Abstract
Zinc (Zn) is a vital trace element that plays a pivotal role in protein synthesis, cellular growth, and differentiation and is involved as a cofactor of metalloenzymes, performing a wide variety of metabolic, immune, and synthesis roles. Zn is required at all stages of an infant’s and child’s development, and severe Zn deficiency has been reported to lead to slower physical, cognitive, and sexual growth. Preterm neonates are at a higher risk of developing zinc deficiency for a variety of reasons, including low Zn intake from enteral feeds containing breast milk, relative malabsorption due to immaturity of the gastrointestinal tract with limited absorptive capacity, increased urinary loss of zinc, and increased demand during the early developmental stages. Moreover, premature infants are at risk of gastrointestinal diseases like necrotizing enterocolitis (NEC), which can limit absorption capacity and potentially lead to malabsorption. TPN is frequently used in preterm infants to provide them with sufficient nutrients and calories. However, it has its own complications, including cholestasis, especially if used for prolonged periods. In this case report, we are presenting the case of a male preterm infant who was delivered by caesarean section at 26 weeks’ gestation. The baby developed an intestinal perforation due to NEC, for which he underwent surgery for resection of the necrotic bowel and the creation of a high ileal stoma and was put on prolonged total parenteral nutrition (TPN), which led to the development of zinc deficiency.
Keywords: diseases, metalloenzymes, total parenteral nutrition (TPN), prematurity, alkaline phosphatase
1. Introduction
Zinc (Zn) is one of the vital trace elements that is required for the efficient growth and development of several tissues as it plays a pivotal role in protein synthesis, cellular growth, and differentiation [1]. Zn plays an important role as a cofactor of metalloenzymes involved in metabolism, immune functions, protein folding, the development of the gastrointestinal tract, and gene expression [2,3]. Currently, around 1000 metalloenzymes and almost 2000 transcription factors are known to be dependent upon Zn for their catalytic activity [3,4,5,6]. Since Zn is required at all stages of a child’s development, severe Zn deficiency has been reported to lead to slower physical, cognitive, and sexual growth. It also manifests in skin disorders, immune compromise, and a higher frequency of acute illnesses in infants and children [1,7,8]. In the worst-case scenarios, Zn deficiency results in childhood stunting with an increased risk of morbidity as well as mortality in children [3,8,9].
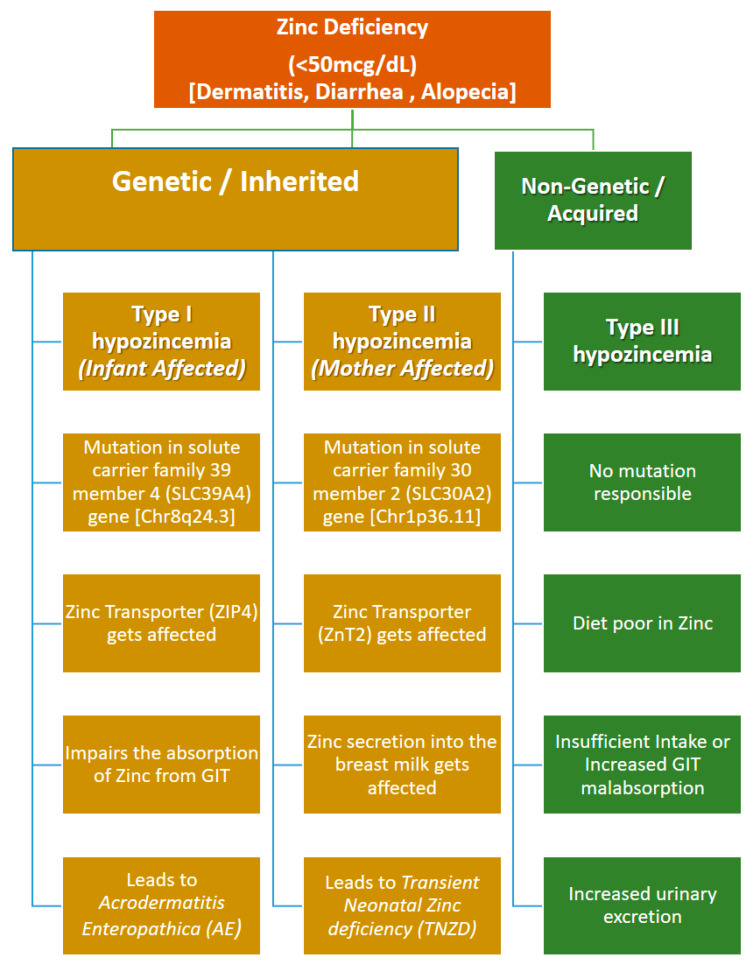
