Abstract
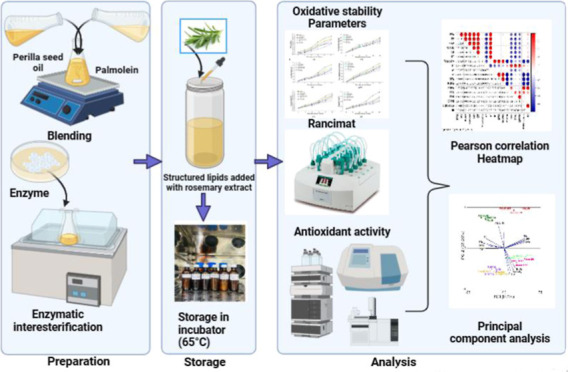
This study investigates the efficacy of rosemary extract (RE) in stabilizing structured lipids (SL) developed using perilla seed oil (PSO) and palmolein (PO) under accelerated storage conditions. The oil samples, comprising PSO, Blend, and SL formulations with and without RE (1500 ppm) and BHT (200 ppm), were studied for their storage stability during a 30 day storage period at 65 °C, with the analysis carried out at 6 day intervals. Oxidative properties were comprehensively assessed, including both physical attributes (color, viscosity, and refractive index) and chemical parameters (peroxide value, free fatty acid (FFA), p-anisidine value, TOTOX value, conjugated dienes, and trienes). The results demonstrated that RE-enriched oil samples exhibited significantly higher oxidative stability (p < 0.05) compared to the control group. SL added with 1500 ppm of RE exhibited notable enhancements in quality parameters, showcasing reductions in FFA, TOTOX value, conjugated diene, and triene value by 44.01%, 35.42, 39.03, and 47.36, respectively, when compared to SL without any antioxidant. The RE at 1500 ppm concentration showed a similar effect as the synthetic antioxidant BHT at 200 ppm. Also, the RE demonstrated potent inhibition of the oxidation of polyunsaturated fatty acids, thereby contributing to the improved oxidative stability of the SLs. Furthermore, SL with RE also exhibited reduced degradation of the tocopherol content and total phenolic content during the storage period. Principal component analysis demonstrated that SL and blend followed similar oxidative characteristics as they fell within the same quadrant. These findings underscore RE as a potent source of antioxidants capable of scavenging free radicals and enhancing the oxidative stability of omega-3 fatty acid-rich SLs.
1. Introduction
Oxidative stability is a critical factor in ensuring the quality and shelf life of fats and oils, and it remains one of the major concerns within the food industry. The presence of polyunsaturated fatty acids (PUFAs) makes lipids highly susceptible to oxidative deterioration, leading to off-flavors, decreased nutritional value, and potential health risks. Perilla seed oil (PSO) is an underutilized oilseed crop and is recognized for its high PUFA content (up to 75%), including linoleic acid (18:2 n-6) and α-linolenic acid (18:3 n-3), which contribute to its nutritional value.1,2 However, the high PUFA levels in PSO make it susceptible to oxidation, limiting its wider application in the fat and oil industries. To counteract the susceptibility of PUFA oils to oxidation, various strategies, including blending, interesterification, encapsulation, and the addition of synthetic and natural antioxidants, are used nowadays. Blending, for instance, involves mixing PUFA-rich oil with saturated fatty acid (SFA)-rich oil to achieve the balance fatty acid composition, thereby improving stability. Yet, challenges arise when blending oils with disparate melting points, leading to phase separation issues that impede the practical application of the blended oil. Conversely, interesterification is a process of randomization of fatty acids and oil triacylglycerols. By strategic localization of specific fatty acids within the lipid structure, interesterification offers improved functional and nutrition properties compared to their unstructured counterparts or blended oil.3
Therefore, the enzymatic interesterification (EIE) of oil for the preparation of structured lipids (SLs) has emerged as a promising approach within this context. Palmolein (PO) emerges as a valuable resource due to its low cost and good oxidation stability.4 With its composition rich in SFA, PO offers enhanced stability against oxidation and rancidity. However, its predominance of SFA in PO raises health implications. Consequently, our prior study has highlighted the EIE of high-saturated fat (PO-70%) with PUFA-rich oil (PSO-30%) as a compelling solution, resulting in improved nutritional and functional properties as well as enhanced applicability.5 Chemical or enzymatic interesterification results in the production of SL, which holds promise for enhancing lipid digestion and energy absorption and providing nutritional and health benefits. Consequently, they find widespread applications in food, health products, and other industries.6−9 However, previous studies have primarily focused on comparing the oxidation stability of oils before and after EIE. These studies reported some limitations, such as the destruction of natural antioxidants in the lipids during the interesterification process, which further results in low oxidative stability.10,11 Therefore, there is a need for further enhancement of the oxidative stability of SL using sustainable sources of the natural antioxidants.
In recent years, researchers have shown growing interest in utilizing antioxidants to improve the thermo-oxidative properties of various edible oils. Synthetic antioxidants have proven to be effective in mitigating oxidation in oils, and there is a growing apprehension surrounding their prolonged use. This apprehension arises from potential adverse health consequences, including skin allergies, gastrointestinal issues, and even a potential association with cancer.12 As a result, researchers have turned their attention toward natural alternatives, such as plant-based extracts, to inhibit lipid oxidation without compromising consumer safety. Rosemary extract (RE), derived from the aromatic herb Rosmarinus officinalis, commonly used as flavoring spices in food, has gained significant attention for its potent antioxidant properties and is commonly used in edible oil as an antioxidant. It contains a range of bioactive compounds, including phenolic diterpenes (carnosic acid, carnosol, and epi-and iso-rosemanaol), flavonoids, and rosmarinic acid, which demonstrate robust capabilities in scavenging free radicals and chelating metals.13,14 These antioxidant compounds have shown promising roles in inhibiting the oxidation of lipids and preserving the quality of various food products. Literature has demonstrated the potential of RE in inhibiting the oxidation process in various oil, such as hazelnut oil, olive oil, and sunflower oil.15−19 These antioxidant compounds have displayed encouraging results in preventing lipid oxidation and maintaining the quality of various fats and oil.
The aim of this research paper is to investigate the potential of RE for improving the oxidation stability of omega-3 rich PSO-based SL. Several studies have reported that the SL has reduced oxidative stability because of the loss of tocopherol and various bioactive compounds due to the processing conditions used during the interesterification process. Therefore, the shelf-life stability of SL was assessed using a Schaal oven test to assess the effectiveness of RE in preventing lipid oxidation of SL under accelerated storage conditions.
2. Materials and Method
2.1. Materials
Perilla seeds were acquired from a local market in Uttarakhand, India, and subsequently subjected to cold pressing using a Sonar oil press machine (Sonar SA2003, Sonar Appliances Private Limited, Delhi, India). The resulting oil underwent physical refining and was then filtered using Whatman filter paper 2, following the method outlined by Mazehari and others.20 RE was procured from Veda Oil in New Delhi, India, and PO was purchased from a local market of Delhi. The lipase enzyme TL IM (Thermomyces lanuginosus) was provided by Novozymes, India, as a gift. All solvents, chemicals, and gases employed in the study met the criteria of analytical grade. Standard FAME mix, BHT (butylated hydroxytoluene), α, δ, and γ tocopherols, DPPH (2,2-diphenyl-1-picrylhydrazyl), and gallic acid standards were sourced from Sigma-Aldrich. To ensure their preservation, the oil samples were stored in securely sealed amber bottles at a temperature of 4 °C.
2.1.1. Preparation of Structured lipid (SL)
In the preparation of SL, a mixture of PO and PSO at a ratio of 70:30 (PO/PSO w/w) was carried out in a dry-capped Erlenmeyer flask. Immobilized Lipozyme, T. lanuginosus (TL IM) enzyme (8% w/w) was used as a biocatalyst. The mixtures were reacted at 45 °C and agitated at 150 rpm for 6.6 h.21 To effectively separate the enzyme from the resulting product, a 0.45 μm PTFE membrane filter was employed. For the purpose of enzyme reusability, a comprehensive washing procedure was carried out using hexane, followed by a drying period in an oven set at 40 °C, spanning 6 h.22
2.1.2. Preparation of Oil with Antioxidant
To evaluate the impact of RE on the oil samples (PSO, blend, and SL), ethanolic RE was introduced into each sample. The RE was incorporated at a concentration of 1500 ppm, as determined based on findings from our previous study,21 and BHT (butylated hydroxytoluene) was added in PSO, blend, and SL at a concentration of 200 ppm. The RE and BHT were appropriately weighed and added to each oil sample separately. Each mixture was thoroughly blended using a magnetic stirrer at 40 °C and 150 rpm for 30 min.
2.1.3. Sachal Oven Test
The Sachal oven test was conducted by placing 100 mL of each sample (PSO, PSO-RE, PSO-BHT, Blend, Blend-RE, Blend-BHT, SL, SL-RE, and SL-BHT) in glass bottles. These bottles were then incubated at a temperature of 65 ± 1 °C in an incubator. A control sample without any additives was also prepared simultaneously. The samples were allowed to remain in the incubator for 30 days. At specific time intervals (0th, 6th, 12th, 18th, 24th, and 30th day), samples were drawn from each bottle for quality analysis of the oil. The fatty acid composition, tocopherol levels, total phenolic content, and DPPH % inhibition of the samples were analyzed at the start of the experiment (0th day) and on the end of the experiment (30th day).
2.2. Oxidative Stability Using Rancimat
To evaluate the oxidative stability of the oil samples on the initial day, an accelerated oxidation test was conducted using the Rancimat professional model 892 (Metrohm Rancimat model 743, Switzerland). This assessment involved measuring the Induction period (IP) in hours. Specifically, 3 g of the oil sample was carefully weighed and subjected to heating at 110 °C while maintaining a 20 L/h airflow rate. The IP value of the sample antioxidant activity of extract and BHT was calculated using the protection factor (PF).
Protection factor (PF) = IP value of sample added with the antioxidant/IP value of control.
2.3. Change in the Physical Quality Parameter during Accelerated Storage
The color characteristics of the oil samples, including L* (lightness), a* (redness), and b* (yellowness), were assessed using a CR400 minolta colorimeter (Konica Minolta Inc., Tokyo, Japan). Refractive index (RI) of the oil samples was measured at 40 °C by ATAGO-RX-5000i-plus refractometer (Japan) according to the AOCS method Cc 7–25.23 The viscosity of oil samples was analyzed using a Brookfield Viscometer using a spindle S18.
2.4. Change in Oxidative Indices during Accelerated Storage
The free fatty acids (FFA), peroxide value (PV), and p-anisidine value (p-AV) for each oil sample were assessed at specific time intervals using the official AOCS methods: Ca 5a-40 for FFA, Cd 8–53 for PV, and Cd 18–90 for p-AV.23 The TOTOX value is calculated using the following formula
The conjugated dienes and trienes of the oil samples were determined by measuring absorbance values at 232 and 268 nm by using a UV–vis spectrophotometer (UV–vis-1800 spectrophotometer, Kyoto, Japan). The oil samples were appropriately diluted with isooctane in accordance with the established standard procedure given by ISO 3656:2011.24 From the absorption value obtained, CD was calculated as follows
where A = Absorbance at λ 232 or 268 nm, l = Optical path of the cuvette (1 cm), and C is the concentration of oil in g/100 mL.
