Abstract
Background
Acute Decompensated Heart Failure (ADHF) is associated with frequent hospitalizations, posing a significant health and economic burden globally. Despite advancements in heart failure management, studies delineating temporal trends in ADHF outcomes are sparse.
Methods: in this retrospective analysis, ADHF patients admitted to Shamir Medical Center from 2007 to 2017 were categorized into two cohorts: early (2007–2011) and recent (2012–2017). Clinical characteristics, in-hospital interventions, and outcomes were compared. Survival analysis was performed using Kaplan-Meier methods with log-rank tests.
Results
8332 admitted patients were analyzed, 4366 (52.4 %) in the early period, and 3966 (47.6 %) in the recent period. In the recent cohort, ischemic heart disease decreased significantly (from 45.2 % to 34.7 %), while hypertension and smoking rates increased. Additionally, a significant increase in coronary artery bypass grafting (from 0.8 % to 3.5 %) and beta-blockers prescription (from 45.5 % to 63.4 %) post-discharge was observed. However, no substantial improvement in in-hospital mortality (8.9 % in early vs. 8.0 % in recent), 30-day (3.2 % in early vs. 3.1 % in recent), 1-year (23.3 % in early vs. 23.8 % in recent), or 5-year survival rates was noted between cohorts. A subset analysis of patients admitted to cardiology departments showed a significant reduction in in-hospital mortality in the recent cohort (12.3 % in early vs. 6.3 % in recent), yet without a corresponding long-term survival benefit.
Conclusions
Advancements in heart failure management over the 11-year study period did not demonstrate an improvement in clinical outcomes for ADHF patients, highlighting the challenge of translating advancements in the medical care of ADHF patients into long-term survival benefits.
Keywords: Temporal trends, Acute decompensated heart failure, Long-term survival
1. Introduction
Heart Failure (HF) remains a significant global health challenge, affecting an increasing number of individuals and leading to substantial healthcare expenditures [1,2]. Acute decompensated heart failure (ADHF), a severe manifestation of HF, frequently necessitates hospital admission, and presents significant management challenges due to its associated high morbidity and mortality rates, alongside the recurrent hospitalizations it often entails [3,4].
Over the years, advancements in both chronic HF and ADHF therapies have emerged, potentially heralding improved patient outcomes [5,6]. Previous publications have explored trends in ADHF outcomes, but often within limited timeframes or without a distinction between earlier and more recent years which could reflect the evolution of therapeutic strategies [7,8]. A comparative analysis juxtaposing outcomes of patients admitted during earlier years to those admitted in more recent years, particularly across a timeframe broad enough to capture potential changes in clinical practice, appears to be less extensively explored.
To address this gap in knowledge, we conducted an eleven-year retrospective cohort analysis of ADHF patients hospitalized from 2007 to 2017, aiming to discern whether outcomes have improved over the years, particularly when comparing admissions during earlier (up to 2011) versus more recent years (from 2012 onwards).
2. Methods
This retrospective study was conducted at Shamir Medical Center (SMC), focusing on patients aged 18 or above hospitalized with ADHF from January 1, 2007, to December 31, 2017. The study included admissions to both internal medicine departments (IMD) and cardiology departments (CD). Included were patients with a primary discharge diagnosis of ADHF, identified by International Classification of Diseases, 9th Revision (ICD-9) codes 428.xx, 429.xx, and 514. In case of multiple ADHF admissions during the time frame, the dataset recorded the first as the index admission, and subsequent admission as readmission outcomes. Data on demographics, clinical characteristics, hospital interventions, and discharge medications were extracted from SMC's digital health archives, while mortality data were cross-verified with the database of Israel's Ministry of Internal Affairs. Ethical approval was obtained from SMC's institutional review board, with patient consent exemption due to the study's retrospective nature.
