Abstract
The theory of “disinhibition” has been very influential in psychiatry and neurology for over a century. Disinhibition has been used to explain clinical findings in many neurological and psychiatric disorders including dementia, traumatic brain injury, attention deficit hyperactive disorder, substance abuse, impulsivity in personality disorders, and neurodevelopmental disorders. In addition, disinhibition has been used as a unifying theory to link clinical observations with cognitive findings, and even cellular findings. This review discusses the origins and history of the theory of disinhibition and its strengths and weaknesses in four domains: face validity, consistency with other brain mechanisms, consistency with evolutionary mechanisms, and empiric support. I assert that the vagueness of the theory, inconsistency with other brain mechanisms, and lack of empiric support limit the usefulness of this theory. Alternative approaches, based on findings in other motor, language, and cognitive functions, are discussed.
Keywords: Disinhibition, dementia, frontal lobes, ADHD, substance abuse
From a person with dementia touching their genitals in a crowded shopping center to excessive rudeness in anonymous online forums (the “online disinhibition effect”) (Suler, 2004), to a child with attention deficit hyperactive disorder (ADHD) who has trouble paying attention in class, to someone who talks excessively to strangers on the subway, disinhibition is commonly invoked to explain the behaviors of both patients with neurological and psychiatric illnesses and neurologically and psychiatrically healthy persons. There is no single accepted definition of disinhibition. A representative definition is “socially inappropriate verbal, physical, or sexual acts, which reflect a loss of inhibition or an inability to conform to social or cultural behavioral norms” (Arciniegas and Wortzel, 2014). However, the concept of disinhibition has been used to unify and explain disparate clinical findings including substance use, conduct disorder, ADHD, novelty seeking, response inhibition on an antisaccade task, and performance on the Stroop task (Young et al., 2009). The questions addressed in this review are the following: “What is the intellectual context and history in which the theory of disinhibition was developed?” “What is the evidence in support of and against this theory?” “Are there other ways we can interpret symptoms currently termed ‘disinhibition’”?
These questions are clinically and scientifically relevant. Managing behavioral symptoms associated with dementia due to neurodegenerative disorders, traumatic brain injury (TBI), and other causes represents a major health crisis throughout the world. As populations age, the prevalence of dementia continues to increase. Currently, over 47 million people suffer from dementia worldwide (World Health Organization, 2016). The majority of these patients will have neuropsychiatric symptoms (NPSs) at some point in their illness (Lyketsos et al., 2011). NPSs, including disinhibition, are a major predictor for placement out of the home and greatly increase the cost of care (Lyketsos et al., 2002). There is great interest in better understanding the neuroanatomical bases of, and developing improved treatments for, NPS in dementia. In psychiatry, disinhibition has been a commonly used explanatory theory for many disparate findings across several common disorders, guiding both therapeutic and research decisions. For example, clinicians and researchers may unnecessarily avoid the use of medications that have been associated with disinhibition, due to fear of exacerbating disinhibition (Gennatas et al., 2012). Newer research technologies, notably functional magnetic resonance imaging, generate enormous amounts of data, requiring theoretical models to simplify and interpret these data. Inaccurate models can result in important misinterpretations of the data (Huey and Lieberman, 2012).
Behavioral and emotional inhibition and disinhibition have been linked to the frontal lobes (Starkstein and Robinson, 1997). Clearly, the human frontal lobes are not uniform structures and are composed of many distinct regions. In this review, the prefrontal cortex or PFC is defined as the frontal cortex anterior to the supplementary motor area and premotor cortex, including the anterior cingulate and orbitofrontal cortexes (OFCs) unless otherwise specified (Kringelbach and Rolls, 2004; Petrides and Pandya, 1994). In the psychiatric literature, the term OFC, encompassing the ventral surface of the prefrontal cortex including parts of BA 10, 11, and 47, is frequently used (Kringelbach and Rolls, 2004). In the neurological literature, this region is more often referred to as the ventral PFC (vPFC), often with a specifier of lateral or medial to capture the differential cytoarchitecture, connectivity, and function of the lateral and medial vPFC (vlPFC and vmPFC). We will utilize the definition of the limbic system as in Morgane et al., 2005, which includes, but is not limited to, the amygdala, hippocampus and entorhinal cortex, vPFC, anterior cingulate cortex, thalamus, hypothalamus, and nucleus accumbens. A review of the differential function of the different frontal regions is beyond the scope of this review. Given the large literature linking damage of the vmPFC to deficits in social cognition, and changes in emotion and behavior, this region will be a focus of this review (Lima-Silva et al., 2015; Mah et al., 2005; Szczepanski and Knight, 2014).
HISTORICAL DEVELOPMENT OF THE THEORY OF DISINHIBITION
Discoveries made in the 19th century laid the groundwork for much of our current understanding of neuroscience. During this period, experimental data gathered from animal research began, for the first time, to allow localizations of some functions to specific nervous system structures. Not surprisingly, the first discoveries related to motor and sensory functions that were easily observable in animals. Charles Bell and Francoise Magendie discovered spinal reflex arcs that control the stretch reflex and reflex withdrawal to injury (Macmillan, 2000b). Motor cortex lesions in animals and humans can result in an increase in these reflexes (hyperreflexia), suggesting that parts of the cortex provide tonic inhibition of spinal reflex arcs (Smith, 1992) and that hyperreflexia can result when this tonic inhibition is removed.
A series of influential animal experiments were performed by David Ferrier in the second half of the 19th century. He mapped out the motor cortex by electrically simulating the motor strip in monkeys (Ferrier, 1876). However, when he stimulated the prefrontal cortex, he found no observable results except for alterations of eye movements with stimulation of dorsal regions of the PFC. He also induced lesions of the PFC in monkeys and observed “no symptoms indicative of affection or impairment of the sensory or motor faculties” (Ferrier, 1876), although he did notice some behavioral changes. Because Ferrier could ascribe no other function to the prefrontal cortex, he hypothesized that the function of the PFC must be an “inhibitory motor” structure whose purpose is to inhibit motor activity in order to facilitate internal mental reflection (Ferrier, 1876; Macmillan, 2000b).
Contemporaneously, in 1848, an influential case report on a neurosurgical patient, Phineas Gage, was published (Harlow, 1848). Mr. Gage suffered a large left frontal injury, including most of the left PFC, from a railroad spike and had subsequent changes in behavior. The case report provides an early use of the theory that the injury induced an imbalance between preserved primitive impulses and impaired control of intellectual control of those impulses. Ferrier was very interested in the case, requesting and examining photographs of Gage's skull. He, and subsequent neuroscientists (Jackson, 1888; Macmillan, 2000a; Smith, 1992), used this case as evidence of the continuity of the mechanism of disinhibition from removal of cortical inhibition resulting in hyperreflexia and that of disinhibited behaviors resulting from focal cortical damage.
