ABSTRACT
Cells use transition metal ions as structural components of biomolecules and cofactors in enzymatic reactions, making transition metal ions integral cellular components. Organisms optimize metal ion concentration to meet cellular needs by regulating the expression of proteins that import and export that metal ion, often in a metal ion concentration-dependent manner. One such regulation mechanism is via riboswitches, which are 5′-untranslated regions of an mRNA that undergo conformational changes to promote or inhibit the expression of the downstream gene, commonly in response to a ligand. The yybP-ykoY family of bacterial riboswitches shares a conserved aptamer domain that binds manganese ions (Mn2+). In Escherichia coli, the yybP-ykoY riboswitch precedes and regulates the expression of two different genes: mntP, which based on genetic evidence encodes an Mn2+ exporter, and alx, which encodes a putative metal ion transporter whose cognate ligand is currently in question. The expression of alx is upregulated by both elevated concentrations of Mn2+ and alkaline pH. With metal ion measurements and gene expression studies, we demonstrate that the alkalinization of media increases the cytoplasmic manganese pool, which, in turn, enhances alx expression. The Alx-mediated Mn2+ export prevents the toxic buildup of the cellular manganese, with the export activity maximal at alkaline pH. We pinpoint a set of acidic residues in the predicted transmembrane segments of Alx that play a critical role in Mn2+ export. We propose that Alx-mediated Mn2+ export serves as a primary protective mechanism that fine tunes the cytoplasmic manganese content, especially during alkaline stress.
IMPORTANCE
Bacteria use clever ways to tune gene expression upon encountering certain environmental stresses, such as alkaline pH in parts of the human gut and high concentration of a transition metal ion manganese. One way by which bacteria regulate the expression of their genes is through the 5′-untranslated regions of messenger RNA called riboswitches that bind ligands to turn expression of genes on/off. In this work, we have investigated the roles and regulation of alx and mntP, the two genes in Escherichia coli regulated by the yybP-ykoY riboswitches, in alkaline pH and high concentration of Mn2+. This work highlights the intricate ways through which bacteria adapt to their surroundings, utilizing riboregulatory mechanisms to maintain Mn2+ levels amidst varying environmental factors.
KEYWORDS: yybP-ykoY, riboswitches, exporters, TerC family, manganese, alx, mntP
INTRODUCTION
Transition metals are essential in all organisms as structural elements of proteins and RNA and as reactive centers in enzymes. Among these metals, iron (Fe2+) acts as a cofactor in many cellular enzymes essential for life, e.g., those involved in respiratory pathways. During aerobic growth or in response to oxidizing agents such as hydrogen peroxide (H2O2), cells generate reactive oxygen species (ROS) that can oxidize Fe2+, thereby inactivating Fe2+-dependent enzymes and leading to cytotoxic effects if not treated. To counter ROS-caused negative consequences, Escherichia coli (E. coli) relies on an Mn2+-dependent isoenzyme of superoxide dismutase (SOD), an enzyme that converts highly reactive superoxide radicals to molecular oxygen and H2O2: cytosolic Mn2+-dependent SodA takes over in aerobic conditions when the activity of Fe2+-dependent SodB is insufficient to scavenge superoxide (1).
To be ready for an impending ROS threat, E. coli maintain a constant cellular pool of manganese through the uptake activity of the only characterized Mn2+ importer, MntH (2, 3). The total cellular concentration of manganese (Mn, free and bound) is estimated to be 15–21 µM by inductively coupled plasma mass spectrometry (ICP-MS) measurements (4–6). MntH uses conserved acidic transmembrane residues to coordinate Mn2+ for import and relies on a proton gradient across the inner membrane of an E. coli cell as a driving force for Mn2+ uptake (7–10). A low-affinity Zn2+ transporter, ZupT, in E. coli was also reported to uptake Mn2+ along with Fe2+, Cd2+, and Co2+ (11, 12). Notwithstanding its critical role within the cell, Mn2+ concentration ([Mn2+]) must be limited as it is toxic to the cell in high concentrations. Excess Mn2+ replaces similarly sized Fe2+ as a cofactor in enzymes and can alter levels of other metal ions (4, 5). To prevent the toxic buildup of Mn2+, the expression of mntH is repressed by elevated [Mn2+] and an Mn2+-dependent transcriptional regulator MntR (3, 13). As an additional protective measure, excess Mn2+ is transported out of E. coli by its only exporter characterized to date, MntP (4, 5, 13, 14). Similar to MntH, several conserved acidic residues within the membrane are implicated in the Mn2+ efflux activity of MntP (14). YiiP, an Fe2+ and Zn2+ exporter, is implicated in protection against Mn2+ intoxication in Salmonella enterica Serovar Typhimurium (15) and is also present in E. coli but not studied for its Mn2+ export potential.
One of the mechanisms by which mntP expression is tuned in response to the changing intracellular [Mn2+] is via the riboswitch in the 5′ untranslated region (UTR) of the mntP gene. Riboswitches are cis-acting elements in the UTRs of mRNAs, meaning that they alter transcriptional and/or translational outcomes for that mRNA. Riboswitches do so by shifting their structural ensembles upon binding to a ligand (16). For example, ligand binding might favor folding of the riboswitch RNA into a hairpin that terminates transcription to attenuate expression of the downstream gene (transcriptional riboswitch) (17, 18). Alternatively, ligand binding can promote the mRNA with a single-stranded ribosome-binding site (RBS), thus enhancing the translation of that mRNA (translational riboswitch). The mntP riboswitch was characterized as a translational riboswitch where the translation is turned on in response to increased intracellular Mn (19, 20). As a member of the ubiquitous yybP-ykoY riboswitch family (21, 22), the mntP riboswitch turns on mntP mRNA translation by binding free Mn2+. A second yybP-ykoY riboswitch in E. coli precedes a gene (alx) that, curiously, is highly induced in response to alkaline pH (23, 24). The expression of both mntP and alx increases in media with elevated [Mn2+] (19, 20). The alx encodes a putative Mn2+ transporter that belongs to the TerC superfamily of proteins (14, 25); however, the function of the Alx protein has not been definitively established.
A prior study indicated that overexpression of Alx results in an increase in the intracellular Mn pool and suggested that Alx may act as an Mn2+ importer (14). This proposal, however, is contradicted by the observations from earlier reports that the expression of alx and mntP (Mn2+ exporter) is increased by supplemented Mn2+ in the media, whereas the expression of mntH (Mn2+ importer) is repressed. If Alx were, indeed, an Mn2+ importer, its expression in response to changing [Mn2+] would have paralleled that of MntH, not MntP. Here, we present evidence that Alx is an exporter of Mn2+ serving as the first line of defense against the potential buildup of cytoplasmic Mn at alkaline pH. By examining the effect of alkaline intracellular pH and increased [Mn2+] on alx expression through transcriptional and translational reporters, we show that these two environmental cues are linked. Additionally, we demonstrate that Alx activity is stimulated by alkaline pH and posit involvement of transmembrane acidic residues of Alx in Mn2+ export.
RESULTS
Increased extracellular pH and Mn enhance alx expression
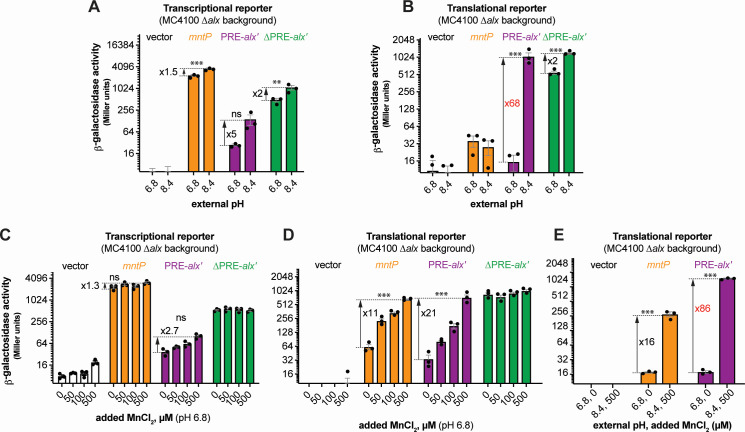
To study the connection between alkaline pH and Mn homeostasis, we employed transcriptional and translational lacZ reporter fusions of alx and mntP cloned with their respective native promoters into single-copy plasmids (Fig. S1A). Effects of extracellular alkaline pH and elevated extracellular [Mn2+] on gene expression were measured by β-galactosidase assays in E. coli strain (MC4100) lacking alx (referred to as ∆alx). Briefly, the ∆alx strain was transformed with plasmids containing either alx or mntP transcriptional or translational reporters (Fig. S1A; Table S2). The strains were cultivated in (i) neutral pH (LBK pH 6.8) or alkaline pH (LBK pH 8.4) media to test the effect of pH on alx transcriptional and translational reporters as described in prior work (24) and (ii) LB (pH 6.8) with supplemented MnCl2 to test the effect of Mn2+ on alx transcriptional and translational reporters. These experimental conditions were tested in parallel on mntP transcriptional and translational reporters (19). We observed that alx transcription increased 5-fold at alkaline pH (Fig. 1A), whereas mntP transcription increased 1.5-fold (Fig. 1A), consistent with an increase in the average rate of nucleotide addition as pH increases (26, 27). The higher increase in the alx vs mntP transcriptional reporter activity at alkaline pH can be explained by the proposed intrinsic terminator in hairpin D forming within the 5′ UTR of alx in neutral but not alkaline pH (19, 24) (Fig. S1B). In contrast, the alx translational reporter produced a striking 68-fold higher signal in alkaline pH—higher than previously observed (24), whereas the mntP translational reporter was unaffected by alkaline pH (Fig. 1B). These results indicate that alx expression is largely regulated post-transcriptionally in alkaline pH, consistent with previous work (24).
Fig 1.
Regulation of alx expression by PRE in response to an increased external pH and [Mn2+]. (A and C) β-galactosidase activities (in Miller units) of mid-log phase grown cultures of ∆alx::Kan derivatives of MC4100 strain of E. coli (RAS31) carrying one of the following plasmids: promoter-less vector with lacZ (pMU2385), transcriptional reporter of mntP (PmntP-5′UTR-mntP′-lacZ, pRA48), transcriptional reporter of alx (Palx-PRE-alx′-lacZ, pRA40), or ∆PRE derivative of alx transcriptional reporter (Palx-alx′-lacZ, pRA41). The above cultures were cultivated in LBK media pH 6.8 or pH 8.4 (panel A) and LB pH 6.8 with and without supplemented MnCl2 (panel C). (B and D) β-galactosidase activities of mid-log phase grown cultures of ∆alx::Kan derivatives of MC4100 strain of E. coli (RAS31) carrying one of the following plasmids: promoter-less vector with lacZ (pMU2386), translational reporter of mntP (PmntP-5′UTR-mntP′-lacZ, pRA57), translational reporter of alx (Palx-PRE-alx′-lacZ, pRA54), or ∆PRE derivative of alx translational reporter (Palx-alx′-lacZ, pRA55). The above cultures were grown in LBK media pH 6.8 or 8.4 (panel B) and LB pH 6.8 with and without supplemented MnCl2 (panel D). A combined effect of alkaline pH and supplemented MnCl2 on translational reporters in LBK media pH 6.8 or LBK media pH 8.4 supplemented with MnCl2 is illustrated in panel E. Each plotted value in a bar graph with standard error of mean (SEM) is an average of three biological replicates of the experiment. The statistical significance of the changes in the reporter signal with growth conditions was assessed by two-way ANOVA (nsP > 0.05, **P < 0.01, ***P < 0.001).
