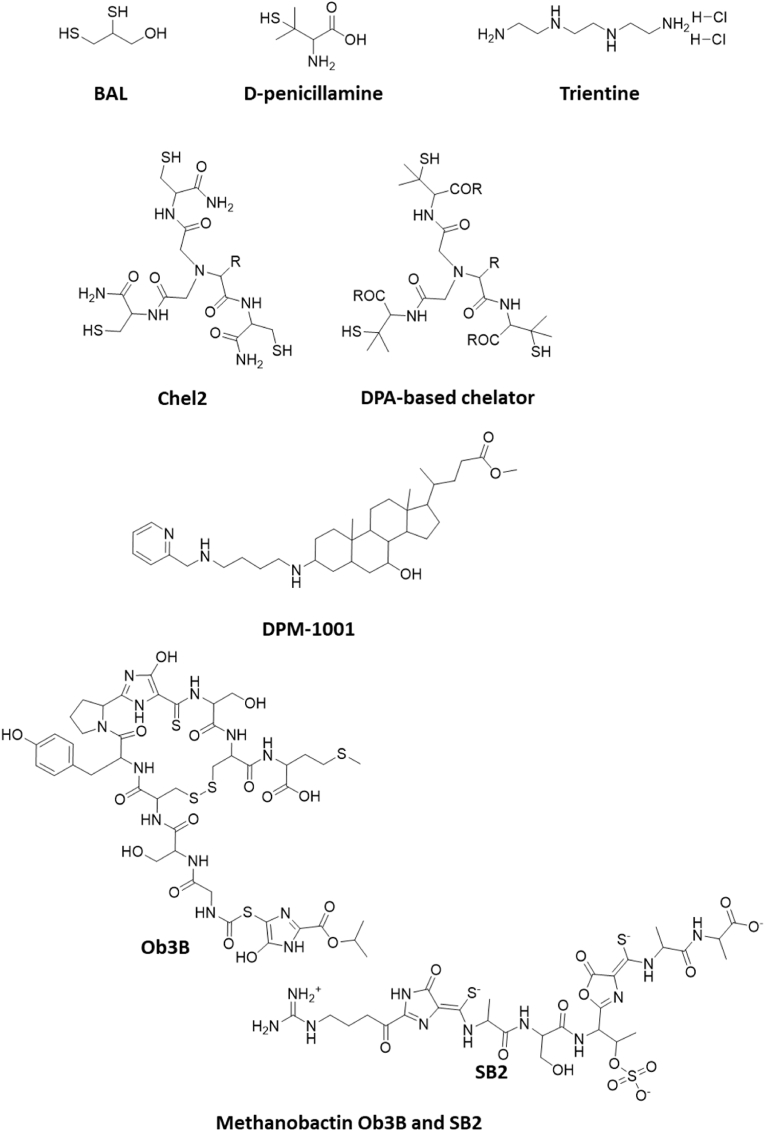
d-penicillamine (DPA, Fig. 4), a non-proteinogenic amino acid with a thiol group, is a non-antibiotic by-product of penicillin and exists in two stereoisomers, of which the D isomer is used as a therapeutic agent [217,218]. Due to its chelating properties against heavy metals (stability of penicillamine-metal complexes in descending order: mercury, lead, nickel, copper, zinc, cadmium, cobalt, iron, manganese), it is used in rheumatoid arthritis and heavy metal poisoning [215,219] and was the first oral drug approved for the treatment of WD in 1956 [220].
Fig. 4.
Representative copper chelators currently in the clinics or in development for clinical use. Structures were illustrated by using ChemDraw Professional, Revity Signals Software. Sources: BAL [214], d-penicillamine [220], trientine [231], Chel2 [256], DPA-based chelator [258], DMP-1001 [259], methanobactins Ob3B and SB2 [264].
Current metal-bound chelators are mainly excreted by the kidneys, with a low fecal excretion resulting from the unabsorbed fraction of the drug [221]. DPA is the first-line drug for WD, prescribed for daily and lifelong administration, although it may present with adverse effects, ranging from rash, nausea, vomiting, thrombocytopenia and proteinuria, to severe effects such as nephron and bone toxicity. Consequences are a poor patient adherence and/or discontinuation of the drug [[222], [223], [224], [225]]. DPA mainly decreases copper serum level, but hardly reduces tissue copper levels even after years of treatment [213,226]. A serious concern with DPA is the aggravation of neurological symptoms, termed neurological worsening, mostly upon treatment initiation [227,228]. Current views attribute this to the profound liberation of excess liver copper upon treatment initiation into blood from where it may reach the brain [229]. Consequently, clinical guidelines recommend a “start low and go slow” treatment strategy [230]. A second form of neurological worsening was recently reported, occurring 12 months after treatment initiation, but the authors associated this form of neurological worsening to treatment non-adherence [228].
Trientine (triethylenetetramine dihydrochloride, TETA, Fig. 4), was developed as an alternative to DPA [231] and is also used as a first-line WD treatment [232,233]. Like DPA, TETA is administered orally lifelong daily [230] and it chelates copper mainly in serum by competition with albumin, followed by renal excretion of the complex. TETA has been reported to be a less potent copper chelator than DPA and also less effective at inducing urinary copper excretion [234,235], but in vitro data contradicts this. In fact, in copper chelating in vitro experiments, TETA appears to be a more potent copper chelator than DPA [236]. However, in another copper binding affinity assay, both chelators had a similar affinity to cuprous copper [237]. TETA further seems to reduce copper uptake in the gut, either by chelating copper directly in the intestinal lumen or by inducing the expression of MTs in enterocytes [238]. Even though it has significantly fewer side effects than DPA, cases of iron deficiency and anemia occurred, and dermatitis and colitis were rarely reported [239,240].
7.3.2. Tissue copper reduction by zinc salts
The efficacy of Zn salts as a de-coppering agent was first described in sheep, suffering from chronic copper toxicosis by elevated copper levels in their diet (up to 30 mg/kg) [241]. The oral administration of zinc leads to the expression of metallothioneins in the enterocytes, which bind the copper taken up with food and prevent its transfer into the bloodstream. During the process of natural turnover of enterocytes, bound copper is excreted with the feces [[242], [243], [244], [245], [246], [247]]. In addition, zinc salts also induce the expression of metallothioneins in the liver and thus strengthen the endogenous defense [248]. However, the therapeutic effect can be observed only after several months, when a negative copper balance has been established [249], thus Zn salts are not suitable in scenarios of acute disease. To that, Zn salts may result in poorer clinical outcomes with respect to liver disease progression and to the prevention of liver injury [[250], [251], [252]], and are therefore considered for maintenance therapy upon initial chelation treatment for patients with mild symptoms and low copper overload/asymptomatic patients [253]. It is usually well tolerated, with the main adverse effect being gastric irritation, which can lead to erosion and ulcers [251,252,254].
One apparent major drawback of all these therapeutic agents is their complex administration schedule, i.e., lifelong twice/trice daily, and to be taken at best 1 h before each meal (trientine and zinc salts) [249] or generally on an empty stomach [255].
7.3.3. New treatment approaches
Due to profound research activities, several new candidate drugs aim to ameliorate the armamentarium against WD. They center on improved copper removal by more effective chelators, nuclear receptor signaling interference, autophagy inducers and gene therapy approaches to reestablish liver copper excretion.
Among the candidate copper chelators, a tripodal metal chelator Chel2 based on three cysteine residues linked via nitriloacetic acid was presented in 2012 (Fig. 4) [256]. It binds Cu+ selectively with a high affinity (KD 10−19 M) and specificity. Furthermore, it has been shown to relocate copper into the feces in Atp7b knockout mice [257]. Based on this concept, several DPA-based chelators containing three DPA molecules linked via nitriloacetic acid have been developed (Fig. 4) [258]. These molecules appeared to have a fairly high copper affinity in vitro, but have not yet been tested in vivo [258].
DPM-1001 (Fig. 4), an allosteric inhibitor of protein tyrosine phosphatase 1B, has been shown to form stable complexes with Cu2+ [259]. This uncharged sterol molecule is orally bioavailable and was therefore also tested in toxic milk mice, another animal model for Wilson's disease. DPM-1001 significantly reduced tissue copper levels and improved hepatic symptoms by oral and intraperitoneal administration, making it another promising candidate in the treatment of Wilson's disease [260].
Methanobactins (Fig. 4), bacterial peptides with very high copper affinity (KD < 10−20 M) [[261], [262], [263], [264]], appeared highly effective in copper depletion in in vitro and in vivo experiments in the LPP rat model of WD without obvious signs of toxicity. Days of intense treatments provoked a most profound fecal copper excretion resulting in wild-type liver copper levels [265]. From that, a new treatment strategy was established in WD rats, consisting of short, intense treatment cycles upon rise of serum liver enzyme levels and prolonged treatment pauses in between [265].
By applying LXR agonists, decreased binding of nuclear receptors to promoter elements (as described in section 3.1.2) was observed in WD mice, which also significantly improved their liver function [97]. However, such treatments also induced steatosis and hypertriglyceridemia in clinical trials for atherosclerosis, requiring further research for a potential use in WD [266].
Another interesting therapeutic approach seems to be the activation of autophagy, as this mechanism appears to be crucial for the cellular defense against copper toxicity. Inhibition of autophagy accelerated cell death in copper-exposed ATP7B knockout cells, while its induction favored survival and significantly reduced apoptosis in treated cells. This result was also confirmed by in vivo data: Inhibition of autophagy led to an increase in liver enzymes of Atp7b knockout mice and thus worsened the phenotype [146].
Finally, gene therapy strategies aim to restore copper metabolism by introducing a functional ATP7B gene via viral vectors. This attempt proved successful using adeno-associated viral vectors and lentiviral vectors in the WD mice [267,268], and clinical trials using two of the tested constructs (rAAV and AAV9) began in 2021 (VTX-801 and UX701) [269,270].
8. Conclusions
Copper is essential for cellular metabolism but, upon excess, is a severe toxin leading to cell death.
In the past, copper toxicity has mainly been attributed to oxidative damage based on its Fenton and Haber Weiss chemistry. However, due to a strong surplus of tight copper binding molecules that also ensure a safe copper transfer, there is no free reactive copper inside cells, thus questioning this mode of toxicity. In addition, cells are protected by extensive cellular antioxidant systems. It is only in specific situations (e.g. under conditions of short-lived spurs of copper overload, upon long-lasting accumulation of very high copper loads, or of a Cu1+/2+ mixture loading by an ionophore) that these systems can be overcome, leading to oxidative cell damage. Rather, thiol-oxidative damage and protein-toxic stress appear to be the major mechanisms of copper toxicity.
A constant routing to supply essential copper to mitochondria predisposes these organelles as prime copper targets. In addition, other intracellular systems like the autophagy/lysosomal pathway are impaired, and a disturbed copper homeostasis also affects the homeostasis of zinc and iron.
The liver is the central regulatory and distribution site and thus the target of excess copper, but it can cope with impressive copper loads before damage manifests, e.g. the long symptom-free period in WD patients. Due to the lack of appropriate animal models, much less is known about copper toxicity in the brain, that appears to be especially sensitive to chronic copper overload. Cases of acute copper intoxication and treatment reports are rare and result from unintentional or suicidal intent with the ingestion of high copper amounts causing direct pathological symptoms, mostly affecting the liver and kidneys. In contrast, there is profound data on chronic copper intoxication in patients with Wilson's disease. Symptoms may appear as late as in the mid 40's and typically affect the liver and brain, but may also damage other organs. While therapeutic options for Wilson disease exist, ongoing research has developed promising and potentially more effective and safer treatments for this devastating disease in the future.
CRediT authorship contribution statement
Judith Sailer: Writing – original draft, Visualization, Formal analysis, Data curation. Judith Nagel: Writing – original draft, Data curation. Banu Akdogan: Data curation. Adrian T. Jauch: Writing – original draft, Validation. Jonas Engler: Writing – original draft, Validation. Percy A. Knolle: Supervision, Conceptualization. Hans Zischka: Writing – review & editing, Validation, Supervision, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors have declared no conflict of interest.
Acknowledgements
HZ acknowledges grants by the Deutsche Forschungsgemeinschaft (DFG) grant ZI 1386/2–1 and by the Bundesministerium für Bildung und Forschung (BMBF) grant 01GM2001B.
