Abstract
Prostate-specific membrane antigen (PSMA) imaging has become a mainstay diagnostic tool in staging unfavorable primary prostate cancer (PC) and identifying sites of recurrence in previously treated PC. One of the biggest pitfalls of PSMA imaging is rapid radionucleotide excretion in the urine via the kidneys, ureters, and bladder. The positron-emission tomography (PET) images obtained show increased radiotracer activity in these structures, which can occlude or even mimic true malignant disease. We describe the diagnostic challenges encountered in differentiating benign versus malignant disease with PSMA scans. A 78-year-old male presented to our outpatient radiation oncology office with high-risk prostate cancer. His medical history was significant for ulcerative colitis (UC). Magnetic resonance imaging (MRI) revealed an enlarged prostate and a Prostate Imaging Reporting and Data System (PI-RADS) class 4 lesion. A subsequent transperineal biopsy confirmed unilateral Gleason 8 adenocarcinoma.
A PSMA PET scan was read as increased uptake in the right prostate and a left external iliac node. The patient, having been initially informed of a positive lymph node metastasis, sought a second opinion,resulting in a CT urogram that revealed physiologic ureteral uptake. We were thus able to avoid lymph node radiation and morbidity to the surrounding bowel, already chronically inflamed with ulcerative colitis. This study demonstrates the potential for misinterpretation of PSMA uptake in the ureter as lymph node metastases. We discuss how peri-uretic activity can hinder accurate visualization of pelvic lymph node metastases. This study highlights the need for careful image interpretation of PSMA uptake patterns in order to avoid diagnostic errors and unnecessary radiation to at-risk organs in prostate cancer management.
Keywords: lymph node, ureter, positron emission tomography, ct urogram, ulcerative colitis, psma, prostate cancer
Introduction
Prostate cancer is the third most prevalent cancer type in the United States, with an estimated 288,300 new cases among American males in 2023 [1]. Imaging techniques play an integral part in the diagnosis and staging of prostate cancer, impacting treatment decisions for patients. For many years, the gold standard of diagnostic imaging modality for prostate cancer was the prostate MRI. The Prostate Imaging Reporting and Data System (PI-RADS) system is a radiological multi-parametric way to stratify how malignant a prostatic lesion appears on MRI by numbering them from 1 to 5. A PI-RADS 1 score indicates very low suspicion that the lesion is malignant, while a PI-RADS 5 score indicates a very high suspicion [2].
Recent advancements in imaging have been facilitated by the development of novel radiotracers. Prostate-specific membrane antigen (PSMA) is a transmembrane protein, the expression of which is significantly increased in prostate cancer, and as such it can be targeted with radiotracers for use in positron-emission tomography (PET) scans [3]. These radiopharmaceuticals have been effective in detecting primary and metastatic prostate cancer. The use of PSMA PET/CT improves the staging of prostate cancer, as it demonstrates superior sensitivity and specificity for detecting metastases compared to conventional imaging [4]. Furthermore, PSMA-targeted theragnostic agents have shown impressive potential in detecting and treating metastatic prostate cancer [5,6]. Agents like Gallium-68 (68Ga)-PSMA-11 and 18F-DCFPyL have been approved by the FDA for staging unfavorable prostate cancer patients [7] and evaluating sites of recurrence following a rising prostate-specific antigen (PSA) after curative treatment [8]. F18-labeled agents provide a higher sensitivity with a higher spatial resolution due to their greater positron yield and lower positron energy [9]. These attributes make PSMA scans a highly effective tool in diagnosing PSMA avid loco-regional and metastatic disease. In addition, PSMA scans may aid in patient-specific design of pelvic radiation treatment volumes [10].
However, despite the diagnostic advances for prostate cancer, PSMA-targeted PET imaging is not without its challenges. Most radiolabeled pharmaceuticals used for PSMA imaging are excreted by the renal system. This can result in increased radiotracer uptake in the kidneys, ureters, bladder, and urethra, which can obscure true malignant lesions adjacent to these structures. This detail becomes especially important when considering that most recurrences after prostatectomy occur in the prostatic fossa at the vesicourethral anastomosis, increasing the risk of missed detection [11]. Conversely, physiological uptake can also result in a false positive, where the reader mistakes bladder or ureteral activity for cancer. This study underscores the importance of detailed clinical understanding and careful interpretation of the imaging characteristics of PSMA scans in order to avoid potential misdiagnoses and unnecessary interventions in prostate cancer management [12].
