Summary
Background
Ethno-racial inequalities are critical determinants of health outcomes. We quantified ethnic-racial inequalities on adverse birth outcomes and early neonatal mortality in Brazil.
Methods
We conducted a cohort study in Brazil using administrative linked data between 2012 and 2019. Estimated the attributable fractions for the entire population (PAF) and specific groups (AF), as the proportion of each adverse outcome that would have been avoided if all women had the same baseline conditions as White women, both unadjusted and adjusted for socioeconomics and maternal risk factors. AF was also calculated by comparing women from each maternal race/skin colour group in different groups of mothers’ schooling, with White women with 8 or more years of education as the reference group and by year.
Findings
21,261,936 newborns were studied. If all women experienced the same rate as White women, 1.7% of preterm births, 7.2% of low birth weight (LBW), 10.8% of small for gestational age (SGA) and 11.8% of early neonatal deaths would have been prevented. Percentages preventable were higher among Indigenous (22.2% of preterm births, 17.9% of LBW, 20.5% of SGA and 19.6% of early neonatal deaths) and Black women (6% of preterm births, 21.4% of LBW, 22.8% of SGA births and 20.1% of early neonatal deaths). AF was higher in groups with fewer years of education among Indigenous, Black and Parda for all outcomes. AF increased over time, especially among Indigenous populations.
Interpretation
A considerable portion of adverse birth outcomes and neonatal deaths could be avoided if ethnic-racial inequalities were non-existent in Brazil. Acting on the causes of these inequalities must be central in maternal and child health policies.
Funding
Bill & Melinda Gates Foundation and Wellcome Trust.
Keywords: Low birth weight, Prematurity, Small for gestational age, Newborn, Health inequalities
Research in context.
Evidence before this study
We searched PubMed from January 1, 2000 to January 1, 2023 for Brazilian studies using the following search terms ((“ethnicity” OR “ethno-racial disparit∗” OR “ethno-racial inequalit∗”) AND (“early neonatal deaths” OR “prematur∗” OR “small for gestational age” OR “low birth weight” OR “SGA” OR “LBW” OR “birth outcomes”)) AND (Brasil OR Brazil). As a result, we identified eight articles that investigated ethnic-racial inequalities on adverse birth outcomes. All the identified studies consistently observed a higher risk for all outcomes among Black and Parda compared to their White counterparts. Only one study included Indigenous ancestry, where it evaluated the occurrence of low birth weight and prematurity in comparison to groups with European ancestry. Our search yielded no studies addressing ethnic-racial inequalities in early neonatal deaths. None used data collected after 2015 nor had nationwide data.
Added value of this study
This study represents a comprehensive analysis of over 21 million births in Brazil. Even after adjusting for mother schooling and maternal factors, we observed racial inequalities in birth outcomes. Notably, among Indigenous women, more than 20% of preterm births and 17.9% of low birth weight (LBW), and over 20.5% of small for gestational age (SGA) and early neonatal deaths, could have been avoided if they had similar baseline risk as their White counterparts. Similarly, among Black women, more than 6% of preterm births, and over 20% of SGA, LBW and early neonatal deaths could have been prevented if they had similar baseline risk as White women. Ethnic-racial inequalities were worse among Indigenous, Black, and Parda women with lower levels of education across all outcomes, and they worsened over the years studied, especially among Indigenous women.
The findings of this study evidence that racial inequalities contribute to significant percentages of adverse birth outcomes.
Implications of all the available evidence
The substantial avoidable ethnic-racial inequalities observed in these four outcomes (prematurity, LBW, SGA and early neonatal mortality) show that actions to reduce race-based inequalities are necessary to improve maternal and child health. Rectifying ethnic-racial inequalities in maternal and child health outcomes demands proactive measures aimed at addressing structural determinants, confronting institutional racism, and implementing effective policies. Sustained efforts toward comprehensive impact evaluations will be instrumental in fostering a healthcare system that upholds principles of equity and social justice for all vulnerable populations.
Introduction
Racial inequalities are a persistent barrier to maternal and child health in Brazil. With adverse outcomes disproportionately affecting Black and Indigenous women and children.1,2 The legacy of slavery and colonialism has left deep-rooted consequences for Black and Indigenous populations in Brazil by defining life conditions, civil rights and access to services. Here, we understand Racism from a systemic perspective, as it encapsulates all its manifestations and processes that create and sustain racial inequalities.3 There is vast documentation of the racialized disparities in socioeconomic conditions,4 healthcare access5,6 and health outcomes in the Brazilian population7 and, even under policies such as the National Policy of Integral Health for Black Population and the National Policy of Attention to the Health of Indigenous Populations, these inequalities seem to persist. This enduring legacy amplifies the susceptibility of systematically discriminated populations to health problems from birth, such as preterm births, low birth weight (LBW) and small-for-gestational-age (SGA), and throughout their life course.8, 9, 10
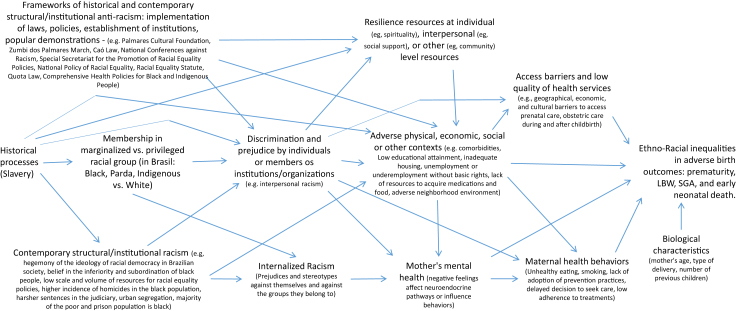
To originate these inequalities, racism operates through various mechanisms (Fig. 1).11 Being part of marginalized racial groups can result in greater exposure to interpersonal discrimination by individuals and/or institutions. In turn, these individuals are subjected to adverse physical effects, and unfavourable economic and social contexts, at an individual or collective level, which can affect access to healthcare services and their quality, culminating in inequalities in adverse birth outcomes.5, 6, 7 Additionally, being part of marginalized racial groups can result in the experience of internalized racism, which operates at the individual level and can specifically affect maternal mental health, health-related behaviours, and ultimately, birth-related health outcomes.12,13 On the other hand, anti-racist policies and resilience resources can be important for maintaining or increasing equity and, consequently, reducing racial inequalities in health outcomes.14
Fig. 1.
Causal mechanisms of racial inequalities in adverse birth outcomes. Adapted from Howe CJ, Bailey ZD, Raifman JR, Jackson JW. Recommendations for Using Causal Diagrams to Study Racial Health Disparities.
Ethnic-racial inequalities in health have been documented in Brazil and worldwide, albeit insufficiently to underscore the multifaceted nature of the issue.2, 3, 4, 5, 6, 7 Investigating racial inequality in health is crucial for developing interventions that promote equity. And understanding how it affects birth outcomes can inform strategies to improve quality of life throughout people's lives.
Preterm and SGA newborns have a higher mortality risk than full-term newborns with adequate size for gestational age.15, 16, 17, 18 According to estimates, one in four (35.3 million) newborns in 2020 were born preterm or small for their gestational age, and over half (55.3%) of the 2.4 million neonatal deaths worldwide were attributed to these conditions. Those who survive are at greater risk of subsequent morbidities, growth and development impairment, and a spectrum of long-term consequences.19, 20, 21 According to previous studies, these adverse birth outcomes are not equally distributed in the population, with a higher prevalence of these conditions being associated with different social markers of vulnerabilities. Notably, ethnic-racial attributes serve as a marker, with a higher burden faced by Black and Parda individuals.22, 23, 24 Consequently, offspring of Black and Parda women are disproportionately affected by the long-life health problems of being born too early or too small than their counterparts, newborns of White women. However, to date, no comprehensive measures communicate the magnitude of these inequalities in pregnancy outcomes, especially regarding Indigenous peoples.
To reduce neonatal mortality and ensure that children have their right to a healthy development protected, ethnic-racial inequalities in health must be better understood, addressed and placed at the centre of public policies. Given the importance of this issue, the Sustainable Development Goals (SDGs) include reducing preventable newborn deaths and ending racial inequalities in health among their targets.25 In this study, we aimed to quantify the magnitude of ethnic-racial inequalities in adverse birth and neonatal outcomes in a cohort of more than 23 million Brazilian newborns.
Methods
Study design, data source, population and linkage process
This nationwide study used linked data from two Brazilian administrative databases: the Information System on Live Births (SINASC) and the Mortality Information System (SIM) from 2012 to 2019.
SINASC records the Declarations of Live Births, a legal document filled out by the health professional who assisted the delivery and registers over 90% of all live births in Brazil.26 The Declaration of Live Births includes maternal information (maternal age, education, marital status and maternal skin colour); pregnancy information (number of prenatal appointments, length of pregnancy and number of fetuses); and information about the newborn, such as birth weight and sex.27
The SIM records the Death Certificate; in 2011, the SIM recorded 97% of all deaths in Brazil.27, 28, 29, 30 It includes information on the deceased such as the name, date of birth, age, education, cause and date of death. There is also information on the deceased mother including maternal name, date of birth and city of residence.