Footnotes
Dedication to Maria C Linder
This review is dedicated to the late Maria C Linder, whose most inspiring work will continuingly guide and encourage researchers all over the world to delve into the mysteries of copper metabolism.
Data availability
Review article
References
- 1.Rae T.D., Schmidt P.J., Pufahl R.A., Culotta V.C., O'Halloran T.V. Undetectable intracellular free copper: the requirement of a copper chaperone for superoxide dismutase. Science. 1999;284(5415):805–808. doi: 10.1126/science.284.5415.805. [DOI] [PubMed] [Google Scholar]
- 2.Lorincz M.T. Neurologic Wilson's disease. Ann. N. Y. Acad. Sci. 2010;1184:173–187. doi: 10.1111/j.1749-6632.2009.05109.x. [DOI] [PubMed] [Google Scholar]
- 3.Cumings J.N. The copper and iron content of brain and liver in the normal and in hepato-lenticular degeneration. Brain. 1948;71(Pt. 4):410–415. doi: 10.1093/brain/71.4.410. [DOI] [PubMed] [Google Scholar]
- 4.Shribman S., Poujois A., Bandmann O., Czlonkowska A., Warner T.T. Wilson's disease: update on pathogenesis, biomarkers and treatments. J. Neurol. Neurosurg. Psychiatry. 2021;92(10):1053–1061. doi: 10.1136/jnnp-2021-326123. [DOI] [PubMed] [Google Scholar]
- 5.Perestrelo A.P., Miranda G., Goncalves M.I., Belino C., Ballesteros R. Chronic copper sulfate poisoning. Eur J Case Rep Intern Med. 2021;8(3) doi: 10.12890/2021_002309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arena M., Auteri D., Barmaz S., Bellisai G., Brancato A., Brocca D., Bura L., Byers H., Chiusolo A., Court Marques D., Crivellente F., De Lentdecker C., Egsmose M., Erdos Z., Fait G., Ferreira L., Goumenou M., Greco L., Ippolito A., Istace F., Jarrah S., Kardassi D., Leuschner R., Lythgo C., Magrans J.O., Medina P., Miron I., Molnar T., Nougadere A., Padovani L., Parra Morte J.M., Pedersen R., Reich H., Sacchi A., Santos M., Serafimova R., Sharp R., Stanek A., Streissl F., Sturma J., Szentes C., Tarazona J., Terron A., Theobald A., Vagenende B., Verani A., Villamar-Bouza L. Peer review of the pesticide risk assessment of the active substance copper compounds copper(I), copper(II) variants namely copper hydroxide, copper oxychloride, tribasic copper sulfate, copper(I) oxide, Bordeaux mixture. EFSA J. 2018;16(1) doi: 10.2903/j.efsa.2018.5152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tchounwou P.B., Newsome C., Williams J., Glass K. Copper-induced cytotoxicity and transcriptional activation of stress genes in human liver carcinoma (HepG(2)) cells. Met Ions Biol Med. 2008;10:285–290. [PMC free article] [PubMed] [Google Scholar]
- 8.Pierson H., Yang H., Lutsenko S. Copper transport and disease: what can we learn from organoids? Annu. Rev. Nutr. 2019;39:75–94. doi: 10.1146/annurev-nutr-082018-124242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trumbo P., Yates A.A., Schlicker S., Poos M. Dietary reference intakes: vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. J. Am. Diet Assoc. 2001;101(3):294–301. doi: 10.1016/s0002-8223(01)00078-5. [DOI] [PubMed] [Google Scholar]
- 10.Turnlund J.R. Copper nutriture, bioavailability, and the influence of dietary factors. J. Am. Diet Assoc. 1988;88(3):303–308. doi: 10.1016/S0002-8223(21)01967-2. [DOI] [PubMed] [Google Scholar]
- 11.Osaki S., Johnson D.A., Frieden E. The possible significance of the ferrous oxidase activity of ceruloplasmin in normal human serum. J. Biol. Chem. 1966;241(12):2746–2751. doi: 10.1016/S0021-9258(18)96527-0. [DOI] [PubMed] [Google Scholar]
- 12.Maio N., Polticelli F., De Francesco G., Rizzo G., Bonaccorsi di Patti M.C., Musci G. Role of external loops of human ceruloplasmin in copper loading by ATP7B and Ccc2p. J. Biol. Chem. 2010;285(27):20507–20513. doi: 10.1074/jbc.M109.090027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Linder M.C. Apoceruloplasmin: abundance, detection, formation, and metabolism. Biomedicines. 2021;9(3) doi: 10.3390/biomedicines9030233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.A. Poujois, J. Poupon, F. Woimant, Chapter 22 - direct determination of non-ceruloplasmin-bound copper in plasma, in: N. Kerkar, E.A. Roberts (Eds.), Clinical and Translational Perspectives on WILSON DISEASE, Academic Press2019, pp. 249-255.
- 15.Linder M.C. Springer Science & Business Media; 2013. Biochemistry of Copper. [Google Scholar]
- 16.Focarelli F., Giachino A., Waldron K.J. Copper microenvironments in the human body define patterns of copper adaptation in pathogenic bacteria. PLoS Pathog. 2022;18(7) doi: 10.1371/journal.ppat.1010617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blockhuys S., Celauro E., Hildesjo C., Feizi A., Stal O., Fierro-Gonzalez J.C., Wittung-Stafshede P. Defining the human copper proteome and analysis of its expression variation in cancers. Metallomics. 2017;9(2):112–123. doi: 10.1039/c6mt00202a. [DOI] [PubMed] [Google Scholar]
- 18.Ghaedamini H., Duanghathaipornsuk S., Onusko P., Binsheheween A.M., Kim D.S. Reduced glutathione-modified electrode for the detection of hydroxyl free radicals. Biosensors. 2023;13(2) doi: 10.3390/bios13020254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arredondo M., Muñoz P., Mura C.V., Nùñez M.T. DMT1, a physiologically relevant apical Cu1+ transporter of intestinal cells. Am. J. Physiol. Cell Physiol. 2003;284(6):C1525–C1530. doi: 10.1152/ajpcell.00480.2002. [DOI] [PubMed] [Google Scholar]
- 20.Zimnicka A.M., Ivy K., Kaplan J.H. Acquisition of dietary copper: a role for anion transporters in intestinal apical copper uptake. Am. J. Physiol. Cell Physiol. 2011;300(3):C588–C599. doi: 10.1152/ajpcell.00054.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zimnicka A.M., Maryon E.B., Kaplan J.H. Human copper transporter hCTR1 mediates basolateral uptake of copper into enterocytes: implications for copper homeostasis. J. Biol. Chem. 2007;282(36):26471–26480. doi: 10.1074/jbc.M702653200. [DOI] [PubMed] [Google Scholar]
- 22.Ohgami R.S., Campagna D.R., McDonald A., Fleming M.D. The Steap proteins are metalloreductases. Blood. 2006;108(4):1388–1394. doi: 10.1182/blood-2006-02-003681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wyman S., Simpson R.J., McKie A.T., Sharp P.A. Dcytb (Cybrd1) functions as both a ferric and a cupric reductase in vitro. FEBS Lett. 2008;582(13):1901–1906. doi: 10.1016/j.febslet.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 24.Kahra D., Kovermann M., Wittung-Stafshede P. The C-terminus of human copper importer Ctr1 acts as a binding site and transfers copper to Atox1. Biophys. J. 2016;110(1):95–102. doi: 10.1016/j.bpj.2015.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nose Y., Wood L.K., Kim B.E., Prohaska J.R., Fry R.S., Spears J.W., Thiele D.J. Ctr1 is an apical copper transporter in mammalian intestinal epithelial cells in vivo that is controlled at the level of protein stability. J. Biol. Chem. 2010;285(42):32385–32392. doi: 10.1074/jbc.M110.143826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kar S., Sen S., Maji S., Saraf D., Ruturaj, Paul R., Dutt S., Mondal B., Rodriguez-Boulan E., Schreiner R., Sengupta D., Gupta A. Copper(II) import and reduction are dependent on His-Met clusters in the extracellular amino terminus of human copper transporter-1. J. Biol. Chem. 2022;298(3) doi: 10.1016/j.jbc.2022.101631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boulet A., Vest K.E., Maynard M.K., Gammon M.G., Russell A.C., Mathews A.T., Cole S.E., Zhu X., Phillips C.B., Kwong J.Q., Dodani S.C., Leary S.C., Cobine P.A. The mammalian phosphate carrier SLC25A3 is a mitochondrial copper transporter required for cytochrome c oxidase biogenesis. J. Biol. Chem. 2018;293(6):1887–1896. doi: 10.1074/jbc.RA117.000265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boyd S.D., Ullrich M.S., Skopp A., Winkler D.D. Copper sources for Sod1 activation. Antioxidants. 2020;9(6) doi: 10.3390/antiox9060500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cobine P.A., Moore S.A., Leary S.C. Getting out what you put in: copper in mitochondria and its impacts on human disease. Biochim. Biophys. Acta Mol. Cell Res. 2021;1868(1) doi: 10.1016/j.bbamcr.2020.118867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruiz L.M., Libedinsky A., Elorza A.A. Role of copper on mitochondrial function and metabolism. Front. Mol. Biosci. 2021;8 doi: 10.3389/fmolb.2021.711227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jett K.A., Leary S.C. Building the Cu(A) site of cytochrome c oxidase: a complicated, redox-dependent process driven by a surprisingly large complement of accessory proteins. J. Biol. Chem. 2018;293(13):4644–4652. doi: 10.1074/jbc.R117.816132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hatori Y., Lutsenko S. The role of copper chaperone Atox1 in coupling redox homeostasis to intracellular copper distribution. Antioxidants. 2016;5(3) doi: 10.3390/antiox5030025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Horn N., Wittung-Stafshede P. ATP7A-Regulated enzyme metalation and trafficking in the menkes disease puzzle. Biomedicines. 2021;9(4) doi: 10.3390/biomedicines9040391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen L., Min J., Wang F. Copper homeostasis and cuproptosis in health and disease. Signal Transduct. Targeted Ther. 2022;7(1):378. doi: 10.1038/s41392-022-01229-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gourdon P., Sitsel O., Karlsen J.L., Møller L.B., Nissen P. Structural models of the human copper P-type ATPases ATP7A and ATP7B. bchm. 2012;393(4):205–216. doi: 10.1515/hsz-2011-0249. [DOI] [PubMed] [Google Scholar]
- 36.Cater M.A., La Fontaine S., Shield K., Deal Y., Mercer J.F. ATP7B mediates vesicular sequestration of copper: insight into biliary copper excretion. Gastroenterology. 2006;130(2):493–506. doi: 10.1053/j.gastro.2005.10.054. [DOI] [PubMed] [Google Scholar]
- 37.Chun H., Catterton T., Kim H., Lee J., Kim B.E. Organ-specific regulation of ATP7A abundance is coordinated with systemic copper homeostasis. Sci. Rep. 2017;7(1) doi: 10.1038/s41598-017-11961-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Polishchuk E.V., Concilli M., Iacobacci S., Chesi G., Pastore N., Piccolo P., Paladino S., Baldantoni D., van I.S.C., Chan J., Chang C.J., Amoresano A., Pane F., Pucci P., Tarallo A., Parenti G., Brunetti-Pierri N., Settembre C., Ballabio A., Polishchuk R.S. Wilson disease protein ATP7B utilizes lysosomal exocytosis to maintain copper homeostasis. Dev. Cell. 2014;29(6):686–700. doi: 10.1016/j.devcel.2014.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fuentealba I., Haywood S., Foster J. Cellular mechanisms of toxicity and tolerance in the copper-loaded rat. II. Pathogenesis of copper toxicity in the liver. Exp. Mol. Pathol. 1989;50(1):26–37. doi: 10.1016/0014-4800(89)90054-3. [DOI] [PubMed] [Google Scholar]