2.5. Fatty Acid Composition
The AOCS Official Method 996.06 was used to analyze the fatty acid content of oil samples.25 Using a gas chromatograph (Agilent 7890 B) equipped with a flame ionization detector (FID) and polyethylene glycol DB wax capillary column (100 mm × 0.25 m × 0.2 m; Agilent Technologies, USA). The fatty acids present in the oil samples were converted into fatty acid methyl esters using BF3. N2 was used as the carrier gas, with flow at a rate of 1.5 mL/min. The following instrumental conditions were used: column pressure of 30 Psi, a sample injection volume of 1 μL, a split ratio of 100:1, and detector and injector temperatures set at 280 °C. The oven temperature program started at 100 °C, maintained for 4 min, and then increased to 240 °C (with a ramp rate of 4 °C/min) and held for 25 min. The H2 and O2 flow rates were 30 and 300 mL/min, respectively. The fatty acid content in the samples was determined by comparing their retention times with Fatty acid methyl ester mix, Sigma-Aldrich standard. To assess the oxidative stability, the COX value was calculated according to the formula given by Cao et al.,26 where
2.6. Total Tocopherol Analysis (TTC)
To assess the total tocopherol content (TTC) of the oil samples, an HPLC system from UFLC Shimadzu in Kyoto, Japan, was employed following the procedure outlined in ISO, (2012) with slight modifications. The HPLC setup was a fluorescence detector (RF-10AXL) with 294 nm excitation and 329 nm emission wavelengths. The separation used a Phenomenex silica column (Spincotech Pvt. Ltd., India) with dimensions 250 × 4.6 mm with 5 μm particle size, and the mobile phase consisted of 3.85% tetrahydrofuran in n-heptane, running at a flow rate of 1 mL/min. For preparing the samples, precisely 100 mg of the oil was combined with n-heptane in a volumetric flask of 10 mL and vortexed. Then, a 20 μL aliquot of this mixture was injected into the HPLC system. Data acquisition and subsequent analysis were conducted using Lab Solution software.27
2.7. Fourier Transform Infrared Spectroscopy (FTIR)
FTIR-ATR analysis was conducted for all the oil samples with and without an antioxidant on the 0th and 30th days of storage. This analysis was performed using an FTIR spectrometer, specifically the Agilent FTIR-Cary 630 model. The measurement encompassed the spectral range from 4500 to 400 cm–1, with a resolution of 2 cm–1 and an average of 30 scans per spectrum. Subsequently, the acquired spectra were subjected to analysis using the Agilent Pro resolution software.28
2.8. Total Phenolic Content (TPC) and DPPH % Inhibition
Utilizing a liquid–liquid extraction method, the polar extracts were obtained from the oil samples. In this procedure, 0.5 g of the oil sample was dissolved in a mixture of methanol and water with an 80:20 ratio (v/v), making up a total of 3 mL. Following thorough mixing, the mixture underwent centrifugation at 6000 rpm for approximately 5 min. The resulting polar extract was isolated and collected. This process of extraction was reiterated three times, and the supernatants collected were combined up to a 10 mL final volume using an 80:20 methanol–water solution (v/v) for analysis in the subsequent steps.29,30 To assess the complete phenolic content (TPC), a modified rendition of the Folin–Ciocalteu method, originally established by singlet and other researchers.31 This procedure involves the interaction between phenolic compounds and the Folin-Ciocalteu reagent, followed by quantifying the absorbance at 765 nm using a spectrophotometer (UV–vis-1800 spectrophotometer, Kyoto, Japan). The outcomes were expressed as milligrams of gallic acid equivalents (GAE) per 100 g of oil.
For DPPH, precisely, 2 mL of the polar oil extract was mixed with 2 mL of a 0.2 mM methanolic DPPH solution. After a 30 min incubation period in darkness, the resulting solution’s absorbance was noted at 517 nm using a Shimadzu UV–vis-1800 spectrophotometer (Kyoto, Japan), with the absorbance of the blank reagent subtracted. The value was calculated as the percentage of inhibition using the given formula
where A0 is the absorbance of the blank sample, and A is the absorbance of the oil sample.
2.9. Statistical Analysis
Each analysis was carried out with a minimum of three replicates. Data analysis was performed using SPSS software (IBM SPSS Statistics Ver. 21, New York), which encompassed ANOVA (Analysis of Variance), standard deviation calculations, and Turkey’s HSD test, with a significance level set at p < 0.05. Additionally, Pearson’s correlation analysis and multivariate analysis, specifically principal component analysis (PCA), were conducted among various parameters using Origin Pro 2023b 10.0.5.157 (OriginLab Corporation, Northampton, MA, USA).
3. Result and Discussion
3.1. Oxidative Stability
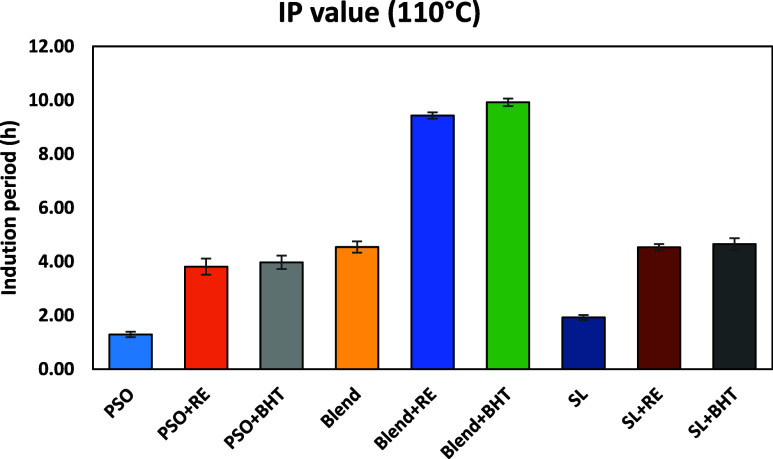
The Rancimat test is a widely used method for evaluating the stability of oils through an accelerated oxidation process. Figure 1 displays the induction period (IP) of all of the oil samples at 110 °C on day 0. The results indicated that PSO had the lowest IP value (1.29 h), while the blend with the addition of BHT and RE exhibited the highest IP value (9.92 h and 9.43, respectively). The primary reason for the low IP value in PSO is its higher level of omega-3 content. Conversely, the blend yielded better results due to the substantial amount of SFA and MUFA from PO, which resulted in its balanced fatty acid composition of SFA, MUFA, and PUFA. The inclusion of RE led to an approximately 3-fold increase in IP values of PSO and SL, whereas 2.5 fold in the case of blend. Notably, BHT and RE demonstrated significantly similar IP values (p < 0.05). While comparing SL and PSO, SL displayed superior IP values, both with and without the antioxidant. The protective factor (PF) for PSO, blend, and SL with and without the antioxidant ranged from 1.05 to 3.07, indicating the potent antioxidative effect of RE against oxidation. Similar results were also reported in our prior studies.32,33 The obtained PF values for the PSO samples were notably higher compared to those in another study where the effectiveness of sage, thyme, rosemary, and bay extracts in canola oil and its triglyceride was assessed. Additionally, the study reported that RE exhibited the highest PF value when compared to other extracts.34
Figure 1.
Induction period (IP) of PSO, Blend, and SL with and without antioxidant.
3.2. Change in Physical Quality Parameters
The study investigated the change in various physical parameters including color (L*, a*, and b* values), viscosity, refractive index (RI) with added RE on PSO, blend, and SL during accelerated storage (Table 1). The statistical analysis revealed a significant effect on the addition of RE, which resulted in increased values of L*, a*, and b* as compared to control samples. The reason behind this could be the migration of colored compounds from the RE to the oil. Similar results were also reported, indicating an increase in the value of L*, a*, and b* in sunflower oil when the ethanolic extract of prickled broom was added.35 During the storage, all the oil samples showed a decrease in the lightness (L*), while the red (a*) and yellow (b*) components intensified over the storage period. These shifts in oil color were attributed to reactions occurring among the oil’s constituents when exposed to elevated storage temperatures because of the polymers formed during the oxidation. A particularly significant change was observed in the a* value, indicating a transition toward red rather than green. This shift was primarily due to the reduced chlorophyll content of the oil samples during storage. The elevated temperatures promote chemical reactions within the oil, leading to the breakdown of chlorophyll molecules and the subsequent alteration of their color properties. A comparable alteration was reported, where they indicated that the extent of color degradation was more pronounced during accelerated storage.36
Table 1. Changes in Color, Viscosity, and Refractive Index of Oil Samples during Accelerated Storage Studya.