For the purposes of analysis, the patient population was divided into two time-based cohorts to ensure a balanced comparison, with both cohorts having similar durations and comparable numbers of patients. An 'early' cohort included admissions between January 1, 2007 and December 31, 2011, and a 'recent' cohort encompassed the period between January 1, 2012 and December 31, 2017. Descriptive statistics for categorical variables are presented as frequency counts and percentages, while continuous variables are presented as either means with standard deviations or medians with interquartile ranges, depending on the normality of data distribution. Data normality was assessed using the Shapiro-Wilk test. Differences between categorical variables were evaluated using Chi-square tests, and continuous variables were analyzed using either two-paired student t-tests or Kruskal-Wallis H tests, based on distribution.
Survival outcomes were analyzed using Kaplan-Meier methods, with log-rank tests employed to compare 5-year survival between the early and recent cohorts. A subset analysis, limited to patients admitted to cardiology departments, was conducted to validate the primary outcomes and identify any distinct departmental trends.
All statistical analyses were performed using R, version 2021, from the R Foundation for Statistical Computing, Vienna, Austria.
3. Results
The analysis included 8332 patients hospitalized for ADHF between December 31, 2006 and December 26, 2017. Among these, 4366 patients were categorized in the "early" cohort (admissions up to 2011), and 3966 were in the "recent" cohort (admissions from 2012 onwards).
The demographic and clinical characteristics of the cohorts are detailed in Table 1. The median age was similar across both cohorts at 78.0 [69.0–84.0] years in the early years' cohort and 79.0 [69.0–86.0] years in the recent years' cohort. A significant reduction in the prevalence of ischemic heart disease (IHD) was noted in the recent cohort (34.7 % vs. 45.2 %, p < 0.001), while hypertension (20.5 % vs. 13.9 %, p < 0.001) and anemia (69.3 % vs. 63.7 %, p < 0.001) were more prevalent in the recent cohort. The rates of diabetes mellitus (49.9 % vs. 49.9 %, p = 0.992) were comparable between the cohorts, as were rates of atrial fibrillation and chronic obstructive pulmonary disease (COPD). Rates of peripheral vascular disease (PVD) were lower in the recent cohort (5.3 % vs. 9 %, p < 0.001). The rate of smoking increased in the recent cohort (18.0 % vs. 14.0 %, p < 0.001). The left ventricular ejection fraction (LVEF) did not differ significantly between the groups, with preserved ejection fraction (EF ≥ 50 %) being 47.8 % in the recent cohort and 44.5 % in the early cohort (p = 0.211 for all EF groups). In-hospital interventions and medications prescribed at discharge are presented in Table 2. A notable increase in coronary artery bypass grafting (CABG) was observed in the recent cohort (3.5 % vs. 0.8 %, p < 0.001). At discharge, the recent cohort had a higher prescription rate of beta-blockers (63.4 % vs. 45.5 %, p < 0.001)
Table 1.
Baseline characteristics of early (2007–2011) and recent (2012–2017) years patients admitted with acute decompensated heart failure.
