Abstract
Cell culture-adapted Sindbis virus strains attach to heparan sulfate (HS) receptors during infection of cultured cells (W. B. Klimstra, K. D. Ryman, and R. E. Johnston, J. Virol. 72:7357–7366, 1998). At least three E2 glycoprotein mutations (E2 Arg 1, E2 Lys 70, and E2 Arg 114) can independently confer HS attachment in the background of the consensus sequence Sindbis virus (TR339). In the studies reported here, we have investigated the mechanism by which the E2 Arg 1 mutation confers HS-dependent binding. Substitution of Arg for Ser at E2 1 resulted in a significant reduction in the efficiency of PE2 cleavage, yielding virus particles containing a mixture of PE2 and mature E2. Presence of PE2 was associated with an increase in HS-dependent attachment to cells and efficient attachment to heparin-agarose beads, presumably because the furin recognition site for PE2 cleavage also represents a candidate HS binding sequence. A comparison of mutants with partially or completely inhibited PE2 cleavage demonstrated that efficiency of cell binding was correlated with the amount of PE2 in virus particles. Viruses rendered cleavage defective due to deletions of portions or all of the furin cleavage sequence attached very poorly to cells, indicating that an intact furin cleavage sequence was specifically required for PE2-mediated attachment to cells. In contrast, a virus containing a partial deletion was capable of efficient binding to heparin-agarose beads, suggesting different requirements for heparin bead and cell surface HS binding. Furthermore, virus produced in C6/36 mosquito cells, which cleave PE2 more efficiently than BHK cells, exhibited a reduction in cell attachment efficiency correlated with reduced content of PE2 in particles. Taken together, these results strongly argue that the XBXBBX (B, basic; X, hydrophobic) furin protease recognition sequence of PE2 can mediate the binding of PE2-containing Sindbis viruses to HS. This sequence is very similar to an XBBXBX heparin-HS interaction consensus sequence. The attachment of furin protease cleavage sequences to HS may have relevance to other viruses whose attachment proteins are cleaved during maturation at positively charged recognition sequences.
The envelope glycoproteins of a number of virus types are produced first as precursors and then are cleaved during virion maturation at short, positively charged sequences (reviewed in references 19 and 26). With several viruses, including alphaviruses, the subtilisin-like host cell protease furin has been identified as mediating this cleavage (6, 19, 23, 42). In mammalian cells, furin is localized to the protein secretory pathway between the trans-Golgi and cell surface (27, 45). The consensus recognition sequence for furin proteases is X-Arg-X-Lys/Arg-Arg-X (i.e., XBXBBX, where B is a basic amino acid and X is a hydrophobic amino acid, with the protein cleaved between the final Arg and X residues); the final X is Ser with many viruses (19, 27, 33, 45).
The Sindbis virus attachment protein E2 is synthesized as a precursor, PE2, which is cleaved at amino acid 64, yielding the mature E2 spike protein. The amino terminus of PE2, E3, contains the XBXBB portion of the cleavage sequence but is not itself retained in virus particles (42). Glycoprotein spikes on mature virus particles are composed of heterodimers of E2 and E1, another viral structural glycoprotein (reviewed in reference 42). Mutagenesis and mutant selection studies have indicated that the efficiency of cleavage at furin recognition sites can be decreased by substitution of amino acids other than Ser at the final X position (9, 14, 35). With Sindbis virus PE2, substitution of Arg at this site results in partial cleavage compared to that with the wild-type Ser, while substitution of Asn, Leu, or Val can result in PE2 that is predominantly cleavage defective (9). Uncleaved PE2 is incorporated into PE2-E1 heterodimers on mutant Sindbis virus particles that are released from infected cells (9, 35).
Surprisingly, the furin recognition sequence is identical (although in opposite orientation) to one of two heparin-heparan sulfate (HS) interaction consensus sequences (XBBXBX or XBXXBBBX) deduced by Cardin and Weintraub through comparison of protein domains known to interact with heparin (4). Two additional heparin-HS consensus sequences have been described more recently (13, 40). In the context of heparin-HS binding proteins, such as vitronectin, fibronectin, and lipoprotein lipase, these sequences promote attachment primarily through ionic interaction with carboxylate and sulfate groups on heparin-HS chains (reviewed in references 16 and 18). It has been proposed that protein-HS interactions can be mediated by single linear consensus sequences as well as binding sites determined by the protein tertiary structure and composed of several linear heparin-HS interaction sequences (13). Biological processes involving protein–heparin-HS interaction include cell adhesion, maintenance of tissue structure, anticoagulation activity, sequestration and concentration of cell signaling factors, and possibly regulation of transcription factor activity (16, 44). In addition, HS attachment plays a role in the in vitro infection of a number of viruses (3, 5, 20, 31, 34, 43, 46).
We have recently shown that Sindbis virus strains adapted to baby hamster kidney (BHK) cells attach to HS during the infection of cultured cells (20). In contrast, the Sindbis virus strain AR339 consensus sequence virus (22) infects cultured cells by a primarily HS-independent mechanism (20). We have identified three loci in the E2 attachment protein (E2 1, E2 70, and E2 114) that mutate to positive charge during the adaptation of Sindbis virus to BHK cells and can independently confer the ability to bind cell surface HS. However, the exact composition of HS binding sites on the Sindbis virus glycoprotein spike remains to be identified.
Several lines of evidence suggest that the furin protease cleavage signal of Sindbis virus PE2 may be involved in HS binding: (i) the E2 Arg 1 mutation occupies the carboxy-terminal position of the XBXBBX PE2 furin protease cleavage site; (ii) as indicated above, Arg at E2 1 results in a reduction in the cleavage efficiency of PE2, resulting in virus particles that contain some PE2 and bind HS (20); and (iii) completely cleavage-defective viruses that maintain the furin protease cleavage signal by incorporating PE2 into virions attach more efficiently to cultured cells than the E2 Arg 1 mutant (10). These observations, in addition to the resemblance of the furin cleavage site to a heparin-HS interaction consensus, have prompted an investigation of whether the XBXBBX furin protease sequence of PE2 could mediate virion attachment to cell surface HS and whether decreased PE2 cleavage efficiency as found with the E2 Arg 1 mutation might be one mechanism by which Sindbis viruses adapt to growth in cultured cells.