The 5′ UTR of alx mRNA referred to as the pH-responsive RNA element (PRE) regulates alx translation in response to a pH change as previously shown (24). We observed that the translational reporter of alx that lacks PRE (∆PRE) exhibited only a 2-fold increase in alkaline pH vs 68-fold increase with PRE present (Fig. 1B). PRE contains two intrinsic transcription terminators (Fig. S1B), and their absence is the dominant cause of higher transcriptional output in the ∆PRE transcriptional reporter. The ∆PRE alx translational reporter displayed high β-galactosidase activity in both pH conditions, with a twofold increase in alkaline vs neutral pH (Fig. 1B), in good agreement with the corresponding twofold increase in ∆PRE alx transcription upon alkalinization (Fig. 1A). Taken together, these results show that PRE regulates both transcription and translation of alx in a pH-responsive manner.
Upon supplementation of MnCl2 to the LB media (pH 6.8), the alx and mntP transcriptional reporter outputs increased 2.7-fold and 1.4-fold, respectively (Fig. 1C). In stark contrast, both alx and mntP translational reporters were progressively induced by increasing [Mn2+], with a 21- and an 11-fold increase, respectively, at the highest MnCl2 concentration tested (500 µM) (Fig. 1D). The ∆PRE translational reporter of alx was unaffected by the added MnCl2 and displayed high reporter activity throughout (Fig. 1D), suggesting that PRE tunes alx expression post-transcriptionally in an [Mn2+]-responsive manner. The response of alx and mntP to alkaline pH or added Mn2+ is also observed in MG1655 strain background, albeit not as pronounced as in MC4100 (Fig. S2).
We next tested the combined effect of alkaline pH and high extracellular [Mn2+], on alx and mntP translational reporters. We found that alx and mntP translational reporters were induced 86- and 16-fold, respectively, in alkaline media with 500 µM MnCl2 (Fig. 1E). Altogether, our results (Fig. 1B, D and E) demonstrate that the effects of elevated extracellular [Mn2+] and alkaline pH on alx expression are additive and may enhance alx expression by independent routes. The mntP expression is induced further if both alkaline pH and extra MnCl2 are provided compared to MnCl2 supplementation alone (Fig. 1E) even though alkaline pH alone had no impact on mntP expression (Fig. 1A and B). Alkaline pH, thus, enhances the Mn2+ effect on mntP expression, consistent with a previously published study (20).
The expression of alx is post-transcriptionally autoregulated by Mn2+ in alkaline pH
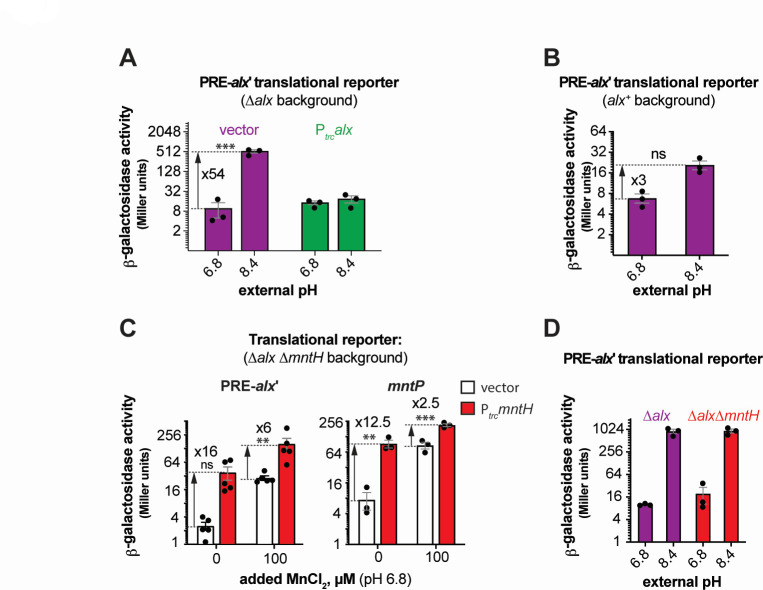
The alx translational reporter activity significantly increased at alkaline pH in the ∆alx strain (Fig. 1B). Intriguingly, this induction was reverted by (i) expressing Alx from a synthetic trc promoter (Ptrc) in the ∆alx strain (Fig. 2A) or (ii) preserving the chromosomally encoded Alx (Fig. 2B). Similarly, induction of alx translation dropped from 21-fold (Fig. 1D) to 11-fold in a strain expressing Alx chromosomally from its native promoter in LB (pH 6.8) with 500 µM MnCl2 (Fig. S3A). Alx, thus, represses its own expression post-transcriptionally at alkaline pH or high [Mn2+]. This repression could be explained by Alx exporting Mn2+ and reducing intracellular [Mn] as described later (see “Alx mediates the export of Mn2+ in alkaline environment” section).
Fig 2.
The induction of alx expression in alkaline pH and its dependence on [Mn2+]. (A and B) β-galactosidase activity (in Miller units) as a reporter of alx translation (Palx-PRE-alx′-lacZ, pRA54). (A) The reporter activity was measured in mid-log phase grown cultures of ∆alx::Kan derivative (RAS31) bearing an empty vector (pHYD5001) or pHYD5001 expressing Alx from Ptrc promoter (pRA27). The cultures were grown in LBK media pH 6.8 or 8.4, supplemented with appropriate concentration of ampicillin, MnCl2, and IPTG. (B) The reporter activity was measured in mid-log phase grown cultures of alx+ strain (MC4100) in LBK media pH 6.8 or 8.4. (C) β-galactosidase activity (in Miller units) as a reporter of alx translation (Palx-PRE-alx′-lacZ, pRA54) and mntP translation (PmntP-5′UTR-mntP′-lacZ, pRA57) was measured in mid-log phase grown cultures of ∆alx ∆mntH::Kan derivative (RAS93) containing vector (pHYD5001) or derivative of pHYD5001 expressing MntH (pRA94) from Ptrc promoter. The cells were cultured in LB pH 6.8 supplemented with appropriate concentration of ampicillin. The statistical significance of the changes in measurements was assessed by two-way ANOVA (nsP > 0.05, **P < 0.01, ***P < 0.001). (D) β-galactosidase activity (in Miller units) as a reporter of alx translation (Palx-PRE-alx′-lacZ, pRA54) was measured in mid-log phase grown cultures of ∆alx mutant (RAS40) and its ∆mntH::Kan derivative (RAS93) in LBK media with pH 6.8 or 8.4. Each plotted value in a bar graph with standard error of mean (SEM) is an average of three biological replicates of the experiment.
To probe if import of trace extracellular Mn2+ is sufficient to upregulate alx expression, we tested the effect of Ptrc-expressed MntH (Mn2+ importer) on alx expression. These measurements were performed in a strain that lacks both chromosomally encoded Alx and MntH (∆alx ∆mntH) in LB (pH 6.8) with and without additional MnCl2. When transcribed from Ptrc, the mntH expression is no longer repressed by MntR in a [Mn2+]-dependent manner like chromosomal mntH (3); therefore, MntH should be continuously expressed from Ptrc to import Mn2+ regardless of the changes in intracellular [Mn2+]. Basal expression of mntH (no added IPTG) from Ptrc enhanced translation of both alx and mntP even without added MnCl2 (Fig. 2C), pointing to an MntH-mediated import of trace Mn2+ from the media at neutral pH and confirming that the import of extra Mn2+ increases alx expression. IPTG-induced expression of mntH was toxic for growth and prevented us from expression measurements in LB containing IPTG.
One possible mechanism by which alkaline pH may increase the intracellular Mn pool via trace Mn2+ import, thereby increasing alx translation, is by directly enhancing the activity of MntH importer. To address this possibility, we measured the alx translational reporter activity in the ∆alx ∆mntH strain. The absence of chromosomal mntH had no impact on the pH-induced increase in the activity of alx translation reporter (Fig. 2D), indicating that pH-driven induction of alx translation is independent of Mn2+ uptake by MntH at alkaline pH and suggesting an MntH-independent route for Mn2+ uptake operating at alkaline pH. At neutral pH, supplemental MnCl2 increased alx translation whether or not the chromosomal mntH was present (Fig. S3B); thus, an alternative, MntH-independent route for Mn2+ into the cell likely exists regardless of pH.
Alx does not participate in maintaining cellular pH
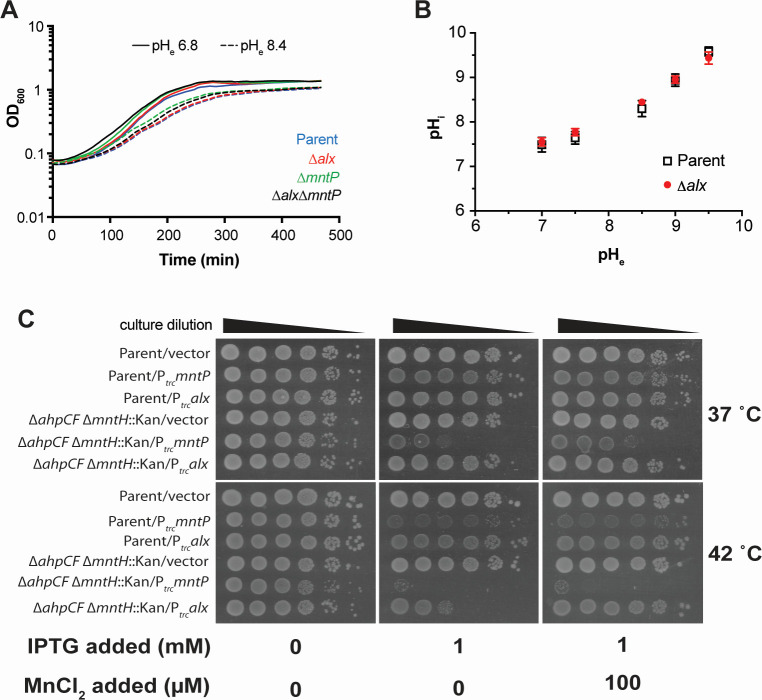
Our data confirm earlier work which demonstrated that the expression of alx is induced in media with alkaline pH or high [Mn2+] [Fig. 1 (19, 20, 23, 24)]. Perhaps, the simplest explanation for the pH stress-induced production of Alx would be its direct involvement in bringing the high intracellular pH back into its neutral physiological range. To probe the contribution of Alx to pH homeostasis, we tested the growth of the parent strain (MC4100) and its ∆alx derivative in LBK media at pH 6.8 or 8.4. The growth of the ∆mntP and the ∆alx ∆mntP mutants was also tested under these conditions. In general, the growth slowed in the pH 8.4 media compared to pH 6.8 (Fig. 3A). Absence of Alx did not affect the growth rate in alkaline pH in comparison to its parent strain, suggesting that Alx does not provide a growth advantage in alkaline pH.
Fig 3.
