Abstract
Patients with obstructive sleep apnea (OSA) experience insulin resistance and its clinical consequences, including hypertriglyceridemia, reduced high density lipoprotein-associated cholesterol (HDL-c), visceral adiposity, hepatic steatosis, increased epicardial fat thickness, essential hypertension, glucose intolerance, increased risk for type 2 diabetes, chronic kidney disease, subclinical vascular damage, and increased risk for cardiovascular events. Obesity is a major contributor to OSA. The prevalence of OSA is almost universal among patients with severe obesity undergoing bariatric surgery. However, insulin resistance and its clinical complications occur in OSA patients irrespective of general obesity (body mass index). In OSA patients, apnea episodes during sleep induce oxyhemoglobin desaturation and tissue hypoxia. Insulin resistance is an adaptive response to tissue hypoxia and develops in conditions with limited tissue oxygen supply, including healthy subjects exposed to hypobaric hypoxia (high altitude) and OSA patients. Indicators of oxyhemoglobin desaturation have been robustly and independently linked to insulin resistance and its clinical manifestations in patients with OSA. Insulin resistance mediates the elevated rate of type 2 diabetes, chronic kidney disease, and cardiovascular disease unexplained with traditional cardiovascular risk factors present in OSA patients. Pathophysiological processes underlying hypoxia-induced insulin resistance involve hypoxia inducible factor-1 upregulation and peroxisome proliferator-activated receptor-gamma (PPAR-) downregulation. In human adipose tissue, PPAR- activity promotes glucose transport into adipocytes, lipid droplet biogenesis, and whole-body insulin sensitivity. Silencing of PPAR- in the adipose tissue reduces glucose uptake and fat accumulation into adipocytes and promotes insulin resistance. In conclusion, tissue hypoxia drives insulin resistance and its clinical consequences in patients with OSA, regardless of body mass index.
Keywords: metabolic syndrome, hypertriglyceridemia, visceral obesity, non-alcoholic fatty liver disease, essential hypertension, diabetes, chronic kidney disease, cardiovascular risk, tissue hypoxia
1. Introduction
Patients with obstructive sleep apnea (OSA) typically suffer from insulin resistance and its clinical manifestations, such as metabolic syndrome, impaired glucose tolerance, reduced cholesterol associated with high density lipoprotein (HDL-c), hypertriglyceridemia, visceral adiposity, non-alcoholic fatty liver disease (NAFLD), increased epicardial fat thickness, subclinical vascular damage including arterial stiffening and increased arterial intima-media thickness (IMT), essential hypertension, glomerulomegaly, and albuminuria. Eventually, clinical complications of insulin resistance may develop, such as type 2 diabetes (T2D), cardiovascular disease (CVD), and chronic kidney disease (CKD) [1, 2, 3, 4, 5]. Obesity is a major determinant of OSA occurrence. OSA prevalence in obese patients undergoing bariatric surgery is strikingly high, having been reported to be 81.1% [6] and 90.07% [7]. However, insulin resistance and its clinical consequences develop in OSA patients irrespective of general obesity. Insulin resistance in OSA patients is an adaptive response to oxyhemoglobin desaturation and tissue hypoxia due to nocturnal apnea episodes. Oxyhemoglobin desaturation has been strongly and independently associated with insulin resistance and its complications in patients with OSA. Both obese and non-obese OSA patients develop this metabolic adaptation (Fig. 1). Molecular mechanisms underlying hypoxia-induced insulin resistance in OSA patients involve hypoxia inducible factor (HIF) upregulation and peroxisome proliferator-activated receptor-gamma (PPAR-) downregulation, which reduces glucose uptake into adipocytes, suppresses adipogenesis, and causes whole-body insulin resistance [1, 2, 8, 9, 10, 11].
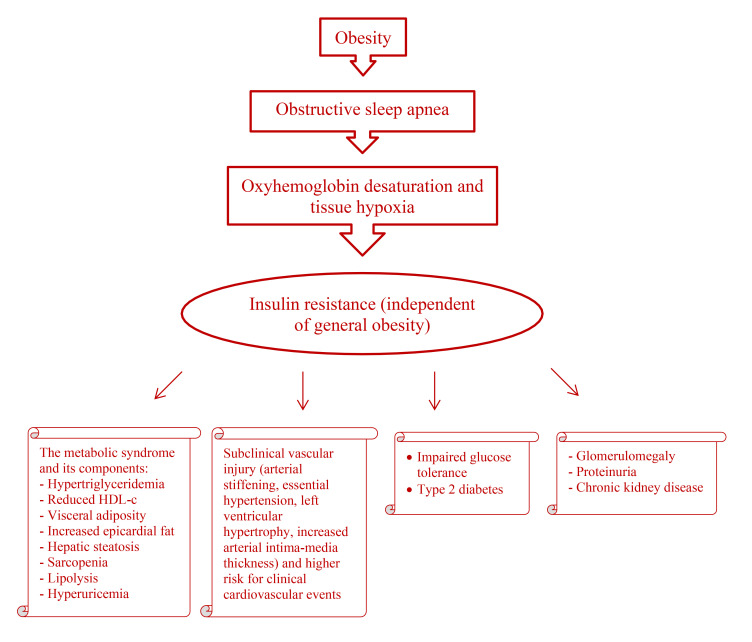
Fig. 1.
Obstructive sleep apnea induces oxyhemoglobin desaturation and tissue hypoxia. Insulin resistance develops as an adaptive process due to tissue hypoxia. Obesity contributes to obstructive sleep apnea, but insulin resistance and its complications (the metabolic syndrome and its components, type 2 diabetes, cardiovascular disease, and kidney disease) occur in patients with obstructive sleep apnea irrespective of general obesity. HDL-c, high density lipoprotein-associated cholesterol.
Information on the connection between hypoxia-induced insulin resistance and its clinical consequences (including CVD, CKD, and T2D) in patients with OSA may help improve the prevention and clinical management of these common conditions in clinical practice. A recent systematic review and meta-analysis that included 9 randomized studies reveals that nocturnal oxygen therapy reduces OSA severity compared to sham, suggesting that this therapy may facilitate the prevention of OSA complications [12].
2. Objectives
Short term exposure to hypobaric hypoxia (high altitude) induces adaptive insulin resistance and a metabolic adjustment that matches reduced oxygen delivery to mitochondrial oxygen consumption. As patients with OSA experience chronic nocturnal oxyhemoglobin desaturation and tissue hypoxia, we investigated the metabolic adaptation that takes place among these patients and its clinical consequences.
3. Methods
This is a comprehensive narrative review pertaining to insulin resistance in patients with OSA. A thorough and exhaustive search of published literature on this topic was performed by using the PubMed database from its inception to February 2024. Only articles written in English and concerning human beings were included. Authors MAA, ADM, ECQ, and RFC contributed to the literature search. MAA approved the final list of included studies.
4. Obstructive Sleep Apnea is Associated with Insulin Resistance, its Clinical Manifestations, and its Clinical Complications
4.1 Obstructive Sleep Apnea is Associated with Insulin Resistance
Compared to control subjects, both children and adults with OSA experience insulin resistance, assessed by a variety of procedures including hyperinsulinemic euglycemic clamps. The prevalence of insulin resistance among OSA patients has been reported to be 64.3% [13]. Cross-sectional studies have observed an independent association between OSA and elevated homeostasis model assessment of insulin resistance (HOMA-IR) values [2, 3, 4, 5, 7, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25], fasting hyperinsulinemia [2, 3, 4, 14, 19, 21, 26, 27, 28], the triglyceride-glucose index, calculated as: In [fasting triglycerides (mg/dL) fasting glucose (mg/dL)/2] [10, 11], and insulin resistance determined by intravenous glucose tolerance tests [29] or steady-state plasma glucose and insulin levels [27, 30]. The cross-sectional association between OSA and insulin resistance is independent of body mass index (BMI) and other confounding factors. OSA patients endure more severe insulin resistance compared to control individuals with comparable BMI. In addition, there is a positive correlation between OSA severity and intensity of insulin resistance (HOMA-IR index). Likewise, the prevalence of insulin resistance (evaluated by the HOMA-IR index and fasting insulin level) is higher among women with polycystic ovary syndrome and OSA compared to women with polycystic ovary syndrome without OSA, after controlling for BMI and other factors. OSA severity is highly correlated with the degree of insulin resistance among these patients [31]. Longitudinal trials establish that OSA precedes the development of insulin resistance. In a prospective community-based study that followed 141 non-diabetic patients with OSA for a mean period of 11 years, a diagnosis of OSA at baseline was independently related to the development of new-onset insulin resistance (evaluated by the HOMA-IR index and oral glucose tolerance tests) at follow-up, after controlling for BMI and other confounders. OSA independently increases the risk of insulin resistance [32]. Systematic reviews and meta-analyses confirm an association between OSA and insulin resistance determined via an elevated HOMA-IR [33] or the atherogenic index of plasma, calculated according to the formula: log (serum triglyceride level/serum HDL-c level) [34]. Compared to usual care, OSA therapy with continuous positive airway pressure (CPAP) [35, 36, 37, 38, 39, 40] or implantation of a mandibular advancement device [41], improves insulin resistance without changing BMI, supporting a role for tissue hypoxia in the pathogenesis of insulin resistance.
4.2 Obstructive Sleep Apnea is Associated with the Metabolic Syndrome and its Components
Correspondingly with the greater degree of insulin resistance, OSA is independently associated with clinical manifestations of this metabolic adaptation to hypoxia, such as metabolic syndrome and its components.
4.2.1 Obstructive Sleep Apnea is Associated with the Metabolic Syndrome
Observational studies [4, 14, 16, 17, 18, 22, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57] and a systematic review and meta-analysis [58] Consistently reveal that the prevalence of metabolic syndrome is increased in OSA patients, compared to control subjects, independently of BMI and other covariates. Metabolic syndrome is approximately 9 times more likely to occur in patients with OSA compared to control subjects. The number of features of metabolic syndrome increases with an increase in OSA severity, regardless of BMI.
4.2.2 Obstructive Sleep Apnea is Associated with Increased Visceral Fat
OSA patients (children and adults) suffer from marked visceral adiposity compared to control subjects, regardless of general obesity (BMI). The excess of visceral fat in OSA patients is determined by ultrasonography, magnetic resonance imaging, bioimpedance analysis, increased waist/hip ratio, widened waist circumference, or the visceral adiposity index (calculated with a formula that includes waist circumference, BMI, triglycerides, and HDL-c). Irrespective of body weight or BMI, OSA patients exhibit increased visceral adipose tissue, reflecting insulin resistance. In addition, OSA severity is strongly predictive of the visceral fat depot, unlike BMI, total fat, or subcutaneous fat [20, 22, 28, 43, 45, 50, 51, 52, 55, 56, 57, 59, 60, 61, 62, 63, 64, 65, 66, 67].
4.2.3 Obstructive Sleep Apnea is Associated with Non-Alcoholic Fatty Liver Disease
NAFLD is a clinical expression of insulin resistance. Therefore, the prevalence of NAFLD is higher in OSA patients compared to control subjects, independent of BMI and other confounding variables. Even in the absence of obesity, the prevalence of NAFLD is increased in OSA patients [7, 15, 68, 69, 70]. NAFLD is present in 81.8% of unselected OSA patients and 96.0% of patients with severe OSA undergoing bariatric surgery [69]. Patients with severe OSA are approximately 53 times more likely to have NAFLD compared to control subjects. There is a positive correlation between the severity of OSA and the degree of hepatic steatosis [70]. Systematic reviews and meta-analyses reveal that OSA is independently related to the development and progression of NAFLD determined by elevated liver enzymes and histological alterations including hepatic fibrosis, both in children and adults [71, 72, 73, 74].
4.2.4 Obstructive Sleep Apnea is Associated with Increased Epicardial Fat Thickness
Increased epicardial fat thickness is an indicator of excessive visceral fat and consequently reflects the presence of insulin resistance. OSA patients show broader epicardial fat thickness compared to control subjects, independent of general obesity (BMI). Both non-obese and obese OSA patients have thicker epicardial fat compared to control individuals. In addition, OSA severity correlates with the magnitude of epicardial fat expansion [50, 54, 75, 76, 77]. These findings are confirmed in a systematic review and meta-analysis that included 9 studies and 1178 subjects [78].
4.2.5 Obstructive Sleep Apnea is Associated with Insulin Resistance-Associated Dyslipidemia
Cross-sectional investigations show an association between OSA and insulin resistance-related dyslipidemia (hypertriglyceridemia and reduced HDL-c). Both adults and children with OSA endure a greater prevalence of hypertriglyceridemia and decreased HDL-c compared to control subjects, after controlling for covariates. Consequently, the triglyceride/HDL-c ratio and its logarithmic transformation (the atherogenic index of plasma) are higher in OSA patients, compared to control subjects. The association of OSA and insulin resistance-mediated dyslipidemia is independent of BMI and therefore occurs in non-obese as well as obese OSA patients. OSA severity is associated with worse lipid abnormalities (decreased HDL-c and increased triglyceride levels) [4, 5, 14, 20, 21, 25, 46, 54, 79, 80, 81, 82, 83]. In contrast, no independent association between OSA and serum levels of total cholesterol or low densitiy lipoprotein-associated choleserol (LDL-c) has been observed [79]. These findings are confirmed in a systematic review that pooled 25 studies for meta-analysis [84].
4.3 Obstructive Sleep Apnea is Associated with Subclinical Vascular Injury and Clinical Cardiovascular Events
It has long been known that patients with OSA endure a high cardiovascular risk that cannot be justified by conventional cardiovascular risk factors. OSA is independently associated with subclinical vascular injury (increased arterial stiffness, left ventricular hypertrophy, increased intima-media thickness, and reduced flow-mediated vasodilation), essential hypertension, and clinical cardiovascular events (coronary artery disease, congestive heart failure, peripheral artery disease, and stroke). Subclinical vascular injury and elevated cardiovascular risk in OSA patients have been regularly associated with insulin resistance, similar to subjects from the general population and other population groups, such as patients with diabetes and patients with CKD.
4.3.1 Obstructive Sleep Apnea is Associated with Increased Arterial Stiffness
Longitudinal trials have shown that increased arterial stiffness (reduced arterial distensibility) precedes the development of essential hypertension and predicts future cardiovascular events among normotensive community-dwelling individuals. Asymptomatic arterial stiffening is an independent risk factor for essential hypertension and CVD. Insulin resistance has consistently been associated with increased arterial stiffness in a number of population groups, including the general population, patients with CKD, and patients with diabetes, independently of other vascular risk factors [85, 86, 87, 88, 89, 90]. Cross-sectional investigations associate OSA with increased arterial stiffness on different arterial areas, evaluated by a variety of procedures, including pulse wave velocity (carotid-femoral, brachial-ankle, or central), the augmentation index, the cardio-ankle vascular index, aortic distensibility calculated from the aortic diameters measured by echocardiography, ultrasound speckle-tracking-based analysis of carotid artery, and cardiovascular magnetic resonance of the aorta and carotid arteries. The association between OSA and arterial stiffening is independent of classical cardiovascular risk factors, such as systemic hypertension and general obesity (BMI). Increased arterial stiffness has been detected in normotensive patients with minimally symptomatic OSA, suggesting that subclinical vascular damage affects OSA patients from the onset of the disorder and precedes the development of arterial hypertension. In addition, OSA severity correlates with the degree of arterial stiffening [51, 53, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108]. Systematic reviews and meta-analyses confirm that OSA is a risk factor for reduced arterial distensibility, independent of conventional cardiovascular factors [109, 110, 111]. Interventional studies show that therapy with positive air pressure (either continuous or autoadaptive) improves arterial stiffening in OSA patients compared to baseline values, ineffective CPAP or conservative therapy, supporting a role for tissue hypoxia in arterial stiffening. The improvement in arterial stiffness occurs early after the initiation of CPAP and reverts quickly with CPAP withdrawal [99, 112, 113, 114, 115, 116, 117, 118]. Systematic reviews and meta-analyses confirm that CPAP therapy reduces arterial stiffening in patients with OSA [109, 110, 119]. Likewise, OSA therapy with the implantation of a mandibular advancement device improves insulin resistance and arterial stiffening compared with baseline values [41].
4.3.2 Obstructive Sleep Apnea is Associated with Essential Hypertension
The prevalence of essential hypertension is higher in patients with OSA (both children and adults) compared to control subjects, regardless of BMI and other risk factors. Further, OSA is associated with poorer blood pressure control among hypertensive patients. In addition, systolic and diastolic blood pressure values increase with OSA severity [4, 14, 26, 42, 43, 45, 46, 47, 49, 53, 54, 57, 60, 80, 120, 121, 122]. Longitudinal surveys on the general population [123] and the elderly [124] reveal that OSA precedes the development of systemic hypertension. Patients with OSA at baseline are at increased risk for developing essential hypertension at follow-up compared to control subjects without baseline OSA. In a systematic review and meta-analysis, pooled data from 6 studies show that OSA patients experience an increased risk for resistant hypertension compared to control subjects, after adjustment for traditional risk factors [125].
Notwithstanding the association between OSA and arterial hypertension, systematic reviews and meta-analyses of placebo-controlled randomized trials show that CPAP therapy reduces only slightly blood pressure values in OSA patients, the effect being more intense in patients with resistant hypertension [126, 127, 128, 129, 130, 131, 132, 133, 134, 135, 136, 137]. Similarly to CPAP, the implantation of mandibular advancement devices mildly reduces systolic and diastolic blood pressure [138].
4.3.3 Obstructive Sleep Apnea is Associated with Left Ventricular Hypertrophy and Left Ventricular Dysfunction
In 1990, a case-control investigation revealed that left ventricular hypertrophy (LVH) was more frequent in normotensive patients with OSA compared to control subjects without OSA. Both the left ventricular mass and the left ventricular mass index (left ventricular mass/body surface area) were larger among normotensive OSA patients, after adjustment for weight, suggesting that OSA is a risk factor for LVH even in normotensive patients [139]. Subsequent cross-sectional studies [50, 93, 140, 141, 142, 143, 144, 145, 146] and a meta-analysis [147] reveal that OSA patients (children and adults) are at increased risk for LVH, after controlling the confounding effects of hypertension, general obesity, and other factors. The prevalence of LVH in the pooled OSA population was 45% [147] while LVH has been reported in 88% of patients with severe OSA. Decreased aortic distensibility present in OSA patients increases left ventricular afterload and may contribute to an increase in left ventricular mass [141]. CPAP therapy in patients with OSA has been reported to reduce LVH compared to usual care [141, 148].
In addition to LVH, both children and adults with OSA may exhibit other echocardiographic abnormalities, including increased left ventricular diameters (diastolic and systolic), decreased left ventricular ejection fraction [149], systolic and diastolic left ventricular dysfunction (assessed by the left ventricle myocardial performance index) [150], left atrial enlargement [97, 141], right atrial enlargement, and right ventricular hypertrophy [140, 141].