| Parameter | Storage time | PSO | PSO + RE | PSO + BHT | Blend | Blend + RE | Blend + BHT | SL | SL + RE | SL + BHT |
|---|---|---|---|---|---|---|---|---|---|---|
| L* | D0 | 72.07 ± 1.05bA | 75.47 ± 0.46aA | 72.44 ± 0.43bA | 69.09 ± 0.73cA | 71.03 ± 0.12bA | 69.09 ± 0.73cA | 69.21 ± 0.21cA | 72.15 ± 0.15bA | 69.21 ± 0.22cA |
| D6 | 70.33 ± 0.10cB | 73.62 ± 0.37AB | 71.80 ± 0.15bB | 67.04 ± 0.41eB | 69.18 ± 1.00dB | 67.64 ± 0.52eB | 67.12 ± 0.12eB | 70.34 ± 0.76cB | 67.61 ± 0.47eB | |
| D12 | 68.22 ± 0.63cC | 72.17 ± 0.17aC | 69.10 ± 0.36bC | 65.44 ± 0.43dC | 68.02 ± 0.49cC | 66.14 ± 0.17dC | 65.46 ± 0.43dC | 69.45 ± 1.06bC | 66.37 ± 0.76dC | |
| D18 | 67.17 ± 0.94cC | 71.15 ± 1.01aD | 68.17 ± 0.17bD | 63.28 ± 0.48fD | 67.53 ± 0.41cC | 65.86 ± 0.74eC | 64.87 ± 0.57eC | 68.88 ± 0.45bC | 65.99 ± 0.19dC | |
| D24 | 66.04 ± 0.49bD | 70.82 ± 0.73aD | 67.01 ± 1.01bE | 62.26 ± 0.27fE | 66.23 ± 0.79bD | 64.13 ± 1.01dD | 63.23 ± 0.67eD | 67.43 ± 0.32bD | 65.14 ± 0.26cC | |
| D30 | 65.96 ± 0.54bD | 69.16 ± 0.25aE | 66.35 ± 0.41bF | 61.35 ± 0.13eF | 65.96 ± 0.61bD | 63.23 ± 0.22dE | 62.40 ± 1.03dE | 66.85 ± 0.53bE | 64.84 ± 0.36cD | |
| a* | D0 | –2.73 ± 0.05bE | –1.13 ± 0.13aD | –2.72 ± 0.07bD | –3.08 ± 0.08cE | –2.64 ± 0.06bE | –3.07 ± 0.08c | –2.96 ± 0.41cE | –2.05 ± 0.05bD | –2.94 ± 0.04cD |
| D6 | –1.09 ± 0.10bD | 0.67 ± 0.04aC | –1.18 ± 0.05bC | –1.66 ± 0.11bD | –2.03 ± 0.02cD | –1.83 ± 0.03b | –1.23 ± 0.07bD | –1.33 ± 0.06bC | –1.63 ± 0.06bC | |
| D12 | –0.23 ± 0.01cC | 1.43 ± 0.13aB | 0.42 ± 0.22bB | –0.34 ± 1.17cC | –1.11 ± 0.09dC | 0.88 ± 0.07b | 0.28 ± 0.06bC | 0.15 ± 0.16bB | –1.03 ± 0.07cC | |
| D18 | 1.15 ± 0.05aB | 1.86 ± 0.14aA | 1.05 ± 0.05aA | 1.05 ± 0.18aB | 0.38 ± 0.08bB | 1.18 ± 0.10a | 1.10 ± 0.05aB | 0.86 ± 0.13bB | 0.23 ± 0.05bB | |
| D24 | 1.35 ± 0.05bB | 2.01 ± 0.42aA | 1.25 ± 0.22bA | 1.44 ± 0.06aA | 1.05 ± 0.05bA | 1.27 ± 0.02b | 1.74 ± 0.11aA | 1.12 ± 0.08bA | 1.03 ± 0.15bA | |
| D30 | 1.66 ± 0.14bA | 2.32 ± 0.08aA | 1.58 ± 0.15bA | 1.96 ± 0.19abA | 1.37 ± 0.05bA | 1.73 ± 0.07bA | 2.07 ± 0.12aA | 1.44 ± 0.07bA | 1.38 ± 0.08bA | |
| b* | D0 | 29.17 ± 0.08eE | 33.09 ± 0.01dD | 29.18 ± 0.08eE | 61.20 ± 0.03bE | 63.62 ± 0.38aE | 61.22 ± 0.12bE | 59.32 ± 0.19cE | 63.04 ± 0.03aE | 59.38 ± 0.38cE |
| D6 | 33.14 ± 0.05eD | 36.05 ± 1.02dC | 32.52 ± 0.19eD | 62.53 ± 0.23bD | 64.33 ± 0.12aD | 62.19 ± 0.02bD | 61.25 ± 0.17cD | 64.46 ± 0.75aC | 60.49 ± 0.36cD | |
| D12 | 35.11 ± 1.01eC | 37.33 ± 0.32dB | 34.71 ± 0.09eC | 65.24 ± 0.15aC | 65.36 ± 0.18aB | 64.20 ± 0.21bC | 63.18 ± 0.21bC | 65.44 ± 0.23aB | 62.09 ± 0.09cC | |
| D18 | 36.51 ± 0.49eB | 37.92 ± 0.01dB | 35.12 ± 0.11eB | 66.96 ± 0.46aB | 65.87 ± 0.53bB | 65.45 ± 0.12bB | 65.00 ± 0.11bB | 66.03 ± 0.11aB | 63.49 ± 0.34cB | |
| D24 | 37.08 ± 0.03eA | 38.41 ± 0.21dA | 36.68 ± 0.30eA | 67.14 ± 0.16aA | 66.19 ± 0.09aA | 66.14 ± 0.13bA | 66.23 ± 0.11bA | 66.93 ± 0.05abA | 64.12 ± 0.11cA | |
| D30 | 37.85 ± 0.12eA | 38.85 ± 0.06dA | 37.06 ± 0.05eA | 67.87 ± 0.02aA | 66.83 ± 0.38bA | 66.45 ± 0.04bA | 66.45 ± 0.13bA | 67.65 ± 0.24aA | 64.91 ± 0.10cA | |
| Viscosity | D0 | 34.3 ± 0.50dE | 33.9 ± 0.40dD | 33.5 ± 0.10dD | 47.8 ± 0.10aE | 47.6 ± 0.10aD | 47.3 ± 0.05aD | 45.9 ± 0.20bD | 44.2 ± 0.20cE | 44.5 ± 0.6cE |
| D6 | 35.5 ± 0.1dD | 34.5 ± 0.15eC | 34.6 ± 0.06eC | 48.6 ± 0.19aD | 48.2 ± 0.04aC | 48.4 ± 0.20aC | 46.8 ± 0.20bC | 45.0 ± 0.23cD | 45.5 ± 0.61cD | |
| D12 | 36.3 ± 0.2dC | 35.2 ± 0.10eB | 35.1 ± 0.06eB | 49.1 ± 0.20aC | 48.8 ± 0.06aC | 48.7 ± 0.09aC | 47.3 ± 0.26bC | 45.8 ± 0.10cD | 46.1 ± 0.07cC | |
| D18 | 37.9 ± 0.06dB | 35.8 ± 0.10eB | 35.7 ± 0.06eB | 50.2 ± 0.06aC | 49.4 ± 0.07aC | 49.2 ± 0.06aC | 48.0 ± 0.23bC | 46.6 ± 0.80cC | 46.8 ± 0.06cB | |
| D24 | 38.5 ± 0.25dB | 36.3 ± 0.06eB | 36.2 ± 0.20eA | 51.6 ± 0.20aB | 50.1 ± 0.09aB | 50.2 ± 0.15aB | 49.6 ± 0.15bB | 47.2 ± 0.05cB | 47.4 ± 0.30cB | |
| D30 | 40.3 ± 0.10eA | 37.1 ± 0.06fA | 36.9 ± 0.06fA | 53.4 ± 0.06aA | 51.2 ± 0.15bA | 51.5 ± 0.36bA | 50.3 ± 0.12cA | 48.2 ± 0.10dA | 48.3 ± 0.00dA | |
| RI | D0 | 1.47488 ± 0.00040aD | 1.47489 ± 0.00023aC | 1.47489 ± 0.00011aC | 1.46314 ± 0.00109bE | 1.46314 ± 0.00037bD | 1.46314 ± 0.00203bB | 1.46327 ± 0.00021bB | 1.46327 ± 0.00020bC | 1.46327 ± 0.00027bC |
| D6 | 1.47522 ± 0.00302aC | 1.47491 ± 0.00053aC | 1.47491 ± 0.00220aC | 1.46387 ± 0.00051bD | 1.46332 ± 0.00060bD | 1.46345 ± 0.00010bB | 1.46387 ± 0.00430bB | 1.46337 ± 0.00028bC | 1.46341 ± 0.00019bC | |
| D12 | 1.47577 ± 0.06002aC | 1.47511 ± 0.00407aB | 1.47511 ± 0.00067aB | 1.46450 ± 0.00107bC | 1.46378 ± 0.00120bC | 1.46369 ± 0.00032bB | 1.46358 ± 0.00300bB | 1.46404 ± 0.00270bB | 1.46412 ± 0.00101bB | |
| D18 | 1.47621 ± 0.00019aB | 1.47537 ± 0.00042aB | 1.47542 ± 0.00011aB | 1.46512 ± 0.00040bB | 1.46415 ± 0.00250bB | 1.46423 ± 0.00063bA | 1.46476 ± 0.00501bA | 1.46421 ± 0.00208bB | 1.46431 ± 0.00165bB | |
| D24 | 1.47675 ± 0.00027aB | 1.47572 ± 0.00036aA | 1.47572 ± 0.00310aA | 1.46583 ± 0.00064bB | 1.46453 ± 0.00071bB | 1.46450 ± 0.00504bA | 1.46521 ± 0.02080bA | 1.46452 ± 0.00014bB | 1.46458 ± 0.00051bA | |
| D30 | 1.47724 ± 0.00062aA | 1.47598 ± 0.05300aA | 1.47579 ± 0.00209aA | 1.46632 ± 0.000643bA | 1.46502 ± 0.00082bA | 1.46491 ± 0.00024bA | 1.46571 ± 0.00034bA | 1.46501 ± 0.00202bA | 1.46481 ± 0.00173bA |
PSO-perilla Seed oil, SL, structured lipids, RE-rosemary extract, BHT-butylated hydroxytoluene, and RI-refractive index. Data are presented as mean (±SD) (n = 3). (a–g) means within each row for each parameter with different superscripts are significantly (p < 0.05) different. (A–B) means within each column for each parameter with different superscripts are significantly (p < 0.05) different.
