Abstract
Cervical cancer (CC) is a malignant tumor of the female reproductive system that typically occurs in cervical cells and has high incidence and mortality rates, strong metastatic ability, and poor prognosis. Asiatic acid (AA) exhibits anti-inflammatory, anti-depressant, and anti-tumor effects. However, the molecular targets and mechanisms underlying AA-mediated inhibition of CC metastasis remain unclear. AA affects the proliferation, metastasis, and epithelial-mesenchymal transition (EMT) process of CC cell lines. MTT experiments verified that AA inhibited the proliferation ability of CC cells, and the effect of AA on the lateral and longitudinal migration ability of CC was evaluated through wound healing and Transwell assays. Western blotting was used to explore whether AA inhibits EMT process in HeLa and C33a cells. Currently, targeting the PI3K/AKT/mTOR pathway as a strategy for cancer treatment remains an evolving field. However, the molecular mechanism by which AA inhibits CC via the PI3K/AKT/mTOR pathway remains unclear and requires further investigation.
Keywords: Cervical cancer, Asiatic acid, Proliferation, Metastasis, PI3K/AKT/mTOR signaling pathway
1. Introduction
The cervix is the part of the uterus that connects to the vagina. Cervical cancer (CC) is usually caused by human papillomavirus (HPV) infection, particularly high-risk HPV [1]. The main risk factors for CC include HPV infection, smoking, multiple sexual partners, impaired immune system, inappropriate genital hygiene, and family history [2,3]. Currently, surgical resection is the preferred treatment method for patients with early CC, but most patients miss the best treatment opportunity because of the lack of obvious symptoms in the early stage and are already in the advanced stage when seeking medical treatment, which substantially impacts patient prognosis [4]. The clinical treatment plan for advanced disease includes a combination of systemic radiotherapy and chemotherapy; however, issues with treatment tolerance reduce efficacy [5]. In recent years, immunotherapy has been considered as a potential and effective treatment for CC, utilizing the immune system to combat cancer [6]. For example, using checkpoint inhibitors to block the inhibitory effect of cancer cells on the immune system enhances the ability of the immune system to attack cancer cells [7]. Therefore, new treatment approaches for CC should be urgently explored to improve the prognosis of patients with CC.
Asiatic acid (AA), first isolated by Bontems in 1941 and structurally confirmed by Polonsky, is a major constituent of the plant Centella asiatica (L.) Urban [8,9]. It has significant therapeutic potential in various aspects, including skin protection, anti-bacterial properties, anti-depressant effects, blood glucose reduction, anti-inflammatory abilities, anti-angiogenic, and anti-tumor properties [[10], [11], [12]]. Recent research has shown that AA notably inhibits prostate cancer proliferation, invasion, and migration and extends patient survival [13]. Consistent with these findings, Chen et al. discovered that AA significantly inhibits CC development both in vivo and in vitro [14]. Zhang et al. found that AA increases tumor cell sensitivity to doxorubicin (DOX) in triple-negative breast cancer and cooperates with DOX to inhibit cell growth and enhance the immune response, thus improving its anti-tumor and anti-metastasis efficiency [15]. Collectively, these results suggest that AA has a pronounced anti-tumor effect.
Tumor development usually involves the influence of multiple signaling pathways which can affect tumor cell proliferation, survival, and metastasis through different mechanisms. The PI3K/AKT/mTOR signaling pathway is abnormally activated in various cancers and an important target for cancer research and treatment [16]. It also plays a crucial role in breast cancer and is closely related to multiple aspects such as tumor cell growth, invasion, and angiogenesis [17]. Iksen et al. found that the PI3K/AKT/mTOR signaling pathway promotes lung cancer cell growth and proliferation, which is associated with poor patient prognosis [18]. Meanwhile, it significantly promotes the ovarian cancer cell proliferation, invasion, and prognosis and inhibits apoptosis [19]. However, the specific mechanism by which AA regulates CC through the PI3K/AKT/mTOR signaling pathway remains unclear.
This study aimed to explore the mechanism by which AA regulates malignant CC progression. Specifically, we determined the concentration-dependent effects of AA on CC cell proliferation and migration. Through in-depth research on AA-mediated PI3K/AKT/mTOR signaling pathway regulation to mediate CC evolution, this study aimed to provide a new perspective and theoretical basis for the treatment of patients with CC. Although AA inhibited the malignant biological processes of CC cells in this study, some shortcomings need to be addressed. The specific mechanisms that affect AA regulation in CC include various factors, such as metabolic reprogramming, signaling pathways, and immunosuppressants, which have not been verified individually. Despite these limitations, we believe that this study has scientific and practical significance. This study is the first to reveal that AA regulates CC cell evolution through the PI3K/AKT/mTOR signaling pathway, providing new targets and guiding principles for better clinical treatment of patients with CC.