4.3.4 Obstructive Sleep Apnea is Associated with Increased Arterial Intima-Media Thickness
Cross-sectional and case-control trials show that OSA patients exhibit increased carotid IMT compared to control individuals. The severity of OSA correlates positively with carotid IMT. Increased carotid IMT is present in OSA patients without hypertension, diabetes or CVD, indicating that subclinical vascular injury occurs early in the evolution of the disorder, independent of traditional risk factors [50, 52, 64, 92, 98, 100, 151, 152, 153, 154, 155, 156]. A population-based, prospective cohort study that followed 790 randomly selected Wisconsin residents from a polysomnogram to a carotid ultrasound (average of 13.5 years) determined that OSA precedes thickening of the carotid artery. Baseline OSA predicted future increased carotid IMT independent of conventional cardiovascular risk factors [157]. In a systematic review and meta-analysis that included 18 studies, OSA was a risk factor for increased carotid IMT, after adjustment for major confounders. OSA patients had a higher carotid IMT compared to healthy controls [158]. CPAP therapy [159] or nasal surgery and uvulo-palato-pharyngoplasty (in OSA patients with narrowing at the nasal cavity and retropalatal airways) [160] have been reported to reduce carotid IMT compared to conservative treatment. However, no change of carotid IMT after CPAP treatment was observed in a meta-analysis that included 7 studies, although carotid IMT was decreased in patients with severe OSA who received CPAP 6 months [161].
4.3.5 Obstructive Sleep Apnea is Associated with Reduced Endothelial Vasodilation
Cross-sectional and case-control investigations show that flow-mediated vasodilation and the reactive hyperemia index are reduced in OSA patients compared to control subjects, suggesting that OSA patients experience asymptomatic vascular damage [92, 95, 96, 115, 156]. In a systematic review and meta-analysis that included 18 articles, flow-mediated vasodilation in patients with OSA was lower compared to control subjects [111].
4.3.6 Obstructive Sleep Apnea is Associated with Increased Clinical Cardiovascular Events
Cross-sectional [17, 42, 103, 162, 163] and longitudinal [164, 165, 166, 167, 168] studies reveal that the rate of clinical cardiovascular events (coronary artery disease, heart failure, peripheral artery disease, and stroke) is increased in OSA patients compared to control subjects. Longitudinal surveys have established that OSA precedes CVD. The diagnosis of OSA at baseline is a predictor of CVD independent of conventional cardiovascular risk factors [164, 165, 166, 167, 168]. Systematic reviews and meta-analyses consistently confirm that OSA is associated with increased cardiovascular risk [169, 170, 171, 172, 173, 174, 175, 176]. A beneficial effect of CPAP on cardiovascular outcomes compared to usual care has not been consistently found among OSA patients, particularly in randomized controlled clinical trials. In a recent systematic review and meta-analysis that included 11 studies (5 randomized controlled trials and 6 observational studies), CPAP therapy was associated with a modest risk reduction in cardiovascular events among patients with OSA and concomitant coronary artery disease. In addition, CPAP reduced the risk of all-cause and cardiovascular death by 23%. Subgroup analysis revealed that a CPAP adherence time 4 hours/night had a greater benefit on preventing cardiovascular events [137].
4.4 Obstructive Sleep Apnea is Associated with Impaired Glucose Tolerance and Increased Risk for Type 2 Diabetes
In OSA patients, insulin resistance causes impaired glucose tolerance and predisposes them to T2D. The prevalence of glucose intolerance is higher in OSA patients compared to control subjects, independent of BMI. Non-diabetic patients with OSA have increased levels of fasting glucose, postprandial glucose, and glycosylated hemoglobin, compared to control subjects, after adjustment for confounding factors [1, 21, 45, 46, 52, 60, 66, 80, 177, 178]. Likewise, cross-sectional [1, 43, 179, 180] and longitudinal [181, 182, 183] studies reveal an independent association between OSA and T2D. The prevalence of T2D is greater in OSA patients compared to control subjects, regardless of general obesity and other risk factors. Longitudinal investigations establish that OSA precedes T2D. Patients with OSA at baseline have a 30% higher risk of developing new-onset T2D at follow-up compared to control individuals, independent of age, race, gender, baseline fasting glucose, and BMI. A meta-analysis of 6 prospective cohort studies and 5953 participants confirms that OSA is an independent risk factor for the development of new-onset T2D, compared with the absence of OSA, over follow-up periods from 2.7 years to 16 years [184]. Longitudinal observational studies show that therapy with positive airway pressure [181, 185] or upper airway surgery [185] reduces the risk of new-onset T2D in OSA patients compared to conservative therapy, independent of confounding factors. Supporting the presence of more severe insulin resistance, T2D patients with OSA endure worse glycemic control compared to T2D patients without OSA, regardless of BMI. Even mild OSA has a negative influence on glycemic control among T2D patients [186, 187, 188]. Investigations on the effect of CPAP on glycemic control (glycosylated hemoglobin) in OSA patients with T2D have yielded inconsistent results. An association between hours of CPAP usage and glycosylated hemoglobin reduction has been observed, such that better adherence to CPAP is associated with greater improvements in glycemic control [189, 190].
4.5 Obstructive Sleep Apnea is Associated with Kidney Disease (Albuminuria, Glomerulomegaly, and Chronic Kidney Disease)
OSA-related insulin resistance has been associated with albuminuria, glomerulomegaly, CKD, and accelerated loss of kidney function toward end-stage kidney disease.
4.5.1 Obstructive Sleep Apnea is Associated with Albuminuria
In 1984, a patient with severe OSA was noted to develop nephrotic syndrome [191]. Subsequent cross-sectional surveys showed that the prevalence of proteinuria (sometimes in nephrotic range) is higher in both children and adults with OSA compared to control subjects, after adjustment for confounding factors. Even OSA patients without diabetes or hypertension experience a higher risk of developing albuminuria (urinary albumin excretion rate or urinary albumin/creatinine ratio) compared to control subjects [192, 193, 194, 195, 196, 197, 198, 199, 200, 201]. In addition, an association between OSA severity and the degree of albuminuria has been observed [202, 203]. Systematic reviews and meta-analyses confirm that OSA is independently associated with higher urinary albumin excretion rate compared to control subjects [204, 205]. Therapy with positive airway pressure reduces albuminuria in OSA patients, either non-diabetic [192, 206, 207] or patients with diabetic kidney disease [208, 209]. CPAP effect on albuminuria is more profound in patients with more severe OSA [197].
4.5.2 Obstructive Sleep Apnea is Associated with Glomerulomegaly and Focal Segmental Glomerulosclerosis
OSA patients with proteinuria exhibit a kidney histopathological picture consistent with insulin resistance, namely glomerulomegaly, focal segmental glomerulosclerosis, and prominent involvement of kidney arterial vessels (hyaline sclerosis of arterioles and fibroelastic thickening of artery walls). The histopathological findings in OSA patients replicate those observed in other conditions associated with tissue hypoxia (and adaptive insulin resistance), such as high-altitude maladaptation, sickle cell disease, and cyanotic congenital heart disease, suggesting a causative role for hypoxia-related insulin resistance in the kidney lesion [206, 210, 211]. On light microscopy, glomeruli are markedly enlarged and focal segmental glomerulosclerosis is observed. Mesangial matrix and mesangial cellularity are usually increased. Arterial involvement is conspicuous with marked arteriolar hyalinosis and arterial intima-media thickening. Correspondingly with arterial damage, focal areas of tubular atrophy and interstitial fibrosis are identified. Immunofluorescence staining is usually negative. Electron microscopy reveals effacement of podocyte foot processes. No subendothelial, mesangial or subepithelial immune complex deposits are noted [206, 210, 211].
4.5.3 Obstructive Sleep Apnea is Associated with Chronic Kidney Disease
OSA-related insulin resistance is an independent risk factor for CKD and accelerated progression to end-stage kidney disease [212]. The prevalence of CKD, defined as estimated glomerular filtration rate (GFR) 60 mL/min/1.73 , is higher among OSA patients compared to control subjects, after adjustment for BMI and other confounders. GFR estimation was assessed with the MDRD (modification of diet in renal disease) equation, the CKD-EPI (CKD Epidemiology Collaboration) equation, or the Cockcroft-Gault formula, the prevalence of CKD remains higher in OSA patients without essential hypertension or T2D. A positive correlation between OSA severity and worse kidney function has been found [200, 201, 202, 213, 214, 215, 216, 217, 218, 219]. Longitudinal studies show that OSA is associated with an increased risk for CKD and end-stage kidney disease compared with the general population, independent of conventional risk factors [212, 220, 221, 222]. In addition, cross-sectional [219] and longitudinal [223, 224] investigations show that OSA patients experience accelerated loss of kidney function, compared to control subjects, after adjustment of risk factors such as age, BMI, diabetes and heart failure. The loss of kidney function is faster in participants with OSA over the follow-up period. Systematic reviews and meta-analyses confirm that OSA patients endure a higher risk of CKD (lower estimated GFR), compared to control subjects. Accordingly, the serum level of cystatin C is increased in OSA patients compared to control individuals, independent of hypertension and diabetes [204, 205]. Likewise, OSA is associated with diabetic kidney disease in T2D patients [225]. Investigations concerning the effect of CPAP therapy on kidney function have rendered conflicting results. Some studies show a slower rate of progression among OSA patients treated with CPAP [226, 227, 228] whereas a systematic review and meta-analysis found no clinically relevant effect of CPAP on estimated GFR [229].
5. Oxyhemoglobin Desaturation (Leading to Tissue Hypoxia) Induces Insulin Resistance in Patients with Obstructive Sleep Apnea
In humans, insulin resistance is an adaptive response to normobaric hypoxia [230, 231, 232, 233, 234] and hypobaric hypoxia (exposure to high altitude) [235, 236, 237]. Likewise, oxyhemoglobin desaturation (leading to tissue hypoxia) has been consistently linked to insulin resistance and its clinical consequences in OSA patients. The severity of oxygen desaturation in these patients may be expressed in a variety of ways, such as the average nocturnal oxygen saturation, the lowest oxygen saturation, the percentage of time spent below 90% oxygen saturation during sleep, and the oxyhemoglobin desaturation index, defined as the average number of desaturation episodes occurring per hour. A desaturation episode is a reduction in oxyhemoglobin saturation 3% that lasts 10 seconds. Oxyhemoglobin saturation is the percentage of hemoglobin bound to oxygen.
5.1 Oxyhemoglobin Desaturation is Independently Associated with Insulin Resistance in Patients with Obstructive Sleep Apnea
Cross-sectional studies show that oxyhemoglobin desaturation is strongly and independently associated with insulin resistance in children and adults with OSA [1, 2, 3, 4, 6, 8, 9, 10, 11, 23, 56, 62, 177, 238, 239, 240, 241]. Likewise, a prospective community-based study with a mean follow-up period of 11 years reveals that more severe oxygen desaturation at baseline is independently associated with more profound insulin resistance at follow-up (evaluated by the HOMA-IR index and the insulin sensitivity index) [32].
5.2 Oxyhemoglobin Desaturation is Independently Associated with the Metabolic Syndrome and its Components in Patients with Obstructive Sleep Apnea
OSA patients with lower oxyhemoglobin saturation experience clinical manifestations of insulin resistance, regardless of BMI and other risk factors. A variety of oxygen desaturation estimates (oxyhemoglobin desaturation index, minimum arterial oxygen saturation, and mean nocturnal oxygen saturation) are independently and robustly associated with metabolic syndrome [49], visceral adiposity [55, 59, 62], non-alcoholic fatty liver disease [242], epicardial fat thickness [77], and dyslipidemia (hypertriglyceridemia and decreased HDL-c) [4, 25, 48, 55, 80, 82] among both obese and non-obese OSA patients.
5.3 Oxyhemoglobin Desaturation is Independently Associated with Cardiovascular Disease in Patients with Obstructive Sleep Apnea
In OSA patients, the hypoxia burden is correlated with subclinical vascular damage. The lowest oxygen saturation, the average oxygen saturation, the oxyhemoglobin desaturation index, and the percentage of time spent below 90% oxygen saturation during sleep, are robustly and independently associated with increased arterial stiffness [96, 243, 244], elevated blood pressure [4, 48, 49, 120, 245], increased carotid IMT [98, 152, 153, 155, 246], and left ventricular dysfunction [146, 245, 247, 248, 249]. Unlike the apnea hypopnea index, oxyhemoglobin desaturation strongly predicts asymptomatic vascular injury and myocardial performance in OSA patients, after adjustment for conventional cardiovascular risk factors. Accordingly, the improvement in oxygen saturation after uvulo-palato-pharyngoplasty showed a correlation with a reduction of arterial IMT [160].
5.4 Oxyhemoglobin Desaturation is Associated with Impaired Glucose Tolerance, Worse Glycemic Control, and Increased Risk of Type 2 Diabetes in Patients with Obstructive Sleep Apnea
OSA patients with more severe nocturnal hypoxemia (assessed by the oxyhemoglobin desaturation index) show an increased risk of T2D [187] and more elevated fasting glucose levels [48, 80], after adjustment for obesity and other confounding factors. In addition, OSA patients with T2D and more pronounced hypoxemia endure worse metabolic control. The percentage of time spent under 90% oxygen saturation and the minimum oxygen saturation are associated with higher glycosylated hemoglobin (HbA1c), independent of general adiposity and other confounders. There is a positive relationship between nocturnal hypoxemia and deficient glycemic control in OSA patients with T2D. Every 10% reduction in minimum oxygen saturation is associated with a 0.3% increase in HbA1c whereas a 10% increase in the percentage of time spent under 90% oxygen saturation is associated with a 0.2% increase in HbA1c [186, 187, 188, 250]. Longitudinal studies reveal that decreased oxygen saturation at baseline predicts incident T2D at follow-up in OSA patients, after adjustment for BMI and other risk factors, suggesting that more severe hypoxemia drives insulin resistance and predisposes to T2D. Unlike the apnea-hypopnea index, the time spent with oxygen saturation of less than 90% is associated with incident diabetes in adjusted models [1, 32, 56, 181, 182, 183].
5.5 Oxyhemoglobin Desaturation is Independently Associated with Kidney Disease in Patients with Obstructive Sleep Apnea
In OSA patients, a more severe hypoxia burden has been consistently associated with increased albuminuria and worse kidney function. Children and adults with more pronounced nocturnal hypoxemia (assessed by the lowest oxygen saturation, the length of sleep time spent at oxygen saturation below 90%, and the desaturation index) experience more severe albuminuria, independent of BMI and other confounders [195, 196, 197, 198, 200, 214, 251, 252]. Accordingly, proteinuria falls after improvement of oxygen saturation with CPAP [252, 253]. The prevalence of CKD (estimated GFR 60 mL/min/1.73 ) is higher in OSA patients with more severe hypoxia burden, after adjusting for confounders. Profounder nocturnal hypoxemia (evaluated by the time spent under 90% oxygen saturation, the mean oxygen saturation, and the lowest nocturnal oxygen saturation) is inversely correlated with estimated GFR, such that OSA patients with more severe hypoxia burden experience worse kidney function (lower estimated GFR). Furthermore, the minimum oxygen saturation is an independent predictor of CKD, indicating that severe hypoxemia, even for a short period of time, is a risk factor for kidney dysfunction [200, 216, 217, 227]. Retrospective cohort trials reveal that oxyhemoglobin desaturation is independently associated with an increased risk for accelerated loss of kidney function and progression to end-stage kidney disease. Patients with more severe hypoxemia experience a faster loss of kidney function [223, 224]. Likewise, more profound nocturnal hypoxemia (time spent under 90% oxygen saturation) is associated with diabetic kidney disease and worse kidney function, after adjusting for confounding variables in patients with OSA and T2D [225, 254]. Accordingly, CPAP therapy improves oxyhemoglobin desaturation and decelerates the rate of CKD progression in patients with CKD and OSA [227]. In OSA patients with advanced CKD, more severe hypoxemia (higher sleep time with oxygen saturation below 90% and lower mean oxygen saturation) is associated with higher mortality over the follow-up period (9 years) [255].
6. Pathophysiological Processes Underlying Insulin Resistance in Patients with Obstructive Sleep Apnea
Pathogenic mechanisms leading to adaptive insulin resistance due to tissue hypoxia in OSA patients involve the upregulation of HIFs and downregulation of PPAR-. Silencing of PPAR- in adipocytes reduces glucose uptake, impairs lipid droplet biogenesis, and induces whole-body insulin resistance [234].
6.1 Hypoxia Induces HIF-1 Upregulation and Subsequent PPAR- Downregulation in Patients with Obstructive Sleep Apnea
HIFs are transcription factors that regulate the transcription of target genes by binding to specific DNA sequences named hypoxia response elements. HIFs are heterodimeric proteins that possess two subunits, and . Three isoforms of the subunit have been identified (HIF-1, HIF-2, and HIF-3) whereas only one subunit has been reported to exist in humans. Each isoform of the subunit may dimerize with the subunit to generate the corresponding HIF (HIF-1, HIF-2, or HIF-3). Formation of human HIFs can only be completed when the oxygen supply to tissues is limited, because, under normoxia, HIF- is continuously degraded and unavailable for dimerization with HIF-. Degradation of HIF- requires initial hydroxylation (catalyzed by prolyl hydroxylase isoenzymes) and subsequent binding to the von Hippel Lindau protein. The hydroxylation of specific proline residues in HIF- permits the binding to the von Hippel Lindau protein. In turn, attachment to the von Hippel Lindau protein labels hydroxylated HIF- to be destroyed [256, 257]. Therefore, inhibition of HIF- prolyl hydroxylases and/or a defective von Hippel Lindau protein block the degradation of HIF- and enable the formation of functional HIFs in humans [258]. HIF- prolyl hydroxylase isoenzymes require oxygen, 2-oxoglutarate, iron, and ascorbate for activity. Consequently, deficiency of either one of them inhibits the hydroxylation of HIF-, blocks its degradation, and allows the dimerization of intact HIF- with the subunit to produce operative HIFs [256, 257] (Fig. 2). Oxygen deprivation (hypoxia) inhibits the activity of prolyl-hydroxylases, promoting the formation of HIFs. In vitro studies using a variety of human cell lines show that HIF-1 expression increases during hypoxia exposure [259, 260, 261, 262, 263, 264]. Similarly to oxygen shortage, the deficiency of 2-oxoglutarate [265], iron [266], and ascorbate [267] suppresses HIF- prolyl hydroxylases and generates HIFs. Once activated, HIFs modulate the transcription of target genes to orchestrate the response to hypoxia or other conditions. The PPARG gene, which codes for PPAR-, has been identified as a target gene for HIF-1 in human cells, including adipocytes. In response to hypoxia, HIF-1 reduces PPARG expression (mRNA and protein) and inhibits PPAR- activity [259, 260, 263, 268, 269, 270, 271].
