Abstract
Objectives
To expand understanding of the pathogenesis, presentations, and treatment of initially idiopathic small fiber polyneuropathy (SFN).
Methods
We longitudinally readministered validated metrics to track disease course and treatment responses in a previously healthy woman with acute, postinfectious, skin biopsy-confirmed, idiopathic SFN.
Results
During 5 years, viral respiratory infections triggered 3 separated episodes of acute, disabling burning hand, foot, and face pain (erythromelalgia). The initial 2 resolved with high-dose prednisone, and the third responded to repeated immunoglobulin treatments. Pregnancy with miscarriage triggered a fourth exacerbation refractory to corticosteroids and cyclosporin. Immunoglobulins restored total remission for 2 months; then, 2 rituximab doses slightly improved later flaring. Subsequently, daratumumab initiated 100-day remission later maintained by belimumab, initiated to permit another pregnancy. Remission continued after gestational week 13 all-treatment withdrawal. A week 30 fifth flare responded to plasmapheresis, with healthy birth at week 40. At 11-week postpartum, as symptoms returned, restarting belimumab restored remission maintained during ≥19 months of breastfeeding.
Discussion
This decade of tracking characterizes a relapsing-remitting course of SFN with initially separated monophasic episodes becoming more confluent, as with multiple sclerosis. This tempo and responsiveness to 5 immunotherapies suggest dysimmune causality. Validated metrics helped define the course and track treatment efficacy, particularly during pregnancy and breastfeeding.
Classification of Evidence
This is a single observational study without controls. This provides Class IV evidence.
Introduction
The latest European prevalence estimate of diagnosed small fiber polyneuropathy (SFN), ≥132 cases/100,000, exceeds that of multiple sclerosis (83/100,000).1 Large series report 1/3-1/2 of SFN cases as initially idiopathic (iiSFN),1-3 whilst providing evidence of dysimmunity/inflammation as the most prevalent comorbidity apart from diabetes. Other associations are accruing with autoantibodies and aberrant innate immunity.4 Satisfying Witebsky postulates for establishing conditions because autoimmune, independent passive transfer experiments reproduced SFN symptoms and pathology in mice injected with IgG from patients with iiSFN.5,6 Population “clinical clues” include excess prevalences of comorbid autoimmune conditions (particularly Sjögren syndrome),7,8 blood inflammatory markers,2 autoantibodies,4,9 and immunoglobulin deficiencies.2,10 Individual clues suggesting dysimmunity include acute-onset of iiSFN,5,11-13 temporal associations with viral infections including COVID-19,5,11,14 non–length-dependent presentations, and responsiveness to immunotherapies in selected patients with apparently dysimmune causality.7,8,10,13
SFN care and research have been hindered by insufficient clinimetrics. The 2 reported tempos for SFN—slowly progressive chronic and acute monophasic—conceptually resemble those of the large-fiber dysimmune neuropathies' chronic inflammatory demyelinating polyneuropathy (CIDP) and Guillain-Barré syndrome.5,10-13 In addition, although young female patients seem preferentially susceptible to iiSFN,1,3,15 we have not seen management during pregnancy and breastfeeding discussed previously.
Methods
We encountered this patient early in Episode 2 and verified she met diagnostic criteria for iiSFN.e1 With IRB consent, we tracked symptoms and examination abnormalities using validated metrics supporting the 2020 global consensus case definition.e1 The patient repeated the online REDCap-validated Small Fiber Symptom Survey (SSS).15
Data Availability
Anonymized data not published within the article will be shared by request from any qualified investigator.
