Abstract
Background
In the past, women have been advised that lowering their salt intake might reduce their risk of developing pre‐eclampsia. Although this practice has largely ceased, it remains important to assess the evidence about possible effects of altered dietary salt intake during pregnancy.
Objectives
The objective of this review was to assess the effects of altered dietary salt during pregnancy on the risk of developing pre‐eclampsia and its complications.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (8 April 2005), the Cochrane Central Register of Controlled Trials (The Cochrane Library, Issue 1, 2005), and EMBASE (2002 to May 2005). We updated the search of the Cochrane Pregnancy and Childbirth Group's Trials Register on 1 October 2009 and added the results to the awaiting classification section.
Selection criteria
Randomised trials evaluating either reduced or increased dietary salt intake during pregnancy.
Data collection and analysis
Two review authors selected trials for inclusion and extracted data independently. Data were entered on Review Manager software for analysis, and double‐checked for accuracy.
Main results
Two trials were included, with 603 women. Both compared advice to reduce dietary salt intake with advice to continue a normal diet. The confidence intervals were wide and crossed the no‐effect line for all the reported outcomes, including pre‐eclampsia (relative risk 1.11, 95% confidence interval 0.46 to 2.66). In other words, there was insufficient evidence for reliable conclusions about the effects of advice to reduce dietary salt.
Authors' conclusions
In the absence of evidence that advice to alter salt intake during pregnancy has any beneficial effect for prevention of pre‐eclampsia or any other outcome, salt consumption during pregnancy should remain a matter of personal preference.
[Note: The citation in the awaiting classification section of the review may alter the conclusions of the review once assessed.]
Keywords: Female; Humans; Pregnancy; Diet, Sodium‐Restricted; Pre‐Eclampsia; Pre‐Eclampsia/prevention & control; Randomized Controlled Trials as Topic; Sodium Chloride, Dietary; Sodium Chloride, Dietary/administration & dosage
Plain language summary
Altered dietary salt for preventing pre‐eclampsia, and its complications
Low salt intake in pregnancy is unlikely to prevent pre‐eclampsia.
Pre‐eclampsia is a serious complication of pregnancy associated with poor health, or even death, for the mother and baby. Pre‐eclampsia is identified by raised blood pressure and protein in the urine during the second half of pregnancy. In the past, a low‐salt diet was often recommended, in the belief that it would help to prevent pre‐eclampsia. However, the review found just two trials that did not show any evidence of benefit for the mother or baby. Salt intake in pregnancy should be a matter of personal preference.
Background
Hypertension (high blood pressure) is common during pregnancy. Around 10% of women will have raised blood pressure at some point before delivery. The hypertensive disorders of pregnancy comprise a spectrum of conditions that is usually classified into four categories: (1) gestational hypertension: a rise in blood pressure during the second half of pregnancy; (2) pre‐eclampsia: usually hypertension with proteinuria (protein in urine) during the second half of pregnancy; (3) chronic hypertension: a rise in blood pressure before pregnancy or before 20 weeks' gestation; and (4) pre‐eclampsia superimposed on chronic hypertension (NHBPEP 2000). For women who have hypertension alone but do not develop proteinuria, pregnancy outcome is similar to that for women with normal blood pressure. Outcome deteriorates once pre‐eclampsia develops. Pre‐eclampsia is a multisystem disorder that affects 2% to 8% of pregnancies (WHO 1988). Complications of pre‐eclampsia are associated with a substantive increase in morbidity and mortality for both the woman and her baby (DH 2002). Pre‐eclampsia is discussed in more detail in the generic protocol of interventions for prevention of pre‐eclampsia (Generic Protocol 2005).
In the early part of the twentieth century, a low‐salt diet was often recommended as treatment for oedema in both pregnant and non‐pregnant people. At that time oedema was included in the definition of pre‐eclampsia, although it is now recognised to be part of normal pregnancy as it affects 80% of pregnant women. This led to the idea that restricting salt intake might treat, and also prevent, pre‐eclampsia (Green 1989). By the 1940s, a low‐salt diet was widely recommended during pregnancy, particularly for women with pre‐eclampsia. In the late 1950s and early 1960s this practice began to be questioned, and it was even suggested that high‐salt intake might prevent or treat pre‐eclampsia (UK 1958; UK 1961). Subsequently, interest in salt consumption during pregnancy has largely faded away.
In most parts of the world, women are no longer advised by clinicians to alter their salt intake during pregnancy. A notable exception is in the Netherlands where, until relatively recently, this practice remained widespread. Nevertheless, some lay literature aimed at pregnant women continues to advocate salt restriction during pregnancy.
Salt is a widely used flavour enhancer. Women who like adding salt in their food may find a low‐salt diet unpalatable. This may be particularly relevant during the first half of pregnancy when nausea and vomiting is common, and taste can also be altered. Low‐salt foods can also be less convenient and more expensive than salted alternatives. Women should not be advised to alter their dietary salt during pregnancy unless there is reasonable evidence of benefit.
This review aims to summarise the evidence about the effects of advice to alter dietary salt intake during pregnancy for prevention of pre‐eclampsia and its consequences. The methods are described in full in the generic protocol of interventions for prevention of pre‐eclampsia (Generic Protocol 2005).