Effect of an increased external pH and combined effect of oxidative stress and perturbed cellular manganese levels on cellular growth. (A) Growth of the parent strain (MC4100) and its derivatives (∆alx::Kan derivative, RAS31; ∆mntP::Kan derivative, RAS32, and ∆alx ∆mntP::Kan derivative, RAS42) in LBK media pH 6.8 or pH 8.4. (B) Cytoplasmic pH (pHi) measured in the parent strain (MC4100) and its ∆alx::Kan derivative (RAS31) expressing pHluorin in M63A media of varying pH. Each plotted value of pHi in the graph with SEM is an average of three biological replicates of the experiment. (C) The spotting assay of tenfold serial dilutions (left to right) of overnight-grown cultures of parent strain (MC4100) and its ∆ahpCF ∆mntH::Kan derivative (RAS95) each bearing an empty vector (pHYD5001) or the same vector expressing MntP (pRA29) or Alx (pRA27) from Ptrc promoter. The serial dilutions were spotted on the surface of LB agar containing the appropriate concentration of ampicillin, MnCl2, and IPTG. Plates were imaged after an incubation at 37 or 42°C for 14 hours. The data shown are representative of three biological replicates of the experiment.
To further rule out the involvement of Alx in pH homeostasis, cytoplasmic pH of the parent strain and its ∆alx derivative were measured over a range of pH values (7, 7.5, 8.5, 9, and 9.5) using genetically encoded ratiometric pHluorin as a reporter (28). Due to interference of LB broth components with the measurements, experiments were performed in M63A media. The cytoplasmic pH of the parent strain increased with an increasing external pH (Fig. 3B), consistent with prior data that showed cytoplasmic pH of E. coli alkalinizing in response to an increased external pH in LB and then recovering partially to 7.8 (29). The cytoplasmic pH of the ∆alx mutant did not differ from its parent strain across the tested pH (Fig. 3B). These results indicate that Alx participation in countering alkalization of cytoplasmic pH is unlikely.
Connection between oxidative stress and Mn2+ export
In line with Mn2+ protecting cells from oxidative damage, the expression of mntH (Mn2+ importer) increases in the presence of high extracellular or endogenously produced H2O2 (2, 10, 30). MntH becomes vital for growth in aerobic conditions of a strain (∆katG ∆katE ∆ahpCF) lacking catalase and peroxidases that would normally clear accumulating H2O2 (2). The ahpCF, in particular, encodes a primary scavenger of endogenously produced H2O2 (31).
To test the role of Alx in the connection between Mn2+ metabolism and oxidative stress, alx and mntP were expressed from Ptrc in the wild-type strain, its ΔmntH, ΔahpCF, and ΔahpCF ΔmntH derivatives at 37 or 42°C (Fig. 3C; Fig. S4A and B). We first confirmed that the ΔahpCF ΔmntH double mutant experiences oxidative stress even upon alx or mntP expression using katG (bifunctional catalase-peroxidase) transcriptional reporter, which is induced in the oxidative environment (32) (Fig. S4C and D). The Ptrc-expressed Alx and MntP only mildly affected the growth of the wild-type strain or its ΔmntH derivative at either temperature (Fig. S4B). In contrast, the growth of ΔahpCF mutant was significantly inhibited by mntP expression at both temperatures (Fig. S4A). The growth inhibition became exacerbated in the ΔahpCF ΔmntH double mutant at 42 vs 37°C (Fig. 3C), likely due to thermal effects on protein folding and function at higher temperatures (33–35). Supplemental MnCl2 did not improve the growth of ΔahpCF ΔmntH strain in the presence of Ptrc-expressed MntP (Fig. 3C), consistent with the lack of MntH importer. The growth of ΔahpCF strain was likewise unaffected by added MnCl2 (Fig. S4A), suggesting that any extra Mn2+ imported by MntH was exported by overexpressed MntP. The Ptrc-expressed Alx did not inhibit the growth of ΔahpCF mutant at either temperature (Fig. S4A). Interestingly, however, alx expression inhibited the growth of ΔahpCF ΔmntH double mutant at 42°C (Fig. 3C), serving as the first indication of a role for Alx in Mn2+ export. MnCl2 supplementation in the presence of Ptrc-expressed Alx rescued ΔahpCF ΔmntH double mutant’s growth (Fig. 3C), in line with the weaker Mn2+ export activity of Alx vs MntP suggested by other data in this work (e.g., Fig. 4D).
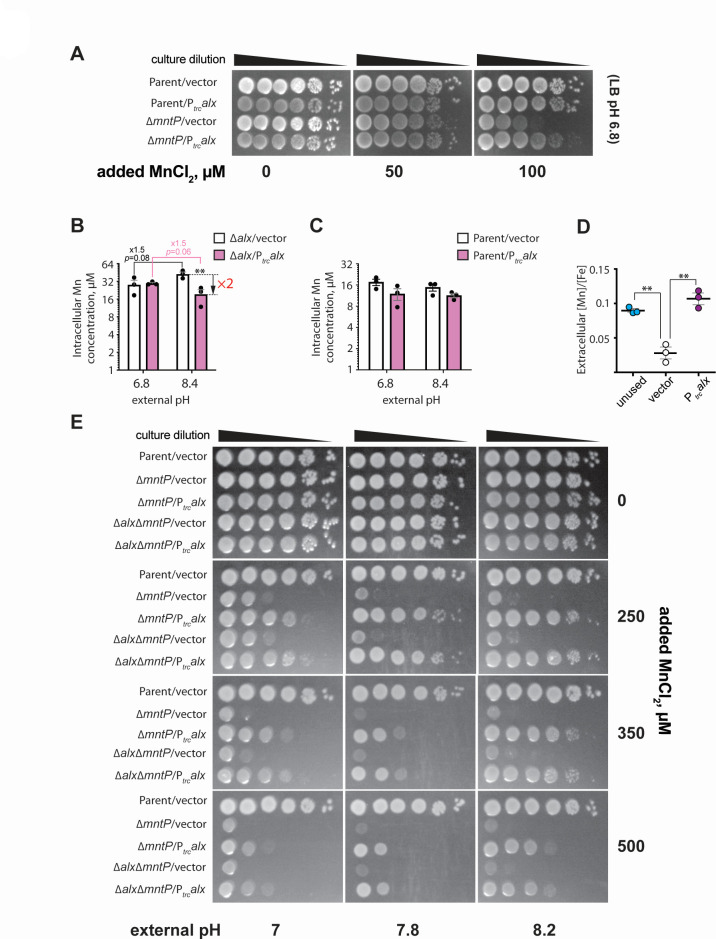
Fig 4.
Alx exports Mn2+ at alkaline pH. (A) The spotting assay of tenfold serial dilutions (left to right) of overnight-grown cultures of parent strain (MC4100) and its ∆mntP::Kan derivative (RAS32), each bearing an empty vector (pHYD5001) or the same vector expressing Alx from Ptrc promoter. The serial dilutions were spotted on the surface of LB agar containing the appropriate concentration of ampicillin, MnCl2, and IPTG. The data shown are representative of three biological replicates of the experiment. Intracellular Mn concentrations measured by ICP-MS in ∆alx mutant (RAS31) (B) or its parent strain (MC4100) (C) carrying a vector (pHYD5001) or a derivative of pHYD5001 expressing Alx from Ptrc promoter (pRA27). The cells were grown to mid-log phase in LBK media pH 6.8 or 8.4 supplemented with 1 mM IPTG and appropriate concentration of ampicillin. The reported metal concentrations with SEM are an average of three biological replicates of the experiment. The statistical significance of the changes in measurements was determined by two-way ANOVA (**P < 0.01). (D) The ratio of extracellular [Mn] to [Fe] measured by ICP-MS in unused LBK media pH 8.4 and in LBK media pH 8.4 after the exponential growth of ∆alx mutant (RAS31) containing a vector (pHYD5001) or a derivative of pHYD5001 expressing Alx from Ptrc promoter (pRA27). The reported [Mn]/[Fe] values with SEM are an average of three biological replicates of the experiment. For unused media, reported [Mn]/[Fe] is an average of three technical replicates. The statistical significance of the changes in measurements was assessed by unpaired Student’s t test (**P < 0.01). (E) The spotting assay of 10-fold serial dilutions (left to right) of overnight-grown cultures of parent strain (MC4100) and its derivatives (∆mntP::Kan derivative, RAS32, and ∆alx ∆mntP::Kan derivative, RAS42), each bearing an empty vector (pHYD5001) or the same vector expressing Alx from Ptrc promoter. The serial dilutions were spotted on the surface of LB agar of varying pH and supplemented with appropriate concentration of ampicillin, MnCl2, and IPTG. The data shown are representative of three biological replicates of the experiment.
Previously, it was noted that alx expression is sensitive to the presence of an oxidizing agent, paraquat (36). Considering that alx expression is induced by alkaline pH, we investigated the connection between alkaline pH and oxidative stress. We assessed the oxidative stress in the parent strain and its ∆alx mutant in alkaline pH and in the presence of high extracellular [Mn2+], using katG transcriptional reporter (32). We measured the katG transcriptional reporter activity in LBK media with pH 6.8 or 8.4 (Fig. S4D). A marginal induction of the katG transcription reporter was observed in both parent and its ∆alx derivative at pH 8.4. The katG transcriptional reporter activity was marginally repressed in both parent strain and ∆alx mutant in LB (pH 6.8) media upon supplementation of MnCl2 (Fig. S4E). Overall, these results suggest that Alx may not be directly participating in the maintenance of a redox stress at alkaline pH or high extracellular [Mn2+].
Alx mediates the export of Mn2+ in alkaline environment
Alx exports excess Mn 2+
The absence of Alx alone did not alter the growth of the wild-type strain or its ∆mntP derivative in media with high [Mn2+] (Table S4). Nonetheless, in light of both alx and mntP expression upregulated by high [Mn2+] [Fig. 1D (19, 20)], we set out to test whether heterologous expression of alx would rescue the Mn2+ sensitivity phenotype of the ∆mntP mutant (RAS32 strain). We found that the expression of alx from Ptrc (no riboswitch control), indeed, partially rescued the growth of ∆mntP mutant in the presence of supplemental Mn2+ (Fig. 4A), whereas the growth of the parent strain was not altered. These results indicate that Alx may mediate the export of Mn2+ in circumstances when cytoplasmic Mn2+ levels are elevated.
To test whether the Mn content of the cells increases at alkaline pH when Alx is most expressed, intracellular concentrations of transition metals ions (Mn, Fe, and Zn) were measured by ICP-MS in the ∆alx mutant (to preclude potential transport of metal ions by the Alx prior to the measurement) under neutral or alkaline pH (Fig. 4B; Fig. S5). Our measured metal ion concentrations agree with previously published values (4–6). A slight increase (1.5-fold) in the total intracellular Mn (from 28 to 42 μM) in the ∆alx mutant was, indeed, noted at pH 8.4 vs 6.8 (Fig. 4B). However, we did not notice an increase in the total intracellular Mn in the parent strain at pH 8.4 vs 6.8 (Fig. 4C). Importantly, we observed that although Ptrc-expressed Alx did not change Mn levels in the ∆alx mutant at pH 6.8, it did reduce the total intracellular Mn 2-fold at pH 8.4 from 42 to 19 μM. In contrast, the total intracellular Mn in the parent strain did not significantly decrease with Ptrc-expressed Alx at pH 6.8 or 8.4 (Fig. 4C). The total intracellular iron (Fe) of the ∆alx mutant increased 2-fold at pH 8.4 vs 6.8, whereas total intracellular zinc (Zn) remained the same at the two tested pH values (Fig. S5). Total intracellular Fe and Zn did not change with Ptrc-expressed Alx. These results suggest that Alx may selectively prevent the buildup of intracellular Mn specifically under alkaline pH.
