Abstract
Introduction
Magnesium sulfate is the most utilized anticonvulsant for treating patients with eclampsia and pre-eclampsia. The purpose of this study is to determine whether the 12-h regimen of magnesium sulfate outweighs the 24-h regimen in both efficacy and safety in the management of patients with mild or severe pre-eclampsia and eclampsia.
Methods
We searched six electronic databases: PubMed, Scopus, Web of Science, Cochrane Library, Ovid, and Google Scholar. This search was conducted to yield any studies that were published until 15 January 2023. We did the statistical analysis plan by Review Manager Software version 5.4.
Results
We included 13 randomized control trials with 2813 patients in this systematic review. Our meta-analysis revealed that there were no statistically significant differences between the 12-h regimen of the magnesium sulfate group and the 24-h regimen of the magnesium sulfate group in our outcome of interest: occurrence of seizure (RD: -0.00, 95% CI [-0.01, 0.00], P = 0.56), diminished deep tendon reflexes (RD: -0.00, 95% CI [-0.01, 0.01], P = 0.80), respiratory depression (RD: -0.00, 95% CI [-0.02, 0.01], P = 0.57), and pulmonary edema (RD: -0.00, 95% CI [-0.01, 0.01], P = 0.85).
Conclusion
Our study showed no statistically significant difference in effectiveness and toxicity risk between the 12-h and 24-h regimens.
Keywords: Magnesium sulfate, Eclampsia, Pre-eclampsia, Seizure, Pritchard, Zuspan
Introduction
Pregnancy may be associated with a group of diseases, such as hypertensive disorders of pregnancy, including pre-eclampsia and eclampsia. Both serious medical conditions affect 10% of pregnancies [1]. Pre-eclampsia and eclampsia put maternal health at risk by increasing maternal morbidity and mortality [2, 3].
Pre-eclampsia is considered a multisystem disease that targets many organs [2]. It is characterized by a hypertensive condition that newly affects women with previously normal blood pressure after twenty weeks of pregnancy [4]. It is caused by cell dysfunction and altered trophoblastic invasion [5].
Pre-eclampsia is a medical condition diagnosed when a pregnant woman’s systolic blood pressure reaches 140 mmHg or higher, or her diastolic blood pressure reaches 90 mmHg or higher, along with proteinuria, which is characterized by a urine protein: creatinine ratio of 30 mg/mmol or more, or albumin: creatinine ratio of 8 mg/mmol or more, or at least 1 g/liter [2 +] on dipstick testing. Renal insufficiency may manifest by a creatinine level of 90 micromole/liter or higher, or a serum creatinine level of 1.02 mg/100 ml or higher. Additionally, liver affection is indicated by elevated transaminases with alanine aminotransferase or aspartate aminotransferase levels above 40 IU/L. Liver involvement may present with right upper quadrant or epigastric abdominal pain. Thrombocytopenia (platelet count < 150,000/µL), disseminated intravascular coagulation or hemolysis may manifest as hematological complications. Furthermore, uteroplacental dysfunction may complicate the pre-eclampsia and manifest by fetal growth restriction, abnormal umbilical artery doppler waveform analysis, or stillbirth [6].
On the other hand, severe pre-eclampsia is a medical condition characterized by persistent and severe hypertension in a patient who does not respond to treatment, as well as ongoing or recurring symptoms such as severe headaches, visual disturbances, nausea or vomiting, epigastric pain, oliguria, and worsening laboratory blood tests, including high levels of creatinine, liver transaminases, decreased platelet count, decreased fetal growth, or abnormal doppler findings [6]. It is crucial to consider it a serious complication, as it correlates with a high risk of maternal death before, during, or after delivery [7].
Although eclampsia in Greece is defined as “a flash of lightning,” which may not match its seriousness, it is defined medically as convulsions related to pre-eclampsia without any obvious neurological as a concurrent cause [8]. Regarding pre-eclampsia and eclampsia, their ideal treatment is primarily to maintain the patient stable, control hypertension, prevent or treat seizures, and arrange for delivery, which is considered the definite cure [9, 10].
Among numerous anticonvulsants, magnesium sulphate (MgSO4) has been used in clinical practice since 1925. For decades, it has still been the most utilized anticonvulsant for controlling patients with eclampsia and pre-eclampsia [1, 2].
Despite this wide use of MgSO4, its exact mechanism is still unclear. However, the most accepted theories suggest that it interferes with calcium homeostasis and controls fits by both neurological and cardiovascular effects [11]. According to the use of MgSO4 in both preventing and treating fits, it is recommended to be administered for 24 h after the latest event, whichever delivery or the last fit [3]. Both Pritchard and Zuspan are considered the standard methods for administering MgSO4. The Zuspan regimen consists of a loading dosage of 4 g of MgSO4 administered IV slowly over five to ten minutes, followed by a maintenance dose of 2 g per hour IV infusion administered for 24 h. The Pritchard regimen consists of a loading dosage of 4 g of MgSO4 given IV slowly over five to ten minutes added to concurrent 10 g IM and a maintenance dose of 5 g of MgSO4 IM each four hours for 24 h [4, 11, 12].
Although preventing or treating fits, MgSO4 administration can cause serious intoxication symptoms like respiratory depression or arrest, absent or minimal deep tendon reflex, and oliguria [5]. Although these serious complications can be considered rare compared to other common, less significant side effects such as flushing, muscle weakness, headache, nausea, vomiting, thirst, confusion, and dizziness, considering these serious intoxication symptoms is still crucial [2]. Based on MgSO4 toxicity, the administration of MgSO4 demands regular checking of patients by experienced doctors, which is cost-consuming, so reducing the duration of MgSO4 administration is expected to be beneficial not only for patients’ health but also for healthcare systems [2, 3].
Although strong evidence indicates magnesium sulfate’s effectiveness in both the prophylaxis and treatment of seizures in pre-eclampsia and eclampsia patients, the ideal duration of MgSo4 administration is not yet known. Magnesium sulfate is typically administered for 24 h following delivery in cases of pre-eclampsia. However, more recent research has suggested the potential benefits of shorter duration postpartum MgSo4 therapy [10]. Many studies have shown that the incidence of seizures is higher in patients who are receiving 24-h MgSo4 [1, 12, 13] while other studies have reported that the incidence of seizures is higher in patients receiving 12-h MgSo4 [2, 8, 14]. Therefore, we conducted this systematic review and meta-analysis to assess if 12- hour MgSo4 could be a promising alternative to 24-h MgSo4 in patients with eclampsia and pre-eclampsia.