2. Materials and methods
2.1. Cell culture and reagents
Human CC cell lines, HeLa and C33a, were purchased from the American Type Culture Collection (ATCC, USA) and maintained in DMEM containing 10 % fetal bovine serum (GIBCO, USA) and 1 % penicillin and streptomycin at 37 °C in a culture chamber containing 5 % CO2. AA was purchased from Nanjing Chunqiu Bioengineering Co., Ltd. (batch number: JXCS20200603; purity >98 %).
2.2. MTT assay
The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was used to detect the viability of cervical cancer cells. Combined HeLa and C33a (1 × 104 cells/well) were added to a 96-well plate and incubated with 0, 10, 20, and 40 μmol/L AA for 24, 48, and 72 h. Then, MTT was added to each well and cell viability was detected using an microplate reader after 4 h.
2.3. Colony formation assay
HeLa and C33a (1000 cells/well) cells were seeded in a 6-well plate and cultured in medium containing 10 % fetal bovine serum for 2 weeks. The cells were then removed, fixed with 4 % paraformaldehyde, stained with hematoxylin for 30 min, observed, and photographed under a microscope.
2.4. 5-Ethyl-2′-deoxyuridine (EdU) incorporation assay
Cell proliferation was monitored using EdU. Briefly, 1 × 105 CC cells were cultured overnight in 96-well plates, using 100μ cells/well mix EdU's A reagent and culture medium (Ruibo Biotechnology Co., Ltd., Guangzhou, Guangdong Province, China) for 2 h. Cells were fixed and washed with PBS according to the manufacturer's instructions. The cells were then stained with Apollo staining solution for 30 min and washed repeatedly. Finally, after repositioning with the F reagent (Hoechst 33342), and observe under a microscope.
2.5. Wound healing assay
HeLa and C33a cells were inoculated in a 6-well culture plate and washed with cold PBS after reaching 80–90 % confluency. Then, the cell surface was scratched using a 200 μL pipette tip and the lateral migration ability was monitored under a microscope at 0 and 48 h.
2.6. Western blot analysis
After incubating HeLa and C33a cells with AA for 48 h, protein samples were collected, and cell lysates were used to lyse adherent cells. The concentration of each protein sample was determined using ELISA and an appropriate amount of concentrated SDS–PAGE protein loading buffer was added. Finally, the experiment was conducted on a standard electrophoresis device (Bio-Rad, Hercules, CA, USA) with a low-voltage setting between 80 and 120 V. Then, the primary antibody was added overnight at 4 °C. On the following day, secondary antibody incubation and strip development were performed using a Bio-Rad machine.
2.7. Transwell assay
The HeLa and C33a cells were grown to 90 % confluency. Then, 104 cells/well was inoculated in the upper part of the chamber and cultured with DMEM without any added serum. The lower chamber was filled with a culture medium containing 20 % FBS. After the cells had adhered to the wall, they were incubated with AA for 48 h. The upper chamber was cleaned with PBS; the cells were fixed with 4 % paraformaldehyde, stained with hematoxylin for 20 min, sealed with a resin adhesive, and imaged using an Olympus BX53 microscope.
2.8. Statistical analysis
The biological process for screening drug-targeted enrichment used the Therapeutic Target Database. Statistical analysis was performed using ImageJ and GraphPad Prism 9.0 softwares. All experiments were repeated at least three times, and the t-test was used to compare the average values between the two groups. p < 0.05 is considered statistically significant.
3. Results
3.1. AA suppressed CC cell growth in vitro
AA is one of the main components of triterpenoid extracts and has a wussane-type skeleton structure (Fig. 1A). To further confirm the effect of AA on the proliferation of CC cells, MTT assay showed that with the increase of AA time and concentration, the CC cell viability of HeLa and C33a cells was significantly inhibited (p < 0.05, Fig. 1B), and the IC50 for HeLa and C33a cells after 24 h of treatment were 13.15 and 25.2 μmol/L, respectively. Plate cloning experiments revealed that different AA doses significantly reduced HeLa and C33a CC cell count (Fig. 1C). In addition, the EdU assay showed that as the concentration of AA increased, the percentage of EdU-positive cells in the AA group gradually decreased than that in the control group. The results showed that The proliferation of CC cells was effectively inhibited in the AA group (p < 0.05; Fig. 1D, Supplementary Fig. 1). These data indicated that AA significantly inhibited CC cell proliferation.
Fig. 1.
AA inhibits the proliferation ability of CC cells. (A) Framework structure of asiatic acid. (B) MTT detection of cell proliferation activity of CC. (C) The clone formation experiment is to measure the proliferation ability of CC cells. (D) Fluorescence microscopy technique for detecting CC cell proliferation-EdU proliferation experiment.