- 40.Yanagimoto C., Harada M., Kumemura H., Abe M., Koga H., Sakata M., Kawaguchi T., Terada K., Hanada S., Taniguchi E., Ninomiya H., Ueno T., Sugiyama T., Sata M. Copper incorporation into ceruloplasmin is regulated by Niemann-Pick C1 protein. Hepatol. Res. 2011;41(5):484–491. doi: 10.1111/j.1872-034X.2011.00788.x. [DOI] [PubMed] [Google Scholar]
- 41.Lenartowicz M., Wieczerzak K., Krzeptowski W., Dobosz P., Grzmil P., StarzyńSki R., LipińSki P. Developmental changes in the expression of theAtp7agene in the liver of mice during the postnatal period. J. Exp. Zool. Part A: Ecological Genetics and Physiology. 2010;9999A doi: 10.1002/jez.586. n/a-n/a. [DOI] [PubMed] [Google Scholar]
- 42.Lutsenko S., Barnes N.L., Bartee M.Y., Dmitriev O.Y. Function and regulation of human copper-transporting ATPases. Physiol. Rev. 2007;87(3):1011–1046. doi: 10.1152/physrev.00004.2006. [DOI] [PubMed] [Google Scholar]
- 43.Barnes N., Tsivkovskii R., Tsivkovskaia N., Lutsenko S. The copper-transporting ATPases, menkes and wilson disease proteins, have distinct roles in adult and developing cerebellum. J. Biol. Chem. 2005;280(10):9640–9645. doi: 10.1074/jbc.M413840200. [DOI] [PubMed] [Google Scholar]
- 44.Przybylkowski A., Gromadzka G., Wawer A., Grygorowicz T., Cybulska A., Czlonkowska A. Intestinal expression of metal transporters in Wilson's disease. Biometals. 2013;26(6):925–934. doi: 10.1007/s10534-013-9668-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim B.E., Turski M.L., Nose Y., Casad M., Rockman H.A., Thiele D.J. Cardiac copper deficiency activates a systemic signaling mechanism that communicates with the copper acquisition and storage organs. Cell Metabol. 2010;11(5):353–363. doi: 10.1016/j.cmet.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xue Q., Kang R., Klionsky D.J., Tang D., Liu J., Chen X. Copper metabolism in cell death and autophagy. Autophagy. 2023;19(8):2175–2195. doi: 10.1080/15548627.2023.2200554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gaetke L.M., Chow C.K. Copper toxicity, oxidative stress, and antioxidant nutrients. Toxicology. 2003;189(1):147–163. doi: 10.1016/S0300-483X(03)00159-8. [DOI] [PubMed] [Google Scholar]
- 48.Uriu-Adams J.Y., Keen C.L. Copper, oxidative stress, and human health. Mol. Aspect. Med. 2005;26(4):268–298. doi: 10.1016/j.mam.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 49.Stohs S.J., Bagchi D. Oxidative mechanisms in the toxicity of metal ions. Free Radic. Biol. Med. 1995;18(2):321–336. doi: 10.1016/0891-5849(94)00159-h. [DOI] [PubMed] [Google Scholar]
- 50.Andrés C.M.C., Pérez de la Lastra J.M., Andrés Juan C., Plou F.J., Pérez-Lebeña E. Impact of reactive species on amino acids-biological relevance in proteins and induced pathologies. Int. J. Mol. Sci. 2022;23(22) doi: 10.3390/ijms232214049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Halliwell B., Adhikary A., Dingfelder M., Dizdaroglu M. Hydroxyl radical is a significant player in oxidative DNA damage in vivo. Chem. Soc. Rev. 2021;50(15):8355–8360. doi: 10.1039/d1cs00044f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Krumova K., Cosa G. vol. 1. The Royal Society of Chemistry; 2016. pp. 1–21. (Chapter 1 Overview of Reactive Oxygen Species, Singlet Oxygen: Applications in Biosciences and Nanosciences). [Google Scholar]
- 53.Girotti A.W. Mechanisms of lipid peroxidation. J. Free Radic. Biol. Med. 1985;1(2):87–95. doi: 10.1016/0748-5514(85)90011-x. [DOI] [PubMed] [Google Scholar]
- 54.Halliwell B., Gutteridge J.M. Role of free radicals and catalytic metal ions in human disease: an overview. Methods Enzymol. 1990;186:1–85. doi: 10.1016/0076-6879(90)86093-b. [DOI] [PubMed] [Google Scholar]
- 55.Banci L., Bertini I., Ciofi-Baffoni S., Kozyreva T., Zovo K., Palumaa P. Affinity gradients drive copper to cellular destinations. Nature. 2010;465(7298):645–648. doi: 10.1038/nature09018. [DOI] [PubMed] [Google Scholar]
- 56.Benitez J.J., Keller A.M., Huffman D.L., Yatsunyk L.A., Rosenzweig A.C., Chen P. Relating dynamic protein interactions of metallochaperones with metal transfer at the single-molecule level. Faraday Discuss. 2011;148:71–82. doi: 10.1039/c004913a. ; discussion 97-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xiao Z., Brose J., Schimo S., Ackland S.M., La Fontaine S., Wedd A.G. Unification of the copper(I) binding affinities of the metallo-chaperones Atx1, Atox1, and related proteins: detection probes and affinity standards. J. Biol. Chem. 2011;286(13):11047–11055. doi: 10.1074/jbc.M110.213074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lichtmannegger J., Leitzinger C., Wimmer R., Schmitt S., Schulz S., Kabiri Y., Eberhagen C., Rieder T., Janik D., Neff F., Straub B.K., Schirmacher P., DiSpirito A.A., Bandow N., Baral B.S., Flatley A., Kremmer E., Denk G., Reiter F.P., Hohenester S., Eckardt-Schupp F., Dencher N.A., Adamski J., Sauer V., Niemietz C., Schmidt H.H., Merle U., Gotthardt D.N., Kroemer G., Weiss K.H., Zischka H. Methanobactin reverses acute liver failure in a rat model of Wilson disease. J. Clin. Invest. 2016;126(7):2721–2735. doi: 10.1172/jci85226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zischka H., Lichtmannegger J., Schmitt S., Jagemann N., Schulz S., Wartini D., Jennen L., Rust C., Larochette N., Galluzzi L., Chajes V., Bandow N., Gilles V.S., DiSpirito A.A., Esposito I., Goettlicher M., Summer K.H., Kroemer G. Liver mitochondrial membrane crosslinking and destruction in a rat model of Wilson disease. J. Clin. Invest. 2011;121(4):1508–1518. doi: 10.1172/JCI45401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Go Y.M., Jones D.P. Redox compartmentalization in eukaryotic cells. Biochim. Biophys. Acta. 2008;1780(11):1273–1290. doi: 10.1016/j.bbagen.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hassett R., Kosman D.J. Evidence for Cu(II) reduction as a component of copper uptake by Saccharomyces cerevisiae. J. Biol. Chem. 1995;270(1):128–134. doi: 10.1074/jbc.270.1.128. [DOI] [PubMed] [Google Scholar]
- 62.Ramos D., Mar D., Ishida M., Vargas R., Gaite M., Montgomery A., Linder M.C. Mechanism of copper uptake from blood plasma ceruloplasmin by mammalian cells. PLoS One. 2016;11(3) doi: 10.1371/journal.pone.0149516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lutsenko S. Human copper homeostasis: a network of interconnected pathways. Curr. Opin. Chem. Biol. 2010;14(2):211–217. doi: 10.1016/j.cbpa.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Palumaa P. Copper chaperones. The concept of conformational control in the metabolism of copper. FEBS Lett. 2013;587(13):1902–1910. doi: 10.1016/j.febslet.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 65.Cope J.D., Valle H.U., Hall R.S., Riley K.M., Goel E., Biswas S., Hendrich M.P., Wipf D.O., Stokes S.L., Emerson J.P. Tuning the copper(II)/copper(I) redox potential for more robust copper-catalyzed C-N bond forming reactions. Eur. J. Inorg. Chem. 2020;14:1278–1285. doi: 10.1002/ejic.201901269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Falcone E., Stellato F., Vileno B., Bouraguba M., Lebrun V., Ilbert M., Morante S., Faller P. Revisiting the pro-oxidant activity of copper: interplay of ascorbate, cysteine, and glutathione. Metallomics. 2023;15(7) doi: 10.1093/mtomcs/mfad040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gu M., Cooper J.M., Butler P., Walker A.P., Mistry P.K., Dooley J.S., Schapira A.H. Oxidative-phosphorylation defects in liver of patients with Wilson's disease. Lancet. 2000;356(9228):469–474. doi: 10.1016/s0140-6736(00)02556-3. [DOI] [PubMed] [Google Scholar]
- 68.Sokol R.J., Twedt D., McKim J.M., Devereaux M.W., Karrer F.M., Kam I., Von Steigman G., Narkewicz M.R., Bacon B.R., Britton R.S., Neuschwander-Tetri B.A. Oxidant injury to hepatic mitochondria in patients with Wilson's disease and Bedlington terriers with copper toxicosis. Gastroenterology. 1994;107(6):1788–1798. doi: 10.1016/0016-5085(94)90822-2. [DOI] [PubMed] [Google Scholar]
- 69.Summer K.H., Eisenburg J. Low content of hepatic reduced glutathione in patients with Wilson's disease. Biochem. Med. 1985;34(1):107–111. doi: 10.1016/0006-2944(85)90067-5. [DOI] [PubMed] [Google Scholar]
- 70.Nair J., Carmichael P.L., Fernando R.C., Phillips D.H., Strain A.J., Bartsch H. Lipid peroxidation-induced etheno-DNA adducts in the liver of patients with the genetic metal storage disorders Wilson's disease and primary hemochromatosis. Cancer Epidemiol. Biomarkers Prev. 1998;7(5):435–440. [PubMed] [Google Scholar]
- 71.Nagasaka H., Inoue I., Inui A., Komatsu H., Sogo T., Murayama K., Murakami T., Yorifuji T., Asayama K., Katayama S., Uemoto S., Kobayashi K., Takayanagi M., Fujisawa T., Tsukahara H. Relationship between oxidative stress and antioxidant systems in the liver of patients with Wilson disease: hepatic manifestation in Wilson disease as a consequence of augmented oxidative stress. Pediatr. Res. 2006;60(4):472–477. doi: 10.1203/01.pdr.0000238341.12229.d3. [DOI] [PubMed] [Google Scholar]
- 72.Kalita J., Kumar V., Misra U.K., Ranjan A., Khan H., Konwar R. A study of oxidative stress, cytokines and glutamate in Wilson disease and their asymptomatic siblings. J. Neuroimmunol. 2014;274(1–2):141–148. doi: 10.1016/j.jneuroim.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 73.Kalita J., Kumar V., Ranjan A., Misra U.K. Role of oxidative stress in the worsening of neurologic wilson disease following chelating therapy. NeuroMolecular Med. 2015;17(4):364–372. doi: 10.1007/s12017-015-8364-8. [DOI] [PubMed] [Google Scholar]
- 74.Zischka H., Lichtmannegger J. Pathological mitochondrial copper overload in livers of Wilson's disease patients and related animal models. Ann. N. Y. Acad. Sci. 2014;1315:6–15. doi: 10.1111/nyas.12347. [DOI] [PubMed] [Google Scholar]
- 75.Ossola J.O., Groppa M.D., Tomaro M.L. Relationship between oxidative stress and heme oxygenase induction by copper sulfate. Arch. Biochem. Biophys. 1997;337(2):332–337. doi: 10.1006/abbi.1996.9788. [DOI] [PubMed] [Google Scholar]
