Abstract
This study examined the safety of intracerebral inoculation of G207, an attenuated, replication-competent herpes simplex virus type 1 (HSV-1) recombinant, in nonhuman primates. Sixteen New World owl monkeys (Aotus nancymae [karyotype 1, formerly believed to be A. trivirgatus]), known for their exquisite susceptibility to HSV-1 infection, were evaluated. Thirteen underwent intracerebral inoculation with G207 at doses of 107 or 109 PFU, two were vehicle inoculated, and one served as an infected wild-type control and received 103 PFU of HSV-1 strain F. HSV-1 strain F caused rapid mortality and symptoms consistent with HSV encephalitis, including fever, hemiparesis, meningitis, and hemorrhage in the basal ganglia. One year after G207 inoculation, seven of the animals were alive and exhibited no evidence of clinical complications. Three deaths resulted from nonneurologic causes unrelated to HSV infection, and three animals were sacrificed for histopathologic examination. Two animals were reinoculated with G207 (107 PFU) at the same stereotactic coordinates 1 year after the initial G207 inoculation. These animals were alive and healthy 2 years after the second inoculation. Cerebral magnetic resonance imaging studies performed both before and after G207 inoculation failed to reveal radiographic evidence of HSV-related sequelae. Despite the lack of outwardly observable HSV pathology, measurable increases in serum anti-HSV titers were detected. Histopathological examination of multiple organ tissues found no evidence of HSV-induced histopathology or dissemination. We conclude that intracerebral inoculation of up to 109 PFU of G207, well above the efficacious dose in mouse tumor studies, is safe and therefore appropriate for human clinical trials.
At present, medical therapy cannot significantly alter the poor prognosis associated with glioblastoma multiforme, the most malignant form of human astrocytic brain tumors (11, 26, 31), making it an excellent target for new therapeutic approaches. Replication-competent, cytotoxic viral vectors offer such an approach, provided that their cytopathic effect is limited to neoplastic tissues. Vectors derived from herpes simplex virus (HSV) are particularly well suited to this approach because HSV infects a broad range of cell types and causes a cytotoxic, lytic infection, yet it can also exist in a latent state within neurons without causing damage (50). However, HSV infection of the central nervous system (CNS) causes encephalitis, with substantial morbidity (∼50%) even with current antiviral therapy (35, 54, 64). In addition to HSV encephalitis, HSV keratoconjunctivis, esophagitis, pneumonitis (inflammation of the lungs), and hepatitis (inflammation of the liver) have been reported (9, 63). Therefore, it is very important that HSV vectors, especially replication-competent vectors, are nonpathogenic in normal tissue.
A large number of HSV genes affect pathogenicity (36, 43). γ34.5 (RL1), located in the long repeat region, enables viral replication in the CNS and is the major viral determinant of neuropathogenicity (8, 65). Mutations in γ34.5 result in decreases in the 50% lethal dose to >106 to 107 PFU after intracerebral injection (8, 30, 61). A number of HSV genes involved in nucleotide metabolism also affect neurovirulence, such as ICP6 (UL39), which encodes the large subunit of ribonucleotide reductase (5, 21, 67), and genes for thymidine kinase (UL23) (15, 17, 57), dUTPase (UL50) (46), uracil DNA glycosylase (UL2) (47), and DNA polymerase (UL30) (44). Attenuated, replication-competent HSV vectors containing mutations in these genes have been shown to effectively inhibit brain tumor growth in animal models (1, 3, 7, 22, 25, 33, 34, 39, 40, 48).
We constructed a second-generation HSV vector, termed G207, containing deletions of both γ34.5 loci (R3616 parent [8]) and an E. coli lacZ insertion that inactivates the ICP6 gene (hrR3 parent [16]) (40). These multiple mutations attenuate neurovirulence and make it unlikely that a pathogenic revertant could arise by recombination or by a second-site suppressor mutation (41). Other favorable properties of G207 are its easy detection due to the lacZ reporter gene, temperature sensitivity, and hypersensitivity to the antiviral drugs ganciclovir and acyclovir (40). As a result of its propensity for replicating in dividing cells, the cytotoxic effects of G207 are preferentially limited to neoplastic cells, resulting in efficacious treatment of established human brain tumors in athymic mice (40, 60, 68).
The use of potentially pathogenic, replication-competent viruses in humans for tumor therapy is dependent upon the demonstration that the mutant viruses will not cause additional disease. The New World owl monkey, Aotus nancymae (karyotype 1, formerly believed to be Aotus trivirgatus [18]), was selected as a nonhuman primate model to test the safety of G207 because of its exquisite sensitivity to HSV (20, 24, 38). Peripheral inoculation of 100 PFU of wild-type HSV-1 or -2 is lethal (37). Owl monkeys have previously been used to test the safety of live, attenuated HSV vaccine strains (37). Using this model, we have now demonstrated the safety of intracerebral inoculation of G207 at doses of up to 109 PFU.
MATERIALS AND METHODS
Virus.
HSV-1 strain F was provided by J. Chou and B. Roizman (University of Chicago) and G207 was constructed as previously described (40). Stocks of G207 were grown in African green monkey kidney (Vero) cells infected at a multiplicity of infection of 0.01 and cultured at 34°C in Dulbecco’s minimal essential medium supplemented with 5% heat-inactivated fetal calf serum (HyClone). Virus was isolated from infected cells when the complete cytopathic effect was observed after a freeze-thaw/sonication regimen and low-speed centrifugation (2,000 × g for 10 min at 4°C) to remove cell debris. Virus was concentrated by high-speed centrifugation (45,000 × g for 150 min at 4°C) and resuspended in virus buffer (150 mM NaCl and 20 mM Tris, pH 7.5), for a final G207 titer of 6 × 109 PFU/ml. The titer of viral stocks was determined by a plaque assay on the Vero cells. Following each surgery, the titer of the inoculum was confirmed.
Animals.
Sixteen New World primates (A. nancymae), 12 males and four females ranging in age from 1 to 6 years and in weight from 500 to 1,000 g, were obtained through the Veterinary Resources Program, National Center for Research Resources, National Institutes of Health (Poolesville, Md.). Seven of the animals were involved in prior experimental studies (T9205 and the others below), two of which had previously undergone splenectomy (T894 and T558). Four of the animals (T1122, T910805, T9307, and T1057) (Table 1) were briefly described in a previous publication (40). The animals were housed separately prior to and after inoculation. Animal procedures were approved by the Georgetown Animal Care and Use Committee and the Institutional Biosafety Committee.
TABLE 1.
