Abstract
We report a case of a 72-year-old female who presented with fever, abdominal pain, and diarrhea accompanied by leukopenia, anemia, and thrombocytopenia. The diagnosis of acute aplastic anemia was confirmed through bone marrow aspiration. Treatment included glucocorticoids, immunoglobulin therapy, and plasma exchange. Subsequently, the patient developed gastrointestinal bleeding and abdominal Computed Tomography (CT) revealed perforation of the transverse colon. Pathological examination of surgically removed diseased tissue confirmed mucor infection. Despite receiving antifungal therapy with amphotericin B, the patient's condition deteriorated due to the sepsis progression. Mucor infection in immunocompromised patients should be vigilant, and early diagnosis may help improve prognosis.
Keywords: Amphotericin B, Colonic perforation, Mucormycosis, Pancytopenia, Sepsis
Invasive fungal infections are often difficult to detect and lead to a worse prognosis, while inadequate clinical diagnosis and a delay in treatment can increase case fatality rates. Mucormycosis is an opportunistic invasive infection caused by fungi of the order Mucorales, exhibiting craniofacial, pulmonary, cutaneous, gastrointestinal and disseminated forms. Immediate treatment is required due to quick progression and tissue destruction characteristics of this infection. Gastrointestinal mucormycosis is a rare but highly lethal disease, histopathologic examination was the most commonly used to detect infection [1]. Extensive surgical debridement along with sequential antifungal drug treatment improved the survival in mucormycosis patients [2].
In our case, glucocorticoid therapy is considered a high risk factor for Mucormold infection in patients. The metagenomic next-generation sequencing (mNGS), that only detected aflatus/Aspergillus oryzae and Candida albicans but not mucor, led to the antifungal therapy of voriconazole and caspofungin. However, both drug failed to prevent intestinal tissue invasion by mucor. Perhaps due to the delayed diagnosis, surgical removal of infected tissue and treatment with amphotericin failed to benefit the patient.
1. Case report
The patient, a 72-year-old married Han Chinese woman residing in Hebei Province, China, presented with symptoms including fever (Temperature 37.7 °C), abdominal pain, diarrhea, sore throat, and severe oral ulcers on August 27th, 2022 while being at home. Despite receiving treatment with cephalosporin antibiotics, acyclovir, interferon, probiotics, and albumin at the local hospital, the patient's symptoms failed to improve and were subsequently followed by hematochezia. Subsequently on September 2nd the patient exhibited bloody stools and abnormal peripheral blood counts including leukopenia (white blood cell count of 0.13 × 109/L), thrombocytopenia (platelet count of 31 × 109/L), and anemia (hemoglobin level of only 68g/L). The patient was admitted to our hospital on September 3rd. She had rheumatoid arthritis for 5 years with irregular oral administration of methotrexate, methylprednisolone, and leflunomide. As well as she had hypertension for 40 years and had a cerebral infarction 20 years earlier without obvious sequelae. Coronary heart disease and angina pectoris were diagnosed one year earlier, and coronary angiography was performed before the onset of the disease On August 26. After admission, the patient was conscious with a temperature of 38.5 °C, a respiratory rate of 28 times/min, a pulse rate of 112 times/min, and blood pressure measuring 105/60 mmHg. She exhibited widespread purpura on her skin, accompanied by oral ulcers that were bleeding and causing pain.
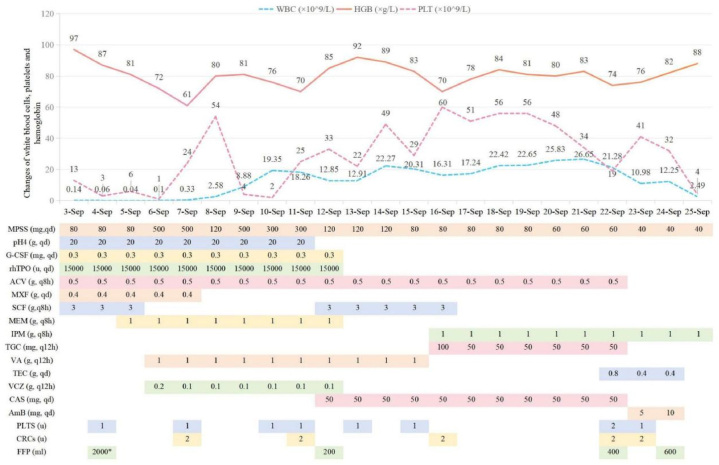
Following transfusions of erythrocytes in suspension, the patient's hemoglobin level increased to 97g/L on September 3rd. However, her platelet (PLT) count had further decreased to 13 × 109/L. Additionally, serum tests revealed elevated levels of interleukin-6 (>4000 pg/mL), procalcitonin (18.8 ng/mL), and myoglobin (119.99 ng/mL). Despite receiving methylprednisolone and gamma globulin as well as granulocyte stimulating factor and thrombopoietin, the patient's PLT count continued to decline to 3 × 109/L on September 4th, while her white blood cell count dropped to 0.06 × 109/L (Fig. 2). The APACHE II score was 17 and SOFA score was 7 evaluated for the patient within 24 hours of admission. She experienced coughing, expectoration with bloody sputum, and showed no significant improvement in symptoms such as oral ulcers and diarrhea. Due to an inadequate response to medical treatment, the patient underwent plasma exchange.