- 76.Nagai M., Vo N.H., Shin Ogawa L., Chimmanamada D., Inoue T., Chu J., Beaudette-Zlatanova B.C., Lu R., Blackman R.K., Barsoum J., Koya K., Wada Y. The oncology drug elesclomol selectively transports copper to the mitochondria to induce oxidative stress in cancer cells. Free Radic. Biol. Med. 2012;52(10):2142–2150. doi: 10.1016/j.freeradbiomed.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 77.Zheng P., Zhou C., Lu L., Liu B., Ding Y. Elesclomol: a copper ionophore targeting mitochondrial metabolism for cancer therapy. J. Exp. Clin. Cancer Res. 2022;41(1):271. doi: 10.1186/s13046-022-02485-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tsvetkov P., Coy S., Petrova B., Dreishpoon M., Verma A., Abdusamad M., Rossen J., Joesch-Cohen L., Humeidi R., Spangler R.D., Eaton J.K., Frenkel E., Kocak M., Corsello S.M., Lutsenko S., Kanarek N., Santagata S., Golub T.R. Copper induces cell death by targeting lipoylated TCA cycle proteins. Science. 2022;375(6586):1254–1261. doi: 10.1126/science.abf0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gao W., Huang Z., Duan J., Nice E.C., Lin J., Huang C. Elesclomol induces copper-dependent ferroptosis in colorectal cancer cells via degradation of ATP7A. Mol. Oncol. 2021;15(12):3527–3544. doi: 10.1002/1878-0261.13079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zulkifli M., Spelbring Amy N., Zhang Y., Soma S., Chen S., Li L., Le T., Shanbhag V., Petris Michael J., Chen T.-Y., Ralle M., Barondeau David P., Gohil Vishal M. FDX1-dependent and independent mechanisms of elesclomol-mediated intracellular copper delivery. Proc. Natl. Acad. Sci. USA. 2023;120(10) doi: 10.1073/pnas.2216722120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hasinoff B.B., Wu X., Yadav A.A., Patel D., Zhang H., Wang D.-S., Chen Z.-S., Yalowich J.C. Cellular mechanisms of the cytotoxicity of the anticancer drug elesclomol and its complex with Cu(II) Biochem. Pharmacol. 2015;93(3):266–276. doi: 10.1016/j.bcp.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 82.Macomber L., Imlay J.A. The iron-sulfur clusters of dehydratases are primary intracellular targets of copper toxicity. Proc. Natl. Acad. Sci. U. S. A. 2009;106(20):8344–8349. doi: 10.1073/pnas.0812808106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Verity M.A., Gambell J.K. Studies of copper ion-induced mitochondrial swelling in vitro. Biochem. J. 1968;108(2):289–295. doi: 10.1042/bj1080289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Garcia N., Martinez-Abundis E., Pavon N., Correa F., Chavez E. Copper induces permeability transition through its interaction with the adenine nucleotide translocase. Cell Biol. Int. 2007;31(9):893–899. doi: 10.1016/j.cellbi.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 85.Borchard S., Bork F., Rieder T., Eberhagen C., Popper B., Lichtmannegger J., Schmitt S., Adamski J., Klingenspor M., Weiss K.-H., Zischka H. The exceptional sensitivity of brain mitochondria to copper. Toxicol. Vitro. 2018;51:11–22. doi: 10.1016/j.tiv.2018.04.012. [DOI] [PubMed] [Google Scholar]
- 86.Saporito-Magriñá C.M., Musacco-Sebio R.N., Andrieux G., Kook L., Orrego M.T., Tuttolomondo M.V., Desimone M.F., Boerries M., Borner C., Repetto M.G. Copper-induced cell death and the protective role of glutathione: the implication of impaired protein folding rather than oxidative stress. Metallomics. 2018;10(12):1743–1754. doi: 10.1039/c8mt00182k. [DOI] [PubMed] [Google Scholar]
- 87.Zuily L., Lahrach N., Fassler R., Genest O., Faller P., Sénèque O., Denis Y., Castanié-Cornet M.P., Genevaux P., Jakob U., Reichmann D., Giudici-Orticoni M.T., Ilbert M. Copper induces protein aggregation, a toxic process compensated by molecular chaperones. mBio. 2022;13(2) doi: 10.1128/mbio.03251-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Burkhead J.L., Gray L.W., Lutsenko S. Systems biology approach to Wilson's disease. Biometals. 2011;24(3):455–466. doi: 10.1007/s10534-011-9430-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bensinger S.J., Bradley M.N., Joseph S.B., Zelcer N., Janssen E.M., Hausner M.A., Shih R., Parks J.S., Edwards P.A., Jamieson B.D., Tontonoz P. LXR signaling couples sterol metabolism to proliferation in the acquired immune response. Cell. 2008;134(1):97–111. doi: 10.1016/j.cell.2008.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Morello F., Saglio E., Noghero A., Schiavone D., Williams T.A., Verhovez A., Bussolino F., Veglio F., Mulatero P. LXR-activating oxysterols induce the expression of inflammatory markers in endothelial cells through LXR-independent mechanisms. Atherosclerosis. 2009;207(1):38–44. doi: 10.1016/j.atherosclerosis.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 91.Repa J.J., Liang G., Ou J., Bashmakov Y., Lobaccaro J.M., Shimomura I., Shan B., Brown M.S., Goldstein J.L., Mangelsdorf D.J. Regulation of mouse sterol regulatory element-binding protein-1c gene (SREBP-1c) by oxysterol receptors, LXRalpha and LXRbeta. Genes Dev. 2000;14(22):2819–2830. doi: 10.1101/gad.844900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wooton-Kee C.R., Jain A.K., Wagner M., Grusak M.A., Finegold M.J., Lutsenko S., Moore D.D. Elevated copper impairs hepatic nuclear receptor function in Wilson's disease. J. Clin. Invest. 2015;125(9):3449–3460. doi: 10.1172/JCI78991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Song M.O., Freedman J.H. Role of hepatocyte nuclear factor 4alpha in controlling copper-responsive transcription. Biochim. Biophys. Acta. 2011;1813(1):102–108. doi: 10.1016/j.bbamcr.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ralle M., Lutsenko S., Blackburn N.J. X-ray absorption spectroscopy of the copper chaperone HAH1 reveals a linear two-coordinate Cu(I) center capable of adduct formation with exogenous thiols and phosphines. J. Biol. Chem. 2003;278(25):23163–23170. doi: 10.1074/jbc.M303474200. [DOI] [PubMed] [Google Scholar]
- 95.Barry A.N., Shinde U., Lutsenko S. Structural organization of human Cu-transporting ATPases: learning from building blocks. J. Biol. Inorg. Chem. 2010;15(1):47–59. doi: 10.1007/s00775-009-0595-4. [DOI] [PubMed] [Google Scholar]
- 96.Meacham K.A., Cortes M.P., Wiggins E.M., Maass A., Latorre M., Ralle M., Burkhead J.L. Altered zinc balance in the Atp7b(-/-) mouse reveals a mechanism of copper toxicity in Wilson disease. Metallomics. 2018;10(11):1595–1606. doi: 10.1039/c8mt00199e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hamilton J.P., Koganti L., Muchenditsi A., Pendyala V.S., Huso D., Hankin J., Murphy R.C., Huster D., Merle U., Mangels C., Yang N., Potter J.J., Mezey E., Lutsenko S. Activation of liver X receptor/retinoid X receptor pathway ameliorates liver disease inAtp7B−/−(Wilson disease) mice. Hepatology. 2016;63(6):1828–1841. doi: 10.1002/hep.28406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kardos J., Heja L., Simon A., Jablonkai I., Kovacs R., Jemnitz K. Copper signalling: causes and consequences. Cell Commun. Signal. 2018;16(1):71. doi: 10.1186/s12964-018-0277-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Solier S., Müller S., Cañeque T., Versini A., Mansart A., Sindikubwabo F., Baron L., Emam L., Gestraud P., Pantoș G.D., Gandon V., Gaillet C., Wu T.-D., Dingli F., Loew D., Baulande S., Durand S., Sencio V., Robil C., Trottein F., Péricat D., Näser E., Cougoule C., Meunier E., Bègue A.-L., Salmon H., Manel N., Puisieux A., Watson S., Dawson M.A., Servant N., Kroemer G., Annane D., Rodriguez R. A druggable copper-signalling pathway that drives inflammation. Nature. 2023;617(7960):386–394. doi: 10.1038/s41586-023-06017-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Travaglia A., La Mendola D., Magri A., Nicoletti V.G., Pietropaolo A., Rizzarelli E. Copper, BDNF and its N-terminal domain: inorganic features and biological perspectives. Chemistry. 2012;18(49):15618–15631. doi: 10.1002/chem.201202775. [DOI] [PubMed] [Google Scholar]
- 101.Wu W., Graves L.M., Jaspers I., Devlin R.B., Reed W., Samet J.M. Activation of the EGF receptor signaling pathway in human airway epithelial cells exposed to metals. Am. J. Physiol. 1999;277(5):L924–L931. doi: 10.1152/ajplung.1999.277.5.L924. [DOI] [PubMed] [Google Scholar]
- 102.Samet J.M., Graves L.M., Quay J., Dailey L.A., Devlin R.B., Ghio A.J., Wu W., Bromberg P.A., Reed W. Activation of MAPKs in human bronchial epithelial cells exposed to metals. Am. J. Physiol. 1998;275(3):L551–L558. doi: 10.1152/ajplung.1998.275.3.L551. [DOI] [PubMed] [Google Scholar]
- 103.Price K.A., Caragounis A., Paterson B.M., Filiz G., Volitakis I., Masters C.L., Barnham K.J., Donnelly P.S., Crouch P.J., White A.R. Sustained activation of glial cell epidermal growth factor receptor by bis(thiosemicarbazonato) metal complexes is associated with inhibition of protein tyrosine phosphatase activity. J. Med. Chem. 2009;52(21):6606–6620. doi: 10.1021/jm9007938. [DOI] [PubMed] [Google Scholar]
- 104.Tonks N.K., Diltz C.D., Fischer E.H. Characterization of the major protein-tyrosine-phosphatases of human placenta. J. Biol. Chem. 1988;263(14):6731–6737. doi: 10.1016/s0021-9258(18)68703-4. [DOI] [PubMed] [Google Scholar]
- 105.Tsai C.Y., Finley J.C., Ali S.S., Patel H.H., Howell S.B. Copper influx transporter 1 is required for FGF, PDGF and EGF-induced MAPK signaling. Biochem. Pharmacol. 2012;84(8):1007–1013. doi: 10.1016/j.bcp.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Turski M.L., Brady D.C., Kim H.J., Kim B.E., Nose Y., Counter C.M., Winge D.R., Thiele D.J. A novel role for copper in Ras/mitogen-activated protein kinase signaling. Mol. Cell Biol. 2012;32(7):1284–1295. doi: 10.1128/MCB.05722-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Brady D.C., Crowe M.S., Turski M.L., Hobbs G.A., Yao X., Chaikuad A., Knapp S., Xiao K., Campbell S.L., Thiele D.J., Counter C.M. Copper is required for oncogenic BRAF signalling and tumorigenesis. Nature. 2014;509(7501):492–496. doi: 10.1038/nature13180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Brady D.C., Crowe M.S., Greenberg D.N., Counter C.M. Copper chelation inhibits BRAF(V600e)-driven melanomagenesis and counters resistance to BRAF(V600E) and MEK1/2 inhibitors. Cancer Res. 2017;77(22):6240–6252. doi: 10.1158/0008-5472.CAN-16-1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mori M., Hattori A., Sawaki M., Tsuzuki N., Sawada N., Oyamada M., Sugawara N., Enomoto K. The LEC rat: a model for human hepatitis, liver cancer, and much more. Am. J. Pathol. 1994;144(1):200–204. [PMC free article] [PubMed] [Google Scholar]