Results
On January 14, 2014, 14-day postonset of uncomplicated viral upper-respiratory infection (URI), a healthy, active married 30-year-old European postdoctoral fellow noted new right toe tingling and reduced sensation (numbness). For 1 week, these spread to both feet and hands and unexplained severe nose itching appeared. She next noted new episodic lancinating foot pain and allodynia discomfort from socks and bed sheets. Foot and hand erythromelalgia (Figure 1) objectively confirmed colocalizing symptoms, including disabling sensations of walking on broken glass (6–8/10 pain). There was never motor, dysautonomic, nor other neurologic symptoms. The only medical history was mild scalp psoriasis and a cousin with CIDP; there was no psychiatric history. Initial neurologic examination (E1-d49) identified normal strength and tendon reflexes with reduced plantar-foot pin sensation. As vibration and proprioception sensations were intact, pure SFN was suspected.1 Electroneuromyography/nerve conduction study, CSF analysis (autoantibody testing was unavailable), head MRI, and testing of heart rate variability during deep breathing and sudomotor skin reflexes (hands and feet) yielded normal results. An E1-d98 PGP5.5-immunolabeled lower-leg skin biopsy pathologically confirmed pure SFN.e1 Measured epidermal neurite density (160/mm2 skin surface area) was 2.28% of predicted by Mass. General Hospital's multivariate algorithm (although a local laboratory applying a flat 100/mm2 cutoff initially gave a false-normal interpretation). Blood testing confirmed idiopathic attribution (Table); again, autoantibody testing was not yet available.4 Owing to symptom severity and recent nonresolving tempo, on d99, oral prednisone 1 mg/kg/d was tried empirically, with major improvement within 2 weeks and 100% remission within 2 months, maintained throughout slow prednisone taper (221-day use).
Figure 1. Foot Erythromelalgia in Small Fiber Neuropathy.
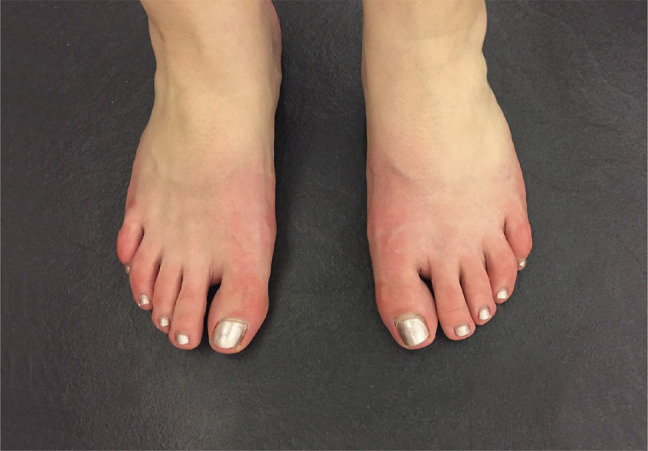
Photograph of patient's feet during second episode. Distal erythromelalgia (Gr; red, painful limb) was provoked by heated rooms, warm footwear, and physical activity. It was severe enough to limit walking. Erythromelalgia is a visible manifestation of the underlying triple flare response of Lewis caused by unprovoked, ectopic firing of damaged C-fiber axon terminals. C-fibers release paracrine inflammatory activators including histamine from their distal terminals, while they send electrical pain signals proximally. The neurogenic flare helps initiate the innate immune response to injury, including microvascular dilation to facilitate immunocyte diapedesis into the tissues. C-fiber activation becomes disabling if it persists chronically.
Table.
Futile Screening Tests for Known Causes of SFN
| Medical conditions | Basic metabolic panel, liver functions, hemoglobin A1c, thyroid function, vitamin B12, folic acid, magnesium, calcium, phosphate, parathormone, heavy metal, blood lipids, complete blood count, coagulation parameters, total protein, serum/urine protein electrophoresis and immunofixation (M-Protein), kappa/lambda light chains, ferritin, beta-2-microglobulin, vATTR amyloid |
| Immune conditions | Erythrocyte sedimentation rate, antinuclear antibodies, Sjögren antibodies, rheumatoid factor, immunoglobulin levels (IgG, IgM, IgA), cryoglobulins (IgM and IgA), anti-transglutaminase IgA and IgG, C-reactive proteins, anti-thyroglobulin and anti-thyroperoxidase antibodies |
| Infections | Campylobacter jejuni (IgG and IgA), Lyme (serum and CSF), EBV (VCA IgG pos, EBNA-1 IgG pos, IgM neg), Helicobacter pylori, HBV antibodies and antigen (neg), HCV antibodies, gonorrhea, syphilis |
| Miscellaneous | Invitae Comprehensive Neuropathies Panel testing for 72 neuropathy-related genes, urine heavy metal screen |
After 3.5 years of off-treatment complete remission, a second same-symptom episode began suddenly in both feet on October 25, 2017, 10-day postonset of viral URI and 4 days after influenza immunization, quickly spreading to the hands and nose. Restarting prednisone (1.2 mg/kg/d) on E2-d2 initiated rapid improvement from SSS = 28/136 pretreatment (E2-d2) to 4/136 on d40 and 0/136 (no symptoms) on E2-d204 (Figure 2). Neurologic examination on d19 revealed only erythromelalgia (Figure 1) and mild paresthesias from touching the left toes. An E2-d21 2nd lower-leg skin biopsy had normalized to 10.7% of predicted, consistent with axonal regeneration during remission and rapidly starting immunotherapy during E2. E2-d22 standard composite autonomic function testing (WR Medical Electronics) yielded normal results. Rapid-then-slow prednisone taper maintained full remission for 16 months including 3-month off-treatment.