Zn deficiency may be either inherited or acquired depending upon the point at which Zn metabolism is being affected [7] (See Figure 1). Inherited Zn deficiency usually arises as secondary to the genetic mutations in the zinc transporter genes. Two of the important inherited Zn disorders have been characterized so far. One is due to mutation in the SLC39A4 gene, which codes for zinc transporter ZIP4 in the affected individual and leads to impairment in the absorption of zinc from the gastrointestinal tract and hence low serum levels of the Zn (<50 mcg/dL); this is referred to as acrodermatitis enteropathica (AE) [7,10]. The second is due to mutation in the mother’s SLC30A2 gene, which codes for the ZnT2 zinc transporter and leads to the reduced transport and secretion of zinc into breast milk; this disease is referred to as transient neonatal zinc deficiency (TNZD) [5,10,11]. The acquired etiologies of Zn deficiency are usually caused by a lack of a sufficient intake of zinc in the diet and may arise because of varied reasons, ranging from milk low in zinc levels, malnutrition, malabsorption, an increased loss of zinc, secondary gastrointestinal illness, increased urinary loss, and high demand [5].
Figure 1.
Different types of zinc deficiency and their etiology [8,11].
Irrespective of the etiology of Zn deficiency, in its early stages all types exhibit the triad of clinical manifestations that include dermatitis, diarrhea, and alopecia [3,5,7,12]. Since, during fetal development, most of the Zn is acquired during the third trimester of the pregnancy, in preterm infants there is a high demand for Zn as a consequence of premature delivery [5,11,12]. Therefore, to avoid this potential risk, zinc is added to the Total Parenteral Nutrition (TPN) provided to premature infants in the neonatal intensive care unit (NICU) till full sufficient oral intake with adequate macro- and micronutrients is achieved [13,14]. After being absorbed in the small intestines via a carrier-mediated mechanism, Zn is taken up and consequently stored in four main organs of the body, the skeletal muscles (60%), bones (30%), liver (5%), and skin (5%). The skin usually contains about 60 µg/g of zinc in the epidermis and 40 µg/g in the upper dermis [4,5,6]. Zinc plays a crucial role in maintaining the structural integrity of skin by being directly involved in anti-inflammatory and wound-healing processes [3,4,5]; thus, skin lesions are common manifestations of Zn deficiency [4,12,14].
The provision of zinc supplementation is a standard intervention for all types of zinc deficiencies, which in the case of preterm newborns is provided via parenteral nutrition (PN) as they are unable to tolerate enteral feeds [12]. The goal of PN should be to provide elemental zinc up to a maximum of 5 mg/kg/day, which is enough to alleviate symptoms within a week and enable clinical improvement within six months of treatment [7,12,15]. Premature and low-birth-weight infants have been reported to develop a complication: PN-associated cholestasis (PNAC), nowadays referred to as intestinal failure-associated liver disease (IFALD), due to the prolonged use of PN (for more than 14 days) deficient in zinc and/or cysteine but rich in soy-based lipid emulsions (SOLEs) [16].