We linked records between SINASC and SIM to obtain information on early neonatal deaths. The linkage process was based on a set of attributes: mother's name and age, date of birth, sex, and place of residence (municipality). It was not possible to use the Live Birth Certificate number as a linking attribute, as it has a very low completion rate.30,31 The relationship was performed by similarity matching, using the algorithm Center for Data and Knowledge Integration for Health-Record Linkage (CIDACS-RL),31 an open-source linkage algorithm from CIDACS that generates a similarity score based on multiple identifiers. The process was conducted in the secure environment of the CIDACS data centre, in strict data protection structure following ethical and legal standards.32 CIDACS-RL applies a combination of indexing and searching algorithms. Indexing searches for the most similar records in the indexed SIM for each SINASC record and submits them to a paired comparison step. Candidate linkage records are ranked according to their scores, and the highest score comparison pair is retained. For this dataset, a sample of 2000 pairs, stratified into three categories of linkage scores (high score, greater than 0.95; intermediate score, values between 0.90 and 0.95; and low score, less than 0.90) was manually reviewed to assess link quality. In this validation process, the overall sensitivity and specificity were greater than 90% in all years, with little variation (for example, 94.0% in 2015; 98.1% in 2017 and 90.4% in 2019) (more information in Appendix p1, Supplementary Material).
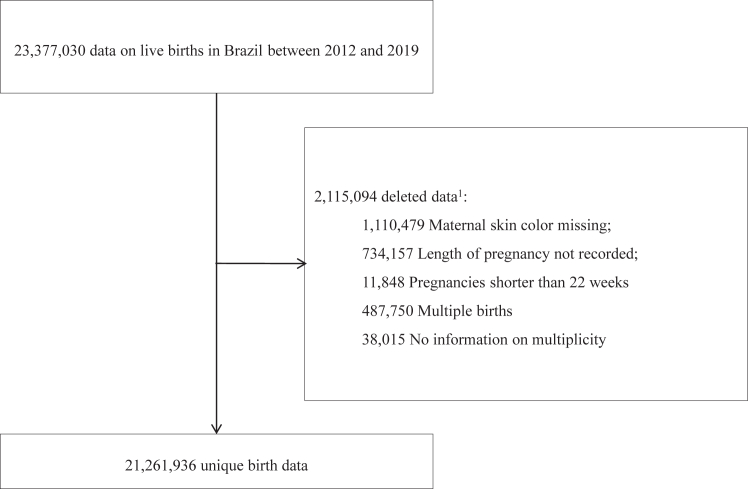
All singleton live births with gestational age between 22 and 42 completed weeks and with complete data on maternal race/skin colour were included in the study (Fig. 2). Pregnancies shorter than 22 weeks, records with no information on the duration of pregnancy and on the number of fetuses were excluded. Multiple births were excluded, as these conditions are strongly associated with adverse birth weight and neonatal mortality.33,34
Fig. 2.
Study design. 1The reasons for exclusion do not sum to 2,115,094 because a record may be missing multiple variables.
Exposures definitions
Maternal race/skin colour, registered at SINASC, is our main exposure. Race/skin colour is the terminology adopted in official Brazilian records and since 2011, the mother who registers the birth of a child has the option of classifying herself as Indigenous, Black, Parda, Yellow (Asian descent) or White.35 We initiated the study from 2012 because that was when the registration of maternal skin colour became systematic, and the final year of our study was 2019, as the next year for which we have data available is 2020, which coincided with the onset of the COVID-19 pandemic. To avoid potential biases stemming from the pandemic's impact, we chose not to include that year. This analysis used White as a reference category, as the children of these women had better health outcomes.31,32 The category “Parda” is defined as the mixture of White with Indigenous; White with Black; Black with Indigenous; or Black with a person of another colour or race.36 This group is part of the Afro-descendant population in Brazil, just like the “Black” group, and, therefore, they also experience the consequences of structural racism. The Asian descendent category was included by the Brazilian Institute of Geography and Statistics (IBGE) at the beginning of the 20th century prompted by the notable influx of Asian immigrants during that period. However, due to the high inconsistency of the self-classification of this category in some areas,37 we chose to present the results for Asian descent in the Supplementary Material (graphs and Tables S1–S3).
We used maternal race/skin colour as a proxy of Racism and its manifestations, understanding that the significance between race/skin colour and the outcomes is due to social differences, since race does not represent biological or cultural differences between groups, but rather a socially constructed category.38,39 Our use of the race/colour variable as a proxy to exposure to racism is justified by the theoretical proposition that, through systemic racism, the inequitable distribution of resources, access to services and health is stratified based on race as a social construct, operationalized in many societies, including Brazil, by phenotypic aspects such as skin colour.
Outcomes definitions
The outcomes investigated were premature birth, term LBW, SGA and early neonatal death. Premature birth was defined as the birth with less than 37 weeks pregnant, those born with less than 22 completed weeks were excluded because it was considered a criterion for foetal viability.40,41 LBW was defined as term live births (≥37 and <42 weeks of gestation) weighing less than 2,500 g. SGA was defined as births to infants at least 24 completed weeks of gestation with birth weight <10th percentile for birth weight for gestational age in completed weeks at birth, by sex, using international birth size standards newborns INTERGROWTH-21st.42,43 Early neonatal deaths were those that occurred from birth to 6 days of life.44
Covariates
Our analysis incorporated maternal education as a stratification variable. Maternal education was classified into four distinct categories: 8 or more years of schooling, 4–7 years, 1–3 years, and no years of schooling. To investigate the intersectionality between maternal race/skin colour and socio-economic conditions, we used white women with higher levels of education (8 or more years of schooling) as the reference group, comparing them with each segment of maternal race/skin colour distributed across different levels of education.
This variable was also utilized as an adjustment factor in the analyses of FAP (Factors Associated with Prevalence) and AF (Factor Analyses), along with maternal variables such as age, parity, and type of delivery. These variables represent biological aspects closely related to childbirth and can act as important determinants.
Statistical analysis
We present descriptive statistics by maternal race/skin colour group. The group of White women was used as a reference category (non-exposed group).
The Population Attributable Fraction (PAF; PAF = [p1 (RR–1)]/RR, where p1 is the prevalence of exposure among cases) was calculated for each outcome using White women as the maternal race/skin colour group of reference, comparing the proportion of outcomes that would occur for the entire population if the rates were the same as in the White group.45 The fraction per population subgroup (AF; AF= (RR-1)/RR) was also calculated, where the results of each maternal race/skin colour group were compared to the White group, obtaining the excess percentage of occurrence of each outcome.
Unadjusted PAF and AF were calculated using the punafcc package in Stata, which uses logistic regression and provides PAF and 95% Confidence Interval (CI). Adjusted PAF and AF included the following confounding factors in the analysis in two steps: first, socioeconomic variable (maternal education) and, finally, in a second model, mother factors variables (age of mother, parity and type of delivery).46
To understand the intersection between race and socioeconomic condition, we estimated the AF of each outcome considering White women with 8 or more years of education as the reference group. This analysis was performed adjusted for mother factors variables (age of mother, parity and type of delivery).
Data analysis was performed using Stata version 15.0. This study is reported as per the REporting of studies Conducted using Observational Routinely collected health Data (RECORD) guidelines (Supplementary Material, Table S2).
Ethics approval
Ethics approval was obtained for this study from the Ethics Committee of the Instituto de Saúde Coletiva at the Federal University of Bahia (registration no. CAAE, 180223194.00005.030).
Role of the funding source
The study's funders had no role in the study design, data collection, data analysis, data interpretation, report writing, or publication submission.
Results
A total of 23,377,030 live births were registered in Brazil between 2012 and 2019. Of those 1,110,479 (4.7%) records did not report maternal race/skin colour, 38,015 (0.2%) were multiple or didn't have information on number of foetus. Gestational age at birth was not reported in 734,157 (3.1%) records, and 11,848 (0.1%) live births had less than 22 completed weeks. After exclusions, 21,261,936 eligible live births were included in the analysis (Fig. 2), of which 2,225,259 (10.5%) were preterm, 1,557,806 (7.3%) were term low birth weight, 1,682,916 (7.9%) were small for gestational age and 103,330 (0.5%) died less than 7 days after birth (Table 1).
Table 1.