Summary of intracerebral inoculations of A. nancymaea
| Primate | First injection
|
Time of MRI | Death
|
Survival (mo) | ||
|---|---|---|---|---|---|---|
| Virus | PFU | Time | Cause | |||
| Group 1 | ||||||
| T1122 | G207 | 107 | 3 mo | Sacrifice | ||
| T9205 | G207 | 107 | Preop | 6 mo | Sacrifice | |
| 1 mo | ||||||
| 6 mo | ||||||
| T9307 | G207 | 107 | 12 mo | Sacrifice | ||
| T344 | G207 | 107 | 41 | |||
| T317 | G207 | 107 | 41 | |||
| T323 | G207 | 107 | 34 | |||
| T432 | G207 | 107 | 7 mo | Inguinal hematoma | ||
| T910805 | G207 | 107 | 4 mo | Pancreatitis | ||
| T894 | G207 | 107 | Preop | 25 days | Fasting | |
| 10 days | ||||||
| Group 2 | ||||||
| T558 | G207 | 109 | 6 mo | 20 mo | Sacrifice | |
| T413 | G207 | 109 | 7 mo | Aortic aneurysm | ||
| Group 3 | ||||||
| T348 | G207 | 107 | 11 mo | 41 | ||
| 25 mo | ||||||
| T442 | G207 | 107 | 12 mo | 41 | ||
| 15 mo | ||||||
| Group 4 | ||||||
| T1057 | F | 103 | 5 days | Encephalitis | ||
| T421 | Mock | 42 | ||||
| T357 | Mock | 30 mo | Acute heart failure | |||
Primates were inoculated with G207 (107 PFU in 10 μl or 109 PFU in 180 μl), strain F, or virus buffer (Mock). Group 3 animals were reinoculated with G207 12 months after the initial inoculation. Preop, preoperatively.
Stereotactic intracerebral inoculations.
The intracerebral inoculations and the surgical time frames for the 16 inoculated animals (13 with G207, 1 with strain F, and 2 with virus buffer) are listed in Table 1. Monkeys were given general anesthesia (propofol drip of 50 to 100 mg/kg of body weight/h with saline in the lateral saphenous vein), and by using a Kopf stereotactic head frame, inoculations were made into the left frontal lobe, 2 mm anterior to the coronal suture and 7 mm lateral to the midline. After manual placement of a burr hole in the calvarium, a sample was stereotactically injected with a Hamilton syringe into the cortex at a depth of 5 mm. Ten microliters of diluted G207 (107 PFU), F, or virus buffer was injected over 5 min, or 180 μl of G207 (109 PFU) was injected over 40 min. Following injection, the burr hole was covered with bone wax and the incision was sutured. No perioperative antibiotics were used. Postoperatively, all animals underwent serial neurologic assessment, ear temperature measurement, and monitoring of food and water intake.
Physiological evaluations.
All animals were screened every 3 months (pre- and postoperatively) with a physical examination, a tuberculin test, and serologic analyses, including blood count with differential, protein measurement, chemistry, and lipase and amylase measurements. Additionally, anti-HSV-1 antibody testing was performed (MA BioServices, Inc., Rockville, Md.) on some blood samples with enzyme-linked immunosorbent assay (ELISA). Since one of the cardinal signs of HSV encephalitis is fever, aural tympanic body temperatures were measured twice daily for the first postoperative week, then daily for 3 weeks, and then at a further reduced frequency. The normal aural tympanic body temperature of the primates ranged from 96.9 to 101.5°F. Primates attempting to elude capture for these temperature measurements experienced a temperature elevation of approximately 2.5°F on average, as determined with a subcutaneously implanted temperature transponder (BioMedic Data Systems Inc., Maywood, N.J.).
MRI.
High-resolution cerebral magnetic resonance imaging (MRI) studies were performed with a Vision 1.5-T MRI unit (Siemens, Erlangen, Germany). Each study consisted of precontrast T1-weighted images in the axial and sagittal planes (time to repeat [TR] = 500, time to echo [TE] = 20, excitations [E] = 4), proton density (TR = 2500, TE = 30, E = 4) and T2-weighted images in the sagittal plane (TR = 3500, TE = 39, E = 2), and postcontrast T1-weighted images in the axial and coronal planes after intravenous administration of gadodiamide (1 ml/kg; Omniscan; Nycomed Inc., Princeton, N.J.). The time frame for MRI imaging is indicated in Table 1.
Necropsy and histopathology.
Animals were sacrificed by intravenous administration of sodium pentobarbital (40 to 60 mg/kg) followed by intracardiac perfusion with 4% paraformaldehyde. Following all deaths, due to euthanasia or otherwise, a complete necropsy was performed. Histologic samples were obtained from the peripheral organs: adrenal gland, heart, intestines, kidney, liver, lymph node, lung, muscle, pancreas, skin, spleen, stomach, and testes or ovaries. Occasionally, the adrenal glands were not found at the time of necropsy. In disseminated HSV disease, usually in neonates and immunocompromised individuals, numerous organs can be involved, including the adrenal glands, pancreas, intestine, spleen, stomach, kidneys, and muscle (9). From the CNS, samples were taken from the frontal, parietal, temporal, and occipital lobes; cerebellum; upper brain stem; medulla; and spinal cord. The injection site in the left frontal lobe was identified at the time of necropsy by the visualization of local scar tissue on the scalp and the burr hole in the skull, and tissue was isolated from the scalp, skull, and left frontal lobe. Specimens were embedded in paraffin, and 10-μm sections were obtained by using a microtome and then dehydrated, stained with hematoxylin and eosin, and coverslipped (29). Additional sections were immunohistochemically stained for HSV-1 antigens with rabbit anti-HSV-1 antibody (Dako) (4), followed by Vectastain indirect immunoperoxidase detection and then counterstained with hematoxylin. Histologic sections were evaluated by a board-certified neuropathologist (H.J.M.).
RESULTS
Intracerebral inoculation of G207.