IFALD is one of the commonest serious complications of long term PN that results in liver injury due to intestinal failure (IF). It has complicated and multivariate causes and consists of a spectrum of liver illnesses, including cholestasis, biliary cirrhosis, and steatohepatitis [17,18,19]. SOLEs used for PN have a high phytosterol content, high ω-6 to ω-3 long-chain polyunsaturated fatty acid (PUFA) ratio, and very low levels of α-tocopherol. Phytosterols play a primary pathogenic role, as they are readily absorbed in the gastrointestinal tract, leading to their accumulation in the hepatocytes when given intravenously [20]. Fish-based lipid emulsions (FILEs) have high levels of α-tocopherol, low levels of phytosterols, and a significant concentration of anti-inflammatory ω-3 fatty acids like eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), which have shown promising results in resolving cholestasis, reversing IFALD, and increasing survival [21,22].
2. Case Report
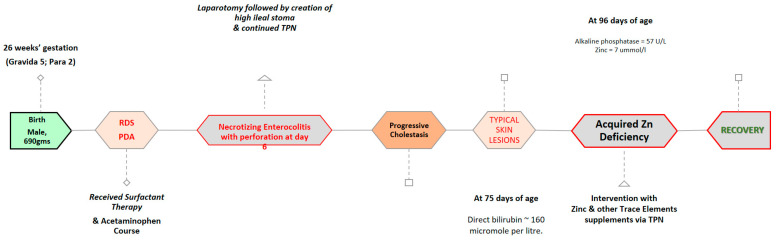
A baby boy was delivered by emergency caesarean section due to abnormal cardiotocography (CTG) at 26 weeks’ gestation to a gravida 5 para 2 mother who earlier had two abortions. The APGAR score was 4 and 8 at 1 and 5 min, respectively. The baby was intubated after birth and received surfactant therapy for respiratory distress syndrome (RDS) and his birth weight was 690 g.
Upon admission to a neonatal intensive care unit (NICU), the baby was sick, hypotensive, and required inotropic support (epinephrine). Echocardiography showed hemodynamically significant patent ductus arteriosus (PDA) for which he was treated with an acetaminophen course.
On day 6, the baby developed clinical and radiological signs of necrotizing enterocolitis, which was complicated by an intestinal perforation, underwent laparotomy, and was found to have multiple bowel perforations, for which a resection of the necrotic bowel and a high ileal stoma were created. There was difficulty in establishing enteral feeds due to significant feeding intolerance and a high stoma output, so the baby required total parental nutrition for a long period and, as a result, he developed cholestasis. His direct bilirubin reached up to 160 micromoles per liter, for which the baby started treatment on ursodeoxycholic acid, and trace elements were reduced to a twice-weekly dose instead of a daily dose in order to manage his worsening cholestasis status.
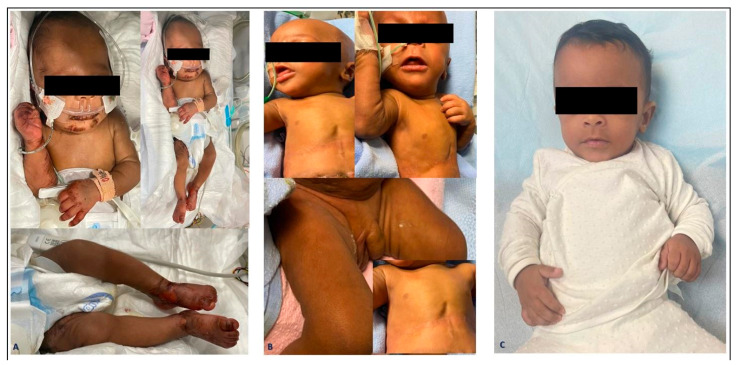
At 96 days of age, he developed skin lesions/changes in the form of excoriation and redness on the face, abdomen, genitalia, and lower limbs (Figure 2 and Figure 3). His lab result at that time showed markedly relatively low alkaline phosphatase (a level of 57 U/L) and his baselines earlier ranged between 200 and 400 U/L, so zinc deficiency was suspected and serum zinc levels were requested, which were 1.7 ummol/L (normal range 9–10 ummol/L) (Table 1). The baby was restarted on oral zinc and resumed daily trace elements of TPN (Peditrace, Fresenius Kabi, Sweden). The recommended dose is 1 mL Peditrace/kg body weight/day (See Table 2 for composition). The repeated zinc level 5 days later was 8 and then 11 ummol/L. The skin changes described earlier had improved dramatically (Figure 2) and his alkaline phosphatase had risen to an acceptable level for a preterm infant.