Maternal characteristics according to maternal race/skin colour (N = 21,261,936).
| Maternal race/skin colour group |
|||||
|---|---|---|---|---|---|
| Indigenous n (%) | Black n (%) | Parda n (%) | White n (%) | Totala n (%) | |
| Children, by maternal skin color | 173,810 (0.8) | 1,205,157 (5.7) | 11,906,994 (56.0) | 7,887,304 (37.1) | 21,261,936 (100) |
| Maternal characteristic at delivery | |||||
| Schooling, years | |||||
| 0 | 19,938 (11.5) | 7683 (0.6) | 71,510 (0.6) | 10,689 (0.1) | 110,147 (0.5) |
| 1–3 | 19,303 (11.1) | 42,350 (3.5) | 402,274 (3.4) | 94,276 (1.2) | 559,718 (2.6) |
| 4–7 | 52,925 (30.5) | 261,045 (21.7) | 2,612,265 (21.9) | 887,659 (11.3) | 3,824,663 (18.0) |
| ≥8 | 74,643 (43.0) | 881,423 (73.1) | 8,652,536 (72.7) | 6,838,915 (86.7) | 16,522,456 (77.7) |
| Missing | 7001 (4.0) | 12,656 (1.1) | 168,409 (1.4) | 55,765 (0.7) | 244,952 (1.2) |
| Marital status | |||||
| Married/Cohabiting | 32,264 (53.1) | 551,062 (45.7) | 6,287,178 (52.0) | 4,877,340 (61.8) | 11,861,934 (55.8) |
| Widow/Separated | 928 (0.5) | 14,699 (1.2) | 124,325 (1.0) | 140,596 (1.8) | 282,406 (1.3) |
| Single | 77,447 (44.6) | 631,330 (0.7) | 5,409,964 (45.4) | 2,835,474 (35.9) | 8,986,383 (42.3) |
| Missing | 3171 (1.8) | 8066 (0.7) | 85,527 (0.7) | 33,894 (0.4) | 131,213 (0.6) |
| Age | |||||
| <20 years | 50,791 (29.2) | 200,301 (16.6) | 2,470,035 (20.7) | 995,186 (12.6) | 3,727,798 (17.5) |
| 20–34 years | 105,245 (60.6) | 839,715 (69.7) | 8,119,485 (68.2) | 5,564,210 (70.5) | 14,689,263 (69.1) |
| 35–49 years | 17,356 (10.0) | 164,938 (13.7) | 1,315,774 (11.0) | 1,326,645 (16.8) | 2,841,263 (13.4) |
| ≥50 years | 375 (0.2) | 137 (0.0) | 1161 (0.0) | 851 (0.0) | 2545 (0.0) |
| Missing | 43 (0.0) | 66 (0.0) | 539 (0.0) | 412 (0.0) | 1067 (0.0) |
| Parity | |||||
| 0 | 45,191 (26.0) | 461,682 (38.3) | 4,486,795 (37.7) | 3,720,977 (47.2) | 8,755,153 (41.2) |
| 1 | 38,248 (22.0) | 354,868 (29.5) | 3,631,954 (30.5) | 2,585,531 (32.8) | 6,639,619 (31.2) |
| 2 | 27,091 (15.6) | 182,734 (15.2) | 1,774,163 (14.9) | 921,574 (11.7) | 2,916,081 (13.7) |
| 3 or more | 57,661 (33.2) | 161,075 (13.4) | 1,379,500 (11.6) | 461,350 (5.9) | 2,065,072 (9.7) |
| Missing | 5619 (3.2) | 44,798 (3.7) | 634,582 (5.3) | 197,872 (2.5) | 886,011 (4.2) |
| Prenatal consultations, number | |||||
| 0 | 9015 (5.2) | 25,675 (2.1) | 219,137 (1.8) | 76,142 (1.0) | 331,038 (1.6) |
| 1–3 | 35,155 (20.2) | 93,945 (7.8) | 919,071 (7.7) | 268,273 (3.4) | 1,321,002 (6.2) |
| 4 or more | 128,345 (73.8) | 1,076,646 (89.3) | 10,702,057 (89.9) | 7,506,203 (95.2) | 19,495,913 (91.7) |
| Missing | 1295 (0.8) | 8891 (0.7) | 66,729 (0.6) | 36,686 (0.5) | 113,983 (0.5) |
| Type of delivery | |||||
| Vaginal | 138,819 (79.9) | 617,837 (51.3) | 5,972,707 (50.2) | 2,642,783 (33.5) | 9,410,116 (44.3) |
| Cesarian | 34,825 (20.0) | 586,509 (48.7) | 5,925,532 (49.8) | 5,241,061 (66.5) | 11,838,569 (55.7) |
| Missing | 166 (0.1) | 811 (0.1) | 8755 (0.1) | 3460 (0.0) | 13,251 (0.1) |
| Pregnancy outcomes | |||||
| Gestational age | |||||
| 22–36 weeks | 25,951 (14.9) | 133,364 (11.1) | 1,266,627 (10.6) | 790,255 (10.0) | 2,225,259 (10.5) |
| 37–41 weeks | 138,500 (79.7) | 1,035,412 (85.9) | 10,189,759 (85.6) | 6,939,887 (88.0) | 18,381,049 (86.4) |
| ≥42 weeks | 9359 (5.4) | 36,381 (3.0) | 450,608 (3.8) | 157,162 (2.0) | 655,628 (3.1) |
| Missing | |||||
| Birthweight | |||||
| <2500 g | 12,712 (7.3) | 104,017 (5.6) | 865,265 (7.3) | 569,119 (7.2) | 1,557,806 (7.3) |
| ≥2500 g | 161,098 (92.7) | 1,101,140 (91.4) | 11,041,729 (92.7) | 7,318,185 (92.8) | 19,704,130 (92.7) |
| Missing | |||||
| Birthweight, centile | |||||
| <3rd | 8649 (5.0) | 40,103 (3.3) | 364,700 (3.1) | 175,481 (2.2) | 591,147 (2.8) |
| ≥3rd a <10th | 11,072 (6.4) | 72,630 (6.0) | 643,253 (5.4) | 360,371 (4.6) | 1,091,769 (5.1) |
| ≥10th a <90th | 116,293 (66.9) | 886,710 (73.6) | 8,708,227 (73.1) | 5,981,894 (75.8) | 15,761,186 (74.1) |
| ≥90th | 34,769 (20.0) | 203,978 (16.9) | 2,176,539 (18.3) | 1,363,090 (17.3) | 3,792,262 (17.8) |
| Missing | 3027 (1.7) | 1736 (0.1) | 14,275 (0.1) | 6468 (0.1) | 25,572 (0.1) |
| Early neonatal death | |||||
| No | 172,494 (99.2) | 1,198,360 (99.4) | 11,844,597 (99.5) | 7,854,799 (99.6) | 21,158,606 (99.5) |
| Yes | 1316 (0.8) | 6797 (0.6) | 62,397 (0.5) | 32,505 (0.4) | 103,330 (0.5) |
The total is the sum of all maternal race/skin colour groups. To access results for people of Asian descent, refer to the appendix.
Indigenous, Black and Parda mothers are proportionally the youngest, with less education, have more children and attend to fewer prenatal appointments (Table 1). Indigenous and Black women had a higher proportion of preterm, term LBW, SGA births and early neonatal deaths (Table 1).
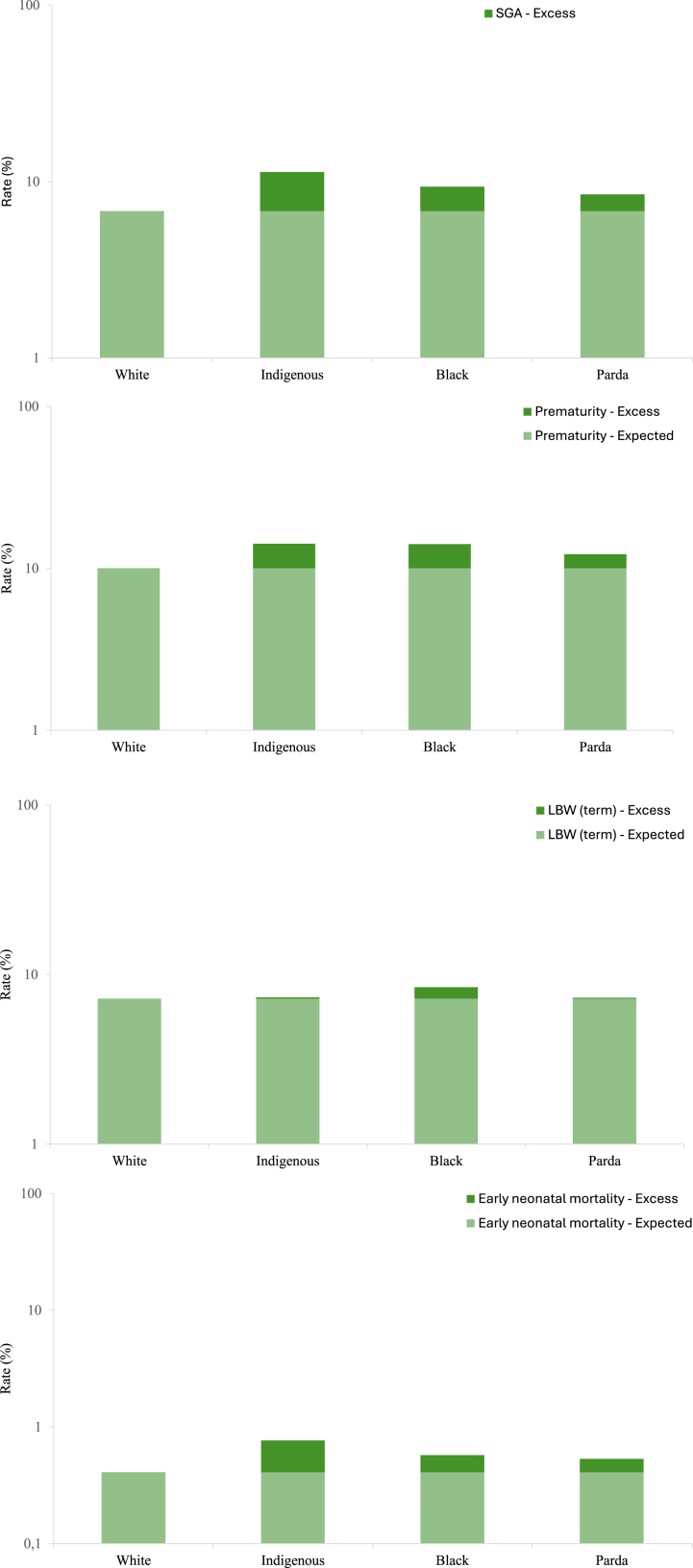
The risk of preterm birth varied according to maternal race/skin colour, ranging from 14.9% among newborns born to Indigenous mothers to 10.0% among newborns from White mothers (Fig. 3; Table S3 in Supplementary Material, p < 0.0001). The PAF using White as the reference group was 2.4% (95% CI = 2.3–2.6) after adjusting for the mother's education, and 1.7% (95% CI = 1.5–1.9) after adjusting for the mother's education and age, parity and type of delivery. Attributable fractions for preterm births were greatly increased among newborns of Indigenous 22.2% (95% CI = 21.2–23.2) and Black 6.0% (95% CI = 5.4–6.5) mothers (Table 2).