Methods
This systematic review and meta-analysis protocol got the PROSPERO registration ID: CRD42023399554 on 25 February 2023. We conducted this study according to the PRISMA guidelines 2020 [15].
Search strategy
We searched six electronic databases: PubMed, Scopus, Web of Science, Cochrane Library, Ovid, and Google Scholar. The search was conducted to yield any studies that were published until 15 January 2023, using the search strategy (("12 h") AND ("24 h") AND ("Magnesium sulfate" OR "Heptahydrate Magnesium Sulfate" OR "Epsom Salt" OR "Sulfamag" OR "English salt" OR "Bitter salts" OR "Bath salt" OR "Concept Ob" OR "Suprep Bowel Prep Kit" OR "Tis- U-sol") AND ("Eclampsia" OR ("pre-eclampsia")) and without performing any filter. We manually searched the reference lists of included studies to include other relevant studies that our strategy could not reach.
On 19 September 2023, we updated our search manually and included a new randomized controlled clinical trial.
Eligibility criteria
The population of interest was pre-eclampsia or eclampsia patients who received no intervention other than a loading dose of MgSO4, even if the cases was diagnosed in their antepartum, intrapartum, or postpartum period. We included eclampsia females who had complications such as HELLP syndrome (a condition in pregnant and postpartum individuals characterized by hemolysis with a microangiopathic blood smear, elevated liver enzymes, and a low platelet count), acute renal failure, gestational diabetes, and MgSO4 hypersensitivity. We included only the randomized controlled clinical trials which compare between 12 h versus 24 h using MgSo4 in managing patients with eclampsia and pre-eclampsia using Pritchard or Zuspan regimen and written in English. Outcomes of interest were the occurrence of seizures, diminished deep tendon reflexes, respiratory depression, and pulmonary edema. Non-original research (i.e., reviews, comments, guidelines, editorials, correspondence, letters to editors, etc.) and any studies that have not compared the effectiveness of 12-h versus 24-h using MgSO4 in treatment of patients with eclampsia or pre-eclampsia were excluded. Additionally, studies in a language other than English were excluded. Patients who experienced recurrent attacks of seizures when already on a MgSO4 regimen, or who had pre-existing DM, renal disease, anuria/oliguria (urine output < 25 ml/h), and patients who had any contraindication to MgSO4 use except drug hypersensitivity (e.g., drug myasthenia gravis) or who experienced coma were excluded.
Study selection
After searching the databases, all retrieved records were imported into EndNote X9 software, and the duplicate studies were identified and deleted. Two authors conducted the two screening phases separately via Microsoft Excel. Title and abstract screening, then, the full text of eligible articles screening. Any conflict was resolved by a third researcher opinion and discussion for final accessibility to meta-analysis.
Data extraction
Data extraction was conducted using Microsoft Excel manually by two independent authors and then reviewed by a third author. If there is any disagreement, a meeting between reviewers could be held to resolve it.
Data extracted from the included study were in three groups: 1—General study characteristics (study ID, country at which the study was performed, and sample size). 2—Baseline patient characteristics (age, gestational age, SBP, DBP, and mode of delivery). 3—Outcome measures (seizure occurrence, respiratory depression, diminished deep tendon reflex, and pulmonary edema).
Quality assessment of included studies
Quality assessment was performed using the Cochrane risk of bias assessment tool by two independent reviewers [16]. Seven domains were studied: sequence generation, allocation sequence concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting, and other risks of bias, in which judgments (high risk, low risk, or unclear risk) for each item in each trial are presented alongside their descriptive justification. The risk of bias was determined across all outcomes within each included study. A meeting between reviewers could be held in case of any disagreement. Potential publication bias was visually assessed using funnel plots.
Data synthesis
We conducted the statistical analysis using the Review Manager (RevMan) software program V.5.4. Results with a P-value < 0.05 were regarded as significant in the Z-test, and the meta-analysis result for dichotomous outcomes was shown as a risk difference with a 95% confidence interval and conducted by using the fixed-effect model. To assess heterogeneity, we employed the Chi-square and I-square tests, as the Chi-square test assesses the presence of heterogeneity, and the I-square test assesses its degree. The result of the I-square test was interpreted as follows: not significant for 0–40 percent, moderate heterogeneity for 30–60 percent, substantial heterogeneity for 50–90 percent, and considerable heterogeneity for 75–100 percent, following the Cochrane handbook (chapter nine). According to the Chi-square test, we considered an alpha level below 0.1 as a significant heterogeneity.
Results
Study selection
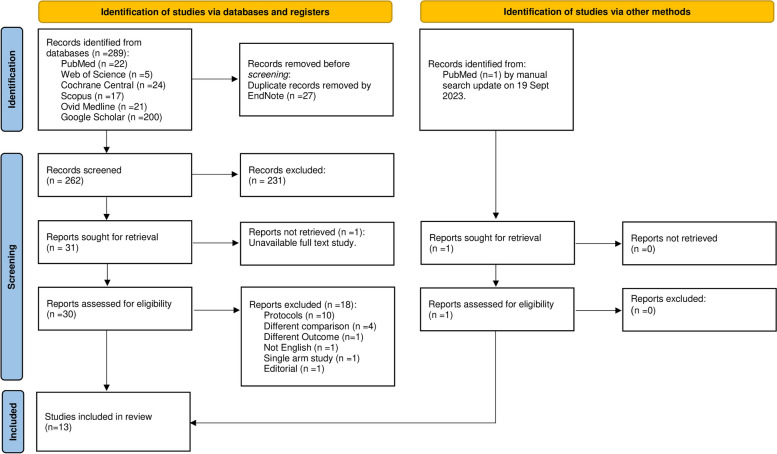
By searching the databases, we reached 289 records. After the removal of 27 duplicates by endnote software, the titles and abstracts of 262 studies were screened. So, the full text of 30 studies was screened according to the eligibility criteria. We excluded 18 studies as they did not meet the eligibility criteria. Finally, 13 studies were included in our systematic review as shown in Fig. 1.
