Abstract
Objectives
To present 4 patients with Erdheim-Chester disease (ECD) based on clinical, radiologic, histopathologic, and molecular genetic findings who had enhancing brainstem lesions and were initially believed to have chronic lymphocytic inflammation with pontine perivascular enhancement responsive to steroids (CLIPPERS).
Methods
Case series.
Results
Although patients with ECD can demonstrate clinical and imaging features similar to CLIPPERS, refractoriness to corticosteroids, lack of fulfillment of specific MRI criteria (i.e., enhancing lesions >3 mm, T2 abnormalities that exceed areas of T1 postgadolinium enhancement), and systemic findings such as “hairy kidney” appearance and metadiaphyseal osteosclerosis on 18F-fluorodeoxyglucose PET-CT help discriminate it from CLIPPERS.
Discussion
ECD is a histiocytic neoplasm characterized by multiorgan infiltration of clonal histiocytes carrying activating variants of the MAPK-ERK pathway. Neurologic involvement occurs in up to 40% of ECD with frequent brainstem lesions that can mimic acquired neuroinflammatory disorders, such as CLIPPERS. ECD is an important CLIPPERS mimic with distinct pathophysiology and targeted treatments. We highlight the need to consider histiocytic disorders among other alternate diagnoses when findings are not classic for CLIPPERS.
Introduction
Erdheim-Chester disease (ECD) is a multisystem histiocytic neoplasm originating from myeloid lineage cells harboring mitogen-activated protein kinase (MAPK) pathway driver alterations.1 Neurologic involvement occurs in up to 40% of ECD with frequent brainstem lesions that mimic acquired neuroinflammatory disorders, such as chronic lymphocytic inflammation with pontine perivascular enhancement responsive to steroids (CLIPPERS).2 These disorders share a propensity for brainstem-predominant punctate and curvilinear gadolinium-enhancing lesions.3 Genetic screening has demonstrated one-third of patients with suspected CLIPPERS carry pathogenic hemophagocytic lymphohistiocytosis (HLH)-related variants suggesting a subset of patients with suspected CLIPPERS are misdiagnosed and alternatively have a histiocytosis requiring distinct treatment.4 Many patients with ECD initially receive suboptimal empiric immunosuppressive treatments for a presumed inflammatory etiology. However, in the era of targeted treatments for ECD, BRAF and MEK inhibitors are efficacious at inducing disease remission.5 We herein describe a series of patients with CLIPPERS-like neurologic syndrome with systemic findings that led to their diagnosis of ECD.
Clinical Cases
Case 1
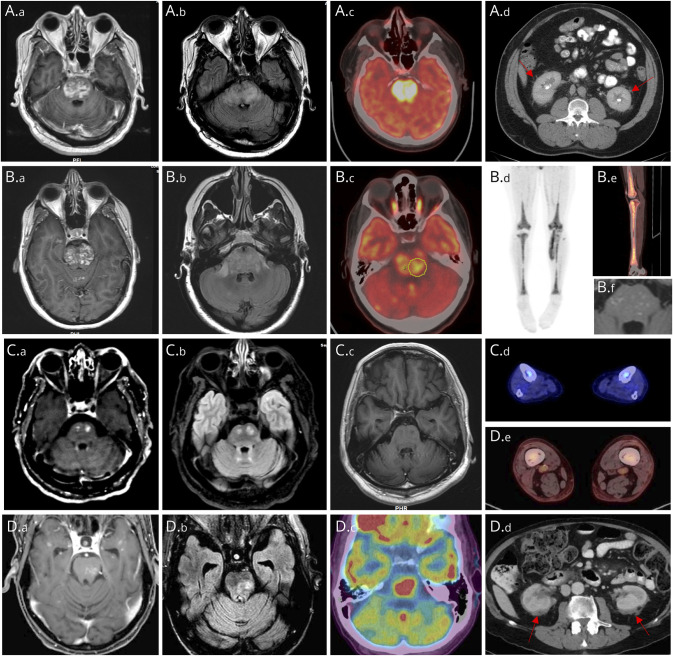
A 52-year-old man presented with progressive dysesthesias, paraparesis, gait imbalance requiring a cane, and hoarseness over 8 months. MRI brain demonstrated punctate/curvilinear enhancement with patchy T2-hyperintensities predominantly involving the pons (Figure, A.a and A.b). He received methylprednisolone 1 g for 5 days without improvement. After 4 months, he developed right face and arm spasms and worsening gait. IVIG 2 g/kg over 5 days had no effect on symptoms. He developed dysarthria, dysphagia, weight loss, worsened imbalance, and bilateral abducens palsies over 4 months. CSF was noninflammatory. Infective studies were negative for JC virus, cryptococcus, West Nile, varicella-zoster virus, cytomegalovirus, herpes simplex, syphilis, HIV, and Mycobacterium tuberculosis. Rheumatologic serologies and complement levels were normal. He underwent stereotactic right cerebellar biopsy that was nondiagnostic demonstrating mild macrophage infiltrate, frequent Rosenthal fibers, and patchy myelin loss, but no evidence of neoplasm, granuloma, or vasculitis. He worsened despite 3 months of mycophenolate and oral dexamethasone for presumed CLIPPERS. Two months later, he was admitted for clinical deterioration, using a wheelchair, needing full assistance to transfer, severely dysarthric with oculomotor dysfunction and quadriparesis. Repeat MRI brain demonstrated persistently enhancing brainstem lesions. 