Objectives
The main objective was to assess the effects of altered dietary salt on the risk of developing pre‐eclampsia and its complications.
If altered salt intake is effective, a secondary objective was to compare the effects of one form of alteration with another, such as restricted salt intake with increased salt intake, and to compare the effects of altered salt intake with other measures for prevention of pre‐eclampsia.
Methods
Criteria for considering studies for this review
Types of studies
Randomised trials evaluating altered salt intake during pregnancy were included.
Studies with a quasi‐random design, such as allocation by alternation or day of week, were excluded as inadequate concealment of the allocation may have introduced bias. For an intervention such as dietary advice where foreknowledge of the allocation might influence recruitment, adequate concealment of the allocation is of particular importance in avoiding bias.
Types of participants
Women who had normal or high blood pressure without proteinuria during pregnancy were included, regardless of gestation at trial entry. Whenever possible women were grouped on the basis of their risk of developing pre‐eclampsia at trial entry as follows:
(1) Women with normal blood pressure
(a) High risk: defined as having one or more of: diabetes, renal disease, thrombophilia, autoimmune disease, previous severe or early onset pre‐eclampsia, or multiple pregnancy. (b) Moderate risk: defined as none of the above, but having either previous pre‐eclampsia that was not severe or early onset (or severity unspecified), or a first pregnancy and at least one of the following: teenager or over 35 years age, family history of pre‐eclampsia, obesity (body mass index greater than or equal to 30), increased sensitivity to Angiotensin II, positive roll over test, abnormal uterine artery doppler scan. (c) Low risk: defined as pregnancy that does not qualify as either high‐ or moderate‐risk. (d) Undefined risk: when the risk is unclear or not specified.
(2) Women with high blood pressure, without proteinuria
(a) Gestational hypertension: hypertension detected for the first time after 20 weeks' gestation, in the absence of proteinuria. (b) Chronic hypertension: essential or secondary hypertension detected prior to pregnancy or before 20 weeks' gestation. Some women with chronic hypertension may have longstanding proteinuria due to their underlying disease. These women will be included, as their proteinuria is not due to pre‐eclampsia.
If a trial included women with pre‐eclampsia as well as those with non‐proteinuric hypertension (gestational or chronic), where possible, we planned to include only the women with non‐proteinuric hypertension in the review. For trials that did not report results separately for the two categories, we planned to include them in the review and present them as a separate subgroup. However, we did not find any such trials.
(3) Undefined: when it is unclear or not specified whether the women have hypertension
Women were excluded if they had established pre‐eclampsia.
Types of interventions
Any comparison of altered dietary salt intake with normal salt intake during pregnancy was included, as were comparisons of one form of alteration with another, such as restricted salt intake with increased salt intake, and comparisons of dietary salt intake with other measures for prevention of pre‐eclampsia.
We included all strategies for altering dietary intake of salt, such as giving advice to alter salt intake, and providing food with either high or low salt content.
Types of outcome measures
The following outcomes were included. To avoid losing valuable data, we planned to include trials that used acceptable variations of the definitions specified below, as well as those that did not state their definitions. If an important outcome was not reported, whenever possible we contacted the authors.
For the woman
Main outcome
(1) Pre‐eclampsia: defined where possible as hypertension (blood pressure at least 140 mmHg systolic or 90 mmHg diastolic) with proteinuria (at least 300 mg protein in a 24 hour urine collection or 30 mg/dL in a single sample or 1+ on dipstick or 30 mg/mmol urine protein/creatinine ratio).
Other outcomes
(2) Death: during pregnancy or up to 42 days after end of pregnancy. (3) Severe morbidity: including eclampsia, liver or renal failure, haemolysis elevated liver enzymes and low platelets syndrome, disseminated intravascular coagulation, stroke, and pulmonary oedema. These outcomes will be reported individually, and as a composite measure where the information is available. (4) Severe pre‐eclampsia: pre‐eclampsia with two or more signs or symptoms of severe disease, such as severe hypertension (blood pressure 160/110 mmHg or more), severe proteinuria (usually 3 g/24h, or 3+ on dipstick), visual disturbances, exaggerated tendon reflexes, upper abdominal pain, impaired liver function tests, high serum creatinine, low platelets, fetal growth restriction, or reduced liquor volume. (5) Gestational hypertension: hypertension after 20 weeks' gestation. (6) Abruption of the placenta or antepartum haemorrhage. (7) Elective delivery: induction of labour or caesarean section. (8) Caesarean section: emergency and elective. (9) Use of hospital resources: visit to day care unit, antenatal hospital admission, intensive care (admission to intensive care unit, length of stay, ventilation, dialysis). (10) Side‐effects, or adverse events, and stopped intervention due to side‐effects. (11) Women's experiences and views: acceptability of the advice, pregnancy and childbirth experience.
For the child
Main outcomes
(1) Death: including all deaths before birth and up to discharge from hospital. (2) Preterm birth: defined as birth at or before 37 completed weeks' gestation. (3) Small‐for‐gestational age: defined as growth below the 3rd centile, or lowest centile reported.