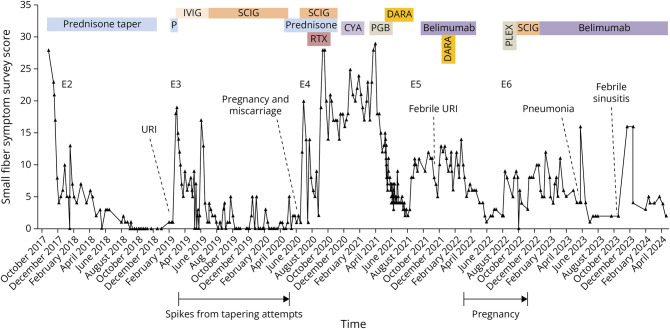
Figure 2. Total Scores on the Small Fiber Symptom Survey During Episodes 2–4.
CyA = cyclosporin A, DARA = daratumumab, E1-6: episodes 1–6, IVIG = IV immunoglobulins, P = prednisone, PLEX = plasmapheresis, PGB = pregabalin, RTX = rituximab, SCIG = subcutaneous immunoglobulins, URI = upper respiratory infection. Scoring details: the Small Fiber Symptom Survey (SSS) is a validated patient-reported questionnaire that measures the presence and frequency of symptoms associated with small fiber neuropathy.15 The 32 items include 5 symptom clusters that cover pain, cardiovascular, gastrointestinal, sweating, and urinary/genital symptoms. Here, the scores for sleep disturbance and headache were excluded because sleep problems were caused by prednisone and headaches by IVIg/SCIg. Dosing details: October 26, 2017: prednisone 1 mg/kg/d for 28 days and then 10 mg/wk tapers. At 20 mg/d, tapering was slowed to 0.25 mg every 4-5 days with successful termination November 29, 2018. February 25, 2019: oral prednisone 1 mg/kg/d for 11 days and then 20 mg for 5 days. March 6, 2019: start of IVIG initial cycle 1.5 g/kg over 3 days and then 1g/kg/4 weeks until June 4, 2019. Doses then tapered to 15 g weekly at a very slow infusion rate due to severe, long-lasting meningeal headaches with nausea. June 12, 2019: SCIG 0.25 g/kg/wk (1 g/kg/4 weeks) until May 16, 2020. May 5, 2020: prednisone 0.5 mg/kg/d for 4 weeks and then taper by 5 mg every 2 weeks. Due to symptom return at 20 mg/d (during 1st pregnancy), prednisone was increased to 40 mg/d for a week, maintained at 25–30 mg/d until September 8, 2020, and then tapered until discontinuation on October 23, 2020. July 3, 2020: SCIG 0.25g/kg/wk until November 29, 2020. Higher single SCIG doses up to 30 g/1 d tried. September 25, 2020: oral methylprednisolone 1g/d pulse therapy for 5 days. August 31 and September 1, 2020: rituximab 1000 mg infusions. December 15, 2020: cyclosporin A initiated at 300 mg orally, then according to therapeutic drug monitoring until January 17, 2021. March 27, 2021: start of pregabalin at 25 mg/day and increase to max of 225 mg/d until June 10, 2021. March 19, April 9, and December 5, 2021: 3 doses of the mRNA anti–SARS-CoV-2 vaccine. May 5, 2021: subcutaneous daratumumab initiated at 1800 mg weekly over 2 months, then biweekly injections through August 3, 2021. October 17, 2021: subcutaneous (SC) belimumab weekly initiated at until April 1, 2022. January 7, 2022: IV infusion (to achieve high initial peak concentration) of daratumumab (16 mg/kg, January 7, 2022) followed by a SC daratumumab (1800 mg January 14, 2022) 7 days after. February. 9, 2022: GW 4 pregnancy confirmed through ultrasound. August 25, 2022: 7 plasmapheresis procedures through September 14, 2022. September 23, 2022: restart of SCIG 1g/kg/4 weeks (approximately) until December 8, 2022. December 9, 2022: restart of SC belimumab 200 mg weekly, continuing in December 2023.