To corroborate the intracellular metal ion analysis, we quantified metal ions (specifically Mn and Fe) in the spent media by ICP-MS (Fig. 4D). The [Mn]/[Fe] ratio is often used as a measure of intracellular Mn in bacterial pathogens (37–39); here, we employed this ratio to assess the changes in extracellular [Mn]. Notably, the ∆alx strain grown in LBK pH 8.4 media exhibited a statistically significant reduction in the media [Mn]/[Fe] compared to unused LBK (Fig. 4D). If Alx were to export Mn2+ at alkaline pH, then spent media Mn would be expected to increase upon Ptrc-driven Alx expression, thus increasing the [Mn]/[Fe]. The Ptrc-expressed Alx in the ∆alx strain, indeed, restored the [Mn]/[Fe] in LBK pH 8.4 media. These results collectively provide evidence for Alx exporting Mn2+ at alkaline pH.
Alx Mn2+ export activity is stimulated by alkaline pH
The Ptrc-driven expression of Alx decreases intracellular [Mn] in the ∆alx mutant at pH 8.4, but not pH 6.8, suggesting that Mn2+ transport by Alx is pH-dependent (Fig. 4B). To test the possibility that the mechanism of Mn2+ export by Alx is proton dependent, we performed assays for detecting substrate-induced proton release in inside-out vesicles using published procedures (40). Everted membrane vesicles were prepared with the ∆alx ∆mntP double mutant containing an empty vector or a vector expressing a human influenza hemagglutinin (HA)-tagged derivative of Alx or MntP (AlxHA or MntPHA). Successful expression of each tagged protein was confirmed by anti-HA immunoblotting. A pH gradient across the vesicle membrane was generated via F0F1 ATPase activity by the addition of ATP to the vesicle suspension (Fig. S6). To monitor the generation of pH gradient, a pH gradient-sensitive, fluorescent dye 9-amino-6-chloro-2-methoxyacridine (ACMA) was employed. An expected quenching of fluorescence occurred upon the addition of ATP, suggesting vesicles were active. If Mn2+ transport by Alx or MntP were dependent on proton release, then a dequenching of ACMA fluorescence upon the addition of MnCl2 would be expected. However, we did not observe a significant change in the fluorescence intensity of ACMA upon the addition of MnCl2 (Fig. S6C and D), suggesting that the transport of Mn2+ by Alx and MntP is unlikely to be accompanied by an H+ antiport.
We speculated that alkaline pH may, thus, stimulate the Mn2+ export activity of Alx directly, perhaps by altering the protonation state of key Alx residues (see “A set of acidic residues in the transmembrane helices are critical for Alx-mediated Mn2+ export” section of the Results). To test this hypothesis, we probed the combined effect of elevated pH and extracellular [Mn2+] on the Mn2+ sensitivity phenotype of the ∆mntP mutant (Fig. 4E). The Mn2+ sensitivity of the ∆mntP mutant was exacerbated by the increasing concentration of MnCl2 in the media or increasing the media pH. This is expected since increasing media [Mn2+] correlates with an increase in the cytoplasmic [Mn] in the ∆mntP mutant (4, 13), and alkalinization of the media likewise increases cytoplasmic [Mn] (Fig. 4B), altogether leading to Mn toxicity. We noted a pH-dependent boost in the ability of Ptrc-expressed Alx to rescue the growth of the ∆mntP mutant in media with increasing [Mn2+] (Fig. 4E), supporting the notion that the Mn2+ export activity of Alx is stimulated by alkaline pH. Alx appears to be a low-activity Mn2+ exporter, in contrast to MntP, because its rescue ability dropped off at particularly high extracellular [Mn2+] (see 350 and 500 µM MnCl2 panels). The growth of the ∆alx ∆mntP strain closely resembled that of the ∆mntP mutant at elevated media [Mn2+] and pH (Fig. 4E). Likewise, the rescue of the growth of the ∆alx ∆mntP double mutant by overexpressed Alx was similar to that in the ∆mntP mutant (Fig. 4E). These observations suggest that chromosomally encoded Alx mitigates the mild perturbations in Mn2+ levels brought about by alkaline pH, and its Mn2+ export activity appears to be milder compared to the chromosomally encoded MntP.
A set of acidic residues in the transmembrane helices is critical for Mn2+ export by Alx
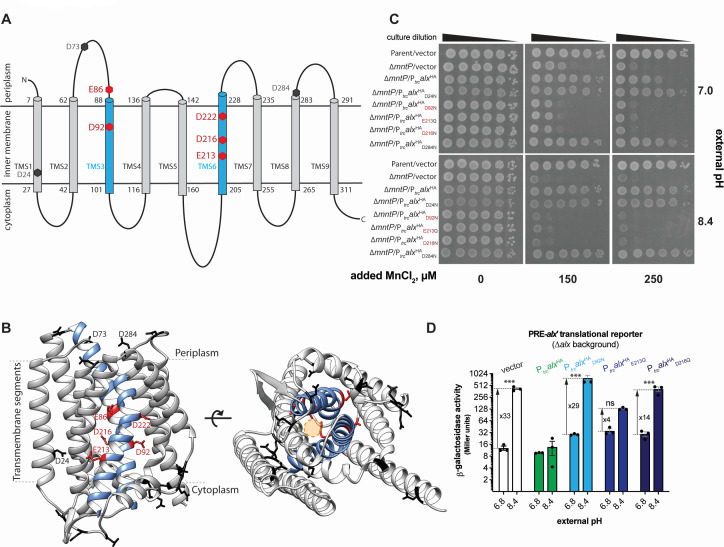
Currently, no experimental three-dimensional (3D) structural information for either Alx or MntP exists. To glean some insight into Alx architecture, its two-dimensional (2D) topology was predicted with multiple web-based tools listed in Table S5. This prediction identified nine Alx transmembrane segments (TMS1-9, Fig. 5A; Table S5), with an overall N-out (periplasmic) and C-in (cytoplasmic) Alx topology. Regardless of the prediction tool used, we noted the presence of acidic residues in the TMS, which is unusual and may suggest the functional importance of these side chains, as demonstrated previously for the export of Mn2+ by MntP (14). Two of these residues (D92 and D222) are conserved across members of the TerC family to which Alx belongs (14). Similar to topology-predicted arrangement, Alx displayed an N-out C-in conformation in the 3D structure predicted by the AlphaFold server (Fig. 5B).
Fig 5.
Structural model of Alx and functional relevance of its acidic residues in Mn2+ export. (A) The 2D topological model of Alx predicted with DeepTMHMM algorithm and relative positions of acidic residues in transmembrane segments (TMS). (B) AlphaFold-predicted 3D structure of Alx and relative positions acidic residues in TMS. A hypothetical channel for the export of Mn2+ is displayed as a circle in the predicted structure. (C) The spotting assay of tenfold serial dilutions (left to right) of overnight-grown cultures of parent strain (MC4100) bearing an empty vector (pHYD5001) and ∆mntP::Kan mutant (RAS32) bearing one of the following plasmids: a vector (pHYD5001), a derivative of pHYD5001 expressing Alx from Ptrc promoter (pRA27), a derivative of pHYD5001 expressing AlxHA from a Ptrc promoter (pRA50), a derivative of pRA50 expressing AlxHAD24N (pRA61), AlxHAD92N (pRA62), AlxHAE213Q (pRA63), AlxHAD216N (pRA64), and AlxHAD284N (pRA58). The serial dilutions were spotted on the surface of LB agar of varying pH and supplemented with appropriate concentration of ampicillin, MnCl2, and IPTG. The data shown are representative of three biological replicates of the experiment. (D) β-galactosidase activity (in Miller units) as a reporter of alx translation (Palx-PRE-alx′-lacZ, pRA54) was measured in mid-log phase grown cultures of ∆alx::Kan strain (RAS31) bearing vector (pHYD5001) and a derivative of pHYD5001 expressing AlxHA (pRA27), AlxHAD92N (pRA62), AlxHAE213Q (pRA63), AlxHAD216N (pRA64) from Ptrc promoter. The cultures were grown in LBK media pH 6.8 or 8.4, supplemented with appropriate concentration of ampicillin and 1 mM IPTG. Each plotted value in a bar graph with standard error of mean (SEM) is an average of three biological replicates of the experiment. The statistical significance of changes in the reporter activity was assessed by two-way ANOVA (nsP > 0.05, *P < 0.05, **P < 0.01, ***P < 0.001).
To probe the importance of the acidic residues predicted to be in the TMS of Alx, the effect of Ptrc-expressed HA-tagged Alx bearing conservative (D to N or E to Q) replacements was tested on the growth of the ∆mntP mutant in LB media with neutral or alkaline pH and added MnCl2 (Fig. 5; Fig. S7). With this strategy, the expression of the wild-type HA-tagged Alx rescued the growth of the ∆mntP mutant like the tag-less version of Alx, suggesting that the HA tag did not alter the activity of Alx. The Ptrc-expressed Alx bearing E86Q, D92N, E213Q, D216N, or D222N replacement (denoted as AlxHAE86Q, AlxHAD92N, AlxHAE213Q, AlxHAD216N, or AlxHAD222N, respectively) did not rescue the growth of the ∆mntP strain in media supplemented with MnCl2, whereas Alx bearing D24N, D73N, or D284N substitution did so (Fig. 5C; Fig. S8). The expression of HA-tagged Alx mutants was unchanged compared to the wild-type Alx (Fig. S8), ruling out Alx expression defects as a cause for the failure to rescue the ∆mntP mutant. These results indicated that E86, D92, E213, D216, and D222 are important for Alx-mediated Mn2+ export.
To expand upon the functional role of inner membrane acidic residues in the Alx protein, we performed a suite of alx translational reporter experiments. Our prior reporter assays demonstrated that Alx displays negative autoregulation, since a translational reporter of alx was not induced by alkaline pH or high [Mn2+] if Alx was expressed from Ptrc, presumably because of Alx-mediated Mn2+ export (Fig. 1D and 2A; Fig. S3A). We, thus, took advantage of this behavior and employed Alx mutants defective in Mn2+ export to link the effects of alkaline pH and [Mn2+] on alx expression. Specifically, we tested the impact of Ptrc-expressed AlxHAD92N, AlxHAE213Q, and AlxHAD216N on alx translational reporter activity in LBK pH 6.8 or 8.4 (Fig. 5D). The expression of wild-type AlxHA from a plasmid repressed the activity of alx translational reporter at alkaline pH in the ∆alx strain (RAS31) as expected. The reason behind the lower (33- vs 68-fold) pH-induced increase in alx translation in this experiment (strain co-transformed with the translational reporter and Alx expression vector) vs earlier experiment (Fig. 1B, strain transformed with translational reporter only) is unclear. Nevertheless, the expression of either AlxHAD92N, AlxHAE213Q, or AlxHAD216N did not repress alx translational reporter activity as wild-type AlxHA did. The fold induction of alx translational reporter activity varied (29, 4, and 14 for AlxHAD92N, AlxHAE213Q, and AlxHAD216N, respectively), indicating that AlxHAE213Q and AlxHAD216N retain partial activity, whereas AlxHAD92N is inactive. Overall, negative autoregulation of alx expression in response to alkaline pH is no longer observed when Alx mutants defective in Mn2+ transport are expressed; in other words, alx expression stays “on.” This suggests a connection between the induction of alx expression and Alx-mediated export of Mn2+ in alkaline pH, where the return of intracellular [Mn2+] back to its “healthy” levels via Alx export shuts down further production of Alx.