Case presentation
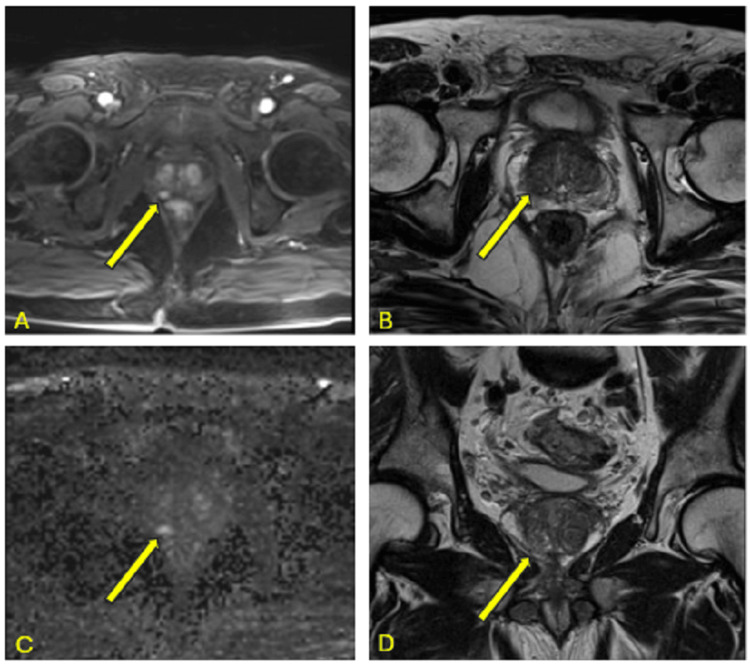
A 78-year-old Caucasian male with a medical history of ulcerative colitis (UC) presented with stage IIC prostate adenocarcinoma (T1cN0Mx). His prostate-specific antigen (PSA) level upon screening was 4.2 ng/mL. Digital rectal examination (DRE) was unremarkable. Magnetic resonance imaging (MRI) showed an enlarged prostate measuring 50 cc with a right mid-peripheral zone (PZ) lesion (0.6 cm), with dynamic post-contrast enhancement and abutment of the capsule without extraprostatic extension, identified as PI-RADS 4 (Figures 1A-1D).
Figure 1. Multiple sequences of MRI prostate.
Images show (A) axial T1-weighted MRI with volumetric interpolated breath-hold, (B) axial T2-weighted MRI, (C) axial MRI with diffusion-weighted imaging, and (D) coronal T2-weighted MRI.
A transperineal (TP) biopsy was performed, where microscopic analysis showed unilateral Gleason 4+4=8 adenocarcinoma involving the right prostate. Notably, three out of 11 sampled regions were involved, with up to 50% core involvement.
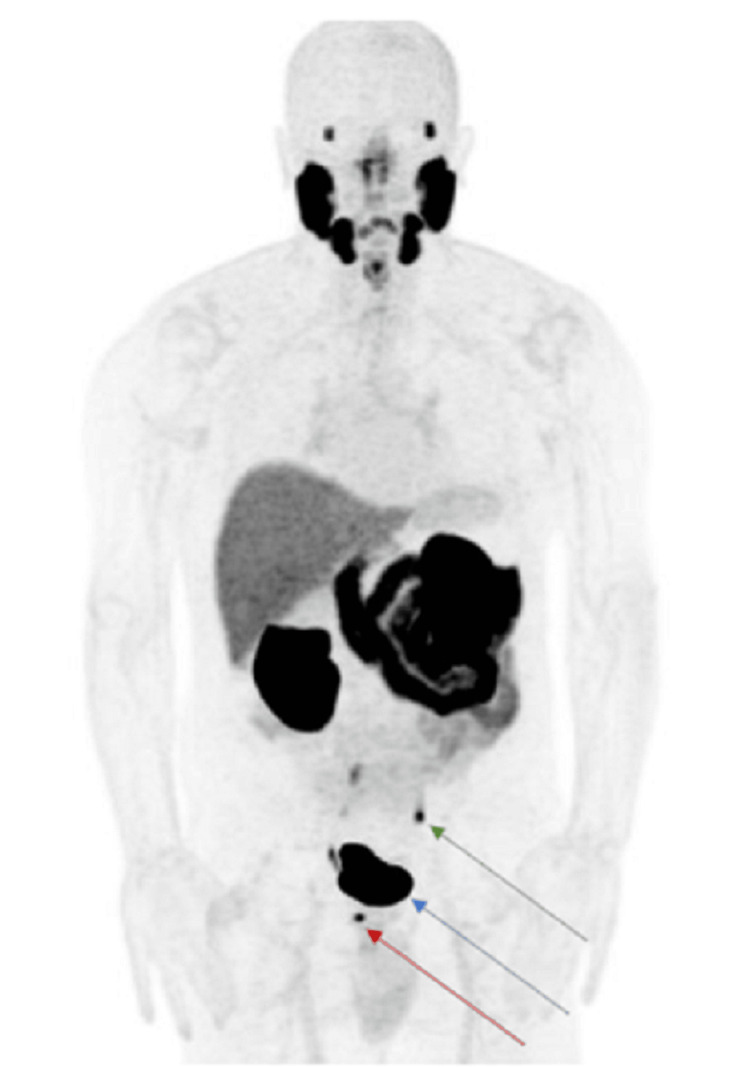
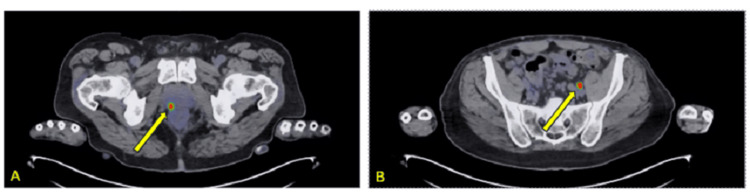
Upon the patient’s initial presentation with high-risk prostate cancer, a staging prostate-specific membrane antigen (PSMA) piflufolastat F 18 (Pylarify; Billerica, MA: Lantheus) scan was obtained [7,8]. The patient had piflufolastat F 18 injected intravenously. Approximately 60 minutes later, PET imaging was performed from the top of the skull to the mid-thighs. Additionally, a limited non-contrast CT was performed for the purposes of attenuation correction and anatomic correlation. Of note, the patient did not eat for 6 hours prior to the scan, though he did remain hydrated. He was not given a diuretic prior to or during the scan. The scan was read by an in-house faculty nuclear medicine physician. The impression was that there was radiotracer accumulation in the right posterior medial peripheral zone, consistent with the known cancer (Figures 2, 3A). There was also increased radiotracer accumulation at a single point adjacent to the left iliac vasculature, thought to represent a prominent, though not enlarged, left internal iliac lymph node (Figure 3B).
Figure 2. Frontal PSMA nuclear scan demonstrating intense radiotracer uptake in the prostate lesion (red arrow), bladder (blue arrow), and left ureter (green arrow).
PSMA: prostate-specific membrane antigen
Figure 3. Axial PSMA scan used to assist with staging.