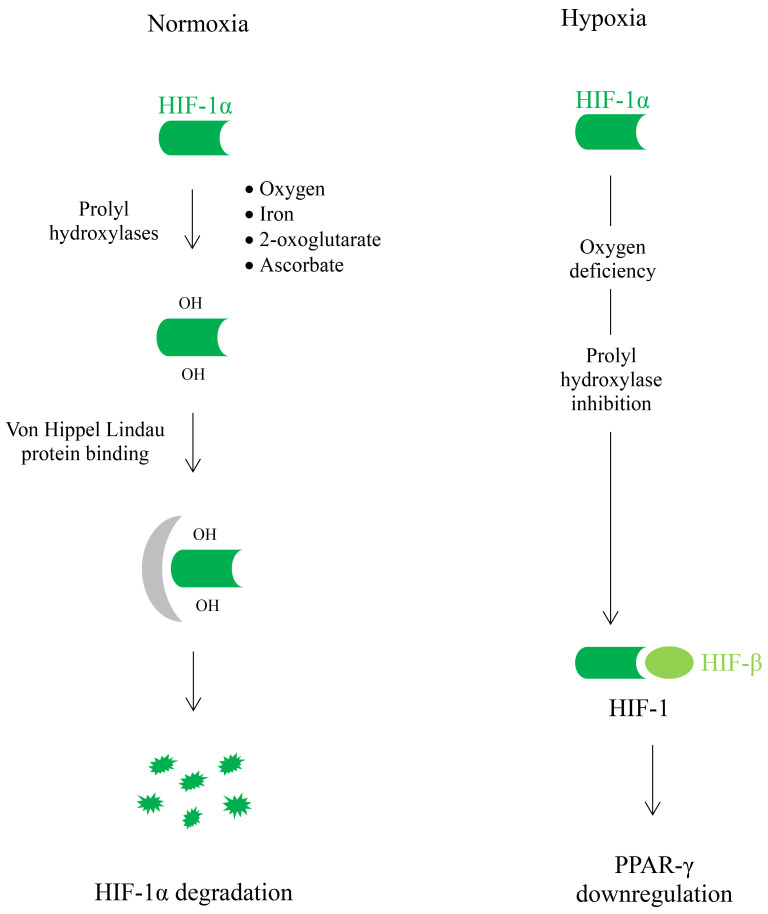
Fig. 2.
In humans, formation of human hypoxia-inducible factors (HIFs) can occur only under hypoxia. Under normal conditions (normoxia), HIF- is continuously degraded and cannot dimerize with HIF-. Breakdown of HIF- requires the action of prolyl hydroxylase isoenzymes and the subsequent attachment to the von Hippel Lindau protein, which labels HIF- for degradation. HIF- prolyl hydroxylase isoenzymes require oxygen to catalyze HIF- hydroxylation. Therefore, hypoxia inhibits this reaction, impedes HIF- degradation and allows the formation of functional HIF-1. Once activated, HIF-1 downregulates the expression of PPARG gene, which codes for peroxisome proliferatoractivated receptor gamma (PPAR-) and suppresses PPAR- activity.
6.2 Patients with Obstructive Sleep Apnea Exhibit HIF1A Upregulation and PPARG Downregulation in the Adipose Tissue
In OSA patients, increased expression of the HIF1A gene (which encodes HIF-1) and downregulation of PPARG have been observed in the subcutaneous adipose tissue, compared to control subjects, independent of BMI [5, 272]. Additionally, OSA patients demonstrate higher serum levels of HIF-1 compared with control subjects. Further, serum HIF-1 level correlates with the number of desaturations during sleep [273].
6.3 PPAR- Silencing Causes Insulin Resistance Due to Defective Glucose Uptake into Adipocytes Leading to Suppression of Adipogenesis
In humans, glucose uptake into adipocytes by the glucose transporter-4 (GLUT-4) facilitates lipid droplet biogenesis, fat accumulation in the subcutaneous adipose tissue, and whole-body insulin sensitivity. These processes are accomplished by normal PPAR- activity. Thiazolidinediones (exogenous PPAR- agonists) replicate PPAR- effects whereas PPAR- silencing (such as occurs due to hypoxia-induced HIF-1 activation) mediates the opposite effects, namely reduction of GLUT-4-mediated glucose transport into adipocytes, suppression of fat deposition and lipid droplet formation in the subcutaneous adipose tissue, and whole-body insulin resistance (Fig. 3).
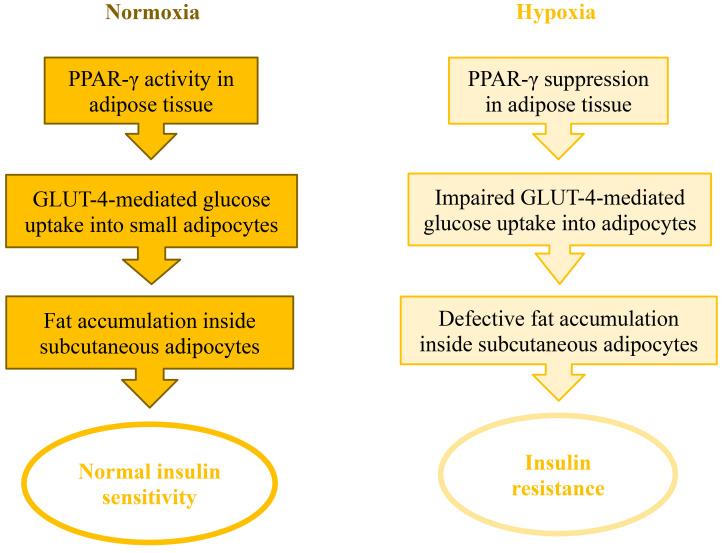
Fig. 3.
In humans, normal PPAR- activity in adipocytes involves glucose uptake mediated by the glucose transporter-4 (GLUT-4) and fat accumulation in the subcutaneous adipose tissue while enhancing whole-body insulin sensitivity. PPAR- silencing during hypoxic conditions induces the opposite effects, namely reduction of GLUT-4-mediated glucose transport into adipocytes, suppression of fat deposition, and whole-body insulin resistance. PPAR-, peroxisome proliferatoractivated receptor gamma.
6.3.1 Glucose Uptake into Adipocytes Promotes Adipocyte Differentiation and Defines Whole-Body Insulin Sensitivity in Normal Humans
6.3.1.1 Glucose Uptake into Adipocytes Mediated by the Glucose Transporter-4 Promotes Lipid Droplet Formation and Fat Deposition in the Adipose Tissue
In normal humans, GLUT-4 proteins carry glucose into adipocytes. The quantity of GLUT-4 declines markedly when adipocytes enlarge due to fat deposition, regardless of BMI and degree of insulin sensitivity, such that large adipocytes contain strikingly fewer GLUT-4 molecules compared to small adipocytes in lean individuals, obese subjects, and T2D patients. Correspondingly, GLUT-4 conveys glucose into small adipocytes while no relevant transport is detected on large cells from human omental and subcutaneous adipose tissue, suggesting that glucose uptake into small adipocytes has a crucial effect on triacylglycerol deposition and subsequent adipocyte enlargement, that is no longer required in large adipocytes [274, 275, 276, 277].
6.3.1.2 The Expression of Glucose Transporter-4 in Adipocytes Correlates with Whole-Body Insulin Sensitivity
The amount of GLUT-4 in small adipocytes correlates with whole-body insulin sensitivity, measured by euglycemic hyperinsulinemic clamps, such that subjects with enhanced insulin sensitivity possess more GLUT-4 whereas patients with insulin resistance possess markedly diminished GLUT-4 (mRNA and protein) levels in small adipocytes. Correspondingly, GLUT-4-mediated glucose uptake into subcutaneous adipocytes is markedly reduced in patients with insulin resistance (including T2D), suggesting that defective glucose uptake into subcutaneous adipocytes contributes to cause whole-body insulin resistance [276, 277, 278, 279, 280, 281, 282, 283].
Similarly to other subjects with insulin resistance, patients with gestational diabetes [284, 285] or polycystic ovary syndrome [286, 287, 288] display reduced GLUT-4 expression and impaired glucose uptake into adipocytes compared to control subjects, independent of obesity. The amount of GLUT-4 in adipocytes is highly correlated with the degree of insulin sensitivity among these patients, such that abundance of GLUT-4 is associated with enhanced insulin sensitivity while scarcity of GLUT-4 is observed in patients with insulin resistance (assessed by insulin-mediated glucose disposal or HOMA-IR index). In a systematic review of the literature, decreased GLUT-4 content in adipocytes (and subsequent impaired glucose transport) is a consistent abnormality in patients with polycystic ovary syndrome, regardless of general adiposity [288].
6.3.1.3 A Reduced Amount of GLUT-4 in Adipocytes and Reduced Fat Accumulation in the Subcutaneous Adipose Tissue Occur at the Onset of Insulin Resistance
Subjects with normal weight, glucose tolerance, and fasting triglyceride level, but with a genetic predisposition for T2D (first-degree relatives of T2D patients) demonstrate a profound reduction of GLUT-4 (mRNA and protein) in adipocytes [282, 289]. The magnitude of GLUT-4 reduction in subcutaneous adipocytes among these subjects is quantitatively similar to that observed in patients with T2D or impaired glucose tolerance [280]. In addition, healthy first-degree relatives of T2D patients exhibit a markedly diminished capability to store fat in the subcutaneous adipose tissue leading to an inappropriate expansion of the adipose cells. In these subjects, insulin resistance is identified by euglycemic hyperinsulinemic clamps [282, 289]. Therefore, reduced GLUT-4 in adipocytes and an impaired ability to store fat in the subcutaneous adipose tissue precedes any other clinical manifestation of insulin resistance, suggesting that these metabolic abnormalities occur at the onset of this metabolic adaptation [289].
6.3.1.4 In vitro Studies Indicate that Glucose Uptake into Adipocytes is Required for Fat Accumulation and Lipid Droplet Biogenesis
The link between glucose uptake into adipocytes and the ability to accumulate fat is further supported by in vitro studies using primary human adipocytes. Silencing of an endoplasmic reticulum protein involved in lipid droplet biogenesis (fat storage-inducing transmembrane protein-2) in primary human adipocytes reduces both glucose uptake and triacylglycerol accumulation, compared with control cells suggests that glucose transport into adipocytes is required for fat accumulation [290]. Consistently, fat storage-inducing transmembrane protein-2 is less abundant in the subcutaneous and omental adipocytes from T2D patients, compared to control subjects [290].
6.3.2 Human PPAR- Facilitates Glucose Uptake into Adipocytes, Subcutaneous Fat Deposition, and Insulin Sensitivity while PPAR- Inhibition has the Converse Effect
In normal humans, PPAR- normally promotes glucose uptake into adipocytes and triglyceride deposition on the subcutaneous adipose tissue while enhancing insulin sensitivity. PPAR- activity is essential for the process of subcutaneous fat deposition and adipocyte differentiation, but is not required for maintenance of the differentiated state. Depletion of PPAR- prevents fat deposition inside subcutaneous adipocytes but does not induce dedifferentiation of mature adipocytes once full differentiation has taken place. In OSA patients, PPAR- suppression is associated with decreased glucose uptake into adipocytes, reduced fat accumulation in the subcutaneous adipose tissue, and whole-body insulin resistance [291, 292, 293]. Thiazolidinediones (such as pioglitazone and rosiglitazone) are exogenous PPAR- ligands that activate PPAR- and reproduce its effects. In non-diabetic patients with insulin resistance (and therefore low GLUT-4 protein in adipose cells), pioglitazone therapy increases GLUT-4 expression in subcutaneous adipose tissue (mRNA and protein) and improves insulin resistance (assessed by euglycemic hyperinsulinemic clamps), strengthening the existence of a mechanistic link between reduced GLUT-4 protein in adipocytes and whole-body insulin resistance [282].
The study’s strengths include a meticulous and unbiased literature search attempting to retrieve all relevant articles on the connection between insulin resistance and OSA. However, some limitations need to be acknowledged. First, we searched only the PubMed database and restricted our search to articles published in English. As a result, some degree of selection bias may have occurred. Second, included studies were heterogenous in terms of study design, classifications for the severity of OSA, population groups, duration of follow-up, and the method of assessing complications of insulin resistance (for instance, blood pressure recordings, arterial stiffness, and kidney function). In addition, except for articles concerning OSA therapy, included investigations were mostly observational, limiting their ability to demonstrate causality.
7. Conclusions
Patients with obstructive sleep apnea experience apnea episodes that induce oxyhemoglobin desaturation and subsequent tissue hypoxia. In turn, tissue hypoxia induces adaptive insulin resistance. In patients with obstructive sleep apnea, oxyhemoglobin desaturation has been consistently associated with insulin resistance and its clinical consequences, independent of body mass index and other confounding factors. Patients with obstructive sleep apnea typically manifest metabolic syndrome, essential hypertension, hypertriglyceridemia, reduced HDL-c, visceral adiposity, non-alcoholic fatty liver disease, increased epicardial fat, subclinical vascular injury (such as reduced arterial distensibility, left ventricular hypertrophy, and increased arterial intima-media thickness), elevated cardiovascular risk, impaired glucose tolerance, predisposition to type 2 diabetes, and kidney disease (glomerulomegaly, albuminuria, and chronic kidney failure). In patients with obstructive sleep apnea, tissue hypoxia upregulates hypoxia-inducible factor-1, which induces peroxisome proliferator-activated receptor-gamma downregulation in the adipose tissue. Attenuation of peroxisome proliferator-activated receptor-gamma reduces glucose transport into adipocytes, impairs fat deposition in the subcutaneous adipose tissue and causes whole-body insulin resistance. Obesity is a major causative factor for obstructive sleep apnea, but insulin resistance develops in these patients irrespective of general adiposity (body mass index). Tissue hypoxia drives insulin resistance in patients with obstructive sleep apnea.
Acknowledgment
Not applicable.
Abbreviations
BMI, body mass index; CKD, chronic kidney disease; CPAP, continuous positive airway pressure; CVD, cardiovascular disease; GFR, glomerular filtration rate; GLUT, glucose transporter; HDL-c, cholesterol associated with high density lipoprotein; HIF, hypoxia-inducible factor; HOMA-IR, homeostasis model assessment-insulin resistance; IMT, intima-media thickness; LVH, left ventricular hypertrophy; NAFLD, non-alcoholic fatty liver disease; OSA, obstructive sleep apnea; PPAR, peroxisome proliferator-activated receptor.
Footnotes
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author Contributions
MAA designed the review, contributed to the literature search and wrote the original draft. ADM, ECQ, and RFC contributed to the literature search and data curation. MAA, CFF, and RFC performed the analysis of the data. ADM, CFF, and ECQ contributed to visualization. All authors contributed to review and editing the manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work. All authors read and approved the final manuscript.
Ethics Approval and Consent to Participate
Not applicable.
Funding
This research received no external funding.
Conflict of Interest
The authors declare no conflict of interest.