18F-FDG-PET-CT showed increased uptake within the pons (Figure, A.c), perirenal fat stranding (Figure, A.d), and metadiaphyseal osteosclerosis. A whole-body technetium-99M methylene diphosphonate bone scan demonstrated increased uptake within the femur. Femur biopsy showed marrow space fibrosis and infiltration by foamy macrophages which labeled CD68+/CD163+/CD1a—compatible with ECD. He received brainstem radiation and dexamethasone with worsening of dysarthria, dysphagia, horizontal ophthalmoparesis, and quadriparesis. He clinically improved after receiving IV high-dose methotrexate but worsened after aspiration pneumonia treated with piperacillin/tazobactam. He switched to oral methotrexate 5 mg daily but deteriorated and died months later. BRAFV600E and other molecular testing was not performed because his case preceded the discovery of somatic mutations in ECD.
Figure. Imaging Findings in Patients With ECD and CLIPPERS-Like Enhancing Brainstem Lesions.
Imaging findings from 4 patients (A–D). MRI brain findings on T1-weighted postgadolinium administration (A.a, B.a, C.a, and D.a), T2-FLAIR (A.b, B.b, C.b, and D.b) sequences, including T1-postgadolinium follow-up scans from patient 2 (B.f) and patient 3 (C.c) demonstrating favorable response to targeted therapies. 18F-FDG PET-CT demonstrated increased uptake within the pons (patient 1 [A.c], patient 2 [B.c], and patient 4 [D.c]) and distal femur and tibia (patient 2 [B.d, B.e], patient 3 [C.d], and patient 4 [D.e]) with wispy infiltration in perinephric region producing a “hairy kidney” appearance (patient 1 [A.d] and patient 4 [D.d]).
Case 2
A 27-year-old woman presented with progressive diplopia, gait imbalance, speech difficulty, and dysphagia over 10 months. She had right abducens, right hemifacial weakness, left > right quadriparesis, appendicular dysmetria, and ataxic gait. MRI brain revealed speckled irregularly enhancing lesions in the pons (Figure, B.a and B.b), cerebellum, and to a lesser extent supratentorial compartment previously believed to be consistent with CLIPPERS. She received methylprednisolone 1 g for 5 days then prednisone 80 mg taper over 8 months without clinical or radiologic response. 18F-FDG-PET-CT demonstrated increased uptake in the pons (Figure, B.c), distal femur, and tibia (Figure, B.d and B.e). Bone biopsy with molecular genetics revealed UBTD2-BRAF fusion confirming ECD. She received cobimetinib 60 mg 21 days on, 7 days off for 1 year with partial clinical and radiographic response (Figure, B.f) without intolerability or toxicities at 1 year follow-up.
Case 3
A 65-year-old man presented with progressive gait imbalance, right leg weakness, dysphagia, and dysarthria over 1 year. He required crutches by 2 years and a walker by 3 years. MRI brain showed multifocal enhancing punctate/nodular lesions interspersed within the pons (Figure, C.a and C.b) and to a lesser extent supratentorium. CSF was unrevealing. He received ocrelizumab, infliximab, cyclophosphamide, and cladribine with multiple steroid courses without clinical or radiologic improvement for presumed neuroinflammatory diagnoses including MS, neurosarcoidosis, and CLIPPERS. On further investigation, 18F-FDG-PET-CT revealed long bone involvement (Figure, C.d) suggestive of ECD and bone marrow biopsy was positive for BRAF-V600E variant by immunohistochemical staining and next-generation sequencing. Approximately 4 years after onset, he started vemurafenib 480 mg twice-a-day for 2 months then combined vemurafenib/cobimetinib (960/40 mg daily/21 days on 7 days off) to augment CNS treatment response with radiologic remission on 18F-FDG-PET-CT and brain MRI (Figure, C.c). Clinical improvement was modest considering long-standing and dense impairments.