In the studies reported here, we have compared the cell and heparin bead binding of viruses with various PE2 cleavage efficiencies (determined by the residue at E2 position 1) as well as those of noncleaving viruses with all or part of the furin protease cleavage site deleted. The results of these studies indicate (i) that furin protease cleavage sequences can mediate attachment of Sindbis viruses containing PE2 to cellular HS and (ii) that the magnitude of cell binding is correlated with the abundance of PE2 and the associated furin cleavage sequence in virus particles. This is in contrast with binding mediated by positive-charge mutations at E2 70 (Lys) or E2 114 (Arg) loci, which is independent of the PE2 furin protease cleavage sequence.
MATERIALS AND METHODS
Cell culture.
Chinese hamster ovary (CHO K1) cells were maintained at 37°C in Ham’s F-12 medium supplemented with 10% fetal bovine serum, 100 U of penicillin per ml, and 0.05 mg of streptomycin per ml. BHK cells were maintained at 37°C in alpha minimal essential medium supplemented with 10% donor calf serum, 10% tryptose phosphate broth, 0.29 mg of l-glutamine per ml, and antibiotics as described above. Mosquito cells (C6/36) were maintained in alpha minimal essential medium supplemented with 10% fetal bovine serum, tryptose phosphate broth, l-glutamine, and antibiotics as described above and were incubated at 28°C.
Viruses.
The construction of the consensus Sindbis virus AR339 clone (pTR339) and clone p3070 has been described previously (20, 22). The “p” prefix indicates the cDNA form of the virus clone. Additional full-length cDNA clones, p39R1, p39N1, p39L1, and p39V1, were constructed by substitution into pTR339 of a StuI-to-BssHII fragment from pTRSB (22), pTRSB-N (9), pTRSB-E2L1 (9), and pTRSB-E2V1 (9), respectively. The cDNA clones pFD, pFD1, pFD2, and pFDN1 were constructed by “megaprimer” PCR mutagenesis (39) of pTR339. Briefly, mutagenesis primers were designed to create the desired PE2 cleavage sequence of each virus. Each mutagenesis primer was used separately, along with a nonmutagenic primer, in a first round of PCR amplification with pTR339 DNA as a template. The nonmutagenic primer was chosen so that the resultant amplicon (megaprimer) would span the StuI restriction site (nucleotide 8571) of pTR339 and be ∼150 to 300 bp in length. After agarose gel purification, each mutant megaprimer was used separately as a primer for a second round of PCR with another nonmutagenic primer. This yielded an amplicon of ∼1.4 kb that spanned from upstream of the StuI restriction site to downstream of the BssHII (nucleotide 9804) restriction site of pTR339. Gel-purified amplicons were then digested with StuI and BssHII and cloned into similarly digested pTR339. p39K70 and pFDK70 were constructed by a similar method but with nonmutagenic primers spanning the same regions and switching template DNA (p3070 in the first round for both and, in the second round, pTR339 for p39K70 and pFD for pFDK70) between the rounds of PCR amplification. This resulted in the combining of mutations present in separate clones. All genetic manipulations were confirmed by DNA sequencing at the University of North Carolina at Chapel Hill Automated DNA Sequencing Facility with a model 373A DNA sequencer (Applied Biosystems) with the Taq DyeDeoxy terminator cycle sequencing kit (Applied Biosystems). Viruses were produced by high-efficiency electroporation of BHK cells with in vitro transcripts of linearized cDNA clones as described previously (20).
Virus purification.
BHK or C6/36 cell monolayers were infected either with TR339, 39R1, 39L1, 39V1, or 39K70 at a multiplicity of infection of >5, or the cells were harvested and electroporated with in vitro transcripts of pFD, pFD1, pFD2, p39N1, pFDN1, or pFDK70, followed by radiolabeling and sucrose gradient purification as previously described (20). Viruses generated by infection were purified on discontinuous 20 to 60% (wt/wt) sucrose gradients in TNE (0.05 M Tris-HCl [pH 7.2], 0.1 M NaCl, 0.001 M EDTA) buffer, followed by continuous 20 to 60% sucrose gradients, and pelleted through 20% sucrose. Due to lower yields from electroporated cells than from infected cells, viruses generated from electroporation were purified on discontinuous sucrose gradients as described above, followed by pelleting through 20% sucrose. The binding phenotypes of virus particles prepared by the two methods were compared by using FD1 (cleavage defective; weak cell binding), 39L1 (cleavage defective; strong cell binding), and TR339 (cleavage competent; weak cell binding) viruses and found not to differ significantly. Specific infectivity (PFU/counts per minute) analysis of radiolabeled virus preparations was performed on BHK cell monolayers as previously described (20).
Virus attachment assay.
CHO and BHK cell binding assays and heparin-agarose and bovine serum albumin (BSA)-agarose bead (both from Sigma) binding assays were done as previously described (20). Virus was allowed to attach to cells or beads for 1 h at 4°C. Heparin-agarose bead binding of virus particles routinely varied between 70 and 90% of added counts per minute between reactions, most likely due to variable loss of beads during the wash steps. For this reason, a positive result from bead binding is reported if >70% of radiolabeled virus particles bound in repeated assays. A negative result is indicated if heparin- or BSA-agarose beads bound <10% of added counts per minute in repeated assays. Heparinase I (Sigma) digestion of CHO cells was done as previously described for BHK cells (20). All binding assays were repeated at least twice.
Polyacrylamide gel analysis of [35S]methionine-labeled viral proteins.
Individual structural proteins from radiolabeled virus particles prepared for binding assays were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (10% acrylamide) under reducing conditions (50 mM 2-mercaptoethanol). The radiolabeled protein bands were visualized with a Storm model PhosphorImager and ImageQuant software (Molecular Dynamics). The relative PE2 cleavage efficiency was determined by dividing the image densities of bands corresponding to PE2 by the total image density of PE2 plus E1 plus E2 plus capsid proteins. The results slightly underestimate the difference between cleaving and noncleaving viruses due to the loss of E3, which contains one methionine (of a total of 27 in PE2), from the PE2-cleaving-virus lanes. PE2 cleavage calculations were performed on at least two gels for each virus with similar results.