Other outcomes
(4) Death, classified by timing of death: miscarriage (fetal loss at or before 19 completed weeks' gestation or however defined in the study), stillbirth (death in utero after 20 weeks' gestation), perinatal death (stillbirth or death in the first seven days of life), neonatal death (death in the first 28 days after birth), infant death (death in the first year of life). (5) Severity of preterm birth: severe (at or before 33 completed weeks' gestation), and extreme (at or before 27 completed weeks' gestation). (6) Apgar score at five minutes: low (less than seven) and very low (less than four) or lowest reported. (7) Neonatal morbidity: respiratory distress syndrome, chronic lung disease, sepsis, necrotising enterocolitis, retinopathy of prematurity, and intraventricular haemorrhage. (8) Endotracheal intubation or use of mechanical ventilation. (9) Long‐term growth and development: blindness, deafness, seizures, poor growth, neurodevelopmental delay, and cerebral palsy. (10) Use of hospital resources: admission to neonatal intensive care unit, duration of hospital stay after delivery.
Economic outcomes
Costs to health service resources: short term and long term for both mother and baby.
Costs to the woman, her family, and society associated with the intervention.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register by contacting the Trials Search Co‐ordinator (8 April, 2005). We updated this search on 1 October 2009 and added the results to Studies awaiting classification.
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL and MEDLINE, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
In addition, we searched CENTRAL (The Cochrane Library, Issue 1, 2005) and EMBASE (2002 to May 2005) using the search strategy listed in the generic protocol (Generic Protocol 2005).
We did not apply any language restrictions.
Data collection and analysis
Selection of studies
Two review authors independently assessed potentially eligible studies for inclusion. Differences in opinion were resolved by discussion.
Assessment of study quality
Two authors independently assessed the quality of each study using the criteria outlined in the Cochrane Reviewers' Handbook (Alderson 2004). Methods used for generation of the randomisation sequence are described for each trial. Each study was assessed for quality of concealment of allocation, completeness of follow up, and blinding.
(1) Allocation concealment
A quality score for concealment of allocation was assigned to each trial, using the following criteria: (A) adequate concealment of allocation, such as telephone randomisation, consecutively numbered sealed opaque envelopes; (B) unclear whether adequate concealment of allocation; (C) inadequate concealment of allocation such as open random number tables, sealed envelopes that are not numbered or opaque.
(2) Completeness of follow up
Completeness of follow up was assessed using the following criteria: (A) less than 5% of participants excluded from analysis; (B) 5% to 10% of participants excluded from analysis; (C) more than 10% and up to and including 20% of participants excluded from analysis.
Studies were excluded if:
more than 20% of participants were excluded from analysis.
more than 10% of participants were not analysed in their randomised groups and it was not possible to restore participants to the correct group.
The generic protocol states that studies should also be excluded if there is more than 10% difference in loss of participants between groups. This is reasonable as a large difference in exclusions between two groups results in the loss of balance created by randomisation, and increases the chance of bias. However, we could not apply this criterion to our review as this would have led to the exclusion of a large study (Netherlands 1997) which is of better quality than most other available data. At present, there is a paucity of data, so we have decided to include this study. In future updates, if further large good quality studies become available, we will consider excluding this study.
We analysed data based on the group to which the participants were randomised, regardless of whether they received the allocated intervention or not.
(3) Blinding
Blinding was assessed using the following criteria:
blinding of participants (yes/no/unclear or unspecified);
blinding of caregiver (yes/no/unclear or unspecified);
blinding of outcome assessment (yes/no/unclear or unspecified).
Data extraction and data entry
Two review authors extracted data independently. They were entered onto the Review Manager software (RevMan 2003), and double‐checked for accuracy.
Statistical analyses
We carried out statistical analysis using the RevMan software (RevMan 2003). Results are presented as summary relative risk with 95% confidence intervals. We used the I2 statistic to assess heterogeneity between trials. In the absence of significant heterogeneity, results were pooled using a fixed‐effect model. If substantial heterogeneity is detected in future updates (I2 more than 50%), we will explore possible causes, and subgroup analyses for the main outcomes will be performed. Heterogeneity that is not explained by subgroup analyses may be modelled using random‐effects analysis, where appropriate.
Sensitivity analyses
Our prespecified sensitivity analysis was to explore the effects of trial quality assessed by concealment of allocation, by excluding studies with clearly inadequate allocation of concealment (rated C). This analysis has not been conducted as there are only two included trials. It will be included in future updates when sufficient data become available.
Subgroup analyses
Our prespecified subgroups were based on:
risk of pre‐eclampsia at trial entry: high risk, moderate risk, low risk, and undefined risk;
gestation at trial entry: before or after 19 completed weeks, or gestation unclear/not specified.
We have not conducted these subgroup analyses due to insufficient data. They will be included in future updates, once sufficient data are available. Only the main outcomes listed above will be included in the subgroup analyses.