Into this theoretical and scientific milieu came Sigmund Freud. The most influential proponent of the theories of behavioral and emotional inhibition and disinhibition, Freud's ideas on this subject have provided much of the current popular and scientific understanding on this topic. Many of Freud's theories, including the concepts of primary versus secondary process, his focus on repression, and his tripartite division of the mind into id, ego, and superego, are fundamentally based on the concepts of inhibition and disinhibition (Freud, 1949). In Freud's tripartite conception of the mind, the id is the unconscious origin of our drives. Self-oriented, irrational, and often in violation of social norms, the id is directed by the ego, which attempts to mesh the id's desires with reality. The superego embodies the moral and societal prohibitions that inhibit the primitive drives of the id (Freud, 1949). Freud did not specifically designate brain structures that are associated with the id, ego, and superego, but later neuroscientists did (MacLean, 1977). Freud was also influenced by physics, in which equal and opposing forces are required to form a stable system.
Freud's theories have had enormous impact on scientific and lay thought about human motivations and behavior. As others have discussed (Smith, 1992), the importance Freud's theories place on the repression of immoral impulses fits well into a larger framework of Western thought heavily influenced by Judeo-Christian theology, which posits a self continually tempted by drives and temptations that must be resisted by rational moral reasoning. Although most modern neuroscientists would be surprised to be described as Freudians, Freud's emphasis on repression and disinhibition has had an enormous influence on past and current neuroscientific conceptions of human behavior (Smith, 1992). We have argued previously that Freud's formulation of the theories of inhibition and disinhibition are accepted implicitly as proven fact in most of the neurosciences rather than as testable and falsifiable theories (Huey et al., 2007). In many other fields such as psychology, anthropology, and the humanities, Freud's theories have been subjected to more scrutiny and criticism than in the neurosciences.
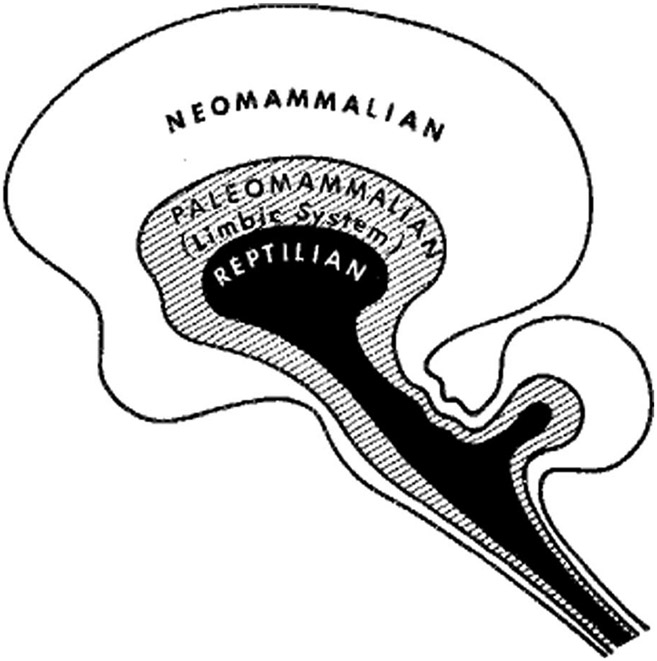
Sigmund Freud did not explicitly map his tripartite mind onto the brain, although he hypothesized special neurons for primary and secondary processes. Paul MacLean was an influential 20th century neuroscientist whose important scientific contributions include a definition and exploration of the limbic system previously outlined by Papez, and a recognition of the importance of the striatum in behavior and emotion (MacLean, 1989). However, he is best known for his theory of the “triune” brain (MacLean, 1989). According to this theory, the brain is composed of the “reptilian” brain including the basal ganglia, defined here as the dorsal striatum (head of the caudate nucleus and putamen), ventral striatum (nucleus accumbens and olfactory tubercle), globus pallidus, ventral pallidum, and subthalamic nucleus, and parts of the midbrain; the “paleomammalian” brain corresponding to the limbic system (defined here as the amygdala, the hypothalamus, and hippocampus, and often including the OFC); and the “neomammalian” brain composed of the neocortex and its thalamic connections (Ploog, 2003) (Fig. 1). In this theory, the reptilian brain is the site of representations of innate species-specific instinctual behaviors, the paleomammalian brain is the “emotional” brain playing important roles in emotional response and functioning in a social setting, and the neomammalian brain is associated with language and complex cognition. MacLean explicitly mapped Freud's concept of the superego anatomically onto the neomammalian brain and Freud's id onto the paleomammalian brain (MacLean, 1977), a concept that has remained popular in scientific and lay thought (Ploog, 2003).
FIGURE 1.
A schematic of MacLean's tripartite view of the brain (MacLean, 1977).
CURRENT INCARNATIONS OF BEHAVIORAL AND EMOTIONAL DISINHIBITION
“Behavioral” and “emotional” disinhibitions have developed as two closely related, but distinct, theories. The theory of behavioral disinhibition refers to the hypothesis that nonfrontal limbic structures, especially the amygdalae, generate the desire to perform societally unacceptable behaviors that are tonically inhibited by the frontal lobes in neurologically intact people. Damage to the frontal lobes releases this tonic inhibition with the resultant emergence of societally unacceptable “disinhibited” behaviors (Bartelet et al., 2014). The theories of behavioral and emotional disinhibition are currently so dominant that it is difficult to choose from ubiquitous examples. Behavioral disinhibition is a diagnostic criterion for frontotemporal dementia (FTD) (Rascovsky et al., 2011), and listed as a symptom of neurocognitive disorders in DSM-5 (American Psychiatric Association, 2013). Commonly used scales of behavioral symptoms associated with dementia and brain injury focus on disinhibition (Cummings et al., 1994); Grace and Malloy, 2001). The symptoms of patients with frontal lobe dysfunction who perform socially inappropriate behaviors are generally described within the theoretical framework of disinhibition. For example, a man with FTD who touches his own genitals in a shopping center would commonly be described as being hypersexual and having disinhibited behaviors.
The closely related theory of “emotional disinhibition” developed from the behavioral inhibition theory. It is utilized mostly in the psychiatric scientific literature and is commonly used to explain the neuroanatomical bases of emotional symptoms in the absence of identifiable brain injury and to interpret functional imaging data collected from psychiatric patients (Huey and Lieberman, 2012; Jovanovic and Ressler, 2010). The emotional disinhibition theory posits that certain limbic structures, notably the amygdala, are the emotional centers of the brain. According to this theory, the frontal lobes are, in contrast, the “cool” cognitive centers of the brain that provide tonic inhibition of the “hot” nonfrontal limbic structures. In the emotional disinhibition theory, certain psychiatric disorders are associated with a failure of the frontal lobes to successfully inhibit excessive emotional output from the amygdalae, resulting in excessive and maladaptive emotional distress and arousal. For example, emotional disinhibition resulting from PFC dysfunction has been invoked to explain the increased amygdalar and vmPFC activation, exacerbated by symptom provocation, observed in patients with posttraumatic stress disorder (PTSD) (Shin et al., 2006) and obsessive-compulsive disorder (OCD) (Chamberlain et al., 2005). Psychotherapy acts to strengthen the “cool” cognitive frontal abilities and counteract posterior limbic input, thus the term “cognitive control” of emotion (Ochsner and Gross, 2005).