- 110.Huster D., Finegold M.J., Morgan C.T., Burkhead J.L., Nixon R., Vanderwerf S.M., Gilliam C.T., Lutsenko S. Consequences of copper accumulation in the livers of the Atp7b-/- (Wilson disease gene) knockout mice. Am. J. Pathol. 2006;168(2):423–434. doi: 10.2353/ajpath.2006.050312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jain S., Cohen J., Ward M.M., Kornhauser N., Chuang E., Cigler T., Moore A., Donovan D., Lam C., Cobham M.V., Schneider S., Hurtado Rúa S.M., Benkert S., Mathijsen Greenwood C., Zelkowitz R., Warren J.D., Lane M.E., Mittal V., Rafii S., Vahdat L.T. Tetrathiomolybdate-associated copper depletion decreases circulating endothelial progenitor cells in women with breast cancer at high risk of relapse. Ann. Oncol. 2013;24(6):1491–1498. doi: 10.1093/annonc/mds654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ramchandani D., Berisa M., Tavarez D.A., Li Z., Miele M., Bai Y., Lee S.B., Ban Y., Dephoure N., Hendrickson R.C., Cloonan S.M., Gao D., Cross J.R., Vahdat L.T., Mittal V. Copper depletion modulates mitochondrial oxidative phosphorylation to impair triple negative breast cancer metastasis. Nat. Commun. 2021;12(1):7311. doi: 10.1038/s41467-021-27559-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.McCall K.A., Huang C., Fierke C.A. Function and mechanism of zinc metalloenzymes. J. Nutr. 2000;130(5S Suppl) doi: 10.1093/jn/130.5.1437S. 1437s-46s. [DOI] [PubMed] [Google Scholar]
- 114.Waalkes M.P., Harvey M.J., Klaassen C.D. Relative in vitro affinity of hepatic metallothionein for metals. Toxicol. Lett. 1984;20(1):33–39. doi: 10.1016/0378-4274(84)90179-6. [DOI] [PubMed] [Google Scholar]
- 115.Jiang L.J., Maret W., Vallee B.L. The glutathione redox couple modulates zinc transfer from metallothionein to zinc-depleted sorbitol dehydrogenase. Proc. Natl. Acad. Sci. U. S. A. 1998;95(7):3483–3488. doi: 10.1073/pnas.95.7.3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Krezel A., Maret W. Thionein/metallothionein control Zn(II) availability and the activity of enzymes. J. Biol. Inorg. Chem. 2008;13(3):401–409. doi: 10.1007/s00775-007-0330-y. [DOI] [PubMed] [Google Scholar]
- 117.Aydemir T.B., Troche C., Kim M.H., Cousins R.J. Hepatic ZIP14-mediated zinc transport contributes to endosomal insulin receptor trafficking and glucose metabolism. J. Biol. Chem. 2016;291(46):23939–23951. doi: 10.1074/jbc.M116.748632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Barber R.G., Grenier Z.A., Burkhead J.L. Copper toxicity is not just oxidative damage: zinc systems and insight from wilson disease. Biomedicines. 2021;9(3) doi: 10.3390/biomedicines9030316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chen H., Huang G., Su T., Gao H., Attieh Z.K., McKie A.T., Anderson G.J., Vulpe C.D. Decreased hephaestin activity in the intestine of copper-deficient mice causes systemic iron deficiency. J. Nutr. 2006;136(5):1236–1241. doi: 10.1093/jn/136.5.1236. [DOI] [PubMed] [Google Scholar]
- 120.Reeves P.G., DeMars L.C. Copper deficiency reduces iron absorption and biological half-life in male rats. J. Nutr. 2004;134(8):1953–1957. doi: 10.1093/jn/134.8.1953. [DOI] [PubMed] [Google Scholar]
- 121.Collins J.F., Franck C.A., Kowdley K.V., Ghishan F.K. Identification of differentially expressed genes in response to dietary iron deprivation in rat duodenum. Am. J. Physiol. Gastrointest. Liver Physiol. 2005;288(5):G964–G971. doi: 10.1152/ajpgi.00489.2004. [DOI] [PubMed] [Google Scholar]
- 122.Ravia J.J., Stephen R.M., Ghishan F.K., Collins J.F. Menkes Copper ATPase (Atp7a) is a novel metal-responsive gene in rat duodenum, and immunoreactive protein is present on brush-border and basolateral membrane domains. J. Biol. Chem. 2005;280(43):36221–36227. doi: 10.1074/jbc.M506727200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chan W.Y., Rennert O.M. The role of copper in iron metabolism. Ann. Clin. Lab. Sci. 1980;10(4):338–344. [PubMed] [Google Scholar]
- 124.El-Shobaki F.A., Rummel W. Binding of copper to mucosal transferrin and inhibition of intestinal iron absorption in rats. Res. Exp. Med. 1979;174(2):187–195. doi: 10.1007/bf01851331. [DOI] [PubMed] [Google Scholar]
- 125.Solomon E.I., Sundaram U.M., Machonkin T.E. Multicopper oxidases and oxygenases. Chem. Rev. 1996;96(7):2563–2606. doi: 10.1021/cr950046o. [DOI] [PubMed] [Google Scholar]
- 126.Hentze M.W., Muckenthaler M.U., Andrews N.C. Balancing acts: molecular control of mammalian iron metabolism. Cell. 2004;117(3):285–297. doi: 10.1016/S0092-8674(04)00343-5. [DOI] [PubMed] [Google Scholar]
- 127.Miyajima H., Nishimura Y., Mizoguchi K., Sakamoto M., Shimizu T., Honda N. Familial apoceruloplasmin deficiency associated with blepharospasm and retinal degeneration. Neurology. 1987;37(5):761–767. doi: 10.1212/wnl.37.5.761. [DOI] [PubMed] [Google Scholar]
- 128.Miyajima H. Aceruloplasminemia, an iron metabolic disorder. Neuropathology. 2003;23(4):345–350. doi: 10.1046/j.1440-1789.2003.00521.x. [DOI] [PubMed] [Google Scholar]
- 129.Mehta K.J., Farnaud S.J., Sharp P.A. Iron and liver fibrosis: mechanistic and clinical aspects. World J. Gastroenterol. 2019;25(5):521–538. doi: 10.3748/wjg.v25.i5.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cobine P.A., Pierrel F., Bestwick M.L., Winge D.R. Mitochondrial matrix copper complex used in metallation of cytochrome oxidase and superoxide dismutase. J. Biol. Chem. 2006;281(48):36552–36559. doi: 10.1074/jbc.M606839200. [DOI] [PubMed] [Google Scholar]
- 131.Cobine P.A., Ojeda L.D., Rigby K.M., Winge D.R. Yeast contain a non-proteinaceous pool of copper in the mitochondrial matrix. J. Biol. Chem. 2004;279(14):14447–14455. doi: 10.1074/jbc.M312693200. [DOI] [PubMed] [Google Scholar]
- 132.Yang L., McRae R., Henary M.M., Patel R., Lai B., Vogt S., Fahrni C.J. Imaging of the intracellular topography of copper with a fluorescent sensor and by synchrotron x-ray fluorescence microscopy. Proc. Natl. Acad. Sci. U. S. A. 2005;102(32):11179–11184. doi: 10.1073/pnas.0406547102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Smeyers-Verbeke J., May C., Drochmans P., Massart D.L. The determination of Cu, Zn, and Mn in subcellular rat liver fractions. Anal. Biochem. 1977;83(2):746–753. doi: 10.1016/0003-2697(77)90080-x. [DOI] [PubMed] [Google Scholar]
- 134.Lal S., Sourkes T.L. Intracellular distribution of copper in the liver during chronic administration of copper sulfate to the rat. Toxicol. Appl. Pharmacol. 1971;18(3):562–572. doi: 10.1016/s0041-008x(71)80009-1. [DOI] [PubMed] [Google Scholar]
- 135.Sternlieb I. Mitochondrial and fatty changes in hepatocytes of patients with Wilson's disease. Gastroenterology. 1968;55(3):354–367. [PubMed] [Google Scholar]
- 136.Sternlieb I., Feldmann G. Effects of anticopper therapy on hepatocellular mitochondria in patients with Wilson's disease: an ultrastructural and stereological study. Gastroenterology. 1976;71(3):457–461. [PubMed] [Google Scholar]
- 137.Sternlieb I. Fraternal concordance of types of abnormal hepatocellular mitochondria in Wilson's disease. Hepatology. 1992;16(3):728–732. doi: 10.1002/hep.1840160319. [DOI] [PubMed] [Google Scholar]
- 138.Sokol R.J., Twedt D., McKim J.M., Jr., Devereaux M.W., Karrer F.M., Kam I., von Steigman G., Narkewicz M.R., Bacon B.R., Britton R.S., et al. Oxidant injury to hepatic mitochondria in patients with Wilson's disease and Bedlington terriers with copper toxicosis. Gastroenterology. 1994;107(6):1788–1798. doi: 10.1016/0016-5085(94)90822-2. [DOI] [PubMed] [Google Scholar]
- 139.Sternlieb I., Quintana N., Volenberg I., Schilsky M.L. An array of mitochondrial alterations in the hepatocytes of Long-Evans Cinnamon rats. Hepatology. 1995;22(6):1782–1787. doi: 10.1016/0270-9139(95)90206-6. [DOI] [PubMed] [Google Scholar]
- 140.Sokol R.J., Deverbaux M., Mierau G.W., Hambidge K.M., Shikes R.H. Oxidant injury to hepatic mitochondrial lipids in rats with dietary copper overload. Gastroenterology. 1990;99(4):1061–1071. doi: 10.1016/0016-5085(90)90627-d. [DOI] [PubMed] [Google Scholar]
- 141.Sokol R.J., Deverbaux M.W., O'Brien K., Khandwala R.A., Loehr J.P. Abnormal hepatic mitochondrial respiration and cytochrome C oxidase activity in rats with long-term copper overload. Gastroenterology. 1993;105(1):178–187. doi: 10.1016/0016-5085(93)90024-7. [DOI] [PubMed] [Google Scholar]
- 142.Vogel F.S., Evans J.W. Morphologic alterations produced by copper in neural tissues with consideration of the role of the metal in the pathogenesis of Wilson's disease. J. Exp. Med. 1961;113(6):997–1004. doi: 10.1084/jem.113.6.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sauer S.W., Merle U., Opp S., Haas D., Hoffmann G.F., Stremmel W., Okun J.G. Severe dysfunction of respiratory chain and cholesterol metabolism in Atp7b(-/-) mice as a model for Wilson disease. Biochim. Biophys. Acta. 2011;1812(12):1607–1615. doi: 10.1016/j.bbadis.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 144.Goldfischer S., Sternlieb I. Changes in the distribution of hepatic copper in relation to the progression of Wilson's disease (hepatolenticular degeneration) Am. J. Pathol. 1968;53(6):883–901. [PMC free article] [PubMed] [Google Scholar]
- 145.Myers B.M., Prendergast F.G., Holman R., Kuntz S.M., Larusso N.F. Alterations in hepatocyte lysosomes in experimental hepatic copper overload in rats. Gastroenterology. 1993;105(6):1814–1823. doi: 10.1016/0016-5085(93)91080-2. [DOI] [PubMed] [Google Scholar]
- 146.Polishchuk E.V., Merolla A., Lichtmannegger J., Romano A., Indrieri A., Ilyechova E.Y., Concilli M., De Cegli R., Crispino R., Mariniello M., Petruzzelli R., Ranucci G., Iorio R., Pietrocola F., Einer C., Borchard S., Zibert A., Schmidt H.H., Di Schiavi E., Puchkova L.V., Franco B., Kroemer G., Zischka H., Polishchuk R.S. Activation of autophagy, observed in liver tissues from patients with wilson disease and from ATP7B-deficient animals, protects hepatocytes from copper-induced apoptosis. Gastroenterology. 2019;156(4):1173–1189 e5. doi: 10.1053/j.gastro.2018.11.032. [DOI] [PubMed] [Google Scholar]
- 147.Klein D., Lichtmannegger J., Heinzmann U., Summer K.H. Dissolution of copper-rich granules in hepatic lysosomes by D-penicillamine prevents the development of fulminant hepatitis in Long-Evans cinnamon rats. J. Hepatol. 2000;32(2):193–201. doi: 10.1016/s0168-8278(00)80063-x. [DOI] [PubMed] [Google Scholar]
- 148.Ruiz L.M., Jensen E.L., Rossel Y., Puas G.I., Gonzalez-Ibanez A.M., Bustos R.I., Ferrick D.A., Elorza A.A. Non-cytotoxic copper overload boosts mitochondrial energy metabolism to modulate cell proliferation and differentiation in the human erythroleukemic cell line K562. Mitochondrion. 2016;29:18–30. doi: 10.1016/j.mito.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 149.Wang Y., Zhao H., Shao Y., Liu J., Li J., Luo L., Xing M. Copper or/and arsenic induces autophagy by oxidative stress-related PI3K/AKT/mTOR pathways and cascaded mitochondrial fission in chicken skeletal muscle. J. Inorg. Biochem. 2018;188:1–8. doi: 10.1016/j.jinorgbio.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 150.Guo M., Wang Y., Zhao H., Mu M., Yang X., Fei D., Liu Y., Zong H., Xing M. Oxidative damage under As(3+) and/or Cu(2+) stress leads to apoptosis and autophagy and may be cross-talking with mitochondrial disorders in bursa of Fabricius. J. Inorg. Biochem. 2020;205 doi: 10.1016/j.jinorgbio.2019.110989. [DOI] [PubMed] [Google Scholar]