Fig. 1.
PRISMA flow diagram
Study characteristics
The summary of the included studies is presented in Table 1. Two studies have been conducted in Egypt [2, 14], three in Nigeria [4, 12, 13], two in India [3, 9], two in Pakistan [11, 17], one in Iran [8], one in Brazil [7], one in Ghana [1], and one in the USA [10]. The current study pools data of 1435 women treated using 12-h MgSO4 and 1374 women treated using 24-h MgSO4. All included studies were published between 2006 and 2023. In the included studies, one study included patients diagnosed with mild pre-eclampsia [10], seven studies included patients diagnosed with severe pre-eclampsia [2, 4, 7–9, 12, 14], two studies reported the treatment of both patients with eclampsia and pre-eclampsia [1, 13], Three studies included patients with eclampsia [3, 11, 17]. According to our included studies, four studies [1, 8, 9, 12] used the Pritchard regimen, while six studies [3, 4, 10, 11, 13, 17] used the Zuspan regimen. The baseline characteristics of the pre-eclampsia and eclampsia patients are shown in Table 2.
Table 1.
Summary of the included studies
| Study ID | Country | 12 h MgSo4 | 24 h MgSo4 | ||||
|---|---|---|---|---|---|---|---|
| Pre-Eclampsia | Sample size | Loading dose | Maintenance dose | Sample size | Loading dose | Maintenance dose | |
| Grillo et al. 2023 [13] | Nigeria | 74 | 4 g on 20% in normal saline IV slowly over 20 min | 1 g/h IV for 12 h | 74 | 4 g on 20% in normal saline IV slowly over 20 min | 1 g/h IV for 24 h |
| Hekal et al. 2020 [2] | Egypt | 80 | 6 g on 250 ml ringer solutions over 20 min by IV drip | 4 g on 250 ml ringer solution over 4 h/4 h by IV drip for 12 h | 80 | 6 g on 250 ml ringer solutions over 20 min by IV drip | 4 g on 250 ml ringer solution over 4 h/4 h by IV drip for 24 h |
| Orisabinone et al. 2020 [12] | Nigeria | 58 | 4 g slow IV bolus + 10 g IM (5g in each buttock) | 5 g IM/ 4 h in alternate buttocks for 12 h | 58 | 4 g slow IV bolus, plus 10 g IM (5g in each buttock) | 5 g IM/4 h in alternate buttocks for 24 h |
| Dixit et al. 2020 [9] | India | 33 | 4 g IV + 10 g IM | 5g /4 h of a 50% in normal saline IM alternate buttocks for 12 h | 34 | 4g IV + 10 g IM | 5g /4 h of a 50% in normal saline IM alternate buttocks for 24 h |
| Unwaha et al. 2020 [4] | Nigeria | 40 | 4 g on 20% in normal saline IV slowly over 10 min | 5 g in 500 mL of normal saline IV 1 g/h for 12 h | 40 | 4 g on 20% in normal saline IV slowly over 10 min | 5 g in 500 mL of normal saline IV 1 g/h, for 24 h |
| Kashanian et al. 2015 [8] | Iran | 79 | 4 g slowly (1 g/m) + 10 g IM | 5 g IM/4 h for 12 h | 91 | 4 g slowly (1 g/m) + 10 g IM | 5 g IM/4 h for 24 h |
| El-Khaya et al. 2014 [14] | Egypt | 80 | 6 g on 250 ml ringer solutions by IV drip over 20 min | 4 g on 250 ml ringer solution IV drip 1 g/h for 12 h | 80 | 6 g on 250 ml ringer solutions by IV drip over 20 min | 4 g on 250 ml ringer solution IV drip 1 g/h for 24 h |
| Maia et al. 2014 [7] | Brazil | 56 | 6 g | 1 g/h IV for 12 h | 56 | 6 g | 1 g/h IV for 24 h |
| Ehrenberg et al. 2006 [10] | USA | 101 | 4 g IV before delivery or within 2 h postpartum | 2 g/h IV for 12 h | 95 | 4 g IV before delivery or within 2 h postpartum | 2 g/h IV for 24 h |
| Eclampsia | |||||||
| Beyuo et al. 2022 [1] | Ghana | 592 | 4 g IV and 10g IM (5g.in each buttock) | 5 g IM/4 h for 12 h | 584 | 4 g IV and 10g IM (5g.in each buttock) | 5 g IM/4 h for 24 h |
| Khan et al. 2021 [17] | Pakistan | 50 | 4 g IV in 10 min | 1 g/h IV for 12 h | 50 | 4 g IV in 10 min | 1 g/h IV for 24 h |
| Anjum et al. 2015 [3] | India | 132 | 4 g IV | 1 g/h for 12 h after the last fit or delivery | 76 | 4 g IV | 1 g/h for 24 h after the last fit or delivery |
| Rao et al. 2015 [11] | Pakistan | 60 | 4 g on 20% in normal saline IV slowly over 15 min | 1 g (20% in normal saline)/h IV for 12 h | 60 | 4 g (20% in normal saline) IV slowly in 15 min | 1 g (20% in normal saline)/h IV for 24 h |
IV Intravenous infusion, IM Intramuscular injection, min minute, h hour, g gram
Table 2.