In psychiatry, disinhibition has been used as a theoretical basis for explaining disparate symptoms including antisocial behaviors in conduct disorders (Simons et al., 2017), socially inappropriate behaviors in neurodevelopmental disorders including autism (Tannan et al., 2008), substance use disorders (Tarter et al., 2003), problematic behaviors in ADHD (Young et al., 2009), and emotional dysregulation in PTSD (Simons et al., 2017). Psychiatric clinicians and researchers have found disinhibition to be a useful concept as an “intermediate trait” that provides a theoretical explanation to explain and link disparate complex behaviors in psychiatric disorders, suggests an anatomical localization of the symptom, and provides laboratory measures of this trait (e.g., the Stroop test).
Current incarnations of the theories of behavioral and emotional disinhibition have integrated recent findings on reward processing. Behaviorally disinhibited patients are often described as excessively reward-seeking (Mendez and Shapira, 2013). The term “acquired sociopathy” has been used to describe patients who have selectively lost their moral cognition due to frontal dysfunction and have thus become disinhibited and violate social rules, similar to sociopaths (i.e., persons with antisocial personality disorder) (Saver and Damasio, 1991). A central assumption of the term “acquired sociopathy” is that healthy people tonically have urges to perform antisocial behaviors that they inhibit because of moral cognition controlled by the PFC. When this inhibition is lost, they are disinhibited or “freed” to perform the antisocial actions they have always wanted to perform but had been previously restrained by moral impulses. In the lay press, disinhibition has been used to explain rudeness in anonymous online forums (the “online disinhibition effect”) (Suler, 2004) and excessive emotional arousal that interferes with rational thought (“amygdalar hijack”) (Goleman, 2006).
EVIDENCE IN SUPPORT OF THE THEORIES OF BEHAVIORAL AND EMOTIONAL DISINHIBITION
Evidence supporting (pro) and against (con) these theories will be discussed into four sections: face validity, consistency with other brain mechanisms, consistency with evolutionary mechanisms, and empiric support.
FACE VALIDITY
Pro
Disinhibition has strong face validity for many people. Neurologically and psychiatrically healthy people have the internal experience of resisting temptation (e.g., if I want to lose weight, I must inhibit the urge to order dessert in a restaurant) and can easily imagine that people could lose that ability and be unable to resist temptation. As has been mentioned, the concept of disinhibition is compatible with strongly held cultural beliefs about the moral importance of inhibiting inherent drives and desires.
Con
The example above of a man with FTD touching his genitals in a shopping center is used to demonstrate hypersexual and disinhibited behavior. However, dysfunction on many levels of cognitive processing could result in the behaviors observed, including the following: the patient not remembering the societal rule that one is not supposed to touch one's genitals in a shopping center, not perceiving that one is in an environment where this behavior is prohibited (there are environments, such as at home, where this behavior may be allowed), not noticing the negative reactions of the people around who are shocked by the behavior (i.e., theory of mind) and altering behavior accordingly, and finally being disinhibited, that is, gratifying the patient's long-standing desire to touch his genitals in public due to hypersexual impulses now disinhibited due to the selective loss of morality despite intact knowledge that this behavior is prohibited (Table 1). Asserting that the behavior is due to disinhibition requires that the more basic elements of behavior listed above are intact. However, there is accumulating evidence that the more basic elements of behavior including knowledge of societal rules (Panchal et al., 2016), theory of mind (Bora et al., 2015), and desire to please others are impaired in most patients with ventral frontal dysfunction. Our laboratory (Fieo et al., 2018) and others (Ahmed et al., 2015; Miller et al., 1995) have found that, contrary to the predictions of the disinhibition theory, most patients with vPFC dysfunction due to FTD or TBI have significantly reduced sexual drive, including patients with inappropriate sexual behaviors.
TABLE 1.
Domains of Dysfunction Associated With the Behavioral Change of Disrobing in a Public Place
| Domain | Example |
|---|---|
| Crystalized knowledge | Does not remember that touching one's genitals in public is socially prohibited |
| Disorientation | Does not recognize that he is in a public setting that is inappropriate for touching one's genitals |
| Theory of mind | Does not understand why touching one's genitals in public would be upsetting to others |
| Perception of social information | Does not perceive that others are upset by this behavior |
| Activation of proper script | Is not executing correct script for shopping at a shopping center |
| Script knowledge | Has trouble remembering script elements or their proper execution or order |
| Social motivation | Perceives, but does not care, that others are upset by his behavior |
| Disinhibition | Has excessive sexual drive due to release of tonic frontal inhibition of libido resulting in hypersexual behavior including disrobing in public |
In the example of the FTD patient touching his genitals, how much does the interpretation of this patient's behavior reflect the cultural biases of the interpreters rather than an objective assessment of the behavioral changes associated with FTD? Inappropriate sexual behaviors are shocking and upsetting to observers. However, the majority of FTD patients have deficits in motivation, cognition, and social interactions that are less upsetting to observers, but these are important symptoms of the illness, profoundly affect behavior, and are often disabling. For example, the majority of FTD patients, including those who perform socially inappropriate behaviors, are also apathetic (Armstrong et al., 2013; Rascovsky et al., 2011).
Finally, the evidence used to support the theory of behavioral disinhibition is subject to base rate bias, that is, the tendency to ignore baseline rates of a symptom in the face of new information. Most healthy persons rarely, if ever, grossly violate social rules, thus even random changes in behavior can present as increases in antisocial behavior from an extremely low baseline prevalence. Most people have never touched their genitals in a shopping center. The disinhibition theory asserts that the emergence of this behavior is evidence that the patient always wanted to perform this activity, there is a region of the brain that existed to tonically inhibit them from doing so, and that region is now damaged. A more parsimonious explanation of newly emerged antisocial behaviors would be that brain circuits involved in the correct performance of social behaviors are malfunctioning, and thus unusual, and sometimes antisocial, behaviors are emerging. As historical evidence for a base rate bias, frontal leukotomies, in which connections to the vPFC are purposely damaged, used to be used as a successful treatment to decrease agitation and antisocial behaviors in persons with a high baseline prevalence of these behaviors (Kucharski, 1984). Clinically, patients with PFC dysfunction usually have executive dysfunction and will display difficulty successfully performing many social and nonsocial tasks, some of which may violate social rules, but most of which do not.
This is not to say that symptoms such as excessive reward-seeking and hypersexuality do not occur. In fact, these symptoms occur frequently, but generally not in association with frontal injury or degeneration. For example, persons using recreational drugs that excessively stimulate the reward system such as cocaine, patients onprodopaminergic medications such as Parkinson disease patients, and manic patients can all exhibit excessive reward-seeking and hypersexuality (Goldstein and Volkow, 2002; Nakum and Cavanna, 2016; Swann, 2009). A relatively intact reward processing system may even be necessary for excessive reward-seeking.