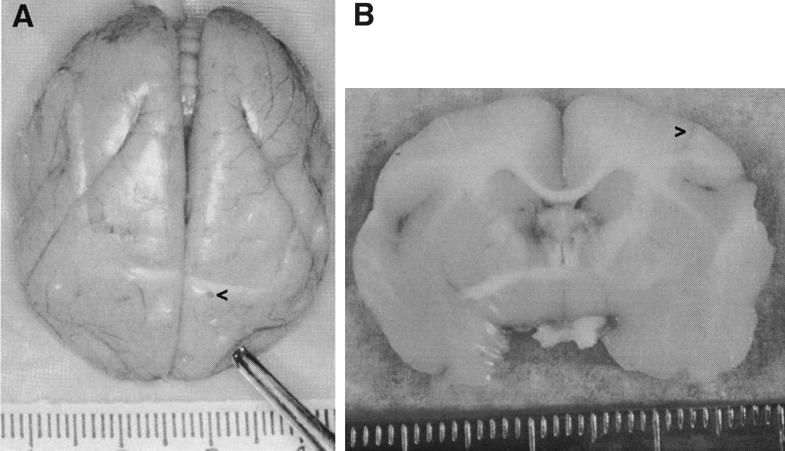
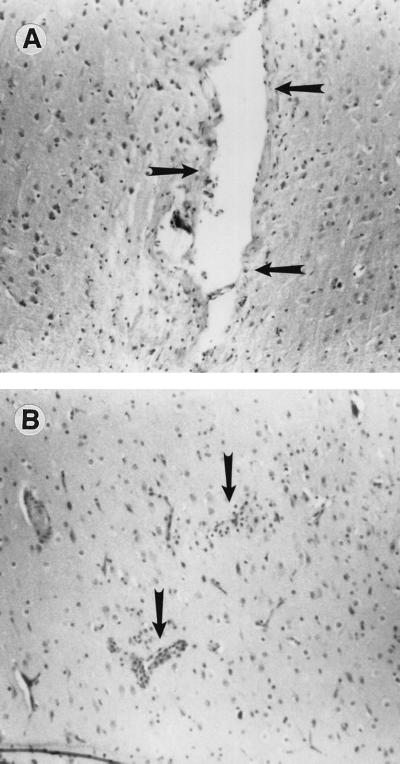
To test the safety of G207 and the extent of neurotoxicity associated with it, we performed stereotactic intracerebral inoculations in the left frontal lobe in three groups of animals: a single inoculation of 107 PFU of G207 in nine animals (group 1), a single inoculation of 109 PFU of G207 in two animals (group 2), and a second inoculation of 107 PFU of G207 1 year after an initial inoculation of 107 PFU in two animals (group 3) (Table 1). In the first group, one animal each was sacrificed at 3, 6, and 12 months for histopathological examination, three animals were alive and healthy more than 2.5 years after inoculation, and three animals died from causes unrelated to HSV. Of the latter deaths, one resulted from extended fasting in preparation for an MRI study (T894), one resulted from an anesthetic complication during surgery to remove an inguinal hematoma (T432), and one resulted from acute pancreatitis (T910805) attributed to natural disease because there was no histologic or immunohistochemical evidence of HSV infection in any of the organs examined, including the pancreas. At the time of necropsy, the injection site was notable in some animals while the brains looked otherwise normal (Fig. 1). Among the three sacrificed animals, neuropathologic examination revealed no abnormalities of the CNS, with the exceptions of sparsely distributed interstitial and/or perivascular inflammatory cells and gliosis at the injection site (Fig. 2). For many animals the injection site was not apparent in histologic sections. The peripheral organs also revealed no evidence of toxicity attributable to G207, and no HSV-immunoreactive cells were seen in any of the examined sections, including those from the injection site. The hearts and kidneys of several monkeys contained mild fibrosis and interstitial nephritis, respectively, a common finding in Aotus (62).
FIG. 1.
Brains from G207-injected animals. (A) Brain from T9205 at time of necropsy, 6 months after injection of 107 PFU of G207, with frontal lobes at the bottom and brain stem at the top. (B) Coronal cut through the brain from T910805 at the injection site 4 months after injection of 107 PFU of G207. In both panels, the left side of the brain is on the right side of the figure, and the injection site is marked (>). Hatch marks on rulers are in millimeters. Photographic slides were scanned with a Polaroid SprintScan 35, and Adobe Photoshop was used to adjust the contrast and brightness of the images.
FIG. 2.
Histology of brain from G207 injection site. Brain tissue was obtained at necropsy from animals T1122 (A) and T894 (B) 3 months and 25 days, respectively, after inoculation of 107 PFU of G207. Ten-micrometer paraffin-embedded sections were stained with hematoxylin and eosin. Mild perivascular inflammation adjacent to the injection site is visible (arrows).
A large inoculation volume (180 μl) was required for group 2, the animals receiving a high dose of G207 (109 PFU). One of these animals (T413) had a tonic/clonic seizure 5 days postinjection and developed mild contralateral weakness, which resolved over 3 days. This animal remained healthy until it died 7 months after inoculation due to a ruptured aortic aneurysm. No histologic evidence of neural, systemic, or vascular HSV infection was found. The other animal (T558) experienced no adverse effects due to the surgery and remained healthy until sacrificed 20 months after injection. T558 had one of the lowest normal body temperatures (98.4 to 100.5°F).
In order to determine whether prior HSV infection might result in an enhanced inflammatory response or allergic encephalomyelitis, animals T442 and T348 were reinoculated with G207 1 year after the first inoculation at the same stereotactic coordinates. There was no evidence of any clinical disease or MRI-detectable changes in these animals and they were alive and healthy more than 2 years after the second inoculation.
Control intracerebral inoculations.
To control for complications due to surgery and to provide a baseline for tissue histopathology, we stereotactically inoculated virus buffer into two animals, T421 and T357 (Table 1). T357 was euthanized 30 months after mock inoculation after becoming moribund due to acute cardiopulmonary failure, and T421 was alive and healthy more than 3 years after surgery.