References
- [1].Punjabi NM, Sorkin JD, Katzel LI, Goldberg AP, Schwartz AR, Smith PL. Sleep-disordered breathing and insulin resistance in middle-aged and overweight men. American Journal of Respiratory and Critical Care Medicine . 2002;165:677–682. doi: 10.1164/ajrccm.165.5.2104087. [DOI] [PubMed] [Google Scholar]
- [2].Ip MSM, Lam B, Ng MMT, Lam WK, Tsang KWT, Lam KSL. Obstructive sleep apnea is independently associated with insulin resistance. American Journal of Respiratory and Critical Care Medicine . 2002;165:670–676. doi: 10.1164/ajrccm.165.5.2103001. [DOI] [PubMed] [Google Scholar]
- [3].Makino S, Handa H, Suzukawa K, Fujiwara M, Nakamura M, Muraoka S, et al. Obstructive sleep apnoea syndrome, plasma adiponectin levels, and insulin resistance. Clinical Endocrinology . 2006;64:12–19. doi: 10.1111/j.1365-2265.2005.02407.x. [DOI] [PubMed] [Google Scholar]
- [4].Lin QC, Zhang XB, Chen GP, Huang DY, Din HB, Tang AZ. Obstructive sleep apnea syndrome is associated with some components of metabolic syndrome in nonobese adults. Sleep & Breathing = Schlaf & Atmung . 2012;16:571–578. doi: 10.1007/s11325-011-0544-7. [DOI] [PubMed] [Google Scholar]
- [5].Thorn CE, Knight B, Pastel E, McCulloch LJ, Patel B, Shore AC, et al. Adipose tissue is influenced by hypoxia of obstructive sleep apnea syndrome independent of obesity. Diabetes & Metabolism . 2017;43:240–247. doi: 10.1016/j.diabet.2016.12.002. [DOI] [PubMed] [Google Scholar]
- [6].Polotsky VY, Patil SP, Savransky V, Laffan A, Fonti S, Frame LA, et al. Obstructive sleep apnea, insulin resistance, and steatohepatitis in severe obesity. American Journal of Respiratory and Critical Care Medicine . 2009;179:228–234. doi: 10.1164/rccm.200804-608OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Zhu C, Tang S, Xiao J, Zhang Y, Sun L, Zhang J, et al. Insulin Resistance, but Not Obstructive Sleep Apnea Is Associated with Hepatic Steatosis in Chinese Patients with Severe Obesity. Obesity Facts . 2023;16:344–355. doi: 10.1159/000528789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Shamsuzzaman A, Szczesniak RD, Fenchel MC, Amin RS. Glucose, insulin, and insulin resistance in normal-weight, overweight and obese children with obstructive sleep apnea. Obesity Research & Clinical Practice . 2014;8:e584–e591. doi: 10.1016/j.orcp.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Isobe Y, Nakatsumi Y, Sugiyama Y, Hamaoka T, Murai H, Takamura M, et al. Severity Indices for Obstructive Sleep Apnea Syndrome Reflecting Glycemic Control or Insulin Resistance. Internal Medicine (Tokyo, Japan) . 2019;58:3227–3234. doi: 10.2169/internalmedicine.3005-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Bikov A, Frent SM, Meszaros M, Kunos L, Mathioudakis AG, Negru AG, et al. Triglyceride-Glucose Index in Non-Diabetic, Non-Obese Patients with Obstructive Sleep Apnoea. Journal of Clinical Medicine . 2021;10:1932. doi: 10.3390/jcm10091932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Sökücü SN, Aydın Ş, Satıcı C, Tural Önür S, Özdemir C. Triglyceride-glucose index as a predictor of obstructive sleep apnoea severity in the absence of traditional risk factors. Arquivos De Neuro-psiquiatria . 2023;81:891–897. doi: 10.1055/s-0043-1776411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Phyu SL, Ercan S, Harriss E, Turnbull C. Nocturnal oxygen therapy in obstructive sleep apnoea: a systematic review and meta-analysis. European Respiratory Review: an Official Journal of the European Respiratory Society . 2024;33:230173. doi: 10.1183/16000617.0173-2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Azman M, Sani A, Kamaruddin NA. Insulin resistance using HOMA model in obstructive sleep apnea: a cross sectional study. Annals of Saudi Medicine . 2014;34:476–481. doi: 10.5144/0256-4947.2014.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Coughlin SR, Mawdsley L, Mugarza JA, Calverley PMA, Wilding JPH. Obstructive sleep apnoea is independently associated with an increased prevalence of metabolic syndrome. European Heart Journal . 2004;25:735–741. doi: 10.1016/j.ehj.2004.02.021. [DOI] [PubMed] [Google Scholar]
- [15].Tanné F, Gagnadoux F, Chazouillères O, Fleury B, Wendum D, Lasnier E, et al. Chronic liver injury during obstructive sleep apnea. Hepatology (Baltimore, Md.) . 2005;41:1290–1296. doi: 10.1002/hep.20725. [DOI] [PubMed] [Google Scholar]
- [16].Peled N, Kassirer M, Shitrit D, Kogan Y, Shlomi D, Berliner AS, et al. The association of OSA with insulin resistance, inflammation and metabolic syndrome. Respiratory Medicine . 2007;101:1696–1701. doi: 10.1016/j.rmed.2007.02.025. [DOI] [PubMed] [Google Scholar]
- [17].Tkacova R, Dorkova Z, Molcanyiova A, Radikova Z, Klimes I, Tkac I. Cardiovascular risk and insulin resistance in patients with obstructive sleep apnea. Medical Science Monitor: International Medical Journal of Experimental and Clinical Research . 2008;14:CR438–CR444. [PubMed] [Google Scholar]
- [18].Nieto FJ, Peppard PE, Young TB. Sleep disordered breathing and metabolic syndrome. WMJ: Official Publication of the State Medical Society of Wisconsin . 2009;108:263–265. [PMC free article] [PubMed] [Google Scholar]
- [19].Kelly A, Dougherty S, Cucchiara A, Marcus CL, Brooks LJ. Catecholamines, adiponectin, and insulin resistance as measured by HOMA in children with obstructive sleep apnea. Sleep . 2010;33:1185–1191. doi: 10.1093/sleep/33.9.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Canapari CA, Hoppin AG, Kinane TB, Thomas BJ, Torriani M, Katz ES. Relationship between sleep apnea, fat distribution, and insulin resistance in obese children. Journal of Clinical Sleep Medicine: JCSM: Official Publication of the American Academy of Sleep Medicine . 2011;7:268–273. doi: 10.5664/JCSM.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bhushan B, Ayub B, Loghmanee DA, Billings KR. Metabolic alterations in adolescents with obstructive sleep apnea. International Journal of Pediatric Otorhinolaryngology . 2015;79:2368–2373. doi: 10.1016/j.ijporl.2015.10.046. [DOI] [PubMed] [Google Scholar]
- [22].Bozkurt NC, Beysel S, Karbek B, Unsal İO, Cakir E, Delibasi T. Visceral Obesity Mediates the Association Between Metabolic Syndrome and Obstructive Sleep Apnea Syndrome. Metabolic Syndrome and Related Disorders . 2016;14:217–221. doi: 10.1089/met.2015.0086. [DOI] [PubMed] [Google Scholar]
- [23].Zhang Y, Xing Y, Yuan H, Gang X, Guo W, Li Z, et al. Impaired Glucose Metabolisms of Patients with Obstructive Sleep Apnea and Type 2 Diabetes. Journal of Diabetes Research . 2018;2018:6714392. doi: 10.1155/2018/6714392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Siriwat R, Wang L, Shah V, Mehra R, Ibrahim S. Obstructive sleep apnea and insulin resistance in children with obesity. Journal of Clinical Sleep Medicine: JCSM: Official Publication of the American Academy of Sleep Medicine . 2020;16:1081–1090. doi: 10.5664/jcsm.8414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Bettini S, Serra R, Fabris R, Dal Prà C, Favaretto F, Dassie F, et al. Association of obstructive sleep apnea with non-alcoholic fatty liver disease in patients with obesity: an observational study. Eating and Weight Disorders: EWD . 2022;27:335–343. doi: 10.1007/s40519-021-01182-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Strohl KP, Novak RD, Singer W, Cahan C, Boehm KD, Denko CW, et al. Insulin levels, blood pressure and sleep apnea. Sleep . 1994;17:614–618. doi: 10.1093/sleep/17.7.614. [DOI] [PubMed] [Google Scholar]
- [27].Stoohs RA, Facchini F, Guilleminault C. Insulin resistance and sleep-disordered breathing in healthy humans. American Journal of Respiratory and Critical Care Medicine . 1996;154:170–174. doi: 10.1164/ajrccm.154.1.8680675. [DOI] [PubMed] [Google Scholar]
- [28].Vgontzas AN, Papanicolaou DA, Bixler EO, Hopper K, Lotsikas A, Lin HM, et al. Sleep apnea and daytime sleepiness and fatigue: relation to visceral obesity, insulin resistance, and hypercytokinemia. The Journal of Clinical Endocrinology and Metabolism . 2000;85:1151–1158. doi: 10.1210/jcem.85.3.6484. [DOI] [PubMed] [Google Scholar]
- [29].Trinh MD, Plihalova A, Gojda J, Westlake K, Spicka J, Lattova Z, et al. Obstructive sleep apnoea increases lipolysis and deteriorates glucose homeostasis in patients with type 2 diabetes mellitus. Scientific Reports . 2021;11:3567. doi: 10.1038/s41598-021-83018-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Liu A, Cardell J, Ariel D, Lamendola C, Abbasi F, Kim SH, et al. Abnormalities of lipoprotein concentrations in obstructive sleep apnea are related to insulin resistance. Sleep . 2015;38:793–799. doi: 10.5665/sleep.4678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Tasali E, Van Cauter E, Hoffman L, Ehrmann DA. Impact of obstructive sleep apnea on insulin resistance and glucose tolerance in women with polycystic ovary syndrome. The Journal of Clinical Endocrinology and Metabolism . 2008;93:3878–3884. doi: 10.1210/jc.2008-0925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Lindberg E, Theorell-Haglöw J, Svensson M, Gislason T, Berne C, Janson C. Sleep apnea and glucose metabolism: a long-term follow-up in a community-based sample. Chest . 2012;142:935–942. doi: 10.1378/chest.11-1844. [DOI] [PubMed] [Google Scholar]
- [33].Iftikhar IH, Hoyos CM, Phillips CL, Magalang UJ. Meta-analyses of the Association of Sleep Apnea with Insulin Resistance, and the Effects of CPAP on HOMA-IR, Adiponectin, and Visceral Adipose Fat. Journal of Clinical Sleep Medicine: JCSM: Official Publication of the American Academy of Sleep Medicine . 2015;11:475–485. doi: 10.5664/jcsm.4610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Behnoush AH, Bahiraie P, Shokri Varniab Z, Foroutani L, Khalaji A. Composite lipid indices in patients with obstructive sleep apnea: a systematic review and meta-analysis. Lipids in Health and Disease . 2023;22:84. doi: 10.1186/s12944-023-01859-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Yang D, Liu Z, Yang H. The impact of effective continuous positive airway pressure on homeostasis model assessment insulin resistance in non-diabetic patients with moderate to severe obstructive sleep apnea. Diabetes/metabolism Research and Reviews . 2012;28:499–504. doi: 10.1002/dmrr.2301. [DOI] [PubMed] [Google Scholar]
- [36].Yang D, Liu Z, Yang H, Luo Q. Effects of continuous positive airway pressure on glycemic control and insulin resistance in patients with obstructive sleep apnea: a meta-analysis. Sleep & Breathing = Schlaf & Atmung . 2013;17:33–38. doi: 10.1007/s11325-012-0680-8. [DOI] [PubMed] [Google Scholar]
- [37].Iftikhar IH, Khan MF, Das A, Magalang UJ. Meta-analysis: continuous positive airway pressure improves insulin resistance in patients with sleep apnea without diabetes. Annals of the American Thoracic Society . 2013;10:115–120. doi: 10.1513/AnnalsATS.201209-081OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Feng Y, Zhang Z, Dong ZZ. Effects of continuous positive airway pressure therapy on glycaemic control, insulin sensitivity and body mass index in patients with obstructive sleep apnoea and type 2 diabetes: a systematic review and meta-analysis. NPJ Primary Care Respiratory Medicine . 2015;25:15005. doi: 10.1038/npjpcrm.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Abud R, Salgueiro M, Drake L, Reyes T, Jorquera J, Labarca G. Efficacy of continuous positive airway pressure (CPAP) preventing type 2 diabetes mellitus in patients with obstructive sleep apnea hypopnea syndrome (OSAHS) and insulin resistance: a systematic review and meta-analysis. Sleep Medicine . 2019;62:14–21. doi: 10.1016/j.sleep.2018.12.017. [DOI] [PubMed] [Google Scholar]
- [40].Cattazzo F, Pengo MF, Giontella A, Soranna D, Bilo G, Zambon A, et al. Effect of Continuous Positive Airway Pressure on Glucose and Lipid Profiles in Patients with Obstructive Sleep Apnoea: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Archivos De Bronconeumologia . 2023;59:370–376. doi: 10.1016/j.arbres.2023.03.012. [DOI] [PubMed] [Google Scholar]
- [41].Galic T, Bozic J, Ivkovic N, Gunjaca G, Ticinovic TK, Dogas Z. Effects of mandibular advancement device treatment on arterial stiffness and glucose metabolism in patients with mild to moderate obstructive sleep apnea: a prospective 1 year study. Sleep & Breathing = Schlaf & Atmung . 2016;20:69–77. doi: 10.1007/s11325-015-1186-y. [DOI] [PubMed] [Google Scholar]
- [42].Grunstein RR, Stenlöf K, Hedner J, Sjöström L. Impact of obstructive sleep apnea and sleepiness on metabolic and cardiovascular risk factors in the Swedish Obese Subjects (SOS) Study. International Journal of Obesity and Related Metabolic Disorders: Journal of the International Association for the Study of Obesity . 1995;19:410–418. [PubMed] [Google Scholar]
- [43].Newman AB, Nieto FJ, Guidry U, Lind BK, Redline S, Pickering TG, et al. Relation of sleep-disordered breathing to cardiovascular disease risk factors: the Sleep Heart Health Study. American Journal of Epidemiology . 2001;154:50–59. doi: 10.1093/aje/154.1.50. [DOI] [PubMed] [Google Scholar]
- [44].Sasanabe R, Banno K, Otake K, Hasegawa R, Usui K, Morita M, et al. Metabolic syndrome in Japanese patients with obstructive sleep apnea syndrome. Hypertension Research: Official Journal of the Japanese Society of Hypertension . 2006;29:315–322. doi: 10.1291/hypres.29.315. [DOI] [PubMed] [Google Scholar]
- [45].Lam JCM, Lam B, Lam CL, Fong D, Wang JKL, Tse HF, et al. Obstructive sleep apnea and the metabolic syndrome in community-based Chinese adults in Hong Kong. Respiratory Medicine . 2006;100:980–987. doi: 10.1016/j.rmed.2005.10.003. [DOI] [PubMed] [Google Scholar]
- [46].Kono M, Tatsumi K, Saibara T, Nakamura A, Tanabe N, Takiguchi Y, et al. Obstructive sleep apnea syndrome is associated with some components of metabolic syndrome. Chest . 2007;131:1387–1392. doi: 10.1378/chest.06-1807. [DOI] [PubMed] [Google Scholar]
- [47].Parish JM, Adam T, Facchiano L. Relationship of metabolic syndrome and obstructive sleep apnea. Journal of Clinical Sleep Medicine: JCSM: Official Publication of the American Academy of Sleep Medicine . 2007;3:467–472. [PMC free article] [PubMed] [Google Scholar]
- [48].Assoumou HGN, Gaspoz JM, Sforza E, Pichot V, Celle S, Maudoux D, et al. Obstructive sleep apnea and the metabolic syndrome in an elderly healthy population: the SYNAPSE cohort. Sleep & Breathing = Schlaf & Atmung . 2012;16:895–902. doi: 10.1007/s11325-011-0593-y. [DOI] [PubMed] [Google Scholar]
- [49].Bonsignore MR, Esquinas C, Barceló A, Sanchez-de-la-Torre M, Paternó A, Duran-Cantolla J, et al. Metabolic syndrome, insulin resistance and sleepiness in real-life obstructive sleep apnoea. The European Respiratory Journal . 2012;39:1136–1143. doi: 10.1183/09031936.00151110. [DOI] [PubMed] [Google Scholar]
- [50].Lubrano C, Saponara M, Barbaro G, Specchia P, Addessi E, Costantini D, et al. Relationships between body fat distribution, epicardial fat and obstructive sleep apnea in obese patients with and without metabolic syndrome. PLoS ONE . 2012;7:e47059. doi: 10.1371/journal.pone.0047059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Iguchi A, Yamakage H, Tochiya M, Muranaka K, Sasaki Y, Kono S, et al. Effects of weight reduction therapy on obstructive sleep apnea syndrome and arterial stiffness in patients with obesity and metabolic syndrome. Journal of Atherosclerosis and Thrombosis . 2013;20:807–820. doi: 10.5551/jat.17632. [DOI] [PubMed] [Google Scholar]
- [52].Apaydin M, Ayik SO, Akhan G, Peker S, Uluc E. Carotid intima-media thickness increase in patients with habitual simple snoring and obstructive sleep apnea syndrome is associated with metabolic syndrome. Journal of Clinical Ultrasound: JCU . 2013;41:290–296. doi: 10.1002/jcu.22040. [DOI] [PubMed] [Google Scholar]
- [53].Seetho IW, Parker RJ, Craig S, Duffy N, Hardy KJ, Wilding JPH. Obstructive sleep apnea is associated with increased arterial stiffness in severe obesity. Journal of Sleep Research . 2014;23:700–708. doi: 10.1111/jsr.12156. [DOI] [PubMed] [Google Scholar]
- [54].Çetin S, Vural MG, Gündüz H, Akdemir R, Fırat H. Epicardial fat thickness regression with continuous positive airway pressure therapy in patients with obstructive sleep apnea: assessment by two-dimensional echocardiography. Wiener Klinische Wochenschrift . 2016;128:187–192. doi: 10.1007/s00508-016-0975-z. [DOI] [PubMed] [Google Scholar]
- [55].Chen GP, Qi JC, Wang BY, Lin X, Zhang XB, Zhao JM, et al. Applicability of visceral adiposity index in predicting metabolic syndrome in adults with obstructive sleep apnea: a cross-sectional study. BMC Pulmonary Medicine . 2016;16:37. doi: 10.1186/s12890-016-0198-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Kim DH, Kim B, Han K, Kim SW. The relationship between metabolic syndrome and obstructive sleep apnea syndrome: a nationwide population-based study. Scientific Reports . 2021;11:8751. doi: 10.1038/s41598-021-88233-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Kim T, Kang J. Relationship between obstructive sleep apnea, insulin resistance, and metabolic syndrome: a nationwide population-based survey. Endocrine Journal . 2023;70:107–119. doi: 10.1507/endocrj.EJ22-0280. [DOI] [PubMed] [Google Scholar]
- [58].Xu S, Wan Y, Xu M, Ming J, Xing Y, An F, et al. The association between obstructive sleep apnea and metabolic syndrome: a systematic review and meta-analysis. BMC Pulmonary Medicine . 2015;15:105. doi: 10.1186/s12890-015-0102-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Grunstein R, Wilcox I, Yang TS, Gould Y, Hedner J. Snoring and sleep apnoea in men: association with central obesity and hypertension. International Journal of Obesity and Related Metabolic Disorders: Journal of the International Association for the Study of Obesity . 1993;17:533–540. [PubMed] [Google Scholar]
- [60].Levinson PD, McGarvey ST, Carlisle CC, Eveloff SE, Herbert PN, Millman RP. Adiposity and cardiovascular risk factors in men with obstructive sleep apnea. Chest . 1993;103:1336–1342. doi: 10.1378/chest.103.5.1336. [DOI] [PubMed] [Google Scholar]