DISCUSSION
In this work, we investigated in depth the effect of increased extracellular pH and [Mn2+] on alx expression and provided multiple pieces of evidence for Alx export of Mn2+ upon alkalinization of the cytoplasm. Our results corroborate earlier findings that alx expression is upregulated by both alkaline pH and elevated [Mn2+] (19, 20, 23, 24, 41) in a riboswitch-dependent manner (Fig. 1). We confirmed that the cytoplasm, indeed, alkalinizes when cells are grown in alkaline media (Fig. 3B); therefore, our observed changes in gene expression and intracellular metal ion content are a consequence of alkaline cytoplasmic pH. The absence of Alx had no impact on cytoplasmic alkalinization with increasing media pH (Fig. 3B) and did not affect cellular growth in alkaline media (Fig. 3A), ruling out direct Alx involvement in pH homeostasis. The expression of Alx did, however, lower total intracellular [Mn], but only at alkaline pH (Fig. 4B), thus implicating Alx as a Mn2+ exporter in alkaline pH. With this newly uncovered function of Alx, our work points to a connection between the two environmental cues: alkaline pH and elevated [Mn2+]. A recent study demonstrated that cytosol alkalinizes in the presence of excess extracellular Mn2+ due to increased ammonia production within an E. coli cell (20); here, we show that the reverse is also true: an alkaline environment promotes the import of Mn2+ into the cell.
Intracellular pH and Mn2+content are linked
We find that alkalinization of the cytoplasm leads to an increase in the intracellular [Mn]. Specifically, our intracellular metal ion measurements show a 1.5-fold increase in [Mn] at pH 8.4 vs 6.8 in the ∆alx strain, from 28 to 42 µM (Fig. 4B). Additional indirect data support this increase. First, the Mn2+ sensitivity of ∆mntP mutant is exacerbated at alkaline pH (Fig. 4D). Second, even though alkaline pH alone did not impact mntP translation, a combination of alkaline pH and extra Mn2+ in the media led to a greater mntP induction than Mn2+ alone (16- and 11-fold, respectively, Fig. 1E). Because upregulation of mntP translation is directly proportional to [Mn2+] (Fig. 1D), the additional increase is likely due to the additional Mn2+ imported into the cell at alkaline vs neutral pH. The fact that alkaline pH alone had no effect on mntP translation suggests that there is a threshold total intracellular [Mn] of >42 µM needed to begin producing additional MntP based on intracellular Mn measurements at alkaline pH (Fig. 4B). Third, the Ptrc-expressed MntH, the only characterized Mn2+ importer in E. coli K12 strain, induced alx and mntP translational reporter at neutral pH (Fig. 2C). The mechanism of the alkaline pH-induced Mn2+ import, on the other hand, is unclear but does not involve MntH (Fig. 2D; Fig. S3B), implicating a potential alternative path for Mn2+ into the cell.
Why would a cell import Mn2+ upon cytosol alkalinization? Among the possible roles that imported Mn2+ could play in an alkaline cytosol is its function as a redox center in the superoxide dismutase SodA and other mononuclear metal enzymes where Mn2+ can replace Fe2+ as a cofactor to prevent protein damage from oxidative stress (1, 2, 42). It may, thus, be an adaptive strategy that cells import Mn2+ in response to elevated ROS in alkaline pH. The expression of MntP (Mn2+ exporter) and Alx slowed the aerobic growth of a sensitized strain that lacks H2O2 degrading enzymes (AhpCF and KatG) (14). The effects of MntP overproduction on the growth of ∆ahpCF ∆katG strain are explained by reduced intracellular [Mn2+] (due to Mn2+ export by MntP) and corresponding reduced protection from ROS. Similar effects of Alx overproduction on this strain’s growth, however, were explained differently by reference (14) as Alx was viewed as an Mn2+ importer. Alx export of Mn2+ by analogy to MntP, on the other hand, better explains the observed slower growth of the ∆ahpCF ∆katG strain upon Alx overexpression because Ptrc-expressed Alx rescues the growth of the ∆mntP mutant in media with extra Mn2+and reduces the intracellular [Mn2+] in alkaline pH.
The alx translational reporter displayed a 68-fold induction in alkaline pH media and a 21-fold induction in neutral pH media with 500 µM MnCl2, with an 86-fold induction when two environmental cues (alkalinity and high [Mn2+]) were combined (Fig. 1). Alkaline pH, thus, augments the effects of elevated [Mn2+] on alx expression. The alkaline pH-induced Mn2+ import also provides an alternate explanation to a recent report where increased cytoplasmic [Mn2+] results in higher activation of mntP riboswitch upon alkalinization in media with extra Mn2+ in contrast to the proposed tighter interaction between Mn2+ and the mntP riboswitch element (20). Differences in the fold induction by the two cues are reflective of a potentially distinct mechanism for modulation of alx expression in alkaline pH that depends on elevated cytoplasmic [Mn2+]. A future direction for deconvoluting the mechanism of pH and Mn2+ control of alx expression will be to examine how pH and Mn2+ differentially affect alx mRNA folding, and specifically folding of its 5′ UTR riboswitch.
Our results contradict Kalita et al.’s (20) assertion that alx and mntP riboswitches necessitate a requirement of both high [Mn2+] and alkaline pH for their optimal activation; however, our results are supportive of the findings that alx riboswitch is responsive to both alkaline pH [(20, 23, 24), Fig. 1; Fig. S2] and Mn2+ [(19, 20), Fig. 1; Fig. S2]. Furthermore, our research supports Kalita et al.’s conclusion that the alx and mntP riboswitches require both increased levels of Mn2+ and an alkaline pH for their full activation.
Mn2+ export by Alx
A previous study proposed that Alx may function as an Mn2+ importer based on the cellular [Mn] measurements in the presence of supplemented Mn2+ (14). Contrary to this earlier study, here, we provided multiple lines of evidence for the Alx-mediated export of Mn2+ in alkaline pH or conditions where cytoplasmic Mn levels go up. First, the Ptrc-expressed Alx inhibited the growth of the ∆ahpCF ∆mntH, oxidatively stressed (2, 14, 31) and Mn-limited strain at 42°C (Fig. 3C). Second, the inability of ∆mntP strain to grow with added Mn2+ was partially rescued by Ptrc-expressed Alx (Fig. 3A). This rescue phenotype was missed in the previous work (14) likely because rescue experiments were performed at neutral pH only. Strikingly, the rescue of ∆mntP mutant’s sensitivity toward Mn2+ by Ptrc-expressed Alx becomes more pronounced with increasing pH, while Mn2+ sensitivity of ∆mntP mutant becomes exacerbated with increasing pH (Fig. 4E). Fourth, the Ptrc-expressed Alx in the ∆alx strain reduced intracellular [Mn2+] ~2-fold but only in alkaline pH, returning intracellular [Mn2+] from 42 to 19 µM (Fig. 4B). Therefore, improved growth of the ∆mntP mutant with Ptrc-expressed Alx can be explained by the increased activity of Alx in alkaline pH.
Mn2+ export by Alx at alkaline pH is also supported by the observed negative feedback regulation of alx expression. Specifically, the alkaline induction of alx translation (68-fold) was repressed by the presence of Alx encoded chromosomally from a native promoter or expressed from Ptrc (Fig. 1B, 2A and B). These results can be explained if Alx exports Mn2+ thereby reducing cytoplasmic [Mn2+] to the levels that no longer stimulate alx translation. The expression of Alx chromosomally resulted in only a 2-fold reduction in alx translation at pH 6.8 and 500 µM added MnCl2: compare Mn-induced increase in alx translation in Fig. S3A (11-fold) in the presence of chromosomal alx to Fig. 1D in the absence of chromosomal alx (21-fold). The mild reporter activity reduction, in this case, can be explained by the lack of alkaline pH-stimulated Mn2+ export activity of Alx.
The driving force and mechanism behind Alx’s export of Mn2+ remain an open direction for future work. A proton gradient is unlikely to drive this transport because we do not observe a loss of pH gradient upon supplementation of Mn2+ to inverted membrane vesicles containing Alx (Fig. S6), ruling out Alx as an Mn2+/H+ antiporter. In another system, a high concentration of potassium ion (K+) in the media was proposed to stimulate the activity of K+ export proteins and inhibit the activity of K+ uptake proteins (43–45). However, in the case of Alx, the stimulation of its activity by alkaline pH is unlikely through just an increase in cellular [Mn2+]. This reasoning is supported by the observation that Ptrc-expressed Alx rescues the growth of ∆mntP mutant at intermediate but not high media [Mn2+] (Fig. 4A and E). The most likely explanation for the stimulation of Alx activity by alkaline pH could be pH-driven structural changes in the Alx protein that affect its Mn2+ export. We identified several acidic residues in TMS3 and TMS6 of Alx (E86, D92, E213, D126, and D222) crucial for Mn2+ transport, by analogy to MntP and MntH [Fig. 5B (8, 14)]. The interaction of positively charged solute (Mn2+), and these acidic side chains may provide a path for Mn2+ transport as depicted in Fig. 5B.
Role of Alx in bacterial physiology
We did not observe a growth phenotype for the ∆alx mutant indicating that chromosomally encoded Alx does not provide a measurable advantage in the tested laboratory conditions of media [Mn2+], pH, and chosen bacterial strain. This observation also suggests that a pH-driven increase in total intracellular [Mn] to 42 µM is not toxic to E. coli. Overexpression of Alx, however, did rescue the growth of the ∆mntP mutant in alkaline media with added Mn2+, which suggests that Alx functions as a weak Mn2+ exporter. To draw a comparison with other bacterial species, Bacillus subtilis (B. subtilis) maintains a higher intracellular [Mn]/[Fe] compared to E. coli (39, 46). A conditional requirement of balancing the excess Mn2+ import in B. subtilis is achieved through transcriptional regulators that repress the expression of Mn2+ importers and the presence of multiple Mn2+ exporters (39, 47–49). Interestingly, an Alx ortholog in B. subtilis regulated by an Mn2+-responsive riboswitch was proposed to detoxify excess Mn and metalate exoenzymes (19, 47, 50). It will be fascinating to understand the riboswitch-controlled regulation of Alx in E. coli that make it both pH and Mn2+ responsive and how it evolved in B. subtilis.