| Early (N = 4366) | Recent (N = 3966) | p-value | |
|---|---|---|---|
| Female sex - n (%) | 2132 (48.8) | 1992 (50.2) | 0.203 |
| Age, years - median [IQR] | 78.0 [69.0–84.0] | 79.0 [69.0–86.0] | <0.001 |
| Medical history - n (%) | |||
| Ischemic heart disease | 1975 (45.2) | 1375 (34.7) | <0.001 |
| Chronic kidney disease | 1614 (37) | 1128 (28.4) | <0.001 |
| Atrial fibrillation | 1387 (31.8) | 1183 (29.8) | 0.056 |
| Hypertension | 607 (13.9) | 814 (20.5) | <0.001 |
| Diabetes mellitus | 2177 (49.9) | 1978 (49.9) | 0.992 |
| Chronic obstructive pulmonary disease | 716 (16.4) | 589 (14.9) | 0.052 |
| Peripheral vascular disease | 393 (9.0) | 209 (5.3) | <0.001 |
| Anemia | 2183 (63.7) | 2709 (69.3) | <0.001 |
| Obesity | 928 (21.3) | 827 (20.9) | 0.652 |
| Smoking | 613 (14.0) | 714 (18.0) | <0.001 |
| Chronic medications - n (%) | |||
| Alpha blockers | 635 (14.5) | 258 (6.5) | <0.001 |
| Beta blockers | 2067 (47.3) | 951 (24.0) | <0.001 |
| Calcium channel blocker | 1554 (35.6) | 565 (14.2) | <0.001 |
| Angiotensin-converting enzyme inhibitors | 1132 (25.9) | 436 (11.0) | <0.001 |
| Angiotensin receptor blockers | 643 (14.7) | 282 (7.1) | <0.001 |
| Aldactone | 160 (3.7) | 45 (1.1) | <0.001 |
| Anti-arrhythmia drugs | 418 (9.6) | 148 (3.7) | <0.001 |
| Antiplatelets | 2578 (59.0) | 978 (24.7) | <0.001 |
| Oral anticoagulants | 851 (19.5) | 328 (8.3) | <0.001 |
| Statins | 2326 (53.3) | 954 (24.1) | <0.001 |
| Other anti-hyperlipidemia drugs | 116 (2.7) | 48 (1.2) | <0.001 |
| Digoxin | 293 (6.7) | 137 (3.5) | <0.001 |
| Diuretics | 2629 (60.2) | 1954 (49.3) | <0.001 |
| Lab indices - median [IQR] | |||
| White blood cells, K/μL | 9.3 [7.3–12.4] | 9.1 [7.1–12.1] | 0.005 |
| Hemoglobin, mg/dL | 11.9 [10.5–13.3] | 11.6 [10.2–12.9] | <0.001 |
| Urea, mg/dL | 52.0 [37.2–78.3] | 49.3 [36.2–73.9] | 0.001 |
| Sodium, mmol/L | 139.0 [136.0–141.0] | 138.0 [134.0–140.0] | <0.001 |
| Creatinine, mg/dL | 1.12 [0.86–1.55] | 1.09 [0.84–1.56] | 0.34 |
| eGFR, mL/min/1.73m2 | 59.1 [40.4–80.1] | 60.1 [40.6–79.8] | 0.56 |
| LV systolic function (echo) - n (%) | 0.211 | ||
| Preserved (EF ≥ 50 %) | 298 (44.5) | 911 (47.8) | |
| Mild Reduced (EF = 40–49 %) | 101 (15.1) | 283 (14.9) | |
| Moderately reduced (EF = 30–39 %) | 175 (26.2) | 495 (26.0) | |
| Severely reduced (EF<30 %) | 95 (14.2) | 216 (11.3) | |
| Admission to cardiology - n (%) | 397 (9.1) | 478 (12.1) | <0.001 |
IQR – interquartile range; eGFR – estimated glomerular filtration rate.
Table 2.
Index admission interventions and discharge medications of early (2007–2011) and recent (2012–2017) years patients admitted with acute decompensated heart failure.