Results
Description of studies
Two trials involving 603 women were included. Both were multicentre studies conducted in The Netherlands, in hospital clinics and midwifery practices. Both trials included women who were healthy and nulliparous. In one study all women were normotensive, in the other (Netherlands 1998) women had a diastolic blood pressure 85 mmHg, or more, at trial entry. Both studies compared advice to restrict dietary salt (to 20 or 50 mmol/day) with advice to continue a normal diet. Compliance was assessed by checking urinary sodium excretion. Although this was higher than the target level for the low sodium group, sodium excretion was still lower than for the normal diet group. (One report from an updated search in October 2009 has been added to Studies awaiting classification.)
Definitions used in trials were similar to those specified for this review, although the definition of hypertension was not clear in one trial (Netherlands 1998).
Six studies were excluded (2992 women). Three of these evaluated a high‐salt diet, but used a quasi‐randomised design (UK 1958; UK 1961; USA 1961). In addition, for two of these the participants had established pre‐eclampsia (UK 1961; USA 1961). The other three studies were excluded because they did not report clinically relevant outcomes (Australia 1986a; Australia 1986b; Belgium 1986).
Details for each trial can be found in the Characteristics of included studies table and the Characteristics of excluded studies table.
Risk of bias in included studies
Allocation concealment
Both trials were multicentre studies. Allocation concealment was adequate in one and unclear in the other.
Follow up
In one study (Netherlands 1998), there was complete follow up for all women. In the other study (Netherlands 1997), 10% of women were excluded from the analyses. There was a substantial difference in the number of excluded participants between the two arms (23 women in the low‐salt arm and five in the normal diet arm). The proportion was higher in the low‐salt group as 17 women (13% of women in the low‐salt arm) did not want to use the recommended diet.
Blinding
Blinding of participants was not possible. In one study, the caregiver was blinded to urinary sodium concentration measurements. As blinding of outcome assessment was not reported in either study, it is unlikely to have been done.
Effects of interventions
The two trials (603 women) compared advice to restrict dietary salt with advice to continue a normal diet. Confidence intervals for all the outcomes reported in this review were wide, and crossed the no‐effect line. For example, the relative risk for pre‐eclampsia was 1.11, 95% confidence interval 0.46 to 2.66. This means that the true effect could be anything from over a halving in the risk of pre‐eclampsia associated with salt restriction to a more than doubling.
No included trials evaluated a high‐salt diet for prevention of pre‐eclampsia and its complications.
Discussion
The aim of this review was to evaluate the effects of altered dietary salt intake for the prevention of pre‐eclampsia. The two trials included in this review evaluated advice to restrict dietary salt. In both, women appear to have taken this advice, as urinary sodium excretion was reduced in the low‐salt group. However, even when taken together, these trials were too small to provide reliable information about the effects of advice to restrict salt intake during normal pregnancy.
Women's preferences were not reported. However, in one study (Netherlands 1997) 13% of women in the salt restriction arm withdrew, as they did not want to follow the diet. In the other study only 24% of women successfully reduced their urinary sodium excretion to below the target level. One possible explanation for this is that women who are not used to a low salt diet may not find it palatable.
No included trials evaluated a high‐salt diet. One excluded study did evaluate a high‐salt diet for prevention of pre‐eclampsia (UK 1958). In this quasi‐random study involving 2077 women the relative risk for pre‐eclampsia was 0.45 (95% confidence interval 0.26 to 0.75), a reduction that appeared to be reflected in fewer complications (UK 1958). In view of the methodological limitations of this study it is difficult to draw any firm conclusions, beyond saying that it seems unlikely that a high‐salt diet will increase the risk of pre‐eclampsia.
Authors' conclusions
Implications for practice.
A low‐salt diet is often unpalatable. In the absence of evidence that advice to alter salt intake during pregnancy has any beneficial effect for prevention of pre‐eclampsia or for any other outcome, salt consumption during pregnancy should remain a matter of personal preference.
Implications for research.
Salt restriction during pregnancy does not seem promising for the prevention of pre‐eclampsia. Very large numbers of women would need to be randomised to detect a moderate to small effect. Such trials might be justified in the context of a setting where this form of dietary advice was already widespread practice, but they would be difficult to justify elsewhere. Unless new and plausible hypotheses emerge, further trials of salt intake are unlikely to be a priority.
[Note: The citation in the awaiting classification section of the review may alter the conclusions of the review once assessed.]
What's new
| Date | Event | Description |
|---|---|---|
| 18 January 2012 | Amended | Contact details updated. |
History
Protocol first published: Issue 2, 2005 Review first published: Issue 4, 2005
| Date | Event | Description |
|---|---|---|
| 1 October 2009 | Amended | Search updated. One report added to Studies awaiting classification (Brown 1985). |
| 28 August 2008 | Amended | Converted to new review format. |
| 8 April 2005 | New search has been performed | The original review has been updated with the following changes. (1) The original review evaluated salt intake for prevention and treatment of pre‐eclampsia (Duley 1999). This review now evaluates salt intake for prevention of pre‐eclampsia only, and the title has been changed to reflect this. A separate review will evaluate salt intake for treatment of pre‐eclampsia. (2) A new co‐author and sources of support have been added. (3) The background, search strategy, and methods sections have been expanded. (4) Additional citations have been added to Netherlands 1997. (5) Belgium 1986 has been added to the list of excluded studies. |
Notes
This review updates the prevention of pre‐eclampsia part of the currently published review 'Reduced salt intake compared to normal dietary salt, or high intake, in pregnancy', which will continue to be published until the review to update altered dietary salt for treating pre‐eclampsia is published.