3.2. AA inhibits CC cell metastasis in vitro
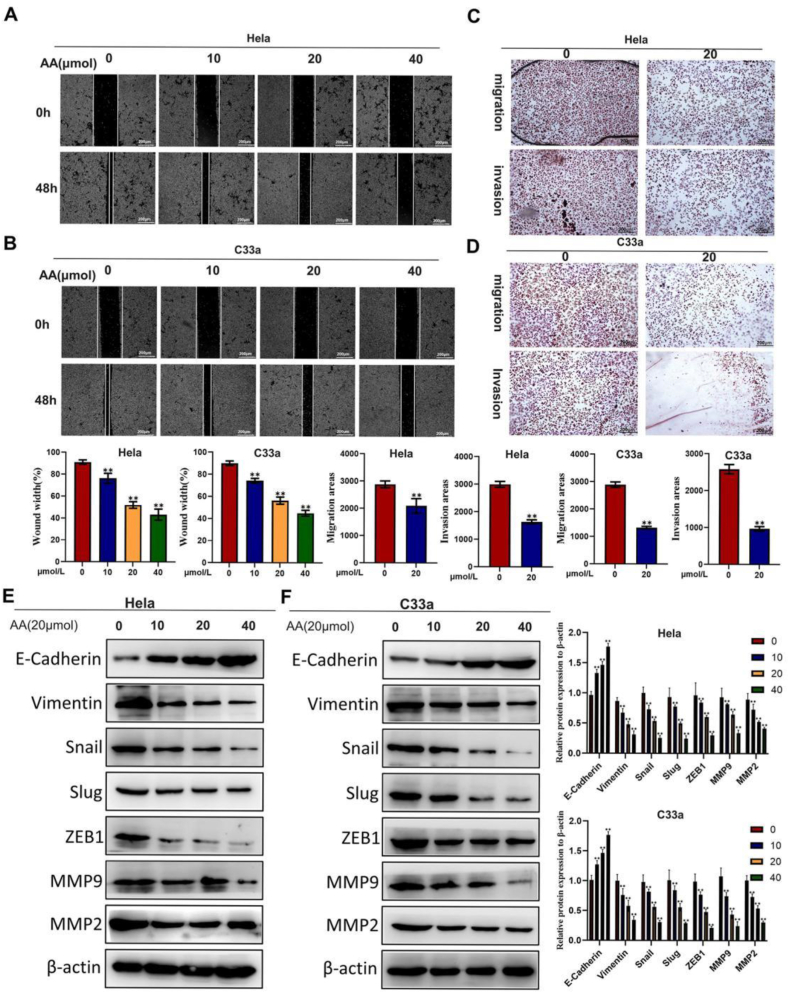
Whether AA affects CC cell migration ability was further investigated using wound healing and Transwell assays. As expected, AA significantly reduced the lateral and vertical CC cell migration in vitro (p < 0.05; Fig. 2A–D). Simultaneously, the epithelial marker E-cadherin expression level significantly increased in CC cells with the increase in AA concentration, while the mesenchymal marker (Vimentin, Snail, Slug, and ZEB1) expression were downregulated and the expression levels of matrix metalloproteinases MMP2 and MMP9 were consistent (p < 0.05, Fig. 2E and F). These results indicated that AA inhibited CC cell invasion, migration, and epithelial–mesenchymal transition (EMT).
Fig. 2.
AA inhibits the migration ability of CC cells. (A–B) The wound healing experiment is a laboratory technique that studies cell migration, repair ability, and intercellular interactions by scratching the monolayer of cells and regularly capturing images through a time-delay microscope. (C–D) To verify the effect of target genes on cell transfer ability by detecting the migration of CC cells into serum containing culture medium in Transwell cells. (E–F) Western blot.
3.3. PI3K/AKT/mTOR signaling pathway regulates CC cell proliferation
To further study the molecular mechanism of AA-induced CC cell proliferation and metastasis, we detected the main biological process of AA enrichment through the network data of the Therapeutic Target Database [20] and found that AA was significantly related to the PI3K/AKT/mTOR signaling pathway (Fig. 3A). To validate the database results, western blotting analysis confirmed that treating AA with CC cells significantly reduced p-PI3K, p-AKT, and p-mTOR expression levels, while the total PI3K, AKT, and mTOR expression levels did not change significantly (p < 0.05, Fig. 3B). We explored the role of the PI3K/AKT/mTOR pathway in AA-mediated CC regulation. Plate cloning experiments showed that the PI3K inhibitor LY294002 significantly inhibited CC cell proliferation (Fig. 3C). However, adding the PI3K activator 740Y–P significantly reversed this phenomenon (Supplementary Fig. 2).