Five-year postonset (February 24, 2019), Episode 3 of identical symptoms began 14-day post-URI. On E3-d2, restarting prednisone 1 mg/kg/d initiated rapid resolution. To minimize corticosteroid exposure, IV immunoglobulin (IVIg, 1.5 g/kg ×1 then 1 g/kg/4 weeks) was added on E3-d12 with remission continuing after 1-week prednisone taper. Owing to severe infusion headaches and end-of-dose wearing-off, subcutaneous Ig (SCIg, 1 g/kg/4 weeks) replaced IVIg in the fourth month. Milder headaches remained, but tapering SCIg precipitated immediate mild relapses (Figure 2), so prednisone (0.5 mg/kg/d) was resumed on E3-d436, SCIg was then discontinued, and on-prednisone remission restored.
6.5-year postonset on June 19, 2020, Episode 4 of identical symptoms began during maintenance prednisone 20 mg/d (0.3 mg/kg), at the time of discovery of a gestational week 5 (GW5) pregnancy that miscarried in GW7. Increasing prednisone to 30 mg/d was ineffective, but reinitiating SCIg on E4-d14 produced full remission within 2 days that lasted ∼50 days. On E4-d64, symptoms flared despite maintaining SCIg (1g/kg/4w) plus 25 mg prednisone/d. During the next 30 days, neuropathic pain and allodynia spread from the feet, hands, and face to affect the entire body. On E4-d73, 1 cycle of rituximab (1,000 mg ×2 at 2-week interval) was administered. This stopped the worsening and provided ∼25% improvement (some symptoms resolved but pain remained insufficiently controlled). A E4-d97 1 g oral methylprednisolone pulse did not further improve symptoms, so on E4-d179, cyclosporin A was initiated. This was stopped on E4-d212 when leg pain and erythromelalgia temporarily worsened, a reported side effect. On E4-d269, distal erythromelalgia flared without apparent trigger; initiating pregabalin gave mild improvement.
Owing to these incomplete responses, on E4-d319, daratumumab was tried (1,800 mg weekly subcutaneously for 8 weeks, then 1,800 mg biweekly). It produced complete symptom resolution within 6 weeks, permitting pregabalin taper-off. On E4-d415, mild hand/foot pain returned, so daratumumab was stopped. Belimumab 200 mg subcutaneous weekly tried on E4-d485 initiated slow symptom improvement with 1 transient exacerbation after febrile viral URI. Daratumumab readministration on E4-d567 (16 mg/kg IV then 1800 mg subcutaneous 7 days later) restored remission within 3 weeks.
On February 9, 2022, a GW4 pregnancy was associated with a GW5 symptom flare (episode 5) that resolved by GW12. For safety, belimumab was stopped during GW13 when maternal antibodies begin crossing the placenta, with full off-medication remission for 17 weeks. In GW30, whole-body symptoms returned without antecedent (episode 6). To avoid medications, plasmapheresis was performed 7× during GW32-34, each producing 2–3 days of complete or partial remission. Given transient benefits, plasmapheresis was stopped at week 35 and SCIg reinstituted. This improved symptoms, but Ig-associated headaches resumed. After uncomplicated GW40 delivery of a healthy newborn, breast-feeding was successful. At 2-month postpartum, exacerbation 3 days after influenza vaccination prompted resumption of weekly belimumab injections, with improvement, so SCIg was discontinued. Breastfeeding and normal infant development continues through 19-month postpartum currently. SFN symptoms remain controlled by belimumab despite occasional transient mild flares with infections.