Figure 2.
Typical skin lesions of zinc deficiency that include dry, scaly, and eczematous patches on the face (periorificial), anogenital areas, and extensor surfaces of the hands and feet (A), and significant improvement after zinc supplementation at 2 weeks (B), and before discharge (C).
Figure 3.
Timeline of the treatment of the patient and the consequent diagnosis of zinc deficiency.
Table 1.
Biochemical profile of the case before and after the treatment.
| Biochemical Variable | 1 Month Before |
2 Weeks Before |
1 Week Before |
Time of Appearance of Skin Rash |
1 Week After |
2 Weeks After |
1 Month After |
|---|---|---|---|---|---|---|---|
| Direct Bilirubin (mmol/L) | 75 | 104 | 161 | - | 112 | 142 | 137 |
| Alkaline Phosphatase (U/L) | 315 | 94 | 57 | - | 480 | 643 | 655 |
| AST (U/L) | 75 | 148 | 115 | - | 142 | 182 | 74 |
| ALT (U/L) | 45 | 178 | 124 | - | 126 | 109 | 96 |
| GGT (U/L) | 43 | 43 | 47 | - | 70 | 51 | 77 |
| Zinc (mcg/dL) | 1.71 | 8.06 | 11.66 | - |
Table 2.
Trace element composition of PEDITRACE (pH of 2.0 and 38 mOsm/kg water).
| Compound | per mL | |
|---|---|---|
| 1 | Zinc chloride | 521 µg |
| 2 | Copper chloride (dihydrate) | 53.7 µg |
| 3 | Manganese chloride (tetrahydrate) | 3.60 µg |
| 4 | Potassium iodide | 1.31 µg |
| 5 | Sodium fluoride | 126 µg |
| 6 | Sodium selenate (anhydrous) | 4.38 µg |
| 7 | Sodium | 70 µg/mL |
| 8 | Potassium | 0.31 µg/mL |
Ref: https://www.fresenius-kabi.com/nz/documents/Peditrace_DataSheet.pdf (accessed on 1 January 2024).
3. Discussion
Total parenteral nutrition (TPN) is commonly used in preterm infants who are not able to receive enteral nutrition via an oral or enteral route [23]. TPN involves providing micro- and macronutrients including carbohydrates, lipids, amino acids, vitamins, trace elements, electrolytes, and water to the preterm infants (UVG, PICC) [23,24]. TPN, however, comes with its own complications—hyperglycemia, hypertriglyceridemia, and Hepatobiliary injury being topmost of them [23]. IFALD is a spectrum of diseases that can range from mild liver enzyme abnormalities to steatosis to eventual fibrosis or cirrhosis [16,23]. The European Society of Paediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) advocates starting PN as soon as possible following surgery, as the early initiation of TPN with all the essential components may improve intestinal function, thereby reducing the risk of IFALD while enhancing nutrition and growth [25,26,27,28].
Here we report the case of a premature male who had to undergo an abdominal surgery for necrotizing enterocolitis and while on prolonged TPN developed cholestasis followed by the appearance of skin lesions/changes in the form of excoriation and redness. The diagnostic decision of an acquired zinc deficiency was made based on the low serum levels of zinc and clinical improvement following zinc supplementation. Similar cases have been reported previously, linking IFALD with the deficiency of dietary zinc, especially for infants born prematurely [3,7,16,29].
Zinc is one of the trace elements which cannot be stored in the human body, and hence a constant supply is to be sustained via diet to maintain its serum level around 700–1200 μg/L for viable functioning [29,30,31]. Poor nutrition, malabsorption, and loss of appetite has been linked to zinc deficiency in both adults and children [5,12,15]. Administration of zinc at doses of 5–10 mg/day is recommended to reduce the risk of zinc deficiency in children with a high demand for nutrients [12,29,32]. Zinc deficiency is more severe in premature infants for several reasons: firstly, zinc is accumulated efficiently only during the late gestation period; secondly, the fetus receives zinc from the mother only during the last ten weeks of gestation; and thirdly, preterm newborns have a high requirement for all trace elements, including zinc, to support rapid growth and development [3,12,16,33,34]. Additionally, human milk has higher zinc levels (>300 mcg/dL) only in the first few weeks of feeding and it gradually declines to <100 mcg/dL at 6 months after delivery [12,34,35].