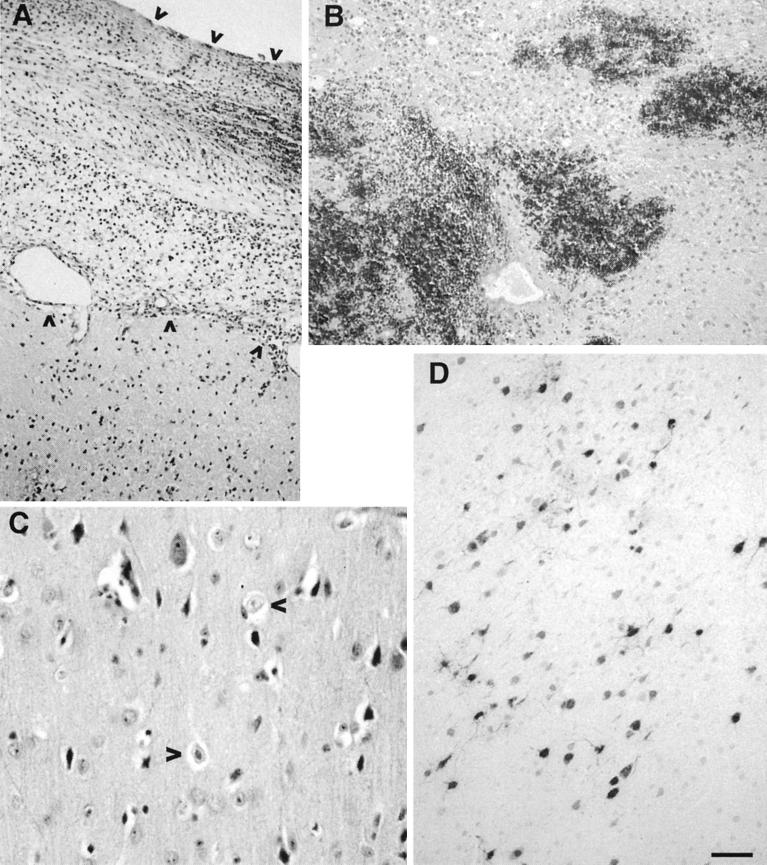
To confirm the exquisite sensitivity of A. nancymae to HSV and to determine the symptoms of HSV encephalitis in this species, one animal (T1057) was inoculated with 103 PFU of strain F, a dose of the wild-type parental strain of G207 4 to 6 log units lower than that of G207. This animal exhibited signs of encephalitis, with a rising fever (103°F on day 3 and 105°F on day 4) and lethargy. On day 5, the animal exhibited hypothermia (97°F), right hemiparesis, and left pupillary enlargement and was euthanized. Histologic signs of HSV encephalitis included meningitis, with thickening of the meninges, subarachnoid hemorrhage, and inflammatory cell infiltrate in the underlying brain parenchyma (Fig. 3A); hemorrhage within the basal ganglia (Fig. 3B) and ventricles; and acute cerebritis, necrotic neurons, and Cowdry A nuclear inclusions (Fig. 3C). Immunohistochemical staining for HSV-1 revealed numerous HSV-immunoreactive neurons in both frontal lobes (Fig. 3D), ipsilateral amygdala, and arachnoid cells. Hemosiderin and erythrophagocytosis were seen in the spleen and in the liver and lymph nodes, respectively, consistent with this animal’s involvement in a previous malaria study. The remaining organs were normal.
FIG. 3.
Histology and immunohistochemistry of brain tissue from HSV-1 strain F injection. Brain tissue was obtained at necropsy from animal T1057 5 days after inoculation of 103 PFU of F. Ten-micrometer paraffin-embedded sections were stained with hematoxylin and eosin (A, B, and C) or were immunohistochemically stained with anti-HSV antibody (D). Changes consistent with HSV encephalitis are evident. (A) Meningitis, with thickened meninges, and inflammatory cell infiltrate (between arrows) as opposed to the normal condition of single-cell thickness. (B) Hemorrhage in the basal ganglia. (C) Cowdry A eosinophilic intranuclear inclusions (10) in the ipsilateral (arrowheads) and contralateral hemispheres. (D) HSV-immunoreactive cells (black) are seen throughout the cortex, in both ipsilateral and contralateral hemispheres. Photographic slides were scanned with a Polaroid SprintScan 35, and Adobe Photoshop was used to adjust the contrast and brightness of the images. Bar, 0.08 mm (A and D), 0.07 mm (B), and 0.03 mm (C).
Cerebral MRI studies.
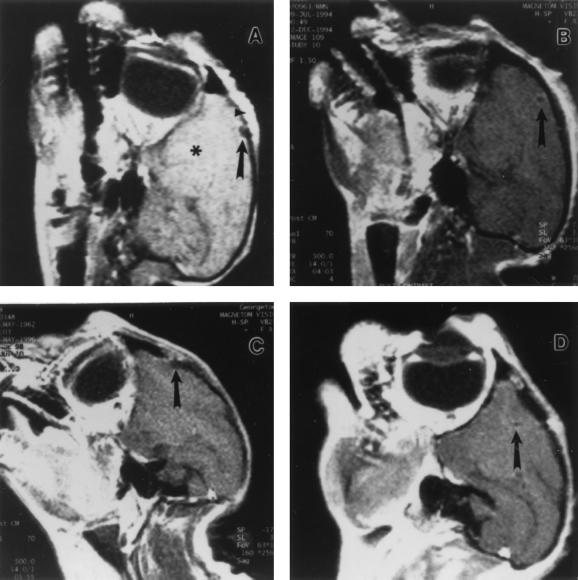
MRI has been shown to be a sensitive noninvasive test for identifying the tissue injury occurring in HSV encephalitis (12, 28, 53, 59). MRIs were obtained 10 days (Fig. 4A) and 1 (Fig. 4B), 6, 10 (Fig. 4C), 11, and 12 months after an initial inoculation of 107 PFU of G207; 3 and 13 months after a second inoculation of 107 PFU of G207; and 6 months (Fig. 4D) after an initial inoculation of 109 PFU of G207 (Table 1). Only subtle radiographic changes at the injection site and in the overlying calvarium, due to burr hole placement, could be identified (Fig. 4). These changes were attributed to the surgery itself since similar subtle findings are often observed after intracranial procedures in humans. No abnormal signal characteristics or contrast enhancements attributable to HSV toxicity were observed in dura or brain parenchyma at the injection site or throughout the rest of the brain.
FIG. 4.
Cerebral MRI studies obtained after G207 inoculation. Sagittal T1-weighted MRIs were obtained after the administration of contrast dye. Images were obtained from T894 (A), T9205 (B), and T348 (C) 10 days, 1 month, and 10 months, respectively, after inoculation of 107 PFU of G207 and from T558 (D) 6 months after inoculation of 109 PFU of G207. The left frontal region of the brain (*) and the site of G207 injection (arrow) are indicated in each panel. Normal postoperative incisional scarring and inflammation of the skin overlying the inoculation site are present in all panels but are best seen in panel C. There were no changes, such as edema, hemorrhage, or necrosis, that would indicate the presence of HSV encephalitis.
Humoral response to G207 injection.
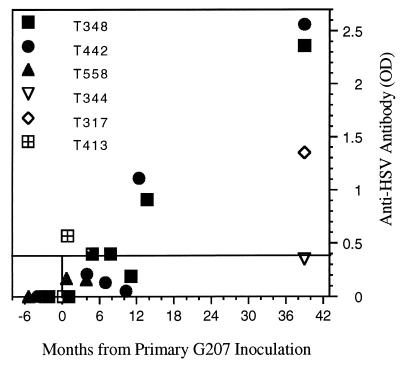
Prior to inoculation, animals T9307, T348, T442, T558, T413, and T421 were found to be seronegative for anti-HSV-1 antibodies by ELISA. A single intracerebral injection of G207 (107 PFU) resulted in low serum anti-HSV titers that decreased over the course of a year, but T317 had a significantly elevated titer after 3 years (Fig. 5). A large increase in antibody titers occurred within 2 months of the second intracerebral injection of G207 in group 3 (1 year after the initial injection), which was still elevated 2 years later (T348 and T442) (Fig. 5). Even with this “booster” effect, no clinical, MRI, or pathological abnormalities were observed.