Discussion
The tempo and immunotherapy responses of this biopsy-confirmed case of SFN inform about pathogenesis. The acute postinfectious onset and multiple post-URI flares suggest dysimmune causality according to the literature.11 Although standard serum inflammatory markers were absent, their predictive value is unknown. Pregnancy and postpartum also appeared to be precipitants, as reported for other autoimmune conditions.e2 The evidence for dysimmune pathology comes from repeated responsiveness to multiple immunotherapies, plus the relapsing/remitting then gradually progressive course akin to multiple sclerosis. The remissions argue against an occult chronic medical cause or primary neurodegenerative disorder, as does normalization of the skin biopsy after a long course of prednisone and 3.5 years of remission. Full remissions were initially sustained for 3.5-year off-treatment, then for 16 months with the last 3 being off-treatment, then for 4-month off-treatment during pregnancy, whereas most later remissions required maintenance immunotherapy (Figure 2). For this patient, an SSS cutoff of 5 helped differentiate exacerbations from baseline. By contrast, no psychosocial contributors were identified; the patient remained full-time university employed and completed a 2-year fellowship abroad during this decade.
One anti–T-cell therapy (cyclosporin) was ineffective, whereas 5 immunotherapies that interfere with B cells among other pleiotropic immune effects were beneficial to varying extents. Corticosteroids are reported as effective in series of apparently dysimmune patients with iiSFN.7,11,13 Therapeutic immunoglobulin therapy, which impedes B-cell activity by binding pathogenic autoantibodies and covering antigenic epitopes while nonspecifically impairing complement and cytokine pathways, may also help.5,8,10 Rituximab was only partly effective, conceivably because it more effectively induces apoptosis of CD20+ precursor B cells, than of mature antibody-producing plasma cells that downregulate CD20 during terminal differentiation. By contrast, daratumumab repeatedly induced rapid, near-total remissions, consistent with case-reported efficacy in more conventional autoantibody-mediated conditions unresponsive to immunoglobulins and rituximab.e3 This CD38-targeting multiple-myeloma treatment has greatest efficacy against antibody-producing plasma B cells, which express the highest levels of CD38, with lesser effects against immunocytes that express less CD38. Plasmapheresis transiently removes antibodies and other soluble immune effectors. Belimumab targets B-cell activating factor (BAFF, aka BlyS) to impair B-cell survival and function. It was chosen for use in early pregnancy and breastfeeding because of preliminary reports of safety during pregnancy.e4 However, this patient's immunotherapy responses cannot confirm relative contributions of innate vs adaptive immunity, specific immunocytes, and immune mediators to her small fiber neuropathy nor predict responses of other patients with apparently dysimmune SFN to specific immunotherapies.
Acknowledgment
Neurologist Martin Landolt, MD, Basel, Switzerland, provided helpful testing and treatment during Episode 1 in 2014.
Appendix. Authors
| Name | Location | Contribution |
| Anne Louise Oaklander, MD, PhD | Department of Neurology, Massachusetts General Hospital and Harvard Medical School; Department of Pathology (Neuropathology), Massachusetts General Hospital, Boston | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
| Julia Allen, PhD, MPH | Harvard T.H. Chan School of Public Health & Harvard Medical School, Boston, MA | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
| Nadja Dietliker, MD | Department of Medical Oncology and Hematology, University Hospital Zurich, Zurich, Switzerland | Drafting/revision of the manuscript for content, including medical writing for content |
| Einar P. Wilder-Smith, MD | Department of Neurology, University of Bern; Department Neruology (Neurozentrum), Luzerner Kantonsspital, Switzerland | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data |
Study Funding
This study was funded in part by the US Public Health Service NIH R01-NS093653 (Oaklander), US DoD GW140169 (Oaklander), The MAYDAY, Curvey and Cutler Foundations (Oaklander), and Harvard Catalyst/REDCapUL1 TR001102.
Disclosure
The authors report no relevant disclosures. Go to Neurology.org/NN for full disclosures.