Acknowledgements
None.
Data and analyses
Comparison 1. Low versus normal salt intake in pregnancy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Gestational hypertension | 1 | 242 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.49, 1.94] |
| 2 Pre‐eclampsia | 2 | 603 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.46, 2.66] |
| 3 Visit to day care unit | 1 | 361 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.48, 2.32] |
| 4 Antenatal hospital admission | 1 | 361 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.56, 1.22] |
| 5 Placental abruption | 1 | 361 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.01, 3.98] |
| 6 Caesarean section | 1 | 361 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.44, 1.27] |
| 7 Perinatal death | 2 | 409 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.92 [0.18, 21.03] |
| 8 Small‐for‐gestational age | 1 | 242 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.73, 3.07] |
| 9 Preterm birth | 1 | 242 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.46, 2.56] |
| 10 Apgar score <7 at 5 minutes | 1 | 361 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.53, 3.53] |
| 11 Admission to neonatal intensive care unit | 1 | 361 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.69, 1.40] |
1.1. Analysis.
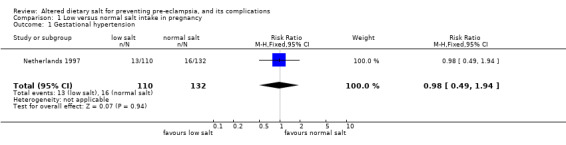
Comparison 1 Low versus normal salt intake in pregnancy, Outcome 1 Gestational hypertension.
1.2. Analysis.
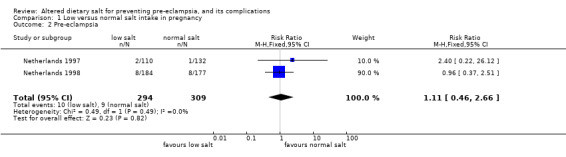
Comparison 1 Low versus normal salt intake in pregnancy, Outcome 2 Pre‐eclampsia.
1.3. Analysis.
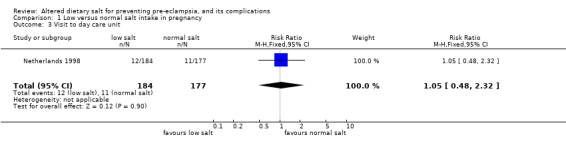
Comparison 1 Low versus normal salt intake in pregnancy, Outcome 3 Visit to day care unit.
1.4. Analysis.
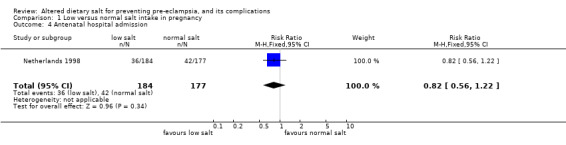
Comparison 1 Low versus normal salt intake in pregnancy, Outcome 4 Antenatal hospital admission.
1.5. Analysis.
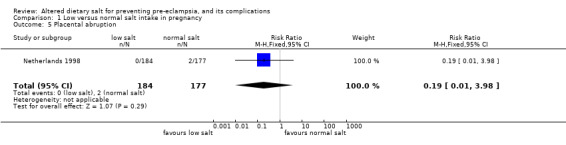
Comparison 1 Low versus normal salt intake in pregnancy, Outcome 5 Placental abruption.
1.6. Analysis.
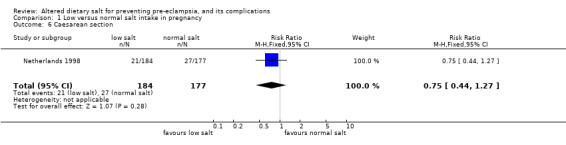
Comparison 1 Low versus normal salt intake in pregnancy, Outcome 6 Caesarean section.
1.7. Analysis.
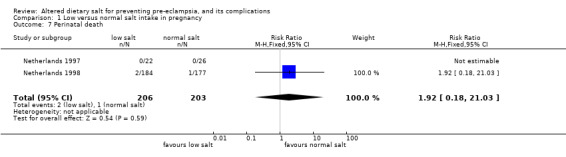
Comparison 1 Low versus normal salt intake in pregnancy, Outcome 7 Perinatal death.
1.8. Analysis.
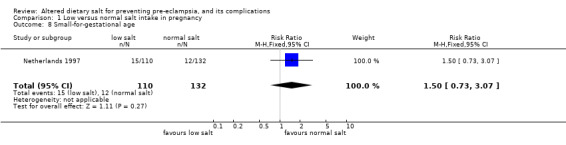
Comparison 1 Low versus normal salt intake in pregnancy, Outcome 8 Small‐for‐gestational age.