RESULTS
To directly evaluate the role of the furin protease cleavage recognition sequence in cell binding, a panel of mutants was created containing substitutions that had previously been shown to affect PE2 cleavage efficiency or that had portions of the XBXBBX sequence deleted (Table 1). Substitution of Arg for Ser at E2 1 is associated with an increase in HS-dependent attachment to cells (20) and partial inhibition of PE2 cleavage (9). Substitution of Val or Leu at E2 1 greatly reduces PE2 cleavage, perhaps due to interference of the aliphatic R group of these amino acids with the furin protease (9). Likewise, substitution of Asn at E2 1, which confers a rapid-penetration phenotype on the related alphavirus S.A.AR86, inhibits PE2 cleavage in the contexts of both Sindbis virus AR339 and S.A.AR86 (9, 35). This residue creates an N-linked glycosylation signal, and the glycosylation of E2 Asn 1 is proposed to interfere with furin activity. For these mutants, uncleaved PE2 would contain an intact BXBB furin recognition and HS binding consensus sequence. In addition, mutants were created with either the entire BXBB sequence or individual positively charged amino acids deleted. With these mutant viruses, the correlation between retention of PE2 in virions, the presence of an intact furin protease cleavage signal, and cell binding could be evaluated.
TABLE 1.
Viruses used in the present studies
| Virusa | Amino acids at PE2 cleavage site (−5−4−3−2−1+1) | Ratio of PE2 to PE2 + E1 + E2 + Cb | BHK specific infectivity (PFU/cpm) |
|---|---|---|---|
| TR339 | GRSKRS | 0.006 | 5.7 |
| 39R1 | GRSKRR | 0.035 | 263 |
| FD | G−−−−S | 0.255 | <0.01 |
| FD1 | GRSK−S | 0.277 | 0.13 |
| FD2 | GRS−−S | 0.260 | 0.05 |
| 39N1 | GRSKRN | 0.270 | 0.74 |
| FDN1 | G−−−−N | 0.256 | 0.05 |
| 39L1 | GRSKRL | 0.179 | 33.5 |
| 39V1 | GRSKRV | 0.197 | 28.4 |
| 39K70c | GRSKRS | 0.004 | 2,000 |
| FDK70d | G−−−−S | 0.243 | 0.51 |
Viruses were derived from cDNA clones that were isogenic except for the indicated loci.
Ratios derived from PhosphorImager quantitation of band densities on SDS-PAGE gels.
Furin cleavage site identical to TR339; contains Glu-to-Lys mutation at E2 70.
Furin cleavage site deleted; contains Glu-to-Lys mutation at E2 70.
PE2 cleavage of viruses produced in BHK cells.
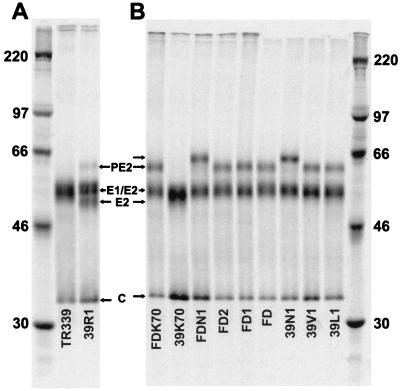
SDS-PAGE analysis of virions with different PE2 cleavage phenotypes is shown in Fig. 1, and the PE2 content of purified virus particles is quantitated in Table 1. A completely noncleaving virus (100% PE2) should give a ratio of PE2 to PE2 plus E2 plus E1 plus capsid of 0.259 (7 methionine residues in PE2 of 27 total methionine residues). Consistent with previous reports (9), Ser at E2 1 (TR339 and 39K70) resulted in nearly complete cleavage of PE2 by the BHK cell furin protease (2.3 and 1.5% PE2, respectively), resulting in virions containing little or no PE2. Also, consistent with previous reports, substitution of Arg at E2 1 (39R1) resulted in significant reduction of cleavage efficiency (∼14% PE2 content in virions). Substitution of Asn at E2 1 (39N1) resulted in near-complete inhibition of PE2 cleavage, suggesting that mature particles of this virus contained spikes composed entirely of PE2-E1 heterodimers. Altered migration of PE2 from 39N1 and FDN1 preparations reflects the additional glycosylation of PE2 with Asn at E2 1 (Fig. 1B) (35). Viruses with the BXBB portion of the cleavage signal deleted (FD, FDN1, or FDK70) or the −1 (FD1) or −1 and −2 (FD2) amino acids of the cleavage signal deleted also failed to cleave PE2. Viruses with Leu (39L1) or Val (39V1) at E2 1 were predominantly cleavage defective; however, Phosphor- Imager analysis suggested that these viruses contained less PE2 (and consequently, more E2) than the deletion mutants or 39N1 (Table 1).
FIG. 1.
SDS-PAGE analysis of purified radiolabeled virus particles; 5.0 × 104 cpm of virus was loaded in each lane. Molecular mass markers (kDa) are shown in the extreme right-hand and left-hand lanes.
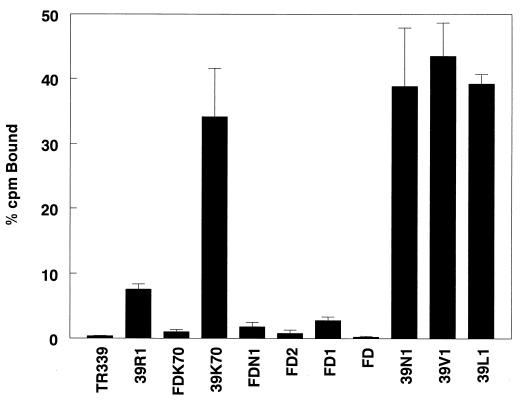
Binding of viruses to CHO cells.
Binding studies with CHO K1 cells indicated that the presence of an intact furin protease cleavage sequence was correlated with efficient attachment to cells (Fig. 2). Consistent with the near-complete cleavage of PE2 and the lack of E2 internal mutations associated with HS binding, TR339 exhibited barely measurable binding to CHO cells. Substitution of Arg for Ser at E2 1 (39R1) resulted in a significant increase in cell attachment (P = <0.01; Student’s t test). As noted above, the enhanced attachment of 39R1 was correlated with partial inhibition of PE2 cleavage and increased retention of PE2 in virions. Further increase in virion PE2 content conferred by substitution of Val (39V1), Leu (39L1), or Asn (39N1) at E2 1 resulted in viruses with four- to fivefold-greater binding to the cells than 39R1, suggesting that the PE2 content of virus particles and, consequently, the presence of the furin cleavage signal was correlated with attachment efficiency. These data also indicate that the individual differences in PE2 content between 39L1, 39V1, and 39N1 virions is not correlated with a difference in attachment, suggesting a threshold of PE2 content above which binding is not increased.
FIG. 2.