CONSISTENCY WITH OTHER BRAIN MECHANISMS
Pro
The theories of behavioral and emotional disinhibition provide a pleasing continuity of mechanism from complex behavior (behavioral inhibition), to cognitive inhibition (e.g., the Stroop test), to motor function (reflex disinhibition), to cellular behavior (neuronal inhibition), and even to physics (opposing forces making a stable system).
Con
Human behavior and emotion are significantly more complex than motor and cellular systems, and it is unlikely that all of these disparate phenomena are understandable by the same simple mechanism (see Aron, 2007; Banich and Depue, 2015 for more complete reviews and critiques of cognitive inhibition; and disinhibition). Recent evidence, too extensive to discuss here but reviewed in Okon-Singer et al. (2015), indicates that the relationship between emotional and cognitive systems is complex and multidirectional. The frontal lobes are essential for the complex cognition, language, emotion, and behavior that characterize humans. Such a complex repertoire of cognition and behavior is unlikely to be fundamentally based on simple inhibition of other brain regions.
An appeal of the disinhibition theory in psychiatry is that it provides a uniting mechanism for symptoms of disparate disorders, including ADHD, autism, substance use disorders, personality disorders, PSTD, and even certain behaviors in healthy persons. In addition, this mechanism unites clinical observations with specific neuropsychological tests, motor inhibition, and even animal and basic research on cellular inhibition and disinhibition (Nigg, 2000). A criticism of this use of the disinhibition theory is that the breath of situations to which it is applied to necessitates that the theory be poorly defined and vague. No single theory can accurately explain the range of phenomenon from center-surround field organization in visual processing to why someone buys a car that is more than he or she can afford. In addition, observed correlations between these phenomena (e.g., ADHD and substance abuse) do not necessarily mean that disinhibition is the underlying cause of both phenomena.
The disinhibition hypothesis is a departure from the way in which we conceptualize most other brain functions. Most of our models of complex brain functions are based on an information processing approach in which different brain structures and regions provide specialized input to contribute to a larger brain system associated with the function. For example, language involves several perisylvian brain regions (Catani et al., 2005). Certain regions are specialized for speech production, others for storage of semantic knowledge, others for speech comprehension, and so on. Rather than existing to simply tonically inhibit each other as asserted by the disinhibition hypothesis, the regions work together, as specialized, interacting, interconnected nodes, each providing its own “expertise,” to permit the function of language. Similar information-processing models are currently in use to attempt to understand other brain functions such as visual processing and working memory (Felleman and Van Essen, 1991; Okon-Singer et al., 2015). It is parsimonious to hypothesize that the brain controls complex and social behavior using similar mechanisms.
When describing dysfunction of other brain functions, we also do not usually initially invoke disinhibition if a simpler cognitive explanation is available. For example, many patients with Alzheimer disease (AD) have word-finding difficulty. Many of these patients will substitute vague terms (“that,” “thing,” “this”) for the words they cannot remember. However, we do not generally describe their use of these words as having become “disinhibited” due to AD, even though their use of these words increased in frequency since the patient developed AD. Instead, we view their increased use of these vague words as a manifestation of, and compensation for, word retrieval dysfunction. In an analogy given by previous authors (Aron, 2007; Gregory, 1961; Nieuwenhuis and Yeung, 2005), if your computer breaks and random colors appear on the screen, you would be unlikely to draw the conclusion that a part of the computer that has the job to prevent random colors from generating on your screen has broken. More likely, you would conclude that some part of the computer related to the display is damaged and this manifests as the random colors you see on the screen. Similarly, we should not use the emergence of behaviors after frontal injury as evidence that there are brain structures with the specific purpose of inhibiting those behaviors. The concepts of inhibition and disinhibition have been “overextended” to explain situations that can be more parsimoniously explained using other mechanisms (Aron, 2007; Munakata et al., 2011). Recent animal, computational modeling, and human imaging studies suggest that much cognitive inhibition may occur through indirect competitive mechanisms, that is, activated representations that suppress competing representations in the PFC (Egner and Hirsch, 2005; Munakata et al., 2011).
CONSISTENCY WITH EVOLUTIONARY MECHANISMS
Pro
The models of behavioral and emotional disinhibition have been presented as consistent with human evolution. The frontal lobes, especially the dorsolateral prefrontal cortex, are the regions of the brain that have most expanded in size in modern humans compared with nonhuman primates and other animals (Roth and Dicke, 2005). Some have suggested that PFC development has allowed humans to develop an ability, unique among animals, to suppress primitive drives for immediate gratification to be able to pursue long-term goals (Hayden, 2016). Disinhibition of these drives has been posited to result in an excessive pursuit of immediately rewarding behaviors in patients with PFC dysfunction (Ainslie, 1975).
Con
In the disinhibition model, humans, with their superior “cool” cognition, can modulate their “hot” emotional desires for immediate gratification in a way that other animals cannot. In this model, patients with PFC injury have lost this ability, and are sometimes described as “animal-like.” Humans excel at pursing long-term goals at the expense of short-term goals, and the frontal lobes are central for this ability. However, could this instead be an emergent feature of the enormous complexity and flexibility of cognition and behavior to conceptualize and pursue goals more globally that the human frontal lobes allow, rather than specifically the ability to inhibit the need for immediate gratification?
The human PFC evolved very rapidly. Within a few million years, the human brain has tripled in size, with the largest proportion of that increase occurring in the frontal regions (Roth and Dicke, 2005). Given this rapid increase in size, it is parsimonious to propose that modern humans utilize similar fundamental brain mechanisms as our closest nonhuman relatives. And indeed, recent research has demonstrated that nonhuman primates have rudimentary forms of many abilities that had been previously thought to be solely the purview of humans such as tool use, self-awareness, and complex communication (Shettleworth, 2012). Many modern evolutionary theorists stress the continuity in behavior between humans and nonhuman primates, asserting that the more fundamental difference between animals and humans is the degree of cognitive and behavioral complexity and flexibility humans possess to pursue goals (MacLean, 2016). There are numerous examples of dissociations between frontal lobe size and complexity and the ability to delay gratification. Birds, with rudimentary PFCs, will migrate thousands of miles with the long-term goal of gaining access to better food resources. Penguins will endure months of cold to incubate eggs. The experimental paradigms that supported the concept that animals have very poor self-control have been questioned recently, with one author concluding that, “animals may be much more patient than is commonly believed” (Hayden, 2016). More fundamental differences between animals and humans may exist in the degree of flexibility, complexity, and planning of behavior (Osvath and Martin-Ordas, 2014). Not surprisingly, patients with PFC damage show early and selective deficits in planning and behavioral and cognitive flexibility (Krueger et al., 2007).