Fig. 3.
AA regulates the PI3K/AKT/mTOR signaling pathway in CC cells. (A) Therapeutic Target Database. (B) The effect of AA on the expression of proteins level on PI3K/AKT/mTOR pathway were assayed by Western blot. (C) Cell viability was detected in CC cells after treatment with AA, LY294002 and 740Y–P by colony formation assays.
3.4. PI3K/AKT/mTOR signaling pathway regulates CC cell migration
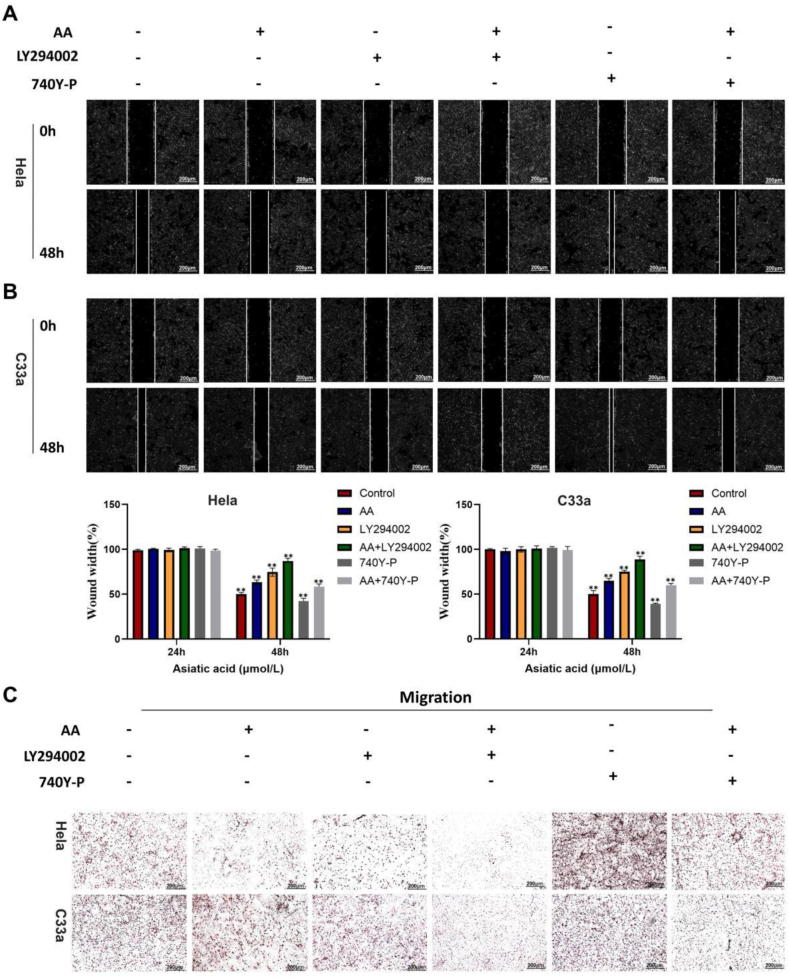
We investigated whether the addition of PI3K inhibitors and activators affected CC cell migration. Wound healing and migration experiments revealed that the PI3K inhibitor LY294002 significantly inhibited the horizontal and vertical CC cell migration (Fig. 4A–C, Supplementary Fig. 3). Compared to the application of AA and LY294002 alone, the combination of LY294002 and AA significantly inhibited CC cell migration. Adding the PI3K activator 740Y–P significantly reversed this phenomenon. These results indicate that AA affects CC cell migration by regulating the PI3K/AKT/mTOR signaling pathway.
Fig. 4.
AA regulates the PI3K/AKT/mTOR signaling pathway in CC cells. Cell migration capacities were detected in CC cells after treatment with AA, LY294002 and 740Y–P.
4. Discussion
CC is a malignant tumor that is generally considered one of the most aggressive and fatal cancers [21,22]. Early CC usually has no obvious symptoms; however, abnormal vaginal bleeding, pain, and abnormal vaginal secretions may occur as the condition progresses [[23], [24], [25]]. Interestingly, CC does not respond well to treatments, particularly in the advanced stages [26]. Therefore, new potential markers and therapeutic targets should be identified for treating CC.