Binding of radiolabeled purified viruses to CHO cells. The bars represent averages of triplicate binding assays with 105 cpm of virus and ∼106 cells per reaction. Each set of triplicates was repeated at least twice. The error bars represent standard deviations.
Binding of FD, which has the BXBB sequence deleted, was not significantly different from that of TR339 (P > 0.4). The hypothesis that HS binding is mediated by the furin cleavage sequence predicts this result, as TR339 contains little or no PE2. Deletion of the BXBB portion of the furin signal in the context of Asn at E2 1 (FDN1) reduced binding by >90%. Deletion of the −1 (FD1) or −1 and −2 (FD2) basic residues from the cleavage site also reduced binding by >90%, although these viruses bound slightly better than FD or TR339. These results indicate that the full BXBB sequence is required for high-affinity attachment to cells and that E3 residues outside the furin recognition sequence are not responsible for cell attachment.
Previously, we reported that Lys at E2 70 is rapidly selected during serial passage of TR339 in BHK cells and is a constituent of many AR339 laboratory strains (20, 22). Similar to the effect of Arg at E2 114 (20), the introduction of Lys at E2 70 into TR339 (39K70 [Table 1]) resulted in a large increase in efficiency of HS-mediated attachment to CHO K1 cells. Binding of 39K70 was similar to that of noncleaving viruses that retained the intact furin cleavage sequence (Fig. 2). As this virus contains little or no PE2, similar to TR339 (Fig. 1A and B and Table 1), this result confirms that internal positive-charge mutations in E2 facilitate HS interaction via binding domains distinct from the furin cleavage sequence. Surprisingly, deletion of the BXBB sequence combined with E2 Lys 70 (FDK70) reduced binding by >90% compared with substitution of E2 Lys 70 alone (39K70). This suggests that E3 residues either block key residues involved in internal HS interaction domains or cause conformational changes that disrupt these domains.
Binding phenotypes of TR339, 39R1, 39L1, 39V1, 39N1, 39K70, and viruses with furin protease site deletions were similar in companion assays with BHK cells (data not shown), suggesting that attachment phenotypes are not limited to CHO K1 cells. In addition, similar to previous results with the cell culture-adapted mutant viruses TRSB and TRSB-R114 (20), the increased cell attachment of partially and completely cleaving viruses 39R1 and 39K70 compared with that of TR339 was correlated with increased specific infectivity for BHK cells (Table 1) and extension of the survival time of infected neonatal mice (data not shown). Viruses with uncleaved PE2 exhibited very low infectivity for BHK cells regardless of attachment efficiency (Table 1), perhaps due to the effects of PE2 on uncoating or other entry processes. Infectivity of 39L1 and 39V1 was increased relative to these viruses, and this may reflect partial alleviation of this inhibition due to limited PE2 cleavage.
Effect of heparinase I digestion on CHO cell binding.
Previously, we showed that the attachment to cells of cell culture-adapted Sindbis virus strains was due to virus interaction with cell surface HS (20). This phenotype was demonstrated by soluble-heparin competition of virus binding and infectivity, reduction of binding and infectivity after heparinase digestion of cell surfaces, evaluation of virus binding and infectivity with HS- or GAG-deficient CHO cells, and direct virus attachment to heparin-agarose beads. In the present studies, we have used digestion of cell surface HS with heparinase I and heparin-agarose bead binding to determine the requirement for HS in cell attachment.
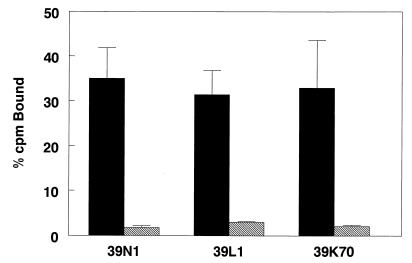
All tested viruses (39L1, 39N1, and 39K70) showed significant (≥90%) reduction in binding affinity after digestion of cell surface HS with heparinase I, indicating that PE2 noncleaving viruses, as well as viruses containing the Glu-to-Lys mutation at E2 70, attach to cell surfaces through interaction with HS (Fig. 3). Viruses differing from TR339 by substitution of Arg at E2 1 are similarly sensitive to digestion of HS with this concentration of heparinase I (20). These results further support the notion that most if not all Sindbis virus strains that exhibit high-efficiency attachment to cells in culture do so through interaction with HS.
FIG. 3.
Binding of viruses to CHO cells without (solid bars) or with (hatched bars) previous digestion with heparinase I. The cells were either digested with 8 U of heparinase I (in phosphate-buffered saline with 0.1% BSA/ml) or mock-digested (phosphate-buffered saline with 0.1% BSA only) for 1 h at 37°C followed by processing for attachment assays as described in Materials and Methods. The bars represent averages of triplicate binding assays with 105 cpm of virus and ∼106 cells per reaction. Each set of triplicates was repeated at least twice. The error bars represent standard deviations.
The binding and infectivity of a virus containing Arg at E2 1 and Arg at E2 114 was insensitive to digestion of HS with heparinase I (20). Preliminary data generated by using viruses with either mutation introduced singly into the TR339 clone suggest that the combination of E21 Arg and E2 114 Arg is required for heparinase I resistance (data not shown). We are currently investigating whether combination of E2 70 Lys with E2 1 Arg similarly results in heparinase I resistance.
Binding of viruses to heparin-agarose and BSA-agarose beads.
Consistent with a direct interaction of efficiently binding viruses with HS, all of these viruses (39R1, 39L1, 39N1, and 39K70) bound to heparin agarose beads (Table 2). Binding of all viruses to BSA-agarose beads ranged from 0.25 to 5% of added counts per minute (data not shown). This result, in combination with our previous report (20), indicates that mutations associated with the PE2 cleavage site (e.g., Arg, Leu, Val, and Asn at the +1 position) and mutations internal to E2 (e.g., E2 Lys 70 and E2 Arg 114) can mediate interaction with heparin. Consistent with our previous report, TR339 attached very poorly to the beads. Similarly, FD exhibited little attachment, suggesting that, similar to cellular HS binding, E3 residues outside of the BXBB signal region are not responsible for the heparin interaction. Deletion of the −1 and −2 basic residues together (FD2) also eliminated the heparin bead interaction; however, deletion of only the −1 basic residue resulted in a virus that bound poorly to cells yet was capable of interaction with the heparin beads. This result indicates that, in the context of the furin cleavage sequence region of the Sindbis virus spike, the XBXBBX or XBXBX sequences are sufficient for interaction with heparin while the full furin site is required for efficient attachment to cellular HS.