FIG. 5.
Serum anti-HSV antibody levels. Anti-HSV antibody titers in serum were determined by ELISA (Table 1) at various times (months) pre- and post-G207 injection. Optical density (OD) values of ≥0.4 are positive, and values between 0.17 and 0.39 are equivocal. All preinjection sera had OD values of 0.
DISCUSSION
Replication-competent viral vectors that are cytotoxic have a number of attractive features for tumor therapy, such as the abilities to generate new infectious virus in situ while killing tumor cells and to spread within the tumor (49, 51). However, as a result of these properties, they are potentially more pathogenic than replication-defective viral vectors. When clinical trials with such viruses are being considered, it is imperative that adequate safeguards be engineered into the vector and that rigorous safety testing be performed. As an example of such a viral vector, we developed an attenuated, replication-competent HSV-1 vector, G207, as a therapeutic agent for brain tumors. G207 is derived from HSV-1 strain F, one of the least virulent laboratory strains (13). It contains deletions of both copies of the γ34.5 gene, the major viral determinant for neurovirulence (6, 8, 56, 58), and a lacZ insertion inactivating the ICP6 gene. ICP6 is required for efficient viral replication in nondividing cells (16, 45) and also is a determinant of neurovirulence (5, 21, 67). The presence of multiple mutations is important for safety, even with deletion mutants, because second-site suppressor mutations (41) or novel recombinants could arise through selective pressure during replication in the tumor or brain.
HSV has evolved elaborate mechanisms to survive in the nervous system, its natural reservoir in humans and the site where it causes the most serious disease. In humans, illness resulting from HSV can be manifested by a variety of syndromes, including disseminated virus; CNS infections; and exanthema localized to the eyes, skin, and mouth (9, 63). CNS infections, usually due to HSV-1, often cause a devastating and rapidly fatal encephalitis. Death occurs in approximately 70% of those who do not receive treatment (66). The signs and symptoms of HSV encephalitis include fever, mental status changes (altered consciousness and personality changes), headache, nausea, and focal neurological findings (i.e., seizures and hemiparesis) indicative of the infected brain area, usually the temporal lobes (9, 54, 66). In neonates, about half the infections involve encephalitis, alone or with disseminated disease, and death is often associated with brain stem involvement (55). Acyclovir is the treatment of choice. However, even when adequate antiviral treatment is rendered, approximately 20% of patients die and the majority of survivors suffer significant neurologic impairment (35, 54, 66). Since G207 is a derivative of HSV-1, it is important to demonstrate that intracerebral inoculation with G207 does not pose a potential health hazard.
A. nancymae is an excellent animal model for characterizing the pathogenesis of HSV. These nonhuman primates are extremely sensitive to HSV infection and disease (24, 38), similar to human neonates and immunocompromised patients (37). They develop clinical symptoms of HSV infection similar to those of humans. Aotus has a somewhat elevated and variable body temperature compared to humans, which might affect the pathogenicity of G207. However, strain F has a temperature sensitivity similar to that of G207 (27), and the animal inoculated with 103 PFU of strain F rapidly developed an encephalitis so severe that euthanasia was required within 5 days of inoculation. An examination of the brain tissues from this animal revealed typical histologic findings of HSV infection, including meningitis, inflammatory infiltrates, neuronal necrosis, Cowdry A intranuclear inclusions, and hemorrhage (24, 38). In contrast, a single intracerebral inoculation with 107 or 109 PFU of G207 produced no neurologic symptoms, and some animals were still alive and healthy 41 months after injection. One animal had a brief seizure 5 days after its inoculation with 109 PFU of G207; this could have been due to cortical irritation along the needle track, the location of the inoculation, the volume inoculated, or the virus. However, the symptoms were self-limiting and required no treatment, and no viral pathology was detected in the brain when it was examined 7 months after inoculation.
Noninvasive diagnostic tests for HSV encephalitis include electroencephalography, computerized tomography, single-photon emission-computed tomography, and MRI (14, 19, 42, 52). MRI is highly effective at detecting the changes in brain water content that accompany brain tissue inflammation and is particularly useful for detecting the early changes associated with HSV encephalitis (28, 53, 59). We obtained MRI scans from 10 days to 1 year following G207 inoculation, and no radiographic evidence of encephalitis was found.
All animals tested developed a detectable but equivocal serum anti-HSV antibody response after intracerebral inoculation of G207. The two animals that received a second G207 inoculation 1 year after the first developed a rapid boost in antibody titer that lasted for at least two more years. In humans experiencing a primary genital infection (HSV-1 or -2), anti-HSV serum antibodies detected by complement fixation, radioimmunoprecipitation, or ELISA are generally not detected in the acute phase of infection (within 1 to 2 weeks) but are present in the early convalescent phase (2 to 8 weeks) after the onset of symptoms (2, 23, 69). In a 9-month-old child that developed HSV encephalitis, neutralizing serum antibody was not detected on day 5 after disease onset but was detected on day 9, increasing to a maximum at days 14 and 26 and then decreasing to about half this level in 14 months (32). While intracerebral inoculation is not a reasonable approach for vaccination, these studies suggest that G207 may be a useful live, attenuated strain for HSV vaccination in the periphery.
These studies demonstrate that G207 can be inoculated safely into the brain at doses of up to 109 PFU, at least 6 log units higher than a lethal dose of wild-type HSV-1. Even animals with humoral immunity due to prior exposure to G207 experienced no adverse consequences, and neither demyelination nor other neurologic sequelae occurred following a repeat intracerebral inoculation. This extensive neural toxicity testing suggests that G207 should be safe for intracerebral use in humans.
ACKNOWLEDGMENTS
This work was partially supported by a fellowship from the American Association of Neurological Surgeons (W.D.H. and F.F.) and a grant from the National Cancer Institute (NS 32677 to R.L.M.). S.D.R. and R.L.M. are consultants for NeuroVir, Inc., which has a license from Georgetown University for G207.
We thank Anu Iyer, Mary Jane Plym, Tina Wilson, Laura Rutter, and Michael Newsome for their technical assistance and the anonymous reviewers for their helpful comments.