Fig. 2.
Blood-count trends and medications(*plasma exchange).
On September 5th, the patient's bone marrow morphology revealed grade V myelodysplasia, which was characterized by the absence of erythrocytes, megakaryocytes and platelets. Immunotyping demonstrated a scarcity of cells with increased fragmentation, granulocytopenia, and no aberrant phenotypic expression. Chromosomal analysis did not reveal any abnormal clones. These findings provide support for the diagnosis of acute aplastic anemia. On September 6th, the dosage of methylprednisolone was escalated from 80mg/day to 500mg/day. Following a two-day course of high-dose methylprednisolone therapy (500mg), the patient's PLT count exhibited a significant increase from 1 × 109/L to 54 × 109/L (with one unit of platelets transfused). Subsequently, in order to mitigate the risk of infection, the dose of methylprednisolone was subsequently tapered down to 120mg/day; however, the PLT count once again declined to 2 × 109/L on September 10th. Consequently, the methylprednisolone dosage was adjusted to 500mg for one day followed by 300mg for two days, resulting in an increase in PLT count to 33 × 109/L on September 12th (Fig. 2). On September 20th, a repeated examination of bone marrow morphology revealed myelodysplasia classified as grade III-VI.
Despite administration of intravenous moxifloxacin, cefoperazone sodium and sulbactam sodium infusions, the patient's procalcitonin levels escalated to 33.74 ng/mL on September 5th. By Metagenomic next-generation sequencing (mNGS), identified Estherichia coli (96 sequences), Enterococcus faecium (62 sequences), Fusobacterium mortiferum (21 sequences), Phocaeicola vulgatus (6 sequences), Bacteroides thetaiotaomicron (2 sequences), Aspergillus aflatus/Aspergillus oryzae (9 sequences), and Candida albicans (1 sequences) in her peripheral blood. The antibiotics were subsequently adjusted to meropenem, vancomycin, and voriconazole for optimal treatment (Fig. 3). The white blood cell (WBC) count gradually rose to 12.45 × 109/L on September 9th and remained at a relatively elevated level thereafter.
Fig. 3.
Timeline for the diagnosis and treatment.
The patient presented with dark red bloody stool on September 10th and was administered haemostatics. She experienced significant discomfort due to the presence of multiple oral ulcers and exhibited a refusal to consume food. After the administration of enteral nutrition via a nasogastric tube, the patient presented with symptoms of nausea and vomiting. During the course of treatment, patients receive calories intravenously through parenteral nutrition.