1.9. Analysis.
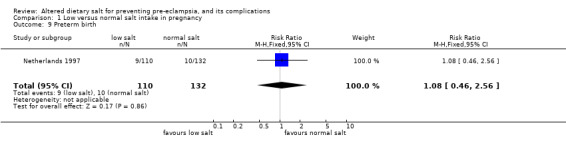
Comparison 1 Low versus normal salt intake in pregnancy, Outcome 9 Preterm birth.
1.10. Analysis.
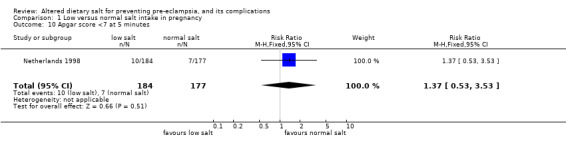
Comparison 1 Low versus normal salt intake in pregnancy, Outcome 10 Apgar score <7 at 5 minutes.
1.11. Analysis.
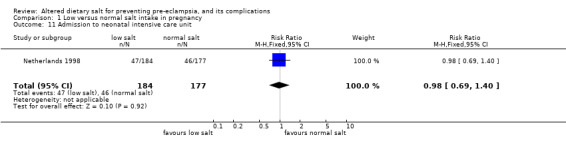
Comparison 1 Low versus normal salt intake in pregnancy, Outcome 11 Admission to neonatal intensive care unit.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Netherlands 1997.
| Methods | Randomisation: method not stated. Allocation concealment: "closed envelope system", no further information. Follow up: 28 women (10%) excluded. Low‐salt 23 excluded: 17 refused diet, 3 social reasons, 2 medical reasons, 1 fetal trisomy. Normal‐salt 5 excluded: 2 social reasons, 3 medical reasons grade (B). Blinding: participants not blinded. Blinding of caregivers and outcome assessment not reported. | |
| Participants | 270 nulliparous women with a singleton pregnancy after 12 weeks, by dates and ultrasound. Excluded if pre‐existing HT, diabetes, renal disease, cardiovascular disease. | |
| Interventions | Low: diet with about 20 mmol sodium per day. Oral and written instruction by dietician, no added salt and ready made foods only if no salt in preparation. Normal: no dietary restriction. | |
| Outcomes | Woman: GH (DBP >/= 90 x 2 or >/= 110 x 1), PE (HT with proteinuria >/= 300 mg/24 hr), severe HT (DBP >/= 110) Baby: death, SGA, preterm delivery. | |
| Notes | Two centre study. Setting: hospital clinics. Mean urinary sodium after randomisation 70 mmol/day in the low sodium group, 135 mmol/day for normal diet. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Netherlands 1998.
| Methods | Randomisation: random numbers in blocks of 10. Stratified by centre. Allocation concealment: sealed, numbered, opaque envelopes. Follow up: no loss to follow up (A). Blinding: participants not blinded. Caregiver blinded only to urinary sodium concentration. Blinding of outcome assessment not reported. | |
| Participants | 361 women booked for midwifery care, nulliparous, DBP < 90 mmHg at booking visit before 20 weeks. Randomised if DBP > 85 x 2 in subsequent visit, or weight gain > 1 kg/week for 3 consecutive weeks, or excess oedema. Excluded if planning to leave the city or risk factors for PIH. | |
| Interventions | Low: sodium‐restricted diet, aimed at less than 50 mmol/day. Written dietary instructions given by midwife. Normal: asked not to change eating habits. | |
| Outcomes | Woman: highest DBP, PE (HT (not defined) with significant proteinuria (not defined)), eclampsia, hospital referrals and admissions for HT, time to delivery, abruption, mode of delivery. Baby: death, gestation at delivery (mean), birthweight, Apgar at 5 minutes, NICU admission. | |
| Notes | Multicentre study: 9 centres. Setting: midwifery practices and hospital clinic. Mean urinary sodium after randomisation 84 mmol/day in low sodium group, 124 mmol/day for normal diet. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
DBP: diastolic blood pressure GH: gestational hypertension HT: hypertension hr: hour NICU: neonatal intensive care unit PE: pre‐eclampsia PIH: pregnancy induced hypertension SGA: small‐for‐gestational age
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Australia 1986a | No clinical outcomes reported. Study design: 'assigned randomly'. Participants: 58 primigravid women. Intervention: one week of either high‐salt or low‐salt diet. Outcomes: plasma arginine vasopressin concentration, urinary sodium excretion. |
| Australia 1986b | No clinical outcomes reported. Study design: 'assigned randomly'. Participants: 40 primigravid women. Intervention: one week of either high‐salt or low‐salt diet. Outcomes: potassium excretion, sodium excretion, plasma progesterone, plasma aldosterone. |
| Belgium 1986 | No clinical outcomes reported in the abstract, and attempts to contact trialists revealed that the trial was stopped because the lead trialist left the institution. Attempts to contact the lead trialist were unsuccessful. Study design: two groups were randomised and matched. No further information. Participants: 30 pregnant women. Intervention: 15 women followed a salt‐poor diet and 15 women followed a salt‐rich diet. Outcomes: body fluid volume, vascular reactivity, clinical evolution, pregnancy outcome (but no data for these outcomes reported in the abstract). |
| UK 1958 | Not a randomised trial. Quasi‐random, using alternate allocation of women attending clinic. Study design: as above. Participants: 2077 women at booking clinic, 58 excluded. Intervention: high‐salt diet with advice to add salt to diet and eat salty food versus low‐salt with advice to avoid adding salt to food and not to eat salty food. Outcomes: toxaemia, oedema, APH, PPH, caesarean section, breech delivery, forceps delivery, perinatal death, deformities, maternal side‐effects. |
| UK 1961 | Not a randomised trial. Allocation by ward and by consultant. Participants were women with established pre‐eclampsia. Study design: as above. Participants: 739 women with high blood pressure, oedema and proteinuria. Excluded if < 24 hours from admission to delivery. Intervention: 2 g salt versus 10 g versus 25 g. Outcomes: surgical induction, caesarean section, number of days in hospital, eclampsia, blood pressure trends, perinatal death, birthweight. |
| USA 1961 | No clinical outcomes reported. Study design: 'assigned randomly'. Participants: 58 primigravid women. Intervention: one week of either high‐salt or low‐salt diet. Outcomes: plasma arginine vasopressin concentration, urinary sodium excretion. |
APH: antepartum haemorrhage PPH: postpartum haemorrhage
Contributions of authors
For the original review, Lelia Duley and David Henderson‐Smart extracted and checked the data. Lelia Duley drafted the text, with comments from David Henderson‐Smart.