Images (A) demonstrated increased uptake in the prostate and (B) demonstrated increased uptake in presumed left pelvic lymph node.
PSMA: prostate-specific membrane antigen
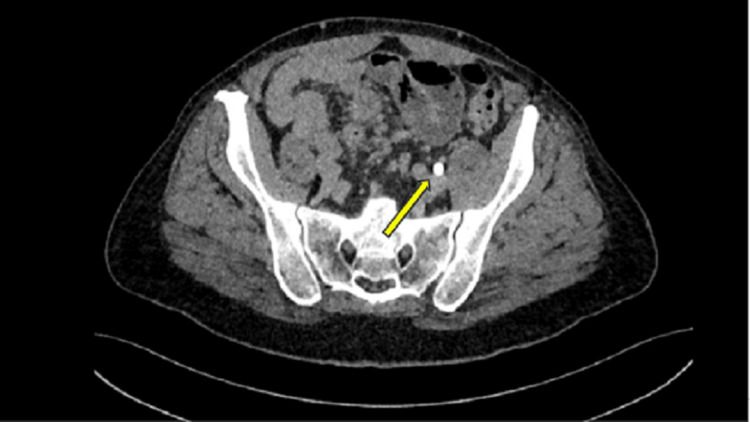
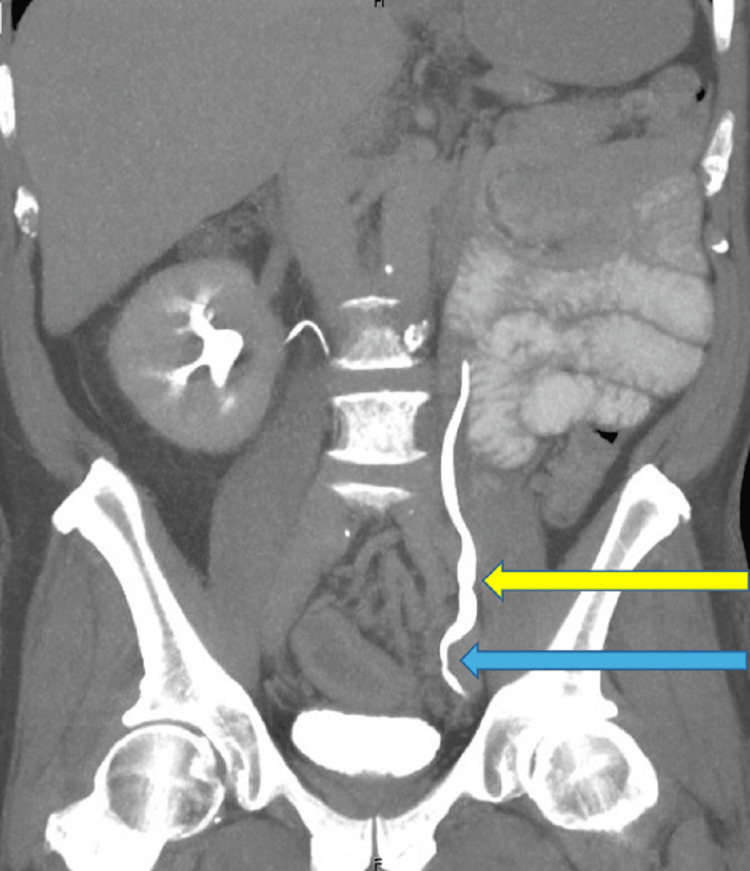
The radiation oncologist (SPC) recommended the patient receive 18 months of androgen deprivation therapy (ADT) combined with stereotactic body radiotherapy (SBRT) with supplemental pelvic radiotherapy, per institutional protocol [13]. The patient was advised that he would be at significant risk of high-grade gastrointestinal toxicity secondary to his ulcerative colitis and consequently sought a second opinion. The physician providing the second opinion ordered a CT Urogram. After injection of iodinated contrast, a dual-phase CT of the abdomen and pelvis was performed, and coronal and sagittal reformations were obtained. The scan revealed no evidence of pelvic lymphadenopathy to correspond with the findings seen on the previous PSMA scan (Figure 4). It showed narrowing of the left distal ureter (likely due to normal physiologic peristalsis), resulting in mild, temporary upstream hydroureter, radiographically corresponding with the increased uptake on the PSMA PET scan. Thus, the CT urogram revealed that what was initially thought to be a positive lymph node for metastasis turned out to be physiologic uptake in the ureter adjacent to the iliac vasculature (Figure 5).
Figure 4. Axial CT with contrast, delayed depiction, with an arrow indicating the ureter.
Figure 5. Coronal CT imaging with delayed contrast, displaying distal ureteral narrowing (blue arrow) resulting in mild hydroureter and tortuosity upstream (yellow arrow).
This patient’s case was reviewed at our institutional genitourinary (GU) tumor board, and the decision was made to proceed with long-course hormonal therapy with prostate SBRT alone to minimize late gastrointestinal side effects. The patient was amenable to this. He underwent placement of fiducials into his prostate for interfractional prostate motion management as well as SpaceOAR Vue Hydrogel between his prostate and his rectum to reduce rectal dose [14,15]. His prostate and organs at risk (OARs) were contoured. The planning treatment volume (PTV) was planned to the 83% isodose line and treated to a total dose of 36.25 Gy over five fractions of 7.25 Gy [16]. Of note, he was concurrently treated with relugolix before, during, and after his radiation treatment [17]. He was seen by the faculty radiation oncologist for his end-of-treatment visit, where he complained of mild urinary symptoms and was advised to stay on his pretreatment tamsulosin and to continue a low-fiber diet. He was scheduled for routine follow-ups moving forward. The patient completed prostate SBRT and to date has not had high-grade GI toxicity. Overall, he was pleased with his care.