| Early (N = 4366) | Recent (N = 3966) | p-value | |
|---|---|---|---|
| Index admission interventions | |||
| Percutaneous coronary intervention | 369 (8.5) | 390 (9.8) | 0.029 |
| Coronary angiography alone | 180 (4.1) | 240 (6.1) | <0.001 |
| Pacemaker implantation | 51 (1.2) | 51 (1.3) | 0.625 |
| Cardiac resynchronization therapy | 12 (0.3) | 9 (0.2) | 0.663 |
| Coronary artery bypass grafting | 37 (0.8) | 140 (3.5) | <0.001 |
| Cardiac nuclear mapping | 0 (0.0) | 1 (0.0) | 0.294 |
| Discharge medications | |||
| Alpha blockers | 616 (14.1) | 581 (14.6) | 0.482 |
| Beta blockers | 1987 (45.5) | 2513 (63.4) | <0.001 |
| Angiotensin-converting enzyme inhibitors | 1219 (27.9) | 1114 (28.1) | 0.864 |
| Angiotensin receptor blockers | 612 (14.0) | 570 (14.4) | 0.643 |
| Aldactone | 102 (2.3) | 118 (3.0) | 0.069 |
| Calcium channel blockers | 1462 (33.5) | 1185 (29.9) | <0.001 |
| Diuretics | 3220 (73.8) | 2940 (74.1) | 0.694 |
| Digoxin | 234 (5.4) | 137 (3.5) | <0.001 |
| Statins | 2463 (56.4) | 2137 (53.9) | 0.02 |
| Other anti-hyperlipidemia drugs | 99 (2.3) | 93 (2.3) | 0.814 |
| Anti-arrhythmia drugs | 416 (9.5) | 358 (9.0) | 0.431 |
| Antiplatelets | 2781 (63.7) | 2097 (52.9) | <0.001 |
| Oral anticoagulants | 1010 (23.1) | 1144 (28.8) | <0.001 |
| Combination pills | 104 (2.4) | 137 (3.5) | 0.004 |
Short and intermediate-term outcomes are presented in Table 3. The median admission length was slightly longer in the recent cohort (6 [3–9] vs. 5 [3–8] days, p < 0.001). However, the in-hospital mortality, 30-day, and 1-year mortality rates did not differ significantly between the cohorts. A 5-year survival analysis, as depicted by Kaplan-Meier survival analysis (Fig. 1), remained consistent across both cohorts (log-rank p = 0.82).
Table 3.
Short and intermediate-term outcomes of early (2007–2011) and recent (2012–2017) years patients admitted with acute decompensated heart failure.
| a. All patients | |||
|---|---|---|---|
| Early (N = 4366) | Recent (N = 3966) | p value | |
| Admission length, days - median [IQR] | 5 [3–8] | 6 [3–9] | <0.001 |
| In hospital mortality - n (%) | 390 (8.9) | 317 (8.0) | 0.124 |
| Readmission within 30 days - n (%) | 854 (21.5) | 745 (20.4) | 0.285 |
| Mortality within 30 days - n (%) | 129 (3.2) | 113 (3.1) | 0.713 |
| Mortality within 1 year - n (%) | 927 (23.3) | 868 (23.8) | 0.627 |
| b. Only patients admitted to cardiology | |||
|---|---|---|---|
| Early (N = 397) | Recent (N = 478) | p value | |
| Admission length, days - median [IQR] | 5 [3–7] | 5 [3–8] | 0.255 |
| In hospital mortality - n (%) | 49 (12.3) | 30 (6.3) | 0.002 |
| Readmission within 30 days - n (%) | 62 (17.8) | 77 (17.2) | 0.817 |
| Mortality within 30 days - n (%) | 6 (1.7) | 11 (2.5) | 0.479 |
| Mortality within 1 year - n (%) | 36 (10.3) | 60 (13.4) | 0.19 |
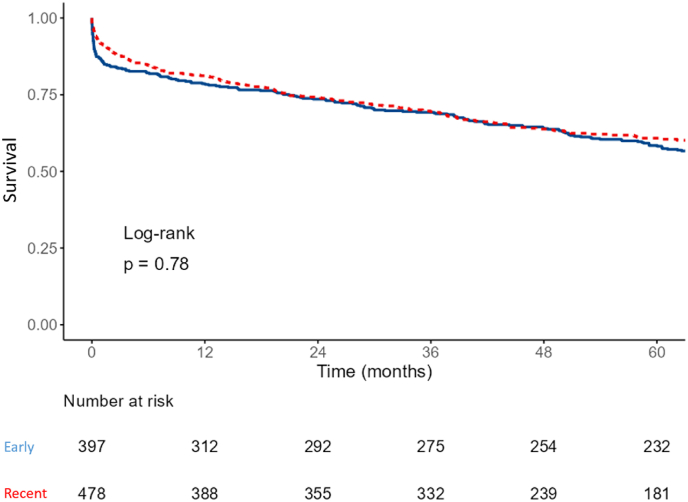
Fig. 1.