Baseline characteristics of the included studies
| Study ID | MgSo4 | Age, y M (SD) |
Gestational age, W M (SD) |
SBP, mmHg M (SD) |
DBP, mmHg M (SD) |
Mode Of Delivery | |||
|---|---|---|---|---|---|---|---|---|---|
| Pre-Eclampsia | No. Vaginal Delivery | No. Cesarean Section | |||||||
| Grillo et al. 2023a [13] | 12 h | 29 (8) | 38 (3) | 170 (30) | 110 (20) | 15 | 59 | ||
| 24 h | 30 (10) | 37 (5) | 170 (23) | 110 (30) | 53 | 21 | |||
| Hekal et al. 2020 [2] | 12 h | 26.6 (5) | 38.2 (2) | 162.5 (14.9) | 105.1 (10.3) | 37 | 43 | ||
| 24 h | 26.6 (5.2) | 38.2 (1.8) | 161.1 (16.9) | 100.3 (13.9) | 35 | 45 | |||
| Orisabinone et al. 2020 [12] | 12 h | 26.7(5.9) | NA | 178.1 (19.3) | 105.1 (23) | 27 | 31 | ||
| 24 h | 27.7(6.7) | NA | 180 (23.3) | 98.4 (32.3) | 26 | 32 | |||
| Dixit et al. 2020 [9] | 12 h | 27.9 (4.8) | 36.7 (2.6) | 166.2 (13.3) | 104.7 (9.5) | 16 | 16 | ||
| 24 h | 27.3 (5.2) | 36.6 (2.7) | 165.2 (12.4) | 104.1 (10.5) | 17 | 18 | |||
| Unwaha et al. 2020 [4] | 12 h | 34.1 (4.5) | 34.4 (4) | 174.5 (27.7) | 110.8 (21.8) | 15 | 25 | ||
| 24 h | 32.3 (6.1) | 34.1 (4.5) | 175.3 (27.7) | 110.9 (15.9) | 10 | 30 | |||
| Kashanian et al. 2015 [8] | 12 h | 28.9 (6.1) | 36.1 (1.2) | 152.2 (12.3) | 95.2 (9.4) | NA | NA | ||
| 24 h | 29.9 (6.1) | 36.2 (1.3) | 158.3 (15.4) | 95.1 (9.5) | NA | NA | |||
| El-Khaya et al. 2014 [14] | 12 h | 26.6 (5) | 35.9 (2.9) | 162.5 (14.9) | 105.1 (10.3) | 37 | 43 | ||
| 24 h | 26.6 (5.2) | 35.6 (2.7) | 161.1 (16.9) | 100.3 (13.9) | 35 | 45 | |||
| Maia et al. 2014 [7] | 12 h | 24.7 (6.3) | 36.8 (3) | 142.4 (16.4) | 92.4 (11.9) | 20 | 36 | ||
| 24 h | 26.3 (7.6) | 37.2 (4.9) | 142.1 (15.1) | 95.4 (10.8) | 23 | 33 | |||
| Ehrenberg et al. 2006 [10] | 12 h | 24.4 (6.5) | 38.7 (1.7) | 143 (13) | 82 (10) | 79 | 22 | ||
| 24 h | 25.2 (6.5) | 38.7 (1.7) | 140 (13) | 82 (11) | 70 | 25 | |||
| Eclampsia | |||||||||
| Khan et al. 2021 [17] | 12 h | ≤ 30 yb | 23 (46%) | 36.7 (1.3) | 138.2 (11.5) | 85.6 (12.4) | 15 | 35 | |
| > 30 yb | 27 (54%) | ||||||||
| 24 h | ≤ 30 yb | 22 (44%) | 36.6 (1.7) | 141.5 (11.8) | 90 (8.1) | 19 | 31 | ||
| > 30 yb | 28 (56%) | ||||||||
| Anjum et al. 2015 [3] | 12 h | 23.8 (3.4) | 35.6 (1.2) | 166.7 (18.8) | 102.2 (8.7) | NA | NA | ||
| 24 h | 24.5 (3.6) | 36.5 (1.6) | 161.6 (21.5) | 100.3 (13.9) | NA | NA | |||
| Rao et al. 2015b [11] | 12 h | 20–24 y | 33 (55%) | NA | ≤ 90 | 6 (10%) | NA | NA | NA |
| 91–100 | 7 (11.6%) | ||||||||
| 25–29 y | 14 (23.3%) | 101–110 | 13 (21.6%) | ||||||
| 30–40 y | 13 (21.6%) | ≥ 110 | 34 (56.6%) | ||||||
| 24 h | 20–24 y | 35 (58.3%) | NA | ≤ 90 | 4 (6.6%) | NA | NA | NA | |
| 91–100 | 9 (15%) | ||||||||
| 25–29 y | 10 (16.6%) | 101–110 | 15 (25%) | ||||||
| 30–40 y | 15(25%) | ≥ 110 | 32 (53.3%) | ||||||
| Beyuo et al. 2022 [1] | 12 h | 31 (6) | 36.5 (4.2) | 168.8 (16.3) | 107.3 (11.1) | 214 | 369 | ||
| 24 h | 31.3 (5.9) | 35.2 (4.4) | 165.7 (21.6) | 109 (14.2) | 172 | 398 | |||
M (SD) mean (standard division), y year, w week, SBP Systolic blood pressure, DBP Diastolic blood pressure
aData of Grillo et al. [13] reported as median (interquartile range)
bData reported as frequency (percentage)
Risk of bias
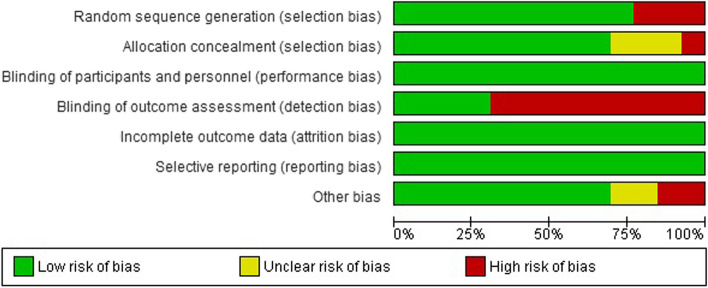
The summary of the quality assessment of the included studies is presented in Figs. 2 and 3. Three studies [7, 13, 14] have a low bias in all domains. Three studies have a high risk of bias in the random sequence generation as they did not mention any information about the randomization process [3, 8, 12]. In allocation concealment, Anjum et al. [3] have a high risk of bias as the data analysts and investigators were not blinded to group assignment, three studies [2, 8, 12] did not mention enough information about the process of concealment, the other studies have a low risk of bias. In the binding outcome assessment, four studies have a low risk of bias [1, 7, 13, 14]. All studies have a low risk of bias in blinding participants and personnel, incomplete outcome data, and selective reporting. Finally, The last domain was low risk of bias in all studies except two [2, 11] as there was insufficient information to determine whether an important risk of bias exists.
Fig. 2.