TABLE 2.
Summary of binding and PE2 cleavage phenotypes
| Virus | Heparin bindinga | Cell binding (% cpm bound) | PE2 content of virus particle (%) | Cleavage site | Substitution mutation |
|---|---|---|---|---|---|
| TR339 | − | 0.4 | 2.3 | Intact | |
| 39R1 | + | 7.6 | 14 | Intact | E2 Arg 1 |
| FDK70 | + | 1.0 | >90 | BXBB deleted | E2 Lys 70 |
| 39K70 | + | 34 | 1.5 | Intact | E2 Lys 70 |
| FDN1 | − | 1.8 | >90 | BXBB deleted | E2 Asn 1 |
| FD2 | − | 0.8 | >90 | −2, −1 deleted | |
| FD1 | + | 2.8 | >90 | −1 deleted | |
| FD | − | 0.2 | >90 | BXBB deleted | |
| 39N1 | + | 39 | >90 | Intact | E2 Asn 1 |
| 39V1 | + | 44 | 76 | Intact | E2 Val 1 |
| 39L1 | + | 39 | 69 | Intact | E2 Leu 1 |
+, present; −, absent.
Correlation of PE2 cleavage efficiency with cell attachment.
The studies described above correlated the attachment of genetically different viruses to HS on cells and heparin-agarose beads with retention of an intact furin protease cleavage sequence in virus particles. Studies in this section evaluated the effect of altered PE2 cleavage efficiency on binding of genetically identical viruses. We initially attempted to produce radiolabeled purified virus in furin protease-deficient CHO cells (23, 24) and BHK cells treated with monensin, which inhibits PE2 cleavage (17, 32). However, virus yields following purification were insufficient (data not shown). Heidner et al. (11) demonstrated that PE2 containing Arg, Leu, or Val at E2 1 was cleaved more efficiently when virus was produced in the mosquito cell line C6/36 than in BHK cells. In these studies, PE2 with Val or Arg at E2 1 was efficiently cleaved in C6/36 cells while PE2 with Leu at E2 1 exhibited partial cleavage. The authors suggested that the host cell protease responsible for PE2 cleavage in arthropod cells was more tolerant of amino acid sequence variation at the +1 position of the furin recognition sequence. Therefore, depending on the cell of origin, stocks of genetically identical viruses could be prepared that differed in PE2 content.
The attachment affinity of C6/36 cell-grown virus to CHO K1 cells was reduced in proportion to the reduction in PE2 in virus particles that was previously reported by Heidner et al. (Fig. 4) (11), with 39V1 and 39R1 reduced 80 to 90% in binding efficiency and 39L1 reduced ∼50% when prepared from C6/36 cells. The 39K70 virus showed no significant difference in cell attachment when prepared from BHK or C6/36 cells. This result supports the hypothesis that positive-charge mutations in E2 that are not associated with the PE2 furin cleavage signal (e.g., E2 Arg 114 and E2 Lys 70) confer HS attachment via a mechanism distinct from alteration of PE2 cleavage efficiency. In addition, this indicates that differential glycosylation of viral glycoproteins in virus particles produced in the two cell types (15) does not significantly affect virus binding. These results are consistent with the studies of Stollar et al. (41), who found that particle-to-PFU ratios of Sindbis virus were not significantly different when the virus was prepared in BHK, C6/36, or chicken embryo fibroblast cells.
FIG. 4.
Binding of radiolabeled viruses prepared in BHK cells or C6/36 cells to CHO cells. The bars represent averages of triplicate binding assays with 105 cpm of virus and ∼106 cells per reaction. Each set of triplicates was repeated at least twice. The error bars represent standard deviations.
DISCUSSION
HS attachment can be mediated by furin protease cleavage sequences.
The results of these studies indicate that the XBXBBX furin protease cleavage signal is directly involved in attachment of partially cleaving or noncleaving Sindbis viruses to cellular HS and that the efficiency of attachment correlates with the content of PE2 in virus particles. While heparin bead attachment can apparently be mediated by XBXBX or XBXBBX sequences, high-affinity attachment to cellular HS requires the XBXBBX motif. This mechanism of HS attachment is distinct from that involving internal E2 residues, such as E2 Lys 70 and E2 Arg 114.
Our results indicate that with Sindbis virus, the presence of PE2 has an enhancing effect on cell attachment that is correlated with the content of PE2 in virus particles. This is in contrast with the studies of Dubuisson and Rice (7) with Sindbis virus and Salminen et al. (38) with Semliki Forest virus, where inhibition of PE2 cleavage resulted in a large reduction in binding efficiency compared with that of parental cleavage-competent viruses. However, in these studies, PE2 cleavage was inhibited by replacing or deleting positively charged amino acids comprising the furin cleavage site. As shown here, deletion of a single basic amino acid from the cleavage consensus results in >90% reduction in PE2 cleavage sequence-mediated HS binding. In addition, results of studies with the FDK70 mutant suggest that the presence of PE2 either causes a conformational change that abrogates E2 Lys 70-mediated HS binding or blocks key residues that cooperate with E2 Lys 70 in promoting HS attachment, although residues sufficient for virus binding to heparin-agarose beads remain exposed. Therefore, the presence of PE2 with a disrupted cleavage sequence prevents HS binding by cell culture-adapted Sindbis viruses. As we have been unable to identify a Sindbis virus strain that exhibits high-affinity attachment to cell surfaces by means other than HS attachment, these results are consistent with and help explain other studies of PE2 cleavage-defective Sindbis viruses. Whether cell culture-adapted Semliki Forest virus strains attach to HS remains to be determined.
All viruses that attached to cell surface HS also attached to heparin-agarose beads; however, heparin bead attachment was not always associated with efficient cell attachment. The partially cleaving viruses, the noncleaving XBXBX (FD1) mutant, and the noncleaving FDK70 mutant failed to attach efficiently to cells. Nevertheless, they attached to heparin beads to a degree similar to the high-affinity cell binding viruses, suggesting that the requirements for heparin bead attachment and cell surface HS attachment are different. Greater stringency in attachment to cellular HS may result from several factors. Since negative-charge density is likely higher on heparin than HS, less concentrated positive charge on the protein ligand may be required for productive interaction. Similarly, specific structural characteristics of the heparin and HS binding sites on protein ligands may differ. Recent studies indicate that there can be specific HS chain structures recognized by individual HS binding domains on protein ligands (reviewed in reference 13). In addition, in vitro binding studies suggest that the spacing of positively charged residues may be more critical to HS binding sites than to heparin binding sites (8). Consequently, heparin binding is likely more promiscuous than HS-mediated binding to cells and may not strictly correlate with HS attachment ability or biologically relevant interactions.