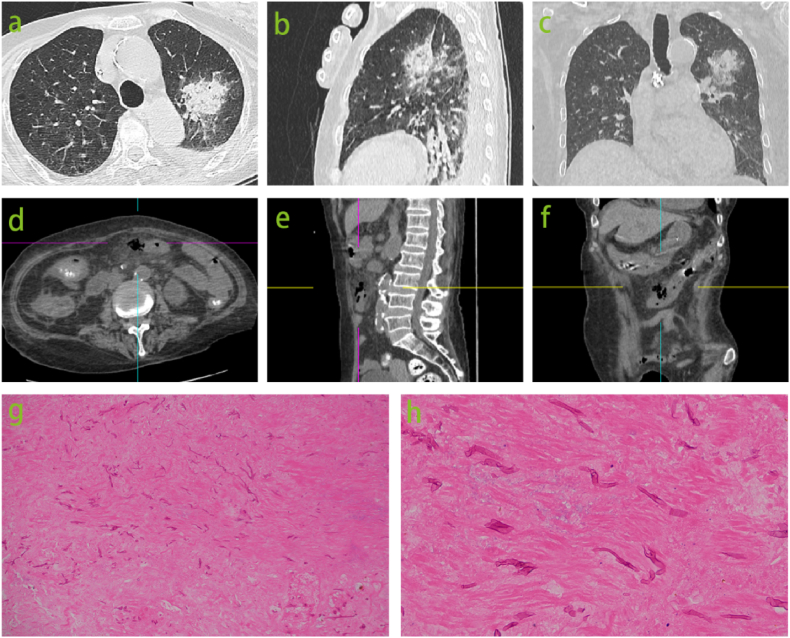
The administration of Somatostatin was discontinued on September 20th, and the patient was advised to resume oral intake. However, the volume of gastrointestinal decompression reached 900mL. The patient presented with an elevated white blood cell count of 26.65 × 109/L on September 21st, accompanied by exacerbated abdominal distention and pain, as well as the presence of approximately 300mL of brown-colored gastric fluid. Additionally, there was a decrease in hemoglobin levels from 83g/L to 74g/L and a decline in platelet count from 34 × 109/L to 19 × 109/L (Fig. 2). These findings were suggestive of stress ulceration with bleeding; however, two fecal occult blood tests yielded negative results. In order to investigate potential infection and assess the condition of the gastrointestinal tract, chest, abdomen, and pelvis CT scans were conducted due to compromised systemic status and thrombocytopenia that rendered gastroenteroscopy unfeasible. CT of the abdomen revealed perforation of the midsection transverse colon. Additionally, CT imaging of the chest identified a new lesion suspected to be caused by fungal infection (Fig. 1 a ∼ f).
Fig. 1.
A newly detected lesion, measuring 38 × 37mm, with an indistinct margin and a reverse halo sign was found in the left upper lobe on chest CT images (ãc). Abdominal CT scans (d ∼ f) revealed local discontinuity of the intestinal wall in the middle segment of transverse colon, encapsulated gas within the abdominal cavity, and surrounding exudative changes. Surgical resection of the bowel demonstrated extensive mucor infiltration causing whole-layer necrosis of the colon wall as evidenced by HE staining at 10 × magnification (g) and 40 × magnification (h).
The concerns raised by the patient's family regarding the potential risks associated with anesthesia and bleeding during the procedure have resulted in the rescheduling of the procedure to September 23rd. During the laparoscopic surgery, it was observed that the middle segment of the transverse colon exhibited a dark brown coloration and was partially obscured by the greater omentum. This region was identified as the site of intestinal perforation, resulting in subsequent partial colectomy and colostomy (Fig. 3). After undergoing surgery, the patient was transferred back to the ICU and continued to receive assisted ventilation in pressure control mode with an oxygen concentration of 50 %. Imipenem and cilastatin sodium and teicoplanin were administered for anti-infection treatment. The histopathological examination of the surgically excised colon tissue revealed full-thickness necrosis with extensive mucor infiltration, invasive vasculitis with thrombosis in the periintestinal and mesentery, and multiple ulcers with granulation tissue proliferation in the surrounding intestinal mucosa of the resected transverse colon (Fig. 1 g ∼ h). The patient was administered amphotericin B treatment based on the CT and surgical findings. The administration of escalating daily doses of amphotericin B was employed to mitigate the drug's adverse effects; nevertheless, the desired therapeutic dosage was not attained during the initial phase of treatment.
On the night of September 24, the drainage fluid from the subhepatic and pelvic areas appeared bright red. Subsequent reexamination revealed a decrease in PLT count from 32 × 109/L to 9 × 109/L, as well as a decrease in hemoglobin (HGB) from 82g/L to 78g/L. In the early morning of September 25, the patient developed wheezing (respiratory rate of 36 breaths per minute), high fever (39.1 °C), bloody sputum and gastric juice. Blood gas analysis indicated metabolic acidosis (pH 7.11, lactic acid at 5.9mmol/L). Laboratory tests showed WBC 2.49 × 109/L, PLT 4 × 109/L, procalcitonin 6.92ng/mL, and creatinine at 187μmol/L. Subsequently, the patient's blood pressure dropped to 47/33 mmHg while serum potassium levels increased to 7.1mmol/L, and lactic acid levels rose to 11mmol/L. An infusion of sodium bicarbonate was administered to correct theacidosis, and norepinephrine and epinephrine were given for elevated blood pressure.