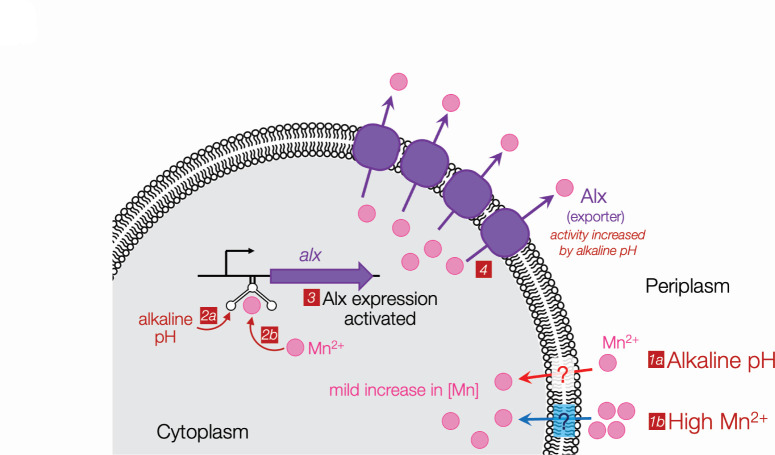
Curiously, an earlier study reported that alx is expressed at both neutral and alkaline pH during anaerobic growth of E. coli where ROS stress is minimal (51). Consequently, a cell no longer needs additional Mn2+ during anaerobic growth and preventing Mn2+ buildup due to its uptake becomes important. This would explain why alx is expressed even at neutral pH in anaerobic conditions (51). We speculate that the expression of alx may provide an advantage in environmental niches where E. coli and other enterobacteria are challenged by both alkaline pH and hypoxia, such as the portion of human gut from duodenum to ileum (52, 53). To cope with the threat of high [Mn2+] in the environment, the Mn2+ export by chromosomally encoded MntP is sufficient to protect the cell. On the other hand, when changes in intracellular [Mn2+] are mild, e.g., as brought about by alkaline pH, Alx fulfills the job of maintaining healthy levels of Mn2+ inside the cell (Fig. 6). We, thus, posit that Alx-mediated Mn2+ export provides a primary protective mechanism that fine tunes the cytoplasmic [Mn2+], especially during alkaline stress.
Fig 6.
A model for the regulation of alx expression in E. coli. Manganese enters the cell by an uncharacterized transport mechanism through the inner membrane during growth in an alkaline environment (1a) or elevated concentration of Mn2+ (1b). The subtle changes in intracellular Mn level are sensed in alkaline (2a) or neutral pH (2b) by the alx riboswitch, triggering an increase in expression of Alx in an Mn-dependent manner (3). The export of excess Mn by Alx, stimulated by alkaline pH, restores cellular Mn levels (4).
MATERIALS AND METHODS
Chemicals
Manganese (II) chloride tetrahydrate and potassium benzoate were purchased from Alfa Aesar. Tris(hydroxymethyl)methyl-3-amino propane sulfonic acid (TAPS) was purchased from Acros Organics. N-(1,1-dimethyl-2-hydroxyethyl)-3-amino-2-hydroxy-propane sulfonic acid (AMPSO), nigericin sodium salt, and valinomycin were purchased from Sigma-Aldrich. 9-Amino-6-chloro-2-methoxyacridine (ACMA) was purchased from Invitrogen. o-nitrophenyl-β-d-galactopyranoside was purchased from Thermo Scientific. Isopropyl-β-d-thiogalactopyranoside (IPTG) and 3-(N-morpholino) propane sulfonic acid (MOPS) were purchased from Fisher Scientific.
Bacterial strain construction and growth conditions
The strains employed in this study are derivatives of E. coli K12 and tabulated in Table S1. The MG1655 ∆ahpCF::Kan strain was constructed by following the procedures of recombineering as described in reference (54). The ∆alx::Kan, ∆mntP::Kan, and ∆mntH::Kan mutations were sourced from appropriate strains from the Keio collection (55) and introduced into recipient strains by P1 transduction. The gene encoding the Kanamycin resistance determinant was excised upon treatment with plasmid pCP20 in ∆alx::Kan (54). All strains were cultivated in LB agar or broth at 37°C. The pH of LB agar or LB broth employed in this study was 7.2 unless stated otherwise. We also employed potassium-modified LB medium (LBK), where equimolar KCl replaced NaCl, buffered with 100 mM N-[tris(hydroxymethyl)methyl]-3-amino propane sulfonic acid (TAPS), and pH adjusted with KOH to 8.4 (41). The pH of unbuffered LBK medium employed was 6.8. In other experiments, LB agar was buffered with HEPES, Tris-Cl, and TAPS (50 mM final concentration), and pH was adjusted to 7, 7.8, and 8.2, respectively.
Plasmid construction
The plasmid pRA40 carrying Palx-PRE-alx' (transcriptional reporter of alx) was constructed by PCR-amplifying MC4100 chromosomal DNA from 473 nucleotides upstream and 53 nucleotides downstream from the translation start site of alx (24) using oligos RAV5 and RAV48 and then cloning the PCR product into the PstI and KpnI sites of a single-copy plasmid pMU2385. The plasmid pRA54 (translational reporter of alx) was constructed similarly by PCR-amplifying MC4100 chromosomal DNA from 473 nucleotides upstream and 99 nucleotides downstream from the translation start site of alx using oligos RAV5 and RAV23 and cloning of PCR product into the PstI and BamHI sites of single-copy plasmid pMU2386, such that the first 33 codons of the ORF are in frame with the 8th codon of lacZ. We extended the length of alx′ in the translational reporter of alx (98 nucleotides downstream from the translation start site), compared to the transcriptional reporter (53 nucleotides downstream from the translation start site) for two reasons: (1) to include the last putative transcriptional pause proximal to the alx translation start site (56) that turned out being important for maximal alx induction and (2) to increase the probability of the Alx′-LacZ hybrid protein localizing in the cytoplasm (refer to the topological analysis of Alx in Fig. 5A). The ∆PRE derivatives of transcriptional (pRA41) and translational (pRA55) reporters of alx were constructed by site-directed mutagenesis of plasmids pRA40 and pRA54, respectively, using oligos RAV15 and RAV16.
The pRA48 plasmid (transcriptional reporter of mntP) was constructed by PCR-amplifying MC4100 chromosomal DNA from 882 nucleotides upstream and 47 nucleotides downstream from the translation start site of mntP (19) using oligos RAV119 and RAV120 and then cloning the PCR product into the PstI and KpnI sites of a single-copy plasmid pMU2385. The plasmid pRA57 (translational reporter of mntP) was constructed by PCR-amplifying MC4100 chromosomal DNA from 882 nucleotides upstream and 47 nucleotides downstream from the translation start sites of mntP (19) using oligos RAV119 and RAV126 and cloning of the PCR product in the PstI and SalI sites of single-copy plasmid pMU2386, such that the first 47 nucleotides of the mntP ORF are in frame with the 8th codon of lacZ.
Oligos RAV65 and RAV66 were employed to PCR-amplify the gene encoding pHluorin from a plasmid (pGFPR01) obtained from a strain JLS1105 (28). The PCR product was cloned into pHYD5001 using Gibson assembly, producing pRA46. Oligos RAV11 and RAV12 were used to PCR-amplify alx from the MC4100 genomic DNA. The PCR product was then cloned into the NdeI and HindIII sites of pHYD5001. The resulting pHYD5001 expressing alx from Ptrc promoter was denoted as pRA27. It was noted upon sequencing of pRA27 that the NdeI site was mutated but alx ORF is still retained. Similarly, oligos pairs RAV13, RAV14 and RAV269, RAV270 were used to PCR-amplify mntP and mntH, respectively, from the MC4100 genomic DNA. The PCR products were cloned into the NdeI and HindIII sites of pHYD5001. The resulting pHYD5001 derivatives expressing mntP and mntH from Ptrc promoter were denoted as pRA29 and pRA94, respectively. The pRA50 plasmid encoding the HA epitope-tagged version of Alx on N-terminus from Ptrc promoter was constructed by PCR amplification with oligos RAV139 and RAV12 and cloning the product into the NdeI and HindIII sites of pHYD5001. Similarly, pRA70 was constructed to express the HA epitope-tagged MntP on their N-terminus from Ptrc promoter. The PCR product was generated by using oligos RAV178 and RAV14 for amplification of mntP from MC4100 genomic DNA as template. PCR products were cloned into the NdeI and HindIII sites of pHYD5001. Other plasmids pRA58, pRA61, pRA62, pRA63, pRA64, pRA68, pRA74, pRA75, and pRA76 were constructed by site-directed mutagenesis of plasmid pRA50 by using oligo pairs stated in Table S3.
β-galactosidase assays
Overnight grown cultures of the strains were inoculated in LBK broth with pH 6.8 and 8.4 or in LB broth with or without appropriate concentration of MnCl2 at 37°C to a mid-log phase. The appropriate concentration of antibiotics (trimethoprim and/or ampicillin) and IPTG (1 mM) were supplemented when needed in the experiments. β-galactosidase assays were carried out by following the method of Miller, and β-galactosidase-specific activity was reported as Miller units (57). Each reported value of Miller units with a standard error of mean is an average of three biological replicates.
Growth rate measurements
Ten microliters of log-phase cultures of appropriate strain was diluted to 1 mL with fresh LB and LBK media with pH 6.8 and 8.4. The appropriate concentration of MnCl2 was added to LB and LBK media as described in the experiments. Two hundred microliters of these diluted cultures was grown in wells of honeycomb multi-well plates at 37°C while shaking. Growth curves were generated using an automated Spark multimode plate reader by Tecan. In a growth curve, the slope of the graph in the exponential phase was used to calculate the growth rate shown in Table S4.
Cytoplasmic pH measurements
The wild-type strains of E. coli and its ∆alx mutant containing a plasmid expressing pHluorin (pRA46) were grown overnight in LBK medium buffered with 50 mM of MOPS (pH 7.5) and an appropriate concentration of ampicillin. Cells were inoculated and grown to mid-log phase in fresh LBK medium at pH 7.5 with an appropriate concentration of ampicillin and 1 mM IPTG at 37°C. Cells were harvested from appropriate volume of the cultures by spinning at 4,000g. Cells were resuspended in 4 mL of M63A minimal medium (0.4 g/L KH2PO4, 0.4 g/L KH2PO4, 2 g/L (NH4)2SO4, 7.45 g/L KCl supplemented with 2 g/L casein hydrolysate) and buffered to the desired pH with 50 mM concentration of the appropriate buffer: pH 7.0 and 7.5, MOPS; 8.5, TAPS, and pH 9 and 9.5, AMPSO. Due to poor growth in extremely alkaline conditions, the initial A600 for cells growing in M63A media with pH 9 and 9.5 was ~0.2, and in M63A media with external pH (pHe) 7, 7.5, and 8.5 was ~ 0.05. The cultures were grown for 2 h at 37°C with mild shaking. To generate a standard curve, 95 µL volume of the culture of parent strain expressing pHluorin from each buffered media was withdrawn and mixed with potassium benzoate to a final concentration of 40 mM in 96-well plates. The cultures were incubated at room temperature for 3 min. Methanol amine was added to the culture at a final concentration of 20 mM. The cultures were incubated for 3 min at room temperature. The 100 µL of the parent strain and its ∆alx mutant expressing pHluorin were withdrawn from each buffered media to 96 well plates and used for the internal pH (pHi) measurements. The measurements with fluorescence emission at 530 nm were taken for the two excitation (410 and 470 nm) wavelengths for each strain expressing pHluorin as described in reference (28). The ratio of fluorescence intensity of pHluorin at two excitation wavelengths against pH was plotted to generate a standard curve for the graph. The slope of the curve was used to calculate the pHi across different pHe. The data obtained were presented as an average of three biological replicates with standard error of mean.