Kaplan Meier 5-year survival estimate for early (2007–2011) and recent (2012–2017) years patients admitted with acute decompensated heart failure.
A subset analysis was performed on patients admitted to cardiology wards, as detailed in Table 3b and Fig. 2. In this subset, a significant reduction in in-hospital mortality was observed in the recent cohort (6.3 % vs. 12.3 %, p = 0.002). However, the Kaplan-Meier survival analysis for this subset over a 5-year period did not exhibit a significant difference (log-rank p = 0.78), indicating that the initial improvement in in-hospital mortality did not translate to a long-term survival benefit.
Fig. 2.
Kaplan Meier 5- year survival estimate for early (2007–2011) and recent (2012–2017) years patients admitted to cardiology with acute decompensated heart failure.
4. Discussion
In our 11-year retrospective study at Shamir Medical Center, encompassing 8332 patients hospitalized for ADHF, we discerned no significant improvement in short or long-term outcomes when comparing early years (2007–2011) to recent years (2012–2017).
Our findings differ from those of Chang PP et al., who documented a temporal trend of improved 1-year survival rates among black women and men hospitalized with ADHF [8]. Several suggestions may explain these differences: First, Chang's study was conducted in the United States (US) while ours took place in Israel. The differences in healthcare systems between the two countries may account for differences in outcomes for HF patients in the US compared with other high-income countries, as demonstrated by Sundaram et al. c [9]. Second, Chang's study's temporal trend improvement was documented in black women and men, while the population in Israel is largely non-black. Paradoxically, as there is evidence that HF prevalence and outcomes are worse in black people [10], they may have a higher potential to show improvement with better treatments over time [11].
An additional factor potentially explaining the divergence from the findings of Chang PP et al. could be our methodology of sampling the initial ADHF admission within the study's timeframe, which may not necessarily represent the patient's first-ever ADHF admission. It's plausible that many patients in the early cohort had already established HF prior to the index admission of our study, whereas in the recent cohort, a greater number of patients might have been experiencing their initial HF presentation. This hypothesis is corroborated by the decreased prevalence of a past medical history of IHD, coupled with an increase in coronary interventions in the recent cohort, hinting at a larger proportion of de novo IHD, i.e., acute coronary syndrome (ACS) events precipitating the ADHF in these patients. Given the known worse prognosis of ADHF due to ACS [12,13], any advancements in ADHF treatment across the cohorts could have been counterbalanced, resulting in an apparent stagnation of outcomes over the years in our dataset.
The complex interplay between comorbidities and the temporal trend observed in our study may also contribute to the apparent stagnation of all-cause mortality outcomes. The recent group exhibited lower rates of CKD and PVD, which would be expected to lead to better outcomes. However, it also had higher rates of hypertension and anemia, and as hinted above, potentially more ACS event. Most of these comorbidities are known negative prognostic factors [[14], [15], [16]] with hypertension standing out as having a by itself complex relation with HF outcomes through different pathways leading to HFrEF or HFpEF [17], and through its impact on CKD occurrence and progression [18]. Overall, the distribution of these comorbidities between the time groups likely offsets the potential benefits of reduced CKD and PVD rates.
Interestingly, our subset analysis of patients admitted to cardiology wards revealed a decline in in-hospital mortality rates in the recent cohort. This suggests that specialized cardiology care may have evolved over time, aligning with the broader transformation within cardiac intensive care units (CICUs) from primarily caring for patients with arrhythmic and other complications of acute coronary syndrome to delivering comprehensive critical care for patients with various cardiovascular diseases [19]. However, it should be acknowledged that this subset cohort has been previously demonstrated to significantly differ from the general ADHF cohort both in characteristics as well as in outcomes [20], rendering extrapolations from it limited. Despite these differences, this subset echoed the prevailing trend of stagnant outcomes, thereby reinforcing our general findings.