Fig. 3.
Preterm birth, LBW, SGA and early neonatal mortality rates by maternal race/skin colour group. Expected: Risk of the outcome if all groups were in similar conditions to white women. Excess: Risk attributed to racial inequality.
Table 2.
Population attributable fractions of preterm birth, LBW, SGA and early neonatal death by maternal race/skin colour group.
| PAF by maternal race/skin colour |
Indigenous people |
AF by maternal race/skin colour |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Black |
Parda |
|||||||||||
| No adjustment | Adjustmenta,b | No adjustment | Adjustmenta,b | No adjustment | Adjustmenta,b | No adjustment | Adjustmenta,b | |||||
| Preterm birth | 4.3% (4.1–4.4) | 2.4% (2.3–2.6) | 1.7% (1.5–1.9) | 33.0% (32.1–33.6) | 25.7% (24.7–26.6) | 22.2% (21.2–23.2) | 9.4% (8.9–9.9) | 6.8% (6.3–7.3) | 6.0% (5.4–6.5) | 5.8% (5.5–6.0) | 3.1% (2.8–3.3) | 1.8% (1.5–2.1) |
| LBW (term) | 9.7% (9.4–10.0) | 7.3% (7.0–7.6) | 7.2% (6.9–7.5) | 37.1% (35.6–38.5) | 22.2% (20.2–24.1) | 17.9% (15.8–20.0) | 25.2% (24.5–26.0) | 21.7% (20.9–22.5) | 21.4% (20.6–22.3) | 13.1% (12.6–13.5) | 9.7% (9.2–10.2) | 9.6% (9.1–10.1) |
| SGA | 14.2% (14.0–14.3) | 12.0% (11.8–12.2) | 10.8% (10.6–11.0) | 40.1% (39.3–40.9) | 26.9% (25.8–27.9) | 20.5% (19.3–21.8) | 27.4% (26.9–27.8) | 24.0% (23.5–24.5) | 22.8% (22.3–23.3) | 19.7% (19.5–20.0) | 16.7% (16.5–17.0) | 15.1% (14.8–15.4) |
| Early neonatal death | 15.2% (14.4–15.9) | 13.0% (12.2–13.8) | 11.8% (11.0–12.7) | 45.6% (42.5–48.5) | 30.7% (26.4–34.7) | 19.6% (14.4–24.5) | 26.9% (25.0–28.8) | 23.3% (21.3–25.4) | 20.1% (17.9–22.3) | 21.3% (20.3–22.4) | 18.3% (17.2–19.4) | 16.7% (15.5–17.9) |
To access results for people of Asian descent, refer to the appendix.
Data are % (95% CI). Compared with White women.
Mother's schooling.
Age of mother, parity, delivery.
The risk of term LBW varied according to maternal race/skin colour ranging from 8.6% for newborns born to Black mothers, to 7.2% for newborns from White mothers (Fig. 3; Table S3 in Supplementary Material, p < 0.0001). The PAF using White as the reference group was 7.2% when adjusted for mother schooling and maternal factors. Attributable fractions for term LBW were greatly increased among newborns of Indigenous 17.9% (95% CI = 15.8–20.0), Black 21.4% (95% CI = 20.6–22.3) and Parda 9.6% (95% CI = 9.1–10.1) mothers (Table 2).
Risk of SGA was 11.3%, 9.4% and 8.5% for newborns born to Indigenous, Black and Parda mothers and 6.8% for Whites (Fig. 3; Table S3 in Supplementary Material, p < 0.0001). PAF using White as the reference group was 10.8% (95% CI = 10.6–11.0) when adjusted for mother schooling and maternal factors. Attributable fractions for SGA were substantially increased among newborns of Indigenous 20.5% (95% CI = 19.3–21.8), Black 22.8% (95% CI = 22.3–23.3) and Parda 15.1% (95% CI = 14.8–15.4) mothers (Table 2).
The risk of early neonatal mortality varied by maternal race/skin colour, from 0.8% among newborns of Indigenous mothers to 0.4% among newborns of White mothers (Fig. 3; Table S3 in the Supplementary Material, p < 0.0001). PAF using White as the reference group was 11.8% (95% CI = 11.0–12.7) when adjusted for socioeconomic and maternal factors. Attributable fractions for early neonatal mortality were substantially increased among newborns of Indigenous 19.6% (95% CI = 14.4–24.5), Black 20.1% (95% CI = 17.9–22.4) and Parda 16.7% (95% CI = 15.5–17.9) mothers (Table 2).
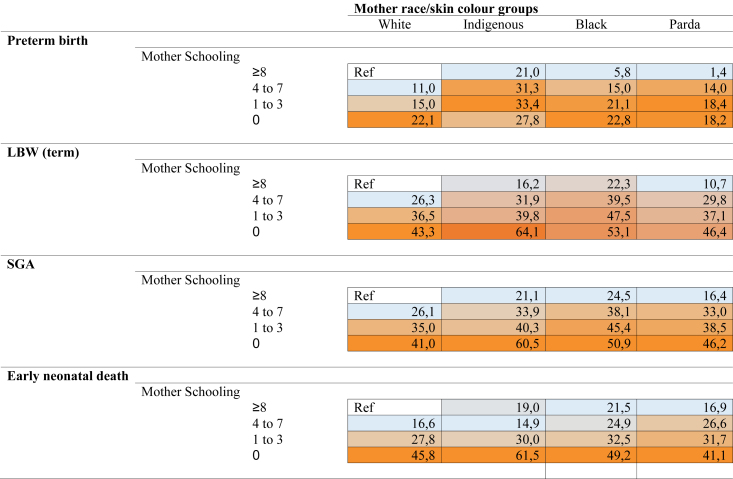
Attributable fractions (AF) for preterm birth were incredibly high in women without any education if they were Indigenous (27.8%, 95% CI = 25.1–30.3) or Black (22.8%, 95% CI = 18.3–27.0) (Fig. 4; Supplementary Material, Table S4). This trend is also observed for LBW among Indigenous, Black, and Parda women in the group with no years of education (64.1%; 95% CI = 62.0–66.0; 53%, 95% CI = 48.4–57.3; 46.4%, 95% CI = 44.4–48.4) (Fig. 4; Supplementary Material, Table S4). Similarly, high AF was found for SGA in women without any education if they were Indigenous (60.5%, 95% CI = 59.2–61.8), Black (50.9%, 95% CI = 47.9–53.6), or Parda (46.2%, 95% CI = 45.0–47.3) (Fig. 4; Supplementary Material, Table S4). In the same way, women without any education from Indigenous, Black and Parda maternal race/skin colour groups had the highest AF for early neonatal death (61.5%, 95% CI = 56.3–66.1; 49.2%, 95% CI = 36.3–59.5; and 41.1%, 95% CI = 35.5–46.3; respectively) (Fig. 4; Supplementary Material, Table S4).
Fig. 4.