Case 4
A 79-year-old man presented with progressive gait impairment after admission for cholecystitis status post robotic-assisted cholecystectomy, initially attributed to deconditioning. His gait worsened over several months associated with falls, right leg weakness, and horizontal diplopia. MRI brain 7 months after onset showed punctate/curvilinear enhancement with corresponding patchy T2-hyperintensities (Figure, D.a and D.b) suggestive of CLIPPERS, in addition to pituitary stalk nodular thickening on T1-weighted imaging. Extensive investigations were negative for infectious, rheumatologic, autoimmune, and metabolic disorders. 18F-FDG-PET-CT disclosed increased uptake in the pons (Figure, D.c), perinephric stranding “hairy kidney” appearance (Figure, D.d), and metadiaphyseal osteosclerosis (Figure, D.e) with avidity in distal femur and tibia. Femur biopsy with molecular genetics detected BRAFV600E and NF1 variants compatible with ECD. The patient has been started on cobimetinib.
Discussion
Originally described in 1930 by Erdheim and Chester, ECD was historically treated as an inflammatory disorder with chemotherapeutic or immunosuppressive agents, and mortality rates were as high as 60% within 3 years.5-7 Interferon α had early positive effect on patient outcomes; however, in 2012, frequent detection of BRAFV600E variant led to use of BRAF inhibitor vemurafenib that induced metabolic radiologic responses in up to 90% of patients who were BRAFV600E sequence variant.5 Further genomic profiling discovered other activating MAPK-ERK pathway alterations (ARAF, MAP2K1, NRAS, and PI3KCA) in BRAF-wild-type patients targetable with MEK inhibitor cobimetinib defining it as a clonal neoplastic disorder.5,7 BRAF and MEK inhibitors are used in moderate to severe disease including all patients with CNS involvement.1,5,7 Intracranially, ECD often involves the posterior fossa with frequent multifocal enhancement involving the pons and cerebellum which can mimic CLIPPERS. Diagnosis of ECD and other histiocytic neoplasms involving the nervous system can be particularly challenging because biopsies can be nonspecific and corroborate a clinical impression of an immune process.8
CLIPPERS is a steroid-responsive brainstem-predominant encephalomyelitis characterized by punctate and curvilinear postgadolinium enhancement first described in 2010.2,7,9-12 Since its original description, several CLIPPERS mimics have been identified in patients with rhombencephalitis and enhancing brainstem lesions including myelin oligodendrocyte glycoprotein antibody-associated disease, autoimmune glial fibrillary acidic protein astrocytopathy, CNS vasculitis, neurosarcoidosis, lymphoprolipherative disorders, and histiocytic disorders including ECD.9,13-15 Diagnostic criteria were proposed in 2017 to distinguish CLIPPERS from non-CLIPPERS etiologies based on clinical, radiologic, and neuropathologic findings.13
Our series emphasizes the importance of considering ECD when findings are not classic for CLIPPERS. In our patients, corticosteroid refractoriness, heterogeneous enhancing lesions >3 mm in diameter, and larger areas of T2-hyperintensity than T1-postgadolinium enhancement argued against CLIPPERS (eTable 1).4,6,13,14 In ECD, enhancing brainstem lesions may accompany osteosclerosis around the knees (95%), perinephric stranding (“hairy kidney”, 65%), diabetes insipidus (50%), periaortic encasement (60%), right atrial pseudotumor (40%), pleural thickening (50%), and orbital masses (30%).2,7,10-12 Systemic features were important clues to the diagnosis of ECD in our series, particularly long bone and perinephric involvement; if ECD is suspected clinically, head-to-toe PET/CT should be performed to evaluate for bone abnormalities and the other findings above.2 This report highlights the importance of following diagnostic criteria for CLIPPERS strictly to reduce risk of misdiagnosis and features that favor ECD.
Appendix. Authors
| Name | Location | Contribution |
| Samir Alkabie, MD, MSc | Department of Neurology, Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, Lenox Hill Hospital, New York | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
| Eli L. Diamond, MD, MPhil | Department of Neurology, Memorial Sloan Kettering Cancer Center, New York | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
Study Funding
This work was supported by the National Institutes of Health/National Cancer Institute (P30 CA008748), as well as the National Cancer Institute (R37CA259260; E.L.D). This work was also supported by the Frame Family Fund (E.L.D), the Joy Family West Foundation (E.L.D), and the Applebaum Foundation (E.L.D).