Further analysis of our data illustrated an increase in the prescription of beta-blockers (BB) in the recent cohort. While ACEI prescription rates at discharge were similar between early and recent cohorts, a detailed examination reveals that the recent cohort had a lower rate of ACEI prescription upon admission (11 %), which significantly increased by discharge to 28.1 %, aligning with the rates in the early cohort (admission: 25.9 %, discharge: 27.9 %). Conversely, BB rates remained stable in the early cohort (admission: 45.5 %, discharge: 47.3 %) but showed a dramatic increase in the recent cohort from 24 % at admission to 63.4 % at discharge, surpassing early years' rates. The lower admission rates of both ACEI and BB in the recent cohort likely reflect a higher proportion of de novo HF patients compared to the early cohort, which had more established HF patients. The significant rise in BB from admission to discharge in recent years likely indicates broader adoption of Guideline-Directed Medical Therapy (GDMT), with ACEI already being relatively established earlier, leading to consistent discharge rates over time, while BB saw a GDMT-driven uptake. Despite improved GDMT adherence, patient outcomes did not correspondingly improve, potentially due to higher prevalence of comorbidities in real-world settings that may temper the effect of GDMT seen in trials [21,22], challenges in achieving optimal GDMT doses [18], and lack of follow-up data on post-discharge adherence, which is crucial for patient outcomes [23,24]. A recent review of Asian HF registries by Balagopalan et al. supports this notion, reporting that while GDMT is often initiated in hospitals across many Asian countries, adherence remains low [25].
Several limitations of our study should be acknowledged. First, the retrospective nature of inclusion relies on a reported diagnosis of ADHF rather than on diagnostic criteria. This limitation is lessened by sampling the primary discharge diagnosis over the admission one, as the former incorporates the clinical course, diagnostic tests, and response to therapy observed during admission. Second, data extracted from hospital records might be prone to entry errors, although we mitigated this by cross-verifying mortality data with external databases. Third, being a single-center study could limit the generalizability of our findings, although the large sample size lends robustness to the observed trends. Fourth, the lack of a multivariable analysis may lower the robustness of the conclusions. However, we elected not to conduct a multivariable analysis as the main outcomes did not differ between the study groups, and although such an analysis would likely demonstrate other independent predictors of outcome, such as age or comorbidities, these factors were already shown for the same cohort in two recent publications [26,27]. Fifth, our study lacked a distinction between HFrEF and HFpEF due to the lack of sufficient echocardiography data and NT-proBNP levels. Nonetheless, there were no differences in the rates of different EF groups, suggesting this distinction would not significantly affect our findings. Lastly, as previously mentioned, our study lacks follow-up data on adherence to therapy. Despite these limitations, our study lays the groundwork for further research, shedding light on the evolving trends in management and outcomes of ADHF patients over a span of 11 years.
In conclusion, despite advancements in HF management, our study did not demonstrate a corresponding improvement in patient outcomes over the study period when comparing early to recent years. The observed disparity in findings with earlier studies accentuates the complex nature of ADHF outcomes and the need for continued research to better understand the influencing factors over time.
Funding
none.
CRediT authorship contribution statement
Gil Marcus: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Mohammad Najjar: Writing – original draft, Methodology, Investigation, Data curation. Antionette Monayer: Writing – review & editing, Investigation, Data curation. Ady Orbach: Visualization, Validation, Supervision, Investigation. Shiri L. Maymon: Visualization, Validation, Data curation. Eran Kalmanovich: Visualization, Validation, Supervision, Investigation. Gil Moravsky: Visualization, Validation, Supervision, Investigation. Avishay Grupper: Visualization, Validation, Supervision, Investigation. Shmuel Fuchs: Visualization, Validation, Supervision, Investigation. Sa'ar Minha: Writing – review & editing, Visualization, Validation, Supervision, Software, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization.
Declaration of competing interest
none.