- 151.Tsang T., Posimo J.M., Gudiel A.A., Cicchini M., Feldser D.M., Brady D.C. Copper is an essential regulator of the autophagic kinases ULK1/2 to drive lung adenocarcinoma. Nat. Cell Biol. 2020;22(4):412–424. doi: 10.1038/s41556-020-0481-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hoshino A., Wang W.J., Wada S., McDermott-Roe C., Evans C.S., Gosis B., Morley M.P., Rathi K.S., Li J., Li K., Yang S., McManus M.J., Bowman C., Potluri P., Levin M., Damrauer S., Wallace D.C., Holzbaur E.L.F., Arany Z. The ADP/ATP translocase drives mitophagy independent of nucleotide exchange. Nature. 2019;575(7782):375–379. doi: 10.1038/s41586-019-1667-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Majima E., Ikawa K., Takeda M., Hashimoto M., Shinohara Y., Terada H. Translocation of loops regulates transport activity of mitochondrial ADP/ATP carrier deduced from formation of a specific intermolecular disulfide bridge catalyzed by copper-o-phenanthroline. J. Biol. Chem. 1995;270(49):29548–29554. doi: 10.1074/jbc.270.49.29548. [DOI] [PubMed] [Google Scholar]
- 154.Feng Y., Forgac M. Inhibition of vacuolar H(+)-ATPase by disulfide bond formation between cysteine 254 and cysteine 532 in subunit A. J. Biol. Chem. 1994;269(18):13224–13230. [PubMed] [Google Scholar]
- 155.Dringen R., Scheiber I.F., Mercer J.F. Copper metabolism of astrocytes. Front. Aging Neurosci. 2013;5:9. doi: 10.3389/fnagi.2013.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Bousleiman J., Pinsky A., Ki S., Su A., Morozova I., Kalachikov S., Wiqas A., Silver R., Sever M., Austin R.N. Function of metallothionein-3 in neuronal cells: do metal ions alter expression levels of MT3? Int. J. Mol. Sci. 2017;18(6) doi: 10.3390/ijms18061133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Koh J.-Y., Lee S.-J. Metallothionein-3 as a multifunctional player in the control of cellular processes and diseases. Mol. Brain. 2020;13(1):116. doi: 10.1186/s13041-020-00654-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hoepken H.H., Korten T., Robinson S.R., Dringen R. Iron accumulation, iron-mediated toxicity and altered levels of ferritin and transferrin receptor in cultured astrocytes during incubation with ferric ammonium citrate. J. Neurochem. 2004;88(5):1194–1202. doi: 10.1046/j.1471-4159.2003.02236.x. [DOI] [PubMed] [Google Scholar]
- 159.Mathiisen T.M., Lehre K.P., Danbolt N.C., Ottersen O.P. The perivascular astroglial sheath provides a complete covering of the brain microvessels: an electron microscopic 3D reconstruction. Glia. 2010;58(9):1094–1103. doi: 10.1002/glia.20990. [DOI] [PubMed] [Google Scholar]
- 160.Nishimuta M., Masui K., Yamamoto T., Ikarashi Y., Tokushige K., Hashimoto E., Nagashima Y., Shibata N. Copper deposition in oligodendroglial cells in an autopsied case of hepatolenticular degeneration. Neuropathology. 2018;38(3):321–328. doi: 10.1111/neup.12456. [DOI] [PubMed] [Google Scholar]
- 161.Dusek P., Litwin T., Czlonkowska A. Neurologic impairment in Wilson disease. Ann. Transl. Med. 2019;7(Suppl 2):S64. doi: 10.21037/atm.2019.02.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Aoyama K. Glutathione in the brain. Int. J. Mol. Sci. 2021;22(9) doi: 10.3390/ijms22095010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Cheng X.Y., Biswas S., Li J., Mao C.J., Chechneva O., Chen J., Li K., Li J., Zhang J.R., Liu C.F., Deng W.B. Human iPSCs derived astrocytes rescue rotenone-induced mitochondrial dysfunction and dopaminergic neurodegeneration in vitro by donating functional mitochondria. Transl. Neurodegener. 2020;9(1):13. doi: 10.1186/s40035-020-00190-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Hayakawa K., Esposito E., Wang X., Terasaki Y., Liu Y., Xing C., Ji X., Lo E.H. Transfer of mitochondria from astrocytes to neurons after stroke. Nature. 2016;535(7613):551–555. doi: 10.1038/nature18928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bantle C.M., Hirst W.D., Weihofen A., Shlevkov E. Mitochondrial dysfunction in astrocytes: a role in Parkinson's disease? Front. Cell Dev. Biol. 2020;8 doi: 10.3389/fcell.2020.608026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Doll D.N., Hu H., Sun J., Lewis S.E., Simpkins J.W., Ren X. Mitochondrial crisis in cerebrovascular endothelial cells opens the blood-brain barrier. Stroke. 2015;46(6):1681–1689. doi: 10.1161/strokeaha.115.009099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Salzano G., Giachin G., Legname G. Structural consequences of copper binding to the prion protein. Cells. 2019;8(8) doi: 10.3390/cells8080770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Singh I., Sagare A.P., Coma M., Perlmutter D., Gelein R., Bell R.D., Deane R.J., Zhong E., Parisi M., Ciszewski J., Kasper R.T., Deane R. Low levels of copper disrupt brain amyloid-β homeostasis by altering its production and clearance. Proc. Natl. Acad. Sci. U. S. A. 2013;110(36):14771–14776. doi: 10.1073/pnas.1302212110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Cheignon C., Tomas M., Bonnefont-Rousselot D., Faller P., Hureau C., Collin F. Oxidative stress and the amyloid beta peptide in Alzheimer's disease. Redox Biol. 2018;14:450–464. doi: 10.1016/j.redox.2017.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Xiao G., Fan Q., Wang X., Zhou B. Huntington disease arises from a combinatory toxicity of polyglutamine and copper binding. Proc. Natl. Acad. Sci. U. S. A. 2013;110(37):14995–15000. doi: 10.1073/pnas.1308535110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Bisaglia M., Bubacco L. Copper ions and Parkinson's disease: why is homeostasis so relevant? Biomolecules. 2020;10(2) doi: 10.3390/biom10020195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Scheuhammer A.M., Cherian M.G. Effects of heavy metal cations, sulfhydryl reagents and other chemical agents on striatal D2 dopamine receptors. Biochem. Pharmacol. 1985;34(19):3405–3413. doi: 10.1016/0006-2952(85)90710-5. [DOI] [PubMed] [Google Scholar]
- 173.Boveris A., Musacco-Sebio R., Ferrarotti N., Saporito-Magrina C., Torti H., Massot F., Repetto M.G. The acute toxicity of iron and copper: biomolecule oxidation and oxidative damage in rat liver. J. Inorg. Biochem. 2012;116:63–69. doi: 10.1016/j.jinorgbio.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 174.Gamakaranage C.S., Rodrigo C., Weerasinghe S., Gnanathasan A., Puvanaraj V., Fernando H. Complications and management of acute copper sulphate poisoning; a case discussion. J. Occup. Med. Toxicol. 2011;6(1):34. doi: 10.1186/1745-6673-6-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Carvalho J.A., Boavida L., Ferreira R., Favas C., Delgado Alves J. Copper-induced haemolytic anaemia. Eur J Case Rep Intern Med. 2021;8(9) doi: 10.12890/2021_002785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Hajimohammadi S., Gharibi S., Pourbarkhordar V., Mousavi S.R., Salmani Izadi H. Acute poisoning of copper sulfate: a case report and review literature. The Egyptian Journal of Internal Medicine. 2022;34(1):84. doi: 10.1186/s43162-022-00168-y. [DOI] [Google Scholar]
- 177.Pfeiffenberger J., Weiss K.-H., Stremmel W. In: Handbook of Clinical Neurology. Członkowska A., Schilsky M.L., editors. Elsevier; 2017. Chapter 17 - wilson disease: symptomatic liver therapy; pp. 205–209. [DOI] [PubMed] [Google Scholar]
- 178.Jantsch W., Kulig K., Rumack B.H. Massive copper sulfate ingestion resulting in hepatotoxicity. J. Toxicol. Clin. Toxicol. 1984;22(6):585–588. doi: 10.3109/15563658408992588. [DOI] [PubMed] [Google Scholar]
- 179.Müller-Höcker J., Meyer U., Wiebecke B., Hübner G., Eife R., Kellner M., Schramel P. Copper storage disease of the liver and chronic dietary copper intoxication in two further German infants mimicking Indian childhood cirrhosis. Pathol. Res. Pract. 1988;183(1):39–45. doi: 10.1016/s0344-0338(88)80157-2. [DOI] [PubMed] [Google Scholar]
- 180.Agarwal B.N., Bray S.H., Bercz P., Plotzker R., Labovitz E. Ineffectiveness of hemodialysis in copper sulphate poisoning. Nephron. 1975;15(1):74–77. doi: 10.1159/000180495. [DOI] [PubMed] [Google Scholar]
- 181.Roy D.N., Mandal S., Sen G., Biswas T. Superoxide anion mediated mitochondrial dysfunction leads to hepatocyte apoptosis preferentially in the periportal region during copper toxicity in rats. Chem. Biol. Interact. 2009;182(2–3):136–147. doi: 10.1016/j.cbi.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 182.Tang H., Xu M., Luo J., Zhao L., Ye G., Shi F., Lv C., Chen H., Wang Y., Li Y. Liver toxicity assessments in rats following sub-chronic oral exposure to copper nanoparticles. Environ. Sci. Eur. 2019;31(1) doi: 10.1186/s12302-019-0214-0. [DOI] [Google Scholar]
- 183.Hosseini M.J., Shaki F., Ghazi-Khansari M., Pourahmad J. Toxicity of copper on isolated liver mitochondria: impairment at complexes I, II, and IV leads to increased ROS production. Cell Biochem. Biophys. 2014;70(1):367–381. doi: 10.1007/s12013-014-9922-7. [DOI] [PubMed] [Google Scholar]
- 184.Kalita J., Kumar V., Misra U.K., Bora H.K. Movement disorder in copper toxicity rat model: role of inflammation and apoptosis in the corpus striatum. Neurotox. Res. 2020;37(4):904–912. doi: 10.1007/s12640-019-00140-9. [DOI] [PubMed] [Google Scholar]
- 185.Wu M., Han F., Gong W., Feng L., Han J. The effect of copper from water and food: changes of serum nonceruloplasmin copper and brain's amyloid-beta in mice. Food Funct. 2016;7(9):3740–3747. doi: 10.1039/c6fo00809g. [DOI] [PubMed] [Google Scholar]
- 186.Liu H., Guo H., Jian Z., Cui H., Fang J., Zuo Z., Deng J., Li Y., Wang X., Zhao L. Copper induces oxidative stress and apoptosis in the mouse liver. Oxid. Med. Cell. Longev. 2020;2020 doi: 10.1155/2020/1359164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Pra D., Franke S.I., Giulian R., Yoneama M.L., Dias J.F., Erdtmann B., Henriques J.A. Genotoxicity and mutagenicity of iron and copper in mice. Biometals. 2008;21(3):289–297. doi: 10.1007/s10534-007-9118-3. [DOI] [PubMed] [Google Scholar]
- 188.Wang X., Wang H., Li J., Yang Z., Zhang J., Qin Z., Wang L., Kong X. Evaluation of bioaccumulation and toxic effects of copper on hepatocellular structure in mice. Biol. Trace Elem. Res. 2014;159(1–3):312–319. doi: 10.1007/s12011-014-9970-2. [DOI] [PubMed] [Google Scholar]
- 189.Sinkovic A., Strdin A., Svensek F. Severe acute copper sulphate poisoning: a case report. Arh. Hig. Rada. Toksikol. 2008;59(1):31–35. doi: 10.2478/10004-1254-59-2008-1847. [DOI] [PubMed] [Google Scholar]