Attributable fractions of preterm birth, LBW, SGA and early neonatal mortality by mother's schooling and maternal race/skin colour group. Data are %; Confidence interval (95% CI): in Suplementary Material, Table S3. The data are attributable fractions (95% CI), calculated by comparing White women with 8 or more years of education. Darker colors indicate a group with a larger attributable fraction. Values adjusted to age of mother, parity and delivery. To access results for people of Asian descent, see Appendix.
Among women with higher education, the risk of LBW, SGA, and early neonatal deaths could be reduced by over 20% among Black women (22.3%, 95% CI = 21.3–23.2; 24.5%, 95% CI = 23.9–25.0; and 21.5%, 95% CI = 18.9–24.0, respectively) and over 10% among Parda women (10.7%, 95% CI = 10.2–11.3; 16.4%, 95% CI = 16.1–16.7; and 16,9%, 95% CI = 15.5–18.2, respectively) if they had the same baseline risk as their White counterparts (Fig. 4; Supplementary Material, Table S4). The analysis of the AF over the years shows an increase in racial inequality across all outcomes, particularly pronounced when considering the Indigenous population (Fig. 5).
Fig. 5.
Attributable fractions trends of preterm birth, LBW, SGA and early neonatal mortality by maternal race/skin colour group.
Regarding descendants of Asians, the risk of preterm birth in this group was 10.2%, the risk of LBW was 7.5%, the risk of SGA was 7.4%, and the risk of early neonatal mortality was 0.4%, with variations of 1% or less compared to children born to White mothers (Fig. 1 and Table S3 in the Supplementary Material, p < 0.0001). AF using Whites as the reference for LBW and SGA was 6.9% (6.9% 95% CI = 3.5–10.2; and 6.9% 95% CI = 4.7–9.1, respectively) (Supplementary Material, Table S2). Similar to other maternal race/skin colour groups, the AF was high in children of Asian descent when maternal education was lower (Table S4 in the Supplementary Material). AF over the years to Asian descent has shown a slight increase in LBW and SGA (Figure S2, Supplementary Material).
Discussion
Our results, which included more than 21 million live births in Brazil between 2012 and 2019, showed important ethnic-racial inequalities in perinatal outcomes. A considerable amount of preterm births, term low birth weight, small-for-gestational-age births and early neonatal deaths could have been avoided had such inequalities not been present, representing a reduction of approximately 1.7% of preterm birth, 7.2% of LBW, 10.8% of SGA and 11.8% of early neonatal deaths in the country. In relation to the intersection between race and mother schooling, we observed that among women with higher education, ethnic-racial inequalities continue to exist, and they are accentuated among women with lower education levels. Among Indigenous women with fewer years of education, the reduction in prematurity would be 27.8%, and in LBW, SGA, and early neonatal mortality, it would exceed 60% if it were not for inequalities. Among Black and Brown women, the reduction in prematurity would be over 18%. In LBW, SGA, and early neonatal mortality, the reduction would be over 40%.
Despite maternal and child health gains and the effect of equity-oriented health and social policies in Brazil over the last few decades,47 racial disparities in pregnancy outcomes persist.22,48,49 Previous studies carried out in Brazil have shown a higher risk of preterm births, SGA and LBW among Black and Parda women compared to White women.22,23,50, 51, 52, 53 The only study that included Indigenous people in the analysis observed a greater risk among Indigenous descents compared to European descents.53 Moreover, a recent analysis of a cohort of Brazilian Indigenous people reported a high prevalence of LBW and prematurity associated with modifiable environmental determinants, such as quality of the home environment, maternal nutritional and health status before and during pregnancy, obstetric history and access to health services.54 In our study, the population attributable fraction decreased after adjusting for mother schooling, suggesting that adverse socioeconomic factors play a role in the ethnic-racial inequalities observed in the studied context.
Corroborating this finding, we observed an important gradient in all outcomes when we analysed the intersection between the mother's race/skin colour and education level. The lower the level of education, the greater the fraction attributable to racial inequality in preterm births, LBW, SGA and early neonatal deaths when compared to white mothers with higher education. We did not find Brazilian studies that had previously carried out this type of analysis. Preterm birth and LBW in more vulnerable populations, such as the Indigenous population, occur as a result of limited access to prenatal care, adverse obstetric conditions, maternal malnutrition, environmental risk and general socioeconomic vulnerabilities.47,55, 56, 57
Different mechanisms could explain the findings of this study. Access to health services is a crucial factor for a healthy pregnancy and birth,58 and this could be affected by structural racism as it systematically shapes access, utilization, and quality of services in the healthcare system, at neighbourhood levels, and by providers. For example, predominantly black neighbourhoods are not prioritized by primary care providers and specialists, for health promotion or disease prevention actions.59 It contributes to a greater probability of potentially preventable diseases and injuries among pregnant women, which, if adequately handled, could avoid preterm births, LBW, uterine growth restriction, and neonatal deaths.60,61
Moreover, some studies show racial inequalities in access to prenatal consultations, essential for a successful pregnancy,62, 63, 64 but also in access and quality of neonatal ICU services, which are necessary for babies with any fragility. In this sense, premature babies may receive differentiated care depending on the maternal race/skin colour to which they belong, with less access to ICUs or accessing ICUs with inferior structure or organisational models and clinical procedures of lower quality.65,66 Studies suggest that improving care provided at this level of service complexity could significantly reduce racial disparities in neonatal deaths.67
This study understands Racism as the “total of ways in which societies promote racial discrimination through systems of housing, education, employment, income, benefits, credit, media, health care and criminal justice that are mutually reinforcing”.68 Structural racism affects Black and Indigenous people in Brazil differently.69 While Indigenous peoples face segregation in rural reserves surrounded by agribusiness, constantly threatened by prospectors and farmers, and under the guise of preserving their rights are actually controlled and penalized, facing barriers to accessing essential basic services for their survival,69 the Black population encounters prejudice, discrimination, and the risk of racist violence in predominantly urban areas.
Racism produces and reinforces the quality and unequal access to health care, the unequal distribution of the social determinants of health and the physical and psychological harm to communities marginalized by racism, resulting in observable inequalities in health.70 It is important to emphasize that, in addition to determining individual exposures to discrimination and structurally impacting societal practices and quality of care, structural racism also plays a role in a complex network of influences, where worse perinatal outcomes may persist disproportionately for Black and Indigenous women, even when access to services is expanded. Therefore, as more recent studies have proposed, equal access (as envisioned and legislated by the Brazilian Universal Healthcare model) may not always ensure equal health outcomes among racial groups.71, 72, 73
Structural racism also operates in the relationships between patients and health professionals. Studies show racial inequalities in the care provided to mothers/families, where mothers belonging to vulnerable maternal race/skin colour groups may receive reduced care based on scientific evidence and experience a greater lack of communication with the medical team.74,75 Finally, structural Racism also operates through stress pathways. Studies show that experiencing Racism can compromise maternal health by affecting the endocrine, immune and vascular systems, increasing susceptibility to diseases and health problems and adverse pregnancy outcomes.76,77 This highlights the link between individuals' health and social institutions and strengthens the conclusion that efforts to eliminate such inequities need to consider the role of Racism as it not only creates direct adverse exposures but indirectly impacts through cultural, commercial and institutional practices.78
The first decades of the 21st century represented a significant shift in the public discourse on racial relations and in public policies aimed at marginalized populations affected by structural racism in Brazil.79 From the establishment of racial quotas to the adoption of a Racial Equality Statute and the creation of specific state agencies to implement public policies, several measures have been taken in this direction.79
The topic of racial inequalities has gained prominence in Brazil as part of a broad set of reflections on social issues, not only identity-based or compensatory, but also from the understanding that they constitute the central axis of social inequality in the country.80 Structural racism acts as both a producer and a reproducer of social hierarchies, limiting social mobility for the majority of the population and becoming a significant barrier to equality of opportunities. Additionally, it serves as a fundamental determinant of the accumulated deficits in Brazilian society.80
Our study is the largest and most recent on ethnic-racial inequalities on perinatal outcomes and the first to include the Indigenous population in the investigation of ethnic-racial inequalities in early neonatal death. However, our study also has limitations and should be interpreted with caution. First, its important to consider the possibility of linkage error. In an evaluation, it was estimated that we were able to capture around 66% (Supplementary Material, Figure S1). This can be explained by the low quality of data, especially in poorer areas,81 where indigenous communities are located, for example, despite recent improvements in the quality of data in the Brazilian Health Information System, and this may underestimate our analysis. Although there has been a national trend of reduction in the incompleteness of the maternal race/skin colour variable in the SINASC over the years of the study,82 this trend is not uniform across the country. In the Northeast region, an area with a significant Black population and a higher proportion of poverty, a significant reduction was not observed between 2012 and 2020 (annual percentage change: −0.4 (95% CI: −3.3; 2.7)).80 In fact, there were some states where the incompleteness of this variable increased during the same period.82 Although, overall, the completeness of the maternal race/skin colour variable in the SINASC is satisfactory, the observed regional disparity may influence the results, limiting the accuracy of our findings and possibly underestimating them. Finally, in addition to the percentage of missing data in the maternal race/colour variable, the use of the race/colour variable as a proxy for Racism is a limitation due to its complexity, and can vary according to whether individual can self-identify or be classified.83 Even so, studies that use race/skin colour as a proxy for racism have shown strong evidence of ethnic-racial inequalities in health.83 Another limitation regarding the self-declaration of maternal race/skin colour is that we cannot control for cases of mixed reports, where the healthcare professional may have filled out this information without consulting the mother. Although the training of these professionals emphasizes a different approach, we do not rule out this possibility. However, we cannot estimate the impact of this on the results of our study. Finally, this is a retrospective, cohort study and the design itself limits our interpretation to associations, precluding any causal inferences.