Regrettably, the patient developed severe sepsis and septic shock, which was accompanied by an increase in APACHE II score to 52 and a SOFA score to 21. Due to the patient's deteriorating condition, the family opted for hospice care and discharged the patient on September 25th.
2. Discussion
Mucor is widely distributed in nature, subsisting on decaying vegetation and diverse organic materials. Mucormycosis has emerged as the third most common invasive mycosis after candidiasis and aspergillosis in patients undergoing hematological and allogeneic stem cell transplantation [3]. Pagano et al. found that the majority of patients diagnosed with mucormycosis succumbed to the disease within 12 weeks [4]. Predisposing risk factors for mucormycosis in patients with hematologic malignancies and/or stem cell transplantation include prolonged and severe neutropenia, monocytopenia, prolonged high-dose systemic corticosteroids, iron overload, high-risk stem cell transplantation (SCT), severe graft-versus-host disease (GVHD) and its treatment, prolonged hyperglycemia, corticosteroid associated hyperglycemia, diabetes mellitus, relapsed leukemia [5]. Gastrointestinal mucormycosis is an exceedingly rare fungal infection caused by mold in the order Mucorales. Among cases of gastrointestinal mucormycosis (12 %), the infection primarily affects the stomach, intestine, and/or colon (74 %), with liver involvement observed in 22 % of cases. The overall mortality rate associated with gastrointestinal mucormycosis remains high at 54 % [6]. Histopathological examination revealed dense infiltration of acute and chronic inflammatory cells, accompanied by epithelioid cell granulomas, foreign body giant cells, and broad aseptate fungal hyphae consistent with mucormycosis [7].
Voriconazole and echinocandins lack anti-Mucorales activity [8]. Liposomal or lipid-complex amphotericin B, in combination with surgical debridement, is recommended as the primary treatment for mucormycosis, while posaconazole is suggested as a salvage therapy for patients who are refractory to or intolerant of previous antifungal treatments [9]. According to the joint clinical guidelines of European Society for Clinical Microbiology and Infectious Diseases (ESCMID) and European Confederation of Medical Mycology (ECMM), it is recommended to discontinue glucocorticosteroid treatment in patients with mucormycosis and maintain antifungal therapy until complete resolution is demonstrated on imaging and permanent reversal of risk factors is achieved [9]. Isavuconazole has been approved for the treatment of invasive aspergillosis and mucormycosis in adults [10]. The efficacy of isavuconazole against mucormycosis was confirmed in a single-arm open-label trial and case-control analysis, demonstrating similar activity to amphotericin B. The use of isavuconazole has demonstrated efficacy and a favorable tolerability profile [11]. According to the guidelines of ECMM together with the Mycoses Study Group Education & Research Consortium (MSG ERC), first-line treatment with high-dose liposomal amphotericin B is strongly recommended, while intravenous isavuconazole and intravenous or delayed release tablet posaconazole are recommended with moderate strength. Both triazoles are strongly recommended salvage treatments. Amphotericin B deoxycholate is not recommended due to significant toxicity, but it may be considered as the only option in resource-limited settings [12].
Our patient received intermittent oral methotrexate, methylprednisolone, and leflunomide for the treatment of rheumatoid arthritis. There was no history of diabetes, hematologic disease or cancer reported by the patient. Voriconazole and caspofungin were administered for the treatment of Aspergillus infection detected by mNGS, but neither proved efficacious against mucormycosis. The histopathological diagnosis achieved through surgery for colonic perforation was crucial in obtaining evidence of mucor infection. Despite receiving treatment with amphotericin B, the patient's condition worsened due to our limited expertise in using drugs for mucormycosis and the limited availability of drug options. The use of high doses of glucocorticoid in the treatment of aplastic anemia may increase the risk of mucor infection in patients and the difficulty of treatment. In conclusion, the diagnosis of gastrointestinal mucormycosis infection poses a significant challenge and relies heavily on pathology. In the absence of surgical indications, colonoscopy may be a useful tool for early diagnosis. Although the patient's treatment achieved some results, a secondary breakthrough fungal infection led to a worsening of the condition. Risk factors for exacerbation included a long history of immunosuppressant use, high-dose glucocorticoid therapy, granulocytosis, enteral dystrophy, steroid diabetes, broad-spectrum antibiotic treatment, voriconazole use, surgical stress, delayed diagnosis of mucor, delayed use of amphotericin and inadequate dose. This deterioration presented here despite the rich work-up approach performed by the treating doctors should encourage all the relevant medical field to have a high index of suspicion of mucor mycosis when dealing with patients having low immunity. It is imperative to augment our comprehension and management of mucoromycosis, given the potential complications posed by corticosteroid therapy. Timely administration of antifungal agents and surgical interventions are pivotal in ameliorating prognosis. The objective of this case is to enhance clinicians' awareness regarding mucor infection and breakthrough fungal infection during the course of treatment. Thorough assessment of risk factors, early screening for mucor, and initiation of first-line antifungal therapy represent strategies to address this clinical challenge.