Disclosure
E. Diamond has received unpaid editorial support from Pfizer Inc and has served on an advisory board for Opna Bio, all outside the submitted work. S. Alkabie reports no relevant disclosures. Go to Neurology.org/NN for full disclosures.
References
- 1.Cohen Aubart F, Idbaih A, Emile JF, et al. Histiocytosis and the nervous system: from diagnosis to targeted therapies. Neuro Oncol. 2021;23(9):1433-1446. doi: 10.1093/neuonc/noab107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goyal G, Young JR, Koster MJ, et al. The Mayo Clinic Histiocytosis Working Group consensus statement for the diagnosis and evaluation of adult patients with histiocytic neoplasms: Erdheim-Chester disease, langerhans cell histiocytosis, and Rosai-Dorfman disease. Mayo Clin Proc. 2019;94(10):2054-2071. doi: 10.1016/j.mayocp.2019.02.023 [DOI] [PubMed] [Google Scholar]
- 3.Taieb G, Duran-Pena A, de Chamfleur NM, et al. Punctate and curvilinear gadolinium enhancing lesions in the brain: a practical approach. Neuroradiology. 2016;58(3):221-235. doi: 10.1007/s00234-015-1629-y [DOI] [PubMed] [Google Scholar]
- 4.Taieb G, Kaphan E, Duflos C, et al. Hemophagocytic lymphohistiocytosis gene mutations in adult patients presenting with CLIPPERS-like syndrome. Neurol Neuroimmunol Neuroinflamm. 2021;8(3):e970. doi: 10.1212/NXI.0000000000000970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vaglio A, Diamond EL. Erdheim-Chester disease: the “targeted” revolution. Blood. 2017;130(11):1282-1284. doi: 10.1182/blood-2017-07-795054 [DOI] [PubMed] [Google Scholar]
- 6.de la Fuente MI, Rosenblum MK, Diamond EL, Tabar VS, Omuro A. Erdheim-Chester disease among neuroinflammatory syndromes: the case for precision medicine. Neurol Neuroimmunol Neuroinflamm. 2020;7(3):e686. doi: 10.1212/NXI.0000000000000686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goyal G, Heaney ML, Collin M, et al. Erdheim-Chester disease: consensus recommendations for evaluation, diagnosis, and treatment in the molecular era. Blood. 2020;135(22):1929-1945. doi: 10.1182/blood.2019003507 [DOI] [PubMed] [Google Scholar]
- 8.Bhatia A, Hatzoglou V, Ulaner G, et al. Neurologic and oncologic features of Erdheim-Chester disease: a 30-patient series. Neuro Oncol. 2020;22(7):979-992. doi: 10.1093/neuonc/noaa008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pittock SJ, Debruyne J, Krecke KN, et al. Chronic lymphocytic inflammation with pontine perivascular enhancement responsive to steroids (CLIPPERS). Brain. 2010;133(9):2626-2634. doi: 10.1093/brain/awq164 [DOI] [PubMed] [Google Scholar]
- 10.Go RS, Jacobsen E, Baiocchi R, et al. Histiocytic neoplasms, Version 2.2021, NCCN clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021;19(11):1277-1303. doi: 10.6004/jnccn.2021.0053 [DOI] [PubMed] [Google Scholar]
- 11.Benson JC, Vaubel R, Ebne BA, et al. Erdheim-chester disease. AJNR Am J Neuroradiol. 2023;44(5):505-510. doi: 10.3174/ajnr.A7832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hazim AZ, Acosta-Medina AA, Young JR, et al. Classical and non-classical phenotypes of Erdheim-Chester disease: correlating clinical, radiographic and genotypic findings. Br J Haematol. 2022;199(3):454-457. doi: 10.1111/bjh.18422 [DOI] [PubMed] [Google Scholar]
- 13.Tobin WO, Guo Y, Krecke KN, et al. Diagnostic criteria for chronic lymphocytic inflammation with pontine perivascular enhancement responsive to steroids (CLIPPERS). Brain. 2017;140(9):2415-2425. doi: 10.1093/brain/awx200 [DOI] [PubMed] [Google Scholar]
- 14.Zalewski NL, Tobin WO. Clippers. Curr Neurol Neurosci Rep. 2017;17(9):65. doi: 10.1007/s11910-017-0773-7 [DOI] [PubMed] [Google Scholar]
- 15.Taieb G, Mulero P, Psimaras D, et al. CLIPPERS and its mimics: evaluation of new criteria for the diagnosis of CLIPPERS. J Neurol Neurosurg Psychiatry. 2019;90(9):1027-1038. doi: 10.1136/jnnp-2018-318957 [DOI] [PubMed] [Google Scholar]