Health indicators are a strong signal of the dramatic racial inequality in Brazil, which persists even among groups with higher levels of education.1 On the other hand, although income transfer programs have reduced poverty and destitution with significant impacts on maternal and child health,49,84,85 it will not be possible to achieve their eradication if racism and its ramifications are not addressed. In addition to existing inequalities, there is a need to be concerned about crisis contexts which can exacerbate them, as was the case with the COVID-19 pandemic. Brazil is known to be the country with the highest number of maternal deaths during this period.86 Among Black women, it was twice as high as among White women.87 Finally, the recent humanitarian crisis faced by the Yanomami people in the country cannot be overlooked.88
Our study showed that a considerable number of adverse birth outcomes and neonatal deaths could be avoided if ethnic-racial inequalities were not present. Urgent action is needed to improve the health of mothers and babies historically excluded by enduring ethnic-racial inequalities and its most extreme form of discrimination–Racism. We will not be able to achieve the Sustainable Development Goals (SDGs) if the gap between ethnic and racial groups is not closed. Therefore, policies in general, and healthcare policies in particular, must address the root causes of structural racism, not just its consequences, in order to tackle racial prejudice through actions that value the Black and Indigenous populations. These policies should complement poverty reduction efforts, being implemented in a specific and targeted manner. It is essential that they confront institutional racism not as a residual issue, but as the core of the political problems faced in Brazil, including with compatible resources. Additionally, there is a need to mobilize more research to continuously investigate the impact of racial inequality on population health and evaluate the effectiveness of policies in addressing it.
Contributors
PR, EFG, ESP, JP, and DR conceptualised the study. PR conducted the analyses. PR, ESP and JP verified the underlying data. PR, DR, MLB, EFG, ESP, and SS wrote the manuscript. EFG, ESP, EPPJ, IRF, RR, LCR, MYI, RV, and MB supervised the analysis and data interpretation. All the authors had access to the data in the study, contributed to the data interpretation and discussion and approved the final manuscript version. We stated that all the autors had access to the data. PR had final responsibility to submit.
Data sharing statement
Data, code book and analytic code: Data described in the manuscript, code book, and analytic code will be made available upon request pending application. All data supporting this study were obtained from Center for Data and Knowledge Integration for Health (CIDACS). Restrictions apply to access these data, which contain sensitive information, were licensed for exclusive use in the current study and, due to privacy regulations from the Brazilian Ethics Committee, are not openly available. Upon reasonable request and with express permission from CIDACS (mail to cidacs.curadoria@fiocruz.br) and approval from an ethics committee, controlled access to the data is possible.
Declaration of interests
We declare no competing interests.
Acknowledgements
We thank all the teams that make up CIDACS: Communication Unit, Operations Unit, Data Production Unit, Curatorship, Information Security Unit, Information Technology Unit. Without this entire team, our study would not be possible.
Funding: This research was supported by the Bill & Melinda Gates Foundation (Brazil PHC Research/INV-006196); the MCTI/CNPq/MS/SCTIE/Decit/Bill & Melinda Gates Foundation's Grandes Desafios Brasil – Desenvolvimento Saudável para Todas as Crianças (Call number 47/2014) and CIDACS received core support from Wellcome Trust (202912/B/16/Z and 225925/Z/22/Z). The views expressed in this Article are those of the authors.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lana.2024.100833.
Appendix A. Supplementary data
References
- 1.Paixao E.S., Ferreira A.J., Pescarini J.M., et al. Maternal and congenital syphilis attributable to ethnoracial inequalities: a national record-linkage longitudinal study of 15 million births in Brazil. Lancet Global Health. 2023;11(11):e1734–e1742. doi: 10.1016/S2214-109X(23)00405-9. [DOI] [PubMed] [Google Scholar]
- 2.Rebouças P., Goes E., Pescarini J., et al. Ethnoracial inequalities and child mortality in Brazil: a nationwide longitudinal study of 19 million newborn babies. Lancet Global Health. 2022;10(10):e1453–e1462. doi: 10.1016/S2214-109X(22)00333-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lett E., Asabor E., Beltrán S., Cannon A.M., Arah O.A. Conceptualizing, contextualizing, and operationalizing race in quantitative health sciences research. Ann Fam Med. 2022;20(2):157–163. doi: 10.1370/afm.2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Osorio R.G. 2021. A desigualdade racial no Brasil nas três últimas décadas: Texto para Discussão. [Google Scholar]
- 5.Cobo B., Cruz C., Dick P.C. Desigualdades de gênero e raciais no acesso e uso dos serviços de atenção primária à saúde no Brasil. Ciência Saúde Coletiva. 2021;26:4021–4032. doi: 10.1590/1413-81232021269.05732021. [DOI] [PubMed] [Google Scholar]
- 6.Leal Mdo C., da Gama S.G., da Cunha C.B. Desigualdades raciais, sociodemográficas e na assistência ao pré-natal e ao parto, 1999-2001. Rev Saude Publica. 2005;39:100–107. doi: 10.1590/s0034-89102005000100013. [DOI] [PubMed] [Google Scholar]
- 7.Tomasiello D.B., Bazzo J.P., Parga J.P., Servo L.M., Pereira R.H.M. 2023. Desigualdades raciais e de renda no acesso à saúde nas cidades brasileiras. [Google Scholar]
- 8.Batista L.E., Rattner D., Kalckmann S., Oliveira MCGd. Humanização na atenção à saúde e as desigualdades raciais: uma proposta de intervenção. Saúde e Sociedade. 2016;25:689–702. [Google Scholar]
- 9.Goes E.F., Nascimento ERd. Mulheres negras e brancas e os níveis de acesso aos serviços preventivos de saúde: uma análise sobre as desigualdades. Saúde em Debate. 2013;37:571–579. [Google Scholar]
- 10.Leal MdC., Gama SGNd, Pereira A.P.E., Pacheco V.E., Carmo CNd, Santos R.V. A cor da dor: iniquidades raciais na atenção pré-natal e ao parto no Brasil. Cad Saúde Pública. 2017;33 doi: 10.1590/0102-311X00078816. [DOI] [PubMed] [Google Scholar]
- 11.Howe C.J., Bailey Z.D., Raifman J.R., Jackson J.W. Recommendations for using causal diagrams to study racial health disparities. Am J Epidemiol. 2022;191(12):1981–1989. doi: 10.1093/aje/kwac140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ding X., Liang M., Wu Y., et al. The impact of prenatal stressful life events on adverse birth outcomes: a systematic review and meta-analysis. J Affect Disord. 2021;287:406–416. doi: 10.1016/j.jad.2021.03.083. [DOI] [PubMed] [Google Scholar]
- 13.Graham J.R., West L.M., Martinez J., Roemer L. The mediating role of internalized racism in the relationship between racist experiences and anxiety symptoms in a Black American sample. Cult Divers Ethnic Minor Psychol. 2016;22(3):369. doi: 10.1037/cdp0000073. [DOI] [PubMed] [Google Scholar]
- 14.Hassen N., Lofters A., Michael S., Mall A., Pinto A.D., Rackal J. Implementing anti-racism interventions in healthcare settings: a scoping review. Int J Environ Res Public Health. 2021;18(6):2993. doi: 10.3390/ijerph18062993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lawn J.E., Ohuma E.O., Bradley E., et al. Small babies, big risks: global estimates of prevalence and mortality for vulnerable newborns to accelerate change and improve counting. Lancet. 2023;401(10389):1707–1719. doi: 10.1016/S0140-6736(23)00522-6. [DOI] [PubMed] [Google Scholar]
- 16.Paixao E.S., Blencowe H., Falcao I.R., et al. Risk of mortality for small newborns in Brazil, 2011-2018: a national birth cohort study of 17.6 million records from routine register-based linked data. Lancet Reg Health Am. 2021;3 doi: 10.1016/j.lana.2021.100045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suárez-Idueta L., Yargawa J., Blencowe H., et al. Vulnerable newborn types: analysis of population-based registries for 165 million births in 23 countries, 2000–2021. BJOG. 2023 doi: 10.1111/1471-0528.17505. [DOI] [PubMed] [Google Scholar]