The data presented in Table 1 illustrates the changes in refractive indices (RI) on PSO, blend, and SL added with and without the antioxidant over a storage period of 30 days at 65 °C. Throughout the storage period, all oil samples exhibited a gradual increase in RI values, indicating a progressive change because of the formation of conjugation products and oil polymerization due to autoxidation.35 After 30 days of storage, a significant difference was observed between the control and oil samples added with RE and BHT (p < 0.05). The obtained result showed that after 30 days of storage, the PSO, blend, and SL demonstrated a higher RI value than the PSO, blend, and SL added with RE or BHT. This suggests that the presence of antioxidants had a notable impact on inhibiting the increase in RI, potentially slowing down the deterioration of the oils. These findings were aligned with a previous study that observed fluctuations in RI values for sunflower and soybean oils when natural antioxidants from fruit leaves (guava, pomegranate, and grapes) were added during accelerated storage.37 However, another study indicated that the RI of sunflowers enriched with ethanolic-based Pterospartum tridentatum extract remained unchanged following 30 days of accelerated storage.35
The viscosity of oils is influenced by the degree of saturation within the oil samples as well as the arrangement of fatty acids on the glycerol backbone within the triglyceride molecule.36,38 Initially, the viscosity of PSO was 34.3 mPa, lower than that of the oil blend (47.6 mPa) and SL (45.9 mPa), which can be ascribed to the high PUFA content in PSO (∼75%). Throughout the storage period, all oil samples exhibited noteworthy changes in viscosity (p < 0.05). However, the addition of RE led to a comparatively lower rate of viscosity increase over time. This implies that the inclusion of RE could potentially mitigate the viscosity changes that typically occur during storage by delaying the accumulation of polymerized compounds formed due to the oxidation of oil. A similar trend in viscosity of Hazelnut oil added with RE was reported.16 Furthermore, studies have also indicated that the inclusion of RE in PO oil during deep frying process resulted in a reduced viscosity compared to the pure PO.39
3.3. Change in Oxidative Indices during Accelerated Storage
3.3.1. Free Fatty Acid (FFA)
Free fatty acid (FFA) is an important parameter for assessing the quality of oil during processing and storage. However, during storage and heating processes, FFA levels tend to increase gradually due to the hydrolysis of fatty acids. The elevation in FFA content leads to a decrease in the smoke point, indicating a decline in the overall oil quality.40
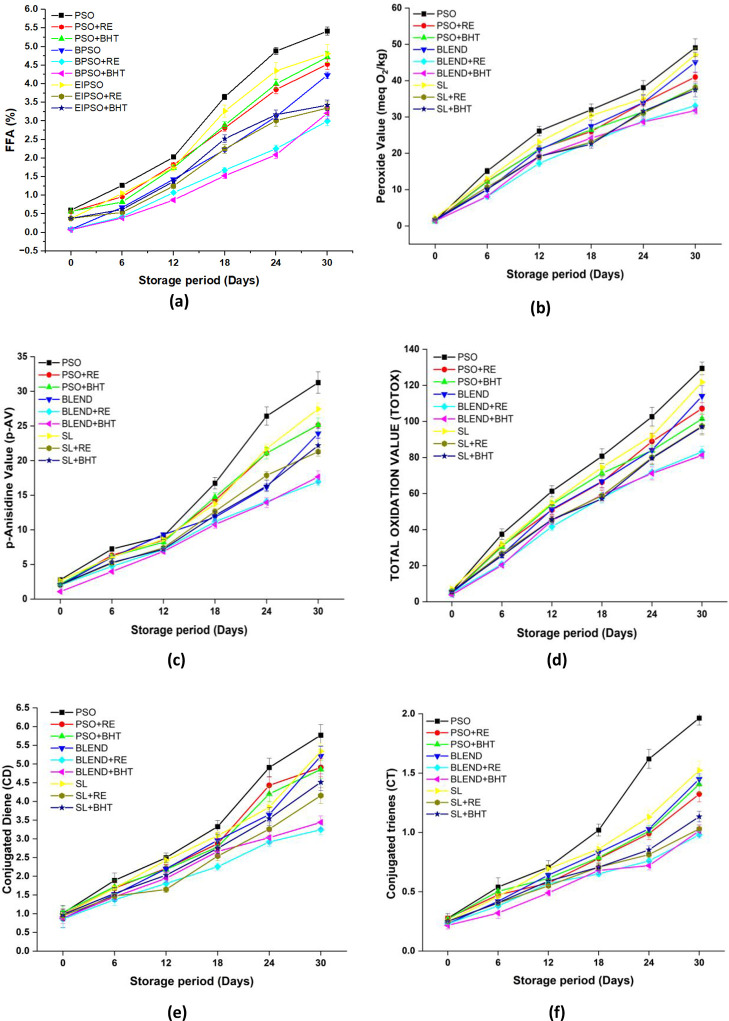
The change in FFA content during the accelerated storage is displayed in Figure 2a. At the onset of the storage period, oil samples displayed an FFA level ranging from 0.08 to 0.60%, within the limits defined by codex Alimentarius41 (≥2%). On the 0th day of storage, the inclusion of RE and BHT to PSO, BPSO, and SL did not yield significant differences in FFA content (p < 0.05). With the progression of storage time, an increase in FFA content was observed across all samples (p < 0.05). This elevation can be attributed to the breakdown of secondary compounds into FFAs and the reduction in the extracts’ capacity to inhibit hydrolysis. However, the addition of RE and BHT to PSO, blend, and SL resulted in a notable decrease in FFA values (p < 0.05) compared to the PSO, blend, and SL without any antioxidants at the end of the storage period. No significant difference was observed among samples containing RE and BHT in PSO, blend, and SL during the storage period. In a previous study, it was found that the addition of Sophora japonica L. extract at 200 ppm to echium oil showed a significant reduction in the increasing acidity trend over a span of 4 weeks at 60 °C as compared to the control sample.42
Figure 2.
Changes in (a) free fatty acid content (FFA), (b) peroxide value (PV), (c) p-anisidine value (p-Av), (d) total oxidation value (TOTOX), (e) conjugated diene (CD), and (f) conjugated trienes (CT) during accelerated storage condition.
3.3.2. Peroxide Value
The assessment of fat and oil’s oxidative deterioration can be effectively conducted using peroxide value (PV), which quantifies the amount of hydroperoxides formed as the primary oxidation products of oil. Figure 2b presents the PV values for PSO, blend, and SL with and without the RE and BHT. Throughout the storage period at 65 °C, the PV of PSO, blend, and SL with and without antioxidants showed an increasing trend over time. This reason behind the rise in PV can be ascribed to the formation of hydroperoxides, which are key indicators of primary lipid oxidation. The PSO consistently displayed the highest PV throughout the storage period (p < 0.05), and the reason behind this could be its high PUFA content, which is highly susceptible to oxidation. At the end of the 30 day storage study, the blend with RE demonstrated the lowest PV as compared to the SL with RE. The reason behind this could be that the SL was subjected to higher exposure to temperature during the EIE process. After the 30th day of storage at 65 °C, the PV of SL added with RE (PV-31.07 mequiv O2/kg) was lower than that of SL without any antioxidant (PV- 35.09 mequiv O2/kg), showing the protective effect of RE. While comparing RE with BHT, RE exhibited better outcomes in all three types of oil samples (PSO, blend, and SL). This may be explained by the increased effectiveness of the extract, resulting in its higher activity, potentially associated with the quick breakdown of BHT at higher temperatures.43 Similar findings were reported in the study, where the researcher compared the efficacy of RE and BHT on palm oil, soybean, cottonseed, and rice bran oil.15,44
Blend and SL with RE and BHT exhibited an acceptable PV level of <10 mequiv/kg oil until the sixth day of storage, while the PV value limit of PSO and SL without antioxidants was exceeded on the sixth day. These observations suggest that the addition of RE in SL effectively controlled PV levels until the sixth day of storage, which is roughly equivalent to six months of real-time storage at 25 °C.45 However, the increasing pattern of PV in the samples with RE was slower, characterized by a less steep slope, compared with samples without any antioxidant. The action of these REs might have hindered the formation of hydroperoxide or enhanced the antioxidants’ ability to neutralize peroxyl radicals. This, in turn, could have restrained the progress of radical chain reactions, leading to the observed slower increase in PV. These results are in line with a prior investigation that highlighted the advantageous impact of incorporating RE in reducing the PV of flaxseed oil in comparison to BHT.46
3.3.3. p-Anisidine Value (p-AV) and Total Oxidation Value (TOTOX)
The quantification of secondary oxidation products, specifically aldehydes and ketones (categorized as carbonyl compounds), formed during the second phase of fat and oil oxidation expressed as p-anisidine values (p-AV). A higher p-AV value indicates a more pronounced oxidative deterioration of the fat, signifying elevated levels of oxidation.40
There was a statistically significant difference observed in the p-AV of oil samples with added RE and BHT (p < 0.05) and followed a similar pattern as peroxide value (PV). However, a significant increase in p-AV was observed in the PSO samples added with RE and BHT after 12 days of accelerated storage, with an increase of 2.1 to 3.8-fold in p-AV. The blend and SL samples with and without antioxidants had p-AV values below 10 until the 12th day of accelerated storage, indicating values within the acceptable limit.47 These observations suggest that the SL and blend with and without RE and BHT have effectively controlled p-AV levels until the 12th day of storage, which is roughly equivalent to 12 months of real-time storage at 25 °C. However, on the 12th day, the control PSO sample and PSO sample with antioxidants exceeded the acceptable limit, suggesting that PSO is more susceptible to oxidation. At the end of the 30th day, the blend added with RE had the lowest p-AV (16.98 ± 0.42), while the highest p-AV was observed in PSO (31.27 ± 1.91) (Figure 2c). Overall, the RE was found to be more effective in terms of thermal and oxidative stability than the BHT at 200 ppm (p < 0.05). These findings are consistent with previous studies, which respectively demonstrated that RE delays the generation of secondary oxidation compounds in hazelnut oil during the frying process.16
The TOTOX value measures the total oxidation compounds formed during the oxidation process, including both primary oxidation compounds, such as hydroperoxides, and their breakdown products (secondary oxidation compounds). The generally accepted empirical threshold for the TOTOX value is 30.48 As the storage duration extended, a noteworthy rise in the TOTOX value was observed in all samples, with statistical significance (p > 0.05) (Figure 2d). Consistent with the findings for PV and p-AV, PSO exhibited the highest TOTOX value among all the oil samples, indicating high oxidative rancidity. Samples containing RE and BHT both showed lower oxidation levels compared to the control sample. Both the blend and SL, with and without antioxidants, maintained TOTOX values within the acceptable limit (30) for up to 6 days of storage. However, beyond this point, there was a significant rise in TOTOX value of almost 50% in SL, primarily attributed to the increased generation of primary and secondary oxidation products. However, PSO samples exceeded the limit on the sixth day of storage. By the 30th day of storage, there was a 37–40% reduction in TOTOX value for the blend added with RE and around a 25% reduction for SL with RE. Furthermore, the results demonstrated that the addition of RE at a concentration of 1500 ppm exhibits protection against oil oxidation during accelerated storage, as evidenced by a lesser increase in TOTOX value compared to samples without RE. These findings align with a previous study in which flaxseed oil was enriched with RE displayed a lower TOTOX value than BHT during storage at 60 °C.49
3.3.4. Conjugated Dienes (CD) and Trienes (CT)
Conjugated dienes (CD) and conjugated triene (CT) are employed for assessing the extent of primary and secondary oxidative processes, respectively. CDs form as a result of the oxidation of unsaturated fatty acids, leading to the formation of more stable radicals. On the other hand, CT is indicative of changes in ketone content.50,51 The CD and CT of oil samples added with antioxidants were significantly different from the sampleds without antioxidants (Figure 2e,f), which displayed an increasing trend as the storage time was extended. Throughout the storage period, all the samples consistently exhibited lower CD and CT values compared to PSO (p < 0.05). By the 30th day of storage, the SL + RE exhibited an approximate 27% reduction in the CD value and a 7% reduction in the CT value compared to PSO + RE. Notably, in the case of SL without any antioxidants, there was a 5.2-fold increase in the CD value from the initial measurement to the 30th day. However, the addition of RE resulted in a comparatively lower increase, with a 3.25-fold rise observed. The results are in line with this previous study, where the CD value of sunflower oil added to RE was significantly lower than that of pure sunflower oil, further highlighting the antioxidant potential of RE.52
3.4. Changes in Fatty Acid Composition of Oil Samples
The fatty acid content of oils can serve as an indicator of their resistance to oxidation and nutritional value. The unsaturated fatty acids in the oil are vulnerable during thermal treatment, which results in a reduction in the content of unsaturated fatty acids and an increase in the content of trans fatty acid or saturated fatty acid. Table 2 represents the fatty acid profile of PSO, blend, and SL with and without antioxidants, comparing the values on day 0 and day 30. The PSO, blend, and SL exhibited a variation in individual fatty acid composition as well as in the proportion of SFA, MUFA, and PUFA. Notably, PSO was characterized by a dominant presence of linolenic acid (C18:3) at 63.54%, while the blend and SL displayed relatively lower levels of linolenic acid compared to PSO. Across all oil types, accelerated storage led to increased levels of SFA like C16:0, C18:0, and C20:0 by day 30, with a statistically significant increase in some of the samples. The elevated storage temperature prompted a reduction in levels of C18:1, C18:2, and C18:3 in PSO, blend, and SL regardless of the presence of antioxidants. A similar reduction in PUFA was reported in a previous study where butter was prepared from cottonseed oil and stability was assessed under an accelerated storage study.53 The incorporation of RE aided in the retention of unsaturated fatty acids during accelerated storage, such as C18:1, C18:2, and C18:3. It was observed that the effectiveness of RE in the retention of unsaturated fatty acids was superior to BHT.