4. Recommendations
The effective early supplementation of TPN as per the ESPGHAN guidelines [26] should be provided within 24 h of surgery if enteral feeding is not possible to prevent complications associated with this deficiency and improve a premature infant’s overall health. TPN should be administered in accordance with the nutrient requirements established based on age and weight, and it should include all necessary elements such as amino acids, carbs, fats, electrolytes, and vitamins. FILE-based TPN containing high quantities of antioxidants is recommended. Finally, an early transition to enteral feeding is advised to enhance gut health and speedy recovery. Ursodeoxycholic acid (UDCA) can be used to enhance bile flow and possibly alleviate symptoms in newborns with cholestasis.
5. Conclusions
This case highlights the importance of monitoring trace element levels in premature infants on long-term TPN, as well as the potential risk of acquired zinc deficiency in those with cholestasis, which may require adjustments of their prolonged provision of TPN. Close monitoring of TPN devoid of SOLEs but enriched with FILEs would help with the early resolution of complications. Effective supplementation as per the ESPGHAN guidelines can prevent complications associated with this deficiency and improve a premature infant’s overall health.
Author Contributions
M.A.Q. conceptualized the project, analyzed the collected data, and edited the manuscript. S.S.A. conceptualized the project, analyzed the collected data, completed the literature survey, and wrote the manuscript. H.M., A.M., J.A., M.A.H. (Mohammed Al Hindi), M.A.H. (Mohammed Al Harbi) and M.H. collected and analyzed the patient data and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and was approved by the Institutional Review Board of KAIMRC (Study No: NRJ24-025-5; IRB No: 7424; Dated: 2 June 2024).
Informed Consent Statement
Informed consent was obtained from the guardians of the subject in this case report for the publication of the report.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Lassi Z.S., Kurji J., de Oliveira C.S., Moin A., Bhutta Z.A. Zinc supplementation for the promotion of growth and prevention of infections in infants less than six months of age. Cochrane Database Syst. Rev. 2020;2020:CD010205. doi: 10.1002/14651858.CD010205.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perks P., Huynh E., Kaluza K., Boullata J.I. Advances in trace element supplementation for parenteral nutrition. Nutrients. 2022;14:1770. doi: 10.3390/nu14091770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prasad A.S. Impact of the discovery of human zinc deficiency on health. J. Trace Elem. Med. Biol. 2014;28:357–363. doi: 10.1016/j.jtemb.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 4.Al-Khafaji Z., Brito S., Bin B. Zinc and zinc transporters in dermatology. Int. J. Mol. Sci. 2022;23:16165. doi: 10.3390/ijms232416165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glutsch V., Hamm H., Goebeler M. Zinc and skin: An update. JDDG J. Dtsch. Dermatol. Ges. 2019;17:589–596. doi: 10.1111/ddg.13811. [DOI] [PubMed] [Google Scholar]
- 6.Ogawa Y., Kinoshita M., Shimada S., Kawamura T. Zinc and skin disorders. Nutrients. 2018;10:199. doi: 10.3390/nu10020199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D’Amico G., De Laet C., Smits G., Salik D., Deprez G., Vilain C., Perlot P., Vicinanza A. Acquired Zinc Deficiency Mimicking Acrodermatitis Enteropathica in a Breast-Fed Premature Infant. Pediatr. Rep. 2021;13:444–449. doi: 10.3390/pediatric13030051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kambe T., Fukue K., Ishida R., Miyazaki S. Overview of inherited zinc deficiency in infants and children. J. Nutr. Sci. Vitaminol. 2015;61:S44–S46. doi: 10.3177/jnsv.61.S44. [DOI] [PubMed] [Google Scholar]