EMPIRIC SUPPORT
Pro
Damage to the vmPFC is more likely to result in behavioral changes than comparable damage to other regions of the brain (Lima-Silva et al., 2015). Patients with vmPFC dysfunction will often pursue immediate gratification in violation of societal rules (Rascovsky et al., 2011). A classic clinical example of this is that patients with vPFC dysfunction will take food from a stranger's plate in a restaurant. Behavioral variant FTD usually initially targets the vmPFC (Seeley et al., 2008). Scales of disinhibited behavior completed by caregivers of patients with FTD are elevated (O'Callaghan et al., 2013). Patients with postsurgical vmPFC damage showed increased amygdala activity in response to aversive images (Motzkin et al., 2015), consistent with the theory that the vmPFC tonically inhibits amygdalar responsiveness. Patients with vmPFC have trouble inhibiting overlearned prepotent behavioral responses.
Many psychiatric imaging studies have been interpreted to be consistent with the theory of emotional disinhibition. Patients with PTSD and OCD demonstrate increased brain activity in the amygadalae and vmPFC compared with healthy controls, exacerbated by symptom provocation (Chamberlain et al., 2005; Jovanovic and Ressler, 2010). Interpreted in the emotional disinhibition framework, the vmPFC is attempting to counter the excessive anxiety and arousal generated by the amygdala (thus the increased activation of both regions), but fails in this task, resulting in excessive arousal and anxiety (i.e., the vmPFC is “overwhelmed” by anxiety and emotional arousal) (Chamberlain et al., 2005; Jovanovic and Ressler, 2010). Psychotherapy can act by strengthening the inhibitory output of the vmPFC to counter the excessive arousal and anxiety (i.e., “cognitive control”) (Ochsner and Gross, 2005).
Con
We have focused on the behavioral and disinhibition theories in humans, but much of the experimental evidence on this topic comes from animal models in which the effects of selective lesions can be studied. According to the theory of behavioral disinhibition, vmPFC lesions in animals (including humans) should result in a decreased ability to inhibit previously rewarded responses when these responses become unrewarded (i.e., positive feedback followed by negative feedback). Instead, macaques with vmPFC lesions have increased difficulty learning from positive feedback, and then will perseverate on the initially rewarded response less than intact macaques when contingencies change (Kennerley et al., 2006; Passingham and Wise, 2012; Rudebeck et al., 2008; Rudebeck and Murray, 2008). There is some evidence that a similar pattern is observed in humans with vmPFC lesions (Camille et al., 2011). These results are more compatible with a global learning deficit in monkeys and humans with vmPFC lesions rather than a specific deficit in inhibition.
As discussed previously, PTSD and OCD, disorders characterized by excessive arousal and anxiety, demonstrate increased activation of the vmPFC and amygdala. These findings are often interpreted as confirmatory of the emotional disinhibition theory: the vmPFC is activated in its insufficient attempt to inhibit the arousal and anxiety generated by the amygdala. But what does this vmPFC activation mean? Does it represent an attempt to suppress or to increase or otherwise modify amygdalar activation? In human lesion studies, damage to the vmPFC was protective against the development of PTSD in brain-injured veterans (Koenigs et al., 2008b) and associated with decreased arousal, dysphoria, and anxiety in patients with dementia and mild cognitive impairment (Huey et al., 2016; Cheran et al., 2018). These findings are the opposite of what is predicted by the emotional disinhibition theory and suggest that the vmPFC plays important roles in the self-perception, rather than simply suppression, of anxiety and arousal.
WHAT IS AN ALTERNATIVE THEORY TO DISINHIBITION?
I do not believe there is a single alternative theory to disinhibition, and certainly not one that gives as simple and satisfying an explanation as the disinhibition theory. Human behavior and emotion are complex, and disorders of these functions will also be complex and multifactorial. I have argued in this article that the disinhibition theory has been overextended to explain behavioral and emotional interactions in the brain that are too complex to be sufficiently explained with a single, simple theory. No single theory can accurately explain behaviors as disparate as behavioral symptoms in ADHD, neuropsychiatric symptoms in dementia, substance use disorders, antisocial behaviors in autism, impulsivity, online rudeness, and poor performance on the Stroop test. Thus, the alternative to disinhibition is not a simple single replacement theory, but rather to espouse multiple alternative approaches to understand complex behavior and emotion informed by our understanding of other brain functions, that is, an alternative approach rather than an alternative theory. This alternative approach will likely be less intuitively appealing as the simple and highly explanatory disinhibition theory, but will hopefully increase accuracy.
Models of motor and cognitive changes that occur with brain dysfunction can be used to develop alternative approaches to understanding behavioral and emotional changes associated with brain dysfunction. For example, apraxia is defined as an impairment of purposeful skilled movements that is not explained by basic motor or perceptual deficits (e.g., paralysis or blindness) (Leiguarda et al., 1994). Patients with apraxia have difficulty integrating basic motor elements into a smooth, successful, well-coordinated movement (Wheaton and Hallett, 2007). Apraxia can be associated with deficits in several domains including cortical integration of sensory information and the execution of complex motor programs (Leiguarda and Marsden, 2000).
Similar to apraxia, brain dysfunction, especially in the PFC, can be associated with a disruption of the ability to successfully learn and perform complex goal-oriented behaviors, which can result in a range of behavioral symptoms including the socially inappropriate behaviors that are currently termed “disinhibition.” Similar to apraxia, the patient is unable to smoothly and correctly perform a learned complex or social behavior such as going to the mall with his or her family because of difficulty organizing and successfully executing the behavior. Also similar to apraxia, abnormal social behaviors associated with PFC damage can be associated with dysfunction on several levels including social knowledge (e.g., not remembering that one is not supposed to touch one's genitals in public places, or an inability to understand why this would be upsetting to others), script knowledge (knowing that he is at the mall to buy clothes, which entails disrobing, but doing it at the wrong time and place), poor perception of social cues (e.g., not recognizing that people are shocked when he touches his genitals in the mall), and deficits in social motivation and reward (upsetting other people is not dysphoric) (Table 1).
In the cognitive realm, information-processing models of aphasia can provide another approach to explain changes in behavior observed with brain dysfunction. These models hypothesize that successful use of language requires an intact network of interconnected specialized brain structures or nodes. The entire network contributes to language function, but each of the nodes has a specialized function (e.g., Broca's and Wernicke's areas), and the symptoms that are associated with dysfunction of specific nodes are related to that function (e.g., expressive aphasia and Broca's area and receptive aphasia and Wernicke's area). Rather than framing the main function of the nodes as tonic inhibition of other nodes, as in the disinhibition theory, the focus of the language model is on information exchange between nodes, determining the relative functions of the nodes, and relating the clinical syndromes observed with node dysfunction with the function of the nodes. Similarly, future models of complex behavior would be based on the assumption that nodes of the network have specialized functions and that the nodes interact in complex ways to successfully perform complex behaviors, rather than based on simple inhibition and disinhibition.
There may be core symptoms that underlie psychiatric disorders such as ADHD, substance use, and autism, and these symptoms may be associated with specific cognitive findings. However, it is likely that these traits and associations will need to be determined empirically, and in manner to minimize bias and preexiting assumptions, for example, the research done on the factor structure underlying DSM diagnoses (Conway et al., 2019).