Discussion
PSMA scans have greatly enhanced our ability to localize newly diagnosed and recurrent prostate cancer and to assess the extent of the malignancy [3,4]. Especially in the early stages of disease, accurate assessments of disease burden can be crucial for determining the appropriate approach to curative therapy. Our case exemplifies one of the common pitfalls of radionucleotide imaging - the false positive read caused by physiologic urinary excretion. Our patient was found to have radiotracer accumulation (standardized uptake value {SUV}: 13.19) in a presumed non-enlarged left-sided pelvic lymph node, which upon further diagnostic investigation, was likely to be urinary stasis caused by normal ureteral peristalsis. Treatment planning based on this scan alone would have resulted in whole-pelvis radiation and hormonal therapy-increasing the bowel toxicity in a male already suffering from ulcerative colitis. Unfortunately, urine radiotracer uptake can yield a similar uptake value as prostate cancer. A recent retrospective study that assessed 57 cases of biochemical recurrence after curative treatment found that the average SUV of recurrent prostate lesions found on 68Ga-PSMA-11 was 14±7.5 [18]. Potential approaches to reducing the impact of urine activity include prophylactic diuretic administration, large delays in scanning and/or multiple scan times, or as in this study, additional imaging (CT urography and/or MRI). However, these approaches may not adequately address the problem as well as add unnecessary costs and delays in management [19].
Inflammatory bowel disease (IBD), such as Crohn's disease or ulcerative colitis, is a chronic idiopathic inflammatory disorder that has historically been considered a soft contraindication to radiation therapy [20,21]. The chronic inflammation of the bowels can result in fistula formation, strictures, poor nutrient absorption, and diarrhea. As such, efforts are traditionally taken to limit the exposure of these patients to pelvic radiation therapy, which would further increase their risk of complications. It is thought that IBD amplifies the toxicity of radiation in a synergistic effect; however, the mechanism by which this occurs remains unclear.