- [61].Degache F, Sforza E, Dauphinot V, Celle S, Garcin A, Collet P, et al. Relation of central fat mass to obstructive sleep apnea in the elderly. Sleep . 2013;36:501–507. doi: 10.5665/sleep.2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Borel AL, Monneret D, Tamisier R, Baguet JP, Faure P, Levy P, et al. The severity of nocturnal hypoxia but not abdominal adiposity is associated with insulin resistance in non-obese men with sleep apnea. PLoS ONE . 2013;8:e71000. doi: 10.1371/journal.pone.0071000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Zhao X, Xu H, Qian Y, Liu Y, Zou J, Yi H, et al. Abdominal Obesity Is More Strongly Correlated with Obstructive Sleep Apnea than General Obesity in China: Results from Two Separated Observational and Longitudinal Studies. Obesity Surgery . 2019;29:2535–2547. doi: 10.1007/s11695-019-03870-z. [DOI] [PubMed] [Google Scholar]
- [64].Çetin N, Güneş Tatar İ, Yüceege M, Ergun O, Hekimoğlu B. Ultrasonographic evaluation of abdominal wall fat index, carotid intima-media thickness and plaque score in obstructive sleep apnea syndrome. Medical Ultrasonography . 2019;21:422–426. doi: 10.11152/mu-1949. [DOI] [PubMed] [Google Scholar]
- [65].Zheng C, Zheng X, Lin X, Ye J, Xu Z, Hu H, et al. Visceral Adipose Tissue Indices Independently Correlated with Obstructive Sleep Apnea in Patients with Type 2 Diabetes. Journal of Diabetes Research . 2022;2022:4950528. doi: 10.1155/2022/4950528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Ma B, Li Y, Wang X, Du L, Wang S, Ma H, et al. Association Between Abdominal Adipose Tissue Distribution and Obstructive Sleep Apnea in Chinese Obese Patients. Frontiers in Endocrinology . 2022;13:847324. doi: 10.3389/fendo.2022.847324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Deng H, Duan X, Huang J, Zheng M, Lao M, Weng F, et al. Association of adiposity with risk of obstructive sleep apnea: a population-based study. BMC Public Health . 2023;23:1835. doi: 10.1186/s12889-023-16695-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Yu JH, Ahn JH, Yoo HJ, Seo JA, Kim SG, Choi KM, et al. Obstructive sleep apnea with excessive daytime sleepiness is associated with non-alcoholic fatty liver disease regardless of visceral fat. The Korean Journal of Internal Medicine . 2015;30:846–855. doi: 10.3904/kjim.2015.30.6.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Zhang YX, Yang L, Yang CC, Wang WY, Shen JH, Shi ML, et al. Correlation between Obstructive Sleep Apnea and Non-Alcoholic Fatty Liver Disease before and after Metabolic Bariatric Surgery. Obesity Surgery . 2020;30:3803–3812. doi: 10.1007/s11695-020-04696-w. [DOI] [PubMed] [Google Scholar]
- [70].Sukahri S, Mohamed Shah FZ, Ismail AI, Koshy M, Johari B, Mohd Razali M, et al. Significantly higher atherosclerosis risks in patients with obstructive sleep apnea and non-alcoholic fatty liver disease. PLoS ONE . 2021;16:e0253298. doi: 10.1371/journal.pone.0253298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Jin S, Jiang S, Hu A. Association between obstructive sleep apnea and non-alcoholic fatty liver disease: a systematic review and meta-analysis. Sleep & Breathing = Schlaf & Atmung . 2018;22:841–851. doi: 10.1007/s11325-018-1625-7. [DOI] [PubMed] [Google Scholar]
- [72].Umbro I, Fabiani V, Fabiani M, Angelico F, Del Ben M. Association between non-alcoholic fatty liver disease and obstructive sleep apnea. World Journal of Gastroenterology . 2020;26:2669–2681. doi: 10.3748/wjg.v26.i20.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Chen LD, Chen MX, Chen GP, Lin XJ, Huang JF, Zeng AM, et al. Association between obstructive sleep apnea and non-alcoholic fatty liver disease in pediatric patients: a meta-analysis. Pediatric Obesity . 2021;16:e12718. doi: 10.1111/ijpo.12718. [DOI] [PubMed] [Google Scholar]
- [74].Hany M, Abouelnasr AA, Abdelkhalek MH, Ibrahim M, Aboelsoud MR, Hozien AI, et al. Effects of obstructive sleep apnea on non-alcoholic fatty liver disease in patients with obesity: a systematic review. International Journal of Obesity (2005) . 2023;47:1200–1213. doi: 10.1038/s41366-023-01378-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Mariani S, Fiore D, Barbaro G, Basciani S, Saponara M, D’Arcangelo E, et al. Association of epicardial fat thickness with the severity of obstructive sleep apnea in obese patients. International Journal of Cardiology . 2013;167:2244–2249. doi: 10.1016/j.ijcard.2012.06.011. [DOI] [PubMed] [Google Scholar]
- [76].Kostopoulos K, Alhanatis E, Pampoukas K, Georgiopoulos G, Zourla A, Panoutsopoulos A, et al. CPAP therapy induces favorable short-term changes in epicardial fat thickness and vascular and metabolic markers in apparently healthy subjects with obstructive sleep apnea-hypopnea syndrome (OSAHS) Sleep & Breathing = Schlaf & Atmung . 2016;20:483–493. doi: 10.1007/s11325-015-1236-5. [DOI] [PubMed] [Google Scholar]
- [77].Derin S, Altun I, Koseoglu S, Sahin C, Yilmaz M, Akin F, et al. Association of epicardial fat thickness with clinical and polysomnographic parameters in non-obese obstructive sleep apnoea patients. The Journal of Laryngology and Otology . 2018;132:439–445. doi: 10.1017/S0022215118000579. [DOI] [PubMed] [Google Scholar]
- [78].Song G, Sun F, Wu D, Bi W. Association of epicardial adipose tissues with obstructive sleep apnea and its severity: A meta-analysis study. Nutrition, Metabolism, and Cardiovascular Diseases: NMCD . 2020;30:1115–1120. doi: 10.1016/j.numecd.2020.03.016. [DOI] [PubMed] [Google Scholar]
- [79].Börgel J, Sanner BM, Bittlinsky A, Keskin F, Bartels NK, Buechner N, et al. Obstructive sleep apnoea and its therapy influence high-density lipoprotein cholesterol serum levels. The European Respiratory Journal . 2006;27:121–127. doi: 10.1183/09031936.06.00131304. [DOI] [PubMed] [Google Scholar]
- [80].Drager LF, Lopes HF, Maki-Nunes C, Trombetta IC, Toschi-Dias E, Alves MJNN, et al. The impact of obstructive sleep apnea on metabolic and inflammatory markers in consecutive patients with metabolic syndrome. PLoS ONE . 2010;5:e12065. doi: 10.1371/journal.pone.0012065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Gündüz C, Basoglu OK, Hedner J, Zou D, Bonsignore MR, Hein H, et al. Obstructive sleep apnoea independently predicts lipid levels: Data from the European Sleep Apnea Database. Respirology (Carlton, Vic.) . 2018;23:1180–1189. doi: 10.1111/resp.13372. [DOI] [PubMed] [Google Scholar]
- [82].Bikov A, Meszaros M, Kunos L, Negru AG, Frent SM, Mihaicuta S. Atherogenic Index of Plasma in Obstructive Sleep Apnoea. Journal of Clinical Medicine . 2021;10:417. doi: 10.3390/jcm10030417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Bajpai J, Pradhan A, Bajaj D, Verma AK, Kant S, Pandey AK, et al. Prevalence of dyslipidaemia in OSA patients at a tertiary care center. American Journal of Cardiovascular Disease . 2023;13:1–9. [PMC free article] [PubMed] [Google Scholar]
- [84].Nadeem R, Singh M, Nida M, Waheed I, Khan A, Ahmed S, et al. Effect of obstructive sleep apnea hypopnea syndrome on lipid profile: a meta-regression analysis. Journal of Clinical Sleep Medicine: JCSM: Official Publication of the American Academy of Sleep Medicine . 2014;10:475–489. doi: 10.5664/jcsm.3690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Hallock P, Benson IC. Studies on the elastic properties of human isolated aorta. The Journal of Clinical Investigation . 1937;16:595–602. doi: 10.1172/JCI100886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Liao D, Arnett DK, Tyroler HA, Riley WA, Chambless LE, Szklo M, et al. Arterial stiffness and the development of hypertension. The ARIC study. Hypertension (Dallas, Tex.: 1979) . 1999;34:201–206. doi: 10.1161/01.hyp.34.2.201. [DOI] [PubMed] [Google Scholar]
- [87].Dernellis J, Panaretou M. Aortic stiffness is an independent predictor of progression to hypertension in nonhypertensive subjects. Hypertension (Dallas, Tex.: 1979) . 2005;45:426–431. doi: 10.1161/01.HYP.0000157818.58878.93. [DOI] [PubMed] [Google Scholar]
- [88].Najjar SS, Scuteri A, Shetty V, Wright JG, Muller DC, Fleg JL, et al. Pulse wave velocity is an independent predictor of the longitudinal increase in systolic blood pressure and of incident hypertension in the Baltimore Longitudinal Study of Aging. Journal of the American College of Cardiology . 2008;51:1377–1383. doi: 10.1016/j.jacc.2007.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Peralta CA, Adeney KL, Shlipak MG, Jacobs D, Jr, Duprez D, Bluemke D, et al. Structural and functional vascular alterations and incident hypertension in normotensive adults: the Multi-Ethnic Study of Atherosclerosis. American Journal of Epidemiology . 2010;171:63–71. doi: 10.1093/aje/kwp319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Duprez DA, Gross MD, Ix JH, Kizer JR, Tracy RP, Shea S, et al. Collagen biomarkers predict new onset of hypertension in normotensive participants: the Multi-Ethnic Study of Atherosclerosis. Journal of Hypertension . 2018;36:2245–2250. doi: 10.1097/HJH.0000000000001793. [DOI] [PubMed] [Google Scholar]
- [91].Phillips C, Hedner J, Berend N, Grunstein R. Diurnal and obstructive sleep apnea influences on arterial stiffness and central blood pressure in men. Sleep . 2005;28:604–609. doi: 10.1093/sleep/28.5.604. [DOI] [PubMed] [Google Scholar]
- [92].Tanriverdi H, Evrengul H, Kara CO, Kuru O, Tanriverdi S, Ozkurt S, et al. Aortic stiffness, flow-mediated dilatation and carotid intima-media thickness in obstructive sleep apnea: non-invasive indicators of atherosclerosis. Respiration; International Review of Thoracic Diseases . 2006;73:741–750. doi: 10.1159/000093531. [DOI] [PubMed] [Google Scholar]
- [93].Drager LF, Bortolotto LA, Figueiredo AC, Silva BC, Krieger EM, Lorenzi-Filho G. Obstructive sleep apnea, hypertension, and their interaction on arterial stiffness and heart remodeling. Chest . 2007;131:1379–1386. doi: 10.1378/chest.06-2703. [DOI] [PubMed] [Google Scholar]
- [94].Tavil Y, Kanbay A, Sen N, Ciftçi TU, Abaci A, Yalçin MR, et al. Comparison of right ventricular functions by tissue Doppler imaging in patients with obstructive sleep apnea syndrome with or without hypertension. The International Journal of Cardiovascular Imaging . 2007;23:469–477. doi: 10.1007/s10554-006-9168-6. [DOI] [PubMed] [Google Scholar]
- [95].Kohler M, Craig S, Nicoll D, Leeson P, Davies RJO, Stradling JR. Endothelial function and arterial stiffness in minimally symptomatic obstructive sleep apnea. American Journal of Respiratory and Critical Care Medicine . 2008;178:984–988. doi: 10.1164/rccm.200805-717OC. [DOI] [PubMed] [Google Scholar]
- [96].Chung S, Yoon IY, Lee CH, Kim JW. The association of nocturnal hypoxemia with arterial stiffness and endothelial dysfunction in male patients with obstructive sleep apnea syndrome. Respiration; International Review of Thoracic Diseases . 2010;79:363–369. doi: 10.1159/000228905. [DOI] [PubMed] [Google Scholar]
- [97].Drager LF, Bortolotto LA, Pedrosa RP, Krieger EM, Lorenzi-Filho G. Left atrial diameter is independently associated with arterial stiffness in patients with obstructive sleep apnea: potential implications for atrial fibrillation. International Journal of Cardiology . 2010;144:257–259. doi: 10.1016/j.ijcard.2009.01.018. [DOI] [PubMed] [Google Scholar]
- [98].Kylintireas I, Craig S, Nethononda R, Kohler M, Francis J, Choudhury R, et al. Atherosclerosis and arterial stiffness in obstructive sleep apnea–a cardiovascular magnetic resonance study. Atherosclerosis . 2012;222:483–489. doi: 10.1016/j.atherosclerosis.2012.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Buchner NJ, Quack I, Stegbauer J, Woznowski M, Kaufmann A, Rump LC. Treatment of obstructive sleep apnea reduces arterial stiffness. Sleep & Breathing = Schlaf & Atmung . 2012;16:123–133. doi: 10.1007/s11325-010-0465-x. [DOI] [PubMed] [Google Scholar]
- [100].Akyuz A, Oran M, Alpsoy S, Mutlu LC, Akkoyun DC, Guzel S, et al. Association between serum fetuin-A levels, carotid artery stiffness, and intima-media thickness in patients with normotensive obstructive sleep apnea syndrome. Angiology . 2014;65:607–613. doi: 10.1177/0003319713497421. [DOI] [PubMed] [Google Scholar]
- [101].Kim T, Lee CS, Lee SD, Kang SH, Han JW, Malhotra A, et al. Impacts of comorbidities on the association between arterial stiffness and obstructive sleep apnea in the elderly. Respiration; International Review of Thoracic Diseases . 2015;89:304–311. doi: 10.1159/000371768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Chen CY, Chen CL, Yu CC. Obstructive sleep apnea is independently associated with arterial stiffness in ischemic stroke patients. Journal of Neurology . 2015;262:1247–1254. doi: 10.1007/s00415-015-7699-2. [DOI] [PubMed] [Google Scholar]
- [103].Schaefer CA, Adam L, Weisser-Thomas J, Pingel S, Vogel G, Klarmann-Schulz U, et al. High prevalence of peripheral arterial disease in patients with obstructive sleep apnoea. Clinical Research in Cardiology: Official Journal of the German Cardiac Society . 2015;104:719–726. doi: 10.1007/s00392-015-0834-3. [DOI] [PubMed] [Google Scholar]
- [104].Tuleta I, Skowasch D, Krycki J, Pizarro C, Hammerstingl C, Weber M, et al. Obstructive Sleep Apnea Is Related to Increased Arterial Stiffness in Ultrasound Speckle-Tracking Analysis. Advances in Experimental Medicine and Biology . 2016;910:9–14. doi: 10.1007/5584_2016_215. [DOI] [PubMed] [Google Scholar]
- [105].Jenner R, Fatureto-Borges F, Costa-Hong V, Lopes HF, Teixeira SH, Marum E, et al. Association of obstructive sleep apnea with arterial stiffness and nondipping blood pressure in patients with hypertension. Journal of Clinical Hypertension (Greenwich, Conn.) . 2017;19:910–918. doi: 10.1111/jch.13008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Luo J, Wang X, Guo Z, Xiao Y, Cao W, Zhang L, et al. Endothelial Function and Arterial Stiffness Should Be Measured to Comprehensively Assess Obstructive Sleep Apnea in Clinical Practice. Frontiers in Cardiovascular Medicine . 2021;8:716916. doi: 10.3389/fcvm.2021.716916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Saeed S, Romarheim A, Mancia G, Saxvig IW, Gulati S, Lehmann S, et al. Characteristics of hypertension and arterial stiffness in obstructive sleep apnea: A Scandinavian experience from a prospective study of 6408 normotensive and hypertensive patients. Journal of Clinical Hypertension (Greenwich, Conn.) . 2022;24:385–394. doi: 10.1111/jch.14425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Nielsen S, Nyvad J, Christensen KL, Poulsen PL, Laugesen E, Grove EL, et al. Obstructive sleep apnea, coronary calcification and arterial stiffness in patients with diabetic kidney disease. Atherosclerosis . 2023:117170. doi: 10.1016/j.atherosclerosis.2023.06.076. [DOI] [PubMed] [Google Scholar]
- [109].Doonan RJ, Scheffler P, Lalli M, Kimoff RJ, Petridou ET, Daskalopoulos ME, et al. Increased arterial stiffness in obstructive sleep apnea: a systematic review. Hypertension Research: Official Journal of the Japanese Society of Hypertension . 2011;34:23–32. doi: 10.1038/hr.2010.200. [DOI] [PubMed] [Google Scholar]
- [110].Vlachantoni IT, Dikaiakou E, Antonopoulos CN, Stefanadis C, Daskalopoulou SS, Petridou ET. Effects of continuous positive airway pressure (CPAP) treatment for obstructive sleep apnea in arterial stiffness: a meta-analysis. Sleep Medicine Reviews . 2013;17:19–28. doi: 10.1016/j.smrv.2012.01.002. [DOI] [PubMed] [Google Scholar]
- [111].Wang J, Yu W, Gao M, Zhang F, Gu C, Yu Y, et al. Impact of Obstructive Sleep Apnea Syndrome on Endothelial Function, Arterial Stiffening, and Serum Inflammatory Markers: An Updated Meta-analysis and Metaregression of 18 Studies. Journal of the American Heart Association . 2015;4:e002454. doi: 10.1161/JAHA.115.002454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Phillips CL, Yee B, Yang Q, Villaneuva AT, Hedner J, Berend N, et al. Effects of continuous positive airway pressure treatment and withdrawal in patients with obstructive sleep apnea on arterial stiffness and central BP. Chest . 2008;134:94–100. doi: 10.1378/chest.07-3121. [DOI] [PubMed] [Google Scholar]
- [113].Shiina K, Tomiyama H, Takata Y, Yoshida M, Kato K, Saruhara H, et al. Effects of CPAP therapy on the sympathovagal balance and arterial stiffness in obstructive sleep apnea. Respiratory Medicine . 2010;104:911–916. doi: 10.1016/j.rmed.2010.01.010. [DOI] [PubMed] [Google Scholar]
- [114].Kartali N, Daskalopoulou E, Geleris P, Chatzipantazi S, Tziomalos K, Vlachogiannis E, et al. The effect of continuous positive airway pressure therapy on blood pressure and arterial stiffness in hypertensive patients with obstructive sleep apnea. Sleep & Breathing = Schlaf & Atmung . 2014;18:635–640. doi: 10.1007/s11325-013-0926-0. [DOI] [PubMed] [Google Scholar]
- [115].Korcarz CE, Benca R, Barnet JH, Stein JH. Treatment of Obstructive Sleep Apnea in Young and Middle-Aged Adults: Effects of Positive Airway Pressure and Compliance on Arterial Stiffness, Endothelial Function, and Cardiac Hemodynamics. Journal of the American Heart Association . 2016;5:e002930. doi: 10.1161/JAHA.115.002930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Domingues CC, Dore FJ, Cho A, Ahmadi N, Kropotova Y, Kundu N, et al. Reassessing the effects of continuous positive airway pressure (CPAP) on arterial stiffness and peripheral blood derived CD34+ progenitor cells in subjects with sleep apnea. Stem Cell Research & Therapy . 2019;10:147. doi: 10.1186/s13287-019-1251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Milicevic T, Katic J, Milovac SN, Matetic A, Aljinovic J, Dogas Z, et al. Auto-adaptive positive airway pressure improves lung function and arterial stiffness parameters in patients with severe obstructive sleep apnea syndrome over a 1 year follow-up. Physiological Measurement . 2020;41:125006. doi: 10.1088/1361-6579/abcdf5. [DOI] [PubMed] [Google Scholar]