ALTERNATIVE THEORIES OF EMOTIONAL DISINHIBITION
Rather than conceptualizing the main purpose of the frontal cortex as suppressing emotional response generated by the amygdala and other posterior limbic structures, I hypothesize that these nodes, among others, form a network that is necessary for the successful generation and perception of emotional states. In support of this hypothesis, emotional blunting and apathy are the most common emotional symptoms of damage to the vmPFC, not emotional disinhibition as predicted by the disinhibition theory (Cheran et al., 2018; Huey et al., 2015; Koenigs et al., 2008a; Koenigs et al., 2008b; Lee et al., 2014). Practically, the study of emotional changes in patients with neurodegenerative disease and dysfunction due to TBI can be valuable to determine which brain regions to target with psychiatric treatments that decrease regional activation such as rTMS to reduce specific psychiatric symptoms.
In summary, I argue that the theory of disinhibition has been overextended. It is used to explain so much that it is now poorly defined and vague. Theories can be useful at one point in time, but can lose their usefulness as we obtain more information. I would argue that the theory of disinhibition has outlived its usefulness. Its continued use as the dominant theory governing our interpretation of behavioral and emotional changes related to frontal dysfunction is no longer warranted and frequently misleading. I have proposed an alternative approach to interpret and investigate behavioral and emotional changes associated with frontal dysfunction based on our current understanding of motor and cognitive changes associated with brain dysfunction. Although this may seem like an esoteric debate, theoretical models can profoundly influence important research and clinical decisions. For example, medications that may be cognitively enhancing, but can worsen disinhibition, have generally been avoided in patients with frontal impairment because of concern for worsening disinhibition, but this may be unnecessary. Adopting a different theoretical model can profoundly alter the interpretation of data (Huey and Lieberman, 2012) and research priorities. The disinhibition theory has had a long and successful run since its inception in the 19th century, but it may be time to replace it, not with an alternative theory, but with different approaches to the problems informed by our findings in related fields.
ACKNOWLEDGMENTS
The author thanks Teal Eich, Stephanie Cosentino, Clara Vivero-Dominguez, and Marie Banich for their valuable and insightful comments on the manuscript; Jordan Grafman, Roland Zahn, and Elizabeth Lindenberger for the formative discussions over the years; the Intramural Program of NIH/NINDS for giving me the time, space, and resources to explore these ideas; and most of all my patients and their families who have been my greatest teachers about the brain.
DISCLOSURE
This work was funded by the following grants and support: NIH/NINDS R01NS076837, NIH/NINDS 5R00NS060766, the Intramural Program of NIH/NINDS, and the Florence and Herbert Irving Clinical Research Career Award.
Footnotes
The author declares no conflict of interest.
REFERENCES
- Ahmed RM, Kaizik C, Irish M, Mioshi E, Dermody N, Kiernan MC, Piguet O, Hodges JR (2015) Characterizing sexual behavior in frontotemporal dementia. J Alzheimers Dis. 46:677–686. [DOI] [PubMed] [Google Scholar]
- Ainslie G (1975) Specious reward: A behavioral theory of impulsiveness and impulse control. Psychol Bull. 82:463–496. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders (5th ed). Washington, DC: American Psychiatric Association. [Google Scholar]
- Arciniegas DB, Wortzel HS (2014) Emotional and behavioral dyscontrol after traumatic brain injury. Psychiatr Clin North Am. 37:31–53. [DOI] [PubMed] [Google Scholar]
- Armstrong N, Schupf N, Grafman J, Huey ED (2013) Caregiver burden in frontotemporal degeneration and corticobasal syndrome. Dement Geriatr Cogn Disord. 36:310–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR (2007) The neural basis of inhibition in cognitive control. Neuroscientist. 13:214–228. [DOI] [PubMed] [Google Scholar]
- Banich MT, Depue BE (2015) Recent advances in understanding neural systems that support inhibitory control. Curr Opin Behav Sci. 1:17–22. [Google Scholar]
- Bartelet M, Waterink W, van Hooren S (2014) Extreme sexual behavior in dementia as a specific manifestation of disinhibition. J Alzheimers Dis. 42:S119–S124. [DOI] [PubMed] [Google Scholar]
- Bora E, Walterfang M, Velakoulis D (2015) Theory of mind in behavioural-variant frontotemporal dementia and Alzheimer's disease: A meta-analysis. J Neurol Neurosurg Psychiatry. 86:714–719. [DOI] [PubMed] [Google Scholar]
- Camille N, Tsuchida A, Fellows LK (2011) Double dissociation of stimulus-value and action-value learning in humans with orbitofrontal or anterior cingulate cortex damage. J Neurosci. 31:15048–15052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M, Jones DK, ffytche DH (2005) Perisylvian language networks of the human brain. Ann Neurol. 57:8–16. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Blackwell AD, Fineberg NA, Robbins TW, Sahakian BJ (2005) The neuropsychology of obsessive compulsive disorder: The importance of failures in cognitive and behavioural inhibition as candidate endophenotypic markers. Neurosci Biobehav Rev. 29:399–419. [DOI] [PubMed] [Google Scholar]
- Cheran G, Silverman H, Manoochehri M, Goldman J, Lee S, Wu L, Wu L, Cines S, Fallon E, Kelly BD, Olszewska DA, Heidebrink J, Shair S, Campbell S, Paulson H, Lynch T, Cosentino S, Huey ED (2018) Psychiatric symptoms in preclinical behavioural-variant frontotemporal dementia in MAPT mutation carriers. J Neurol Neurosurg Psychiatry. 89:449–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway CC, Forbes MK, Forbush KT, Fried EI, Hallquist MN, Kotov R, Mullins-Sweatt SN, Shackman AJ, Skodol AE, South SC, Sunderland M, Waszczuk MA, Zald DH, Afzali MH, Bornovalova MA, Carragher N, Docherty AR, Jonas KG, Krueger RF, Patalay P, Pincus AL, Tackett JL, Reininghaus U, Waldman ID, Wright AGC, Zimmermann J, Bach B, Bagby RM, Chmielewski M, Cicero DC, Clark LA, Dalgleish T, DeYoung CG, Hopwood CJ, Ivanova MY, Latzman RD, Patrick CJ, Ruggero CJ, Samuel DB, Watson D, Eaton NR (2019) A hierarchical taxonomy of psychopathology can transform mental health research. Perspect Psychol Sci. 14:419–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J (1994) The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 44:2308–2314. [DOI] [PubMed] [Google Scholar]
- Egner T, Hirsch J (2005) Cognitive control mechanisms resolve conflict through cortical amplification of task-relevant information. Nat Neurosci. 8:1784–1790. [DOI] [PubMed] [Google Scholar]