- 190.Franchitto N., Gandia-Mailly P., Georges B., Galinier A., Telmon N., Ducassé J.L., Rougé D. Acute copper sulphate poisoning: a case report and literature review. Resuscitation. 2008;78(1):92–96. doi: 10.1016/j.resuscitation.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 191.Gunay N., Yildirim C., Karcioglu O., Gunay N.E., Yilmaz M., Usalan C., Kose A., Togun I. A series of patients in the emergency department diagnosed with copper poisoning: recognition equals treatment. Tohoku J. Exp. Med. 2006;209(3):243–248. doi: 10.1620/tjem.209.243. [DOI] [PubMed] [Google Scholar]
- 192.Mansouri A., Gaou I., Fromenty B., Berson A., Lettéron P., Degott C., Erlinger S., Pessayre D. Premature oxidative aging of hepatic mitochondrial DNA in Wilson's disease. Gastroenterology. 1997;113(2):599–605. doi: 10.1053/gast.1997.v113.pm9247482. [DOI] [PubMed] [Google Scholar]
- 193.Shawky R.M., Abdel-Gaffar T.Y., Eladawy M.S., El-Etriby M.A., ElMoneiri M.S., Elhefnawy N.G., Elsherif R., Nour El-Din S.M. Mitochondrial alterations in children with chronic liver disease. Egyptian Journal of Medical Human Genetics. 2010;11(2):143–151. doi: 10.1016/j.ejmhg.2010.10.006. [DOI] [Google Scholar]
- 194.Walshe J.M., Potter G. The pattern of the whole body distribution of radioactive copper (67Cu, 64Cu) in Wilson's Disease and various control groups. Q. J. Med. 1977;46(184):445–462. [PubMed] [Google Scholar]
- 195.Brewer G.J., Yuzbasyan-Gurkan V. Wilson disease. Medicine. 1992;71(3):139–164. doi: 10.1097/00005792-199205000-00004. [DOI] [PubMed] [Google Scholar]
- 196.Ala A., Walker A.P., Ashkan K., Dooley J.S., Schilsky M.L. Wilson's disease. Lancet. 2007;369(9559):397–408. doi: 10.1016/s0140-6736(07)60196-2. [DOI] [PubMed] [Google Scholar]
- 197.Dzieżyc K., Litwin T., Członkowska A. Other organ involvement and clinical aspects of Wilson disease. Handb. Clin. Neurol. 2017;142:157–169. doi: 10.1016/b978-0-444-63625-6.00013-6. [DOI] [PubMed] [Google Scholar]
- 198.Machado A., Chien H.F., Deguti M.M., Cancado E., Azevedo R.S., Scaff M., Barbosa E.R. Neurological manifestations in Wilson's disease: report of 119 cases. Mov. Disord. 2006;21(12):2192–2196. doi: 10.1002/mds.21170. [DOI] [PubMed] [Google Scholar]
- 199.Wilson S.A.K. Progressive lenticular degeneration: a familial nervous disease associated with cirrhosis of the LIVER1. Brain. 1912;34(4):295–507. doi: 10.1093/brain/34.4.295. [DOI] [PubMed] [Google Scholar]
- 200.Poujois A., Mikol J., Woimant F. Wilson disease: brain pathology. Handb. Clin. Neurol. 2017;142:77–89. doi: 10.1016/B978-0-444-63625-6.00008-2. [DOI] [PubMed] [Google Scholar]
- 201.Richter R. The pallial component in hepato-lenticular degeneration. J. Neuropathol. Exp. Neurol. 1948;7(1):1–18. doi: 10.1097/00005072-194801000-00001. [DOI] [PubMed] [Google Scholar]
- 202.Mikol J., Vital C., Wassef M., Chappuis P., Poupon J., Lecharpentier M., Woimant F. Extensive cortico-subcortical lesions in Wilson's disease: clinico-pathological study of two cases. Acta Neuropathol. 2005;110(5):451–458. doi: 10.1007/s00401-005-1061-1. [DOI] [PubMed] [Google Scholar]
- 203.Goldman J.E. Alzheimer type I astrocytes: still mysterious cells. J. Neuropathol. Exp. Neurol. 2022;81(8):588–595. doi: 10.1093/jnen/nlac043. [DOI] [PubMed] [Google Scholar]
- 204.Spielmeyer W. Die histopathologische zusammengehörigkeit der Wilsonschen Krankheit und der pseudosklerose. Zeitschrift für die gesamte Neurologie und Psychiatrie. 1920;57(1):312–351. [Google Scholar]
- 205.Bertrand E., Lewandowska E., Szpak G.M., Hoogenraad T., Blaauwgers H.G., Członkowska A., Dymecki J. Neuropathological analysis of pathological forms of astroglia in Wilson's disease. Folia Neuropathol. 2001;39(2):73–79. [PubMed] [Google Scholar]
- 206.Meenakshi-Sundaram S., Mahadevan A., Taly A.B., Arunodaya G.R., Swamy H.S., Shankar S.K. Wilson's disease: a clinico-neuropathological autopsy study. J. Clin. Neurosci. 2008;15(4):409–417. doi: 10.1016/j.jocn.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 207.Shribman S., Bocchetta M., Sudre C.H., Acosta-Cabronero J., Burrows M., Cook P., Thomas D.L., Gillett G.T., Tsochatzis E.A., Bandmann O., Rohrer J.D., Warner T.T. Neuroimaging correlates of brain injury in Wilson's disease: a multimodal, whole-brain MRI study. Brain. 2022;145(1):263–275. doi: 10.1093/brain/awab274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Glazebrook A.J. Wilson's disease. Edinb. Med. J. 1945;52(2):83–87. [Google Scholar]
- 209.Litwin T., Gromadzka G., Szpak G.M., Jabłonka-Salach K., Bulska E., Członkowska A. Brain metal accumulation in Wilson's disease. J. Neurol. Sci. 2013;329(1–2):55–58. doi: 10.1016/j.jns.2013.03.021. [DOI] [PubMed] [Google Scholar]
- 210.Czlonkowska A., Litwin T., Dusek P., Ferenci P., Lutsenko S., Medici V., Rybakowski J.K., Weiss K.H., Schilsky M.L. Wilson disease. Nat. Rev. Dis. Prim. 2018;4(1):21. doi: 10.1038/s41572-018-0018-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Litwin T., Dušek P., Członkowska A. Symptomatic treatment of neurologic symptoms in Wilson disease. Handb. Clin. Neurol. 2017;142:211–223. doi: 10.1016/b978-0-444-63625-6.00018-5. [DOI] [PubMed] [Google Scholar]
- 212.Squitti R., Tecchio F., Ventriglia M. The role of copper in human diet and risk of dementia. Current Nutrition Reports. 2015;4 doi: 10.1007/s13668-015-0121-y. [DOI] [Google Scholar]
- 213.Scheinberg I.H., Sternlieb I., Schilsky M., Stockert R.J. Penicillamine may detoxify copper in Wilson's disease. Lancet. 1987;2(8550):95. doi: 10.1016/s0140-6736(87)92753-x. [DOI] [PubMed] [Google Scholar]
- 214.Denny-Brown D., Porter H. The effect of BAL (2,3-dimercaptopropanol) on hepatolenticular degeneration (Wilson's Disease) N. Engl. J. Med. 1951;245(24):917–925. doi: 10.1056/NEJM195112132452401. [DOI] [PubMed] [Google Scholar]
- 215.Chelating Agents, LiverTox . National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda (MD): 2012. Clinical and Research Information on Drug-Induced Liver Injury. [Google Scholar]
- 216.Purchase R. The treatment of Wilson's disease, a rare genetic disorder of copper metabolism. Sci. Prog. 2013;96(Pt 1):19–32. doi: 10.3184/003685013x13587771579987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Abraham E.P., Chain E., Baker W., Robinson R. Penicillamine, a Characteristic Degradation Product of Penicillin. Nature. 1943;151(3821) doi: 10.1038/151107a0. 107-107. [DOI] [Google Scholar]
- 218.Netter P., Bannwarth B., Péré P., Nicolas A. Clinical pharmacokinetics of D-penicillamine. Clin. Pharmacokinet. 1987;13(5):317–333. doi: 10.2165/00003088-198713050-00003. [DOI] [PubMed] [Google Scholar]
- 219.Vahabzadeh M., Balali-Mood M., Banagozar Mohammadi A., Moshiri M. Efficacy and expenses of succimer vs. d-penicillamine plus garlic in the treatment of lead poisoning: a retrospective cross-sectional study. Daru. 2021;29(2):477–481. doi: 10.1007/s40199-021-00407-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Walshe J.M. WILSON'S disease: new oral therapy. Lancet. 1956;267(6906):25–26. doi: 10.1016/S0140-6736(56)91859-1. [DOI] [PubMed] [Google Scholar]
- 221.Litin R.B., Goldstein N.P., Randall R.V., Power M.H., Diessner G.R. Effect of D,L-penicillamine on the urinary excretion of copper and calcium in hepatolenticular degeneration (Wilson's disease) Neurology. 1960;10:123–126. doi: 10.1212/wnl.10.2.123. [DOI] [PubMed] [Google Scholar]
- 222.Czlonkowska A., Gajda J., Rodo M. Effects of long-term treatment in Wilson's disease with D-penicillamine and zinc sulphate. J. Neurol. 1996;243(3):269–273. doi: 10.1007/bf00868525. [DOI] [PubMed] [Google Scholar]
- 223.Starosta-Rubinstein S., Young A.B., Kluin K., Hill G., Aisen A.M., Gabrielsen T., Brewer G.J. Clinical assessment of 31 patients with Wilson's disease. Correlations with structural changes on magnetic resonance imaging. Arch. Neurol. 1987;44(4):365–370. doi: 10.1001/archneur.1987.00520160007005. [DOI] [PubMed] [Google Scholar]
- 224.Merle U., Schaefer M., Ferenci P., Stremmel W. Clinical presentation, diagnosis and long-term outcome of Wilson's disease: a cohort study. Gut. 2007;56(1):115–120. doi: 10.1136/gut.2005.087262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Kumar S., Patra B.R., Irtaza M., Rao P.K., Giri S., Darak H., Gopan A., Kale A., Shukla A. Adverse Events with D-penicillamine Therapy in Hepatic Wilson's Disease: A Single-Center Retrospective Audit. Clin. Drug Invest. 2022;42(2):177–184. doi: 10.1007/s40261-022-01117-x. [DOI] [PubMed] [Google Scholar]
- 226.Stremmel W., Weiskirchen R. Therapeutic strategies in Wilson disease: pathophysiology and mode of action. Ann. Transl. Med. 2021;9(8):732. doi: 10.21037/atm-20-3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Borchard S., Raschke S., Zak K.M., Eberhagen C., Einer C., Weber E., Müller S.M., Michalke B., Lichtmannegger J., Wieser A., Rieder T., Popowicz G.M., Adamski J., Klingenspor M., Coles A.H., Viana R., Vendelbo M.H., Sandahl T.D., Schwerdtle T., Plitz T., Zischka H. Bis-choline tetrathiomolybdate prevents copper-induced blood-brain barrier damage. Life Sci. Alliance. 2022;5(3) doi: 10.26508/lsa.202101164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Mohr I., Pfeiffenberger J., Eker E., Merle U., Poujois A., Ala A., Weiss K.H. Neurological worsening in Wilson disease – clinical classification and outcome. J. Hepatol. 2023;79(2):321–328. doi: 10.1016/j.jhep.2023.04.007. [DOI] [PubMed] [Google Scholar]
- 229.Chen D.B., Feng L., Lin X.P., Zhang W., Li F.R., Liang X.L., Li X.H. Penicillamine increases free copper and enhances oxidative stress in the brain of toxic milk mice. PLoS One. 2012;7(5) doi: 10.1371/journal.pone.0037709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.EASL Clinical Practice Guidelines: Wilson's disease. J. Hepatol. 2012;56(3):671–685. doi: 10.1016/j.jhep.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 231.Walshe J.M. Treatment of Wilson's disease with trientine (triethylene tetramine) dihydrochloride. Lancet. 1982;1(8273):643–647. doi: 10.1016/s0140-6736(82)92201-2. [DOI] [PubMed] [Google Scholar]
- 232.Walshe J.M. Copper chelation in patients with Wilson's disease. A comparison of penicillamine and triethylene tetramine dihydrochloride. Q. J. Med. 1973;42(167):441–452. [PubMed] [Google Scholar]
- 233.Sarkar B., Sass-Kortsak A., Clarke R., Laurie S.H., Wei P. A comparative study of in vitro and in vivo interaction of D-penicillamine and triethylenetetramine with copper. Proc. Roy. Soc. Med. 1977;70(Suppl 3):13–18. doi: 10.1177/00359157770700S307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Morita J., Yoshino M., Watari H., Yoshida I., Motohiro T., Yamashita F., Okano Y., Hashimoto T. Wilson's disease treatment by triethylene tetramine dihydrochloride (trientine, 2HCl): long-term observations. Dev. Pharmacol. Ther. 1992;19(1):6–9. doi: 10.1159/000457456. [DOI] [PubMed] [Google Scholar]