REFERENCES
- 1.Andreansky S S, He B, Gillespie G Y, Soroceanu L, Markert J, Chou J, Roizman B, Whitley R J. The application of genetically engineered herpes simplex viruses to the treatment of experimental brain tumors. Proc Natl Acad Sci USA. 1996;93:11313–11318. doi: 10.1073/pnas.93.21.11313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ashley R, Benedetti J, Corey L. Humoral immune response to HSV-1 and HSV-2 viral proteins in patients with primary genital herpes. J Med Virol. 1985;17:153–166. doi: 10.1002/jmv.1890170208. [DOI] [PubMed] [Google Scholar]
- 3.Boviatsis E J, Scharf J M, Chase M, Harrington K, Kowall N W, Breakefield X O, Chiocca E A. Antitumor activity and reporter gene transfer into rat brain neoplasms inoculated with herpes simplex virus vectors defective in thymidine kinase or ribonucleotide reductase. Gene Ther. 1994;1:323–331. [PubMed] [Google Scholar]
- 4.Budka H, Popow-Kraupp T. Rabies and herpes simplex virus encephalitis. An immunohistological study on site and distribution of viral antigens. Virchows Arch Abt A. 1981;390:353–364. doi: 10.1007/BF00496565. [DOI] [PubMed] [Google Scholar]
- 5.Cameron J M, McDougall I, Marsden H S, Preston V G, Ryan D M, Subak-Sharpe J H. Ribonucleotide reductase encoded by herpes simplex virus is a determinant of the pathogenicity of the virus in mice and a valid antiviral target. J Gen Virol. 1988;69:2607–2612. doi: 10.1099/0022-1317-69-10-2607. [DOI] [PubMed] [Google Scholar]
- 6.Centifanto-Fitzgerald Y M, Yamaguchi T, Kaufman H E, Tognon M, Roizman B. Ocular disease pattern induced by herpes simplex virus is genetically determined by a specific region of viral DNA. J Exp Med. 1982;155:475–489. doi: 10.1084/jem.155.2.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chambers R, Gillespie G Y, Soroceanu L, Adreansky S, Chatterjee S, Chou J, Roizman B, Whitley R J. Comparison of genetically engineered herpes simplex viruses for the treatment of brain tumors in a scid mouse model of human malignant glioma. Proc Natl Acad Sci USA. 1995;92:1411–1415. doi: 10.1073/pnas.92.5.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chou J, Kern E R, Whitley R J, Roizman B. Mapping of herpes simplex virus-1 neurovirulence to gamma 34.5, a gene nonessential for growth in culture. Science. 1990;250:1262–1266. doi: 10.1126/science.2173860. [DOI] [PubMed] [Google Scholar]
- 9.Corey L, Spear P G. Infections with herpes simplex viruses (2) N Engl J Med. 1986;314:749–757. doi: 10.1056/NEJM198603203141205. [DOI] [PubMed] [Google Scholar]
- 10.Cowdry E V, Nicholson F M. Inclusion bodies in experimental herpetic infection of rabbits. J Exp Med. 1923;38:695–706. doi: 10.1084/jem.38.6.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daumas-Duport C, Scheithauer B, O’Fallon J, Kelly P. Grading of astrocytomas. A simple and reproducible method. Cancer. 1988;62:2152–2165. doi: 10.1002/1097-0142(19881115)62:10<2152::aid-cncr2820621015>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 12.Demaerel P, Wilms G, Robberecht W, Johannik K, Van Hecke P, Carton H, Baert A L. MRI of herpes simplex encephalitis. Neuroradiology. 1992;34:490–493. doi: 10.1007/BF00598957. [DOI] [PubMed] [Google Scholar]
- 13.Dix R D, McKendall R R, Baringer J R. Comparative neurovirulence of herpes simplex virus type 1 strains after peripheral or intracerebral inoculation of BALB/c mice. Infect Immun. 1983;40:103–112. doi: 10.1128/iai.40.1.103-112.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dutt M K, Johnston I D. Computed tomography and EEG in herpes simplex encephalitis. Their value in diagnosis and prognosis. Arch Neurol. 1982;39:99–102. doi: 10.1001/archneur.1982.00510140033008. [DOI] [PubMed] [Google Scholar]
- 15.Field H J, Wildy P. The pathogenicity of thymidine kinase-deficient mutants of herpes simplex virus in mice. J Hyg. 1978;81:267–277. doi: 10.1017/s0022172400025109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldstein D J, Weller S K. Herpes simplex virus type 1-induced ribonucleotide reductase activity is dispensable for virus growth and DNA synthesis: isolation and characterization of an ICP6 lacZ insertion mutant. J Virol. 1988;62:196–205. doi: 10.1128/jvi.62.1.196-205.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gordon Y J, Gilden D M, Becker Y. HSV-1 thymidine kinase promotes virulence and latency in the mouse. Investig Ophthalmol Vis Sci. 1983;24:599–602. [PubMed] [Google Scholar]
- 18.Hershkovitz P. Two new species of night monkeys, genus Aotus (Cebidae, Platyrhini): a preliminary report on Aotus taxonomy. Am J Primatol. 1983;4:209–243. doi: 10.1002/ajp.1350040302. [DOI] [PubMed] [Google Scholar]
- 19.Hindmarsh T, Lindqvist M, Olding-Stenkvist E, Skoldenberg B, Forsgren M. Accuracy of computed tomography in the diagnosis of herpes simplex encephalitis. Acta Radiol Suppl. 1986;369:192–196. [PubMed] [Google Scholar]
- 20.Hunt R D. Herpesvirus simplex infection. In: Jones T C, Mohr U, Hunt R D, editors. Nonhuman primates I. Berlin, Germany: Springer-Verlag KG; 1993. pp. 82–86. [Google Scholar]
- 21.Jacobson J G, Leib D A, Goldstein D J, Bogard C L, Schaffer P A, Weller S K, Coen D M. A herpes simplex virus ribonucleotide reductase deletion mutant is defective for productive acute and reactivatable latent infections of mice and for replication in mouse cells. Virology. 1989;173:276–283. doi: 10.1016/0042-6822(89)90244-4. [DOI] [PubMed] [Google Scholar]
- 22.Jia W W, McDermott M, Goldie J, Cyander M, Tan J, Tufaro F. Selective destruction of gliomas in immunocompetent rats by thymidine kinase-defective herpes simplex virus type 1. J Natl Cancer Inst. 1994;86:1209–1215. doi: 10.1093/jnci/86.16.1209. [DOI] [PubMed] [Google Scholar]