Tests of the Mn2+-sensitive phenotype and its rescue
Strains were inoculated in LB broth overnight at 37°C in a shaker. Five microliters of tenfold serial dilutions of an overnight grown culture of each strain was spotted on LB agar supplemented with MnCl2 as described in the Results. Whenever required, LB broth or agar media were supplemented with an appropriate concentration of antibiotics and IPTG. LB agar plates were imaged after incubation at 37°C for 14–16 h.
ICP-MS measurement of cellular and media metal ions
The total Mn, Fe, and Zn were quantified from 5 mL cultures. Cells were grown overnight in LB broth and then inoculated in LBK pH 6.8 or LBK pH 8.4 media supplemented with 1 mM IPTG and appropriate concentration of ampicillin. After growth to the mid-log phase at 37°C, cells were harvested using centrifugation at 4,000g for 10 min. Cell pellets were washed with 10 mM N-2-hydroxyethylpiperazine-N-2-ethane sulfonic acid (HEPES) pH 7.5, containing 2 mM EDTA, and then washed twice with 10 mM HEPES as described in reference (14). Cell pellets were dried for 1 h in a centrifuge evaporator. Dried cell pellets were solubilized in 400 µL of 30% (vol/vol) HNO3 and incubated at 95°C for 10 min. Samples were centrifuged at 20,000g for 5 min, prepared for ICP-MS by diluting 300 µL of supernatant of lysed cells into 2.7 mL of 2.5% (vol/vol) HNO3, and analyzed on an iCAP RQ ICP-MS (Thermo Scientific). Metal ion concentrations are presented as intracellular levels after correction for mean cell volume determined from total protein content (4). The data obtained were presented as an average of three biological replicates with standard error of mean.
For media metal ion measurements, cells containing appropriate plasmids were inoculated in LBK pH 8.4 media supplemented with 1 mM IPTG and appropriate concentration of ampicillin. Cells were harvested after growth to mid-exponential phase (0.4–0.5 OD600) and separated from the media by centrifugation at 4,000g for 15 min at room temperature. The media supernatant was filtered using a 0.25-µm filter, and 2.5% (vol/vol) HNO3 was added to the supernatant. The samples prepared for ICP-MS were diluted before analysis 1:50 to avoid interference from LBK media components with MS. From metal ion measurements in the spent media after exponential cell growth, the [Mn] to [Fe] ratio was calculated. The data plotted for spent media were an average of three biological replicates with standard error of mean. In the case of unused media, three technical replicates were employed to determine an average with standard error of mean.
Preparation and storage of inside-out vesicles
Inside-out vesicles were prepared from the strain RAS42 that lacks alx and mntP. RAS42 containing an empty vector and its derivatives that express AlxHA or MntPHA from the trc promoter were cultivated in 1 L of LB broth with an appropriate concentration of ampicillin at 37°C. The cultures were grown to an OD600 of 0.2 and then induced with 1 mM IPTG for Ptrc-driven expression of AlxHA and MntPHA at 18°C for 12 h. The inside-out vesicles were prepared using procedures identical to those described in reference (58) except aliquots were stored in Buffer B with 10% glycerol, frozen in liquid nitrogen, and stored at –80°C until further use.
Detection of substrate-induced proton release in inside-out membrane vesicles
Kinetic measurements were performed as described in reference (40). Frozen vesicles were thawed on ice. Forty microliters of membrane vesicles was diluted to 2 mL with solution of 50 mM KCl and 10 mM MgSO4. ACMA (10 µM) and valinomycin (0.05 µM) were added at the beginning of the kinetic measurement. The fluorescence measurements of ACMA (λEx 409 nm, λEm 474 nm) were recorded for 250 s with continuous stirring of the samples. ATP (0.25 mM, final concentration) was added after 50 s to generate the pH gradient across the membrane as estimated by quenching of ACMA’s fluorescence. MnCl2 (1 mM, final concentration) was added after 150 s. Any significant change in pH due to substrate-induced proton release was measured by dequenching of ACMA fluorescence. The measurements were terminated by the addition of nigericin (4 µM) after 200 s.
Immunoblotting
The cultures were grown to the mid-log phase. Cells were harvested by centrifugation at 20,000g for 1 min. Cell pellets (OD600 of 1) were solubilized in 200 µL of SDS-PAGE loading dye. The whole cell extracts were loaded on 12% SDS-PAGE gel after incubation at 37°C for 10 min. Proteins were transferred to the PVDF membrane (Bio-Rad) by Trans-Blot Turbo, a semi-dry transfer apparatus (Bio-Rad). The PVDF membrane was treated with a blocking buffer (Tris-HCl buffer saline with 5% fat-free milk powder) for 30 min. The membrane was probed with an anti-HA rabbit monoclonal antibody (Invitrogen) at a dilution of 1:5,000 overnight at 4°C and with an anti-rabbit, horseradish peroxidase-conjugated antibody (Promega) at a dilution of 1:5,000 for 2 h at room temperature. The blot was developed using a Clarity Western ECL substrate (Bio-Rad), and the signal was detected by the ChemiDoc imaging system. The PVDF membrane was stained with 0.1% amido black solution to confirm equal loading of samples across the lanes. Following staining for 15 s, the membrane was destained with a solution of 45% methanol, 45% water, and 10% glacial acetic acid.
Statistical analysis
Calculations for mean with standard error of mean (SEM) and statistical analyses were performed using GraphPad Prism version 9.5.1 for Windows. The two-way ANOVA test was used for statistical analysis. A P-value greater than 0.05 is not considered statistically significant (ns), whereas a P-value less that 0.05 is considered significant and indicated by an *. Unless specified in the figures, fold changes are not statistically significant.
ACKNOWLEDGMENTS
We thank Terry Hwa, Abhijit A. Sardesai, James Imlay, and Robert Browne for sharing strains and plasmids for this study. We are grateful to Neal Arakawa at the Environmental and Complex Analysis Laboratory (ECAL) at UCSD for helping with metal ion measurements. We also thank Mark Herzik, Galia Debelouchina, Itay Budin, Elizabeth Komives, and Terry Hwa for providing access to their lab instruments. We thank Iman Saeed for preparing several clones for this study and members of the Mishanina lab for critically reading the manuscript.
This work was supported by the National Institutes of Health (NIGMS ESI grant R35GM142785), UCSD institutional support, and Yinan Wang Memorial Chancellor’s Endowed Junior Faculty Fellowship to T.V.M.
Contributor Information
Tatiana V. Mishanina, Email: tmishanina@ucsd.edu.
Conrad W. Mullineaux, Queen Mary University of London, London, United Kingdom
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/jb.00168-24.
Figures S1 to S8; Tables S1 to S5.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Hopkin KA, Papazian MA, Steinman HM. 1992. Functional differences between manganese and iron superoxide dismutases in Escherichia coli K-12. J Biol Chem 267:24253–24258. [PubMed] [Google Scholar]
- 2. Anjem A, Varghese S, Imlay JA. 2009. Manganese import is a key element of the OxyR response to hydrogen peroxide in Escherichia coli. Mol Microbiol 72:844–858. doi: 10.1111/j.1365-2958.2009.06699.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Patzer SI, Hantke K. 2001. Dual repression by Fe2+-Fur and Mn2+-MntR of the mntH gene, encoding an NRAMP-like Mn2+ transporter in Escherichia coli. J Bacteriol 183:4806–4813. doi: 10.1128/JB.183.16.4806-4813.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Martin JE, Waters LS, Storz G, Imlay JA. 2015. The Escherichia coli small protein MntS and exporter MntP optimize the intracellular concentration of manganese. PLoS Genet 11:e1004977. doi: 10.1371/journal.pgen.1004977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kaur G, Kumar V, Arora A, Tomar A, Sur R, Dutta D. 2017. Affected energy metabolism under manganese stress governs cellular toxicity. Sci Rep 7:11645. doi: 10.1038/s41598-017-12004-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kaur G, Sengupta S, Kumar V, Kumari A, Ghosh A, Parrack P, Dutta D. 2014. Novel MntR-independent mechanism of manganese homeostasis in Escherichia coli by the ribosome-associated protein HflX. J Bacteriol 196:2587–2597. doi: 10.1128/JB.01717-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Makui H, Roig E, Cole ST, Helmann JD, Gros P, Cellier MFM. 2000. Identification of the Escherichia coli K-12 Nramp orthologue (MntH) as a selective divalent metal ion transporter. Mol Microbiol 35:1065–1078. doi: 10.1046/j.1365-2958.2000.01774.x [DOI] [PubMed] [Google Scholar]
- 8. Haemig HAH, Brooker RJ. 2004. Importance of conserved acidic residues in MntH, the Nramp homolog of Escherichia coli. J Membr Biol 201:97–107. doi: 10.1007/s00232-004-0711-x [DOI] [PubMed] [Google Scholar]
- 9. Bozzi AT, Zimanyi CM, Nicoludis JM, Lee BK, Zhang CH, Gaudet R. 2019. Structures in multiple conformations reveal distinct transition metal and proton pathways in an Nramp transporter. Elife 8:e41124. doi: 10.7554/eLife.41124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kehres DG, Zaharik ML, Finlay BB, Maguire ME. 2000. The NRAMP proteins of Salmonella typhimurium and Escherichia coli are selective manganese transporters involved in the response to reactive oxygen. Mol Microbiol 36:1085–1100. doi: 10.1046/j.1365-2958.2000.01922.x [DOI] [PubMed] [Google Scholar]
- 11. Grass G, Franke S, Taudte N, Nies DH, Kucharski LM, Maguire ME, Rensing C. 2005. The metal permease ZupT from Escherichia coli is a transporter with a broad substrate spectrum. J Bacteriol 187:1604–1611. doi: 10.1128/JB.187.5.1604-1611.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Taudte N, Grass G. 2010. Point mutations change specificity and kinetics of metal uptake by ZupT from Escherichia coli. Biometals 23:643–656. doi: 10.1007/s10534-010-9319-z [DOI] [PubMed] [Google Scholar]
- 13. Waters LS, Sandoval M, Storz G. 2011. The Escherichia coli MntR miniregulon includes genes encoding a small protein and an efflux pump required for manganese homeostasis. J Bacteriol 193:5887–5897. doi: 10.1128/JB.05872-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zeinert R, Martinez E, Schmitz J, Senn K, Usman B, Anantharaman V, Aravind L, Waters LS. 2018. Structure–function analysis of manganese exporter proteins across bacteria. J Biol Chem 293:5715–5730. doi: 10.1074/jbc.M117.790717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ouyang A, Gasner KM, Neville SL, McDevitt CA, Frawley ER. 2022. MntP and YiiP contribute to manganese efflux in Salmonella enterica serovar Typhimurium under conditions of manganese overload and nitrosative stress. Microbiol Spectr 10:e0131621. doi: 10.1128/spectrum.01316-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Serganov A, Nudler E. 2013. A decade of riboswitches. Cell 152:17–24. doi: 10.1016/j.cell.2012.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cromie MJ, Shi Y, Latifi T, Groisman EA. 2006. An RNA sensor for intracellular Mg2+. Cell 125:71–84. doi: 10.1016/j.cell.2006.01.043 [DOI] [PubMed] [Google Scholar]