Relationship of binding to infectivity.
Cleavage of exterior viral spike glycoproteins is normally associated with activation of the viral spike complex for infection (19). During virus particle assembly, uncleaved spike proteins are presumed to be “protected” from premature fusion with infected host cell membranes. After cleavage, which usually occurs as a late event in morphogenesis, virus particles are released from the cell in a fusion-competent state. This also appears to be the case with Sindbis virus strain AR339. Completely cleavage defective Sindbis virus AR339 particles, while attaching very efficiently to cells, are poorly infectious, perhaps due to inhibition of fusion or other uncoating processes (36). In contrast, the Sindbis virus-like alphavirus S.A.AR86 (in which E2 Asn 1 was selected by adaptation to BHK cells [35]) exhibits enhanced infectivity when cleavage is inhibited by glycosylation at E2 Asn 1. With this virus, uncoating and fusion may be allowed in the presence of uncleaved PE2, with greater infectivity potentially arising from HS attachment mediated by the furin cleavage sequence. With Sindbis virus strain AR339, partial inhibition of cleavage (e.g., 39R1), may allow the virus to increase binding affinity due to the interaction of the furin site with HS while maintaining uncoating and fusion competence, resulting in an overall increase in infection efficiency. Similarly, internal mutations in E2 (Lys 70 and Arg 114) increase cell attachment while potentially maintaining or increasing uncoating and fusion efficiency, resulting in a large infectivity increase. Indeed, second-site-reverting mutations (e.g., Glu to Gly at E2 216), that promote enhanced infection efficiency can be selected in the cleavage-defective background (12). The E2 Gly 216 mutation does not alter the efficiency of binding mediated by the furin cleavage sequence (data not shown). Therefore, this mutation may act by improving the efficiency of uncoating and entry (36). In addition, the significant difference in infectivity among 39L1, 39V1, and 39N1 (Table 1), although these viruses bind similarly to cells (Fig. 2), may also result from uncoating and entry efficiency. While there are very likely different cellular HS structures bound by different Sindbis virus mutants (20), our data suggest that cell culture-adaptive mutations that increase virus attachment efficiency while maintaining uncoating and fusion competence result in enhanced infection efficiency for fibroblast cells such as BHK.
Can furin protease sequences of other virus types bind HS?
The results of these studies raise the possibility that furin protease cleavage sequences could participate in an HS-dependent attachment by other virus types. Spike proteins of the Togaviridae, Orthomyxoviridae, Paramyxoviridae, Flaviviridae, Coronaviridae, Toroviridae, Retroviridae, and Herpesviridae families are cleaved by furin-like host proteases at XBXBBX sequences during maturation (reviewed in reference 19). Several studies with human immunodeficiency virus (HIV) have suggested that some T-cell-tropic strains attach to HS during infection of cultured cells (29, 31, 34). At least one of the HS attachment domains has been mapped to the V3 loop of HIV gp120 (34). Interestingly, this region of the glycoprotein contains what has been termed an “occult” furin protease cleavage sequence that has been proposed to be cleaved during entry into cells (25). Additionally, two colocalized furin consensus sites in the immature gp160 can be cleaved during virus maturation to yield the mature gp120 (2). Short, branched-chain synthetic peptides comprising these gp160 furin protease cleavage sequences inhibit HIV replication in a dose-dependent manner (1). The antiviral effect is associated with peptide attachment to and internalization by cells; however, the mechanism of attachment and internalization of these peptides is unknown (1). It is possible that these positively charged peptides bind to cellular HS and are internalized as proteoglycan-peptide complexes.
The ability of the Sindbis virus furin protease cleavage sequence to mediate HS attachment may result from several factors: (i) as repeating clusters of positive charge are commonly found in protein HS binding motifs (13), the rigid icosahedral structure of alphavirus particles and consequent repeating structure of glycoprotein spikes may provide an appropriate constellation of positive charges for HS interaction, and (ii) fortuitous location of the cleavage sequence in the fully formed viral spike complex may promote access of cellular HS to the cleavage sequence. The putative location of E3 (bearing the XBXBBX cleavage sequence) in mature glycoprotein spikes containing PE2 is on the outer edge near the apical surface of the spike (30). In addition, E3 on adjacent spikes may be in close proximity (30), perhaps providing a repeating structure capable of interacting with HS. The likelihood that HS attachment can be mediated by furin protease recognition sequences of spike proteins of other viruses will depend upon factors such as location of the sequence in the mature spike, structural integrity of viral spikes containing uncleaved or partially cleaved precursor proteins, requirements for repeating or rigid structure in the HS binding domain, and whether infection competence can be maintained in the presence of partially or completely uncleaved spike proteins.
An additional factor may be whether a significant selective advantage is conferred by increasing HS-dependent attachment to cells. We have been unable to demonstrate efficient attachment of TR339 to cultured cells by using many different cell types and binding assay conditions (20), yet this virus is more virulent for neonatal mice than any of the cell culture-adapted Sindbis virus strains. During cultured-cell passage of TR339, mutations that increase binding and infection efficiency through HS attachment are rapidly selected. These results suggest that with cultured cells, the absence of an efficient attachment receptor results in selection for alternative attachment strategies. A similar mechanism may operate with type O strains of foot-and-mouth disease virus (FMDV). On susceptible cultured cells, and presumably in animal hosts, FMDV initiates infection through interaction with the αvβ3 integrin complex (28). With CHO cells, this complex is not present, and infection of native particles is inefficient (21). Passage in these cells rapidly selects for HS attachment (37), suggesting that the absence of a high-affinity receptor may promote strong selective advantage for viruses that can increase cell attachment. However, this in vitro selective advantage apparently compromises virus replication competence in animal hosts, as mutations conferring HS attachment are highly attenuating for both Sindbis virus and FMDV (20, 37).