References
- 1.Bitzi LM, Lehnick D, Wilder-Smith EP. Small fiber neuropathy: Swiss cohort characterization. Muscle Nerve. 2021;64(3):293-300. doi: 10.1002/mus.27340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lang M, Treister R, Oaklander AL. Diagnostic value of blood tests for occult causes of initially idiopathic small-fiber polyneuropathy. J Neurol. 2016;263(12):2515-2527. doi: 10.1007/s00415-016-8270-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Greef BTA, Hoeijmakers JGJ, Gorissen-Brouwers CML, Geerts M, Faber CG, Merkies ISJ. Associated conditions in small fiber neuropathy—a large cohort study and review of the literature. Eur J Neurol. 2018;25(2):348-355. doi: 10.1111/ene.13508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Daifallah O, Farah A, Dawes JM. A role for pathogenic autoantibodies in small fiber neuropathy? Front Mol Neurosci. 2023;16:1254854. doi: 10.3389/fnmol.2023.1254854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yuki N, Chan AC, Wong AHY, et al. Acute painful autoimmune neuropathy: a variant of Guillain-Barré syndrome. Muscle Nerve. 2018;57(2):320-324. doi: 10.1002/mus.25738 [DOI] [PubMed] [Google Scholar]
- 6.Fujii T, Lee EJ, Miyachi Y, et al. Antiplexin D1 antibodies relate to small fiber neuropathy and induce neuropathic pain in animals. Neurol Neuroimmunol Neuroinflamm. 2021;8(5):e1028. doi: 10.1212/NXI.0000000000001028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oaklander AL, Klein MM. Evidence of small-fiber polyneuropathy in unexplained, juvenile-onset, widespread pain syndromes. Pediatrics. 2013;131(4):e1091-e1100. doi: 10.1542/peds.2012-2597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pindi Sala T, Villedieu M, Damian L, et al. Long-term efficacy of immunoglobulins in small fiber neuropathy related to Sjogren's syndrome. J Neurol. 2020;267(12):3499-3507. doi: 10.1007/s00415-020-10033-z [DOI] [PubMed] [Google Scholar]
- 9.Chan ACY, Wong HY, Chong YF, et al. Novel autoantibodies in idiopathic small fiber neuropathy. Ann Neurol. 2022;91(1):66-77. doi: 10.1002/ana.26268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu X, Treister R, Lang M, Oaklander AL. IVIg for apparently autoimmune small-fiber polyneuropathy: first analysis of efficacy and safety. Ther Adv Neurol Disord. 2018;11:1756285617744484. doi: 10.1177/1756285617744484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gendre T, Lefaucheur JP, Nordine T, et al. Characterizing acute-onset small fiber neuropathy. Neurol Neuroimmunol Neuroinflamm. 2024;11(2):e200195. doi: 10.1212/NXI.0000000000200195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dabby R, Gilad R, Sadeh M, Lampl Y, Watemberg N. Acute steroid responsive small-fiber sensory neuropathy: a new entity? J Peripher Nerv Syst. 2006;11(1):47-52. doi: 10.1111/j.1085-9489.2006.00062.x [DOI] [PubMed] [Google Scholar]
- 13.Paticoff J, Valovska A, Nedeljkovic SS, Oaklander AL. Defining a treatable cause of erythromelalgia: acute adolescent autoimmune small-fiber axonopathy. Anesth Analg. 2007;104(2):438-441. doi: 10.1213/01.ane.0000252965.83347.25 [DOI] [PubMed] [Google Scholar]
- 14.Oaklander AL, Mills AJ, Kelley M, et al. Peripheral neuropathy evaluations of patients with prolonged Long COVID. Neurol Neuroimmunol Neuroinflamm. 2022;9(3):e1146. doi: 10.1212/NXI.0000000000001146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Treister R, Lodahl M, Lang M, Tworoger SS, Sawilowsky S, Oaklander AL. Initial development and validation of a patient-reported symptom survey for small-fiber polyneuropathy. J Pain. 2017;18(5):556-563. doi: 10.1016/j.jpain.2016.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- eReferences are available as supplemental digital content.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data not published within the article will be shared by request from any qualified investigator.