- [118].Picard F, Panagiotidou P, Weinig L, Steffen M, Tammen AB, Klein RM. Effect of CPAP therapy on nocturnal blood pressure fluctuations, nocturnal blood pressure, and arterial stiffness in patients with coexisting cardiovascular diseases and obstructive sleep apnea. Sleep & Breathing = Schlaf & Atmung . 2021;25:151–161. doi: 10.1007/s11325-020-02075-4. [DOI] [PubMed] [Google Scholar]
- [119].Lin X, Chen G, Qi J, Chen X, Zhao J, Lin Q. Effect of continuous positive airway pressure on arterial stiffness in patients with obstructive sleep apnea and hypertension: a meta-analysis. European Archives of Oto-rhino-laryngology: Official Journal of the European Federation of Oto-Rhino-Laryngological Societies (EUFOS): Affiliated with the German Society for Oto-Rhino-Laryngology - Head and Neck Surgery . 2016;273:4081–4088. doi: 10.1007/s00405-016-3914-8. [DOI] [PubMed] [Google Scholar]
- [120].Lavie P, Herer P, Hoffstein V. Obstructive sleep apnoea syndrome as a risk factor for hypertension: population study. BMJ (Clinical Research Ed.) . 2000;320:479–482. doi: 10.1136/bmj.320.7233.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Nieto FJ, Young TB, Lind BK, Shahar E, Samet JM, Redline S, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA . 2000;283:1829–1836. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- [122].Kirk V, Midgley J, Giuffre M, Ronksley P, Nettel-Aguirre A, Al-Shamrani A. Hypertension and obstructive sleep apnea in Caucasian children. World Journal of Cardiology . 2010;2:251–256. doi: 10.4330/wjc.v2.i8.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. The New England Journal of Medicine . 2000;342:1378–1384. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- [124].Guillot M, Sforza E, Achour-Crawford E, Maudoux D, Saint-Martin M, Barthélémy JC, et al. Association between severe obstructive sleep apnea and incident arterial hypertension in the older people population. Sleep Medicine . 2013;14:838–842. doi: 10.1016/j.sleep.2013.05.002. [DOI] [PubMed] [Google Scholar]
- [125].Ahmed AM, Nur SM, Xiaochen Y. Association between obstructive sleep apnea and resistant hypertension: systematic review and meta-analysis. Frontiers in Medicine . 2023;10:1200952. doi: 10.3389/fmed.2023.1200952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Alajmi M, Mulgrew AT, Fox J, Davidson W, Schulzer M, Mak E, et al. Impact of continuous positive airway pressure therapy on blood pressure in patients with obstructive sleep apnea hypopnea: a meta-analysis of randomized controlled trials. Lung . 2007;185:67–72. doi: 10.1007/s00408-006-0117-x. [DOI] [PubMed] [Google Scholar]
- [127].Haentjens P, Van Meerhaeghe A, Moscariello A, De Weerdt S, Poppe K, Dupont A, et al. The impact of continuous positive airway pressure on blood pressure in patients with obstructive sleep apnea syndrome: evidence from a meta-analysis of placebo-controlled randomized trials. Archives of Internal Medicine . 2007;167:757–764. doi: 10.1001/archinte.167.8.757. [DOI] [PubMed] [Google Scholar]
- [128].Fava C, Dorigoni S, Dalle Vedove F, Danese E, Montagnana M, Guidi GC, et al. Effect of CPAP on blood pressure in patients with OSA/hypopnea a systematic review and meta-analysis. Chest . 2014;145:762–771. doi: 10.1378/chest.13-1115. [DOI] [PubMed] [Google Scholar]
- [129].Schein ASO, Kerkhoff AC, Coronel CC, Plentz RDM, Sbruzzi G. Continuous positive airway pressure reduces blood pressure in patients with obstructive sleep apnea; a systematic review and meta-analysis with 1000 patients. Journal of Hypertension . 2014;32:1762–1773. doi: 10.1097/HJH.0000000000000250. [DOI] [PubMed] [Google Scholar]
- [130].Iftikhar IH, Valentine CW, Bittencourt LRA, Cohen DL, Fedson AC, Gíslason T, et al. Effects of continuous positive airway pressure on blood pressure in patients with resistant hypertension and obstructive sleep apnea: a meta-analysis. Journal of Hypertension . 2014;32:2341–2350. doi: 10.1097/HJH.0000000000000372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Hu X, Fan J, Chen S, Yin Y, Zrenner B. The role of continuous positive airway pressure in blood pressure control for patients with obstructive sleep apnea and hypertension: a meta-analysis of randomized controlled trials. Journal of Clinical Hypertension (Greenwich, Conn.) . 2015;17:215–222. doi: 10.1111/jch.12472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Liu L, Cao Q, Guo Z, Dai Q. Continuous Positive Airway Pressure in Patients With Obstructive Sleep Apnea and Resistant Hypertension: A Meta-Analysis of Randomized Controlled Trials. Journal of Clinical Hypertension (Greenwich, Conn.) . 2016;18:153–158. doi: 10.1111/jch.12639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Schwarz EI, Schlatzer C, Rossi VA, Stradling JR, Kohler M. Effect of CPAP Withdrawal on BP in OSA: Data from Three Randomized Controlled Trials. Chest . 2016;150:1202–1210. doi: 10.1016/j.chest.2016.07.012. [DOI] [PubMed] [Google Scholar]
- [134].Lei Q, Lv Y, Li K, Ma L, Du G, Xiang Y, et al. Effects of continuous positive airway pressure on blood pressure in patients with resistant hypertension and obstructive sleep apnea: a systematic review and meta-analysis of six randomized controlled trials. Jornal Brasileiro De Pneumologia: Publicacao Oficial Da Sociedade Brasileira De Pneumologia E Tisilogia . 2017;43:373–379. doi: 10.1590/S1806-37562016000000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Labarca G, Schmidt A, Dreyse J, Jorquera J, Enos D, Torres G, et al. Efficacy of continuous positive airway pressure (CPAP) in patients with obstructive sleep apnea (OSA) and resistant hypertension (RH): Systematic review and meta-analysis. Sleep Medicine Reviews . 2021;58:101446. doi: 10.1016/j.smrv.2021.101446. [DOI] [PubMed] [Google Scholar]
- [136].Shang W, Zhang Y, Liu L, Chen F, Wang G, Han D. Benefits of continuous positive airway pressure on blood pressure in patients with hypertension and obstructive sleep apnea: a meta-analysis. Hypertension Research: Official Journal of the Japanese Society of Hypertension . 2022;45:1802–1813. doi: 10.1038/s41440-022-00954-9. [DOI] [PubMed] [Google Scholar]
- [137].Yang D, Li L, Dong J, Yang W, Liu Z. Effects of continuous positive airway pressure on cardiac events and metabolic components in patients with moderate to severe obstructive sleep apnea and coronary artery disease: a meta-analysis. Journal of Clinical Sleep Medicine: JCSM: Official Publication of the American Academy of Sleep Medicine . 2023;19:2015–2025. doi: 10.5664/jcsm.10740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Bratton DJ, Gaisl T, Wons AM, Kohler M. CPAP vs Mandibular Advancement Devices and Blood Pressure in Patients With Obstructive Sleep Apnea: A Systematic Review and Meta-analysis. JAMA . 2015;314:2280–2293. doi: 10.1001/jama.2015.16303. [DOI] [PubMed] [Google Scholar]
- [139].Hedner J, Ejnell H, Caidahl K. Left ventricular hypertrophy independent of hypertension in patients with obstructive sleep apnoea. Journal of Hypertension . 1990;8:941–946. doi: 10.1097/00004872-199010000-00009. [DOI] [PubMed] [Google Scholar]
- [140].Amin RS, Kimball TR, Bean JA, Jeffries JL, Willging JP, Cotton RT, et al. Left ventricular hypertrophy and abnormal ventricular geometry in children and adolescents with obstructive sleep apnea. American Journal of Respiratory and Critical Care Medicine . 2002;165:1395–1399. doi: 10.1164/rccm.2105118. [DOI] [PubMed] [Google Scholar]
- [141].Cloward TV, Walker JM, Farney RJ, Anderson JL. Left ventricular hypertrophy is a common echocardiographic abnormality in severe obstructive sleep apnea and reverses with nasal continuous positive airway pressure. Chest . 2003;124:594–601. doi: 10.1378/chest.124.2.594. [DOI] [PubMed] [Google Scholar]
- [142].Sukhija R, Aronow WS, Sandhu R, Kakar P, Maguire GP, Ahn C, et al. Prevalence of left ventricular hypertrophy in persons with and without obstructive sleep apnea. Cardiology in Review . 2006;14:170–172. doi: 10.1097/01.crd.0000184455.52778.00. [DOI] [PubMed] [Google Scholar]
- [143].Usui Y, Takata Y, Inoue Y, Shimada K, Tomiyama H, Nishihata Y, et al. Coexistence of obstructive sleep apnoea and metabolic syndrome is independently associated with left ventricular hypertrophy and diastolic dysfunction. Sleep & Breathing = Schlaf & Atmung . 2012;16:677–684. doi: 10.1007/s11325-011-0557-2. [DOI] [PubMed] [Google Scholar]
- [144].Sekizuka H, Osada N, Akashi YJ. Impact of obstructive sleep apnea and hypertension on left ventricular hypertrophy in Japanese patients. Hypertension Research: Official Journal of the Japanese Society of Hypertension . 2017;40:477–482. doi: 10.1038/hr.2016.170. [DOI] [PubMed] [Google Scholar]
- [145].Hanlon CE, Binka E, Garofano JS, Sterni LM, Brady TM. The association of obstructive sleep apnea and left ventricular hypertrophy in obese and overweight children with history of elevated blood pressure. Journal of Clinical Hypertension (Greenwich, Conn.) . 2019;21:984–990. doi: 10.1111/jch.13605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Huang Z, Wang L, Liu Y, Huang K, Xu Y, Chen P, et al. Impact of obstructive sleep apnea on left ventricular mass index in men with coronary artery disease. Journal of Clinical Sleep Medicine: JCSM: Official Publication of the American Academy of Sleep Medicine . 2020;16:1675–1682. doi: 10.5664/jcsm.8642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Cuspidi C, Tadic M, Sala C, Gherbesi E, Grassi G, Mancia G. Obstructive sleep apnoea syndrome and left ventricular hypertrophy: a meta-analysis of echocardiographic studies. Journal of Hypertension . 2020;38:1640–1649. doi: 10.1097/HJH.0000000000002435. [DOI] [PubMed] [Google Scholar]
- [148].Ohira A, Yamakawa T, Iwahashi N, Tanaka S, Sugiyama M, Harada M, et al. Association of continuous positive airway pressure therapy on cardiac hypertrophy in patients with sleep apnea comorbid with type 2 diabetes mellitus. Endocrine Journal . 2023;70:47–58. doi: 10.1507/endocrj.EJ22-0308. [DOI] [PubMed] [Google Scholar]
- [149].Yu L, Li H, Liu X, Fan J, Zhu Q, Li J, et al. Left ventricular remodeling and dysfunction in obstructive sleep apnea: Systematic review and meta-analysis. Herz . 2020;45:726–738. doi: 10.1007/s00059-019-04850-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Varol E, Akcay S, Ozaydin M, Ozturk O, Cerci SS, Sahin U. Influence of obstructive sleep apnea on left ventricular mass and global function: sleep apnea and myocardial performance index. Heart and Vessels . 2010;25:400–404. doi: 10.1007/s00380-009-1225-3. [DOI] [PubMed] [Google Scholar]
- [151].Li C, Zhang XL, Liu H, Wang ZG, Yin KS. Association among plasma interleukin-18 levels, carotid intima- media thickness and severity of obstructive sleep apnea. Chinese Medical Journal . 2009;122:24–29. [PubMed] [Google Scholar]
- [152].Tan TY, Liou CW, Friedman M, Lin HC, Chang HW, Lin MC. Factors associated with increased carotid intima-media thickness in obstructive sleep apnea/hypopnea syndrome. The Neurologist . 2012;18:277–281. doi: 10.1097/NRL.0b013e3182675344. [DOI] [PubMed] [Google Scholar]
- [153].Ozdemir C, Conkbayır I, Kuru A, Fırat H, Sökücü SN, Dalar L, et al. Correlation between the intima-media thickness and Framingham risk score in patients with sleep apnea syndrome. Journal of Thoracic Disease . 2013;5:751–757. doi: 10.3978/j.issn.2072-1439.2013.11.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Fox N, Ayas N, Park JE, Fleetham J, Frank Ryan C, Lear SA, et al. Carotid intima media thickness in patients with obstructive sleep apnea: comparison with a community-based cohort. Lung . 2014;192:297–303. doi: 10.1007/s00408-014-9556-y. [DOI] [PubMed] [Google Scholar]
- [155].Salepci B, Fidan A, Ketenci SC, Parmaksiz ET, Comert SS, Kiral N, et al. The effect of obstructive sleep apnea syndrome and snoring severity to intima-media thickening of carotid artery. Sleep & Breathing = Schlaf & Atmung . 2015;19:239–246. doi: 10.1007/s11325-014-1002-0. [DOI] [PubMed] [Google Scholar]
- [156].Farooqui FA, Sharma SK, Kumar A, Soneja M, Mani K, Radhakrishnan R, et al. Endothelial function and carotid intima media thickness in obstructive sleep apnea without comorbidity. Sleep & Breathing = Schlaf & Atmung . 2017;21:69–76. doi: 10.1007/s11325-016-1371-7. [DOI] [PubMed] [Google Scholar]
- [157].Gunnarsson SI, Peppard PE, Korcarz CE, Barnet JH, Aeschlimann SE, Hagen EW, et al. Obstructive sleep apnea is associated with future subclinical carotid artery disease: thirteen-year follow-up from the Wisconsin sleep cohort. Arteriosclerosis, Thrombosis, and Vascular Biology . 2014;34:2338–2342. doi: 10.1161/ATVBAHA.114.303965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Zhou M, Guo B, Wang Y, Yan D, Lin C, Shi Z. The Association Between Obstructive Sleep Apnea and Carotid Intima-Media Thickness: A Systematic Review and Meta-Analysis. Angiology . 2017;68:575–583. doi: 10.1177/0003319716665985. [DOI] [PubMed] [Google Scholar]
- [159].Hui DS, Shang Q, Ko FW, Ng SS, Szeto CC, Ngai J, et al. A prospective cohort study of the long-term effects of CPAP on carotid artery intima-media thickness in obstructive sleep apnea syndrome. Respiratory Research . 2012;13:22. doi: 10.1186/1465-9921-13-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Peng Y, Zhang L, Hu D, Dai Y, Wang S, Liao H, et al. Reduction of internal carotid artery intima-media thickness in patients with moderate-to-severe obstructive sleep apnea syndrome after nasal surgery and uvulopalatopharyngoplasty. Acta Oto-laryngologica . 2016;136:514–521. doi: 10.3109/00016489.2015.1130856. [DOI] [PubMed] [Google Scholar]
- [161].Chen LD, Lin L, Lin XJ, Ou YW, Wu Z, Ye YM, et al. Effect of continuous positive airway pressure on carotid intima-media thickness in patients with obstructive sleep apnea: A meta-analysis. PLoS ONE . 2017;12:e0184293. doi: 10.1371/journal.pone.0184293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Hung J, Whitford EG, Parsons RW, Hillman DR. Association of sleep apnoea with myocardial infarction in men. Lancet (London, England) . 1990;336:261–264. doi: 10.1016/0140-6736(90)91799-g. [DOI] [PubMed] [Google Scholar]
- [163].Walia HK, Khosla AA, Saxena A, Aneni E, Ali SS, Valero-Elizondo J, et al. Atherosclerotic plaque in individuals without known cardiovascular disease but with established obstructive sleep apnea and at high risk of obstructive sleep apnea. American Journal of Preventive Cardiology . 2023;14:100497. doi: 10.1016/j.ajpc.2023.100497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Peker Y, Hedner J, Norum J, Kraiczi H, Carlson J. Increased incidence of cardiovascular disease in middle-aged men with obstructive sleep apnea: a 7-year follow-up. American Journal of Respiratory and Critical Care Medicine . 2002;166:159–165. doi: 10.1164/rccm.2105124. [DOI] [PubMed] [Google Scholar]
- [165].Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. The New England Journal of Medicine . 2005;353:2034–2041. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- [166].Nagayoshi M, Lutsey PL, Benkeser D, Wassel CL, Folsom AR, Shahar E, et al. Association of sleep apnea and sleep duration with peripheral artery disease: The Multi-Ethnic Study of Atherosclerosis (MESA) Atherosclerosis . 2016;251:467–475. doi: 10.1016/j.atherosclerosis.2016.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Cai X, Hu J, Wen W, Wang J, Wang M, Liu S, et al. Associations of the Cardiometabolic Index with the Risk of Cardiovascular Disease in Patients with Hypertension and Obstructive Sleep Apnea: Results of a Longitudinal Cohort Study. Oxidative Medicine and Cellular Longevity . 2022;2022:4914791. doi: 10.1155/2022/4914791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Yang W, Cai X, Hu J, Wen W, Mulalibieke H, Yao X, et al. The Metabolic Score for Insulin Resistance (METS-IR) Predicts Cardiovascular Disease and Its Subtypes in Patients with Hypertension and Obstructive Sleep Apnea. Clinical Epidemiology . 2023;15:177–189. doi: 10.2147/CLEP.S395938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Wang X, Ouyang Y, Wang Z, Zhao G, Liu L, Bi Y. Obstructive sleep apnea and risk of cardiovascular disease and all-cause mortality: a meta-analysis of prospective cohort studies. International Journal of Cardiology . 2013;169:207–214. doi: 10.1016/j.ijcard.2013.08.088. [DOI] [PubMed] [Google Scholar]
- [170].Dong JY, Zhang YH, Qin LQ. Obstructive sleep apnea and cardiovascular risk: meta-analysis of prospective cohort studies. Atherosclerosis . 2013;229:489–495. doi: 10.1016/j.atherosclerosis.2013.04.026. [DOI] [PubMed] [Google Scholar]
- [171].Zhao Y, Yu BYM, Liu Y, Liu Y. Meta-Analysis of the Effect of Obstructive Sleep Apnea on Cardiovascular Events After Percutaneous Coronary Intervention. The American Journal of Cardiology . 2017;120:1026–1030. doi: 10.1016/j.amjcard.2017.06.035. [DOI] [PubMed] [Google Scholar]
- [172].Qu H, Guo M, Zhang Y, Shi DZ. Obstructive sleep apnea increases the risk of cardiac events after percutaneous coronary intervention: a meta-analysis of prospective cohort studies. Sleep & Breathing = Schlaf & Atmung . 2018;22:33–40. doi: 10.1007/s11325-017-1503-8. [DOI] [PubMed] [Google Scholar]
- [173].Wang X, Fan JY, Zhang Y, Nie SP, Wei YX. Association of obstructive sleep apnea with cardiovascular outcomes after percutaneous coronary intervention: A systematic review and meta-analysis. Medicine . 2018;97:e0621. doi: 10.1097/MD.0000000000010621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Yang SH, Xing YS, Wang ZX, Liu YB, Chen HW, Ren YF, et al. Association of Obstructive Sleep Apnea With the Risk of Repeat Adverse Cardiovascular Events in Patients With Newly Diagnosed Acute Coronary Syndrome: A Systematic Review and Meta-Analysis. Ear, Nose, & Throat Journal . 2021;100:260–270. doi: 10.1177/0145561321989450. [DOI] [PubMed] [Google Scholar]
- [175].Salari N, Khazaie H, Abolfathi M, Ghasemi H, Shabani S, Rasoulpoor S, et al. The effect of obstructive sleep apnea on the increased risk of cardiovascular disease: a systematic review and meta-analysis. Neurological Sciences: Official Journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology . 2022;43:219–231. doi: 10.1007/s10072-021-05765-3. [DOI] [PubMed] [Google Scholar]