Table 2. Changes in the Fatty Acid Composition and Total Tocopherol Composition in Oil Samples on 0th and 30th Day of Accelerated Storagea.
| Fatty acid composition | Storage time | PSO | PSO + RE | PSO + BHT | Blend | Blend + RE | Blend + BHT | SL | SL + RE | SL + BHT |
|---|---|---|---|---|---|---|---|---|---|---|
| Palmitic (C16:0) | D0 | 8.24 ± 0.07bB | 8.24 ± 0.07bB | 8.24 ± 0.07bB | 32.64 ± 0.07aB | 32.64 ± 0.07aB | 32.64 ± 0.07aB | 31.96 ± 0.09aB | 31.96 ± 0.09aB | 31.96 ± 0.09aB |
| D30 | 9.98 ± 0.91bA | 9.43 ± 0.32bA | 9.31 ± 0.24bA | 33.76 ± 0.07aA | 33.25 ± 0.32aA | 33.09 ± 0.43aA | 32.89 ± 0.53aA | 32.40 ± 0.97aA | 32.37 ± 0.18aA | |
| Stearic (C18:0) | D0 | 3.01 ± 0.03bB | 3.01 ± 0.03bB | 3.01 ± 0.03bB | 4.28 ± 0.11aB | 4.28 ± 0.11aA | 4.28 ± 0.11aB | 3.58 ± 0.14bB | 3.58 ± 0.14bA | 3.58 ± 0.14bA |
| D30 | 4.01 ± 0.15bA | 3.87 ± 0.23bA | 3.81 ± 0.04bA | 5.29 ± 0.08aA | 4.98 ± 0.12aA | 5.13 ± 0.09aA | 4.26 ± 0.21bA | 3.96 ± 0.32bA | 4.01 ± 0.13bA | |
| Arachidic acid(C20:0) | D0 | 0.26 ± 0.01bB | 0.26 ± 0.01bA | 0.26 ± 0.01bA | 0.35 ± 0.01aB | 0.35 ± 0.01aB | 0.35 ± 0.01aB | n.d | n.d | n.d |
| D30 | 0.42 ± 0.01bA | 0.37 ± 0.01bA | 0.38 ± 0.01bA | 0.53 ± 0.01aA | 0.48 ± 0.01aA | 0.50 ± 0.01aA | n.d | n.d | n.d | |
| Oleic (C18:1) | D0 | 9.96 ± 0.32cA | 9.96 ± 0.32cA | 9.96 ± 0.32cA | 31.73 ± 0.08bA | 31.73 ± 0.08bA | 31.73 ± 0.08bA | 34.58 ± 0.17aA | 34.58 ± 0.17aA | 34.58 ± 0.17aA |
| D30 | 9.03 ± 0.07cB | 8.84 ± 0.18cA | 8.97 ± 0.14cA | 30.74 ± 0.08bB | 31.10 ± 0.21bA | 30.98 ± 0.08bA | 34.02 ± 0.19aA | 34.27 ± 0.32aA | 34.31 ± 0.54aA | |
| Linoleic (C18:2) | D0 | 14.85 ± 0.19bA | 14.85 ± 0.19bA | 14.85 ± 0.19bA | 11.60 ± 0.11aA | 11.60 ± 0.11aA | 11.60 ± 0.11aA | 12.63 ± 0.29aA | 12.63 ± 0.29aA | 12.63 ± 0.29aA |
| D30 | 13.53 ± 0.14bB | 14.06 ± 0.31aA | 13.76 ± 0.27bB | 10.88 ± 0.18dB | 11.23 ± 0.33dB | 11.15 ± 0.43dA | 10.58 ± 0.17eB | 12.12 ± 0.32cA | 12.24 ± 0.14cA | |
| Linolenic (C18:3) | D0 | 63.54 ± 0.54aA | 63.54 ± 0.54aA | 63.54 ± 0.54aA | 18.94 ± 0.43bA | 18.94 ± 0.43bA | 18.94 ± 0.43bA | 17.21 ± 0.22Ac | 17.21 ± 0.22cA | 17.21 ± 0.22cA |
| D30 | 61.91 ± 0.29bB | 62.24 ± 0.34aA | 63.01 ± 0.32aA | 18.09 ± 0.39cA | 18.16 ± 0.19cA | 18.32 ± 0.43cA | 16.14 ± 0.46dB | 16.92 ± 0.23dA | 16.87 ± 0.21dA | |
| SFA | D0 | 11.24cB | 11.24cB | 11.24cB | 36.92aB | 36.92aB | 36.92aB | 35.54bB | 35.54bA | 35.54bA |
| D30 | 13.99cA | 13.18cA | 13.12cA | 39.04aA | 38.23aA | 38.22aA | 37.18bA | 36.36bB | 36.38bB | |
| MUFA | D0 | 10.22cA | 10.22cA | 10.22cA | 32.08aA | 32.08aA | 32.08aA | 29.85bA | 29.85bA | 29.85bA |
| D30 | 9.44cA | 9.21cB | 9.34cB | 31.27aA | 31.58aA | 31.49aA | 26.72bB | 29.04bB | 29.11bB | |
| PUFA | D0 | 78.40aA | 78.40aA | 78.40aA | 30.55bA | 30.55bA | 30.55bA | 29.85bA | 29.85bA | 29.85bA |
| D30 | 75.46aB | 76.31aB | 76.77aB | 28.97bB | 29.39bA | 29.46bA | 26.72cB | 29.04bA | 29.11bA | |
| COX value | D0 | 15.36aA | 15.36aA | 14.98aA | 5.60bA | 5.60bA | 5.60bA | 5.37bA | 5.37bA | 5.37bA |
| D30 | 14.86aA | 14.98aA | 15.36aA | 5.34bA | 5.39bA | 5.41bA | 4.92bA | 5.37bA | 5.25bA | |
| Total tocopherol | D0 | 497.44±0.76aA | 502.86 ± 0.88aA | 496.90 ± 0.65aA | 158.18 ± 0.81bA | 161.09 ± 0.88bA | 154.67 ± 0.63cA | 147.50 ± 0.54dA | 154.20 ± 0.79cA | 146.60 ± 0.39dA |
| D30 | 11.89 ± 0.13fB | 171.52 ± 0.8aB | 76.57 ± 0.48bB | 47.58 ± 0.67aB | 94.18 ± 0.49aB | 69.52 ± 0.65cB | 22.27 ± 0.41eB | 79.09 ± 0.75bB | 38.85 ± 0.31dB | |
| α-tocopherol | D0 | 35.50 ± 0.38bA | 38.30 ± 0.79aA | 35.98 ± 0.35bA | 11.91 ± 0.03fA | 15.78 ± 0.06dA | 11.28 ± 0.04fA | 13.25 ± 0.12eA | 17.08 ± 0.17cA | 13.66 ± 0.19eA |
| D30 | 0.0 | 6.95 ± 0.12aB | 2.15 ± 0.16cB | 0.00 | 6.53 ± 0.04aB | 2.26 ± 0.12cB | 0.0 | 6.57 ± 0.38aB | 3.24 ± 0.05bB | |
| γ-tocopherol | D0 | 456.87 ± 0.57aA | 458.82 ± 0.51aA | 456.12 ± 0.25aA | 143.40 ± 0.02bA | 141.36 ± 0.23bA | 140.69 ± 0.50bA | 131.44 ± 0.47Ac | 133.44 ± 0.47cA | 130.16 ± 0.06cA |
| D30 | 11.34 ± 0.06g | 162.26 ± 0.10aB | 73.15 ± 0.15cB | 47.15 ± 0.81dB | 85.59 ± 0.43bB | 66.08 ± 0.38dB | 21.93 ± 0.40fB | 71.48 ± 0.22cB | 35.05 ± 0.56eB | |
| δ-tocopherol | D0 | 4.63 ± 0.45bA | 5.74 ± 0.02aA | 4.81 ± 0.02bA | 2.87 ± 0.11dA | 3.96 ± 0.16cA | 2.70 ± 0.14dA | 2.83 ± 0.13dA | 3.68 ± 0.06cA | 2.79 ± 0.04dA |
| D30 | 0.55 ± 0.01cB | 2.30 ± 0.16aB | 1.27 ± 0.17bB | 0.43 ± 0.06cB | 2.06 ± 0.05aB | 1.19 ± 0.13bB | 0.33 ± 0.02cB | 1.04 ± 0.02bB | 0.56 ± 0.05cB |
PSO-perilla seed oil; SL, structured lipid; RE-rosemary extract; BHT-butylated hydroxytoluene. Data are presented as mean (±SD) (n = 3). (a–g) means within each column for each parameter with different superscripts are significantly (p < 0.05) different. (A–B) means within each row for each parameter with different superscripts are significantly (p < 0.05) different.
In the case of SL, by the 30th day of storage, there was an evident increase of 3.15% in SFA content. In parallel, there was an approximate 1% reduction in MUFA and a notable decrease of 12% in PUFA content. This pronounced decline in PUFA content signifies a significant degradation of the oils. These outcomes were found to be in line with prior research, which supports the observed results.49 However, the addition of RE exhibited a remarkable effect in limiting the oxidation of SL. With the addition of RE, the PUFA content reduction was limited to 2.75%, marking an approximate 10% decrease compared to the scenario without an antioxidant (Pure SL). This result underscores the scavenging activity of RE, which effectively counteracted the degradation of PUFA in the SL. In essence, the findings highlight that the introduction of RE can act as a potent antioxidant by inhibiting the propagation of free radicals or limiting the formation of free radicals, mitigating the decline in PUFA content in SL during storage.