For the 2005 update, Lelia Duley and Shireen Meher updated the background section, search strategy, and methodology of the review, with comments from David Henderson‐Smart. Lelia Duley and Shireen Meher assessed trial reports for inclusion/exclusion.
Sources of support
Internal sources
Centre for Perinatal Health Services Research, University of Sydney, Australia.
The University of Liverpool, UK.
Nuffield Department of Medicine, University of Oxford, UK.
External sources
Medical Research Council, UK.
Health Technology Assessment, UK.
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
Netherlands 1997 {published data only}
- Delemarre FM, Leest LA, Jongsma HW, Steegers EA. Effects of low‐sodium diet on uteroplacental circulation. Journal of Maternal‐Fetal Medicine 2000;9(4):197‐200. [DOI] [PubMed] [Google Scholar]
- Delemarre FMC, Thomas CMG, Berg RJ, Jongsma HW, Steegers EAP. Urinary prostaglandin excretion in pregnancy: the effect of dietary sodium restriction. Prostaglandins, Leukotrienes, and Essential Fatty Acids 2000;63(4):209‐15. [DOI] [PubMed] [Google Scholar]
- Steegers EAP, Lakwijk HPJM, Jongsma HW, Fast JH, Boo T, Eskes TKAB, et al. (Patho)physiological implications of chronic dietary sodium restriction during pregnancy; a longitudinal prospective randomized study. British Journal of Obstetrics and Gynaecology 1991;98:980‐7. [DOI] [PubMed] [Google Scholar]
- Steegers EAP, Buul EJA. Chronic dietary sodium restriction in the prevention of hypertension during pregnancy: preliminary results of a Dutch multicentered trial. Proceedings of the 9th International Congress, International Society for the Study of Hypertension in Pregnancy; 1994 March 15‐18; Sydney, Australia. 1994:54.
- Maten GD. Low sodium diet in pregnancy: effects on maternal nutritional status. European Journal of Obstetrics & Gynecology and Reproductive Biology 1995;61:63‐4. [DOI] [PubMed] [Google Scholar]
- Buul BJA, Steegers EAP, Jongsma HW, Rijpkema AL, Eskes TKAB, Thomas CMG, et al. Dietary sodium restriction in the prophylaxis of hypertensive disorders of pregnancy: effects on the intake of other nutrients. American Journal of Clinical Nutrition 1995;62:49‐57. [DOI] [PubMed] [Google Scholar]
- Buul BJA, Steegers EAP, Maten GD, Delemarre FMC, Jongsma HW, Oosterbaan HP, et al. Dietary sodium restriction does not prevent gestational hypertension: a Dutch two‐center randomized trial. Hypertension in Pregnancy 1997;16:335‐46. [Google Scholar]
- Buul E, Rijpkema A, Steegers E, Jongsma T, Eskes P. Chronic dietary sodium restriction in pregnancy reduces calcium intake. Journal of Perinatal Medicine 1992;20(Suppl 1):216. [Google Scholar]
- Buul EJA, Steegers EAP, Jongsma HW, Thomas CMG, Hein PR. Chronic dietary sodium restriction in pregnancy: effects on urinary prostaglandin excretion. Proceedings of 2nd European Congress on Prostaglandins in Reproduction; 1991; The Hague, The Netherlands. 1991:186.