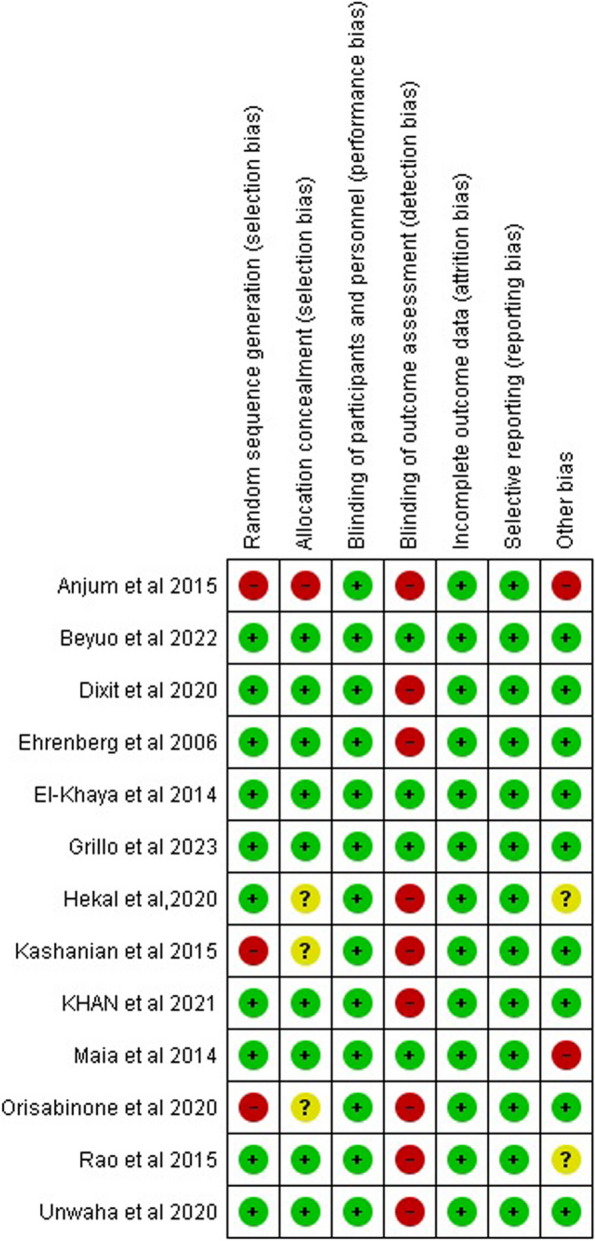
Summary of risk of bias in each study
Fig. 3.
Graph of risk of bias
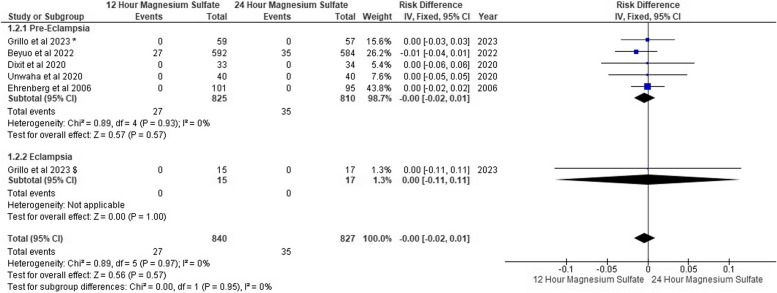
Occurrence of seizure
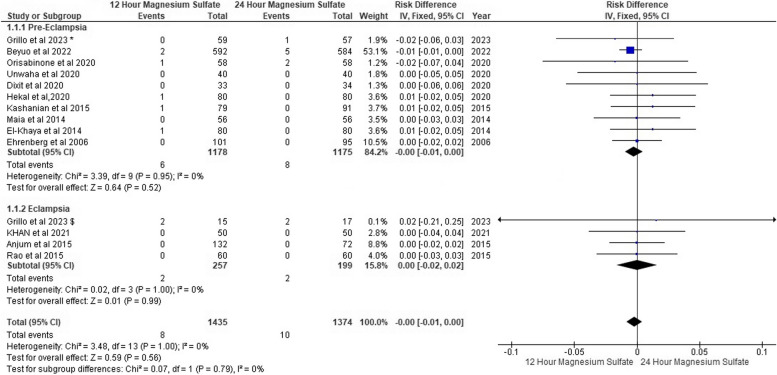
We found that seizures had occurred in six pre-eclampsia patients on 12-h MgSo4 versus eight patients on 24-h MgSo4. On the other hand, in eclampsia patients, seizures occurred in two patients on 12-h MgSo4 and two patients on 24-h MgSo4. However, we found no seizure risk difference between both MgSo4 regime groups.
For pre-eclampsia, the pooled risk difference was (RD: -0.00, 95% CI [-0.01, 0.00], P = 0.52), and the pooled risk difference for eclampsia was (RD: -0.00, 95% CI [-0.02, 0.02], P = 0.99). The pooled results were homogeneous in both subgroups (P = 0.95, I2: 0%) and (P = 1.00, I2: 0%) Fig. 4. Additionally, funnel plots and Egger’s test found no evidence of publication bias, as shown in Fig. 5.
Fig. 4.
Occurrence of seizures forest plot
Fig. 5.