Handling editor: D Levy
References
- 1.Savarese G., Becher P.M., Lund L.H., Seferovic P., Rosano G.M.C., Coats A.J.S. Global burden of heart failure: a comprehensive and updated review of epidemiology. Cardiovasc. Res. 2023;118(17):3272–3287. doi: 10.1093/cvr/cvac013. [DOI] [PubMed] [Google Scholar]
- 2.Wang S.Y., Valero-Elizondo J., Ali H.-J., et al. Out-of-Pocket annual health expenditures and financial toxicity from healthcare costs in patients with heart failure in the United States. J. Am. Heart Assoc. 2021;10(14) doi: 10.1161/JAHA.121.022164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Teerlink J.R., Alburikan K., Metra M., Rodgers J.E. Acute decompensated heart failure update. Curr. Cardiol. Rev. 2015;11(1):53–62. doi: 10.2174/1573403X09666131117174414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raj L., Maidman S.D., Adhyaru B.B. Inpatient management of acute decompensated heart failure. Postgrad Med J. 2020;96(1131):33–42. doi: 10.1136/postgradmedj-2019-136742. [DOI] [PubMed] [Google Scholar]
- 5.Onwuanyi A., Taylor M. Acute decompensated heart failure: pathophysiology and treatment. Am. J. Cardiol. 2007;99(6B):25D–30D. doi: 10.1016/j.amjcard.2006.12.017. [DOI] [PubMed] [Google Scholar]
- 6.Kalmanovich E., Audurier Y., Akodad M., et al. Management of advanced heart failure: a review. Expert Rev. Cardiovasc Ther. 2018;16(11):775–794. doi: 10.1080/14779072.2018.1530112. [DOI] [PubMed] [Google Scholar]
- 7.Fonarow G.C., Heywood J.T., Heidenreich P.A., Lopatin M., Yancy C.W., ADHERE Scientific Advisory Committee and Investigators Temporal trends in clinical characteristics, treatments, and outcomes for heart failure hospitalizations, 2002 to 2004: findings from Acute Decompensated Heart Failure National Registry (ADHERE) Am. Heart J. 2007;153(6):1021–1028. doi: 10.1016/j.ahj.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 8.Chang P.P., Wruck L.M., Shahar E., et al. Trends in hospitalizations and survival of acute decompensated heart failure in four US communities (2005-2014): ARIC study community surveillance. Circulation. 2018;138(1):12–24. doi: 10.1161/CIRCULATIONAHA.117.027551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sundaram V., Nagai T., Chiang C.-E., et al. Hospitalization for heart failure in the United States, UK, taiwan, and Japan: an international comparison of administrative health records on 413,385 individual patients. J. Card. Fail. 2022;28(3):353–366. doi: 10.1016/j.cardfail.2021.08.024. [DOI] [PubMed] [Google Scholar]
- 10.Nayak A., Hicks A.J., Morris A.A. Understanding the complexity of heart failure risk and treatment in black patients. Circ Heart Fail. 2020;13(8) doi: 10.1161/CIRCHEARTFAILURE.120.007264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Vries T.I., Stam-Slob M.C., Peters R.J.G., van der Graaf Y., Westerink J., Visseren F.L.J. Impact of a patient's baseline risk on the relative benefit and harm of a preventive treatment strategy: applying trial results in clinical decision making. J. Am. Heart Assoc. 2022;11(1) doi: 10.1161/JAHA.120.017605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steg P.G., Dabbous O.H., Feldman L.J., et al. Determinants and prognostic impact of heart failure complicating acute coronary syndromes: observations from the Global Registry of Acute Coronary Events (GRACE) Circulation. 2004;109(4):494–499. doi: 10.1161/01.CIR.0000109691.16944.DA. [DOI] [PubMed] [Google Scholar]