If it is absolutely necessary to administer pelvic radiation to someone with IBD, the existing literature suggests that doing so while the IBD is dormant may offer the best chance of sparing extra toxicity. Data has emerged assessing the outcomes of these patients after definitive stereotactic body radiotherapy (SBRT) for prostate cancer. A recent single-institutional report of 31 patients with IBD who were treated with SBRT for prostate cancer had excellent quality of life outcomes at one year [22]. Moreover, a control-matched review of 39 males with inactive IBD who were treated with prostate SBRT showed that IBD was not associated with increased odds of late grade ≥2 GI or GU toxicity (median follow-up time of 89 months). There was, however, an increased acute risk of low-grade GI/GU toxicity in those with dormant IBD [23]. This data offers encouraging insight into the outcomes of those who received radiation with dormant IBD; however, it is still generally recommended to avoid pelvic radiation, if possible, during an acute IBD flare-up.
The ureter is a retroperitoneal structure that is susceptible to radiation toxicity. Radiation-induced changes to the ureters include endarteritis, ischemia, tissue contraction, and fibrosis. This can lead to significant genitourinary impairment in the form of ureteral atrophy and stenosis. These conditions impair tissue healing and lead to ureteral atrophy and contraction, ultimately resulting in ureteral stenosis [24-26]. Additionally, case reports have shown evidence that inflammation of the retroperitoneal bowel structures (e.g., ascending or descending colon) can increase risk of ureteral strictures [25]. Though there is a paucity of literature assessing the odds of developing ureteral strictures with IBD, it can be extrapolated that inflammation of the retroperitoneal colon, adjacent to the ureter, can increase the risk of ureteral strictures. There is limited data assessing the rates of ureteral injury with definitive prostate SBRT; however, there is evidence to suggest that traditional or moderately hypofractionated external beam radiotherapy (EBRT) can convey an increased risk of ureteral injury by 0.25% per year for up to 25 years [26]. It is therefore prudent to avoid pelvic radiotherapy if possible, especially when the patient’s comorbidities can increase the risk of radiation-associated toxicity to pelvic structures such as the bowel and ureters.
Definitive treatment of prostate cancer that has spread to the pelvic lymph nodes requires higher doses of radiation to the pelvis, sometimes incorporating a simultaneous-integrated boost (SIB) to the PET-positive lymph node [27]. A false-positive lymph node on a PSMA, as laid out in the present case, can therefore expose the patient to unnecessary treatment risk and morbidity. Instead of radiation to the prostate alone, these patients usually receive radiation to the prostate and the pelvic lymph nodes [28]. Specifically, they can receive anywhere between 45 Gy and 54 Gy to the pelvic lymph nodes as well as 19.5-21 Gy to the prostate with SBRT [14,29]. A positive pelvic lymph node can result in hormonal therapy for 24 months or longer, as opposed to the more tolerable 18 months if the cancer is confined to the prostate [30]. In addition, intensification with secondary hormonal agents would be considered [30]. Because we were able to discern that our patient’s PSMA scan yielded a false-positive lymph node, we were only able to avoid potentially morbid pelvic treatment and limit systemic therapy to a shorter, less intense course of hormonal therapy [30]. Due to adverse side effects of androgen deprivation therapy, it is advisable to avoid extended courses of hormonal therapy in elderly males.
There are currently three PSMA-targeting radiopharmaceuticals approved by the FDA, the most recent of which is 18F-rhPSMA-7.3 (Posluma; Oxford, UK: Blue Earth Diagnostics Ltd). A 2021 phase 1, open-label study assessed the biodistribution of 8F-rhPSMA-7.3 in six healthy volunteers at different time points after injection. Blood and urine volumes of the radiotracer were taken in regular increments between 30 seconds and 255 minutes after injection. The first post-injection urine sample taken 90 minutes after injection showed an average urinary excretion of 7.2% (range: 4.4-9.0%) [31]; this is significantly lower than the values noted for 18F-DCFPyL (11%) and 68Ga-PSMA-11 (11%) in the first 2 hours [32]. Because most PET protocols suggest imaging approximately 1 hour after administration of the radiotracer, we can infer that PET/CT performed with 18F-rhPSMA-7.3 has less diagnostic interference with urinary structures due to slower urinary excretion of 18F-rhPSMA-7.3 compared to other radiopharmaceuticals.
The efficacy of 18F-rhPSMA-7.3 PET/CT scans in detecting prostate cancer has been assessed in two phase III clinical trials. The LIGHTHOUSE trial looked at males with newly diagnosed unfavorable prostate cancer who were planned to undergo a prostatectomy with pelvic lymph node dissection (PLND) [33,34], and the SPOTLIGHT trial assessed the detection rate of biochemically recurrent prostate cancer [35-37]. As in the present case, patients in these trials were not given diuretics, and PET imaging was performed 50-70 minutes after administration of the radiopharmaceutical. A post-hoc analysis of these two trials examined 712 of the PSMA PET scans performed on their patients. By expert opinion, 96% of the scans were considered to be unobscured by physiologic urinary activity, and ureteric activity was absent in >50% of scans altogether [38,39]. While these results are promising, it is important to bear in mind that these studies were not designed to prove 18F-rhPSMA-7.3 superiority over other radiopharmaceuticals, and so SUV comparisons between modalities must be made with caution.