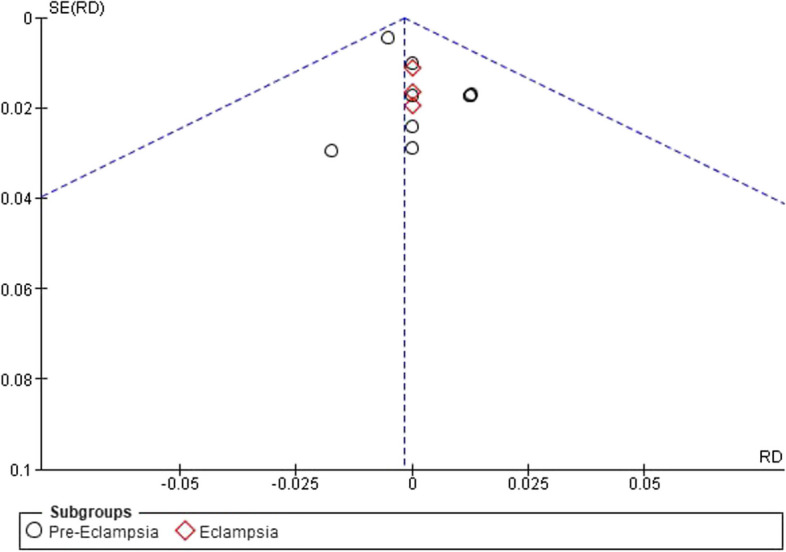
Occurrence of seizures funnel plot
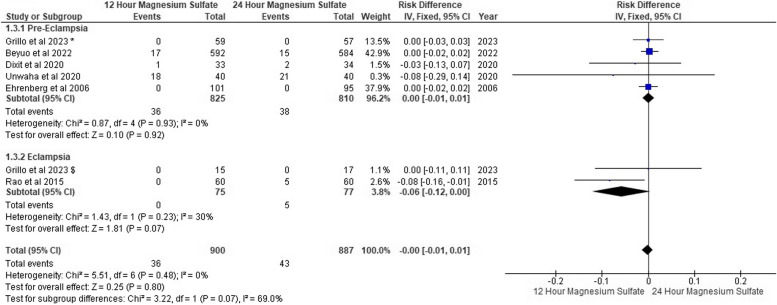
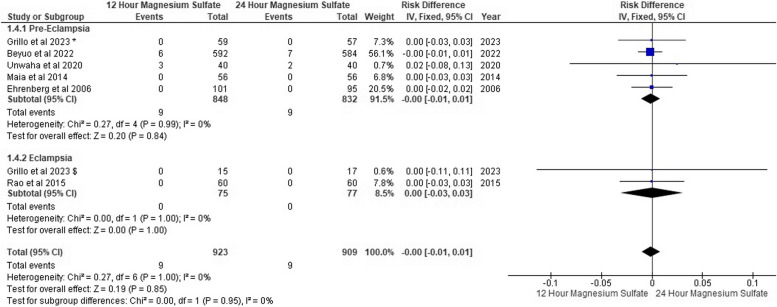
Diminished deep tendon reflexes
Five trials of pre-eclampsia reported that 36 patients in the 12-h MgSo4 group were complicated with diminished deep tendon reflexes versus 38 patients in the 24-h MgSo4 group. Also, eclampsia trials showed that five patients were complicated with diminished deep tendon reflexes in the 24-h MgSo4 group, although no patients were affected in the 12-h MgSo4 group. We found no risk difference between both MgSo4 regimen groups with overall pooled risk difference (RD: -0.00, 95% CI [-0.01, 0.01], P = 0.48) with no heterogeneity (P = 0.48, I2: 0%) as shown in Fig. 6.
Fig. 6.
Diminished deep tendon reflexes forest plot
Respiratory depression
Four studies of pre-eclampsia reported no respiratory depression in both groups. However, Beyuo et al. [1] reported that 27 patients were complicated with respiratory depression in 12-h MgSo4 versus 35 patients in 24-h MgSo4. According to Grillo et al. [13], no eclamptic patients were complicated with respiratory depression.
We found no respiratory depression risk difference between both MgSo4 regimen groups in both pre-eclampsia patients and eclampsia (RD: -0.00, 95% CI [-0.02, 0.01], P = 0.57), (RD: -0.00, 95% CI [-0.11, 0.11], P = 0.57), respectively and the pooled results were homogeneous (P = 0.93, I2: 0%), (P = 0.97, I2: 0%) as shown in Fig. 7.
Fig. 7.
Respiratory depression forest plot
Pulmonary edema
In the pre-eclampsia subgroup, nine patients in the 12-h MgSo4 group suffered pulmonary edema, and nine in the 24-h MgSo4 group. In the eclampsia subgroup, no patients suffered from pulmonary edema in both groups. Overall, both MgSo4 regimen groups showed the same number of patients complicated with pulmonary edema with no risk deference (RD: -0.00, 95% CI [-0.01, 0.01], P = 0.85). The pooled results were homogeneous (P = 1, I2: 0%) as shown in Fig. 8.
Fig. 8.
Pulmonary Edema forest plot
Discussion
We conducted this systematic review and meta-analysis to condense the evidence on clinical efficacy and safety of 12-h MgSO4 compared to 24-h MgSo4 in the prophylaxis and treatment of seizures in pre-eclampsia and eclampsia patients. We included 13 RCTS with 2809 patients (12-h MgSo4 = 1435 and 24-h MgSo4 = 1374). We found an overall low risk of bias in nine trials, some concern in two trials, and high risk in another two studies. The pooled findings confirmed that there is no statistically significant difference between both regimens in occurrence of seizures, diminished deep tendon reflexes, respiratory depression, and pulmonary edema in pre-eclampsia and eclampsia patients. There is no heterogeneity in all outcomes. There was no evidence of publication bias for occurrence of seizures.
MgSo4 is the preferred and standard treatment for managing seizures events in eclampsia patients and as a prophylaxis for pre-eclampsia patients [18]. Convulsions have been observed to occur with greater frequency during or following the first pregnancy, and these episodes have been associated with a poor prognosis [19]. A crucial question remains as to whether they represent epilepsy or specific pregnancy complications [19]. It has been suggested that eclampsia differs from epilepsy as epilepsy is chronic and recurs throughout the lifespan, whereas eclampsia does not [20]. Eclampsia can occur in prepartum, intrapartum, or postpartum [21]. According to our included studies, three studies [1, 3, 13] included patients who developed eclampsia ever prepartum, intrapartum, or postpartum. Khan et al. [17] included patients who developed eclampsia postpartum, while Rao et al. [11] did not specify the period of convulsion occurrence. The studies that included patients with pre-eclampsia did not specify when convulsion occurred as it is considered only as a complication in un/maltreated patients. Our primary outcome is the prevention of the occurrence of seizures in pre-eclampsia patients and its recurrence in eclampsia patients. Our findings showed no difference between both 12-h and 24-h MgSo4 groups in the occurrence of seizure (N = 8, N = 10), respectively, in both pre-eclampsia and eclampsia patients.