- [176].Sun B, Ma Q, Shen J, Meng Z, Xu J. Up-to-date advance in the relationship between OSA and stroke: a narrative review. Sleep & Breathing = Schlaf & Atmung . 2024;28:53–60. doi: 10.1007/s11325-023-02904-2. [DOI] [PubMed] [Google Scholar]
- [177].Punjabi NM, Shahar E, Redline S, Gottlieb DJ, Givelber R, Resnick HE, et al. Sleep-disordered breathing, glucose intolerance, and insulin resistance: the Sleep Heart Health Study. American Journal of Epidemiology . 2004;160:521–530. doi: 10.1093/aje/kwh261. [DOI] [PubMed] [Google Scholar]
- [178].Kurosawa H, Saisho Y, Fukunaga K, Haraguchi M, Yamasawa W, Kurihara I, et al. Association between severity of obstructive sleep apnea and glycated hemoglobin level in Japanese individuals with and without diabetes. Endocrine Journal . 2018;65:121–127. doi: 10.1507/endocrj.EJ17-0356. [DOI] [PubMed] [Google Scholar]
- [179].Reichmuth KJ, Austin D, Skatrud JB, Young T. Association of sleep apnea and type II diabetes: a population-based study. American Journal of Respiratory and Critical Care Medicine . 2005;172:1590–1595. doi: 10.1164/rccm.200504-637OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Koh TK, Kang EJ, Bae WY, Kim SW, Kim CH, Koo SK, et al. Quantitative analysis of carotid arterial calcification using airway CT in obstructive sleep apnea. Auris, Nasus, Larynx . 2019;46:559–564. doi: 10.1016/j.anl.2018.11.015. [DOI] [PubMed] [Google Scholar]
- [181].Botros N, Concato J, Mohsenin V, Selim B, Doctor K, Yaggi HK. Obstructive sleep apnea as a risk factor for type 2 diabetes. The American Journal of Medicine . 2009;122:1122–1127. doi: 10.1016/j.amjmed.2009.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Kendzerska T, Gershon AS, Hawker G, Tomlinson G, Leung RS. Obstructive sleep apnea and incident diabetes. A historical cohort study. American Journal of Respiratory and Critical Care Medicine . 2014;190:218–225. doi: 10.1164/rccm.201312-2209OC. [DOI] [PubMed] [Google Scholar]
- [183].Wojeck BS, Inzucchi SE, Qin L, Yaggi HK. Polysomnographic predictors of incident diabetes and pre-diabetes: an analysis of the DREAM study. Journal of Clinical Sleep Medicine: JCSM: Official Publication of the American Academy of Sleep Medicine . 2023;19:703–710. doi: 10.5664/jcsm.10414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Wang X, Bi Y, Zhang Q, Pan F. Obstructive sleep apnoea and the risk of type 2 diabetes: a meta-analysis of prospective cohort studies. Respirology (Carlton, Vic.) . 2013;18:140–146. doi: 10.1111/j.1440-1843.2012.02267.x. [DOI] [PubMed] [Google Scholar]
- [185].O’Connor-Reina C, Alcala LR, Ignacio JM, Iriarte MTG, Llatas MC, Morente JCC, et al. Risk of diabetes in patients with sleep apnea: comparison of surgery versus CPAP in a long-term follow-up study. Journal of Otolaryngology - Head & Neck Surgery = Le Journal D’oto-rhino-laryngologie et De Chirurgie Cervico-faciale . 2023;52:16. doi: 10.1186/s40463-022-00616-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Aronsohn RS, Whitmore H, Van Cauter E, Tasali E. Impact of untreated obstructive sleep apnea on glucose control in type 2 diabetes. American Journal of Respiratory and Critical Care Medicine . 2010;181:507–513. doi: 10.1164/rccm.200909-1423OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Kent BD, Grote L, Ryan S, Pépin JL, Bonsignore MR, Tkacova R, et al. Diabetes mellitus prevalence and control in sleep-disordered breathing: the European Sleep Apnea Cohort (ESADA) study. Chest . 2014;146:982–990. doi: 10.1378/chest.13-2403. [DOI] [PubMed] [Google Scholar]
- [188].Leong WB, Banerjee D, Nolen M, Adab P, Thomas GN, Taheri S. Hypoxemia and glycemic control in type 2 diabetes mellitus with extreme obesity. The Journal of Clinical Endocrinology and Metabolism . 2014;99:E1650–E1654. doi: 10.1210/jc.2014-1260. [DOI] [PubMed] [Google Scholar]
- [189].Herth J, Sievi NA, Schmidt F, Kohler M. Effects of continuous positive airway pressure therapy on glucose metabolism in patients with obstructive sleep apnoea and type 2 diabetes: a systematic review and meta-analysis. European Respiratory Review: an Official Journal of the European Respiratory Society . 2023;32:230083. doi: 10.1183/16000617.0083-2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Labarca G, Reyes T, Jorquera J, Dreyse J, Drake L. CPAP in patients with obstructive sleep apnea and type 2 diabetes mellitus: Systematic review and meta-analysis. The Clinical Respiratory Journal . 2018;12:2361–2368. doi: 10.1111/crj.12915. [DOI] [PubMed] [Google Scholar]
- [191].Chaudhary B, Dennis A, Chaudhary T, Speir W. Sleep Apnea, Pulmonary Hypertension and Nephrotic Syndrome. Journal of the Islamic Medical Association of North America . 1984;16:26–28. [Google Scholar]
- [192].Chaudhary BA, Sklar AH, Chaudhary TK, Kolbeck RC, Speir WA., Jr Sleep apnea, proteinuria, and nephrotic syndrome. Sleep . 1988;11:69–74. doi: 10.1093/sleep/11.1.69. [DOI] [PubMed] [Google Scholar]
- [193].Chaudhary BA, Rehman OU, Brown TM. Proteinuria in patients with sleep apnea. The Journal of Family Practice . 1995;40:139–141. [PubMed] [Google Scholar]
- [194].Faulx MD, Storfer-Isser A, Kirchner HL, Jenny NS, Tracy RP, Redline S. Obstructive sleep apnea is associated with increased urinary albumin excretion. Sleep . 2007;30:923–929. doi: 10.1093/sleep/30.7.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Ursavas A, Karadag M, Gullulu M, Demirdogen E, Coskun F, Onart S, et al. Low-grade urinary albumin excretion in normotensive/non-diabetic obstructive sleep apnea patients. Sleep & Breathing = Schlaf & Atmung . 2008;12:217–222. doi: 10.1007/s11325-008-0169-7. [DOI] [PubMed] [Google Scholar]
- [196].Tsioufis C, Thomopoulos C, Dimitriadis K, Amfilochiou A, Tsiachris D, Selima M, et al. Association of obstructive sleep apnea with urinary albumin excretion in essential hypertension: a cross-sectional study. American Journal of Kidney Diseases: the Official Journal of the National Kidney Foundation . 2008;52:285–293. doi: 10.1053/j.ajkd.2008.05.001. [DOI] [PubMed] [Google Scholar]
- [197].Canales MT, Paudel ML, Taylor BC, Ishani A, Mehra R, Steffes M, et al. Sleep-disordered breathing and urinary albumin excretion in older men. Sleep & Breathing = Schlaf & Atmung . 2011;15:137–144. doi: 10.1007/s11325-010-0339-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Varlami V, Malakasioti G, Alexopoulos EI, Theologi V, Theophanous E, Liakos N, et al. Low-grade albuminuria in children with obstructive sleep apnea. Journal of Sleep Research . 2013;22:289–294. doi: 10.1111/jsr.12021. [DOI] [PubMed] [Google Scholar]
- [199].Ricardo AC, Chen J, Singh M, Heiss G, Raij L, Ramos A, et al. Sleep-Disordered Breathing and Prevalent Albuminuria in Hispanics/Latinos. Kidney International Reports . 2018;3:1276–1284. doi: 10.1016/j.ekir.2018.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Jackson CL, Umesi C, Gaston SA, Azarbarzin A, Lunyera J, McGrath JA, et al. Multiple, objectively measured sleep dimensions including hypoxic burden and chronic kidney disease: findings from the Multi-Ethnic Study of Atherosclerosis. Thorax . 2021;76:704–713. doi: 10.1136/thoraxjnl-2020-214713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Jung SM, Lee MR. Association between Obstructive Sleep Apnea and Chronic Kidney Disease According to Sex, Long Working Hours: The Korean National Health and Nutrition Examination Survey (2019-2020) Life (Basel, Switzerland) . 2023;13:1625. doi: 10.3390/life13081625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Stadler S, Zimmermann T, Franke F, Rheinberger M, Heid IM, Böger CA, et al. Association of sleep-disordered breathing with diabetes-associated kidney disease. Annals of Medicine . 2017;49:487–495. doi: 10.1080/07853890.2017.1306100. [DOI] [PubMed] [Google Scholar]
- [203].Nishimura A, Kasai T, Kikuno S, Nagasawa K, Okubo M, Narui K, et al. Effect of Sleep-Disordered Breathing on Albuminuria in 273 Patients With Type 2 Diabetes. Journal of Clinical Sleep Medicine: JCSM: Official Publication of the American Academy of Sleep Medicine . 2018;14:401–407. doi: 10.5664/jcsm.6986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Hwu DW, Lin KD, Lin KC, Lee YJ, Chang YH. The association of obstructive sleep apnea and renal outcomes-a systematic review and meta-analysis. BMC Nephrology . 2017;18:313. doi: 10.1186/s12882-017-0731-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Liu T, Zhan Y, Wang Y, Li Q, Mao H. Obstructive sleep apnea syndrome and risk of renal impairment: a systematic review and meta-analysis with trial sequential analysis. Sleep & Breathing = Schlaf & Atmung . 2021;25:17–27. doi: 10.1007/s11325-020-02090-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Hall IE, Kashgarian M, Moeckel GW, Dahl NK. Resolution of proteinuria in a patient with focal segmental glomerulosclerosis following BiPAP initiation for obesity hypoventilation syndrome. Clinical Nephrology . 2012;77:62–65. doi: 10.5414/cn106859. [DOI] [PubMed] [Google Scholar]
- [207].Parmaksız E, Torun Parmaksız E. Reversibility of microalbuminuria with continuous positive airway pressure treatment in obstructive sleep apnea syndrome. International Urology and Nephrology . 2020;52:1719–1724. doi: 10.1007/s11255-020-02519-6. [DOI] [PubMed] [Google Scholar]
- [208].Zamarrón E, Jaureguizar A, García-Sánchez A, Díaz-Cambriles T, Alonso-Fernández A, Lores V, et al. Continuous Positive Airway Pressure Effect on Albuminuria Progression in Patients with Obstructive Sleep Apnea and Diabetic Kidney Disease: A Randomized Clinical Trial. American Journal of Respiratory and Critical Care Medicine . 2023;207:757–767. doi: 10.1164/rccm.202206-1091OC. [DOI] [PubMed] [Google Scholar]
- [209].Chen R, Huang ZW, Lin XF, Lin JF, Yang MJ. Effect of continuous positive airway pressure on albuminuria in patients with obstructive sleep apnea: a meta-analysis. Sleep & Breathing = Schlaf & Atmung . 2022;26:279–285. doi: 10.1007/s11325-021-02393-1. [DOI] [PubMed] [Google Scholar]
- [210].Jennette JC, Charles L, Grubb W. Glomerulomegaly and focal segmental glomerulosclerosis associated with obesity and sleep-apnea syndrome. American Journal of Kidney Diseases: the Official Journal of the National Kidney Foundation . 1987;10:470–472. doi: 10.1016/s0272-6386(87)80196-8. [DOI] [PubMed] [Google Scholar]
- [211].Bailey RR, Lynn KL, Burry AF, Drennan C. Proteinuria, glomerulomegaly and focal glomerulosclerosis in a grossly obese man with obstructive sleep apnea syndrome. Australian and New Zealand Journal of Medicine . 1989;19:473–474. doi: 10.1111/j.1445-5994.1989.tb00310.x. [DOI] [PubMed] [Google Scholar]
- [212].Full KM, Jackson CL, Rebholz CM, Matsushita K, Lutsey PL. Obstructive Sleep Apnea, Other Sleep Characteristics, and Risk of CKD in the Atherosclerosis Risk in Communities Sleep Heart Health Study. Journal of the American Society of Nephrology: JASN . 2020;31:1859–1869. doi: 10.1681/ASN.2020010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Iseki K, Tohyama K, Matsumoto T, Nakamura H. High Prevalence of chronic kidney disease among patients with sleep related breathing disorder (SRBD) Hypertension Research: Official Journal of the Japanese Society of Hypertension . 2008;31:249–255. doi: 10.1291/hypres.31.249. [DOI] [PubMed] [Google Scholar]
- [214].Chou YT, Lee PH, Yang CT, Lin CL, Veasey S, Chuang LP, et al. Obstructive sleep apnea: a stand-alone risk factor for chronic kidney disease. Nephrology, Dialysis, Transplantation: Official Publication of the European Dialysis and Transplant Association - European Renal Association . 2011;26:2244–2250. doi: 10.1093/ndt/gfq821. [DOI] [PubMed] [Google Scholar]
- [215].Kanbay A, Buyukoglan H, Ozdogan N, Kaya E, Oymak FS, Gulmez I, et al. Obstructive sleep apnea syndrome is related to the progression of chronic kidney disease. International Urology and Nephrology . 2012;44:535–539. doi: 10.1007/s11255-011-9927-8. [DOI] [PubMed] [Google Scholar]
- [216].Leong WB, Nolen M, Thomas GN, Adab P, Banerjee D, Taheri S. The impact of hypoxemia on nephropathy in extremely obese patients with type 2 diabetes mellitus. Journal of Clinical Sleep Medicine: JCSM: Official Publication of the American Academy of Sleep Medicine . 2014;10:773–778. doi: 10.5664/jcsm.3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Marrone O, Battaglia S, Steiropoulos P, Basoglu OK, Kvamme JA, Ryan S, et al. Chronic kidney disease in European patients with obstructive sleep apnea: the ESADA cohort study. Journal of Sleep Research . 2016;25:739–745. doi: 10.1111/jsr.12426. [DOI] [PubMed] [Google Scholar]
- [218].Yayan J, Rasche K, Vlachou A. Obstructive Sleep Apnea and Chronic Kidney Disease. Advances in Experimental Medicine and Biology . 2017;1022:11–18. doi: 10.1007/5584_2017_35. [DOI] [PubMed] [Google Scholar]
- [219].Beaudin AE, Raneri JK, Ahmed SB, Hirsch Allen AJM, Nocon A, Gomes T, et al. Risk of chronic kidney disease in patients with obstructive sleep apnea. Sleep . 2022;45:zsab267. doi: 10.1093/sleep/zsab267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].Lee YC, Hung SY, Wang HK, Lin CW, Wang HH, Chen SW, et al. Sleep apnea and the risk of chronic kidney disease: a nationwide population-based cohort study. Sleep . 2015;38:213–221. doi: 10.5665/sleep.4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Chu H, Shih CJ, Ou SM, Chou KT, Lo YH, Chen YT. Association of sleep apnoea with chronic kidney disease in a large cohort from Taiwan. Respirology (Carlton, Vic.) . 2016;21:754–760. doi: 10.1111/resp.12739. [DOI] [PubMed] [Google Scholar]
- [222].Lin YS, Liu PH, Lin SW, Chuang LP, Ho WJ, Chou YT, et al. Simple obstructive sleep apnea patients without hypertension or diabetes accelerate kidney dysfunction: a population follow-up cohort study from Taiwan. Sleep & Breathing = Schlaf & Atmung . 2017;21:85–91. doi: 10.1007/s11325-016-1376-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [223].Ahmed SB, Ronksley PE, Hemmelgarn BR, Tsai WH, Manns BJ, Tonelli M, et al. Nocturnal hypoxia and loss of kidney function. PLoS ONE . 2011;6:e19029. doi: 10.1371/journal.pone.0019029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [224].Sakaguchi Y, Hatta T, Hayashi T, Shoji T, Suzuki A, Tomida K, et al. Association of nocturnal hypoxemia with progression of CKD. Clinical Journal of the American Society of Nephrology: CJASN . 2013;8:1502–1507. doi: 10.2215/CJN.11931112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Leong WB, Jadhakhan F, Taheri S, Thomas GN, Adab P. The Association between Obstructive Sleep Apnea on Diabetic Kidney Disease: A Systematic Review and Meta-Analysis. Sleep . 2016;39:301–308. doi: 10.5665/sleep.5432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [226].Puckrin R, Iqbal S, Zidulka A, Vasilevsky M, Barre P. Renoprotective effects of continuous positive airway pressure in chronic kidney disease patients with sleep apnea. International Urology and Nephrology . 2015;47:1839–1845. doi: 10.1007/s11255-015-1113-y. [DOI] [PubMed] [Google Scholar]
- [227].Li X, Liu C, Zhang H, Zhang J, Zhao M, Sun D, et al. Effect of 12-month nasal continuous positive airway pressure therapy for obstructive sleep apnea on progression of chronic kidney disease. Medicine . 2019;98:e14545. doi: 10.1097/MD.0000000000014545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [228].Rimke AN, Ahmed SB, Turin TC, Pendharkar SR, Raneri JK, Lynch EJ, et al. Effect of CPAP Therapy on Kidney Function in Patients With Chronic Kidney Disease: A Pilot Randomized Controlled Trial. Chest . 2021;159:2008–2019. doi: 10.1016/j.chest.2020.11.052. [DOI] [PubMed] [Google Scholar]
- [229].Fu Y, Lin J, Chen L, Chen X, Chen Q. Meta-analysis of the effects of CPAP therapy on estimated glomerular filtration rate in patients with obstructive sleep apnea. Sleep & Breathing = Schlaf & Atmung . 2023;27:2155–2163. doi: 10.1007/s11325-023-02811-6. [DOI] [PubMed] [Google Scholar]
- [230].Oltmanns KM, Gehring H, Rudolf S, Schultes B, Rook S, Schweiger U, et al. Hypoxia causes glucose intolerance in humans. American Journal of Respiratory and Critical Care Medicine . 2004;169:1231–1237. doi: 10.1164/rccm.200308-1200OC. [DOI] [PubMed] [Google Scholar]
- [231].Louis M, Punjabi NM. Effects of acute intermittent hypoxia on glucose metabolism in awake healthy volunteers. Journal of Applied Physiology (Bethesda, Md.: 1985) . 2009;106:1538–1544. doi: 10.1152/japplphysiol.91523.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [232].D’Hulst G, Jamart C, Van Thienen R, Hespel P, Francaux M, Deldicque L. Effect of acute environmental hypoxia on protein metabolism in human skeletal muscle. Acta Physiologica (Oxford, England) . 2013;208:251–264. doi: 10.1111/apha.12086. [DOI] [PubMed] [Google Scholar]
- [233].D’Hulst G, Sylow L, Hespel P, Deldicque L. Acute systemic insulin intolerance does not alter the response of the Akt/GSK-3 pathway to environmental hypoxia in human skeletal muscle. European Journal of Applied Physiology . 2015;115:1219–1231. doi: 10.1007/s00421-015-3103-2. [DOI] [PubMed] [Google Scholar]
- [234].Van Meijel RLJ, Wang P, Bouwman F, Blaak EE, Mariman ECM, Goossens GH. The Effects of Mild Intermittent Hypoxia Exposure on the Abdominal Subcutaneous Adipose Tissue Proteome in Overweight and Obese Men: A First-in-Human Randomized, Single-Blind, and Cross-Over Study. Frontiers in Physiology . 2022;12:791588. doi: 10.3389/fphys.2021.791588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [235].Larsen JJ, Hansen JM, Olsen NV, Galbo H, Dela F. The effect of altitude hypoxia on glucose homeostasis in men. The Journal of Physiology . 1997;504 (Pt 1):241–249. doi: 10.1111/j.1469-7793.1997.241bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [236].Braun B, Rock PB, Zamudio S, Wolfel GE, Mazzeo RS, Muza SR, et al. Women at altitude: short-term exposure to hypoxia and/or alpha(1)-adrenergic blockade reduces insulin sensitivity. Journal of Applied Physiology (Bethesda, Md.: 1985) . 2001;91:623–631. doi: 10.1152/jappl.2001.91.2.623. [DOI] [PubMed] [Google Scholar]