- 18.Victora C.G., Adair L., Fall C., et al. Maternal and child undernutrition: consequences for adult health and human capital. Lancet. 2008;371(9609):340–357. doi: 10.1016/S0140-6736(07)61692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mwaniki M.K., Atieno M., Lawn J.E., Newton C.R. Long-term neurodevelopmental outcomes after intrauterine and neonatal insults: a systematic review. Lancet. 2012;379(9814):445–452. doi: 10.1016/S0140-6736(11)61577-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saigal S., Doyle L.W. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet. 2008;371(9608):261–269. doi: 10.1016/S0140-6736(08)60136-1. [DOI] [PubMed] [Google Scholar]
- 21.Teune M.J., Bakhuizen S., Bannerman C.G., et al. A systematic review of severe morbidity in infants born late preterm. Am J Obstet Gynecol. 2011;205(4):374.e1–374.e9. doi: 10.1016/j.ajog.2011.07.015. [DOI] [PubMed] [Google Scholar]
- 22.Fonseca J., Silva A., Rocha P., et al. Racial inequality in perinatal outcomes in two Brazilian birth cohorts. Braz J Med Biol Res. 2021;54:e10120. doi: 10.1590/1414-431X202010120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silveira M.F., Victora C.G., Barros A.J., Santos I.S., Matijasevich A., Barros F.C. Determinants of preterm birth: pelotas, Rio Grande do Sul state, Brazil, 2004 birth cohort. Cad Saúde Pública. 2010;26:185–194. doi: 10.1590/s0102-311x2010000100019. [DOI] [PubMed] [Google Scholar]
- 24.Silveira M.F., Victora C.G., Horta B.L., da Silva B.G., Matijasevich A., Barros F.C. Low birthweight and preterm birth: trends and inequalities in four population-based birth cohorts in Pelotas, Brazil, 1982–2015. Int J Epidemiol. 2019;48(Supplement_1):i46–i53. doi: 10.1093/ije/dyy106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.UN . United Nations; New York, NY, USA: 2015. Transforming our world: the 2030 agenda for sustainable development. [Google Scholar]
- 26.Szwarcwald C.L., Leal MdC., Esteves-Pereira A.P., et al. Avaliação das informações do Sistema de Informações sobre Nascidos Vivos (SINASC), Brasil. Cad Saúde Pública. 2019;35 doi: 10.1590/0102-311X00214918. [DOI] [PubMed] [Google Scholar]
- 27.Maia LTdS., Souza WVd, Mendes AdCG., Silva AGSd. Uso do linkage para a melhoria da completude do SIM e do Sinasc nas capitais brasileiras. Rev Saude Publica. 2017;51 [Google Scholar]
- 28.Lino R.R.G. 2018. Série temporal da completitude das estatísticas vitais no período neonatal, estado do Rio de Janeiro, 1999 a 2014. [DOI] [PubMed] [Google Scholar]
- 29.Marques L.J.P., Pimentel DdR., Oliveira CMd, Vilela M.B.R., Frias P.G., Bonfim CVd. Concordância da causa básica e da evitabilidade dos óbitos infantis antes e após a investigação no Recife, Pernambuco, 2014. Epidemiol Serv Saúde. 2018;27 doi: 10.5123/s1679-49742018000100007. [DOI] [PubMed] [Google Scholar]
- 30.Morais RMd, Costa A.L. An evaluation of the Brazilian mortality information system. Saúde Debate. 2017;41:101–117. [Google Scholar]
- 31.Barbosa G.C., Ali M.S., Araujo B., et al. CIDACS-RL: a novel indexing search and scoring-based record linkage system for huge datasets with high accuracy and scalability. BMC Med Inf Decis Mak. 2020;20:1–13. doi: 10.1186/s12911-020-01285-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barreto M.L., Ichihara M., Almeida B.A., et al. The centre for data and knowledge integration for health (CIDACS): linking health and social data in Brazil. Int J Popul Data Sci. 2019;4(2):1140. doi: 10.23889/ijpds.v4i2.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith L.K., Manktelow B.N., Draper E.S., Boyle E.M., Johnson S.J., Field D.J. Trends in the incidence and mortality of multiple births by socioeconomic deprivation and maternal age in England: population-based cohort study. BMJ Open. 2014;4(4) doi: 10.1136/bmjopen-2013-004514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Woodhouse C., Lopez Camelo J., Wehby G.L. A comparative analysis of prenatal care and fetal growth in eight South American countries. PLoS One. 2014;9(3) doi: 10.1371/journal.pone.0091292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brazil GCoEI, Analyses–CGIAE DoHS, Health Md . 2013. Consolidação Sistema de Informações Sobre Nascidos Vivos: 2011. [Google Scholar]
- 36.IBGE . Rio de Janeiro; 2023. Censo demográfico 2022: identificação étnico-racial da população, por sexo e idade: resultados do universo. [Google Scholar]
- 37.Petruccelli J.L., Saboia A.L. 2013. Características étnico-raciais da população. Classificações e identidades Rio de Janeiro: Instituto Brasileiro de Geografia e Estatística. [Google Scholar]
- 38.Adkins-Jackson P.B., Chantarat T., Bailey Z.D., Ponce N.A. Measuring structural racism: a guide for epidemiologists and other health researchers. Am J Epidemiol. 2022;191(4):539–547. doi: 10.1093/aje/kwab239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mays V.M., Ponce N.A., Washington D.L., Cochran S.D. Classification of race and ethnicity: implications for public health. Annu Rev Public Health. 2003;24(1):83–110. doi: 10.1146/annurev.publhealth.24.100901.140927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ecker J.L., Kaimal A., Mercer B.M., et al. Periviable birth. Obstet Gynecol Surv. 2016;71(3):137–139. [Google Scholar]
- 41.Patel R.M., Rysavy M.A., Bell E.F., Tyson J.E. Survival of infants born at periviable gestational ages. Clin Perinatol. 2017;44(2):287–303. doi: 10.1016/j.clp.2017.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Villar J., Giuliani F., Fenton T.R., Ohuma E.O., Ismail L.C., Kennedy S.H. INTERGROWTH-21st very preterm size at birth reference charts. Lancet. 2016;387(10021):844–845. doi: 10.1016/S0140-6736(16)00384-6. [DOI] [PubMed] [Google Scholar]
- 43.Villar J., Ismail L.C., Victora C.G., et al. International standards for newborn weight, length, and head circumference by gestational age and sex: the newborn cross-sectional study of the INTERGROWTH-21st Project. Lancet. 2014;384(9946):857–868. doi: 10.1016/S0140-6736(14)60932-6. [DOI] [PubMed] [Google Scholar]
- 44.Organization WH . Vol. 1. 1992. ICD-10. International statistical classification of diseases and related health problems: tenth revision 1992, volume 1= CIM-10. (Classification statistique internationale des maladies et des problèmes de santé connexes: Dixième Révision). [Google Scholar]
- 45.Mansournia M.A., Altman D.G. Population attributable fraction. BMJ. 2018;360 doi: 10.1136/bmj.k757. [DOI] [PubMed] [Google Scholar]
- 46.Newson R.B. Attributable and unattributable risks and fractions and other scenario comparisons. STATA J. 2013;13(4):672–698. [Google Scholar]
- 47.Cardoso A.M., Horta B.L., Coimbra Junior C.E.A., Follér M.-L., Souza MCd. 2009. Inquérito nacional de saúde e nutrição dos povos indígenas. [DOI] [PubMed] [Google Scholar]
- 48.Hone T., Rasella D., Barreto M.L., Majeed A., Millett C. Association between expansion of primary healthcare and racial inequalities in mortality amenable to primary care in Brazil: a national longitudinal analysis. PLoS Med. 2017;14(5) doi: 10.1371/journal.pmed.1002306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ramos D., da Silva N.B., Ichihara M.Y., et al. Conditional cash transfer program and child mortality: a cross-sectional analysis nested within the 100 Million Brazilian Cohort. PLoS Med. 2021;18(9) doi: 10.1371/journal.pmed.1003509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barros F.C., Victora C.G., Horta B.L. Ethnicity and infant health in Southern Brazil. A birth cohort study. Int J Epidemiol. 2001;30(5):1001–1008. doi: 10.1093/ije/30.5.1001. [DOI] [PubMed] [Google Scholar]
- 51.Nyarko K.A., Lopez-Camelo J., Castilla E.E., Wehby G.L. Explaining racial disparities in infant health in Brazil. Am J Public Health. 2013;103(9):1675–1684. doi: 10.2105/AJPH.2012.301021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Silva L., Silva R., Silva A., Bettiol H., Barbieri M.A. Racial inequalities and perinatal health in the southeast region of Brazil. Braz J Med Biol Res. 2007;40:1187–1194. doi: 10.1590/s0100-879x2006005000144. [DOI] [PubMed] [Google Scholar]