In the present studies, we have characterized one of the sites in the Sindbis virus attachment protein that can mediate virion attachment to HS. Our work suggests that by altering the efficiency of cleavage of the PE2 precursor, Sindbis virus mutants can enhance attachment affinity and increase infectivity for cultured cells. In this instance, the virus has utilized a preexisting attribute of the attachment protein in adapting to cultured cells. Positive-charge mutations at E2 70 or E2 114 also dramatically enhance the HS-dependent attachment of Sindbis virus; however this mechanism is independent of PE2 cleavage (20). Further research will be required to determine if these internal mutations operate in concert with preexisting HS binding domains or create these domains de novo and whether the interaction of such domains with HS plays any role in the natural history of Sindbis virus populations.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grant AI22186. W.B.K. was supported by an NIH Predoctoral Traineeship (T32 AI07419) and by the U.S. Army Research Office (DAAH04-95-1-0224). H.W.H. was supported by a National Research Service Award (F32-AI09015) and by training grant (AI07151).
REFERENCES
- 1.Barbouche R, Sabatier J M, Fenouillet E. An anti-HIV peptide construct derived from the cleavage region of the Env precursor acts on Env fusogenicity through the presence of a functional cleavage sequence. Virology. 1998;247:137–143. doi: 10.1006/viro.1998.9239. [DOI] [PubMed] [Google Scholar]
- 2.Bosch V, Pawlita M. Mutational analysis of the human immunodeficiency virus type I env gene product proteolytic site. J Virol. 1990;64:2337–2344. doi: 10.1128/jvi.64.5.2337-2344.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Byrnes A P, Griffin D E. Binding of Sindbis virus to cell surface heparan sulfate. J Virol. 1998;72:7349–7356. doi: 10.1128/jvi.72.9.7349-7356.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cardin A D, Weintraub H J R. Molecular modeling of protein-glycosaminoglycan interactions. Arteriosclerosis. 1989;9:21–32. doi: 10.1161/01.atv.9.1.21. [DOI] [PubMed] [Google Scholar]
- 5.Chen Y, Maguire T, Hileman R E, Fromm J R, Esko J D, Linhardt R J, Marks R M. Dengue virus infectivity depends on envelope protein binding to target cell heparan sulfate. Nat Med. 1997;3:866–871. doi: 10.1038/nm0897-866. [DOI] [PubMed] [Google Scholar]
- 6.de Curtis I, Simons K. Dissection of Semliki Forest virus glycoprotein delivery from the trans-golgi network to the cell surface in permeablized BHK cells. Proc Natl Acad Sci USA. 1988;85:8052–8056. doi: 10.1073/pnas.85.21.8052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dubuisson J, Rice C M. Sindbis virus attachment: isolation and characterization of mutants with impaired binding to vertebrate cells. J Virol. 1993;67:3363–3374. doi: 10.1128/jvi.67.6.3363-3374.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fromm J R, Hileman R E, Caldwell E E O, Weiler J M, Linhardt R J. Pattern and spacing of basic amino acids in heparin binding sites. Arch Biochem Biophys. 1997;343:92–100. doi: 10.1006/abbi.1997.0147. [DOI] [PubMed] [Google Scholar]
- 9.Heidner H W, Johnston R E. The amino-terminal residue of Sindbis virus glycoprotein E2 influences virus maturation, specific infectivity for BHK cells, and virulence in mice. J Virol. 1994;68:8064–8070. doi: 10.1128/jvi.68.12.8064-8070.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heidner, H. W., and R. E. Johnston. Unpublished observations.
- 11.Heidner H W, Knott T A, Johnston R E. Differential processing of Sindbis virus glycoprotein PE2 in cultured vertebrate and arthropod cells. J Virol. 1996;70:2069–2073. doi: 10.1128/jvi.70.3.2069-2073.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heidner H W, McKnight K L, Davis N L, Johnston R E. Lethality of PE2 incorporation into Sindbis virus can be suppressed by second-site mutations in E3 and E2. J Virol. 1994;68:2683–2692. doi: 10.1128/jvi.68.4.2683-2692.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hileman R E, Fromm J R, Weiler J M, Linhardt R J. Glycosaminoglycan-protein interactions: definition of consensus sites in glycosaminoglycan binding proteins. Bioessays. 1998;20:156–167. doi: 10.1002/(SICI)1521-1878(199802)20:2<156::AID-BIES8>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 14.Horimoto T, Kawaoka Y. The hemagglutinin cleavability of a virulent avian influenza virus by subtilisin-like endoproteases is influenced by the amino acid immediately downstream of the cleavage site. Virology. 1995;210:466–470. doi: 10.1006/viro.1995.1363. [DOI] [PubMed] [Google Scholar]
- 15.Hsieh P, Robbins P W. Regulation of asparagine-linked oligosaccharide processing: oligosaccharide processing in Aedes albopictus mosquito cells. J Biol Chem. 1984;259:2375–2382. [PubMed] [Google Scholar]
- 16.Jackson R L, Busch S J, Cardin A D. Glycosaminoglycans: molecular properties, protein interactions, and role in physiological processes. Physiol Rev. 1991;71:481–539. doi: 10.1152/physrev.1991.71.2.481. [DOI] [PubMed] [Google Scholar]
- 17.Kaariainen L, Hashimoto K, Saraste J, Virtanen I, Penttinen K. Monensin and FCCP inhibit the intracellular transport of alphavirus membrane glycoproteins. J Cell Biol. 1980;87:783–791. doi: 10.1083/jcb.87.3.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kjellen L, Lindahl U. Proteoglycans: structures and interactions. Annu Rev Biochem. 1991;60:443–475. doi: 10.1146/annurev.bi.60.070191.002303. [DOI] [PubMed] [Google Scholar]
- 19.Klenk H-D, Garten W. Activation cleavage of viral spike proteins by host proteases. In: Wimmer E, editor. Cellular receptors for animal viruses. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1994. pp. 241–280. [Google Scholar]
- 20.Klimstra W B, Ryman K D, Johnston R E. Adaptation of Sindbis virus to BHK cells selects for use of heparan sulfate as an attachment receptor. J Virol. 1998;72:7357–7366. doi: 10.1128/jvi.72.9.7357-7366.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mason P W, Baxt B, Brown F, Harber J, Murdin A, Wimmer E. Antibody complexed foot-and-mouth disease virus, but not poliovirus, can infect cells by the Fc receptor. Virology. 1993;192:568–577. doi: 10.1006/viro.1993.1073. [DOI] [PubMed] [Google Scholar]