- 18. Dann CE, Wakeman CA, Sieling CL, Baker SC, Irnov I, Winkler WC. 2007. Structure and mechanism of a metal-sensing regulatory RNA. Cell 130:878–892. doi: 10.1016/j.cell.2007.06.051 [DOI] [PubMed] [Google Scholar]
- 19. Dambach M, Sandoval M, Updegrove TB, Anantharaman V, Aravind L, Waters LS, Storz G. 2015. The ubiquitous yybP-ykoY riboswitch is a manganese-responsive regulatory element. Mol Cell 57:1099–1109. doi: 10.1016/j.molcel.2015.01.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kalita A, Mishra RK, Kumar V, Arora A, Dutta D. 2022. An intrinsic alkalization circuit turns on mntP riboswitch under manganese stress in Escherichia coli. Microbiol Spectr 10:e0336822. doi: 10.1128/spectrum.03368-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Meyer MM, Hammond MC, Salinas Y, Roth A, Sudarsan N, Breaker RR. 2011. Challenges of ligand identification for riboswitch candidates. RNA Biol 8:5–10. doi: 10.4161/rna.8.1.13865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Breaker RR. 2022. The biochemical landscape of riboswitch ligands. Biochemistry 61:137–149. doi: 10.1021/acs.biochem.1c00765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bingham RJ, Hall KS, Slonczewski JL. 1990. Alkaline induction of a novel gene locus, alx, in Escherichia coli. J Bacteriol 172:2184–2186. doi: 10.1128/jb.172.4.2184-2186.1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nechooshtan G, Elgrably-Weiss M, Sheaffer A, Westhof E, Altuvia S. 2009. A pH-responsive riboregulator. Genes Dev 23:2650–2662. doi: 10.1101/gad.552209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Anantharaman V, Iyer LM, Aravind L. 2012. Ter-dependent stress response systems: novel pathways related to metal sensing, production of a nucleoside-like metabolite, and DNA-processing. Mol Biosyst 8:3142–3165. doi: 10.1039/c2mb25239b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mishanina TV, Palo MZ, Nayak D, Mooney RA, Landick R. 2017. Trigger loop of RNA polymerase is a positional, not acid-base, catalyst for both transcription and proofreading. Proc Natl Acad Sci U S A 114:E5103–E5112. doi: 10.1073/pnas.1702383114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stephen C, Mishanina TV. 2022. Alkaline pH has an unexpected effect on transcriptional pausing during synthesis of the Escherichia coli pH-responsive riboswitch. J Biol Chem 298:102302. doi: 10.1016/j.jbc.2022.102302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Martinez KA, Kitko RD, Mershon JP, Adcox HE, Malek KA, Berkmen MB, Slonczewski JL. 2012. Cytoplasmic pH response to acid stress in individual cells of Escherichia coli and Bacillus subtilis observed by fluorescence ratio imaging microscopy. Appl Environ Microbiol 78:3706–3714. doi: 10.1128/AEM.00354-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hartmann FSF, Weiß T, Shen J, Smahajcsik D, Savickas S, Seibold GM. 2022. Visualizing the pH in Escherichia coli colonies via the sensor protein mCherryEA allows high-throughput screening of mutant libraries. mSystems 7:e0021922. doi: 10.1128/msystems.00219-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kehres DG, Janakiraman A, Slauch JM, Maguire ME. 2002. Regulation of Salmonella enterica serovar Typhimurium mntH transcription by H2O2, Fe2+, and Mn2+. J Bacteriol 184:3151–3158. doi: 10.1128/JB.184.12.3151-3158.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Seaver LC, Imlay JA. 2001. Alkyl hydroperoxide reductase is the primary scavenger of endogenous hydrogen peroxide in Escherichia coli. J Bacteriol 183:7173–7181. doi: 10.1128/JB.183.24.7173-7181.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li X, Imlay JA. 2018. Improved measurements of scant hydrogen peroxide enable experiments that define its threshold of toxicity for Escherichia coli. Free Radic Biol Med 120:217–227. doi: 10.1016/j.freeradbiomed.2018.03.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gottesman S. 1996. Proteases and their targets in Escherichia coli. Annu Rev Genet 30:465–506. doi: 10.1146/annurev.genet.30.1.465 [DOI] [PubMed] [Google Scholar]
- 34. Laskowska E, Kuczyńska-Wiśnik D, Skórko-Glonek J, Taylor A. 1996. Degradation by proteases Lon, Clp and HtrA, of Escherichia coli proteins aggregated in vivo by heat shock; HtrA protease action in vivo and in vitro. Mol Microbiol 22:555–571. doi: 10.1046/j.1365-2958.1996.1231493.x [DOI] [PubMed] [Google Scholar]
- 35. Moon S, Ham S, Jeong J, Ku H, Kim H, Lee C. 2023. Temperature matters: bacterial response to temperature change. J Microbiol 61:343–357. doi: 10.1007/s12275-023-00031-x [DOI] [PubMed] [Google Scholar]
- 36. Pomposiello PJ, Bennik MHJ, Demple B. 2001. Genome-wide transcriptional profiling of the Escherichia coli responses to superoxide stress and sodium salicylate. J Bacteriol 183:3890–3902. doi: 10.1128/JB.183.13.3890-3902.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Veyrier FJ, Boneca IG, Cellier MF, Taha MK. 2011. A novel metal transporter mediating manganese export (MntX) regulates the Mn to Fe intracellular ratio and Neisseria meningitidis virulence. PLoS Pathog 7:e1002261. doi: 10.1371/journal.ppat.1002261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Daly MJ, Gaidamakova EK, Matrosova VY, Vasilenko A, Zhai M, Venkateswaran A, Hess M, Omelchenko MV, Kostandarithes HM, Makarova KS, Wackett LP, Fredrickson JK, Ghosal D. 2004. Accumulation of Mn(II) in Deinococcus radiodurans facilitates gamma-radiation resistance. Science 306:1025–1028. doi: 10.1126/science.1103185 [DOI] [PubMed] [Google Scholar]
- 39. Helmann JD. 2014. Specificity of metal sensing: iron and manganese homeostasis in Bacillus subtilis. J Biol Chem 289:28112–28120. doi: 10.1074/jbc.R114.587071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dubey S, Majumder P, Penmatsa A, Sardesai AA. 2021. Topological analyses of the L-lysine exporter LysO reveal a critical role for a conserved pair of intramembrane solvent-exposed acidic residues. J Biol Chem 297:101168. doi: 10.1016/j.jbc.2021.101168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Stancik LM, Stancik DM, Schmidt B, Barnhart DM, Yoncheva YN, Slonczewski JL. 2002. pH-dependent expression of periplasmic proteins and amino acid catabolism in Escherichia coli. J Bacteriol 184:4246–4258. doi: 10.1128/JB.184.15.4246-4258.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Whittaker MM, Mizuno K, Bächinger HP, Whittaker JW. 2006. Kinetic analysis of the metal binding mechanism of Escherichia coli manganese superoxide dismutase. Biophys J 90:598–607. doi: 10.1529/biophysj.105.071308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Li Y, Moe PC, Chandrasekaran S, Booth IR, Blount P. 2002. Ionic regulation of MscK, a mechanosensitive channel from Escherichia coli. EMBO J 21:5323–5330. doi: 10.1093/emboj/cdf537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sharma R, Shimada T, Mishra VK, Upreti S, Sardesai AA. 2016. Growth inhibition by external potassium of Escherichia coli lacking PtsN (EIIANtr) is caused by potassium limitation mediated by YcgO. J Bacteriol 198:1868–1882. doi: 10.1128/JB.01029-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Roe AJ, McLaggan D, O’Byrne CP, Booth IR. 2000. Rapid inactivation of the Escherichia coli Kdp K+ uptake system by high potassium concentrations. Mol Microbiol 35:1235–1243. doi: 10.1046/j.1365-2958.2000.01793.x [DOI] [PubMed] [Google Scholar]
- 46. Bosma EF, Rau MH, van Gijtenbeek LA, Siedler S. 2021. Regulation and distinct physiological roles of manganese in bacteria. FEMS Microbiol Rev 45:fuab028. doi: 10.1093/femsre/fuab028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Paruthiyil S, Pinochet-Barros A, Huang X, Helmann JD. 2020. Bacillus subtilis TerC family proteins help prevent manganese intoxication. J Bacteriol 202:e00624-19. doi: 10.1128/JB.00624-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Guedon E, Helmann JD. 2003. Origins of metal ion selectivity in the DtxR/MntR family of metalloregulators. Mol Microbiol 48:495–506. doi: 10.1046/j.1365-2958.2003.03445.x [DOI] [PubMed] [Google Scholar]
- 49. McGuire AM, Cuthbert BJ, Ma Z, Grauer-Gray KD, Brunjes Brophy M, Spear KA, Soonsanga S, Kliegman JI, Griner SL, Helmann JD, Glasfeld A. 2013. Roles of the A and C sites in the manganese-specific activation of MntR. Biochemistry 52:701–713. doi: 10.1021/bi301550t [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. He B, Sachla AJ, Helmann JD. 2023. TerC proteins function during protein secretion to metalate exoenzymes. Nat Commun 14:6186. doi: 10.1038/s41467-023-41896-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hayes ET, Wilks JC, Sanfilippo P, Yohannes E, Tate DP, Jones BD, Radmacher MD, BonDurant SS, Slonczewski JL. 2006. Oxygen limitation modulates pH regulation of catabolism and hydrogenases, multidrug transporters, and envelope composition in Escherichia coli K-12. BMC Microbiol 6:89. doi: 10.1186/1471-2180-6-89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Litvak Y, Byndloss MX, Bäumler AJ. 2018. Colonocyte metabolism shapes the gut microbiota. Science 362:eaat9076. doi: 10.1126/science.aat9076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rogers AWL, Tsolis RM, Bäumler AJ. 2020. Salmonella versus the microbiome. Microbiol Mol Biol Rev 85:e00027-19. doi: 10.1128/MMBR.00027-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A 97:6640–6645. doi: 10.1073/pnas.120163297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, Datsenko KA, Tomita M, Wanner BL, Mori H. 2006. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol 2:2006.0008. doi: 10.1038/msb4100050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Larson MH, Mooney RA, Peters JM, Windgassen T, Nayak D, Gross CA, Block SM, Greenleaf WJ, Landick R, Weissman JS. 2014. A pause sequence enriched at translation start sites drives transcription dynamics in vivo. Science 344:1042–1047. doi: 10.1126/science.1251871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Miller JH. 1992. A short course in bacterial genetics: a laboratory manual and handbook for Escherichia coli and related bacteria. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY. [Google Scholar]
- 58. Verkhovskaya M. 2017. Preparation of everted membrane vesicles from Escherichia coli cells. Bio Protoc 7:e2254. doi: 10.21769/BioProtoc.2254 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figures S1 to S8; Tables S1 to S5.