Ethics approval and consent to participate
The study was reviewed and approved by the Ethics Committees of the Aerospace Center Hospital. Written consent was obtained from the patient's guardian.
Consent for publication
Written informed consent was obtained from the patient's guardian to publish this case report and any accompanying images.
Funding source
This study was sponsored by Science Foundation of Aerospace Medical Health Technology (AMHT) under grant number 2020YK03.
Limitations of the study
We speculated that the utilization of immunosuppressants and glucocorticoids, along with the presence of agranulocytosis and impairment of mucosal barriers, may serve as primary factors contributing to the heightened incidence of disseminated fungal infections in patients. Since blood mNGS only screen for Aspergillus, we have neglected effective intervention for mucor infection. The delay in the diagnosis and surgical intervention may contribute to a suboptimal clinical prognosis. The significance of this case lies in enhancing our comprehension of mucormycosis and using more testing methods in clinical practice to promptly identify the type and focus of infection.
Data availability statement
The data associated with the study has not been deposited into a publicly available repository. Data will be made available on request.
CRediT authorship contribution statement
Zhou-ping Li: Writing – review & editing, Writing – original draft, Project administration, Formal analysis, Data curation, Conceptualization. Jing-cheng Yang: Writing – review & editing, Writing – original draft, Investigation, Formal analysis, Data curation, Conceptualization. Tao Ma: Writing – review & editing, Writing – original draft, Investigation, Formal analysis, Data curation, Conceptualization. Xiao-xu He: Writing – review & editing, Writing – original draft, Investigation, Formal analysis, Data curation, Conceptualization. Yi-fan Gong: Writing – review & editing, Writing – original draft, Investigation, Formal analysis, Data curation, Conceptualization. Jing Xue: Writing – review & editing, Writing – original draft, Investigation, Formal analysis, Data curation, Conceptualization. Xiao-yan Xue: Writing – review & editing, Writing – original draft, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank the patients and their families.
List of abbreviations
- MPSS
methylprednisolone sodium succinate
- pH4
Intravenous Immunoglobin
- G-CSF
Human Granulocyte Colony-stimulating Factor Injection
- rhTPO
Recombinant Human Thrombopoietin Injection
- ACV
Aciclovir
- MXF
Moxifloxacin Hydrochloride and Sodium Chloride Injection
- SCF
Cefoperazone Sodium and Sulbactam Sodium for Injection
- MEM
Meropenem for Injection
- IPM
Imipenem And Cilastatin Sodium For lnjection
- TGC
Tigecycline for Injection
- VA
Vancomycin Hydrochloride for Injection
- TEC
Teicoplanin for Injection
- VCZ
Voriconazole for Injection
- CAS
Caspofungin Acetate for Injection
- AmB
Amphotericin B for Injection
- CRCs
Red Blood Cells Suspension
- FFP
fresh frozen plasma
- PLTS
Platelets
- PE
plasma exchange
- CRRT
continuous renal replacement therapy
- MPPT
Methylprednisolne pluse therapy
- PPI
proton pump inhibitor
- EN
enteral nutrition
References
- 1.Didehdar M., Chegini Z., Moradabadi A., Anoushirvani A.A., Tabaeian S.P., Yousefimashouf M., et al. Gastrointestinal mucormycosis: a periodic systematic review of case reports from 2015 to 2021. Microb. Pathog. 2022;163 doi: 10.1016/j.micpath.2022.105388. https://pubmed.ncbi.nlm.nih.gov/34995749 [DOI] [PubMed] [Google Scholar]