- 9.Penny M.E. Zinc supplementation in public health. Ann. Nutr. Metab. 2013;62((Suppl. 1)):31–42. doi: 10.1159/000348263. [DOI] [PubMed] [Google Scholar]
- 10.Jagadeesan S., Kaliyadan F. Acrodermatitis Enteropathica. In StatPearls [Internet]. StatPearls Publishing. [(accessed on 20 January 2024)];2023 Available online: https://www.ncbi.nlm.nih.gov/books/NBK441835/ [PubMed]
- 11.Yang W.L., Hsu C.K., Chao S.C., Huang C.Y., Lee J.Y.Y. Transient zinc deficiency syndrome in a breast-fed infant due to decreased zinc in breast milk (type II hypozincemia of infancy): A case report and review of the literature. Dermatol. Sin. 2012;30:66–70. doi: 10.1016/j.dsi.2011.09.013. [DOI] [Google Scholar]
- 12.Terrin G., Berni Canani R., Di Chiara M., Pietravalle A., Aleandri V., Conte F., De Curtis M. Zinc in early life: A key element in the fetus and preterm neonate. Nutrients. 2015;7:10427–10446. doi: 10.3390/nu7125542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carducci B., Keats E.C., Bhutta Z.A. Zinc supplementation for improving pregnancy and infant outcome. Cochrane Database Syst. Rev. 2021;2021:CD000230. doi: 10.1002/14651858.CD000230.pub6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taylor A.J., Jones L.J., Osborn D.A. Zinc supplementation of parenteral nutrition in newborn infants. Cochrane Database Syst. Rev. 2017;2017:CD012561. doi: 10.1002/14651858.CD012561. [DOI] [Google Scholar]
- 15.Hawrysz Z., Woźniacka A. Zinc: An undervalued microelement in research and treatment. Adv. Dermatol. Allergol./Postępy Dermatol. I Alergol. 2023;40:208–214. doi: 10.5114/ada.2023.127639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lauriti G., Zani A., Aufieri R., Cananzi M., Chiesa P.L., Eaton S., Pierro A. Incidence, prevention, and treatment of parenteral nutrition–associated cholestasis and intestinal failure–associated liver disease in infants and children: A systematic review. J. Parenter. Enter. Nutr. 2014;38:70–85. doi: 10.1177/0148607113496280. [DOI] [PubMed] [Google Scholar]
- 17.Di Dato F., Iorio R., Spagnuolo M.I. IFALD in children: What’s new? A narrative review. Front. Nutr. 2022;9:928371. doi: 10.3389/fnut.2022.928371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zafirovska M., Zafirovski A., Rotovnik Kozjek N. Current insights regarding intestinal failure-associated liver disease (IFALD): A narrative review. Nutrients. 2023;15:3169. doi: 10.3390/nu15143169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khalaf R.T., Sokol R.J. New insights into intestinal failure–associated liver disease in children. Hepatology. 2020;71:1486–1498. doi: 10.1002/hep.31152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huff K.A., Breckler F., Cruse W., Szeszycki E., Vanderpool C. Pediatric smoflipid therapy: Patient response and safety concerns. J. Parenter. Enter. Nutr. 2021;45:792–799. doi: 10.1002/jpen.1929. [DOI] [PubMed] [Google Scholar]