Conclusions
This study reinforces the challenges of singularly using PSMA PET scans in prostate cancer management. It draws attention to the diagnostic difficulties clinicians may face when differentiating between periureteral activity and malignancy, which can hinder accurate visualization of pelvic lymph node metastases. Erroneous interpretations can impact healthcare outcomes regarding radiation therapy toxicities. A thorough understanding of the various patterns of PSMA uptake is vital for minimizing errors in image interpretation. An approach that concurrently utilizes CT urograms can offer a valuable adjunct to assist in cases of a difficult diagnosis. The case presented here underlines the necessity of careful interpretation and the importance of developing multimodal approaches to ensure proper treatment for patients with prostate cancer.
Acknowledgments
The authors would like to acknowledge the significant contributions of Deepak Kumar, MD, of North Carolina Central University for his assistance in project design and manuscript writing. The authors would also like to acknowledge Dr. Johanna Cecelic, MD, of the University of Maryland for further elucidating the urologic elements of this paper and for manuscript editing.
Disclosures
Human subjects: Consent was obtained or waived by all participants in this study. Institutional Review Board of MedStar Georgetown University issued approval #2009-510.
Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following:
Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work.
Financial relationships: Sean Collins declare(s) personal fees from Accuray. Clinical consultant for Accuray.
Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Author Contributions
Concept and design: Tim Kearney, Sean Collins, Lauren Ching, Alan L. Zwart, Simeng Suy, Giuseppe Esposito
Acquisition, analysis, or interpretation of data: Tim Kearney, Sean Collins, Lauren Ching, Matthew Bourne, Karbi Choudhury, Alan L. Zwart, Malika T. Danner, Simeng Suy
Drafting of the manuscript: Tim Kearney, Sean Collins, Lauren Ching, Alan L. Zwart, Malika T. Danner, Simeng Suy
Critical review of the manuscript for important intellectual content: Tim Kearney, Sean Collins, Matthew Bourne, Karbi Choudhury, Alan L. Zwart, Malika T. Danner, Simeng Suy, Giuseppe Esposito
Supervision: Sean Collins
References
- 1.Cancer statistics, 2023. Siegel RL, Miller KD, Wagle NS, Jemal A. CA Cancer J Clin. 2023;73:17–48. doi: 10.3322/caac.21763. [DOI] [PubMed] [Google Scholar]
- 2.Prostate imaging reporting and data system version 2.1: 2019 update of prostate imaging reporting and data system version 2. Turkbey B, Rosenkrantz AB, Haider MA, et al. Eur Urol. 2019;76:340–351. doi: 10.1016/j.eururo.2019.02.033. [DOI] [PubMed] [Google Scholar]
- 3.PSMA PET/CT for primary staging of prostate cancer - an updated overview. Jochumsen MR, Bouchelouche K. Semin Nucl Med. 2024;54:39–45. doi: 10.1053/j.semnuclmed.2023.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Traditional and novel imaging modalities for advanced prostate cancer: a critical review. Hill S, Kassam F, Verma S, Sidana A. Urol Ann. 2023;15:249–255. doi: 10.4103/UA.UA_170_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.PSMA theragnostics for metastatic castration resistant prostate cancer. Song H, Guja KE, Iagaru A. Transl Oncol. 2022;22 doi: 10.1016/j.tranon.2022.101438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.FDA approves second PSMA-targeted PET imaging drug for men with prostate cancer. 2021. https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-second-psma-targeted-pet-imaging-drug-men-prostate-cancer. https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-second-psma-targeted-pet-imaging-drug-men-prostate-cancer [DOI] [PubMed]
- 7.A phase 2/3 prospective multicenter study of the diagnostic accuracy of prostate specific membrane antigen PET/CT with (18)F-DCFPyL in prostate cancer patients (OSPREY) Pienta KJ, Gorin MA, Rowe SP, et al. J Urol. 2021;206:52–61. doi: 10.1097/JU.0000000000001698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diagnostic performance of (18)F-DCFPyL-PET/CT in men with biochemically recurrent prostate cancer: results from the CONDOR phase III, multicenter study. Morris MJ, Rowe SP, Gorin MA, et al. Clin Cancer Res. 2021;27:3674–3682. doi: 10.1158/1078-0432.CCR-20-4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prostate cancer-PET imaging update. Jetty S, Loftus JR, Patel A, Gupta A, Puri S, Dogra V. Cancers (Basel) 2023;15 doi: 10.3390/cancers15030796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.(68) Ga-PSMA-PET for radiation treatment planning in prostate cancer recurrences after surgery: individualized medicine or new standard in salvage treatment. Habl G, Sauter K, Schiller K, Dewes S, Maurer T, Eiber M, Combs SE. Prostate. 2017;77:920–927. doi: 10.1002/pros.23347. [DOI] [PubMed] [Google Scholar]