- Felleman DJ, Van Essen DC (1991) Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex. 1:1–47. [DOI] [PubMed] [Google Scholar]
- Ferrier D (1876) The Functions of the Brain. London: Smith, Elder. [Google Scholar]
- Fieo RA, Silverman H, O'Shea D, Manoochehri M, Grafman J, Huey ED (2018) Establishing dimensionality of sexual behaviours in patients with regional brain dysfunction. Brain Inj. 32:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freud S (1949) The Ego and the Id. London, United Kingdom: The Hogarth Press Ltd. [Google Scholar]
- Gennatas ED, Cholfin JA, Zhou J, Crawford RK, Sasaki DA, Karydas A, Boxer AL, Bonasera SJ, Rankin KP, Gorno-Tempini ML, Rosen HJ, Kramer JH, Weiner M, Miller BL, Seeley WW (2012) COMT Val158Met genotype influences neurodegeneration within dopamine-innervated brain structures. Neurology. 78:1663–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND (2002) Drug addiction and its underlying neurobiological basis: Neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 159:1642–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goleman D (2006) Emotional intellegence. New York: Bantam. [Google Scholar]
- Grace J, Malloy P (2001) Frontal Systems Behavior Scale (FrSBe): Professional Manual. Lutz, FL: Psychological Assessment Resources. [Google Scholar]
- Gregory RL (1961) The brain as an engineering problem. In Thorpe WH, Zangwill OL (Eds), Current problems in animal behavior. Cambridge, United Kingdom: Cambridge University Press. [Google Scholar]
- Harlow JM (1848) Passage of an iron bar through the head. The Boston Medical and Surgical Journal. 39:389–393. [Google Scholar]
- Hayden BY (2016) Time discounting and time preference in animals: A critical review. Psychon Bull Rev. 23:39–53. [DOI] [PubMed] [Google Scholar]
- Huey ED, Lee S, Brickman AM, Manoochehri M, Griffith E, Devanand DP, Stern Y, Grafman J (2015) Neuropsychiatric effects of neurodegeneration of the medial versus lateral ventral prefrontal cortex in humans. Cortex. 73:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huey ED, Lee S, Cheran G, Grafman J, Devanand DP, Alzheimer's Disease Neuroimaging Initiative (2016) Brain regions involved in arousal and reward processing are associated with apathy in Alzheimer's disease and frontotemporal dementia. J Alzheimers Dis. 55:551–558. [DOI] [PubMed] [Google Scholar]
- Huey ED, Lieberman JA (2012) From known to unknown; old to new: Can lesion studies inform psychiatry about mental illness in the 21st century? Neuropsychiatry. 2:369–372. [Google Scholar]
- Huey ED, Zahn R, Grafman J (2007) "[H]E is no more a person now but a whole climate of opinion" (Auden, 1940). Cortex. 43:1097–1098; discussion 1116–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson JH (1888) Discussion of Dr. Mercier's "Inhibition". Brain. 11:386–393. [Google Scholar]
- Jovanovic T, Ressler KJ (2010) How the neurocircuitry and genetics of fear inhibition may inform our understanding of PTSD. Am J Psychiatry. 167:648–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennerley SW, Walton ME, Behrens TE, Buckley MJ, Rushworth MF (2006) Optimal decision making and the anterior cingulate cortex. Nat Neurosci. 9:940–947. [DOI] [PubMed] [Google Scholar]
- Koenigs M, Huey ED, Calamia M, Raymont V, Tranel D, Grafman J (2008a) Distinct regions of prefrontal cortex mediate resistance and vulnerability to depression. J Neurosci. 28:12341–12348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigs M, Huey ED, Raymont V, Cheon B, Solomon J, Wassermann EM, Grafman J (2008b) Focal brain damage protects against post-traumatic stress disorder in combat veterans. Nat Neurosci. 11:232–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kringelbach ML, Rolls ET (2004) The functional neuroanatomy of the human orbitofrontal cortex: Evidence from neuroimaging and neuropsychology. Prog Neurobiol. 72:341–372. [DOI] [PubMed] [Google Scholar]
- Krueger F, Rostami E, Huey ED, Snyder A, Grafman J (2007) Evidence of an inferior total-order planning strategy in patients with frontotemporal dementia. Neurocase. 13:426–437. [DOI] [PubMed] [Google Scholar]
- Kucharski A (1984) History of frontal lobotomy in the United States, 1935–1955. Neurosurgery. 14:765–772. [DOI] [PubMed] [Google Scholar]
- Lee GJ, Lu PH, Mather MJ, Shapira J, Jimenez E, Leow AD, Thompson PM, Mendez MF (2014) Neuroanatomical correlates of emotional blunting in behavioral variant frontotemporal dementia and early-onset Alzheimer's disease. J Alzheimers Dis. 41:793–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiguarda R, Lees AJ, Merello M, Starkstein S, Marsden CD (1994) The nature of apraxia in corticobasal degeneration. J Neurol Neurosurg Psychiatry. 57:455–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiguarda RC, Marsden CD (2000) Limb apraxias: Higher-order disorders of sensorimotor integration. Brain. 123(pt 5):860–879. [DOI] [PubMed] [Google Scholar]
- Lima-Silva TB, Bahia VS, Carvalho VA, Guimarães HC, Caramelli P, Balthazar ML, Damasceno B, Bottino CM, Brucki SM, Nitrini R, Yassuda MS (2015) Neuropsychiatric symptoms, caregiver burden and distress in behavioral-variant frontotemporal dementia and Alzheimer's disease. Dement Geriatr Cogn Disord. 40:268–275. [DOI] [PubMed] [Google Scholar]
- Lyketsos CG, Carrillo MC, Ryan JM, Khachaturian AS, Trzepacz P, Amatniek J, Cedarbaum J, Brashear R, Miller DS (2011) Neuropsychiatric symptoms in Alzheimer's disease. Alzheimers Dement. 7:532–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyketsos CG, Lopez O, Jones B, Fitzpatrick AL, Breitner J, DeKosky S (2002) Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: Results from the cardiovascular health study. JAMA. 288:1475–1483. [DOI] [PubMed] [Google Scholar]
- MacLean EL (2016) Unraveling the evolution of uniquely human cognition. Proc Natl Acad Sci U S A. 113:6348–6354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean P (1989) The Triune Brain in Evolution. New York, NY: Plenum. [Google Scholar]
- MacLean PD (1977) The triune brain in conflict. Psychother Psychosom. 28:207–220. [DOI] [PubMed] [Google Scholar]
- Macmillan M (2000a) Nineteenth-century inhibitory theories of thinking: Bain, Ferrier, Freud (and Phineas Gage). Hist Psychol. 3:187–217. [DOI] [PubMed] [Google Scholar]
- Macmillan M (2000b) An Odd Kind of Fame. Cambridge, MA: The MIT Press. [Google Scholar]
- Mah LW, Arnold MC, Grafman J (2005) Deficits in social knowledge following damage to ventromedial prefrontal cortex. J Neuropsychiatry Clin Neurosci. 17:66–74. [DOI] [PubMed] [Google Scholar]
- Mendez MF, Shapira JS (2013) Hypersexual behavior in frontotemporal dementia: a comparison with early-onset Alzheimer's disease. Arch Sex Behav. 42:501–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BL, Darby AL, Swartz JR, Yener GG, Mena I (1995) Dietary changes, compulsions and sexual behavior in frontotemporal degeneration. Dementia. 6:195–199. [DOI] [PubMed] [Google Scholar]