- 53.Wehby G.L., Gili J.A., Pawluk M., Castilla E.E., López-Camelo J.S. Disparities in birth weight and gestational age by ethnic ancestry in South American countries. Int J Public Health. 2015;60:343–351. doi: 10.1007/s00038-014-0639-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barreto C.T.G., Tavares F.G., Theme-Filha M., Farias Y.N., Pantoja LdN., Cardoso A.M. Low birthweight, prematurity, and intrauterine growth restriction: results from the baseline data of the first indigenous birth cohort in Brazil (Guarani Birth Cohort) BMC Pregnancy Childbirth. 2020;20(1):1–19. doi: 10.1186/s12884-020-03491-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Anderson I., Robson B., Connolly M., et al. Indigenous and tribal peoples' health (the lancet–lowitja Institute global collaboration): a population study. Lancet. 2016;388(10040):131–157. doi: 10.1016/S0140-6736(16)00345-7. [DOI] [PubMed] [Google Scholar]
- 56.Goldenberg R.L., Culhane J.F., Iams J.D., Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371(9606):75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Leal MdC., Esteves-Pereira A.P., Nakamura-Pereira M., et al. Prevalence and risk factors related to preterm birth in Brazil. Reprod Health. 2016;13:163–174. doi: 10.1186/s12978-016-0230-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Leal MdC., Esteves-Pereira A.P., Viellas E.F., Domingues R.M.S.M., Gama SGNd. Prenatal care in the Brazilian public health services. Rev Saude Publica. 2020;54:8. doi: 10.11606/s1518-8787.2020054001458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Beck A.F., Edwards E.M., Horbar J.D., Howell E.A., McCormick M.C., Pursley D.M. The color of health: how racism, segregation, and inequality affect the health and well-being of preterm infants and their families. Pediatr Res. 2020;87(2):227–234. doi: 10.1038/s41390-019-0513-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dominguez T.P. Race, racism, and racial disparities in adverse birth outcomes. Clin Obstet Gynecol. 2008;51(2):360–370. doi: 10.1097/GRF.0b013e31816f28de. [DOI] [PubMed] [Google Scholar]
- 61.Lu M.C., Halfon N. Racial and ethnic disparities in birth outcomes: a life-course perspective. Matern Child Health J. 2003;7:13–30. doi: 10.1023/a:1022537516969. [DOI] [PubMed] [Google Scholar]
- 62.Byrd D.R., Katcher M.L., Peppard P., Durkin M., Remington P.L. Infant mortality: explaining black/white disparities in Wisconsin. Matern Child Health J. 2007;11:319–326. doi: 10.1007/s10995-007-0183-6. [DOI] [PubMed] [Google Scholar]
- 63.Kitsantas P., Gaffney K.F. 2010. Racial/ethnic disparities in infant mortality. [DOI] [PubMed] [Google Scholar]
- 64.Lorch S.A., Enlow E. The role of social determinants in explaining racial/ethnic disparities in perinatal outcomes. Pediatr Res. 2016;79(1):141–147. doi: 10.1038/pr.2015.199. [DOI] [PubMed] [Google Scholar]
- 65.Howell E.A. Racial disparities in infant mortality: a quality of care perspective. Mt Sinai J Med. 2008;75(1):31–35. doi: 10.1002/msj.20018. [DOI] [PubMed] [Google Scholar]
- 66.Howell E.A., Janevic T., Hebert P.L., Egorova N.N., Balbierz A., Zeitlin J. Differences in morbidity and mortality rates in black, white, and Hispanic very preterm infants among New York City hospitals. JAMA Pediatr. 2018;172(3):269–277. doi: 10.1001/jamapediatrics.2017.4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Howell E.A., Hebert P., Chatterjee S., Kleinman L.C., Chassin M.R. Black/white differences in very low birth weight neonatal mortality rates among New York City hospitals. Pediatrics. 2008;121(3):e407–e415. doi: 10.1542/peds.2007-0910. [DOI] [PubMed] [Google Scholar]
- 68.Bailey Z.D., Krieger N., Agénor M., Graves J., Linos N., Bassett M.T. Structural racism and health inequities in the USA: evidence and interventions. Lancet. 2017;389(10077):1453–1463. doi: 10.1016/S0140-6736(17)30569-X. [DOI] [PubMed] [Google Scholar]
- 69.Milanez F., Sá L., Krenak A., et al. Existência e diferença: o racismo contra os povos indígenas. Revista Direito e Práxis. 2019;10(3):2161–2181. [Google Scholar]
- 70.Phelan J.C., Link B.G. Is racism a fundamental cause of inequalities in health? Annu Rev Sociol. 2015;41:311–330. [Google Scholar]
- 71.Assari S. Social epidemiology of perceived discrimination in the United States: role of race, educational attainment, and income. Int J Epidemiol Res. 2020;7(3):136–141. doi: 10.34172/ijer.2020.24. [DOI] [PubMed] [Google Scholar]
- 72.Assari S., Zare H. Beyond access, proximity to care, and healthcare use: sustained racial disparities in perinatal outcomes due to marginalization-related diminished returns and racism. J Pediatr Nurs. 2022;63:e161–e163. doi: 10.1016/j.pedn.2021.09.021. [DOI] [PubMed] [Google Scholar]
- 73.Woods-Giscombe C., Robinson M.N., Carthon D., Devane-Johnson S., Corbie-Smith G. Superwoman schema, stigma, spirituality, and culturally sensitive providers: factors influencing African American women's use of mental health services. J Best Pract Health Prof Divers. 2016;9(1):1124. [PMC free article] [PubMed] [Google Scholar]
- 74.Lee H.C., Lyndon A., Blumenfeld Y.J., Dudley R.A., Gould J.B. Antenatal steroid administration for premature infants in California. Obstet Gynecol. 2011;117(3):603. doi: 10.1097/aog.0b013e31820c3c9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sigurdson K., Morton C., Mitchell B., Profit J. Disparities in NICU quality of care: a qualitative study of family and clinician accounts. J Perinatol. 2018;38(5):600–607. doi: 10.1038/s41372-018-0057-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jackson F.M., Phillips M.T., Hogue C.J.R., Curry-Owens T.Y. Examining the burdens of gendered racism: implications for pregnancy outcomes among college-educated African American women. Matern Child Health J. 2001;5:95–107. doi: 10.1023/a:1011349115711. [DOI] [PubMed] [Google Scholar]
- 77.Wadhwa P.D., Culhane J.F., Rauh V., Barve S.S. Stress and preterm birth: neuroendocrine, immune/inflammatory, and vascular mechanisms. Matern Child Health J. 2001;5:119–125. doi: 10.1023/a:1011353216619. [DOI] [PubMed] [Google Scholar]
- 78.Gee G.C., Walsemann K.M., Brondolo E. A life course perspective on how racism may be related to health inequities. Am J Public Health. 2012;102(5):967–974. doi: 10.2105/AJPH.2012.300666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jaccoud L. A Construção de uma política de promoção da igualdade racial: uma análise dos últimos 20 anos. 2009. A construção de uma política de promoção da igualdade racial: uma análise dos últimos 20 anos; p. 233. [Google Scholar]
- 80.Theodoro M. Relações raciais, racismo e políticas públicas no Brasil contemporâneo. Revista de Estudos e Pesquisas sobre as Américas. 2014;8(1):205–219. [Google Scholar]
- 81.Estatística IBdGe, de Oliveira A.T.R. IBGE; 2018. Sistemas de estatísticas vitais no Brasil: avanços, perspectivas e desafios. [Google Scholar]
- 82.Santana B.E.F., Andrade ACdS., Muraro A.P. Trend of incompleteness of maternal schooling and race/skin color variables held on the Brazilian Live Birth Information System, 2012-2020. Epidemiol Serv Saúde. 2023;32 doi: 10.1590/S2237-96222023000100013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chor D., Lima CRdA. Aspectos epidemiológicos das desigualdades raciais em saúde no Brasil. Cad Saúde Pública. 2005;21(5):1586–1594. doi: 10.1590/s0102-311x2005000500033. [DOI] [PubMed] [Google Scholar]
- 84.Falcão I.R., Ribeiro-Silva RdC., Alves F.J.O., et al. Evaluating the effect of bolsa familia, Brazil's conditional cash transfer programme, on maternal and child health: a study protocol. PLoS One. 2022;17(5) doi: 10.1371/journal.pone.0268500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rasella D., Alves F.J.O., Rebouças P., et al. Long-term impact of a conditional cash transfer programme on maternal mortality: a nationwide analysis of Brazilian longitudinal data. BMC Med. 2021;19:1–9. doi: 10.1186/s12916-021-01994-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Orellana J., Jacques N., Leventhal D.G.P., Marrero L., Morón-Duarte L.S. Excess maternal mortality in Brazil: regional inequalities and trajectories during the COVID-19 epidemic. PLoS One. 2022;17(10) doi: 10.1371/journal.pone.0275333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Takemoto M.L., Menezes MdO., Andreucci C.B., et al. The tragedy of COVID-19 in Brazil: 124 maternal deaths and counting. Int J Gynecol Obstet. 2020;151(1):154–156. doi: 10.1002/ijgo.13300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Watts J. Health emergency over Brazil's Yanomami people. Lancet. 2023;401(10377):631. doi: 10.1016/S0140-6736(23)00384-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.