3.5. Changes in Tocopherol Content
The tocopherol content in the oil samples on days 0 and 30 of storage is shown in Table 2. Tocopherols are monophenolic compounds that highly soluble in oil and are the most potent antioxidant that are commonly found in edible oils.54 Among the PSO, blend, and SL, PSO contains the most abundant amount of total tocopherol (497.44 mg/100 g), followed by the blend containing 158.25 mg/100 g and the SL containing 154.67 mg/100 g of total tocopherol. However, the tocopherol content in SL was lower compared to the blend due to the processing conditions (such as temperature) used during EIE processing.5,55
The rate of degradation in the tocopherol content after 30 days of storage varies in each sample. Samples without any antioxidants showed higher degradation of tocopherol compared to RE and BHT. The inclusion of RE in oils slowed the rate of deterioration of tocopherols in PSO, blend, and SL up to 30–35%. Whereas, BHT exhibited 12–15% reduction compared to the sample without any antioxidants. A parallel reduction in tocopherol content was examined in different edible oil, such as rice bran, cottonseed, and soybean oil, when enriched with RE.44
3.6. Changes in DPPH % Inhibition and Total Phenolic Content (TPC)
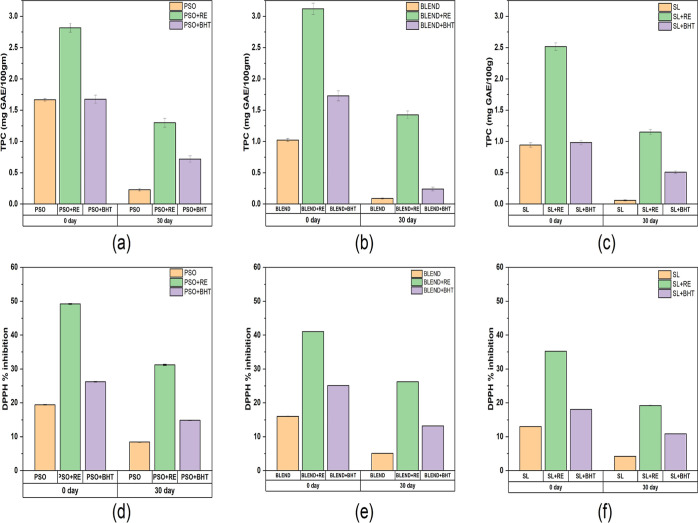
DPPH % inhibition and the TPC content of all the oil samples with and without the addition of antioxidants are depicted in Figure 3. When comparing PSO, blend, and SL, PSO demonstrated the highest TPC (1.67 mgGAE/100 g) and DPPH content (19.45%), followed by blend (1.02 mgGAE/100 gm; 16.04%) and SL (0.94 mgGAE/100 mg; 13.04%). The lower DPPH % inhibition and TPC content in the SL sample can be attributed to the specific processing conditions (temperature) employed during its preparation, which may have led to a reduction in the phenolic compounds and antioxidant properties. Despite PSO containing a substantial amount of TPC, DPPH, and tocopherol content, its OSI was comparatively low. This could be ascribed to the higher PUFA content in PSO, which is more susceptible to oxidation. Additionally, the initial peroxide value (PV) of PSO was higher, indicating that the oil may have been more prone to oxidation from the outset.
Figure 3.
Change in total phenolic content (TPC) on 0th and 30th day (a) PSO, (b) Blend, and (c) SL and changes in the DPPH % inhibition on 0th and 30th day (d) PSO, (e) Blend, and (f) SL.
At the end of the accelerated storage period (30th day), the addition of RE significantly reduced the degradation of TPC and DPPH content in the PSO, blend, and SL, hence preserving the antioxidant activity. The addition to the RE in SL resulted in a 62.6% increase in TPC content in the SL enriched with RE. Various studies reported that the addition of RE resulted in enhanced TPC and DPPH % inhibition.44 The RE also showed better antioxidant activity compared to BHT. By the 30th day, it was observed that the blend with RE exhibited superior antioxidant activity. This can be attributed to the fact that initially it had better antioxidant capacity, and further addition of RE helped in the retention of higher levels of phenolics within the blend. These compounds are capable of donating hydrogen atoms, which in turn aids in the stabilization of free radicals. This, in effect, reduces the formation of peroxides and mitigates their subsequent decomposition into volatile organic acids, ultimately leading to enhanced oxidative stability.56
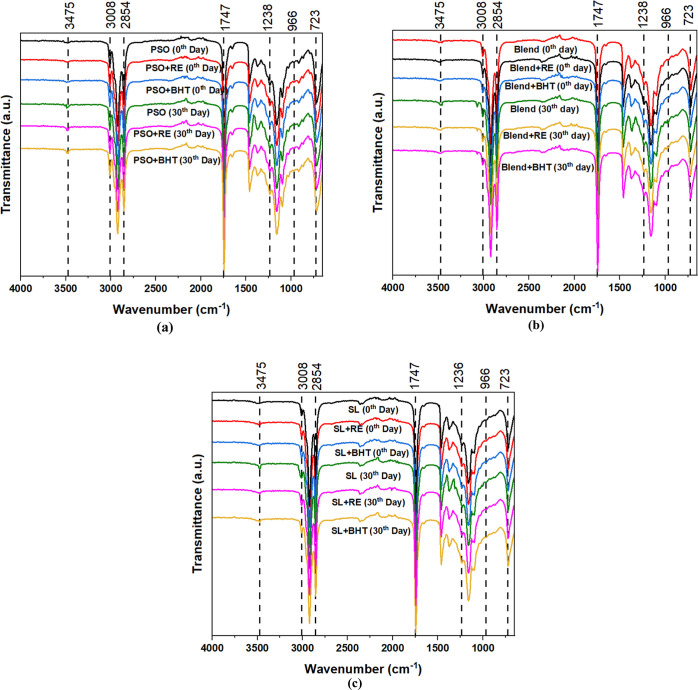
3.7. Fourier Transform Infrared Spectroscopy (FTIR)
The FTIR spectra of the oil samples taken on day 0 and day 30 exhibit a remarkable similarity, suggesting that the primary C-functional groups forming the backbone of the oil components remained stable during storage at 65 °C. This finding aligns with the previous observations in FTIR studies examining the oxidative stability of edible oil.57,58 However, subtle variations in the peak intensities are noticeable on the 30th day of accelerated storage (Figure 4). These variations are primarily because of the emergence of oxidation byproducts, such as hydroperoxides, aldehydes, ketones, and free fatty acids, which are common outcomes of the oxidative process in oil samples without any antioxidants.59 Notably, after 30 days of accelerated storage, there is a pronounced increase in peak intensities of PSO, blend, and SL (without RE and BHT) in the range of 3070–3800 cm–1, indicating the generation of hydroperoxides (–OOH) as a result of oxidation. Additionally, variations are observed in the spectral range spanning from 2800 to 3800 cm–1, which encompasses hydroperoxides and their decomposition products, constituting the primary byproducts of lipid oxidation.
Figure 4.
FTIR spectrum of oil samples at the 0th and 30th day (a) PSO, (b) Blend, and (c) SL.
Furthermore, there is an expansion in the spectral span of 930–990 cm–1, signifying the formation of double bond trans groups toward the end of the storage period. Conversely, the peak intensities at 723 cm–1 decrease, suggesting a reduction in the number of double bonds (–HC=CH−) as an effect of the oxidation process in the oil sample. In other words, the absorbance at this wavelength directly correlates with the formation of oxidation by-products originating from PUFA, including conjugated aldehydes and double-bond systems. In a detailed investigation, Wen et al. (2022) reported a consistent downward trend in peaks at 723 cm–1 of walnut oil samples during a 7 day period of storage at 60 °C.
3.8. Correlation and Principal Component Analysis (PCA)
A frequently employed visualization technique in scientific research papers is the heatmap, particularly when illustrating sample correlations using various parameters.56 We conducted a correlation heatmap analysis, and the Pearson’s correlation coefficients were employed to quantify the strength of various parameter correlations, falling within the range of −1 to 1. When these values approach 0, it signifies weak correlations, whereas values nearing −1 or 1 signify strong negative or positive correlations, respectively. The intensity of color within the correlation heatmap effectively communicates the magnitude of the correlations among various indicators. The dark red color represents the positive correlation, and the dark blue color represents the negative correlation. As a result of correlation analysis, a positive correlation between FFA, PV, p-AV, TOTOX, CD, and CT at the level of p < 0.001 can be seen. Also, DPPH/TPC, viscosity/SFA showed a positive correlation. The DPPH showed a negative correlation with FFA, PV, p-AV, and TOTOX at the level of p < 0.05. The negative correlation can be seen in the case of the MUFA/COX value and the SFA/COX value at p < 0.001. The obvious negative relationship between MUFA and PUFA is also shown in Figure 5a.
Figure 5.
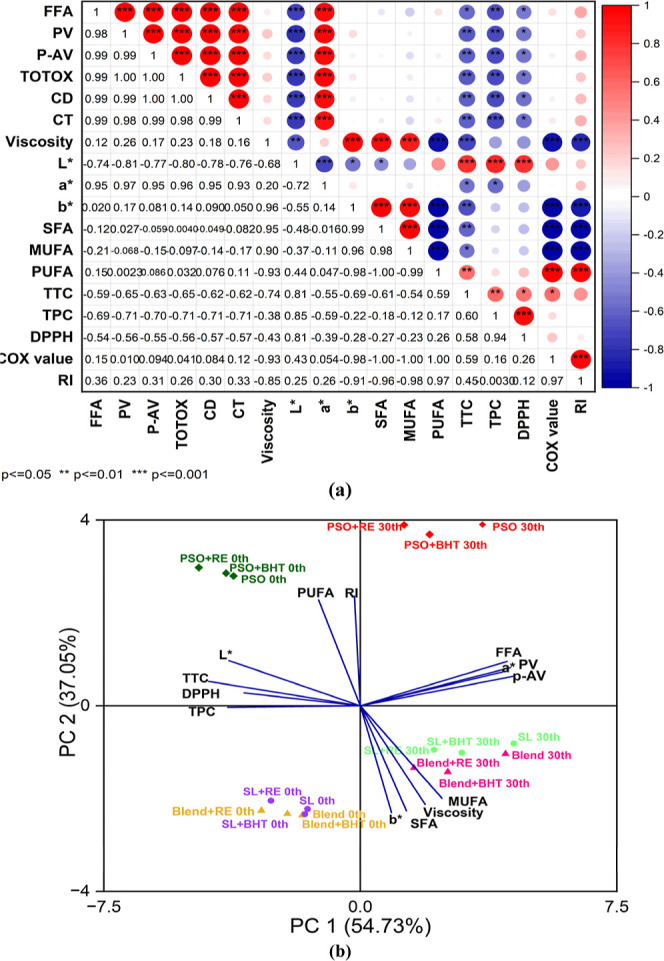
(a) Heatmap Pearson’s correlation of the different parameters of oil samples. Significant difference at p < 0.05, p < 0.01, and p < 0.001. The Red color indicates positive correlation, and the blue color indicates negative correlation, as well as the number on the lower triangular side shows the Pearson correlation coefficient. (b) Biplot of the principal component analysis (PCA) for various oil parameters in oil samples on 0th and 30th day.
The results of the PCA analysis showed that the first principal component accounted for 54.73% and the second principal component accounted for 37.05% of the variability (Figure 5b). The biplot generated from the PCA analysis provided insights into the relationships between the parameters and the principal components. FFA, PV, and p-AV displayed positive loadings on PC1, indicating that they contributed positively to the separation along this axis. In contrast, TPC had negative loadings on PC1, implying that it had an inverse relationship with the other parameters. The PCA analysis unveiled distinct clusters among the samples on the 0th and 30th days of storage. Specifically, four main clusters of samples were observed, suggesting that there were underlying factors driving the separation among these groups. A noteworthy observation was that all the samples of SL and the blend fell within the same cluster. This outcome suggests that SL exhibited comparable stability to the blend, reinforcing the notion that these two sample types share similar characteristics, possibly related to stability factors.