AA is extracted from plants such as Centella asiatica and has been widely studied because it has various medicinal properties, including skin-protective, anti-bacterial, anti-depressant, and anti-tumor effects [[27], [28], [29], [30]]. Recent research has suggested that AA may have anti-tumor effects in certain types of cancer and inhibits tumor cell proliferation, invasion, and migration, thereby reducing tumor growth and spread [31]. AA also inhibits angiogenesis, which is an important process in tumor growth and metastasis [32]. Lai et al. found that AA inhibits malignant processes such as human prostate cancer cell proliferation and migration by mediating the MZF-1/Elk-1/Snail signaling axis, providing new potential targets for treating human prostate cancer [33]. By reducing new blood vessel formation, AA can inhibit tumor blood supply, reducing the likelihood of its growth and spread [34]. In this study, AA concentration-dependently inhibited CC cell proliferation, migration, and EMT. Although some preliminary research results indicate that AA has anti-tumor potential, more research is still needed to confirm its effectiveness and safety should be further confirmed. In addition, AA may have different effects on different types of cancer. Therefore, further research is needed to understand its specific mechanisms of action in different cancer types.
The PI3K/AKT/mTOR signaling pathway plays an important role in various tumor types and is closely related to tumor cell growth, proliferation, survival, and metabolism [35]. Alves et al. found that the PI3K/AKT/mTOR pathway plays a crucial role in regulating cell cycle and growth. Abnormally activated pathways can promote the division and proliferation of tumor cells, leading to tumor growth [36]. The PI3K/AKT/mTOR pathway plays an important role in angiogenesis. The abnormally activated pathway can promote new blood vessel formation around tumor cells, providing nutrients and oxygen and promoting tumor growth and diffusion [37]. Miricescu et al. found that abnormal PI3K/AKT/mTOR pathway activation is associated with tumor resistance to radiotherapy, chemotherapy, and targeted therapy [38]. The abnormal activation of cancer cell pathways can reduce drug efficacy, making tumors more difficult to treat [39]. AA inhibits colorectal cancer cell proliferation and migration and promotes apoptosis by regulating the PI3K/Akt/mTOR/p70S6K signaling axis [40]. However, the mechanism by which AA regulates the PI3K/Akt/mTOR signaling axis-mediated CC progression is not yet clear, and further research is needed. In this study, AA regulated malignant CC progression by mediating the PI3K/AKT/mTOR signaling pathway. Overall, the PI3K/AKT/mTOR pathway plays an important role in tumor occurrence and development, making it a focal point for tumor research and treatment. Understanding and intervening in this pathway should provide new strategies for tumor treatment.
In this study, CC cells were treated with different AA concentrations. Compared to that in the control cells, AA significantly inhibited the growth of CC cells. Simultaneously, we found that the PI3K/AKT/mTOR signaling pathway is closely related to AA-regulated CC progression. This was further verified by adding a PI3K inhibitor and activator, which also provided a new direction for using AA to treat CC. This study demonstrated the anti-tumor activity of AA, which helps inhibit CC cell proliferation and migration, thereby inhibiting the malignant progression of tumors.
The research on the treatment of CC with AA has certain potential. It has been confirmed that AA has anti cervical cancer activity in vitro and animal experiments, including inhibiting tumor cell proliferation, inducing apoptosis, and blocking angiogenesis; AA also exhibits anti-inflammatory and immunomodulatory effects, which can affect the tumor microenvironment, inhibit tumor growth and spread; AA can be used as a monotherapy or in combination with other chemotherapy drugs, radiotherapy, etc., forming diverse treatment strategies and improving the treatment options and efficacy of cervical cancer patients. However, there are still some knowledge gaps in the research on the treatment of cervical cancer with AA: for example, there is insufficient clinical validation: currently, there is still insufficient evidence of the efficacy and safety of AA in clinical treatment of cervical cancer, and more clinical trials are needed to verify its effectiveness and safety in clinical applications; The mechanism research is not in-depth enough: Although some of the mechanisms of action of AA in the treatment of cervical cancer have been preliminarily understood, the in-depth study of its mechanism of action is still insufficient, especially in the research of signaling pathways and molecular mechanisms related to cervical cancer, which still needs further exploration; Drug resistance issue: Like other chemotherapy drugs, the therapeutic effect of AA may be affected by the drug resistance of tumor cells, so it is necessary to conduct more in-depth research on the mechanism of drug resistance in order to find effective strategies to reverse drug resistance. In the next five years, the therapeutic effect of AA on cervical cancer can be further studied by increasing clinical trials, deepening mechanism research, and exploring combination therapy strategies.
However, this study also has limitations, such as a lack of clinical evidence. Currently, most research on the treatment of CC with AA mainly focuses on cell experiments, lacking sufficient clinical research to support its safety and effectiveness in the human body. Second, as a natural herb, the content of active ingredients in AA may vary, and further research on drug purity and standardization is needed to ensure consistent and reliable therapeutic effects. Therefore, we should strengthen clinical research by conducting more clinical studies to evaluate the safety and effectiveness of AA in CC treatment and determine the best treatment plan and dosage. We should also optimize drug extraction and preparation techniques to ensure the purity and consistency of AA drugs, and improve the reliability of their therapeutic effects.