Although the MgSo4 effectively treats seizures, there is a risk of developing hypermagnesemia toxicity. The therapeutic level of Mg to maintain its treating effect ranges between 3.5 – 7 mEq/L, and when it exceeds that, toxicity symptoms begin to appear [22]. Affection of deep tendon reflexes represents one of the MgSo4 serious toxicity effects. Diminished deep tendon reflex is the first sign of MgSo4 toxicity as the patellar deep tendon reflex disappears when the concentration of serum Mg ranges between 8–10 mEq/L [22]. This study found no difference between 12-h and 24-h MgSO4 groups in the affection of deep tendon reflexes with (N = 36, N = 43), respectively, in pre-eclampsia and eclampsia patients.
Respiratory depression is also considered one of the serious MgSo4 toxicity signs. It is reported when the level of Mg reaches > 13 mEq/L [22, 23]. Based on our analysis findings, there is no difference between 12-h and 24-h MgSO4 groups in the occurrence of respiratory depression with (N = 27, N = 35), respectively, in both pre-eclampsia and eclampsia patients.
Pulmonary edema may present in patients by shortness of breath, cough, chest pain, and frothy sputum [24]. The analysis of our study showed no difference between both 12-h and 24-h MgSO4 groups in the occurrence of respiratory depression with (N = 9, N = 9), respectively, in both pre-eclampsia and eclampsia patients.
Although Sullivan et al. [25] is the last systematic review that published and discussed a similar comparison, it compares 24-h MgSO4 and less than 24-h MgSO4. So, it is difficult to compare our results to Sullivan et al. [25] as they did not specify the duration of therapy in the intervention group. Additionally, Yifu et al. [26] also compare less than 24-h MgSO4, which includes 12-h MgSO4, and other regimens with 24-h MgSO4, so dependently, it cannot be compared to our study. Upon that, our study is considered to be the first systematic review and meta-analysis that compares only the 12-h MgSO4 with 24-h MgSO4 therapy with more detailed data and analysis of signs of toxicity of MgSO4.
Our study had many strengths; it is considered the first systematic review and meta-analysis to include patients with eclampsia, severe pre-eclampsia, and mild pre-eclampsia. All our 13 included studies were RCTs. We updated the search on PubMed and included the last RCT published in March 2023. This study analyzed data from 2809 patients and performed a subgroup meta-analysis on whether patients were diagnosed with eclampsia or pre-eclampsia in all reported outcomes.
However, there were also some limitations, our sample size was 2813 while only 2809 patients were included in the analysis as there were four patients excluded from the analysis in Anjum et al. [3] and accordingly excluded from our study analysis. Beyuo et al. [1] included both patients of pre-eclampsia and eclampsia without separating their data; we preferred including it in the pre-eclampsia subgroup, as 90.1% of its patients were diagnosed as patients with pre-eclampsia. Only English papers were included because we did not have access to a reliable method to translate non- English papers. Ehrenberg et al. [10] with mild pre-eclampsia patients is included in our pre-eclampsia subgroup, so we could not conduct a subgroup while other studies represent patients with severe pre-eclampsia.
We totally recommend establishing more clinical trials in industrialized countries as we only found one paper from the USA. We couldn’t compare between the two periods of MgSo4 regarding the cost effectiveness because there are not any included studies discuss the cost effectiveness of the two period of MgSo4. So that, we recommend the future research to compare between the regimes regarding the cost and to be powered enough to investigate the safety of both regimens.
Conclusion
MgSO4 is the most effective anticonvulsant for prophylaxis and treatment of fits in patients with pre-eclampsia and eclampsia, considered serious medical conditions affecting pregnant females. Our Systematic review and meta-analysis showed no significant difference between the 12-h and 24-h regimens in the occurrence or recurrence of seizures, respiratory depression, absence or decrease of deep tendon reflex, and pulmonary edema.
Acknowledgements
None.
Abbreviations
- DBP
Diastolic blood pressure
- IM
Intra-muscular
- IV
Intra-venous
- MgSO4
Magnesium sulphate
- RCTs
Randomized controlled trials
- SBP
Systolic blood pressure
- USA
United States of America
Authors’ contributions
RSS and RAI: Conceptualization, Methodology. RSS, RAI, EYS, and SMK: Data curation, Writing- Original draft preparation. RSS, ASE: Visualization, Investigation. ASE: Supervision. RSS: Software, Validation. ASE, RSS, RAI, EYS, and SMK: Writing—Reviewing and Editing.
Funding
This research received no specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Availability of data and materials
Data will be provided upon request from Rahma Sameh Shaheen (rahma193226@fmed.bu.edu.eg).
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rahma Sameh Shaheen and Rahma Abdelaziz Ismail contributed equally to this work.
References
- 1.Beyuo TK, Lawrence ER, Kobernik EK, Oppong SA. A novel 12-hour versus 24-hour magnesium sulfate regimen in the management of eclampsia and preeclampsia in Ghana (MOPEP Study): a randomized controlled trial. Int J Gynecology Obstet. 2022;159:495–504. 10.1002/ijgo.14181 [DOI] [PubMed] [Google Scholar]
- 2.A. Hekal M, A. Wafa Y, I. El-Mohandes M. Different regimens of magnesium sulfate for management of women with severe preeclampsia. Al-Azhar Med J. 2020;49:861–71. 10.21608/amj.2020.91612 [DOI] [Google Scholar]
- 3.Anjum S, Goel N, Sharma R, Mohsin Z, Garg N. Maternal outcomes after 12 hours and 24 hours of magnesium sulfate therapy for eclampsia. Int J Gynecol Obstet. 2015;132:68–71. 10.1016/j.ijgo.2015.06.056. 10.1016/j.ijgo.2015.06.056 [DOI] [PubMed] [Google Scholar]
- 4.Unwaha EA, Bello FA, Bello OO, Oladokun A. Intravenous magnesium sulfate in the management of severe pre-eclampsia: a randomized study of 12-hour versus 24-hour maintenance dose. Int J Gynecol Obstet. 2020;149:37–42. 10.1002/ijgo.13082 [DOI] [PubMed] [Google Scholar]
- 5.Farahani F, Zare S, Seydoshohadaei F, Rahmani K, Soufizadeh N. Comparison of the 12-hour and 24-hour magnesium sulfate therapy regimens after delivery in patients with severe preeclampsia. J Pharm Negat Results. 2022;13:2136–40. [Google Scholar]
- 6.Recommendations | Hypertension in pregnancy: diagnosis and management | Guidance | NICE. 2019. https://www.nice.org.uk/guidance/ng133/chapter/Recommendations#management-of-pre-eclampsia. Accessed 15 Jun 2024.