4. Conclusions
In conclusion, the comprehensive evaluation of various oxidative indices, including Rancimat, PV, p-AV, TOTOX, CD, and CT, has unequivocally demonstrated the robust protective effect of RE at the concentration of 1500 ppm in PSO-based SLs during accelerated storage conditions. Also, the PCA plot confirmed that SL and blend had similar oxidative indices and fell in the same quadrant. The application of RE has emerged as one of the most potent and sustainable alternatives to BHT in preserving the quality of interesterified SLs subjected to extended temperature during EIE and accelerated storage conditions. Also, the addition of RE resulted in promising natural sources of antioxidants that improved the shelf life and overall quality of SL. Furthermore, this research underscores the underutilized potential of PSO as a valuable resource for the production of omega-3 rich SL with a wider application in the food industry. By incorporating natural antioxidants like RE, the shelf life and oxidative stability of SL contribute to the preservation and restoration of naturally occurring bioactive components. In essence, the findings of this study highlight the promising role of RE in lipid preservation and endorse its use as a greener and more sustainable alternative to synthetic antioxidants like BHT. Moreover, the extended storage capability of interesterified omega-3 fatty acid-rich SL opens up exciting opportunities for innovative product development with improved thermo-oxidative stability.
Acknowledgments
First author is thankful to University Grant Commission (UGC), New Delhi, to provide fellowship for conducting this research.
Glossary
Abbreviation
- PSO
perilla seed oil
- SL
structured lipid
- TPC
total phenolic compounds
- RE
rosemary extract
- MUFA
monounsaturated fatty acids
- PUFA
polyunsaturated fatty acids
- ALA
alpha-linolenic acid
- PC
principal component
- PCA
principal component analysis
- ANOVA
analysis of variance
- BHT
butylated hydroxytoluene
- IP
induction period
- RI
refractive index
- PV
peroxide value
- p-AV
para-anisidine value
- FFA
free fatty acid
- TOTOX
total oxidation
- CD
conjugated diene
- CT
conjugated trienes
- FTIR
Fourier transform infrared
Author Contributions
Priyanka Kumari Singh: conceptualization, methodology, formal analysis, data curation, writing, funding acquisition, and writing—original draft preparation. Rajni Chopra: project administration and resources; Meenakshi Garg: Project administration, resources, and editing. Komal Chauhan—review and editing; Neha Singh: writing—review and editing and software; Snigdha Homroy: methodology, formal analysis, data curation; Aparna Aggarwal: writing—review and editing; Awdhesh Kumar Mishra: writing—review and editing; Madhu Kamle: methodology, formal analysis; Dipendra Kumar Mahato, writing—review and editing. Abhishek Dutt Tripathi—review and editing; All authors have read and agreed to the published version of the manuscript.
No funding is provided for this research article.
The authors declare no competing financial interest.
References
- Lee K.-Y.; Rahman M. S.; Kim A.-N.; Jeong E.-J.; Kim B.-G.; Lee M.-H.; Kim H.-J.; Choi S.-G. Effect of Superheated Steam Treatment on Yield, Physicochemical Properties and Volatile Profiles of Perilla Seed Oil. LWT 2021, 135, 110240. 10.1016/j.lwt.2020.110240. [DOI] [Google Scholar]
- Torri L.; Bondioli P.; Folegatti L.; Rovellini P.; Piochi M.; Morini G. Development of Perilla Seed Oil and Extra Virgin Olive Oil Blends for Nutritional, Oxidative Stability and Consumer Acceptance Improvements. Food Chem. 2019, 286 (February), 584–591. 10.1016/j.foodchem.2019.02.063. [DOI] [PubMed] [Google Scholar]
- Singh P. K.; Chopra R.; Garg M.; Dhiman A.; Dhyani A. Enzymatic Interesterification of Vegetable Oil:A Review on Physicochemical and Functional Properties, and Its Health Effects. J. Oleo Sci. 2022, 71 (12), 1697–1709. 10.5650/jos.ess22118. [DOI] [PubMed] [Google Scholar]
- Habi Mat Dian N. L. PALM OIL AND PALM KERNEL OIL: VERSATILE INGREDIENTS FOR FOOD APPLICATIONS. J. Oil Palm Res. 2018, 29 (4), 487–511. 10.21894/jopr.2017.00014. [DOI] [Google Scholar]
- Singh P. K.; Chopra R.; Dhiman A.; Chuahan K.; Garg M. Development of Omega-3-Rich Structured Lipids Using Perilla Seed Oil and Palm Olein: Optimization and Characterization. Biomass Convers. Biorefin. 2023, 10.1007/s13399-023-04422-3. [DOI] [Google Scholar]
- Berry S. E. E. E. Triacylglycerol Structure and Interesterification of Palmitic and Stearic Acid-Rich Fats: An Overview and Implications for Cardiovascular Disease. Nutr. Res. Rev. 2009, 22 (1), 3–17. 10.1017/S0954422409369267. [DOI] [PubMed] [Google Scholar]
- Huang Z.; Guo Z.; Xie D.; Cao Z.; Chen L.; Wang H.; Jiang L.; Shen Q. Rhizomucor Miehei Lipase-Catalysed Synthesis of Cocoa Butter Equivalent from Palm Mid-Fraction and Stearic Acid: Characteristics and Feasibility as Cocoa Butter Alternative. Food Chem. 2021, 343 (July 2020), 128407. 10.1016/j.foodchem.2020.128407. [DOI] [PubMed] [Google Scholar]
- Innis S. M. Dietary Triacylglycerol Structure and Its Role in Infant Nutrition. Adv. Nutr. 2011, 2 (3), 275–283. 10.3945/an.111.000448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traul K.; Driedger A.; Ingle D.; Nakhasi D. Review of the Toxicologic Properties of Medium-Chain Triglycerides. Food Chem. Toxicol. 2000, 38 (1), 79–98. 10.1016/S0278-6915(99)00106-4. [DOI] [PubMed] [Google Scholar]
- Pang M.; Ge Y.; Cao L.; Cheng J.; Jiang S. Physicochemical Properties, Crystallization Behavior and Oxidative Stabilities of Enzymatic Interesterified Fats of Beef Tallow, Palm Stearin and Camellia Oil Blends. J. Oleo Sci. 2019, 68 (2), 131–139. 10.5650/jos.ess18201. [DOI] [PubMed] [Google Scholar]
- Brzezińska R.; Bryś J.; Giers O.; Bryś A.; Górska A.; Ostrowska-Ligęza E.; Wirkowska-Wojdyła M. Quality Evaluation of Plant Oil Blends Interesterified by Using Immobilized Rhizomucor Miehei Lipase. Appl. Sci. 2022, 12 (21), 11148. 10.3390/app122111148. [DOI] [Google Scholar]
- Lourenço S. C.; Moldão-Martins M.; Alves V. D. Antioxidants of Natural Plant Origins: From Sources to Food Industry Applications. Molecules 2019, 24 (22), 4132. 10.3390/molecules24224132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couto R. O.; Conceição E. C.; Chaul L. T.; Oliveira E. M. S.; Martins F. S.; Bara M. T. F.; Rezende K. R.; Alves S. F.; Paula J. R. Spray-Dried Rosemary Extracts: Physicochemical and Antioxidant Properties. Food Chem. 2012, 131 (1), 99–105. 10.1016/j.foodchem.2011.08.036. [DOI] [Google Scholar]
- Quintana S. E.; Villanueva-Bermejo D.; Reglero G.; García-Risco M. R.; Fornari T. Supercritical Antisolvent Particle Precipitation and Fractionation of Rosemary (Rosmarinus Officinalis L.) Extracts. J. CO2 Util. 2019, 34, 479–489. 10.1016/j.jcou.2019.07.032. [DOI] [Google Scholar]
- Guo Q.; Gao S.; Sun Y.; Gao Y.; Wang X.; Zhang Z. Antioxidant Efficacy of Rosemary Ethanol Extract in Palm Oil during Frying and Accelerated Storage. Ind. Crops Prod. 2016, 94, 82–88. 10.1016/j.indcrop.2016.08.032. [DOI] [Google Scholar]
- Tohma S.; Turan S. Rosemary Plant (Rosmarinus Officinalis L.), Solvent Extract and Essential Oil Can Be Used to Extend the Usage Life of Hazelnut Oil during Deep Frying. Eur. J. Lipid Sci. Technol. 2015, 117 (12), 1978–1990. 10.1002/ejlt.201400382. [DOI] [Google Scholar]
- Bañares C.; Martin D.; Reglero G.; Torres C. F. C. F. Protective Effect of Hydroxytyrosol and Rosemary Extract in a Comparative Study of the Oxidative Stability of Echium Oil. Food Chem. 2019, 290 (March), 316–323. 10.1016/j.foodchem.2019.03.141. [DOI] [PubMed] [Google Scholar]
- Tohma S.; Günal-Köroğlu D.; Turan S.; Ramadan M. F. Efficacy of Rosemary (Rosmarinus Officinalis L.) Powder and Extracts in the Protection of Refined and Stripped Hazelnut Oil. Rend. Lincei 2021, 32 (3), 585–598. 10.1007/s12210-021-01002-3. [DOI] [Google Scholar]
- Moczkowska M.; Karp S.; Horbanczuk O. K.; Hanula M.; Wyrwisz J.; Kurek M. A. EFFECT OF ROSEMARY EXTRACT ADDITION ON OXIDATIVE STABILITY AND QUALITY OF HEMP SEED OIL. Food Bioprod. Process. 2020, 124, 33–47. 10.1016/j.fbp.2020.08.002. [DOI] [Google Scholar]
- Mazaheri Y.; Torbati M.; Azadmard-Damirchi S.; Savage G. P. Oil Extraction from Blends of Sunflower and Black Cumin Seeds by Cold Press and Evaluation of Its Physicochemical Properties. J. Food Process. Preserv. 2019, 43 (10), e14154 10.1111/jfpp.14154. [DOI] [Google Scholar]
- Singh P. K.; Chopra R.; Garg M. Comparative Study on the Use of Rosemary Bioactive for Enhancing the Oxidative Stability of Blended Perilla Seed Oil: A Multivariant Kinetic Approach. Food Chem. Adv. 2023, 3, 100447. 10.1016/j.focha.2023.100447. [DOI] [Google Scholar]
- Zou X.-G.; Hu J.-N.; Zhao M.-L.; Zhu X.-M.; Li H.-Y.; Liu X.-R.; Liu R.; Deng Z.-Y. Lipozyme RM IM-Catalyzed Acidolysis of Cinnamomum Camphora Seed Oil with Oleic Acid To Produce Human Milk Fat Substitutes Enriched in Medium-Chain Fatty Acids. J. Agric. Food Chem. 2014, 62 (43), 10594–10603. 10.1021/jf503691p. [DOI] [PubMed] [Google Scholar]
- AOCS . Official Methods and Recommended Practices of the American Oil Chemists’ Society, 7th edn.; AOCS Press: Champaign, IL, 2017. [Google Scholar]
- ISO 3656 . Animal and Vegetable Fats and Oils—Determination of Ultraviolet Absorbance Expressed as Specific UV Extinction. 2011, https://www.iso.org/obp/ui/#iso:std:iso:3656:ed-4:v1:en (accessed September 27, 2023).