- 21.Raphael B.P., Duggan C. Seminars in Liver Disease. Volume 32. Thieme Medical Publishers; New York, NY, USA: 2012. Prevention and treatment of intestinal failure–associated liver disease in children; pp. 341–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Puder M., Valim C., Meisel J.A., Le H.D., De Meijer V.E., Robinson E.M., Zhou J., Duggan C., Gura K.M. Parenteral fish oil improves outcomes in patients with parenteral nutrition-associated liver injury. Ann. Surg. 2009;250:395–402. doi: 10.1097/SLA.0b013e3181b36657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berlana D. Parenteral Nutrition Overview. Nutrients. 2022;14:4480. doi: 10.3390/nu14214480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boullata J.I., Gilbert K., Sacks G., Labossiere R.J., Crill C., Goday P. American Society for Parenteral and Enteral Nutrition. ASPEN clinical guidelines: Parenteral nutrition ordering, order review, compounding, labeling, and dispensing. JPEN J. Parenter. Enter. Nutr. 2014;38:334–377. doi: 10.1177/0148607114521833. [DOI] [PubMed] [Google Scholar]
- 25.Shakeel F., Newkirk M., Sellers A., Shores D.R. Postoperative feeding guidelines improve outcomes in surgical infants. J. Parenter. Enter. Nutr. 2020;44:1047–1056. doi: 10.1002/jpen.1726. [DOI] [PubMed] [Google Scholar]
- 26.ESPGHAN Guidelines on Parenteral Nutrition in Children. [(accessed on 20 January 2024)]. Available online: https://espghan.info/published-guidelines/
- 27.Hartman C., Shamir R., Simchowitz V., Lohner S., Cai W., Decsi T., Braegger C., Bronsky J., Campoy C., Carnielli V., et al. ESPGHAN/ESPEN/ESPR/CSPEN guidelines on pediatric parenteral nutrition: Complications. Clin. Nutr. 2018;37:2418–2429. doi: 10.1016/j.clnu.2018.06.956. [DOI] [PubMed] [Google Scholar]
- 28.Domellöf M., Szitanyi P., Simchowitz V., Franz A., Mimouni F., Braegger C., Bronsky J., Cai W., Campoy C., Carnielli V., et al. ESPGHAN/ESPEN/ESPR/CSPEN guidelines on pediatric parenteral nutrition: Iron and trace minerals. Clin. Nutr. 2018;37:2354–2359. doi: 10.1016/j.clnu.2018.06.949. [DOI] [PubMed] [Google Scholar]
- 29.Abdelhamid N., Wahby A., Kandil M.E. Zinc status in infants and children with cholestatic liver diseases and its effect on growth. Bull. Natl. Res. Cent. 2019;43:121. doi: 10.1186/s42269-019-0166-y. [DOI] [Google Scholar]
- 30.Sultan S., Irfan S.M., Kakar J., Zeeshan R. Effect of iron chelator desferrioxamine on serum zinc levels in patients with beta thalassemia major. Malays. J. Pathol. 2015;37:35. [PubMed] [Google Scholar]
- 31.Tapiero H., Tew K.D. Trace elements in human physiology and pathology: Zinc and metallothioneins. Biomed. Pharmacother. 2003;57:399–411. doi: 10.1016/S0753-3322(03)00081-7. [DOI] [PubMed] [Google Scholar]
- 32.Norton S.A., Soghier L., Hatfield J., Lapinski J., Barfield W.D. Zinc Deficiency Dermatitis in Cholestatic Extremely Premature Infants after a Nationwide Shortage of Injectable Zinc—Washington, DC, December 2012. MMWR Morb. Mortal. Wkly. Rep. 2013;62:136. [PMC free article] [PubMed] [Google Scholar]
- 33.Petry N., Olofin I., Boy E., Donahue Angel M., Rohner F. The effect of low dose iron and zinc intake on child micronutrient status and development during the first 1000 days of life: A systematic review and meta-analysis. Nutrients. 2016;8:773. doi: 10.3390/nu8120773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zaladonis C.A., Safeer L.Z., Hanson D.C., Erickson-Parsons L., Krakowski A.C. Zinc Deficiency in a Preterm Infant. J. Pediatr. 2022;240:304–306. doi: 10.1016/j.jpeds.2021.08.020. [DOI] [PubMed] [Google Scholar]
- 35.Vázquez-Gomis R., Bosch-Gimenez V., Juste-Ruiz M., Vázquez-Gomis C., Izquierdo-Fos I., Pastor-Rosado J. Zinc concentration in preterm newborns at term age, a prospective observational study. BMJ Paediatr. Open. 2019;3:e000527. doi: 10.1136/bmjpo-2019-000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are contained within the article.