- Morgane PJ, Galler JR, Mokler DJ (2005) A review of systems and networks of the limbic forebrain/limbic midbrain. Prog Neurobiol. 75:143–160. [DOI] [PubMed] [Google Scholar]
- Motzkin JC, Philippi CL, Wolf RC, Baskaya MK, Koenigs M (2015) Ventromedial prefrontal cortex is critical for the regulation of amygdala activity in humans. Biol Psychiatry. 77:276–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munakata Y, Herd SA, Chatham CH, Depue BE, Banich MT, O'Reilly RC (2011) A unified framework for inhibitory control. Trends Cogn Sci. 15:453–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakum S, Cavanna AE (2016) The prevalence and clinical characteristics of hypersexuality in patients with Parkinson's disease following dopaminergic therapy: A systematic literature review. Parkinsonism Relat Disord. 25:10–16. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Yeung N (2005) Neural mechanisms of attention and control: Losing our inhibitions? Nat Neurosci. 8:1631–1633. [DOI] [PubMed] [Google Scholar]
- Nigg JT (2000) On inhibition/disinhibition in developmental psychopathology: Views from cognitive and personality psychology and a working inhibition taxonomy. Psychol Bull. 126:220–246. [DOI] [PubMed] [Google Scholar]
- O'Callaghan C, Hodges JR, Hornberger M (2013) Inhibitory dysfunction in frontotemporal dementia: A review. Alzheimer Dis Assoc Disord. 27:102–108. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ (2005) The cognitive control of emotion. Trends Cogn Sci. 9:242–249. [DOI] [PubMed] [Google Scholar]
- Okon-Singer H, Hendler T, Pessoa L, Shackman AJ (2015) The neurobiology of emotion-cognition interactions: Fundamental questions and strategies for future research. Front Hum Neurosci. 9:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osvath M, Martin-Ordas G (2014) The future of future-oriented cognition in non-humans: Theory and the empirical case of the great apes. Philos Trans R Soc Lond B Biol Sci. 369:20130486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panchal H, Paholpak P, Lee G, Carr A, Barsuglia JP, Mather M, Jimenez E, Mendez MF (2016) Neuropsychological and neuroanatomical correlates of the social norms questionnaire in frontotemporal dementia versus Alzheimer's disease. Am J Alzheimers Dis Other Demen. 31:326–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passingham D, Wise SP (2012) The neurobiology of the prefrontal cortex. Oxford, United Kingdom: Oxford University Press. [Google Scholar]
- Petrides M, Pandya DN (1994) Comparative architonic analysis of the human and macaque frontal cortex. In Boller F, Grafman J (Eds), Handbook of neuropsychology (pp 17–58). Amsterdam, the Netherlands: Elsevier. [Google Scholar]
- Ploog DW (2003) The place of the Triune Brain in psychiatry. Physiol Behav. 79:487–493. [DOI] [PubMed] [Google Scholar]
- Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, van Swieten JC, Seelaar H, Dopper EG, Onyike CU, Hillis AE, Josephs KA, Boeve BF, Kertesz A, Seeley WW, Rankin KP, Johnson JK, Gorno-Tempini ML, Rosen H, Prioleau-Latham CE, Lee A, Kipps CM, Lillo P, Piguet O, Rohrer JD, Rossor MN, Warren JD, Fox NC, Galasko D, Salmon DP, Black SE, Mesulam M, Weintraub S, Dickerson BC, Diehl-Schmid J, Pasquier F, Deramecourt V, Lebert F, Pijnenburg Y, Chow TW, Manes F, Grafman J, Cappa SF, Freedman M, Grossman M, Miller BL (2011) Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 134(pt 9):2456–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth G, Dicke U (2005) Evolution of the brain and intelligence. Trends Cogn Sci. 9:250–257. [DOI] [PubMed] [Google Scholar]
- Rudebeck PH, Behrens TE, Kennerley SW, Baxter MG, Buckley MJ, Walton ME, Rushworth MF (2008) Frontal cortex subregions play distinct roles in choices between actions and stimuli. J Neurosci. 28:13775–13785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudebeck PH, Murray EA (2008) Amygdala and orbitofrontal cortex lesions differentially influence choices during object reversal learning. J Neurosci. 28:8338–8343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saver JL, Damasio AR(1991) Preserved access and processing of social knowledge in a patient with acquired sociopathy due to ventromedial frontal damage. Neuropsychologia. 29:1241–1249. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Crawford R, Rascovsky K, Kramer JH, Weiner M, Miller BL, Gorno-Tempini ML (2008) Frontal paralimbic network atrophy in very mild behavioral variant frontotemporal dementia. Arch Neurol. 65:249–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shettleworth SJ (2012) Modularity, comparative cognition and human uniqueness. Philos Trans R Soc Lond B Biol Sci. 367:2794–2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin LM, Rauch SL, Pitman RK (2006) Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Ann N Y Acad Sci. 1071:67–79. [DOI] [PubMed] [Google Scholar]
- Simons JS, Simons RM, O'Brien C, Stoltenberg SF, Keith JA, Hudson JA (2017) PTSD, alcohol dependence, and conduct problems: Distinct pathways via lability and disinhibition. Addict Behav. 64:185–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R (1992) Inhibition. Berkeley, CA: University of California Press. [Google Scholar]
- Starkstein SE, Robinson RG (1997) Mechanism of disinhibition after brain lesions. J Nerv Ment Dis. 185:108–114. [DOI] [PubMed] [Google Scholar]
- Suler J (2004) The online disinhibition effect. Cyberpsychol Behav. 7:321–326. [DOI] [PubMed] [Google Scholar]
- Swann AC (2009) Impulsivity in mania. Curr Psychiatry Rep. 11:481–487. [DOI] [PubMed] [Google Scholar]
- Szczepanski SM, Knight RT (2014) Insights into human behavior from lesions to the prefrontal cortex. Neuron. 83:1002–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannan V Holden JK, Zhang Z, Baranek GT, Tommerdahl MA (2008) Perceptual metrics of individuals with autism provide evidence for disinhibition. Autism Res. 1:223–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarter RE, Kirisci L, Mezzich A, Cornelius JR, Pajer K, Vanyukov M, Gardner W, Blackson T, Clark D (2003) Neurobehavioral disinhibition in childhood predicts early age at onset of substance use disorder. Am J Psychiatry. 160:1078–1085. [DOI] [PubMed] [Google Scholar]
- Wheaton LA, Hallett M (2007) Ideomotor apraxia: A review. J Neurol Sci 260(1–2):1–10. [DOI] [PubMed] [Google Scholar]
- World Health Organization (2016) Dementia fact sheet. Available at: https://www.who.int/news-room/fact-sheets/detail/dementia.
- Young SE, Friedman NP, Miyake A, Willcutt EG, Corley RP, Haberstick BC, Hewitt JK (2009) Behavioral disinhibition: Liability for externalizing spectrum disorders and its genetic and environmental relation to response inhibition across adolescence. J Abnorm Psychol. 118:117–130. [DOI] [PMC free article] [PubMed] [Google Scholar]