- 22.McKnight K L, Simpson D A, Lin S C, Knott T A, Polo J M, Pence D F, Johannsen D B, Heidner H W, Davis N L, Johnston R E. Deduced consensus sequence of Sindbis virus strain AR339: mutations contained in laboratory strains which affect cell culture and in vivo phenotypes. J Virol. 1996;70:1981–1989. doi: 10.1128/jvi.70.3.1981-1989.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moehring J M, Inocencio N M, Robertson B J, Moehring T J. Expression of mouse furin in a Chinese hamster cell resistant to Pseudomonas exotoxin A and viruses complements the genetic lesion. J Biol Chem. 1993;268:2590–2594. [PubMed] [Google Scholar]
- 24.Moehring J M, Moehring T J. Strains of CHO-K1 cells resistant to Pseudomonas exotoxin A and cross-resistant to diphtheria toxin and viruses. Infect Immun. 1983;41:998–1009. doi: 10.1128/iai.41.3.998-1009.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morikawa Y, Barsov E, Jones I. Legitimate and illegitimate cleavage of human immunodeficiency virus glycoproteins by furin. J Virol. 1993;67:3601–3604. doi: 10.1128/jvi.67.6.3601-3604.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagai Y. Protease-dependent virus tropism and pathogenicity. Trends Microbiol. 1993;1:81–87. doi: 10.1016/0966-842X(93)90112-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakayama K. Furin: a mammalian subtilisin/Kex2p-like endoprotease involved in processing of a wide variety of precursor proteins. Biochem J. 1997;327:625–635. doi: 10.1042/bj3270625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neff S, Sa-Carvalho D, Rieder E, Mason P W, Blystone S D, Brown E J, Baxt B. Foot-and-mouth disease virus virulent for cattle utilizes the integrin alpha(v)beta3 as its receptor. J Virol. 1998;72:3587–3594. doi: 10.1128/jvi.72.5.3587-3594.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ohshiro Y, Murakami T, Matsuda K, Nishioka K, Yoshida K, Yamamoyo N. Role of cell surface glycosaminoglycans of human T cells in human immunodeficiency virus type-1 (HIV-1) infection. Microbiol Immunol. 1996;40:827–835. doi: 10.1111/j.1348-0421.1996.tb01148.x. [DOI] [PubMed] [Google Scholar]
- 30.Paredes A M, Heidner H, Thuman-Commike P, Venkataram Prasad B V, Johnston R E, Chiu W. Structural localization of the E3 glycoprotein in attenuated Sindbis virus mutants. J Virol. 1998;72:1534–1541. doi: 10.1128/jvi.72.2.1534-1541.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patel M, Yanagishita M, Roderiquez G, Bouhabib D C, Oravecz T, Hascall V C, Norcross M A. Cell-surface heparan sulfate proteoglycan mediates HIV-1 infection of T-cell lines. AIDS Res Hum Retroviruses. 1993;9:167–174. doi: 10.1089/aid.1993.9.167. [DOI] [PubMed] [Google Scholar]
- 32.Presely J F, Brown D T. The proteolytic cleavage of PE2 to envelope glycoprotein E2 is not strictly required for the maturation of Sindbis virus. J Virol. 1989;63:1975–1980. doi: 10.1128/jvi.63.5.1975-1980.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rice C M, Strauss J H. Nucleotide sequence of the 26S mRNA of Sindbis virus and deduced sequence of the encoded virus structural proteins. Proc Natl Acad Sci USA. 1981;78:2062–2066. doi: 10.1073/pnas.78.4.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roderiquez G, Oravecz T, Yanagishita M, Bou-Habib D C, Mostowski H, Norcross M A. Mediation of human immunodeficiency virus type 1 binding by interaction of cell surface heparan sulfate proteoglycans with the V3 region of envelope gp120-gp41. J Virol. 1995;69:2233–2239. doi: 10.1128/jvi.69.4.2233-2239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Russell D L, Dalrymple J M, Johnston R E. Sindbis virus mutations which coordinately affect glycoprotein processing, penetration, and virulence in mice. J Virol. 1989;63:1619–1629. doi: 10.1128/jvi.63.4.1619-1629.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ryman, K. D., and R. E. Johnston. 1997. Unpublished observations.
- 37.Sa-Carvalho D, Reider E, Baxt B, Rodarte R, Tanuri A, Mason P. Tissue culture adaptation of foot-and-mouth disease virus selects viruses that bind to heparin and are attenuated in cattle. J Virol. 1997;71:5115–5123. doi: 10.1128/jvi.71.7.5115-5123.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salminen A, Wahlberg J M, Lobigs M, Liljestrom P, Garoff H. Membrane fusion process of Semliki Forest virus II: cleavage-dependent reorganization of the spike protein complex controls virus entry. J Cell Biol. 1992;116:349–357. doi: 10.1083/jcb.116.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sarkar G, Sommer S S. The “megaprimer” method of site-directed mutagenesis. BioTechniques. 1990;8:404–407. [PubMed] [Google Scholar]
- 40.Sobel M, Soler D F, Kermode J C, Harris R B. Localisation and characterization of a heparin binding domain peptide of human von Willebrand factor. J Biol Chem. 1992;267:8857–8862. [PubMed] [Google Scholar]
- 41.Stollar V, Stollar B D, Koo R, Harrap K A, Schlesinger R W. Sialic acid contents of sindbis virus from vertebrate and mosquito cells. Equivalence of biological and immunological viral properties. Virology. 1976;69:104–115. doi: 10.1016/0042-6822(76)90198-7. [DOI] [PubMed] [Google Scholar]
- 42.Strauss J H, Strauss E G. The alphaviruses: gene expression, replication, and evolution. Microbiol Rev. 1994;58:491–562. doi: 10.1128/mr.58.3.491-562.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Summerford C, Samulski R J. Membrane-associated heparan sulfate proteoglycan is a receptor for adeno-associated virus type 2 virions. J Virol. 1998;72:1438–1445. doi: 10.1128/jvi.72.2.1438-1445.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Templeton D M. Proteoglycans in cell regulation. Crit Rev Clin Lab Sci. 1992;29:141–184. doi: 10.3109/10408369209114599. [DOI] [PubMed] [Google Scholar]
- 45.Van de Ven W J, Creemers J W, Roebroek A J. Furin: the prototype mammalian subtilisin-like proprotein-processing enzyme. Endoproteolytic cleavage at paired basic residues of proproteins of the eukaryotic secretory pathway. Enzyme. 1991;45:257–270. doi: 10.1159/000468900. [DOI] [PubMed] [Google Scholar]
- 46.WuDunn D, Spear P G. Initial interaction of herpes simplex virus with cells is binding to heparan sulfate. J Virol. 1989;63:52–58. doi: 10.1128/jvi.63.1.52-58.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]