5. Conclusions
In conclusion, we confirmed the anti-cancer effect of AA, discussed how it regulates the molecular mechanism of CC through the PI3K/AKT/mTOR signaling pathway, and further examined the molecular mechanism of the PI3K/AKT/mTOR signaling pathway in CC cells by treating CC cells with PI3K inhibitors and activators in combination with AA, providing a reliable theoretical basis for CC treatment.
Ethical approval
None required.
Funding
The research was funded by the Department of Science and Technology of Jilin Province, with the project number: 20230204036YY & 20230203051SF & YDZJ202301ZYTS113 & YDZJ202301ZYTS119 &YDZJ202102CXJD076.
Data availability statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study. And additional information in the supplementary figure.
CRediT authorship contribution statement
Xiuying Lin: Writing – review & editing, Writing – original draft, Funding acquisition. Yanqiu Fang: Writing – review & editing, Writing – original draft, Formal analysis, Conceptualization. Xuguang Mi: Data curation. jianhua Fu: Visualization, Methodology. Shiling Chen: Writing – review & editing. Mengxue Wu: Writing – original draft. Ningyi Jin: Writing – review & editing, Writing – original draft, Funding acquisition, Formal analysis, Conceptualization.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:Ningyi Jin reports financial support was provided by Yanbian University Medical College. All authors declare that they have no known competing financial interests or personal relationships. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
I am grateful for Yanbian University and Jilin Province People's Hospital's help.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e34047.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Weyers S., Garland S.M., Cruickshank M., Kyrgiou M., Arbyn M. Cervical cancer prevention in transgender men: a review. BJOG. 2021;128(5):822–826. doi: 10.1111/1471-0528.16503. [DOI] [PubMed] [Google Scholar]
- 2.Chargari C., Peignaux K., Escande A., Renard S., Lafond C., Petit A., Lam Cham Kee D., Durdux C., Haie-Méder C. Radiotherapy of cervical cancer. Cancer Radiother. 2022;26(1–2):298–308. doi: 10.1016/j.canrad.2021.11.009. [DOI] [PubMed] [Google Scholar]
- 3.Mayadev J.S., Ke G., Mahantshetty U., Pereira M.D., Tarnawski R., Toita T. Global challenges of radiotherapy for the treatment of locally advanced cervical cancer. Int. J. Gynecol. Cancer. 2022;32(3):436–445. doi: 10.1136/ijgc-2021-003001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poddar P., Maheshwari A. Surgery for cervical cancer: consensus & controversies. Indian J. Med. Res. 2021;154(2):284–292. doi: 10.4103/ijmr.IJMR_4240_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mollica V., Rizzo A., Marchetti A., et al. The impact of ECOG performance status on efficacy of immunotherapy and immune-based combinations in cancer patients: the MOUSEION-06 study. Clin. Exp. Med. 2023;23(8):5039–5049. doi: 10.1007/s10238-023-01159-1. [DOI] [PubMed] [Google Scholar]
- 6.Rizzo A., Mollica V., Tateo V., et al. Hypertransaminasemia in cancer patients receiving immunotherapy and immune-based combinations: the MOUSEION-05 study. Cancer Immunol. Immunother. 2023;72(6):1381–1394. doi: 10.1007/s00262-023-03366-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dall'Olio F.G., Rizzo A., Mollica V., et al. Immortal time bias in the association between toxicity and response for immune checkpoint inhibitors: a meta-analysis. Immunotherapy. 2021;13(3):257–270. doi: 10.2217/imt-2020-0179. [DOI] [PubMed] [Google Scholar]
- 8.Fang M., Wan W., Li Q., Wan W., Long Y., Liu H., Yang X. Asiatic acid attenuates diabetic retinopathy through TLR4/MyD88/NF-κB p65 mediated modulation of microglia polarization. Life Sci. 2021;277 doi: 10.1016/j.lfs.2021.119567. [DOI] [PubMed] [Google Scholar]
- 9.Ding L., Liu T., Ma J. Neuroprotective mechanisms of Asiatic acid. Heliyon. 2023;9(5) doi: 10.1016/j.heliyon.2023.e15853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qiu W., Zhang X., Pang X., Huang J., Zhou S., Wang R., Tang Z., Su R. Asiatic acid alleviates LPS-induced acute kidney injury in broilers by inhibiting oxidative stress and ferroptosis via activation of the Nrf2 pathway. Food Chem. Toxicol. 2022;170 doi: 10.1016/j.fct.2022.113468. [DOI] [PubMed] [Google Scholar]