- 11.Endorectal magnetic resonance imaging at 1.5 Tesla to assess local recurrence following radical prostatectomy using T2-weighted and contrast-enhanced imaging. Cirillo S, Petracchini M, Scotti L, et al. Eur Radiol. 2009;19:761–769. doi: 10.1007/s00330-008-1174-8. [DOI] [PubMed] [Google Scholar]
- 12.Revisiting prostate cancer recurrence with PSMA PET: atlas of typical and atypical patterns of spread. Barbosa FG, Queiroz MA, Nunes RF, Viana PC, Marin JF, Cerri GG, Buchpiguel CA. Radiographics. 2019;39:186–212. doi: 10.1148/rg.2019180079. [DOI] [PubMed] [Google Scholar]
- 13.Intensity modulated radiation therapy with stereotactic body radiation therapy boost for unfavorable prostate cancer: five-year outcomes. Carrasquilla M, Sholklapper T, Pepin AN, et al. Front Oncol. 2023;13 doi: 10.3389/fonc.2023.1240939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Utilization of iodinated SpaceOAR Vue during robotic prostate stereotactic body radiation therapy (SBRT) to identify the rectal-prostate interface and spare the rectum: a case report. Conroy D, Becht K, Forsthoefel M, et al. Front Oncol. 2020;10 doi: 10.3389/fonc.2020.607698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ano-rectal wall dose-surface maps localize the dosimetric benefit of hydrogel rectum spacers in prostate cancer radiotherapy. Vanneste BG, Buettner F, Pinkawa M, Lambin P, Hoffmann AL. Clin Transl Radiat Oncol. 2019;14:17–24. doi: 10.1016/j.ctro.2018.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stereotactic body radiation therapy (SBRT) for clinically localized prostate cancer: the Georgetown University experience. Chen LN, Suy S, Uhm S, et al. Radiat Oncol. 2013;8 doi: 10.1186/1748-717X-8-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Early biochemical outcomes following neoadjuvant/adjuvant relugolix with stereotactic body radiation therapy for intermediate to high risk prostate cancer. Gallagher L, Xiao J, Hsueh J, et al. Front Oncol. 2023;13 doi: 10.3389/fonc.2023.1289249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.(68)Ga PSMA-11 PET with CT urography protocol in the initial staging and biochemical relapse of prostate cancer. Iravani A, Hofman MS, Mulcahy T, Williams S, Murphy D, Parameswaran BK, Hicks RJ. Cancer Imaging. 2017;17 doi: 10.1186/s40644-017-0133-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Comparison of early imaging and imaging 60 min post-injection after forced diuresis with furosemide in the assessment of local recurrence in prostate cancer patients with biochemical recurrence referred for 68Ga-PSMA-11 PET/CT. Bayerschmidt S, Uprimny C, Kroiss AS, et al. Diagnostics (Basel) 2021;11 doi: 10.3390/diagnostics11071191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Low toxicity in inflammatory bowel disease patients treated with abdominal and pelvic radiation therapy. White EC, Murphy JD, Chang DT, Koong AC. Am J Clin Oncol. 2015;38:564–569. doi: 10.1097/COC.0000000000000010. [DOI] [PubMed] [Google Scholar]
- 21.Acute and late radiotherapy toxicity in patients with inflammatory bowel disease. Song DY, Lawrie WT, Abrams RA, Kafonek DR, Bayless TM, Welsh JS, DeWeese TL. Int J Radiat Oncol Biol Phys. 2001;51:455–459. doi: 10.1016/s0360-3016(01)01629-7. [DOI] [PubMed] [Google Scholar]
- 22.Stereotactic body radiation therapy for the treatment of localized prostate cancer in men with underlying inflammatory bowel disease. Lischalk JW, Blacksburg S, Mendez C, et al. Radiat Oncol. 2021;16 doi: 10.1186/s13014-021-01850-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Toxicity after stereotactic body radiation therapy for prostate cancer in patients with inflammatory bowel disease: a multi-institutional matched case-control series. Juarez JE, Romero T, Mantz CA, et al. Adv Radiat Oncol. 2021;6 doi: 10.1016/j.adro.2021.100759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.The association between inflammatory bowel disease and prostate cancer risk: a meta-analysis. Ge Y, Shi Q, Yao W, Cheng Y, Ma G. Prostate Cancer Prostatic Dis. 2020;23:53–58. doi: 10.1038/s41391-019-0177-7. [DOI] [PubMed] [Google Scholar]