- Maten GD, Raaij JM, Visman L, Heijden LJ, Oosterbaan HP, Boer R, et al. Low‐sodium diet in pregnancy: effects on blood pressure and maternal nutritional status. British Journal of Nutrition 1997;77(5):703‐20. [DOI] [PubMed] [Google Scholar]
- Maten GD, Hammen RM, Visman L, Oosterban HP, Boer R, Heijden LJM, et al. Effect of sodium restricted diet during pregnancy on maternal blood pressure and zinc status. Journal of Perinatal Medicine 1992;20(Suppl 1):49. [Google Scholar]
- Post JA, Buul BJ, Hart AA, Heerikhuize JJ, Pesman G, Legros JJ, et al. Vasopressin and oxytocin levels during normal pregnancy: effects of chronic dietary sodium restriction. Journal of Endocrinology 1997;152:345‐54. [DOI] [PubMed] [Google Scholar]
Netherlands 1998 {published data only}
- Knuist M, Bonsel GJ, Zondervan HA, Treffers PE. Low sodium diet and pregnancy‐induced hypertension: a multi‐centre randomised controlled trial. British Journal of Obstetrics and Gynaecology 1998;105:430‐4. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Australia 1986a {published data only}
- Brown MA, Gallery EDM. Sodium excretion in human pregnancy: a role for arginine vasopressin. American Journal of Obstetrics and Gynecology 1986;154:914‐9. [DOI] [PubMed] [Google Scholar]
Australia 1986b {published data only}
- Brown MA, Sinosich MJ, Saunders DM, Gallery EDM. Potassium regulation and progesterone‐aldosterone interrelationships in human pregnancy: a prospective study. American Journal of Obstetrics and Gynecology 1986;155:349‐53. [DOI] [PubMed] [Google Scholar]
Belgium 1986 {published and unpublished data}
- Loquet P, Rutsaert R, Buytaert P, Broe ME. The influence of salt intake on some parameters that may be involved in the pathogenesis of pre‐eclampsia. 5th International Congress for the International Society for the Study of Hypertension in Pregnancy; 1986 July 7‐10; Nottingham, England. 1986:50.
UK 1958 {published data only}
- Robinson M. Salt in pregnancy. Lancet 1958;1:178‐81. [DOI] [PubMed] [Google Scholar]
UK 1961 {published data only}
- Bower D. The influence of dietary salt intake on pre‐eclampsia. Journal of Obstetrics and Gynaecology of the British Commonwealth 1961;63:123‐6. [DOI] [PubMed] [Google Scholar]
USA 1961 {published data only}
- Mengert WF, Tacchi DA. Pregnancy toxemia and sodium chloride ‐ preliminary report. American Journal of Obstetrics and Gynecology 1961;81:601‐5. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Brown 1985 {published data only}
- Brown MA, Nicholson E, Prendergast JS, Ross MR, Gallery EDM. Control of the renin‐aldosterone system in primigravid human pregnancy [abstract]. Kidney International 1985;28:861. [Google Scholar]
Additional references
Alderson 2004
- Alderson P, Green S, Higgins JPT, editors. Cochrane Reviewers' Handbook 4.2.2 [updated March 2004]. In: The Cochrane Library, Issue 2, 2004. Oxford: Update Software. Updated quarterly.
DH 2002
- Department of Health, Scottish Executive Health Department and Department of Health, Social Services, Public Safety. Northern Ireland. Why Mothers Die. The sixth report on confidential enquiries into maternal deaths in the United Kingdom 2000‐2002. London: RCOG Press, 2002. [Google Scholar]
Generic Protocol 2005
- Meher S, Duley L, on behalf of the Prevention of Pre‐eclampsia Cochrane Review authors. Interventions for prevention of pre‐eclampsia and its consequences: generic protocol. Cochrane Database of Systematic Reviews 2005, Issue 2. [Google Scholar]
Green 1989
- Green J. Diet and the prevention of pre‐eclampsia. In: Chalmers I, Enkin MW, Keirse MJNC editor(s). Effective care in pregnancy and childbirth. Oxford: Oxford University Press, 1989:281‐300. [Google Scholar]
NHBPEP 2000
- Gifford RW Jr, August PA, Cunningham G, Green LA, Lindhemier MD, McNellis D, et al. Report of the national high blood pressure education program working group on high blood pressure in pregnancy. American Journal of Obstetrics and Gynecology 2000;183(Suppl):1‐22. [PubMed] [Google Scholar]
RevMan 2003 [Computer program]
- The Cochrane Collaboration. Review Manager (RevMan). Version 4.2 for Windows. Oxford, England: The Cochrane Collaboration, 2003.
WHO 1988
- World Health Organization International Collaborative Study of Hypertensive Disorders in Pregnancy. Geographic variation in the incidence of hypertension in pregnancy. American Journal of Obstetrics and Gynecology 1988;158:80‐3. [PubMed] [Google Scholar]
References to other published versions of this review
Duley 1995
- Duley L. Low vs high salt intake in pregnancy. Keirse MJNC, Renfrew MJ, Neilson JP, Crowther C (eds.) Pregnancy and Childbirth Module. In: The Cochrane Pregnancy and Childbirth Database. Oxford: Update Software, 1995, issue 2.
Duley 1999
- Duley L, Henderson‐Smart D. Reduced salt intake compared to normal dietary salt, or high intake, in pregnancy. Cochrane Database of Systematic Reviews 1999, Issue 3. [DOI] [PMC free article] [PubMed] [Google Scholar]