- 11.Zhu Z., Cui L., Yang J., Vong C.T., Hu Y., Xiao J., Chan G., He Z., Zhong Z. Anticancer effects of asiatic acid against doxorubicin-resistant breast cancer cells via an AMPK-dependent pathway in vitro. Phytomedicine. 2021;92 doi: 10.1016/j.phymed.2021.153737. [DOI] [PubMed] [Google Scholar]
- 12.Yi C., Song M., Sun L., Si L., Yu D., Li B., Lu P., Wang W., Wang X. Asiatic acid alleviates myocardial ischemia-reperfusion injury by inhibiting the ROS-mediated mitochondria-dependent apoptosis pathway. Oxid. Med. Cell. Longev. 2022;2022 doi: 10.1155/2022/3267450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Islam M.T., Ali E.S., Uddin S.J., Khan I.N., Shill M.C., de Castro E Sousa J.M., de Alencar M.V.O.B., Melo-Cavalcante A.A.C., Mubarak M.S. Anti-cancer effects of asiatic acid, a triterpene from Centilla asiatica L: a review. Anti Cancer Agents Med. Chem. 2020;20(5):536–547. doi: 10.2174/1871520619666191211103006. [DOI] [PubMed] [Google Scholar]
- 14.Chen Y.H., Wu J.X., Yang S.F., Hsiao Y.H. Synergistic combination of luteolin and asiatic acid on cervical cancer in vitro and in vivo. Cancers. 2023;15(2):548. doi: 10.3390/cancers15020548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Y., Wang Y., Zhang H., et al. Replacing cholesterol with asiatic acid to prolong circulation and enhance anti-metastatic effects of non-PEGylated liposomes. J. Contr. Release. 2024;366:585–595. doi: 10.1016/j.jconrel.2024.01.009. [DOI] [PubMed] [Google Scholar]
- 16.Tewari D., Patni P., Bishayee A., Sah A.N., Bishayee A. Natural products targeting the PI3K-Akt-mTOR signaling pathway in cancer: a novel therapeutic strategy. Semin. Cancer Biol. 2022;80:1–17. doi: 10.1016/j.semcancer.2019.12.008. [DOI] [PubMed] [Google Scholar]
- 17.Peng Y., Wang Y., Zhou C., Mei W., Zeng C. PI3K/Akt/mTOR pathway and its role in cancer therapeutics: are we making headway? Front. Oncol. 2022;12 doi: 10.3389/fonc.2022.819128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iksen Pothongsrisit S., Pongrakhananon V. Targeting the PI3K/AKT/mTOR signaling pathway in lung cancer: an update regarding potential drugs and natural products. Molecules. 2021;26(13):4100. doi: 10.3390/molecules26134100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou J., Jiang Y.Y., Chen H., Wu Y.C., Zhang L. Tanshinone I attenuates the malignant biological properties of ovarian cancer by inducing apoptosis and autophagy via the inactivation of PI3K/AKT/mTOR pathway. Cell Prolif. 2020;53(2) doi: 10.1111/cpr.12739. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Zhou Y., Zhang Y., Zhao D., Yu X., Shen X., Zhou Y., Wang S., Qiu Y., Chen Y., Zhu F. TTD: therapeutic Target Database describing target druggability information. Nucleic Acids Res. 2023;15 doi: 10.1093/nar/gkad751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Musunuru H.B., Pifer P.M., Mohindra P., Albuquerque K., Beriwal S. Advances in management of locally advanced cervical cancer. Indian J. Med. Res. 2021;154(2):248–261. doi: 10.4103/ijmr.IJMR_1047_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhatla N., Aoki D., Sharma D.N., Sankaranarayanan R. Cancer of the cervix uteri: 2021 update. Int. J. Gynaecol. Obstet. 2021;155(Suppl 1):28–44. doi: 10.1002/ijgo.13865. Suppl 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gopu P., Antony F., Cyriac S., Karakasis K., Oza A.M. Updates on systemic therapy for cervical cancer. Indian J. Med. Res. 2021;154(2):293–302. doi: 10.4103/ijmr.IJMR_4454_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manrriquez E.N., Zakhour M., Salani R. Precision medicine for cervical cancer. Curr. Opin. Obstet. Gynecol. 2022;34(1):1–5. doi: 10.1097/GCO.0000000000000755. [DOI] [PubMed] [Google Scholar]