- AOCS . AOCS Official Method Ce 2–66: Preparation of Methyl Esters of Fatty Acids, 1997. [Google Scholar]
- Cao J.; Li H.; Xia X.; Zou X.-G.; Li J.; Zhu X.-M.; Deng Z.-Y. Effect of Fatty Acid and Tocopherol on Oxidative Stability of Vegetable Oils with Limited Air. Int. J. Food Prop. 2015, 18 (4), 808–820. 10.1080/10942912.2013.864674. [DOI] [Google Scholar]
- ISO . International Standard International Standard—ISO 2062; ISO, 2012; Vol. 2012, p 16. [Google Scholar]
- Manzoor S.; Masoodi F. A.; Akhtar G.; Rashid R. Production of Trans-Free Shortening by Lipase Catalysed Interesterification Using Mustard Oil and Palm Stearin: Optimisation and Characterisation. Biomass Convers. Biorefin. 2022, 10.1007/s13399-022-03315-1. [DOI] [Google Scholar]
- Pan F.; Wen B.; Luo X.; Wang C.; Wang X.; Guan X.; Xu Y.; Dang W.; Zhang M. Influence of Refining Processes on the Bioactive Composition, in Vitro Antioxidant Capacity, and Their Correlation of Perilla Seed Oil. J. Food Sci. 2020, 85 (4), 1160–1166. 10.1111/1750-3841.15070. [DOI] [PubMed] [Google Scholar]
- Durmaz G.; Gökmen V. Effect of Refining on Bioactive Composition and Oxidative Stability of Hazelnut Oil. Food Res. Int. 2019, 116, 586–591. 10.1016/j.foodres.2018.08.077. [DOI] [PubMed] [Google Scholar]
- Singleton V. L.; Orthofer R.; Lamuela-Raventós R. M. [14] Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. 10.1016/S0076-6879(99)99017-1. [DOI] [Google Scholar]
- Singh P. K.; Chopra R.; Garg M.; Chauhan K.; Agarwal A. Stability of Perilla Seed Oil Based PUFA-Rich Structured Lipids Using Enzymatic Interesterification: A Thermo-Oxidative Kinetic Study. Ind. Crops Prod. 2024, 209, 118029. 10.1016/j.indcrop.2024.118029. [DOI] [Google Scholar]
- Chandra P.; Enespa; Singh R.; Arora P. K. Microbial Lipases and Their Industrial Applications: A Comprehensive Review. Microb. Cell Factories 2020, 19 (1), 169. 10.1186/s12934-020-01428-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turan S. Effects of Some Plant Extracts on the Oxidative Stability of Canola Oil and Its Purified Triacylglycerols. J. Food Qual. 2014, 37 (4), 247–258. 10.1111/jfq.12086. [DOI] [Google Scholar]
- Neves M.; Miranda A.; Lemos M. F. L.; Silva S.; Tecelão C. Enhancing Oxidative Stability of Sunflower Oil by Supplementation with Prickled Broom (Pterospartum Tridentatum) Ethanolic Extract. J. Food Sci. 2020, 85 (9), 2812–2821. 10.1111/1750-3841.15378. [DOI] [PubMed] [Google Scholar]
- Naik M.; Natarajan V.; Thangaraju S.; Modupalli N.; Rawson A. Assessment of Storage Stability and Quality Characteristics of Thermo-sonication Assisted Blended Bitter Gourd Seed Oil and Sunflower Oil. J. Food Process. Eng. 2023, 46 (6), e14070 10.1111/jfpe.14070. [DOI] [Google Scholar]
- Mansour H. M. M.; El-Sohaimy S. A.; Zeitoun A. M.; Abdo E. M. Effect of Natural Antioxidants from Fruit Leaves on the Oxidative Stability of Soybean Oil during Accelerated Storage. Antioxidants 2022, 11 (9), 1691. 10.3390/antiox11091691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos J. C. O.; Santos I. M. G.; Souza A. G. Effect of Heating and Cooling on Rheological Parameters of Edible Vegetable Oils. J. Food Eng. 2005, 67 (4), 401–405. 10.1016/j.jfoodeng.2004.05.007. [DOI] [Google Scholar]
- Jaswir I.; Man Y. B. C.; Kitts D. D. Synergistic Effects of Rosemary, Sage, and Citric Acid on Fatty Acid Retention of Palm Olein during Deep-Fat Frying. J. Am. Oil Chem. Soc. 2000, 77 (5), 527–533. 10.1007/s11746-000-0084-7. [DOI] [Google Scholar]
- Shahidi F.; Zhong Y.. Lipid Oxidation: Measurement Methods. Bailey’s Industrial Oil and Fat Products; Wiley, 2005. [Google Scholar]
- FAO . Codex Alimentarius—Joint FAO/WHO Food Standards Programme, 2013; Vol. 21. [Google Scholar]
- Mihaylova D.; Gandova V.; Deseva I.; Tschuikowa S.; Schalow S.; Westphal G. Arrhenius Equation Modeling for the Oxidative Stability Evaluation of Echium Oil Enriched with a Natural Preservative. Eur. J. Lipid Sci. Technol. 2020, 122 (11), 2000118. 10.1002/ejlt.202000118. [DOI] [Google Scholar]
- Hamama A. A.; Nawar W. W. Thermal Decomposition of Some Phenolic Antioxidants. J. Agric. Food Chem. 1991, 39 (6), 1063–1069. 10.1021/jf00006a012. [DOI] [Google Scholar]
- Yang Y.; Song X.; Sui X.; Qi B.; Wang Z.; Li Y.; Jiang L. Rosemary Extract Can Be Used as a Synthetic Antioxidant to Improve Vegetable Oil Oxidative Stability. Ind. Crops Prod. 2016, 80, 141–147. 10.1016/j.indcrop.2015.11.044. [DOI] [Google Scholar]
- Sharma S.; Chakkaravarthi S.; Bhattacharya B. Enhancement of Oxidative Stability of Soybean Oil via Nano-Emulsification of Eggplant Peel Extract: Process Development and Application. Food Chem. 2023, 402, 134249. 10.1016/j.foodchem.2022.134249. [DOI] [PubMed] [Google Scholar]
- Wang Y. Z.; Fu S.-G. G.; Wang S.-Y. Y.; Yang D.-J. J.; Wu Y.-H. S. H. S.; Chen Y.-C. C. Effects of a Natural Antioxidant, Polyphenol-Rich Rosemary (Rosmarinus Officinalis L.) Extract, on Lipid Stability of Plant-Derived Omega-3 Fatty-Acid Rich Oil. LWT 2018, 89 (November 2017), 210–216. 10.1016/j.lwt.2017.10.055. [DOI] [Google Scholar]
- Talbot G.The Stability and Shelf Life of Fats and Oils. The Stability and Shelf Life of Food; Elsevier, 2016; pp 461–503. [Google Scholar]
- Sun-Waterhouse D.; Zhou J.; Miskelly G. M.; Wibisono R.; Wadhwa S. S. Stability of Encapsulated Olive Oil in the Presence of Caffeic Acid. Food Chem. 2011, 126 (3), 1049–1056. 10.1016/j.foodchem.2010.11.124. [DOI] [Google Scholar]
- Wang Y.; Fu S. G.; Wang S. Y.; Yang D. J.; Wu Y. H. S.; Chen Y. C. Effects of a Natural Antioxidant, Polyphenol-Rich Rosemary (Rosmarinus Officinalis L.) Extract, on Lipid Stability of Plant-Derived Omega-3 Fatty-Acid Rich Oil. Lwt 2018, 89 (November 2017), 210–216. 10.1016/j.lwt.2017.10.055. [DOI] [Google Scholar]
- Umeda W. M.; Jorge N. Oxidative Stability of Soybean Oil Added of Purple Onion (Allium Cepa L.) Peel Extract during Accelerated Storage Conditions. Food Control 2021, 127, 108130. 10.1016/j.foodcont.2021.108130. [DOI] [Google Scholar]
- Yu K. S.; Cho H.; Hwang K. T. Physicochemical Properties and Oxidative Stability of Frying Oils during Repeated Frying of Potato Chips. Food Sci. Biotechnol. 2018, 27 (3), 651–659. 10.1007/s10068-017-0292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbančič S.; Kolar M. H.; Dimitrijević D.; Demšar L.; Vidrih R. Stabilisation of Sunflower Oil and Reduction of Acrylamide Formation of Potato with Rosemary Extract during Deep-Fat Frying. LWT--Food Sci. Technol. 2014, 57 (2), 671–678. 10.1016/j.lwt.2013.11.002. [DOI] [Google Scholar]
- He Z.; Nam S.; Klasson K. T. Oxidative Stability of Cottonseed Butter Products under Accelerated Storage Conditions. Molecules 2023, 28 (4), 1599. 10.3390/molecules28041599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golmakani M. T.; Mansouri Z.; Ansari S.; Alavi N. Improving Oxidative Stability of Pomegranate Seed Oil Using Oliveria Decumbens Essential Oil. J. Food Process. Preserv. 2021, 45 (6), 1–8. 10.1111/jfpp.15483. [DOI] [Google Scholar]
- Zhang Z.; Ye J.; Lee W. J.; Akoh C. C.; Li A.; Wang Y. Modification of Palm-Based Oil Blend via Interesterification: Physicochemical Properties, Crystallization Behaviors and Oxidative Stabilities. Food Chem. 2021, 347, 129070. 10.1016/j.foodchem.2021.129070. [DOI] [PubMed] [Google Scholar]
- Boungo Teboukeu G.; Tonfack Djikeng F.; Klang M. J.; Houketchang Ndomou S.; Karuna M. S. L.; Womeni H. M. Polyphenol Antioxidants from Cocoa Pods: Extraction Optimization, Effect of the Optimized Extract, and Storage Time on the Stability of Palm Olein during Thermoxidation. J. Food Process. Preserv. 2018, 42 (5), e13592 10.1111/jfpp.13592. [DOI] [Google Scholar]
- Guillen M. D.; Cabo N. Some of the Most Significant Changes in the Fourier Transform Infrared Spectra of Edible Oils under Oxidative Conditions. J. Sci. Food Agric. 2000, 80 (14), 2028–2036. . [DOI] [Google Scholar]
- Wen Y.; Zhou S.; Wang L.; Li Q.; Gao Y.; Yu X. New Method for the Determination of the Induction Period of Walnut Oil by Fourier Transform Infrared Spectroscopy. Food Anal. Methods 2022, 15 (3), 833–843. 10.1007/s12161-021-02170-6. [DOI] [Google Scholar]
- Poiana M.-A.; Moigradean D.; Dumbrava D.-G.; Radulov I.; Raba D. N.; Rivis A. Exploring the Potential of Grape Pomace Extract to Inhibit Thermo-Oxidative Degradation of Sunflower Oil: From Routine Tests to ATR-FTIR Spectroscopy. Foods 2022, 11 (22), 3674. 10.3390/foods11223674. [DOI] [PMC free article] [PubMed] [Google Scholar]