- 23.Kahlon J, Lakeman F D, Ackermann M, Whitley R J. Human antibody response to herpes simplex virus-specific polypeptides after primary and recurrent infection. J Clin Microbiol. 1986;23:725–730. doi: 10.1128/jcm.23.4.725-730.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Katzin D S, Connor J D, Wilson L A, Sexton R S. Experimental herpes simplex infection in the owl monkey. Proc Soc Exp Biol Med. 1967;125:391–398. doi: 10.3181/00379727-125-32100. [DOI] [PubMed] [Google Scholar]
- 25.Kesari S, Randazzo B P, Valyi-Nagy T, Huang Q S, Brown S M, MacLean A R, Lee V, Trojanowski J Q, Fraser N W. Therapy of experimental human brain tumors using a neuroattenuated herpes simplex virus mutant. Lab Investig. 1995;73:636–648. [PubMed] [Google Scholar]
- 26.Kim T S, Halliday A L, Hedley-Whyte E T, Convery K. Correlates of survival and the Daumas-Duport grading system for astrocytomas. J Neurosurg. 1991;74:27–37. doi: 10.3171/jns.1991.74.1.0027. [DOI] [PubMed] [Google Scholar]
- 27.Knipe D M, Batterson W, Nosal C, Roizman B, Buchan A. Molecular genetics of herpes simplex virus. VI. Characterization of a temperature-sensitive mutant defective in the expression of all early viral gene products. J Virol. 1981;38:539–547. doi: 10.1128/jvi.38.2.539-547.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koelfen W, Freund M, Guckel F, Rohr H, Schultze C. MRI of encephalitis in children: comparison of CT and MRI in the acute stage with long-term follow-up. Neuroradiology. 1996;38:73–79. doi: 10.1007/BF00593228. [DOI] [PubMed] [Google Scholar]
- 29.Luna L G. Manual of histological staining methods of the Armed Forces Institute of Pathology. 3rd ed. New York, N.Y: McGraw-Hill; 1968. [Google Scholar]
- 30.MacLean A R, ul-Fareed M, Robertson L, Harland J, Brown S M. Herpes simplex virus type 1 deletion variants 1714 and 1716 pinpoint neurovirulence-related sequences in Glasgow strain 17+ between immediate early gene 1 and the ‘a’ sequence. J Gen Virol. 1991;72:631–639. doi: 10.1099/0022-1317-72-3-631. [DOI] [PubMed] [Google Scholar]
- 31.Mahaley M S, Jr, Mettlin C, Natarajan N, Laws E R, Jr, Peace B B. National survey of patterns of care for brain-tumor patients. J Neurosurg. 1989;71:826–836. doi: 10.3171/jns.1989.71.6.0826. [DOI] [PubMed] [Google Scholar]
- 32.Mann D R, Hilty M D. Antibody response to herpes simplex virus type 1 polypeptides and glycoproteins in primary and recurrent infection. Pediatr Res. 1982;16:176–180. doi: 10.1203/00006450-198203000-00003. [DOI] [PubMed] [Google Scholar]
- 33.Markert J M, Malick A, Coen D M, Martuza R L. Reduction and elimination of encephalitis in an experimental glioma therapy model with attenuated herpes simplex mutants that retain susceptibility to acyclovir. Neurosurgery. 1993;32:597–603. doi: 10.1227/00006123-199304000-00016. [DOI] [PubMed] [Google Scholar]
- 34.Martuza R L, Malick A, Markert J M, Ruffner K L, Coen D M. Experimental therapy of human glioma by means of a genetically engineered virus mutant. Science. 1991;252:854–856. doi: 10.1126/science.1851332. [DOI] [PubMed] [Google Scholar]
- 35.McGrath N, Anderson N E, Croxson M C, Powell K F. Herpes simplex encephalitis treated with acyclovir: diagnosis and long term outcome. J Neurol Neurosurg Psychiatry. 1997;63:321–326. doi: 10.1136/jnnp.63.3.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meignier B, Longnecker R, Mavromara-Nazos P, Sears A E, Roizman B. Virulence of and establishment of latency by genetically engineered deletion mutants of herpes simplex virus 1. Virology. 1988;162:251–254. doi: 10.1016/0042-6822(88)90417-5. [DOI] [PubMed] [Google Scholar]
- 37.Meignier B, Martin B, Whitley R J, Roizman B. In vivo behavior of genetically engineered herpes simplex viruses R7017 and R7020. II. Studies in immunocompetent and immunosuppressed owl monkeys (Aotus trivirgatus) J Infect Dis. 1990;162:313–321. doi: 10.1093/infdis/162.2.313. [DOI] [PubMed] [Google Scholar]
- 38.Melendez L V, Espana C, Hunt R D, Daniel M D, Garcia F G. Natural herpes simplex infection in the owl monkey (Aotus trivirgatus) Lab Anim Care. 1969;19:38–45. [PubMed] [Google Scholar]
- 39.Mineta T, Rabkin S D, Martuza R L. Treatment of malignant gliomas using ganciclovir-hypersensitive, ribonucleotide reductase-deficient herpes simplex viral mutant. Cancer Res. 1994;54:3963–3966. [PubMed] [Google Scholar]
- 40.Mineta T, Rabkin S D, Yazaki T, Hunter W D, Martuza R L. Attenuated multi-mutated herpes simplex virus-1 for the treatment of malignant gliomas. Nat Med. 1995;1:938–943. doi: 10.1038/nm0995-938. [DOI] [PubMed] [Google Scholar]
- 41.Mohr I, Gluzman Y. A herpesvirus genetic element which affects translation in the absence of the viral GADD34 function. EMBO J. 1996;15:4759–4766. [PMC free article] [PubMed] [Google Scholar]
- 42.Neils E W, Lukin R, Tomsick T A, Tew J M. Magnetic resonance imaging and computerized tomography scanning of herpes simplex encephalitis. Report of two cases. J Neurosurg. 1987;67:592–594. doi: 10.3171/jns.1987.67.4.0592. [DOI] [PubMed] [Google Scholar]
- 43.Nishiyama Y. Herpesvirus genes: molecular basis of viral replication and pathogenicity. Nagoya J Med Sci. 1996;59:107–119. [PubMed] [Google Scholar]
- 44.Pelosi E, Rozenberg F, Coen D M, Tyler K L. A herpes simplex virus DNA polymerase mutation that specifically attenuates neurovirulence in mice. Virology. 1998;252:364–372. doi: 10.1006/viro.1998.9447. [DOI] [PubMed] [Google Scholar]
- 45.Preston V G, Palfreyman J W, Duita B M. Identification of a herpes simplex virus type 1 polypeptide which is a component of the virus-induced ribonucleotide reductase. J Gen Virol. 1984;65:1457–1466. doi: 10.1099/0022-1317-65-9-1457. [DOI] [PubMed] [Google Scholar]