- 25.Narasimhamurthy M., Kafle S.U. Cervical cancer in Nepal: current screening strategies and challenges. Front. Public Health. 2022;10 doi: 10.3389/fpubh.2022.980899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Volkova L.V., Pashov A.I., Omelchuk N.N. Cervical carcinoma: oncobiology and biomarkers. Int. J. Mol. Sci. 2021;22(22) doi: 10.3390/ijms222212571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qiu F., Yuan Y., Luo W., Gong Y.S., Zhang Z.M., Liu Z.M., Gao L. Asiatic acid alleviates ischemic myocardial injury in mice by modulating mitophagy- and glycophagy-based energy metabolism. Acta Pharmacol. Sin. 2022;43(6):1395–1407. doi: 10.1038/s41401-021-00763-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kukula O., Kırmızıkan S., Tiryaki E.S., Çiçekli M.N., Günaydın C. Asiatic acid exerts an anti-psoriatic effect in the imiquimod-induced psoriasis model in mice. Immunopharmacol. Immunotoxicol. 2022;44(3):367–372. doi: 10.1080/08923973.2022.2048849. [DOI] [PubMed] [Google Scholar]
- 29.Sycz Z., Tichaczek-Goska D., Wojnicz D. Anti-planktonic and anti-biofilm properties of pentacyclic triterpenes-asiatic acid and ursolic acid as promising antibacterial future pharmaceuticals. Biomolecules. 2022;12(1):98. doi: 10.3390/biom12010098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen X., Han D., Liu T., Huang C., Hu Z., Tan X., Wu S. Asiatic acid improves high-fat-diet-induced osteoporosis in mice via regulating SIRT1/FOXO1 signaling and inhibiting oxidative stress. Histol. Histopathol. 2022;37(8):769–777. doi: 10.14670/HH-18-446. [DOI] [PubMed] [Google Scholar]
- 31.Niu K., Bai P., Yang B., Feng X., Qiu F. Asiatic acid alleviates metabolism disorders in ob/ob mice: mechanistic insights. Food Funct. 2022;13(13):6934–6946. doi: 10.1039/d2fo01069k. [DOI] [PubMed] [Google Scholar]
- 32.Tian M., Chen K., Huang J., Chu D., Li J., Huang K., Ma C. Asiatic acid inhibits angiogenesis and vascular permeability through the VEGF/VEGFR2 signaling pathway to inhibit the growth and metastasis of breast cancer in mice. Phytother Res. 2021;35(11):6389–6400. doi: 10.1002/ptr.7292. [DOI] [PubMed] [Google Scholar]
- 33.Lai Y.W., Wang S.W., Lin C.L., Chen S.S., Lin K.H., Lee Y.T., Chen W.C., Hsieh Y.H. Asiatic acid exhibits antimetastatic activity in human prostate cancer cells by modulating the MZF-1/Elk-1/Snail signaling axis. Eur. J. Pharmacol. 2023;951 doi: 10.1016/j.ejphar.2023.175770. [DOI] [PubMed] [Google Scholar]
- 34.Zou W., Zhang J., Zhang K., Peng Z., Xin R., Wang L., Li J. Asiatic acid attenuates inflammation induced by Salmonella via upregulating LncRNA TVX1 in microglia. Int. J. Mol. Sci. 2022;23(18) doi: 10.3390/ijms231810978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu L., Wei J., Liu P. Attacking the PI3K/Akt/mTOR signaling pathway for targeted therapeutic treatment in human cancer. Semin. Cancer Biol. 2022;85:69–94. doi: 10.1016/j.semcancer.2021.06.019. [DOI] [PubMed] [Google Scholar]
- 36.Alves C.L., Ditzel H.J. Drugging the PI3K/AKT/mTOR pathway in ER+ breast cancer. Int. J. Mol. Sci. 2023;24(5):4522. doi: 10.3390/ijms24054522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barzegar Behrooz A., Talaie Z., Jusheghani F., Łos M.J., Klonisch T., Ghavami S. Wnt and PI3K/Akt/mTOR survival pathways as therapeutic targets in glioblastoma. Int. J. Mol. Sci. 2022;23(3):1353. doi: 10.3390/ijms23031353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miricescu D., Totan A., Stanescu-Spinu I.I., Badoiu S.C., Stefani C., Greabu M. PI3K/AKT/mTOR signaling pathway in breast cancer: from molecular landscape to clinical aspects. Int. J. Mol. Sci. 2020;22(1):173. doi: 10.3390/ijms22010173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu K., Wu Y., He P., Fan Y., Zhong X., Zheng H., Luo T. PI3K/AKT/mTOR-Targeted therapy for breast cancer. Cells. 2022;11(16):2508. doi: 10.3390/cells11162508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hao Y., Huang J., Ma Y., Chen W., Fan Q., Sun X., Shao M., Cai H. Asiatic acid inhibits proliferation, migration and induces apoptosis by regulating Pdcd4 via the PI3K/Akt/mTOR/p70S6K signaling pathway in human colon carcinoma cells. Oncol. Lett. 2018;15(6):8223–8230. doi: 10.3892/ol.2018.8417. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study. And additional information in the supplementary figure.