- 13.McManus D.D., Chinali M., Saczynski J.S., et al. 30-year trends in heart failure in patients hospitalized with acute myocardial infarction. Am. J. Cardiol. 2011;107(3):353–359. doi: 10.1016/j.amjcard.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Campbell R.C., Sui X., Filippatos G., et al. Association of chronic kidney disease with outcomes in chronic heart failure: a propensity-matched study. Nephrol Dial Transplant Off Publ Eur Dial Transpl Assoc - Eur Ren Assoc. 2009;24(1):186–193. doi: 10.1093/ndt/gfn445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones W.S., Clare R., Ellis S.J., et al. Effect of peripheral arterial disease on functional and clinical outcomes in patients with heart failure (from HF-ACTION) Am. J. Cardiol. 2011;108(3):380–384. doi: 10.1016/j.amjcard.2011.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Magrì D., De Martino F., Moscucci F., Agostoni P., Sciomer S. Anemia and iron deficiency in heart failure: clinical and prognostic role. Heart Fail. Clin. 2019;15(3):359–369. doi: 10.1016/j.hfc.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 17.Messerli F.H., Rimoldi S.F., Bangalore S. The transition from hypertension to heart failure: contemporary update. JACC Heart Fail. 2017;5(8):543–551. doi: 10.1016/j.jchf.2017.04.012. [DOI] [PubMed] [Google Scholar]
- 18.Diaz J., Calderon J.M., Martínez F., et al. Impact of previous diagnosis of hypertension on renal function in heart failure patients. J. Hypertens. 2023;41(Suppl 3) [Google Scholar]
- 19.Morrow D.A. Trends in cardiac critical care: reshaping the cardiac intensive care unit. Circ Cardiovasc Qual Outcomes. 2017;10(8) doi: 10.1161/CIRCOUTCOMES.117.004010. [DOI] [PubMed] [Google Scholar]
- 20.Maymon SL, Moravsky G, Marcus G. Disparities in the characteristics and outcomes of patients hospitalized with acute decompensated heart failure admitted to internal medicine and cardiology departments: a single‐centre, retrospective cohort study. ESC Heart Fail. 2021;8:390–398. doi: 10.1002/ehf2.13084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seo W.-W., Park J.J., Park H.A., et al. Guideline-directed medical therapy in elderly patients with heart failure with reduced ejection fraction: a cohort study. BMJ Open. 2020;10(2) doi: 10.1136/bmjopen-2019-030514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tan Y.Y., Papez V., Chang W.H., Mueller S.H., Denaxas S., Lai A.G. Comparing clinical trial population representativeness to real-world populations: an external validity analysis encompassing 43 895 trials and 5 685 738 individuals across 989 unique drugs and 286 conditions in England. Lancet Healthy Longev. 2022;3(10):e674–e689. doi: 10.1016/S2666-7568(22)00186-6. [DOI] [PubMed] [Google Scholar]
- 23.McCullough P.A., Mehta H.S., Barker C.M., et al. Mortality and guideline‐directed medical therapy in real‐world heart failure patients with reduced ejection fraction. Clin. Cardiol. 2021;44(9):1192–1198. doi: 10.1002/clc.23664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee S.-H., Hyun D., Choi J., et al. Adherence to guideline-directed medical therapy and 3-year clinical outcome following acute myocardial infarction. Eur Heart J Open. 2023;3(2) doi: 10.1093/ehjopen/oead029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Balagopalan J.P., Abdullakutty J. Heart failure registries in asia – what have we learned? Cardiovasc Innov Appl. 2024;9:953. [Google Scholar]
- 26.Marcus G., Kofman N., Maymon S.L., et al. Marital status impact on the outcomes of patients admitted for acute decompensation of heart failure: a retrospective, single-center, analysis. Clin. Cardiol. 2023;46(8):914–921. doi: 10.1002/clc.24053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Monayer A., Minha S., Maymon S.L., et al. Statin therapy impact on Long-Term outcomes in acute heart Failure: retrospective analysis of hospitalized patients. IJC Heart Vasc. 2024;53 doi: 10.1016/j.ijcha.2024.101431. [DOI] [PMC free article] [PubMed] [Google Scholar]