- [237].Mekjavic IB, Amon M, Simpson EJ, Kölegård R, Eiken O, Macdonald IA. Energy Intake of Men With Excess Weight During Normobaric Hypoxic Confinement. Frontiers in Physiology . 2022;12:801833. doi: 10.3389/fphys.2021.801833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [238].Steiropoulos P, Papanas N, Nena E, Tsara V, Fitili C, Tzouvelekis A, et al. Markers of glycemic control and insulin resistance in non-diabetic patients with Obstructive Sleep Apnea Hypopnea Syndrome: does adherence to CPAP treatment improve glycemic control. Sleep Medicine . 2009;10:887–891. doi: 10.1016/j.sleep.2008.10.004. [DOI] [PubMed] [Google Scholar]
- [239].Perantoni E, Filos D, Archontogeorgis K, Steiropoulos P, Chouvarda IC. Pre-diabetic patients with severe obstructive sleep apnea: novel parameters of hypoxia during sleep correlate with insulin resistance. Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society. Annual International Conference . 2019;2019:5002–5005. doi: 10.1109/EMBC.2019.8857457. [DOI] [PubMed] [Google Scholar]
- [240].Kang HH, Kim SW, Lee SH. Association between triglyceride glucose index and obstructive sleep apnea risk in Korean adults: a cross-sectional cohort study. Lipids in Health and Disease . 2020;19:182. doi: 10.1186/s12944-020-01358-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [241].Michalek-Zrabkowska M, Macek P, Martynowicz H, Gac P, Mazur G, Grzeda M, et al. Obstructive Sleep Apnea as a Risk Factor of Insulin Resistance in Nondiabetic Adults. Life (Basel, Switzerland) . 2021;11:50. doi: 10.3390/life11010050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [242].Schwenger KJP, Ghorbani Y, Li C, Fischer SE, Jackson TD, Okrainec A, et al. Obstructive Sleep Apnea and Non-alcoholic Fatty Liver Disease in Obese Patients Undergoing Bariatric Surgery. Obesity Surgery . 2020;30:2572–2578. doi: 10.1007/s11695-020-04514-3. [DOI] [PubMed] [Google Scholar]
- [243].Drager LF, Diegues-Silva L, Diniz PM, Bortolotto LA, Pedrosa RP, Couto RB, et al. Obstructive sleep apnea, masked hypertension, and arterial stiffness in men. American Journal of Hypertension . 2010;23:249–254. doi: 10.1038/ajh.2009.246. [DOI] [PubMed] [Google Scholar]
- [244].Theorell-Haglöw J, Hoyos CM, Phillips CL, Yee BJ, Melehan KL, Liu PY, et al. Associations Between Obstructive Sleep Apnea and Measures of Arterial Stiffness. Journal of Clinical Sleep Medicine: JCSM: Official Publication of the American Academy of Sleep Medicine . 2019;15:201–206. doi: 10.5664/jcsm.7616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [245].Noda A, Okada T, Yasuma F, Nakashima N, Yokota M. Cardiac hypertrophy in obstructive sleep apnea syndrome. Chest . 1995;107:1538–1544. doi: 10.1378/chest.107.6.1538. [DOI] [PubMed] [Google Scholar]
- [246].Suzuki T, Nakano H, Maekawa J, Okamoto Y, Ohnishi Y, Yamauchi M, et al. Obstructive sleep apnea and carotid-artery intima-media thickness. Sleep . 2004;27:129–133. doi: 10.1093/sleep/27.1.129. [DOI] [PubMed] [Google Scholar]
- [247].Lisi E, Faini A, Bilo G, Lonati LM, Revera M, Salerno S, et al. Diastolic dysfunction in controlled hypertensive patients with mild-moderate obstructive sleep apnea. International Journal of Cardiology . 2015;187:686–692. doi: 10.1016/j.ijcard.2015.02.037. [DOI] [PubMed] [Google Scholar]
- [248].Yamaguchi T, Takata Y, Usui Y, Asanuma R, Nishihata Y, Kato K, et al. Nocturnal Intermittent Hypoxia Is Associated With Left Ventricular Hypertrophy in Middle-Aged Men With Hypertension and Obstructive Sleep Apnea. American Journal of Hypertension . 2016;29:372–378. doi: 10.1093/ajh/hpv115. [DOI] [PubMed] [Google Scholar]
- [249].Kim SE, Seo J, Kwon Y, Cho I, Shim CY, Ha JW, et al. Effects of continuous positive airway pressure therapy on left ventricular performance in patients with severe obstructive sleep apnea. Scientific Reports . 2023;13:5335. doi: 10.1038/s41598-023-32274-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [250].Mahmoud MI, Alotaibi RK, Almusally R, Shafiek H, Elamin Y, Alhaj Z, et al. Effect of nocturnal hypoxemia on glycemic control among diabetic Saudi patients presenting with obstructive sleep apnea. Frontiers in Endocrinology . 2023;13:1020617. doi: 10.3389/fendo.2022.1020617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [251].Iliescu EA, Lam M, Pater J, Munt PW. Do patients with obstructive sleep apnea have clinically significant proteinuria. Clinical Nephrology . 2001;55:196–204. [PubMed] [Google Scholar]
- [252].Yaşar ZA, Ucar ZZ, Demir AU, Kirakli C, Kalenci D, Tibet G. Does CPAP therapy alter urinary albumin level in adult patients with moderate to severe obstructive sleep apnea syndrome. Sleep & Breathing = Schlaf & Atmung . 2014;18:525–532. doi: 10.1007/s11325-013-0914-4. [DOI] [PubMed] [Google Scholar]
- [253].Sklar AH, Chaudhary BA. Reversible proteinuria in obstructive sleep apnea syndrome. Archives of Internal Medicine . 1988;148:87–89. [PubMed] [Google Scholar]
- [254].Zhang R, Zhang P, Zhao F, Han X, Ji L. Association of Diabetic Microvascular Complications and Parameters of Obstructive Sleep Apnea in Patients with Type 2 Diabetes. Diabetes Technology & Therapeutics . 2016;18:415–420. doi: 10.1089/dia.2015.0433. [DOI] [PubMed] [Google Scholar]
- [255].Jhamb M, Ran X, Abdalla H, Roumelioti ME, Hou S, Davis H, et al. Association of Sleep Apnea with Mortality in Patients with Advanced Kidney Disease. Clinical Journal of the American Society of Nephrology: CJASN . 2020;15:182–190. doi: 10.2215/CJN.07880719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [256].Semenza GL, Nejfelt MK, Chi SM, Antonarakis SE. Hypoxia-inducible nuclear factors bind to an enhancer element located 3’ to the human erythropoietin gene. Proceedings of the National Academy of Sciences of the United States of America . 1991;88:5680–5684. doi: 10.1073/pnas.88.13.5680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [257].Maxwell PH, Pugh CW, Ratcliffe PJ. Inducible operation of the erythropoietin 3’ enhancer in multiple cell lines: evidence for a widespread oxygen-sensing mechanism. Proceedings of the National Academy of Sciences of the United States of America . 1993;90:2423–2427. doi: 10.1073/pnas.90.6.2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [258].Ang SO, Chen H, Gordeuk VR, Sergueeva AI, Polyakova LA, Miasnikova GY, et al. Endemic polycythemia in Russia: mutation in the VHL gene. Blood Cells, Molecules & Diseases . 2002;28:57–62. doi: 10.1006/bcmd.2002.0488. [DOI] [PubMed] [Google Scholar]
- [259].Li X, Kimura H, Hirota K, Sugimoto H, Kimura N, Takahashi N, et al. Hypoxia reduces the expression and anti-inflammatory effects of peroxisome proliferator-activated receptor-gamma in human proximal renal tubular cells. Nephrology, Dialysis, Transplantation: Official Publication of the European Dialysis and Transplant Association - European Renal Association . 2007;22:1041–1051. doi: 10.1093/ndt/gfl766. [DOI] [PubMed] [Google Scholar]
- [260].Wang B, Wood IS, Trayhurn P. Hypoxia induces leptin gene expression and secretion in human preadipocytes: differential effects of hypoxia on adipokine expression by preadipocytes. The Journal of Endocrinology . 2008;198:127–134. doi: 10.1677/JOE-08-0156. [DOI] [PubMed] [Google Scholar]
- [261].Cavadas MAS, Mesnieres M, Crifo B, Manresa MC, Selfridge AC, Scholz CC, et al. REST mediates resolution of HIF-dependent gene expression in prolonged hypoxia. Scientific Reports . 2015;5:17851. doi: 10.1038/srep17851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [262].Blum JI, Bijli KM, Murphy TC, Kleinhenz JM, Hart CM. Time-dependent PPARγ Modulation of HIF-1α Signaling in Hypoxic Pulmonary Artery Smooth Muscle Cells. The American Journal of the Medical Sciences . 2016;352:71–79. doi: 10.1016/j.amjms.2016.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [263].Wang M, Li G, Yang Z, Wang L, Zhang L, Wang T, et al. Uncoupling protein 2 downregulation by hypoxia through repression of peroxisome proliferator-activated receptor γ promotes chemoresistance of non-small cell lung cancer. Oncotarget . 2017;8:8083–8094. doi: 10.18632/oncotarget.14097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [264].Martinez CA, Kerr B, Jin C, Cistulli PA, Cook KM. Obstructive Sleep Apnea Activates HIF-1 in a Hypoxia Dose-Dependent Manner in HCT116 Colorectal Carcinoma Cells. International Journal of Molecular Sciences . 2019;20:445. doi: 10.3390/ijms20020445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [265].Locatelli F, Ravera M, Esposito C, Grandaliano G, Gesualdo L, Minutolo R. A novel scenario in the therapeutic management of anemia of chronic kidney disease: placement and use of roxadustat. Journal of Nephrology . 2024 doi: 10.1007/s40620-023-01849-9. (online ahead of print) [DOI] [PubMed] [Google Scholar]
- [266].Ren X, Dorrington KL, Maxwell PH, Robbins PA. Effects of desferrioxamine on serum erythropoietin and ventilatory sensitivity to hypoxia in humans. Journal of Applied Physiology (Bethesda, Md.: 1985) . 2000;89:680–686. doi: 10.1152/jappl.2000.89.2.680. [DOI] [PubMed] [Google Scholar]
- [267].Satawiriya M, Khongphatthanayothin A, Limsuwan A. Reversible severe pulmonary hypertension related to scurvy in children. BMC Cardiovascular Disorders . 2024;24:24. doi: 10.1186/s12872-023-03629-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [268].Sharma S, Malur A, Marshall I, Huizar I, Barna BP, Pories W, et al. Alveolar macrophage activation in obese patients with obstructive sleep apnea. Surgery . 2012;151:107–112. doi: 10.1016/j.surg.2011.06.035. [DOI] [PubMed] [Google Scholar]
- [269].Lu X, Bijli KM, Ramirez A, Murphy TC, Kleinhenz J, Hart CM. Hypoxia downregulates PPARγ via an ERK1/2-NF-κB-Nox4-dependent mechanism in human pulmonary artery smooth muscle cells. Free Radical Biology & Medicine . 2013;63:151–160. doi: 10.1016/j.freeradbiomed.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [270].Karasaki Y, Kashiwazaki H. The difference of mutation in the peroxisome proliferator activated receptor gamma2 gene among people at high altitudes and low altitudes in Bolivia. Journal of UOEH . 2005;27:243–248. doi: 10.7888/juoeh.27.243. [DOI] [PubMed] [Google Scholar]
- [271].Liu XL, Zhang FL, Zhou ZY, Zhao HL, Shen GM, Baohan WY, et al. Analysis of two sequence variants in peroxisome proliferator activated receptor gamma gene in Tajik population at high altitudes and Han population at low altitudes in China. Molecular Biology Reports . 2010;37:179–184. doi: 10.1007/s11033-009-9581-8. [DOI] [PubMed] [Google Scholar]
- [272].Gharib SA, Hayes AL, Rosen MJ, Patel SR. A pathway-based analysis on the effects of obstructive sleep apnea in modulating visceral fat transcriptome. Sleep . 2013;36:23–30. doi: 10.5665/sleep.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [273].Gabryelska A, Szmyd B, Szemraj J, Stawski R, Sochal M, Białasiewicz P. Patients with obstructive sleep apnea present with chronic upregulation of serum HIF-1α protein. Journal of Clinical Sleep Medicine: JCSM: Official Publication of the American Academy of Sleep Medicine . 2020;16:1761–1768. doi: 10.5664/jcsm.8682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [274].Karnieli E, Chernow B, Hissin PJ, Simpson IA, Foley JE. Insulin stimulates glucose transport in isolated human adipose cells through a translocation of intracellular glucose transporters to the plasma membrane: a preliminary report. Hormone and Metabolic Research = Hormon- Und Stoffwechselforschung = Hormones et Metabolisme . 1986;18:867–868. doi: 10.1055/s-2007-1012458. [DOI] [PubMed] [Google Scholar]
- [275].Karnieli E, Barzilai A, Rafaeloff R, Armoni M. Distribution of glucose transporters in membrane fractions isolated from human adipose cells. Relation to cell size. The Journal of Clinical Investigation . 1986;78:1051–1055. doi: 10.1172/JCI112660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [276].Garvey WT, Huecksteadt TP, Matthaei S, Olefsky JM. Role of glucose transporters in the cellular insulin resistance of type II non-insulin-dependent diabetes mellitus. The Journal of Clinical Investigation . 1988;81:1528–1536. doi: 10.1172/JCI113485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [277].Koester AM, Geiser A, Bowman PRT, van de Linde S, Gadegaard N, Bryant NJ, et al. GLUT4 translocation and dispersal operate in multiple cell types and are negatively correlated with cell size in adipocytes. Scientific Reports . 2022;12:20535. doi: 10.1038/s41598-022-24736-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [278].Foley JE, Kashiwagi A, Verso MA, Reaven G, Andrews J. Improvement in in vitro insulin action after one month of insulin therapy in obese noninsulin-dependent diabetics. Measurements of glucose transport and metabolism, insulin binding, and lipolysis in isolated adipocytes. The Journal of Clinical Investigation . 1983;72:1901–1909. doi: 10.1172/JCI111153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [279].Kashiwagi A, Verso MA, Andrews J, Vasquez B, Reaven G, Foley JE. In vitro insulin resistance of human adipocytes isolated from subjects with noninsulin-dependent diabetes mellitus. The Journal of Clinical Investigation . 1983;72:1246–1254. doi: 10.1172/JCI111080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [280].Garvey WT, Maianu L, Huecksteadt TP, Birnbaum MJ, Molina JM, Ciaraldi TP. Pretranslational suppression of a glucose transporter protein causes insulin resistance in adipocytes from patients with non-insulin-dependent diabetes mellitus and obesity. The Journal of Clinical Investigation . 1991;87:1072–1081. doi: 10.1172/JCI115068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [281].Maianu L, Keller SR, Garvey WT. Adipocytes exhibit abnormal subcellular distribution and translocation of vesicles containing glucose transporter 4 and insulin-regulated aminopeptidase in type 2 diabetes mellitus: implications regarding defects in vesicle trafficking. The Journal of Clinical Endocrinology and Metabolism . 2001;86:5450–5456. doi: 10.1210/jcem.86.11.8053. [DOI] [PubMed] [Google Scholar]
- [282].Hammarstedt A, Sopasakis VR, Gogg S, Jansson PA, Smith U. Improved insulin sensitivity and adipose tissue dysregulation after short-term treatment with pioglitazone in non-diabetic, insulin-resistant subjects. Diabetologia . 2005;48:96–104. doi: 10.1007/s00125-004-1612-3. [DOI] [PubMed] [Google Scholar]
- [283].Kampmann U, Christensen B, Nielsen TS, Pedersen SB, Ørskov L, Lund S, et al. GLUT4 and UBC9 protein expression is reduced in muscle from type 2 diabetic patients with severe insulin resistance. PLoS ONE . 2011;6:e27854. doi: 10.1371/journal.pone.0027854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [284].Garvey WT, Maianu L, Zhu JH, Hancock JA, Golichowski AM. Multiple defects in the adipocyte glucose transport system cause cellular insulin resistance in gestational diabetes. Heterogeneity in the number and a novel abnormality in subcellular localization of GLUT4 glucose transporters. Diabetes . 1993;42:1773–1785. doi: 10.2337/diab.42.12.1773. [DOI] [PubMed] [Google Scholar]
- [285].Colomiere M, Permezel M, Lappas M. Diabetes and obesity during pregnancy alter insulin signalling and glucose transporter expression in maternal skeletal muscle and subcutaneous adipose tissue. Journal of Molecular Endocrinology . 2010;44:213–223. doi: 10.1677/JME-09-0091. [DOI] [PubMed] [Google Scholar]
- [286].Rosenbaum D, Haber RS, Dunaif A. Insulin resistance in polycystic ovary syndrome: decreased expression of GLUT-4 glucose transporters in adipocytes. The American Journal of Physiology . 1993;264:E197–E202. doi: 10.1152/ajpendo.1993.264.2.E197. [DOI] [PubMed] [Google Scholar]
- [287].Ezeh U, Chen IYD, Chen YH, Azziz R. Adipocyte expression of glucose transporter 1 and 4 in PCOS: Relationship to insulin-mediated and non-insulin-mediated whole-body glucose uptake. Clinical Endocrinology . 2019;90:542–552. doi: 10.1111/cen.13931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [288].Bril F, Ezeh U, Amiri M, Hatoum S, Pace L, Chen YH, et al. Adipose Tissue Dysfunction in Polycystic Ovary Syndrome. The Journal of Clinical Endocrinology and Metabolism . 2023;109:10–24. doi: 10.1210/clinem/dgad356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [289].Arner P, Arner E, Hammarstedt A, Smith U. Genetic predisposition for Type 2 diabetes, but not for overweight/obesity, is associated with a restricted adipogenesis. PLoS ONE . 2011;6:e18284. doi: 10.1371/journal.pone.0018284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [290].Agrawal M, Yeo CR, Shabbir A, Chhay V, Silver DL, Magkos F, et al. Fat storage-inducing transmembrane protein 2 (FIT2) is less abundant in type 2 diabetes, and regulates triglyceride accumulation and insulin sensitivity in adipocytes. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology . 2019;33:430–440. doi: 10.1096/fj.201701321RR. [DOI] [PubMed] [Google Scholar]
- [291].Barroso I, Gurnell M, Crowley VE, Agostini M, Schwabe JW, Soos MA, et al. Dominant negative mutations in human PPARgamma associated with severe insulin resistance, diabetes mellitus and hypertension. Nature . 1999;402:880–883. doi: 10.1038/47254. [DOI] [PubMed] [Google Scholar]
- [292].Catalano PM, Nizielski SE, Shao J, Preston L, Qiao L, Friedman JE. Downregulated IRS-1 and PPARgamma in obese women with gestational diabetes: relationship to FFA during pregnancy. American Journal of Physiology. Endocrinology and Metabolism . 2002;282:E522–E533. doi: 10.1152/ajpendo.00124.2001. [DOI] [PubMed] [Google Scholar]
- [293].Liao W, Nguyen MTA, Yoshizaki T, Favelyukis S, Patsouris D, Imamura T, et al. Suppression of PPAR-gamma attenuates insulin-stimulated glucose uptake by affecting both GLUT1 and GLUT4 in 3T3-L1 adipocytes. American Journal of Physiology. Endocrinology and Metabolism . 2007;293:E219–E227. doi: 10.1152/ajpendo.00695.2006. [DOI] [PubMed] [Google Scholar]