- 2.Sahu M., Shah M., Mallela V.R., Kola V.R., Boorugu H.K., Punjani A., et al. COVID-19 associated multisystemic mucormycosis from India: a multicentric retrospective study on clinical profile, predisposing factors, cumulative mortality and factors affecting outcome. Infection. 2023;51(2):407–416. doi: 10.1007/s15010-022-01891-y. https://pubmed.ncbi.nlm.nih.gov/35922704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petrikkos G., Skiada A., Lortholary O., Roilides E., Walsh T.J., Kontoyiannis D.P. Epidemiology and clinical manifestations of mucormycosis. Clin. Infect. Dis. 2012;54(Suppl 1):S23–S34. doi: 10.1093/cid/cir866. https://pubmed.ncbi.nlm.nih.gov/22247442 [DOI] [PubMed] [Google Scholar]
- 4.Pagano L., Girmenia C., Mele L., Ricci P., Tosti M.E., Nosari A., et al. Infections caused by filamentous fungi in patients with hematologic malignancies. A report of 391 cases by GIMEMA Infection Program. Haematologica. 2001;86(8):862–870. https://pubmed.ncbi.nlm.nih.gov/11522544 [PubMed] [Google Scholar]
- 5.Kontoyiannis D.P., Lewis R.E. How I treat mucormycosis. Blood. 2011;118(5):1216–1224. doi: 10.1182/blood-2011-03-316430. https://pubmed.ncbi.nlm.nih.gov/21622653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jeong W., Keighley C., Wolfe R., Lee W.L., Slavin M.A., Kong D., et al. The epidemiology and clinical manifestations of mucormycosis: a systematic review and meta-analysis of case reports. Clin. Microbiol. Infect. 2019;25(1):26–34. doi: 10.1016/j.cmi.2018.07.011. https://pubmed.ncbi.nlm.nih.gov/30036666 [DOI] [PubMed] [Google Scholar]
- 7.Budhiraja R., Bhargava S., Sood N. Jejunal stricture due to mucormycosis. Trop. Doct. 2019;49(4):318–320. doi: 10.1177/0049475519864250. https://pubmed.ncbi.nlm.nih.gov/31324130 [DOI] [PubMed] [Google Scholar]
- 8.Boucher H.W., Groll A.H., Chiou C.C., Walsh T.J. Newer systemic antifungal agents : pharmacokinetics, safety and efficacy. Drugs. 2004;64(18):1997–2020. doi: 10.2165/00003495-200464180-00001. https://pubmed.ncbi.nlm.nih.gov/15341494 [DOI] [PubMed] [Google Scholar]
- 9.Cornely O.A., Arikan-Akdagli S., Dannaoui E., Groll A.H., Lagrou K., Chakrabarti A., et al. ESCMID and ECMM joint clinical guidelines for the diagnosis and management of mucormycosis 2013. Clin. Microbiol. Infect. 2014;20(Suppl 3):5–26. doi: 10.1111/1469-0691.12371. https://pubmed.ncbi.nlm.nih.gov/24479848 [DOI] [PubMed] [Google Scholar]
- 10.Shirley M., Scott L.J. Isavuconazole: a review in invasive aspergillosis and mucormycosis. Drugs. 2016;76(17):1647–1657. doi: 10.1007/s40265-016-0652-6. https://pubmed.ncbi.nlm.nih.gov/27766566 [DOI] [PubMed] [Google Scholar]
- 11.Marty F.M., Ostrosky-Zeichner L., Cornely O.A., Mullane K.M., Perfect J.R., Thompson G.R., 3rd, et al. Isavuconazole treatment for mucormycosis: a single-arm open-label trial and case-control analysis. Lancet Infect. Dis. 2016;16(7):828–837. doi: 10.1016/S1473-3099(16)00071-2. https://pubmed.ncbi.nlm.nih.gov/26969258 [DOI] [PubMed] [Google Scholar]
- 12.Cornely O.A., Alastruey-Izquierdo A., Arenz D., Chen S., Dannaoui E., Hochhegger B., et al. Global guideline for the diagnosis and management of mucormycosis: an initiative of the European confederation of medical Mycology in cooperation with the Mycoses study Group education and Research Consortium. Lancet Infect. Dis. 2019;19(12):e405–e421. doi: 10.1016/S1473-3099(19)30312-3. https://pubmed.ncbi.nlm.nih.gov/31699664 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data associated with the study has not been deposited into a publicly available repository. Data will be made available on request.