- 235.Saito H., Watanabe K., Sahara M., Mochizuki R., Edo K., Ohyama Y. Triethylene-tetramine (trien) therapy for Wilson's disease. Tohoku J. Exp. Med. 1991;164(1):29–35. doi: 10.1620/tjem.164.29. [DOI] [PubMed] [Google Scholar]
- 236.Říha M., Karlíčková J., Filipský T., Macáková K., Hrdina R., Mladěnka P. Novel method for rapid copper chelation assessment confirmed low affinity of D-penicillamine for copper in comparison with trientine and 8-hydroxyquinolines. J. Inorg. Biochem. 2013;123:80–87. doi: 10.1016/j.jinorgbio.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 237.Smirnova J., Kabin E., Järving I., Bragina O., Tõugu V., Plitz T., Palumaa P. Copper(I)-binding properties of de-coppering drugs for the treatment of Wilson disease. α-Lipoic acid as a potential anti-copper agent. Sci. Rep. 2018;8(1):1463. doi: 10.1038/s41598-018-19873-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.F.T. Kirk, D.E. Munk, E.S. Swenson, A.M. Quicquaro, M.H. Vendelbo, M.L. Schilsky, P. Ott, T.D. Sandahl, Effects of trientine and penicillamine on intestinal copper uptake: A mechanistic 64Cu PET/CT study in healthy humans, Hepatology (9900) 10.1097/HEP.0000000000000708. [DOI] [PMC free article] [PubMed]
- 239.Dahlman T., Hartvig P., Löfholm M., Nordlinder H., Lööf L., Westermark K. Long-term treatment of Wilson's disease with triethylene tetramine dihydrochloride (trientine) QJM. 1995;88(9):609–616. [PubMed] [Google Scholar]
- 240.Brewer G.J., Askari F., Lorincz M.T., Carlson M., Schilsky M., Kluin K.J., Hedera P., Moretti P., Fink J.K., Tankanow R., Dick R.B., Sitterly J. Treatment of Wilson disease with ammonium tetrathiomolybdate: IV. Comparison of tetrathiomolybdate and trientine in a double-blind study of treatment of the neurologic presentation of Wilson disease. Arch. Neurol. 2006;63(4):521–527. doi: 10.1001/archneur.63.4.521. [DOI] [PubMed] [Google Scholar]
- 241.Bremner I., Young B.W., Mills C.F. Protective effect of zinc supplementation against copper toxicosis in sheep. Br. J. Nutr. 1976;36(3):551–561. doi: 10.1079/bjn19760108. [DOI] [PubMed] [Google Scholar]
- 242.Hall A.C., Young B.W., Bremner I. Intestinal metallothionein and the mutual antagonism between copper and zinc in the rat. J. Inorg. Biochem. 1979;11(1):57–66. doi: 10.1016/s0162-0134(00)80054-9. [DOI] [PubMed] [Google Scholar]
- 243.Menard M.P., McCormick C.C., Cousins R.J. Regulation of intestinal metallothionein biosynthesis in rats by dietary zinc. J. Nutr. 1981;111(8):1353–1361. doi: 10.1093/jn/111.8.1353. [DOI] [PubMed] [Google Scholar]
- 244.Yuzbasiyan-Gurkan V., Grider A., Nostrant T., Cousins R.J., Brewer G.J. Treatment of Wilson's disease with zinc: X. Intestinal metallothionein induction. J. Lab. Clin. Med. 1992;120(3):380–386. [PubMed] [Google Scholar]
- 245.Brewer G.J., Fink J.K., Hedera P. Diagnosis and treatment of Wilson's disease. Semin. Neurol. 1999;19(3):261–270. doi: 10.1055/s-2008-1040842. [DOI] [PubMed] [Google Scholar]
- 246.Garla R., Kango P., Gill N.K., Garg M.L. Induction of Metallothionein in Rat Liver by Zinc Exposure: A Dose and Time Dependent Study. Protein J. 2017;36(5):433–442. doi: 10.1007/s10930-017-9737-7. [DOI] [PubMed] [Google Scholar]
- 247.Schilsky M.L., Blank R.R., Czaja M.J., Zern M.A., Scheinberg I.H., Stockert R.J., Sternlieb I. Hepatocellular copper toxicity and its attenuation by zinc. J. Clin. Invest. 1989;84(5):1562–1568. doi: 10.1172/jci114333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Suzuki C.A., Ohta H., Albores A., Koropatnick J., Cherian M.G. Induction of metallothionein synthesis by zinc in cadmium pretreated rats. Toxicology. 1990;63(3):273–284. doi: 10.1016/0300-483x(90)90190-r. [DOI] [PubMed] [Google Scholar]
- 249.Hedera P. Clinical management of Wilson disease. Ann. Transl. Med. 2019;7(Suppl 2):S66. doi: 10.21037/atm.2019.03.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Brewer G.J. Zinc therapy induction of intestinal metallothionein in Wilson's disease. Am. J. Gastroenterol. 1999;94(2):301–302. doi: 10.1111/j.1572-0241.1999.00301.x. [DOI] [PubMed] [Google Scholar]
- 251.Weiss K.H., Gotthardt D.N., Klemm D., Merle U., Ferenci-Foerster D., Schaefer M., Ferenci P., Stremmel W. Zinc monotherapy is not as effective as chelating agents in treatment of Wilson disease. Gastroenterology. 2011;140(4):1189–1198 e1. doi: 10.1053/j.gastro.2010.12.034. [DOI] [PubMed] [Google Scholar]
- 252.Camarata M.A., Ala A., Schilsky M.L. Zinc Maintenance Therapy for Wilson Disease: A Comparison Between Zinc Acetate and Alternative Zinc Preparations. Hepatol Commun. 2019;3(8):1151–1158. doi: 10.1002/hep4.1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.European Association for Study of L. EASL Clinical Practice Guidelines: Wilson's disease. J. Hepatol. 2012;56(3):671–685. doi: 10.1016/j.jhep.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 254.Medici V., Trevisan C.P., D'Incà R., Barollo M., Zancan L., Fagiuoli S., Martines D., Irato P., Sturniolo G.C. Diagnosis and management of Wilson's disease: results of a single center experience. J. Clin. Gastroenterol. 2006;40(10):936–941. doi: 10.1097/01.mcg.0000225670.91722.59. [DOI] [PubMed] [Google Scholar]
- 255.Schuna A., Osman M.A., Patel R.B., Welling P.G., Sundstrom W.R. Influence of food on the bioavailability of penicillamine. J. Rheumatol. 1983;10(1):95–97. [PubMed] [Google Scholar]
- 256.Pujol A.M., Cuillel M., Jullien A.S., Lebrun C., Cassio D., Mintz E., Gateau C., Delangle P. A sulfur tripod glycoconjugate that releases a high-affinity copper chelator in hepatocytes. Angew Chem. Int. Ed. Engl. 2012;51(30):7445–7448. doi: 10.1002/anie.201203255. [DOI] [PubMed] [Google Scholar]
- 257.Monestier M., Pujol A.M., Lamboux A., Cuillel M., Pignot-Paintrand I., Cassio D., Charbonnier P., Um K., Harel A., Bohic S., Gateau C., Balter V., Brun V., Delangle P., Mintz E. A liver-targeting Cu(i) chelator relocates Cu in hepatocytes and promotes Cu excretion in a murine model of Wilson's disease. Metallomics. 2020;12(6):1000–1008. doi: 10.1039/d0mt00069h. [DOI] [PubMed] [Google Scholar]
- 258.Jullien A.S., Gateau C., Lebrun C., Kieffer I., Testemale D., Delangle P. D-Penicillamine tripodal derivatives as efficient copper(I) chelators. Inorg. Chem. 2014;53(10):5229–5239. doi: 10.1021/ic5004319. [DOI] [PubMed] [Google Scholar]
- 259.Krishnan N., Konidaris K.F., Gasser G., Tonks N.K. A potent, selective, and orally bioavailable inhibitor of the protein-tyrosine phosphatase PTP1B improves insulin and leptin signaling in animal models. J. Biol. Chem. 2018;293(5):1517–1525. doi: 10.1074/jbc.C117.819110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Krishnan N., Felice C., Rivera K., Pappin D.J., Tonks N.K. DPM-1001 decreased copper levels and ameliorated deficits in a mouse model of Wilson's disease. Genes Dev. 2018;32(13–14):944–952. doi: 10.1101/gad.314658.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.El Ghazouani A., Baslé A., Firbank S.J., Knapp C.W., Gray J., Graham D.W., Dennison C. Copper-binding properties and structures of methanobactins from Methylosinus trichosporium OB3b. Inorg. Chem. 2011;50(4):1378–1391. doi: 10.1021/ic101965j. [DOI] [PubMed] [Google Scholar]
- 262.Kim H.J., Graham D.W., DiSpirito A.A., Alterman M.A., Galeva N., Larive C.K., Asunskis D., Sherwood P.M. Methanobactin, a copper-acquisition compound from methane-oxidizing bacteria. Science. 2004;305(5690):1612–1615. doi: 10.1126/science.1098322. [DOI] [PubMed] [Google Scholar]
- 263.Choi D.W., Zea C.J., Do Y.S., Semrau J.D., Antholine W.E., Hargrove M.S., Pohl N.L., Boyd E.S., Geesey G.G., Hartsel S.C., Shafe P.H., McEllistrem M.T., Kisting C.J., Campbell D., Rao V., de la Mora A.M., DiSpirito A.A. Spectral, Kinetic, and Thermodynamic Properties of Cu(I) and Cu(II) Binding by Methanobactin from Methylosinus trichosporium OB3b. Biochemistry. 2006;45(5):1442–1453. doi: 10.1021/bi051815t. [DOI] [PubMed] [Google Scholar]
- 264.Bandow N., Gilles V.S., Freesmeier B., Semrau J.D., Krentz B., Gallagher W., McEllistrem M.T., Hartsel S.C., Choi D.W., Hargrove M.S., Heard T.M., Chesner L.N., Braunreiter K.M., Cao B.V., Gavitt M.M., Hoopes J.Z., Johnson J.M., Polster E.M., Schoenick B.D., Umlauf A.M., DiSpirito A.A. Spectral and copper binding properties of methanobactin from the facultative methanotroph Methylocystis strain SB2. J. Inorg. Biochem. 2012;110:72–82. doi: 10.1016/j.jinorgbio.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 265.Einer C., Munk D.E., Park E., Akdogan B., Nagel J., Lichtmannegger J., Eberhagen C., Rieder T., Vendelbo M.H., Michalke B., Wimmer R., Blutke A., Feuchtinger A., Dershwitz P., DiSpirito A.M., Islam T., Castro R.E., Min B.-K., Kim T., Choi S., Kim D., Jung C., Lee H., Park D., Im W., Eun S.-Y., Cho Y.-H., Semrau J.D., Rodrigues C.M.P., Hohenester S., Damgaard Sandahl T., DiSpirito A.A., Zischka H. ARBM101 (Methanobactin SB2) Drains Excess Liver Copper via Biliary Excretion in Wilson's Disease Rats. Gastroenterology. 2023;165(1):187–200.e7. doi: 10.1053/j.gastro.2023.03.216. [DOI] [PubMed] [Google Scholar]
- 266.Fessler M.B. The challenges and promise of targeting the Liver X Receptors for treatment of inflammatory disease. Pharmacol. Ther. 2018;181:1–12. doi: 10.1016/j.pharmthera.2017.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Roybal J.L., Endo M., Radu A., Gray L., Todorow C.A., Zoltick P.W., Lutsenko S., Flake A.W. Early gestational gene transfer with targeted ATP7B expression in the liver improves phenotype in a murine model of Wilson's disease. Gene Ther. 2012;19(11):1085–1094. doi: 10.1038/gt.2011.186. [DOI] [PubMed] [Google Scholar]
- 268.Murillo O., Luqui D.M., Gazquez C., Martinez-Espartosa D., Navarro-Blasco I., Monreal J.I., Guembe L., Moreno-Cermeño A., Corrales F.J., Prieto J., Hernandez-Alcoceba R., Gonzalez-Aseguinolaza G. Long-term metabolic correction of Wilson's disease in a murine model by gene therapy. J. Hepatol. 2016;64(2):419–426. doi: 10.1016/j.jhep.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 269.Sas V.T. A Phase I/II Study of VTX-801 in Adult Patients With Wilson's Disease. 2021. https://classic.clinicaltrials.gov/show/NCT04537377
- 270.U.P. Inc Study of UX701 Gene Transfer for the Treatment of Wilson Disease. 2021. https://classic.clinicaltrials.gov/show/NCT04884815
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Review article