- 25.Inflammatory stricture of the right ureter following perforated appendicitis: the first Indian report. Rajkumar JS, Ganesh D, Rajkumar A. J Minim Access Surg. 2016;12:375–377. doi: 10.4103/0972-9941.181324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Urinary adverse effects of pelvic radiotherapy. Liberman D, Mehus B, Elliott SP. Transl Androl Urol. 2014;3:186–195. doi: 10.3978/j.issn.2223-4683.2014.04.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Impact of (68)Ga-PSMA PET/CT on salvage radiotherapy planning in patients with prostate cancer and persisting PSA values or biochemical relapse after prostatectomy. Bluemel C, Linke F, Herrmann K, et al. EJNMMI Res. 2016;6 doi: 10.1186/s13550-016-0233-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patient-reported persistent symptoms after radiotherapy and association with quality of life for prostate cancer survivors. Spampinato S, Rancati T, Waskiewicz JM, et al. Acta Oncol. 2023;62:1440–1450. doi: 10.1080/0284186X.2023.2259597. [DOI] [PubMed] [Google Scholar]
- 29.Stereotactic body radiation therapy (SBRT) for high-risk prostate cancer: where are we now? Gonzalez-Motta A, Roach M 3rd. Pract Radiat Oncol. 2018;8:185–202. doi: 10.1016/j.prro.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 30.Prostate cancer, version 4.2023, NCCN Clinical Practice Guidelines in Oncology. Schaeffer E, Srinivas S, Nabil A, et al. J Natl Compr Canc Net. 2023;21:1067–1096. doi: 10.6004/jnccn.2023.0050. [DOI] [PubMed] [Google Scholar]
- 31.Safety, biodistribution, and radiation dosimetry of (18)F-rhPSMA-7.3 in healthy adult volunteers. Tolvanen T, Kalliokoski K, Malaspina S, et al. J Nucl Med. 2021;62:679–684. doi: 10.2967/jnumed.120.252114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Comparative study of the most commonly-used radiopharmaceuticals for PSMA prostate PET/CT imaging. Pasini E, Panagiotidis E, Zoglopitou LA, Kalathas T, Makridou A, Chatzipavlidou V. Hell J Nucl Med. 2022;25:83–87. doi: 10.1967/s002449912437. [DOI] [PubMed] [Google Scholar]
- 33.Diagnostic performance and safety of positron emission tomography with (18)F-rhPSMA-7.3 in patients with newly diagnosed unfavourable intermediate-to-very-high-risk prostate cancer: results from a phase 3, prospective, multicentre study (LIGHTHOUSE) Surasi DS, Eiber M, Maurer T, et al. Eur Urol. 2023;84:361–370. doi: 10.1016/j.eururo.2023.06.018. [DOI] [PubMed] [Google Scholar]
- 34.Utility of (18)F-rhPSMA-7.3 PET for imaging of primary prostate cancer and preoperative efficacy in N-staging of unfavorable intermediate-to-very high-risk patients validated by histopathology. Langbein T, Wang H, Rauscher I, et al. J Nucl Med. 2022;63:1334–1342. doi: 10.2967/jnumed.121.263440. [DOI] [PubMed] [Google Scholar]
- 35.Detection efficacy of (18)F-rhPSMA-7.3 PET/CT and impact on patient management in patients with biochemical recurrence of prostate cancer after radical prostatectomy and prior to potential salvage treatment. Rauscher I, Karimzadeh A, Schiller K, et al. J Nucl Med. 2021;62:1719–1726. doi: 10.2967/jnumed.120.260091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Diagnostic performance and safety of (18)F-rhPSMA-7.3 positron emission tomography in men with suspected prostate cancer recurrence: results from a phase 3, prospective, multicenter study (SPOTLIGHT) Jani AB, Ravizzini GC, Gartrell BA, et al. J Urol. 2023;210:411–412. doi: 10.1097/JU.0000000000003598. [DOI] [PubMed] [Google Scholar]
- 37.Quantitative and qualitative assessment of urinary activity of 18F-flotufolastat-PET/CT in patients with prostate cancer: a post hoc analysis of the lighthouse and spotlight studies. Kuo PH, Hermsen R, Penny R, Postema EJ. Mol Imaging Biol. 2024;26:53–60. doi: 10.1007/s11307-023-01867-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.PSMA PET/CT: joint EANM procedure guideline/SNMMI procedure standard for prostate cancer imaging 2.0. Fendler WP, Eiber M, Beheshti M, et al. Eur J Nucl Med Mol Imaging. 2023;50:1466–1486. doi: 10.1007/s00259-022-06089-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Early injection of furosemide increases detection rate of local recurrence in prostate cancer patients with biochemical recurrence referred for (68)Ga-PSMA-11 PET/CT. Uprimny C, Bayerschmidt S, Kroiss AS, et al. J Nucl Med. 2021;62:1550–1557. doi: 10.2967/jnumed.120.261866. [DOI] [PMC free article] [PubMed] [Google Scholar]