- 7.Maia SB, Katz L, Neto CN, Caiado BVR, Azevedo APRL, Amorim MMR. Abbreviated (12-hour) versus traditional (24-hour) postpartum magnesium sulfate therapy in severe pre-eclampsia. Int J Gynecol Obstet. 2014;126:260–4. 10.1016/j.ijgo.2014.03.024 [DOI] [PubMed] [Google Scholar]
- 8.Kashanian M, Koohpayehzadeh J, Sheikhansari N, Bararpour F, Sahraian G, Asadolla S. A comparison between the two methods of magnesium sulfate administration for duration of 12 versus 24 h after delivery in patients with severe preeclampsia. J Matern Fetal Neonatal Med. 2016;29:2282–7. 10.3109/14767058.2015.1083547 [DOI] [PubMed] [Google Scholar]
- 9.Dixit DrP, Thakur DrS, Singh DrK, Sharma DrJ, Singh DrS. To compare abbreviated regimens of single dose and 12 hours magnesium sulphate administration with the conventional 24 hours postpartum in severe preeclampsia: a randomized clinical trial. Int J Clin Obstet Gynaecol. 2020;4:205–10. 10.33545/gynae.2020.v4.i4d.645 [DOI] [Google Scholar]
- 10.Ehrenberg HM, Mercer BM. Abbreviated postpartum magnesium sulfate therapy for women with mild preeclampsia: a randomized controlled trial. Obstet Gynecol. 2006;108:833–8. 10.1097/01.AOG.0000236493.35347.d8 [DOI] [PubMed] [Google Scholar]
- 11.Rao SI, Hussna S. Comparison of efficacy and safety of magnesium sulphate in 12 hours versus 24 hours after last fit in eclamptic patients. Med Forum Monthly. 2015;26:7–10. [Google Scholar]
- 12.Orisabinone IB, Onwudiegwu U, Adeyemi AB, Oriji CP, Makinde OI. Shortened versus standard post-partum maintenance therapy of magnesium sulphate in severe pre-eclampsia: a randomised control trial. Int J Reprod Contracept Obstet Gynecol. 2020;9:1646. 10.18203/2320-1770.ijrcog20201239 [DOI] [Google Scholar]
- 13.Grillo EO, Awonuga DO, Dedeke IOF, Abiodun O, Imaralu JO, Sotunsa JO, et al. Comparison of Zuspan regimen and its 12-hour modification in women with severe pre-eclampsia and eclampsia in two hospitals in Abeokuta. Pregnancy Hypertens. 2023;32:22–7. 10.1016/j.preghy.2023.03.001 [DOI] [PubMed] [Google Scholar]
- 14.El-Khayat W, Atef A, Abdelatty S, El-semary A. A novel protocol for postpartum magnesium sulphate in severe pre-eclampsia: a randomized controlled pilot trial. J Matern Fetal Neonatal Med. 2016;29:154–8. 10.3109/14767058.2014.991915 [DOI] [PubMed] [Google Scholar]
- 15.Page MJ, Mckenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;37:n71. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higgins JPT, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khan S, Humayun P, Awan SN, Naqvi SB, Mohsin R, Jawad Z. Comparison of 12 hours versus 24 hours intravenous administration of MgSO4 in the management of eclampsia. Pakistan J Med Health Sci. 2021;15:365–7. [Google Scholar]
- 18.Laskowska M. Prevalence, diagnosis, and management of eclampsia and the need for improved maternal care: a review. Med Sci Monit. 2023;29:e939919. 10.12659/MSM.939919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Erez O, Romero R, Jung E, Chaemsaithong P, Bosco M, Suksai M, et al. Preeclampsia/eclampsia: the conceptual evolution of a syndrome. Am J Obstet Gynecol. 2022;226 2 Suppl:S786-803. 10.1016/j.ajog.2021.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chesley LC. History and epidemiology of preeclampsia-eclampsia. Clin Obstet Gynecol. 1984;27:801–20. 10.1097/00003081-198412000-00004 [DOI] [PubMed] [Google Scholar]
- 21.Pratt JJ, Niedle PS, Vogel JP, Oladapo OT, Bohren M, Tunçalp Ö, et al. Alternative regimens of magnesium sulfate for treatment of preeclampsia and eclampsia: a systematic review of non-randomized studies. Acta Obstet Gynecol Scand. 2016;95:144–56. 10.1111/aogs.12807 [DOI] [PubMed] [Google Scholar]
- 22.Euser AG, Cipolla MJ. Magnesium sulfate for the treatment of eclampsia: a brief review. Stroke. 2009;40:1169–75. 10.1161/STROKEAHA.108.527788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith JM, Lowe RF, Fullerton J, Currie SM, Harris L, Felker-Kantor E. An integrative review of the side effects related to the use of magnesium sulfate for pre-eclampsia and eclampsia management. BMC Pregnancy Childbirth. 2013;13:34. 10.1186/1471-2393-13-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barbieri RL. Individualize the duration of postpartum magnesium treatment for patients with preeclampsia to best balance the benefits and harms of treatment. OBGM. 2022;34(1):7–9. 10.12788/obgm.0165 [DOI] [Google Scholar]
- 25.Sullivan M, Cunningham K, Angras K, Mackeen AD. Duration of postpartum magnesium sulfate for seizure prophylaxis in women with preeclampsia: a systematic review and meta-analysis. J Matern Fetal Neonatal Med. 2024;35:7188–93. 10.1080/14767058.2021.1946505 [DOI] [PubMed] [Google Scholar]
- 26.Yifu P, Lei Y, Yujin G, Xingwang Z, Shaoming L. Shortened postpartum magnesium sulfate treatment vs traditional 24h for severe preeclampsia: a systematic review and meta-analysis of randomized trials. Hypertens Pregnancy. 2020;39:186–95. 10.1080/10641955.2020.1753067 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be provided upon request from Rahma Sameh Shaheen (rahma193226@fmed.bu.edu.eg).