- 46.Pyles R B, Sawtell N M, Thompson R L. Herpes simplex virus type 1 dUTPase mutants are attenuated for neurovirulence, neuroinvasiveness, and reactivation from latency. J Virol. 1992;66:6706–6713. doi: 10.1128/jvi.66.11.6706-6713.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pyles R B, Thompson R L. Evidence that the herpes simplex virus type 1 uracil DNA glycosylase is required for efficient viral replication and latency in the murine nervous system. J Virol. 1994;68:4963–4972. doi: 10.1128/jvi.68.8.4963-4972.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pyles R B, Warnick R E, Chalk C L, Szanti B E, Parysek L M. A novel multiply-mutated HSV-1 strain for the treatment of human brain tumors. Hum Gene Ther. 1997;8:533–544. doi: 10.1089/hum.1997.8.5-533. [DOI] [PubMed] [Google Scholar]
- 49.Rabkin S D, Mineta T, Miyatake S, Yazaki T. Gene therapy: targeting tumor cells for destruction. Hum Cell. 1996;9:265–276. [PubMed] [Google Scholar]
- 50.Roizman B, Sears A E. Herpes simplex viruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1995. pp. 2231–2296. [Google Scholar]
- 51.Russell S J. Replicating vectors for gene therapy of cancer: risks, limitations and prospects. Eur J Cancer. 1994;30:1165–1171. doi: 10.1016/0959-8049(94)90477-4. [DOI] [PubMed] [Google Scholar]
- 52.Schmidbauer M, Podreka I, Wimberger D, Oder W, Koch G, Wenger S, Goldenberg G, Asenbaum S, Deecke L. SPECT and MR imaging in herpes simplex encephalitis. J Comput Assisted Tomogr. 1991;15:811–815. doi: 10.1097/00004728-199109000-00016. [DOI] [PubMed] [Google Scholar]
- 53.Schroth G, Gawehn J, Thron A, Vallbracht A, Voigt K. Early diagnosis of herpes simplex encephalitis by MRI. Neurology. 1987;37:179–183. doi: 10.1212/wnl.37.2.179. [DOI] [PubMed] [Google Scholar]
- 54.Sköldenberg B. Herpes simplex encephalitis. Scand J Infect Dis Suppl. 1996;100:8–13. [PubMed] [Google Scholar]
- 55.Soong S J, Watson N E, Caddell G R, Alford C A, Jr, Whitley R J. Use of brain biopsy for diagnostic evaluation of patients with suspected herpes simplex encephalitis: a statistical model and its clinical implications. NIAID Collaborative Antiviral Study Group. J Infect Dis. 1991;163:17–22. doi: 10.1093/infdis/163.1.17. [DOI] [PubMed] [Google Scholar]
- 56.Taha M Y, Clements G B, Brown S M. A variant of herpes simplex virus type 2 strain HG52 with a 1.5 kb deletion in RL between 0 to 0.02 and 0.81 to 0.83 map units is non-neurovirulent for mice. J Gen Virol. 1989;70:705–716. doi: 10.1099/0022-1317-70-3-705. [DOI] [PubMed] [Google Scholar]
- 57.Tenser R B. Intracerebral inoculation of newborn and adult mice with thymidine kinase-deficient mutants of herpes simplex virus type 1. J Infect Dis. 1983;147:956. doi: 10.1093/infdis/147.5.956. [DOI] [PubMed] [Google Scholar]
- 58.Thompson R L, Wagner E K, Stevens J G. Physical location of a herpes simplex virus type-1 gene function(s) specifically associated with a 10 million-fold increase in HSV neurovirulence. Virology. 1983;131:180–192. doi: 10.1016/0042-6822(83)90544-5. [DOI] [PubMed] [Google Scholar]
- 59.Tien R D, Felsberg G J, Osumi A K. Herpesvirus infections of the CNS: MR findings. AJR Am J Roentgenol. 1993;161:167–176. doi: 10.2214/ajr.161.1.8390790. [DOI] [PubMed] [Google Scholar]
- 60.Toda M, Rabkin S D, Martuza R L. Treatment of human breast cancer in a brain metastatic model by G207, a replication-competent multimutated herpes simplex virus 1. Hum Gene Ther. 1998;9:2177–2185. doi: 10.1089/hum.1998.9.15-2177. [DOI] [PubMed] [Google Scholar]
- 61.Valyi-Nagy T, Fareed M U, O’Keefe J S, Gesser R M, MacLean A R, Brown S M, Spivack J G, Fraser N W. The herpes simplex virus type 1 strain 17+ gamma 34.5 deletion mutant 1716 is avirulent in SCID mice. J Gen Virol. 1994;75:2059–2063. doi: 10.1099/0022-1317-75-8-2059. [DOI] [PubMed] [Google Scholar]
- 62.Weller R E. Infectious and noninfectious disease of owl monkeys. In: Baer J F, Weller R E, Kakoma I, editors. Aotus: the owl monkey. San Diego, Calif: Academic Press; 1994. pp. 177–215. [Google Scholar]
- 63.Whitley R J. Herpes simplex viruses. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1995. pp. 2297–2342. [Google Scholar]
- 64.Whitley R J, Alford C A, Hirsch M S, Schooley R T, Luby J P, Aoki F Y, Hanley D, Nahmias A J, Soong S J. Vidarabine versus acyclovir therapy in herpes simplex encephalitis. N Engl J Med. 1986;314:144–149. doi: 10.1056/NEJM198601163140303. [DOI] [PubMed] [Google Scholar]
- 65.Whitley R J, Kern E R, Chatterjee S, Chou J, Roizman B. Replication, establishment of latency, and induced reactivation of herpes simplex virus gamma 1 34.5 deletion mutants in rodent models. J Clin Investig. 1993;91:2837–2843. doi: 10.1172/JCI116527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Whitley R J, Lakeman F. Herpes simplex virus infections of the central nervous system: therapeutic and diagnostic considerations. Clin Infect Dis. 1995;20:414–420. doi: 10.1093/clinids/20.2.414. [DOI] [PubMed] [Google Scholar]
- 67.Yamada Y, Kimura H, Morishima T, Daikoku T, Maeno K, Nishiyama Y. The pathogenicity of ribonucleotide reductase-null mutants of herpes simplex virus type 1 in mice. J Infect Dis. 1991;164:1091–1097. doi: 10.1093/infdis/164.6.1091. [DOI] [PubMed] [Google Scholar]
- 68.Yazaki T, Manz H J, Rabkin S D, Martuza R L. Treatment of human malignant meningiomas by G207, a replication-competent multimutated herpes simplex virus 1. Cancer Res. 1995;55:4752–4756. [PubMed] [Google Scholar]
- 69.Zweerink H J, Corey L. Virus-specific antibodies in sera from patients with genital herpes simplex virus infection. Infect Immun. 1982;37:413–421. doi: 10.1128/iai.37.2.413-421.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]